
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 05 July 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.867490
This article is part of the Research TopicLeveraging Pharmacovigilance Data Mining with “The Patient” in MindView all 12 articles
 Kanyawan Tiwattanon1†
Kanyawan Tiwattanon1† Shobana John2,3†
Shobana John2,3† Napatrupron Koomdee2,3*
Napatrupron Koomdee2,3* Pimonpan Jinda2,3
Pimonpan Jinda2,3 Jiratha Rachanakul2,3
Jiratha Rachanakul2,3 Thawinee Jantararoungtong2,3
Thawinee Jantararoungtong2,3 Nutthan Nuntharadthanaphong2,3
Nutthan Nuntharadthanaphong2,3 Chiraphat Kloypan4,5
Chiraphat Kloypan4,5 Mohitosh Biswas2,3,6
Mohitosh Biswas2,3,6 Apisit Boongird1,7*
Apisit Boongird1,7* Chonlaphat Sukasem2,3,7,8,9
Chonlaphat Sukasem2,3,7,8,9Objective: This study aimed to investigate the clinical impact of HLA-B*15:02 pharmacogenomics (PGx) testing before carbamazepine (CBZ)/oxcarbazepine (OXC) prescriptions and to determine whether this PGx testing was associated with the reduction of CBZ/OXC-induced cutaneous adverse drug reactions (CADRs) in Thailand.
Methods: This retrospective observational cohort study was conducted by obtaining relevant HLA-B*15:02 PGx-testing and clinical data from electronic medical records during 2011–2020. 384 patient data were included in this study to investigate the clinical decision on CBZ/OXC usage before and after the HLA-B*15:02 PGx testing, and 1,539 patient data were included in this study to demonstrate the incidence of CBZ/OXC-induced SCARs and SJS between HLA-B*15:02 tested and non-tested patients. To analyze and summarize the results, descriptive statistics were employed, and Fisher exact test was used to compare the clinical difference between the HLA-B*15:02 positive and negative groups and to compare the differences of SCARs incidence.
Results: 384 patients were included in this study as per the inclusion criteria. Of these, 70 patients carried HLA-B*15:02, of which 63 and 65 patients were not prescribed with CBZ/OXC before and after the availability of genotyping results, respectively. In the remaining HLA-B*15:02 non-carriers, 48, and 189 patients were prescribed CBZ/OXC before and after genotyping results were available, respectively. The findings of this study showed that the incidence of SCARs of CBZ/OXC was significantly lower (p < 0.001) in the HLA-B*15:02 screening arm than in the non-screening arm.
Conclusion: HLA-B pharmacogenetics testing influenced the selection of appropriate AEDs. The presence of mild rash in the HLA-B*15:02 negative group indicates that other genetic biomarker (HLA-A*31:01) and/or non-genetic variables are involved in CBZ/OXC-induced CADRs, emphasizing that CBZ/OXC prescriptions necessitate CADR monitoring. The hospital policy and clinical decision support (CDS) alert system is essential to overcome the barriers associated with the utilization of PGx guidelines into clinical practice.
Carbamazepine (CBZ) is a first-generation antiepileptic drugs (AEDs) used to treat a variety of neurological and psychiatric problems, including epilepsy, trigeminal neuralgia, and bipolar disorders. In Thais, there has been a well-documented association between HLA-B*15:02 and CBZ-induced Stevens-Johnson syndrome (SJS) and toxic epidermal necrolysis (TEN) (Hung et al., 2006; Locharernkul et al., 2008; Tassaneeyakul et al., 2010; Tangamornsuksan et al., 2013; Sukasem et al., 2018; Sukasem et al., 2021b). In 2013, the government launched a policy requiring HLA-B*15:02 pharmacogenetic (PGx) testing before the start of CBZ in Bangkok as a pilot study (Department of Medical Sciences, 2013). Subsequently, national policy screening for HLA-B*15:02 was reinforced by the National Health Security Office (NHSO) in 2018 throughout Thailand (Ang et al., 2017; Chang et al., 2020; Sukasem et al., 2021a; Jantararoungtong et al., 2021). Besides HLA-B*15:02, some other variants such as HLA-B*15:08, HLA-B*15:11 and HLA-B*15:21 may also affect the safety of CBZ and found to be other HLA-B risk alleles associated with CBZ-induced cutaneous adverse drug reactions (CADRs) (Jaruthamsophon et al., 2017; Volpi et al., 2018; Kloypan et al., 2021).
In addition, Chen et al. reported a strong association between the HLA-B*15:02 allele and oxcarbazepine (OXC)-induced SJS/TEN in Chinese and Thai populations (Chen et al., 2017). Consequently, the Clinical Pharmacogenetics Implementation Consortium (CPIC) guideline recommended to avoid the prescription of CBZ and OXC in HLA-B*15:02 carrier (Phillips et al., 2018).
Although knowledge and research on PGx testing in Thailand have been rapidly expanding in recent years (Jaruthamsophon et al., 2017), there has been no longitudinal research on the application of HLA-B*15:02 genotype results in daily clinical practice, including documentation of severe cutaneous adverse drug reactions (SCARs) and new adverse drug reactions (ADRs) in electronic medical records, patterns of AEDs prescriptions based on genotype results, and assessment of the CADRs. This is the first research of its kind in Thailand, and it focuses on these fascinating topics.
The goal of this study was to investigate how PGx testing (HLA-B*15:02) affected the CBZ/OXC prescriptions and the reduction of CBZ/OXC-induced SCARs in Thais. The findings of this study have the potential to help physicians regarding the prescription of appropriate AEDs in clinical practice for those patients carrying HLA-B*15:02 CBZ/OXC-risk alleles and to support NHSO Thailand’s HLA-B*15:02 screening as the national policy.
This was a retrospective cohort study conducted at the Ramathibodi Hospital of the Faculty of Medicine. In order to determine the influence of HLA-B*15:02 PGx testing in clinical practice, 1,020 patients with performed HLA-B*15:02 PGx testing between 2011 and 2020 were included. Among 1,020 patients, 636 patients were excluded due to incomplete data (n = 266), PGx tested for other AEDs (n = 51), repeated PGx order (n = 53), and less than 15 years of age (n = 266) (Figure 1). This study did not include HLA-B genotyping that was performed to test the association of drug induced CADRs. Subsequently, 384 patients were enrolled for analysis. Patients’ demographic information, such as age, gender, diagnosis, and drug-related information, such as the number of CBZ prescriptions and their association to HLA-B*15:02 genotyping results, CADRs types, drug allergic history, PGx testing, number of comorbidities, types of comorbidities, and alternative drugs utilized were recorded. All CADRs were diagnosed and confirmed by a dermatologist at Ramathibodi Hospital in Thailand.

