
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 17 June 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.888073
This article is part of the Research Topic Treatment of Infectious Diseases with Bioactive Compounds from Medicinal Plants: Their Mechanisms and Applications View all 11 articles
 Peiying Huang1,2
Peiying Huang1,2 Yin Li3
Yin Li3 Bixuan Huang4
Bixuan Huang4 Shuai Zhao2,5
Shuai Zhao2,5 Li Chen2,5
Li Chen2,5 Hansu Guan6
Hansu Guan6 Yan Chen5
Yan Chen5 Yuchao Feng2,5
Yuchao Feng2,5 Xiaoyan Huang2,5
Xiaoyan Huang2,5 Yi Deng2,5
Yi Deng2,5 Sisi Lei1,2
Sisi Lei1,2 Qihua Wu1,2
Qihua Wu1,2 Haobo Zhang1,2
Haobo Zhang1,2 Zhongyi Zeng7
Zhongyi Zeng7 Linsheng Zeng7
Linsheng Zeng7 Bojun Chen1,2,5*
Bojun Chen1,2,5*Background: Acute tonsillitis has high morbidity. Chinese herbal injections (CHIs) were reported to be useful in treating acute tonsillitis and might reduce the probability of antibiotic resistance. Nevertheless, the optimal strategy for combining CHIs with western medicine (WM) to treat acute tonsillitis remains unclear.
Methods: We retrieved data from the following databases with retrieval time from inception to 11 January 2022: PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure, Wanfang Database, Weipu Journal Database, and Chinese Biomedical Literature Database. Version 2 of the Cochrane risk-of-bias tool (ROB2) was used for evaluating the quality of the included studies. R 4.1.2, STATA 14.0, and Python 3.10.4 were employed for network meta-analysis, with 5-dimensional K-means cluster analysis, meta-regression analyses, sensitivity analyses, and subgroup analyses.
Results: A total of 110 randomized controlled trials including 12,152 patients were included. All the studies were rated as “high risk” and “some concerns”. In terms of improving clinical effectiveness rate, Qingkailing injection + WM ranked ahead of other interventions (89.51%). Regarding reducing antipyretic time, Reduning injection + WM had the highest-ranking probability (68.48%). As for shortening sore throat relief time, Shuanghuanglian injection + WM ranked first (76.82%). Concerning shortening red and swollen tonsils relief time, Yanhuning injection + WM possessed the highest-ranking probability (89.17%). In terms of reducing tonsillar exudate relief time, Xuebijing injection + WM ranked ahead of the other interventions (94.82%). Additionally, the results of the cluster analysis suggested that Xuebijing injection + WM, Reduning injection + WM, and Yanhuning injection + WM were probably the best interventions. Furthermore, adverse drug reactions rate of Xuebijing injection + WM, Reduning injection + WM, Yanhuning injection + WM, Qingkailing injection + WM, and Shuanghuanglian injection + WM were individually 0.00%, 3.11%, 3.08%, 4.29%, and 4.62%.
Conclusions: CHIs + WM have a better impact on patients with acute tonsillitis than WM alone. Xuebijing injection, Reduning injection, and Yanhuning injection might have potential advantages in treating the disease. Concerning adverse drug reactions, Xuebijing injection is presumably the optimal CHI. More high-quality studies are needed to further confirm our findings.
Systematic Review Registration: CRD42022303243; URL= https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=303243
Acute tonsillitis is a type of acute upper respiratory tract infection, with acute sore throat as the principal symptom, accompanied by fever, red and swollen tonsils, enlarged cervical lymph nodes, and may be associated with tonsil exudation (Bartlett et al., 2015; Windfuhr et al., 2016). The disease affects both sexes and all age groups, predominantly in school-aged children (Sidell and Shapiro, 2012). It is estimated that acute tonsillitis makes up approximately 1.3% of outpatient visits (Kocher and Selby, 2014), which generates a substantial workload for primary care physicians and places huge financial pressures on medical budget (Bird et al., 2014).
In 50%–80% of acute tonsillitis patients, the causative pathogens are viruses (e.g., Epstein–Barr virus, rhinovirus, respiratory syncytial virus, adenovirus, and coronavirus), while 5%–36% of cases are caused by bacteria, for the most part, Group A beta-haemolytic streptococci (Ebell et al., 2000; Bird et al., 2014). In western medicine (WM), symptomatic and supportive treatment is the mainstay of viral cases, such as fluid rehydration, antipyretic analgesics, local anesthetics, and corticosteroids. Antibiotics are used as prescribed when there is a possibility of bacterial infection. Although pathogen detection and Centor score/McIsaac score are helpful in the pathogen diagnosis, it remains difficult to distinguish between a bacterial or viral etiology clinically (Bird et al., 2014; Windfuhr et al., 2016). Standard use of antibiotics, therefore, is difficult to achieve for clinicians, which may bring the promotion of bacterial resistance as well as adverse drug reactions. Additionally, antipyretic analgesics, anesthetics, or corticosteroids are classically restrained in some patient populations due to their certain side effects, such as non-steroidal anti-inflammatory drugs in gastrointestinal bleeding and opioids in airway compromise (Bird et al., 2014). Tonsillectomy, a way to deal with recurrent acute tonsillitis, also be restrained during the acute phase and has limited long-term benefits (Morad et al., 2017).
Compared with WM, Traditional Chinese medicine presents the following advantages in treating acute tonsillitis: multiple mechanisms of action (e.g., antimicrobial, anti-inflammatory, antipyretic, pain relief), few contraindications, and low-cost treatment (Fan et al., 2017). Chinese herbal injections (CHIs) are intravenous injections prepared by extracting the active ingredients of traditional Chinese medicine, with the characteristics of rapid onset and improved bioavailability. Research showed that contrasted with WM alone, CHI combined with WM has better clinical efficiency, including more preferable relief of symptoms and shorter disease duration, which may reduce the adverse drug reactions of WM and decrease the risk of antibiotic resistance (Zhou et al., 2020). However, there is a wide variety of CHIs used for acute tonsillitis with few studies comparing them. We thus initiated a network meta-analysis to achieve these comparisons.
This study was conducted following the PRISMA extension statement (Hutton et al., 2015) with a PRISMA checklist which is provided in Supplementary File S1.
We searched relevant databases including PubMed, Embase, Web of Science, Cochrane Library, China National Knowledge Infrastructure, Wanfang Database, Weipu Journal Database, and Chinese Biomedical Literature Database from database inception to 11 January 2022. The search strategies are provided in Supplementary File S2.
Only randomized controlled trials (RCTs) which targeted the treatment of CHIs to acute tonsillitis were included. In the selected studies, CHIs plus WM should be compared with WM alone or/and another type of CHIs plus WM. Notably, each group within one included trial received the same treatment regimen of WM. No limitations were defined by age, sex, or race whereas patients with concurrent acute tonsillitis and infections at other sites (e.g., pneumonia) were excluded. The outcome of interest included clinical effectiveness rate (proportion of patients improving after treatment), antipyretic time, sore throat relief time, red and swollen tonsils relief time, tonsillar exudate relief time, and adverse drug reactions (ADRs). A study was admitted according to the inclusion criteria independently by two reviewers. Discrepancies were resolved by consensus between the two reviewers or arbitrated by a third reviewer.
