- 1Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
- 2Institute of Acupuncture and Moxibustion, China Academy of Chinese Medical Sciences, Beijing, China
- 3School of Life Science, Beijing University of Chinese Medicine, Beijing, China
- 4Beijing Emergency Medical Center, Beijing, China
- 5Beijing Tiantan Hospital, Capital Medical University, Beijing, China
- 6Beijing Hospital of Traditional Chinese Medicine, Beijing, China
- 7Institute for Brain Disorders, Beijing University of Chinese Medicine, Beijing, China
Background: As the only traditional Chinese medicine injection approved by the China Food and Drug Administration for use as stroke first aid in ambulances, Xingnaojing Injection (XNJI) has been widely used in cases of both acute ischemic stroke (IS) and intracerebral hemorrhage (ICH). However, there is no robust clinical evidence regarding the efficacy and safety of the early use of XNJI during stroke first aid. The main purpose of this trial is to observe whether XNJI, intravenously administered within 24 h of onset in the prehospital ambulance setting, protects against early neurological deterioration (END) on the third day of onset in patients with acute stroke.
Methods: The Trial of a prehospital intervention with traditional Chinese medicine for acute stroke (TRACE) is a Mixed-Methods research (MMR) study that involves a combination of quantitative and qualitative research. The quantitative research part of this project is a prospective, multicenter, observational, clinical registry study, for which we aimed to recruit 1,000 patients with acute stroke (IS and ICH). Based on our observation of whether XNJI was intravenously administered within 24 h of onset in the prehospital ambulance setting, patients with acute stroke will be divided into two groups: the exposure group comprising patients who were intravenously administered XNJI and the nonexposure group comprising patients who were not. The primary outcome is early neurological deterioration (END) on the third day of onset defined as an increase of 2 or more points in the National Institute of Health Stroke Scale score between baseline and day 3. In addition, based on the aforementioned quantitative research, qualitative research will be conducted by interviewing emergency doctors about their knowledge and attitude regarding XNJI used for stroke first aid.
Discussion: The results of the TRACE study will provide preliminary evidence for the relationship between XNJI used within 24 h of onset and the presence of END on the third day after stroke onset; it will aid in improving the current knowledge regarding the early use of XNJI for stroke first aid.
Clinical Trial Registration: clinicaltrials.gov, identifier NCT04275349
Introduction
Stroke is the first cause of death and disability among adults in China and the second leading cause of death worldwide (Group, 2020). In 2019, among all the subtypes of stroke, ischaemic stroke (IS) had a prevalence of 62.4%, and intracerebral brain hemorrhage (ICH), of 27.9% (GBD 2019 Stroke Collaborators, 2021). Stroke, a life-threatening disease, often leads to severe central nervous system damage in case of untimely treatment (Saver, 2006; Chen et al., 2019). Early neurological deterioration (END) is a frequent and serious complication of acute stroke, with an incidence of 5%–40% (Thanvi et al., 2008; Geng et al., 2017). It is characterized by progressive deterioration of neurological function within 48-72 h after stroke (Celik et al., 2019; Kang et al., 2021). END is strongly associated with an increased risk of functional disability, mortality, and poor 3-month clinical outcomes (Kwan and Hand, 2006; Geng et al., 2017). Currently, vascular recanalization strategies, including intravenous thrombolysis (IVT) and endovascular therapy (EVT), are recommended first-line therapy options for acute ischemic stroke (AIS) (Ma et al., 2019; Powers et al., 2019). However, they only benefit a small number of patients due to narrow time windows, imaging dependence, high risk of bleeding, and limited repass rates (Wang et al., 2019). The treatment options for ICH are also limited (Schrag and Kirshner, 2020). There are no specific drugs for treating ICH, and the benefits and risks of surgical treatment are controversial (Deng and Wu, 2021; Zhang and Zhou, 2022)).
Xingnaojing injection (XNJI) is made using an extract from the An Gong Niu Huang pill, a popular and effective first-aid TCM used for acute stroke, which has been clinically applied for more than 200 years (Deng et al., 2010; Wang, 2022; Wang W. et al., 2022). Using modern pharmaceutical technology, dangerous ingredients, such as cinnabar and realgar, are removed, and four ingredients including musk, gardenia, Yujin, and borneol are retained and refined to obtain a convenient and effective water-soluble intravenous injection (Yue et al., 2019). Many studies have demonstrated the effectiveness and safety of XNJI for acute stroke (Ma et al., 2013; Shen et al., 2020; Wang et al., 2021), and the guidelines and consensus in China have recommended the use of XNJI as stroke first aid (Gao, 2016; Department of Neurology, 2018).
