- 1Ganzhou Key Laboratory of Hematology, Department of Hematology, the First Affiliated Hospital of Gannan Medical University, Ganzhou, China
- 2Department of Oral Pathology, SGT Dental College Hospital and Research Institute, Gurugram, India
- 3College of Pharmacy and Health Sciences, St. John’s University, New York, NY, United States
- 4Department of Oral Pathology, Manipal College of Dental Sciences, Manipal Academy of Higher Education, Manipal, India
A burning sensation on eating spicy foods purportedly supports the role of capsaicin, an active component of chili peppers, in the etiology of oral submucous fibrosis (OSF). Although the mast cell mediators and activated P2X receptors induce a constant burning sensation through an ATP-dependent mechanism, it is the activation of the transient receptor potential vanilloid 1 (TRPV-1) receptor by capsaicin that aggravates it. The molecular basis for the burning pain in OSF is thus attributable to the activation of TRPV1. There is overwhelming evidence that confirms capsaicin has more of a protective role in attenuating fibrosis and is potentially therapeutic in reversing conditions linked to collagen accumulation. The activation of TRPV-1 by capsaicin increases intracellular calcium ([Ca2+]i), upregulates AMP-activated protein kinase (AMPK) and Sirtuin-1 (SIRT-1), to enrich endothelium-dependent vasodilation via endothelial nitric oxide synthase (eNOS). The induction of vasodilation induces antifibrotic effects by alleviating hypoxia. The antifibrotic effects of capsaicin are mediated through the upregulation of antioxidant enzymes, downregulation of inflammatory genes and suppression of new collagen fibril formation. Capsaicin also demonstrates an anticarcinogenic effect by upregulating the cytotoxic T cells and downregulating regulatory T cells through the inhibition of angiogenesis and promotion of apoptosis. Judicious administration of capsaicin with an appropriate delivery mechanism may have therapeutic benefits in reducing pain sensation, rendering antifibrotic effects, and preventing the malignant transformation of OSF. This paper provides an overview of the molecular basis of capsaicin and its therapeutic application as an antifibrotic and anticarcinogenic agent for the treatment of OSF.
Introduction
One of the telltale symptoms of oral submucous fibrosis (OSF) is a burning sensation in the mouth when eating spicy foods. Vesicular stomatitis/blister formation following the intake of foods laced with chilies (Capsicum annum and Capsicum frutescence) has led to the hypothesis that supports the etiological role of chilies in the development of OSF. This was reinforced by Sirsat and Khanolkar in 1960 (Sirsat and Khanolkar, 1960a; Sirsat and Khanolkar, 1960b), who were able to produce a mucosal connective tissue response similar to OSF by topical application of capsaicin (8-methyl-N-vanillyl-6-nonenamide), the active ingredient in chilies, in protein depleted and vitamin B deficient Wistar rats (Sirsat and Khanolkar, 1960a). Histologically, the epithelial changes and sub-epithelial hyalinization were reminiscent of OSF, but no changes were noted in the deeper connective tissue characteristic of fibrosis (Sirsat and Khanolkar, 1960a; Sirsat and Khanolkar, 1960b). Since these alterations were non-specific and indicative of local reaction to irritants, the authors were guarded in proposing chilies as the causative factor in OSF (Sirsat and Khanolkar, 1960a; Murti et al., 1995). Thus, they reported that ‘conditioned mucosa’ owing to vitamin and protein deficiency might make the mucosa susceptible to OSF. However, it has been observed that the dietary state may not be the primary factor as patients with advanced OSF have difficulty having a normal diet. Also, there are no interventional studies that support the protective effects of nutritional supplements against OSF (Cox and Walker, 1996).
Several investigators have supported the chili hypothesis (Sirsat and Khanolkar, 1960a; Sirsat and Pindborg, 1967; Shiau and Kwan, 1979) with some suggesting an allergic reaction to chilies (Sirsat and Khanolkar, 1960a; Pindborg et al., 1964; Pindborg and Singh, 1964; Pindborg and Sirsat, 1966; Sirsat and Pindborg, 1967; Pindborg et al., 1968; Shiau and Kwan, 1979). Hypersensitivity to chilies and a susceptible mucosa result in increased eosinophilia in tissue and serum suggesting that OSF arises as a result of an allergic reaction to chilies (Desa, 1957; Sirsat and Khanolkar, 1960a; Rao, 1962; Pindborg et al., 1964; Pindborg and Singh, 1964; Pindborg and Sirsat, 1966; Wahi et al., 1966; Sirsat and Pindborg, 1967; Pindborg et al., 1968; Hamner et al., 1971; Shiau and Kwan, 1979). In this paper, we provide an explanation for the continuous burning pain in OSF and attempt to correct the myth that chili is an etiologic factor in the development of OSF. We explain the molecular mechanisms of capsaicin-mediated antifibrotic and anticarcinogenic effects and draw attention to the therapeutic use of capsaicin in reversing fibrosis and preventing the malignant transformation of OSF.
Alternative Chili Hypothesis in the Development of OSF
The role of chilies in the development of OSF has been widely discussed in the literature. While the formation of vesicles and secondary eosinophilia has been considered to be the manifestation of an allergic response to chili (Pindborg and Singh, 1964; Pindborg and Sirsat, 1966), several investigators have refuted this association (Desa, 1957; Rao, 1962; Wahi et al., 1966; Hamner et al., 1971; Shiau and Kwan, 1979; Gupta et al., 1980). Even though the formation of vesicles occurred within 3 hours of consumption of food strongly laced with chili (Pindborg and Sirsat, 1966), only 30% of OSF patients showed above normal absolute eosinophil counts (Wahi et al., 1966).
In a case series analysis by Desa (1957) only 6/64 patients with OSF (9.4%) reported excessive use of chilies (Desa, 1957). Contesting the chili hypothesis, Wahi et al. (1966) showed that neither the amount nor the duration of chili consumption contributed to OSF progression (Wahi et al., 1966). Likewise, Hamner et al. (1971) failed to produce OSF in hamster cheek cells with topical application of 2% capsaicin dissolved in mineral oil or freshly ground chili powder 3 times per week for 2-years (Hamner et al., 1971). Furthermore, OSF is not reported in Mexico and South America, where the dietary intake of chilies equals or even exceeds that in India (Cox and Walker, 1996). Shiau and Kwan (1979) in their report on 35 OSF patients in Taiwan did not see any causal association between chili consumption and OSF (Shiau and Kwan, 1979). Gupta et al. (1980) in their 10-years follow-up of Indian villagers could not correlate the disease process to any other dietary factors except the use of areca nut (AN) (Gupta et al., 1980). Seedat and van Wyk (1988) could not find any association between OSF and the use of tobacco, lime, and chili among those who chewed AN (Seedat and van Wyk, 1988).
Continuous Burning Pain in OSF: Molecular Mechanisms
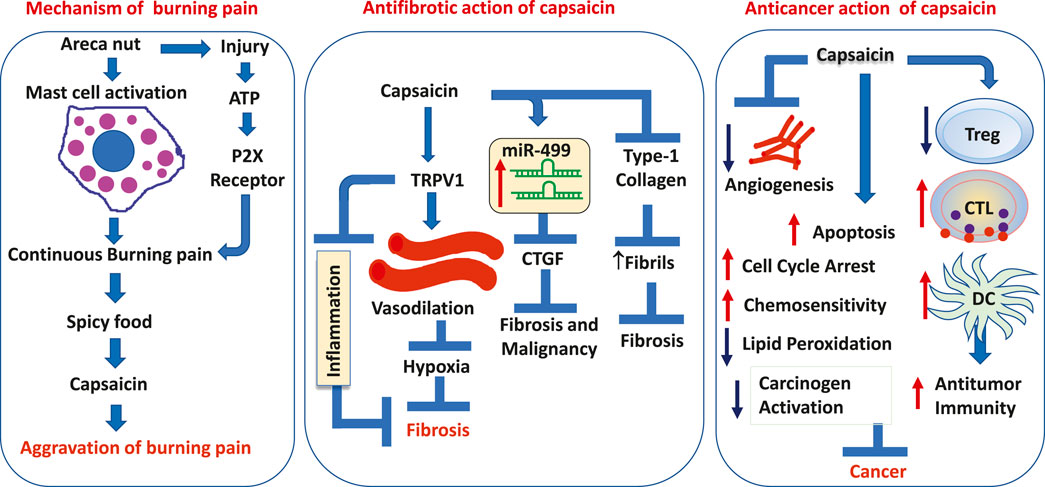
Patients with OSF suffer constant burning pain which is often aggravated by the intake of spicy food (Desa, 1957; Murti et al., 1995; Kumar et al., 2020). The burning pain is present in every stage of the disease and is significantly worse in clinical stage III (Kumar et al., 2020). Several attempts have been made to address the plausible mechanism and active agents that cause the burning mucosa in OSF (Lu and Insel, 2014; Feller et al., 2017; Yu et al., 2018; Mendivil et al., 2019). The burning pain that the patients experience in OSF, particularly when the buccal and labial mucosa is involved is alleviated with the use of local anesthetic rinses. It was thus suggested that the burning pain in OSF was due to the irritation of nerve endings and inflammation of the nerve secondary to changes in the connective tissue (Desa, 1957; Kumar et al., 2020). Such an altered mucosa showed a decrease in the number of small-diameter nerve fibers and the activation of transient receptor potential vanilloid 1 (TRPV1) receptors, P2X3 receptors, and nerve growth factor (NGF) (Feller et al., 2017).
