Abstract
The herb-pair ginseng-Fuzi (the root of Aconitum carmichaelii) is the material basis of Shenfu prescriptions and is popular in traditional Chinese medicine for the treatment of heart failure, and even shock with severe-stage of COVID-19. A narrow therapeutic window of Fuzi may cause significant regional loss of property and life in clinics. Therefore, systemic elucidation of active components is crucial to improve the safety dose window of Shenfu oral prescriptions. A high performance liquid chromatography-mass spectrometry method was developed for quantification of 10 aconitines in SD rat plasma within 9 min. The limit of detection and the limit of quantification were below 0.032 ng/ml and 0.095 ng/ml, respectively. Furthermore, a systemic comparison with their pharmacokinetic characteristics after oral administration of a safe dosage of 2 g/kg of Fuzi and ginseng-Fuzi decoction for 24 h was conducted. Eight representative diester, monoester, and non-ester aconitines and two new active components (i.e., songorine and indaconitine) were all adopted to elucidating the differences of the pharmacokinetic parameters in vivo. The compatibility of Fuzi and ginseng could significantly increase the in vivo exposure of active components. The terminal elimination half-life and the area under the concentration-time curve of mesaconitine, benzoylaconitine, benzoylmesaconitine, benzoylhypaconitine, and songorine were all increased significantly. The hypaconitine, benzoylmesaconitine, and songorine were regarded as the main active components in vivo, which gave an effective clue for the development of new Shenfu oral prescriptions.
1 Introduction
Toxic-efficient dual Chinese medicines have remarkable efficacy and certain toxicity or side effects. If used improperly, it will cause unavoidable toxic side effects, and even endanger patients’ lives in serious cases (Wei et al., 2019). Due to the irreplaceability in the potent effects, toxic-efficient dual Chinese medicines are still widely used in clinics. The lateral root of Aconitum carmichaelii Debx (named Fuzi) is commonly used for the treatment of rheumatism, heart failure, and renal failure (Wang et al., 2007; Li et al., 2017a; Shuo et al., 2017; Chen et al., 2021). However, it often triggers aconitine poisoning events due to a narrow therapeutic window (Singhuber et al., 2009; Huang et al., 2018; Qiu et al., 2021a). The main active components in Fuzi are aconitines, including diester alkaloids, i.e., aconitine, mesaconitine and hypaconitine, and monoester alkaloids, i.e., benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine (Qiu et al., 2021a; Qiu et al., 2021b). However, the diester alkaloids are considered to be the main toxic components for the cardiac and central nervous systems. The toxicity of diester alkaloids is 200–500 times and 2000–4,000 times of monoester alkaloids and non-ester alkaloids, i.e., aconine, mesaconine, and hypaconine, respectively (Liu et al., 2017). The cardiotoxicity target of diester alkaloids is the site 2 of sodium channel. Their cardiotoxicity mechanism is a large influx of Na+ causes persistent malignant arrhythmias (Chan et al., 1994; Fu et al., 2006; Chen et al., 2013).
Compatibility has been often used to reduce toxicity and increase efficacy (Zhang et al., 2012; Zhang et al., 2013; Liu et al., 2014; Liu et al., 2017; Sun et al., 2018; Qiu et al., 2020a). The pharmacokinetic characterizations of herb-pairs Fuzi-Gancao (Zhang et al., 2013; Zhang H. et al., 2015), Fuzi-ginger (Peng et al., 2013; Zhang W. et al., 2015), Fuzi-Beimu (Yang et al., 2016; Xu et al., 2017), and ginseng-Fuzi (Shenfu) (Li Z. et al., 2015; Yang et al., 2018), and formula [e.g., Wutou Decoction (Dai et al., 2014), Sini Decoction (He et al., 2009; Zhang H. et al., 2015; Zhang W. et al., 2015; Zhou et al., 2019), Dahuang Fuzi Decoction (Liu et al., 2014; Li YX. et al., 2015; Li et al., 2017b), and Shenfu injectable powder (Zhang et al., 2008; Li Z. et al., 2015; Zhang et al., 2016)] have been elucidated (He et al., 2015; Chen et al., 2019; Wei et al., 2019). Currently, the pharmacokinetic studies of aconites were usually reported on Shenfu injection (Zhang et al., 2008; Zhang et al., 2016; Shen et al., 2021). There is no other relevant study with simultaneous quantification of three types of aconitines for oral preparations containing Fuzi-ginseng herb pair (Xu et al., 2020). Shenfu preparations have been commonly used in the treatment of heart failure, and even the shock patient of severe-stage COVID-19. In daily life, the oral drugs are easily accepted by patients and have a promising market prospect in the treatment of chronic heart failure. However, their narrow oral safety dose window makes it difficult to effectively balance cardiac efficacy and cardiotoxicity, resulting in extremely low market share and difficulty in applying new oral drugs. It is urgent to elucidate the in vivo active components and compatibility mechanism to develop new oral drugs further.
High performance liquid chromatography coupled with tandem mass spectrometry (HPLC-MS/MS) contains the advantages of high throughput, high sensitivity, and high resolution for analysis of complex matrix samples (Garran et al., 2019). In this study, an HPLC-MS/MS method for quantification of 10 aconitines components with all three types of structure in rat plasma was developed (Figure 1). The method had a lower limit of detection (LOD) and limit of quantification (LOQ) than other methods (Zhang et al., 2014; Xu et al., 2020). The comparative pharmacokinetic study between Fuzi decoction with ginseng-Fuzi decoction was conducted to screen out the in vivo active components of Shenfu oral prescriptions. Moreover, the pharmacokinetic parameters of songorine and indaconitine were studied in Shenfu decoction for the first time. The aim is to lay the foundation for the scientific design of the prescription, dosage, and controlled active/toxic combinatorial components of Shenfu oral prescriptions.
FIGURE 1
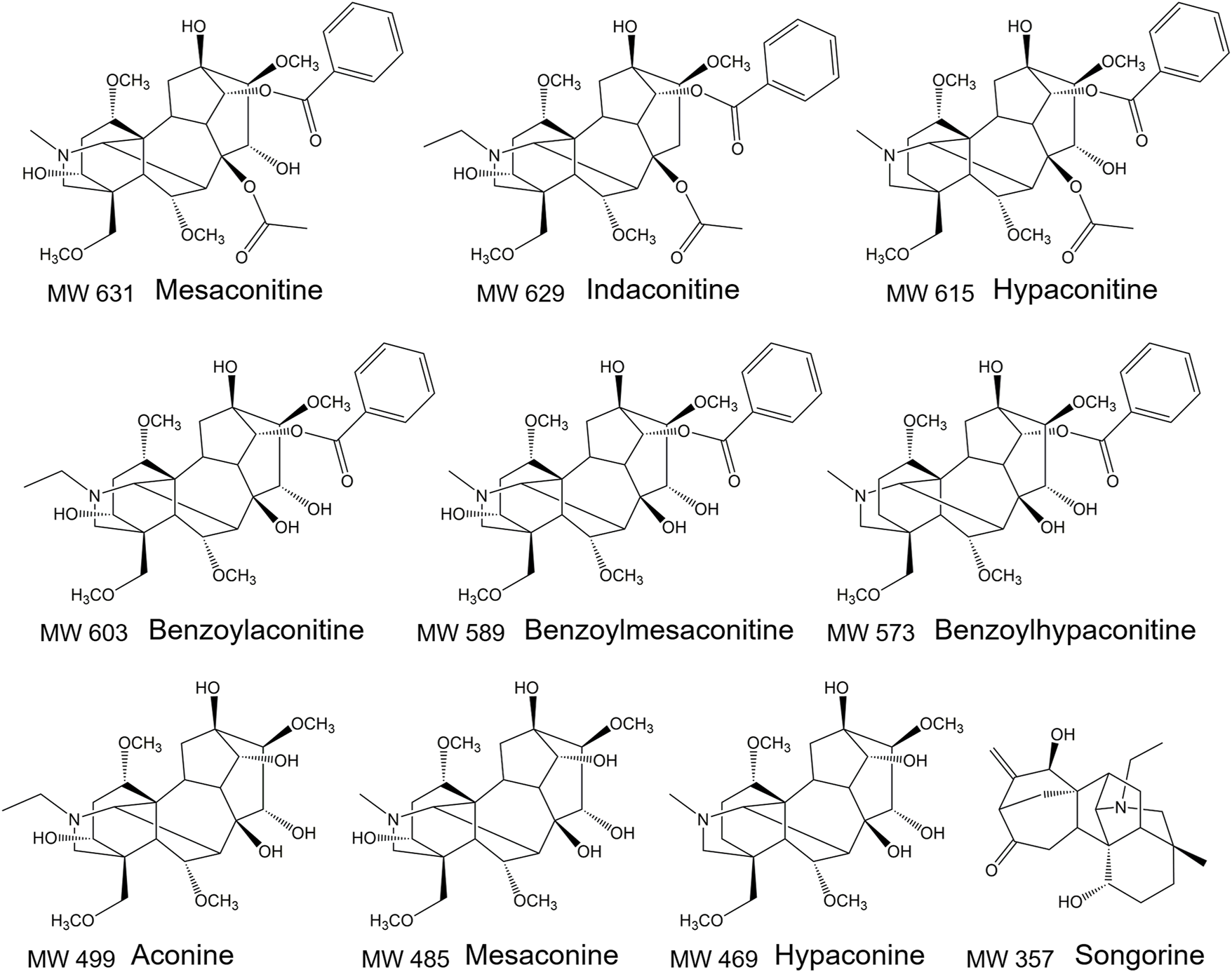
The structure of 10 aconitines.
