- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3No. 1 Orthopedics Hospital of Chengdu, Chengdu, China
- 4Sichuan Nursing Vocational College, Chengdu, China
Objectives: To evaluate the efficacy and safety of Chinese herbal medicine (CHM) for type 2 diabetes mellitus (T2DM) with nonalcoholic fatty liver disease (NAFLD) with current evidence.
Methods: This study was registered in PROSPERO as CRD42021271488. A literature search was conducted in eight electronic databases from inception to December 2021. The primary outcomes were lipid indices and liver functions, including triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine transaminase (ALT), and aspartate transaminase (AST). Review Manager 5.2 and Stata v14.0 were applied for analysis.
Results: The research enrolled 18 RCTs with 1,463 participants. Results showed CHM combined with western medicine (WM) was more effective than WM alone in TG (weighted mean differences (WMD) = −0.35.95% confidence interval (CI) [−0.51, −0.19], p < 0.0001), TC (WMD = −0.58.95%CI [−0.80, −0.36], p < 0.00001), LDL-C (WMD = −0.37, 95%CI [−0.47, −0.26], p < 0.00001), HDL-C (WMD = 0.20, 95%CI [0.10, 0.29], p < 0.0001), ALT (WMD = −4.99, 95%CI [−6.64, −3.33], p < 0.00001), AST (WMD = −4.76, 95%CI [−6.35, −3.16], p < 0.00001), homeostatic model assessment of insulin resistance (WMD = −1.01, 95%CI [−1.22, −0.79], p < 0.00001), fasting blood glucose (WMD = −0.87, 95%CI [−1.13, −0.61], p < 0.00001), 2-h postprandial glucose (WMD = −1.45.95%CI [−2.00, −0.91], p < 0.00001), body mass index (WMD = −0.73.95%CI [−1.35, −0.12], p = 0.02), and overall effective rate (risk ratio (RR) = 1.37.95%CI [1.29, 1.46], p < 0.00001).
Conclusion: The CHM in combination with WM seems to be more beneficial in T2DM with NAFLD patients in improving lipid and glucose metabolism, liver function, and insulin resistance as well as improving overall efficiency and reducing body weight. Given the poor quality of reports from these studies and uncertain evidence, these findings should be interpreted cautiously.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021271488, identifier CRD42021271488.
1 Introduction
Nonalcoholic fatty liver disease (NAFLD), also known as metabolism-associated fatty liver disease (MAFLD), represents a clinical syndrome characterized by steatosis and accumulation of fat in liver parenchymal cells (Eslam et al., 2020). NAFLD includes simple fatty liver, nonalcoholic steatohepatitis (NASH), and cirrhosis (Younossi et al., 2018). The prevalence of NAFLD is as high as 75% in population diagnosed with type 2 diabetes mellitus (T2DM) (Hua et al., 2014; Younossi et al., 2016). T2DM and NAFLD can accelerate disease progression in a reciprocal manner, thereby becoming an important social public health burden (Loria et al., 2013; Mantovani et al., 2018). NAFLD could increase glycemic excursions, making it more challenging to glycemic control glycemic (Bril and Cusi, 2017), as a result significantly increase the risk of macrovascular and microangiopathy (Hazlehurst et al., 2016; Lomonaco et al., 2016). T2DM, on the other hand, exacerbates the risk of liver fibrosis and cancer in NAFLD (Targher et al., 2021). The coexistence of the two pathologies leads to a significant increase in the risk of aggravated metabolic disorders and cardiovascular disease, culminating in death related to liver disease (Portillo-Sanchez et al., 2015). Most T2DM patients with NAFLD experience “late detection, late treatment, and difficulty in recovery” because of the lack of typical or serious clinical symptoms or signs in the early stages, thereby impacting the physical and mental health and quality of life (Hu et al., 2017).
Obesity and insulin resistance (IR) are major pathogenic drivers in NAFLD and T2DM (Buzzetti et al., 2016; Wei et al., 2021), and these two pathological conditions usually coexist (Tilg et al., 2017; Targher et al., 2018). Due to the prevalence of T2DM with NAFLD and the many potential health risks associated with their coexistence, active and effective prevention measures should be employed to protect this population (Wong et al., 2018; Lee et al., 2020). At present, there is no effective pharmacotherapy for NAFLD approved by the international authorities, regardless of T2DM status. (European Association for the Study of the Liver, 2016). Lifestyle modifications are key to the clinical management of NAFLD across the disease spectrum (Nguyen and George, 2015). Both T2DM and NAFLD promote deterioration of each other physiologically and pathologically, and there are many restrictions in treatment, thereby precluding an effective interventional strategy. Therefore, alternative therapy is urgently needed.
