- 1AgrobioSciences Research, Mohammed VI Polytechnic University, Ben-Guerir, Morocco
- 2African Sustainable Agriculture Research Institute (ASARI), Mohammed VI Polytechnic University (UM6P), Laayoune, Morocco
Caper (Capparis spinosa L.) is a perennial shrub of the family Capparaceae, endemic to circum-Mediterranean countries. Caper carries a renowned nutritional value, especially in terms of vitamins and antioxidants related to the occurrence of flavonoids, alkaloids, and glucosinolates as main secondary metabolites. Caper extracts have also shown to display antibacterial, antifungal, analgesic, antitumor, hepatoprotective, antioxidant, anti-inflammatory, and neuroprotective effects which correlate the uses of the plant in folk medicine against both metabolic and infectious diseases. The present review aims to provide exhaustive phytochemistry and pharmacological properties survey on Caper constituents. Attention has also been given to the nutritional values and traditional uses of main organs to pinpoint research gaps for future investigations on the plant.
Introduction
Spices constitute one of the valuable ingredients for making dishes worldwide. The spread of spices is related to the diversity and cultural groups around the world leading to versatile dishes (Dinesha and Chikkanna, 2014). Spices are hot, salty, sweet, or sharp inducing secretion of saliva, promoting digestion, preventing cold, and avoiding nausea and vomiting (Kuete et al., 2011). Most of these ingredients are also used by traditional healers to cure several diseases including cancer, microbial infections, and gastrointestinal diseases (Sharma and Sharma, 2012).
Caper (Capparis spinosa L.) is a perennial spiny shrub of the family of Capparaceae with fleshy leaves and big white to pinkish-white flowers. Caper is salt tolerant and resistant to drought, grows up to 4 m in height in warm and dry weather, and has extensive root systems which can extend up to 6–10 m (Manikandaselvi and Brindha, 2014; Manikandaselvi et al., 2016). The plant is native to the Mediterranean basin but is distributed around Southern Europe, the Northern and Eastern Africa including Madagascar, Southwestern and Central Asia, Indonesia, Australia, Papua New Guinea, and Oceania (Rivera et al., 2003; Nabavi et al., 2016). Caper is plesiomorphic supporting results of studies which reported the polymorphic aspects of the plant and the heterogeneity of its morphological characters. Accordingly, C. spinosa are found under 22 variety names when searched in the database (Fici, 2014; Fici, 2015).
Caper is of great interest both as food additives and complementary drugs. The literature abounds with more than 11 reviews on the plant emphasizing the nutraceutical merits, biological effects, or chemical and pharmacological wealth (Mingzhang et al., 1994; Ji et al., 2006; Geng et al., 2007; Xie et al., 2007; Li et al., 2008; Satyanarayana et al., 2008; Yang et al., 2008; Zhao et al., 2014; Manikandaselvi et al., 2016; Rahnavard and Razavi, 2017; Vahid et al., 2017; Zhang and Ma, 2018). These research surveys are not less than 5 years old and most of them are not accessible though they are from Chinese research journals (engines). The present review aimed to provide a detailed exploration of the chemistry and pharmacology of Caper to pinpoint research gaps for future investigations. Data collected in the frame of this work were generated by common research engines such as Web of Science, SciFinder-n, PubMed, ScienceDirect, and Scopus, when entering the references “Caper”, “Capparis spinosa” and refining with keywords “chemistry”, “biological”, “antioxidant”, and “anticancer”. A total of 1612 research items were examined out of which 215 fall into the scope of the review, thus, constituting the baseline of the present survey.
Summary of Bibliometric Analysis of Caper Research
A total of 835 documents were retrieved from the Scopus database between 2000 and 2021. Most of the documents were original research articles, followed by reviews and other types of documents comprising proceedings papers, book reviews, and meeting abstracts. Noteworthy, there was a gradual rise in annual output from the early 2010s and the highest number of articles were recorded in the United States (Guesmi et al., 2021), Italy (Hameed et al., 2021), China (Mickymaray and Al Aboody, 2019), Turkey (Gull et al., 2015), Iran (Bakr et al., 2016) and Spain (Fu et al., 2008). Furthermore, the over-visualization of the bibliometric analysis shows the trend from the year related to Caper research (Figure 1A). Using VOSviewer, a term map was also created using the occurrences of relevant words. A total of 1432 words were identified from all keyword fields. With the number of occurrences, different research themes were assigned by different clusters (Figure 1B).
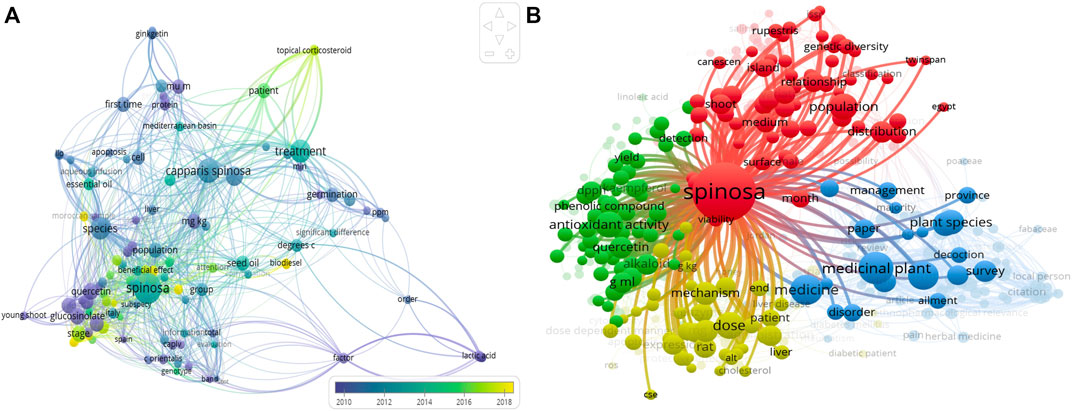
FIGURE 1. (A) Visualization of topic areas of Caper research using overlay visualization. (B) Term map generated from all keyword’s fields on Caper and representing the different research themes that defined different clusters. Cluster 1 is colored in red on the term map and represents the botanical and geographical distribution of the plant. This cluster represents research publications on the relationship between the genetic variation and distribution of the plant in different areas. Cluster 2 is colored in green and represents the antioxidant activity of Caper and its correlation with compounds, especially quercetin and kaempferol. Cluster 3 is colored in blue on the map. This cluster represents the ethnopharmacological properties of aper in traditional medicine. The different uses depend on the local zones of distribution. Cluster 4 is colored in yellow. The cluster is broadly classified as publications linked with the in vivo activity of the plant related to liver disease and diabetes, with the mechanism of the plant on these human diseases.
Taxonomical and Vernacular Names
The taxonomical aspect of C. spinosa is characterized by high variability due to different factors such as phenotypical plasticity, hybridization processes, selection of cultivated forms, and eco–geographical differentiation. Two recent taxonomic revisions were carried out by Fici, (2014); Fici, (2015) based on the variation of Caper species over a wide geographical range. The taxonomic revision conducted on the geographical area extending from the Mediterranean to central Asia recognized the Capparis spinosa as a single species divided into two subspecies, subsp. Spinosa, widespread in habitats distinguished by clays, marls, and evaporates, extended from the Mediterranean eastwards to central Asia and Nepal, and subsp. rupestris (Sm.) Nyman (1878: 68), a Steno–Mediterranean element extending to the central Saharian massifs, where the habitat is characterized by rocky outcrops and cliffs (Fici, 2014). The other taxonomic revision was conducted in eastern Africa, Madagascar, southern Asia, Australia, and Oceania where four subspecies were referred to, himalayensis stat. nov, Nummularia, Cordifolia, and Cartilaginea. (Fici, 2015). The distribution of C. spinosa worldwide makes it difficult to list every vernacular name. However, it is obvious to find the plant under popular names like Caper (also known as Mariana caper-bush, Mariana caper) in English; kabar, alaf-e-mar in late Persia region; kabbar in the Arabic language; câprier in French; alcaparro in Spain; cappero in Italy; melada in Malaysia; alcaparras in the Philippines or even himsraa, kaakdaani, and kabara in India (Zohary, 1960; Jacobs, 1964; Tutin et al., 1976; Metcalfe, 2006; Ezzeddine et al., 2007).
Traditional Uses
Caper drains a long history of ethnomedicinal practices worldwide, each organ is concerned (Table 1) (Eddouks et al., 2002). In Iran, fruits and roots bark are used as diuretics and tonics against malaria and hemorrhoids (Hooper and Field, 1937; Afsharypuor et al., 1998). The leaves of the plant serve as an analgesic, aperients, and depurative in Pakistan (Sher et al., 2012). The whole plant and roots of the plant are employed to relieve paralysis, against rheumatism, toothache, and to kill worms in the ear against coughs (Tlili et al., 2011). The decoction of the root bark or the infusion as the tea of young shoots is applied in China against rheumatism, stomachache, anemia, and to treat dropsy (Miraldi et al., 2001; Feng et al., 2011). Moroccans use the buds or leaves as herbal tea or decoction to alleviate eye infections, gastrointestinal infections, diabetes, and the removal of kidney stones. Caper is also deeply involved in Mediterranean gastronomy, it is used as pickles in salads and sauces, as well as condiments and seasoning spices (Gamble, 1972). In addition, the Caper is used in cosmetics, where roots extract is beneficial in treating rose-colored rashes and capillary weaknesses (Chiej, 1984).
Nutraceutical Values of Caper
Caper is known as rich in vitamins and fibers, with a minimal amount of fats and calories (Tlili et al., 2009). The plant is considered a good source of vitamins B1, B3, B6, and B9 and moderate in vitamin E. Caper flowering buds also contain a good amount of vitamins A, C, and K (Tlili et al., 2010; Nabavi et al., 2016; Ulukapi et al., 2016). Minerals also abound in Caper mainly calcium, iron, potassium, phosphorus, magnesium, zinc, and manganese, which play a very important role in maintaining proper metabolic activities (Özcan et al., 2004; Arslan and Ozcan, 2007).
Chemical Constituent’s Synopsis of Caper
Different organs of Caper have been investigated to clear the plant’s chemical constituents. Overall, as awaited, the fruits of the plant were the most screened organ. Caper produces diverse secondary metabolites including alkaloids, sulfur-containing indoles, flavonoids, furan and pyrrole derivatives, tetraterpenes, phenolic acids, and sterols (Moufid et al., 2015; Zhang and Ma, 2018). Rare primary metabolites like nucleosides and nucleic acids have been highlighted to occur in Caper alongside other classes of compounds but with at most two members each. Benzidane et al. (2020) compared the chemical composition through a comprehensive analytical TLC diagram of both aqueous and methanol extracts from fruits, leaves, roots, flowers, seeds, root bark, and twigs. All in all, the methanol extracts sound more chemically rich than aqueous extracts. In addition, except for the fruit, leaves, and flowers, the other plant organs are chemically poor (Benzidane et al., 2020). This statement was typically confirmed looking at reports in the literature since works concentrated on either fruit, leaves, or flowers of the plant.
Nucleotides and Nucleic Acids
The occurrence of nucleosides (2-4) and nucleic acids (1-5) (Figure 2) are quite comprehensive in the animal kingdom since they intervene in the energy production through ATP formation; however, their presence in the plant kingdom is not common. Uracil (1) and adenosine (3) are the most abundant members of this group reported in fruits of the plant (Fu et al., 2007; Li et al., 2014). Nucleosides and nucleic acids could be extracted from plants following the total alkaloid extraction method and recovered with n-butanol (Fu et al., 2007).
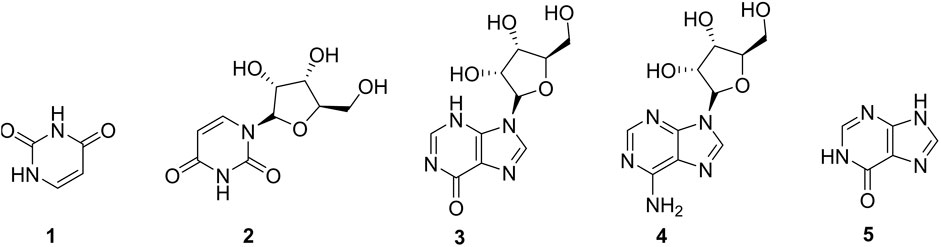
FIGURE 2. Nucleosides and nucleic acids from Caper. 1 = Uracil, 2 = Uridine, 3 = Inosine, 4 = Adenosine, 6 = Hypoxanthine.
Alkaloids
Alkaloids (6-22) (Figure 3) are one of the largest groups of compounds in Caper. They constitute 0.91 and 0.86% of mass material from root bark and fruits, respectively. Alkaloids have not yet been reported in the leaves of the studied plant. Two main classes of alkaloids have been isolated so far, indoles (7-14) and spermidines (16-22). Spermidines occur almost exclusively in the roots in the yield of 3.5 mg/g of dried material (Khatib et al., 2016), while indoles are abundant in the fruits. The total alkaloid fraction of the fruits retrieved with n-butanol afforded the cyanidoindol alkaloid, cappariloside A (8), as part of other compounds carried over (Fu et al., 2007). The 6′-glucopyranosyl isomer of compound 8, cappariloside B (9), was rather found in the MeOH extract of dried mature fruits of the plant alongside compound 8 (Çaliş et al., 1999). Both compounds have also been found in the water-soluble fraction of the same plant material as part of the complex mixture of compounds obtained (Çalış et al., 2002). One of the scarce amino acids also abundant in every organ of the plant namely stachydrine (6), has been always isolated in its zwitterion form. Mukhamedova et al. (1969) and Sadykov et al. (1981) evaluated by HPLC/DAD the yield of stachydrine to 87.43% of the total alkaloid extract also quantified to 7.4% of the roots of the plant dried material (Sadykov et al., 1981); (Mukhamedova et al., 1969); (Fu et al., 2008).
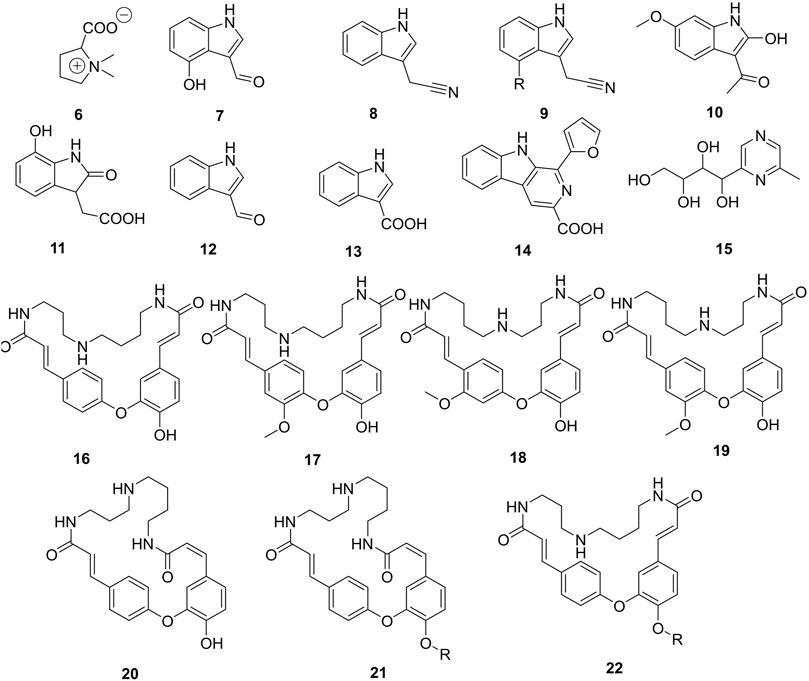
FIGURE 3. Alkaloids isolated from roots and fruits of Caper. R = β-D-glucopyranosyl, 6 = (-)-Stachydrine, 7 = 4-Hydroxy-1H-indole-3-carboxaldehyde, 8 = Cappariloside A, 9 = Cappariloside B, 10 = 1-(2-Hydroxy-6-methoxy-1H-indol-3-yl)ethanone, 11 = 2,3-Dihydro-7-hydroxy-2-oxo-1H-indole-3-acetic acid, 12 = Indole-3-carbaldehyde, 13 = Indole-3-carboxylic acid, 14 = Flazin, 15 = 1-(6-Methyl-2-pyrazinyl)-1,2,3,4-butanetetrol, 16 = Cadabicine, 17 = Isocodonocarpine, 18 = Capparisine, 19 = Codonocarpine, 20 = Capparispine, 21 = Capparispine 26-O-β-D-glucose, 22 = Cadabicine 26-O-β-D-glucose.
