
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 04 August 2022
Sec. Cardiovascular and Smooth Muscle Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.883600
This article is part of the Research Topic Cardiovascular Toxicity Associated with Cancer Treatment: Strategies for Diagnosis, Management and Cardioprotection View all 20 articles
 Ping Huang1†
Ping Huang1† Jia-huan Huang1,2†
Jia-huan Huang1,2† Ya-bing Zheng1
Ya-bing Zheng1 Wen-ming Cao1
Wen-ming Cao1 Xi-ying Shao1
Xi-ying Shao1 Jun-qing Chen1
Jun-qing Chen1 Yuan Huang1
Yuan Huang1 Guang-liang Li1
Guang-liang Li1 K Sharma3
K Sharma3 Huan-huan Zhou1
Huan-huan Zhou1 Xiao-jia Wang1*
Xiao-jia Wang1* Hong-chuan Jin4*
Hong-chuan Jin4* Zhan-hong Chen1*
Zhan-hong Chen1*Background: Cardiotoxicity associated with the sequential use of anthracyclines followed by trastuzumab is common in adjuvant therapy of patients with HER2-positive early breast cancer (eBC). However, the cardiac safety of trastuzumab concurrent with pegylated liposomal doxorubicin (PLD) is relatively less studied.
Method: Clinical data of patients with HER2-positive eBC treated with PLD and cyclophosphamide (PLD-C) followed by taxanes plus trastuzumab ± pertuzumab (TH or TPH) who then completed standard anti-HER2 treatment for 12 months from June 2012 to August 2021 were retrospectively collected. The primary endpoints were clinical and subclinical cardiotoxicity.
Result: In total, 70 eligible patients were enrolled. Among them, 55 patients (78.6%) received PLD-C → TH and 15 patients (21.4%) received PLD-C → TPH. The median follow-up time was 41.8 months. Until August 2021, only two patients had recurrent or metastatic diseases, with 2-year and 5-year disease-free survivals of 98.6% and 96.8%, respectively. Clinical cardiotoxicity occurred in six patients (8.6%), and all of them had an absolute decline of ≥16% from baseline left ventricular ejection fraction (LVEF) but not below the lower limit of normal (LLN = 50%). Subclinical cardiotoxicity events occurred in 17 patients (24.3%), and all of them had absolute declines of ≥10% and <16% from baseline LVEF but not below the LLN. No patients were interrupted from treatment, and all patients completed anti-HER2 treatment for 12 months. The sharpest decrease in LVEF was observed at 18 months after the start of PLD treatment. The cumulative incidences of clinical and subclinical cardiotoxicity were 9.8% and 28.3%, respectively. In the univariate analysis, body mass index, age, left chest wall radiotherapy, and ongoing cardiovascular risk factors were not significantly associated with clinical or subclinical cardiotoxicity (p > 0.05). No patients had congestive heart failure or death caused by PLD or anti-HER2 treatment.
Conclusion: The sequential use of PLD and trastuzumab showed a lower incidence of clinical cardiotoxicity, presented as asymptomatic decreased LVEF, compared with the results obtained in previous clinical studies using conventional anthracycline, taxanes and trastuzumab. The study regimen demonstrated good cardiac tolerance and is an alternative strategy for cardioprotection in patients with HER2-positive eBC.
Breast cancer is the most common type of malignant tumor and the leading cause of death in women worldwide (Sung et al., 2021). The use of chemotherapy has significantly improved both mortality and morbidity outcomes in breast cancer patients. Anthracyclines are widely used in the treatment of hematological malignancies and solid tumors, with powerful antitumor effects and indispensability (Nicolazzi et al., 2018). However, cardiotoxicity is a serious side effect of these kinds of drugs, which limits their clinical application (Schwentner et al., 2016). Pegylated liposomal doxorubicin (PLD) is a new dosage form of doxorubicin confined in liposomes, which can form a stable three-dimensional structure when polyethylene glycol is grafted onto the surface (stealth liposome) (Gabizon et al., 2008). Pegylated liposomal encapsulation reduces the plasma levels of free doxorubicin and may reduce drug delivery to normal tissues, which can in turn reduce cardiotoxicity (Gil-Gil et al., 2021). Results from clinical data revealed that PLD showed a similar efficacy to doxorubicin with a lower incidence of cardiotoxicity when administered in either advanced or (neo)adjuvant stages of treatment (O’Brien et al., 2004; Liu et al., 2021).