2 Experimental
2.1 Materials and Reagents
Processed Aconitum (Heishunpian) was purchased from Sichuan, China. Ginseng was purchased from Tongrentang Chinese Medicine. The specimens were stored in the National Resource Center for Chinese Materia Medica, Chinese Academy of Chinese Medical Sciences. Berberine (internal standard) was purchased from ANPEL Laboratory Technologies (Shanghai) Inc. (Shanghai, China, purity >98%). Eight authentic components including mesaconitine, indaconitine, hypaconitine, benzoylaconitine, benzoylmesaconitine, benzoylhypaconitine, aconine, and songorine were supplied by Beijing Rongcheng Xinde Technology Development Co., Ltd. (Beijing, China, HPLC purity >98%). Another two authentic components including mesaconine and mesaconine were acquired from Chengdu Must Biotechnology Co., Ltd. (Chengdu, Sichuan, China). The purity of each component was >98%, as determined by HPLC analysis. Pure water was prepared from Mill-Q water purification system (Billerica, MA, United States). Methanol and acetonitrile (HPLC grade) were purchased from ThermoFisher Scientific (San Jose, CA, United States). Ammonium chloride (AR) was purchased from Aladdin Industrial Corporation (Shanghai, China).
2.2 Animals
Male Sprague-Dawley (SD) rats (n = 12) weighted 180–220 g were supplied by Laboratory Animal Science and Technology Center, Jiangxi University of Traditional Chinese Medicine (Nanchang, Jiangxi, China). Animals were housed under standard conditions for a week of adjustable feeding. All animal experiments were carried out according to the Guidelines for the Care and Use of Laboratory Animals and were approved by the Animal Ethics Committee of Jiangxi University of Traditional Chinese Medicine.
2.3 Preparation of Standard Solutions
A series of mixed working solutions at gradient concentrations were prepared by dissolving appropriate amounts of 10 aconitines with methanol and gradient dilution. The frozen plasma samples were thawed naturally at room temperature, 10 μL of mixed working solutions and 90 μL plasma were mixed and vortexed for 1 min with sufficient mixing. The 10 μL of internal standard solution (berberine, 500 ng/ml) and 300 μL of methanol were added. All samples were vortexed at 2,500 rpm for 3 min and centrifuged at 10,000 rpm for 10 min at 4°C. The supernatant was collected and was then dried under nitrogen at 40°C. The 100 μL of methanol was added to redissolve the residue. After vortexing for 1 min, the resolution was centrifuged at 14,000 rpm for 10 min at 4°C, and the supernatant was collected and stored at −20°C until analysis.
2.4 Sample Preparation
The 12.5 g of processed Fuzi powder were weighed and soaked for 30 min in water (1:10, w/v), then was decocted for 30 min. The filtrate through 8 layers of gauze was collected. The residues were re-decocted by 8 times of water for 30 min. The two filtrates were combined and concentrated by rotary evaporator at 40°C to 0.175 g/ml (in terms of Fuzi) of Fuzi extract was prepared, containing mesaconitine 0.03 μg/ml, indaconitine 0.12 μg/ml, hypaconitine 1.04 μg/ml, benzoylaconitine 10.16 μg/ml, benzoylmesaconitine 36.40 μg/ml, benzoylhypaconitine 14.28 μg/ml, aconine 2.04 μg/ml, mesaconine 6.02 μg/ml, hypaconine 2.74 μg/ml, songorine 9.04 μg/mL. As for Shenfu extract, the mass ratio of Fuzi and ginseng was 1:1. The other preparation steps were the same as those of Fuzi extract, containing 0.10 μg/ml of mesaconitine, 4.85 μg/ml of hypaconitine, 11.70 μg/ml of benzoylaconitine, 28.30 μg/ml of benzoylmesaconitine, 12.10 μg/ml of benzoylhypaconitine, 1.71 μg/ml of aconine, 5.31 μg/ml of mesaconine, 1.68 μg/ml of hypaconine, and 6.25 μg/ml of songorine. The quantification of alkaloids was performed according to our validated HPLC-MS/MS method. All extracts were stored at 4°C.
2.5 Liquid Chromatography With Tandem Mass Spectrometry Conditions
The Shimadzu LC-30AD (Kyoto, Japan) consisted of a binary pump and a sample manager was applied as the LC system. Gradient elution was performed on a Waters ACQUITY UPLC BEH C18 column (1.7 μm, 2.1 mm × 100 mm) protected by a Van Guard BEH C18 column (1.7 μm, 2.1 mm × 5 mm). The column temperature was maintained at 35°C. The experiment was carried out at a flow rate of 0.4 ml/min. The injection volume was 2 μL. The mobile phase was acetonitrile (solvent B)—water (solvent A) containing 0.5 mM ammonium chloride. Gradient elution was performed as follow: 0–2 min 35% B, 2–4 min 35–85% B, 4–6 min 85–90% B, 6–7 min 90–100% B, 7–9 min 100% B, 9–9.5 min 100–35% B, and 9.5–12.5 min 35% B. QTRAP 4500 mass spectrometer (Applied Bio-systems, AB Sciex, United States) coupled with ESI source was employed in the MS/MS analysis. Mass spectrum parameters were set as follows: Curtain Gas = 35 psi, Collision Gas = Medium, IonSpray Voltage = 4500 V, Temperature = 550°C, and Gas1 = Gas2 = 55 psi. MRM mode was adopted to detect the target components and internal standard (50 ng/ml). The DP and CE were automatically optimized to enhance the intensity of ion pairs of all the target components. All samples were analyzed by LC-MS in positive ion mode.
2.6 Method Validation
2.6.1 Specificity
The specificity was investigated by comparing the chromatograms of blank rat plasma, corresponding spiked plasma, and rat plasma sample at 45 min after oral administration of Fuzi, to exclude the interference of endogenous substances and metabolites.
2.6.2 Linearity, Limit of Detection, and Limit of Quantification
For the calibration curve, the gradient dilution was used to obtain a series of solutions with gradient concentrations (0.001–125 ng/ml) for LC-MS analysis. The regression equation and correlation coefficient (R2) were calculated using the concentration of the component as the horizontal coordinate (X, ng/mL) and the ratio of the integrated peak area of the component to the internal standard as the vertical coordinate (Y). The concentration was used as the LOD and the LOQ at the signal-to-noise ratio (S/N) equal to 3 and 10, respectively.
2.6.3 Precision and Stability
The QC samples of high, medium, and low concentrations were injected six times consecutively and replicated for three consecutive days, and the intra-day precision and precision were calculated and expressed as relative standard deviation (RSD). The stability assay of the high, medium, and low concentrations of mixed standards in plasma samples was conducted. All prepared samples were stored for 12 h at room temperature to evaluate their room temperature stability. As for freeze-thaw stability, the plasma samples were stored for 12 h at room temperature and then 12 h at −20°C, and repeated three times. The plasma samples of the long-term stability analysis should be stored for 15 days at −20°C. All samples were injected under the same conditions for LC-MS analysis and their mean concentration, standard deviation (SD), and RSD were calculated.
2.6.4 Recovery and Matrix Effect
The pre-extraction samples were prepared according to the preparation of standard solutions. The blank plasma was prepared with the same method, and then 10 μL of 500 ng/ml internal standard solution and 90 μL of mixed standard solution were added to redissolve the residue. These samples were recorded as post-extraction samples. All samples were analyzed by the same LC-MS conditions and the extraction recoveries were calculated according to formula 1.
The mixed working solutions were prepared in methanol with high, medium, and low concentrations respectively and analyzed by the same LC-MS conditions. The matrix effect was calculated according to formula 2.
2.7 Pharmacokinetics
Male SD rats were randomly divided into two groups of six rats each for Fuzi and Shenfu groups. The animals were acclimatized and fed for 7 days. Before the experiment, the animals fasted for 12 h without water. The animals were administered by the same dosage (equal to 2 g/kg of Fuzi). The blood was collected into heparinized tubes before (0 h), 0.25, 0.5, 0.75, 1, 1.5, 2.5, 4, 6, 8, 12, and 24 h after administration, and centrifuged at 4,000 rpm for 10 min at 4°C. 100 μL of supernatant was obtained and stored at −80°C before analysis. The corresponding peak area integration values were recorded. The concentration was calculated using the corresponding calibration equation.
2.8 Statistical Analysis
The drug concentration-time curve was plotted using time as the horizontal coordinate and the mean value of the blood concentration corresponding to each time point as the vertical coordinate. The relevant pharmacokinetic parameters, including terminal elimination half-life (T1/2), area under the concentration-time curve (AUC0-t), mean residence time (MRT0-t), time to achieve maximum concentration (Tmax), and maximum plasma concentration (Cmax), were calculated using non-compartment analysis with DAS software and expressed as mean ± standard deviation. The relative bioavailability was calculated by formula 3. The comparison of the main pharmacokinetic parameters between the Fuzi group and the Shenfu group was performed by independent samples t-test with SPSS software.
