- 1Department of Clinical and Experimental Medicine, Dermatology, University of Messina, Messina, Italy
- 2Department of Clinical and Experimental Medicine, Pharmacology, University of Messina, Messina, Italy
- 3Department of Dermatology, University of Modena and Reggio Emilia, Modena, Italy
- 4Department of Human Pathology in Adult and Developmental Age “Gaetano Barresi, Pediatryˮ, University of Messina, Messina, Italy
Photodynamic therapy (PDT) is a photochemotherapy based on local application of a photosensitive compound and subsequent exposure to a light source of adequate wavelength. It is a non-invasive therapeutic procedure widely used in oncodermatology for treatment of numerous skin cancers, but in the last years its use has been gradually extended to an increasing list of skin diseases of both infectious and inflammatory nature. Although PDT is proven as a safe and effective therapeutic option in adults, its use is not well standardized in the pediatric population. In this review, we will focus on clinical applications, mechanisms of action, protocols, and adverse events in children and adolescents. Most of pediatric experiences concerned treatment of skin cancers in Gorlin syndrome and xeroderma pigmentosum, acne vulgaris, and viral warts, but other applications emerged, such as cutaneous lymphoma and pseudo-lymphomas, necrobiosis lipoidica, hidradenitis suppurativa, dissecting cellulitis, leishmaniasis, angiofibromas, verrucous epidermal nevus, and linear porokeratosis. In these pediatric diseases, PDT appeared as an effective therapeutic alternative. The results on vitiligo were limited and not fully encouraging. Although highly versatile, PDT is not a therapy for all skin diseases, and a deeper knowledge of its mechanisms of action is required to better define its spectrum of action and safety in pediatric patients.
Introduction
Photodynamic therapy (PDT) is an attractive, non-invasive therapeutic procedure widely used in adult patients for treatment of tumoral, inflammatory, and infectious skin diseases. PDT is a photochemotherapy based on local application of a photosensitive compound and subsequent exposure to a light source of adequate wavelength. The most employed photosensitizers commonly used in dermatology are the 5-aminolaevulinic acid (ALA, an intermediate of the heme biosynthetic pathway) and its methyl ester 5-aminolevulinate (MAL), which are converted inside the target cells to photo-active protoporphyrin IX (PpIX). After an incubation period (generally 3 h), PpIX is activated by an artificial light source (conventional PDT) or by sunlight (daylight PDT), thus leading to the production of reactive oxygen species (ROS), triggering both apoptosis and necrosis of target cells as well as stimulation of an immune modulating response. Different light sources with varying wavelengths can be used in PDT. The absorption spectrum of protoporphyrin IX shows maximal absorption peaks at approximately 410 nm, namely, at the wavelength of blue light, but it also shows smaller absorption peaks at 506, 532, 580, and 630 nm as well, namely, within the red light wavelength (Prieto et al., 2005). Nevertheless, the effect of red light appears to be stronger than that observed with blue light because of the greater depth of penetration of red light into dermis, thus explaining its diffuse use worldwide with respect to blue light. DL-PDT is a novel procedure in which the activation of the topical photosensitizer is induced by exposure to natural daylight, without requiring preliminary occlusion and dedicated instrumental equipment. With respect to the conventional one, DL-PDT has a more superficial depth of penetration, so its use is reserved to thin lesions (Borgia et al., 2020a).
The heterogeneous mechanisms of action and the multiple targets hit by PDT have allowed to progressively extend its use from the treatment of non-melanoma skin cancer to an always increasing list of skin diseases of both infectious and inflammatory nature. PDT displays several major strengths: it is a non-invasive, easily repeatable, outpatient treatment that can be applied to wide areas of affected skin with an overall good profile of safety. PDT can be used in fragile patients, that is, elderly subjects in whom surgery is contraindicated, in immuno-depressed subjects, or to treat large or multiple lesions localized in poor healing areas. Moreover, PDT shows superior cosmetic outcome compared with more invasive therapeutic approaches such as surgery and cryotherapy, with no scarring and pigmentary changes. Although PDT is proven as a safe and effective therapeutic option in several dermatologic diseases in adults, its use is not well standardized in pediatric population. For this reason, we performed a review about the employment of PDT in the pediatric age to provide an overview of the current state of art and to explore new potential fields of use of this technique.
Methods
We checked the PubMed (https://ncbi.nlm.nih.gov/PubMed) database using the string “photodynamic therapy” [All Fields] AND “skin” [All Fields].
Only the research works written in English language, concerning humans and child (birth to 18 years), and with no time limits were included. A systematic literature search was led according to the PRISMA flowchart, also reviewing the abstracts of the articles whose title suggested this association. The references retrieved were critically examined by two experts in the field of dermatology to select those pertinent to our research, namely, clinical trials, retrospective studies, case series, and case reports. Reviews were excluded, but their reference lists were also examined to find other relevant articles, which were eventually revised and included if appropriate.
Figure 1 summarizes the publication screening scheme used according to PRISMA guidelines.
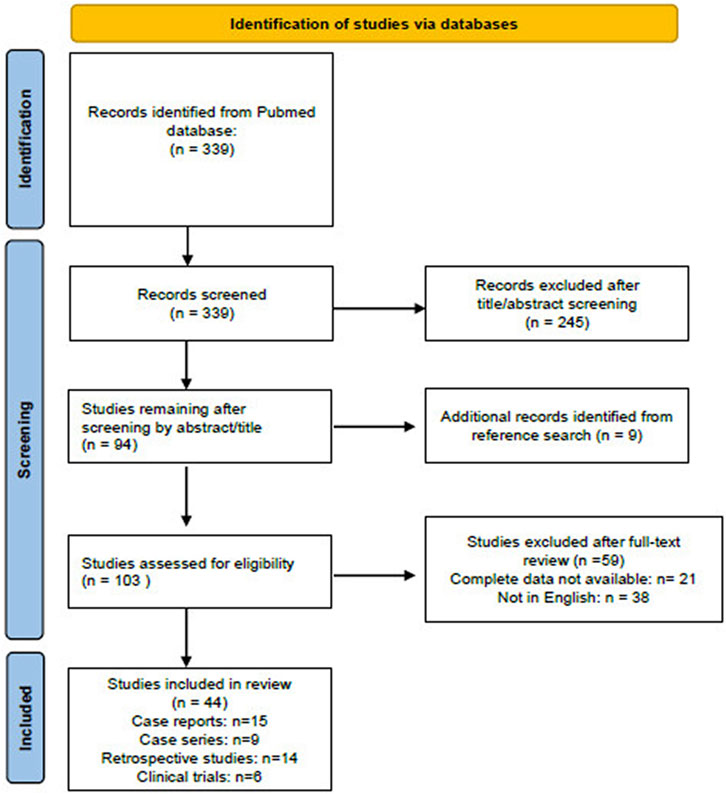
FIGURE 1. Flow diagram of the literature screened using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. The figure is adapted from http://prisma-statement.org.
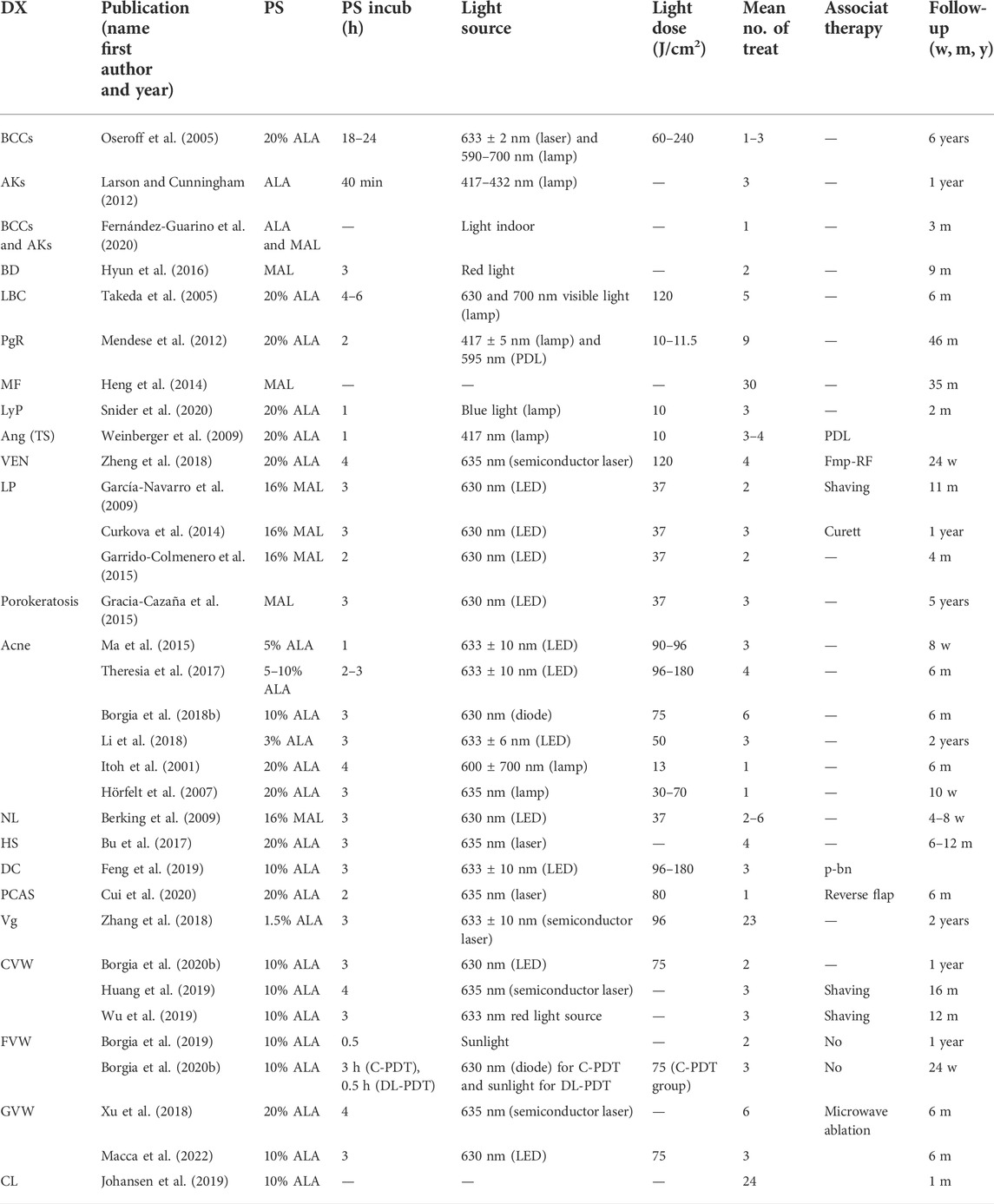
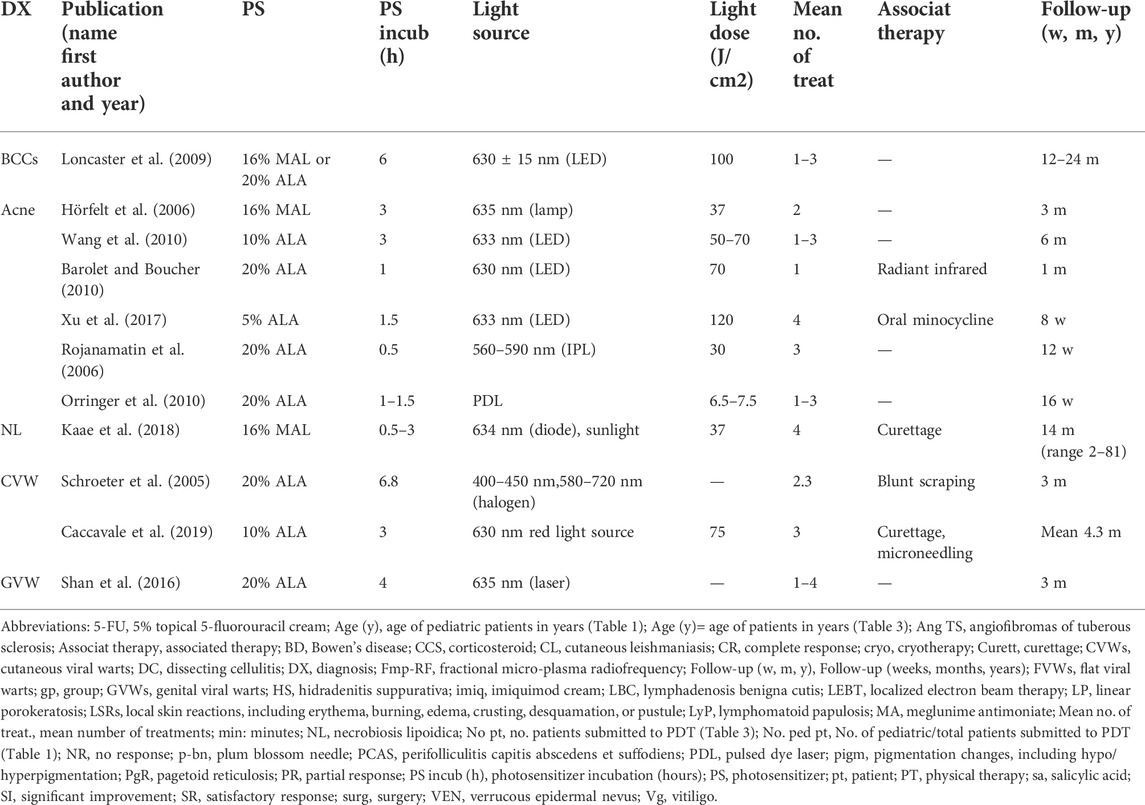
We included only studies on patients treated with topical ALA-PDT or MAL-PDT, the photosensitizers widely available and therefore most commonly used by dermatologists. As of 6 January 2022, 44 articles were identified. Studies exclusively focusing on pediatric patients were 33. Parameters of these studies, including patients’ features, type of topical photosensitizers and light sources used, conditions of treatment, number of treatments, and outcomes and adverse events, are summarized in Tables 1, 2. Studies including both adults and children with no specific data on pediatric patients were 11. They were also included in results and summarized in Tables 3, 4. For convenience, the results have been categorized into three main topics (oncologic, anti-inflammatory, and antimicrobial), with a fourth paragraph including a miscellanea mainly dealing with disorders of keratinization.