Data regarding trial information (title, first-author, publication year, sample size, trial duration, interventions, and control), population characteristics (sex, age, and consistency of baseline), reported outcomes (response rate in categorical variables and means/standard deviation in continuous variables), information on methodology (blinding, random methods, and measurement of each indicator), and sponsorship from pharmaceutical companies, were extracted by two independent reviewers using Excel 356 software. The reviewers further used Version 2 of the Cochrane risk-of-bias tool for randomized trials (RoB 2) to assess the risk of bias for each outcome of the included RCTs through the following aspects: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, and selection of the reported result (Sterne et al., 2019). Any discrepancies were resolved by discussions between the two reviewers, and if necessary, by arbitration by a third reviewer.
In this study, the program was analyzed by a random-effects network meta-analysis within a Bayesian framework (Salanti, 2012; Mavridis and Salanti, 2013). Based on four Monte Carlo Markov Chains, 200,000 in iterations and 10,000 in annealing were set. Risk Ratio (RR)with 95% confidence interval (CI) was calculated as pooled effect measure for categorical variables while pooled effect measures of continuous variables were expressed as Mean Differences (MD) with 95%CI. A league table was generated to present the comparisons between each pair of interventions within each outcome. Surface under the cumulative ranking area curves (SUCRA) with mean ranking probabilities were used to summarize treatment hierarchy (Dias et al., 2013). Additionally, node-splitting method was performed to assess the inconsistency of the model by separating evidence on a particular comparison into direct and indirect evidence in outcome(s) with at least one closed loop (van Valkenhoef et al., 2016). A heatmap was closely employed to measure the contribution degree of each pair of interventions for overall inconsistency. Moreover, Global I2-statistic was used to evaluate the heterogeneity of the estimated effect size (Higgins and Thompson, 2002). Network meta-regression in the context of a Bayesian framework was further conducted to examine the potential modification effects for outcome(s) with significant heterogeneity. Furthermore, subgroup network meta-analyses and sensitivity analyses were conducted to assess the robustness of the results and deal with heterogeneity. Comparison-adjusted funnel plots and Egger’s test were used to explore potential publication bias in the outcomes with greater than or equal to 10 RCTs (Begg and Mazumdar, 1994; Stuck et al., 1998).
Additionally, for a comprehensive assessment of treatment effect of CHIs + WM, a 5-dimensional K-means cluster analysis based on the SUCRA values of the selected CHIs + WM within each outcome (clinical effectiveness rate, antipyretic time, sore throat relief time, red and swollen tonsils relief time, and tonsillar exudate relief time) was performed (Wan et al., 1988). Missing values were replaced with the mean of the SUCRA values of each outcome. The steps of clustering were as follows: 1) Randomly selected K objects in the data space as the initial cluster centers. 2) According to the Euclidean distances among the SUCRA values and the pre-set cluster centers, the SUCRA values were divided into the cluster center (category) closest to them. 3) A value of the objective function was calculated using the mean of the SUCRA values in each category. Determine whether the values of the pre-set cluster center and the objective function were consistent. If so, output the result; if not, return to the second step to continue the iteration. Subsequently, principal component analysis (Karhunen-Loeve Transform) was used to convert the results of the 5-dimensional K-means cluster analysis into three dimensions via mapping and then visualize the results on a 3-dimensional axis (Bro and Smilde, 2014).
All analyses were performed using R 4.1.2 (gemtc package: network meta-analysis, heterogeneity, inconsistency, network meta-regression, subgroup analysis, and sensitivity analysis; ggplot2 package: SUCRA graphs), STATA 14.0 (publication bias), and Python 3.10.4 (sklearn package: 5-dimensional K-means cluster analysis, principal component analysis; matplotlib package: visualization of the results of principal component analysis).
Overall, 869 records were retrieved, in which 110 trials were finally included in the current analysis according to the predesigned criteria (see Supplementary File S3 for the citations of the included studies). A flow chart of the literature search is provided in Supplementary File S4. All the selected trials were two-arm studies with publication years from 1998 to 2021, involving nine kinds of CHIs: Reduning injection (RDN, 31 RCTs), Tanreqing injection (TRQ, 23 RCTs), Xiyanping injection (XYP, 36 RCTs), Yanhuning injection (YHN, seven RCTs), Chuanhuning injection (CHN, one RCTs), Qingkailing injection (QKL, three RCTs), Shuanghuanglian injection (SHL, four RCTs), Xuebijing injection (XBJ, three RCTs), and Yuxingcao injection (YXC, four RCTs) (see Supplementary File S5 for characteristics of the included CHIs). A total of 12,152 patients were included in the entire analysis, of whom 6,772 were male patients (56.78%). Overall, 103 (93.64%), 48 (43.64%), 28 (25.45%), 18 (16.36%), 33 (30.00%), and 44 (40.00%) studies, separately, contributed to the six outcomes, i.e., clinical effectiveness rate, antipyretic time, sore throat relief time, red and swollen tonsils relief time, tonsillar exudate relief time, and ADRs. The selected trails possessed consistent baselines and treatment duration of them ranging from 2 to 10 days. The details of the selected RCTs are shown in Supplementary File S6. Of the 110 RCTs included, the connections among the interventions were visualized as a network diagram within each outcome. The network graphs are depicted in Figure 1, in which the size of node represents the sample size and the thickness of the line between nodes represents the volume of studies.
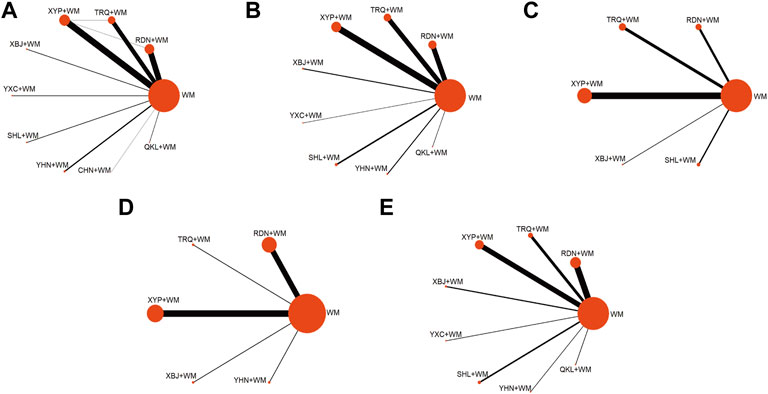
FIGURE 1. Network graph of different interventions (A) Clinical effectiveness rate (B) Antipyretic time (C) Sore throat relief time (D) Red and swollen tonsils relief time (E) Tonsillar exudate relief time; WM, Western Medicine; RDN, Reduning injection; TRQ, Tanreqing injection; QKL, Qingkailing injection; XBJ, Xuebijing injection; SHL, Shuanghuanglian injection; YHN, Yanhuning injection; CHN, Chuanhuning injection; YXC, Yuxingcao injection; XYP, Xiyanping injection.
