
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 11 April 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.875384
This article is part of the Research Topic Novel Modalities in Cancer Diagnostics and Therapeutics View all 18 articles
Although most thyroid cancers have a good and predictable prognosis, the anaplastic, medullary, and refractory thyroid cancers still prone to recurrence and metastasis, resulting in poor prognosis. Although a number of newly developed targeted therapies have begun to be indicated for the above types of thyroid cancer in recent years, their ability to improve overall survival remain hindered by low efficacy. As the largest component of immune cells in tumor microenvironment, tumor-associated macrophages play a key role in the invasion and metastasis of thyroid cancer. There is much evidence that the immune system, tumor microenvironment and cancer stem cell interactions may revolutionize traditional therapeutic directions. Tumor-associated macrophages have been extensively studied in a variety of tumors, however, research on the relationship between thyroid cancer and macrophages is still insufficient. In this review, we summarize the functions of tumor-associated macrophages in different types of thyroid cancer, their cytokines or chemokines effect on thyroid cancer and the mechanisms that promote tumor proliferation and migration. In addition, we discuss the mechanisms by which tumor-associated macrophages maintain the stemness of thyroid cancer and potential strategies for targeting tumor-associated macrophages to treat thyroid cancer.
In recent years, thyroid cancer (TC) has attracted more and more attention due to its rapid increase in incidence. TC ranks the fifth in the incidence of all malignant tumors and has become the most common endocrine tumor (Cabanillas et al., 2016). It is important to note that the incidence of TC is also increased in children and adolescents (Person et al., 2021). The incidence of TC continues to increase not only because of environmental pollution, radiation exposure and other external factors, but also because of the improvement of imaging or ultrasound diagnostic technology and biopsy diagnosis (La Vecchia et al., 2015). Papillary thyroid carcinoma (PTC) and follicular thyroid carcinoma (FTC) account for 80%–85% and 10%–15% of TCs respectively (LiVolsi, 2011; Sowder et al., 2018). Patients with these differentiated TCs can benefit from surgery, radioiodine therapy, and thyroid stimulating hormone (TSH) inhibition therapy, and thus have a better prognosis. In contrast, medullary thyroid carcinoma (MTC) is one of the relatively rare thyroid malignant tumors, accounting for 1%–2% of all TCs, but it has a worse prognosis with a 10-year overall survival (OS) of only 81.2% (Cabanillas et al., 2016; Kotwal et al., 2021). Notably, there are approximately 2% of anaplastic thyroid cancer (ATC) (Smallridge et al., 2012), though rare, is often associated with resistance to radioiodine treatment and is characterized by an aggressive phenotype and poor prognosis, with an average survival time of few months (Cabanillas et al., 2016; Veschi et al., 2020). Currently, the clinical treatment for ATC is a great challenge.
Macrophages are highly plastic cells with multiple functions and these cells originate in the bone marrow from myeloid derived progenitor cells (Varol et al., 2015). Tumor-associated macrophages (TAMs) are derived from tumor-infiltrating monocytes in peripheral blood. Current studies indicate that TAM population is in a constant transition between M1 and M2 type (Pan et al., 2020). M1-macrophages have antitumor effects, through the identification of tumor cells and finally kill tumor cells, the study revealed M1-macrophages by two different mechanism of killing tumor cells, one is mediated cytotoxicity to kill tumor cells directly, and the other is antibody dependent cell-mediated cytotoxicity to kill tumor cells. M2-macrophages are divided into multiple subtypes, M2a is involved in tissue fiber formation, M2b contributes to tumor progression, M2c is responsible for tissue remodeling, and M2d promotes angiogenesis (Wang et al., 2019a). TAMs can promote tumor progression by secreting cytokines, such as vascular endothelial growth factor (VEGF) or fibroblast growth factor (FGF) (Mantovani et al., 2002). Therefore TAMs promote angiogenesis and are often associated with high density of blood vessels (Coffelt et al., 2010). In TCs, TAMs mainly exhibit the M2 phenotype (Solinas et al., 2010). Therefore, how to control the phenotypic transformation of TAMs may be the focus of future research. These cytokines or chemokines originate from cancer cells and macrophages, but the mechanisms by which these cytokines or chemokines act on tumors have not been fully elucidated. TAMs maintains the survival, proliferation and invasion of cancer cells and may be a potential therapeutic target (Bolli et al., 2017; Mantovani et al., 2017; Cassetta and Pollard, 2018).
Tumor microenvironment (TME) is mainly composed of tumor cells, innate and adaptive immune cells, fibroblasts, vascular, cytokines and chemokines, et al. TME can not only provide a suitable environment for tumor to promote tumor growth and metastasis, but also inhibit the occurrence and development of tumor through changes in metabolism, secretion, immunity and other conditions (Ben-Baruch, 2006; de Visser et al., 2006). TME is a complex and interactive network regulated by immune cells, cytokines and other elements. Tumor cells are regulated by their own genes and external factors, which jointly promote tumor development and phenotypic changes, such as EMT of tumor cells (Pradella et al., 2017; Weng et al., 2019). TC cells can maintain growth by secreting a variety of cytokines and also by secreting chemokines to recruit different immune cells (Lumachi et al., 2010; Fozzatti and Cheng, 2020). These cytokines or chemokines affect the progression of TC and changes in the immune microenvironment (Menicali et al., 2020). Innate immune cells, especially natural-killer cells (NK cells) and macrophages play a key role in the regulation of tumor progression and inhibition (Goldberg and Sondel, 2015). Similarly, these immune cells can also secrete some cytokines and angiogenic factors to remodeling the TME of TC (Poschke et al., 2011). In addition, tumor progression can also destroy the balance of the original microenvironment, making it conducive to tumor proliferation and migration. Immune surveillance is the function of the immune system to identify, kill and remove mutated cells from the body to prevent tumor development. Tumor immune escape is a phenomenon in which tumor cells evade recognition and attack by the immune system through various mechanisms, thus allowing them to survive and proliferate in vivo (Prendergast et al., 2010). Although immune cells tend to kill tumor cells in the early stage of tumor genesis, they seem to evade immune surveillance eventually, and even inhibit the cytotoxic effect of immune cells on tumor through various mechanisms (Lei et al., 2020).
At present, it is believed that TC is closely associated with the occurrence and development of inflammation, and about 20% of PTC is caused by chronic thyroiditis (Fugazzola et al., 2011). Chronic inflammatory is an important factor mediating tumorigenesis (Noy and Pollard, 2014). The original concept of “inflammation causes tumors” is gradually changed to “inflammation causes tumors, and tumors cause inflammation (Allavena et al., 2011).” In this review, we discuss the types of TAMs and their effects on TCs, the interaction between cytokines and chemokines in TME, cancer stem cell (CSC), and new strategies for immunotherapy of TCs.
Similar to other malignant tumors, the thyroid-TME is composed of immune cells (macrophages, mast cells, neutrophils, and lymphocytes) and soluble mediators (chemokines, cytokines, and growth factors) that are active in and around cancer cells (Yapa et al., 2017). Currently, TAMs infiltration in TME is believed to be through recruitment of circulating monocytes. Cytokines and chemokines in TME promote polarization of macrophages and affect the functions of TAMs, such as promoting tumor proliferation, stemness, gene instability, blood vessel and lymphatic proliferation, immunosuppression, et al. (Mantovani and Allavena, 2015). A schematic diagram of the interaction between TC and TAM is shown in Figure 1.
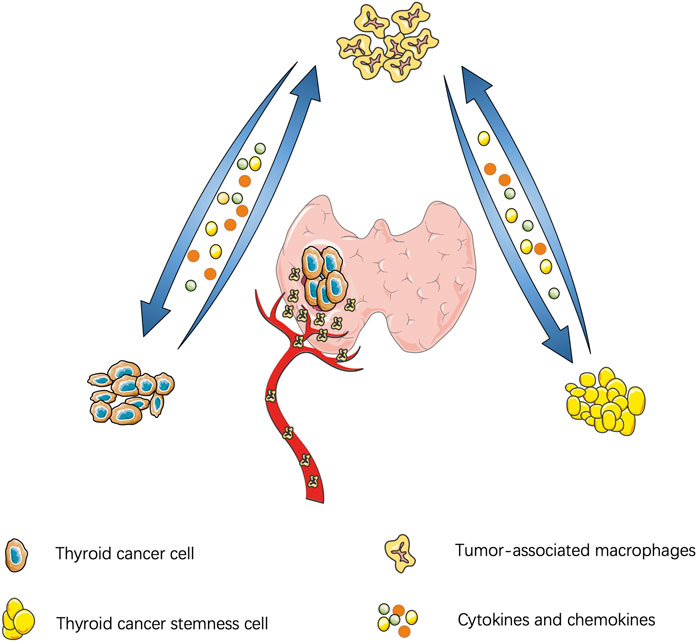
FIGURE 1. Interaction between thyroid cancer and tumor-associatated macrophages: Thyroid cancer cells secrete cytokines to manitain the stemness of cancer cells and cancer stem cells feedback to promote tumor proliferation and migration. Thyroid cancer can also secrete chemokines to recruit macrophages and polarize into M2 subtype. Similarly, cytokines derived from tumor-association macrophages promote tumor migration.
TC is commonly associated with a complex genetic background and gene mutations, that could be involved in mutations in BRAF, RAS and PI3K signaling pathways. Losing or inhibiting the functions of PTEN, p53 and B-catenin is commonly seen in poorly differentiated TCs (Hou et al., 2007). BRAFV600E mutation is most frequent in PTCs or ATCs (Cancer Genome Atlas Research, 2014). While RAS mutation mostly exist in FTCs and other variant of PTCs (Cabanillas et al., 2016). RET proto-oncogene mutation is thought to be the cause of most MTC, while a low proportion of TCs is caused by sporadic RAS mutation (Ciampi et al., 2013; Wells et al., 2015). In an in vivo study of nude mice, high CXCL16 expression is associated with M2-macrophage infiltration in BRAFV600E mutated PTC, promoting tumor angiogenesis and resulting in poor prognosis (Kim et al., 2019). In another study, In BRAFV600E and BRAFWT, the average ratio of immune cell populations CD68 +/CD163 + cells tends to decrease, resulting in extensive immunosuppression of TC in BRAFV600E (Angell et al., 2014).
Macrophages recruited from circulating monocytes into tumor tissues and endowed with tumor-promoting or suppressive effects are called TAMs (Pathria et al., 2019; Cassetta and Pollard, 2020). Once TAMs from peripheral blood monocytes are recruited to TME by tumor secreted chemokines and polarized into M1/M2 macrophages under various stimuli. TAMs can also show significant plasticity within the TME, transforming from one phenotype to another in response to certain stimuli (Sica and Mantovani, 2012).
M1-TAMs are involved in the activation of Th1 type immune response and they have a high capacity of antigen presentation, secreting some cytokines and chemokines such as IL-1β, IL-6, IL-12, IL-23, CXCL9, CXCL10, TNF-α, etc., (Porta et al., 2015; Wu et al., 2020). In addition, Some surface proteins of M1-TAMs, CD68, CD80 and CD86, are also upregulated (de Sousa et al., 2016). In contrast, M2-TAMs primarily upregulated the expression of anti-inflammatory cytokines and chemokines, including IL-10, TGF-β, CCL17, CCL18, CCL22, and CCL24. Similarly, some surface proteins of M2-TAMs are upregulated including CD163, CD204 and CD206 (Biswas et al., 2013; Jayasingam et al., 2019).