Another explanation for the persistent burning pain in OSF relates to mast cell activation by areca nut and tobacco chewing (Pujari and Vidya, 2013; Kumar et al., 2020) (Figures 1A, B). With increased mast cell density (MCD) noted with the progression of the disease, it was proposed that areca nut by-products cause the release of mast cell mediators like histamine, serotonin, kallikrein, TNF-1α, IL-1, tryptase, and chymase. The release of mast cell mediators was reported to produce a burning sensation secondary to stomatitis, glossitis, and vesicle formation (Pujari and Vidya, 2013; Kumar et al., 2020) (Figure 1A). Further mast cell activity and degranulation resulted in fibrotic change, characterized by limited mouth opening (Pujari and Vidya, 2013) (Figure 1B).
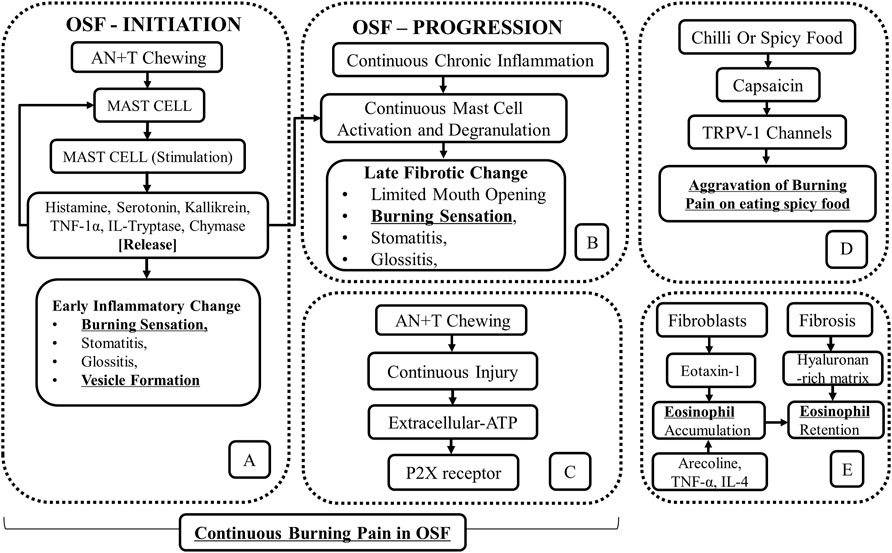
FIGURE 1. Schematic outline of the role of (A,B) mast cells in continuous burning pain and vesicles formation in OSF (Adapted and Modified from Pujari and Vidya, 2013), (C) P2X receptors in a continuous burning pain in OSF, (D) chili or spicy food in aggravation of burning pain in OSF. (E) The accumulation of eosinophils in OSF as a consequence of the process of fibrosis and as a result of irritation by areca nut byproducts.
The molecular mechanism for the burning sensation in OSF has been attributed to the activation of the P2X receptor. While it was noted that fibrosis is mediated by P2X receptors present on fibroblasts in an autocrine manner (Lu and Insel, 2014), their presence on nerve fibers (Feller et al., 2017), evoked a continuous burning pain due to the activation of the P2X receptor by extracellular ATP (Lu and Insel, 2014; Feller et al., 2017). The latter is released through connexin (Cxs)/pannexin (Panx) hemichannels in areas of chronic tissue injury (Figure 1C) (Lu and Insel, 2014).
TRPV-1 Activation and the Aggravation of Burning Pain
Heat, capsaicin, and H+ ions present in acidic food are the known agonists of TRPV1 receptors, which mediate the sensation of burning pain (Yu et al., 2018). The best-known TRPV-1 activator among these stimulants is capsaicin, present in chilies and peppers (McCarty et al., 2015; Mendivil et al., 2019). The aggravation of burning pain in OSF by hot, spicy, and acidic foods has been ascribed to TRPV1 stimulation (Yu et al., 2018; Mendivil et al., 2019). It has been established that the TRPV-1 receptor activation by capsaicin may result in the aggravation of burning pain than capsaicin/spicy food being a precursor for the disease (Figure 1D)
Targetting the Pathways of Burning Pain in OSF
The burning pain in OSF can be alleviated by targetting the NGF pathway (Watson et al., 2008) and the P2X receptor pathway (Bernier et al., 2018). The drugs specifically targetting the P2X3 pathway like A317491, AF-219, IP5, and RO-85 can be envisaged as novel pharmacological agents for treating burning pain in OSF (Bernier et al., 2018). Several mast cell released factors like serotonin, kallikrein, histamine, tryptase, Substance-p, prostaglandins, TNF-α, IL-1 are the mediators of inflammatory pain (Sharma, 2005; Roman et al., 2014; Kempuraj et al., 2019; Paredes et al., 2019; Mailhot et al., 2020; Alexander et al., 2021; Salvo et al., 2021). The use of mast cell stabilizers like ketotifen (Meloto et al., 2021) can reduce the burning pain in OSF, by countering these mediators, besides having a beneficial effect on afflicted mucosa. The use of AZD5213 the inverse agonist of histamine three receptor and INCB38579 as an antagonist of histamine three receptor are the other therapeutic modalities for the control of pain in OSF (Shin et al., 2012; Alexander et al., 2021). The repeated application of capsaicin itself can lead to depletion of substance-p and receptor desensitization (Friedman et al., 2018).
Eosinophilia and its Significance in OSF
The accumulation of eosinophils in OSF tissues has been attributed to the presence of a hyaluronan-rich matrix, which tends to retain eosinophils in the areas of fibrosis (Wight and Potter-Perigo, 2011). The eosinophil accumulation in OSF is attributable to the release of eotaxin-1, a potent eosinophil chemoattractant, from the gingival fibroblasts due to areca nut chewing along with the release of TNF-α and IL-4. However, the fact that eosinophilia is present in only 30% of OSF cases does not support the role of chili as an allergen causing OSF. Instead, increased eosinophilia in OSF is a result of the fibrotic process rather than an allergic reaction. This explains the association between asthma and decreased lung function among betel chewers at an adjusted odds ratio of 2.05 with a 95% confidence interval (Wang TN et al., 2014) (Figure 1E).