FIGURE 1. The flowchart of patients recruitment and clinical decision in HLA-B*15:02 positive (n = 70) and HLA-B*15:02 negative (n = 314) in Ramathibodi Hospital, Bangkok.
To demonstrate how prospective HLA-B*15:02 testing likely reduces the occurrence of hypersensitivity reactions to CBZ/OXC, we recruited 1,539 patients data who were prescribed CBZ/OXC for a period of 2013–2021 and further classified them as tested for HLA-B*15:02 group or not tested for HLA-B*15:02 group. We retrospectively collected CBZ/OXC prescribing data, HLA-B*15:02 testing data, and SCARs occurrence data for this part of the study.
To exemplify the overall trend of HLA-B*15:02 testing requests within and outside of Ramathibodi Hospital, we collected overall HLA-B*15:02 PGx testing for CBZ/OXC that was performed during 2011–2020 and presented them in percentage as per year (Figure 2).
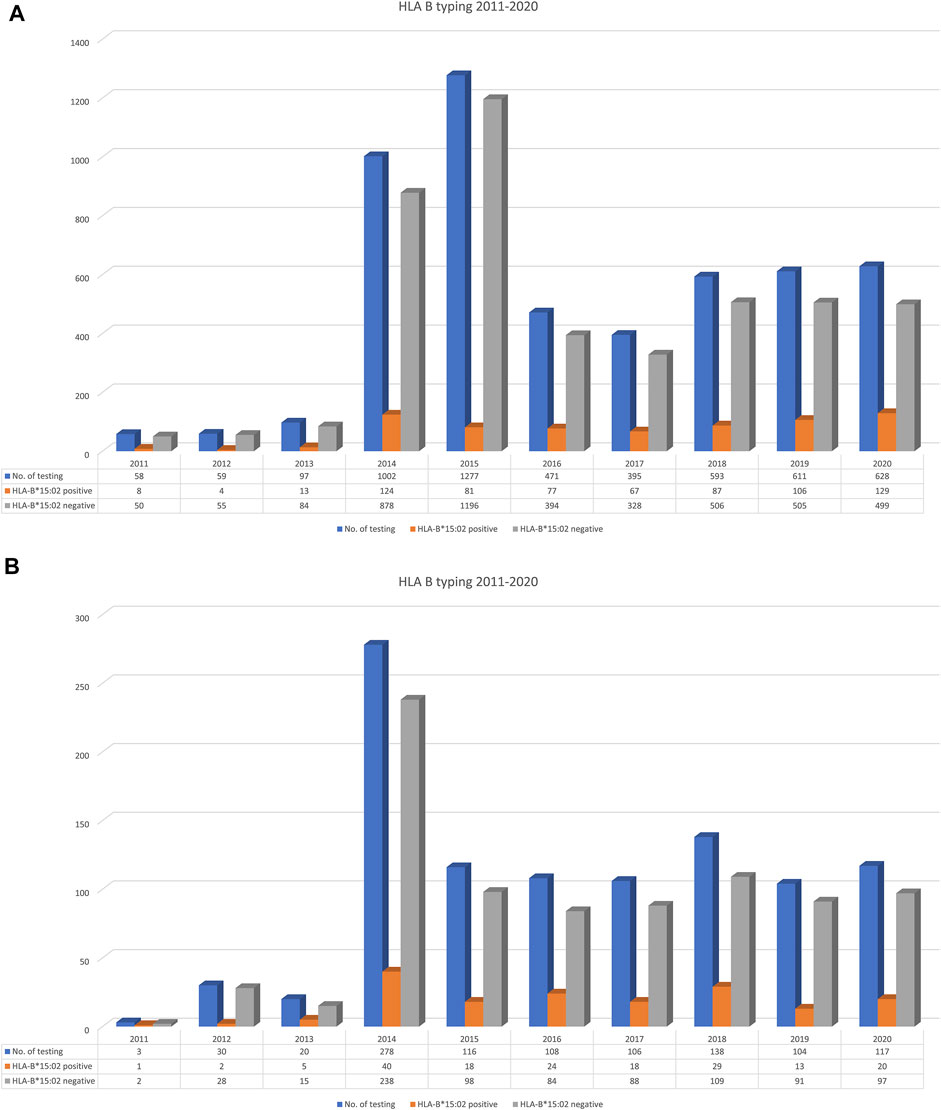
FIGURE 2. Trend of HLA-B*15:02 testing requested by out-side [n = 5,191; (A)] and in-side [n = 1,020; (B)] of Ramathibodi Hospital (19.64%) during 2011–2020.
The studies involving human participants were reviewed and approved by the Ethical Review Committee on Research Involving Human Subjects, Faculty of Medicine Ramathibodi Hospital, Mahidol University. The patients provided written informed consent to participate in this study.
Genomic DNA samples were isolated from EDTA blood, using the MagNAprue Compact Nucleic Acid Isolation kits (Roche Applied Science, Mannheim, Germany). The quality of genomic DNA was measured by NanoDrop® ND-1000 (Thermo Scientific, Wilmington, United States). HLA-B alleles were analyzed by the polymerase chain reaction-sequence specific oligonucleotide probe (PCR-SSOP) assay and Luminex™ Multiplex Technology with well established protocols.
In brief, the DNA sample obtained from patients was amplified by polymerase chain reaction (PCR). The PCR product was then hybridized against a panel of sequence specific oligonucleotide probes on coated polystyrene microspheres that had sequences complementary to stretches of polymorphism within the target HLA-B. The amplicon-probe complex was then visualized using a colorimetric reaction and fluorescence detection technology by the Luminex®IS 100 system (Luminex Corporation, Austin, Texas, United States). Analysis of the HLA class I alleles were performed using HLA fusion software version 2.0 (One Lambda, Canoga Park, CA, United States). For each allele, the results were reported as either HLA-B*15:02 positive or negative.