Regarding methodologies of the selected trials, 17 RCTs (15.45%) reported specific details of randomized approaches. Allocation concealment was reported in 13.64% of the cases, and these trials were evaluated as “low risk” in “randomization process”. By contrast, no clear information was reported in all the trials about a predesigned protocol or appropriate analysis that was used to estimate the effect of assignment to intervention, which made both “selection of the reported result” and “deviation from intended interventions” rated as “some concerns”. “Missing outcomes data” was generally a low risk of bias as all the outcomes were comprehensively described with specific number of patients involved in the assessment. Additionally, one RCT (0.91%) showed itself as a double-blind trial while three RCTs (3.60%) blinded trial performers and 10 RCTs (10.00%) blinded trial participants. The differences in blinding among the included trials arrived at the results that in “measurement of the outcome”, severally, 99.1%, 0.00%, 90.00%, 96.4%, and 96.4% of the selected studies in clinical effectiveness rate, antipyretic time, sore throat relief time, red and swollen tonsils relief time, and tonsillar exudate relief time were rated as “high risk” and thus this part of the studies were assessed as “high risk” in “overall bias” (see Figure 2 for risk of bias assessment).
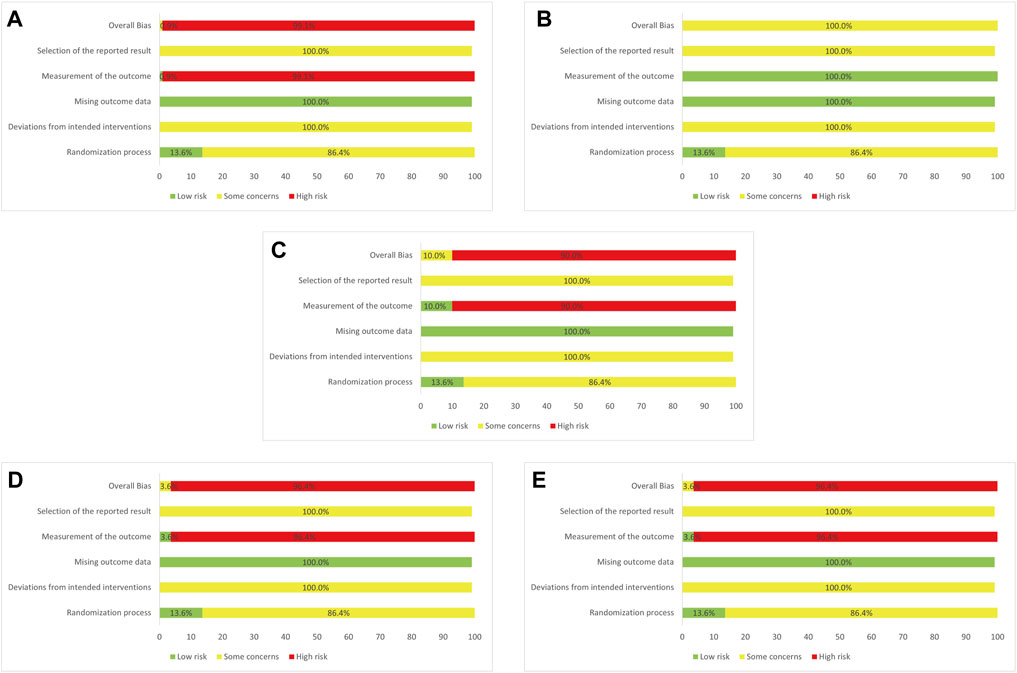
FIGURE 2. Assessment of risk bias (A) Clinical effectiveness rate (B) Antipyretic time (C) Sore throat relief time (D) Red and swollen tonsils relief time (E) Tonsillar exudate relief time.
Nine CHIs (QKL, RDN, SHL, TRQ, XBJ, XYP, YHN, YXC, and CHN) were involved in the evaluation of clinical effectiveness rate. An improved effect of clinical effectiveness rate was detected for all types of the included CHIs + WM (apart from CHN + WM) vs. WM, while CHN + WM obtained a worse effect than other interventions. XYP + WM improved clinical effectiveness rate as compared with RDN + WM. No such evident effect was observed in any other comparison (see Table 1 for between-intervention differences). According to SUCRA, QKL + WM (89.51%), XBJ + WM (87.39%), and XYP + WM (69.15%) ranked first, second, and third, respectively, whereas RDN + WM (38.25%), WM (11.13%), and CHN + WM (0.03%) separately ranked eighth, ninth, and 10th. More details about SUCRA and its rank probability are individually shown in Figure 3 and Table 2.
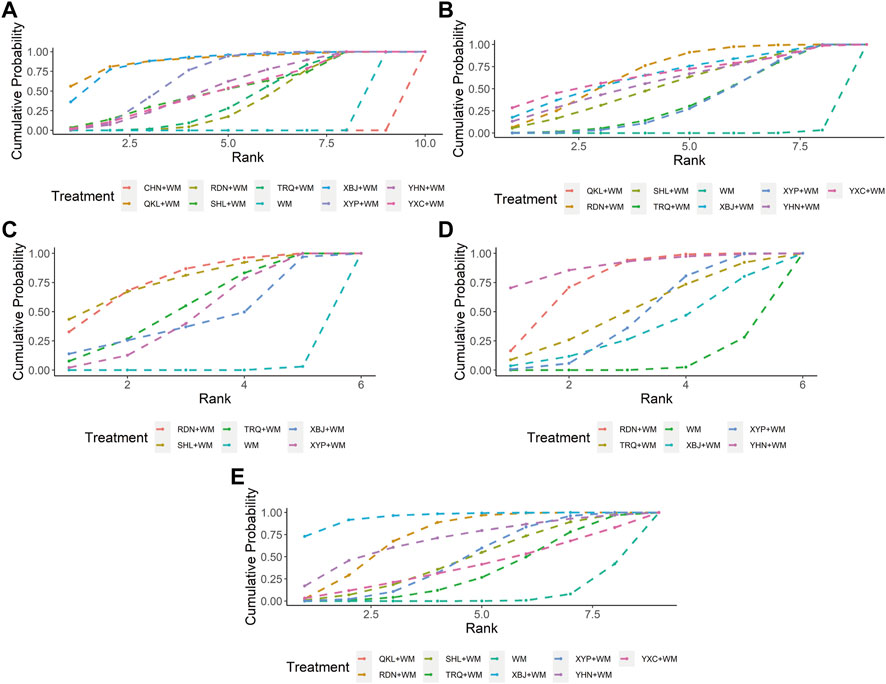
FIGURE 3. Plots of the surface under the cumulative ranking curves for all interventions (A) Clinical effectiveness rate (B) Antipyretic time (C) Sore throat relief time (D) Red and swollen tonsils relief time (E) Tonsillar exudate relief time; WM, Western Medicine; RDN, Reduning injection; TRQ, Tanreqing injection; QKL, Qingkailing injection; XBJ, Xuebijing injection; SHL, Shuanghuanglian injection; YHN, Yanhuning injection; CHN, Chuanhuning injection; YXC, Yuxingcao injection; XYP, Xiyanping injection.
