- 1School of Traditional Chinese Medicine, Southern Medical University, Guangzhou, China
- 2School of Pharmacy, Guilin Medical University, Guilin, China
- 3Nephrology, Guilin TCM Hospital of China, Guilin, China
Objective: To investigate the therapeutic effect of petroleum ether extract of P. aculeate Miller (PEEP) on rheumatoid arthritis (RA).
Methods: In vitro: The Cell Counting Kit-8 (CCK-8) was used to detect cell activity and select the optimal concentration of the extract; the effective site was screened by nitric oxide (NO) colorimetric method and Q-PCR method; the expression of p38, p-p38, p-MK2, and Tristetraprolin (TTP) in RAW 264.7 cells were detected by Western blot. In vivo: The rat model was established by complete Freund’s adjuvant (CFA). The different doses of PEEP on CFA rats were observed with life status, paw swelling, spleen index, X-ray, Hematoxylin eosin (HE) staining; the secretion of Tumor necrosis factor α (TNF-α), interleukin-6 (IL-6) and Prostaglandin E2 (PGE2) were detected by Enzyme linked immunosorbent assay (ELISA); the expressions of p38, p-p38, p-MK2, and TTP in the ankle joints of CFA rats were detected by Western blot.
Result: In vitro: PEEP, Ethyl Acetate Extract of P. aculeate Miller (EEEP), N-butanol Extract of P. aculeate Miller (BEEP) have no toxic effects on RAW264.7 macrophages. PEEP, EEEP, and BEEP reduce the secretion of NO in RAW264.7 cells induced by lipopolysaccharide (LPS), only PEEP significantly inhibited the mRNA expression levels of inflammatory factors TNF-α and IL-6; PEEP-dependently reduce the secretion of TNF-α and IL-6, decrease the expression of p-p38 and p-MK2, and the level of TTP phosphorylation in LPS-induced RAW264.7 cells. In vivo: PEEP improve the living conditions of CFA rats, reduce foot swelling, spleen index, bone surface erosion and joint space narrowing; reduce the formation of synovial cells, inflammatory cells and pannus in the foot and ankle joints. PEEP reduce the secretion of TNF-α, IL-6, PGE2 in rat serum, downregulate the expression of p-p38 and p-MK2 in the ankle joint, and reduce the phosphorylation of TTP.
Conclusion: PEEP improve the living conditions of CFA rats, reduce the degree of foot swelling, protect immune organs, reduce inflammatory cell infiltration, cartilage damage, pannus formation, reduce inflammation and RA damage. The mechanism through regulating the signal pathway of p38 mitogen-activated protein kinase (p38/MAPK), which reduces the release of TNF-α, IL-6, and PGE2 in the serum.
1 Introduction
Rheumatoid arthritis is an autoimmune disease characterized by polyarthritis, progressive joint damage, and swelling deformities (Pu et al., 2016; Sparks, 2019; Shi et al., 2020). The pathological features of RA are joint synovial lesions, including excessive synovial cell proliferation, inflammatory cell infiltration, pannus formation, joint bone and cartilage destruction (Aletaha et al., 2011; Xing et al., 2018). According to relevant investigations and studies, the incidence of this disease worldwide is as high as 1%–2%. There are more than 5 million RA patients in China, most of whom are in the 30–50 years old age group. It is the second largest cause of disability in the Chinese population (van der Woude and van der Helm-van Mil, 2018). It can quickly develop into multi-system inflammation and irreversible joint damage, leading to a decline in quality of life, disability and death without proper treatment (Malmstrom et al., 2017). Because the etiology and pathogenesis of RA are not clearly understood (Mcinnes and Schett, 2011). The targets and methods of clinical treatment are not clear, which brings certain difficulties to treatment. There are no highly targeted therapeutic drugs in clinical practice, the treatment of RA still from the aspects of analgesia, anti-inflammatory, preventing or reduce joint deformation and damage and increasing joint mobility (Yuan et al., 2015; Shams et al., 2021). These drugs reduce joint inflammation, inhibit the development of lesions and irreversible bone destruction, but long-term use of these drugs lead to immune system decline, bone marrow suppression, liver and kidney function damage, gastrointestinal function decline, cartilage degeneration, infection (Abbasi et al., 2019; Koller et al., 2019). Therefore, it is clinically necessary to find effective drugs with low toxicity to treat RA. In recent years, ethnomedicine and some natural medicine products have attracted more and more researchers’ attention because of their unique safe and effective pharmacological activities (Jang et al., 2014). Ethnomedicine can be a new resource for the treatment of rheumatoid arthritis with more and more evidences.
P. aculeata Mill is a plant of the genus Pereskia in the Cactaceae family, also known as leaf cactus and tiger thorn. It is mainly produced in Yunnan, Guangdong, Guangxi and other regions south of the Yangtze River. It has a long history of folk medicine, with low side effects, and anti-inflammatory, antioxidant, antibacterial and other pharmacological activities (Tan et al., 2005; Kazama et al., 2012; Pinto and Scio, 2014; Pinto et al., 2015). Its medicinal liquor can treat bruises and rib pain. According to reports in the literature, there are as many as 30 chemical components in the essential oil of P. aculeata Mill leaves, among which the higher content includes phytol, palmitic acid, linoleic acid (Souza et al., 2014). In addition, the plant essential oil has the highest content of oxidized sesquiterpenes (44.92%), acorus spirenone comes next. The current research on the pharmacological effects of P. aculeata Mill mainly focuses on analgesia, cardiovascular protection and other related aspects. There are few studies on its anti-RA effect (Peng et al., 2018). The previous research show that P. aculeata Mill ethanol extract inhibited acetic acid-induced increase in vascular permeability and writhing behavior of mice in acetic acid-induced writhing reaction experiment, reduce the swelling of the feet of CFA rats and the level of IL-6 and other inflammatory cytokines in the rat serum, inhibit LPS-induced inflammatory response in RAW264.7 macrophages (Kanno et al., 2006).
This study aimed to screen effective sites by establishing an LPS-induced RAW264.7 macrophage inflammation model, and through the FCA rat model to evaluate the therapeutic effect and possible mechanism of PEEP, provide a more adequate theoretical basis for the development and utilization of PEEP.
2 Materials and Methods
2.1 Cell Culture and Laboratory Animals
Mouse macrophages RAW264.7 were from the Pharmacology Laboratory of Guilin Medical University, China, and were preserved by our laboratory for future generations.
Healthy female SD rats, SPF grade, 60 rats, weight (200 ± 20 g), provided by Hunan SJA Laboratory Animal Co., Ltd., license SCXK Xiang 2016-0002. Rats were kept at a temperature of 21°C–25°C and a relative humidity of 50%–60%. Rats in each group eat and drink freely.
2.2 Main Experimental Reagents
2.2.1 Main Drugs and Reagents
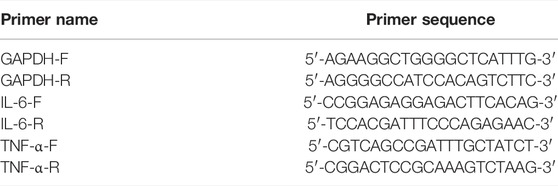
P. aculeata Mill petroleum ether extract; NO kit (Wuhan, Elabscience Biotechnology Co., Ltd.); p38MAPK antibody, p-p38MAPK antibody, p-MK2 antibody and TTP antibody (CST, Miami, FL, United States); β-Actin (Proteintech company, Chicago, IL, United States); Primary antibody and secondary antibody diluent (China, Beyotime Biotechnology); Rainbow marker (Thermo Fisher Scientific, Waltham, MA, United States); Fetal Bovine Serum (GIBCO Company, Waltham, MA, United States); Cell protein lysate, Phosphatase inhibitor (Beijing, CWBIO Biotechnology Co., Ltd.); Lipopolysaccharide (Sigma-Aldrich Reagent Company, United States); CCK-8 kit (Japan, DOJIDO company); AceQ qPCR Green Master Mix, HiScript QRT SuperMIX (Nanjing, Vazyme Biotech Co., Ltd.); 1M Tirs-Hcl 6.8, 1M Tirs-Hcl 8.8, PBS (Phosphate buffer asline), Glycine, SDS, Tris (China, Solarbio Biotechnology Co., Ltd.); Primer (Shenzhen, BGI Technology Co., Ltd.). PEEP (Laboratory Preparation); Freund’s complete adjuvant (SIGMA-ALDRICH, St. Louis, MI, United States); Dexamethasone (DEX) (Guangdong, Sancai Shiqi Pharmaceutical Co., Ltd.); Rat PGE2, IL-6 and TNF-α kits (Wuhan, Elabscience Biotechnology Co., Ltd.); 4% tissue fixative (China, Solarbio Life Sciences Co., Ltd.) (Tables 1, 2).
2.3 Main Experimental Equipment
Epoch microplate reader (Biotek, Winooski, VT, United States); Small desktop refrigerated centrifuge, Liquid nitrogen storage tank (Thermo Fisher Scientific, Waltham, MA, United States); Protein electrophoresis, Electric transfer system, Gel imaging analysis system (Bio-Rad, Hercules, CA, United States); Constant temperature water bath (Shanghai, Baoling Instrument Equipment Co., Ltd.); Carbon dioxide constant temperature incubator, Ice machine (Sanyo, Japan); Ultra-clean workbench, High Pressure Steam Sterilizer (Shandong, BIOBASE); Fluorescence quantitative PCR machine (IT-IS company, United Kingdom); Foot swelling degree measuring instrument (Ugo Basile company, Italy); Small animal live optical imaging system (Shanghai, PerkinElmer Enterprise Management Co., Ltd.); Pipette gun (Eppendorf, Germany); Electric heating constant temperature sink (Shanghai, Huitai Instrument Manufacturing Co., Ltd.).
2.4 Experimental Method
2.4.1 Preparation of Effective Parts of P. aculeata Mill
We took 1,000 ml of P. aculeata Mill ethanol extract, filtered with a qualitative filter paper circle, and then concentrated it with a rotary evaporator, added water to suspend the extract. Extract with petroleum ether, ethyl acetate and n-butanol, recover the solvent and evaporate to dryness to obtain PEEP 2.415 g, EEEP 3.598 g and BEEP 3.473 g.