The Role of Capsaicin as an Antifibrotic Agent
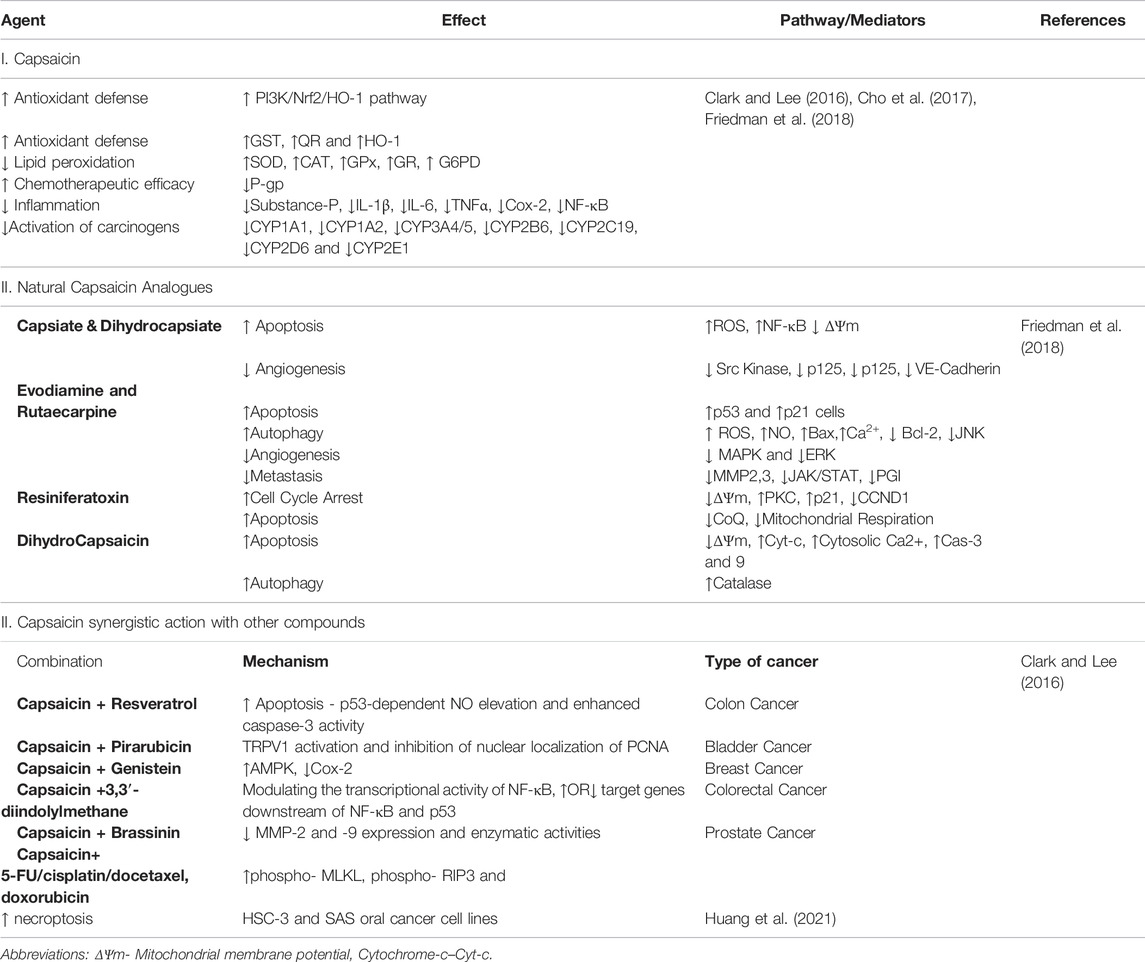
While the role of capsaicin in fibrosis has been challenged, several studies have shown that capsaicin has proven antifibrotic properties (Wang Q et al., 2014; Perumal et al., 2015; Choi et al., 2017; Mendivil et al., 2019; Sheng et al., 2020). Capsaicin activation of TRPV-1 increases intracellular calcium ([Ca2+]i), upregulates AMP-activated protein kinase (AMPK), and SIRT-1, which then upregulates endothelial nitric oxide synthase (eNOS) (McCarty et al., 2015), thus improving the endothelium-dependent vasodilation (EDV). Improved EDV could lead to alleviation of hypoxia and inhibition of fibrosis (Figure 2A).
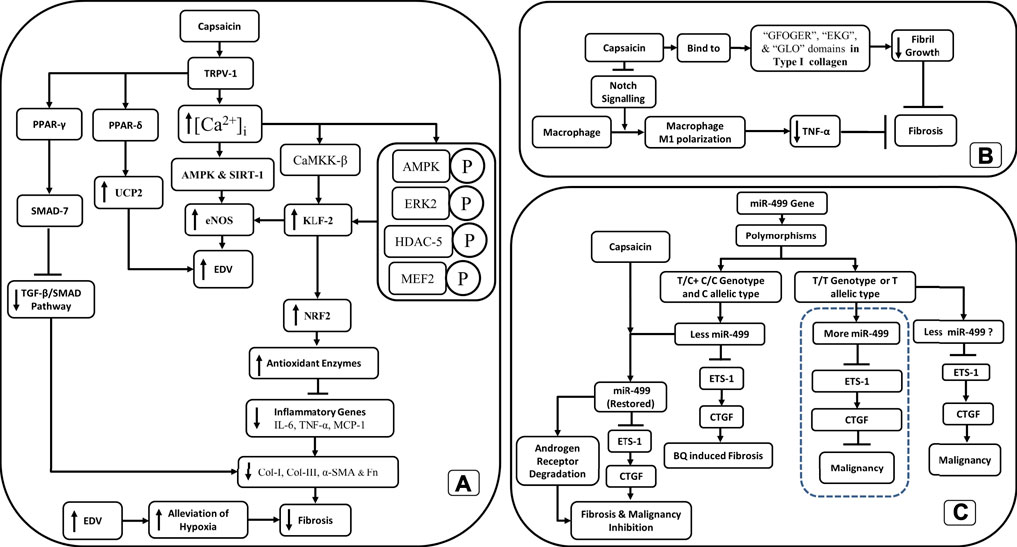
FIGURE 2. Schematic outline of the antifibrotic action of capsaicin through (A) TRPV-1 channels, (B) inhibition of fibril growth and decreased M1 macrophage polarization. (C) The restoration of miR-499 through capsaicin exposure inhibits both fibrosis and malignancy in OSF.
Increased [Ca2+]i also upregulates endothelial-specific transcription factor Krüppel-like Factor 2 (KLF2) through the activation of Ca2+/calmodulin-dependent protein kinase kinase-β (CaMKK-β) and downstream phosphorylation of AMP-activated protein kinase (AMPK), extracellular signal-regulated kinase 2 (ERK2), histone deacetylase-5 (HDAC-5), and myocyte enhancer factor-2 (MEF2) (McCarty et al., 2015). KLF2 also upregulates eNOS, antioxidant enzymes responsive to nuclear factor erythroid 2-related factor 2 (NRF2), and thrombomodulin. The upregulation of these antioxidant enzymes downregulates inflammatory genes like nuclear factor-kappa B (NF-κB), IL-6, TNF-α, and Monocyte Chemoattractant Protein-1 (MCP-1) (McCarty et al., 2015; Choi et al., 2017). TRPV-1 upregulates uncoupling protein 2 (UCP2) through peroxisome proliferator-activated receptor delta (PPAR-δ). Augmented UCP2 has proven to be important in promoting EDV in mice (McCarty et al., 2015). Besides that, peroxisome proliferator-activated receptor gamma (PPAR-γ) is upregulated (McCarty et al., 2015), which mediates its antifibrotic effects by inhibiting the TGF-β1/Smad pathway through SMAD-7 (Choi et al., 2017). Inhibition of the TGF-β1/Smad pathway decreases collagen I (Col-I), collagen III, a-SMA, and fibronectin (Fn) deposits in the tissue (Wang Q et al., 2014; Choi et al., 2017) (Figure 2A).
In the liver, capsaicin has been shown to attenuate fibrosis through the inhibition of notch signaling, inhibiting M1-polarization of macrophages, and reducing TNF-α secretion (Sheng et al., 2020). Capsaicin along with sulforaphane synergistically inhibited fibrosis of the liver by upregulating PPAR-γ and Nrf2. This, in turn, upregulated catalase and downregulated proinflammatory cytokines like TGF-β1, TNF-α, IL-6, and Type I collagen (Mendivil et al., 2019). Nrf2 interacts with the antioxidant response element of the catalase gene to enhance its production (Mendivil et al., 2019).
Similarly, in benzo(a)pyrene-induced lung cancer the levels of ECM components like collagen, elastin, uronic acid, and glycosaminoglycans such as hyaluronic acid, chondroitin sulfate, keratan sulfate, dermatan sulfate were elevated, but upon treatment with capsaicin, they were significantly reduced (Cho et al., 2017). TRPV-1 activation protects against renal and cardiac fibrosis through inhibition of the TGF-β/Smad2/3 signaling pathway (Wang Q. et al., 2014; Yu et al., 2018). In the peritoneal macrophages, the anti-inflammatory activity of capsaicin is facilitated by IkB-α degradation (Kim et al., 2003). In the retina, capsaicin renders a protective effect on ischemia-induced injury (Wang et al., 2017). On the contrary, conjunctival epithelial cells possessing TRPV1 channels seem to be profibrotic and proinflammatory (Yang et al., 2013; Khajavi et al., 2014). This is attributable to corneal epithelial cells containing the highest density of capsaicin-sensitive TRPV1 channels in the human body (Muller et al., 2003; Marfurt et al., 2010).
In oral mucosa TRPV1 channels functions as an environment sensing protein (Takahashi et al., 2020). However, the effect of capsaicin is dose-dependent with lower doses being antifibrotic, and higher doses being profibrotic (Yu et al., 2018). The antifibrotic mechanism of capsaicin and the pathways involved have been discussed in Table 1.