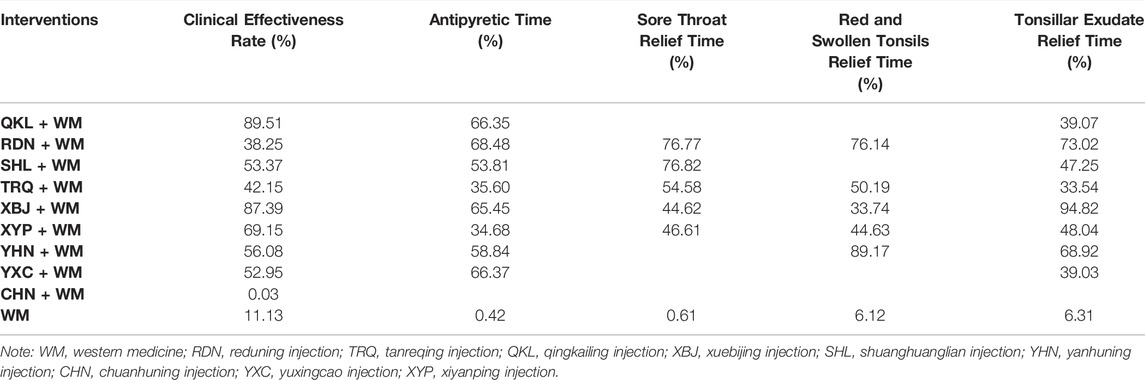
TABLE 2. Ranking probabilities of surface under the cumulative ranking area curves (SUCRA) for five outcomes.
Eight CHIs (QKL, RDN, SHL, TRQ, XBJ, XYP, YHN, and YXC) were involved in the evaluation of antipyretic time. In this clinical indicator, shortening was statistically significant for QKL + WM, RDN + WM, SHL + WM, TRQ + WM, XBJ + WM, XYP + WM, YHN + WM, and YXC + WM, as compared with WM. In addition, XBJ + WM was superior to XYP + WM, YHN + WM, and YXC + WM. No significant association was found with other comparators (see Table 1 for between-intervention differences). Based on SUCRA, RDN + WM (68.48%), YXC + WM (66.37%), and QKL + WM (66.35%) ranked first, second, and third, respectively, whereas TRQ + WM (35.60%), XYP + WM (34.68%), and WM (0.42%) separately ranked seventh, eighth, and ninth. More details about SUCRA and its rank probability are individually shown in Figure 3 and Table 2.
Five CHIs (RDN, SHL, TRQ, XBJ, and XYP) were involved in the evaluation of sore throat relief time. RDN + WM, SHL + WM, TRQ + WM, and XYP + WM statistically reduced sore throat relief time as compared with WM. No such evident effect was observed with other pairwise interventions (see Table 1 for between-intervention differences). According to SUCRA, SHL + WM (76.82%), RDN + WM (76.77%), and TRQ + WM (54.58%) ranked first, second, and third, respectively, whereas XYP + WM (46.61%), XBJ + WM (44.62%), and WM (0.61%) severally ranked fourth, fifth, and sixth. More details about SUCRA and its rank probability are individually shown in Figure 3 and Table 2.
Five CHIs (RDN, TRQ, XBJ, YHN, and XYP) were involved in the evaluation of red and swollen tonsils relief time. RDN + WM, XYP + WM, and YHN + WM showed a significant decrease in red and swollen tonsils relief time as compared with WM, whereas no significant association was found in any other comparison (see Table 1 for between-intervention differences). According to SUCRA, YHN + WM (89.17%), RDN + WM (76.14%), and TRQ + WM (50.19%) ranked first, second, and third, respectively, whereas XYP + WM (44.63%), XBJ + WM (33.74%), and WM (6.12%) individually ranked fourth, fifth, and sixth. More details about SUCRA and its rank probability are individually shown in Figure 3 and Table 2.
Eight CHIs (QKL, RDN, SHL, TRQ, XBJ, XYP, YHN, and YXC) were involved in the appraisal of tonsillar exudate relief time. RDN + WM, XBJ + WM, and XYP + WM were associated with a significant reduction in tonsillar exudate relief time as compared with WM. In comparison with XBJ + WM, both TRQ + WM and XYP + WM obtained a worse effect (see Table 1 for between-intervention differences). According to SUCRA, XBJ + WM (94.82%), RDN + WM (73.02%), and YHN + WM (68.92%) ranked first, second, and third, respectively, whereas YXC + WM (39.03%), TRQ + WM (33.54%), and WM (6.31%) separately ranked seventh, eighth, and ninth. More details about SUCRA and its rank probability are individually shown in Figure 3 and Table 2.
ADRs were monitored in 44 RCTs (40.00%), of which 24 studies (21.82%) reported the number of affected patients in detail whereas 20 studies (18.18%) presented no ADRs during the treatment. No ADRs were observed in the reported 52 patients using XBJ (0.00%). The ADRs rate for RDN, TRQ, XYP, YHN, YXC, QKL, and SHL were 3.11%, 0.88%, 2.99%, 3.08%, 2.78%, 4.29%, and 4.62%, respectively, without fatal reactions. The ADRs are further detailed in Table 3.
A 5-dimensional K-means cluster analysis was conducted to comprehensively compare the effects of the interventions on the five outcomes (clinical effectiveness rate, antipyretic time, sore throat relief time, red and swollen tonsils relief time, and tonsillar exudate relief time). The results reduced to three dimensions by principal component analysis are shown in Figure 4. Upon visual inspection, all interventions were clustered into three categories, in which XBJ + WM, RDN + WM, and YHN + WM were classified as a category with optimal treatment effect while WM alone was as a category with worst curative effect.
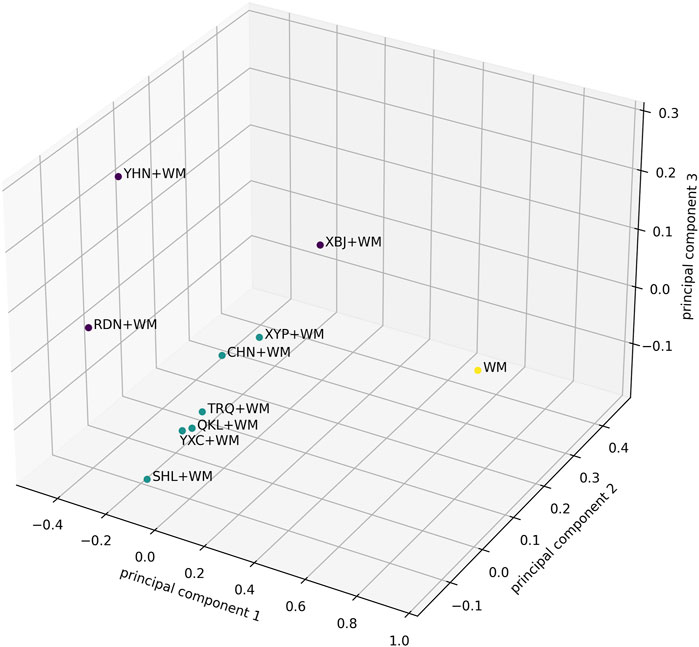
FIGURE 4. The results of the 5-Dimensional K-means cluster analysis were reduced to three dimensions by principal component analysis. The purple dots represent the best category of curative effect, while the green dots are the second and the yellow dots are the worst. The axes represent the three principal components in the principal component analysis.