2.4.2 Screening of Effective Parts of P. aculeata Mill
RAW264.7 macrophages were used to screen the effective site of P. aculeata Mill. The concentration of each extraction part of P. aculeata Mill is setting as follows: PEEP: 0, 15, 30, 60, 120, and 240 μg/ml; EEEP: 0, 5, 15, 30, and 60 μg/ml, BEEP: 0, 10, 20, 40, and 80 μg/ml. The effects of PEEP, EEEP, and BEEP on the viability of RAW264.7 macrophages were detected by CCK-8, calculate the survival rate of each group of cells according to the formula.
The experiment set up normal group, LPS group (1 μg/ml), LPS + DEX group (1 × 10−4 M), LPS + PEEP (60 μg/ml), LPS + EEEP (30 μg/ml), LPS + BEEP (40 μg/ml). The effect of each extract part on NO content in RAW264.7 macrophages stimulated by LPS was detected according to the instructions in Wuhan Elabscience Biotechnology Co., Ltd. NO kit, and Q-PCR was used to detect the levels of TNF-α and IL-6 in RAW264.7 macrophages stimulated by LPS in each extraction site to screen the most effective site.
(1) The configuration of the Q-PCR reaction system:
(2) The relative expression formula of the target gene is:
According to the previous experimental results, the effect of PEEP (15, 30, and 60 μg/ml) on NO content in RAW264.7 macrophages stimulated by LPS was detected. Q-PCR was used to detect the effect of PEEP (15, 30 and 60 μg/ml) on the expression levels of TNF-α and IL-6 in LPS-induced RAW264.7 macrophages. And the effect of PEEP (15, 30 and 60 μg/ml) on p38/MAPK signaling pathway in LPS-induced RAW264.7 macrophages was detected by western blot.
2.4.3 Preparation and Grouping of CFA Rat Model
After adaptive feeding for 7 days, 60 rats were randomly divided into six groups: blank group, model group, L-PEEP (16 mg/kg) group, M-PEEP (32 mg/kg) group, H-PEEP (64 mg/kg) group, DEX (0.5 mg/kg) group. Except for the 6 rats in the blank group, the rats in the other groups all used Freund’s complete adjuvant to prepare CFA models. After routine disinfection, straighten the right paw of the rat, and use a 1 ml sterile syringe to inject 0.1 ml of Freund’s complete adjuvant into the center of the plantar of each rat.
2.4.4 Method of Administration
According to the characteristics of the CFA model (Chang et al., 2011), the foot swelling at the model site reached its peak (acute inflammatory response) at 18–27 h, and it continued for 10 days and then gradually reduced; secondary lesions generally appeared at 11 days after inflammation. Combined with pre-experimental results, in this experiment, the drug was started 14 days after the model was established, and the drug was administered continuously for 10 days.
2.4.5 Measurement of Rat Foot Swelling Degree
Observe each group at 0, 9, 18, and 27 h, d 3, d 5, d 9, d 11, d 13, d 15, d 18, d 20, d 22, and d 24 after inflammation. The swelling of the hind paw of the rat was measured, and the swelling degree of the right hind paw and the swelling degree of the left hind paw on the non-inflammatory side were measured with a foot swelling meter.
2.4.6 X-Rays to Observe Changes in Cartilage and Bone Structure
After 10 days of administration, the rats in each group were observed to undergo changes in cartilage and bone structure by X-ray. The rats in each group were intraperitoneally injected with 10% chloral hydrate. After anesthesia, take the supine position and transfer to the small animal in vivo imaging device, and take pictures of the left and right feet of each group of rats in turn.
2.4.7 Determination of Spleen Index
After the rats were sacrificed, the abdominal cavity of the rats was cut open quickly and the spleens were cut out. The spleens were quickly rinsed with cold PBS, and the remaining liquid was soaked up with filter paper and weighed to calculate the spleen index of the rats.
2.4.8 Determination of Serum TNF-α, IL-6, and PGE2 Levels in Rats
After the last administration, the rats were fasted for 24 h. After weighing, the rats in each group were blood collected from the vein behind the eyeball and centrifuged to extract serum. They were allowed to stand in a refrigerator at 4°C for 2 h, centrifuged at 4,000 rpm for 10 min, and the supernatant was collected and used for experiment. The concentration of TNF-α, IL-6, and PGE2 in serum was detected by ELISA method, and the operation was performed according to the kit instructions.
2.4.9 HE Staining to Observe the Pathological Changes of the Foot and Ankle Joints
After removing the soft tissue around the right hind ankle of the rat, it was immersed in 4% tissue fixative for 24 h, and sent to the Department of Pathology, Affiliated Hospital of Guilin Medical College to make HE stained sections. The slices were taken to capture images under a 200×, 400× field of view.
2.4.10 Western Blot Detection
Total protein extracted from cells and ankle joints, and then separated by SDS-PAGE gel electrophoresis to bromophenol blue to the bottom of the separation gel. The protein was transferred to PVDF membrane and blocked with 5% skimmed milk powder for 1.5 h. Incubate overnight with primary antibodies β-actin, p38, p-p38, p-MK2, and TTP at 4°C. Incubate for 1.5 h with goat anti-mouse IgG and goat anti-rabbit IgG. All experiments were repeated 3 times.
2.5 Statistical Analysis
SPSS 20.0 was used for data analysis, GraphPad Prism 6.0 was used for graphing, and the data were expressed as mean ± standard deviation (mean ± S.E.M). The comparison between groups was performed by one-way analysis of variance. p < 0.05 and p < 0.01 were considered statistically different.
3 Results
3.1 CCK-8 Detects the Effect of Each Extraction Part of P. aculeata Mill on the Viability of RAW264.7 Macrophages
The CCK-8 experiment was used to detect the effects of PEEP, EEEP, and BEEP on the survival rate of RAW264.7 macrophages. PEEP (0, 15, 30, and 60 μg/ml), EEEP (0, 5, 15, and 30 μg/ml) and BEEP (0, 10, 20, and 40 μg/ml) concentration survival rate is similar to the blank control group (Figures 1A–C). The results illustrated that each extraction site has no toxic and side effects on RAW264.7 macrophages within the designed concentration. Therefore, PEEP (60 μg/ml), EEEP (30 μg/ml), and BEEP (40 μg/ml) were selected as the highest concentrations for subsequent experiments.
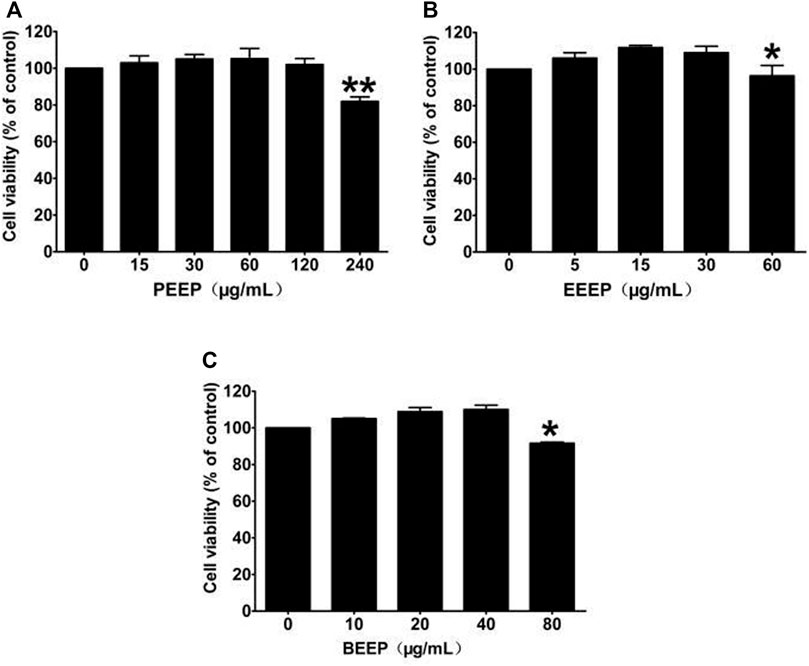
FIGURE 1. Effects of different concentrations of PEEP, EEEP, and BEEP on cell viability in RAW264.7 cells. Results were exhibited as the means ± S.E.M. of three independent experiments. *p < 0.05, **p < 0.01 vs. Normal group.
3.2 The Effect of the Extracts of P. aculeata Mill on the Expression of Nitric Oxide in RAW264.7 Macrophages Induced by Lipopolysaccharide
LPS stimulate macrophages to express nitric oxide synthase (iNOS) and release NO (Chen et al., 2014). Therefore, the effect of each extraction site of P. aculeata Mill on LPS-induced NO expression in RAW264.7 macrophages was investigated by NO colorimetry. The expression level of No in RAW264.7 macrophages induced by LPS was significantly reduced (p < 0.01) through the intervention of each extraction site of P. aculeata Mill (Figure 2A). The inhibitory effect of PEEP on NO is stronger than other parts, and it has the most significant anti-inflammatory potential. PEEP (15, 30 and 60 μg/ml) significantly reduce the expression of NO in RAW264.7 macrophages induced by LPS when compared with the model group, and in concentration-dependent (Figure 2B).
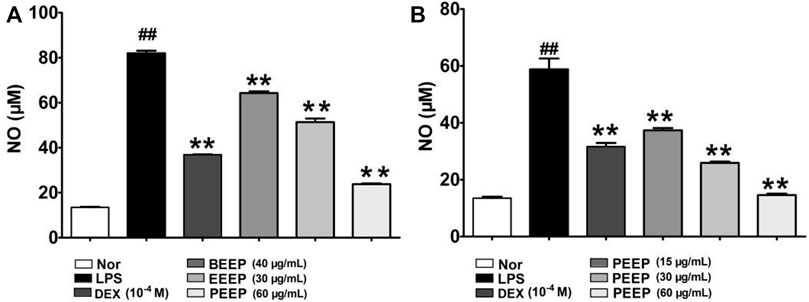
FIGURE 2. Effects of different concentrations of PEEP, EEEP and BEEP on cell viability in RAW264.7 macrophages. (A) Effect of PEEP (60 μg/ml), EEEP (30 μg/ml) and BEEP (40 μg/ml) on the content of NO induced by LPS in RAW264.7 macrophages. (B) Effect of PEEP (15, 30 and 60 μg/ml) on the content of NO induced by LPS in RAW264.7 macrophages. Results were exhibited as the means ± S.E.M. of three independent experiments. ##p < 0.01 vs. Normal; *p < 0.05, **p <0.01 vs. LPS group.