3 Result and Discussion
3.1 Optimization of High Performance Liquid Chromatography Coupled With Tandem Mass Spectrometry Conditions
The ion spray voltage (3.5–5.0 kV) and source temperature (350–550°C) were optimized in the positive ion mode to determine the mass spectrometry conditions in terms of the response intensity and noise intensity of the components. The final mass spectrometry conditions were determined as follows: positive ion mode Curtain Gas = 35 psi, Collision Gas = Medium, Ion Spray Voltage = 4500 V, Temperature = 550°C, Gas1 = Gas2 = 55 psi. The ESI-MS was injected at a flow rate of 7 μL/min and the optimized mass spectrometry conditions were used for the analysis. The collision energy (CE) and declustering potential (DP) were automatically optimized by the instrument. The final ion pairs and related parameters were determined as Table 1. The 0.5 mM ammonium chloride in the study had no significant inhibitory effect on aconitines with LOD below 0.03 ng/ml. Column temperatures were optimized at 30, 35, 40, and 45°C, and small differences were found. To avoid degradation of aconitines, which are easily decomposed by heat, during the analysis and resulting in reduced accuracy, 35°C was chosen as the analytical column temperature in this experiment and the sample tray temperature was set at 4°C. Ultimately, the 10 aconitines were all determined within 9 min (Figure 2).
TABLE 1
| Components | Retention time (min) | Q1 | Q3 | Time (ms) | DP (V) | CE (V) |
|---|---|---|---|---|---|---|
| Aconine | 0.70 | 500.1 | 450.4 | 25 | 100 | 48 |
| Mesaconine | 0.70 | 486.1 | 436.0 | 25 | 100 | 49 |
| Hypaconine | 0.70 | 470.2 | 438.4 | 25 | 120 | 44 |
| Songorine | 1.13 | 358.1 | 340.1 | 25 | 129 | 36 |
| Benzoylmesaconitine | 1.17 | 590.0 | 540.1 | 25 | 120 | 50 |
| Benzoylaconitine | 1.42 | 604.1 | 554.2 | 25 | 124 | 50 |
| Berberine | 1.54 | 336.0 | 320.1 | 25 | 115 | 41 |
| Benzoylhypaconitine | 1.58 | 574.1 | 542.0 | 25 | 120 | 45 |
| Mesaconitine | 2.96 | 632.4 | 572.1 | 25 | 120 | 47 |
| Hypaconitine | 3.44 | 616.1 | 556.2 | 25 | 80 | 44 |
| Indaconitine | 3.55 | 630.2 | 570.1 | 25 | 80 | 47 |
Ion pairs and the detailed parameters in MRM mode.
FIGURE 2
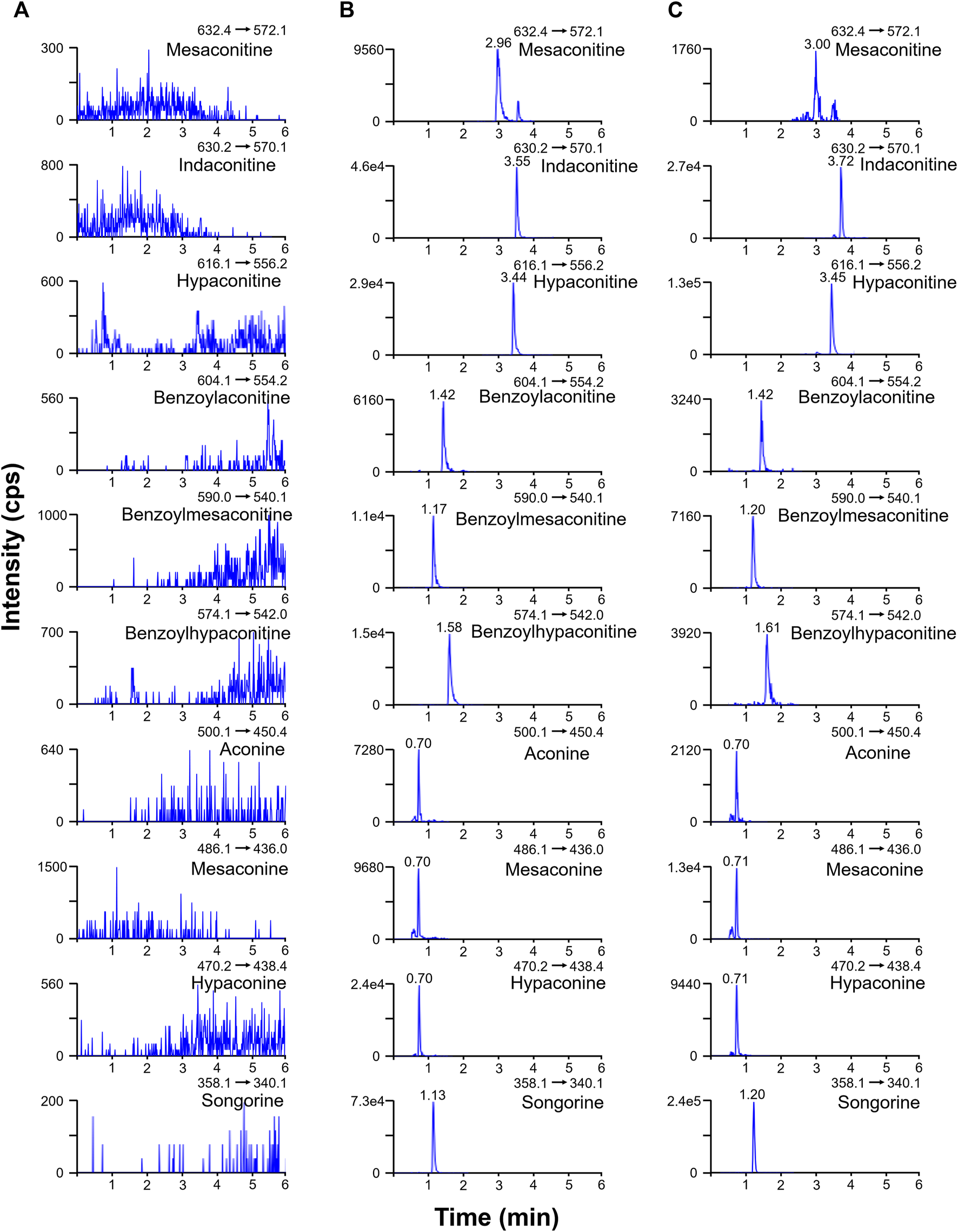
Multiple reaction monitoring chromatograms of blank plasma (A), spiked standard solution in blank plasma (B), and the rat plasma sample at 45 min after oral administration of Fuzi (C).
3.2 Results of Method Validation
Representative chromatograms of blank rat plasma, corresponding spiked plasma, and plasma samples from rats 45 min after oral administration are shown in Figure 2, indicating good specificity and no interference from endogenous substances and metabolites in the experiment. In blank plasma, all 10 aconitines were not detected. The calibration curve of the target components exhibited good linearity in the range of 0.0156–125 ng/ml, with R2 > 0.99 (Table 2). The LOD and LOQ of the target components were of 0.002–0.032 ng/ml and 0.006–0.095 ng/ml, respectively. For most components, the inter-day and intra-day precision with RSD were between 80 and 120% (Table 3) and were stable in short-term, long-term, and freeze-thaw experiments with RSD less than 10% (Table 4). The extraction recoveries and matrix effects for most components were in the range of 85–115% (Table 5), indicating that the pretreatment method met the requirements and the matrix had no significant effect on the target components. Generally, the quantification results were deemed accurate and reliable. Since its many measurement points of aconitine, a major diester alkaloid in raw Fuzi, were significantly below the lower limit of the linear calibration range of 0.125 ng/ml, it would be difficult to determine its concentration in real plasma samples. Therefore, the subsequent comparative pharmacokinetic study of aconitine was not carried out in this study.
TABLE 2
| Components | Calibration equation | R 2 | Linearity range (ng/ml) | LOD (ng/ml) | LOQ (ng/ml) |
|---|---|---|---|---|---|
| Mesaconitine | Y = 0.012X−3.44e−5 | 0.9974 | 0.0156–31.3 | 0.0020 | 0.0059 |
| Indaconitine | Y = 0.0271X+1.57e−4 | 0.9985 | 0.0625–31.3 | 0.0159 | 0.0477 |
| Hypaconitine | Y = 0.0171X+1.53e−3 | 0.9995 | 0.125–62.5 | 0.0318 | 0.0953 |
| Benzoylaconitine | Y = 0.00591X+0.14e−3 | 0.9930 | 0.0625–31.3 | 0.0079 | 0.0238 |
| Benzoylmesaconitine | Y = 0.00469X+5.87e−4 | 0.9962 | 0.0313–62.5 | 0.0040 | 0.0119 |
| Benzoylhypaconitine | Y = 0.0112X+4.74e−4 | 0.9960 | 0.0156–62.5 | 0.0020 | 0.0059 |
| Aconine | Y = 0.00317X−3.79e−5 | 0.9913 | 0.0625–7.81 | 0.0159 | 0.0477 |
| Mesaconine | Y = 0.00333X+8.12e−4 | 0.9964 | 0.0625–125 | 0.0079 | 0.0238 |
| Hypaconine | Y = 0.00835X+0.01e−1 | 0.9956 | 0.0625–31.3 | 0.0079 | 0.0238 |
| Songorine | Y = 0.0176X+6.58e−4 | 0.9956 | 0.0625–62.5 | 0.0079 | 0.0238 |
Linearity, LOD, and LOQ of target components.