Descriptive statistics were employed to summarize and analyze the collected data. The Fisher exact test was used to compare the clinical characteristics of the HLA-B*15:02 positive (positive group) and HLA-B*15:02 negative (negative group) and to calculate the difference of the SCARs incidence between the HLA-B*15:02 tested group and non-tested groups.
Out of 1,020 patients undertaking HLA-B*15:02 genotyping tests during 2011–2020, only 384 patients were included as per the inclusion criteria in this study (Figure 1). Incomplete data (n = 266), tested for other AEDs (n = 51), Patients’ data under the age of 15 (n = 266), and repeatedly requested data (n = 53) were excluded from this study. Our study population consists of 243 (63.28%) females and 141 (36.72%) males. The age range of this population was 15–95, with a mean age of 49.23 ± 19.79 (SD). Out of 384 patients, 112 (29.16%) were diagnosed with different types of epilepsy. The second most common disease for the CBZ indication was trigeminal neuralgia (n = 95, 24.74%). Neuropathic pain (n = 64, 16.67%), hemifacial spasm (n = 14, 3.65%), tonic spasm (n = 13, 3.39%), and bipolar disorder (n = 23, 5.99%) were the other commonly diagnosed diseases for which CBZ was prescribed in this study. Sixty-three cases were in miscellaneous categories (Table 1).
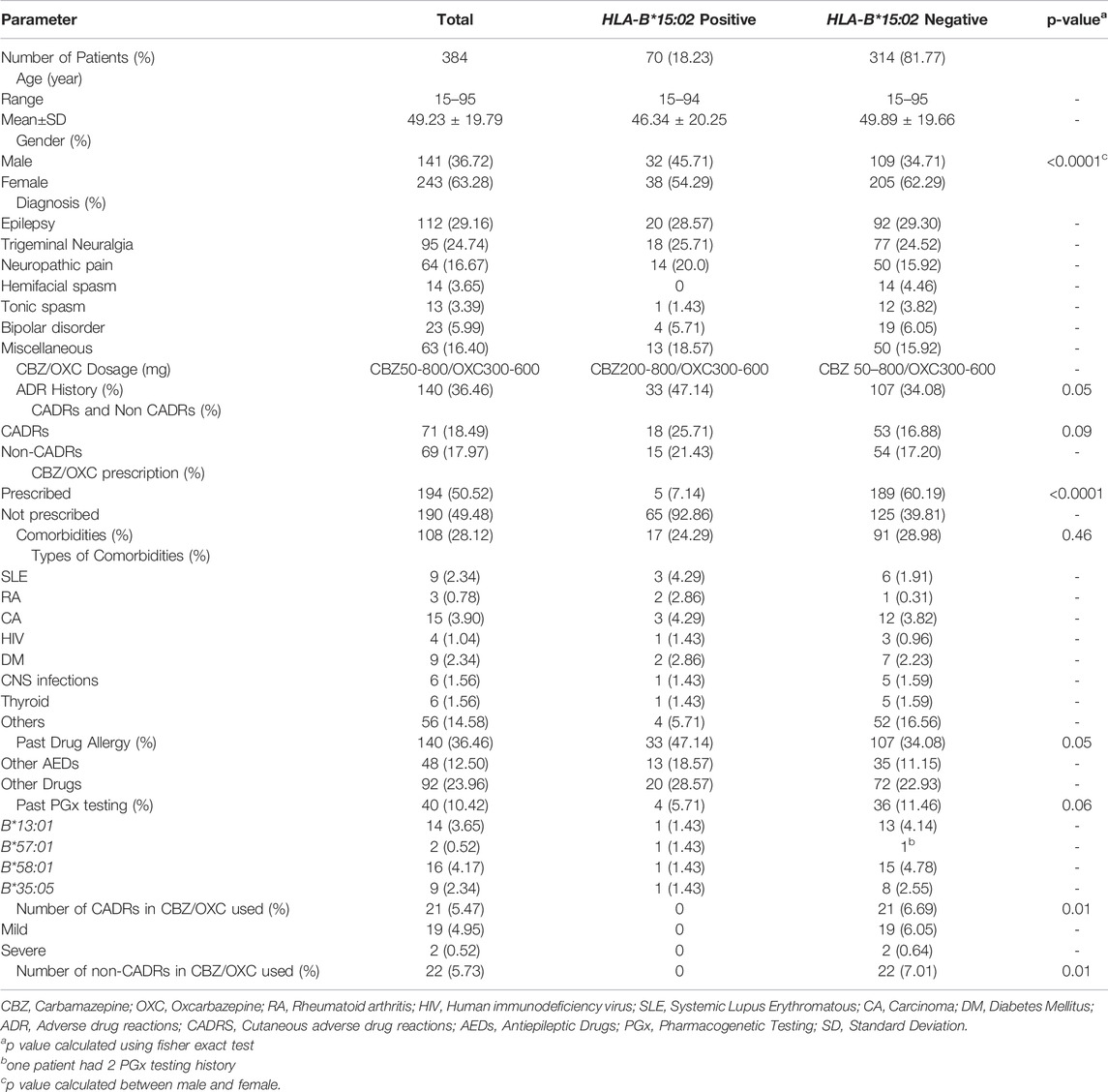
TABLE 1. Patients characteristics, diagnosis, comorbidities, adverse drug reactions (ADRs), drug allergy.
Comorbidities were discovered in 108 (28.12%) patients, 17 (17/70, 24.29%) of them were HLA-B*15:02 positive and the remaining 91 (91/314, 28.98%) were HLA-B*15:02 negative. The CBZ was prescribed in doses ranging from 50 to 800 mg, while the OXC was prescribed in doses ranging from 300 to 600 mg.
According to the findings, 36.46% (n = 140) of the patients had previous ADR history, while 18.49% (n = 71) had previously experienced CADRs. HLA-B*15:02 positive patients had higher CADRs than HLA-B*15:02 negative patients (18/70, 25.71% vs 53/314, 16.88%, p = 0.09), however, the difference was insignificant. 48 of the 384 patients had a prior history of AED allergy, with 18.57% (13/70) and 11.15% (35/314) belonging to the HLA-B*15:02 positive and negative groups, respectively (p = 0.05).