Glucosinolates and Other Sulfur-Containing Compounds
Other alkaloids from indole-type but containing sulfur abundant in the plant are termed glucosinolates (Figure 4A). In this group, sulfur occurs either as a thiol group (27-28) or as part of the sulfate fragment. The later mentioned moiety could be essential in the standardization of either an extract, fraction, or pure compounds as it helps to increase the bioavailability of the drugs. Schraudolf (1988) was the first to highlight the occurrence of glucosinolates in the roots with the isolation of compounds (23-26) (Schraudolf, 1989). Glucocapparin is one of the main representatives of glucosinolates in Caper (29) (Meiliwan et al., 2008; Jiménez-López et al., 2018), and methyl-isothiocyanate and benzyl-isothiocyanate are the main compounds in the series of isothiocyanate derivatives from the fruits (Romeo et al., 2007). They have not been isolated from any plant organ yet, rather the indole derivative (28) has been isolated with an integrated isocyanate moiety to the core indole (Zhou et al., 2010).
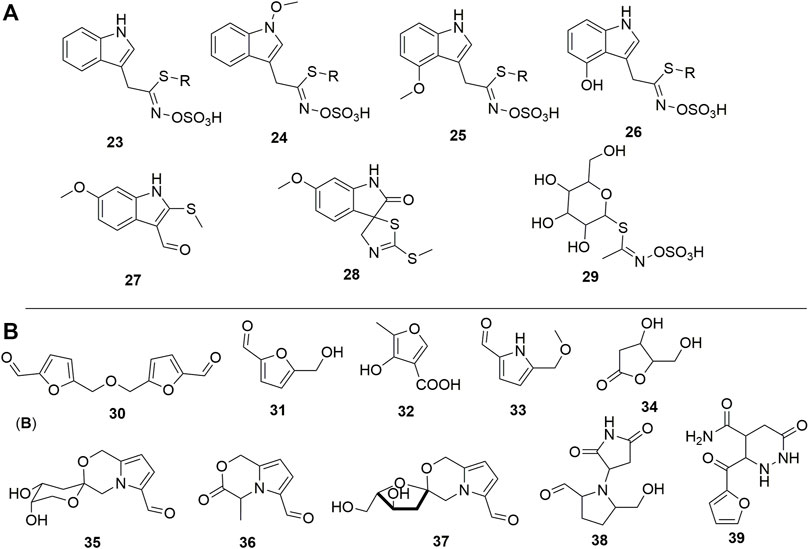
FIGURE 4. (A) Sulfur-containing compounds from Caper. (B) Furan and pyrrole analogues reported in Caper. R = β-D-glucopyranosyl, 23 = Glucobrassicin, 24 = Neoglucobrassicin, 25 = 4-Methoxyglucobrassicin, 26 = 4-Hydroxyglucobrassicin, 27 = 6-Methoxy-2-(methylthio)-1H-indole-3-carboxaldehyde, 28 = (3S)-(-)-6-Methoxy-2'-(methylthio)spiro[3H-indole-3,5′(4′H)-thiazol]-2(1H)-one, 29 = Glucocapparin, 30 = 5,5'-[Oxybis(methylene)]bis[2-furancarboxaldehyde], 31 = 5-(Hydroxymethyl)furfural, 32 = 4-Hydroxy-5-methyl-3-furancarboxylic acid, 33 = 5-(Methoxymethyl)-1H-pyrrole-2-carbaldehyde, 34 = 3,4,5-trihydroxypentanoic acid γ-lactone, 35 = Capparisine B, 36 = 2-(5-hydroxymethyl-2-formylpyrrol-1-yl) propionic acid lactone, 37 = Capparisine A, 38 = N-(30-maleimidy1)-5-hydroxymethyl-2-pyrrole formaldehyde, 39 = Capparisine C.
Furans and Pyrroles
Furan and pyrrole derivatives (30-39), (Figure 4B) constitute another most important group of compounds of Caper. Compounds 30 and 31 are the most representative of this group in the plant since they have been highlighted by many authors (Li et al., 2014; Hu et al., 2017). The differential extraction of the ethanol extract of dried fruits with petroleum ether and ethyl acetate in water yields pyrrole derivatives capparisines A (35), B (37), and C (39), 2-(5-hydroxymethyl-2-formylpyrrol-1-yl) propionic acid lactone (36) and N-(30-maleimidy1)-5-hydroxymethyl-2-pyrrole formaldehyde (38) (Yang et al., 2010a). This is also the unique report of the occurrence of these compounds in Caper.
Flavonoids
The total flavonoid content of Caper ranged from 4.71 to 72.79 mg equivalent to quercetin per Gram of dried material (QE/g DR) including compounds 40–52 (Figure 5) (Tlili et al., 2015). Benzidane et al. (2020) studied the occurrence of rutin, quercetin, catechin, and gallic acid in both methanol and aqueous extracts of Caper organs. The methanol extract of the leaves contained rutin in a larger amount compared to the other extracts. However, the methanol extracts of fruits and flowers contained rutin with a similar yield (Benzidane et al., 2020). Ramezani et al. (2008) and Musallam et al. (2012) have come to the same conclusion, assessing the amount of rutin in hydroalcoholic extracts of these three main organs of Caper (Ramezani et al., 2008; Musallam et al., 2012). Rutin was found in large amounts in leaves (62 mg/100 g) followed by flowers (44 mg/100 g) and fruits (6 mg/100 g). Comparing microwave, Soxhlet, and decoction methods in the extraction of rutin, Mollica et al. (2019) indicated how effective the Soxhlet extraction method was in the targeted compound compared to the others (Mollica et al., 2019). Likewise, both methanol and aqueous extracts of the fruits showed to contain either catechin or gallic acid since these standards travel with the same frontal report on the plate. Quercetin was almost absent in the extracts examined (Benzidane et al., 2020).
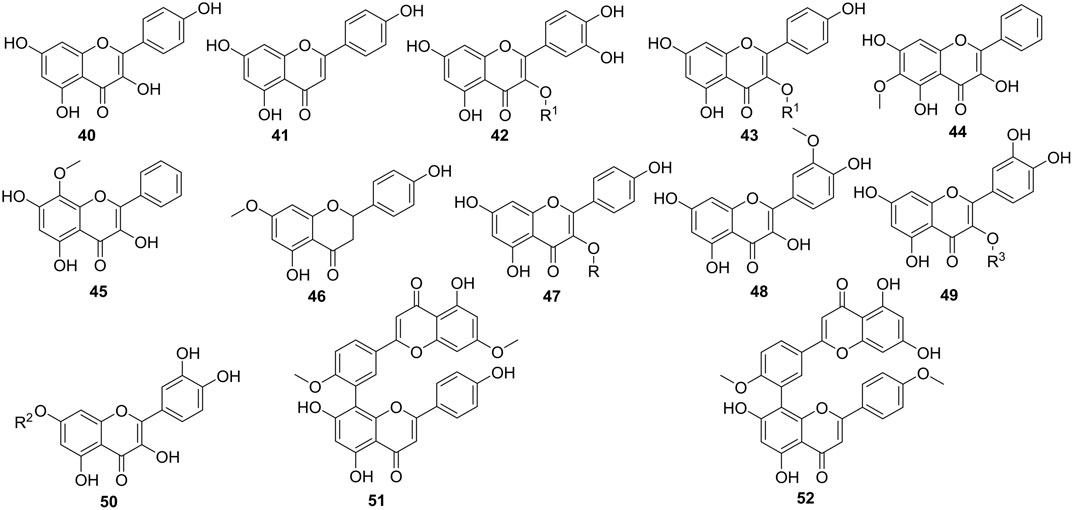
FIGURE 5. Flavonoids isolated in Caper organs. R = β-D-glucopyranosyl, R1 = rutinosyl, R2 = β-D-glucorhamnoside, R3 = 6‘-O-rutinosyl-O-β-D-glucoside, 40 = Kampferol, 41 = Apigenin, 42 = Rutin, 43 = kaempferol 3-O-rutinoside, 44 = Oroxylin A, 45 = Wogonin, 46 = Sakuranetin, 47 = Astragalin, 48 = quercetin 3-O-[6‴-α-L-rhamnosyl-6″-β-D-glucosyl]-β-D-glucoside, 49 = Isorhamnetin, 50 = Quercetin 7-O-β-D-glucorhamnoside, 51 = Ginkgetin, 52 = Isoginkgetin.
Other methods have been applied to isolate and identify flavonoids in Caper. Results confirm the absence of quercetin in the plant organs. Rather, methoxylated flavonoids (44-45, 48) have also been isolated from Caper organs. In addition, a quercetin triglucoside (49) has been mentioned to occur in the 80% hydromethanolic extract of the aerial part together with quercetin 3-O-β-D-glucopyranoside (47) (Sharaf et al., 2000). Other flavonoid glycosides, mainly quercetin 7-O-β-D-glucorhamnoside (50), have also been reported from the n-butanol fraction of the aerial part of the plant (Artemeva et al., 1981). To date, only two biflavonoids, isoginkgetin, and ginkgetin, amongst polyflavonoids have been reported from the fruits of the studied plant or one of the other organs (Zhou et al., 2011).
Phenolic Acids and Fatty Acids
The yield of an extract in phenolic acids could be linked to the extraction methods. The maceration method has shown higher levels of phenolic acids and flavonoids compared to ultrasonic-assisted extraction and reflux (Yahia et al., 2020). Quinic, gallic, and protocatechuic acids were the most abundant phenolic acids in the leaf extracts (Yahia et al., 2020). These acids and others which include chlorogenic, p-hydroxybenzoic, vanillic, caffeic, syringic, p-coumaric, ferulic or rosmarinic acids, and vanillin, protocatechuic aldehyde, and syringaldehyde were not detected in similar research on the fruits of the plant. However, gentisic, sinapic and benzoic acids were identified (Aliyazicioglu et al., 2013). Quinic acid, chlorogenic acid, and p-coumaroyl quinic acid were checked by LC-ESI-MS/MS from the aerial part of the plant (Bakr et al., 2016). By all, the total phenolic content (including phenolic acids, flavonoids, and coumarins) of the plant seeds ranged from 1.31 mg gallic acid equivalents per g of dry residue (GAE/g DR) to 8.14 mg GAE/g DR (Tlili et al., 2015). Leaves content in phenolic acids corresponded to 427.27 mg GAE/g DR (Mansour et al., 2016) while fruits carry 1.43 mg GAE/g DR and small buds, 5.97 mg GAE/g DR (Ghafoor et al., 2020). Nonetheless, the content of a plant extract in phenolic compounds could be drastically affected by fermentation (Sonmezdag et al., 2019). For instance, fresh buds of the plant showed to contain 18.43 mg/g DR which drops to 11.98–15.39 mg/g DR in fermented buds (Aksay et al., 2021) (Table 2). Likewise, polyunsaturated fatty acids constituted 50% of accounted fatty oil content (also representing 1.6%) of the plant (Rodrigo et al., 1992). The most abundant in this series is oleic acid (45.82%) followed by linoleic acid (25.37%), palmitic acid (15.93%), palmitoleic acid (4.55%) and stearic acid (4.06%) (Tlili et al., 2009).
Terpenes and Miscellaneous
C-15 sesquiterpene analogs (53-60) and steroids (63-65) (Figure 6) are two members of terpenes reported to have occured in Caper organs. The water-soluble fraction of the dried mature fruit material showed to contain the degraded tetraterpene analogs corchoionoside C, spionosides A and B, and phaseic acid along with the glucopyranosyl derivative (61) (Çalış et al., 2002). Moreover, Tlili et al. (2010) reported roughly 2.24 mg/g of phytosterols in the extracted lipids of the seeds plant. β-sitosterol was the most abundant in this series accounting for 1.39 mg/g of lipid followed by campesterol (0.382 mg/g) and stigmasterol (0.265 mg/g) (Tlili et al., 2010). However, only three sterols have been isolated by chromatographic methods including compound 63 and saponins (64-65). Two other sterol derivatives namely spinosols A and B have been highlighted to occur in Caper but there is no available information on their structures (Razaq et al., 2017).
FIGURE 6. Terpenes and other compounds from Caper. R = β-D-glucopyranosyl, R2 = 6‘-stearyl-β-D-glucopyranosyl, 53 = (6S,7E)-6,9-Dihydroxymegastigma-4,7-dien-3-one, 54 = 4-Hydroxy-4-(3-hydroxy-1-buten-1-yl)-3,5,5-trimethyl-2-cyclohexen-1-one, 55 = (4S)-4-[(1E,3S)-3-(ß-D-Glucopyranosyloxy)-1-buten-1-yl]-4-hydroxy-3-(hydroxymeth…, 56 = Corchoionoside C, 57 = (2R)-2-[(1E,3R)-3-(ß-D-Glucopyranosyloxy)-1-buten-1-yl]-2-hydroxy-1,3-dimethyl-5…, 58 = (1R,5R,8S)-8-[(1E,3S)-3-(ß-D-Glucopyranosyloxy)-1-buten-1-yl]-8-hydroxy-1,5-dime…, 59 = (1R,5R,8S)-8-Hydroxy-8-[(1E,3S)-3-hydroxy-1-buten-1-yl]-1,5-dimethyl-6-oxabicycl…, 60 = Phaseic acid, 61 = Methyl α-D-fructofuranoside, 62 = 1-prenylglucopyranoside, 63 = β-Sitosterol, 64 = Daucosterol, 65 = Daucosterol 6'-O-stearate, 66 = alpha-tocopherol, 67 = (2R,4aR,8aR)-3,4,4a,8a-Tetrahydro-4a-hydroxy-2,6,7,8a-tetramethyl-2-(4,8,12-trimethyltridecyl)-2H-chromene-5,8-dione, 68 = myo-Inositol, 69 = (R)-2,4-Dimethoxy-2-methyl-6H-pyran-3-one, 70 = Isopsoralen, 71 = Sporalen.
Tlili et al. (2009) have also evaluated the wealth of the plant in carotenoids and tocopherols. The latter is present in plant seeds and constitutes 628 mg/100 g of the fatty oil extracted, made up of γ-tocopherol (92%), α-tocopherol (4%), and δ-tocopherol (2%) (Tlili et al., 2009). However, only α-tocopherol (66) and an oxidized analog (67) have been isolated to date but not γ-tocopherol although it is said to occur in higher amounts (Hu et al., 2017). In addition, the seed oil contained a significant level of β-carotene evaluated at 375 μg/100 g out of 457 μg/100 g of the plant carotenoids (Tlili et al., 2009). Caper also contains saccharides including sucrose, compounds 61 and 62, pyran (69), polyol (68), and coumarins (70-71).
Nonetheless, leaves, seeds, roots, and fruits of Caper drain significant levels (0.02–48.7 mg/kg) of essential and nonessential heavy metals including iron (Fe), nickel (Ni), manganese (Mn), zinc (Zn), copper (Cu), cadmium (Cd), chromium (Cr), titanium (Ti), barium (Ba), strontium (Sr), aluminum (Al), magnesium (Mg), potassium (K), sodium (Na) and lead (Pb). These metals could originate from the in-situ bioremediation potency of the plant rather than its natural predisposition to produce them. However, the occurrence of a metal element in an organ varies from one research work to another. For instance, heavy metal content of Caper leaves found to decrease in the order of Fe > Zn > Mn > Cu > Pb > Ni > Cr > Cd by (Niaz et al., 2013) and of Cu > Ti > Cr > Ba > Zn > Sr > Mn > Al > Fe > Mg > K > Ca by (Vaidya et al., 2017), both in polluted and unpolluted aerials. The control of heavy metals in medicinal plants like Caper is essential to prevent their adverse effects on the human health system.