Although many markers related to TAMs polarization have been discovered, some markers of M1 or M2 phenotype can be co-expressed on an individual cell (Chong et al., 2015). Therefore, the anti-tumor or pro-tumor effects of M1 or M2 macrophages are still controversial. Their effects can be determined not only by the high and low expression of markers, but also by the complex activation mechanism of the mutual transformation between the two phenotypes (Figure 2).
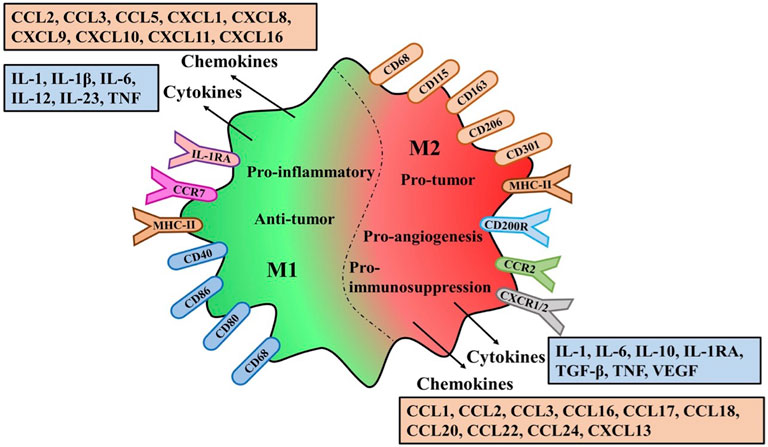
FIGURE 2. Polarization characteristics surface markers receptors and secretions of TAMs.The variation of M1/M2-TAMS in the Tumor microenviroment the same or different markers and receptors on the membrane surface are explained. Different types of Polarization secreted different cytokines and chemokines and different effects on tumors
Hypoxia is an effective means to induce TME to recruit TAMs, and imbalance of TME oxygen supply may inhibit TAMs migration. Hypoxia inducible factor-1α (HIF-1α) is a key transcription factor regulating hypoxia-induced gene expression, and its high expression can induce TAMs to enter hypoxia region continuously. HIF-1α upregulates CXCR4 and its ligand CXCL12 in monocytes/macrophages and induces these chemotactic responses (Kumar and Gabrilovich, 2014).
After recruitment and polarization, M2-TAMs can secrete a series of cytokines to stimulate tumor cell proliferation and survival including epithelial growth factor (EGF), epithelial growth ligands of the factor receptor (EGFR) and basic fibroblast growth factor (bFGF) (Yin et al., 2016). Polarization of TAMs has been confirmed to be associated with proliferation and metastasis of various malignant tumors. In ovarian cancer, TAMs can promote tumor proliferation and migration by upregulating insulin like growth factor (IGF1) signaling pathway (Liu et al., 2018a). Gastric cancer derived mesenchymal cells secrete IL-6 and IL-8 (CXCL8) through JAK2/STAT3 signaling pathway and induce polarization of gastric cancer M2-macrophages. M2-macrophages significantly promote gastric cancer metastasis through promoting epithelial-mesenchymal transition (EMT) mechanism (Li et al., 2019). In a study on exosomes and TME, Mir-183-5p level of M2-TAMs exosomes was significantly increased, which targeted THEM4-mediated Akt/NF-κB and inhibited the mRNA and protein expression, promoted the proliferation and invasion of colon cancer cells, and inhibited apoptosis. Downregulation of Mir-183-5p reversed the M2-TAMs mediated tumor promotion (Zhang et al., 2021a). In some clinical studies of non-small cell lung cancer, M2-TAMs infiltrate induce tumor cell invasion and progression, resulting in poor prognosis (Sumitomo et al., 2019; Sedighzadeh et al., 2021). Pulmonary macrophage accumulation is one of the important factors leading to lung metastasis of ATC (Li et al., 2016). Recent studies have found that some gene mutations can cause imbalance of M1/M2-TAMs. For example, a recent animal study revealed that Mettl3-deficient mice showed increased infiltration of M1/M2-TAMs. Deletion of METTL3 disrupts YTHDF1-mediated translation of SPRED2, thereby enhancing NF-kB and STAT3 activation through the ERK pathway, leading to increased tumor growth and metastasis (Yin et al., 2021). When the mTOR pathway is activated, the monocyte macrophage phenotype is converted to M2-TAMs, enhancing pro-angiogenic capacity. On the contrary, when the mTOR pathway is inhibited, the cell phenotype is transformed into M1-TAMs, and its decreased angiogenesis ability can inhibit tumor growth (Li et al., 2020). Therefore, under the structure of TME, M2-TAMs can not only secrete cytokines or chemokines to induce tumor development, but also promote tumor proliferation and migration through cross talk with tumor cells.
There is substantial evidence that inflammatory responses at tumor sites can promote tumor growth and progression. Inflammation and immune evasion are considered to be hallmarks of cancer.
CSC is a subpopulation of cancer cells with the ability to self-renew and proliferate heterogeneous cancer cells. CSCs have an important role in tumor survival, proliferation, metastasis, drug resistance and recurrence (Ayob and Ramasamy, 2018). CSCs are primarily found inside tumors, named stem cell niches, under specific microenvironmental conditions, that consist of multiple types of stromal cells, including the vascular system, mesenchymal cells, immune cells, extracellular matrix, and cytokines (Borovski et al., 2011). In 2005, Takano and his colleagues proposed that TC cells were derived from stem cells or precursor cells of fetal origin in normal thyroid gland rather than from differentiated thyroid follicular cells.
Based on this hypothesis, many scholars believe that TC is a CSC-driven disease that may originate from the transformation of stem cells or from the dedifferentiation process of cancer cells (Zhang et al., 2006; Zito et al., 2008). CSCs are predominantly found in different specific TME, where the dynamic balance between intracellular and extracellular cytokines produced by the TME allows the maintenance of a stem cell phenotype characterized by a lack of tissue-specific differentiation, slow cell cycle rate, quiescence and a theoretically unlimited capacity for self-renewal (Pietras et al., 2014; Chan et al., 2019; Grassi et al., 2021). A growing number of studies have shown that CSCs are closely associated with radiotherapy tolerance, tumor metastasis and recurrence (Magee et al., 2012; Mertins, 2014). The transition of stem cells to differentiated cancer cells is stimulated by growth factors and cytokines that are present in the TME in which the stem cells are located (Gianì et al., 2020). TAMs secretes multiple cytokines or chemokines to support self-renewal and maintenance of stemness. Similarly, CSCs secrete pro-tumor signals to activate TAMs, which further promotes tumor development (Sainz et al., 2016). The expression of EMT biomarkers was strongly correlated with the presence of CSCs in TC and the role of TAMs (Lan et al., 2013; Hardin et al., 2018). CSCs are currently thought to drive TC heterogeneity, contributing to their metastatic potential and therapeutic resistance (Grassi et al., 2021). CSCs can also secrete exosomes to regulate the polarization of TAMs toward the M2 phenotype, inhibit NK-cell activity, and promote suppression of the immune microenvironment in CSC niches (Caruso Bavisotto et al., 2019; Baig et al., 2020). The current view is that eradication of CSCs may inhibit tumor recurrence, while failure to completely remove CSCs eventually leads to tumor recurrence (Nguyen et al., 2012). Although current research is limited, there is evidence that TAMs can enhance the stemness of CSC (Chanmee et al., 2014). In a study of head and neck cancer, TAMs can affect CD44 signal through PI3K-4EBP1-SOX2 pathway to mediate stemness enhancement, and In vivo experiments, Targeting CD44 reduced PI3K-4EBP1-SOX2 signal, inhibited tumor growth, and attenuated stemness (Gomez et al., 2020). Periostin secreted by glioma stem cells recruited peripheral blood mononuclear cells into glioblastoma tissue. Moreover, TAMs and glioma stem cells co-distribute in perivascular niches to maintain the characteristics of M2 macrophages and secrete tumour-supportive factors to promote glioblastoma growth and progression (Zhou et al., 2015).
Although the current research results on thyroid CSCs and TAMs are still insufficient. However, existing studies can show that there is a mutual promotion between thyroid CSCs, TAMs and TME, that is, TAMs can maintain the stemness of thyroid CSCs, and CSCs can also promote polarization of TAMs to M2 macrophages. Finally, CSCs and TAMSs establish a positive loop by a cross talk mechanism.
TAMs accounts for a large proportion of tumor-infiltrating immune cells in TME and are highly plastic (Chen et al., 2019). TAMs are infiltrating immune cells that can occupy up to 50% of the total volume of thyroid tumors (Ryder et al., 2008). TAMs respond to cytokines or chemokines secreted by tumor cells and polarize into M1 or M2-TAMs. but in TME, M2-TAMs are usually predominant. M1-TAMs play a key role in killing tumor cells by producing reactive oxygen/nitrogen species (ROS/RNS) and pro-inflammatory cytokines such as IL-1β, IL-6, and TNF-α, and thus M1-TAMs are macrophages with anti-tumor effects (Jeannin et al., 2018; Chen et al., 2019). Typically, most TAMs are M2-TAMs with a Th2 immune response, which can enhance tumor cell immune escape (Galdiero et al., 2013; Ferrari et al., 2019).
TC cells and M2-macrophages can promote each other. M2-TAMs promotes TC dedifferentiation, proliferation and metastasis by secreting Wnt1 and Wnt3 ligands, that activate the Wnt signaling pathway and promote β-catenin activation (Lv et al., 2021a). Inhibition of the Akt-mTOR signaling pathway inhibits TC-induced cytokines production by macrophages (Sloot et al., 2019a). Mazzoni et al. found that TC cells activate PGE2 secretion to promote M2 polarization, enhancing tumor invasiveness (Mazzoni et al., 2019). On the contrary, TCs can induce macrophages polarization, which in turn acts on tumor cells to lead to tumor progression and metastasis. For example, senescent thyroid tumor cells can induce M2-macrophages polarization with increased expression of CCL17,CCL18,IL-18,TGFβ1, that ultimately promotes tumor migration by activating NF-κB signaling pathway (Zhang et al., 2021b). In addition, gene mutations also have an impact on TAM polarization, such as BRAFV600E mutation, which can lead to an increase in the proportion of M2-TAMs and promote tumor growth (Ryder et al., 2013). Some drugs or compounds can alter polarization of M2. For example, a recent study by Juan LV et al. found that Zoledronic acid inhibits M2-induced TC proliferation, stemness and metastasis by inhibiting M2 polarization and suppressing the Wnt/β-catenin signaling pathway (Lv et al., 2020). TNF-α derived from TAMs can induce IL-32β expression in TC cells. Although IL-32β does not directly affect TC cells migration, alternative splicing of IL-32 towards the IL-32β isoform may be beneficial for TC cell survival through induction of the pro-survival chemokine IL-8 (CXCL8) (Sloot et al., 2019b).
M1-macrophages can inhibit tumor proliferation and migration in a variety of malignant tumors. However, due to the limitations of various factors and conditions, studies on TC are still insufficient. Reversing the polarization of M2-macrophages has also emerged as one of the potential new strategies for TC treatment. Metabolic reprogramming of M1-macrophages has been found in autoimmune diseases to enhance glycolysis and inhibit oxidative phosphorylation, which may be one of the clues of autoimmune thyroiditis (Murray et al., 2014).