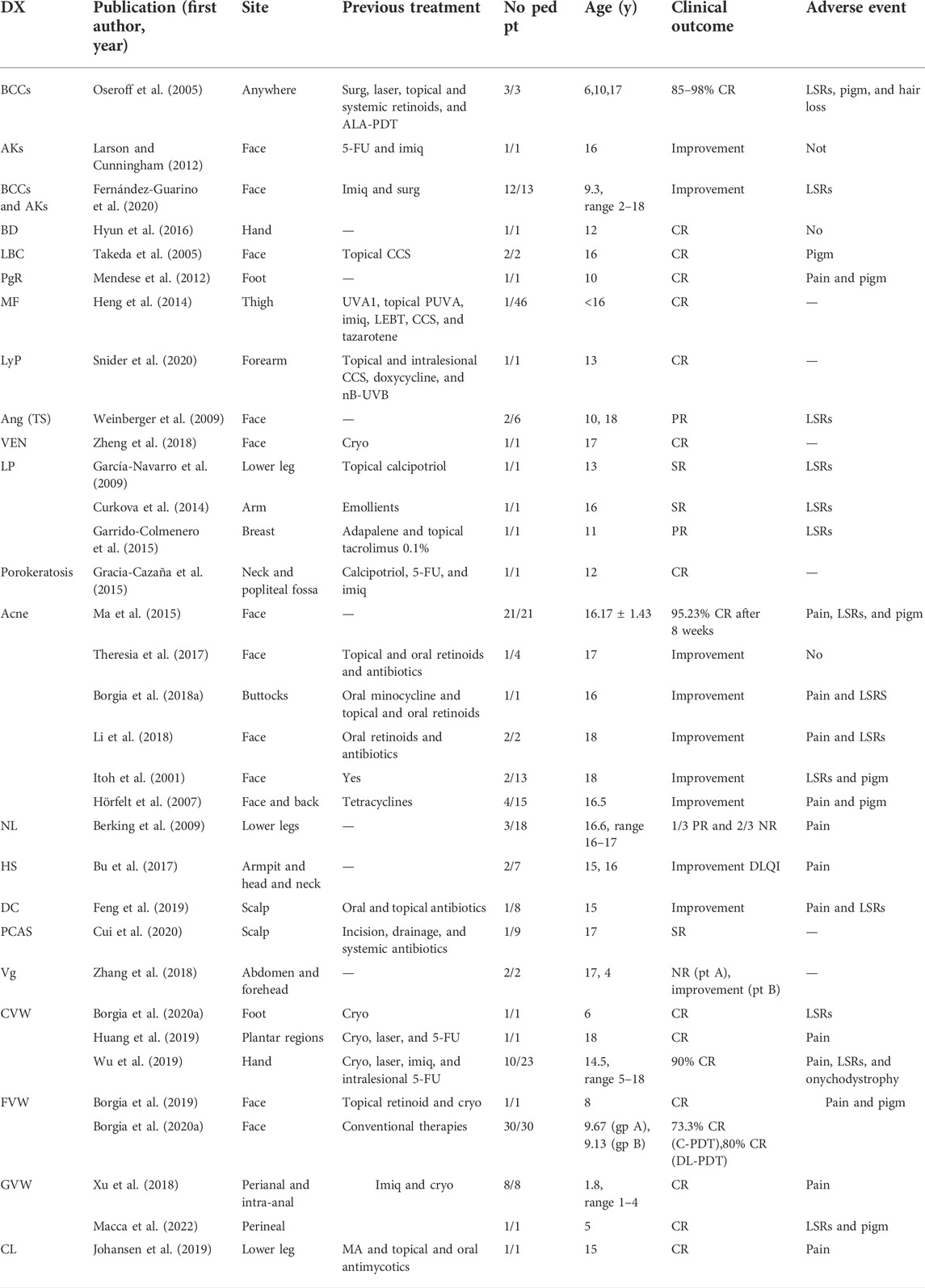
TABLE 1. Studies with specific data on pediatric patients. Patients’ age, clinical features, and PDT clinical outcomes.
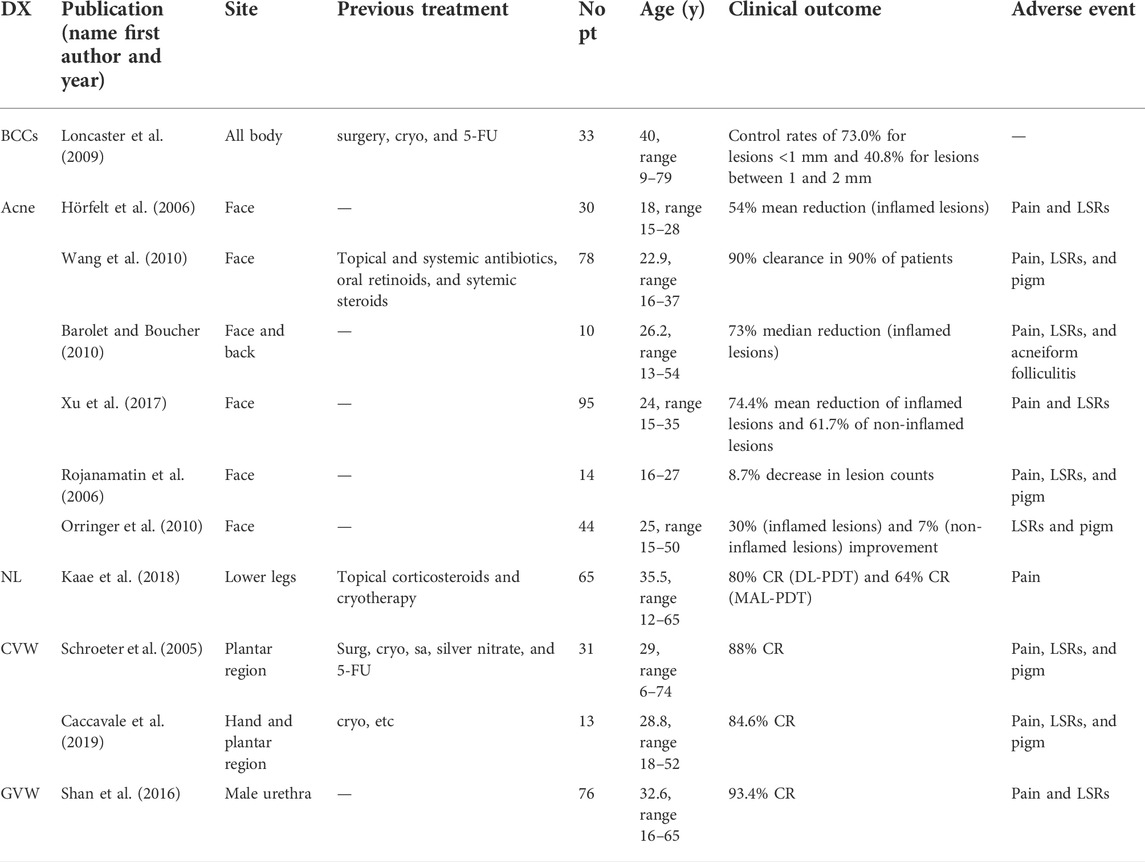
TABLE 3. Studies including both adults and children with no specific data on pediatric patients. Patients’ age, clinical features, and PDT clinical outcomes.
PDT and pediatric skin cancers
The onset of skin cancers in pediatric age is a rare event. Nevertheless, some genetic syndromes, such as Gorlin syndrome or xeroderma pigmentosum, may predispose to the development of skin tumors since childhood.
Gorlin syndrome
Gorlin syndrome, or nevoid basal cell carcinoma syndrome (NBCCS), is an autosomal dominant syndrome caused by mutations in the PTCH1 (Patched 1) gene, characterized by multiple basal cell carcinomas (BCCs) occurring from puberty, in addition to various dental, osseous, ophthalmic, neurological, and sex organ abnormalities (Jawa et al., 2009).
A total of three Gorlin patients <18 years old (6, 10, and 17 years) with diffused BCCs were treated by means of ALA-PDT, achieving 85 to 98% complete response (Oseroff et al., 2005).
Oseroff et al. (2005) performed several sessions of 10% ALA-PDT on the children with a red light laser for areas with lower diameter (from 2 to 7 cm) and a lamp for those with larger diameter (up to a 16 cm diameter). The patients presented BCCs on 12–25% of their body surface, and four to seven sessions were needed for each patient but individual areas received one to three treatments. The patients reported excellent cosmetic outcomes with no evidence of new BCCs in the treated areas up to 6 years of follow-up.
Loncaster et al. (2009) treated 33 Gorlin patients of all ages (range 9–79 years) with ALA-PDT and MAL-PDT, obtaining different control rates depending on the thickness of lesions. They performed ultrasound investigation to assess lesion thickness and used topical PDT to treat only superficial lesions (<2 mm thick). Thicker lesions were treated with a systemic photosensitizer. At 12 months, local control rates were 73.0% for lesions <1 mm, 40.8% for lesions measuring between 1 and 2 mm, and 59.3% for lesions >2 mm.