3.3 Effects of the Extracts of P. aculeata Mill on the Expression of TNF-α and IL-6 in RAW264.7 Macrophages Induced by Lipopolysaccharide
Macrophages play an important role in the pathogenesis of RA by secreting TNF-α, IL-6 and other inflammatory cytokines (Kanno et al., 2006). The related expression results are shown in Figures 3A,B. PEEP reduce the mRNA expression levels of TNF-α and IL-6 in RAW264.7 macrophages induced by LPS when compared with the model group (p < 0.05, p < 0.01). However, the inhibition of TNF-α and IL-6 by EEEP and BEEP was not statistically significant (p > 0.05). PEEP (15, 30 and 60 μg/ml) decreased the mRNA levels of TNF-α and IL-6 in LPS-induced RAW264.7 macrophages (p < 0.05, p < 0.01) in a concentration-dependent manner (Figures 3C,D). The above results indicate that PEEP significantly reduce the level of TNF-α and IL-6 mRNA in activated RAW264.7 macrophages, and the concentration increases, the lower the mRNA level. Therefore, based on the experimental results, PEEP (15, 30 and 60 μg/ml) was selected for subsequent experiments.
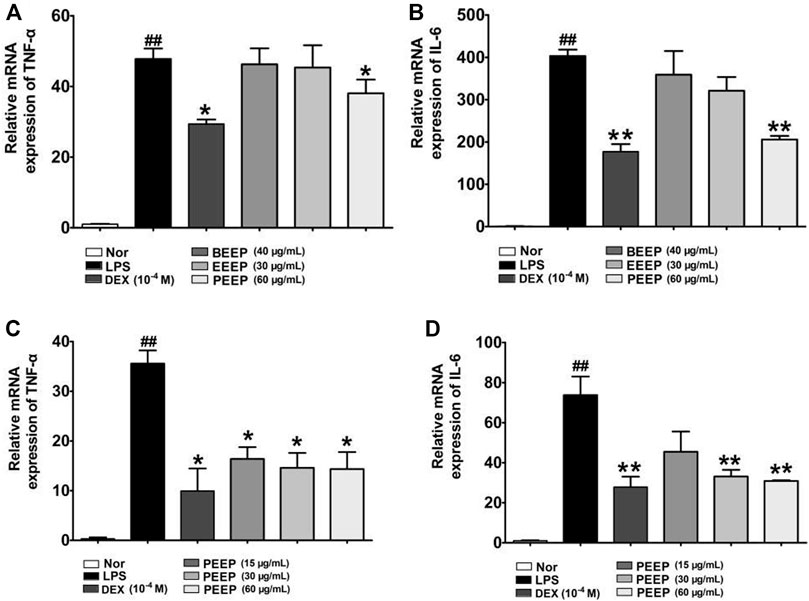
FIGURE 3. Effect of PEEP, EEEP and BEEP on the expressions of inflammatory factors in RAW264.7 macrophages induced by LPS. (A) The effect of PEEP (60 μg/ml), EEEP (30 μg/ml) or BEEP (40 μg/ml) on mRNA expressions of TNF-α were detected using Q-PCR assays. (B) The effect of PEEP (60 μg/ml), EEEP (30 μg/ml) or BEEP (40 μg/ml) on mRNA expressions of IL-6 were detected using Q-PCR assays. (C) The effect of PEEP (15, 30 and 60 μg/ml) on mRNA expressions of TNF-α were detected using Q-PCR assays. (D) The effect of PEEP (15, 30 and 60 μg/ml) on mRNA expressions of IL-6 were detected using Q-PCR assays. The data were expressed as the means ± S.E.M. of three independent experiments. ##p < 0.01 vs. Normal group; *p < 0.05, **p < 0.01 vs. LPS group.
3.4 Effect of PEEP on the p38/MAPK Signaling Pathway of Lipopolysaccharide-Induced RAW264.7 Macrophages
The p38/MAPK pathway is closely related to the regulation of inflammatory response. It is an important mediator involved in the expression of a variety of genes in the stress process. After activation, it moves from the cytoplasm to the nucleus, and interacts with the corresponding transcription factors to control the transcription of a variety of genes (Roussel et al., 2010), it plays a key role in the signal transduction of a variety of cytokine synthesis induced by LPS. Therefore, we analyzed the expression of p38, p-p38, p-MK2, and TTP protein in order to further explore the molecular mechanism of PEEP anti-inflammatory. PEEP downregulated the expression levels of p-p38, p-MK2 and increased the expression level of TTP in LPS-induced RAW264.7 macrophages when compared with the model group (p < 0.01, p < 0.05) in a concentration-dependent (Figure 4).
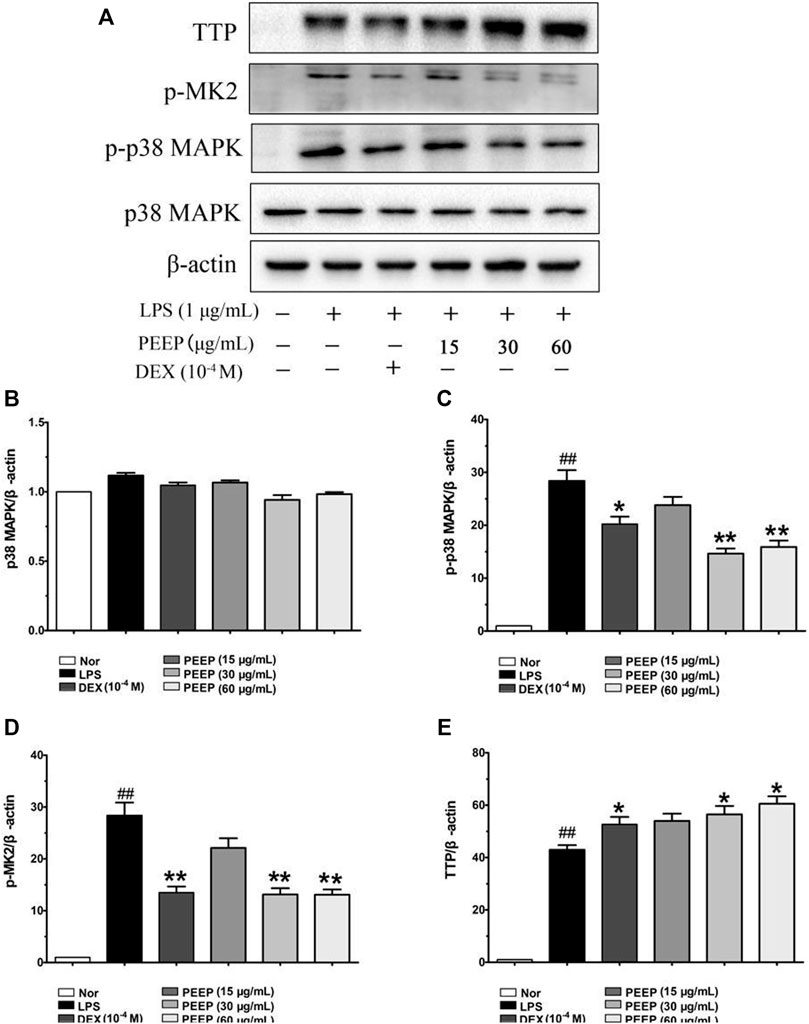
FIGURE 4. Effect of PEEP on p38, p-p38, p-MK2, and TTP expressions induced by LPS in RAW264.7 macrophages. (A) The protein expressions of p38, p-p38, p-MK2, and TTP were detected by using western blot analysis. (B) Quantitative analysis of gray value of p38 was performed in several groups with β-actin as loading control. (C) Quantitative analysis of gray value of p-p38 was performed in several groups with β-actin as loading control. (D) Quantitative analysis of gray value of p-MK2 was performed in several groups with β-actin as loading control. (E) Quantitative analysis of gray value of TTP was performed in several groups 27 with β-actin as loading control. Data represent the mean ± S.E.M. of three independent experiments. ##p < 0.01 vs. Normal group; *p < 0.05, **p < 0.01. vs. LPS group.
3.5 General Observation of Rats in Each Group
The rats with normal appetite and active behavior before CFA injection. The skin of the feet of CFA rats began to become red, and the modeled feet were swollen after inflammation for 2 days. And the primary paws of CFA rats were significantly more swollen, and movement was significantly restricted, the secondary paws were not significantly swollen, and appetite decreased slightly after inflammation for 7 days when compared with the control group. Rats in the PEEP medium and high dose group had better eating conditions, and relieved the swelling of the feet after 10 days of administration when compared with the model group. No abnormally dead rats were found.
3.6 The Effect of PEEP on the Swelling Degree of Complete Freund’s Adjuvant Rats’ Feet
Combined with the characteristics of the CFA model (Chang et al., 2011). The swelling degree of the right hind foot of the model group CFA rats gradually reached a peak after 15 days of modeling when compared with the blank group (p < 0.01). The swelling of the right hind foot of rats in the M-PEEP (32 mg/kg) group and H-PEEP (64 mg/kg) group decreased significantly after 10 days of administration when compared with the model group (p < 0.01). The CFA rats in the model group developed secondary swelling of the left hind foot 11 days after modeling when compared with the blank group (p < 0.01). The swelling of the left hind foot of the rats in the M-PEEP (32 mg/kg) group and H-PEEP (64 mg/kg) group decreased significantly after 10 days of administration when compared with the model group (p < 0.01). The results show that the petroleum ether site significantly reduce the degree of foot swelling in CFA model rats (Figures 5A,B).