However, on conducting an overview of systematic reviews and meta-analyses (Tian et al., 2021), we found that none of the included studies reported on the different medication timings of XNJI use in acute stroke and on stroke severity. We also conducted a mixed-methods research (MMR) study (Tian, 2021) and found that early XNJI use within 6 h of stroke onset maybe associated with greater functional improvement. However, the appropriate initiation time of XNJI treatment is also unclear due to medical insurance restrictions and concerns regarding possible incompatibility between XNJI and IVT. A recent systematic review and meta-analysis (Wang L. et al., 2022) have also suggested that the optimum XNJI initiation time during the acute phase might be within the first 72 h after stroke onset, preferably within the first 6 h. However, due to insufficient evidence, it remains unknown whether XNJI should ideally be administered immediately after stroke onset or within 24 h. In addition, in real-world settings, the knowledge and attitude of emergency doctors regarding XNJI used for stroke first aid are also very critical. Therefore, we designed this MMR study to explore the relationship between XNJI used within 24 h of onset and the presence of END 3 days after symptom onset in patients with acute stroke.
Methods and analysis
Study design
The Trial of a prehospital intervention with traditional Chinese medicine for acute stroke (TRACE) study (registered with ClinicalTrials.gov, ID: NCT04275349) will be designed as an MMR study aimed at exploring the relationship of XNJI used within 24 h of onset with the presence of END on the third day after symptom onset in patients with acute stroke. We will compare the presence of END on the third day after stroke onset between the exposure group and the nonexposure group. An overview of the study flowchart is presented in Figure 1. The registry protocol was approved by the Ethics Committee of Dongzhimen Hospital Affiliated with Beijing University of Chinese Medicine, Beijing, China (Approval number: DZMEC-KY-2019-153), and will be approved based on the requirements of the local institutional review boards of all the participating sites.
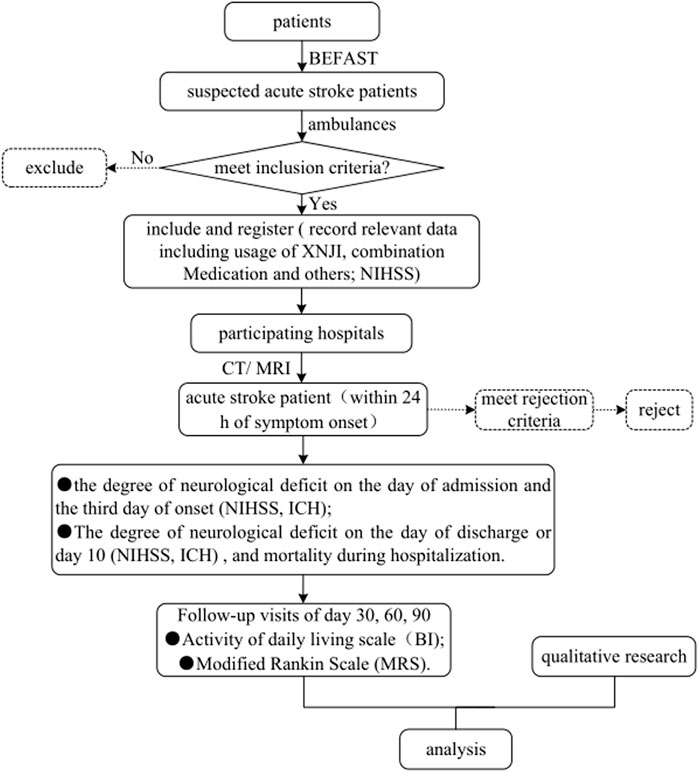
FIGURE 1. Flowchart of the TRACE study. Abbreviations: TRACE, Trial of a prehospital intervention with traditional Chinese medicine for acute stroke; BEFAST, Balance-Eyes-Face-Arms-Speech-Time; XNJI, Xingnaojing Injection; NIHSS, National Institutes of Health Stroke Scale; CT, Computed Tomography; MRI, Magnetic Resonance Imaging; ICH, intracerebral hemorrhage; BI, Barthel Index; mRS, modified Rankin Scale.