Table 2 shows the status of a clinical decision on CBZ/OXC prescription. Because all of the patients were scheduled to receive CBZ/OXC therapy, HLA-B*15:02 testing was performed as per the national policy. Out of 384 patients, the clinical decision on CBZ/OXC was put on hold for 85.67% of patients and requested the HLA-B*15:02 testing prior prescription. Because of the clinical urgency, 55 patients were prescribed CBZ/OXC while the HLA-B*15:02 testing was ordered. HLA-B*15:02 carriers were found in 70 of the 384 cases. This accounts for 18.23% of the overall study population. Fortunately, CBZ was not prescribed in 63 of 70 patients with HLA-B*15:02 carriers before the genotyping results were available; and after receiving the HLA-B*15:02 results, CBZ/OXC was not prescribed in 65 of 70 patients. Only 48 patients in the negative group received CBZ/OXC before the HLA-B*15:02 requested, but 189 patients with HLA-B*15:02 negative were prescribed CBZ/OXC following the results available in the electronic medical record (EMRs) (Table 2). In this cohort, five patients were remain treated with CBZ/OXC-based regimen since they were received this regimen for longer than 3 months and had no CADRs. On the other hand, 189 patients in HLA-B*15:02 negative group were administered CBZ/OXC as recommended. Surprisingly, 21 patients with negative HLA-B*15:02 had CADRs, of which 2 patients (9.52%) were SCARs (one DRESS and one SJS cases) and 19 patients (90.48%) were mild rash. Non-CADRs were also observed in 22 patients with negative HLA-B*15:02, with dizziness being the most common ADRs (Table 1).
In this study, 13 cases were reported as CBZ-induced SCARs after received CBZ without PGx testing during 2013–2021 (Table 3). Among them, the patients were diagnosed as SJS/TEN (n = 10), Drug rash with eosinophilia and systemic symptoms (DRESS, n = 2) and fix-drug eruption (FDE, n = 1). Remarkably, HLA-B*15:02 were positive in all SJS/TEN cases (10/10, 100%), where as negative in DRESS and FDE cases. Two patients with CBZ-induced DRESS showed no HLA-B*15:02 positive (HLA-B*27:06/58:01 and HLA-B*40:01/58:01).
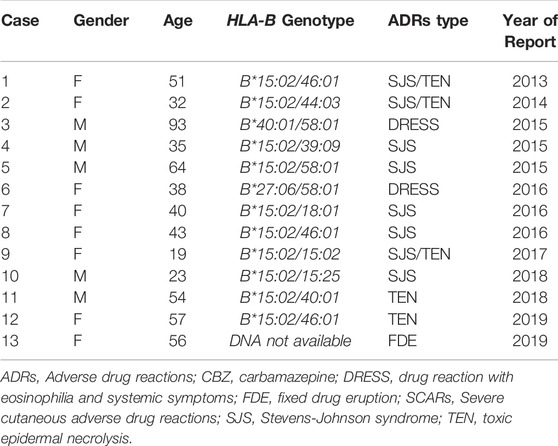
TABLE 3. Cases of CBZ/OXC-induced SCARs and fixed drug eruption after received CBZ without PGx testing.
Available data demonstrated that prospective HLA-B*15:02 probably reduces the incidence of hypersensitivity reactions to CBZ/OXC (Table 4). Of the 1,539 patients who were prescribed for CBZ/OXC, 916 (59.5%) were tested for the HLA-B*15:02 allele and 623 (40.5%) were not tested. The incidence of clinically diagnosed SCARs to CBZ/OXC was significantly lower (p < 0.001) in the screening arm (0.22%) when compared to the non-screening arm (1.93%). Moreover, 10 of 623 (1.61%) of non-tested patients suffered from SJS/TEN, while only 1 of 916 (0.22%) was observed in tested patients (p < 0.001).
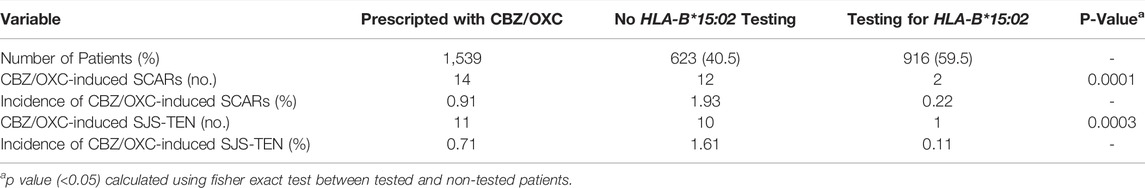
TABLE 4. Incidence of CBZ/OXC-induced SCAR and SJS between HLA-B*15:02 tested patients and non-tested patients.
The Laboratory for Pharmacogenomics (PPM), Ramathibodi Hospital has been established as the first reference laboratory for PGx testing in Thailand. Figure 2 illustrates the trend of HLA-B*15:02 testing in the country and also hospital during 2011–2020. Totally, 5,191 PGx testing for CBZ/OXC were performed during 2011–2020 in Thailand whereas 1,020 (19.64%) patients were requested HLA-B*15:02 for CBZ/OXC treatment in Ramathibodi Hospital. Interestingly, only 58 samples were requested by clinicians in 2011, but the number of PGx testing was escalated to 1,002 and 1,277 in 2014 and 2015, respectively. The similar trend was found in Ramathibodi Hospital; it started with three patients in 2011 and has gradually risen in 2014 (n = 278) and dropped in 2015 (n = 116), 2016 (n = 108), 2017 (n = 106), 2018 (n = 138), 2019 (n = 104) and 2020 (n = 117). Totally, 696 (13.27%) and 170 (16.67%)HLA-B*15:02 carriers were detected in Thailand and Ramathibodi Hospital, respectively.
In order to prevent the SJS/TEN from CBZ/OXC, the alternative AEDs choices were prescribed in HLA-B*15:02 carriers as per the Thailand Clinical Practice Guidelines for Epilepsy and also NHSO policies. Figure 3 depicts the alternative medications that were utilised in place of CBZ/OXC. The most widely utilised alternative AEDs were levetiracetam (30%), valproate (28%) and lamotrigine (LTG) (16%).