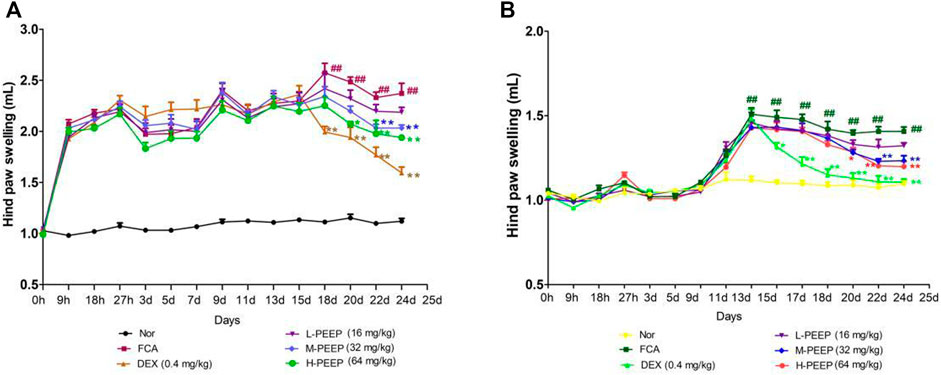
FIGURE 5. Effects of different doses of PEEP on swelling of paw in CFA rats. (A)Effects of different doses of PEEP on swelling of right hind paw in CFA rats. (B) Effects of different doses of PEEP on swelling of left hind paw in CFA rats. ##p < 0.01 vs. Normal group; *p < 0.05, **p < 0.01 vs. CFA group.
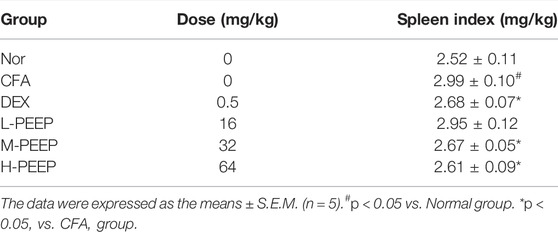
3.7 The Effect of PEEP on the Spleen Index of Complete Freund’s Adjuvant Rats
The results are presented in Table 3, the spleen index of the model group increased when compared with the blank group (p < 0.05). The spleen index of M-PEEP (32 mg/kg) group and H-PEEP (64 mg/kg) group was significantly lower than model group (p < 0.05). The spleen index of the L-PEEP (16 mg/kg) group was lower than model group, but there is no significant difference (p > 0.05).
3.8 Effect of PEEP on the Levels of IL-6, PGE2 and TNF-α in Serum of Complete Freund’s Adjuvant Rats
The serum levels of IL-6, PGE2 and TNF-α in the model group increased significantly when compared with the blank group (p < 0.01). The expressions of IL-6 and PGE2 in the serum of rats in the L-PEEP (16 mg/kg) group, M-PEEP (32 mg/kg) group, and H-PEEP (64 mg/kg) group were significantly lower than the model group (p < 0.05). the expression of TNF-α in the serum of rats in the M-PEEP (32 mg/kg) group and H-PEEP (64 mg/kg) group was significantly reduced (p < 0.05), but the L-PEEP (16 mg/kg) group only had a decreasing trend, and the difference was not statistically significant (p > 0.05). Therefore, it can be considered that medium and high doses of PEEP can reduce the expression of IL-6, PGE2, and TNF-α, which can inhibit inflammation and control the development of the disease. The treatment effect of L-PEEP group was slightly worse than that of M-PEEP group and H-PEEP group (Figure 6).
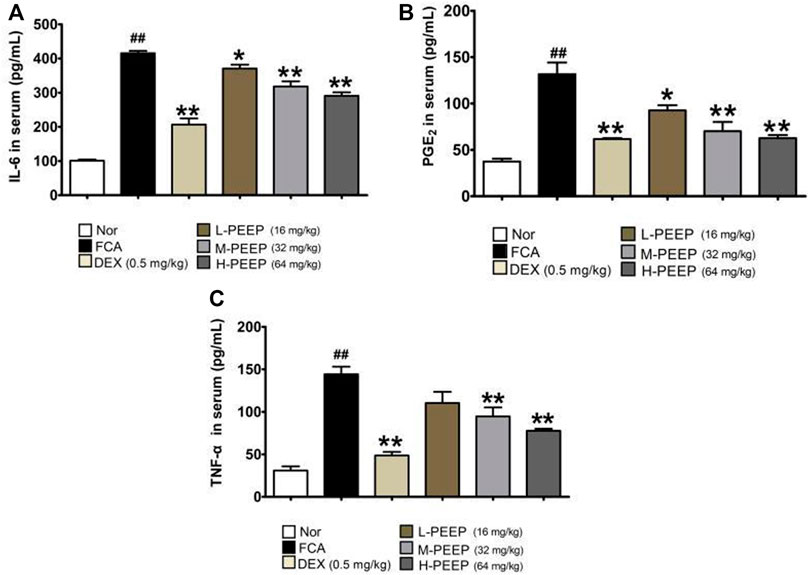
FIGURE 6. Effects of different doses of PEEP on the expression of inflammatory cytokines in CFA rat serum. (A) The concentrations of IL-6 in serum were determined by ELISA. (B) The concentrations of PGE2 in serum were determined by ELISA. (C) The concentrations of TNF-α in serum were determined by ELISA. The data were expressed as the means ± S.E.M. (n = 5). ##p < 0.05 vs. Normal group; *p < 0.05, **p < 0.01 vs. CFA group.
3.9 X-Ray Observation Results of Rat Feet in Each Group
The paws of the rats in the model group were swollen, the joint space was narrow, and the articular surface was blurred. M-PEEP (32 mg/kg) group, H-PEEP (64 mg/kg) group and DEX group (0.5 mg/kg) reduced the swelling of soft tissue around the ankle joint and improved the bone surface erosion in rats and joint space narrowing after 10 days of administration by observing the X-ray of the right hind paw of the rats in each group. The left foot swelling of CFA rats in each group was not obvious, and there was no bone destruction (Figure 7).
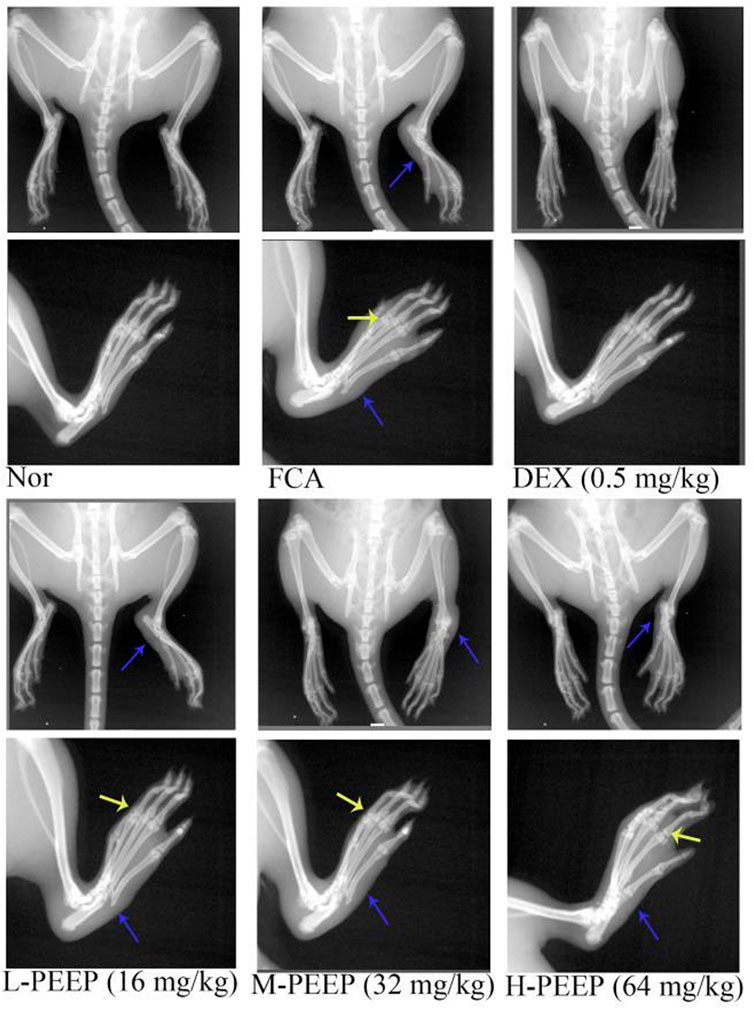
FIGURE 7. X-ray evaluation of joint injury in CFA rats. X-rays showed the effects of different doses of PEEP on hind paw and right paw of CFA rats. Blue arrow, swelling of soft tissue; yellow arrow, narrow joint space, blurred joint surface.
3.10 Observation of PEEP on HE Staining of Ankle Joint of Complete Freund’s Adjuvant Rats
HE staining of the blank group showed complete and clear structure, no inflammatory cell infiltration and synovial hyperplasia, and no damage to cartilage and bone joints. Cartilage destruction, inflammatory cell infiltration, proliferation of a large number of synovial cells, and pannus formation occurred in the foot and ankle joints in the model group. HE staining in the DEX (0.5 mg/kg) group showed slight synovial hyperplasia and less inflammatory cell infiltration when compared with the model group. The degree of synovial hyperplasia or inflammatory cell infiltration in the M-PEEP (32 mg/kg) group and H-PEEP (64 mg/kg) group was lighter, and the cartilage erosion was lighter than the model group (Figure 8).
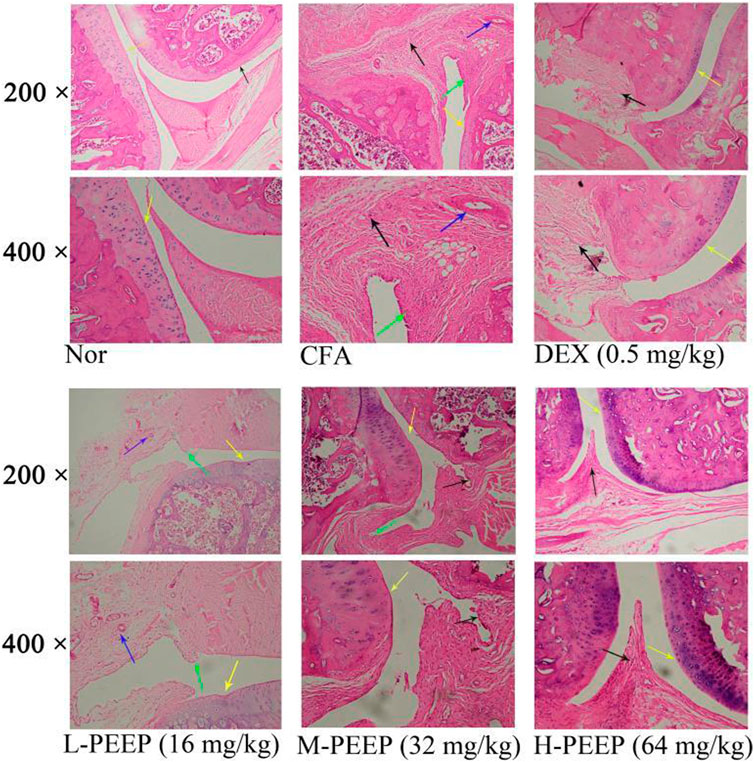
FIGURE 8. The pathologial slice pictures of the ankle joint of CFA rats treated with various doses of. PEEP. Red arrow, articular cartilage; black arrow, synovial hyperplasia; green arrow, inflammatory cell infiltration; blue arrow, pannus.