Recruitment
Patients will be recruited consecutively from 20 participating sites, including both Western medicine hospitals and TCM hospitals in Beijing, China, which have the capability of conducting qualified research and express a proven commitment to the study. The recruitment started in March 2018 and is estimated to end in December 2022. Eligible adult patients with suspected acute stroke within 24 h of onset will be sent to the hospital by ambulance. We plan to recruit 1,000 patients with confirmed acute stroke based on the inclusion and rejection criteria. The time of stroke onset was defined as the time at which the patient was last observed to be well. Only those who meet the inclusion criteria and willingly provide written informed consent will be recruited. Since this is an observational study that involves a specific prehospital emergency intervention, it is difficult to obtain written informed consent from the patient in the prehospital ambulance setting. The patient will be asked to sign a written informed consent form in the hospital. The researchers will inform patients of the benefits and risks of participating in the research and of the need to analyze their prehospital information in addition to their in-hospital information; they will also be assured that this will not interfere with the actual clinical diagnosis and treatment. Legal guardians will sign the informed consent form on behalf of patients in a coma. If the patient or his legal guardian does not agree to the use of the diagnosis and treatment-related information in this study, the prehospital information will not be used. The participation of patients will be entirely voluntary, and their data will be strictly protected to the extent permitted by the law.
In this study, to increase the rate of identifying suspected stroke patients, the prehospital assessment scale for identifying these patients was changed from the Face-Arms-Speech-Time (FAST) scale to the Balance-Eyes-Face-Arms-Speech-Time (BEFAST) scale. Once meeting the inclusion criteria, patients will be included and registered immediately in an ambulance. However, due to the busy task and high tension in the prehospital ambulance setting, ambulance doctors are unable to exclude patients in detail. The ineligible patients will be rejected after admission. Therefore, this study has changed the exclusion criterion to the rejection criterion.
Inclusion criteria
•Diagnosis of suspected acute stroke
•Within 24 h of symptom onset
•Age≥18 years
Rejection criteria
•Other diseases that lead to motor dysfunction (e.g., claudication, severe osteoarthritis, rheumatoid arthritis, and gouty arthritis)
•Other diseases or psychosis that renders outcome assessments or follow-up unlikely to be possible
•Known to be pregnant or lactating
•Non-acute stroke [result from Computed Tomography (CT) or Magnetic Resonance Imaging (MRI)].
Intervention measures
The quantitative research part of this study will be observational. Based on our observation of whether XNJI was intravenously administered within 24 h of onset in the prehospital ambulance setting, patients with acute stroke will be divided into two groups: the exposure group comprising patients who were intravenously administered XNJI and the nonexposure group comprising patients who were not. During the study period, all relevant data will be collected at the following timepoints to facilitate a continuous dynamic observation and evaluation: during the prehospital ambulance service; within 24 h of admission; day 3 after stroke onset; day 10 after stroke onset or the day of discharge; and 30 ± 3, 60 ± 3, and 90 ± 7 days after stroke onset. This research will not interfere with the actual diagnosis and treatment of the participants. The clinicians will make decisions regarding routine treatment options based on the patient’s specific circumstances. The routine stroke treatment will follow the current national guidelines for acute Is and ICH from the Chinese Society of Neurology.
Main variables
All variables used for outcomes evaluation of efficacy and safety are abstracted from all relevant data collected during the observation period. The main variables of this study are listed in Table 1.
Data collection and management
Through a prospectively designed electronic case registration form (eCRF), trained ambulance doctors will collect the prehospital diagnosis- and treatment-related information of the patient and will perform assessments using the National Institute of Health Stroke Scale (NIHSS) score through a face-to-face interview within 24 h after stroke onset during the prehospital ambulance service. Trained researchers appointed at each participating site will collect in-hospital data as follows: conditions state of consciousness, and survival or death on the day of admission; baseline information on the day of admission; the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification of AIS; major treatment methods; in-hospital medication; rehabilitation; auxiliary examination; complication; in-hospital mortality; discharge diagnosis and Indicators on health economics; they will also perform assessments using the NIHSS scale, ICH scale, Glasgow Coma Scale (GCS), and mRS during a face-to-face interview during hospitalization.