TABLE 3
| Compound | Spiked (ng/ml) | Inter-day precision | Intra-day precision | ||||
|---|---|---|---|---|---|---|---|
| Mean (ng/ml) | RSD (%) | Accuracy (%) | Mean (ng/ml) | RSD (%) | Accuracy (%) | ||
| Mesaconitine | 0.5 | 0.47 | 7.72 | 94.33 | 0.46 | 2.08 | 91.17 |
| 2 | 2.17 | 6.40 | 108.50 | 2.00 | 2.76 | 99.83 | |
| 20 | 21.17 | 4.23 | 105.83 | 20.00 | 1.50 | 100.00 | |
| Indaconitine | 0.5 | 0.57 | 2.32 | 113.28 | 0.57 | 0.34 | 114.80 |
| 2 | 1.71 | 5.14 | 85.49 | 1.60 | 2.86 | 80.16 | |
| 20 | 16.73 | 5.54 | 83.66 | 15.90 | 2.86 | 79.49 | |
| Hypaconitine | 0.5 | 0.59 | 8.11 | 118.36 | 0.59 | 0.79 | 117.74 |
| 2 | 1.80 | 3.92 | 90.04 | 1.63 | 1.86 | 81.70 | |
| 20 | 19.41 | 5.75 | 97.05 | 18.83 | 0.61 | 94.15 | |
| Benzoylaconitine | 0.5 | 0.47 | 7.75 | 94.09 | 0.48 | 4.61 | 95.84 |
| 2 | 2.28 | 1.12 | 113.96 | 2.13 | 1.84 | 106.56 | |
| 20 | 21.08 | 8.41 | 105.38 | 20.77 | 1.32 | 103.84 | |
| Benzoylmesaconitine | 0.5 | 0.58 | 2.26 | 115.31 | 0.57 | 4.18 | 113.31 |
| 2 | 1.72 | 5.51 | 85.81 | 1.68 | 2.56 | 83.90 | |
| 20 | 20.23 | 7.15 | 101.14 | 18.33 | 3.98 | 91.67 | |
| Benzoylhypaconitine | 0.5 | 0.45 | 2.94 | 90.00 | 0.42 | 3.88 | 84.50 |
| 2 | 2.16 | 4.70 | 108.16 | 2.03 | 1.59 | 101.33 | |
| 20 | 21.89 | 5.20 | 109.44 | 20.95 | 0.71 | 104.75 | |
| Aconine | 0.5 | 0.57 | 4.98 | 113.53 | 0.59 | 5.64 | 117.85 |
| 2 | 1.86 | 12.01 | 92.89 | 1.62 | 3.52 | 81.15 | |
| 5 | 4.86 | 7.46 | 97.14 | 4.77 | 2.18 | 95.31 | |
| Mesaconine | 0.5 | 0.61 | 16.76 | 122.50 | 0.56 | 3.36 | 111.83 |
| 2 | 2.19 | 3.20 | 109.26 | 2.12 | 12.80 | 106.02 | |
| 20 | 21.33 | 4.92 | 106.67 | 21.20 | 4.72 | 106.00 | |
| Hypaconine | 0.5 | 0.56 | 8.90 | 112.97 | 0.54 | 1.47 | 107.06 |
| 2 | 2.20 | 9.06 | 110.02 | 1.94 | 2.84 | 96.85 | |
| 20 | 21.79 | 4.69 | 108.96 | 21.36 | 0.71 | 106.82 | |
| Songorine | 0.5 | 0.43 | 4.62 | 86.50 | 0.43 | 0.34 | 85.33 |
| 2 | 2.18 | 3.05 | 109.17 | 2.17 | 3.33 | 108.67 | |
| 20 | 21.87 | 2.68 | 109.33 | 22.03 | 1.46 | 110.17 | |
Precision of target compounds.
TABLE 4
| Compound | Spiked ng/mL | Short-term | Long-term | 3 times freeze-thaw | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD (ng/ml) | RSD (%) | Mean ± SD (ng/ml) | RSD (%) | Mean ± SD (ng/ml) | RSD (%) | ||
| Mesaconitine | 0.5 | 0.47 ± 0.04 | 7.63 | 0.46 ± 0.01 | 1.90 | 0.45 ± 0.01 | 2.11 |
| 2 | 2.16 ± 0.15 | 6.98 | 2.01 ± 0.08 | 3.89 | 2.05 ± 0.08 | 3.70 | |
| 20 | 21.03 ± 1.02 | 4.86 | 20.13 ± 0.51 | 2.55 | 20.43 ± 0.38 | 1.85 | |
| Indaconitine | 0.5 | 0.57 ± 0.01 | 0.92 | 0.57 ± 0.01 | 2.30 | 0.56 ± 0.01 | 1.97 |
| 2 | 1.70 ± 0.10 | 5.92 | 1.61 ± 0.05 | 3.09 | 1.63 ± 0.07 | 4.07 | |
| 20 | 16.77 ± 0.90 | 5.34 | 15.86 ± 0.39 | 2.43 | 15.96 ± 0.41 | 2.58 | |
| Hypaconitine | 0.5 | 0.59 ± 0.05 | 8.07 | 0.59 ± 0.01 | 1.41 | 0.58 ± 0.03 | 5.54 |
| 2 | 1.78 ± 0.10 | 5.70 | 1.65 ± 0.06 | 3.64 | 1.72 ± 0.11 | 6.68 | |
| 20 | 19.44 ± 1.09 | 5.63 | 18.80 ± 0.13 | 0.70 | 18.75 ± 0.04 | 0.23 | |
| Benzoylaconitine | 0.5 | 0.48 ± 0.02 | 4.95 | 0.47 ± 0.03 | 7.45 | 0.46 ± 0.02 | 4.41 |
| 2 | 2.25 ± 0.08 | 3.42 | 2.16 ± 0.8 | 3.81 | 2.21 ± 0.11 | 4.94 | |
| 20 | 21.30 ± 1.67 | 7.85 | 20.55 ± 0.27 | 1.34 | 20.33 ± 0.53 | 2.60 | |
| Benzoylmesaconitine | 0.5 | 0.58 ± 0.01 | 2.54 | 0.57 ± 0.02 | 4.15 | 0.58 ± 0.01 | 2.26 |
| 2 | 1.73 ± 0.09 | 5.22 | 1.67 ± 0.04 | 2.25 | 1.65 ± 0.02 | 1.44 | |
| 20 | 19.92 ± 1.58 | 7.94 | 18.64 ± 1.25 | 6.72 | 18.99 ± 1.03 | 5.42 | |
| Benzoylhypaconitine | 0.5 | 0.45 ± 0.01 | 3.22 | 0.42 ± 0.02 | 4.50 | 0.43 ± 0.02 | 4.73 |
| 2 | 2.14 ± 0.13 | 6.02 | 2.05 ± 0.05 | 2.25 | 2.09 ± 0.03 | 1.54 | |
| 20 | 21.67 ± 1.29 | 5.97 | 21.16 ± 0.32 | 1.52 | 21.21 ± 0.27 | 1.26 | |
| Aconine | 0.5 | 0.55 ± 0.04 | 6.75 | 0.55 ± 0.03 | 5.19 | 0.53 ± 0.02 | 3.54 |
| 2 | 1.70 ± 0.11 | 6.65 | 1.73 ± 0.28 | 16.39 | 1.73 ± 0.24 | 13.63 | |
| 5 | 4.82 ± 0.36 | 7.49 | 4.72 ± 0.09 | 1.95 | 4.51 ± 0.18 | 4.02 | |
| Mesaconine | 0.5 | 0.57 ± 0.07 | 13.05 | 0.59 ± 0.04 | 6.25 | 0.58 ± 0.10 | 18.00 |
| 2 | 2.24 ± 0.11 | 4.83 | 2.15 ± 0.12 | 5.79 | 2.22 ± 0.04 | 1.88 | |
| 20 | 21.63 ± 1.16 | 5.36 | 20.90 ± 0.61 | 2.91 | 20.93 ± 0.55 | 2.63 | |
| Hypaconine | 0.5 | 0.55 ± 0.05 | 8.89 | 0.55 ± 0.03 | 5.29 | 0.54 ± 0.04 | 7.17 |
| 2 | 2.07 ± 0.12 | 5.66 | 2.07 ± 0.15 | 7.23 | 2.10 ± 0.26 | 12.32 | |
| 20 | 21.64 ± 1.05 | 4.85 | 21.52 ± 0.30 | 1.41 | 21.26 ± 0.53 | 2.50 | |
| Songorine | 0.5 | 0.42 ± 0.01 | 2.45 | 0.44 ± 0.02 | 3.50 | 0.43 ± 0.02 | 4.69 |
| 2 | 2.19 ± 0.07 | 3.20 | 2.17 ± 0.07 | 3.07 | 2.18 ± 0.08 | 3.57 | |
| 20 | 21.97 ± 0.67 | 3.03 | 21.93 ± 0.15 | 0.70 | 21.73 ± 0.47 | 2.17 | |
Stability of target compounds.
