
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
EDITORIAL article
Front. Pharmacol. , 16 March 2022
Sec. Renal Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.864117
This article is part of the Research Topic Receptor Biology and Cell Signaling in Diabetes View all 9 articles
Editorial on the Research Topic
Receptor Biology and Cell Signaling in Diabetes
Diabetes mellitus affects almost half a billion people around the globe, and nearly 25% of diabetic people eventually develop proteinuria, vascular dysfunction, and related kidney disease (Cooper and Warren, 2019). Organ fibrosis among diabetic patients is a leading cause of death worldwide. The cellular mechanisms of organ fibrosis and the origin of these fibroblasts are poorly understood (Srivastava et al.). Diabetes linked receptor dysfunctions result in defective central metabolism, disrupted cytoskeleton, and compromised organelle function, and it is likely associated with activation of cellular transdifferentiation processes causing fibrogenesis in organs such as the kidneys, heart, and blood vessels, ultimately leading to organ failure (Kang et al., 2015; Srivastava et al., 2018). Myofibroblasts metabolic shifts, cytokine and chemokine reprogramming, and autophagy defects are key examples of a critical phenomenon that influence the destruction of cellular structures, deposition of extracellular matrix, and fibrogenic pathways.
Using contemporary cutting-edge technology, we have demonstrated the importance of glucocorticoid receptor (GR), fibroblast growth factor receptor 1, and sirtuin 3 mediated processes, which include suppression in myofibroblasts metabolic reprogramming, cytokine and chemokine reprogramming, inhibition in the levels of endothelial-to-mesenchymal transitions, and epithelial-to-mesenchymal processes in the diabetic kidneys (Figure 1) (Li et al., 2020a; Srivastava et al., 2021a; Srivastava et al., 2021c). Dipeptidyl transpeptidase-4 (DPP-4) and low-density lipoprotein receptor-related proteins 5 and 6 are key mesenchymal activators in diabetic tubules and endothelial cells. However, their interactions and their relation with myofibroblasts’ metabolic reprogramming is a matter of ongoing research. Improved understanding of the pathogenesis of diabetes and associated kidney disease is urgently needed to catalyze the development of new therapeutics. Available therapies are neither tissue- nor cell-specific and are ineffective in reversing kidney fibrosis and diabetic complications.
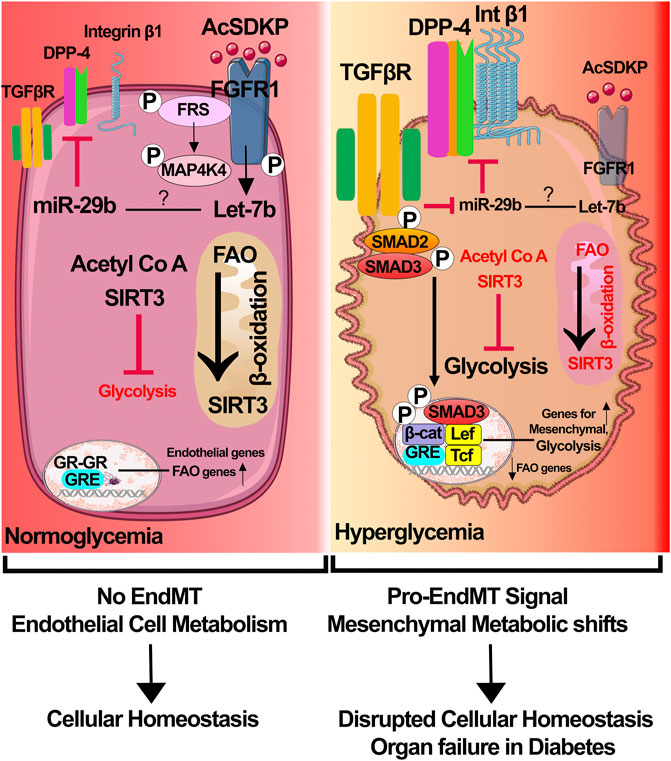
FIGURE 1. Receptor dysfunctions and metabolic myofibroblasts shifts in diabetic endothelial cells. In healthy endothelial cells, 1) physiological level of N-seryl-acetyl-lysyl-proline (AcSDKP)/fibroblasts growth factor receptor 1 (FGFR1) interaction leads to activation of signaling cascade that is important for expression of anti-mesenchymal microRNAs miR-29 and miR-let-7; 2) sirtuins 3 (SIRT3), which regulates the levels of fatty acid oxidation in mitochondria and glycolysis in cytosol, balances the endothelial cell metabolism; and 3) glucocorticoid receptor is key for the transcription of endothelial genes and fatty acid oxidation genes. Cumulative effects contribute to endothelial cell health and homeostasis. In diabetic endothelial cells, 1) suppression of AcSDKP/FGFR1 interaction causes downregulation of expression levels of miR-29 and miR-let-7, which leads to the elevation in the levels of mesenchymal transforming growth factor receptor 1 (TGFβR1), dipeptidyl peptidase-4 (DPP-4), and integrin β1; 2) SIRT3 deficiency causes suppression in the fatty acid oxidation and induction in the aberrant glycolysis; GR deficiency results in activation of Wnt signaling, endothelial-to-mesenchymal transition processes and suppression in the level of fatty acid oxidation. Accumulative effects of all these lead to myofibroblast metabolic reprogramming. Components of this figure were created using Servier Medical Art templates.