In this study, the data entry process will be refined, and different responsibilities and authorities of prehospital and in-hospital personnel will be emphasized. In the prehospital ambulance setting, the researcher will need to record the prehospital ambulance data of the patient promptly and fill in, complete, and submit the prehospital module before arriving at the hospital to hand over to the patient. Once the patient is admitted to the hospital, the information collected in the prehospital ambulance setting will be submitted by default and will not be modified again. After admission, the researcher in the receiving hospital will record the diagnosis and treatment data in the hospital timely, accurately, completely, and clearly in the eCRF according to the original observation record. Case registration must be completed for each selected patient; data collection and management will be conducted using an electronic data capture (EDC) system (http://1-dao.net:13579); the researchers and research assistants are responsible for data entry and submission by logging in the mobile terminal information platform. If the clinical supervisor has questions regarding the data recorded in the eCRF, the research assistants will consult with the researchers, who will answer and return as soon as possible, and then modify, confirm, and input the data. The research assistant will assist in completing the q & A work and fill out the data entry Q & A form. If the data collected after admission are found to be incorrect, modifications can be made with the help of a form comprising online questions and answers; the supervisor will thoroughly check all the data, raises questions, and coordinate with the researchers to resolve any concerns. The main goal of the verification process is to confirm whether data collection via the eCRF is timely, complete, objective, and accurate. The consistency of data and source files (photo of uploaded laboratory examination/function check report) will be checked, and a clinical explanation will be sought in case of any laboratory abnormalities. The supervisor will also check whether modifications of data collected through the eCRF comply with the specifications and note the reason for the modification; further, the collected data will be reconfirmed to ensure no logic errors. The electronic data entry system is set up with the necessary legal values and logical verification rules, whereby it alerts the researcher in case of possible outliers or data logic errors. The supervisor will regularly send data-related questions to the researchers in the participating centers and will follow up regarding the completion of the responses. After completing the data entry, the researcher will upload the data to the data center. The study data will be downloaded from the data center and transferred to the research team leader, sponsor, and statistical analyst. Written records of all the data handover processes will be maintained. The responsible unit and statistical analyst will lock the data, and no further revisions will be performed. Problems found after data locking will be corrected in the statistical analysis program after confirmation. Finally, after all the research data are entered and locked, the database will be submitted to the statistical analysts, who will perform statistical analyses according to the requirements outlined in the statistical plan.
Follow-up procedures
Independent interviewers with a background in neurology will be trained in a standard operating procedure (SOP) for contacting patients. The interviewers will regularly follow up with the patients over the telephone using standard interview forms in the eCRF at 30 ± 3, 60 ± 3, and 90 ± 7 days after stroke onset. The standard interview forms in the eCRF include the mRS, BI, basic medication-related questions, and rehabilitation-related questions. All interviews will be recorded and saved. Patients will have the right to withdraw from the trial at any time without reason, punishment, or loss of interest, and their medical treatments will not be affected. Participants who do not formally withdraw from the trial but no longer attend follow-up sessions will also be regarded as having withdrawn from the trial. The data of these participants will be considered valid and will not be replaced. While respecting the individual rights of the participants, researchers should try their best to understand the reasons for withdrawal to better protect the participants. The participants who drop out due to AEs should be followed up until their physical status is stable, while the other participants who dropped out should not be followed up.
Types of outcomes
Efficacy outcomes
The primary outcome is END on the third day of onset defined as an increase of 2 or more points in the NIHISS score between baseline and day 3. The secondary outcomes include the rates of stroke-related and any-cause deaths during hospitalization, differences in the changes in the neurological deficit degree, risk grade of ICH evaluated through changes in the ICH scale score from baseline to day 10 or the day of discharge, the proportion of patients with activities of daily living with a Barthel Index score of ≥90, and the proportion of independent patients defined by a modified Rankin Scale score ≤2 at 30 ± 3, 60 ± 3, and 90 ± 7 days after stroke onset. A complete list of the efficacy outcomes analyzed in the TRACE study is presented in Table 2.
Safety outcomes
We defined an AE as any unfavorable and unintended sign, symptom, or disease that develops in the participants in both groups during the trial period regardless of whether or not it is related to the XNJI treatment (Lai et al., 2017). Safety outcomes will be assessed by routine physical and laboratory examinations and AEs recording during the observation period. Because the methodology of the study does not interfere with the actual clinical diagnosis and treatment, we changed our approach from AEs processing to AEs recording. If AEs occur during hospitalization, it will not need to be reported; however, relevant information (start time; symptoms/signs; intensity; attack frequency, duration, and termination time; laboratory indicators; treatment methods and outcomes; follow-up results; etc.) will have to be accurately recorded. The use of all concomitant medications should be recorded in detail. If any treatment is required, a record should be made regarding the treatment administered.
Quality control and monitoring
Before registration, several approaches will be implemented to ensure coordination among the different centers to avoid the potential heterogeneity of data. First, all the research personnel at each center and independent interviewers will be trained so that they understand the clinical trial protocol and comply with the SOP for consecutive patient screening and recruitment, obtaining informed consent, and collecting data (scale filling, scale evaluation, eCRF filling), etc; this will be achieved through centralized on-site training, videoconferences, and training videos. Second, special quality auditors will be set up in each subcenter and will conduct regular in-institution quality audits, which will include the subject registration process, the implementation of registration standards, data authenticity and traceability, data collection integrity, etc. Third, an independent and professional third-party supervision agency will perform the main supervision task of the research group. The monitoring personnel will set up the monitoring task, frequency, and time points according to the research risks; they will regularly analyze and deal with the research risks under the guidance or approval of the group leadership unit. The data monitoring process is divided into two parts: on-site monitoring and remote network center monitoring. Remote monitoring will be carried out based on the project management platform and EDC system. In this study, the data quality monitoring will mainly be conducted remotely, and standardization, compliance, and data authenticity monitoring during the research process will mainly be conducted on-site. The supervisor will contact the research assistants, researchers, and central quality control personnel from time to time to ensure the implementation of the research and quality control process. A clinical research associate (equivalent to a clinical study monitor, CRA) will visit the research centers regularly or irregularly for site inspection. The CRA will be responsible for tasks such as the following: to check whether the documents recorded are timely, complete, accurate, and true; to check whether the signing of the informed consent form complies with good clinical practice (GCP) and relevant regulatory requirements; to ensure that all the data collected via the eCRF are consistent with the original data, such as those in the hospitalization medical records; and to ensure that all AEs are recorded.