This is the first retrospective cohort study in Thailand to determine the clinical impact of pharmacogenomics (PGx) testing on carbamazepine/oxcarbazepine (CBZ/OXC) prescribing. The female preponderance was seen in the PGx testing population in this study, and it may be because of the higher incidence of hypersensitivity reactions among females (Saksit et al., 2017). Many earlier research studies support this fact. For example, a study conducted by Alvestad et al. (2007) reported that a striking difference in the incidence of hypersensitivity was seen between males (8%) and females (19%) with p < 0.001. This association is highly dependent on the age group; reproductive years 20–50 are the most significant age group. The explanation for this is that sex hormones alter the immunological process of rash. Androgens limit the inflammatory response more than endogenous glucocorticoids, whereas female sex hormones enhance the immunological response both pathologically and physiologically (Talal, 1987; Da Silva, 1999; Osman, 2003).
Pharmacogenomic testing for HLA-B*15:02 is now standard practice in Thailand. In this study, physicians requested the PGx testing for CBZ/OXC and hold the prescription until received the PGx report for 85.67% of 384 patients. This was complying with Clinical Practice Guidelines for Epilepsy (2018) of Epilepsy Society of Thailand and the Clinical Pharmacogenetics Implementation Consortium Guideline for HLA Genotype and Use of Carbamazepine and Oxcarbazepine: 2017 Update (Epilepsy Society of Thailand, 2018; Phillips et al., 2018). Do not use CBZ in naïve-patients that are positive for HLA-B*15:02. If patient used CBZ for longer than 3 months without incidence of adverse reactions, consider use with caution (Phillips et al., 2018). However, seven patients were found to receive CBZ/OXC while requesting the HLA-B*15:02 PGx testing. Two of them were CBZ naïve-patients and prescribed with CBZ due to clinical urgency and five were identified as CBZ/OXC-treated patients (used CBZ/OXC for longer than 3 months). Therefore, alternative drugs were prescribed in 65 (92.86%) patients who carried HLA-B*15:02 (positive group).
Among the 314, negative HLA-B*15:02 group, 60% were prescribed with CBZ/OXC, whereas the remaining 40% switched over to other AEDs overall before and after the PGx test. The retrospective follow up analysis showed that lengthy HLA-B testing results turnaround time and/or lengthy reach out time of the available PGx testing results to physicians were the two important reasons for it. This is a very common barrier while translating the PGx into the real clinical world (Krebs and Milani, 2019). Thailand has recently seen a lot of technological improvement in genetic analysis but we used samples from 2011 in our research. This could explain why clinician prescribed CBZ/OXC without waiting for the PGx results in 55 (14.32%) patients. Physicians, on the other hand, awaited for the PGx results of 329 patients before making a clinical decision. This supports our hypothesis that physician CBZ/OXC prescribing decisions were influenced by HLA-B*15:02 genotyping. Moreover, 92.85% (65/70) of HLA-B*15:02 risk allele carriers were avoided by CBZ/OXC, so it can be assumed that the risk population is separated and SCARs are prevented in this population.
However, the screening for HLA-B*15:02 is not a compulsory testing in Thailand prior to initiation of CBZ treatment. During 2013–2019, CBZ-induced SCARs were reported from 13 patients who received CBZ without PGx screening in Thai-SCARs cohort. Remarkably, HLA-B*15:02 were positive in all SJS/TEN cases (n = 10, 100%), where as negative HLA-B*15:02 in DRESS and FDE. Assumably, these patients can be prevented from SJS/TEN if the HLA-B*15:02 PGx testing was required as a mandatory testing for CBZ prescription. Additionally, the hospital policy and clinical decision support (CDS) alert system is essential to overcome the barriers associated with the utilization of PGx guidelines into clinical practice. The PGx-CDS should be integrated into EMRs and pop-up alert in the therapeutics order system recommending the clinician to order a PGx screening test prior initiation of preventable drug-induced SCARs such as CBZ/OXC (HLA-B*15:02), allopurinol (HLA-B*58:01), abacavir (HLA-B*57:01) and cotrimoxazole/dapsone (HLA-B*13:01) (Sukasem et al., 2016; Sukasem et al., 2020; Kloypan et al., 2021; Satapornpong et al., 2021).
An earlier study in Thailand reported that out of 214 SCARs-treated patients over a period of 10 years, 23 patients (10.6%) were treated with CBZ, and this shows that CBZ is the major culprit for SCARs. If MPE and other mild-moderate level reactions were included, this number could go higher than this. However, it cannot be assumed that all the risk allele carriers will have CBZ-induced CADRs. In this study, 7.14% of patients who carried HLA-B*15:02 allele did not manifest any CADRs. Because the frequency of SJS/TEN is substantially lower than the frequency of the risk allele in these groups, previous studies have shown that the test’s positive predictive value is poor (1–5%). Although having HLA-B*15:02 increases the risk of CBZ/OXC induced SJS/TEN by up to >700-fold compared to non-carriers, the vast majority of patients with HLA-B*15:02 (95%–99%) do not develop SJS/TEN from CBZ/OXC (Amstutz et al., 2014).
However, in this study if the mild reactions are included this rate goes up to 11%. The reason could be that 29% of the negative patients were reported with multiple comorbidities and most of them were immune related disorders like Systemic lupus erythematosus (SLE), hypo/hyperthyroid, cancer, rheumatoid arthritis (RA), diabetes mellitus (DM), hypertension (HTN), and central nervous system (CNS) infections. Earlier studies showed that some non-clinical risk factors may be involved in the initiation of CADRs (Campos-Fernández Mdel et al., 2005; Patel et al., 2014; John et al., 2021).
Unexpectedly, 2 cases with CBZ-induced SCARs (DRESS and SJS) were reported among 189 patients with HLA-B*15:02 negative after CBZ/OXC treated patients. Likewise, 2 cases with CBZ-induced DRESS were reported after CBZ treatment without HLA-B*15:02 PGx testing. Commonly, the HLA-B*15:02 allele has been reported to be specifically associated with CBZ-induced SJS/TEN in Asian populations, and no associations have been reported for drug-induced MPE and DRESS (phenotype-specific biomarker) (Sukasem et al., 2021b). In contrast, HLA-A*31:01 was reported to be associated with CBZ-induced DRESS and MPE (Mockenhaupt et al., 2019; Ahmed et al., 2021). US-FDA recommended that HLA-B*15:02 and HLA-A*31:01 genotypes should be detected to stratify the high risk patients prior CBZ prescription. The use of CBZ should be avoided in patients who test positive for the HLA-A*31:01 or HLA-B*15:02 alleles (US Food and Drug Administration, 2009). However, conflicting evidence has been found in this study, one SJS patient was reported from negative of HLA-B*15:02 group. This suggested that other genetic and clinical factors may also influence a patient’s risk for adverse reactions.