The effect of capsaicin on the properties of collagen was studied using a combination of biophysical and computational tools. It was found that capsaicin strongly suppressed collagen fibril formation, increased the stability of collagen fibers in tendons, and had no effect on the stability of collagen. While capsaicin did not promote the disassembly of collagen fibrils it moderately protected the collagen fibrils from enzymatic degradation. However, computational studies have revealed the functions of the aromatic and amide regions of capsaicin in the capsaicin-collagen interaction. Capsaicin is bound to the “GFOGER”, “EKG”, and “GLO” triple-helical conformational sequences within Collagen-I to inhibit collagen fibril formation (Perumal et al., 2015) (Figure 2B).
Capsaicin as an Anticarcinogenic Agent
The pro-carcinogenic effect of capsaicin in higher doses has been previously described (Murti et al., 1995) (Friedman et al., 2019). However, several studies have also demonstrated the potential anticancer effects of capsaicin (Ip et al., 2012; Gonzales et al., 2014; Clark and Lee, 2016; Cho et al., 2017; Dai et al., 2018; Friedman et al., 2018; Friedman et al., 2019; Mosqueda-Solis et al., 2021) and its non-genotoxic properties (Cho et al., 2017). Capsaicin was shown to reduce the incidence of oral epithelial dysplasia (OED) in a 4-nitroquinoline 1-oxide (4-NQO) induced tongue cancer model in male rats (Tanaka et al., 2002). Capsaicin prevents the transformation of OED to oral squamous cell carcinoma (OSCC) in mice through the reduction of anti-apoptotic marker B-cell lymphoma 2 (BCL2) (Mohamed and AlQarni, 2019). In a recent systematic review by Mosqueda-Solís et al., on capsaicin intake and oral carcinogenesis, a definitive protective effect of capsaicin on the development of oral cancer in mice was demonstrated (Mosqueda-Solis et al., 2021). Additional evidence of capsaicin being an anticarcinogenic agent comes from the fact that stomach cancer is less common in the Indian subcontinent where the consumption of spicy food is not unusual (Barad et al., 2014). This is particularly beneficial among cisplatin-resistant stomach cancer cells whereby cisplatin inducible Aurora-A kinase is degraded by capsaicin to induce apoptosis (Huh et al., 2011).
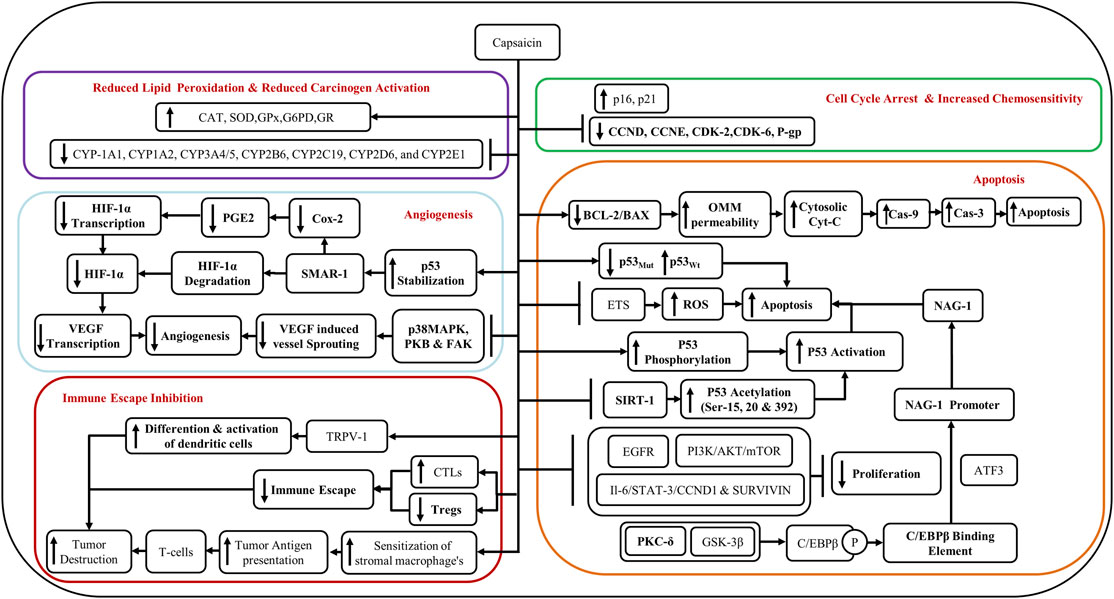
Capsaicin restores the wild-type p53 (p53Wt) and degrades mutant p53 (p53Mut) (Cho et al., 2017), and promotes apoptosis. Capsaicin decreases the BCL2/BAX ratio which in turn increases the permeability of the outer mitochondrial membrane (OMM) causing the release of cytochrome-c (Cyt-C) and its cytosolization. The cytosolic Cyt-C activates Caspase 9 (Cas-9) and Caspase 3 (Cas-3) which then cause apoptosis of the cell (Cho et al., 2017) (Figure 3).
Gonzales et al., in 2014 reported the anticancer effect of capsaicin on oral cancer cells through enhanced apoptosis, independent of the TRPV-1 receptor (Gonzales et al., 2014). Capsaicin has been shown to induce apoptosis in human tongue cancer cell lines SCC4, SCC25, and HSC3 via mitochondria-dependent and independent pathways (Ip et al., 2012; Gonzales et al., 2014). The apoptosis was mediated by p53 through enhanced phosphorylation at serine residues 15, 20, 392 and increased acetylation (through the inhibition of SIRT1) (Clark and Lee, 2016).
Another mechanism supporting the anticancer activity of capsaicin is via glycogen synthase kinase-3β (GSK3β)- and protein kinase C-δ (PKC-δ)-dependent phosphorylation of CCAAT enhancer-binding proteinβ (C/EBPβ). Phosphorylation of C/EBPβ augments the binding affinity of C/EBPβ onto non-steroidal anti-inflammatory drug (NSAID)-activated gene-1 (NAG-1) promoter, which activates the transcription of NAG-1 genes. Activating transcription factor 3 (ATF3) enhances the recruitment of C/EBPβ to the NAG-1 promoter by acting either as a bridge protein or by helping in the formation of a supramolecular complex along with other transcription factors. The activation of NAG-1 genes then triggers apoptosis (Lee et al., 2010; Clark and Lee, 2016) (Figure 3).
Additionally, capsaicin-induced a G0/G1 phase arrest in oral cancer cells through the reduction of cyclin-D (CCND), cyclin-dependent kinase 2 (CDK2), and cyclin-dependent kinase 6 (CDK6), and an increase in the levels of p21 and p16 (Ip et al., 2012). Furthermore, the anti-cancer effects of capsaicin have been demonstrated by the inhibition of Epidermal Growth Factor Receptor (EGFR) and PI3K/Akt/mTOR signaling pathways (Dai et al., 2018), and through IL-6/Signal Transducer and Activator of Transcription- 3 (STAT-3)/Cyclin D1 (CCND1) and survivin pathways (Cho et al., 2017) (Figure 3).
Capsaicin inhibits angiogenesis both in vitro and in vivo. It has been shown to cause the degradation of HIF-1α through the scaffold/matrix-associated region-binding protein 1 (SMAR1) and by the stabilization of p53 (Clark and Lee, 2016; Cho et al., 2017). SMAR1 upregulation leads to downregulation of Cox-2 and PGE-2 and reduced transcription of HIF-1α (Cho et al., 2017). Reduction in HIF-1α leads to reduced transcription of vascular endothelial growth factor (VEGF) and suppression of angiogenesis (Clark and Lee, 2016; Cho et al., 2017). Capsaicin inhibits VEGF-induced vessel sprouting and angiogenesis by the suppression of p38 MAPK, protein kinase B (PKB), and FAK pathways (Clark and Lee, 2016) (Figure 3).
The expression of the growth modulator, TRPV-1, was investigated in the pre-malignant and malignant lesions of the tongue. TRPV-1 expression was dramatically increased in all grades of oral squamous cell carcinoma (OSCC). An increase in TRPV-1 was also noted in the OSCC cell lines (Marincsak et al., 2009). This was, however, not due to the pro-carcinogenic effects of capsaicin as Ca++ channels were triggered minimally (evidence for the activation of the receptor) upon engagement of the receptor (Gonzales et al., 2014). Additionally, TRPV1 channels were inactive in primary tongue cancer-derived cell lines SCC4, SCC25, and HSC3 (Gonzales et al., 2014). Capsaicin seemingly had an anti-cancer effect in already transformed oral cells through a mechanism independent of TRPV1 (Gonzales et al., 2014), by acting as an antagonist of coenzyme-Q (Co-Q), inhibiting the electron transport system (ETS), thereby generating ROS, and inducing apoptosis (Figure 3). On the contrary, the immortalized keratinocyte cell line OKF6-TERT2 did demonstrate cytotoxicity to capsaicin via TRPV1 channels (Gonzales et al., 2014). Therefore, capsaicin has anticancer effects in the background of OSF via a TRPV1-dependent mechanism.