Sample size calculation
According to the definition of the dominant population, at least one particular subgroup will have a positive outcome in terms of the primary efficacy index. A previous study conducted in Germany reported that approximately 13% of patients exhibited END on the third day after stroke onset (an increase of 2 or more points in the NIHSS score). Data obtained from our previous research as well as other domestic research showed that the rate of END on the third day after stroke onset was approximately 6–8%. The sample size was calculated on the following assumptions: the END rate in patients who did not receive the TCM intervention was 13% based on data from studies conducted overseas, and the END rate in patients who received the TCM intervention was 7% based on domestic data. Since there is no formula meant specifically for calculating the sample size in registry studies, we decided to use the one-sided difference test based on the statistical analysis method and formula for calculating the sample size in cohort studies. Consequently, the following formula, where α = 0.05 and β = 0.2, was used:
The smallest sample size for a single cohort was estimated to be 426 patients; therefore, a minimum of 852 patients must be included. Considering the uneven distribution of exposure factors among patients and the inability of some patients to attend all the visits through the 90 days, the sample size was increased to 1,000 cases, which is an increase of 17% on the original base size.
Qualitative research
Qualitative research will be conducted based on the results of the quantitative research. The emergency doctors participating in this study will be interviewed via one-to-one semi-structured interviews or group interviews, lasting approximately 60 min/person. The number of interviewers will be determined based on the results of three to five pre-interviews. According to the degree of information saturation, approximately 20 doctors will be interviewed. The interview will be mainly about the knowledge and attitude of the emergency doctors regarding XNJI used for stroke first aid. The process is as follows:
• Interview sampling: Purposive sampling. Depending on the degree of information saturation, approximately 20 individuals will be interviewed based on the results of the pre-interviews.
• Interview place: Beijing 120 Emergency Center.
• Interview time: Approximately 60 min/person.
• Data collection: A basic personal questionnaire will be used to collect data, such as the name, sex, age, education background, professional title, years of first aid work, professional physician certificate category, etc. During this research process, the content of the interviews of participating doctors will be documented in detail to help researchers in understanding the respondent’s basic information.
Data processing and analysis
The mean (Mean), standard mean difference (SD), median (Median), interquartile ranges, minimum (Min), and maximum (Max) will be used to summarize continuous data, while rate or percentage will be used for categorical data. Between-group comparisons will be conducted using parametric or non-parametric tests, as appropriate. Pearson’s chi-squared test is used for the count data, and the results are expressed as rate or percentage (%). Measured data, if data are normally distributed and the variance is homogeneous, the parametric test (t-test) will be used and results will be expressed as mean ± SD; If the data are not normally distributed and still do not meet the requirements for the parametric test after data conversion, the non-parametric test (Wilcoxon rank sum test or Mann-Whitney U test) will be used and results will be expressed as median (Median).
As the primary analysis, a comparison of the presence of END on the third day after stroke onset will be conducted between the two groups of patients—the exposure group and the nonexposure group—using Pearson’s chi-squared test. The secondary outcome analyses will involve the rate of stroke-related and any-cause deaths, differences in the NIHISS scores, differences in the ICH scale scores, proportion of patients with BI scores ≥90, and proportion of patients with mRS scores ≤2. These analyses will be performed according to the standard statistical principles for comparing parametric or non-parametric distributions, as appropriate. During the analysis, multivariate linear regression analysis is performed for the influencing factors that are statistically significant in the univariate analysis. The full analysis set will be used in the efficacy correlation analysis in this study. The safety analysis set will be used in the safety analysis. The full analysis set refers to the set of eligible cases and detached cases but not eliminated cases. The safety analysis set will consist of safety indicators for all enrolled subjects with post-medication safety evaluation data; the number of subjects with missing data will be reported. All statistical tests will be one-sided, and statistical significance will be set at p < 0.05. SAS software, version 9.2 (SAS Institute, Inc., Cary, NC, United States), will be used to perform the statistical analyses.
