
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 30 May 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.881787
 Meiyu Wu1,2
Meiyu Wu1,2 Shuxia Qin1,2
Shuxia Qin1,2 Liting Wang1,2
Liting Wang1,2 Chongqing Tan1,2
Chongqing Tan1,2 Ye Peng1,2
Ye Peng1,2 Xiaohui Zeng3
Xiaohui Zeng3 Xia Luo1,2
Xia Luo1,2 Lidan Yi1,2
Lidan Yi1,2 Xiaomin Wan1,2*
Xiaomin Wan1,2*Objective: Pembrolizumab plus chemotherapy is recommended as the first-line treatment for advanced oesophageal cancer. The objective of this study is to evaluate the cost-effectiveness of pembrolizumab plus chemotherapy as first-line therapy for advanced oesophageal cancer from the healthcare system perspective in China.
Methods: Based on the KEYNOTE-590 trial, a Markov model was constructed to estimate the cost and effectiveness of pembrolizumab plus chemotherapy and placebo plus chemotherapy, respectively. Total costs, life years (LYs), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were calculated. One-way, probabilistic sensitivity analyses (PSA), and subgroup analyses were adapted to test the model robustness.
Result: Compared with the placebo group, pembrolizumab group obtained an additional 1.05 QALY, but the cost was also increased by $121,478.76. The ICER was $115,391.84 per QALY gained, which was higher than the willingness-to-pay (WTP) of $31,304.31. The results of One-way sensitivity analyses showed that the ICER was sensitive to the hazard ratio of PFS and per cycle cost of pembrolizumab. At a WTP threshold of $31,304.31, the probability of pembrolizumab plus chemotherapy being cost-effective was 0%.
Conclusion: From the perspective of China healthcare system, pembrolizumab plus chemotherapy as first-line treatment is not cost-effective for patients with advanced oesophageal cancer compared with placebo plus chemotherapy.
Oesophageal cancer ranks seventh in incidence and sixth in mortality worldwide. Eastern Asia occupies the highest incidence rate, partly because of the heavy burden in China (Sung et al., 2021). In 2019, the number of new cases and deaths of esophageal cancer in China are about 278,120 and 257,315 respectively. The total number of disability-adjusted life years (DALYs) is 5,759,997, which accounted for 8.6% of all cancer DALYs (Institute for Health Metrics and Evaluation, 2019; Qiu et al., 2021). The histologic subtypes of oesophageal cancer are divided into squamous cell carcinoma, adenocarcinoma, and other subtypes. In China, about 90% of patients with esophageal cancer are diagnosed with squamous cell carcinoma, and most patients are diagnosed at advanced stages (Chen et al., 2016; Li et al., 2017; Abnet et al., 2018). The 5-year survival rate of patients with advanced oesophageal cancer remains at a low level (Enomoto et al., 2021).
At present, fluorouracil combined with platinum, or paclitaxel combined with platinum, are the most recommended first-line treatment options for patients with advanced or metastatic oesophageal cancer (Muro et al., 2019; National Comprehensive Cancer Network, 2022). However, these treatments have little effect on improving overall survival (OS) in patients with advanced esophageal cancer. Compared with chemotherapy alone, the combination of immune checkpoint inhibitors (ICIs) and chemotherapy showed more effective antitumor activity in several studies (Gandhi et al., 2018; Cortes et al., 2020; Sun et al., 2021).
Pembrolizumab is a humanized monoclonal antibody that blocks the interaction between PD-1 and its ligands, PD-L1 and PD-L2. The phase Ⅲ KEYNOTE-590 trial evaluated the efficacy and safety of pembrolizumab plus chemotherapy (pembrolizumab group) compare with placebo plus chemotherapy (placebo group) as first-line treatment in advanced oesophageal cancer and Siewert type 1 gastro-oesophageal junction cancer (Sun et al., 2021). In that study, 749 patients were randomized to the pembrolizumab group or placebo group and were followed up for a median of 22·6 months. The result showed that compared with chemotherapy alone, pembrolizumab plus chemotherapy prolonged median OS [12.4 months vs. 9.8 months; hazard ratio (HR) 0.64] and median progression-free survival (PFS; 6.3 months vs. 5.8 months; HR 0.65).