Gorlin patients are highly susceptible to DNA damage from therapies such as ionizing radiation. However, Oseroff et al. (2005) found no evidence of ALA-PDT inducing or promoting BCCs in pediatric patients. Gorlin patients have increased risk of medulloblastoma and may develop multiple to thousands BCCs in the site of radiotherapy, namely, on the anterior surface of abdomen and on the back. In these cases, the carcinomas are so numerous that surgical excision is impractical. There are reports of pediatric Gorlin patients who benefited from PDT for the treatment of radiotherapy-induced BCCs (Walter et al., 1997; Oseroff et al., 2005). In adult patients, MAL-PDT or nanoemulsion ALA-PDT are considered for non-aggressive, low-risk BCC, that is, small superficial and nodular types not exceeding 2 mm tumor thickness, where surgery is impractical or contraindicated, or avoidance of scarring is a priority (Rhodes et al., 2004; Peris et al., 2019). Ultrasound may be useful to assess BCC thickness prior to treatment and assign Gorlin patients to a PDT treatment, as demonstrated by Loncaster et al. (2009). An international experts consensus established recommendations for the use of MAL-PDT in patients with Gorlin syndrome (Basset-Seguin et al., 2014). Although MAL-PDT is not approved for children, three of the seven expert panel members had pediatric experience and all agreed that MAL-PDT might also be considered in the pediatric age (Basset-Seguin et al., 2014).
A recent study in adult patients observed higher recurrence rates in BCCs of neck and head treated with MAL-PDT (Condorelli et al., 2022). Vinciullo et al. highlighted that lesions located in the H-zone, whether large or not, had unfavorable CCR following MAL-PDT (Vinciullo et al., 2005). For these reasons, according to the most recent European guidelines for treatment of BCC, less common histologic variants of BCC, such as morphoeic, pigmented, and micronodular types, as well as areas with higher risk of tumor survival and deep penetration (facial ‘H’-zone) should not be treated with PDT (Peris et al., 2019). Usually, PDT for pigmented BCC treatment is not performed because of the lower penetration of the light, possibly due to the melanin content of these variants (Salvio et al., 2021). During the treatment of their pediatric patients with Gorlin syndrome, Oseroff et al. (2005) noted that a patient presented a lower response rate probably due to the varying pigmentation of his BCCs, which reduced the effective light dose. A prior debulking of pigmented BCC with removal of pigmented component may be an option for the treatment of these subtypes. Its suitability should also be investigated in pediatric population. In a group of adult patients, Salvio et al. (2021) performed debulking of 30 pigmented BCC before MAL-PDT and obtained complete response in 100% of cases, with no recurrence at mean 24-month follow-up.
Xeroderma pigmentosum and Bowen’s disease
Xeroderma pigmentosum (XP) is a rare autosomal recessive disorder of defective UV-radiation–induced damage repair that is characterized by photosensitivity and higher risk for developing skin cancer at an early age (Black, 2016). Reports of PDT in XP are scarce because of the fear of developing skin cancers following illumination (Fernández-Guarino et al., 2020). Larson and Cunningham successfully treated facial actinic keratosis of a 16-year-old girl with type C XP, by means of three sessions of ALA-PDT with blue light. Before starting treatment, Larson and Cunningham tested skin photosensitivity of their patient performing a 3 cm2 test treatment on her left arm, not noticing adverse cutaneous reactions of the exposed area. The treatment was repeated for 1 year and did not cause any adverse events (Larson and Cunningham, 2012). Fernández-Guarino et al. successfully treated 13 young African XP patients, 12 of whom were younger than 18, affected by facial AKs and BCCs, by using one session of DL-PDT. They used indoor DL-PDT because the window blocked UVB radiation, responsible for DNA damage, thus limiting treatment-related skin cancer induction. After two days of treatment, the patients presented with crusting and scaling, but a week later the cutaneous reaction resolved. No adverse events were noted at 3-month follow-up (Fernández-Guarino et al., 2020). Despite these encouraging results, the use of PDT in pediatric patients with XP is still limited and its safety should be confirmed by further studies considering long-term follow-up. Keratinocyte-derived tumors are rare in the pediatric age with a single case of periungueal Bowen’s disease treated with PDT in a 12-year-old boy. Hyun et al. treated the child with two sessions of MAL-PDT at an interval of 3 weeks. The authors did not observe any sign of recurrence 9 months after treatment, and no adverse events were reported (Hyun et al., 2016).
Cutaneous lymphomas
Cutaneous lymphomas have a heterogeneous clinical presentation, ranging from pink, red, or violaceous solitary papules or nodules to widespread infiltrative lesions. There is an emerging interest about the antitumor properties of PDT applied to cutaneous lymphomas, particularly T-cell lymphomas, although only a few cases in adults, and even less in children, are described in literature.
Overall, five pediatric patients with various cutaneous lymphomas have been treated with MAL- or ALA-PDT, achieving a complete response in all cases (Takeda et al., 2005; Mendese et al., 2012; Heng et al., 2014; Snider et al., 2020).
Takeda et al. observed complete resolution of periocular lymphadenosis benigna cutis (also called lymphocytoma cutis) in two 16-year-old girls after five sessions of ALA-PDT. They noted only a transient hyperpigmentation and no recurrences at 6-months follow-up (Takeda et al., 2005). Mendese et al. treated a 10-year-old boy with pagetoid reticulosis, a rare variant of mycosis fungoides (MF), on his right foot by nine sessions of PDT over 13 months. They applied ALA topical solution with subsequent illumination with blue light or pulsed day laser. On three occasions, ALA was injected intralesionally to ensure adequate penetration. The patients reported treatment-related moderate pain and post-inflammatory pigmentation. At the end of the treatment period, he was clinically disease-free. At a follow-up visit 15 months after the last PDT session, two punch biopsies confirmed the absence of atypical lymphocytes in the treated area. Clearance was maintained at 46 months follow-up (Mendese et al., 2012). Heng et al. also reported a case of successful treatment of solitary mycosis fungoides after 30 sessions of MAL-PDT. After two months of stopping PDT, the patient showed no evidence of MF. The patient was disease-free at 35-months follow-up (Heng et al., 2014). Snider et al. used PDT in combination with narrow-band UVB to treat a 13-year-old boy with multiple lesions of lymphomatoid papulosis (LyP) over his elbows, forearms, proximal thighs, and right hip. They used 20% ALA topical solution and LED illumination to treat right forearm nodules resistant to nb-UVB treatment. He achieved the clearance of all lesions on his right arm within 2 months of combination therapy. Nevertheless, 2 years later new lesions appeared, but further PDT sessions were not attempted and subsequently the patient was lost at follow-up (Snider et al.,. 2020).
PDT and pediatric inflammatory skin diseases
Acne vulgaris
Acne is a chronic inflammatory disease of the sebaceous-pilosebaceous unit. In the last years, it has been clearly demonstrated that acne development is linked to the combination of predisposing genetic factors and environmental triggers, among which a prominent role is played by the follicular colonization by Propionibacterium acnes (P. acnes) (Antiga et al., 2015). Studies focusing exclusively on acne and PDT in pediatric population are scarce. In total, three possible ways of action have been proposed to explain the improvement of acne by PDT: photodynamic killing of Propionibacterium acnes, which sterilizes the sebaceous follicle; direct photodynamic injury of sebaceous glands inhibiting sebum production; and reduction of follicular obstruction by an effect on keratinocyte shedding and hyperkeratosis. Nevertheless, these mechanisms do not appear to occur simultaneously in all cases of acne improvement. In a study on 10 patients involving a 16-year-old female, Pollock et al. (2004) found a statistically significant reduction of inflammatory acne lesions following ALA-PDT but failed to demonstrate changes in P. acnes numbers or in sebum excretion in the same patients. It has been hypothesized that light destroys P. acnes by targeting its endogenous porphyrins, including coproporphyrin III and protoporphyrin (Yeung et al., 2007). Nevertheless, according to some studies, PDT may determine a functional damage of P. acnes rather than a quantitative reduction (Pollock et al., 2004; Hörfelt et al., 2007). Moreover, irreversible damage of sebaceous glands may be reached only with repeated session of PDT (Pollock et al., 2004). So, other PDT effects, such as the reduction of follicular obstruction or anti-inflammatory effects, may play a more important role for acne improvement than destruction of sebaceous glands or killing of P. acnes (Pollock et al., 2004; Hörfelt et al., 2007).
Overall 31 pediatric patients with acne vulgaris were treated with various concentrations of ALA.
Ma et al. (2015) carried out a prospective study to evaluate ALA-PDT in severe acne of 21 adolescent patients. They treated patients with an average of three PDT sessions. They obtained high rates of effective response (85.7%) and observed that the efficacy of ALA-PDT tends to increase even after, reaching 95.23% of effective response after 8 weeks. PDT appears particularly useful in acne treatment when other conventional therapies have failed. Theresia et al. (2017) treated a 17-year-old male patient with severe multiple nodulocystic acne lesions on the face with ALA-PDT, who failed treatment with numerous topical and oral retinoids and antibiotics. The authors noticed a mild reduction in inflammatory acne lesions and sebum secretion already after the first session of PDT. After the fourth session of ALA-PDT, the patient had long-term remission with no new lesions during the 6 months follow-up. Borgia et al. (2018a) obtained improvement and a sustained good response in a case of acne conglobata on the buttocks in a 16-year-old boy, who did not respond to oral minocycline, topical retinoid, and systemic isotretinoin. They used ALA-PDT for a total of six sessions. The patient experienced intense pain and inflammation during the first two sessions, then discomfort was milder. At the end of the treatment period, healing of the cutaneous nodules was observed, and at 6 months follow-up, the patient maintained good cosmetic results with no side effects. In total, two 18-year-old identical male twins with severe nodulocystic facial acne resistant to oral retinoids, antibiotics, and previous physical therapies experienced decrease in acne lesions with sustained response at 2-year follow-up after three sessions of ALA-PDT at 2 weeks interval. Both the patients suffered transient moderate pain and mild erythema during treatment with no residual pigmentation. They evaluated this method better than the previous medications (Li et al., 2018). In literature, several clinical trials or case series on PDT efficacy for acne treatment included both children and adults. Overall, 271 adult and pediatric patients with acne vulgaris were treated with various concentrations of ALA, achieving improvement of various degree, ranging from resolution of 30% of inflamed lesions and 7% of non-inflamed lesions (Orringer et al., 2010) to 90% clearance in 90% of patients (Wang et al., 2010). Itoh et al. tested the effects of 20% ALA oil-in-water emulsion and subsequent illumination by polychromatic visible light source on three men and 10 women with intractable facial acne. Among these, two 18-year-old males were also treated with one session of ALA-PDT and obtained excellent control of their acne and transient side effects. One of them experienced subsequent hyperpigmentation and was therefore treated with 4% hydroquinone cream for 10 days (Itoh et al., 2001). In a study on the efficacy of 20% ALA-PDT involving 15 patients with facial and dorsal acne vulgaris, four of them were pediatric (two of 16 years and two of 17 years). Improvement of acne lesions were recorded in all pediatric patients with facial acne after one session of ALA-PDT. There were no data about the 17-year-old male affected by dorsal acne because he was lost at 20-weeks follow-up (Hörfelt et al., 2007).