Biological Potential of Caper
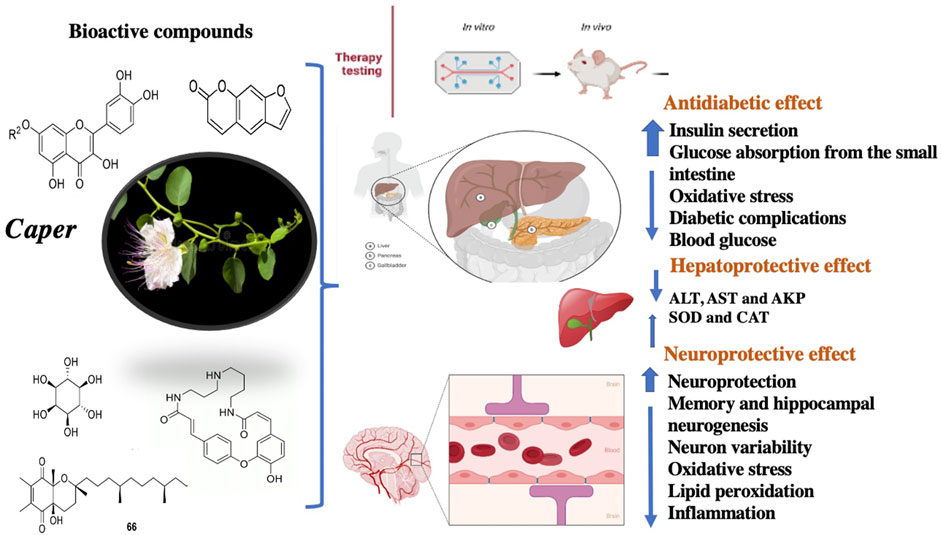
Extracts and isolated compounds from various organs of Caper have been assessed for their biological potential. The activities were evaluated using common methodology and standards. Overall, Caper organs are preferably good antidiabetic, hepatoprotective, and neuroprotective agents (Figure 7). In addition, they also showed considerable antimicrobial, antioxidant, anti-inflammatory, and anticancer activities.
Antimicrobial Activity
Caper extracts furnished promising antimicrobial activities in different experimental models (Supplementary Table S1). For instance, the methanol extract of the fruits showed a dose-dependent degree of quorum sensing, expressing 70–79% of biofilm inhibition and 46–67% reduction of exopolysaccharide production (EPS) production in Serratia marcescens, Pseudomonas aeruginosa, Escherichia coli and Proteus mirabilis at 0.5 and 2 mg/mL. The plant also reduced the swimming and swarming mobility of bacterial pathogens (Issac Abraham et al., 2011). Likewise, the antibacterial activity of the 80% hydroalcoholic (methanol, ethanol, and acetone) extracts from stem bark, shoots, fruits, flowers, and roots were investigated towards Staphylococcus aureus NCTC6571, E. coli ATCC8739, Bacillus subtilis NCTC10400, and Pasteurella multocida (isolated strains). The methanol extracts were the most significant to slow down the growth of the microorganisms with diameter zones of inhibition (DZI) around 24 mm compared to 27–32 mm for the controls, amoxicillin, and ciprofloxacin. The activity of the ethanol and acetone extracts was comparable with DZI of about 15 mm. None of the organs was most active than the others toward the tested microorganisms (Gull et al., 2015). Similar results were described by Adwan and Omar, (2021), stem and leaf ethanol extracts exhibited MIC of 6.25–100 mg/mL against relatively identical bacterial strains (Gull et al., 2015; Mickymaray and Al Aboody, 2019; Adwan and Omar, 2021). Similarly, the ethanol fruit extract of Caper featured strong antibacterial activity with MIC of 1.73 mg/mL against Listeria monocytogenes and 6.25 mg/mL against E. coli and Pseudomonas aeruginosa. The extract was moderately active against S. aureus sensitive to methicillin (SASM), S. aureus resistant to methicillin (SARM) Klebsiella pneumoniae, and Salmonella sp. with MIC 21–32 mg/mL. The ethanol flower extract was not as strong as the fruit extract against the same microorganisms disclosing MIC ranging from 10–15 mg/mL (Ennacerie et al., 2017; Hameed et al., 2021). Caper extract also showed differential antibacterial activity against Pasteurella multocida, K. pneumonia, Acinetobacter baumannii, Enterobacter aerogenes, and Proteus mirabilis with DZI values of 24.9, 36, 21, 26 and 27 mm, respectively (Gull et al., 2015; Mickymaray and Al Aboody, 2019; Adwan and Omar, 2021). From other perspectives, the copper nanoparticles prepared from the aqueous extract from the fruits showed significant antibacterial activity with MIC of 5–10 mg/mL against S. aureus PTCC1112, B. cereus PTCC1556, E. coli PTCC1330, and K. pneumoniae PTCC1053 (Ebrahimi et al., 2017). Likewise, silver nanoparticles prepared from aqueous fruit extract disclosed significant antifungal activity with MIC values ranging from 5 mg/mL to 0.625 mg/mL (Ebrahimi et al., 2019). Caper extracts rather were active against some fungal strains including Aspergillus flavus, Candida albicans, Candida glabrata, and Kluyveromyces marxianus. Overall, tested water-soluble or alcoholic extracts displayed moderate activity with either DZI around 19 mm or MIC <12.5 μg/mL (Sherif et al., 2013). All these promising antimicrobial results from Caper extracts are considered a good starting point for further studies to better understand the mode of action of these extracts (Supplementary Table S1).
Antioxidant Potency
Antioxidants include all molecules able to inhibit free radical reactions produced naturally in the body from metabolic processes or by extrinsic agents such as exposure to X-rays, environmental pollution, ultraviolet light, drugs, and pesticides (Rahman, 2007). These free radicals cause damage to DNA, cell membranes, and other cell tissues leading to various diseases. Several studies revealed in vitro antioxidant potential of Caper extracts using DPPH, ABTS, FRAP, CUPRAC, phosphomolybdenum, metal chelating power, and TAC assays (Supplementary Table S2). These activities have been related to the occurrence of high levels of phenolic acids and flavonoids (Rahnavard and Razavi, 2017). Indeed, aqueous leaf extracts prepared by either maceration, reflux, or ultrasound-assisted extraction were assessed for their antioxidant activity. The reflux extract showed the highest capacity to reduce DPPH with IC50 of 36.6 mg/mL; the ultrasound-assisted extract showed the highest ABTS scavenging activity with 258.77 mg of ascorbic acid equivalent/g dry weight (DW), while the macerated extract showed the highest FRAP activity with EC50 equal to 120.2 mg/mL (Yahia et al., 2020). Likewise, the acetone 80% extract of fresh buds showed significant DPPH scavenging activity with IC50 = 5.90 μg/mL (El amri et al., 2019) as do the hydro-ethanolic extracts of different tissues towards DPPH radical with IC50 values of 1.41 mg/ml, 1.56 mg/ml, and 2.49 mg/mL, for leaves, fruits, and buds extracts, respectively (Assadi et al., 2021). In the same line, Saleem et al. (2021) compared the antioxidant activity of the methanol and dichloromethane (DCM) extracts of plant aerial parts and roots. The DCM extract showed the highest activity towards FRAP, CUPRAC, phosphomolybdenum, and metal chelating power assays with 50.37 mg TE/g extract, and 118.45 mg TE/g extract, 75.79 mg TE/g extract, and 2.51 mg EDTA/g endpoints, respectively. The methanol aerial parts and root extracts showed strong activity against DPPH and ABTS, instead. The administration of hydro-alcoholic extract of fruits demonstrated protective effects on tissue function through oxidative stress alleviation and antioxidant mechanism restoration (Mirzakhani et al., 2020).
Hepatoprotective Activity
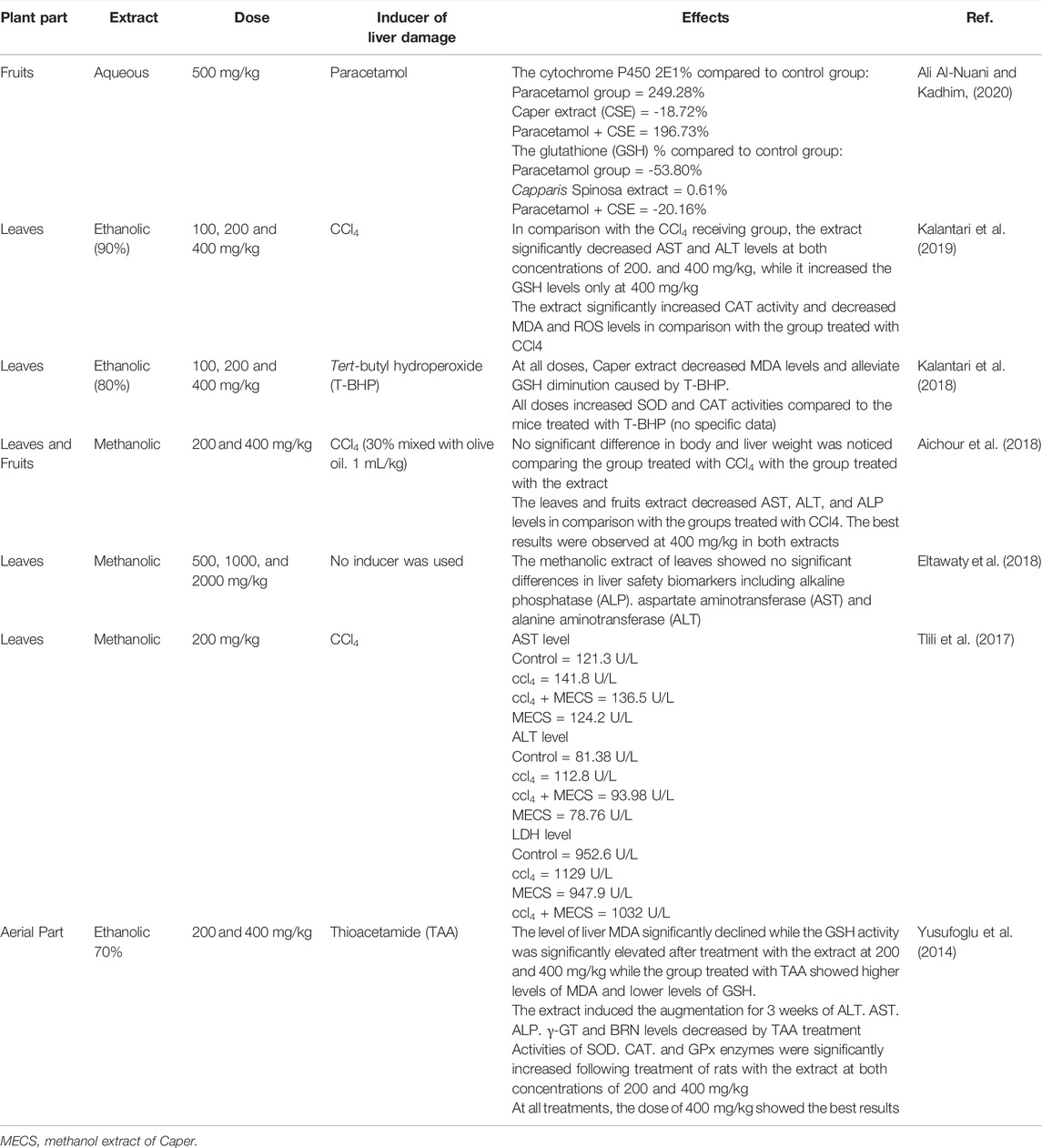
The liver plays an immense role in our body, it helps digest food, store energy, and get rid of poisons and toxins. Furthermore, the liver ensures the detoxification of a wide range of toxic molecules present in the organism. Most of these molecules increase the production of reactive oxygen species (ROS), which have exhibited their hepatotoxic effect in several experimental models (Sobeh et al., 2019; Sobeh et al., 2020a). Table 3 summarizes the hepatoprotective activity of Caper extracts.
The methanol leaf and fruit extracts of Caper were reported to display a significant hepatoprotective effect that may lead to the stoppage of the extension of liver damage by increasing levels of phase I detoxification enzymes namely cytochrome P450 enzymes (CYP) and phase II detoxification enzymes such as glutathione S-transferase (GST), quinone reductase (QR), UDP-glucuronosyltransferase (UGT), amino acid transferases, N-acetyl transferases, and methyltransferases. In addition, Caper extracts were able to decrease other enzyme levels that the liver releases in response to damage or disease including ALT, AST, AKP, γ-glutamyltransferase (γ-GT), and lactate dehydrogenase (LDH). Aichour et al. (2018), for instance, pointed out that methanol extracts from leaves and fruits of Caper reduced the elevated serum enzyme (AST, ALT, ALP, and bilirubin) levels induced by CCl4 at doses of 200 and 400 mg/kg for the highest effect encountered at 400 mg/kg (Aichour et al., 2018). Similarly, the hepatoprotective activity of the ethanol leaf extract of Caper was evaluated against tert-butyl hydroperoxide (T-BHP) as a liver damage inducer. The extract mitigated the diminution of detoxification enzyme GSH and reduced MDA levels while SOD and CAT levels increased significantly in the group treated with the extract (Kalantari et al., 2018).
The protective effects of Caper seed extract on the toxicity induced by CCl4 and cisplatin were demonstrated by Tir et al. (2019). Mainly, the pretreatment of animals restored the biomarkers of liver and kidney injuries alongside an increase in antioxidant enzymes, which was confirmed by the histopathological studies with a decrease in the degree of tissue fibrosis (Tir et al., 2019). Recently, Ali Al-Nuani and Kadhim, (2020) investigated the effect of aqueous fruit extract of Caper on two detoxification enzymes in comparison with the paracetamol effect. The extract furnished a reduction of the cytochrome P450 2E1 percentage from 249.28 to 196.73% when paracetamol is injected first then the extract and from 249.28 to 200.59% when the extract is injected prior to paracetamol. The glutathione (GSH) levels also increased to 53.80% with paracetamol and 20.16 and 40.49% when paracetamol was injected followed by extract and when the extract was injected first followed by paracetamol, respectively (Ali Al-Nuani and Kadhim, 2020). Nevertheless, further studies on the mechanism of action of Caper in liver diseases are required to shed light on developing methods in clinical practices.
Anti-Inflammatory Activity
Inflammation occurs in the body as a natural defense mechanism against xenobiotics and harmful compounds, but it can also cause diseases (Sobeh et al., 2020b). Various studies reported the anti-inflammatory effect of Caper extract including in vivo and in vitro assays. Kernouf et al. (2018) revealed the anti-inflammatory activity of the 80% hydromethanolic extract of buds at 200 and 400 mg/kg doses. The extract reduced the in vitro paw edema inflammation by 52–69% compared to the control, while 1 mg/pouch of the extract revealed inhibition of 48.92% of leukocytes infiltration. In addition, 100 μg/ml of the extract mitigated the production of inflammatory mediators TNF-α, IL-1β, LTB4, and superoxide anion by 21.28, 38.04, 20.84, and 71.16%, respectively (Kernouf et al., 2018). Likewise, the methanol extract and the resulted hexane fraction of the plant leaves comparably reduced contact hypersensitivity response in mice by approximately 73.44% of edema inhibition percentage using 1.07 g/kg dose and inhibited IFNγ, IL-17, and IL-4 as cytokine gene expression (El Azhary et al., 2017).