Hou et al. showed that the T/C + C/C genotypes and C allelic type for miR-499a produced reduced amounts of miR-499a when compared to the TT genotype or T allelic type (Hou et al., 2015). miR-499a negatively regulated the proto-oncogene ETS-1, which in turn upregulated the connective tissue growth factor (CTGF), a well-known orchestrator of fibrosis (Hou et al., 2015). The T/C + C/C genotypes and C allelic type for miR-499a had a greater risk for BQ-induced OSF than the TT genotype or T allelic type (Hou et al., 2015). The higher risk of fibrosis in T/C + C/C genotypes and C allelic type was due to decreased expression of miR-499a, which in turn caused the activation of ETS-1 and the upregulation of CTGF. The reduction in miR-499 expression was correlated with advanced stage and larger tumor size (Hou et al., 2015). Incidentally, mir-499a levels were restored by capsaicin (Zheng et al., 2015), which in turn caused a significant reduction in ETS-1 and CTGF. Thus, the restoration of miR-499 expression through capsaicin may avert the malignancy arising in OSF through downregulation of the ETS-1/CTGF pathway and degradation of androgen receptor (Wu et al., 2015; Zheng et al., 2015) (Figure 2C).
A reduction in the dendritic cell (DC) population in pre-malignant and malignant oral lesions with higher grades (Pellicioli et al., 2017), may promote malignancy through immune evasion. Several studies have shown the downregulation of immune response among those who chew areca nut (Wang et al., 2011; Wang et al., 2012; Chang et al., 2014; Lee et al., 2014). Areca nut extract (ANE) inhibits the differentiation and function of DCs (Wang et al., 2012; Lee et al., 2014). Furthermore, augmentation of myeloid-derived suppressor cells (MDSC) via continued areca nut use fosters malignancy through auxiliary downregulation of the immune response (Dolcetti et al., 2010; Wang et al., 2011). Notably, several studies have shown that capsaicin can restore the function of the immune system and stimulate the DCs (Gilardini Montani et al., 2015; Lee et al., 2016). Intertumoral depletion of CD8+T-cells/cytotoxic T cells (CTLs) and upregulation of regulatory T cells (Tregs) are also responsible for the immune escape of tumors (Cho et al., 2017). Capsaicin could counter the immune escape of tumors by upregulating the CTLs and downregulating Tregs. Moreover, capsaicin could also lead to enhanced sensitization of stromal macrophages to tumors and increase tumor antigen presentation thereby enhancing the destruction of tumors by T cells (Cho et al., 2017) (Figure 3).
Future Perspectives
The debate over whether capsaicin is pro-carcinogenic or anti-carcinogenic as well as if it exerts pro-fibrotic or anti-fibrotic effects continue. There is overwhelming evidence confirming that capsaicin, when used in its purest form and at an appropriate dose, is anti-fibrotic and anti-carcinogenic. Repeated application of capsaicin causes the depletion of substance-P (a tumor promoter), thereby reducing inflammation. It has been established that capsaicin activates PPARγ through TRPV-independent mechanisms by activating the liver X receptors (LXR), which inhibit the activation of the NF-κB pathway via LXR response elements (LXREs). Blocking NF-κB activation suppresses the production of inflammatory cytokines like IL-1β, IL-6, and TNFα (Cho et al., 2017).
Capsaicin is anti-carcinogenic and thereby inhibits microsomal phase-I xenobiotic-metabolizing enzymes like CYP-1A1, CYP1A2, CYP3A4/5, CYP2B6, CYP2C19, CYP2D6, and CYP2E1, leading to reduced activation of carcinogens. Capsaicin inhibits the mutagenicity of tobacco-specific carcinogen 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK) by blocking its metabolic activation by microsomal enzymes (Cho et al., 2017). Reduced lipid peroxidation through the restoration of antioxidant enzymes like catalase (CAT), superoxide dismutase (SOD), glutathione peroxidase (GPx), glucose-6-phosphate dehydrogenase (G6PD), and glutathione reductase (GR) represent some other novel anti-cancer mechanisms mediated by capsaicin (Cho et al., 2017). Additionally, capsaicin and evodiamine restore chemosensitivity by inhibiting the P-Glycoprotein (P-gp) and ATP-binding cassette transporter G2 (ABCG2), which is an important drug efflux pump mediating chemoresistance (Cho et al., 2017; Friedman et al., 2018). The treatment of HSC-3 and SAS oral cancer cell lines expressing a mutant p53 gene with capsaicin, augmented chemosensitivity of chemotherapeutic agents like 5-FU, cisplatin, docetaxel and doxorubicin through increased autophagy and deceased ribophorin-II. This resulted in increased necroptosis of these cells as evidenced by elevated levels of necroptotic markers, the phospho-Mixed Lineage Kinase domain-like (MLKL) and phospho-Receptor interacting protein kinase 3 (RIP3) (Huang et al., 2021). The anticancer potential of capsaicin and its analogs is discussed in detail in Table 2 and their chemical structure have been shown in Figure 4. Structurally capsaicin has been divided into three regions aromatic, amide and hydrophobic regions which are designated region A, region B and region C, respectively. Capsaicin has been shown to act as a classic agonist of the TRPV-1 channel, while evodiamine is a potent selective agonist and differs from capsaicin in region C. Capsiate and Dihydrocapsiate differ from capsaicin in region B. The occurrence of a carbon-carbon double bond in region C in capsaicin and the lack of it dihydrocapsaicin, chemically differentiate from each other (Friedman et al., 2018) (Figure 4).

FIGURE 4. Chemical structure and activity relationship of capsaicin and its analogy (A). Capsaicin, (B). Dihydrocapsaicin, (C). Capsiate, (D). Dihydrocapsiate, (E). Evodiamine, (F). Rutaecarpine.
Conclusion
The role of chilies or spicy food in the genesis of OSF has long been debated. Capsaicin has a protective role in attenuating fibrosis and is potentially therapeutic in reversing conditions linked to collagen accumulation. The continuous burning pain observed in the mucosa of OSF patients is due to the activation of mast cells and P2X receptors. This pain further gets aggravated upon the activation of TRPV-1 channels by capsaicin. Higher doses of capsaicin, however, would result in the deactivation of TRPV-1 channels. An appropriate dosage of capsaicin may ameliorate fibrosis and prevent the malignant progression of OSF. Judicious use of capsaicin and its delivery as a topical agent may have therapeutic benefits in reducing the sensation of pain through receptor desensitization, by optimizing anti-fibrotic function, thus preventing the malignant transformation of OSF.
Author Contributions
ZH and MS-Contributed to the conception and design of the study, and drafting of the article. AD-Contributed to the analysis and interpretation of data, revising it for important intellectual content. Z-SC and RR-Contributed to the conception and design of the study, analyzed and interpretation of data, and critically revised it for intellectual content. All authors approved the final version to be submitted.
Funding
This work was supported by the Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India sanction order No. “EMR/2017/002792".
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to Poonam Soi, for helping us with the language editing. (Career Vision®–SAT Prep, Gurugram, INDIA).
References
Alexander, R. C., Raudibaugh, K., Spierings, E. L. H., and Katz, N. (2021). A 3-way Cross-Over Study of Pregabalin, Placebo, and the Histamine 3 Receptor Inverse Agonist AZD5213 in Combination with Pregabalin in Patients with Painful Diabetic Neuropathy and Good Pain-Reporting Ability. Clin. J. Pain 37 (1), 38–42. doi:10.1097/AJP.0000000000000886
Barad, A. K., Mandal, S. K., Harsha, H. S., Sharma, B. M., and Singh, T. S. (2014). Gastric Cancer-A Clinicopathological Study in a Tertiary Care centre of North-eastern India. J. Gastrointest. Oncol. 5 (2), 142–147. doi:10.3978/j.issn.2078-6891.2014.003
Bernier, L. P., Ase, A. R., and Séguéla, P. (2018). P2X Receptor Channels in Chronic Pain Pathways. Br. J. Pharmacol. 175 (12), 2219–2230. doi:10.1111/bph.13957
Chang, L. Y., Lai, Y. L., Yu, T. H., Chen, Y. T., and Hung, S. L. (2014). Effects of Areca Nut Extract on Lipopolysaccharides-Enhanced Adhesion and Migration of Human Mononuclear Leukocytes. J. Periodontol. 85 (6), 859–867. doi:10.1902/jop.2013.130198
Cho, S. C., Lee, H., and Choi, B. Y. (2017). An Updated Review on Molecular Mechanisms Underlying the Anticancer Effects of Capsaicin. Food Sci. Biotechnol. 26 (1), 1–13. doi:10.1007/s10068-017-0001-x
Choi, J. H., Jin, S. W., Choi, C. Y., Kim, H. G., Lee, G. H., Kim, Y. A., et al. (2017). Capsaicin Inhibits Dimethylnitrosamine-Induced Hepatic Fibrosis by Inhibiting the TGF-β1/Smad Pathway via Peroxisome Proliferator-Activated Receptor Gamma Activation. J. Agric. Food Chem. 65 (2), 317–326. doi:10.1021/acs.jafc.6b04805
Clark, R., and Lee, S. H. (2016). Anticancer Properties of Capsaicin against Human Cancer. Anticancer Res. 36 (3), 837–843.