In a RCT on both pediatric and adult patients (median 18 years, range 15–28), two sessions of MAL-PDT, 2 weeks apart, determined a median reduction of 54% in the total inflammatory lesion count at week 12. Clinical response in MAL-PDT–treated patients was significantly better with respect to placebo-treated patients. Nevertheless, MAL-PDT was associated with more pain than placebo-PDT (Hörfelt et al., 2006). Wang et al. evidenced a 90% clearance rate after three sessions of ALA-PDT in a group of patients with mean age 22.9 years (range 16–37). Only 10% of patients had clearance rates between 50 and 90%. Side effects were well tolerated and transient, except for a patient who left the study because of excessive pain and discomfort (Wang et al., 2010). In a study involving patients with an age range 13–54 years, Barolet and Boucher et al. increased skin temperature of 10 patients with radiant infrared (IR) prior to ALA-PDT application to enhance the PS penetration. The authors observed a significant difference in median reduction of inflammatory lesions on the IR pre-treated vs. the control side 1 month after PDT. The authors did not report unusual treatment–related adverse effects (Barolet and Boucher, 2010). Also the combination of PDT and antibacterial therapies for acne has been investigated with encouraging early results. In a clinical trial, Xu et al. compared the effects of minocycline plus ALA-PDT and minocycline alone on moderate-to-severe facial acne of 95 patients aged 15–35 years. The authors observed a greater mean percentage reduction of lesion counts in the minocycline plus PDT group compared to the minocycline-alone group at 8 weeks follow-up for both inflammatory and non-inflammatory lesions and only mild and transient adverse events in the minocycline plus PDT group (Xu et al., 2017). Intense pulsed light (IPL) has been investigated as a light source in PDT for juvenile acne. Rojanamatin et al. treated 14 patients (range 16–27 years) with topical ALA plus IPL for three sessions. The combination determined a decrease in lesions counts of 87.7% at 12 weeks (Rojanamatin et al., 2006). Also, a pulsed dye laser (PDL) has been evaluated as a light source for ALA-PDT. Orringer et al. treated 44 patients, including pediatric patients (mean age 25 years, range 15–50) with three PDL treatments after a 60–90 min ALA application time. Nevertheless, the results with this light source were not particularly exciting with only transient decrease in mean inflammatory papule counts but no statistically significant differences in lesion counts (papules, pustules, and open and closed comedones) between treated and untreated control skin at the conclusion of the study on week 16 (Orringer et al., 2010). However, compared to topical or systemic treatments for juvenile acne, PDT did not show clear advantages in efficacy, and for this reason, it should be reserved for severe, recalcitrant cases, resistant to antibiotics and/or hormonal conventional treatments and/or not eligible to treatment with isotretinoin. In a systematic review on light therapies for acne, 25 trials on a total of 694 patients, also including pediatric patients, were analyzed. The authors observed that PDT did not show better efficacy than topical 1% adapalene gel. Nevertheless, PDT showed a benefit over light therapy alone (Hamilton et al., 2009).
Necrobiosis lipoidica
Necrobiosis lipoidica (NL) is a rare granulomatous disease strongly, but not exclusively, associated with diabetes mellitus characterized by yellowish-brown telangiectatic plaques with central atrophic area and erythematous edge, usually localized on the pretibial skin of females. Treatment of NL is often not satisfactory. PDT exerts positive effects in controlling the disease. In addition to its general anti-inflammatory effects, PDT seems to influence positively the course of NL by remodeling the collagen matrix, thus stimulating the wound healing and improving sclerosis (Borgia et al., 2014). PDT is able to induce matrix metalloproteinases (MMP) production in fibroblasts (Motta and Monti, 2007) with higher production of MMP-1, MMP-9, and transforming growth factor (TGF)-β3 in wounds treated with MAL-PDT compared to untreated wounds (Mills et al., 2014). Berking et al. obtained partial results from the treatment of three adolescents among 18 patients with necrobiosis lipoidica of lower legs. The patients received two to six sessions of MAL-PDT. Pain was measured by using a 10 cm visual analog scale and pediatric patients referred a pain level ranging from 4/10 to 10/10. One of them stopped the treatment after two sessions because of treatment-related pain achieving no benefit. Of the remaining two patients, one presented partial response, whereas the other was not responder (Berking et al., 2009). Kaae et al. (2018) conducted a retrospective study on 65 NL patients treated with DL-PDT and C-PDT, including pediatric patients (median age at first treatment 35.5 years, range 12–65) and observed complete response in 66% of cases, with similar rates between C-PDT and DL-PDT. MAL-PDT was in median performed four times. The authors observed no correlation between clinical response and gender, age at first PDT treatment, duration of NL prior to PDT treatment, number of NL elements, or diabetes.
Hidradenitis suppurativa and dissecting cellulitis (perifolliculitis capitis abscedens et suffodiens or PCAS)
HS, often designed as acne inversa, is a chronic follicular inflammatory skin disease characterized by characterized by occlusion of hairs follicles and inflammation, which clinically leads to painful nodules, abscesses, and interconnecting sinus tracts involving the axillary, inguinal, anogenital, and inframammary regions. It usually manifests after puberty, but it can affect young patients especially those with familial history of HS. Bu et al. used 20% ALA-PDT as adjuvant therapy post-surgery for HS in seven patients, including two adolescents of 15 and 16 years, with Hurley grade II and III, respectively. At 5 months, they observed a marked improvement of the Dermatology Life Quality Index (DLQI) in all patients, including pediatric patients, with no recurrences at 6–12 months follow-up. Pain during illumination was well tolerated in all cases, but the 16-year-old patient, with Hurley Grade III lesions on craniofacial and neck areas, took the analgesic 30 min before PDT due to the moderate pain (Bu et al., 2017).
PCAS is considered an inflammatory bacterial process frequently caused by Staphylococcus aureus and Staphylococcus epidermidis. Cui et al. speculated that PDT ameliorated PCAS by inhibiting bacterial infection. In their case series of nine patients treated with combination of surgical reverse flaps and PDT, they included a 17-year-old-boy, who reported satisfied outcome after a single session (Cui et al., 2020). Feng et al. (2019) treated a 15-year-old boy among eight male patients with dissecting cellulitis by using ALA-PDT. Before 10% ALA application and red light illumination, they cut hair and performed micropunctures in skin lesions by using a plum blossom needle, a kind of micro-needle. After 3 months, the authors observed a significant improvement in the pediatric patient, with a clearance rate > 70% and marked relief of symptoms. Treatment-related pain was tolerable and previous analgesia was not needed.
PDT and pediatric infectious skin diseases
PDT exerts antimicrobial effects on viruses, bacteria, fungi, and parasites. First, such activity is related to its ability to form high amount of reactive oxygen species (ROS), which damage biomolecules of all type of microorganisms, including viruses (Pérez-Laguna et al., 2018). In addition, as for tumoral cells, PDT stimulates the recognition of microorganisms by the immune system and mediates a local immune response against them. HPV is one of the most frequently targeted viruses in pediatric PDT. Cells infected by HPV are ideal targets of PDT because high-proliferating and can selectively accumulate PpIX compared to the surrounding non-infected cells. Moreover, selective photosensitization occurs not only in clinical HPV lesions but also in subclinical infection (Shan et al., 2016). Viruses have no capacity for PPIX production, but it has been demonstrated that the addition of exogenous ALA and its derivatives induces selective accumulation of PPIX in HPV-infected cells. PDT is able to significantly reduce HPV viral loads and to promote viral inactivation via cell necrosis and induction of T lymphocyte–mediated immune response against infected keratinocytes by increasing levels of IFN-α and IFN-ß (Borgia et al., 2020a). In bacterial infection, PDT-induced oxidative stress may damage multiple targets such as DNA, membrane integrity, protease activity, and lipopolysaccharide (LPS). Nevertheless, Gram-positive bacteria appear more sensitive to PDT than Gram-negative. Peptidoglycans and lipid acids in wall of Gram-positive allow penetration of cationic, anionic, and even neutral PS, while the double membrane in Gram-negative is particularly hampering and only cationic PS are active against them. Nevertheless, new technologies, including nanoparticle-based PDT, have significantly increased the PS penetration. The onset of resistance to PDT in bacteria is very unlikely because PDT-induced oxidative stress does not have a specific target but causes destruction of cell in different ways. Therefore, PDT has several advantages to antibiotics and may be considered a valid therapeutic alternative to them (Pérez-Laguna et al., 2018).
Cutaneous viral warts
PDT is indicated for treatment of cutaneous viral warts when other therapies have failed or in difficult to treat cases.
Overall 12 pediatric patients with cutaneous viral warts were treated with various concentrations of ALA, achieving complete response in almost all cases (Borgia et al., 2020b; Huang et al., 2019; Wu et al., 2019).
Borgia et al. described the case of a 6-year-old girl with multiple viral warts on the dorsal left foot. After failure of cryotherapy, the authors performed ALA-PDT for two sessions, 1 month apart. In each session, the patient experienced mild burning sensation. Complete clearance of the treated warts was seen 6 weeks after the second treatment with no recurrences at 1-year follow-up (Borgia et al., 2020b). Huang et al. described the exciting case of an 18-year-old female who completely resolved her 2 year history of resistant multiple warts in the right foot after curettage plus PDT. The patient was treated with ALA-PDT for a total of three sessions, but superficial shaving was applied only for the first session. At 3-months follow-up, the warts disappeared with no residual scar (Huang et al., 2019). Wu et al. performed on average three sessions of ALA-PDT on 10 pediatric patients ranging from 5 to 18 years among 23 total patients with multi-resistant periungual warts. The patients underwent superficial shaving before the first PDT, not performed in the following additional sessions of PDT. The authors observed an overall complete clearance in 61% of patients with a higher rate in the pediatric group (9/10 young patients had complete response, namely, 90%). All patients completed the treatment and satisfactory cosmetic outcome was obtained in almost all patients (96%). A significant decrease in DLQI at 12-month follow-up was reported. Pain was the most common adverse event, followed by secondary onychodystrophy, mild itching, and blisters (Wu et al., 2019). Also, in case of cutaneous viral warts, many studies on PDT efficacy include both children and adults.
Overall, 44 adult and pediatric patients with cutaneous viral warts were treated with 10–20% ALA, achieving complete response in 84.6–88% of cases (Schroeter et al., 2005; Caccavale et al., 2019).
Schroeter et al. (2005) treated 48 plantar warts from 31 patients (mean age 29 years, range 6–74) with 20% ALA cream and red light, observing a complete response in 88% of cases and no significant side effects. Caccavale et al. (2019) proven the efficacy of combination of curettage plus microneedling plus topical ALA-PDT for the treatment of acral resistant warts in young patients (mean age 28.8 years, range 18–52). They performed a thorough curettage on palmar and plantar warts of 13 patients, subsequent application of 10% ALA cream and microneedling. After 3 h of incubation, the warts were irradiated with a red light source. After three sessions of treatment, at 3-week intervals, the authors observed complete remission in 84.6% of cases and partial remission in further 7.7%.
Flat viral warts
PDT should be considered a useful option in treatment of flat warts, particularly in aesthetically sensitive areas such as the face of children (Borgia et al., 2020a). Overall 31 pediatric patients with flat viral warts were treated with C-PDT or DL-PDT, achieving complete response in one case (Borgia et al., 2019) and various degree of complete response ranging from 73.3% (C-PDT) to 80% (DL-PDT) in a study involving 30 patients (Borgia et al., 2020a).