For patients with unresectable, metastatic oesophageal cancer, the combination of pembrolizumab could be considered as the first-line treatment (Sun et al., 2021). However, the high cost of pembrolizumab may increase the economic burden of society. This study aimed to evaluate the cost-effectiveness of Pembrolizumab plus chemotherapy as first-line therapy for advanced oesophageal cancer from the perspective of the Chinese healthcare system based on KEYNOTE-590 trial data.
We constructed a Markov cohort model to compare two first-line among patients with oesophageal cancer: 1) pembrolizumab, 5-fluorouracil, and cisplatin; and 2) placebo, 5-fluorouracil, and cisplatin. The model contained three mutually exclusive health states: PFS, progressive disease (PD), and death (Figure 1). All patients were in state PFS at the beginning and the patients could maintain the health state of the previous cycle or enter another health state in each cycle. We assumed that patients would receive second-line treatment after disease progression. The analysis used a lifetime horizon and the cycle length was 3 weeks. Costs and benefits were discounted at an annual rate of 3% (Sanders et al., 2016), and the half-cycle correction was used. Total costs, life years (LYs), quality-adjusted life years (QALYs), and incremental cost-effectiveness ratios (ICERs) were the primary outputs in the model. According to the recommendation of the World Health Organization (WHO), we applied 3 times per capita gross domestic product (GDP) of China as the willingness-to-pay (WTP) threshold, to judge whether pembrolizumab combined chemotherapy is cost-effective compared with chemotherapy alone ($31,304.31) (The World Bank, 2022). Model establishment and data analysis were performed in the R software (R version 4.0.5; http://www.r-project.org).
The characteristics of the simulated population are similar to the patients of the KEYNOTE-590 trial, which enrolled patients aged 18 years or older with previously untreated, locally advanced, unresectable oesophageal cancer or Siewert type 1 gastro-oesophageal junction adenocarcinoma. We assumed that the weight and height of the simulated population were 60 kg and 1.64 m, respectively, meaning a body surface area (BSA) of 1.21 (Zhang et al., 2021).
The estimation of the transition probability between different health states is mainly based on the KEYNOTE-590 trial. We obtained points on the PFS and OS curves through the GetData Graph Digitizer software (version 2.26; http://getdata-graph-digitizer.com) and then reconstructed the individual patient data (IPD) using R software according to the algorithm proposed by Guyot et al. (2012). Exponential, Weibull, log-normal, log-logistic and gompertz distribution were used to fit these data. Log-logistic distribution was selected as the best fit distribution because of its minimum Akaike information criterion (AIC) value. Natural death probabilities at different ages were also included, which were derived from the Chinese life tables (Word health Organization, 2022).
In this study, we only considered the cost of drugs, routine follow-up, supportive care, treatment-related grade 3 or worse adverse events (AEs). In the first-line treatment, the usage and dosage of drugs are as follows: pembrolizumab 200 mg once every 3 weeks for up to 35 cycles, 5-fluorouracil 800 mg/m2 on days 1–5 plus cisplatin 80 mg/m2 on day 1 once every 3 weeks for up to 6 cycles. After disease progressed, patients who received subsequent anticancer therapy were 43% in the pembrolizumab group and 47% in the placebo group, respectively (Sun et al., 2021). We assumed that patients who received subsequent anticancer therapy all used paclitaxel monotherapy as second-line treatment in the model. The prices of drugs were obtained from The Second Xiangya Hospital of Central South University, and the rest of the costs were derived from previously published studies (Chongqing et al., 2014; Bai et al., 2017; Yang et al., 2021; Zhang et al., 2021). The costs of AEs were calculated by multiplying the incidence of AEs by the costs of managing the AEs per event. All costs were converted to 2020 United States dollars (USD) using the local Consumer Price Index ($1 = ¥6.9) (National Bureau of Statistics, 2021). The utility values of health states were from a published study, which assigned 0.74to PFS and 0.58 to PD (Cai et al., 2021) (Table1).