Human epidermal growth factor receptor 2 (HER2) is overexpressed in approximately 20–25% of breast cancer cases, which is associated with poor prognosis (Lynce et al., 2017). However, remarkable progress of trastuzumab plus chemotherapy as adjuvant treatment has significantly improved the survival rates of female breast cancer patients (Slamon et al., 2011; Goldhirsch et al., 2013; Perez et al., 2014; Cameron et al., 2017). Further development in the administration of dual anti-HER2 therapy with trastuzumab and pertuzumab shows great outcomes in (neo)adjuvant as well as metastatic settings (Baselga et al., 2012; Gianni et al., 2012; von Minckwitz et al., 2017). Trastuzumab is also associated with an increased risk of cardiotoxicity, particularly when administered in combination with anthracycline-based therapy. Cardiotoxicity, in this instance, would be manifested as symptomatic congestive heart failure (CHF) or asymptomatic decline of left ventricular ejection fraction (LVEF) (Seidman et al., 2002).
Previous studies have reported that 3–7% of patients who were receiving trastuzumab exhibited cardiac dysfunction in various forms (Seidman et al., 2002). When pertuzumab was combined with trastuzumab and chemotherapy, its cardiac safety was similar to that of trastuzumab alone (Swain et al., 2013; Yu et al., 2016). Nevertheless, limited data are available on the cardiac safety of PLD-based treatment administered sequentially with trastuzumab ± pertuzumab for the adjuvant treatment of HER2-positive early breast cancer (eBC) patients. Therefore, in this study, we aimed to explore strategies to reduce the cardiotoxicity of anthracycline sequential trastuzumab treatment and to clarify the cardiac safety and efficacy of PLD sequential trastuzumab treatment in adjuvant therapy.
Baseline clinical data as well as posttreatment patient assessment data were retrospectively collected, including the LVEF, electrocardiogram (ECG) status, and efficacy, in patients with HER2-positive eBC treated with PLD-C (PLD plus cyclophosphamide) followed by TH (taxanes with trastuzumab) or TPH (taxanes with trastuzumab and pertuzumab) who then completed standard anti-HER2 treatment for a total of 12 months from June 2012 to August 2021.
Inclusion criteria were patients with HER2-positive eBC diagnosed by pathology who had been treated with radical surgery, age of ≥18 years, and sequential use of PLD and trastuzumab ± pertuzumab. LVEF was evaluated at least twice at our center before and after treatment by 2D echocardiography.
Patients were excluded if they had metastatic disease or severe CHF (NYHA III–IV).
LVEF monitoring was performed before chemotherapy and every 3 months, where ECG including preanthracycline and pretrastuzumab was collected at multiple timepoints and sequentially throughout the therapy.
Clinical cardiotoxicity caused by cancer therapy is defined as ① symptomatic CHF; ② an asymptomatic absolute decline of ≥16% from baseline LVEF; ③ an asymptomatic absolute decline of 10–15% from baseline LVEF to below the lower limit of normal (LLN = 50%); and ④ LVEF<45% (in these situations, antitumor therapy should be halted for more than 4 weeks, and LVEF needs to be rechecked at an interval of 3–4 weeks). Signs associated with CHF consist of S3 rhythm gallops and/or tachycardia (Yu et al., 2015). Subclinical cardiotoxicity is defined as ① an asymptomatic absolute decline of ≥10% and <16% from baseline LVEF and ② an asymptomatic absolute decline of <10% to below the LLN (LLN = 50%) (in these situations, antitumor therapy continued, and LVEF needs to be rechecked at an interval of 3–4 weeks) (Yu et al., 2015). Adverse events (AEs) were also monitored continuously and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) v4.0.
Interruption of anti-HER2 treatment was defined as interruption of one or more doses or ≥6 weeks between doses.
The efficacy endpoint was disease-free survival (DFS), defined as the time from the first date of surgery to the first date of disease progression or August 2021.
Patients received PLD-C (PLD 25 mg/m2 on day 1 plus cyclophosphamide 600 mg/m2 on day 1) every 3 weeks for four cycles, followed by taxanes (paclitaxel 80 mg/m2 on day 1 or docetaxel 75–100 mg/m2 on day 1 or nab-paclitaxel 125 mg/m2 on day 1, 8) every 3 weeks for four cycles plus anti-HER2 therapy (trastuzumab was given at an initial dose of 8 mg/kg, followed by 6 mg/kg; pertuzumab was given at an initial dose of 840 mg, followed by 420 mg) every 3 weeks. Patients were allowed to complete 12 months of trastuzumab ± pertuzumab maintenance treatment until unacceptable toxicity levels were registered, or if/when there were signs of disease progression.
The normally distributed continuous data were expressed as mean ± standard deviation. Qualitative data were expressed as frequency and percentage. Chi-square (χ2) test of significance was used to compare proportions between qualitative parameters. The Kaplan–Meier method was used to estimate the percentage of DFS at 2 and 5 years. A p-value of <0.05 was considered statistically significant. Data were analyzed using Statistical Program for Social Science version 25.0.