Cox, S. C., and Walker, D. M. (1996). Oral Submucous Fibrosis. A Review. Aust. Dent. J. 41 (5), 294–299. doi:10.1111/j.1834-7819.1996.tb03136.x
Dai, N., Ye, R., He, Q., Guo, P., Chen, H., and Zhang, Q. (2018). Capsaicin and Sorafenib Combination Treatment Exerts Synergistic Anti-hepatocellular Carcinoma Activity by Suppressing EGFR and PI3K/Akt/mTOR Signaling. Oncol. Rep. 40 (6), 3235–3248. doi:10.3892/or.2018.6754
Desa, J. V. (1957). Submucous Fibrosis of the Palate and Cheek. Ann. Otol. Rhinol. Laryngol. 66 (4), 1143–1159. doi:10.1177/000348945706600420
Dolcetti, L., Peranzoni, E., Ugel, S., Marigo, I., Fernandez Gomez, A., Mesa, C., et al. (2010). Hierarchy of Immunosuppressive Strength Among Myeloid-Derived Suppressor Cell Subsets is Determined by GM-CSF. Eur. J. Immunol. 40 (1), 22–35. doi:10.1002/eji.200939903
Feller, L., Fourie, J., Bouckaert, M., Khammissa, R. A. G., Ballyram, R., and Lemmer, J. (2017). Burning Mouth Syndrome: Aetiopathogenesis and Principles of Management. Pain Res. Manag. 2017, 1926269. doi:10.1155/2017/1926269
Friedman, J. R., Nolan, N. A., Brown, K. C., Miles, S. L., Akers, A. T., Colclough, K. W., et al. (2018). Anticancer Activity of Natural and Synthetic Capsaicin Analogs. J. Pharmacol. Exp. Ther. 364 (3), 462–473. doi:10.1124/jpet.117.243691
Friedman, J. R., Richbart, S. D., Merritt, J. C., Brown, K. C., Denning, K. L., Tirona, M. T., et al. (2019). Capsaicinoids: Multiple Effects on Angiogenesis, Invasion and Metastasis in Human Cancers. Biomed. Pharmacother. 118, 109317. doi:10.1016/j.biopha.2019.109317
Gilardini Montani, M. S., D'Eliseo, D., Cirone, M., Di Renzo, L., Faggioni, A., Santoni, A., et al. (2015). Capsaicin-mediated Apoptosis of Human Bladder Cancer Cells Activates Dendritic Cells via CD91. Nutrition 31 (4), 578–581. doi:10.1016/j.nut.2014.05.005
Gonzales, C. B., Kirma, N. B., De La Chapa, J. J., Chen, R., Henry, M. A., Luo, S., et al. (2014). Vanilloids Induce Oral Cancer Apoptosis Independent of TRPV1. Oral Oncol. 50 (5), 437–447. doi:10.1016/j.oraloncology.2013.12.023
Gupta, P. C., Mehta, F. S., Daftary, D. K., Pindborg, J. J., Bhonsle, R. B., Jalnawalla, P. N., et al. (1980). Incidence Rates of Oral Cancer and Natural History of Oral Precancerous Lesions in a 10-year Follow-Up Study of Indian Villagers. Community Dent. Oral Epidemiol. 8 (6), 283–333. doi:10.1111/j.1600-0528.1980.tb01302.x
Hamner, J. E., Mehta, F. S., Pindborg, J. J., and Daftary, D. K. (1971). Altered Staining Reaction of Connective Tissues in 53 Submucous Fibrosis Patients. J. Dent. Res. 50 (2), 388–392. doi:10.1177/00220345710500024601
Hou, Y. Y., Lee, J. H., Chen, H. C., Yang, C. M., Huang, S. J., Liou, H. H., et al. (2015). The Association between miR-499a Polymorphism and Oral Squamous Cell Carcinoma Progression. Oral Dis. 21 (2), 195–206. doi:10.1111/odi.12241
Huang, Y. C., Yuan, T. M., Liu, B. H., Liu, K. L., Wung, C. H., and Chuang, S. M. (2021). Capsaicin Potentiates Anticancer Drug Efficacy through Autophagy-Mediated Ribophorin II Downregulation and Necroptosis in Oral Squamous Cell Carcinoma Cells. Front. Pharmacol. 12, 676813. doi:10.3389/fphar.2021.676813
Huh, H. C., Lee, S. Y., Lee, S. K., Park, N. H., and Han, I. S. (2011). Capsaicin Induces Apoptosis of Cisplatin-Resistant Stomach Cancer Cells by Causing Degradation of Cisplatin-Inducible Aurora-A Protein. Nutr. Cancer 63 (7), 1095–1103. doi:10.1080/01635581.2011.607548
Ip, S. W., Lan, S. H., Huang, A. C., Yang, J. S., Chen, Y. Y., Huang, H. Y., et al. (2012). Capsaicin Induces Apoptosis in SCC-4 Human Tongue Cancer Cells through Mitochondria-dependent and -independent Pathways. Environ. Toxicol. 27 (6), 332–341. doi:10.1002/tox.20646
Kempuraj, D., Mentor, S., Thangavel, R., Ahmed, M. E., Selvakumar, G. P., Raikwar, S. P., et al. (2019). Mast Cells in Stress, Pain, Blood-Brain Barrier, Neuroinflammation and Alzheimer's Disease. Front. Cel Neurosci. 13, 54. doi:10.3389/fncel.2019.00054
Khajavi, N., Reinach, P. S., Skrzypski, M., Lude, A., and Mergler, S. (2014). L-carnitine Reduces in Human Conjunctival Epithelial Cells Hypertonic-Induced Shrinkage through Interacting with TRPV1 Channels. Cell Physiol. Biochem. 34 (3), 790–803. doi:10.1159/000363043
Kim, C. S., Kawada, T., Kim, B. S., Han, I. S., Choe, S. Y., Kurata, T., et al. (2003). Capsaicin Exhibits Anti-inflammatory Property by Inhibiting IkB-A Degradation in LPS-Stimulated Peritoneal Macrophages. Cell Signal. 15 (3), 299–306. doi:10.1016/s0898-6568(02)00086-4
Kumar, L. B., Mathew, P., Madhavan, N., Siddique, S., Kshetrimayum, N., and Iyer, K. (2020). Evaluation of Mast Cells and Burning Sensation in Various Stages of Oral Submucous Fibrosis. J. Oral. Biol. Craniofac. Res. 10 (4), 430–434. doi:10.1016/j.jobcr.2020.07.005
Lee, S. H., Krisanapun, C., and Baek, S. J. (2010). NSAID-activated Gene-1 as a Molecular Target for Capsaicin-Induced Apoptosis through a Novel Molecular Mechanism Involving GSK3beta, C/EBPbeta and ATF3. Carcinogenesis 31 (4), 719–728. doi:10.1093/carcin/bgq016
Lee, Y. Y., Lin, M. B., Cheng, C. F., Chang, L. Y., Liu, T. Y., and Hung, S. L. (2014). Inhibitory Effects of Areca Nut Extract on Expression of Complement Receptors and Fc Receptors in Human Neutrophils. J. Periodontol. 85 (8), 1096–1106. doi:10.1902/jop.2013.130498
Lee, Y. H., Im, S. A., Kim, J. W., and Lee, C. K. (2016). Vanilloid Receptor 1 Agonists, Capsaicin and Resiniferatoxin, Enhance MHC Class I-Restricted Viral Antigen Presentation in Virus-Infected Dendritic Cells. Immune Netw. 16 (4), 233–241. doi:10.4110/in.2016.16.4.233
Lu, D., and Insel, P. A. (2014). Cellular Mechanisms of Tissue Fibrosis. 6. Purinergic Signaling and Response in Fibroblasts and Tissue Fibrosis. Am. J. Physiol. Cel Physiol. 306 (9), C779–C788. doi:10.1152/ajpcell.00381.2013
Mailhot, B., Christin, M., Tessandier, N., Sotoudeh, C., Bretheau, F., Turmel, R., et al. (2020). Neuronal Interleukin-1 Receptors Mediate Pain in Chronic Inflammatory Diseases. J. Exp. Med. 217 (9), e20191430. doi:10.1084/jem.20191430