Assessment of inconsistency by node-splitting method indicated that inconsistency was not detected in clinical effectiveness rate as p-values in all the comparisons were greater than 0.05 (Supplementary File S7). The heatmap revealed that the pooled effect size in “RDN + WM vs. XYP + WM” had the greatest contribution to the inconsistency of clinical effectiveness rate (see Supplementary File S8 for contribution degree of inconsistency). Regarding heterogeneity, Global I2-statistic was 18.07%, 96.54%, 94.12%, 95.96%, and 96.63% for clinical effectiveness rate, antipyretic time, sore throat relief time, red and swollen tonsils relief time, and tonsillar exudate relief time, individually. Upon visual inspection, the funnel plots showed unremarkable asymmetry on both sides of the centerline, which did not suggest a significant risk of publication bias in our sample of the included studies. Nevertheless, quantitative detection of publication bias (Egger’s test) demonstrated that the p value for antipyretic time was 0.006 (<0.05), while the p values for clinical effectiveness rate, sore throat relief time, red and swollen tonsils relief time, and tonsillar exudate relief time were respectively 0.434, 0.360, 0.424 and 0.400, suggesting that there were small-study effects in the outcome of antipyretic time. The funnel plots are shown in Figure 5 and the results of Egger’s test are provided in Supplementary File S9.
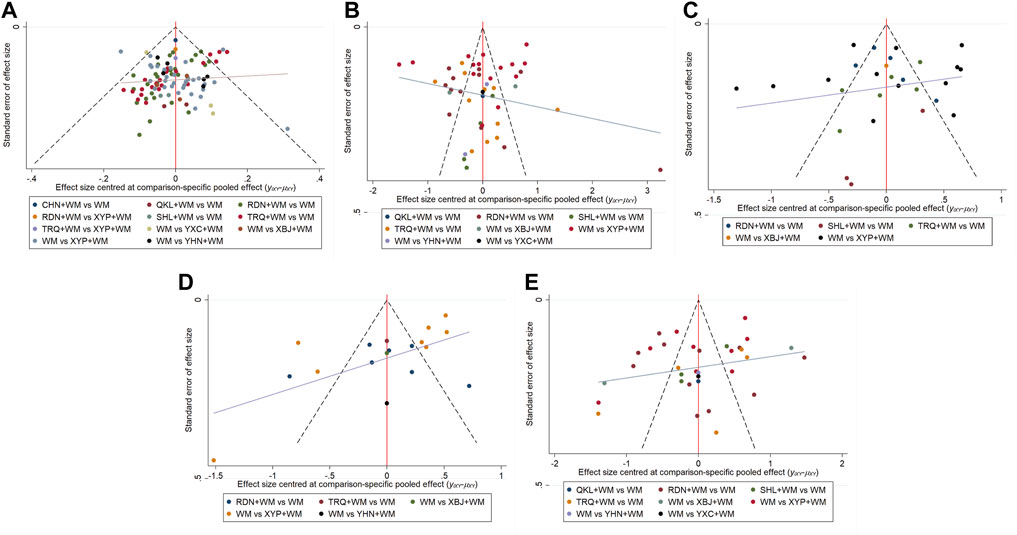
FIGURE 5. Funnel plots (A) Clinical effectiveness rate (B) Antipyretic time (C) Sore throat relief time (D) Red and swollen tonsils relief time (E) Tonsillar exudate relief time.
Since there are statistical heterogeneities according to the Global I2, four outcomes (antipyretic time, sore throat relief time, red and swollen tonsils relief time, and tonsillar exudate relief time) were analyzed by network meta-regression with patients’ age and publication year as the covariates. The results suggested that red and swollen tonsils relief time would decrease by 0.7 days per year’s change in patients’ age, whereas the covariates were not statistically significant in the remaining outcomes (Supplementary File S10). Sensitivity analysis, a network meta-analysis in selected studies published after 2010, indicated that the overall results were robust (Supplementary File S11). Subgroup analyses were performed according to patients’ age, tonsil suppuration, and treatment regimen of WM. As the number of studies targeting adult patients, patients without suppurative tonsillitis, and patients treated with WM apart from penicillins/cephalosporins was too small to achieve subgroup analyses, the subgroup analyses were finally conducted in studies with pediatric patients, patients with suppurative tonsillitis, patients treated by penicillins, and patients received cephalosporins as the treatment regimen of WM. In the subgroup of children, compared to the overall results, XBJ + WM ranked first in clinical effectiveness rate (78.30%) while QKL + WM was not included in this outcome analysis (Supplementary File S12). The subgroup of patients with suppurative tonsillitis demonstrated that, as compared with the overall results, XBJ + WM ranked first (94.87%) in decreasing antipyretic time (Supplementary File S13). Compared to the overall results, the subgroup for patients who received penicillins as the treatment regimen of WM indicated that XYP + WM ranked first (81.19%) in reducing red and swollen tonsils relief time and YHN + WM possessed the highest-ranking probability (89.78%) in decreasing tonsillar exudate relief time, while XBJ + WM was not included in the tonsillar exudate relief time analysis (Supplementary File S14). In the subgroup for patients who received cephalosporins as the treatment regimen of WM, as compared with the overall results, XBJ + WM ranked ahead of other interventions in the outcomes of clinical effectiveness rate (99.35%) and antipyretic time (95.41%); TRQ + WM ranked first (77.97%) in reducing sore throat relief time; RDN + WM has the highest-ranking probability (85.13%) in decreasing red and swollen tonsils relief time; SHL + WM and YHN + WM were individually not included in the sore throat relief time analysis and red and swollen tonsils relief time analysis (Supplementary File S15).
In the theory of Traditional Chinese medicine, acute tonsillitis is classified as acute nippled moth, which is predominantly caused by pathogenic qi that is associated with heat-toxicity (Gao et al., 2017). Therefore, in the position of the Chinese medicine theory, the main strategy of treating acute tonsillitis is to clear heat and detoxify (Gao et al., 2017). In the current study, all the included CHIs have the efficacy of clearing heat or detoxifying and thus are used for the treatment of acute tonsillitis clinically. Among the CHIs, this network meta-analysis (SUCRA) suggested that QKL, XBJ, RDN, SHL, and YHN might have potential advantages in treating the disease, in which XBJ, RDN, and YHN deserved more attention based on the cluster analysis. Simultaneously, XBJ may be the optimal CHI for acute tonsillitis considering ADRs.
According to our findings, QKL + WM showed good performance in improving clinical effectiveness rate as well as resolving fever through pairwise comparison and ranking probability. QKL is mainly prepared from baicalin, Isatis tinctoria L [Brassicaceae], Lonicera japonica Thunb [Caprifoliaceae], and Gardenia jasminoides J.Ellis [Rubiaceae]. Similar to our study, some studies found that QKL has a significant antipyretic effect, which was associated with repairing the perturbed pathways of lipid metabolism and amino acid metabolism (Gao et al., 2013; Qin et al., 2016). An in vitro experiment confirmed an inhibitory efficacy on resistant bacteria containing blaNDM-1 by QKL and especially its active ingredient, baicalin; an experiment also indicated the significant inhibition of QKL plus antibiotics to multidrug-resistant bacteria (Shang et al., 2013). Besides, QKL was confirmed to possess its potent reduction in inhibiting damage caused by infection, e.g., leucopenia and thrombocytopenia (Yi et al., 2021). These pharmacological mechanisms may be tied to the efficacies of QKL to acute tonsillitis.