In total, 70 eligible patients were enrolled. The mean age was 54.0 years (range 30–70 years), and 63 patients (90.0%) were younger than 65 years. All patients underwent breast surgery, either modified radical mastectomy (88.6%) or conservative surgery (11.4%). Of note, 41 patients (58.6%) received radiation therapy, of which 20 patients (48.8%) received left chest wall radiotherapy. Among all the patients, 55 patients (78.6%) received PLD-C followed by TH and 15 patients (21.4%) received PLD-C followed by TPH. Of all cases, 14 patients (20.0%) had at least one cardiovascular risk factor, including hypertension, diabetes, or dyslipidemia. The concrete baseline characteristics of patients are listed in Table 1.
During the observation period, clinical cardiotoxicity occurred in six patients (8.6%), and all of them were observed to have an absolute decline of ≥16% from baseline LVEF but not below the LLN (LLN = 50%). Subclinical cardiotoxicity events occurred in 17 patients (24.3%), and all these patients had absolute declines of ≥10% and <16% from baseline LVEF but not below the LLN (LLN = 50%).
One patient developed syncope after the first cycle of PLD-C, and ECG studies further showed evidence of third-degree atrioventricular block, which was considered to be related to previous bradycardia. The patient underwent cardiac pacemaker implantation soon after but still completed the remaining seven cycles of chemotherapy and 1-year trastuzumab therapy. There was no cardiotoxicity manifested as a decrease in LVEF in this patient. No other patients were observed to have CHF, and there were no deaths caused by PLD or anti-HER2 drugs.
By August 2021, no patients were interrupted from treatment, and all patients completed anti-HER2 treatment for 12 months.
The mean baselines of pre-AC and pre-H/HP LVEF were 71.7 ± 4.4% and 70.3 ± 4.1%, respectively. During the treatment period, the sharpest decrease in LVEF was observed within 18 months after the start of PLD treatment, which was equivalent to that within 15 months after the start of 12-month trastuzumab treatment (Figures 1 and 2). At each monitoring timepoint, the incidence of cardiotoxicity was the highest at the 18th month after the start of PLD or at the 15th month after the start of trastuzumab treatment, which was 8.7% (Table 2). LVEF began to recover at the 21st month after starting PLD treatment, which was the same as that observed at the 18th month after patients started trastuzumab treatment. The cumulative incidence of clinical cardiotoxicity-related events within 6 years was 9.8% and that of subclinical cardiotoxicity-related events within 6 years was 28.3% (Figure 3).
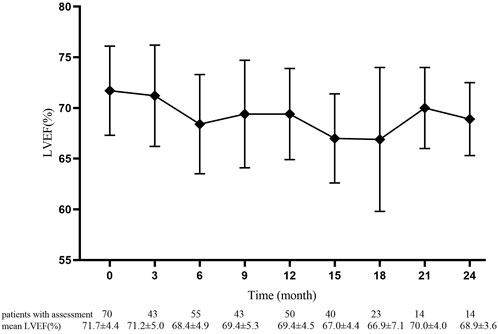
FIGURE 1. Pre-anthracycline baseline and value of left ventricular ejection fraction in 70 patients at each monitoring timepoint. Data are presented as mean ± standard deviation.
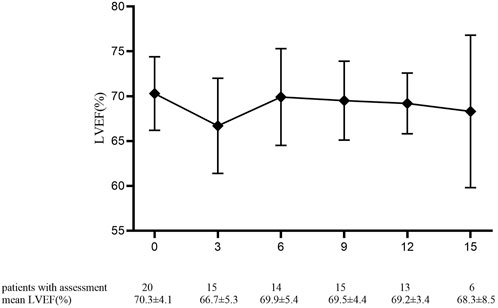
FIGURE 2. Pre-trastuzumab baseline and value of left ventricular ejection fraction percentage in 20 patients at each monitoring timepoint. Data are presented as mean ± standard deviation.

TABLE 2. Changes in left ventricular ejection fraction are divided into two groups, and the number of cases is listed.
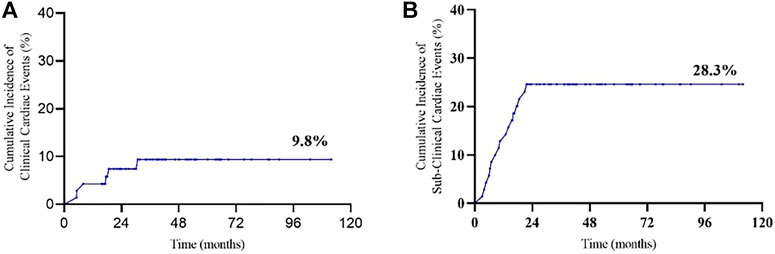
FIGURE 3. Cumulative incidence of cardiac events: (A) clinical cardiotoxicity and (B) subclinical cardiotoxicity.