Flat viral warts appear to be responsive not only to conventional PDT but also to DL-PDT. In the case of an 8-year-old female child with multiple facial flat warts resistant to previous topical tretinoin and cryosurgery, Borgia et al. performed two sessions of DL-PDT with 10% ALA ointment obtaining complete response with no recurrence at 1 year follow-up (Borgia et al., 2019). Borgia et al. also compared efficacy and safety of C-PDT and DL-PDT for treatment of facial flat warts in pediatric patients. They studied 30 young patients, who were divided in two group, with mean age of 9.67 ± 4.48 years (range 4–17) in group A and 9.13 ± 2.77 years (range 5–15) in group B. The two groups were randomly assigned to receive treatment with C-PDT or DL-PDT. The authors noted that in the early 12 weeks the treatment with DL-PDT seemed to fail. In fact, none of patients treated with DL-PDT reached an excellent response (75–100% reduction of total wart count), compared to 53.3% of patients treated with C-PDT. Nevertheless, this gap was filled in the following 12 weeks. At 24 weeks follow-up 80% of patients of DL-PDT group showed excellent response compared to 73.3% of patients of C-PDT group. So, in the long-term follow-up DL-PDT and C-PDT showed similar clinical efficacy for the treatment of pediatric facial flat warts. Adverse effects were also similar in the two group, with transient pain, irritation and hyperpigmentation reported (Borgia et al., 2020a). At 1-year follow-up, 60% of patients of both group (DL-PDT and C-PDT) maintained excellent response (75–100% reduction of total wart count compared with baseline). After 24 weeks, among the responders, 13.3% of C-PDT–treated patients and 20% of those treated with DL-PDT experienced mild relapses in terms of lesions’ number and size. None of non-responders at 24 weeks achieved improvement at 1-year follow-up. No long-term side effects were reported in both groups (Borgia et al., 2021).
Genital viral warts
PDT may be particularly useful for genital warts difficult to treat due to their localization.
Shan et al. (2016) described the efficacy of PDT in the treatment of genital viral warts in male urethra. They treated 76 men including pediatric patients (mean age 32.6 years, range 16–65) applying 20% ALA solution with a thin cotton swab gently inserted into the urethra. After a 3 h incubation period, they irradiated the lesions with a urethral cylindrical semiconductor laser fiber emitting light of 635 nm wavelength. The treatment was repeated once every week for 4 weeks. At the 3 months follow-up, almost all patients had a complete response and only five (6.6%) patients relapsed. Of these, three received four more sessions of PDT resulting in clearance of lesions without further recurrence.
Overall nine pediatric patients with genital viral warts were treated with ALA-PDT, achieving complete response in all cases (Xu et al., 2018; Macca et al., 2022).
Xu et al. (2018) reported eight treatment-resistant cases of pediatric genital warts successfully treated with 20% ALA-PDT. They irradiated perianal and intra-anal areas with red light from a semiconductor laser. Pretreatment by using microwave ablation was applied for lesions larger 5 cm. The patients were sedated with oral chloral hydrate (0.5–0.8 mg/kg) half an hour before light exposure. The majority of patients achieved complete response after three to six PDT sessions, but one patient required up to 12 sessions. The patients experienced mild to moderate pain during light exposure, according to a pain score. At a 6-month follow-up, neither other side effects nor recurrences were detected.
Macca et al. (2022) proven the effectiveness of ALA-PDT on non-sexually transmitted genital warts of a 5-year-old female. They applied 10% ALA ointment and irradiated with red light after an incubation period of 3 h. At 3 months follow-up, only a few flat elements were still visible but after further 3 months complete clearance was detected. The patient experienced mild to moderate burning sensation during light exposure and a transient hyperpigmentation, with no long-term side effects at 6 months follow-up. In conclusion, PDT may be considered as first-line therapy in patients with high number of genital warts, for which other topical therapies are excessively expensive or painful, or in patients with warts in urethral or anal and perianal areas. Xu et al. (2018) proven that the rapid healing of PDT makes it an optimal therapeutic option for pediatric genital warts in perianal and intra-anal areas, where other invasive treatments may cause anal stenosis and difficult defecation. Similarly, Shan et al. (2016) successfully used PDT for urethral genital warts in patients of all ages, including children.
Cutaneous leishmaniasis
In addition to treatment of viral and bacterial disease, PDT in pediatric age has been studied for management of parasitic infection from Leishmania. Mechanisms underlying effects of PDT on cutaneous leishmaniasis are largely unknown. Amastigotes are proven to accumulate very low amounts of protoporphyrin IX and some species of Leishmania lack enzymes of heme synthesis. Despite this, PDT may exert its effects on this type of protozoa by increasing the local temperature of the skin: hyperthermia treatment has been described as an effective therapeutic option for cutaneous leishmaniasis (Fink et al., 2016). Johansen et al. described an ulcerative resistant case of cutaneous leishmaniasis by Leishmania major in a 15-year-old boy successfully treated by 10% ALA-PDT. They performed conventional PDT twice weekly for 12 weeks. The patient had previously received unsuccessful treatments, including meglumime antimoniate, topical ketoconazole, and oral fluconazole. The authors noted full healing of the ulcer 1 month after PDT. Pain experienced during treatment was controlled by topical application of lidocaine (Johansen et al., 2019). Leishmania species that can cause mucocutaneous (L. braziliensis complex) or visceral leishmaniasis (L. donovani complex) should not be treated with PDT.
However, currently PDT is indicated only for cutaneous leishmaniasis resistant to other treatments and in aesthetically sensitive parts of the body (Morton et al., 2020).
Miscellanea
Vitiligo
Vitiligo is a common progressive depigmentation of the skin due to selective destruction of melanocytes (Vaccaro et al., 2015; Vaccaro et al., 2017a). Although the pathogenesis remains scarcely known, it seems to be related to genetic predisposing factors, oxidative stress and autoimmune dysregulation (Vaccaro et al., 2016; Vaccaro et al., 2017b; Custurone et al., 2021). PDT is proven to inhibit melanogenesis in vitro, reducing melanocytes melanin content and tyrosinase activity. In vivo, PDT reduces mottled hyperpigmentation of photoaged patient skin (Kim et al., 2018). These observations may partially explain why PDT did not achieve brilliant results in treatment of vitiligo in pediatric patients. Zhang et al. (2018) conducted a study to determine the effective PS concentration, PS application duration, irradiation duration, and irradiation dosage for the treatment of vitiligo. They selected ALA concentration of 1.5%, PS application duration of 3 h, irradiation duration of 20 min, and irradiation dosage of 80 mw/cm2 as the better parameters for treating their patients. They treated vitiligo in two pediatric patients (4 and 17 years). Nevertheless, the results were contrasting and characterized by alternating periods of worsening and improvement. In the 17-year-old patient, pigment islands around skin follicles of vitiliginous areas of abdomen increased significantly with the number of early treatments. Nevertheless, during subsequent treatments at long intervals, pigment islands decreases progressively and, at the end of follow-up, no significant changes in pigmentation were detected compared to baseline. In the 4-year-old patient, vitiligo was found on forehead. During PDT treatment, pigment islands increased significantly first near the left eyebrow, then on the forehead and finally near the hairline, with an apparent general improvement of vitiligo area compared to baseline.
When compared to topical corticosteroids, a standard treatment for vitiligo, PDT does not demonstrate any additional therapeutic effects (Rahimi et al., 2021). For that reason, there are no reasons to prefer it to other available therapies.
Angiofibromas of tuberous sclerosis
Weinberger et al. (2009) associated ALA-PDT with pulsed dye laser (PDL) to treat angiofibromas of tuberous sclerosis of six young patients. Two of these were in pediatric age (10 and 18 years). They combined 417-nm blue light with 595-nm PDL after application of 20% ALA solution and obtained decrease of lesions number and size. Transient side effects included erythema, swelling and superficial desquamation.
Verrucous epidermal nevus
Zheng et al. (2018) combined fractional micro-plasma radiofrequency (RF) technology and PDT to treat a facial verrucous epidermal nevus (VEN) in a 17-year-old girl. They carried out local anesthesia by using lidocaine cream under occlusion an hour before the therapy. Then, they performed the fractional micro-plasma RF treatment, which caused a temporary volume decrease of verrucous papules, and the first treatment of ALA-PDT 4 h later. Other three ALA-PDT session without RF pretreatment were performed. After treatment, the patient achieved complete disappearance of warty lesions on her face and no recurrences of VEN were detected at 24 weeks follow-up.
Linear porokeratosis
MAL-PDT has proven to be very effective in the treatment of Linear porokeratosis (LP), is a disorder of keratinization typically occurring in pediatric age. García-Navarro et al. (2009) obtained a cosmetic and clinical improvement of a LP on lower leg of a 13-year-old boy by using 16% MAL-PDT and red light. They performed two PDT sessions 1 month apart. No recurrences were observed after 11 months.
Curkova et al. (2014) performed three MAL-PDT sessions on extensive LP of a 16-year-old girl. They noted a progressive improvement of her multiple reddish-brown macules and depressions on right arm and at 1-year follow-up the cosmetic and clinical response was considered satisfactory. They did not performed pretreatment except for removal of superficial scale before second PDT session. The patient experienced only a transient burning sensation during illumination.
Garrido-Colmenero et al. (2015) reported the case of an 11-year-old girl with LP on her left breast. They did not obtain clinical improvement by using topical therapies, including adapalene and tacrolimus 0.1% ointment so the patient underwent two sessions of MAL-PDT with good result. Four months after PDT, only few lesions remained.
Gracia-Cazaña et al. (2015) used MAL-PDT to treat porokeratosis in children with bone marrow transplant. A 12-year-old boy presented three round lesions in the right popliteal fossa and two in the cervical region, histologically diagnosed as porokeratosis. After three sessions of MAL-PDT, he achieved complete clearance and remained free of disease after 5 years follow-up.