Recently, many renal protective agents that modulate the receptor physiology and improve cellular health and homeostasis, including sodium-glucose cotransporter inhibitors (SGLT-2), DPP-4 inhibitors, and N-seryl-acetyl-lysyl-proline, have been evaluated in preclinical settings and some in controlled clinical trials with promising outcomes (Kanasaki et al., 2014; Srivastava et al., 2016; Stavropoulos et al., 2018; Srivastava et al., 2020a; Srivastava et al., 2020b). Moreover, larger sample size is necessary to optimize their use in human beings.
In this Research Topic of Frontiers in Pharmacology, we discuss new pathophysiologic mechanisms related to receptor dysfunction and cell signaling in diverse cell types in diabetic kidneys, and targeting receptor dysfunction is one of the driving mechanisms in the development of future therapies against vascular dysfunction and fibrogenesis in diabetic kidney disease. In addition, we discuss the effect of modulators that inhibit the aberrant cell signaling in diabetic conditions. The knowledge gained from this issue will enhance the understanding of the complex nature of this disease which can be utilized to develop future therapeutics against diabetic kidney disease.
We focused on two sections.
Podocytes are specialized differentiated cells, and loss of podocyte cells or podocyte foot process effacement are prominent features of diabetic nephropathy (Clement et al., 2011). GR and circulatory angiopoietin-like 4 proteins regulate podocyte-endothelium health and GR loss in podocytes leading to proteinuria and glomerulosclerosis in diabetes (Clement et al., 2014; Srivastava et al., 2021b). Epigenetic alterations, DNA methylation, and inability in DNA damage repair in diabetic podocytes are directly associated with diabetic nephropathy (DN) phenotypes. Vermon et al. study demonstrated the critical role of podocyte VEGF-A in the diffused glomerulosclerosis in diabetic and eNOS mutant mice. Deletion of VEGF-A in podocytes induced the abnormal S-nitrosylation of specific proteins, including β-integrin and laminin, which are involved in the development of diffused glomerulosclerosis, suggesting the essential role of VEGF-A and nitric oxide in the glomerular homeostasis and pathogenic roles of S-nitrosylated β-integrin and laminin in the glomerular disease, implicating the significant advances in podocyte cell biology (Vermon et al.). In addition, aberrant levels of ferroptosis, pyroptosis, necroptosis, and apoptosis are important phenomena in DN, linked with the activation of fibrogenic pathways in tubules and podocytes. Understanding gasdermin D mediated pyroptosis provides valuable insights into DN research. Another part of the study describes the role of mineralocorticoid receptor (MR) in diabetic kidney disease (DKD). MR antagonism reversed DKD phenotypes by suppressing fibrosis, oxidative stress, and inflammation (Kawanami et al.). Therefore, an enhanced understanding of MR biology in diverse cell types is important for DKD management.