Until August 2021, only two patients developed local recurrent or metastatic diseases. In total, 57 patients were followed up for 2 years, and the 2-year DFS was 98.6%. Moreover, 18 patients were followed up for 5 years, and the 5-year DFS was 96.8% (Figure 4).
The main cardiac safety and efficacy results of this study were compared with other pivotal adjuvant treatments of eBC using anti-HER2 monoclonal therapy and summarized, as presented in Table 3.
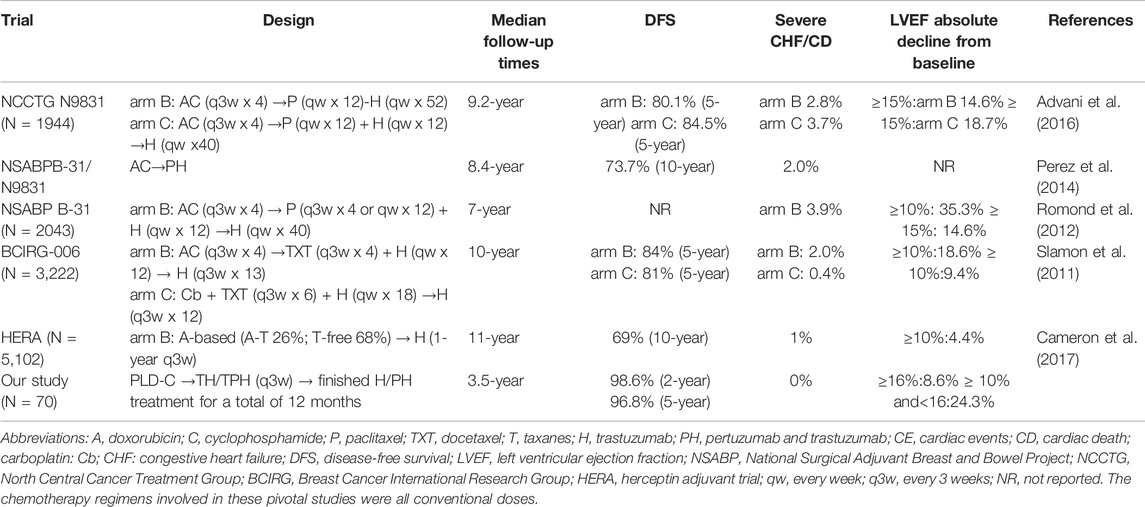
TABLE 3. Summary of the cardiac safety and efficacy results of our study compared with other pivotal adjuvant trastuzumab trials.
In the univariate analysis, body mass index (BMI; <25, ≥25 kg/m2), age (<60, ≥60 years), left chest wall radiotherapy, and ongoing cardiovascular risk factors were not significantly associated with clinical or subclinical cardiotoxicity (p > 0.05); the results are listed in Tables 4 and 5.
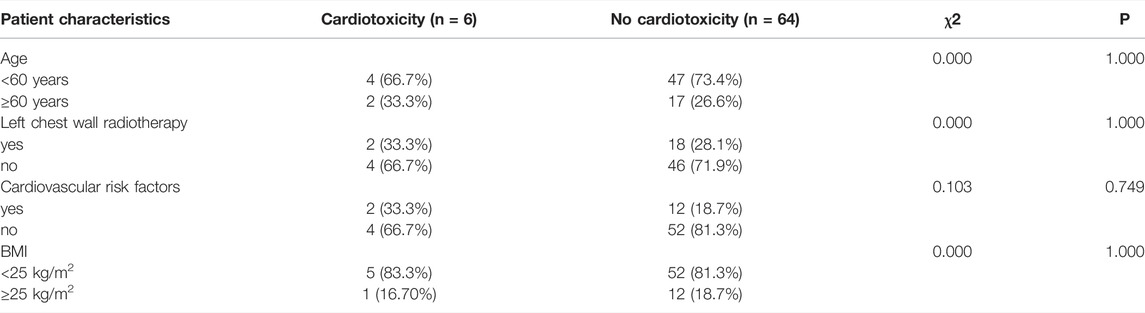
TABLE 4. Univariate chi-square analyses for influencing factors of clinical cardiotoxicity according to patients’ characteristics.