To evaluate the robustness of the model, one-way and probabilistic sensitivity analyses (PSA) were performed in this study. One-way sensitivity analyses is to observe the influence of different parameters on ICER by using the maximum and minimum values of the parameter. In this research, pembrolizumab fluctuated within ± 50% of the current price value, and other parameters changed within 95% confidence interval or ± 20% of the base value (Table1). The discount rate ranged between 0 and 5%. In the PSA, a 1000 times Monte Carlo simulation was performed by randomly generating different parameter values according to the specific distribution. Our study assumed that the utility parameter conformed to beta distribution and the cost parameter conformed to gamma distribution (Briggs et al., 2012). In addition, the threshold analysis for cycle cost of Pembrolizumab was performed.
The base-case analyses showed that patients in the pembrolizumab group had 5.36 LYs and 3.37 QALYs for $135,890.90. In the placebo group, patients had 3.71 LYs and 2.32 QALYs for $14,412.14. Compared with the placebo group, the mean incremental effect and cost were 1.05 QALYs and $121,478.76 for the pembrolizumab group. ICER was calculated by dividing the incremental cost by the incremental effect and was estimated to be $115,391.84 per QALY gained (Table2). At the WTP threshold of $31,304.31 in China, pembrolizumab plus chemotherapy as the first-line therapy for advanced oesophageal cancer was not a cost-effective strategy compared with placebo-chemotherapy.
The results of the one-way sensitivity analyses demonstrated that the ICER was most sensitive to HR of PFS between pembrolizumab and placebo groups. The ICER was as high as $254,146.49 when the HR of PFS was 0.78, which far exceeded the WTP threshold of $31,304.31 per QALY gained in China. The cost of pembrolizumab per cycle, HR of OS, initial age of the patient cohort, and utility of PFS also had a significant impact on the model results. If the cost of pembrolizumab per cycle was reduced by 50%, the ICER was decreased to $59,482.61. However, all results indicated that pembrolizumab combined chemotherapy was not cost-effective compared with chemotherapy alone, with a WTP of $31,304.31 (Figure2). To make the ICER below $31,304.31 per QALY, the cost of pembrolizumab per cycle should be reduced by 74% (see Supplementary Table S2 in supplementary material).
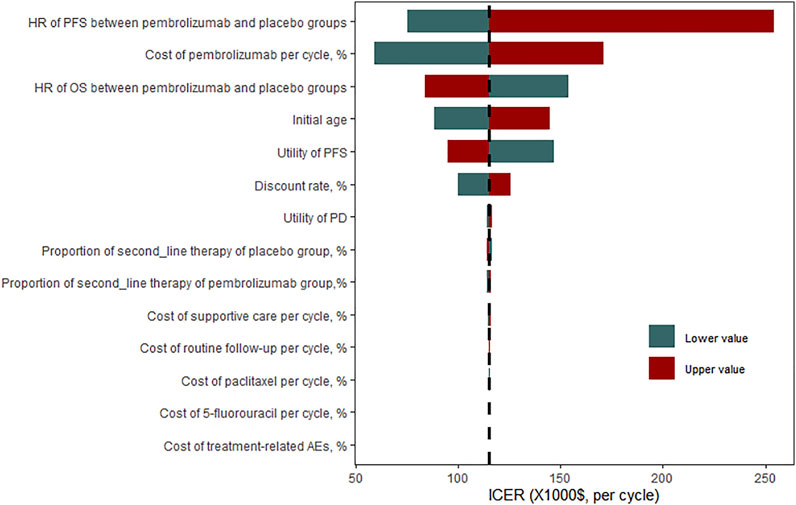
FIGURE 2. One-way sensitivity analysis for pembrolizumab versus placebo group. HR, hazard ratio; OS overall survival; PFS, progression-free survival; ICER, incremental cost-effectiveness ratio.