PGx testing has been implemented in many hospitals throughout the world over the last decade to guarantee that the best treatment is chosen. For example, 27 institutions in the United States have established numerous PGx implementation programmes (Dunnenberger et al., 2015; Volpi et al., 2018), while other PGx implementation programmes in Europe and East Asia include EU-funded Ubiquitous Pharmacogenomics (U-PGx), PREemptive Pharmacogenomic testing for the prevention of Adverse Drug Reactions (PREPARE) (van der Wouden et al., 2017), and the Southeast Asian Pharmacogenomics Research Network (SEAPharm) (RIKEN, 2012; Chumnumwat et al., 2019).
We discovered that the number of HLA-B*15:02 tests per year has increased significantly since 2013 in Thailand. The reason for the high PGx testing could be because of the implementation of a pilot project for HLA-B*15:02 PGx testing before prescribing CBZ in Bangkok by the Department of Medical Sciences, Ministry of Public Health, Thailand. This phenomenon was emphasised later by NHSO announcement for implementation of national screening policy in 2018.
In the HLA-B*15:02 positive group, levetiracetam, sodium valproate, LTG, gabapentin, and phenytoin (PHT) sodium were the most prescribed as alternative AEDs in epilepsy. The reason that levetiracetam and sodium valproate were used is that they are non-aromatic AEDs with a low rate of cross-reactivity with CADRs. In 2014, Li et al. (2015) reported a significant association between HLA-B*15:02 and PHT or LTG-induced SJS/TEN. Nevertheless, our study found that LTG and PHT were prescribed in HLA-B*15:02 positive patients without any ADRs. The reason could be the strength of the association is much weaker than it was for CBZ/OXC-related SJS/TEN. A study from Thailand reported a moderate association (limited evidence) (Locharernkul et al., 2008) and a case report of Han Chinese also showed a weak association (insufficient evidence) (Shi et al., 2012).
Although the retrospective nature of this study is its limitation, this is the first large cohort study in Thailand that provides valuable information on the real clinical impact of HLA-B*15:02 PGx testing for CBZ prescription and associated CADRs in Thai patients.
The selection of appropriate AEDs was influenced by pharmacogenomic testing of HLA-B*15:02 risk alleles. HLA-B genotyping can guide the physician in selecting CBZ/OXC and other AEDs prescriptions rationally. The number of HLA-B*15:02 screening has increased significantly in Thailand. In 85% of cases, physicians waited for HLA-B*15:02 testing to make clinical decisions on CBZ/OXC prescribing. However, physicians have administered CBZ/OXC without PGx testing in the urgent cases. Though there were no CADRs in the HLA-B*15:02 positive group, CBZ-induced SJS/TEN were reported from HLA-B*15:02 carriers who received CBZ without PGx screening before prescription. The presence of mild rash in the HLA-B*15:02 negative group indicates that other genetic biomarker such as HLA-A*31:01 and/or non-genetic variables are involved in CBZ/OXC-induced CADRs, emphasizing that CBZ/OXC prescriptions necessitate CADRs monitoring even in patient without HLA-B*15:02 risk alleles.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the ethics committee of Ramathibodi Hospital. The patients/participants provided their written informed consent to participate in this study.
KT, SJ, and CS: wrote the manuscript; AB, NK and CS designed and conceptualization the research; All authors performed the research; KT and SJ formal analysis and curation the data; CS, NK and AB: Validation and finalized the study.
This study was supported by grants from the 1) Mahidol University International Postdoctoral Fellowship, Mahidol University 2) Faculty of Medicine, Ramathibodi Hospital, Mahidol University 3) the Health System Research Institute under Genomics Thailand Strategic Fund, 4) The International Research Network-The Thailand Research Fund (IRN60W003).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ahmed, A. F., Sukasem, C., Sabbah, M. A., Musa, N. F., Mohamed Noor, D. A., and Daud, N. A. A. (2021). Genetic Determinants in HLA and Cytochrome P450 Genes in the Risk of Aromatic Antiepileptic-Induced Severe Cutaneous Adverse Reactions. J. Pers. Med. 11 (5), 383. doi:10.3390/jpm11050383
Alvestad, S., Lydersen, S., and Brodtkorb, E. (2007). Rash from Antiepileptic Drugs: Influence by Gender, Age, and Learning Disability. Epilepsia 48 (7), 1360–1365. doi:10.1111/j.1528-1167.2007.01109.x
Amstutz, U., Shear, N. H., Rieder, M. J., Hwang, S., Fung, V., Nakamura, H., et al. (2014). Recommendations for HLA-B*15:02 and HLA-A*31:01 Genetic Testing to Reduce the Risk of Carbamazepine-Induced Hypersensitivity Reactions. Epilepsia 55 (4), 496–506. doi:10.1111/epi.12564
Ang, H. X., Chan, S. L., Sani, L. L., Quah, C. B., Brunham, L. R., Tan, B. O. P., et al. (2017). Pharmacogenomics in Asia: A Systematic Review on Current Trends and Novel Discoveries. Pharmacogenomics 18 (9), 891–910. doi:10.2217/pgs-2017-0009
Campos-Fernández Mdel, M., Ponce-De-León-Rosales, S., Archer-Dubon, C., and Orozco-Topete, R. (2005). Incidence and Risk Factors for Cutaneous Adverse Drug Reactions in an Intensive Care Unit. Rev. Invest. Clin. 57 (6), 770–774. https://www.medigraphic.com/pdfs/revinvcli/nn-2005/nn056b.pdf. https://pubmed.ncbi.nlm.nih.gov/16708902/
Chang, W. C., Abe, R., Anderson, P., Anderson, W., Ardern-Jones, M. R., Beachkofsky, T. M., et al. (2020). SJS/TEN 2019: From Science to Translation. J. Dermatol Sci. 98 (1), 2–12. doi:10.1016/j.jdermsci.2020.02.003
Chen, C. B., Hsiao, Y. H., Wu, T., Hsih, M. S., Tassaneeyakul, W., Jorns, T. P., et al. (2017). Risk and Association of HLA with Oxcarbazepine-Induced Cutaneous Adverse Reactions in Asians. Neurology 88 (1), 78–86. doi:10.1212/wnl.0000000000003453
Chumnumwat, S., Lu, Z. H., Sukasem, C., Winther, M. D., Capule, F. R., Abdul Hamid, A. A. A. T., et al. (2019). Southeast Asian Pharmacogenomics Research Network (SEAPharm): Current Status and Perspectives. Public Health Genomics 22 (3-4), 132–139. doi:10.1159/000502916
Da Silva, J. A. (1999). Sex Hormones and Glucocorticoids: Interactions with the Immune System. Ann. N. Y. Acad. Sci. 876, 102–108. doi:10.1111/j.1749-6632.1999.tb07628.x
Department of Medical Sciences (2013). กรมวิทย์ฯนำร่องป้องกันผื่นแพ้ยารุนแรงในเขตกรุงเทพฯ. [Online]. Bangkok, Thailand: Department of Medical Sciences. Available at: http://www.dmsc.moph.go.th/secretary/pr/mass-news/mass-news_2556/10_Oct/กรมวิทย์ฯป้องกันผื่นแพ้ยารุนแรง.pdf.