TABLE 5
| Compound | Spiked ng/mL | Recovery | Matrix effect | ||
|---|---|---|---|---|---|
| Mean (%) | RSD (%) | Mean (%) | RSD (%) | ||
| Mesaconitine | 0.5 | 105.22 | 2.96 | 90.38 | 3.81 |
| 2 | 104.61 | 4.49 | 87.66 | 4.92 | |
| 20 | 109.87 | 2.19 | 90.09 | 7.72 | |
| Indaconitine | 0.5 | 101.55 | 4.56 | 97.81 | 8.15 |
| 2 | 105.44 | 6.74 | 95.51 | 5.93 | |
| 20 | 110.96 | 3.15 | 97.29 | 7.00 | |
| Hypaconitine | 0.5 | 109.50 | 3.08 | 106.81 | 3.93 |
| 2 | 107.67 | 4.24 | 108.32 | 7.45 | |
| 20 | 109.67 | 2.17 | 86.04 | 7.05 | |
| Benzoylaconitine | 0.5 | 111.88 | 9.46 | 95.65 | 3.69 |
| 2 | 109.76 | 7.71 | 110.54 | 9.95 | |
| 20 | 106.18 | 2.43 | 97.94 | 8.38 | |
| Benzoylmesaconitine | 0.5 | 103.44 | 4.52 | 83.35 | 2.12 |
| 2 | 102.78 | 4.95 | 88.45 | 3.03 | |
| 20 | 108.75 | 4.82 | 109.16 | 5.42 | |
| Benzoylhypaconitine | 0.5 | 107.03 | 2.10 | 90.72 | 0.84 |
| 2 | 104.04 | 2.96 | 105.93 | 7.47 | |
| 20 | 105.69 | 2.93 | 94.86 | 5.77 | |
| Aconine | 0.5 | 104.41 | 7.08 | 87.82 | 6.21 |
| 2 | 106.76 | 4.91 | 105.70 | 13.84 | |
| 5 | 100.69 | 5.53 | 116.04 | 10.92 | |
| Mesaconine | 0.5 | 101.67 | 10.84 | 110.47 | 14.64 |
| 2 | 105.51 | 14.35 | 108.25 | 7.43 | |
| 20 | 109.49 | 7.67 | 108.84 | 9.95 | |
| Hypaconine | 0.5 | 98.20 | 9.88 | 97.60 | 11.17 |
| 2 | 100.90 | 8.22 | 83.20 | 7.74 | |
| 20 | 100.82 | 11.04 | 116.22 | 9.86 | |
| Songorine | 0.5 | 83.24 | 12.37 | 87.25 | 4.35 |
| 2 | 85.17 | 2.16 | 102.45 | 7.06 | |
| 20 | 95.18 | 1.75 | 93.01 | 7.81 | |
Recovery and matrix effect of PK method.
3.3 Comparative Pharmacokinetic Study
As can be seen from Figure 3, in terms of the overall trend, a distinct peak shape is visible in both the Fuzi and Shenfu groups. In the plasma concentration-time curve of the diester alkaloids and monoester alkaloids, double peaks were evident (e.g., benzoylaconitine). This has been similar in other studies (Liu et al., 2014; Li et al., 2016; Zhang et al., 2020). This biabsorption phenomenon may come from multiple-sites absorption and enterohepatic circulation (Zhang et al., 2020). In Fuzi and Shenfu decoctions, the monoester alkaloids benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine were the main class of components. However, the monoester alkaloids, non-ester alkaloids, and hypaconitine were the main components in vivo. This compatibility had a significant decrease of in vivo exposure of an active diester alkaloid indaconitine (Yu et al., 2021). This may be because some components of ginseng prevent the dissolution of indaconitine in Shenfu decoction. The short Tmax and T1/2 of the aconitines (Table 6) exhibited the distinct characteristics of fast absorption and rapid elimination after oral administration of the extract of Fuzi (Song et al., 2015; Xu et al., 2017) and Shenfu. In contrast, the long T1/2 of some components (e.g., benzoylmesaconitine in the Shenfu group) may be since half of the Cmax had not yet been reached at the end of the 24 h experiment. It is noteworthy that in this study, the minor diester alkaloid yunaconitine was not detected in Fuzi and Shenfu decoctions and the rat plasma.
FIGURE 3
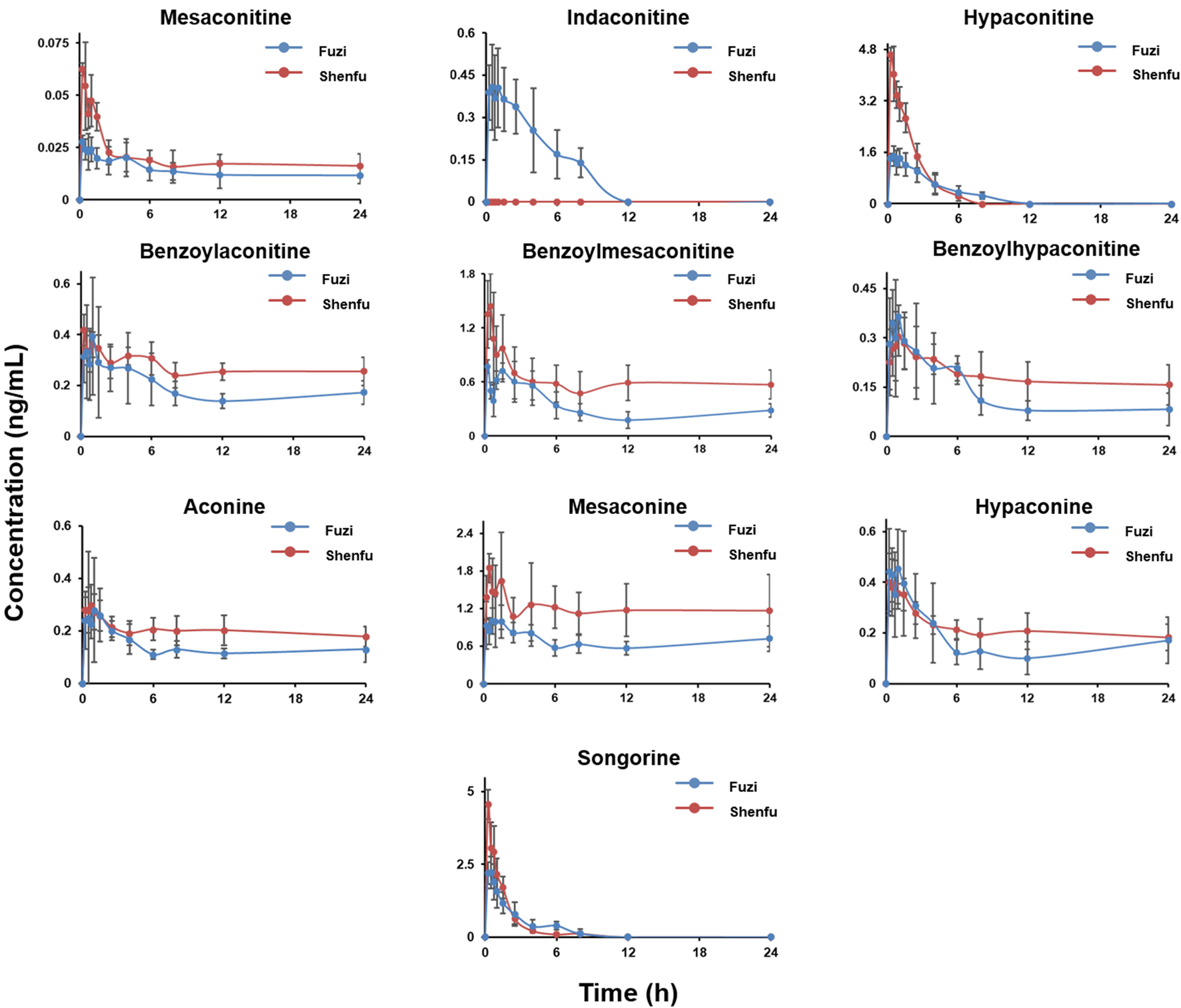
The concentration-time profile of aconitines after oral administration of Fuzi and Shenfu. The dosage of Fuzi is all 2 g/kg in two groups.