Discussion
A wealth of active clinical research is being conducted in stroke medicine driven by the significant public health and socioeconomic implications of this common disease (Hurford et al., 2020). Given that current guideline-recommended treatments for AIS and ICH are limited, there remains a need for a more effective and safe prehospital treatment, which could reduce the impact of treatment delay on efficacy. At the same time, to close the gap in stroke care between countries, we should emphasize the importance of expanding evidence-based stroke first aid strategies that are culturally and environmentally appropriate. For thousands of years, traditional Chinese medicine (TCM) has been widely used as an important and effective treatment for stroke in China, but the evidence supporting its use is insufficient (Wu et al., 2007; Wu et al., 2019). Many previous studies have demonstrated the efficacy and safety of XNJI in acute stroke. However, due to the low level of evidence, the optimum timing for initiating XNJI treatment during stroke first aid is still unclear. Therefore, we designed this MMR study to explore the relationship between XNJI used within 24 h after stroke onset and the presence of END on the third day after symptom onset in patients with acute stroke. Evaluating the efficacy and safety of complex TCM interventions involves multiple associations (Feng et al., 2021). Fortunately, the MMR design is suitable for an individualized evaluation of TCM in the actual clinical setting and enables us to explore and interpret research-related concerns from multiple dimensions in the real-world setting (Qiu et al., 2020). A potential advantage of the MMR design is that the effect and safety of XNJI in our trial will resemble those in clinical practice.
However, there are several limitations to this study. First, it will remain unknown whether XNJI can improve prognosis and reduce mortality in patients with acute stroke over longer periods due to the relatively short follow-up period of 3 months. Second, the eCRF that will be used in this study is a pre-designed module, which may lead to the loss of some key information; this form will need to be improved in future studies. Furthermore, because this study will be performed in Beijing China, it is uncertain whether the clinical efficacy of XNJI would be similar in other regions in China and other countries. Therefore, future randomized controlled trials with more rigorous study design, robust quality control, longer follow-up periods, larger cohort sizes, and multicenter or international collaboration are still needed to obtain additional high-quality evidence supporting the use of XNJI as stroke first aid.
To the best of our knowledge, the TRACE study will provide preliminary evidence for the relationship between XNJI used within 24 h of onset and the presence of END on the third day after stroke onset in real-world settings; consequently, it will aid in improving the current knowledge regarding the early use of XNJI for stroke first aid.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee (Dongzhimen Hospital affiliated with Beijing University of Chinese Medicine). The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YC, ZT, and SW have contributed equally to the drafting of the manuscript and are the co-first author. LK and YG proposed the clinical question and designed the study. All co-authors have read and approved the final manuscript.
Funding
This research is financially supported by Capital’s Funds for Health Improvement and Research (CFH 2018-1-4191).
Acknowledgments
The authors acknowledge contributions from the TRACE team members all over China. We would like to thank Editage (www.editage.com) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AEs, adverse events; AIS, acute ischemic stroke; BEFAST, Balance-Eyes-Face-Arms-Speech-Time; BI, Barthel Index; EDC, electronic data capture; END, early neurological deterioration; EVT, endovascular therapy; GCS, Glasgow Coma Scale; IS, ischemic stroke; ICH, intracerebral brain hemorrhage; IVT, intravenous thrombolysis; MMR, mixed-methods research; eCRF, electronic case registration form; NIHSS, National Institute of Health Stroke Scale; ECG, electrocardiographic; CRA, clinical research associate; mRS, modified Rankin Scale; SOP, standard operating procedure; TIFs, telephone interview forms; TCM, traditional Chinese medicine; TRACE, Trial of a prehospital intervention with traditional Chinese medicine for acute stroke; TOAST, Trial of Org 10172 in Acute Stroke Treatment; XNJI, Xingnaojing Injection.
References
Celik, O., Cil, C., Biteker, F. S., Gokcek, A., and Dogan, V. (2019). Early neurological deterioration in acute ischemic stroke. J. Chin. Med. Assoc. 82 (3), 245. doi:10.1097/JCMA.0000000000000022
Chen, H., Gong, X., Xu, D., Wang, Z., Hu, H., Wu, C., et al. (2019). Advanced treatment time improves outcomes of patients with ischemic stroke undergoing reperfusion therapy. Zhejiang Univ. ( Med. Sci.) 48 (3), 247–253. doi:10.3785/j.issn.1008-9292.2019.06.03
Deng, L., Tian, L., and Wang, H. (2010). Research progress on clinical application of angong niuhuangwan and its derivative prescription. Chin. J. Exp. Traditional Med. Formulae 16, 215–219. doi:10.13422/j.cnki.syfjx.2010.12.053
Deng, L., and Wu, B. (2021). Key points and interpretation of the updated guide for diagnosis and treatment of cerebral bleeding in china 2019. Cardio-Cerebrovasc. Dis. Prev. Treat. 21 (1), 13–17+34.