Apart from QKL, the injections that were prepared from traditional heat-clearing and detoxifying Chinese herbs in our study also included RDN, SHL, and YHN. We found that RDN + WM, SHL + WM, and YHN + WM exerted superior effects in lowering body temperature, shortening sore throat relief time, and reducing red and swollen tonsils relief time, separately. The antipyretic mechanism of RDN might be related to the regulation of biosynthesis as well as sphingolipid metabolism of valine, leucine, and isoleucine (Gao et al., 2020). In addition, RDN was reported to possess anti-inflammatory and antiviral effects (Cao et al., 2015; Xie et al., 2020; Xu et al., 2021), which might work in treating acute tonsillitis. SHL is made from active ingredients of Forsythia suspensa (Thunb.) Vahl [Oleaceae], Lonicera japonica Thunb [Caprifoliaceae], and Scutellaria baicalensis Georgi [Lamiaceae]. Under some in vitro and in vivo experiments, the injection also had benefit of inhibiting viruses, in which the pathogens might be the perpetrator of acute tonsillitis, e.g., SARS-CoV-2 and influenza A virus H5N1(Tang et al., 2018; Su et al., 2020). Moreover, SHL could inhibit NF-kappaB-mediated production of pro-inflammatory cytokines and chemokines, thereby reducing the inflammatory response to microbial infection (Chen et al., 2002). YHN originates from Andrographis paniculata (Burm.f.) Nees [Acanthaceae], a Chinese herbal medicine possessing primary effects of clearing heat and detumescence. Pharmacological research showed that YHN has strong inhibitory effects on respiratory syncytial virus, Coxsackie virus, Epstein-Barr virus, and rotavirus (Liu et al., 2007; Han, 2012; Guan and Cao, 2013; Huang et al., 2013), among which some viruses might cause acute tonsillitis. Additionally, YHN has therapeutic effects on SD rats with upper respiratory tract infection modeled by beta-hemolytic streptococcus via inhibiting the expression of IL-1β, IL-6β, and TNF-α(Liang et al., 2012), while beta-hemolytic streptococcus is the main bacterium causing acute tonsillitis.
Unlike the CHIs mentioned above consisting of heat-clearing and detoxifying Chinese herbs as raw materials, XBJ, another included intravenous Chinese medicine preparation, derived from a traditional formulation called “Xuefuzhuyu Decoction” which does not contain any heat-clearing and detoxifying Chinese herb but Chinese herb activating blood circulation and removing stasis, whereas has the functions of dispelling blood stasis and detoxification. Pharmacological analysis research had demonstrated that the main constituents of XBJ including paeoniflorin, senkyunolide I, safflor yellow A, danshensu, uridine, rosmarinic acid, beta-ocimene-X, gallic acid, protocatechualdehyde, hydroxysafflor yellow A, and oxypaeoniflorin, etc. via ultra-high-performance liquid chromatography (Ji et al., 2010; Jiang et al., 2013), in which the active ingredients play anti-infection and immunomodulatory effects by acting on targets/pathways such as COX-2, IKK-2, 5-LOX, NF-κB, MAPK, eNOS, iNOS, A2AR, and MIF(Ma et al., 2009; Jiang et al., 2013). These pharmacological mechanisms may be related to the treatment of acute tonsillitis with XBJ. Indeed, XBJ has played a vital role in treating sepsis or septic shock as a result of its anti-inflammatory effect as well as immunomodulatory function, and thus the injection has been included in the treatment guidelines of sepsis in China (Branch, 2015). In clinical, XBJ has also been used to treat acute tonsillitis, a disease that is classified as an infectious disease as sepsis. As indicated in our study, XBJ was revealed as the potential optimal CHIs in shortening tonsillar exudate relief time and possibly even the best CHI for the comprehensive treatment of acute tonsillitis, which was consistent with the results of a previous network meta-analysis targeting CHIs plus WM in the treatment of acute tonsillitis in children (involving 65 RCTs as well as six CHIs). In that study, XBJ possessed the highest-ranking probability regarding antipyretic time, sore throat relief time, and red and swollen tonsils relief time, whereas had a similar ranking for clinical effectiveness rate and tonsillar exudate relief time with our subgroup analyses of children (Zhou et al., 2020).
In addition to clinical efficacy, the adverse reactions of CHIs are also attention-worthy. In the current study, we reported both the incidence and types of ADRs for seven CHIs. Although the studies we included did not monitor the occurrence of fatal ADRs, the safety of CHIs remains a concern; how to reduce the occurrence of ADRs in CHIs deserves our attention. Risk factors for ADRs in CHIs, in this case, may provide some recommendations. A retrospective study showed that ADRs are more likely to occur in children or combine with cephalosporin when using QKL (Wu et al., 2018). In addition, ADRs to XBJ are related to vehicle type, dosage, older age, and drug combination (e.g., reduced glutathione, aspirin-dl-lysine, and torsemide) (Wang et al., 2019), while the history of drug allergy, abnormal liver and kidney function, traditional Chinese medicine dialectical medication, dispensing time, drip rate, and drug combination might play roles in ADRs of SHL through multi-factor analysis (Pang and Zhang, 2018). Besides, children are a high-risk group for ADRs with YHN, and off-label drug use is responsible for ADRs in RDN (Huang, 2018; Yu et al., 2019). Anyhow, CHIs should be used more regulated and cautiously, especially in children.
The major strength of the current study included comprehensive search strategies and analyses. Furthermore, we performed sensitivity analyses to assess the robustness of the results and carried out network meta-regression as well as subgroup analyses to address the heterogeneity of the selected studies. Meanwhile, a 5-dimensional K-means cluster analysis was employed to comprehensively compare the treatment effects of the selected CHIs on the five outcomes. However, some limitations to this study should be mentioned. First, all the outcomes were rated as “high risk” and “some concerns”, for which the results should be interpreted cautiously. Secondary, the WM treatment regimens of the included studies were inconsistent; hence, the results should be interpreted with caution. Third, all the studies were conducted in China and the results may not be generalizable. Finally, several CHIs were associated with small numbers of RCTs (CHN, one RCTs; QKL, three RCTs; SHL, four RCTs; XBJ, three RCTs; YXC, four RCTs) and the interpretation of the results might be restricted.
CHIs combined with WM have more favorable effects than WM alone in treating acute tonsillitis. QKL, XBJ, RDN, SHL, and YHN deserve more attention when facing patients with acute tonsillitis. Taking ADRs into consideration, XBJ was probably the best CHI for the disease. More evidence, however, is required to support these suggestions.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
PH, and BC done conception and design of the study. YF and SL performed the literature search, screening, and extraction. XH, YC, and YD verified the data. QW and HZ evaluated the quality of the included RCTs. PH, YL, BH, LC, HG, ZZ, and LZ performed the network meta-analysis. PH wrote the original draft. BC and SZ interpreted the results, incorporated comments for the co-authors, and finalized the manuscript. All the authors approved the final version of the manuscript.