Traditional Chinese medicine (TCM) is one of the major complementary and alternative medicine systems that have been developed in China for thousands of years, including Chinese herbal medicine (CHM), acupuncture, massage, and other therapies. In addition to China, some Asian countries also have high acceptance of CHM. CHM is a traditional botanical, animal, and mineral medicine used in China, of which botanical is the most common. CHM, based on the principles of syndrome differentiation and holistic treatment, has a long history in the treatment of diabetes and its complications, which can provide individualized treatment for patients. Existing studies (Chen et al., 2016; Shi et al., 2019; Wu and Wei, 2019) have described CHM as effective in the treatment of T2DM with NAFLD and is different from the forced hypoglycemic and lipid-lowering effects of Western medicine (WM), thereby providing a therapeutic advantage of “all-round, multi-faceted, and multi-target.” More recent traditional Chinese medicine (TCM) scholars have studied CHM purely based on theoretical considerations, clinical applications, and scientific experiments and demonstrated that CHM can effectively regulate glucose and lipid metabolism, improve insulin resistance and hemorheology (Gao et al., 2020), repair liver histopathological injury (Chen C. et al., 2021), inhibit oxidative stress (Zhang L. L. et al., 2019), and delay the progression of T2DM with NAFLD. Nevertheless, to date, there is no systematic review or meta-analysis to evaluate the efficacy and safety of CHM. In this study, we addressed the efficacy and safety of CHM in relation to the management of T2DM with NAFLD using an evidence-based approach, with the aim of providing scientific references for improving the therapeutic strategy.
2 Methods
This study was conducted in accordance with the Cochrane Handbook on Systematic Review of Interventions, the guidelines of Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 (Moher et al., 2015) (Supplementary File S1). Additionally, the review was registered at PROSPERO (CRD42021271488).
2.1 Search Strategies
We performed a comprehensive search of eight electronic databases from inception to December 2021, including PubMed, Cochrane Library, Embase, Web of Science, China National Knowledge Infrastructure (CNKI), Wanfang Database, China Biomedical Medicine database (CBM), and the VIP information resource integration service platform (cqvip). In addition, the Chinese Clinical Trial Registry (CHiCTR) (http://www.chictr.org.cn/index.aspx) and ClinicalTrials.gov were also searched for randomized controlled trials (RCTs) that were either ongoing or completed but unpublished. We included all RCTs that examined the efficacy of CHM in the management of T2DM with NAFLD. Search terms included were as follows: “Traditional Chinese Medicine,” “Traditional Tongue Diagnosis,” “Zhong Yi Xue,” “Chung I Hsueh,” “Diabetes Mellitus, Type 2,” “Diabetes Mellitus, Noninsulin-Dependent,” “Stable Diabetes Mellitus,” “Diabetes Mellitus, Type II,” and “Maturity-Onset Diabetes,” etc. Comprehensive search strategies for the databases are shown in the Supplementary Files (Supplementary Table S1). No restrictions were applied on language.
2.2 Inclusion and Exclusion Criteria
All RCTs evaluating the effects of CHM on T2DM with NAFLD were included in the meta-analysis. The inclusion criteria were as follows:
1) Study design: RCTs; 2) Participants: patients with a definite diagnosis of T2DM with NAFLD and no limitations relating to gender, nationality, ethnicity, and education level. 3) Interventions: patients in the intervention group should receive CHM (including decoction, pills, and granules, regardless of duration) plus WM, and the control group should be treated with WM the same as the intervention group; 4) Outcomes: the primary outcomes of the study were triglyceride (TG), total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), alanine transaminase (ALT), and aspartate transaminase (AST). The secondary outcomes included were homeostatic model assessment of insulin resistance (HOMA-IR), fasting blood glucose (FBG), 2-h postprandial glucose (2hPG), body mass index (BMI), overall effective rate, and adverse effects. Studies that met any of the following criteria were excluded: 1) non-RCTs, such as retrospective studies, animal experiments, case reports, reviews, and conference abstracts. 2) Patients received other TCM interventions, including acupuncture, massage, or moxibustion. 3) Studies that lacked sufficient details on outcomes.