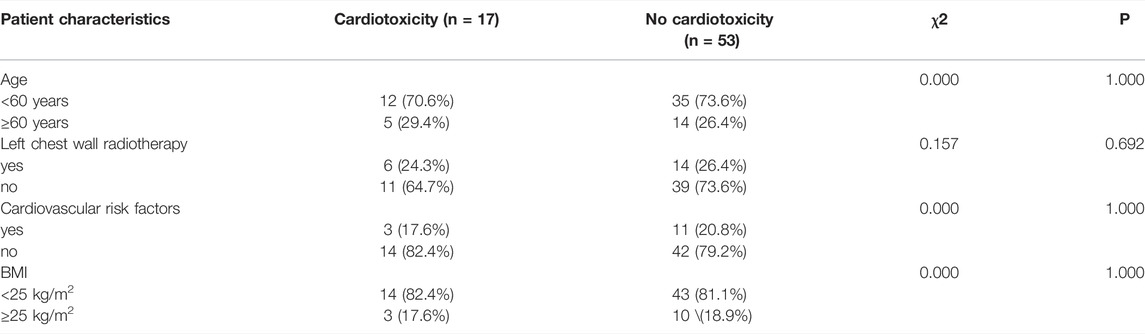
TABLE 5. Univariate chi-square analyses for influencing factors of subclinical cardiotoxicity according to patients’ characteristics.
The most frequent cardiac disorder reported by echocardiogram was left ventricular diastolic dysfunction, which was more common in the no-cardiotoxicity group (70.3%) than in the cardiotoxicity group (66.7%). Left ventricular systolic dysfunction events had an incidence of 33.3% in the cardiotoxicity group (Table 6).
In the cardiotoxicity group, ECG changes mainly included T-wave changes (83.3%), sinus bradycardia (50.0%), ST-TU segment changes (33.3%), ST-T segment changes (16.7%), and T-U segment changes (16.7%). The concrete disorders are listed in Table 6.
Over recent years, the application of anthracyclines sequentially with anti-HER2 therapy has shown to significantly improve the survival rates and prognoses of patients (Slamon et al., 2011). However, cardiotoxicity, as the major cause of breast cancer deaths, even without symptoms, may significantly limit the possibility of tumor treatment (Patnaik et al., 2011). In clinical practice, LVEF measured by echocardiography is used as an index to evaluate cardiotoxicity due to its accessibility.
PLD has been demonstrated to have equivalent efficacy but significantly less cardiotoxicity than conventional doxorubicin. In a phase III study, the cardiotoxicity rates were 3.9% and 18.8% with PLD and doxorubicin, respectively (O'Brien et al., 2004). A retrospective study compared the use of traditional anthracyclines and PLD in neoadjuvant therapy for patients with breast cancer. The study revealed higher pathologic complete response rates and lower incidences of cardiotoxicity in PLD-based cohorts (Liu et al., 2021). Therefore, it can be considered that the risk of cardiac toxicity after PLD sequential trastuzumab therapy is slightly higher than that of PLD monotherapy, but it is acceptable. Dual-target therapy with the addition of pertuzumab did not increase the incidence of cardiac-related AEs in either metastatic or neoadjuvant studies (Gianni et al., 2012; Schneeweiss et al., 2013).
PLD-C is not a standard protocol for breast cancer in adjuvant chemotherapy. However, due to the individualized treatment of cancer, some patients choose PLD as an alternative drug to anthracycline in adjuvant therapy. Through the narration of the physician in charge of these cases, the main reasons for these patients to choose PLD in our center include the following: 1) to avoid obvious hair loss caused by initial chemotherapy, 2) to avoid stronger nausea and vomiting symptoms, 3) to avoid the potential risk of developing febrile neutropenia, and 4) consideration of the potential to reduce the incidence of lifetime cardiotoxicity.
In this retrospective cohort study, PLD-C followed by anti-HER2 therapy as adjuvant therapy was effective and safe in a population of HER2-positive eBC patients. This study demonstrated that these schemes had a low risk for cardiotoxicity with slight clinical cardiotoxicity compared with treatments in previous clinical studies containing conventional anthracycline, taxanes, and trastuzumab (Table 3).