Based on the Monte Carlo PSA, the probability of pembrolizumab plus chemotherapy being a cost-effective regime for patients with advanced esophageal cancer would be 50% if the WTP threshold was $100,500 per QALY gained. As the WTP threshold increases, the greater the probability that pembrolizumab plus chemotherapy would be cost-effective. When the WTP threshold was set at $340,000, the probability of pembrolizumab plus chemotherapy being cost-effective is 100% (Figure3).
We performed subgroup analysis based on histology and PD-L1 status. In the subgroup analysis, the incremental cost and incremental QALY of patients with PD-L1 combined positive score (CPS) of 10 or more were $126,693.97 and 1.56, respectively. Resulting in an ICER of $81,236.42 per QALY gained, which was the lowest in all groups, but still higher than $31,304.31. More details are shown in Table3.
Base on the results of our results, the ICER of pembrolizumab plus chemotherapy for patients with advanced oesophageal cancer as first-line treatment in China is $115,391.84 per QALY gained, which was above the WTP threshold of $31,304.31 per QALY, indicating that pembrolizumab plus chemotherapy was not a cost-effective regime compared with placebo plus chemotherapy.
In September 2021, pembrolizumab was approved by the National Medical Products Administration (NMPA) to combine chemotherapy for the first-line treatment of patients with unresectable, locally advanced or metastatic oesophageal cancer, or gastro-oesophageal junction cancer, which means that pembrolizumab had become the first and only PD-1 monoclonal antibody approved for the first-line treatment of oesophageal cancer in China (National Medical Products Administration, 2022). However, pembrolizumab with higher price than a series of Chinese domestic ICIs (Camrelizumab: $424.35/200 mg, Toripalimab: $1088.04/240 mg, Sintilimab: $412.03/100 mg) (DRUGDATAEXPY, 2022). In the one-way sensitivity analyses, the cost of pembrolizumab per cycle played a very important role in affecting ICER. The cycle cost of pembrolizumab ranged from $2812.87 to $8438.62, the ICER varied from $59,482.61 to $171,301.30, which was still above $31,304.31.
But it does not mean that we can only choose chemotherapy alone with low safety and effectiveness. Clinically, the choice of treatment scheme needs to consider a variety of factors. In order to reduce the economic pressure of cancer patients and make the limited resources meet the needs of more people, China has rolled out many preferential policies related to anti-tumor drugs in recent years. For example, the price of camrelizumab per 200 mg has dropped from $2,802 in 2020 to $424.35 in 2021. Therefore, it is expected to reduce the price of pembrolizumab and improve its cost-effectiveness. Pembrolizumab plus chemotherapy would be cost-effective when the cycle cost of pembrolizumab is reduced to $1332 (74%), with the ICER of $30,996.13. The effectiveness of pembrolizumab had the greatest impact on ICER, the HR of PFS ranged from 0.52 to 0.78, the ICER varied from $75,487.06 to $254,146.49. Indeed, in the subgroup analysis, patients with PD-L1 CPS of 10 or more had the lowest HR and generated the lowest ICER. It seems that they can benefit most from the regimen of pembrolizumab plus chemotherapy. Value-based drug pricing would benefit the sustainability of the health care system and promote drug development. Our result shows that when the cost of pembrolizumab per cycle was halved, the ICER was above the $31,304.31 per QALY, which was not cost-effective. If the cost of pembrolizumab per cycle decreased by 74%, pembrolizumab would be cost-effective as a first-line strategy for advanced oesophageal cancer. In addition, selecting patients with PD-L1 CPS of 10 or more for treatment could improve the pharmacoeconomic profile of pembrolizumab. Reducing prices through negotiations on the trade-off between drug prices and coverage may be an appropriate and effective way to improve the cost-effectiveness.