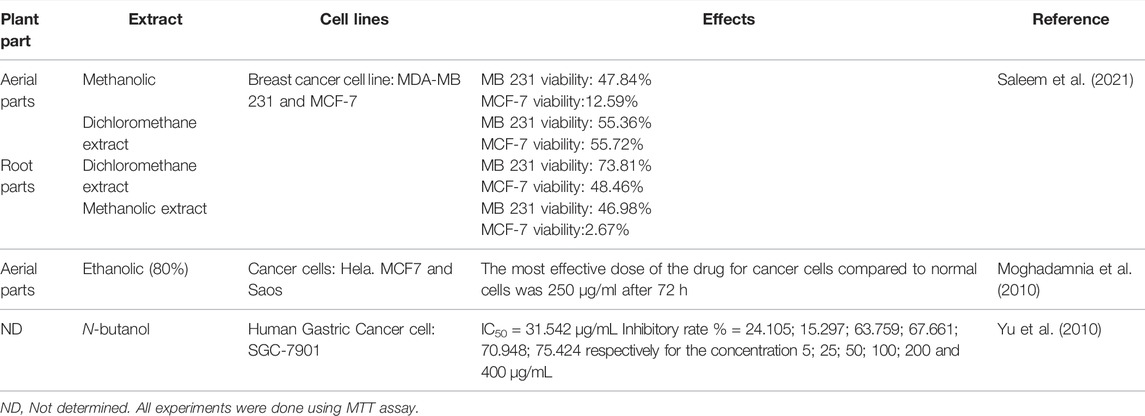
Anticancer Activity
Cancer is an abnormal growth and proliferation of cells and affects any human organ. Lung, prostate, colorectal, stomach, and liver are the most common organs likely to develop cancer in men, while breast, colorectal, lung, cervical, and thyroid cancers are the most common types of tumors found among women. Natural products and related constituents could serve as promising alternatives to chemotherapeutic and chemopreventive agents (El-Hossary et al., 2020; Tawfeek et al., 2021). A study has been conducted earlier by Yu et al. (2010) against human gastric cancer cell SGC-790 with MTT assay, the n-butanol extract showed potential effect against cell proliferation, the lowest and the highest inhibitory rate percentages were 24.1% at 5 μg/mL and 75.4% at 400 μg/mL, respectively, with an IC50 of 31.5 μg/mL (Yu et al., 2010). Saleem et al. (2021) investigated the cytotoxicity of the methanol and dichloromethane extracts of aerial parts and roots against breast cancer cell lines MDA-MB 231 and MCF-7. The dichloromethane extract of the roots showed the highest activity against MB 231 cells with a viability percentage of 73.81%, while the dichloromethane extract of the aerial part was active against both cells with a viability yields of 55.36 and 55.72% against MB 231 and MCF-7, respectively (Saleem et al., 2021). Moreover, Caper isothiocyanates are well known as cancer preventive agents and different extracts have hypoglycemic properties and protective effects against hepatotoxic substances. These promising in vitro results noted by different extracts (Table 4) need further in vivo studies to prove their efficiency and uncover the underpinning mechanisms of antitumor activity.
Neuroprotective Effect
Studies have highlighted the relationship between inflammation and memory impairments (Geng et al., 2018; Lin et al., 2018). Systemic inflammation results in learning and memory impairment through the activation of microglia. Caper reduced brain inflammation by an increase in anti-inflammatory mediators (IL-10) and a decrease of inflammatory mediators (TNF-α, IL-1β) in LPS-induced cognitive impairment (Rahimi et al., 2020). In the same line, Goel et al. (2016) investigated the effect of Caper on learning and memory damage after administration of LPS. Results demonstrated a reductive effect of the aqueous extract of the plant buds on the neurodegeneration in the hippocampal circuit region of the hippocampus (Goel et al., 2016). The inhibition capacity of Caper (200 mg/kg b.w.) attenuated cognitive impairment induced by D-galactose in mice (Turgut et al., 2015). Furthermore, Khorrami et al. (2021) investigated the effect of Caper extract in the middle cerebral artery occlusion (MCAO) model of ischemic stroke. As a result, the pretreatment with Caper reduced MVAO injury and the neurological deficit score through the suppression of oxidative stress (Khorrami et al., 2021). Moreover, Caper extract demonstrated a good effect to regulate inflammation-involved genes in Alzheimer’s, especially on the amyloid-beta peptide (Aβ)-injected rats. This activity could be attributed to the high level of flavonoids in the plant (Mohebali et al., 2018). The findings demonstrated the neuroprotective effect of Caper and provide evidence that the plant could be considered for the treatment of neurodegenerative disorders such as Alzheimer’s disease.
Antidiabetic Activity
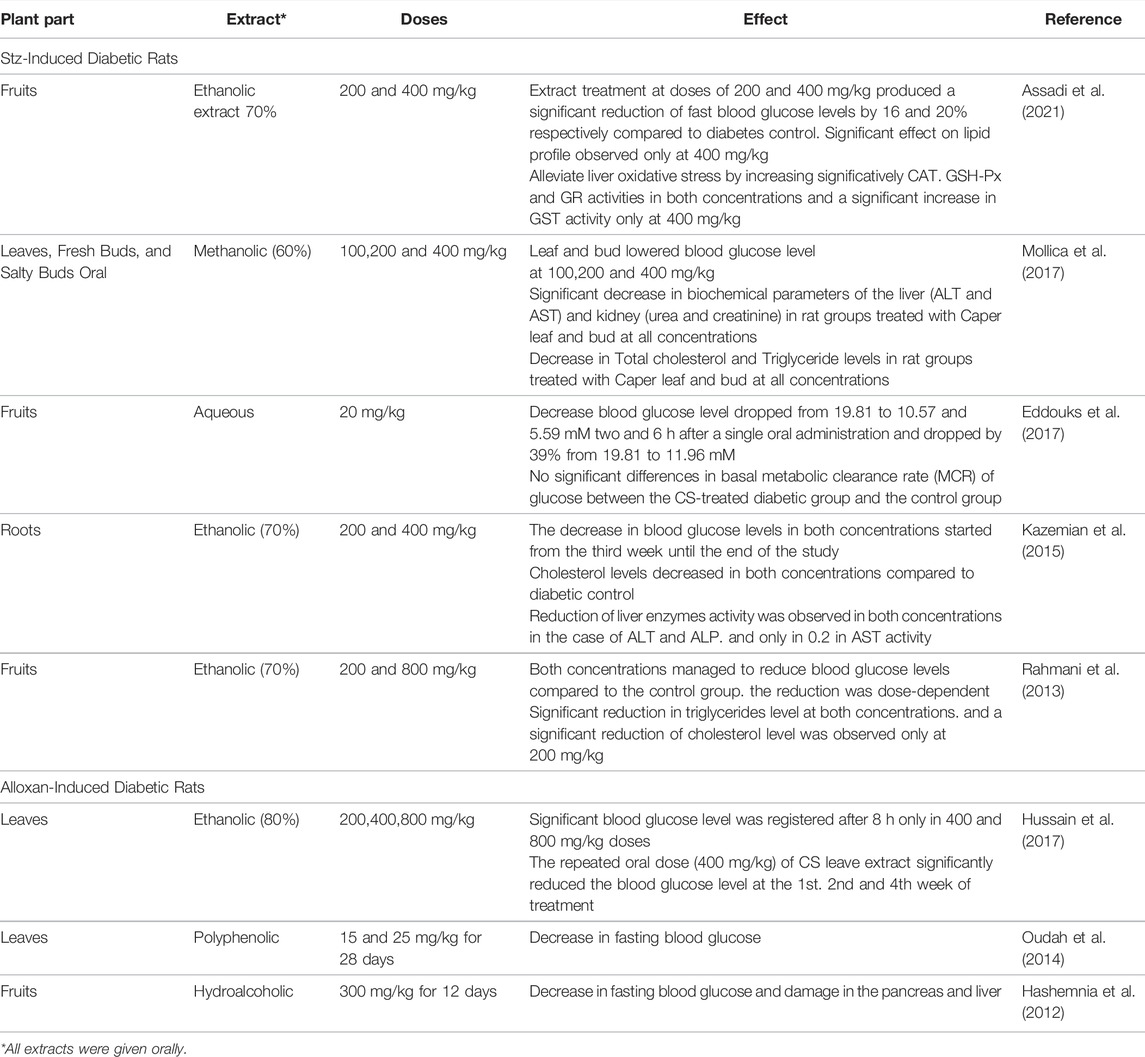
The antidiabetic properties of Caper extracts have been well-documented in the literature and are summarized in Table 5. Studies were carried out in vivo using animal models and clinical trials in patients and demonstrated the antihyperglycemic effects of Caper at various doses starting from 15 mg/kg up to 800 mg/kg and between 12 and 60 days of treatment (Rahmani et al., 2013; Kazemian et al., 2015; Eddouks et al., 2017).
The hydroethanolic fruit extract of Caper produced a significant reduction of fasting blood glucose levels in type-2 diabetic rats by 16% at a concentration of 200 mg/kg and by 20% at 400 mg/kg compared to streptozotocin (STZ). However, a significant effect on lipid profile was observed only at the concentration of 400 mg/kg, mitigating liver oxidative stress and increasing detoxification enzyme levels (Assadi et al., 2021). Similar results were found earlier by (Kazemian et al., 2015) using 70% ethanolic extract of the roots of Caper at the same doses (200 and 400 mg/kg). In the same line, Jalali et al. investigated the antidiabetic effects of aqueous extract of the fruits and revealed the oral administration of 20 mg/kg of the extract decreased the fasting blood glucose (FBG) in STZ-induced diabetic rats (Jalali et al., 2016).
Another study showed a better hypoglycemic effect using a lower dose of aqueous extract (20 mg/kg) with a decrease in blood glucose level from 19.81 to 5.59 mM after a single oral administration and to 11.96 mM after a daily repeated administration (Eddouks et al., 2017). A mixture of plant materials including Caper was used to assess their efficacy on patients with Type-2 diabetes mellitus, the plant mixture reduced fasting plasma glucose and glycated hemoglobin (HbA1c) compared to the patients treated with placebo and showed similar results compared to the metformin-treated patients (Mehrzadi et al., 2020). In addition, Huseini et al. (2013), evaluated the antihyperglycemic effect of Caper in patients with type-2 diabetes and the results showed a significant decrease in FBG and HbA1c levels in the patients treated with 400 mg of hydroalcoholic extract of the plant. Based on these findings, Caper could be considered an adjuvant agent in diabetes.
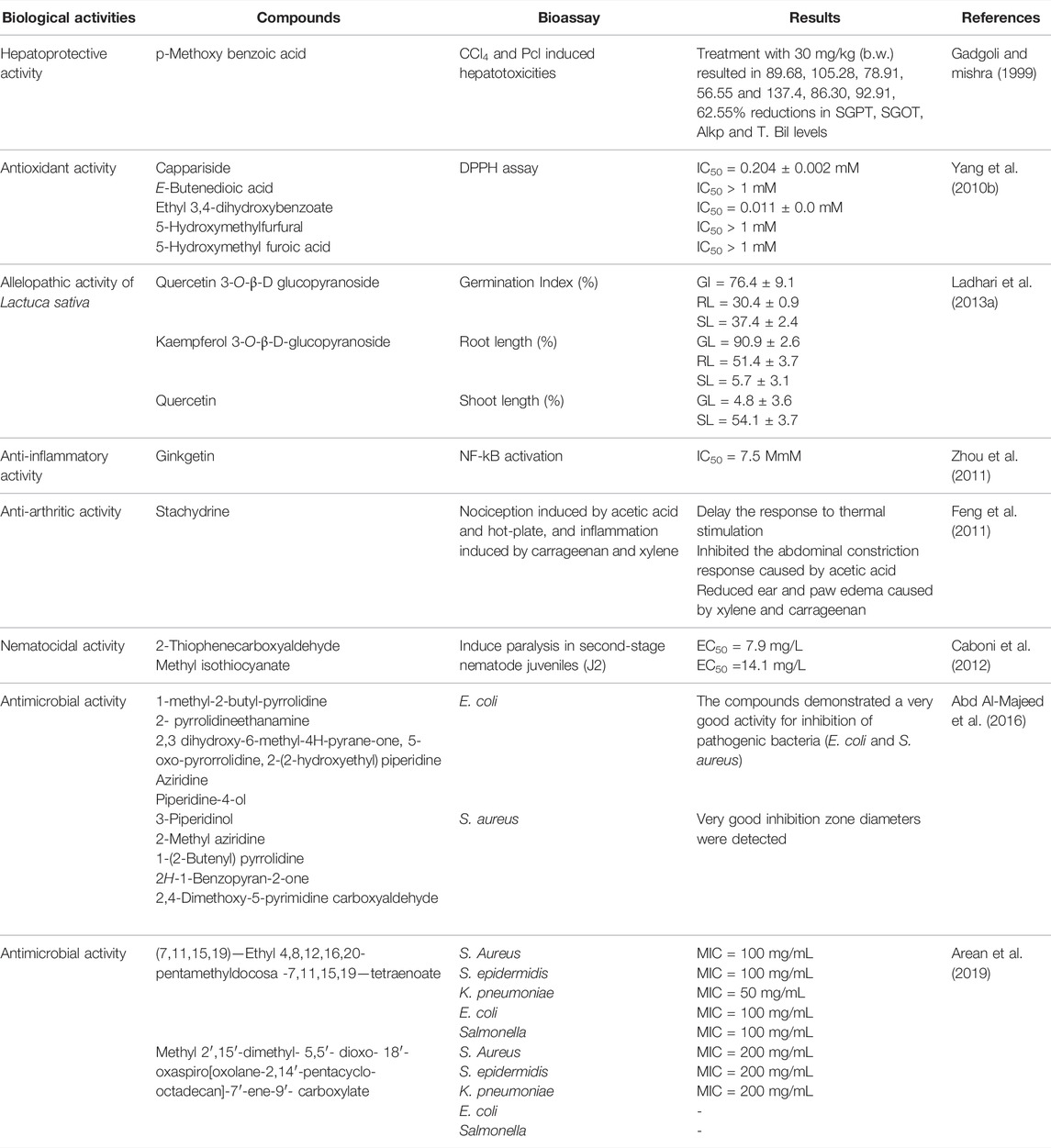
Biological Activities of the Phytocompounds Isolated From Caper
Different parts of Caper comprise a wide variety of active secondary metabolites with several biological activities. Many bioactive compounds were isolated from Caper. Although phytochemicals act synergistically with other compounds in the plant, some of the identified compounds demonstrated a variety of biological activities. Table 6 shows a summary of bioactive compounds isolated from Caper.
Toxicity Studies
Only a few reports described the side effects of Caper (Taghavi et al., 2014; Kazemian et al., 2015). All in all, Caper is safe for consumption. Fruits induce no side effects on the liver and no signs of nephrotoxicity in rats (Heidari et al., 2010; Huseini et al., 2013). Ouadah Hamam et.al. (2019) investigated the acute toxicity effect of polyphenolic extract of the plant leaves and the extract was nontoxic at doses up to 100 mg/kg b.w (Oudah hamam et al., 2019). The hydroalcoholic extract of Caper fruits in rats showed an LD50 value of 400 mg/kg supporting results encountered by El-Hawary et al. (2018) who demonstrated no mortality in rats within 24 h of pretreatment with Caper methanol extract at doses of 1000–4000 mg/kg, suggesting an LD50 > 4000 mg/kg (El-Hawary et al., 2018).
Other Biological Activities
Caper extracts were also screened for other biological activities including insecticidal effects. Caper roots are submerged in water all night and then dispersed on plant seeds to protect the seeds from pests all over the year (Mahboubi and Mahboubi, 2014). In addition, Caper leaf extracts displayed strong insecticidal activity with 100% mortality, while stem extracts showed moderate activity (50% of mortality) (Ladhari et al., 2013b). The acetone extract of Caper showed insecticidal activity against third instar larvae of Aedes aegypti inducing 40% mortality at 2 mg/ml concentration and an LC50 value of 1.77 mg/ml. In addition, the hydro-alcoholic extract of Caper showed significant in vivo hypnotic activity in mice in comparison with diazepam at a 3 mg/kg body weight dose inducing no cytotoxic effect and an LD50 value of 2.4 g/kg (Rakhshandeh et al., 2020).