As described in Table 3, the NCCTG N9831 trial randomized patients between two arms in the adjuvant setting with AC followed by paclitaxel either with sequential or concurrent trastuzumab, and at a median follow-up of 9.2 years, the reported incidence of CHF or cardiac death (CD) was 2.8% in arm B (AC followed by paclitaxel and then trastuzumab) and 3.7% in arm C (AC followed by paclitaxel concurrent with trastuzumab) (Advani et al., 2016). In the NSABP-B31 study, at a median follow-up of 7 years, the incidence of severe CHF/CD was 3.9% in the arm with AC followed by TH. An independent retrospective review of the B-31 and N9831 trials demonstrated a 2.0% incidence of symptomatic CHF or CD in the trastuzumab-containing arm (Romond et al., 2012). In the BCIRG-006 study, at a median follow-up of 10 years, the incidence of CHF or CD was 2.0% in the arm with AC followed by docetaxel and trastuzumab (Slamon et al., 2011). While in our study, at a median follow-up of 3.5 years, the incidence of CHF or CD was 0% in the groups with PLD-C followed by TH/TPH (q3w). In the NCCTGN9831 and NSABPB-31 trials, LVEF reductions ≥15% from baseline were 14.6% (NCCTG N9831 arm B) and 18.7% (NCCTG N9831 arm C), respectively (Romond et al., 2012; Advani et al., 2016). While in our study, LVEF reduction ≥16% from baseline was 8.6%, where cardiotoxicity halved in value. Subclinical cardiotoxicity in the NSABP B-31 and BCIRG-006 studies using anthracyclines and trastuzumab was similar to that in our study (Slamon et al., 2011; Romond et al., 2012). This indicates that although PLD is used, cardiotoxicity monitoring is still necessary. In the HERA study, because 68% of patients did not use taxanes, the subclinical cardiotoxicity was relatively low (4.4%) (Cameron et al., 2017). In the NCCTGN9831 study, the 5-year DFS rates were 80.1% (arm B) and 84.5% (arm C) (Advani et al., 2016). In the BCIRG-006 study, the 5-year DFS rates were 84% (arm B:AC-TH group) and 81% (arm C:TCH group), respectively (Slamon et al., 2011). While in our study, the 5-year DFS was 96.8%, suggesting that patients had improved survival. It is worth noting that all patients completed PLD-C followed by TH or TPH every 3 weeks, which reflects the excellent tolerance; this may be related to higher DFS and efficiency.
The NCCTG N9831 trial demonstrated that age ≥60 years, registration LVEF <65%, and use of antihypertensive medications were associated with an increased risk of cardiac events, while radiation therapy, BMI, or ethnicity were not statistically significant (Advani et al., 2016). In the NSABP-B31 trial, LVEF (50–54%), age (≥60 years), and receiving antihypertensive medications and radiotherapy were also not statistically significant (Romond et al., 2012). The CANTO study indicated that obesity appeared to be associated with an important increase in risk-related cardiotoxicity in eBC patients (Kaboré and Guenancia, 2019). In our study, since the basal LVEF of the patients was not low and the incidence of cardiotoxicity was low, it was less possible to conclude that cardiotoxicity was related to BMI (<25, ≥25 kg/m2), age (<60, ≥60 years), radiotherapy, ongoing cardiovascular risk factors, or LVEF at baseline.
Our study had several limitations as follows. This study was a retrospective study, the test population was of a small sample size, and the LVEF values were not available for all patients at all corresponding observation points, leading to the possibility that the results showed a reduced incidence of cardiotoxicity. Due to the lack of necessary baseline or follow-up LVEF measurements, many patients had to be excluded, resulting in some selection bias. Therefore, as a future consideration, a prospective cohort study to further clarify the precise incidence and outcome of PLD sequential trastuzumab cardiotoxicity is worth carrying out.
PLD sequential trastuzumab treatment reduces cardiotoxicity by nearly half compared with traditional anthracyclines, which is well tolerated. Most patients can complete the established treatment plans, which may have a positive effect on survival; this merits further exploration and may become an alternative strategy for cardioprotection in patients with HER2-positive eBC.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Board of Zhejiang Cancer Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and institutional requirements.
PH and J-HH were involved in conception of the study, participated in the design of the study, collected cases, and performed statistical analysis. PH, JH-H, and KS wrote the first draft of the manuscript. Z-HC, Y-BZ, W-MC, X-YS, J-QC, YH, G-LL, and H-HZ collected cases and performed statistical analysis. Z-HC, H-CJ, and X-JW designed this study. All authors contributed to the manuscript and revised, read, and approved the submitted version.