At present, there are few studies on the pharmacoeconomic evaluation of oesophageal cancer. To our knowledge, this is the first cost-effectiveness analysis of pembrolizumab plus chemotherapy as first-line therapy for advanced oesophageal cancer. One recent study evaluated the cost-effectiveness of camrelizumab plus chemotherapy as first-line therapy for advanced or metastatic esophageal squamous cell carcinoma in China (Zhang et al., 2021). Camrelizumab combined with paclitaxel and cisplatin is recommended by the Chinese Society of Clinical Oncology (CSCO) Guidelines for the Diagnosis and Treatment of Esophageal Cancer in 2021 as the first-line treatment of advanced or metastatic esophageal squamous cell carcinoma (Guidelines Working Committee of Chinese society of clinical oncology, 2021). This study showed that the ICER of camrelizumab plus chemotherapy versus placebo plus chemotherapy was $46,671.10 per QALY gained, which was higher than the WTP threshold of $31,498.70 per QALY gained. Therefore camrelizumab plus chemotherapy is not a cost-effective strategy as the first-line treatment for advanced or metastatic esophageal squamous from the perspective of the Chinese healthcare system, which was basically consistent with our results. Another study is the cost-effectiveness analysis of nivolumab as the second-line treatment from the perspective of Chinese society (Zhang et al., 2020). The results of this study demonstrated that the ICER was $136,709.35 per QALY gained, and nivolumab is not a cost-effective treatment option based on the WTP threshold of $29,306.43 per QALY gained. On the basis of previous studies, although ICIs can prolong the OS of patients with advanced oesophageal cancer, ICIs are still not cost-effective due to the high price, whether it is a first-line treatment or second-line treatment.
Several limitations in our study must be acknowledged. First, the results of this study are obtained by modeling, which might not completely reflect the real world. However, we tested several distributions and selected the best fitting (log-logistic), which could reduce the inaccuracy of the model. Second, the utility values in the model were derived from previously published studies, which might not accurately reflect the utility of the KEYNOTE-590 trial. Although one-way sensitivity analyses showed that the utility used in the model had an impact on ICER, the ICER was still far above the WTP threshold of $31304.31, whether increasing or decreasing the utility. Third, in this model, we only consider paclitaxel as a second-line treatment. In reality, we should choose a suitable second-line treatment according to the actual situation. Luckily, the cost of second-line had a minor influence on economic outcomes. Fourth, we did not consider the cost of grade 1–2 AEs, but the results were not sensitive to the cost of treatment-related AEs based on the one-way sensitivity analyses.
Based on our study, at the WTP threshold of $31,304.31 per QALY gained, pembrolizumab plus chemotherapy as first-line therapy is not a cost-effective regime for patients with advanced oesophageal cancer compared with placebo plus chemotherapy from the perspective of the Chinese healthcare system. However, the cost-effectiveness of pembrolizumab plus chemotherapy for patients with advanced oesophageal cancer is expected to improve as the price of pembrolizumab decreases.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MW, SQ, CT, YP, and XW developed the economic model, performed the analyses and interpreted the results. LW, XZ, and XL collected and reviewed data. MW and LY drafted the manuscript. XW contributed to the conception, design of the primary model. All authors read and approved the final article.