TABLE 6
| Components | Fuzi | Shenfu | Relative bioavailability/(Frel, %) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| T 1/2/h | AUC 0-t/(ng/mL·min) | MRT 0-t/(ng/mL·min) | T max/h | C max/(ng/ml) | T 1/2/h | AUC 0-t/(ng/mL·min) | MRT 0-t/(ng/mL·min) | T max/h | C max/(ng/ml) | ||
| Mesaconitine | 21.74 ± 3.85 | 0.33 ± 0.07 | 10.73 ± 1.29 | 0.50 ± 0.09 | 0.02 ± 0.00 | 89.71 ± 9.32*** | 0.46 ± 0.07** | 10.58 ± 1.80 | 0.50 ± 0.07 | 0.05 ± 0.00 | 144.00 ± 35.83 |
| Indaconitine | 2.36 ± 0.35 | 2.29 ± 0.62 | 3.88 ± 0.58 | 0.50 ± 0.10 | 0.41 ± 0.15 | - | - | - | - | - | - |
| Hypaconitine | 2.07 ± 0.53 | 6.16 ± 0.64 | 3.29 ± 0.50 | 0.50 ± 0.10 | 1.48 ± 0.34 | 2.65 ± 0.50 | 8.93 ± 1.10*** | 1.94 ± 0.26** | 0.50 ± 0.06 | 4.04 ± 0.16*** | 146.12 ± 22.30 |
| Benzoylaconitine | 9.28 ± 0.18 | 4.47 ± 0.54 | 10.79 ± 2.16 | 1.00 ± 0.07 | 0.39 ± 0.22 | 80.39 ± 16.01 | 6.43 ± 0.90** | 11.59 ± 1.39 | 1.00 ± 0.12 | 0.37 ± 0.05 | 144.94 ± 23.37 |
| Benzoylmesaconitine | 6.48 ± 1.03 | 7.56 ± 0.91 | 10.02 ± 0.80 | 1.50 ± 0.21 | 0.83 ± 0.07 | 524.59 ± 99.67*** | 14.55 ± 3.64** | 11.38 ± 1.71 | 0.50 ± 0.06*** | 1.44 ± 0.36** | 195.96 ± 53.55 |
| Benzoylhypaconitine | 4.65 ± 0.65 | 3.11 ± 0.53 | 8.79 ± 1.41 | 1.00 ± 0.11 | 0.36 ± 0.03 | 32.55 ± 5.35*** | 4.42 ± 0.51** | 10.91 ± 1.31* | 1.00 ± 0.15 | 0.30 ± 0.05* | 147.07 ± 37.80 |
| Aconine | - | 3.30 ± 0.43 | 11.05 ± 2.80 | 1.00 ± 0.04 | 0.28 ± 0.12 | 89.78 ± 14.78 | 4.78 ± 1.04* | 11.49 ± 1.42 | 0.75 ± 0.08*** | 0.30 ± 0.07 | 147.42 ± 43.85 |
| Mesaconine | 124.13 ± 18.62 | 16.31 ± 2.61 | 11.74 ± 2.00 | 0.75 ± 0.09 | 1.00 ± 0.16 | 304.61 ± 57.87*** | 28.64 ± 3.44*** | 11.81 ± 1.65 | 0.50 ± 0.10** | 1.85 ± 0.22*** | 177.17 ± 17.37 |
| Hypaconine | 18.31 ± 2.01 | 3.98 ± 1.35 | 10.61 ± 3.60 | 1.00 ± 0.12 | 0.45 ± 0.17 | 75.83 ± 13.64 | 5.15 ± 0.77 | 10.98 ± 2.74 | 0.75 ± 0.07** | 0.39 ± 0.11 | 141.60 ± 47.45 |
| Songorine | 2.17 ± 0.31 | 5.56 ± 0.67 | 2.77 ± 0.66 | 0.50 ± 0.07 | 2.22 ± 0.53 | 2.56 ± 0.53 | 5.69 ± 0.71 | 2.00 ± 0.38* | 0.50 ± 0.02 | 3.07 ± 0.33** | 103.05 ± 20.96 |
Comparison with the pharmacokinetic parameters of aconitines in Fuzi before and after compatibility of ginseng (mean ± SD, n = 6).
*p < 0.05; **p < 0.01; ***p < 0.001.
The minimum toxic doses of mesaconitine and hypaconitine in humans have been reported as 0.0035 and 0.0162 mg/kg, respectively (Qiu et al., 2020b), which can be converted to 21.88 and 101.25 μg/kg in rats (Huang et al., 2004). The doses of the two diester alkaloids in this experiment were 0.34 μg/kg for mesaconitine and 11.86 μg/kg for hypaconitine in Fuzi decoction, and 1.80 μg/kg for mesaconitine and 87.03 μg/kg for hypaconitine in Shenfu decoction. After the application of our developed toxicity prediction method (Qiu et al., 2020a; Qiu et al., 2020b), it was found that the in vivo holistic weighted toxicity (HWT) value was less than 1, indicating all alkaloids were below the minimum toxic doses. Therefore, the three diester alkaloids showed no toxicity but only medicinal effects under the present conditions. As Table 6 shown, in vivo exposure of Fuzi and Shenfu groups, the hypaconitine and benzoylmesaconitine are representatively active components of the diester alkaloid and monoester alkaloid, respectively. As for compatibility, the mechanism of drug interactions is complicated. This study attempts to clarify these interactions between aconitines and ginsenosides. Comparing the drug concentration-time curves of Fuzi group and Shenfu group (Figure 3), it was found that in some alkaloids with higher absorption (e.g., hypaconitine and songorine), the Shenfu group decreased to plateau more quickly than the Fuzi group. However, their AUC0-t values of Shenfu groups (5.69 for songorine, 8.93 for hypaconitine) were higher than those of Fuzi group (5.56 for songorine, 6.16 for hypaconitine), indicating the hypaconitine and songorine in the Shenfu group was significantly faster than those of Fuzi group in the elimination phase. The main reason might be that the ginsenoside Rg1 could promote absorption of aconitines (Xu et al., 2020) and up-regulate in vivo expression of CYP450 for accelerating the metabolism of hypaconitine and songorine (Li et al., 2019). The exposure concentrations of other aconitines were higher in Shenfu group, especially for the diester alkaloid (i.e., mesaconitine), monoester alkaloids (i.e., benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine) (He et al., 2015; Xie et al., 2021), and non-ester alkaloids (i.e., aconine and mesaconine). Their T1/2 and AUC0-t would be significantly increased in Shenfu group (Table 6), which may be caused by the inhibitory of the P-glycoprotein (P-gp)-mediated aconitines efflux by in vivo metabolites of ginsenosides (Chen et al., 2009; Tang et al., 2012; Li et al., 2014). These phenomena were consistent with Xu’s study (Xu et al., 2020). Among all exposed components, songorine, a non-ester alkaloid with good anti-arrhythmic effects (Dzhakhangirov et al., 1997; Khan et al., 2018) and cardioprotection efficacy (Li et al., 2021), showed a larger Cmax within 1 h, which could effectively eliminate the potential cardiotoxicity of the diester alkaloids (e.g., mesaconitine and hypaconitine). In short, the compatibility of Fuzi and ginseng could significantly increase the in vivo exposure of the active ingredients.
4 Conclusion
An HPLC-MS-based method was developed for the quantification of 10 aconitines in rat plasma within 9 min, with the LOD of 0.002–0.032 ng/ml and LOQ of 0.006–0.095 ng/ml. A comparative pharmacokinetic study was conducted in SD rats orally administered with the Fuzi and Shenfu decoction. Under safe dosage, it was found that for most alkaloids, including diester type alkaloids (mesaconitine and hypaconitine) and monoester alkaloids (benzoylaconitine, benzoylmesaconitine, and benzoylhypaconitine), were exposed more in Shenfu group (AUC0-t were 0.46–14.55 ng/mlmin) than in Fuzi group (AUC0-t were 0.33–7.56 ng/mlmin). Except for the hypaconitine, other components were metabolized more slowly in Shenfu group than in Fuzi group. Therefore, the compatibility of Fuzi and ginseng could significantly increase the bioavailability (103.05–195.96%) and efficiency of active components in vivo. songorine containing a potential anti-cardiotoxicity ability showed a larger Cmax. Ultimately, the hypaconitine, benzoylmesaconitine, and songorine could be considered as the main active components in Shenfu oral prescriptions. This study aims to achieve clinical “efficacy enhancement” and lay the foundation for the scientific design of new Shenfu oral prescriptions.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The animal study was reviewed and approved by the experimental animal ethics committee of Jiangxi University of traditional Chinese Medicine.
Author contributions
C-J-SL and L-QH designed and supported the research. Z-YC, X-YW, Z-DQ, TT and Y-LF conducted the research. X-YW, Z-YC, YH, JG, G-HC and C-J-SL analyzed the data and wrote the manuscript. C-J-SL and L-QH had primary responsibility for the final content.
Funding
This work was supported by the National Natural Science Foundation of China (No. 82074012), the CACMS Innovation Fund (No. CI2021A05051), and the Fundamental Research Funds for the Central Public Welfare Research Institutes (No. ZZ13-YQ-090-C1).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Chan T. Y. Tomlinson B. Tse L. K. Chan J. C. Chan W. W. Critchley J. A. (1994). Aconitine Poisoning Due to Chinese Herbal Medicines: A Review. Vet. Hum. Toxicol.36 (5), 452–455.
2
Chen L. Yang J. Davey A. K. Chen Y. X. Wang J. P. Liu X. Q. (2009). Effects of Diammonium Glycyrrhizinate on the Pharmacokinetics of Aconitine in Rats and the Potential Mechanism. Xenobiotica39 (12), 955–963. 10.3109/00498250903271997
3
Chen L. L. Lai C. J. Mao L. Y. Yin B. W. Tian M. Jin B. L. et al (2021). Chemical Constituents in Different Parts of Seven Species of Aconitum Based on UHPLC-Q-TOF/MS. J. Pharm. Biomed. Anal.193, 113713. 10.1016/j.jpba.2020.113713
4
Chen R. C. Sun G. B. Zhang Q. Ye Z. G. Sun X. B. (2013). Advances in Studies on Toxicity of Aconite. Zhongguo Zhong Yao Za Zhi38 (8), 1126–1129.