In PTC, the normal thyroid tissue had higher density of M1-macrophages than the cancer tissue. And the proportion of M1-macrophages in stage I and II is higher than that in other stages (Xie et al., 2020). More importantly, CIBERSORT results showed that some anti-tumor immune cells such as M1-macrophages, plasma cells and CD8+ T cells were positively correlated. Similarly, A the cancer genome atlas (TCGA) prognostic model of TC revealed that a higher proportion of M1-macrophages and dendritic resting cells in the low-risk group (Zhang et al., 2021c). In addition, the endocrine system is also involved in regulating the polarization of macrophages. In vitro results showed that triiodothyronine (T3) had a negative effect on triggering the differentiation of bone marrow derived monocytes into nonpolarized macrophages. M1-macrophages polarization of macrophages induced by T3 (Perrotta et al., 2014). In vitro experiments, the co-culture system of TPC-1 cells and bleomycin revealed that low-dose bleomycin could transform M2-TAMs into M1-TAMs, and the proliferation, migration and invasion abilities of TPC-1 cells were significantly reduced (Liu et al., 2018b). Another in vitro study found that in Graves disease (GD), activated NK-cells drive macrophages to differentiate to M1 phenotype, which in turn is cytotoxic to cancer cells and downregulates the M2 phenotype (Imam et al., 2019). This will open up new prospects for immunotherapy of TC. Macrophage/innate immunity can modulate from M2 phenotype to M1 phenotype to help treat TC as naturally done by GD.
The most common route of metastasis for PTC is lymphatic metastasis. Current studies have found that PTC can achieve the purpose of macrophage recruitment in TME by upregulation of some chemokine transcription programs (Guarino et al., 2010; Muzza et al., 2010). Previous studies have demonstrated that high density of M2-TAMs leads to lymph node metastasis in PTC (Gulubova and Ivanova, 2019). It was reported that M2 macrophages accumulated around the lymphatic vessels at the PTC tumor margins implicated lymphatic invasion of cancer cells (Kabasawa et al., 2021). M2-TAMs around lymphatic vessels enhance lymphatic invasion by upregulating MMP2. Yang et al. found that Mir-324-5p/PTPRD/CEBPD axis induces Human Umbilical Vein Endothelial Cells (HUVEC) invasion/migration and M2- macrophages polarization through VEGF and IL4/IL13, respectively, and participates in the progression of PTC (Yang et al., 2020a). TAMs conditioned medium co-cultured with PTC cell enhanced the invasion of PTC. More importantly, this study found that CXCL8 promotes PTC metastasis in vivo, confirming that TAMs may promote PTC metastasis through the interaction between CXCL8 and its receptor, CXCr1/2 (Fang et al., 2014). In addition, other cytokines secreted by TAMs also promote the invasion and metastasis of PTC. For example, The combination of CXCL16 derived from M2-TAMs with angiogenic genes leads to high aggressiveness of PCT, and knockout of endogenous CXCL16 delayed tumor growth in athymic mice (Cho et al., 2016). M2-macrophages also have immunosuppressive function, which can mediate immune escape and promote the proliferation and metastasis of TC. M2-TAMs promote the proliferation and metastasis of TC through a variety of paracrine methods. In addition, the upregulated level of VEGF and EGF secreted by M2-macrophages can promote tumor microangiogenesis by recruiting endothelial cells (Chandler et al., 2019). Hypoxia is also a major driver of tumor angiogenesis, and a large number of TAMs have been found in hypoxia areas of tumors, especially necrotic tissues (Yang et al., 2020b).
The density of M2-macrophages varied among thyroid tumors, with higher density in PTC and highest in ATC. The density of M2-macrophages is closely associated with tumor prognosis (Jung et al., 2015; Ferrari et al., 2019). The presence of a high density of macrophages in ATC, which are dominated by polarized M2-TAMs, is associated with tumor aggressiveness, and therefore M2-TAMs can be used as a biomarker to predict prognosis (Sica et al., 2006; Caillou et al., 2011). However, the high density of TAMs aggregation is not sufficient to evaluate the prognosis. It is critical to evaluate their function and signaling pathways (MacDonald et al., 2020). The infiltrating cells of ATCs include lymphocyte infiltrate (mainly CD8+ cytotoxic T cells) and TAMs (Cameselle-García et al., 2021; Prete et al., 2021). M2-TAMs can secrete insulin-like growth factors (IGF) to activate IR-A/IGF1R and mediate PI3K/AKT/mTOR signaling pathway to accelerate ATC cells metastasis and enhance stem cell viability and stemness (Lv et al., 2021b). A recent study revealed that blocking CD47 inhibits the growth of ATCs and reduces TAMs density, thereby suppressing tumor growth (Schürch et al., 2019). Caluo and his colleagues identified a specific type of TAM in ATC, called “ramified TAM” (RTAM), which is not present in other types of TCs, and forms a high dense network interlinked with tumor cells in ATC, providing signals and nutrients for tumor growth (Caillou et al., 2011).
A recent in vitro study showed that CCL15 derived from FTC cells can effectively recruit TAMs to TME, thus providing a favorable microenvironment for tumor growth (Huang et al., 2016). MALAT1-mediated TAMs secretes FGF2 (bFGF) to inhibit the release of inflammatory cytokines, promote the proliferation, migration and invasion of FTC133 cells, and induce angiogenesis (Huang et al., 2017). In transgenic mouse models of FTC, a mechanism was found that advanced cancer in emasculated men was due to increased expression of tumor suppressor genes:GLIPR1 and SFRP1, resulting in increased tumor invasion of M1-macrophages and CD8 cells. In addition, GLIPR1 slows down the growth of cancer cells and increases the secretion of CCL5, which leads to the activation of immune cells (Zhang et al., 2015). However, studies on TAMs in FTC is still lacking due to tumor samples limitations, and large samples of clinical and basic studies are needed to confirm this function. Interestingly, there is still no study or report on MTC and TAMs, and the relationship between the two is still unclear.
TAMs not only provide structural support for tumor growth, but also participate in tumor development by secreting signaling molecules (Kogure et al., 2019). Although TAMs have insufficient secretory function, they can secrete cytokines or directly stimulate TC cells. Though some of the macrophage phenotypes will have anti-tumor immune function, but in recent years, there are evidence that TAMs can reconstruct TME, promote tumor cell proliferation and survival, promote angiogenesis and lymphatic vessel formation, and suppress T cell response to tumors (Mantovani and Allavena, 2015).
TAMs can also increase angiogenic factors to promote tumor angiogenesis. A recent study confirmed that TAMs promote tumor angiogenesis by upregulating VEGF (Hughes et al., 2015; Osterberg et al., 2016). In addition, VEGF secreted by TAMs increased the growth and activity of tumor microvasculature, providing suitable microenvironment for TC infiltration and metastasis. Examples include VEGF, platform-derived growth factor (PDGF), bFGF (Melaccio et al., 2021). In addition, it can also produce the matrix metalloproteases (MMPS) (Ojalvo et al., 2010; Cheng et al., 2021). Angiogenesis is related to tumor growth and metastasis and plays an important role in tumor development. Therefore, TAMs are one of the important factors in tumor angiogenesis (Chen et al., 2019).
In a BRAFV600E mutation-induced mouse tumor model, TC cells and TAMs secrete TGF-β, which leads to tumorigenic EMT and increases the invasiveness of TC cells (Knauf et al., 2011). TAMs not only secretes some cytokines to promote the EMT of TC, but also maintains the stemness of TC. In TC, IL-6 is dependent on the activation of the IL6/JAK1/STAT3 signaling pathway to promote thyroid CSC proliferation and colony formation and to enhance the properties of thyroid CSCs and EMT (Zheng et al., 2019). IL-10 is secreted by both TAMs and malignant tumor cells. IL-10 has various effects in the immune system, most notably in the form of immunosuppression. IL-10 is found to be overexpressed in TCs and its expression was shown to be correlated with tumor size and tumor extension, suggesting that IL-10 might enhance the function of tumor progression (Mocellin et al., 2005; Cunha et al., 2017).
In summary, after monocytes are recruited near the tumor and differentiate into TAMs, TAMs secrete a variety of molecules, including growth factors, cytokines, and proteases, that promote tumor angiogenesis, immunosuppression, and tumor metastasis.
Chemokines are classified into four subtypes: C, CC, CXC, and CX3C, and CC in the predominant one of these subtypes (Yapa et al., 2017). The role of cytokines is to create a concentration gradient to induce motility or chemotaxis for specific receptor cells, inducing the migration of immune cells such as NK-cells, dendritic cells, and others (Mukaida et al., 2014). Previous studies have identified a variety of chemokine-mediated immune cells that promote progression of TCs (Yapa et al., 2017). However, the signaling pathways by which these chemokines mediate immune cells are diverse. TC cells or normal thyroid cells secrete a variety of chemokines in the basal state or under stimulation (Mironska et al., 2019).
In the studies on chemokine-mediated macrophages, cellular CXCL8 was the first chemokines found to be associated with TC, and cell lines from PTC and ATC were found to contain high levels of CXCL8 and TAMs, leading to tumor proliferation and metastasis (Kobawala et al., 2011). When CXCL8 is blocked, the metastatic potential of PTC is significantly reduced, and conversely, increasing exogenous CXCL8 promotes the metastatic potential of PTC (Fang et al., 2014). In addition, Visciano and his colleagues used in vitro cell culture to demonstrate that (IL-8) CXCL8 induces EMT in TC and enhances the stemness of thyroid CSCs through the Akt-Slug signaling pathway (Visciano et al., 2015). These chemokines also have the function of promoting EMT in TC (Pradella et al., 2017).
In a study of another chemokine receptor, CXCR4, whose ligand is CXCL12, activates G protein-mediated signaling pathways, including AKT, JAK/STAT, and MAPK (Pawig et al., 2015), and promotes migration and invasion of a variety of malignancies, including TCs. High expression of CXCR4 promotes cell proliferation and lymph node metastasis in PTCs and ATCs (Hwang et al., 2003; Wagner et al., 2008). In addition, there are many chemokines that can enhance the proliferation and invasion of TC. For example, CCL2/MCP-1 (monocyte chemotactic protein-1) promotes lymph node metastasis in PTC by recruiting TAMs expressing CCR2, and patients with high CCL2 expression are more likely to recurrence (Tanaka et al., 2009). The chemokine CCL21 and its receptor CCR7 were found to play crucial roles in the proliferation and migration of PTC and MTC by Sancho et al. (2006). In PTC, patients with elevated CCL2 are more likely to develop lymph metastases and have a high recurrence risk (Tanaka et al., 2009). In another experiment found that PTC overexpression of CXCL16 is correlated with M2 polarization and promote tumor angiogenesis (Kim et al., 2019). A recent study confirmed that high CXCR6 expression was positively associated with PTC lymph metastasis and that CXCL16 mediated macrophages invasion of PTC and altered the macrophages phenotype to M2-TAMs in the TME (Cho et al., 2016).
For all types of TC, surgical treatment is still the best means of treatment, and TSH inhibition therapy could be performed according to the postoperative pathological results such as lymph node metastasis of patients and the risk of recurrence (Haugen et al., 2015). If necessary, 131I therapy, external radiation or targeted therapy should be performed (Wang et al., 2019b). Tumor immunotherapy is an effective anti-tumor therapy strategy developed in recent years. There is considerable evidence that macrophages are polarized into the M2 phenotype in the development of many solid tumors (Komohara et al., 2016; Movahedi and Van Ginderachter, 2016). Therefore, targeting TAMs is a potential therapeutic strategy for solid tumors. The transformation of M2 to M1-macrophages is a potential new antitumor immunotherapy, and its mechanism is related to the upregulation of macrophage phagocytosis. Current strategies for macrophage therapy are mainly through two approaches: inhibition of recruitment and/or clearance, and reversal of differentiation. Blocking macrophages recruitment has been extensively studied in preclinical models, and is being evaluated for feasibility of clinical application. Several monoclonal antibodies that have been approved for clinical use in the treatment of tumors have been shown to work therapeutically, primarily by increasing the phagocytic activity of macrophages. Targeted regulation of TAMs plays a key role in the activity of antitumor drugs (Germano et al., 2013).