Marfurt, C. F., Cox, J., Deek, S., and Dvorscak, L. (2010). Anatomy of the Human Corneal Innervation. Exp. Eye Res. 90 (4), 478–492. doi:10.1016/j.exer.2009.12.010
Marincsak, R., Tóth, B. I., Czifra, G., Márton, I., Rédl, P., Tar, I., et al. (2009). Increased Expression of TRPV1 in Squamous Cell Carcinoma of the Human Tongue. Oral Dis. 15 (5), 328–335. doi:10.1111/j.1601-0825.2009.01526.x
McCarty, M. F., DiNicolantonio, J. J., and O'Keefe, J. H. (2015). Capsaicin May Have Important Potential for Promoting Vascular and Metabolic Health. Open Heart 2 (1), e000262. doi:10.1136/openhrt-2015-000262
Meloto, C. B., Ingelmo, P., Perez, E. V., Pitt, R., González Cárdenas, V. H., Mohamed, N., et al. (2021). Mast Cell Stabilizer Ketotifen Fumarate Reverses Inflammatory but Not Neuropathic-Induced Mechanical Pain in Mice. Pain Rep. 6 (2), e902. doi:10.1097/PR9.0000000000000902
Mendivil, E. J., Sandoval-Rodriguez, A., Zuñiga-Ramos, L. M., Santos-Garcia, A., and Armendariz-Borunda, J. (2019). Capsaicin and Sulforaphane Prevent Experimental Liver Fibrosis via Upregulation of Peroxisome Proliferator-Activated Receptor Gamma and Nuclear Factor (Erythroid-derived 2)-like 2. J. Funct. Foods 52, 382–388. doi:10.1016/j.jff.2018.11.014
Mohamed, M., and AlQarni, A. (2019). Chemopreventive Effect of Capsaicin in Experimentally Induced Hamster Buccal Pouch Carcinogenesis (Immunohistochemical Study Bcl-2). Egypt. Dental J. 65 (2), 1237–1243. doi:10.21608/edj.2019.72201
Mosqueda-Solis, A., Lafuente-Ibáñez de Mendoza, I., Aguirre-Urizar, J. M., and Mosqueda-Taylor, A. (2021). Capsaicin Intake and Oral Carcinogenesis: A Systematic Review. Med. Oral Patol. Oral Cir. Bucal 26 (2), e261–e268. doi:10.4317/medoral.24570
Muller, L. J., Marfurt, C. F., Kruse, F., and Tervo, T. M. (2003). Corneal Nerves: Structure, Contents and Function. Exp. Eye Res. 76 (5), 521–542. doi:10.1016/s0014-4835(03)00050-2
Murti, P. R., Bhonsle, R. B., Gupta, P. C., Daftary, D. K., Pindborg, J. J., and Mehta, F. S. (1995). Etiology of Oral Submucous Fibrosis with Special Reference to the Role of Areca Nut Chewing. J. Oral Pathol. Med. 24 (4), 145–152. doi:10.1111/j.1600-0714.1995.tb01156.x
Paredes, S., Cantillo, S., Candido, K. D., and Knezevic, N. N. (2019). An Association of Serotonin with Pain Disorders and its Modulation by Estrogens. Int. J. Mol. Sci. 20 (22), 5729. doi:10.3390/ijms20225729
Pellicioli, A. C. A., Bingle, L., Farthing, P., Lopes, M. A., Martins, M. D., and Vargas, P. A. (2017). Immunosurveillance Profile of Oral Squamous Cell Carcinoma and Oral Epithelial Dysplasia through Dendritic and T-Cell Analysis. J. Oral Pathol. Med. 46 (10), 928–933. doi:10.1111/jop.12597
Perumal, S., Dubey, K., Badhwar, R., George, K. J., Sharma, R. K., Bagler, G., et al. (2015). Capsaicin Inhibits Collagen Fibril Formation and Increases the Stability of Collagen Fibers. Eur. Biophys. J. 44 (1-2), 69–76. doi:10.1007/s00249-014-1002-9
Pindborg, J. J., and Singh, B. (1964). Formation of Vesicles in Oral Submucous Fibrosis. Acta Pathol. Microbiol. Scand. 62, 562–566. doi:10.1111/apm.1964.62.4.562
Pindborg, J. J., and Sirsat, S. M. (1966). Oral Submucous Fibrosis. Oral Surg. Oral Med. Oral Pathol. 22 (6), 764–779. doi:10.1016/0030-4220(66)90367-7
Pindborg, J. J., Chawla, T. N., Srivastava, A. N., Gupta, D., and Mehrotra, M. L. (1964). Clinical Aspects of Oral Submucous Fibrosis. Acta Odontol. Scand. 22, 679–691. doi:10.3109/00016356409058581
Pindborg, J. J., Mehta, F. S., Gupta, P. C., and Daftary, D. K. (1968). Prevalence of Oral Submucous Fibrosis Among 50,915 Indian Villagers. Br. J. Cancer 22 (4), 646–654. doi:10.1038/bjc.1968.76
Pujari, R., and Vidya, N. (2013). Mast Cell Density in Oral Submucous Fibrosis: a Possible Role in Pathogenesis. Int. J. Health Sci. 7 (1), 23–29. doi:10.12816/0006017
Roman, K., Done, J. D., Schaeffer, A. J., Murphy, S. F., and Thumbikat, P. (2014). Tryptase-PAR2 axis in Experimental Autoimmune Prostatitis, a Model for Chronic Pelvic Pain Syndrome. Pain 155 (7), 1328–1338. doi:10.1016/j.pain.2014.04.009
Salvo, E., Tu, N. H., Scheff, N. N., Dubeykovskaya, Z. A., Chavan, S. A., Aouizerat, B. E., et al. (2021). TNFα Promotes Oral Cancer Growth, Pain, and Schwann Cell Activation. Sci. Rep. 11 (1), 1840. doi:10.1038/s41598-021-81500-4
Seedat, H. A., and van Wyk, C. W. (1988). Betel Chewing and Dietary Habits of Chewers without and with Submucous Fibrosis and with Concomitant Oral Cancer. S Afr. Med. J. 74 (11), 572–575.
Sharma, J. N. (2005). The Kallikrein-Kinin System: from Mediator of Inflammation to Modulator of Cardioprotection. Inflammopharmacology 12 (5-6), 591–596. doi:10.1163/156856005774382760
Sheng, J., Zhang, B., Chen, Y., and Yu, F. (2020). Capsaicin Attenuates Liver Fibrosis by Targeting Notch Signaling to Inhibit TNF-α Secretion from M1 Macrophages. Immunopharmacol. Immunotoxicol. 42 (6), 556–563. doi:10.1080/08923973.2020.1811308
Shiau, Y. Y., and Kwan, H. W. (1979). Submucous Fibrosis in Taiwan. Oral Surg. Oral Med. Oral Pathol. 47 (5), 453–457. doi:10.1016/0030-4220(79)90128-2
Shin, N., Covington, M., Bian, D., Zhuo, J., Bowman, K., Li, Y., et al. (2012). INCB38579, a Novel and Potent Histamine H₄ Receptor Small Molecule Antagonist with Anti-inflammatory Pain and Anti-pruritic Functions. Eur. J. Pharmacol. 675 (1-3), 47–56. doi:10.1016/j.ejphar.2011.11.027
Sirsat, S. M., and Khanolkar, V. R. (1960a). Submucous Fibrosis of the Palate in Diet-Preconditioned Wistar Rats. Induction by Local Painting of Capsaicin-Aan Optical and Electron Microscopic Study. Arch. Pathol. 70, 171–179.
Sirsat, S. M., and Khanolkar, V. R. (1960b). Submucous Fibrosis of the Palate. Induction by Local Painting of Capsaicin in Wistar Rats Treated with Desoxycorticosterone Acetate-Aan Optical and Electron Microscopic Study. Arch. Pathol. 70, 180–187.