Dunnenberger, H. M., Crews, K. R., Hoffman, J. M., Caudle, K. E., Broeckel, U., Howard, S. C., et al. (2015). Preemptive Clinical Pharmacogenetics Implementation: Current Programs in Five US Medical Centers. Annu. Rev. Pharmacol. Toxicol. 55, 89–106. doi:10.1146/annurev-pharmtox-010814-124835
Epilepsy Society of Thailand (2018). Clinical Practice Guidelines for Epilepsy. [Online]. Bangkok, Thailand: Epilepsy Society of Thailand. Available at: http://thaiepilepsysociety.com/wp-content/uploads/2013/07/CPG_guidelines-for-epilepsy_Edited-at-page-55_Nov-2018.pdf.
Hung, S. I., Chung, W. H., Jee, S. H., Chen, W. C., Chang, Y. T., Lee, W. R., et al. (2006). Genetic Susceptibility to Carbamazepine-Induced Cutaneous Adverse Drug Reactions. Pharmacogenet Genomics 16 (4), 297–306. doi:10.1097/01.fpc.0000199500.46842.4a
Jantararoungtong, T., Tempark, T., Koomdee, N., Medhasi, S., and Sukasem, C. (2021). Genotyping HLA Alleles to Predict the Development of Severe Cutaneous Adverse Drug Reactions (SCARs): State-Of-The-Art. Expert Opin. Drug Metab. Toxicol. 17 (9), 1049–1064. doi:10.1080/17425255.2021.1946514
Jaruthamsophon, K., Tipmanee, V., Sangiemchoey, A., Sukasem, C., and Limprasert, P. (2017). HLA-B*15:21 and Carbamazepine-Induced Stevens-Johnson Syndrome: Pooled-Data and In Silico Analysis. Sci. Rep. 7, 45553. doi:10.1038/srep45553
John, S., Canyuk, B., Anand, T. C. V., Sukasem, C., and Pattharachayakul, S. (2021). Patient, Disease, and Drug-Related Risk Factors Associated with Phenytoin-Induced Cutaneous Adverse Drug Reactions in South Indian Epileptic Patients - A Prospective Case-Control Study. Curr. Drug Saf. 17, 241–249. doi:10.2174/157488631602211118122907
Kloypan, C., Koomdee, N., Satapornpong, P., Tempark, T., Biswas, M., and Sukasem, C. (2021). A Comprehensive Review of HLA and Severe Cutaneous Adverse Drug Reactions: Implication for Clinical Pharmacogenomics and Precision Medicine. Pharm. (Basel) 14 (11), 1077. doi:10.3390/ph14111077
Krebs, K., and Milani, L. (2019). Translating Pharmacogenomics into Clinical Decisions: Do Not Let the Perfect Be the Enemy of the Good. Hum. Genomics 13 (1), 39. doi:10.1186/s40246-019-0229-z
Li, X., Yu, K., Mei, S., Huo, J., Wang, J., Zhu, Y., et al. (2015). HLA-B*1502 Increases the Risk of Phenytoin or Lamotrigine Induced Stevens-Johnson Syndrome/toxic Epidermal Necrolysis: Evidence from a Meta-Analysis of Nine Case-Control Studies. Drug Res. (Stuttg) 65 (2), 107–111. doi:10.1055/s-0034-1375684
Locharernkul, C., Loplumlert, J., Limotai, C., Korkij, W., Desudchit, T., Tongkobpetch, S., et al. (2008). Carbamazepine and Phenytoin Induced Stevens-Johnson Syndrome Is Associated with HLA-B*1502 Allele in Thai Population. Epilepsia 49 (12), 2087–2091. doi:10.1111/j.1528-1167.2008.01719.x
Mockenhaupt, M., Wang, C. W., Hung, S. I., Sekula, P., Schmidt, A. H., Pan, R. Y., et al. (2019). HLA-B*57:01 Confers Genetic Susceptibility to Carbamazepine-Induced SJS/TEN in Europeans. Allergy 74 (11), 2227–2230. doi:10.1111/all.13821
Osman, M. (2003). Therapeutic Implications of Sex Differences in Asthma and Atopy. Arch. Dis. Child. 88 (7), 587–590. doi:10.1136/adc.88.7.587
Patel, T. K., Thakkar, S. H., and Sharma, D. (2014). Cutaneous Adverse Drug Reactions in Indian Population: A Systematic Review. Indian Dermatol Online J. 5 (Suppl. 2), S76–S86. doi:10.4103/2229-5178.146165
Phillips, E. J., Sukasem, C., Whirl-Carrillo, M., Müller, D. J., Dunnenberger, H. M., Chantratita, W., et al. (2018). Clinical Pharmacogenetics Implementation Consortium Guideline for HLA Genotype and Use of Carbamazepine and Oxcarbazepine: 2017 Update. Clin. Pharmacol. Ther. 103 (4), 574–581. doi:10.1002/cpt.1004
RIKEN (2012). Collaboration with Asian Institutes and SEAPharm. [Online]. Yokohama, Japan: IMS RIKEN Center for Integrative Medical Sciences. Available at: https://www.ims.riken.jp/english/projects/pj09.php.