Department of Neurology, C.S.o.T.C.a.W.M. (2018). Guidelines to combining chinese and western medicine for the treatment of cerebral infarction in china (2017). CJITWM 38 (2), 136–144. doi:10.3969/j.ISSN.1007–9572.2016.30.001
Feng, L., Kong, L., Dong, X., Lai, X., Zhang, D., Ren, B., et al. (2021). China stroke registry for patients with traditional Chinese medicine (CASES-TCM): rationale and design of a prospective, multicenter, observational study. Front. Pharmacol. 12, 743883. doi:10.3389/fphar.2021.743883
Gao, L. (2016). Expert consensus on hypertensive intracerebral hemorrhage in acute stage in diagnosis and treatment combining traditional Chinese medicine and Western medicine. Chin. Gen. Pract. 19 (30), 3641–3648. doi:10.3969/j.ISSN.1007–9572.2016.30.001
GBD 2019 Stroke Collaborators (2021). Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the global burden of disease study 2019. Lancet. Neurol. 20 (10), 795–820. doi:10.1016/s1474-4422(21)00252-0
Geng, H. H., Wang, Q., Li, B., Cui, B. B., Jin, Y. P., Fu, R. L., et al. (2017). Early neurological deterioration during the acute phase as a predictor of long-term outcome after first-ever ischemic stroke. Med. Baltim. 96 (51), e9068. doi:10.1097/MD.0000000000009068
Group, R.o.s.p.a.t.i.C.W. (2020). Brief report on stroke prevention and treatment in China, 2019. Chin. J. Cerebrovasc. Dis. 17 (5), 272–281. doi:10.3969/j.issn.1672-5921.2020.05.008
Hurford, R., Sekhar, A., Hughes, T. A. T., and Muir, K. W. (2020). Diagnosis and management of acute ischaemic stroke. Pract. Neurol. 20 (4), 304–316.
Kang, L., Kong, L., and Gao, Y. (2021). TCM pathogenesis of early neurological deterioration in acute ischemic stroke based on the theory of toxin and Xuanfu. J. Beijing Univ. Trad. Chin. Med. 44, 625–630. doi:10.3969/j.issn.1006-2157.2021.07.007
Kwan, J., and Hand, P. (2006). Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. Qjm 99 (9), 625–633. doi:10.1093/qjmed/hcl082
Lai, X., Cao, K., Kong, L., Liu, Q., Gao, Y., investigators, X. s., et al. (2017). Xingnaojing for moderate-to-severe acute ischemic stroke (XMAS): study protocol for a randomized controlled trial. Trials 18 (1), 479. doi:10.1186/s13063-017-2222-y
Ma, R., Xu, H., Yang, X., and Wang, G. (2019). Research progress in the treatment of acute stroke. Chongqing Med. 48, 1010–1013. doi:10.3969/j.issn.1671-8348.2019.06.029
Ma, L., Li, D., and Li, K. (2013). The clinical curative effect of Xingnaojing injection for acute ischemic stroke: A systematic review. Liaoning J. Traditonal Chin. Med. 40 (4), 734–735. doi:10.13192/j.ljtcm.2013.04.132.malh.007
Powers, W. J., Rabinstein, A. A., Ackerson, T., Adeoye, O. M., Bambakidis, N. C., Becker, K., et al. (2019). Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke 50 (12), e344–e418. doi:10.1161/STR.0000000000000211
Qiu, R., Zhao, C., Zhong, C., Hu, J., Guan, M., Li, M., et al. (2020). Application of mixed methods research in the individualized therapeutic evaluation of clinical trials of traditional Chinese medicine. Chin. J. Evid.-Based Med. 20 (8), 973–978. doi:10.7507/1672-2531.201911079
Saver, J. L. (2006). Time is brain--quantified. Stroke 37 (1), 263–266. doi:10.1161/01.STR.0000196957.55928.ab
Schrag, M., and Kirshner, H. (2020). Management of intracerebral hemorrhage: JACC focus seminar. J. Am. Coll. Cardiol. 75 (15), 1819–1831. doi:10.1016/j.jacc.2019.10.066
Shen, P., Cheng, S., Fu, S., Ding, H., and Li, Z. (2020). The application of Xingnaojing injection in acute cerebral infarction. J. JIANGXI UNIV. TCM 32 (6), 113–115.