The use of SGLT2 inhibitors as safe therapeutics in diabetic kidney disease has recently been widely studied in preclinical settings and randomized clinical trials. The function of SGLT2 is to reabsorb urine glucose filtered from the glomerulus. The EMPA-REG trial has demonstrated that empagliflozin suppressed diabetic nephropathy phenotypes, and this study represents a crucial development in the clinical practice of diabetes management (Mayer et al., 2019). Data in mouse models suggest that SGLT2 inhibitors suppress the pathogenic abnormal glycolysis levels and restore the fatty acid oxidation levels in the tubules, improving tubular health (Li et al., 2020b).
One of the studies included in this issue imparts significant information on dapagliflozin in tubule protection. However, high salt diet intake compromised the tubular protection of dapagliflozin in diabetic patients and mouse models of DKD. These data demonstrate that high-salt diet intake impairs the tubule fatty acid metabolism, a key contributor of renal fibrosis in diabetes (Zou et al.). In addition, SGLT2 inhibitors are effective against autophagic defects in metabolic diseases. Autophagic improvement by SGLT2 inhibitors would be of great significance and usher the need for future randomized clinical trials to evaluate the SGLT2 inhibitors in autophagy defects related metabolic diseases. Zhang et al. tested the recombinant anti-IL6R fusion proteins in the mouse models of diabetic nephropathy and found that reducing DN phenotypes by mitigating the JAK2/STAT3 signal transduction pathway is effective, suggesting the pathobiology of IL-6R/JAK2/STAT3 pathway in DN (Zhang et al.). In recent years, ROCK inhibitors have been shown to effectively improve renal outcomes of diabetic patients suggesting the possibility of a potential lead in DKD.
This Research Topic discussed the crucial pathways that will enlighten possible future therapeutics against fibrogenesis and vascular dysfunction in diabetes. The information gained from this Research Topic will be useful for clinicians and basic science researchers to guide novel therapeutic approaches and future research directions.
SS proposed the idea, conceptualized, contributed to writing, and provided intellectual input. KK provided intellectual output.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Clement, L. C., Avila-Casado, C., Macé, C., Soria, E., Bakker, W. W., Kersten, S., et al. (2011). Podocyte-secreted Angiopoietin-Like-4 Mediates Proteinuria in Glucocorticoid-Sensitive Nephrotic Syndrome. Nat. Med. 17, 117–122. doi:10.1038/nm.2261
Clement, L. C., Macé, C., Avila-Casado, C., Joles, J. A., Kersten, S., and Chugh, S. S. (2014). Circulating Angiopoietin-like 4 Links Proteinuria with Hypertriglyceridemia in Nephrotic Syndrome. Nat. Med. 20, 37–46. doi:10.1038/nm.3396
Cooper, M., and Warren, A. M. (2019). A Promising Outlook for Diabetic Kidney Disease. Nat. Rev. Nephrol. 15, 68–70. doi:10.1038/s41581-018-0092-5
Kanasaki, K., Shi, S., Kanasaki, M., He, J., Nagai, T., Nakamura, Y., et al. (2014). Linagliptin-Mediated DPP-4 Inhibition Ameliorates Kidney Fibrosis in Streptozotocin-Induced Diabetic Mice by Inhibiting Endothelial-To-Mesenchymal Transition in a Therapeutic Regimen. Diabetes 63, 2120–2131. doi:10.2337/db13-1029
Kang, H. M., Ahn, S. H., Choi, P., Ko, Y. A., Han, S. H., Chinga, F., et al. (2015). Defective Fatty Acid Oxidation in Renal Tubular Epithelial Cells Has a Key Role in Kidney Fibrosis Development. Nat. Med. 21, 37–46. doi:10.1038/nm.3762
Li, J., Liu, H., Srivastava, S. P., Hu, Q., Gao, R., Li, S., et al. (2020a). Endothelial FGFR1 (Fibroblast Growth Factor Receptor 1) Deficiency Contributes Differential Fibrogenic Effects in Kidney and Heart of Diabetic Mice. Hypertension 76, 1935–1944. doi:10.1161/HYPERTENSIONAHA.120.15587
Li, J., Liu, H., Takagi, S., Nitta, K., Kitada, M., Srivastava, S. P., et al. (2020b). Renal Protective Effects of Empagliflozin via Inhibition of EMT and Aberrant Glycolysis in Proximal Tubules. JCI Insight 5, e129034. doi:10.1172/jci.insight.129034
Mayer, G. J., Wanner, C., Weir, M. R., Inzucchi, S. E., Koitka-Weber, A., Hantel, S., et al. (2019). Analysis from the EMPA-REG OUTCOME® Trial indicates Empagliflozin May Assist in Preventing the progression of Chronic Kidney Disease in Patients with Type 2 Diabetes Irrespective of Medications that Alter Intrarenal Hemodynamics. Kidney Int. 96, 489–504. doi:10.1016/j.kint.2019.02.033
Srivastava, S. P., Goodwin, J. E., Kanasaki, K., and Koya, D. (2020a). Inhibition of Angiotensin-Converting Enzyme Ameliorates Renal Fibrosis by Mitigating DPP-4 Level and Restoring Antifibrotic MicroRNAs. Genes (Basel). 11, 211. doi:10.3390/genes11020211
Srivastava, S. P., Goodwin, J. E., Kanasaki, K., and Koya, D. (2020b). Metabolic Reprogramming by N-Acetyl-Seryl-Aspartyl-Lysyl-Proline Protects against Diabetic Kidney Disease. Br. J. Pharmacol. 177, 3691–3711.