5
Chen Y. Liang X. G. Liu H. N. Xiong Y. L. Sun R. J. Yan X. J. et al (2019). Different Compatibility Ratio and Clinical Application of Shenfutang. Chin. J. Exp. Tradit. Med. Form.25 (3), 220–225. 10.13422/j.cnki.syfjx.20190331
6
Dai P. M. Wang Y. Ye L. Zeng S. Zheng Z. J. Li Q. et al (2014). Pharmacokinetic Comparisons of Benzoylmesaconine in Rats Using Ultra-performance Liquid Chromatography-Tandem Mass Spectrometry after Administration of Pure Benzoylmesaconine and Wutou Decoction. Molecules19 (10), 16757–16769. 10.3390/molecules191016757
7
Dzhakhangirov F. N. Sultankhodzhaev M. N. Tashkhodzhaev B. Salimov B. T. (1997). Diterpenoid Alkaloids as a New Class of Antiarrhythmic Agents. Structure-Activity Relationship. Chem. Nat. Compd.33 (2), 190–202. 10.1007/bf02291540
8
Fu M. Wu M. Qiao Y. Wang Z. Wang Z. (2006). Toxicological Mechanisms of Aconitum Alkaloids. Pharmazie61 (9), 735–741.
9
Garran T. A. Ji R. Chen J. L. Xie D. Guo L. Huang L. Q. et al (2019). Elucidation of Metabolite Isomers of Leonurus Japonicus and Leonurus Cardiaca Using Discriminating Metabolite Isomerism Strategy Based on Ultra-high Performance Liquid Chromatography Tandem Quadrupole Time-Of-Flight Mass Spectrometry. J. Chromatogr. A.1598, 141–153. 10.1016/j.chroma.2019.03.059
10
He J. L. Zhao J. W. Ma Z. C. Wang Y. G. Liang Q. D. Tan H. L. et al (20152015). Serum Pharmacochemistry Analysis Using UPLC-Q-TOF/MS after Oral Administration to Rats of Shenfu Decoction. Evid. Based Complement. Alternat Med.2015, 973930. 10.1155/2015/973930
11
He L. P. Di B. Du Y. X. Yan F. Su M. X. Liu H. Q. et al (2009). Development and Validation of a High-Performance Liquid Chromatography-Tandem Mass Spectrometry Method for the Rapid Simultaneous Quantification of Aconitine, Mesaconitine, and Hypaconitine in Rat Plasma after Oral Administration of Sini Decoction. J. Anal. Toxicol.33, 588–594. 10.1093/jat/33.9.588
12
Huang G. Yang L. Zhou W. Tang X. Wang Y. Ma Z. et al (2018). Study on Cardiotoxicity and Mechanism of "Fuzi" Extracts Based on Metabonomics. Int. J. Mol. Sci.19 (11), 3506. 10.3390/ijms19113506
13
Huang J. H. Huang X. H. Chen Z. Y. Zheng Q. S. Sun R. Y. (2004). Dose Conversion Among Different Animals and Healthy Volunteers in Pharmacological Study. Chin. J. Clin. Pharmacol. Ther.9, 1069–1072.
14
Khan H. Nabavi S. M. Sureda A. Mehterov N. Gulei D. Berindan-Neagoe I. et al (2018). Therapeutic Potential of Songorine, a Diterpenoid Alkaloid of the Genus Aconitum. Eur. J. Med. Chem.153 (10), 29–33. 10.1016/j.ejmech.2017.10.065
15
Li H. Zhang G. Ma M. Su P. Yang Y. Chen T. et al (2019). Study on Regulation of CYP450 Enzyme System to Reduce Liver Toxicity through the Compatibility of Radix Aconiti Lateralis Praeparata with Panax Ginseng C. A. Mey and Glycyrrhiza Uralensis Fisch. Chin. J. New Drug28 (24), 2948–2953.
16
Li N. Wang D. Ge G. Wang X. Liu Y. Yang L. (2014). Ginsenoside Metabolites Inhibit P-Glycoprotein In Vitro and In Situ Using Three Absorption Models. Planta Med.80 (04), 290–296. 10.1055/s-0033-1360334
17
Li Y. Feng Y. F. Liu X. T. Li Y. C. Zhu H. M. Sun M. R. et al (2021). Songorine Promotes Cardiac Mitochondrial Biogenesis via Nrf2 Induction during Sepsis. Redox Biol.38, 101771. 10.1016/j.redox.2020.101771
18
Li Y. Li Y. X. Dang J. Luo L. Yuan A. Zhao M. J. et al (2017a). Simultaneous Determination and Comparative Pharmacokinetics of Fuzi Water-Soluble Alkaloids between Normal and Acute Heart Failure Rats by Ultra Performance Liquid Chromatography Method. J. Chromatogr. Sci.55 (7), 719–728. 10.1093/chromsci/bmx026
19
Li Y. Li Y. X. Zhao M. J. Yuan A. Gong X. H. Zhao M. J. et al (2017b). The Effects of Rheum Palmatum L. On the Pharmacokinetic of Major Diterpene Alkaloids of Aconitum Carmichaelii Debx. in Rats. Eur. J. Drug Metab. Pharmacokinet.42 (3), 441–451. 10.1007/s13318-016-0356-z
20
Li Y. X. Gong X. H. Li Y. Zhang R. Q. Yuan A. Zhao M. J. et al (2015b). The Influence of Aconitum Carmichaelii Debx. On the Pharmacokinetic Characteristics of Main Components in Rheum Palmatum L. Phytother. Res.29 (8), 1259–1264. 10.1002/ptr.5369
21
Li Y. Zhao M. J. Yuan A. Gong X. H. Peng C. Li Y. X. (2016). Effect of Dosage on Pharmacokinetic Characteristics of Total Alkaloids from Aconiti Lateralis Radix Praeparata in Rats. Chin. J. Exp. Tradit. Med. Form.22 (22), 82–85. 10.13422/j.cnki.syfjx.2016220082
22
Li Z. Zhang R. Wang X. Hu X. Chen Y. Liu Q. (2015a). Simultaneous Determination of Seven Ginsenosides in Rat Plasma by High-Performance Liquid Chromatography Coupled to Time-Of-Flight Mass Spectrometry: Application to Pharmacokinetics of Shenfu Injection. Biomed. Chromatogr.29 (2), 167–175. 10.1002/bmc.3272
23
Liu S. Li F. Li Y. Li W. Xu J. Du H. (2017). A Review of Traditional and Current Methods Used to Potentially Reduce Toxicity of Aconitum Roots in Traditional Chinese Medicine. J. Ethnopharmacol.207, 237–250. 10.1016/j.jep.2017.06.038
24
Liu X. Li H. Song X. Qin K. Guo H. Wu L. et al (2014). Comparative Pharmacokinetics Studies of Benzoylhypaconine, Benzoylmesaconine, Benzoylaconine and Hypaconitine in Rats by LC-MS Method after Administration of Radix Aconiti Lateralis Praeparata Extract and Dahuang Fuzi Decoction. Biomed. Chromatogr.28 (7), 966–973. 10.1002/bmc.3102
25
Peng W. W. Li W. Li J. S. Cui X. B. Zhang Y. X. Yang G. M. et al (2013). The Effects of Rhizoma Zingiberis on Pharmacokinetics of Six Aconitum Alkaloids in Herb Couple of Radix Aconiti Lateralis-Rhizoma Zingiberis. J. Ethnopharmacol.148 (2), 579–586. 10.1016/j.jep.2013.04.056
26
Qiu Z. D. Chen J. L. Zeng W. Ma Y. Chen T. Tang J. F. et al (2020a). Real-time Toxicity Prediction of Aconitum Stewing System Using Extractive Electrospray Ionization Mass Spectrometry. Acta Pharm. Sin. B10 (5), 903–912. 10.1016/j.apsb.2019.08.012
27
Qiu Z. D. Wei X. Y. Sun R. Q. Chen J. L. Tan T. Xu J. Q. et al (2020b). Limitation Standard of Toxic Aconitines in Aconitum Proprietary Chinese Medicines Using On-Line Extraction Electrospray Ionization Mass Spectrometry. Acta Pharm. Sin. B10 (8), 1511–1520. 10.1016/j.apsb.2019.12.009
28
Qiu Z. D. Wei X. Y. Wang Y. N. Chen J. L. Tan T. Zhang X. P. et al (2021b). Quality Tracing Evaluation Strategies of Compatible Materials in Aconitum Proprietary Chinese Medicines. J. Pharm. Biomed. Anal.192, 113654. 10.1016/j.jpba.2020.113654
29
Qiu Z. D. Zhang X. Wei X. Y. Chingin K. Xu J. Q. Gao W. et al (2021a). Online Discovery of the Molecular Mechanism for Directionally Detoxification of Fuzi Using Real-Time Extractive Electrospray Ionization Mass Spectrometry. J. Ethnopharmacol.277, 114216. 10.1016/j.jep.2021.114216
30
Shen B. Q. Qu C. Mi L. Wang H. Y. Yang H. (2021). Simultaneous Quantification of Twenty-Eight Components of Shenfu Injection in Rat Plasma by UHPLC-QQQ MS and its Application to a Pharmacokinetic Study. J. Pharm. Biomed. Anal.203, 114211. 10.1016/j.jpba.2021.114211
31
Shuo X. U. Liang X. Qiong L. I. Jin P. (2017). Advances on Chinese Herbal Medicine Aconiti Lateralis Radix Praeparata. Northwest. Pharm. J.32 (2), 248–254.