Discussion
Despite its worldwide use in adult patients that has provided strong evidence about efficacy and safety not only for oncologic conditions but also for inflammatory and infectious diseases, PDT applied to the pediatric population appears to be a substantially unexplored continent. Our review has in fact evidenced that this peculiar kind of photochemotherapy has been investigated in few diseases, with a very limited number of RCT and small case series, while most of our knowledge in children originates from sporadic case reports on single patients. This find an obvious justification regards to skin tumors, which occurrence in pediatric age is very rare, mainly represented by keratinocyte cancer in syndromic patients. The consequent almost complete absence of data about its effectiveness in the face of the not estimable risk of worsening or relapse strongly suggest, for ethical reason, its use only in exceptional cases. PDT could be considered therapeutic alternative in case of benign lymphocytic infiltration of the skin, while its use in cutaneous lymphomas may be hypothesized in a near future, as proposed for adult patients, only in localized form when other therapies are contraindicated or have failed (Morton et al., 2020). Moreover, as demonstrated in adults patients (Hooper et al., 2021) and suggested for children by Heng et al. and Mendese et al., repeated sessions of PDT are needed to obtain a clinical response in cutaneous lymphomas such as MF, thus potentially limiting the feasibility of this type of treatment. The anti-inflammatory effects of PDT have been studied in diseases mainly affecting the pilosebaceous unit, such as acne and HS. Both acne and HS have a dramatic burden, negatively conditioning the everyday life of the patients, especially in a delicate period such as adolescence, with disastrous effect on affective, social and sexual aspects resulting in low self-esteem feelings and depression. The point of strength of PDT in such cases seems to be its ability to hit with one shot three different pathogenic mechanisms, inhibiting the proliferation of P. acnes, targeting activated T lymphocytes thus reducing the release of cytokines which attract leucocytes to dermis and improving follicular hyperkeratosis acting on keratinocytes differentiation and proliferation. Examining the available data on this topic, no significant advantages emerge respect to both topical and/or systemic conventional therapies. To date, PDT may be considered as a valid second-line treatment in patients resistant to antibiotics and/or retinoids or when such therapies are contraindicated. Some types of acne, especially the nodulocystic form of the face and trunk, may largely benefit from PDT not only to achieve sustained clinical improvement but also to reduce the risk of permanent scarring. PDT is known to promote the remodeling of the dermal matrix architecture via keratinocyte photoactivation with subsequent paracrine induction of matrix metalloproteinases production in fibroblasts. Its use at an early stage of the disease may accelerate resolution of the cystic lesions, reducing the risk and the severity of disfiguring scars. On the basis of these consideration, PDT could find growing application also in pediatric HS patients, a highly disabling disease with an always increasing incidence in pre-puberal and puberal patients, especially in those affected by concomitant predisposing factors such as obesity. More than in acne, PDT could help to control inflammation in a non-invasive way lowering the necessity to recur to prolonged systemic therapies not free from long-term side effects (antibiotic resistance), partially contraindicated (tetracyclines) and often not well accepted by both little patients and their parents. Moreover, its use since the onset of the disease may reduce the frequency and the severity of inflammatory episodes, preventing the development of undesirable scars with both cosmetic and functional impairment. An interesting field of application of PDT in pediatric patients is undoubtedly the antimicrobial one, with particular regards to HPV infection. With respect to the aforementioned indications, there are enough experimental and clinical experiences to affirm that PDT can be considered an effective, safe and well tolerated solution for both cutaneous and mucosal warts. Conventional therapies, including topical keratolytic agents, electrosurgery, cryotherapy and carbon dioxide laser may cause scars, inflammatory reactions and hyper- or hypopigmentation, with high risk of treatment failure and recurrence. Furthermore, such treatments are often contraindicated or not tolerated, especially in children. In general, lesion-directed therapies are not fully effective to eradicate HPV infection, in particular in subclinical and latent conditions. Conventional PDT has been used in children with good cosmetic results, better compliance and lower recurrence rates. However, it shows some limits: it is accompanied by pain during illumination, is time-consuming and requires dedicated equipment. DL-PDT offers advantages over C-PDT in terms of tolerability, time and cost, making this procedure more suited to the pediatric setting. DL-PDT is a novel procedure in which the activation of the topical photosensitizer is induced by exposure to natural daylight, without requiring preliminary occlusion. The absence of occlusion, with consequent less time spent at the clinic and the possibility to perform the treatment in an outdoor setting, may increase the compliance of young patients. In addition, pain intensity during DL-PDT is significantly lower than with C-PDT, probably because of gradual and continuous production and photoactivation of smaller amounts of protoporphyrin IX, minimizing the little patient’s discomfort during irradiation.
With regards to the safety profile, as reported by all aforementioned studies, PDT is associated with only transient and mild to moderate adverse effects in pediatric population, such as those observed in the adult population. PDT side effects can be classified in early (immediately or within days after treatment) and late (after weeks or months) onset side effects. Early onset side effects include pain and local skin reactions (LSRs), namely, erythema, burning, edema, crusting, desquamation, or pustules (Borgia et al., 2018b). Pain is the more common adverse effects, but rarely requires analgesia. DL-PDT is considerably and statistically significantly less associated with pain than C-PDT. Borgia et al. observed that post-irradiation pain was similar in DL-PDT and C-PDT groups of pediatric patients, but further studies are needed to compare the two modalities and to evaluate any differences in terms of safety (Borgia et al., 2020a). Pain during PDT in pediatric patients may be also related to disease localization. Head and neck district is one of sites most associated with treatment-related pain. In their study on treatment of HS, Bu et al. proven that PDT did not requires previous analgesia except in the case of a 16-year-old boy who presented HS lesion in craniofacial and neck areas (Bu et al., 2017). Hyperpigmentation is a worrisome side effect, especially in children with high phototype skin, but may be avoided using appropriate photosensitizer concentration and incubation time and most often is transient and disappears spontaneously after a few weeks or months. Other side effects have been described following PDT, such as onychodystrophy, hair loss, erosive pustular dermatosis of the scalp and urticarial reaction urticarial reaction (Guarneri and Vaccaro 2009). Urticaria-like reaction was reported in two pediatric patients following a few minutes of light exposure. The first patient was an 11-year-old girl with Gorlin syndrome who was treated for BCC, while the second patient was a 4-year-old girl who was treated for porokeratosis. In the first patient, a subsequent provocation skin test confirmed that the reaction was produced by the combination of MAL and illumination while in the second patient, provocation testing was not carried out due to the her young age (Miguélez et al., 2013). No information regarding long-term safety of PDT in pediatric patients are available (Snider et al., 2020). The main concern is the development of PDT-induced skin cancers, as reported in some cases in adult patients (Borgia et al., 2018b). To date, there is no evidence that PDT can stimulate skin carcinogenesis in children but a continuous and careful follow-up of PDT-treated patients is needed to verify this hypothesis.
Conclusion
Clinical trials focusing on PDT treatment in children are rare. There is a general reluctance about involving children in trials by parents and adults, especially because of fears of unpredictable side effects in the pediatric population. Moreover, trials on children involve more ethical concerns because children lack the capacity to understand the risks underlying trials and informed consent is difficult to obtain by parents (Joseph et al., 2015). Nevertheless, the review of the available data has showed promising results, with some points of strength but even a bigger number of uncertainties. It appears as a safe therapeutic procedure. Pain may limit the compliance of pediatric patients but previous local analgesia or more tolerable PDT settings, for example, daylight PDT, lower PS concentration, shorter incubation times or lower light fluences, may be useful in more sensitive patients. These parameters should be modulated in the same way to avoid the risk of hyperpigmentation, which represents a particularly worrisome side effect on a child’s face. Nevertheless, PDT-induced hyperpigmentation is generally transient and responsive to local treatment. To date, there are no reports of PDT-induced skin cancers in pediatric age, so this possible adverse events remains only theoretical. PDT may be a soft procedure in children because it does not require daily treatments but limited in number and spaced in time. Nevertheless, it is time-consuming because the patients have to wait a number of hours at the hospital between PS application and illumination. Daylight PDT may be useful to reduce waiting times and it better fits to pediatric patients who would rather spend time outside than within the walls of an hospital. Data on efficacy of DL-PDT in pediatric patients are still limited, and further comparison between C-PDT and DL-PDT is needed in this population. PDT has the advantage that it can be easily combined with other therapies, thus increasing its effectiveness rates. The majority of local combination therapies, such as curettage, microneedling, fractional micro-plasma radiofrequency, radiant infrared or surgical debulking, were used to improve penetration of photosensitizer in the skin. Instead, other therapies with mechanism of action other than PDT, such as cryotherapy for viral warts or oral antibiotics for juvenile acne, can also be associated without increased risk of side effects. Nevertheless, the analysis of the literature has evidenced a number of questions that need to be addressed relatively to the most appropriate type, concentrations, and incubation period of photosensitizers, and optimal parameters of illumination sources in the different pathologies, adapting them in the light of the clinical characteristic of each single patient including age, disease severity, extent of the disease and its localization in different areas of the body. Unfortunately, the great heterogeneity of light sources, formulations, and photosensitizer types reported in literature makes comparison and analysis difficult. Moreover, PDT has been used with satisfying results in adults for other dermatologic conditions which may be explored also in pediatric patients, including connective tissue disorders, such as chronic lupus erythematosus or morphea/scleroderma, genital and oral lichen planus, lichen sclerosus, and several types of scars (Kvaal et al., 2013; Gordon Spratt et al., 2015; Wennberg, 2015; Borgia et al., 2016b; Gerkowicz et al., 2021). Specific protocols for pediatric patients as well as the length of follow-up intervals must be better standardized in larger RCT studies in order to draw up shared guidelines taking full advantage from such versatile treatment.
Author contributions
DL: conceptualization, data curation, formal analysis, writing—original draft, and first authorship. AD: methodology, data curation, formal analysis, validation, writing—review and editing, and first authorship. VM: conceptualization, data curation, investigation, supervision, and writing—review and editing. VF: data curation, investigation, methodology, and validation. SV: data curation, investigation, methodology, and validation. SF: data curation, investigation, methodology, supervision, and writing—review and editing. BF: conceptualization, data curation, investigation, supervision, and writing—review and editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor FS is currently organizing a research topic with the author DA.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Antiga, E., Verdelli, A., Bonciani, D., Bonciolini, V., Caproni, M., and Fabbri, P. (2015). Acne: A new model of immune-mediated chronic inflammatory skin disease. G. Ital. Dermatol. Venereol. 150, 247–254.