3.11 Effect of PEEP on p38/MAPK Signal Pathway in Complete Freund’s Adjuvant Rats
The results are presented in Figure 9, the expression of p38 in the ankle joint has no significant difference (p > 0.05). The expression of p-p38 and p-MK2 in the M-PEEP (32 mg/kg) group and the H-PEEP (64 mg/kg) group was reduced when compared with the model group (p < 0.01), while the expression of p-p38 and p-MK2 in the L-PEEP (16 mg/kg) group has a decreasing trend, no significant difference (p > 0.05). The protein expression of TTP in the ankle joints increased when compared with the model group (p < 0.01). Overall, the results show that PEEP can inhibit the expression of p-p38 and p-MK2 proteins in the foot and ankle joints of CFA rats, and reduce the phosphorylation of TTP. There is a certain dose relationship, and the best effect is shown at high doses.
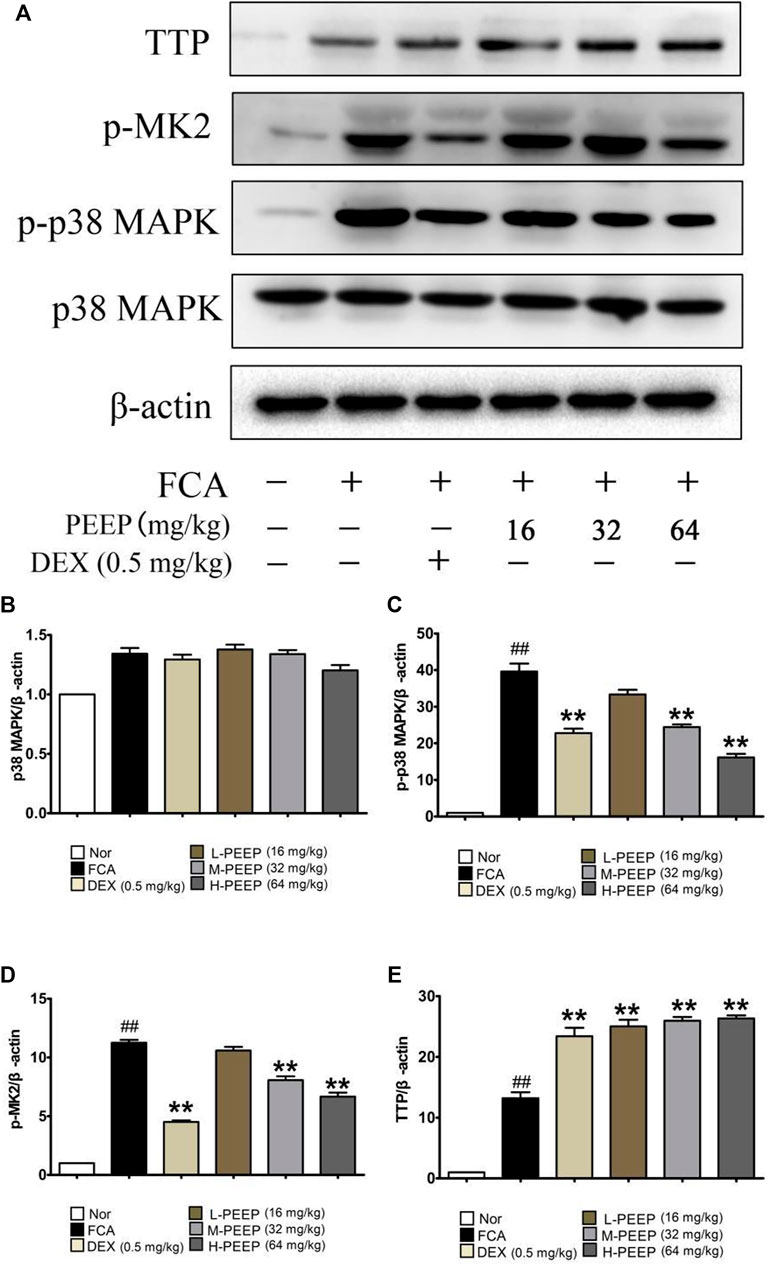
FIGURE 9. Effects of different doses of BGE on the p38/MAPK signaling pathway. (A) The expression of p-38, p-p38, p-MK2, and TTP obtained from ankle joints of several groups was detected by western blot analysis. (B) Quantitative analysis of gray value of p38 was performed in several groups with β-actin as loading control. (C) Quantitative analysis of gray value of p-p38 was performed in several groups with β-actin as loading control. (D) Quantitative analysis of gray value of p-MK2 was performed in several groups with β-actin as loading control. (E) Quantitative analysis of gray value of TTP was performed in several groups with β-actin as loading control. Data represent the mean ± S.E.M. of three independent experiments. p < 0.05, ##p < 0.01 vs. Normal group; **p < 0.01 vs. CFA group.
4 Discussion
The etiology and pathogenesis of RA have not been clearly elucidated. The number of macrophages is one of the main factors in RA because the severity of the disease and the degree of joint damage are significantly related to the number of macrophages (Kinne et al., 2007). When macrophages are stimulated by LPS, they will activate MAPK and other related signal pathways, and then secrete a large number of inflammatory factors, such as TNF-α, IL-6, IL-1β, and PGE2 (Xu et al., 2017; Yonezawa et al., 2018). RAW264.7 mouse macrophages are a type of macrophages. Because of their strong adhesion and ability to swallow antigens, they are generally used to build inflammation models and conduct research on inflammatory responses (Adams and Hamilton, 1984; Yang et al., 2014). LPS was used for stimulation to establish a macrophage inflammation model to study the effect of PEEP on inflammation and its molecular mechanism. According to the literature, the preliminary experimental results of the research group, 1 μg/ml LPS was used to stimulate RAW264.7 macrophages to construct as vitro inflammation model. The CCK-8 method was used to detect the effects of different concentrations of P. aculeata Mill on the proliferation of RAW264.7 macrophages. The results showed that, the concentrations of PEEP (0, 15, 30, and 60 μg/ml), EEEP (0, 5, 15, and 30 μg/ml), and BEEP (0, 10, 20, and 40 μg/ml) no significant effect on the survival rate of RAW264.7 macrophages when compared with the blank group. Therefore, PEEP (60 μg/ml), EEEP (30 μg/ml), and BEEP (40 μg/ml) are selected as the highest concentrations for subsequent experiments.
In the early stage of inflammation, inflammatory cytokines such as TNF-α and IL-6 released by activated macrophages play an extremely important role in initiating local inflammation (Ueda et al., 2015). TNF-α is a potent pro-inflammatory cytokine released by macrophages, and its secretion reflect the severity of inflammation in the body (Caldwell et al., 1999; Su et al., 2015), and the excessive release of TNF-α can induce the production of other cytokines (such as IL-6), to further amplify the damage and destruction of TNF-α to inflamed tissues. Therefore, inhibiting the expression of TNF-α has a good control effect on the inflammatory response (Jayaraman et al., 2008). IL-6 plays an important role in chronic inflammatory diseases, and promotes the proliferation and differentiation of cells in inflammation (Tanaka and Kishimoto, 2014; Takeuchi et al., 2021). It is associated with high levels of IL-6 in a variety of inflammation-related diseases. Therefore, IL-6 is used as an indicator of anti-inflammatory efficacy (Mesquida et al., 2014). NO is a highly reactive oxidative free radical in organisms, which is generated by endogenous L-arginine under the catalysis of iNOS (Raij, 2006). It has been reported in the literature that NO is directly involved in the inflammatory response and joint damage process of RA (Mccartney-Francis et al., 2001). High expression levels of NO can cause hyperdilatation and increased exudation of blood, accompanied by the release of inflammatory cytokines (TNF-α, IL-6). TNF-α induces the synthesis of iNOS and further increases the level of NO (Cohen et al., 2017). Therefore, the level of NO can also be used as one of the important indicators for observing the inflammatory response. The NO colorimetric method to detect the effect of different concentrations of P. aculeata Mill extracts on the LPS-induced NO expression in RAW264.7 macrophages. The results show that the extracts from P. aculeata Mill significantly reduce NO. PEEP has the most significant effect which is dose-dependent. It is suggested that PEEP more effectively reduce the production of NO, and play a better therapeutic effect on intracellular inflammation and oxidative stress. Next, the Q-PCR experiment was conducted to investigate the effects of each extraction site of P. aculeata Mill on the expression of inflammatory factors in RAW264.7 macrophages induced by LPS. The results showed that PEEP significantly inhibit TNF-α and IL-6. EEEP and BEEP have no significant effect on the levels of TNF-α and IL-6 in cells. Based on the experimental results, PEEP significantly reduce the levels of NO, TNF-α, and IL-6 in activated RAW264.7 macrophage expression level and inhibit cell inflammatory response.