Inhibition of cytokines or chemokines, such as CCL2 and colony stimulating factor (CSF-1), that promote the polarization of macrophages into M2 phenotype, is a promising immunotherapy (Kim et al., 2013; Richards et al., 2013). Tyrosinase inhibitors for TC and immunotherapy drugs under development are listed in Table 1. A recent study showed that PLX4720 (The main active molecule is 7-azaindole, which is a potent and selective inhibitor of B-Raf V600E) combined with anti-PD-L1/PD-1 antibody significantly reduced ATC tumor volume, increased the density and cytotoxicity of CD8+ T cells and NK cells, increased M1-TAMs, prolonged survival, improved TME, and enhanced anti-tumor immune effect (Gunda et al., 2018). In addition, Rituximab is a chimeric monoclonal antibody that specifically binds to the transmembrane antigen CD20 and inhibit the growth of non-Hodgkin’s lymphoma cells by promoting macrophage phagocytosis (Chao et al., 2010). Trastuzumab blocks the attachment of human EGF to Her2 by attaching itself to Her2 and mediate macrophage killing of Her-2 overexpressing in breast cancer cells (Shi et al., 2015). Similarly, bleomycin mainly interferes with DNA synthesis, but low dose bleomycin can reverse the M2 to M1-macrophages, and the proliferation, migration and invasion of TPC-1 cells are significantly reduced, suggesting that bleomycin can inhibit the progression of PTC by modulating macrophages polarization (Liu et al., 2018b). Similarly, selective elimination of M2-TAMs inhibits PTC growth, and CSF-1 signaling may serve as a potential therapeutic target for inhibiting BRAFV600E mutation-induced PTC (Ryder et al., 2013). More interestingly, A bisphosphonate (zoledronic acid) binds to microcalcification in tumor tissue and is subsequently phagocytized by TAM to induce apoptosis, and also promotes polarization of M2 towards M1-macrophages (Hattori et al., 2015). A mouse model of combined anti-macrophage zoledronic acid and tumor cytotoxic docetaxel showed that the combined strategy significantly inhibited tumor growth and pulmonary metastasis (Sun et al., 2015). Blocking and targeting the CCL-2/CCR2 and CSF-1/CSF-1R pathways is a promising approach in ATC. This approach can not only inhibit the recruitment of tumor M2-macrophages, but also re-polarize them into the M1 phenotype (Naoum et al., 2018).
Blocking common TAM-related chemokines such as CCL2 and CXCL8 and their associated receptors may also be a potential therapeutic strategy. Since ELR- can counteract the effect of ELR+, targeting ELR + CXC chemokines can also inhibit tumorigenesis (Mukaida et al., 2014). Stassi et al. found that IL-4 and IL-10 promoted the progression of TC cells and resistance to chemotherapy through upregulation of anti-apoptotic proteins. Therefore, IL-4 and IL-10 may be new therapeutic targets for TC (Stassi et al., 2003). In addition, TC cells have some functional receptors that receive signals of chemokines for proliferation, immunosuppression, angiogenesis and other activities to maintain the growth and metastatic potential of tumor cells (Mantovani et al., 2010; Allavena et al., 2011). In a BRAFV600E mutant PTC mouse model, IL-12 treatment significantly reduced tumor size and weight and improved OS (Parhar et al., 2016). Another study identified IL-12 as a pro-inflammatory cytokine with anti-tumor activity, demonstrating inhibition of ATC growth (Lu, 2017). Possible molecular mechanisms are associated with NF-κB activation and NF-κ B-dependent MMP-3 upregulation. Therefore, molecular therapies targeting CCL20 and CCR6 may provide promising intervention strategies for TC (Cunha et al., 2012; Zeng et al., 2014). In a animal model experiment, the change of secretory CXCL8 in the treatment of MTC can be used as a biomarker for the clinical efficacy of sunitinib (Broutin et al., 2011). In addition to MTC and ATC, the prognosis of PTC is also closely related to TME. Researchers are looking for an immunotherapy to modify TME to enhance the outcome of surgery and RAI (Na and Choi, 2018). Balkwill et al. demonstrated that CXCR7, when downregulated in PTC, inhibits cell growth and invasion, leads to S-phase arrest, and promotes apoptosis, suggesting that CXCR7 may be a promising target for therapeutic intervention of PTC (Balkwill, 2012).
The plasticity of macrophages highlights the potential of macrophage reprogramming as a therapeutic strategy to inhibit tumor progression, enabling these cells to adapt their functions to meet the needs of tumor resistance. In order to better understand the activation status of TAMs in TME, further studies on specific markers are needed to distinguish the different functions of antitumor or pro-tumor TAMs.
In recent decades, there has been no significant improvement in the survival of patients with progressive or recurrent TCs, despite systematic and multidisciplinary treatments. Therefore, for the postoperative management of TC patients, in addition to histological classification, other pathological parameters such as mutational status, activation of molecular signaling pathways, tumor cell differentiation, and associated immunophenotype need to be considered. TME plays an important role in TC development, metastasis, stemness, and TAMs account for the largest proportion of cells in TME. Although it is currently believed that high infiltration of M2-TAMs supports the TME and promotes the growth of TC, it has also been suggested that mixed immune cell infiltration may be associated with a good prognosis in differentiated TC. The study of TAMs in TC is a novel field and more research is needed to unravel the complex and dynamic crosstalk between TC cells and their microenvironment. Although current research has evidence to support that targeting TAMs can significantly improve the efficacy of conventional therapies and immunotherapy, there is still a long way to go to understand the role and mechanisms of TAMs in TC progression and the use of TAM-based immunotherapy.
QL: Conceptualization, Writing—Original draft preparation. WS: Writing—Reviewing and Editing, Validation. HZ: Supervision.
This works was funded by National Natural Science Foundation of China (81902726), Science and Technology Project of Shenyang City (21-173-9-31).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Allavena, P., Germano, G., Marchesi, F., and Mantovani, A. (2011). Chemokines in Cancer Related Inflammation. Exp. Cel Res 317 (5), 664–673. doi:10.1016/j.yexcr.2010.11.013
Angell, T. E., Lechner, M. G., Jang, J. K., Correa, A. J., LoPresti, J. S., and Epstein, A. L. (2014). BRAF V600E in Papillary Thyroid Carcinoma Is Associated with Increased Programmed Death Ligand 1 Expression and Suppressive Immune Cell Infiltration. Thyroid 24 (9), 1385–1393. doi:10.1089/thy.2014.0134
Ayob, A. Z., and Ramasamy, T. S. (2018). Cancer Stem Cells as Key Drivers of Tumour Progression. J. Biomed. Sci. 25 (1), 20. doi:10.1186/s12929-018-0426-4
Baig, M. S., Roy, A., Rajpoot, S., Liu, D., Savai, R., Banerjee, S., et al. (2020). Tumor-derived Exosomes in the Regulation of Macrophage Polarization. Inflamm. Res. 69 (5), 435–451. doi:10.1007/s00011-020-01318-0
Balkwill, F. R. (2012). The Chemokine System and Cancer. J. Pathol. 226 (2), 148–157. doi:10.1002/path.3029
Ben-Baruch, A. (2006). Inflammation-associated Immune Suppression in Cancer: the Roles Played by Cytokines, Chemokines and Additional Mediators. Semin. Cancer Biol. 16 (1), 38–52. doi:10.1016/j.semcancer.2005.07.006
Bible, K. C., Suman, V. J., Molina, J. R., Smallridge, R. C., Maples, W. J., Menefee, M. E., et al. (2014). A Multicenter Phase 2 Trial of Pazopanib in Metastatic and Progressive Medullary Thyroid Carcinoma: MC057H. J. Clin. Endocrinol. Metab. 99 (5), 1687–1693. doi:10.1210/jc.2013-3713
Biswas, S. K., Allavena, P., and Mantovani, A. (2013). Tumor-associated Macrophages: Functional Diversity, Clinical Significance, and Open Questions. Semin. Immunopathol 35 (5), 585–600. doi:10.1007/s00281-013-0367-7
Bolli, E., Movahedi, K., Laoui, D., and Van Ginderachter, J. A. (2017). Novel Insights in the Regulation and Function of Macrophages in the Tumor Microenvironment. Curr. Opin. Oncol. 29 (1), 55–61. doi:10.1097/CCO.0000000000000344
Borovski, T., De Sousa E Melo, F., Vermeulen, L., and Medema, J. P. (2011). Cancer Stem Cell Niche: the Place to Be. Cancer Res. 71 (3), 634–639. doi:10.1158/0008-5472.CAN-10-3220
Broutin, S., Ameur, N., Lacroix, L., Robert, T., Petit, B., Oumata, N., et al. (2011). Identification of Soluble Candidate Biomarkers of Therapeutic Response to Sunitinib in Medullary Thyroid Carcinoma in Preclinical Models. Clin. Cancer Res. 17 (7), 2044–2054. doi:10.1158/1078-0432.CCR-10-2041
Cabanillas, M. E., McFadden, D. G., and Durante, C. (2016). Thyroid Cancer. Lancet 388 (10061), 2783–2795. doi:10.1016/S0140-6736(16)30172-6
Caillou, B., Talbot, M., Weyemi, U., Pioche-Durieu, C., Al Ghuzlan, A., Bidart, J. M., et al. (2011). Tumor-associated Macrophages (TAMs) Form an Interconnected Cellular Supportive Network in Anaplastic Thyroid Carcinoma. PLoS One 6 (7), e22567. doi:10.1371/journal.pone.0022567
Cameselle-García, S., Abdulkader-Sande, S., Sánchez-Ares, M., Rodríguez-Carnero, G., Garcia-Gómez, J., Gude-Sampedro, F., et al. (2021). PD-L1 Expression and Immune Cells in Anaplastic Carcinoma and Poorly Differentiated Carcinoma of the Human Thyroid Gland: A Retrospective Study. Oncol. Lett. 22 (1), 553. doi:10.3892/ol.2021.12814