32
Singhuber J. Zhu M. Prinz S. Kopp B. (2009). Aconitum in Traditional Chinese Medicine: a Valuable Drug or an Unpredictable Risk?J. Ethnopharmacol.126 (1), 18–30. 10.1016/j.jep.2009.07.031
33
Song S. Tang Q. Huo H. Li H. Xing X. Luo J. (2015). Simultaneous Quantification and Pharmacokinetics of Alkaloids in Herba Ephedrae-Radix Aconiti Lateralis Extracts. J. Anal. Toxicol.39 (1), 58–68. 10.1093/jat/bku113
34
Sun W. Yan B. Wang R. Liu F. Hu Z. Zhou L. et al (2018). In Vivo acute Toxicity of Detoxified Fuzi (Lateral Root of Aconitum Carmichaeli) after a Traditional Detoxification Process. EXCLI J.17, 889–899. 10.17179/excli2018-1607
35
Tang L. Gong Y. Lv C. Ye L. Liu L. Liu Z. (2012). Pharmacokinetics of Aconitine as the Targeted Marker of Fuzi (Aconitum Carmichaeli) Following Single and Multiple Oral Administrations of Fuzi Extracts in Rat by UPLC/MS/MS. J. Ethnopharmacol.141 (2), 736–741. 10.1016/j.jep.2011.08.070
36
Wang Z. Wang Z. Wen J. He Y. (2007). Simultaneous Determination of Three Aconitum Alkaloids in Urine by LC-MS-MS. J. Pharm. Biomed. Anal.45 (1), 145–148. 10.1016/j.jpba.2007.04.016
37
Wei X. Y. Qiu Z. D. Chen J. L. Sun R. Q. Huang L. Q. Lai C. J. (2019). Research Advancement in Mechanisms of Processing and Compatibility for Detoxication of Aconitums. Zhongguo Zhong Yao Za Zhi44 (17), 3695–3704. 10.19540/j.cnki.cjcmm.20190629.301
38
Xie G. Ma Z. Mei Y. Zhang X. Tan H. Gao Y. (2021). Evaluation of Pharmacokinetics of Aconiti Lateralis Radix of Shenfu Prescription in Rats with Heart Failure. Chin. J. Pharm.18 (07), 632–636. 10.19803/j.1672-8629.2021.07.08
39
Xu Y. Li Y. Zhang P. Yang B. Wu H. Guo X. et al (2017). Sensitive UHPLC-MS/MS Quantitation and Pharmacokinetic Comparisons of Multiple Alkaloids from Fuzi- Beimu and Single Herb Aqueous Extracts Following Oral Delivery in Rats. J. Chromatogr. B Analyt Technol. Biomed. Life Sci.1058, 24–31. 10.1016/j.jchromb.2017.05.016
40
Xu Y. Yang L. Liang K. An R. Wang X. Zhang H. (2020). Pharmacokinetic Effects of Ginsenoside Rg1 on Aconitine, Benzoylaconine and Aconine by UHPLC-MS/MS. Biomed. Chromatogr.34 (4), e4793. 10.1002/bmc.4793
41
Yang B. Xu Y. Wu Y. Wu H. Wang Y. Yuan L. et al (2016). Simultaneous Determination of Ten Aconitum Alkaloids in Rat Tissues by UHPLC-MS/MS and its Application to a Tissue Distribution Study on the Compatibility of Heishunpian and Fritillariae Thunbergii Bulbus. J. Chromatogr. B Analyt Technol. Biomed. Life Sci.1033, 242–249. 10.1016/j.jchromb.2016.08.033
42
Yang L. Wang Y. Huang G. Li J. Zhang Z. Ma Z. et al (20182018). Simultaneous Evaluation of the Influence of Panax Ginseng on the Pharmacokinetics of Three Diester Alkaloids after Oral Administration of Aconiti Lateralis Radix in Rats Using UHPLC/QQQ-MS/MS. Evid. Based Complement. Alternat Med.2018, 6527549. 10.1155/2018/6527549
43
Yu X. Liu H. Xu X. Hu Y. Wang X. Wen C. (2021). Pharmacokinetics of Yunaconitine and Indaconitine in Mouse Blood by UPLC-MS/MS. J. Chromatogr. B1179, 122840. 10.1016/j.jchromb.2021.122840
44
Zhang F. Tang M. H. Chen L. J. Li R. Wang X. H. Duan J. G. et al (2008). Simultaneous Quantitation of Aconitine, Mesaconitine, Hypaconitine, Benzoylaconine, Benzoylmesaconine and Benzoylhypaconine in Human Plasma by Liquid Chromatography-Tandem Mass Spectrometry and Pharmacokinetics Evaluation of "SHEN-FU" Injectable Powder. J. Chromatogr. B Analyt Technol. Biomed. Life Sci.873 (2), 173–179. 10.1016/j.jchromb.2008.08.008
45
Zhang H. Liu M. Zhang W. Chen J. Zhu Z. Cao H. et al (2015a). Comparative Pharmacokinetics of Three Monoester-Diterpenoid Alkaloids after Oral Administration of Acontium Carmichaeli Extract and its Compatibility with Other Herbal Medicines in Sini Decoction to Rats. Biomed. Chromatogr.29 (7), 1076–1083. 10.1002/bmc.3394
46
Zhang J. Gao W. Hu X. Liu Z. Liu C. (2012). The Influence of Compatibility of Traditional Chinese Medicine on the Pharmacokinetic of Main Components in Fructus Aurantii. J. Ethnopharmacol.144 (2), 277–283. 10.1016/j.jep.2012.09.009
47
Zhang J. M. Liao W. He Y. X. He Y. Yan D. Fu C. M. (2013). Study on Intestinal Absorption and Pharmacokinetic Characterization of Diester Diterpenoid Alkaloids in Precipitation Derived from Fuzi-Gancao Herb-Pair Decoction for its Potential Interaction Mechanism Investigation. J. Ethnopharmacol.147 (1), 128–135. 10.1016/j.jep.2013.02.019
48
Zhang Q. Ma Y. M. Wang Z. T. Wang C. H. (2014). Pharmacokinetics Difference of Multiple Active Constituents from Decoction and Maceration of Fuzi Xiexin Tang after Oral Administration in Rat by UPLC-MS/MS. J. Pharm. Biomed. Anal.92, 35–46. 10.1016/j.jpba.2013.12.038
49
Zhang W. Zhang H. Sun S. Sun F. F. Chen J. Zhao L. et al (2015b). Comparative Pharmacokinetics of Hypaconitine after Oral Administration of Pure Hypaconitine, Aconitum Carmichaelii Extract and Sini Decoction to Rats. Molecules20 (1), 1560–1570. 10.3390/molecules20011560
50
Zhang Y. Tian D. Huang Y. Li L. Mao J. Tian J. et al (2016). Pharmacokinetic Evaluation of Shenfu Injection in Beagle Dogs after Intravenous Drip Administration. Acta Pharm. Sin. B6 (6), 584–592. 10.1016/j.apsb.2016.05.006
51
Zhang Y. Zong X. Wu J. L. Liu Y. Liu Z. Zhou H. et al (2020). Pharmacokinetics and Tissue Distribution of Eighteen Major Alkaloids of Aconitum Carmichaelii in Rats by UHPLC-QQQ-MS. J. Pharm. Biomed. Anal.185, 113226. 10.1016/j.jpba.2020.113226
52
Zhou Q. Meng P. Wang H. Dong X. Tan G. (2019). Pharmacokinetics of Monoester-Diterpenoid Alkaloids in Myocardial Infarction and normal Rats after Oral Administration of Sini Decoction by Microdialysis Combined with Liquid Chromatography-Tandem Mass Spectrometry. Biomed. Chromatogr.33 (1), e4406. 10.1002/bmc.4406
Summary
Keywords
Aconitum carmichaelii, ginseng, pharmacokinetics, aconitine, high performance liquid chromatography-mass spectrometry, COVID-19
Citation
Chen Z-Y, Wei X-Y, Qiu Z-D, Huang Y, Tan T, Feng Y-L, Guo J, Cui G-H, Huang L-Q and Lai C-J-S (2022) Compatibility of Fuzi and Ginseng Significantly Increase the Exposure of Aconitines. Front. Pharmacol. 13:883898. doi: 10.3389/fphar.2022.883898
Received
25 February 2022
Accepted
05 April 2022
Published
26 April 2022
Volume
13 - 2022
Edited by
Zipeng Gong, Guizhou Medical University, China
Reviewed by
Caisheng Wu, Xiamen University, China
Guo Ma, Fudan University, China
Jiangeng Huang, Huazhong University of Science and Technology, China
Updates
Copyright
© 2022 Chen, Wei, Qiu, Huang, Tan, Feng, Guo, Cui, Huang and Lai.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lu-Qi Huang, huangluqi01@126.com; Chang-Jiang-Sheng Lai, laichangjiang44@126.com
†These authors have contributed equally to this work
This article was submitted to Drug Metabolism and Transport, a section of the journal Frontiers in Pharmacology
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.