Clinical Studies
Various studies conducted on Caper showed high promising results, which led to clinical studies to confirm previous in vivo and in vitro results. A study by Banerjee et al. (2011) carried out using Caper as a part of the polyherbal formulation to assess its efficiency and safety profile as an antioxidant for geriatric patients. A remarkable restoration of antioxidant properties was reported among the patients treated with the polyherbal formulation in comparison with the control group (Banerjee et al., 2011). Another clinical trial investigated the anti-hyperglycemic effects of the Caper fruit extract in type 2 diabetic patients and significant results were seen in patients treated with 400 mg extract with no side effects on the kidney or liver (Huseini et al., 2013).
Commercial Formulation and Patented Products of Caper
Caper is one of the most important economical species in the Capparaceae family. Caper and its berries are the main products with economic importance at an international level. They are used as a flavor in food industries or as condiments (Rivera et al., 2002; Reyahi-Khoram and Reyahi-Khoram, 2018). In countries such as Tunisia, Saudi Arabia, Lebanon, and Syria, the species is suggested to raise the socioeconomic value (Sher and Alyemeni, 2010). In China, Caper constituted an annual contribution to the economy of the country of about 3 million USD. In the Balkans region, the total production costs of Caper represent less than 10% only of its selling price in the US markets (Saadaoui et al., 2011; Saadaoui et al., 2013). In addition, the plant has high nutritional value and demonstrated efficacy in medicines and cosmetics manufacturing (Aytac et al., 2009). For instance, Gatuline® Derma-Sensitive is a natural soothing active prepared from Caper fruit extract. The product is an anti-aging agent claiming skin protection and reducing inflammation. Moreover, the product is commercialized under SKIN MOON®; SKIN SAVE® and certified by ECOCERT and NSF. Caper buds are also typically commercialized as delicatessen products and used as pickles. The buds are categorized consistent with their sizes, the smallest is the most expensive in the market due to their concentrated flavor (Peregrin, 1985). Moreover, the young shoots of Caper are cooked in the same way as asparagus (Facciola, 1990).
Bioavailability and Pharmacokinetics of Caper Phytoconstituents
Caper furnished numerous biological activities, among them antioxidant, antibacterial, antifungal, anti-inflammatory, analgesic, antitumor, hepatoprotective, and neuroprotective effects. These activities are attributed to the presence of diverse classes of secondary metabolites, such as phenolic acids, glucosinolates, furans, pyrroles, flavonoids, alkaloids, and terpenoids. To draw insight into the possible mechanisms, several molecular descriptors were calculated using SwissADME (Daina et al., 2017), among them several drug-likeness rules (Lipinski, Veber, Egan, and Muegge). Interestingly, out of 16 alkaloids identified from the plant, 14 compounds fulfilled all criteria of Lipinski, Egan, and Veber rules, and 7 and 3 compounds fulfilled Muegge and Ghose rules, Supplementary Table S3 and Figure 8. Also, all furans and pyrroles (10 compounds) satisfied all criteria of Lipinski, Egan, and Veber rules, and 5 and 2 compounds satisfied Muegge and Ghose rules. Most of the terpenoids and the other miscellaneous compounds fulfilled the criteria of the five tested drug-likeness rules as well. However, most of the flavonoids did not obey the rules due to their high molecular weights, and high numbers of hydrogen bond donors and acceptors. Glucosinolates showed some violations of the tested rules and this was partially due to their high numbers of hydrogen bond donors and acceptors, Supplementary Table S3 and Figure 8.
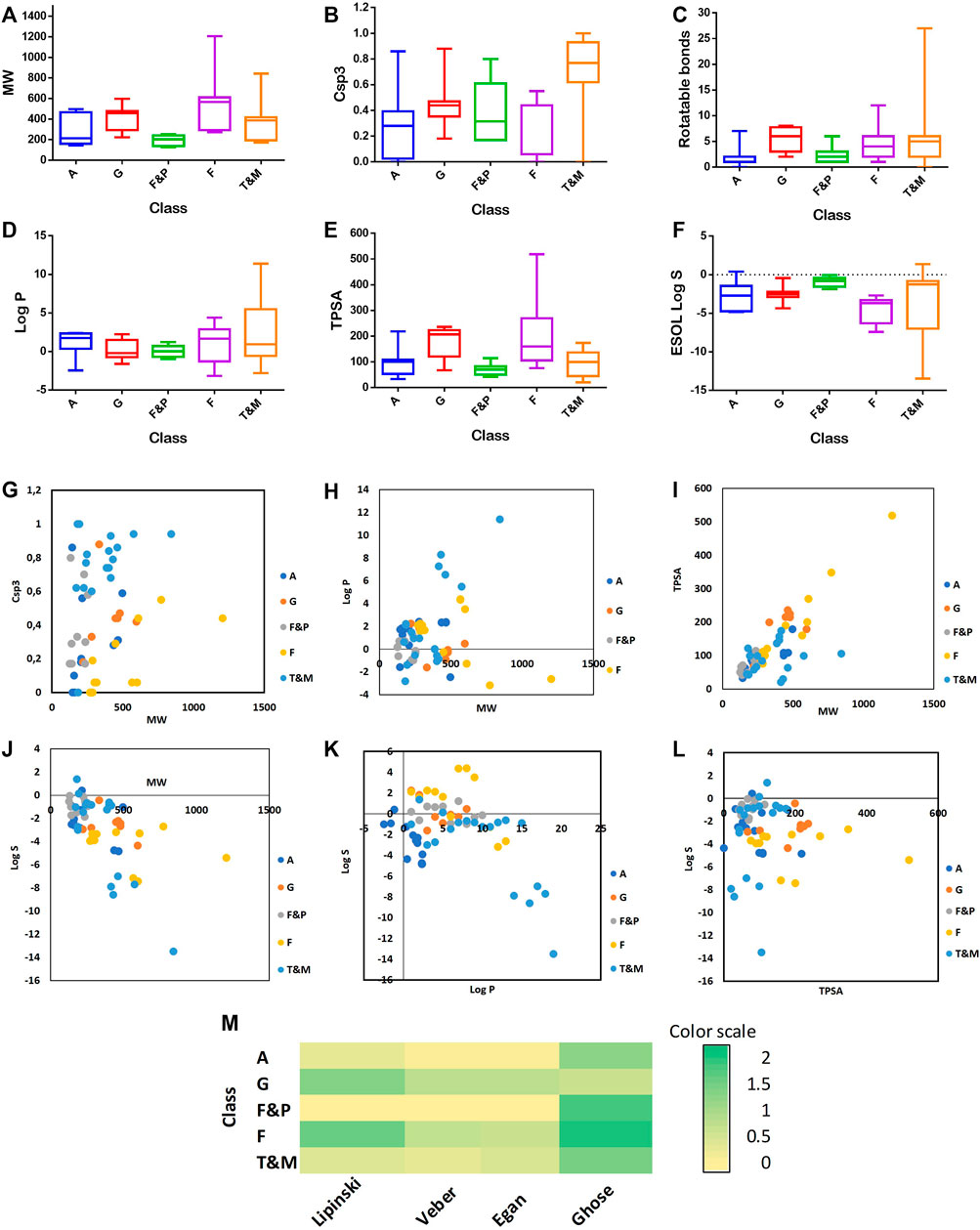
FIGURE 8. Distribution of MW (A); F Csp3 (B); number of RBs (C); Log P (D); TPSA (E); and Log S (F) accordingly to the class of compounds. Comparison between the values of F Csp3 carbons and MW (G); log P and MW (H); MW and TPSA (I); MW and log S (J); log P and log S (K); TPSA and log S (L). Heatmap of the compliance with rules of drug-likeness for the compound’s classes (M). A (Alkaloids), G (Glucosinolates), F&P (Furans), F (Flavonoids), and T&M (terpenoids and miscellaneous).
Topological polar surface area (TPSA), another descriptor, is the sum of the surfaces of all the polar atoms present in a molecule, which mainly are the oxygen and nitrogen atoms including the attached hydrogens. Apart from the molecular weight, TPSA has a great impact on the ability of a molecule to penetrate through the cell membranes and blood-brain barrier. Veber states that molecules with TPSA ≤140 A2 tend to be well absorbed and able to reach their molecular target within the body cells. Veber also stated that a molecule should have no more than 10 rotatable bonds for good oral absorption. Egan considered chemical compounds with TPSA not more than 132 A2 and log-P between -1 and 6 as leads with high drug-likeness potential and good oral bioavailability. Muegge utilized a pharmacophore point filter based on very simple structural rules to differentiate between drug-like and nondrug-like molecules, among them TPSA not greater than 150 A2 as well as no more than 15 rotatable bonds. Noteworthy, all furans and pyrroles, terpenoids and miscellaneous compounds and alkaloids, except compounds 9 and 21, had TPSA ≤150 A2. All Caper compounds, except one terpenoid (compound 65) had rotatable bonds less than 15, Supplementary Table S4 and Figure 8.
Another indicator for oral bioavailability that we tested is the bioavailability score which indicates the possibility of a molecule to be more than 10% bioavailable in the absorption assays. Generally, compounds satisfying the Lipinski rule with a bioavailability score of 0.55 are considered to be orally bioavailable. Out of the identified compounds from the plant, 50 phytoconstituents showed a bioavailability score of 0.55. Compounds 11, 13, 14, and 32, are of special interest as they showed good bioavailability scores of 0.56, 0.85, 0.56, and 0.85, respectively, Supplementary Table S4 and Figure 8.
Oral bioavailability depends as well on the degree of the molecular flexibility of a given compound. Compounds with very a high degree of flexibility do not usually show good oral bioavailability as they tend to be less planar and with very complex 3D shapes. The sp3 carbons fraction (Fraction Csp3) and the number of rotatable bonds (RB) are two crucial measures for molecular flexibility. Csp3 is the ratio of the sp3 carbon atoms to the total carbons present in a given compound. It assigns the degree of carbon saturation, characterizes the space complexity, and also correlates to the solubility of the compound. A Csp3 score between 0.25 and 1 is considered optimum for drug likeness. In the studied case, 48 phytoconstituents showed a Csp3 score ranging between 0.28 and 1. The water solubility, expressed as log S, is another essential measure for drug bioavailability. Compounds with poor water solubility have poor absorption and oral bioavailability, and low formulation potential. Caper phytoconstituents furnished different solubility orders as furans and pyrroles, were the most soluble class (mean value of −0.8), while flavonoids were the most poorly soluble class (mean value of −3.7), Supplementary Table S4 and Figure 8.
To elucidate the pharmacokinetic behavior of the identified secondary metabolites from Caper, various descriptors were explored. These include gastrointestinal absorption (GI), blood-brain barrier permeation (BBB), P-glycoprotein substrate (P-gp), skin permeation (Log Kp), and potential inhibitors of cytochrome P450 members, Supplementary Table S5 and Supplementary Figure S1. Interestingly 14 compounds displayed high GI absorption, passively crossed the blood membrane barrier, and did not show any potential for P-glycoprotein substrate, Supplementary Table S5, and Supplementary Figure S1. Also, Caper constituents revealed potential inhibition for some CYP 450 isoforms which require attention when coadministered with possible substrates of these enzymes, Supplementary Table S5.
Altogether, Caper is rich in phytoconstituents that fulfilled all the criteria of several drug-likeness rules with promising pharmacokinetic behavior which promotes its utilization as well as further research to isolate its phytoconstituents and evaluate their biological activities.
Discussion
This review aimed to summarize the scientific literature on the nonconventional edible Caper plant (C. spinosa) and evaluate the phytochemistry, safety, and biological activities of its extracts and/or phytocompounds. The major phytochemicals identified in Caper were flavonoids (rutin, quercetin, and catechin), alkaloids (indoles and spermidines), and glucosinolates (glucocapparin). Other constituents such as furan and pyrrole derivatives as well as polyunsaturated fatty acids represented mainly by oleic acid, linoleic acid, and palmitic acid were also among the most important group of chemicals found in Caper. This plant is well renowned for its ethnopharmacological interests mainly in Iran, China, and India but also in Morocco with very diversified applications ranging from rheumatism and correlated infections to diabetes and kidney stones (Table 1). Several studies supported these traditional uses via in vitro and in vivo studies. Given its biosafety both traditionally and through scientific studies, Caper’s different extracts have been shown to elicit strong antioxidant activities in vitro and related disorders due to the occurrence of high levels of phenolic acids and flavonoids. Compounds isolated from Caper such as 5-hydroxymethylfurfural, E-butenedioic acid, and 5-hydroxymethyl furoic acid were demonstrated as DPPH scavengers (Table 6). This could be behind the protective effect of Caper extracts in alleviating the dysregulation of hepatic enzymatic parameters and in boosting the antioxidant machinery following stress induction. Neuroprotective effects were also demonstrated using Caper aqueous extract which attenuated the cognitive impairment and reduced the middle cerebral artery occlusion (Khorrami et al., 2021). This was noticed in the inflammation process involving Alzheimer’s genes as well. However, more in deep studies should address the underpinning mechanisms and uncover the targets of Caper antioxidant compounds within the cells and explore their downstream effects.
The most well-documented effect of Caper is the antidiabetic activity. It was shown in vivo using animal models and clinical trials in patients. A decrease in FBG and HbA1c were the main induced effects (Huseini et al., 2013). These encouraging results strongly support the use of Caper extracts as adjuvant agents in diabetes treatments. Caper also furnished anti-inflammatory responses both in vitro and in vivo as it inhibited the edema inflammation, reduced leukocyte infiltration, mitigated the production of pro-inflammatory mediators (TNF-α, IL-1β, and LTB4), and increased anti-inflammatory mediators (IL-10) (Kernouf et al., 2018). It has been shown that Nf-KB activation by ginkgetin, a compound isolated from Caper, is an interesting mechanism of anti-inflammatory responses (Zhou et al., 2011). However, more studies on other Caper extracts and/or essential oils on inflammation using cell-based lines and animal models are necessary.
Additionally, an exhaustive number of in vitro studies have shown that Caper extracts had anticancer and antimicrobial properties and compounds like 1-methyl-2-butyl-pyrrolidine, 2-methyl aziridine, aziridine, 7,11,15,19−ethyl 4,8,12,16,20-pentamethyldocosa-7,11,15,19−tetraenoate were identified as the bioactive agents (Table 6). As suggested, these plant extracts/compounds could trigger the bacterial cell membrane, coagulate the cytoplasm and bind lipids and proteins (Viuda-Martos et al., 2011). The cytotoxic effect of phytochemicals has been reported to be a result of many mechanisms including the activation of the apoptosis-inducing enzymes (Caspases 3, 8, and 9) of cancer cell lines and the expression of death receptors (Khan et al., 2020; Guesmi et al., 2021). However, given the complexity of biological systems, it would be very difficult to extrapolate these data on Caper without resorting to animal and clinical experiments. Moreover, the potential synergetic effect between plant bioactive molecules should be considered as well as possible drug interactions and toxicity issues.
Similarly, findings on the neuroprotective effect of Caper extracts are promising but evidenced in vivo studies on their use in the treatment of Alzheimer’s disease, chronic neuropathic pain, and anticholinesterases activities are lacking. Although the traditional uses of Caper include gastrointestinal and diuretic activities, no in vitro nor in vivo studies are available to date. Thus, giving scientific impetus to the traditional uses of this plant through in vitro, in vivo, and clinical studies is still needed as it has large and promising applications in disease prevention and treatment.