Barolet, D., and Boucher, A. (2010). Radiant near infrared light emitting Diode exposure as skin preparation to enhance photodynamic therapy inflammatory type acne treatment outcome. Lasers Surg. Med. 42, 171–178. doi:10.1002/lsm.20886
Basset-Seguin, N., Bissonnette, R., Girard, C., Haedersdal, M., Lear, J. T., and Paul, C. (2014). Consensus recommendations for the treatment of basal cell carcinomas in Gorlin syndrome with topical methylaminolaevulinate-photodynamic therapy. J. Eur. Acad. Dermatol. Venereol. 28, 626–632. doi:10.1111/jdv.12150
Berking, C., Hegyi, J., Arenberger, P., Ruzicka, T., and Jemec, G. B. (2009). Photodynamic therapy of necrobiosis lipoidica--a multicenter study of 18 patients. Dermatology 218, 136–139. doi:10.1159/000182259
Black, J. O. (2016). Xeroderma pigmentosum. Head. Neck Pathol. 10, 139–144. doi:10.1007/s12105-016-0707-8
Borgia, F., Coppola, M., Giuffrida, R., and Cannavò, S. P. (2019). Excellent cosmetic result of daylight photodynamic therapy for facial flat warts in a child. Photodiagnosis Photodyn. Ther. 26, 27–28. doi:10.1016/j.pdpdt.2019.02.021
Borgia, F., Giuffrida, R., Caradonna, E., Vaccaro, M., Guarneri, F., and Cannavò, S. P. (2018b). Early and late onset side effects of photodynamic therapy. Biomedicines 6, 12. doi:10.3390/biomedicines6010012
Borgia, F., Giuffrida, R., Coppola, M., and Cannavò, S. P. (2020b). Successful photodynamic therapy in a pediatric patient with difficult warts. Dermatol. Ther. 33, e13391. doi:10.1111/dth.13391
Borgia, F., Giuffrida, R., Coppola, M., Princiotta, R., Vaccaro, M., Guarneri, F., et al. (2020a). Efficacy and safety of conventional versus daylight photodynamic therapy in children affected by multiple facial flat warts. Photodiagnosis Photodyn. Ther. 31, 101819. doi:10.1016/j.pdpdt.2020.101819
Borgia, F., Giuffrida, R., Vaccaro, M., Lentini, M., and Cannavò, S. P. (2016a). Photodynamic therapy in lupus miliaris disseminatus faciei's scars. Dermatol. Ther. 29, 320–324. doi:10.1111/dth.12367
Borgia, F., Macca, L., Giuffrida, R., Coppola, M., Princiotta, R., Vaccaro, M., et al. (2021). Recurrence after conventional versus daylight photodynamic therapy in children effected by multiple facial flat warts. Photodiagnosis Photodyn. Ther. 36, 102579. doi:10.1016/j.pdpdt.2021.102579
Borgia, F., Vaccaro, M., Cantavenera, L. G., Aragona, E., and Cannavò, S. P. (2014). Ulcerative necrobiosis lipoidica successfully treated with photodynamic therapy: Case report and literature review. Photodiagnosis Photodyn. Ther. 11, 516–518. doi:10.1016/j.pdpdt.2014.08.002
Borgia, F., Vaccaro, M., Foti, A., Giuffrida, R., and Cannavò, S. P. (2016b). Zoon's balanitis successfully treated with photodynamic therapy: Case report and literature review. Photodiagnosis Photodyn. Ther. 13, 347–349. doi:10.1016/j.pdpdt.2015.08.010
Borgia, F., Vaccaro, M., Giuffrida, R., and Cannavò, S. P. (2018a). Photodynamic therapy for acne conglobata of the buttocks: Effective antiinflammatory treatment with good cosmetic outcome. Indian J. dermatol. Venereol. Leprol. 84, 617–619. doi:10.4103/ijdvl.IJDVL_683_17
Bu, W., Xu, X., Wang, Y., Huang, L., Zeng, R., Chen, X., et al. (2017). Surgery combined with photodynamic therapy for the treatment of hidradenitis suppurativa: A report of 7 cases. Photodiagnosis Photodyn. Ther. 18, 46–49. doi:10.1016/j.pdpdt.2017.01.176
Caccavale, S., Iocco, A., Pieretti, G., Alfano, R., and Argenziano, G. (2019). Curettage + microneedling + topical ALA-PDT for the treatment of acral resistant warts: Our experience. Photodiagnosis Photodyn. Ther. 27, 276–279. doi:10.1016/j.pdpdt.2019.04.008
Condorelli, A. G., Motolese, A., Borgia, F., Bartolomeo, L. D., Bianchi, L., Rossi, P. G., et al. (2022). Photodynamic therapy for superficial basal cell carcinomas: Clinical features of partial responses and recurrences. Photodiag. Photodyn. Ther. 37, 102727. doi:10.1016/j.pdpdt.2022.102727
Cui, X., Zhu, J., Yao, X., Zhu, W., Xu, P., and Wu, X. (2020). Photodynamic therapy combined with dermatosurgical approach for Perifolliculitis Capitis Abscedens et Suffodiens. Photodiagnosis Photodyn. Ther. 30, 101767. doi:10.1016/j.pdpdt.2020.101767
Curkova, A. K., Hegyi, J., Kozub, P., Szep, Z., D'Erme, A. M., and Simaljakova, M. (20142014). A case of linear porokeratosis treated with photodynamic therapy with confocal microscopy surveillance. Dermatol. Ther. 27, 144–147. doi:10.1111/dth.12097
Custurone, P., Di Bartolomeo, L., Irrera, N., Borgia, F., Altavilla, D., Bitto, A., et al. (2021). Role of cytokines in vitiligo: Pathogenesis and possible targets for old and new treatments. Int. J. Mol. Sci. 22, 11429. doi:10.3390/ijms222111429
Feng, Y., Zhang, Y., Guo, H., Lin, Z., Chen, H., Wu, Y., et al. (2019). Treatment of dissecting cellulitis of the scalp with 10% ALA-PDT. Lasers Surg. Med. 51, 332–338. doi:10.1002/lsm.23016
Fernández-Guarino, M., Mavura, D., Fernández-González, P., Chapa, P., Ravazzano, C., Jaén, L., et al. (2020). Daylight photodynamic therapy is an option for the treatment of actinic keratosis in patients with xeroderma pigmentosum in Africa. Photodiagnosis Photodyn. Ther. 29, 101631. doi:10.1016/j.pdpdt.2019.101631
Fink, C., Toberer, F., Enk, A., and Gholam, P. (2016). Effective treatment of cutaneous leishmaniasis caused by Leishmania tropica with topical photodynamic therapy. J. Dtsch. Dermatol. Ges. 14, 836–838. doi:10.1111/ddg.13082
García-Navarro, X., Garcés, J. R., Baselga, E., and Alomar, A. (2009). Linear porokeratosis: Excellent response to photodynamic therapy. Arch. Dermatol. 145, 526–527. doi:10.1001/archdermatol.2009.45
Garrido-Colmenero, C., Ruiz-Villaverde, R., Martínez-García, E., Aneiros-Fernández, J., and Tercedor-Sánchez, J. (2015). Photoletter to the editor: Response of linear porokeratosis to photodynamic therapy in an 11-year-old girl. J. Dermatol. Case Rep. 9, 118–119. doi:10.3315/jdcr.2015.1220
Gerkowicz, A., Szczepanik-Kułak, P., and Krasowska, D. (2021). Photodynamic therapy in the treatment of vulvar lichen sclerosus: A systematic review of the literature. J. Clin. Med. 10, 5491. doi:10.3390/jcm10235491
Gordon Spratt, E., Gorcey, L., Soter, N., and Brauer, J. (2015). Phototherapy, photodynamic therapy and photophoresis in the treatment of connective-tissue diseases: A review. Br. J. Dermatol. 173, 19–30. doi:10.1111/bjd.13544
Gracia-Cazaña, T., Vera-Álvarez, J., García-Patos, V., and Gilaberte, Y. (2015). Imiquimod and photodynamic therapy are useful in the treatment of porokeratosis in children with bone marrow transplantation. Pediatr. Dermatol. 32, e291–293. doi:10.1111/pde.12654
Guarneri, C., and Vaccaro, M. (2009). Erosive pustular dermatosis of the scalp following topical methylaminolaevulinate photodynamic therapy. J. Am. Acad. Dermatol. 60, 521–522. doi:10.1016/j.jaad.2008.09.006
Hamilton, F. L., Car, J., Lyons, C., Car, M., Layton, A., and Majeed, A. (2009). Laser and other light therapies for the treatment of acne vulgaris: Systematic review. Br. J. Dermatol. 160, 1273–1285. doi:10.1111/j.1365-2133.2009.09047.x
Heng, Y. K., Koh, M. J., Giam, Y. C., Tang, M. B., Chong, W. S., and Tan, S. H. (2014). Pediatric mycosis fungoides in Singapore: A series of 46 children. Pediatr. Dermatol. 31, 477–482. doi:10.1111/pde.12352
Hooper, M., Hatch, L., and Seminario-Vidal, L. (2021). Photodynamic therapy of mycosis fungoides: A systematic review of case studies. Photodermatol. Photoimmunol. Photomed. 37, 549–552. doi:10.1111/phpp.12698
Hörfelt, C., Funk, J., Frohm-Nilsson, M., Wiegleb Edström, D., and Wennberg, A. M. (2006). Topical methyl aminolaevulinate photodynamic therapy for treatment of facial acne vulgaris: Results of a randomized, controlled study. Br. J. Dermatol. 155, 608–613. doi:10.1111/j.1365-2133.2006.07340.x
Hörfelt, C., Stenquist, B., Larkö, O., Faergemann, J., and Wennberg, A. M. (2007). Photodynamic therapy for acne vulgaris: A pilot study of the dose-response and mechanism of action. Acta Derm. Venereol. 84, 325–329. doi:10.2340/00015555-0243
Huang, K., Li, Y., Zeng, W., Jiang, Z., Zhu, W., Chen, M., et al. (2019). Successful treatment of recalcitrant plantar warts: Pretreatment with superficial shaving is vital before photodynamic therapy. Photodiagnosis Photodyn. Ther. 27, 216–217. doi:10.1016/j.pdpdt.2019.05.040
Hyun, D. J., Seo, S. R., Kim, D. H., Yoon, M. S., and Lee, H. J. (2016). Periungual Bowen's disease in a 12-year-old boy treated with photodynamic therapy. Pediatr. Dermatol. 33, e82–83. doi:10.1111/pde.12753
Itoh, Y., Ninomiya, Y., Tajima, S., and Ishibashi, A. (2001). Photodynamic therapy of acne vulgaris with topical delta-aminolaevulinic acid and incoherent light in Japanese patients. Br. J. Dermatol. 144, 575–579. doi:10.1046/j.1365-2133.2001.04086.x
Jawa, D. S., Sircar, K., Somani, R., Grover, N., Jaidka, S., and Singh, S. (2009). Gorlin-Goltz syndrome. J. Oral Maxillofac. Pathol. 13, 89–92. doi:10.4103/0973-029X.57677
Johansen, M. B., Jemec, G. B. E., and Fabricius, S. (2019). Effective treatment with photodynamic therapy of cutaneous leishmaniasis: A case report. Dermatol. Ther. 32, e13022. doi:10.1111/dth.13022
Joseph, P. D., Craig, J. C., and Caldwell, P. H. (2015). Clinical trials in children. Br. J. Clin. Pharmacol. 79, 357–369. doi:10.1111/bcp.12305
Kaae, J., Philipsen, P. A., and Wulf, H. C. (2018). Photodynamic therapy of necrobiosis lipoidica using methyl aminolevulinate: A retrospective follow-up study. Photodiagnosis Photodyn. Ther. 22, 223–226. doi:10.1016/j.pdpdt.2018.04.020
Kim, S. K., Oh, S. J., Park, S. Y., Kim, W. J., Kim, Y. S., and Kim, Y. C. (2018). Photodynamic therapy inhibits melanogenesis through paracrine effects by keratinocytes and fibroblasts. Pigment Cell Melanoma Res. 31, 277–286. doi:10.1111/pcmr.12658
Kvaal, S. I., Angell-petersen, E., and Warloe, T. (2013). Photodynamic treatment of oral lichen planus. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 115, 62–70. doi:10.1016/j.oooo.2012.08.448
Larson, D. M., and Cunningham, B. B. (2012). Photodynamic therapy in a teenage girl with xeroderma pigmentosum type C. Pediatr. Dermatol. 29, 373–374. doi:10.1111/j.1525-1470.2011.01657.x
Li, S. S., Zhang, L. L., Nie, S., Lv, T., and Wang, H. W. (2018). Severe acne in monozygotic twins treated with photodynamic therapy. Photodiagnosis Photodyn. Ther. 23, 235–236. doi:10.1016/j.pdpdt.2018.06.016
Loncaster, J., Swindell, R., Slevin, F., Sheridan, L., Allan, D., and Allan, E. (2009). Efficacy of photodynamic therapy as a treatment for Gorlin syndrome-related basal cell carcinomas. Clin. Oncol. R. Coll. Radiol. 21, 502–508. doi:10.1016/j.clon.2009.03.004