Cancer Genome Atlas Research, N. (2014). Integrated Genomic Characterization of Papillary Thyroid Carcinoma. Cell 159 (3), 676–690. doi:10.1016/j.cell.2014.09.050
Carr, L. L., Mankoff, D. A., Goulart, B. H., Eaton, K. D., Capell, P. T., Kell, E. M., et al. (2010). Phase II Study of Daily Sunitinib in FDG-PET-Positive, Iodine-Refractory Differentiated Thyroid Cancer and Metastatic Medullary Carcinoma of the Thyroid with Functional Imaging Correlation. Clin. Cancer Res. 16 (21), 5260–5268. doi:10.1158/1078-0432.CCR-10-0994
Caruso Bavisotto, C., Cipolla, C., Graceffa, G., Barone, R., Bucchieri, F., Bulone, D., et al. (2019). Immunomorphological Pattern of Molecular Chaperones in Normal and Pathological Thyroid Tissues and Circulating Exosomes: Potential Use in Clinics. Int. J. Mol. Sci. 20 (18). doi:10.3390/ijms20184496
Cassetta, L., and Pollard, J. W. (2018). Targeting Macrophages: Therapeutic Approaches in Cancer. Nat. Rev. Drug Discov. 17 (12), 887–904. doi:10.1038/nrd.2018.169
Cassetta, L., and Pollard, J. W. (2020). Tumor-associated Macrophages. Curr. Biol. 30 (6), R246–R248. doi:10.1016/j.cub.2020.01.031
Chan, T. S., Shaked, Y., and Tsai, K. K. (2019). Targeting the Interplay between Cancer Fibroblasts, Mesenchymal Stem Cells, and Cancer Stem Cells in Desmoplastic Cancers. Front. Oncol. 9, 688. doi:10.3389/fonc.2019.00688
Chandler, K. B., Costello, C. E., and Rahimi, N. (2019). Glycosylation in the Tumor Microenvironment: Implications for Tumor Angiogenesis and Metastasis. Cells 8 (6). doi:10.3390/cells8060544
Chanmee, T., Ontong, P., Konno, K., and Itano, N. (2014). Tumor-associated Macrophages as Major Players in the Tumor Microenvironment. Cancers (Basel) 6 (3), 1670–1690. doi:10.3390/cancers6031670
Chao, M. P., Alizadeh, A. A., Tang, C., Myklebust, J. H., Varghese, B., Gill, S., et al. (2010). Anti-CD47 Antibody Synergizes with Rituximab to Promote Phagocytosis and Eradicate Non-hodgkin Lymphoma. Cell 142 (5), 699–713. doi:10.1016/j.cell.2010.07.044
Chen, Y., Song, Y., Du, W., Gong, L., Chang, H., and Zou, Z. (2019). Tumor-associated Macrophages: an Accomplice in Solid Tumor Progression. J. Biomed. Sci. 26 (1), 78. doi:10.1186/s12929-019-0568-z
Cheng, N., Bai, X., Shu, Y., Ahmad, O., and Shen, P. (2021). Targeting Tumor-Associated Macrophages as an Antitumor Strategy. Biochem. Pharmacol. 183, 114354. doi:10.1016/j.bcp.2020.114354
Cho, S. W., Kim, Y. A., Sun, H. J., Kim, Y. A., Oh, B. C., Yi, K. H., et al. (2016). CXCL16 Signaling Mediated Macrophage Effects on Tumor Invasion of Papillary Thyroid Carcinoma. Endocr. Relat. Cancer 23 (2), 113–124. doi:10.1530/ERC-15-0196
Chong, B. F., Tseng, L. C., Hosler, G. A., Teske, N. M., Zhang, S., Karp, D. R., et al. (2015). A Subset of CD163+ Macrophages Displays Mixed Polarizations in Discoid Lupus Skin. Arthritis Res. Ther. 17, 324. doi:10.1186/s13075-015-0839-3
Ciampi, R., Mian, C., Fugazzola, L., Cosci, B., Romei, C., Barollo, S., et al. (2013). Evidence of a Low Prevalence of RAS Mutations in a Large Medullary Thyroid Cancer Series. Thyroid 23 (1), 50–57. doi:10.1089/thy.2012.0207
Coffelt, S. B., Lewis, C. E., Naldini, L., Brown, J. M., Ferrara, N., and De Palma, M. (2010). Elusive Identities and Overlapping Phenotypes of Proangiogenic Myeloid Cells in Tumors. Am. J. Pathol. 176 (4), 1564–1576. doi:10.2353/ajpath.2010.090786
Cohen, E. E., Tortorici, M., Kim, S., Ingrosso, A., Pithavala, Y. K., and Bycott, P. (2014). A Phase II Trial of Axitinib in Patients with Various Histologic Subtypes of Advanced Thyroid Cancer: Long-Term Outcomes and Pharmacokinetic/pharmacodynamic Analyses. Cancer Chemother. Pharmacol. 74 (6), 1261–1270. doi:10.1007/s00280-014-2604-8
Cunha, L. L., Morari, E. C., Guihen, A. C., Razolli, D., Gerhard, R., Nonogaki, S., et al. (2012). Infiltration of a Mixture of Immune Cells May Be Related to Good Prognosis in Patients with Differentiated Thyroid Carcinoma. Clin. Endocrinol. (Oxf) 77 (6), 918–925. doi:10.1111/j.1365-2265.2012.04482.x
Cunha, L. L., Morari, E. C., Nonogaki, S., Marcello, M. A., Soares, F. A., Vassallo, J., et al. (2017). Interleukin 10 Expression Is Related to Aggressiveness and Poor Prognosis of Patients with Thyroid Cancer. Cancer Immunol. Immunother. 66 (2), 141–148. doi:10.1007/s00262-016-1924-4
de Sousa, J. R., de Sousa, R. P., Aarão, T. L., Dias, L. B., Carneiro, F. R., Fuzii, H. T., et al. (2016). In Situ expression of M2 Macrophage Subpopulation in Leprosy Skin Lesions. Acta Trop. 157, 108–114. doi:10.1016/j.actatropica.2016.01.008
de Visser, K. E., Eichten, A., and Coussens, L. M. (2006). Paradoxical Roles of the Immune System during Cancer Development. Nat. Rev. Cancer 6 (1), 24–37. doi:10.1038/nrc1782
Fang, W., Ye, L., Shen, L., Cai, J., Huang, F., Wei, Q., et al. (2014). Tumor-associated Macrophages Promote the Metastatic Potential of Thyroid Papillary Cancer by Releasing CXCL8. Carcinogenesis 35 (8), 1780–1787. doi:10.1093/carcin/bgu060
Ferrari, S. M., Fallahi, P., Galdiero, M. R., Ruffilli, I., Elia, G., Ragusa, F., et al. (2019). Immune and Inflammatory Cells in Thyroid Cancer Microenvironment. Int. J. Mol. Sci. 20 (18). doi:10.3390/ijms20184413
Fozzatti, L., and Cheng, S. Y. (2020). Tumor Cells and Cancer-Associated Fibroblasts: A Synergistic Crosstalk to Promote Thyroid Cancer. Endocrinol. Metab. (Seoul) 35 (4), 673–680. doi:10.3803/EnM.2020.401
Fugazzola, L., Colombo, C., Perrino, M., and Muzza, M. (2011). Papillary Thyroid Carcinoma and Inflammation. Front. Endocrinol. (Lausanne) 2, 88. doi:10.3389/fendo.2011.00088
Galdiero, M. R., Garlanda, C., Jaillon, S., Marone, G., and Mantovani, A. (2013). Tumor Associated Macrophages and Neutrophils in Tumor Progression. J. Cel Physiol 228 (7), 1404–1412. doi:10.1002/jcp.24260
Germano, G., Frapolli, R., Belgiovine, C., Anselmo, A., Pesce, S., Liguori, M., et al. (2013). Role of Macrophage Targeting in the Antitumor Activity of Trabectedin. Cancer Cell 23 (2), 249–262. doi:10.1016/j.ccr.2013.01.008
Gianì, F., Vella, V., Tumino, D., Malandrino, P., and Frasca, F. (2020). The Possible Role of Cancer Stem Cells in the Resistance to Kinase Inhibitors of Advanced Thyroid Cancer. Cancers (Basel) 12 (8). doi:10.3390/cancers12082249
Goldberg, J. L., and Sondel, P. M. (2015). Enhancing Cancer Immunotherapy via Activation of Innate Immunity. Semin. Oncol. 42 (4), 562–572. doi:10.1053/j.seminoncol.2015.05.012
Gomez, K. E., Wu, F., Keysar, S. B., Morton, J. J., Miller, B., Chimed, T. S., et al. (2020). Cancer Cell CD44 Mediates Macrophage/Monocyte-Driven Regulation of Head and Neck Cancer Stem Cells. Cancer Res. 80 (19), 4185–4198. doi:10.1158/0008-5472.CAN-20-1079
Grassi, E. S., Ghiandai, V., and Persani, L. (2021). Thyroid Cancer Stem-like Cells: From Microenvironmental Niches to Therapeutic Strategies. J. Clin. Med. 10 (7). doi:10.3390/jcm10071455
Guarino, V., Castellone, M. D., Avilla, E., and Melillo, R. M. (2010). Thyroid Cancer and Inflammation. Mol. Cel Endocrinol 321 (1), 94–102. doi:10.1016/j.mce.2009.10.003
Gulubova, M. V., and Ivanova, K. V. (2019). The Expression of Tumor-Associated Macrophages and Multinucleated Giant Cells in Papillary Thyroid Carcinoma. Open Access Maced J. Med. Sci. 7 (23), 3944–3949. doi:10.3889/oamjms.2019.715
Gunda, V., Gigliotti, B., Ndishabandi, D., Ashry, T., McCarthy, M., Zhou, Z., et al. (2018). Combinations of BRAF Inhibitor and Anti-PD-1/pd-L1 Antibody Improve Survival and Tumour Immunity in an Immunocompetent Model of Orthotopic Murine Anaplastic Thyroid Cancer. Br. J. Cancer 119 (10), 1223–1232. doi:10.1038/s41416-018-0296-2
Hardin, H., Helein, H., Meyer, K., Robertson, S., Zhang, R., Zhong, W., et al. (2018). Thyroid Cancer Stem-like Cell Exosomes: Regulation of EMT via Transfer of lncRNAs. Lab. Invest. 98 (9), 1133–1142. doi:10.1038/s41374-018-0065-0
Hattori, Y., Shibuya, K., Kojima, K., Miatmoko, A., Kawano, K., Ozaki, K., et al. (2015). Zoledronic Acid Enhances Antitumor Efficacy of Liposomal Doxorubicin. Int. J. Oncol. 47 (1), 211–219. doi:10.3892/ijo.2015.2991
Haugen, B. R., Alexander, E. K., Bible, K. C., Doherty, G. M., Mandel, S. J., Nikiforov, Y. E., et al. (2015). 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26 (1), 1–133. doi:10.1089/thy.2015.0020
Hou, P., Liu, D., Shan, Y., Hu, S., Studeman, K., Condouris, S., et al. (2007). Genetic Alterations and Their Relationship in the Phosphatidylinositol 3-kinase/Akt Pathway in Thyroid Cancer. Clin. Cancer Res. 13 (4), 1161–1170. doi:10.1158/1078-0432.CCR-06-1125
Huang, F. J., Zhou, X. Y., Ye, L., Fei, X. C., Wang, S., Wang, W., et al. (2016). Follicular Thyroid Carcinoma but Not Adenoma Recruits Tumor-Associated Macrophages by Releasing CCL15. BMC Cancer 16, 98. doi:10.1186/s12885-016-2114-7
Huang, J. K., Ma, L., Song, W. H., Lu, B. Y., Huang, Y. B., Dong, H. M., et al. (2017). LncRNA-MALAT1 Promotes Angiogenesis of Thyroid Cancer by Modulating Tumor-Associated Macrophage FGF2 Protein Secretion. J. Cel Biochem 118 (12), 4821–4830. doi:10.1002/jcb.26153
Hughes, R., Qian, B. Z., Rowan, C., Muthana, M., Keklikoglou, I., Olson, O. C., et al. (2015). Perivascular M2 Macrophages Stimulate Tumor Relapse after Chemotherapy. Cancer Res. 75 (17), 3479–3491. doi:10.1158/0008-5472.CAN-14-3587