Sirsat, S. M., and Pindborg, J. J. (1967). Subepithelial Changes in Oral Submucous Fibrosis. Acta Pathol. Microbiol. Scand. 70 (2), 161–173. doi:10.1111/j.1699-0463.1967.tb01278.x
Takahashi, N., Tsuzuno, T., Mineo, S., Yamada-Hara, M., Aoki-Nonaka, Y., and Tabeta, K. (2020). Epithelial TRPV1 Channels: Expression, Function, and Pathogenicity in the Oral Cavity. J. Oral Biosci. 62 (3), 235–241. doi:10.1016/j.job.2020.05.005
Tanaka, T., Kohno, H., Sakata, K., Yamada, Y., Hirose, Y., Sugie, S., et al. (2002). Modifying Effects of Dietary Capsaicin and Rotenone on 4-nitroquinoline 1-Oxide-Induced Rat Tongue Carcinogenesis. Carcinogenesis 23 (8), 1361–1367. doi:10.1093/carcin/23.8.1361
Wahi, P. N., Kapur, V. L., Luthra, U. K., and Srivastava, M. C. (1966). Submucous Fibrosis of the Oral Cavity. 2. Studies on Epidemiology. Bull. World Health Organ. 35 (5), 793–799.
Wang, C. C., Lin, H. L., Liang, H. J., and Jan, T. R. (2011). Areca Nut Extracts Enhance the Development of CD11b(+) Gr-1(+) Cells with the Characteristics of Myeloid-Derived Suppressor Cells in Antigen-Stimulated Mice. J. Oral Pathol. Med. 40 (10), 769–777. doi:10.1111/j.1600-0714.2011.01043.x
Wang, C. C., Chen, T. Y., Wu, H. Y., Liu, T. Y., and Jan, T. R. (2012). Areca Nut Extracts Suppress the Differentiation and Functionality of Human Monocyte-Derived Dendritic Cells. J. Periodontal Res. 47 (2), 198–203. doi:10.1111/j.1600-0765.2011.01421.x
Wang, J., Tian, W., Wang, S., Wei, W., Wu, D., Wang, H., et al. (2017). Anti-inflammatory and Retinal Protective Effects of Capsaicin on Ischaemia-Induced Injuries through the Release of Endogenous Somatostatin. Clin. Exp. Pharmacol. Physiol. 44 (7), 803–814. doi:10.1111/1440-1681.12769
Wang Q, Q., Ma, S., Li, D., Zhang, Y., Tang, B., Qiu, C., et al. (2014). Dietary Capsaicin Ameliorates Pressure Overload-Induced Cardiac Hypertrophy and Fibrosis through the Transient Receptor Potential Vanilloid Type 1. Am. J. Hypertens. 27 (12), 1521–1529. doi:10.1093/ajh/hpu068
Wang TN, T. N., Huang, M. S., Lin, M. C., Duh, T. H., Lee, C. H., Wang, C. C., et al. (2014). Betel Chewing and Arecoline Affects Eotaxin-1, Asthma and Lung Function. PLoS One 9 (3), e91889. doi:10.1371/journal.pone.0091889
Watson, J. J., Allen, S. J., and Dawbarn, D. (2008). Targeting Nerve Growth Factor in Pain: What is the Therapeutic Potential? BioDrugs 22 (6), 349–359. doi:10.2165/0063030-200822060-00002
Wight, T. N., and Potter-Perigo, S. (2011). The Extracellular Matrix: an Active or Passive Player in Fibrosis? Am. J. Physiol. Gastrointest. Liver Physiol. 301 (6), G950–G955. doi:10.1152/ajpgi.00132.2011
Wu, T. F., Luo, F. J., Chang, Y. L., Huang, C. M., Chiu, W. J., Weng, C. F., et al. (2015). The Oncogenic Role of Androgen Receptors in Promoting the Growth of Oral Squamous Cell Carcinoma Cells. Oral Dis. 21 (3), 320–327. doi:10.1111/odi.12272
Yang, Y., Wang, Z., Yang, H., Wang, L., Gillespie, S. R., Wolosin, J. M., et al. (2013). TRPV1 Potentiates TGFβ-Induction of Corneal Myofibroblast Development through an Oxidative Stress-Mediated P38-SMAD2 Signaling Loop. PLoS One 8 (10), e77300. doi:10.1371/journal.pone.0077300
Yu, S. Q., Ma, S., and Wang, D. H. (2018). Activation of TRPV1 Prevents Salt-Induced Kidney Damage and Hypertension after Renal Ischemia-Reperfusion Injury in Rats. Kidney Blood Press. Res. 43 (4), 1285–1296. doi:10.1159/000492412
Zheng, L., Chen, J., Ma, Z., Liu, W., Yang, F., Yang, Z., et al. (2015). Capsaicin Causes Inactivation and Degradation of the Androgen Receptor by Inducing the Restoration of miR-449a in Prostate Cancer. Oncol. Rep. 34 (2), 1027–1034. doi:10.3892/or.2015.4055
Glossary
4-NQO: 4-nitroquinoline 1-oxide
ABCG2: ATP-binding cassette transporter G2
AMPK: AMP-activated protein kinase
AN: areca nut
ANE: Arecanut extract
ATF3: Activating transcription factor 3
BAX: BCL2 Associated X, Apoptosis Regulator
BCL2: B-cell lymphoma 2,
C/EBPβ: CCAAT enhancer-binding proteinβ
CaMKK-β: Ca2+/calmodulin-dependent protein kinase kinase-β
Cas-3: Caspase three
Cas-9: Caspase 9,
CAT: Catalase
CCND: Cyclin-D
CCND1: Cyclin D1
CCNE: Cyclin-E
CDK2: Cyclin-dependent kinase
CDK6: Cyclin-dependent kinase
Col-I: Collagen I
Co-Q: Coenzyme-Q
CTLs: Cytotoxic T cells
Cxs: Connexin
Cyt-C: Cytochrome-c
DC: Dendritic cell
EDV: Endothelium-dependent vasodilation
eNOS: Endothelial nitric oxide synthase
ERK2: Extracellular signal-regulated kinase
ETS: Electron transport system
Fn: Fibronectin
G6PD: Glucose-6-phosphate dehydrogenase
GPx: Glutathione peroxidase
GR: Glutathione reductase
GSK3β: Glycogen synthase kinase-3β
HDAC-5: Histone deacetylase-5
KLF2: Krüppel-like Factor 2
LXR: Liver X receptors,
LXRE: LXT response elements
MCD: Mast cell density
MCP-1: Monocyte Chemoattractant Protein-1
MDSC: Myeloid-derived suppressor cells
MEF-2: Myocyte enhancer factor-2
NAG-1: Non-steroidal anti-inflammatory drug (NSAID)-activated gene-1
NF-κB: Nuclear factor-kappa B
NGF: Nerve growth factor
NNK: 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone
NRF2: Nuclear factor erythroid 2-related factor 2
OMM: Outer mitochondrial membrane
OSF: Oral submucous fibrosis
p53Mut: Mutant p53
p53Wt: Wild-type p53
Panx: Pannexin
P-gp: P-Glycoprotein
PKB: Protein kinase B
PKC-δ: Protein kinase C-δ
PPAR-γ: Proliferator-activated receptor γ
PPAR-δ: Proliferator-activated receptor δ
SIRT-1: Sirtuin-1
SMAR1: Scaffold/matrix-associated region-binding protein 1
SOD: Superoxide dismutase
STAT-3: Signal Transducer and Activator of Transcription- three
Tregs: Regulatory T cells
TRPV-1: Transient receptor potential vanilloid
UCP2: Uncoupling protein 2
VEGF: Vasculoendothelial growth factor
Keywords: capsaicin, burning pain, chili, oral submucous fibrosis, antifibrotic, anticarcinogen
Citation: Huang Z, Sharma M, Dave A, Yang Y, Chen Z-S and Radhakrishnan R (2022) The Antifibrotic and the Anticarcinogenic Activity of Capsaicin in Hot Chili Pepper in Relation to Oral Submucous Fibrosis. Front. Pharmacol. 13:888280. doi: 10.3389/fphar.2022.888280
Received: 02 March 2022; Accepted: 19 April 2022;
Published: 04 May 2022.
Edited by:
Uraiwan Panich, Mahidol University, ThailandReviewed by:
Jian-Bo Wan, University of Macau, ChinaStefan Mergler, Charité Universitätsmedizin Berlin, Germany
Copyright © 2022 Huang, Sharma, Dave, Yang, Chen and Radhakrishnan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhe-Sheng Chen, Y2hlbnpAc3Rqb2hucy5lZHU=; Raghu Radhakrishnan, cmFnaHUuYXJAbWFuaXBhbC5lZHU=
†These authors have contributed equally to the work
 Zoufang Huang
Zoufang Huang Mohit Sharma
Mohit Sharma Aparna Dave2
Aparna Dave2 Yuqi Yang
Yuqi Yang Zhe-Sheng Chen
Zhe-Sheng Chen Raghu Radhakrishnan
Raghu Radhakrishnan