Srivastava, S. P., Li, J., Kitada, M., Fujita, H., Yamada, Y., Goodwin, J. E., et al. (2018). SIRT3 Deficiency Leads to Induction of Abnormal Glycolysis in Diabetic Kidney with Fibrosis. Cell Death Dis 9, 997. doi:10.1038/s41419-018-1057-0
Srivastava, S. P., Li, J., Takagaki, Y., Kitada, M., Goodwin, J. E., Kanasaki, K., et al. (2021a). Endothelial SIRT3 Regulates Myofibroblast Metabolic Shifts in Diabetic Kidneys. iScience 24, 102390. doi:10.1016/j.isci.2021.102390
Srivastava, S. P., Zhou, H., Setia, O., Dardik, A., Fernandez-Hernando, C., and Goodwin, J. (2021b). Podocyte Glucocorticoid Receptors Are Essential for Glomerular Endothelial Cell Homeostasis inDiabetes Mellitus. J. Am. Heart Assoc. 10, e019437. doi:10.1161/JAHA.120.019437
Srivastava, S. P., Shi, S., Kanasaki, M., Nagai, T., Kitada, M., He, J., et al. (2016). Effect of Antifibrotic MicroRNAs Crosstalk on the Action of N-Acetyl-Seryl-Aspartyl-Lysyl-Proline in Diabetes-Related Kidney Fibrosis. Sci. Rep. 6, 29884. doi:10.1038/srep29884
Srivastava, S. P., Zhou, H., Setia, O., Liu, B., Kanasaki, K., Koya, D., et al. (2021c). Loss of Endothelial Glucocorticoid Receptor Accelerates Diabetic Nephropathy. Nat. Commun. 12, 2368. doi:10.1038/s41467-021-22617-y
Stavropoulos, K., Imprialos, K. P., Stavropoulos, N., Bouloukou, S., Kerpiniotis, G., Dimitriadis, K., et al. (2018). Sodium-glucose Cotransporter 2 Inhibitors: Nephroprotective Impact on Diabetic Kidney Disease. Cardiovasc. Hematol. Disord. Drug Targets 18, 120–126. doi:10.2174/1871529X18666180206155349
Keywords: endothelial cells, diabetes, fatty acid oxidation, glycolysis, mesenchymal gene expression, fibrosis, organ fibrosis, diabetic kidney disease
Citation: Srivastava SP and Kanasaki K (2022) Editorial: Receptor Biology and Cell Signaling in Diabetes. Front. Pharmacol. 13:864117. doi: 10.3389/fphar.2022.864117
Received: 28 January 2022; Accepted: 24 February 2022;
Published: 16 March 2022.
Edited and reviewed by:
Giuseppe Remuzzi, Mario Negri Pharmacological Research Institute (IRCCS), ItalyCopyright © 2022 Srivastava and Kanasaki. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Swayam Prakash Srivastava, c3dheWFtLmNkcmlAZ21haWwuY29t, c3dheWFtLnNyaXZhc3RhdmFAeWFsZS5lZHU=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.