Hwang, J. H., Hwang, J. H., Chung, H. K., Kim, D. W., Hwang, E. S., Suh, J. M., et al. (2003). CXC Chemokine Receptor 4 Expression and Function in Human Anaplastic Thyroid Cancer Cells. J. Clin. Endocrinol. Metab. 88 (1), 408–416. doi:10.1210/jc.2002-021381
Imam, S., Dar, P., Paparodis, R., Almotah, K., Al-Khudhair, A., Hasan, S. A., et al. (2019). Nature of Coexisting Thyroid Autoimmune Disease Determines success or Failure of Tumor Immunity in Thyroid Cancer. J. Immunother. Cancer 7 (1), 3. doi:10.1186/s40425-018-0483-y
Jayasingam, S. D., Citartan, M., Thang, T. H., Mat Zin, A. A., Ang, K. C., and Ch'ng, E. S. (2019). Evaluating the Polarization of Tumor-Associated Macrophages into M1 and M2 Phenotypes in Human Cancer Tissue: Technicalities and Challenges in Routine Clinical Practice. Front. Oncol. 9, 1512. doi:10.3389/fonc.2019.01512
Jeannin, P., Paolini, L., Adam, C., and Delneste, Y. (2018). The Roles of CSFs on the Functional Polarization of Tumor-Associated Macrophages. FEBS J. 285 (4), 680–699. doi:10.1111/febs.14343
Jung, K. Y., Cho, S. W., Kim, Y. A., Kim, D., Oh, B. C., Park, D. J., et al. (2015). Cancers with Higher Density of Tumor-Associated Macrophages Were Associated with Poor Survival Rates. J. Pathol. Transl Med. 49 (4), 318–324. doi:10.4132/jptm.2015.06.01
Kabasawa, T., Ohe, R., Aung, N. Y., Urano, Y., Kitaoka, T., Tamazawa, N., et al. (2021). Potential Role of M2 TAMs Around Lymphatic Vessels during Lymphatic Invasion in Papillary Thyroid Carcinoma. Sci. Rep. 11 (1), 1150. doi:10.1038/s41598-020-80694-3
Kim, M. J., Sun, H. J., Song, Y. S., Yoo, S. K., Kim, Y. A., Seo, J. S., et al. (2019). CXCL16 Positively Correlated with M2-Macrophage Infiltration, Enhanced Angiogenesis, and Poor Prognosis in Thyroid Cancer. Sci. Rep. 9 (1), 13288. doi:10.1038/s41598-019-49613-z
Kim, S., Cho, S. W., Min, H. S., Kim, K. M., Yeom, G. J., Kim, E. Y., et al. (2013). The Expression of Tumor-Associated Macrophages in Papillary Thyroid Carcinoma. Endocrinol. Metab. (Seoul) 28 (3), 192–198. doi:10.3803/EnM.2013.28.3.192
Knauf, J. A., Sartor, M. A., Medvedovic, M., Lundsmith, E., Ryder, M., Salzano, M., et al. (2011). Progression of BRAF-Induced Thyroid Cancer Is Associated with Epithelial-Mesenchymal Transition Requiring Concomitant MAP Kinase and TGFβ Signaling. Oncogene 30 (28), 3153–3162. doi:10.1038/onc.2011.44
Kobawala, T. P., Patel, G. H., Gajjar, D. R., Patel, K. N., Thakor, P. B., Parekh, U. B., et al. (2011). Clinical Utility of Serum Interleukin-8 and Interferon-Alpha in Thyroid Diseases. J. Thyroid Res. 2011, 270149. doi:10.4061/2011/270149
Koehler, V. F., Adam, P., Frank-Raue, K., Raue, F., Berg, E., Hoster, E., et al. (2021). Real-World Efficacy and Safety of Cabozantinib and Vandetanib in Advanced Medullary Thyroid Cancer. Thyroid 31 (3), 459–469. doi:10.1089/thy.2020.0206
Kogure, A., Kosaka, N., and Ochiya, T. (2019). Cross-talk between Cancer Cells and Their Neighbors via miRNA in Extracellular Vesicles: an Emerging Player in Cancer Metastasis. J. Biomed. Sci. 26 (1), 7. doi:10.1186/s12929-019-0500-6
Komohara, Y., Fujiwara, Y., Ohnishi, K., and Takeya, M. (2016). Tumor-associated Macrophages: Potential Therapeutic Targets for Anti-cancer Therapy. Adv. Drug Deliv. Rev. 99 (Pt B), 180–185. doi:10.1016/j.addr.2015.11.009
Kotwal, A., Erickson, D., Geske, J. R., Hay, I. D., and Castro, M. R. (2021). Predicting Outcomes in Sporadic and Hereditary Medullary Thyroid Carcinoma over Two Decades. Thyroid 31 (4), 616–626. doi:10.1089/thy.2020.0167
Kumar, V., and Gabrilovich, D. I. (2014). Hypoxia-inducible Factors in Regulation of Immune Responses in Tumour Microenvironment. Immunology 143 (4), 512–519. doi:10.1111/imm.12380
La Vecchia, C., Malvezzi, M., Bosetti, C., Garavello, W., Bertuccio, P., Levi, F., et al. (2015). Thyroid Cancer Mortality and Incidence: a Global Overview. Int. J. Cancer 136 (9), 2187–2195. doi:10.1002/ijc.29251
Lam, E. T., Ringel, M. D., Kloos, R. T., Prior, T. W., Knopp, M. V., Liang, J., et al. (2010). Phase II Clinical Trial of Sorafenib in Metastatic Medullary Thyroid Cancer. J. Clin. Oncol. 28 (14), 2323–2330. doi:10.1200/JCO.2009.25.0068
Lan, L., Luo, Y., Cui, D., Shi, B. Y., Deng, W., Huo, L. L., et al. (2013). Epithelial-mesenchymal Transition Triggers Cancer Stem Cell Generation in Human Thyroid Cancer Cells. Int. J. Oncol. 43 (1), 113–120. doi:10.3892/ijo.2013.1913
Lei, X., Lei, Y., Li, J. K., Du, W. X., Li, R. G., Yang, J., et al. (2020). Immune Cells within the Tumor Microenvironment: Biological Functions and Roles in Cancer Immunotherapy. Cancer Lett. 470, 126–133. doi:10.1016/j.canlet.2019.11.009
Li, W., Zhang, X., Wu, F., Zhou, Y., Bao, Z., Li, H., et al. (2019). Gastric Cancer-Derived Mesenchymal Stromal Cells Trigger M2 Macrophage Polarization that Promotes Metastasis and EMT in Gastric Cancer. Cell Death Dis 10 (12), 918. doi:10.1038/s41419-019-2131-y
Li, X., Shan, C., Wu, Z., Yu, H., Yang, A., and Tan, B. (2020). Emodin Alleviated Pulmonary Inflammation in Rats with LPS-Induced Acute Lung Injury through Inhibiting the mTOR/HIF-1α/VEGF Signaling Pathway. Inflamm. Res. 69 (4), 365–373. doi:10.1007/s00011-020-01331-3
Li, X. J., Gangadaran, P., Kalimuthu, S., Oh, J. M., Zhu, L., Jeong, S. Y., et al. (2016). Role of Pulmonary Macrophages in Initiation of Lung Metastasis in Anaplastic Thyroid Cancer. Int. J. Cancer 139 (11), 2583–2592. doi:10.1002/ijc.30387
Liu, H., Dong, H., Jiang, L., Li, Z., and Ma, X. (2018). Bleomycin Inhibits Proliferation and Induces Apoptosis in TPC-1 Cells through Reversing M2-Macrophages Polarization. Oncol. Lett. 16 (3), 3858–3866. doi:10.3892/ol.2018.9103
Liu, L., Wang, X., Li, X., Wu, X., Tang, M., and Wang, X. (2018). Upregulation of IGF1 by Tumor-Associated Macrophages Promotes the Proliferation and Migration of Epithelial Ovarian Cancer Cells. Oncol. Rep. 39 (2), 818–826. doi:10.3892/or.2017.6148
LiVolsi, V. A. (2011). Papillary Thyroid Carcinoma: an Update. Mod. Pathol. 24 (Suppl. 2), S1–S9. doi:10.1038/modpathol.2010.129
Lu, X. (2017). Impact of IL-12 in Cancer. Curr. Cancer Drug Targets 17 (8), 682–697. doi:10.2174/1568009617666170427102729
Lumachi, F., Basso, S. M., and Orlando, R. (2010). Cytokines, Thyroid Diseases and Thyroid Cancer. Cytokine 50 (3), 229–233. doi:10.1016/j.cyto.2010.03.005
Lv, J., Chen, F. K., Liu, C., Liu, P. J., Feng, Z. P., Jia, L., et al. (2020). Zoledronic Acid Inhibits Thyroid Cancer Stemness and Metastasis by Repressing M2-like Tumor-Associated Macrophages Induced Wnt/β-Catenin Pathway. Life Sci. 256, 117925. doi:10.1016/j.lfs.2020.117925
Lv, J., Feng, Z. P., Chen, F. K., Liu, C., Jia, L., Liu, P. J., et al. (2021). M2-like Tumor-Associated Macrophages-Secreted Wnt1 and Wnt3a Promotes Dedifferentiation and Metastasis via Activating β-catenin Pathway in Thyroid Cancer. Mol. Carcinog 60 (1), 25–37. doi:10.1002/mc.23268
Lv, J., Liu, C., Chen, F. K., Feng, Z. P., Jia, L., Liu, P. J., et al. (2021). M2-like T-umour-associated M-acrophage-secreted IGF P-romotes T-hyroid C-ancer S-temness and M-etastasis by A-ctivating the PI3K/AKT/mTOR P-athway. Mol. Med. Rep. 24 (2). doi:10.3892/mmr.2021.12249
MacDonald, L., Jenkins, J., Purvis, G., Lee, J., and Franco, A. T. (2020). The Thyroid Tumor Microenvironment: Potential Targets for Therapeutic Intervention and Prognostication. Horm. Cancer 11 (5-6), 205–217. doi:10.1007/s12672-020-00390-6
Magee, J. A., Piskounova, E., and Morrison, S. J. (2012). Cancer Stem Cells: Impact, Heterogeneity, and Uncertainty. Cancer Cell 21 (3), 283–296. doi:10.1016/j.ccr.2012.03.003
Mantovani, A., and Allavena, P. (2015). The Interaction of Anticancer Therapies with Tumor-Associated Macrophages. J. Exp. Med. 212 (4), 435–445. doi:10.1084/jem.20150295
Mantovani, A., Marchesi, F., Malesci, A., Laghi, L., and Allavena, P. (2017). Tumour-associated Macrophages as Treatment Targets in Oncology. Nat. Rev. Clin. Oncol. 14 (7), 399–416. doi:10.1038/nrclinonc.2016.217
Mantovani, A., Savino, B., Locati, M., Zammataro, L., Allavena, P., and Bonecchi, R. (2010). The Chemokine System in Cancer Biology and Therapy. Cytokine Growth Factor. Rev. 21 (1), 27–39. doi:10.1016/j.cytogfr.2009.11.007
Mantovani, A., Sozzani, S., Locati, M., Allavena, P., and Sica, A. (2002). Macrophage Polarization: Tumor-Associated Macrophages as a Paradigm for Polarized M2 Mononuclear Phagocytes. Trends Immunol. 23 (11), 549–555. doi:10.1016/s1471-4906(02)02302-5
Markham, A. (2020). Pralsetinib: First Approval. Drugs 80 (17), 1865–1870. doi:10.1007/s40265-020-01427-4
Mazzoni, M., Mauro, G., Erreni, M., Romeo, P., Minna, E., Vizioli, M. G., et al. (2019). Senescent Thyrocytes and Thyroid Tumor Cells Induce M2-like Macrophage Polarization of Human Monocytes via a PGE2-dependent Mechanism. J. Exp. Clin. Cancer Res. 38 (1), 208. doi:10.1186/s13046-019-1198-8
Melaccio, A., Sgaramella, L. I., Pasculli, A., Di Meo, G., Gurrado, A., Prete, F. P., et al. (2021). Prognostic and Therapeutic Role of Angiogenic Microenvironment in Thyroid Cancer. Cancers (Basel) 13 (11). doi:10.3390/cancers13112775
Menicali, E., Guzzetti, M., Morelli, S., Moretti, S., and Puxeddu, E. (2020). Immune Landscape of Thyroid Cancers: New Insights. Front. Endocrinol. (Lausanne) 11, 637826. doi:10.3389/fendo.2020.637826
Mertins, S. D. (2014). Cancer Stem Cells: a Systems Biology View of Their Role in Prognosis and Therapy. Anticancer Drugs 25 (4), 353–367. doi:10.1097/CAD.0000000000000075