p38/MAPK is an important member of the MAPK family. It triggers a chain reaction of intracellular protein kinases under the stimulation of inflammatory factors and external factors, thereby affecting biological effects such as cell transcription, protein synthesis and cell surface receptor expression (Munoz and Ammit, 2010). The p38/MAPK signaling pathway is one of the most important family members that control inflammation, and plays an important role in the physiological and pathological processes such as inflammation, cell stress, apoptosis, cell cycle and growth (Niranjan et al., 2012). Under LPS stimulation, macrophages and the p38/MAPK signaling pathway be activated, and cause the secretion of a large number of inflammatory factors (TNF-α, IL-6) (Zhu et al., 2016; Dong et al., 2018). The activation of p38/MAPK lead to the overexpression of p-MK2 which downstream substrate of the pathway, and activated MK2 phosphorylate zinc finger protein 36 (Tristetraprolin, TTP), thereby over-promoting and regulating cytokines such as TNF-α and IL-6 hyperplasia leads to the continuous expression of inflammatory factors and causes a series of inflammatory reactions. Therefore, the p38-MK2-TTP axis mediate pro-inflammatory signals, which also explains why the knockout mouse MK2 gene cannot be successfully prepared into experimental arthritis (Hegen et al., 2006). We explored the effects of different concentrations of PEEP on the expression of p-p38, p-MK2 and TTP in RAW264.7 macrophags stimulated by LPS in order to further explore the molecular mechanism of PEEP inhibiting inflammation. Western blot was used to detect the effect of PEEP on the p38/MAPK signaling pathway of RAW264.7 macrophages stimulated by LPS. The results showed that the expression of p38 in activated RAW264.7 macrophages was not significantly different The levels of p-p38 and p-MK2 in the cells induced by LPS increased significantly when compared with the blank group. The expressions of p-p38 and p-MK2 in PEEP (30 and 60 μg/ml) were lower than those in the model group. The expression of TTP in each PEEP administration group increased when compared with the model group. The results suggest that PEEP exerts anti-inflammatory effects by regulating the p38/MAPK signaling pathway. PEEP can reduce the expression of NO, TNF-α, and IL-6 in RAW264.7 macrophages induced by LPS. The mechanism may be through regulating the p38/MAPK signaling pathway, thereby inhibiting LPS-induced inflammatory response in RAW264.7 macrophages.
CFA-induced arthritis model is the basic method of the immune arthritis rat model. It is an inflammatory response mediated by immune complexes and its pathogenesis is mainly based on molecular simulation theory. Intradermal injection of CFA delays the body’s absorption and causes continuous stimulation of the body to produce a secondary autoimmune response (Shakeel et al., 2021). The primary lesion of adjuvant arthritis model rats is an acute stress response, it will appear red and swollen joints and foot soles after sensitization. The secondary lesions appeared about 10 days ago, which was an autoimmune reaction, generally manifested as the swelling of joints and plantar joints on the inflamed side and non-inflamed side after modeling. The rat adjuvant arthritis model established by CFA is a simple method, with high success rate and stable model after establishment. Its pathological process and laboratory indicators are similar to those of RA and it is an ideal model for screening and researching drugs for the treatment of RA (Wang et al., 2019). Therefore, we established an CFA-induced arthritis rat model in order to further verify the anti-RA effective part of P. aculeata Mill. The results of this experiment show that, rats in the model group were injected with CFA intracutaneously on the right hind foot plantar. The inflamed right paw of the rat began to swell within 5–9 h, and the swelling degree of the rat’s foot gradually became swollen. It swelled again 14 days after modeling, secondary lesions generally appear at about 11 days after inflammation, and the swelling of the contralateral foot gradually increases to a peak. P. aculeata Mill petroleum ether had a significant inhibitory effect on the swelling of the left and right paws of arthritis after 10 days of administration when compared with the model group.
RA mainly manifests as persistent joint and synovial inflammation, accompanied by bone and bone erosion, joint adhesion, pannus formation, and inflammatory cell infiltration (Turesson et al., 2003; Lee et al., 2012). HE staining and X-ray observation of rat foot and ankle joints showed that the synovial tissue of the model group was proliferated, inflammatory cells infiltrated, and pannus formed. X-rays showed that the soft tissues of the model group were swollen, the joint space was narrow, and the articular surface was blurred. PEEP significantly improve synovial hyperplasia, inflammatory cell infiltration and pannus formation in rat foot and ankle joint disease, reduce the swelling of soft tissue around the foot joint, and improve the bone surface erosion in rats and the degree of joint space stenosis after administration when compared with the model group.
The spleen is the body’s immune organ and plays a very important role in the body’s immune function (Xu et al., 2021). The results of this experiment showed that the spleen index of the model group increased. PEEP reduce the spleen index of CFA rats when compared with the model group. It shows that CFA rats have immune disorders and damaged immune organs. A certain dose of PEEP has a protective effect on the immune function of CFA rats.
In the process of inflammation in CFA rats, the release of inflammatory cytokines (TNF-α, IL-6, IL-8, etc.) played an important role in the occurrence and development of inflammation, the disintegration of articular cartilage matrix and bone destruction (Scott et al., 2010; Wang et al., 2017; Uttra et al., 2018). TNF-α is one of the earliest and most important inflammatory mediators in the process of inflammation. It can activate neutrophils and lymphocytes, increase the permeability of vascular endothelial cells, regulate the metabolic activity of other tissues and promote the synthesis and release of other cytokines (Moelants et al., 2013). PGE2 is an important inflammatory mediator as a second messenger. The inflammatory response is closely related to the part PGE2 expression level. Studies have shown that high concentrations of PGE2 will dilate capillaries, increase vascular permeability, tissue congestion and edema, aggravate inflammation, and even produce collagenase, erode cartilage, and destroy bone (Akaogi et al., 2006; Chen et al., 2012). Further studies have found that PGE2 induce the production of IL-6, and these cytokines usually interact with each other to form a network and jointly participate in the pathogenesis of RA (Matsuno et al., 2002; Axmann et al., 2009; Takeuchi et al., 2020). Therefore, it can help determine the improvement or deterioration of the inflammatory state in rats when studying the levels of TNF-α, IL-6, and PGE2. The results showed that the expression levels of inflammatory factors TNF-α, IL-6, and PGE2 in the serum of the model group increased significantly. PEEP reduce the levels of inflammatory factors TNF-α, IL-6, and PGE2 in the serum of rats when compared with the model group.
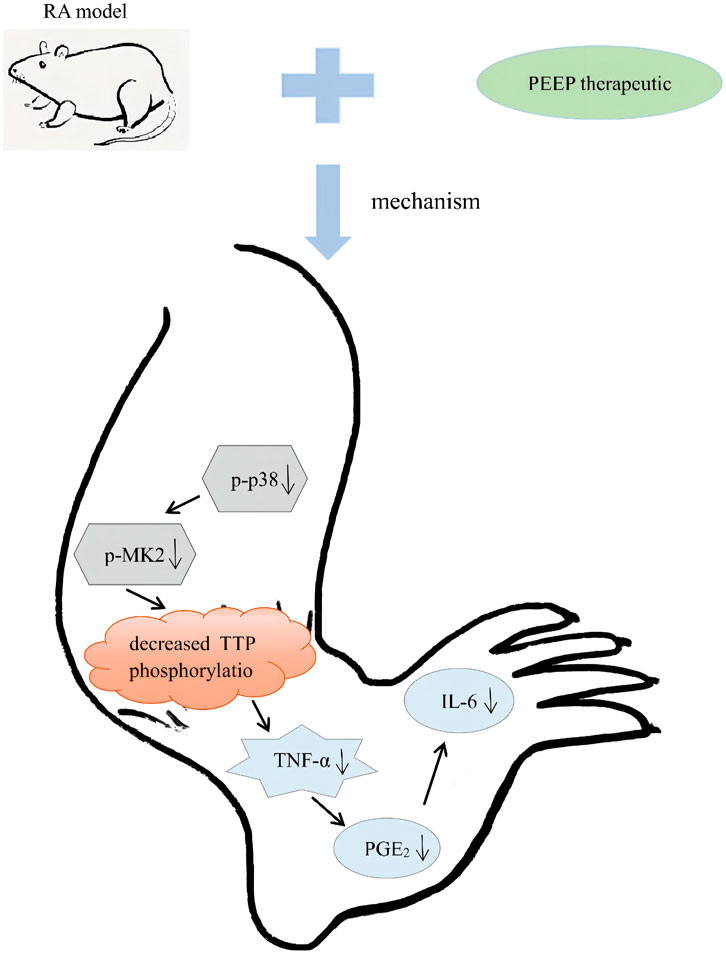
We explored the regulation of PEEP on the p38/MAPK signaling pathway by detecting the protein expressions of p38, p-p38, p-MK2, and TTP in the ankle joints. It preliminarily proves that PEEP regulate the p38/MAPK pathway by inhibiting the expression levels of p-p38 and p-MK2 and reduce the phosphorylation of TTP, and ultimately slow down the inflammatory response and the progression of RA disease.
Each group of PEEP can reduce the index of the spleen of the immune organ, significantly improve the synovial hyperplasia, inflammatory cell infiltration and pannus formation in the foot and ankle joint disease of rats, reduce the swelling of the soft tissue around the foot and ankle joint, and improve the bone of the foot and ankle joint in rats surface erosion and joint space stenosis alleviate various inflammations in rats with adjuvant arthritis. The mechanism of action is that PEEP downregulates the levels of TNF-α, IL-6, and PGE2 in rat serum by regulating the p38/MAPK pathway, thereby alleviating the RA inflammation and damage.
The pharmacodynamic evaluation in vitro and in vivo strongly demonstrated that the P. aculeata Mill petroleum ether site is the active site for anti-rheumatoid arthritis. P. aculeata Mill petroleum ether site regulate the p38/MAPK pathway by inhibiting the expression levels of p-p38 and p-MK2 and reduce the phosphorylation of TTP, and further inhibit the secretion of NO, TNF-α, IL-6, and PGE2 inflammatory cytokines, eventually slow down the inflammatory response and the progression of RA disease (Figure 10).
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the Guilin Medical University Laboratory Animal Ethics Committee.