2.3 Study Selection and Data Extraction
Three investigators (LL, ZX, and X-YZ) searched and screened for appropriate studies according to the predefined inclusion and exclusion criteria. In the case of discrepancies, the final decision was made through consensus agreement. To manage literature, Endnote V.X9 software was used. Two reviewers (HW and XL) independently extracted relevant data from the eligible studies using standardized extraction forms, including: the first author, year of publication, country, sample size, average age, gender, duration of disease, interventions, details of CHM (name of the prescription and composition), adverse events, and outcomes. The extracted data were cross-checked by HW and XL, and a third reviewer (SY) was available to resolve any conflicts.
2.4 Risk of Bias Assessment
Two reviewers (CX and X-GZ) independently assessed the risk of bias according to the Cochrane Collaboration’s Risk of Bias tool (Higgins et al., 2019), which included the following criteria: random sequence generation, allocation concealment, incomplete data, blinding, selective reporting, and other bias. The results were judged as ‘low,’ ‘high,’ or ‘unclear,’ and any disagreements were resolved by the third investigator (SY).
2.5 Data Synthesis and Statistical Analysis
Review Manager 5.2 was applied to analyze and assess the effect of CHM on T2DM with NAFLD patients from the aspects of lipid indices, liver functions, insulin, glycemic indices, and so on. For dichotomous data, a risk ratio (RR) with a 95% confidence interval (CI) was used to measure the results. Continuous variables, such as TG, TC, ALT, AST, FBG, and BMI, were evaluated by weighted mean differences (WMDs) and 95%CI. The heterogeneity of data was investigated by the X2 test and I2 test. A fixed effects model was applied if there was homogeneity (p > 0.05, I2<50%) (Higgins et al., 2003); otherwise, the random effects model was used. A p-value of less than 0.05 was considered statistically significant. To explore the potential sources of heterogeneity, the factors that contributed to heterogeneity were analyzed through subgroup analysis. In addition, publication bias was assessed by funnel plots and investigated statistically by Egger’s test with Stata v14.0.
2.6 Sensitivity Analysis
To assess the robustness and reliability of the combined results in meta-analysis, we used sensitivity analysis as an important method. Sensitivity analysis was conducted by excluding individual studies in-turn and re-performing the meta-analysis of the remaining studies. We evaluated whether the results obtained were significantly different from those before the exclusion to ensure the robustness of the results.
3 Results
3.1 Literature Selection
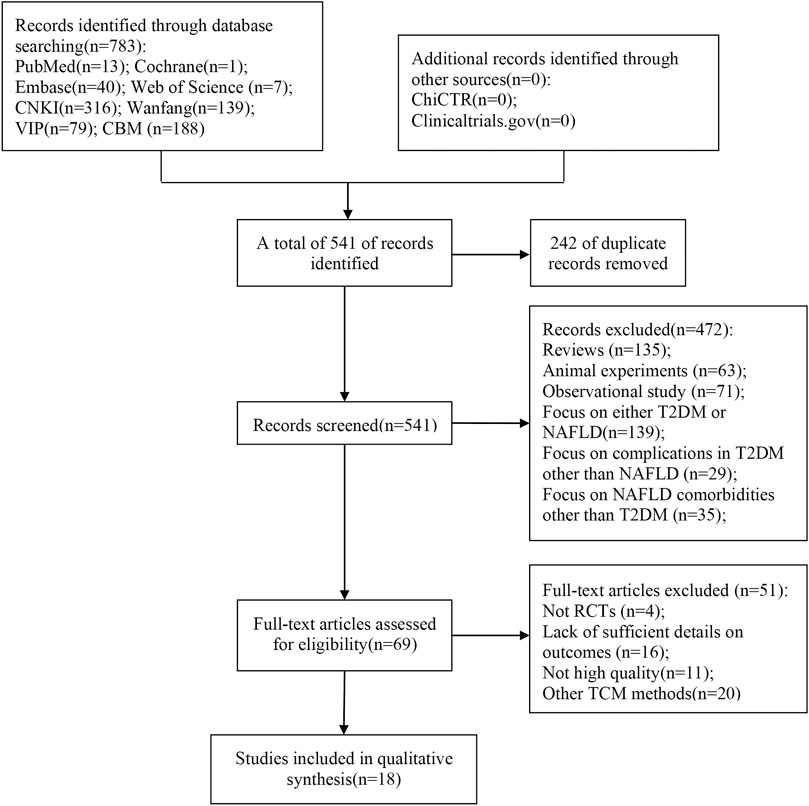
Through our search strategy, 783 relevant articles were initially evaluated. After removing duplicates, the remaining 541 articles were screened by title and abstract, and 69 articles required further screening after excluding articles that did not meet the inclusion criteria, such as reviews and animal experiments. After careful full-text reading of the 69 articles, 51 articles were excluded for the following reasons: not RCTs (n = 4), other TCM methods (n = 20), lack of sufficient details on outcomes (n = 16), and lack of high quality (n = 11). Finally, 18 articles (Wang, 2010; Kang et al., 2016; Zheng et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Wang et al., 2018; Li, 2019; Liu, 2019; Wang, 2019; Zou, 2019; Li et al., 2020; Liu, 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) were included in this meta-analysis. The study selection process is shown in Figure 1.