Conclusions and Future Perspectives
Capers have been widely used in traditional medicine. It was reported as a good source of flavonoids, alkaloids, phenolic acids, fatty acids, and glucosinolates derivatives. Mostly, Caper is endowed with a plethora of notable biological activities mainly antibacterial, antioxidant, hepatoprotection, and anticancer. Additionally, it is an excellent candidate for the development of antidiabetic drugs. Although the large amount of literature on Caper is related to its health benefits, there are still no conclusive clinical studies regarding the association between the plant extracts/compounds and their effect on human health. Moreover, studies on the individual components of the plant are limited. Given the growing demand for natural, sustainable, and safe treatments, further studies are recommended to explore the diverse biological activities of the plant and its individual secondary metabolites both in vitro, in vivo, and through cell-based assays as well as animal studies. Noteworthily, a deep understanding of Caper chemotypes and cultivars would be essential for the selection of high-quality Caper genotypes that may be of interest for further pharmaceutical studies. Considering the phytochemical constituents and the data reported in this review, we recommend the bioprospection of Caper as a promising source of bioactive molecules to be tested in clinical experiments to evaluate their biosafety and clinical efficacy in modern pharmaceutical applications. Subsequently, Caper-based formulations should be characterized and tested with respective purposes.
Author Contributions
HA, YS, GB, and WB reviewed the literature and wrote the manuscript. BD, IM, and ME revised the manuscript. MS revised the manuscript and designed and conceived the work. All authors approved the final version.
Funding
The APC was funded by UM6P.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.878749/full#supplementary-material
References
Abd Al-Majeed, M. I., Al-Ghizawi, G. J., Dawwas, B. H. A. A. A., and Al-Maliki, M. (2016). Isolation and Identification of Alkaloidic Extract of Capparis spinosaL Buds and Study of its Cytoxicity and Antibacterial Activity. J. Nat. Sci. Res. 6 6.
Adwan, G. M., and Omar, G. I. (2021). Evaluation of Antimicrobial Activity and Genotoxic Potential of Capparis Spinosa (L.) Plant Extracts. Microbiol. Res. J. Int. 31 (1), 48–57. doi:10.9734/mrji/2021/v31i130297
Afsharypuor, S., Jeiran, K., and Jazy, A. A. (1998). First Investigation of the Flavour Profiles of the Leaf, Ripe Fruit and Root of Capparis Spinosa Var. Mucronifolia from Iran. Pharm. Acta Helvetiae 72 (5), 307–309. doi:10.1016/s0031-6865(97)00023-x
Aichour, R., Benzidane, N., Arrar, L., Charef, N., and Baghiani, A. (2018). Hepatoprotective and Anti-inflammatory Activities of Algerian Capparis Spinosa. L. Annu. Res. Rev. Biol. 25 (3), 1–12. doi:10.9734/arrb/2018/40410
Aksay, O., Selli, S., and Kelebek, H. (2021). LC-DAD-ESI-MS/MS-based Assessment of the Bioactive Compounds in Fresh and Fermented Caper (Capparis Spinosa) Buds and Berries. Food Chem. 337, 127959. doi:10.1016/j.foodchem.2020.127959
Ali Al-Nuani, R. M., and Kadhim, N. J. (2020). The Effect of Capparis Spinosa L. Plant on the Cytochrome and Glutathione to Reduce the Hepatotoxicity Induced by Paracetamol in Mice. J. Phys. Conf. Ser. 1664 (1), 178. doi:10.1088/1742-6596/1664/1/012121
Aliyazicioglu, R., Eyupoglu, O. E., Sahin, H., Yildiz, O., and Baltas, N. (2013). Phenolic Components , Antioxidant Activity , and Mineral Analysis of Capparis Spinosa L. Afr. J. Biotechnol. 12 (47), 6643–6649. doi:10.5897/AJB2013.13241
Ao, M. Z., Gao, Y-Y., and Yu, L. J. (2007). Advances in Studies on Constituents and Their Pharmacological Activities of Capparis Spinosa. Chin. Traditional Herb. Drugs 38 (3), 463.
Arean, A. G., Ali, T. H., and Muraih, J. K. (2019). Extracted Chemical Compounds from Capparis Spinosa Leaves and Their Antibacterial Activity on Pathogenic Bacteria. J. Pharm. Sci. Res. 11 (2), 603–608.
Arslan, D., and Ozcan, M. M. (2007). Effect of Some Organic Acids, Yoghurt, Starter Culture and Bud Sizes on the Chemical Properties of Pickled Caper Buds. J. Food Sci. Technol. 44 (1), 66.
Artemeva, M., Karryev, M., Meshcheryakov, A., and Gordienko, V. (1981). A New Flavonol Glycoside, Quercetin 7-O-Glucorhamnoside from Capparis Spinosa. Izk Akad. Nauk. Turk SSSR, Ser. Fizl Tekh. 3, 123.
Assadi, S., Shafiee, S. M., Erfani, M., and Akmali, M. (2021). Antioxidative and Antidiabetic Effects of Capparis Spinosa Fruit Extract on High-Fat Diet and Low-Dose Streptozotocin-Induced Type 2 Diabetic Rats. Biomed. Pharmacother. 138 (March), 111391. doi:10.1016/j.biopha.2021.111391
Aytac, Z., Kinaci, G., and Ceylan, A. (2009). Yield and Some Morphological Characteristics of Caper (Capparis Spinosa L.) Population Cultivated at Various Slopes in Aegean Ecological Conditions. Pak. J. Bot. 41 (2), 591–596.
Bakr, R. O., El Bishbishy, M. H., and Helmy, M. (2016). Profile of Bioactive Compounds of Capparis Spinosa Var. Aegyptiaca Growing in Egypt. Rev. Bras. Farmacogn. 26, 514–520. doi:10.1016/j.bjp.2016.04.001
Banerjee, P., Maity, S., Das, T., and Mazumder, S. (2011). A Double-Blind Randomized Placebo-Controlled Clinical Study to Evaluate the Efficacy and Safety of a Polyherbal Formulation in Geriatric Age Group: a Phase IV Clinical Report. J. Ethnopharmacol. 134 (2), 429–433. doi:10.1016/j.jep.2010.12.044
Benzidane, N., Aichour, R., Guettaf, S., Laadel, N., Khennouf, S., Baghiani, A., and Arrar, L. (2020). Chemical Investigation, the Antibacterial and Antifungal Activity of Different Parts of Capparis Spinosa Extracts. J. Drug Deliv. Ther. 10 (5), 118–125. doi:10.22270/jddt.v10i5.4388
Caboni, P., Sarais, G., Aissani, N., Tocco, G., Sasanelli, N., Liori, B., Carta, A., and Angioni, A. (2012). Nematicidal Activity of 2-thiophenecarboxaldehyde and Methylisothiocyanate from Caper (Capparis Spinosa) against Meloidogyne incognita. J. Agric. Food Chem. 60 (30), 7345–7351. doi:10.1021/jf302075w
Çaliş, İ., Kuruüzüm, A., and Rüedi, P. (1999). 1H-Indole-3 Acetonitrile Glycosides from Capparis Spinosa Fruits. Phytochemistry 50 (7), 1205–1208. doi:10.1016/S0031-9422(98)00669-4
Çalış, İ., Kuruüzüm-Uz, A., Lorenzetto, P. A., and Rüedi, P. (2002). (6S)-Hydroxy-3-oxo-α-ionol Glucosides from Capparis Spinosa Fruits. Phytochemistry 59 (4), 451–457. doi:10.1016/S0031-9422(01)00399-5
Daina, A., Michielin, O., and Zoete, V. (2017). SwissADME: a Free Web Tool to Evaluate Pharmacokinetics, Drug-Likeness and Medicinal Chemistry Friendliness of Small Molecules. Sci. Rep. 7 (1), 42717. doi:10.1038/srep42717
Dinesha, R., and Chikkanna, D. (2014). Antioxidant Activities of Pippali (Piper Longum) Proteins. Indian J. Pharm. Drug Anal. 2, 811–814.
Ebrahimi, K., Shiravand, S., and Mahmoudvand, H. (2017). Biosynthesis of Copper Nanoparticles Using Aqueous Extract of Capparis Spinosa Fruit and Investigation of its Antibacterial Activity. Marmara Pharm. J. 21 (4), 866–871. doi:10.12991/mpj.2017.31
Ebrahimi, K., Madani, M., Ashrafi, B., Shiravand, S., and Sepahvand, A. (2019). Antifungal Properties of Silver Nanoparticles Synthe sized from Capparis Spinosa Fruit. Res. Mol. Med. 98 (916), 43–50. doi:10.32598/rmm.7.4.43
Eddouks, M., Maghrani, M., Lemhadri, A., Ouahidi, M. L., and Jouad, H. (2002). Ethnopharmacological Survey of Medicinal Plants Used for the Treatment of Diabetes Mellitus, Hypertension and Cardiac Diseases in the South-East Region of Morocco (Tafilalet). J. Ethnopharmacol. 82 (2-3), 97–103. doi:10.1016/s0378-8741(02)00164-2
Eddouks, M., Lemhadri, A., and Michel, J. B. (2004). Caraway and Caper: Potential Anti-hyperglycaemic Plants in Diabetic Rats. J. Ethnopharmacol. 94 (1), 143–148. doi:10.1016/j.jep.2004.05.006
Eddouks, M., Lemhadri, A., Hebi, M., El Hidani, A., Zeggwagh, N. A., El Bouhali, B., Hajji, L., and Burcelin, R. (2017). Capparis spinosa L. Aqueous Extract Evokes Antidiabetic Effect in Streptozotocin-Induced Diabetic Mice. Avicenna J. Phytomed 7 (2), 191–198.
El amri, N., Errachidi, F., Bour, A., and Chabir, R. (2019). Characterization of Moroccan Raw and Processed Caper Berries. Mater. Today Proc. 13, 841–849. doi:10.1016/j.matpr.2019.04.047
El Azhary, K., Jouti, N. T., Khachibi, M. E., Moutia, M., Tabyaoui, I., Hou, A. E., et al. (2017). Anti-inflammatory Potential of Capparis Spinosa L . In Vivo in Mice through Inhibition of Cell Infiltration and Cytokine Gene Expression. BMC Complement. Altern. Med. 17 (1), 18. doi:10.1186/s12906-017-1569-7
El-Hawary, S. S., Taha, K. F., Kirillos, F. N., Dahab, A. A., El-Mahis, A. A., and El-Sayed, S. H. (2018). Complementary Effect of Capparis Spinosa L. And Silymarin With/without Praziquantel on Mice Experimentally Infected with Schistosoma Mansoni. Helminthologia 55 (1), 21–32. doi:10.1515/helm-2017-0055
El-Hossary, E. M., Abdel-Halim, M., Ibrahim, E. S., Pimentel-Elardo, S. M., Nodwell, J. R., Handoussa, H., Abdelwahab, M. F., Holzgrabe, U., and Abdelmohsen, U. R. (2020). Natural Products Repertoire of the Red Sea. Mar. Drugs 18 (9), 457. doi:10.3390/md18090457
Eltawaty, S. I., Omare, M. A., Almagboul, A. Z., Tarig, M., Mohammad, A. E., and Bofarwa, S. M. (2018). The Potential Antioxidant and Hepatotoxicity of Methanolic Extract of Leaves of Libyan capparis Spinosa Subsp Orientalis (duh.) Jafri in Rats. World J. Pharm. Res. 7 (5), 101–112. doi:10.20959/wjpr20185-11274
Ennacerie, F., Moukrad, N., Filali, F., Moukrad, N., and Dra., A. (2017). Antibacterial Synergistic Effect of Extracts of the Organs of capparis Spinosa and in Combination with Antibiotics. Int. J. Adv. Res. 5 (9), 1238–1247. doi:10.21474/ijar01/5445
Ezzeddine, S., Abdelhamid, K., and Larbi, K. M. (2007). Etude de la variabilité morphologique du câprier (Capparis spp.) en Tunisie. Rev. Des. régions Arid., 523–527.
Facciola, S. (1990). Cornucopia: A Source Book of Edible Plants. United States, US:Kampong publications.
Feng, X., Lu, J., Xin, H., Zhang, L., Wang, Y., and Tang, K. (2011). Anti-arthritic Active Fraction of Capparis Spinosa L. Fruits and its Chemical Constituents. Yakugaku Zasshi 131 (3), 423–429. doi:10.1248/yakushi.131.423
Fici, S. (2014). A Taxonomic Revision of the Capparis Spinosa Group (Capparaceae) from the Mediterranean to Central Asia. Phytotaxa 174 (1), 1–24. doi:10.11646/phytotaxa.174.1.1
Fici, S. (2015). A Taxonomic Revision of the Capparis Spinosa Group (Capparaceae) from Eastern Africa to Oceania. Phytotaxa 203 (1), 24–36. doi:10.11646/phytotaxa.203.1.2
Fu, X. P., Aisa, H. A., Abdurahim, M., Yili, A., Aripova, S. F., and Tashkhodzhaev, B. (2007). Chemical Composition of Capparis Spinosa Fruit. Chem. Nat. Compd. 43 (2), 181–183. doi:10.1007/s10600-007-0074-5
Fu, X. P., Wu, T., Abdurahim, M., Su, Z., Hou, X. L., and Aisa, H. A. (2008). New Spermidine Alkaloids from Capparis spinosa Roots. Phytochemi. Lett.. 1 (1), 59–62. doi:10.1016/j.phytol.2008.01.001
Gadgoli, C., and Mishra, S. H. (1999). Antihepatotoxic Activity of P-Methoxy Benzoic Acid from Capparis Spinosa. J. Ethnopharmacol. 66 (2), 187–192. doi:10.1016/s0378-8741(98)00229-3
Gamble, J. (1972). Manual of Indian Timbers. 2nd edn. Dehradun, India: Bishen Singh Mahendra Pal Singh.
Geng, D. S., Wu, J., and Liang, J. (2007). Chemical Constituents and Pharmacological Activity of Capparis Spinosa Linn. Jiefangjun Yaoxue Xuebao 23 (5), 369–371.
Geng, J., Wang, L., Zhang, L., Qin, C., Song, Y., Ma, Y., Chen, Y., Chen, S., Wang, Y., Zhang, Z., and Yang, G. Y. (2018). Blood-brain Barrier Disruption Induced Cognitive Impairment Is Associated with Increase of Inflammatory Cytokine. Front. Aging Neurosci. 10, 129. doi:10.3389/fnagi.2018.00129
Ghafoor, K., Juhaimi, F. A., Özcan, M. M., Uslu, N., Babiker, E. E., and Ahmed, I. A. M. (2020). Bioactive Properties and Phenolic Compounds in Bud, Sprout, and Fruit of Capparis Spp. Plants. J. Food Process. Preserv. 44 (3), e14357. doi:10.1111/jfpp.14357
Goel, A., Garg, A., and Kumar, A. (2016). Effect of Capparis Spinosa Linn. Extract on Lipopolysaccharide-Induced Cognitive Impairment in Rats.
Guesmi, F., Prasad, S., Ali, M. B., Ismail, I. A., and Landoulsi, A. (2021). Thymus Hirtus Sp. Algeriensis Boiss. And Reut. Volatile Oil Enhances TRAIL/Apo2L Induced Apoptosis and Inhibits Colon Carcinogenesis through Upregulation of Death Receptor Pathway. Aging (Albany NY) 13 (18, 21990), 21975. doi:10.18632/aging.203552
Gull, T., Sultana, B., Bhatti, I. A., and Jamil, A. (2015). Antibacterial Potential of Capparis Spinosa and Capparis Decidua Extracts. Int. J. Adv. Biol. 17, 727–733. doi:10.17957/ijab/14.0007
Hameed, A. T., Zaidan, D. H., and Dawd, S. M. (2021). The Phytochemical Constituent of Capparis Spinosa L . And Phenolic Activity on Pathogenic Bacteria and Blood Parameters. Syst. Rev. Pharm. 12 (1), 1193–1198.