Author Contributions
YiC directed the experimental process and revised the manuscript. KL participated in the experimental process and revised the manuscript. YuC collected materials, performed experiments, and wrote the manuscript. YQ and SC gave design suggestions for the experiment. GG and YH participated in the experimental process. ZM gave constructive comments on the experiment.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82160832, 81873030 and 81673628) and the Natural Science Foundation of Guangxi Zhuang Autonomous Region (Nos. 2017GXNSFAA198255 and 2018GXNSFBA138028) and Guangxi Key Laboratory of Brain and Cognitive Neuroscience Open Project Funding (No. GKLBCN-20180105-03) and the Fourth Training Plan for Thousands of Young and Mid-aged Mainstay Teachers in Guangxi Colleges and Universities and 2020 College Student Innovation and Entrepreneurship Project Training Plan (No. 202010601099) and 2022 Annual Scientific Research Project of Guangdong Provincial Administration of Traditional Chinese Medicine (No. 20222138).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BEEP, N-butanol Extract of Pereskia aculeate Miller; CCK-8, Cell Counting Kit-8; DEX, Dexamethasone; DMSO, DimethylsμLfoxide; EEEP, Ethyl Acetate Extract of Pereskia aculeate Miller; ELISA, Enzyme linked immunosorbent assay; CFA, Complete Freund’s adjuvant; IL-6, βinterleukin-6; LPS, Lipopolysaccharide; NO, Nitric oxide; PBS, Phosphate buffer asline; PEEP, Petroleum Ether Extract of Pereskia aculeate Miller; PGE2, Prostaglandin E2; p38MAPK, p38 mitogen-activated protein kinase; RA, Rheumatoid arithritis; TNF-α, Tumor necrosis factor α; TTP, Tristetraprolin.
References
Abbasi, M., Mousavi, M. J., Jamalzehi, S., Alimohammadi, R., Bezvan, M. H., Mohammadi, H., et al. (2019). Strategies toward Rheumatoid Arthritis Therapy; the Old and the New. J. Cel Physiol 234, 10018–10031. doi:10.1002/jcp.27860
Adams, D. O., and Hamilton, T. A. (1984). The Cell Biology of Macrophage Activation. Annu. Rev. Immunol. 2, 283–318. doi:10.1146/annurev.iy.02.040184.001435
Akaogi, J., Nozaki, T., Satoh, M., and Yamada, H. (2006). Role of PGE2 and EP Receptors in the Pathogenesis of Rheumatoid Arthritis and as a Novel Therapeutic Strategy. Endocr. Metab. Immune Disord. Drug Targets 6, 383–394. doi:10.2174/187153006779025711
Aletaha, D., Funovits, J., and Smolen, J. S. (2011). Physical Disability in Rheumatoid Arthritis Is Associated with Cartilage Damage rather Than Bone Destruction. Ann. Rheum. Dis. 70, 733–739. doi:10.1136/ard.2010.138693
Axmann, R., Böhm, C., Krönke, G., Zwerina, J., Smolen, J., and Schett, G. (2009). Inhibition of Interleukin-6 Receptor Directly Blocks Osteoclast Formation In Vitro and In Vivo. Arthritis Rheum. 60, 2747–2756. doi:10.1002/art.24781
Caldwell, S. H., Oelsner, D. H., Iezzoni, J. C., Hespenheide, E. E., Battle, E. H., and Driscoll, C. J. (1999). Cryptogenic Cirrhosis: Clinical Characterization and Risk Factors for Underlying Disease. Hepatology 29, 664–669. doi:10.1002/hep.510290347
Chang, Y., Wu, Y., Wang, D., Wei, W., Qin, Q., Xie, G., et al. (2011). Therapeutic Effects of TACI-Ig on Rats with Adjuvant-Induced Arthritis via Attenuating Inflammatory Responses. Rheumatology (Oxford) 50, 862–870. doi:10.1093/rheumatology/keq404
Chen, W. P., Wang, Y. L., Tang, J. L., Hu, P. F., Bao, J. P., and Wu, L. D. (2012). Morin Inhibits Interleukin-1β-Induced Nitric Oxide and Prostaglandin E2 Production in Human Chondrocytes. Int. Immunopharmacol 12, 447–452. doi:10.1016/j.intimp.2011.12.024
Chen, H., Sohn, J., Zhang, L., Tian, J., Chen, S., and Bjeldanes, L. F. (2014). Anti-inflammatory Effects of Chicanine on Murine Macrophage by Down-Regulating LPS-Induced Inflammatory Cytokines in IκBα/MAPK/ERK Signaling Pathways. Eur. J. Pharmacol. 724, 168–174. doi:10.1016/j.ejphar.2013.12.016
Cohen, S. B., Tanaka, Y., Mariette, X., Curtis, J. R., Lee, E. B., Nash, P., et al. (2017). Long-term Safety of Tofacitinib for the Treatment of Rheumatoid Arthritis up to 8.5 Years: Integrated Analysis of Data from the Global Clinical Trials. Ann. Rheum. Dis. 76, 1253–1262. doi:10.1136/annrheumdis-2016-210457
Dong, J., Li, J., Cui, L., Wang, Y., Lin, J., Qu, Y., et al. (2018). Cortisol Modulates Inflammatory Responses in LPS-Stimulated RAW264.7 Cells via the NF-Κb and MAPK Pathways. BMC Vet. Res. 14, 30. doi:10.1186/s12917-018-1360-0
Hegen, M., Gaestel, M., Nickerson-Nutter, C. L., Lin, L. L., and Telliez, J. B. (2006). MAPKAP Kinase 2-deficient Mice Are Resistant to Collagen-Induced Arthritis. J. Immunol. 177, 1913–1917. doi:10.4049/jimmunol.177.3.1913
Jang, M., Jeong, S. W., Cho, S. K., Ahn, K. S., Lee, J. H., Yang, D. C., et al. (2014). Anti-inflammatory Effects of an Ethanolic Extract of Guava (Psidium Guajava L.) Leaves In Vitro and In Vivo. J. Med. Food 17, 678–685. doi:10.1089/jmf.2013.2936
Jayaraman, T., Paget, A., Shin, Y. S., Li, X., Mayer, J., Chaudhry, H., et al. (2008). TNF-alpha-mediated Inflammation in Cerebral Aneurysms: a Potential Link to Growth and Rupture. Vasc. Health Risk Manag. 4, 805–817. doi:10.2147/vhrm.s2700
Kanno, S., Shouji, A., Tomizawa, A., Hiura, T., Osanai, Y., Ujibe, M., et al. (2006). Inhibitory Effect of Naringin on Lipopolysaccharide (LPS)-induced Endotoxin Shock in Mice and Nitric Oxide Production in RAW 264.7 Macrophages. Life Sci. 78, 673–681. doi:10.1016/j.lfs.2005.04.051
Kazama, C. C., Uchida, D. T., Canzi, K. N., De Souza, P., Crestani, S., Gasparotto, A., et al. (2012). Involvement of Arginine-Vasopressin in the Diuretic and Hypotensive Effects of Pereskia Grandifolia Haw. (Cactaceae). J. Ethnopharmacol 144, 86–93. doi:10.1016/j.jep.2012.08.034
Kinne, R. W., Stuhlmüller, B., and Burmester, G. R. (2007). Cells of the Synovium in Rheumatoid Arthritis. Macrophages. Arthritis Res. Ther. 9, 224. doi:10.1186/ar2333
Koller, G., Cusnir, I., Hall, J., and Ye, C. (2019). Reversible Alopecia Areata: a Little Known Side Effect of Leflunomide. Clin. Rheumatol. 38, 2015–2016. doi:10.1007/s10067-019-04577-3
Lee, S. W., Kim, J. H., Park, M. C., Park, Y. B., Chae, W. J., Morio, T., et al. (2012). Alleviation of Rheumatoid Arthritis by Cell-Transducible Methotrexate upon Transcutaneous Delivery. Biomaterials 33, 1563–1572. doi:10.1016/j.biomaterials.2011.10.079
Malmström, V., Catrina, A. I., and Klareskog, L. (2017). The Immunopathogenesis of Seropositive Rheumatoid Arthritis: from Triggering to Targeting. Nat. Rev. Immunol. 17, 60–75. doi:10.1038/nri.2016.124
Matsuno, H., Yudoh, K., Katayama, R., Nakazawa, F., Uzuki, M., Sawai, T., et al. (2002). The Role of TNF-Alpha in the Pathogenesis of Inflammation and Joint Destruction in Rheumatoid Arthritis (RA): a Study Using a Human RA/SCID Mouse Chimera. Rheumatology (Oxford) 41, 329–337. doi:10.1093/rheumatology/41.3.329
Mccartney-Francis, N. L., Song, X., Mizel, D. E., and Wahl, S. M. (2001). Selective Inhibition of Inducible Nitric Oxide Synthase Exacerbates Erosive Joint Disease. J. Immunol. 166, 2734–2740. doi:10.4049/jimmunol.166.4.2734
Mcinnes, I. B., and Schett, G. (2011). The Pathogenesis of Rheumatoid Arthritis. N. Engl. J. Med. 365, 2205–2219. doi:10.1056/NEJMra1004965
Mesquida, M., Leszczynska, A., Llorenç, V., and Adán, A. (2014). Interleukin-6 Blockade in Ocular Inflammatory Diseases. Clin. Exp. Immunol. 176, 301–309. doi:10.1111/cei.12295
Moelants, E. A., Mortier, A., Van Damme, J., and Proost, P. (2013). Regulation of TNF-α with a Focus on Rheumatoid Arthritis. Immunol. Cel Biol 91, 393–401. doi:10.1038/icb.2013.15
Munoz, L., and Ammit, A. J. (2010). Targeting P38 MAPK Pathway for the Treatment of Alzheimer's Disease. Neuropharmacology 58, 561–568. doi:10.1016/j.neuropharm.2009.11.010
Niranjan, R., Nath, C., and Shukla, R. (2012). Melatonin Attenuated Mediators of Neuroinflammation and Alpha-7 Nicotinic Acetylcholine Receptor mRNA Expression in Lipopolysaccharide (LPS) Stimulated Rat Astrocytoma Cells, C6. Free Radic. Res. 46, 1167–1177. doi:10.3109/10715762.2012.697626
Peng, Z. J., Lai, Q., Teng, Y. F., Qiao, Z. Y., and Chen, Y. F. (2018). The Paw Swelling Effect of Pereskia Aculeate miller on Adjuvant Arthritisin Rats. Sci. Tech. Vis. 14, 124–126. doi:10.19694/j.cnki.issn2095-2457.2018.14.055