This work was supported by a grant from the National Natural Science Foundation of China (grant number 71874209, 82073818); the research project of the Health Commission of Hunan province (grant number202113050283); and the Postgraduate Scientific Research Innovation Project of Hunan Province (grant number CX20210350). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.881787/full#supplementary-material
Abnet, C. C., Arnold, M., and Wei, W. Q. (2018). Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 154, 360–373. doi:10.1053/j.gastro.2017.08.023
Bai, Y., Xu, Y., and Wu, B. (2017). Cost-Effectiveness and Budget Impact Analysis of Apatinib for Advanced Metastatic Gastric Cancer from the Perspective of Health Insurance System. Gastroenterol. Res. Pract. 2017, 2816737. doi:10.1155/2017/2816737
Briggs, A. H., Weinstein, M. C., Fenwick, E. A., Karnon, J., Sculpher, M. J., and Paltiel, A. D. (2012). Model Parameter Estimation and Uncertainty Analysis: a Report of the ISPOR-SMDM Modeling Good Research Practices Task Force Working Group-6. Med. Decis. Mak. 32, 722–732. doi:10.1177/0272989X12458348
Cai, H., Xu, B., Li, N., Zheng, B., Zheng, Z., and Liu, M. (2021). Cost-Effectiveness Analysis of Camrelizumab versus Chemotherapy as Second-Line Treatment of Advanced or Metastatic Esophageal Squamous Cell Carcinoma. Front. Pharmacol. 12, 732912. doi:10.3389/fphar.2021.732912
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer Statistics in China, 2015. CA Cancer J. Clin. 66, 115–132. doi:10.3322/caac.21338
Chongqing, T., Liubao, P., Xiaohui, Z., Jianhe, L., Xiaomin, W., Gannong, C., et al. (2014). Cost-utility Analysis of the Newly Recommended Adjuvant Chemotherapy for Resectable Gastric Cancer Patients in the 2011 Chinese National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines in Oncology: Gastric Cancer. Pharmacoeconomics 32, 235–243. doi:10.1007/s40273-013-0065-2
Cortes, J., Cescon, D. W., Rugo, H. S., Nowecki, Z., Im, S. A., Yusof, M. M., et al. (2020). Pembrolizumab Plus Chemotherapy versus Placebo Plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): a Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet 396, 1817–1828. doi:10.1016/S0140-6736(20)32531-9
DRUGDATAEXPY, (2022). https://db.yaozh.com/yaopinzhongbiao (Accessed on February 6, 2022).
Enomoto, N., Yamada, K., Terayama, M., Kato, D., Yagi, S., Wake, H., et al. (2021). Current Status of Immune Checkpoint Inhibitor Therapy for Advanced Esophageal Squamous Cell Carcinoma. Glob. Health Med. 3, 378–385. doi:10.35772/ghm.2020.01112
Gandhi, L., Rodríguez-Abreu, D., Gadgeel, S., Esteban, E., Felip, E., De Angelis, F., et al. (2018). Pembrolizumab Plus Chemotherapy in Metastatic Non-small-cell Lung Cancer. N. Engl. J. Med. 378, 2078–2092. doi:10.1056/NEJMoa1801005
Guidelines Working Committee of Chinese society of clinical oncology (2021). Guidelines for the Diagnosis and Treatment of Esophageal Cancer (Vertion 2021). USA: People's Medical Publishing House.
Guyot, P., Ades, A. E., Ouwens, M. J., and Welton, N. J. (2012). Enhanced Secondary Analysis of Survival Data: Reconstructing the Data from Published Kaplan-Meier Survival Curves. BMC Med. Res. Methodol. 12, 9. doi:10.1186/1471-2288-12-9
Institute for Health Metrics and Evaluation (2019). Global Burdenof Disease project[EB/OL]. Available at http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/f8a0d9c0a3f20728ed41900616580915 (Accessed on Feb 6, 2022).