Saksit, N., Tassaneeyakul, W., Nakkam, N., Konyoung, P., Khunarkornsiri, U., Chumworathayi, P., et al. (2017). Risk Factors of Allopurinol-Induced Severe Cutaneous Adverse Reactions in a Thai Population. Pharmacogenet Genomics 27 (7), 255–263. doi:10.1097/fpc.0000000000000285
Satapornpong, P., Pratoomwun, J., Rerknimitr, P., Klaewsongkram, J., Nakkam, N., Rungrotmongkol, T., et al. (2021). HLA-B*13 :01 is a Predictive Marker of Dapsone-Induced Severe Cutaneous Adverse Reactions in Thai Patients. Front. Immunol. 12, 661135. doi:10.3389/fimmu.2021.661135
Shi, Y. W., Min, F. L., Qin, B., Zou, X., Liu, X. R., Gao, M. M., et al. (2012). Association between HLA and Stevens-Johnson Syndrome Induced by Carbamazepine in Southern Han Chinese: Genetic Markers besides B*1502? Basic Clin. Pharmacol. Toxicol. 111 (1), 58–64. doi:10.1111/j.1742-7843.2012.00868.x
Sukasem, C., Jantararoungtong, T., Kuntawong, P., Puangpetch, A., Koomdee, N., Satapornpong, P., et al. (2016). HLA-B (*) 58:01 for Allopurinol-Induced Cutaneous Adverse Drug Reactions: Implication for Clinical Interpretation in Thailand. Front. Pharmacol. 7, 186. doi:10.3389/fphar.2016.00186
Sukasem, C., Chaichan, C., Nakkrut, T., Satapornpong, P., Jaruthamsophon, K., Jantararoungtong, T., et al. (2018). Association between HLA-B Alleles and Carbamazepine-Induced Maculopapular Exanthema and Severe Cutaneous Reactions in Thai Patients. J. Immunol. Res. 2018, 2780272. doi:10.1155/2018/2780272
Sukasem, C., Pratoomwun, J., Satapornpong, P., Klaewsongkram, J., Rerkpattanapipat, T., Rerknimitr, P., et al. (2020). Genetic Association of Co-trimoxazole-induced Severe Cutaneous Adverse Reactions is Phenotype-specific: HLA Class I Genotypes and Haplotypes. Clin. Pharmacol. Ther. 108 (5), 1078–1089. doi:10.1002/cpt.1915
Sukasem, C., Jantararoungtong, T., and Koomdee, N. (2021a). Pharmacogenomics Research and its Clinical Implementation in Thailand: Lessons Learned from the Resource-Limited Settings. Drug Metab. Pharmacokinet. 39, 100399. doi:10.1016/j.dmpk.2021.100399
Sukasem, C., Sririttha, S., Chaichan, C., Nakkrut, T., Satapornpong, P., Jaruthamsophon, K., et al. (2021b). Spectrum of Cutaneous Adverse Reactions to Aromatic Antiepileptic Drugs and Human Leukocyte Antigen Genotypes in Thai Patients and Meta-Analysis. Pharmacogenomics J. 21 (6), 682–690. doi:10.1038/s41397-021-00247-3
Talal, N. (1987). Autoimmune Mechanisms in Patients and Animal Models. Toxicol. Pathol. 15 (3), 272–275. doi:10.1177/019262338701500303
Tangamornsuksan, W., Chaiyakunapruk, N., Somkrua, R., Lohitnavy, M., and Tassaneeyakul, W. (2013). Relationship between the HLA-B*1502 Allele and Carbamazepine-Induced Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis: A Systematic Review and Meta-Analysis. JAMA Dermatol 149 (9), 1025–1032. doi:10.1001/jamadermatol.2013.4114
Tassaneeyakul, W., Tiamkao, S., Jantararoungtong, T., Chen, P., Lin, S. Y., Chen, W. H., et al. (2010). Association between HLA-B*1502 and Carbamazepine-Induced Severe Cutaneous Adverse Drug Reactions in a Thai Population. Epilepsia 51 (5), 926–930. doi:10.1111/j.1528-1167.2010.02533.x
US Food and Drug Administration (2009). Official Drug Label: Trileptal (Oxcarbazepine). [Online]. East Hanover, New Jersey: Novartis Pharmaceuticals Corporation. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021014s036lbl.pdf.
van der Wouden, C. H., Cambon-Thomsen, A., Cecchin, E., Cheung, K. C., Dávila-Fajardo, C. L., Deneer, V. H., et al. (2017). Implementing Pharmacogenomics in Europe: Design and Implementation Strategy of the Ubiquitous Pharmacogenomics Consortium. Clin. Pharmacol. Ther. 101 (3), 341–358. doi:10.1002/cpt.602
Keywords: carbamazepine, HLA-B risk alleles, pharmacogenomics, cutaneous adverse drug reactions, precision medicine
Citation: Tiwattanon K, John S, Koomdee N, Jinda P, Rachanakul J, Jantararoungtong T, Nuntharadthanaphong N, Kloypan C, Biswas M, Boongird A and Sukasem C (2022) Implementation of HLA-B*15:02 Genotyping as Standard-of-Care for Reducing Carbamazepine/Oxcarbazepine Induced Cutaneous Adverse Drug Reactions in Thailand. Front. Pharmacol. 13:867490. doi: 10.3389/fphar.2022.867490
Received: 01 February 2022; Accepted: 02 June 2022;
Published: 05 July 2022.
Edited by:
Maxine Deborah Gossell-Williams, University of the West Indies, JamaicaReviewed by:
Chuang-Wei Wang, Linkou Chang Gung Memorial Hospital, TaiwanCopyright © 2022 Tiwattanon, John, Koomdee, Jinda, Rachanakul, Jantararoungtong, Nuntharadthanaphong, Kloypan, Biswas, Boongird and Sukasem. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Napatrupron Koomdee, bmFwYXRydXBvcm4ua29tQG1haGlkb2wuYWMudGg=; Apisit Boongird, YXBpc2l0LmJvbkBtYWhpZG9sLmFjLnRo
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.