Thanvi, B., Treadwell, S., and Robinson, T. (2008). Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms, and management. Postgrad. Med. J. 84 (994), 412–417. doi:10.1136/pgmj.2007.066118
Tian, Z. (2021). A mixed-method study on the evidence-based evaluation of early intervention of Xingnaojing injection in acute stroke and its prognostic impact on patients. Beijing: Beijing University of Chinese Medicine Press.
Tian, Z., Feng, L., Xie, Y., Xu, D., Zhang, C., Kong, L., et al. (2021). Chinese herbal medicine xingnaojing injection for acute ischemic stroke: an overview of systematic reviews and meta-analyses. Front. Pharmacol. 12, 659408. doi:10.3389/fphar.2021.659408
Wang, J. (2022). Clinical observation on the treatment of acute cerebral hemorrhage with Angong Niuhuang pill. CJGMCM 37 (9), 1532–1534. doi:10.3969/j.ISSN.1003-8914.2022.09.009
Wang, J., Zhou, X., and Wang, C. (2019). Clinical effect of thrombolytic therapy with recombinant tissue plasminogen activator at different time on acute ischemic stroke. Clin. Med. 39 (11), 26–28. doi:10.19528/j.issn.1003-3548.2019.11.010
Wang, L., Fan, X., Chen, Y., Liang, X., Shen, W., Zhang, Y., et al. (2022). Efficacy and safety of xingnaojing injection for emergency treatment of acute ischemic stroke: a systematic review and meta-analysis. Front. Pharmacol. 13, 839305. doi:10.3389/fphar.2022.839305
Wang, M., Jia, M., Du, W., Zhang, X., Jiao, W., Chen, Q., et al. (2021). Overview of systematic reviews/Meta-analysis of Xingnaojing Injection in treatment of intracerebral hemorrhage. Chin. J. Trad. Chin. Med. 46 (18), 4633–4643. doi:10.19540/j.cnki.cjcmm.20210622.501
Wang, W., Zhang, H., Wang, S., Xue, X., Zhao, X., Xiang, X., et al. (2022). Treatment of acute progressive cerebral infarction with Angong Niuhuang pill. Guide China Med. 20 (10), 22–25. doi:10.15912/j.cnki.gocm.2022.10.004
Wu, B., Liu, M., Liu, H., Li, W., Tan, S., Zhang, S., et al. (2007). Meta-analysis of traditional Chinese patent medicine for ischemic stroke. Stroke 38 (6), 1973–1979. doi:10.1161/strokeaha.106.473165
Wu, S., Wu, B., Liu, M., Chen, Z., Wang, W., Anderson, C. S., et al. (2019). Stroke in China: Advances and challenges in epidemiology, prevention, and management. Lancet Neurol. 18 (4), 394–405. doi:10.1016/s1474-4422(18)30500-3
Yue, M., Li, Q., Lu, C., and Jiang, L. (2019). Expert consensus on the clinical application of Xingnaojing injection in the treatment of acute and critical diseases (symptom). Chin. J. Hyg. Rescue ( Electron. Ed.) 5 (02), 65–70.
Keywords: prehospital intervention, traditional chinese medicine, xingnaojing injection, acute stroke, study protocol, mixed methods research
Citation: Chen Y, Tian Z, Wang S, Liu H, Liu Y, Peng W, Lai X, Qi D, Kong L and Gao Y (2022) Trial of a prehospital intervention with traditional Chinese medicine for acute stroke (TRACE): Protocol for a mixed-methods research study. Front. Pharmacol. 13:879282. doi: 10.3389/fphar.2022.879282
Received: 19 February 2022; Accepted: 13 July 2022;
Published: 29 August 2022.
Edited by:
Jon Wardle, Southern Cross University, AustraliaReviewed by:
Ethan Brandler, Stony Brook Medicine, United StatesWenxing Chen, Nanjing University of Chinese Medicine, China
Kuo-Lun Huang, Linkou Chang Gung Memorial Hospital, Taiwan
Copyright © 2022 Chen, Tian, Wang, Liu, Liu, Peng, Lai, Qi, Kong and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lingbo Kong, a2xiMTk4NEAxNjMuY29t; Ying Gao, Z2FveWluZzk3M0AxMjYuY29t
†These authors have contributed equally to this work and share the first authorship
 Yuanyuan Chen
Yuanyuan Chen Ziyu Tian
Ziyu Tian Shuyan Wang
Shuyan Wang Hongmei Liu4
Hongmei Liu4 Yanfang Liu
Yanfang Liu Xinxing Lai
Xinxing Lai