Mironska, A., Łukaszewicz-Zajac, M., and Mroczko, B. (2019). Clinical Significance of Selected Chemokines in Thyroid Cancer. Anticancer Res. 39 (6), 2715–2720. doi:10.21873/anticanres.13397
Mocellin, S., Marincola, F. M., and Young, H. A. (2005). Interleukin-10 and the Immune Response against Cancer: a Counterpoint. J. Leukoc. Biol. 78 (5), 1043–1051. doi:10.1189/jlb.0705358
Movahedi, K., and Van Ginderachter, J. A. (2016). The Ontogeny and Microenvironmental Regulation of Tumor-Associated Macrophages. Antioxid. Redox Signal. 25 (14), 775–791. doi:10.1089/ars.2016.6704
Mukaida, N., Sasaki, S., and Baba, T. (2014). Chemokines in Cancer Development and Progression and Their Potential as Targeting Molecules for Cancer Treatment. Mediators Inflamm. 2014, 170381. doi:10.1155/2014/170381
Murray, P. J., Allen, J. E., Biswas, S. K., Fisher, E. A., Gilroy, D. W., Goerdt, S., et al. (2014). Macrophage Activation and Polarization: Nomenclature and Experimental Guidelines. Immunity 41 (1), 14–20. doi:10.1016/j.immuni.2014.06.008
Muzza, M., Degl'Innocenti, D., Colombo, C., Perrino, M., Ravasi, E., Rossi, S., et al. (2010). The Tight Relationship between Papillary Thyroid Cancer, Autoimmunity and Inflammation: Clinical and Molecular Studies. Clin. Endocrinol. (Oxf) 72 (5), 702–708. doi:10.1111/j.1365-2265.2009.03699.x
Na, K. J., and Choi, H. (2018). Immune Landscape of Papillary Thyroid Cancer and Immunotherapeutic Implications. Endocr. Relat. Cancer 25 (5), 523–531. doi:10.1530/ERC-17-0532
Naoum, G. E., Morkos, M., Kim, B., and Arafat, W. (2018). Novel Targeted Therapies and Immunotherapy for Advanced Thyroid Cancers. Mol. Cancer 17 (1), 51. doi:10.1186/s12943-018-0786-0
Nguyen, L. V., Vanner, R., Dirks, P., and Eaves, C. J. (2012). Cancer Stem Cells: an Evolving Concept. Nat. Rev. Cancer 12 (2), 133–143. doi:10.1038/nrc3184
Noy, R., and Pollard, J. W. (2014). Tumor-associated Macrophages: from Mechanisms to Therapy. Immunity 41 (1), 49–61. doi:10.1016/j.immuni.2014.06.010
Ojalvo, L. S., Whittaker, C. A., Condeelis, J. S., and Pollard, J. W. (2010). Gene Expression Analysis of Macrophages that Facilitate Tumor Invasion Supports a Role for Wnt-Signaling in Mediating Their Activity in Primary Mammary Tumors. J. Immunol. 184 (2), 702–712. doi:10.4049/jimmunol.0902360
Osterberg, N., Ferrara, N., Vacher, J., Gaedicke, S., Niedermann, G., Weyerbrock, A., et al. (2016). Decrease of VEGF-A in Myeloid Cells Attenuates Glioma Progression and Prolongs Survival in an Experimental Glioma Model. Neuro Oncol. 18 (7), 939–949. doi:10.1093/neuonc/now005
Pan, Y., Yu, Y., Wang, X., and Zhang, T. (2020). Tumor-Associated Macrophages in Tumor Immunity. Front. Immunol. 11, 583084. doi:10.3389/fimmu.2020.583084
Parhar, R. S., Zou, M., Al-Mohanna, F. A., Baitei, E. Y., Assiri, A. M., Meyer, B. F., et al. (2016). IL-12 Immunotherapy of Braf(V600E)-Induced Papillary Thyroid Cancer in a Mouse Model. Lab. Invest. 96 (1), 89–97. doi:10.1038/labinvest.2015.126
Pathria, P., Louis, T. L., and Varner, J. A. (2019). Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 40 (4), 310–327. doi:10.1016/j.it.2019.02.003
Pawig, L., Klasen, C., Weber, C., Bernhagen, J., and Noels, H. (2015). Diversity and Inter-connections in the CXCR4 Chemokine Receptor/Ligand Family: Molecular Perspectives. Front. Immunol. 6, 429. doi:10.3389/fimmu.2015.00429
Perrotta, C., Buldorini, M., Assi, E., Cazzato, D., De Palma, C., Clementi, E., et al. (2014). The Thyroid Hormone Triiodothyronine Controls Macrophage Maturation and Functions: Protective Role during Inflammation. Am. J. Pathol. 184 (1), 230–247. doi:10.1016/j.ajpath.2013.10.006
Person, L., Lacour, B., Faure, L., Guissou, S., Poulalhon, C., Orbach, D., et al. (2021). Childhood Head and Neck Cancer in France: Incidence, Survival and Trends from 2000 to 2015. Int. J. Pediatr. Otorhinolaryngol. 150, 110858. doi:10.1016/j.ijporl.2021.110858
Pietras, A., Katz, A. M., Ekström, E. J., Wee, B., Halliday, J. J., Pitter, K. L., et al. (2014). Osteopontin-CD44 Signaling in the Glioma Perivascular Niche Enhances Cancer Stem Cell Phenotypes and Promotes Aggressive Tumor Growth. Cell Stem Cell 14 (3), 357–369. doi:10.1016/j.stem.2014.01.005
Porta, C., Riboldi, E., Ippolito, A., and Sica, A. (2015). Molecular and Epigenetic Basis of Macrophage Polarized Activation. Semin. Immunol. 27 (4), 237–248. doi:10.1016/j.smim.2015.10.003
Poschke, I., Mougiakakos, D., and Kiessling, R. (2011). Camouflage and Sabotage: Tumor Escape from the Immune System. Cancer Immunol. Immunother. 60 (8), 1161–1171. doi:10.1007/s00262-011-1012-8
Pradella, D., Naro, C., Sette, C., and Ghigna, C. (2017). EMT and Stemness: Flexible Processes Tuned by Alternative Splicing in Development and Cancer Progression. Mol. Cancer 16 (1), 8. doi:10.1186/s12943-016-0579-2
Prendergast, G. C., Metz, R., and Muller, A. J. (2010). Towards a Genetic Definition of Cancer-Associated Inflammation: Role of the Ido Pathway. Am. J. Pathol. 176 (5), 2082–2087. doi:10.2353/ajpath.2010.091173
Prete, A., Matrone, A., Gambale, C., Torregrossa, L., Minaldi, E., Romei, C., et al. (2021). Poorly Differentiated and Anaplastic Thyroid Cancer: Insights into Genomics, Microenvironment and New Drugs. Cancers (Basel) 13 (13). doi:10.3390/cancers13133200
Richards, D. M., Hettinger, J., and Feuerer, M. (2013). Monocytes and Macrophages in Cancer: Development and Functions. Cancer Microenviron 6 (2), 179–191. doi:10.1007/s12307-012-0123-x
Ryder, M., Ghossein, R. A., Ricarte-Filho, J. C., Knauf, J. A., and Fagin, J. A. (2008). Increased Density of Tumor-Associated Macrophages Is Associated with Decreased Survival in Advanced Thyroid Cancer. Endocr. Relat. Cancer 15 (4), 1069–1074. doi:10.1677/ERC-08-0036
Ryder, M., Gild, M., Hohl, T. M., Pamer, E., Knauf, J., Ghossein, R., et al. (2013). Genetic and Pharmacological Targeting of CSF-1/CSF-1R Inhibits Tumor-Associated Macrophages and Impairs BRAF-Induced Thyroid Cancer Progression. PLoS One 8 (1), e54302. doi:10.1371/journal.pone.0054302
Sainz, B., Carron, E., Vallespinós, M., and Machado, H. L. (2016). Cancer Stem Cells and Macrophages: Implications in Tumor Biology and Therapeutic Strategies. Mediators Inflamm. 2016, 9012369. doi:10.1155/2016/9012369
Sancho, M., Vieira, J. M., Casalou, C., Mesquita, M., Pereira, T., Cavaco, B. M., et al. (2006). Expression and Function of the Chemokine Receptor CCR7 in Thyroid Carcinomas. J. Endocrinol. 191 (1), 229–238. doi:10.1677/joe.1.06688
Schürch, C. M., Roelli, M. A., Forster, S., Wasmer, M. H., Brühl, F., Maire, R. S., et al. (2019). Targeting CD47 in Anaplastic Thyroid Carcinoma Enhances Tumor Phagocytosis by Macrophages and Is a Promising Therapeutic Strategy. Thyroid 29 (7), 979–992. doi:10.1089/thy.2018.0555
Sedighzadeh, S. S., Khoshbin, A. P., Razi, S., Keshavarz-Fathi, M., and Rezaei, N. (2021). A Narrative Review of Tumor-Associated Macrophages in Lung Cancer: Regulation of Macrophage Polarization and Therapeutic Implications. Transl Lung Cancer Res. 10 (4), 1889–1916. doi:10.21037/tlcr-20-1241
Shi, Y., Fan, X., Deng, H., Brezski, R. J., Rycyzyn, M., Jordan, R. E., et al. (2015). Trastuzumab Triggers Phagocytic Killing of High HER2 Cancer Cells In Vitro and In Vivo by Interaction with Fcγ Receptors on Macrophages. J. Immunol. 194 (9), 4379–4386. doi:10.4049/jimmunol.1402891
Sica, A., and Mantovani, A. (2012). Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Invest. 122 (3), 787–795. doi:10.1172/JCI59643
Sica, A., Schioppa, T., Mantovani, A., and Allavena, P. (2006). Tumour-associated Macrophages Are a Distinct M2 Polarised Population Promoting Tumour Progression: Potential Targets of Anti-cancer Therapy. Eur. J. Cancer 42 (6), 717–727. doi:10.1016/j.ejca.2006.01.003
Sloot, Y. J. E., Rabold, K., Netea, M. G., Smit, J. W. A., Hoogerbrugge, N., and Netea-Maier, R. T. (2019). Effect of PTEN Inactivating Germline Mutations on Innate Immune Cell Function and Thyroid Cancer-Induced Macrophages in Patients with PTEN Hamartoma Tumor Syndrome. Oncogene 38 (19), 3743–3755. doi:10.1038/s41388-019-0685-x
Sloot, Y. J. E., Rabold, K., Ulas, T., De Graaf, D. M., Heinhuis, B., Händler, K., et al. (2019). Interplay between Thyroid Cancer Cells and Macrophages: Effects on IL-32 Mediated Cell Death and Thyroid Cancer Cell Migration. Cel Oncol (Dordr) 42 (5), 691–703. doi:10.1007/s13402-019-00457-9
Smallridge, R. C., Ain, K. B., Asa, S. L., Bible, K. C., Brierley, J. D., Burman, K. D., et al. (2012). American Thyroid Association Guidelines for Management of Patients with Anaplastic Thyroid Cancer. Thyroid 22 (11), 1104–1139. doi:10.1089/thy.2012.0302
Solinas, G., Schiarea, S., Liguori, M., Fabbri, M., Pesce, S., Zammataro, L., et al. (2010). Tumor-conditioned Macrophages Secrete Migration-Stimulating Factor: a New Marker for M2-Polarization, Influencing Tumor Cell Motility. J. Immunol. 185 (1), 642–652. doi:10.4049/jimmunol.1000413
Sowder, A. M., Witt, B. L., and Hunt, J. P. (2018). An Update on the Risk of Lymph Node Metastasis for the Follicular Variant of Papillary Thyroid Carcinoma with the New Diagnostic Paradigm. Head Neck Pathol. 12 (1), 105–109. doi:10.1007/s12105-017-0835-9
Stassi, G., Todaro, M., Zerilli, M., Ricci-Vitiani, L., Di Liberto, D., Patti, M., et al. (2003). Thyroid Cancer Resistance to Chemotherapeutic Drugs via Autocrine Production of Interleukin-4 and Interleukin-10. Cancer Res. 63 (20), 6784–6790.