This work was funded by the National Natural Science Foundation of China (Grant NO.81273961 and NO.81303117), Science and Technology Foundation of Shenzhen City (Grant No. JCYJ20190812164009243), and Guangdong Medical Research Foundation (Grant No. B2020135).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.888073/full#supplementary-material
Bartlett, A., Bola, S., and Williams, R. (2015). Acute Tonsillitis and its Complications: An Overview. J. R. Nav. Med. Serv. 101 (1), 69–73. doi:10.1136/jrnms-101-69
Begg, C. B., and Mazumdar, M. (1994). Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 50 (4), 1088–1101. doi:10.2307/2533446
Bird, J. H., Biggs, T. C., and King, E. V. (2014). Controversies in the Management of Acute Tonsillitis: An Evidence-Based Review. Clin. Otolaryngol. 39 (6), 368–374. doi:10.1111/coa.12299
Branch, C. M. A. I. M. (2015). Guidelines for the Treatment of Severe Sepsis/Septic Shock in China (2014). Chin. Crit. Care Med. 27 (6), 401–426. doi:10.3760/j.issn.2095-4352.2015.06.001
Bro, R., and Smilde, A. K. (2014). Principal Component Analysis. Anal. Methods 6 (9), 2812–2831. doi:10.1039/C3AY41907J
Cao, Z. Y., Chang, X. J., Zhao, Z. P., Cao, L., and Xiao, W. (2015). Antiviral Effects of Reduning Injection against Enterovirus 71 and Possible Mechanisms of Action. Chin. J. Nat. Med. 13 (12), 881–888. doi:10.1016/s1875-5364(15)30093-5
Chen, X., Howard, O. M., Yang, X., Wang, L., Oppenheim, J. J., and Krakauer, T. (2002). Effects of Shuanghuanglian and Qingkailing, Two Multi-Components of Traditional Chinese Medicinal Preparations, on Human Leukocyte Function. Life Sci. 70 (24), 2897–2913. doi:10.1016/s0024-3205(02)01541-2
Dias, S., Sutton, A. J., Ades, A. E., and Welton, N. J. (2013). Evidence Synthesis for Decision Making 2: A Generalized Linear Modeling Framework for Pairwise and Network Meta-Analysis of Randomized Controlled Trials. Med. Decis. Mak. 33 (5), 607–617. doi:10.1177/0272989x12458724
Ebell, M. H., Smith, M. A., Barry, H. C., Ives, K., and Carey, M. (2000). The Rational Clinical Examination. Does This Patient Have Strep Throat? JAMA 284 (22), 2912–2918. doi:10.1001/jama.284.22.2912
Fan, C. Z., Miao, Q., Zhang, Q., Fan, M. R., Liao, X., Liu, J., et al. (2017). Advantages and Evidence of Chinese Medicine in Prevention and Treatment of Adult Acute Tonsillitis. Zhongguo Zhong Yao Za Zhi 42 (8), 1430–1438. doi:10.19540/j.cnki.cjcmm.2017.0039
Gao, G., Jin, J., Liu, N., Zhao, P., Wang, C., and Liu, Q. (2017). The Progress of Traditional Chinese Medicine in the Treatment of Acute Tonsillitis in Children. J. Emerg. Traditional Chin. Med. 26 (02), 268–270. doi:10.3969/j.issn.1004-745X.2017.02.026
Gao, X., Guo, M., Peng, L., Zhao, B., Su, J., Liu, H., et al. (2013). UPLC Q-TOF/MS-Based Metabolic Profiling of Urine Reveals the Novel Antipyretic Mechanisms of Qingkailing Injection in a Rat Model of Yeast-Induced Pyrexia. Evid. Based Complement. Altern. Med. 2013, 864747. doi:10.1155/2013/864747
Gao, X., Huang, C., Geng, T., Chen, X., Wang, J., Liu, J., et al. (2020). Serum and Urine Metabolomics Based on UPLC-Q-TOF/MS Reveals the Antipyretic Mechanism of Reduning Injection in a Rat Model. J. Ethnopharmacol. 250, 112429. doi:10.1016/j.jep.2019.112429
Guan, Y., and Cao, S. (2013). Observation on the Curative Effect of Yanhuning Injection in the Treatment of Rotavirus Diarrhea in Children. Chin. Med. Innov. 10 (7), 2. doi:10.3969/j.issn.1674-4985.2013.07.012
Han, H. (2012). In Andrographolide Study on the Effect of Ribavirin Reducing the CVA16 Replication. J. Community Med. 10 (12), 4. CNKI:SUN:SQYX.0.2012-12-016.
Higgins, J. P., and Thompson, S. G. (2002). Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Huang, K., Xu, D., Cao, Y., Ma, F., Zhang, G., Liu, R., et al. (2013). Comparison of the Effects of Recombinant Human Interferon α1b, Yanhuning and Xiyanping against Respiratory Syncytial Virus Infection. Chin. J. Exp. Clin. Infect. Dis. (Electronic Version) 7 (6), 5. doi:10.3877/cma.j.issn.1674-1358.2013.06.004
Huang, S. (2018). Related Factors and Countermeasures of Adverse Reactions in Yanhuning Injection. Smart Healthc. 4 (26), 2. doi:10.19335/j.cnki.2096-1219.2018.26.061
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern Med. 162 (11), 777–784. doi:10.7326/m14-2385
Ji, L., Huang, H., Jiang, M., Bai, G., and Luo, G. (2010). Simultaneous HPLC Determination of 11 Essential Compounds in Xuebijing Injection. China J. Chin. Materia Medica 35 (18), 2395–2398. doi:10.4268/cjcmm20101807
Jiang, M., Zhou, M., Han, Y., Xing, L., Zhao, H., Dong, L., et al. (2013). Identification of NF-κB Inhibitors in Xuebijing Injection for Sepsis Treatment Based on Bioactivity-Integrated UPLC-Q/TOF. J. Ethnopharmacol. 147 (2), 426–433. doi:10.1016/j.jep.2013.03.032
Kocher, J. J., and Selby, T. D. (2014). Antibiotics for Sore Throat. Am. Fam. Physician 90 (1), 23–24.
Liang, L., Pu, J., Gao, H., and Tai, G. (2012). Effect of Yanhuning Injection on Animal Models with Acute Pharyngitis and its Mechanism. Drug Eval. Res. 35 (003), 165–168. CNKI:SUN:YWPJ.0.2012-03-007.