Ma, Y., Liu, Y., Wang, Q., Ren, J., and Xiang, L. (2015). Prospective study of topical 5-aminolevulinic acid photodynamic therapy for the treatment of severe adolescent acne in Chinese patients. J. Dermatol. 42, 504–507. doi:10.1111/1346-8138.12836
Macca, L., Li Pomi, F., Custurone, P., Vaccaro, M., and Borgia, F. (2022). Photodynamic therapy for pediatric genital warts: A case report. Dermatol. Ther. 12, e15313. doi:10.1111/dth.15313
Mendese, G. W., Beckford, A., Krejci, N., Mahalingam, M., Goldberg, L., and Gilchrest, B. A. (2012). Pagetoid reticulosis in a prepubescent boy successfully treated with photodynamic therapy. Clin. Exp. Dermatol. 37, 759–761. doi:10.1111/j.1365-2230.2012.04352.x
Miguélez, A., Martín-Santiago, A., Bauzá, A., and Gilaberte, Y. (2013). Urticaria-like reaction secondary to photodynamic therapy in 2 pediatric patients. Actas Dermosifiliogr. 104, 727–729. doi:10.1016/j.adengl.2012.12.010
Mills, S. J., Farrar, M. D., Ashcroft, G. S., Griffiths, C. E., Hardman, M. J., and Rhodes, L. E. (2014). Topical PDT following excisional wounding of humanskin increases production of TGF-β3, MMP-1 and MMP-9 with associated improvement in dermal matrix organisation. Br. J. Dermatol. 171, 55–62. doi:10.1111/bjd.12843
Morton, C. A., Szeimies, R. M., Basset-Séguin, N., Calzavara-Pinton, P. G., Gilaberte, Y., Haedersdal, M., et al. (2020). European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 2: Emerging indications - Field cancerization, photorejuvenation and inflammatory/infective dermatoses. J. Eur. Acad. Dermatol. Venereol. 34, 17–29. doi:10.1111/jdv.16044
Motta, S., and Monti, M. (2007). Photodynamic therapy — a promising treatment option for autoimmune skin ulcers: A case report. Photochem. Photobiol. Sci. 6, 1150–1151. doi:10.1039/b711920h
Orringer, J. S., Sachs, D. L., Bailey, E., Kang, S., Hamilton, T., and Voorhees, J. J. (2010). Photodynamic therapy for acne vulgaris: A randomized, controlled, split-face clinical trial of topical aminolevulinic acid and pulsed dye laser therapy. J. Cosmet. Dermatol. 9, 28–34. doi:10.1111/j.1473-2165.2010.00483.x
Oseroff, A. R., Shieh, S., Frawley, N. P., Cheney, R., Blumenson, L. E., Pivnick, E. K., et al. (2005). Treatment of diffuse basal cell carcinomas and basaloid follicular hamartomas in nevoid basal cell carcinoma syndrome by wide-area 5-aminolevulinic acid photodynamic therapy. Arch. Dermatol. 141, 60–67. doi:10.1001/archderm.141.1.60
Pérez-Laguna, V., García-Malinis, A. J., Aspiroz, C., Rezusta, A., and Gilaberte, Y. (2018). Antimicrobial effects of photodynamic therapy. G. Ital. Dermatol. Venereol. 153, 833–846. doi:10.23736/S0392-0488.18.06007-8
Peris, K., Fargnoli, M. C., Garbe, C., Kaufmann, R., Bastholt, L., Seguin, N. B., et al. (2019). European dermatology forum (EDF), the European association of dermato-oncology (EADO) and the European organization for research and treatment of cancer (EORTC) (2019) diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur. J. Cancer. 118, 10–34. doi:10.1016/j.ejca.2019.06.003
Pollock, B., Turner, D., Stringer, M. R., Bojar, R. A., Goulden, V., Stables, G. I., et al. (2004). Topical aminolaevulinic acid-photodynamic therapy for the treatment of acne vulgaris: A study of clinical efficacy and mechanism of action. Br. J. Dermatol. 151, 616–622. doi:10.1111/j.1365-2133.2004.06110.x
Prieto, V. G., Zhang, P. S., and Sadick, N. S. (2005). Evaluation of pulsed light and radiofrequency combined for the treatment of acne vulgaris with histologic analysis of facial skin biopsies. J. Cosmet. Laser Ther. 7, 63–68. doi:10.1080/14764170500231848
Rahimi, H., Zeinali, R., and Tehranchinia, Z. (2021). Photodynamic therapy of vitiligo: A pilot study. Photodiagnosis Photodyn. Ther. 36, 102439. doi:10.1016/j.pdpdt.2021.102439
Rhodes, L. E., de Rie, M., Enström, Y., Groves, R., Morken, T., Goulden, V., et al. (2004). Photodynamic therapy using topical methyl aminolevulinate vs surgery for nodular basal cell carcinoma: Results of a multicenter randomized prospective trial. Arch. Dermatol. 140, 17–23. doi:10.1001/archderm.140.1.17
Rojanamatin, J., and Choawawanich, P. (2006). Treatment of inflammatory facial acne vulgaris with intense pulsed light and short contact of topical 5-aminolevulinic acid: A pilot study. Dermatol. Surg. 32, 991–996. doi:10.1111/j.1524-4725.2006.32221.x
Salvio, A. G., Requena, M. B., Stringasci, M. D., and Bagnato, V. S. (2021). Photodynamic therapy as a treatment option for multiple pigmented basal cell carcinoma: Long-term follow-up results. Photodiagnosis Photodyn. Ther. 33, 102154. doi:10.1016/j.pdpdt.2020.102154
Schroeter, C. A., Pleunis, J., van Nispen tot Pannerden, C., Reineke, T., and Neumann, H. A. (2005). Photodynamic therapy: New treatment for therapy-resistant plantar warts. Dermatol. Surg. 31, 71–75. doi:10.1111/j.1524-4725.2005.31011
Shan, X., Wang, N., Li, Z., Hou, J., Zheng, R., Tian, H., et al. (2016). An open uncontrolled trial of topical 5-aminolevulinic acid photodynamic therapy for the treatment of urethral condylomata acuminata in male patients. Indian J. dermatol. Venereol. Leprol. 82, 65–67. doi:10.4103/0378-6323.171649
Snider, S., Costello, C. M., Ederaine, S., Besch-Stokes, J., Severson, K. J., DiCaudo, D. J., et al. (2020). A case of pediatric lymphomatoid papulosis treated with photodynamic therapy and narrowband ultraviolet B. Pediatr. Dermatol. 37, 881–883. doi:10.1111/pde.14244
Takeda, H., Kaneko, T., Harada, K., Matsuzaki, Y., Nakano, H., and Hanada, K. (2005). Successful treatment of lymphadenosis benigna cutis with topical photodynamic therapy with delta-aminolevulinic acid. Dermatology 211, 264–266. doi:10.1159/000087021
Theresia, C., Zheng, J., and Chen, X. Y. (2017). Topical ALA-PDT as alternative therapeutic option in treatment-recalcitrant dermatosis: Report of 4 cases. Photodiagnosis Photodyn. Ther. 20, 189–192. doi:10.1016/j.pdpdt.2017.10.010
Vaccaro, M., Bagnato, G., Cristani, M., Borgia, F., Spatari, G., Tigano, V., et al. (2017a). Oxidation products are increased in patients affected by non-segmental generalized vitiligo. Arch. Dermatol. Res. 309, 485–490. doi:10.1007/s00403-017-1746-z
Vaccaro, M., Cannavò, S. P., Imbesi, S., Cristani, M., Barbuzza, O., Tigano, V., et al. (2015). Increased serum levels of interleukin-23 circulating in patients with non-segmental generalized vitiligo. Int. J. Dermatol. 54, 672–674. doi:10.1111/ijd.12392
Vaccaro, M., Cicero, F., Mannucci, C., Calapai, G., Spatari, G., Barbuzza, O., et al. (2016). IL-33 circulating serum levels are increased in patients with non-segmental generalized vitiligo. Arch. Dermatol. Res. 308, 527–530. doi:10.1007/s00403-016-1675-2
Vaccaro, M., Irrera, N., Cutroneo, G., Rizzo, G., Vaccaro, F., Anastasi, G. P., et al. (2017b). Differential expression of nitric oxide synthase isoforms nNOS and iNOS in patients with non-segmental generalized vitiligo. Int. J. Mol. Sci. 18, 2533. doi:10.3390/ijms18122533
Vinciullo, C., Elliott, T., Francis, D., Gebauer, K., Spelman, L., Nguyen, R., et al. (2005). Photodynamic therapy with topical methyl aminolaevulinate for “difficult-to-treat” basal cell carcinoma. Br. J. Dermatol. 152, 765–772. doi:10.1111/j.1365-2133.2005.06484.x
Walter, A. W., Pivnick, E. K., Bale, A. E., and Kun, L. E. (1997). Complications of the nevoid basal cell carcinoma syndrome: A case report. J. Pediatr. Hematol. Oncol. 19, 258–262. doi:10.1097/00043426-199705000-00016
Wang, X. L., Wang, H. W., Zhang, L. L., Guo, M. X., and Huang, Z. (2010). Topical ALA PDT for the treatment of severe acne vulgaris. Photodiagnosis Photodyn. Ther. 7, 33–38. doi:10.1016/j.pdpdt.2010.01.003
Weinberger, C. H., Endrizzi, B., Hook, K. P., and Lee, P. K. (2009). Treatment of angiofibromas of tuberous sclerosis with 5-aminolevulinic acid blue light photodynamic therapy followed by immediate pulsed dye laser. Dermatol. Surg. 35, 1849–1851. doi:10.1111/j.1524-4725.2009.01304.x
Wennberg, A. (2015). Vulvovaginal photodynamic therapy for genital erosive lichen planus. Br. J. Dermatol. 173, 1119–1120. doi:10.1111/bjd.14181
Wu, L., Chen, W., Su, J., Li, F., Chen, M., Zhu, W., et al. (2019). Efficacy of the combination of superficial shaving with photodynamic therapy for recalcitrant periungual warts. Photodiagnosis Photodyn. Ther. 27, 340–344. doi:10.1016/j.pdpdt.2019.06.021
Xu, M., Lin, N., Li, J., Jiang, L., and Zeng, K. (2018). Photodynamic therapy as an alternative therapeutic option for pediatric condyloma acuminata: A case series. Photodiagnosis Photodyn. Ther. 24, 179–181. doi:10.1016/j.pdpdt.2018.09.010
Xu, X., Zheng, Y., Zhao, Z., Zhang, X., Liu, P., and Li, C. (2017). Efficacy of photodynamic therapy combined with minocycline for treatment of moderate to severe facial acne vulgaris and influence on quality of life. Med. Baltim. 96, e9366. doi:10.1097/MD.0000000000009366
Yeung, C. K., Shek, S. Y., Bjerring, P., Yu, C. S., Kono, T., and Chan, H. H. (2007). A comparative study of intense pulsed light alone and its combination with photodynamic therapy for the treatment of facial acne in Asian skin. Lasers Surg. Med. 39, 1–6. doi:10.1002/lsm.20469
Zhang, Y., Lin, H., Guo, X., and Zou, X. (2018). A case series pilot study on the combination of 5-aminolevulinic acid and photodynamic therapy (ALA-PDT) for treatment of vitiligo. An. Bras. Dermatol. 93, 539–545. doi:10.1590/abd1806-4841.20187014
Keywords: photodynamic therapy, child, pediatric dermatology, acne vulgaris, viral warts, Gorlin syndrome, necrobiosis lipoidica, hidradenitis suppurativa
Citation: Di Bartolomeo L, Altavilla D, Vaccaro M, Vaccaro F, Squadrito V, Squadrito F and Borgia F (2022) Photodynamic therapy in pediatric age: Current applications and future trends. Front. Pharmacol. 13:879380. doi: 10.3389/fphar.2022.879380
Received: 19 February 2022; Accepted: 27 June 2022;
Published: 16 August 2022.
Edited by:
Yolanda Gilaberte, Hospital Universitario Miguel Servet, SpainReviewed by:
Francesca Prignano, Università di Firenze, ItalyRony Shreberk-Hassidim, Hadassah Medical Center, Israel
Copyright © 2022 Di Bartolomeo, Altavilla, Vaccaro, Vaccaro, Squadrito, Squadrito and Borgia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Luca Di Bartolomeo, bHVjYWRpYmFydG9sb21lb0BsaXZlLml0
†These authors equally contributed to this work and share the first authorship
 Luca Di Bartolomeo
Luca Di Bartolomeo Domenica Altavilla
Domenica Altavilla Mario Vaccaro
Mario Vaccaro Federico Vaccaro
Federico Vaccaro Violetta Squadrito
Violetta Squadrito Francesco Squadrito
Francesco Squadrito Francesco Borgia
Francesco Borgia