Li, M., Wan, X., Wang, Y., Sun, Y., Yang, G., and Wang, L. (2017). Time Trends of Esophageal and Gastric Cancer Mortality in China, 1991-2009: an Age-Period-Cohort Analysis. Sci. Rep. 7, 6797. doi:10.1038/s41598-017-07071-5
Muro, K., Lordick, F., Tsushima, T., Pentheroudakis, G., Baba, E., Lu, Z., et al. (2019). Pan-Asian Adapted ESMO Clinical Practice Guidelines for the Management of Patients with Metastatic Oesophageal Cancer: a JSMO-ESMO Initiative Endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann. Oncol. 30, 34–43. doi:10.1093/annonc/mdy498
National Bureau of Statistics (2021). China Statistical Yearbook. Available at http://www.stats.gov.cn/tjsj/ndsj/2021/indexch.htm (Accessed Feb 6, 2022).
National Comprehensive Cancer Network (2022). NCCN Guidelines in Esophageal and Esophagogastric Junction Cancers. Version 1. Available at https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1433 (Accessed on Feb 6, 2022).
National Medical Products Administration (2022). National Medical Products Administration. Available at https://www.nmpa.gov.cn/(Accessed on Feb 6, 2022).
Qiu, H., Cao, S., and Xu, R. (2021). Cancer Incidence, Mortality, and Burden in China: a Time-Trend Analysis and Comparison with the United States and United Kingdom Based on the Global Epidemiological Data Released in 2020. Cancer Commun. (Lond) 41, 1037–1048. doi:10.1002/cac2.12197
Sanders, G. D., Neumann, P. J., Basu, A., Brock, D. W., Feeny, D., Krahn, M., et al. (2016). Recommendations for Conduct, Methodological Practices, and Reporting of Cost-Effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA 316, 1093–1103. doi:10.1001/jama.2016.12195
Sun, J. M., Shen, L., Shah, M. A., Enzinger, P., Adenis, A., Doi, T., et al. (2021). Pembrolizumab Plus Chemotherapy versus Chemotherapy Alone for First-Line Treatment of Advanced Oesophageal Cancer (KEYNOTE-590): a Randomised, Placebo-Controlled, Phase 3 Study. Lancet 398, 759–771. doi:10.1016/S0140-6736(21)01234-4
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
The World Bank (2022). GDP Per Capita (Current US$). Available at https://data.worldbank.org/indicator/NY.GDP.PCAP.CD (Accessed on Feb 6, 2022).
Word health Organization (2022). Life Tables by Country (GHE: Life Tables). Available at https://www.who.int/data/gho/data/indicators/indicator-details/GHO/gho-ghe-life-tables-by-country (Accessed on Feb 6, 2022).
Yang, F., Fu, Y., Kumar, A., Chen, M., Si, L., and Rojanasorot, S. (2021). Cost-effectiveness Analysis of Camrelizumab in the Second-Line Treatment for Advanced or Metastatic Esophageal Squamous Cell Carcinoma in China. Ann. Transl. Med. 9, 1226. doi:10.21037/atm-21-1803
Zhang, P. F., Xie, D., and Li, Q. (2020). Cost-effectiveness Analysis of Nivolumab in the Second-Line Treatment for Advanced Esophageal Squamous Cell Carcinoma. Future Oncol. 16, 1189–1198. doi:10.2217/fon-2019-0821
Keywords: pembrolizumab, oesophageal cancer, cost-effectiveness, markov model, chemotherapy
Citation: Wu M, Qin S, Wang L, Tan C, Peng Y, Zeng X, Luo X, Yi L and Wan X (2022) Cost-Effectiveness of Pembrolizumab Plus Chemotherapy as First-Line Therapy for Advanced Oesophageal Cancer. Front. Pharmacol. 13:881787. doi: 10.3389/fphar.2022.881787
Received: 23 February 2022; Accepted: 13 May 2022;
Published: 30 May 2022.
Edited by:
Tin Wui Wong, Universiti Teknologi MARA Puncak Alam, MalaysiaReviewed by:
Jie Zhao, First Affiliated Hospital of Zhengzhou University, ChinaCopyright © 2022 Wu, Qin, Wang, Tan, Peng, Zeng, Luo, Yi and Wan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaomin Wan, d2FueGlhb21pbkBjc3UuZWR1LmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.