Liu, X., Zhan, S., Wang, Q., Ye, S., Zhou, L., Li, L., et al. (2007). Comparative Study on the Inhibition of EB Virus Antigen Expression by Common Andrographis Herb Extract versus Aciclovir. Chin. J. Infect. Chemother. 7 (6), 3. doi:10.3321/j.issn:1009-7708.2007.06.015
Ma, S.-T., Liu, P.-X., Long, W., Yu, J., and Xu, Y. (2009). Effects of the Multi-Target Capability of Xuebijing and its Inflammatory Pharmacodynamic Material Basis. Acta Physico-Chimica Sin. 25 (10), 2080–2086. doi:10.3866/PKU.WHXB20090907
Mavridis, D., and Salanti, G. (2013). A Practical Introduction to Multivariate Meta-Analysis. Stat. Methods Med. Res. 22 (2), 133–158. doi:10.1177/0962280211432219
Morad, A., Sathe, N. A., Francis, D. O., McPheeters, M. L., and Chinnadurai, S. (2017). Tonsillectomy Versus Watchful Waiting for Recurrent Throat Infection: A Systematic Review. Pediatrics 139 (2), e20163490. doi:10.1542/peds.2016-3490
Pang, S., and Zhang, X. (2018). Incidence of Adverse Reactions of Traditional Chinese Medicine Injection and its Influencing Factors. Int. Med. Health Guid. News 024 (020), 3179–3182. doi:10.3760/cma.j.issn.1007-1245.2018.20.042
Qin, L., Zhang, Z., Guo, M., Zhang, Q., Wang, Q., Lu, Z., et al. (2016). Plasma Metabolomics Combined with Lipidomics Profiling Reveals the Potential Antipyretic Mechanisms of Qingkailing Injection in a Rat Model. Chem. Biol. Interact. 254, 24–33. doi:10.1016/j.cbi.2016.05.022
Salanti, G. (2012). Indirect and Mixed-Treatment Comparison, Network, or Multiple-Treatments Meta-Analysis: Many Names, Many Benefits, Many Concerns for the Next Generation Evidence Synthesis Tool. Res. Synth. Methods 3 (2), 80–97. doi:10.1002/jrsm.1037
Shang, W., Wang, X. S., Zou, D. Y., Zhang, Z. N., Liao, X. R., and Yuan, J. (2013). Antimicrobial Effects of Qingkailing Injection Extract and Combination Therapy of Qingkailing Injection and Antibiotics on Bacteria Carrying blaNDM-1 Resistance Gene. Zhongguo Zhong Xi Yi Jie He Za Zhi 33 (4), 506–509. CNKI:SUN:ZZXJ.0.2013-04-024.
Sidell, D., and Shapiro, N. L. (2012). Acute Tonsillitis. Infect. Disord. Drug Targets 12 (4), 271–276. doi:10.2174/187152612801319230
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A Revised Tool for Assessing Risk of Bias in Randomised Trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Stuck, A. E., Rubenstein, L. Z., and Wieland, D. (1998). Bias in Meta-Analysis Detected by a Simple, Graphical Test. Asymmetry Detected in Funnel Plot Was Probably Due to True Heterogeneity. BMJ 316 (7129), 469–461. doi:10.1136/bmj.316.7129.469
Su, H. X., Yao, S., Zhao, W. F., Li, M. J., Liu, J., Shang, W. J., et al. (2020). Anti-SARS-CoV-2 Activities In Vitro of Shuanghuanglian Preparations and Bioactive Ingredients. Acta Pharmacol. Sin. 41 (9), 1167–1177. doi:10.1038/s41401-020-0483-6
Tang, Y., Wang, Z., Huo, C., Guo, X., Yang, G., Wang, M., et al. (2018). Antiviral Effects of Shuanghuanglian Injection Powder against Influenza a Virus H5N1 In Vitro and In Vivo. Microb. Pathog. 121, 318–324. doi:10.1016/j.micpath.2018.06.004
van Valkenhoef, G., Dias, S., Ades, A. E., and Welton, N. J. (2016). Automated Generation of Node-Splitting Models for Assessment of Inconsistency in Network Meta-Analysis. Res. Synth. Methods 7 (1), 80–93. doi:10.1002/jrsm.1167
Wan, S. J., Wong, S. K. M., and Prusinkiewicz, P. (1988). An Algorithm for Multidimensional Data Clustering. ACM Trans. Math. Softw. 14 (2), 153–162. doi:10.1145/45054.45056
Wang, C., Shi, Q. P., Ding, F., Jiang, X. D., Tang, W., Yu, M. L., et al. (2019). Reevaluation of the Post-Marketing Safety of Xuebijing Injection Based on Real-World and Evidence-Based Evaluations. Biomed. Pharmacother. 109, 1523–1531. doi:10.1016/j.biopha.2018.10.190
Windfuhr, J. P., Toepfner, N., Steffen, G., Waldfahrer, F., and Berner, R. (2016). Clinical Practice Guideline: Tonsillitis I. Diagnostics and Nonsurgical Management. Eur. Arch. Otorhinolaryngol. 273 (4), 973–987. doi:10.1007/s00405-015-3872-6
Wu, B. L., He, W. X., Ke, M., Shang-Guan, X. F., He, G. F., and Huang, R. (2018). A Retrospective Analysis on 1330 Adverse Event Reports of Qingkailing in China: Further Perception of its Risks and Rational Use. Curr. Med. Sci. 38 (6), 1103–1108. doi:10.1007/s11596-018-1990-2
Xie, F., Xie, M., Yang, Y., Zhang, M., Xu, X., Liu, N., et al. (2020). Assessing the Anti-Inflammatory Mechanism of Reduning Injection by Network Pharmacology. Biomed. Res. Int. 2020, 6134098. doi:10.1155/2020/6134098
Xu, X., Zhang, J., Zheng, W., Yang, Z., Zhao, X., Wang, C., et al. (2021). Efficacy and Safety of Reduning Injection in the Treatment of COVID-19: A Randomized, Multicenter Clinical Study. Ann. Palliat. Med. 10 (5), 5146–5155. doi:10.21037/apm-20-2121
Yi, Y., Li, C. Y., Zhao, Y., Tian, J. Z., Wang, L. M., Pan, C., et al. (2021). Study on Synergistic Effect of Qingkailing Injection and Shengmai Injection on Organ Injury in Endotoxemia Rats. Zhongguo Zhong Yao Za Zhi 46 (16), 4193–4200. doi:10.19540/j.cnki.cjcmm.20210524.404
Yu, S., Wang, M., Ding, Y., Ye, L., and Chen, D. (2019). Analysis of 1452 Cases of Adverse Drug Reactions of Reduning Injection in Jiangsu Province. Chin. J. Pharmacoepidemiol. 28 (12), 800–804. CNKI:SUN:YWLX.0.2019-12-006.
Keywords: acute tonsillitis, Chinese herbal injections, western medicine, efficacy, 5-dimensional network meta-analysis
Citation: Huang P, Li Y, Huang B, Zhao S, Chen L, Guan H, Chen Y, Feng Y, Huang X, Deng Y, Lei S, Wu Q, Zhang H, Zeng Z, Zeng L and Chen B (2022) A Five-Dimensional Network Meta-Analysis of Chinese Herbal Injections for Treating Acute Tonsillitis Combined With Western Medicine. Front. Pharmacol. 13:888073. doi: 10.3389/fphar.2022.888073
Received: 02 March 2022; Accepted: 06 May 2022;
Published: 17 June 2022.
Edited by:
Carlos L. Cespedes-Acuña, University of Bio Bio Chillan Chile, ChileReviewed by:
Rao Sun, Huazhong University of Science and Technology, ChinaCopyright © 2022 Huang, Li, Huang, Zhao, Chen, Guan, Chen, Feng, Huang, Deng, Lei, Wu, Zhang, Zeng, Zeng and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bojun Chen, NzE5NTIzNDc2QHFxLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.