This work was partly supported by Zhejiang Province’s Scientific Research Foundation of Traditional Chinese Medicine, China (2020ZB034), and Medical Science and Technology Project of Zhejiang Province, China (2020KY492).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Advani, P. P., Ballman, K. V., Dockter, T. J., Colon-Otero, G., and Perez, E. A. (2016). Long-Term Cardiac Safety Analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. J. Clin. Oncol. 34, 581–587. doi:10.1200/JCO.2015.61.8413
Baselga, J., Cortés, J., Kim, S. B., Im, S. A., Hegg, R., Im, Y. H., et al. (2012). Pertuzumab Plus Trastuzumab Plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 366, 109–119. doi:10.1056/NEJMoa1113216
Cameron, D., Piccart-Gebhart, M. J., Gelber, R. D., Procter, M., Goldhirsch, A., de Azambuja, E., et al. (2017). 11 Years' Follow-Up of Trastuzumab after Adjuvant Chemotherapy in HER2-Positive Early Breast Cancer: Final Analysis of the HERceptin Adjuvant (HERA) Trial. Lancet 389, 1195–1205. doi:10.1016/S0140-6736(16)32616-2
Gabizon, A., Isacson, R., Rosengarten, O., Tzemach, D., Shmeeda, H., and Sapir, R. (2008). An Open-Label Study to Evaluate Dose and Cycle Dependence of the Pharmacokinetics of Pegylated Liposomal Doxorubicin. Cancer Chemother. Pharmacol. 61, 695–702. doi:10.1007/s00280-007-0525-5
Gianni, L., Pienkowski, T., Im, Y. H., Roman, L., Tseng, L. M., Liu, M. C., et al. (2012). Efficacy and Safety of Neoadjuvant Pertuzumab and Trastuzumab in Women with Locally Advanced, Inflammatory, or Early HER2-Positive Breast Cancer (NeoSphere): a Randomised Multicentre, Open-Label, Phase 2 Trial. Lancet Oncol. 13, 25–32. doi:10.1016/S1470-2045(11)70336-9
Gil-Gil, M. J., Bellet, M., Bergamino, M., Morales, S., Barnadas, A., Manso, L., et al. (2021). Long-Term Cardiac Safety and Survival Outcomes of Neoadjuvant Pegylated Liposomal Doxorubicin in Elderly Patients or Prone to Cardiotoxicity and Triple Negative Breast Cancer. Final Results of the Multicentre Phase II CAPRICE Study. Front. Oncol. 11, 645026. doi:10.3389/fonc.2021.645026
Goldhirsch, A., Gelber, R. D., Piccart-Gebhart, M. J., de Azambuja, E., Procter, M., Suter, T. M., et al. (2013). 2 Years versus 1 Year of Adjuvant Trastuzumab for HER2-Positive Breast Cancer (HERA): an Open-Label, Randomised Controlled Trial. Lancet 382, 1021–1028. doi:10.1016/S0140-6736(13)61094-6
Kaboré, E. G., Guenancia, C., Vaz-Luis, I., Di Meglio, A., Pistilli, B., Coutant, C., et al. (2019). Association of Body Mass Index and Cardiotoxicity Related to Anthracyclines and Trastuzumab in Early Breast Cancer: French CANTO Cohort Study. PLoS Med. 16, e1002989. doi:10.1371/journal.pmed.1002989
Liu, W., Chen, W., Zhang, X., Zhao, P., Fan, Z., Bi, L., et al. (2021). Higher Efficacy and Reduced Adverse Reactions in Neoadjuvant Chemotherapy for Breast Cancer by Using Pegylated Liposomal Doxorubicin Compared with Pirarubicin. Sci. Rep. 11, 199. doi:10.1038/s41598-020-80415-w
Lynce, F., Barac, A., Tan, M. T., Asch, F. M., Smith, K. L., Dang, C., et al. (2017). SAFE-HEaRt: Rationale and Design of a Pilot Study Investigating Cardiac Safety of HER2 Targeted Therapy in Patients with HER2-Positive Breast Cancer and Reduced Left Ventricular Function. Oncologist 22, 518–525. doi:10.1634/theoncologist.2016-0412
Nicolazzi, M. A., Carnicelli, A., Fuorlo, M., Scaldaferri, A., Masetti, R., Landolfi, R., et al. (2018). Anthracycline and Trastuzumab-Induced Cardiotoxicity in Breast Cancer. Eur. Rev. Med. Pharmacol. Sci. 22, 2175–2185. doi:10.26355/eurrev_201804_14752
O'Brien, M. E., Wigler, N., Inbar, M., Rosso, R., Grischke, E., Santoro, A., et al. (2004). Reduced Cardiotoxicity and Comparable Efficacy in a Phase III Trial of Pegylated Liposomal Doxorubicin HCl (CAELYX/Doxil) versus Conventional Doxorubicin for First-Line Treatment of Metastatic Breast Cancer. Ann. Oncol. 15, 440–449. doi:10.1093/annonc/mdh097