Hashemnia, M., Oryan, A., Hamidi, A. R., and Mohammadalipour, A. (2012). Blood Glucose Levels and Pathology of Organs in Alloxan-Induced Diabetic Rats Treated with Hydro-Ethanol Extracts of Allium Sativum and Capparis Spinosa. Afr. J. Pharm. Pharmacol. 6 (21), 1559–1564. doi:10.5897/ajpp12.330
Heidari, M., Mirshamsi, M., Naghibi, B., and Vafazade, J. (2010). Evaluation of Hepatotoxicity and Renal Toxicity of Methanolic Extract of capparis Spinosa in Rats. SSU_Journals 18 (1), 47–55.
Hooper, D., and Field, H. (1937). Useful plants and drugs of Iran and Iraq/by David Hooper. With notes by Henry Field. doi:10.5962/bhl.title.2281
Hu, D., Zhang, S., Li, M., and Wu, X. (2017). Chemical Constituents of Capparis Spinosa. Chem. Nat. Compd. 53 (3), 557–558. doi:10.1007/s10600-017-2048-6
Huseini, H. F., Hasani-Rnjbar, S., Nayebi, N., Heshmat, R., Sigaroodi, F. K., Ahvazi, M., Alaei, B. A., and Kianbakht, S. (2013). Capparis Spinosa L. (Caper) Fruit Extract in Treatment of Type 2 Diabetic Patients: a Randomized Double-Blind Placebo-Controlled Clinical Trial. Complement. Ther. Med. 21 (5), 447–452. doi:10.1016/j.ctim.2013.07.003
Hussain, J., Sarhan, H., and Bassal, M. (2017). Effect of Syrian Capparis Spinosa Leave Extract on Alloxan-Induced Diabetes in Rats. Int. J. Pharm. Phytopharm. Res. 7, 31–43.
Issac Abraham, S. V., Palani, A., Ramaswamy, B. R., Shunmugiah, K. P., and Arumugam, V. R. (2011). Antiquorum Sensing and Antibiofilm Potential of Capparis Spinosa. Arch. Med. Res. 42 (8), 658–668. doi:10.1016/j.arcmed.2011.12.002
Jacobs, M. (1964). The Genus Capparis (Capparaceae) from the Indus to the Pacific. Blumea Biodivers. Evol. Biogeogr. Plants 12 (3), 385–541.
Jalali, M. T., Mohammadtaghvaei, N., and Larky, D. A. (2016). Investigating the Effects of Capparis Spinosa on Hepatic Gluconeogenesis and Lipid Content in Streptozotocin-Induced Diabetic Rats. Biomed. Pharmacother. 84, 1243–1248. doi:10.1016/j.biopha.2016.10.061
Ji, Y., Guo, S., and Ji, C. (2006). Progress of Study on Capparis Spinosa L. J. Harbin Univ. Commer. 22, 5–10.
Jiménez-López, J., Ruiz-Medina, A., Ortega-Barrales, P., and Llorent-Martínez, E. J. (2018). Phytochemical Profile and Antioxidant Activity of Caper Berries (Capparis Spinosa L.): Evaluation of the Influence of the Fermentation Process. Food Chem. 250, 54–59. doi:10.1016/j.foodchem.2018.01.010
Jouad, H., Haloui, M., Rhiouani, H., El Hilaly, J., and Eddouks, M. (2001). Ethnobotanical Survey of Medicinal Plants Used for the Treatment of Diabetes, Cardiac and Renal Diseases in the North Centre Region of Morocco (Fez-Boulemane). J. Ethnopharmacol. 77 (2-3), 175–182. doi:10.1016/s0378-8741(01)00289-6
Kalantari, H., Foruozandeh, H., Khodayar, M. J., Siahpoosh, A., Saki, N., and Kheradmand, P. (2018). Antioxidant and Hepatoprotective Effects of Capparis Spinosa L. Fractions and Quercetin on Tert-Butyl Hydroperoxide- Induced Acute Liver Damage in Mice. J. Tradit. Complement. Med. 8 (1), 120–127. doi:10.1016/j.jtcme.2017.04.010
Kalantari, H., Alijani, A., Kheradmand, P., Goodarzian, M., and Zeidooni, L. (2019). Hydroalcoholic Extract of Iranian Caper Leaves Protects Hepatic Toxicity by Suppressing Oxidative Stress in Mice. Pharm. Biomed. Res. 5 (3), 8–14. doi:10.18502/pbr.v5i3.2112
Kazemian, M., Abad, M., Haeri, M. R., Ebrahimi, M., and Heidari, R. (2015). Anti-diabetic Effect of Capparis Spinosa L. Root Extract in Diabetic Rats. Avicenna J. Phytomed 5 (4), 325–332.
Kernouf, N., Bouriche, H., Kada, S., Messaoudi, D., Assaf, A., and Senator, A. (2018). Anti-Inflammatory and Immuno-Modulatory Effects of Capparis Spinosa Flower Bud Extract. Annu. Res. Rev. Biol. 25 (3), 1–11. doi:10.9734/arrb/2018/40189
Khorrami, M. B., Abbassian, H., and Forouzanfar, F. (2021). Hypnotic Activity of Capparis spinosa Hydro-Alcoholic Extract in Mice. Recent Pat. food, Nutr. Agric. 12 (1), 58–62. doi:10.2174/2212798411666200727151142
Khan, T., Ali, M., Khan, A., Nisar, P., Jan, S. A., Afridi, S., and Shinwari, Z. K. (2020). Anticancer Plants: A Review of the Active Phytochemicals, Applications in Animal Models, and Regulatory Aspects. Biomolecules 10 (1), 47. doi:10.3390/biom10010047
Khatib, M., Pieraccini, G., Innocenti, M., Melani, F., and Mulinacci, N. (2016). An Insight on the Alkaloid Content of Capparis Spinosa L. Root by HPLC-DAD-MS, MS/MS and (1)H qNMR. J. Pharm. Biomed. Anal. 123, 53–62. doi:10.1016/j.jpba.2016.01.063
Kuete, V., Krusche, B., Youns, M., Voukeng, I., Fankam, A. G., Tankeo, S., Lacmata, S., and Efferth, T. (2011). Cytotoxicity of Some Cameroonian Spices and Selected Medicinal Plant Extracts. J. Ethnopharmacol. 134 (3), 803–812. doi:10.1016/j.jep.2011.01.035
Ladhari, A., Omezzine, F., Dellagreca, M., Zarrelli, A., and Haouala, R. (2013a). Phytotoxic Activity of Capparis Spinosa L. And its Discovered Active Compounds. Allelopathy J. 32 (2), 175–190.
Ladhari, A., Omezzine, F., Chaieb, I., and Haouala, R. (2013b). Antifeeding and Insecticidal Effects of Capparis Spinosa L. On Spodoptera Littoralis (Boisduval) Larvae. Afr. J. Agric. Res. 8 (42), 5232–5238.
Li, M., Liu, W., Gan, L., Wang, Y., and Yu, L. (2008). Progress on the Botanical Characteristic of Capparis Spinosa L. Xiandai Shengwuyixue Jinzhan 8 (11), 2194-7–2178.
Li, W. L., Yu, L., and Ji, Y. B. (2014). Chemical Constituents of n-Butanol Extract of Capparis spinosa L. 711. Asian J. Chem. 26 (11), 3435. doi:10.7314/apjcp.2014.15.21.9153
Lin, T., Liu, G. A., Perez, E., Rainer, R. D., Febo, M., Cruz-Almeida, Y., and Ebner, N. C. (2018). Systemic Inflammation Mediates Age-Related Cognitive Deficits. Front. Aging Neurosci. 10, 236. doi:10.3389/fnagi.2018.00236
Mahboubi, M., and Mahboubi, A. (2014). Antimicrobial Activity of Capparis Spinosa as its Usages in Traditional Medicine. Herba Pol. 60 (1), 39. doi:10.2478/hepo-2014-0004
Manikandaselvi, S., and Brindha, P. (2014). Chemical Standardization Studies on Capparis Spinosa L. Int. J. Pharm. Pharm. Sci. 6 (Suppl. 1), 47–54.
Manikandaselvi, S., Vadivel, V., and Brindha, P. (2016). Review on Ethnobotanical Studies of Nutraceutical Plant: Capparis Spinosa L.(Caper). Asian J. Pharm. Clin. Res. 9 (3), 123–126. https://innovareacademics.in/journals/index.php/ajpcr/article/view/10448.
Mansour, R. B., Jilani, I. B. H., Bouaziz, M., Gargouri, B., Elloumi, N., Attia, H., Ghrabi-Gammar, Z., and Lassoued, S. (2016). Phenolic Contents and Antioxidant Activity of Ethanolic Extract of Capparis Spinosa. Cytotechnology 68 (1), 135–142. doi:10.1007/s10616-014-9764-6
Mehrzadi, S., Mirzaei, R., Heydari, M., Sasani, M., Yaqoobvand, B., and Huseini, H. F. (2020). Efficacy and Safety of a Traditional Herbal Combination in Patients with Type II Diabetes Mellitus: A Randomized Controlled Trial. J. Diet. Suppl. 0 (0), 1–13. doi:10.1080/19390211.2020.1727076
Meiliwan, A., Jiang, L., and Aisa, H. (2008). Determination of Glucocapparin in Capparis Spinosa L. 19, 2084–2085.
Metcalfe, S. E. (2006). Late Quaternary Environments of the Northern Deserts and Central Transvolcanic Belt of Mexico1. Ann. Mo. Botanical Gard. 93 (2), 258–273. doi:10.3417/0026-6493(2006)93[258:lqeotn]2.0.co;2
Mickymaray, S., and Al Aboody, M. S. (2019). In vitro Antioxidant and Bactericidal Efficacy of 15 Common Spices: Novel Therapeutics for Urinary Tract Infections. Medicina 55 (6), 289. doi:10.3390/medicina55060289
Mingzhang, A., Gao, Y., and Longjiang, Y. (1994). Advances in Studies on Constituents and Their Pharmacological Activities of Capparis Spinosa, 03. China: Chinese Traditional and Herbal Drugs.
Miraldi, E., Ferri, S., and Mostaghimi, V. (2001). Botanical Drugs and Preparations in the Traditional Medicine of West Azerbaijan (Iran). J. Ethnopharmacol. 75 (2-3), 77–87. doi:10.1016/s0378-8741(00)00381-0
Mirzakhani, N., Farshid, A. A., Tamaddonfard, E., Tehrani, A., and Imani, M. (2020). “Comparison of the Effects of Hydroalcoholic Extract of Capparis Spinosa Fruit, Quercetin and Vitamin E on Monosodium Glutamate-Induced Toxicity in Rats,” in Faculty of Veterinary Medicine (Urmia, Iran: Urmia University), 127.
Moghadamnia, Y., Mousavi Kani, S. N., Ghasemi-Kasman, M., Kazemi Kani, M. T., and Kazemi, S. (2019). The Anti-cancer Effects of Capparis Spinosa Hydroalcoholic Extract. Avicenna J. Med. Biotechnol. 11 (1), 43–47.
Mohebali, N., Shahzadeh Fazeli, S. A., Ghafoori, H., Farahmand, Z., MohammadKhani, E., Vakhshiteh, F., et al. (2018). Effect of Flavonoids Rich Extract of Capparis Spinosa on Inflammatory Involved Genes in Amyloid-Beta Peptide Injected Rat Model of Alzheimer's Disease. Nutr. Neurosci. 21 (2), 143–150. doi:10.1080/1028415X.2016.1238026
Mollica, A., Zengin, G., Locatelli, M., Stefanucci, A., Mocan, A., Macedonio, G., et al. (2017). Anti-diabetic and Anti-hyperlipidemic Properties of Capparis Spinosa L.: In Vivo and In Vitro Evaluation of its Nutraceutical Potential. J. Funct. Foods 35, 32–42. doi:10.1016/j.jff.2017.05.001
Mollica, A., Stefanucci, A., Macedonio, G., Locatelli, M., Luisi, G., Novellino, E., and Zengin, G. (2019). Chemical Composition and Biological Activity of Capparis Spinosa L. From Lipari Island. South Afr. J. Bot. 120, 135–140. doi:10.1016/j.sajb.2018.02.397
Moufid, A., Farid, O., and Eddouks, M. (2015). Pharmacological Prop-Erties of Capparis Spinosa Linn. Int. J. Diabetol. Vasc. Dis. Res. 3 (5), 99–104. doi:10.19070/2328-353X-1500020
Mukhamedova, K. S., Akramov, S. T., and Yunusov, S. Y. (1969). Stachydrine fromCapparis Spinosa. Chem. Nat. Compd. 5 (1), 60. doi:10.1007/bf00564943
Musallam, I., Duwayri, M., Shibli, R., and Alali, F. (2012). Investigation of Rutin Content in Different Plant Parts of Wild Caper (Capparis Spinosa L.) Populations from Jordan. Res. J. Med. Plant 6 (1), 27–36. doi:10.3923/rjmp.2012.27.36
Nabavi, S. F., Maggi, F., Daglia, M., Habtemariam, S., Rastrelli, L., and Nabavi, S. M. (2016). Pharmacological Effects of Capparis Spinosa L. Phytother. Res. 30 (11), 1733–1744. doi:10.1002/ptr.5684
Niaz, A., Ullah, N., Rehman, A., Ahmad, I., Ikhlaq, M., and Rehman, H. U. (2013). Pollution Based Study of Heavy Metals in Some Selected Medicinal Plants by Dry Digestion Method. Int. J. Pharma Sci. Res. 4 (2), 17–24.
Oudah, S. K., Al-Salih, R. M., and Gusar, S. H. (2014). Study the Role of Polyphenolic Extract of Capparis spinosa L. Leaves as a Hypoglycemic Agent. Int. J. SciEng Res. 5 (5), 1561–1575.
Oudah hamam, K., AL-Salih, R. M., Gusar, S. H., and Roomi, A. B. (2019). Study of the Role of Polyphenolic Extract of capparis Spinosa L. Leaves as Acute Toxicity and Antibacterial Agent. Plant Arch. 19, 3821–3829.
Özcan, M., Hacıseferoğulları, H., and Demi̇r, F. (2004). Some Physico-Mechanic and Chemical Properties of Capers (Capparis Ovata Desf. Var. Canescens (Coss.) Heywood) Flower Buds. J. Food Eng. 65 (1), 151–155. doi:10.1016/j.jfoodeng.2004.01.006
Rahimi, V. B., Rajabian, A., Rajabi, H., Vosough, E. M., Mirkarimi, H. R., Hasanpour, M., et al. (2020). The Effects of Hydro-Ethanolic Extract of Capparis Spinosa (C. Spinosa) on Lipopolysaccharide (LPS)-induced Inflammation and Cognitive Impairment: Evidence from In Vivo and In Vitro Studies. J. Ethnopharmacol. 256, 112706. doi:10.1016/j.jep.2020.112706
Rahman, K. (2007). Studies on Free Radicals, Antioxidants, and Co-factors. Clin. Interv. Aging 2 (2), 219–236.
Rahmani, R., Mahmoodi, M., Karimi, M., Hoseini, F., Heydari, R., Salehi, M., et al. (2013). Effect of Hydroalcoholic Extract of Capparis Spinosa Fruit on Blood Sugar and Lipid Profile of Diabetic and Normal Rats. Zahedan J. Res. Med. Sci. 15 (11), 34–38.
Rahnavard, R., and Razavi, N. (2017). A Review on the Medical Effects of Capparis Spinosa L. Adv. Herb. Med. 3 (1), 44–53.