Pinto, N. C., Machado, D. C., Da Silva, J. M., Conegundes, J. L., Gualberto, A. C., Gameiro, J., et al. (2015). Pereskia Aculeata Miller Leaves Present In Vivo Topical Anti-inflammatory Activity in Models of Acute and Chronic Dermatitis. J. Ethnopharmacol 173, 330–337. doi:10.1016/j.jep.2015.07.032
Pinto, N. C., and Scio, E. (2014). The Biological Activities and Chemical Composition of Pereskia Species (Cactaceae)-Aa Review. Plant Foods Hum. Nutr. 69, 189–195. doi:10.1007/s11130-014-0423-z
Pu, J., Fang, F. F., Li, X. Q., Shu, Z. H., Jiang, Y. P., Han, T., et al. (2016). Matrine Exerts a Strong Anti-arthritic Effect on Type II Collagen-Induced Arthritis in Rats by Inhibiting Inflammatory Responses. Int. J. Mol. Sci. 17, 410. doi:10.3390/ijms17091410
Raij, L. (2006). Nitric Oxide in the Pathogenesis of Cardiac Disease. J. Clin. Hypertens. (Greenwich) 8, 30–39. doi:10.1111/j.1524-6175.2006.06025.x
Roussel, L., Houle, F., Chan, C., Yao, Y., Bérubé, J., Olivenstein, R., et al. (2010). IL-17 Promotes P38 MAPK-dependent Endothelial Activation Enhancing Neutrophil Recruitment to Sites of Inflammation. J. Immunol. 184, 4531–4537. doi:10.4049/jimmunol.0903162
Scott, D. L., Wolfe, F., and Huizinga, T. W. (2010). Rheumatoid Arthritis. Lancet 376, 1094–1108. doi:10.1016/S0140-6736(10)60826-4
Shakeel, F., Alam, P., Ali, A., Alqarni, M. H., Alshetaili, A., Ghoneim, M. M., et al. (2021). Investigating Antiarthritic Potential of Nanostructured Clove Oil (Syzygium Aromaticum) in CFA-Induced Arthritic Rats: Pharmaceutical Action and Delivery Strategies. Molecules 26, 7327. doi:10.3390/molecules26237327
Shams, S., Martinez, J. M., Dawson, J. R. D., Flores, J., Gabriel, M., Garcia, G., et al. (2021). The Therapeutic Landscape of Rheumatoid Arthritis: Current State and Future Directions. Front. Pharmacol. 12, 680043. doi:10.3389/fphar.2021.680043
Shi, L., Hu, F., Zhu, L., Xu, C., Zhu, H., Li, Y., et al. (2020). CD70-mediated CD27 Expression Downregulation Contributed to the Regulatory B10 Cell Impairment in Rheumatoid Arthritis. Mol. Immunol. 119, 92–100. doi:10.1016/j.molimm.2020.01.016
Souza, L. F., De Barros, I. B., Mancini, E., De Martino, L., Scandolera, E., and De Feo, V. (2014). Chemical Composition and Biological Activities of the Essential Oils from Two Pereskia Species Grown in Brazil. Nat. Prod. Commun. 9, 1805–1808. doi:10.1177/1934578x1400901237
Sparks, J. A. (2019). Rheumatoid Arthritis. Ann. Intern. Med. 170, ITC1–ITC16. doi:10.7326/AITC201901010
Su, X., Yu, Y., Zhong, Y., Giannopoulou, E. G., Hu, X., Liu, H., et al. (2015). Interferon-γ Regulates Cellular Metabolism and mRNA Translation to Potentiate Macrophage Activation. Nat. Immunol. 16, 838–849. doi:10.1038/ni.3205
Takeuchi, T., Yoshida, H., and Tanaka, S. (2021). Role of Interleukin-6 in Bone Destruction and Bone Repair in Rheumatoid Arthritis. Autoimmun. Rev. 20, 102884. doi:10.1016/j.autrev.2021.102884
Takeuchi, Y., Hirota, K., and Sakaguchi, S. (2020). Impaired T Cell Receptor Signaling and Development of T Cell-Mediated Autoimmune Arthritis. Immunol. Rev. 294, 164–176. doi:10.1111/imr.12841
Tan, M. L., Sulaiman, S. F., Najimuddin, N., Samian, M. R., and Muhammad, T. S. (2005). Methanolic Extract of Pereskia Bleo (Kunth) DC. (Cactaceae) Induces Apoptosis in Breast Carcinoma, T47-D Cell Line. J. Ethnopharmacol 96, 287–294. doi:10.1016/j.jep.2004.09.025
Tanaka, T., and Kishimoto, T. (2014). The Biology and Medical Implications of Interleukin-6. Cancer Immunol. Res. 2, 288–294. doi:10.1158/2326-6066.CIR-14-0022
Turesson, C., O'Fallon, W. M., Crowson, C. S., Gabriel, S. E., and Matteson, E. L. (2003). Extra-articular Disease Manifestations in Rheumatoid Arthritis: Incidence Trends and Risk Factors over 46 Years. Ann. Rheum. Dis. 62, 722–727. doi:10.1136/ard.62.8.722
Ueda, F., Iizuka, K., Tago, K., Narukawa, Y., Kiuchi, F., Kasahara, T., et al. (2015). Nepetaefuran and Leonotinin Isolated from Leonotis Nepetaefolia R. Br. Potently Inhibit the LPS Signaling Pathway by Suppressing the Transactivation of NF-Κb. Int. Immunopharmacol 28, 967–976. doi:10.1016/j.intimp.2015.08.015
Uttra, A. M., AlamgeerSHAHZAD, M., Shahzad, M., Shabbir, A., and Jahan, S. (2018). Ephedra Gerardiana Aqueous Ethanolic Extract and Fractions Attenuate Freund Complete Adjuvant Induced Arthritis in Sprague Dawley Rats by Downregulating PGE2, COX2, IL-1β, IL-6, TNF-α, NF-kB and Upregulating IL-4 and IL-10. J. Ethnopharmacol 224, 482–496. doi:10.1016/j.jep.2018.06.018
van der Woude, D., and van der Helm-van Mil, A. H. M. (2018). Update on the Epidemiology, Risk Factors, and Disease Outcomes of Rheumatoid Arthritis. Best Pract. Res. Clin. Rheumatol. 32, 174–187. doi:10.1016/j.berh.2018.10.005
Wang, X., He, X., Zhang, C. F., Guo, C. R., Wang, C. Z., and Yuan, C. S. (2017). Anti-arthritic Effect of Berberine on Adjuvant-Induced Rheumatoid Arthritis in Rats. Biomed. Pharmacother. 89, 887–893. doi:10.1016/j.biopha.2017.02.099
Wang, Y., Chen, Z., Liu, C., Lu, X., Yang, C., and Qiu, S. (2019). Distributive differences of P2Xs between the forelimb and hind limb of adjuvant arthritis rats and intervention by Notopterygh rhizoma et radix. Pharm. Biol. 57, 82–89. doi:10.1080/13880209.2018.1561730
Xing, R., Sun, L., Wu, D., Jin, Y., Li, C., Liu, X., et al. (2018). Autoantibodies against Interleukin-21 Correlate with Disease Activity in Patients with Rheumatoid Arthritis. Clin. Rheumatol. 37, 75–80. doi:10.1007/s10067-017-3862-8
Xu, G., Feng, Y., Li, H., Chen, C., Li, H., Wang, C., et al. (2021). Molecular Mechanism of the Regulatory Effect of Schisandrol A on the Immune Function of Mice Based on a Transcription Factor Regulatory Network. Front. Pharmacol. 12, 785353. doi:10.3389/fphar.2021.785353
Xu, J., Zhao, Y., and Aisa, H. A. (2017). Anti-inflammatory Effect of Pomegranate Flower in Lipopolysaccharide (LPS)-stimulated RAW264.7 Macrophages. Pharm. Biol. 55, 2095–2101. doi:10.1080/13880209.2017.1357737
Yang, Y., Li, S., Yang, Q., Shi, Y., Zheng, M., Liu, Y., et al. (2014). Resveratrol Reduces the Proinflammatory Effects and Lipopolysaccharide- Induced Expression of HMGB1 and TLR4 in RAW264.7 Cells. Cell Physiol Biochem 33, 1283–1292. doi:10.1159/000358696
Yonezawa, Y., Miyashita, T., Nejishima, H., Takeda, Y., Imai, K., and Ogawa, H. (2018). Anti-inflammatory Effects of Olive-Derived Hydroxytyrosol on Lipopolysaccharide-Induced Inflammation in RAW264.7 Cells. J. Vet. Med. Sci. 80, 1801–1807. doi:10.1292/jvms.18-0250
Yuan, H. Y., Zhang, X. L., Zhang, X. H., Meng, L., and Wei, J. F. (2015). Analysis of Patents on Anti-rheumatoid Arthritis Therapies Issued in China. Expert Opin. Ther. Pat 25, 909–930. doi:10.1517/13543776.2015.1044972
Zhu, Z., Gu, Y., Zhao, Y., Song, Y., Li, J., and Tu, P. (2016). GYF-17, a Chloride Substituted 2-(2-Phenethyl)-Chromone, Suppresses LPS-Induced Inflammatory Mediator Production in RAW264.7 Cells by Inhibiting STAT1/3 and ERK1/2 Signaling Pathways. Int. Immunopharmacol 35, 185–192. doi:10.1016/j.intimp.2016.03.044
Keywords: petroleum ether extract of P. aculeate Miller, rheumatoid arthritis, p38/MAPK signaling pathway, inflammation, ethnomedicine
Citation: Chen Y, Liu K, Qin Y, Chen S, Guan G, Huang Y, Chen Y and Mo Z (2022) Effects of Pereskia aculeate Miller Petroleum Ether Extract on Complete Freund’s Adjuvant-Induced Rheumatoid Arthritis in Rats and its Potential Molecular Mechanisms. Front. Pharmacol. 13:869810. doi: 10.3389/fphar.2022.869810
Received: 05 February 2022; Accepted: 18 April 2022;
Published: 09 May 2022.
Edited by:
Tao Xu, Anhui Medical University, ChinaReviewed by:
Jia-Xin Shi, The First People’s Hospital of Lianyungang, ChinaSolomon Umukoro, University of Ibadan, Nigeria
Copyright © 2022 Chen, Liu, Qin, Chen, Guan, Huang, Chen and Mo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Chen, NjI0NjU3MzA1QHFxLmNvbQ==; Zhixian Mo, Y2hlcnJ5bW9Ac211LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship.
 Yifei Chen
Yifei Chen Kaifei Liu
Kaifei Liu Yingyuan Qin3
Yingyuan Qin3