Patnaik, J. L., Byers, T., Diguiseppi, C., Dabelea, D., and Denberg, T. D. (2011). Cardiovascular Disease Competes with Breast Cancer as the Leading Cause of Death for Older Females Diagnosed with Breast Cancer: a Retrospective Cohort Study. Breast Cancer Res. 13, R64. doi:10.1186/bcr2901
Perez, E. A., Romond, E. H., Suman, V. J., Jeong, J. H., Sledge, G., Geyer, C. E., et al. (2014). Trastuzumab Plus Adjuvant Chemotherapy for Human Epidermal Growth Factor Receptor 2-positive Breast Cancer: Planned Joint Analysis of Overall Survival from NSABP B-31 and NCCTG N9831. J. Clin. Oncol. 32, 3744–3752. doi:10.1200/JCO.2014.55.5730
Romond, E. H., Jeong, J. H., Rastogi, P., Swain, S. M., Geyer, C. E., Ewer, M. S., et al. (2012). Seven-year Follow-Up Assessment of Cardiac Function in NSABP B-31, a Randomized Trial Comparing Doxorubicin and Cyclophosphamide Followed by Paclitaxel (ACP) with ACP Plus Trastuzumab as Adjuvant Therapy for Patients with Node-Positive, Human Epidermal Growth Factor Receptor 2-positive Breast Cancer. J. Clin. Oncol. 30, 3792–3799. doi:10.1200/JCO.2011.40.0010
Schneeweiss, A., Chia, S., Hickish, T., Harvey, V., Eniu, A., Hegg, R., et al. (2013). Pertuzumab Plus Trastuzumab in Combination with Standard Neoadjuvant Anthracycline-Containing and Anthracycline-free Chemotherapy Regimens in Patients with HER2-Positive Early Breast Cancer: a Randomized Phase II Cardiac Safety Study (TRYPHAENA). Ann. Oncol. 24, 2278–2284. doi:10.1093/annonc/mdt182
Schwentner, L., Harbeck, N., Singer, S., Eichler, M., Rack, B., Forstbauer, H., et al. (2016). Short Term Quality of Life with Epirubicin-Fluorouracil-Cyclophosphamid (FEC) and Sequential Epirubicin/cyclophosphamid-Docetaxel (EC-DOC) Chemotherapy in Patients with Primary Breast Cancer - Results from the Prospective Multi-Center Randomized ADEBAR Trial. Breast 27, 69–77. doi:10.1016/j.breast.2016.03.003
Seidman, A., Hudis, C., Pierri, M. K., Shak, S., Paton, V., Ashby, M., et al. (2002). Cardiac Dysfunction in the Trastuzumab Clinical Trials Experience. J. Clin. Oncol. 20, 1215–1221. doi:10.1200/JCO.2002.20.5.1215
Slamon, D., Eiermann, W., Robert, N., Pienkowski, T., Martin, M., Press, M., et al. (2011). Adjuvant Trastuzumab in HER2-Positive Breast Cancer. N. Engl. J. Med. 365, 1273–1283. doi:10.1056/NEJMoa0910383
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Swain, S. M., Ewer, M. S., Cortés, J., Amadori, D., Miles, D., Knott, A., et al. (2013). Cardiac Tolerability of Pertuzumab Plus Trastuzumab Plus Docetaxel in Patients with HER2-Positive Metastatic Breast Cancer in CLEOPATRA: a Randomized, Double-Blind, Placebo-Controlled Phase III Study. Oncologist 18, 257–264. doi:10.1634/theoncologist.2012-0448
von Minckwitz, G., Procter, M., de Azambuja, E., Zardavas, D., Benyunes, M., Viale, G., et al. (2017). Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 377, 122–131. doi:10.1056/NEJMoa1703643
Yu, A. F., Manrique, C., Pun, S., Liu, J. E., Mara, E., Fleisher, M., et al. (2016). Cardiac Safety of Paclitaxel Plus Trastuzumab and Pertuzumab in Patients with HER2-Positive Metastatic Breast Cancer. Oncologist 21, 418–424. doi:10.1634/theoncologist.2015-0321
Keywords: cardiotoxicity, early breast cancer, HER2-positive, pegylated liposomal doxorubicin, trastuzumab
Citation: Huang P, Huang J-h, Zheng Y-b, Cao W-m, Shao X-y, Chen J-q, Huang Y, Li G-l, Sharma K, Zhou H-h, Wang X-j, Jin H-c and Chen Z-h (2022) Cardiac Safety in Breast Cancer Patients Receiving Pegylated Liposome Doxorubicin Sequential Anti-HER2 Monoclonal Antibody Therapy. Front. Pharmacol. 13:883600. doi: 10.3389/fphar.2022.883600
Received: 25 February 2022; Accepted: 23 June 2022;
Published: 04 August 2022.
Edited by:
Zhi-Ren Zhang, Harbin Medical University, ChinaReviewed by:
Tanaya Vaidya, AbbVie (United States), United StatesCopyright © 2022 Huang, Huang, Zheng, Cao, Shao, Chen, Huang, Li, Sharma, Zhou, Wang, Jin and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhan-hong Chen, Y3pyZWRAc2luYS5jb20=; Hong-chuan Jin, amluaGNAemp1LmVkdS5jbg==; Xiao-jia Wang, d3hpYW9qaWEwODAzQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.