Rakhshandeh, H., Rashidi, R., Vahedi, M. M., Khorrami, M. B., Abbassian, H., and Forouzanfar, F. (2020). Hypnotic Activity of Capparis Spinosa Hydro-Alcoholic Extract in Mice. Recent Pat. Food Nutr. Agric., 11. doi:10.2174/2212798411666200727151142
Ramezani, Z., Aghel, N., and Keyghobadi, H. (2008). Rutin from Different Parts of Capparis Spinosa Growing Wild in Khuzestan/Iran. Pak J. Biol. Sci. 11 (5), 768–772. doi:10.3923/pjbs.2008.768.772
Razaq, A., Ahmed, E., Sharif, A., Rasool, M. A., Arshad, M., Anjum, M. I., et al. (2017). Lipoxygenase Inhibiting Two New Sterols from Capparis Spinosa. J. Chem. Soc. Pak. 39 (6), 1080.
Reyahi-Khoram, M., and Reyahi-Khoram, R. (2018). Early Feasibility Study on Capparis Production and Processing in Hamedan Province in Iran. J. Adv. Agric. Technol. 5 (4), 313. doi:10.18178/joaat.5.4.313-317
Rivera, D., Inocencio, C., Obón, C., Carreño, E., Reales, A., and Alcaraz, F. (2002). Archaeobotany of Capers ( Capparis ) (Capparaceae). Veg. Hist. Archaeobotany 11 (4), 295–314. doi:10.1007/s003340200042
Rivera, D., Inocencio, C., Obón, C., and Alcaraz, F. (2003). Review of Food and Medicinal Uses of Capparis L. Subgenus Capparis (Capparidaceae). Econ. Bot. 57 (4), 515–534. doi:10.1663/0013-0001(2003)057[0515:rofamu]2.0.co;2
Rodrigo, M., Lazaro, M. J., Alvarruiz, A., and Giner, V. (1992). Composition of Capers (Capparis Spinosa): Influence of Cultivar, Size and Harvest Date. J Food Sci. 57 (5), 1152–1154. doi:10.1111/j.1365-2621.1992.tb11286.x
Romeo, V., Ziino, M., Giuffrida, D., Condurso, C., and Verzera, A. (2007). Flavour Profile of Capers (Capparis Spinosa L.) from the Eolian Archipelago by HS-SPME/GC-MS. Food Chem. 101 (3), 1272–1278. doi:10.1016/j.foodchem.2005.12.029
Saadaoui, E., Guetat, A., Tlili, N., El Gazzah, M., and Khaldi, A. (2011). Subspecific Variability of Tunisian Wild Populations of Capparis Spinosa L. J. Med. Plants Res. 5 (17), 4339–4348.
Saadaoui, E., Gómez, J. J. M., and Cervantes, E. (2013). Intraspecific Variability of Seed Morphology in Capparis Spinosa L. Acta Biol. Cracoviensia Ser. Bot. 55 (2), 1. doi:10.2478/abcsb-2013-0027
Sadykov, L., Yu, D., and Khodzhimatov, M. (1981). Alkaloids of Capparis Spinosa. Dokl. Akad. Nauk. Tadzhikskoj SSR 24 (10), 617–620.
Saleem, H., Khurshid, U., Sarfraz, M., Ahmad, I., Alamri, A., Anwar, S., et al. (2021). Investigation into the Biological Properties, Secondary Metabolites Composition, and Toxicity of Aerial and Root Parts of Capparis Spinosa L.: An Important Medicinal Food Plant. Food Chem. Toxicol. 155, 112404. doi:10.1016/j.fct.2021.112404
Satyanarayana, T., Anjana, A., and Vijetha, P. (2008). PHCOG REV.: Plant Review Phytochemical and Pharmacological Review of Some Indian Capparis Species. Pharmacogn. Rev. 2 (4), 36–45.
Schraudolf, H. (1989). Indole Glucosinolates of Capparis Spinosa. Phytochemistry 28 (1), 259–260. doi:10.1016/0031-9422(89)85051-4
Sharaf, M., El-Ansari, M. A., and Saleh, N. A. (2000). Quercetin Triglycoside from Capparis Spinosa. Fitoterapia 71 (1), 46–49. doi:10.1016/s0367-326x(99)00116-1
Sharma, M. M., and Sharma, R. K. (2012). “Coriander,” in Handbook of Herbs and Spices [Internet] (Elsevier), 216–249. doi:10.1533/9780857095671.216
Sher, H., and Alyemeni, M. N. (2010). Ethnobotanical and Pharmaceutical Evaluation of Capparis Spinosa L, Validity of Local Folk and Unani System of Medicine. J. Med. Plants Res. 4 (17), 1751–1756.
Sher, H., ALMutairi, K., and Mansoor, M. (2012). Study on the Ethnopharmaceutical Values and Traditional Uses of Capparis Spinosa L. Afr. J. Pharm. Pharmacol. 6 (16), 1255–1259. doi:10.5897/ajpp10.160
Sherif, M. M., El-Shiekh, H. H., Elaasser, M. M., and Elbadry, M. (2013). In Vitro evaluation of Antimicrobial and Cytotoxic Effects of Caper (Capparis Spinosa). J. Appl. Sci. Res, 9 4, 2944-2950.
Sobeh, M., Mahmoud, M. F., Hasan, R. A., Abdelfattah, M. A. O., Osman, S., Rashid, H. O., et al. (2019). Chemical Composition, Antioxidant and Hepatoprotective Activities of Methanol Extracts from Leaves of Terminalia Bellirica and Terminalia Sericea (Combretaceae). PeerJ 7, e6322. doi:10.7717/peerj.6322
Sobeh, M., Hamza, M. S., Ashour, M. L., Elkhatieb, M., El Raey, M. A., Abdel-Naim, A. B., et al. (2020a). A Polyphenol-Rich Fraction from Eugenia Uniflora Exhibits Antioxidant and Hepatoprotective Activities In Vivo. Pharm. (Basel) 13 (5), 84. doi:10.3390/ph13050084
Sobeh, M., Rezq, S., Cheurfa, M., Abdelfattah, M. A. O., Rashied, R. M. H., El-Shazly, A. M., et al. (2020b). Thymus Algeriensis and Thymus Fontanesii: Chemical Composition, In Vivo Antiinflammatory, Pain Killing and Antipyretic Activities: A Comprehensive Comparison. Biomolecules 10 (4), 599. doi:10.3390/biom10040599
Sonmezdag, A. S., Kelebek, H., and Selli, S. (2019). Characterization of Aroma-Active Compounds, Phenolics, and Antioxidant Properties in Fresh and Fermented Capers (Capparis Spinosa) by GC-MS-Olfactometry and LC-DAD-ESI-MS/MS. J. Food Sci. 84, 2449–2457. doi:10.1111/1750-3841.14777
Taghavi, M., Nazari, M., Rahmani, R., Sayadi, A., Hajizadeh, M., Mirzaei, M., et al. (2014). Outcome of Capparisspinosa Fruit Extracts Treatment on Liver, Kidney, Pancreas and Stomach Tissues in Normal and Diabetic Rats. Med. Chem. 4, 717–721. doi:10.4172/2161-0444.1000218
Tawfeek, N., Mahmoud, M. F., Hamdan, D. I., Sobeh, M., Farrag, N., Wink, M., et al. (2021). Phytochemistry, Pharmacology and Medicinal Uses of Plants of the Genus Salix: An Updated Review. Front. Pharmacol. 12, 593856. doi:10.3389/fphar.2021.593856
Tir, M., Feriani, A., Labidi, A., Mufti, A., Saadaoui, E., Nasri, N., et al. (2019). Protective Effects of Phytochemicals of Capparis Spinosa Seeds with Cisplatin and CCl4 Toxicity in Mice. Food Biosci. 28, 42–48. doi:10.1016/j.fbio.2019.01.002
Tlili, N., Munne-bosch, S., Nasri, N., Saadaoui, E., Khaldi, A., and Triki, S. D. (2009). Fatty Acids, Tocopherols and Carotenoids from Seeds of Tunisian Caper  Œcapparis Spinosaâ. J. Food Lipids 16 (4), 452–464. doi:10.1111/j.1745-4522.2009.01158.x
Tlili, N., Khaldi, A., Triki, S., and Munné-Bosch, S. (2010). Phenolic Compounds and Vitamin Antioxidants of Caper (Capparis Spinosa). Plant Foods Hum. Nutr. 65 (3), 260–265. doi:10.1007/s11130-010-0180-6
Tlili, N., Nasri, N., Khaldi, A., Triki, S., and Munné-bosch, S. (2011). Phenolic Compounds, Tocopherols, Carotenoids and Vitamin C of Commercial Caper. J. Food Biochem. 35 (2), 472–483. doi:10.1111/j.1745-4514.2010.00396.x
Tlili, N., Mejri, H., Anouer, F., Saadaoui, E., Khaldi, A., and Nasri, N. (2015). Phenolic Profile and Antioxidant Activity of Capparis Spinosa Seeds Harvested from Different Wild Habitats. Industrial Crops Prod. 76, 930–935. doi:10.1016/j.indcrop.2015.07.040
Tlili, N., Feriani, A., Saadoui, E., Nasri, N., and Khaldi, A. (2017). Capparis Spinosa Leaves Extract: Source of Bioantioxidants with Nephroprotective and Hepatoprotective Effects. Biomed. Pharmacother. 87, 171–179. doi:10.1016/j.biopha.2016.12.052
Turgut, N. H., Kara, H., Arslanbaş, E., Mert, D. G., Tepe, B., and Güngör, H. (2015). Effect of Capparis Spinosa L. On Cognitive impairment Induced by D-Galactose in Mice via Inhibition of Oxidative Stress. Turk J. Med. Sci. 45 (5), 1127–1136. doi:10.3906/sag-1405-95
Tutin, T. G., Heywood, V. H., Burges, N. A., and Valentine, D. H. (1976). Flora Europaea: Plantaginaceae to Compositae (And Rubiaceae), 4. London: Cambridge University Press.
Ulukapi, K., Özdemir, B., Kulcan, A. A., Tetik, N., Ertekin, C., and Onus, A. N. (2016). Evaluation of Biochemical and Dimensional Properties of Naturally Grown capparis Spinosa Var. Spinosa and capparis Ovata Var. Palaestina. Int. J. Agric. Innovations Res. 5 (2), 2319–1473.
Vahid, H., Rakhshandeh, H., and Ghorbani, A. (2017). Antidiabetic Properties of Capparis Spinosa L. And its Components. Biomed. Pharmacother. 92, 293–302. doi:10.1016/j.biopha.2017.05.082
Vaidya, V., Shinde, M., and Pradhan, P. (2017). Proximate Analysis and Heavy Metal Determination of Leaf of Capparis Spinosa L. Int. J. Res. Pharm. Chem. 7 (4), 382–386.
Viuda-Martos, M., Mohamady, M. A., Fernández-López, J., Abd ElRazik, K. A., Omer, E. A., Pérez-Alvarez, J. A., and Sendra, E. (2011). In Vitro antioxidant and Antibacterial Activities of Essentials Oils Obtained from Egyptian Aromatic Plants. Food control. 22 (11), 1715–1722. doi:10.1016/j.foodcont.2011.04.003
Xie, L. q., Xue, S. y., Yan, X., and Tian, C. (2007). Chemical Constituents and Pharmacological Study of Uygur Herb Medicine Capparis Spinosa L. Xibei Yaoxue Zazhi 22 (4).
Yahia, Y., Benabderrahim, M. A., Tlili, N., Hannachi, H., Ayadi, L., and Elfalleh, W. (2020). Comparison of Three Extraction Protocols for the Characterization of Caper ( Capparis Spinosa L .) Leaf Extracts : Evaluation of Phenolic Acids and Flavonoids by Liquid Chromatography – Electrospray Ionization – Tandem Mass Spectrometry ( LC – ESI – MS ). Anal. Lett. 0 (0), 1–12. doi:10.1080/00032719.2019.1706546
Yang, T., Liu, Y. Q., Wang, C. H., and Wang, Z. T. (2008). Advances on Investigation of Chemical Constituents, Pharmacological Activities and Clinical Applications of Capparis Spinosa. Zhongguo Zhong Yao Za Zhi 33 (21), 2453–2458.
Yang, T., Wang, C.-h., Chou, G.-x., Wu, T., Cheng, X.-m., and Wang, Z.-t. (2010a). New Alkaloids from Capparis Spinosa: Structure and X-Ray Crystallographic Analysis. Food Chem. 123 (3), 705–710. doi:10.1016/j.foodchem.2010.05.039
Yang, T., Wang, C., Liu, H., Chou, G., Cheng, X., and Wang, Z. (2010b). A New Antioxidant Compound from Capparis Spinosa. Pharm. Biol. 48 (5), 589–594. doi:10.3109/13880200903214231
Yu, Y., Gao, H., Tang, Z., Song, X., and Wu, L. (2006). Several Phenolic Acids from the Fruit of Capparis Spinosa. 亚洲传统医药. 1 (2), 101–104.
Yu, L., Xie, L, and Ji, Y. (2010). Preliminary Study on Apoptotic Effect Induced by N-Butanol Extract in Capparis spinosa L. on SGC-7901," in 2010 4th International Conference on Bioinformatics and Biomedical Engineering. IEEE, , 1–4. doi:10.1109/icbbe.2010.5516478
Yusufoglu, H., Soliman, G., Abdel-Rahman, R., and Tatli-Çankaya, I. (2014). The Potential Hepatoprotective Activity of Allium Paniculatum and Capparis Spinosa on Thioacetamide Induced Hepatotoxicity in Rats. Planta Medica 80 (10). doi:10.1055/s-0034-1382524
Zhang, H., and Ma, Z. F. (2018). Phytochemical and Pharmacological Properties of Capparis Spinosa as a Medicinal Plant. Nutrients 10 (2), 116. doi:10.3390/nu10020116
Zhao, J., Dong, F., Yu, M., and Dong, C. (2014). Pharmacological Effects and Clinical Applications Research Progress of Capparis Spinosa L. Heilongjiang Yiyao 27 (1), 58–61.
Zhou, H., Jian, R., Kang, J., Huang, X., Li, Y., Zhuang, C., et al. (2010). Anti-inflammatory Effects of Caper (Capparis Spinosa L.) Fruit Aqueous Extract and the Isolation of Main Phytochemicals. J. Agric. Food Chem. 58 (24), 12717–12721. doi:10.1021/jf1034114
Zhou, H. F., Xie, C., Jian, R., Kang, J., Li, Y., Zhuang, C. L., et al. (2011). Biflavonoids from Caper (Capparis Spinosa L.) Fruits and Their Effects in Inhibiting NF-Kappa B Activation. J. Agric. Food Chem. 59 (7), 3060–3065. doi:10.1021/jf105017j
Keywords: capparaceae, antidiabetic, hepatoprotective, flavonoids, glucosinolates, indoles, caper bush, flinders rose
Citation: Annaz H, Sane Y, Bitchagno GTM, Ben Bakrim W, Drissi B, Mahdi I, El Bouhssini M and Sobeh M (2022) Caper (Capparis spinosa L.): An Updated Review on Its Phytochemistry, Nutritional Value, Traditional Uses, and Therapeutic Potential. Front. Pharmacol. 13:878749. doi: 10.3389/fphar.2022.878749
Received: 18 February 2022; Accepted: 25 May 2022;
Published: 22 July 2022.
Edited by:
Gokhan Zengin, Selcuk University, TurkeyCopyright © 2022 Annaz, Sane, Bitchagno, Ben Bakrim, Drissi, Mahdi, El Bouhssini and Sobeh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hassan Annaz, aGFzc2FuLmFubmF6QHVtNnAubWE=; Mansour Sobeh, bWFuc291ci5zb2JlaEB1bTZwLm1h
 Hassan Annaz
Hassan Annaz Yaya Sane
Yaya Sane Gabin Thierry M. Bitchagno
Gabin Thierry M. Bitchagno Widad Ben Bakrim
Widad Ben Bakrim Badreddine Drissi
Badreddine Drissi Ismail Mahdi
Ismail Mahdi Mustapha El Bouhssini1
Mustapha El Bouhssini1 Mansour Sobeh
Mansour Sobeh