Subbiah, V., Kreitman, R. J., Wainberg, Z. A., Cho, J. Y., Schellens, J. H. M., Soria, J. C., et al. (2018). Dabrafenib and Trametinib Treatment in Patients with Locally Advanced or Metastatic BRAF V600-Mutant Anaplastic Thyroid Cancer. J. Clin. Oncol. 36 (1), 7–13. doi:10.1200/JCO.2017.73.6785
Sumitomo, R., Hirai, T., Fujita, M., Murakami, H., Otake, Y., and Huang, C. L. (2019). M2 Tumor-Associated Macrophages Promote Tumor Progression in Non-small-cell Lung Cancer. Exp. Ther. Med. 18 (6), 4490–4498. doi:10.3892/etm.2019.8068
Sun, X., Gao, D., Gao, L., Zhang, C., Yu, X., Jia, B., et al. (2015). Molecular Imaging of Tumor-Infiltrating Macrophages in a Preclinical Mouse Model of Breast Cancer. Theranostics 5 (6), 597–608. doi:10.7150/thno.11546
Sun, Y., Du, F., Gao, M., Ji, Q., Li, Z., Zhang, Y., et al. (2018). Anlotinib for the Treatment of Patients with Locally Advanced or Metastatic Medullary Thyroid Cancer. Thyroid 28 (11), 1455–1461. doi:10.1089/thy.2018.0022
Tahara, M., Kiyota, N., Yamazaki, T., Chayahara, N., Nakano, K., Inagaki, L., et al. (2017). Lenvatinib for Anaplastic Thyroid Cancer. Front. Oncol. 7, 25. doi:10.3389/fonc.2017.00025
Tanaka, K., Kurebayashi, J., Sohda, M., Nomura, T., Prabhakar, U., Yan, L., et al. (2009). The Expression of Monocyte Chemotactic Protein-1 in Papillary Thyroid Carcinoma Is Correlated with Lymph Node Metastasis and Tumor Recurrence. Thyroid 19 (1), 21–25. doi:10.1089/thy.2008.0237
Varol, C., Mildner, A., and Jung, S. (2015). Macrophages: Development and Tissue Specialization. Annu. Rev. Immunol. 33, 643–675. doi:10.1146/annurev-immunol-032414-112220
Veschi, V., Verona, F., Lo Iacono, M., D'Accardo, C., Porcelli, G., Turdo, A., et al. (2020). Cancer Stem Cells in Thyroid Tumors: From the Origin to Metastasis. Front. Endocrinol. (Lausanne) 11, 566. doi:10.3389/fendo.2020.00566
Visciano, C., Liotti, F., Prevete, N., Cali', G., Franco, R., Collina, F., et al. (2015). Mast Cells Induce Epithelial-To-Mesenchymal Transition and Stem Cell Features in Human Thyroid Cancer Cells through an IL-8-Akt-Slug Pathway. Oncogene 34 (40), 5175–5186. doi:10.1038/onc.2014.441
Wagner, P. L., Moo, T. A., Arora, N., Liu, Y. F., Zarnegar, R., Scognamiglio, T., et al. (2008). The Chemokine Receptors CXCR4 and CCR7 Are Associated with Tumor Size and Pathologic Indicators of Tumor Aggressiveness in Papillary Thyroid Carcinoma. Ann. Surg. Oncol. 15 (10), 2833–2841. doi:10.1245/s10434-008-0064-2
Wang, J. R., Zafereo, M. E., Dadu, R., Ferrarotto, R., Busaidy, N. L., Lu, C., et al. (2019). Complete Surgical Resection Following Neoadjuvant Dabrafenib Plus Trametinib in BRAFV600E-Mutated Anaplastic Thyroid Carcinoma. Thyroid 29 (8), 1036–1043. doi:10.1089/thy.2019.0133
Wang, L. X., Zhang, S. X., Wu, H. J., Rong, X. L., and Guo, J. (2019). M2b Macrophage Polarization and its Roles in Diseases. J. Leukoc. Biol. 106 (2), 345–358. doi:10.1002/JLB.3RU1018-378RR
Wells, S. A., Asa, S. L., Dralle, H., Elisei, R., Evans, D. B., Gagel, R. F., et al. (2015). Revised American Thyroid Association Guidelines for the Management of Medullary Thyroid Carcinoma. Thyroid 25 (6), 567–610. doi:10.1089/thy.2014.0335
Weng, Y. S., Tseng, H. Y., Chen, Y. A., Shen, P. C., Al Haq, A. T., Chen, L. M., et al. (2019). MCT-1/miR-34a/IL-6/IL-6R Signaling axis Promotes EMT Progression, Cancer Stemness and M2 Macrophage Polarization in Triple-Negative Breast Cancer. Mol. Cancer 18 (1), 42. doi:10.1186/s12943-019-0988-0
Wirth, L. J., Sherman, E., Robinson, B., Solomon, B., Kang, H., Lorch, J., et al. (2020). Efficacy of Selpercatinib in RET-Altered Thyroid Cancers. N. Engl. J. Med. 383 (9), 825–835. doi:10.1056/NEJMoa2005651
Wu, K., Lin, K., Li, X., Yuan, X., Xu, P., Ni, P., et al. (2020). Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front. Immunol. 11, 1731. doi:10.3389/fimmu.2020.01731
Xie, Z., Li, X., He, Y., Wu, S., Wang, S., Sun, J., et al. (2020). Immune Cell Confrontation in the Papillary Thyroid Carcinoma Microenvironment. Front. Endocrinol. (Lausanne) 11, 570604. doi:10.3389/fendo.2020.570604
Yang, L., Shi, P., Zhao, G., Xu, J., Peng, W., Zhang, J., et al. (2020). Targeting Cancer Stem Cell Pathways for Cancer Therapy. Signal. Transduct Target. Ther. 5 (1), 8. doi:10.1038/s41392-020-0110-5
Yang, Y., Xia, S., Zhang, L., Wang, W., Chen, L., and Zhan, W. (2020). MiR-324-5p/PTPRD/CEBPD axis Promotes Papillary Thyroid Carcinoma Progression via Microenvironment Alteration. Cancer Biol. Ther. 21 (6), 522–532. doi:10.1080/15384047.2020.1736465
Yapa, S., Mulla, O., Green, V., England, J., and Greenman, J. (2017). The Role of Chemokines in Thyroid Carcinoma. Thyroid 27 (11), 1347–1359. doi:10.1089/thy.2016.0660
Yin, H., Zhang, X., Yang, P., Zhang, X., Peng, Y., Li, D., et al. (2021). RNA m6A Methylation Orchestrates Cancer Growth and Metastasis via Macrophage Reprogramming. Nat. Commun. 12 (1), 1394. doi:10.1038/s41467-021-21514-8
Yin, M., Li, X., Tan, S., Zhou, H. J., Ji, W., Bellone, S., et al. (2016). Tumor-associated Macrophages Drive Spheroid Formation during Early Transcoelomic Metastasis of Ovarian Cancer. J. Clin. Invest. 126 (11), 4157–4173. doi:10.1172/JCI87252
Zeng, W., Chang, H., Ma, M., and Li, Y. (2014). CCL20/CCR6 Promotes the Invasion and Migration of Thyroid Cancer Cells via NF-Kappa B Signaling-Induced MMP-3 Production. Exp. Mol. Pathol. 97 (1), 184–190. doi:10.1016/j.yexmp.2014.06.012
Zhang, C., Gu, X., Pan, M., Yuan, Q., and Cheng, H. (2021). Senescent Thyroid Tumor Cells Promote Their Migration by Inducing the Polarization of M2-like Macrophages. Clin. Transl Oncol. 23 (6), 1253–1261. doi:10.1007/s12094-020-02516-2
Zhang, L. J., Xiong, Y., Nilubol, N., He, M., Bommareddi, S., Zhu, X., et al. (2015). Testosterone Regulates Thyroid Cancer Progression by Modifying Tumor Suppressor Genes and Tumor Immunity. Carcinogenesis 36 (4), 420–428. doi:10.1093/carcin/bgv001
Zhang, P., Zuo, H., Ozaki, T., Nakagomi, N., and Kakudo, K. (2006). Cancer Stem Cell Hypothesis in Thyroid Cancer. Pathol. Int. 56 (9), 485–489. doi:10.1111/j.1440-1827.2006.01995.x
Zhang, S., Chen, S., Wang, Y., Zhan, Y., Li, J., Nong, X., et al. (2021). Association of a Novel Prognosis Model with Tumor Mutation Burden and Tumor-Infiltrating Immune Cells in Thyroid Carcinoma. Front. Genet. 12, 744304. doi:10.3389/fgene.2021.744304
Zhang, S., Li, D., Zhao, M., Yang, F., Sang, C., Yan, C., et al. (2021). Exosomal miR-183-5p Shuttled by M2 Polarized Tumor-Associated Macrophage Promotes the Development of Colon Cancer via Targeting THEM4 Mediated PI3K/AKT and NF-Κb Pathways. Front. Oncol. 11, 672684. doi:10.3389/fonc.2021.672684
Zheng, R., Chen, G., Li, X., Wei, X., Liu, C., and Derwahl, M. (2019). Effect of IL-6 on Proliferation of Human Thyroid Anaplastic Cancer Stem Cells. Int. J. Clin. Exp. Pathol. 12 (11), 3992–4001.
Zhou, W., Ke, S. Q., Huang, Z., Flavahan, W., Fang, X., Paul, J., et al. (2015). Periostin Secreted by Glioblastoma Stem Cells Recruits M2 Tumour-Associated Macrophages and Promotes Malignant Growth. Nat. Cel Biol 17 (2), 170–182. doi:10.1038/ncb3090
Keywords: thyroid cancer, cancer stem cell, tumor microenvironment, tumor associated macrophages, cytokines
Citation: Liu Q, Sun W and Zhang H (2022) Roles and new Insights of Macrophages in the Tumor Microenvironment of Thyroid Cancer. Front. Pharmacol. 13:875384. doi: 10.3389/fphar.2022.875384
Received: 14 February 2022; Accepted: 21 March 2022;
Published: 11 April 2022.
Edited by:
Dong-Hua Yang, St. John’s University, United StatesReviewed by:
Zhenhai Li, Shenzhen University, ChinaCopyright © 2022 Liu, Sun and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Zhang, aGFvemhhbmdAY211LmVkdS5jbg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.