3.2 Study Characteristics
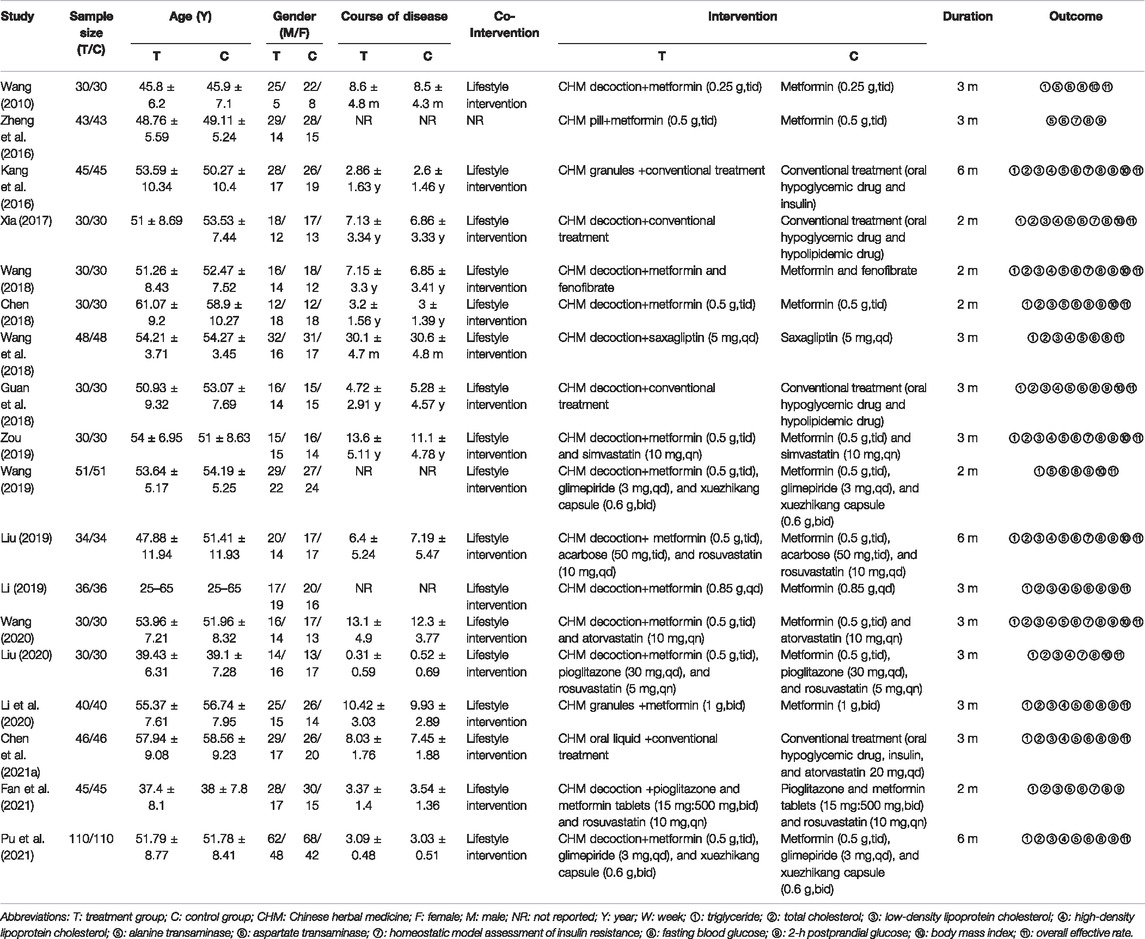
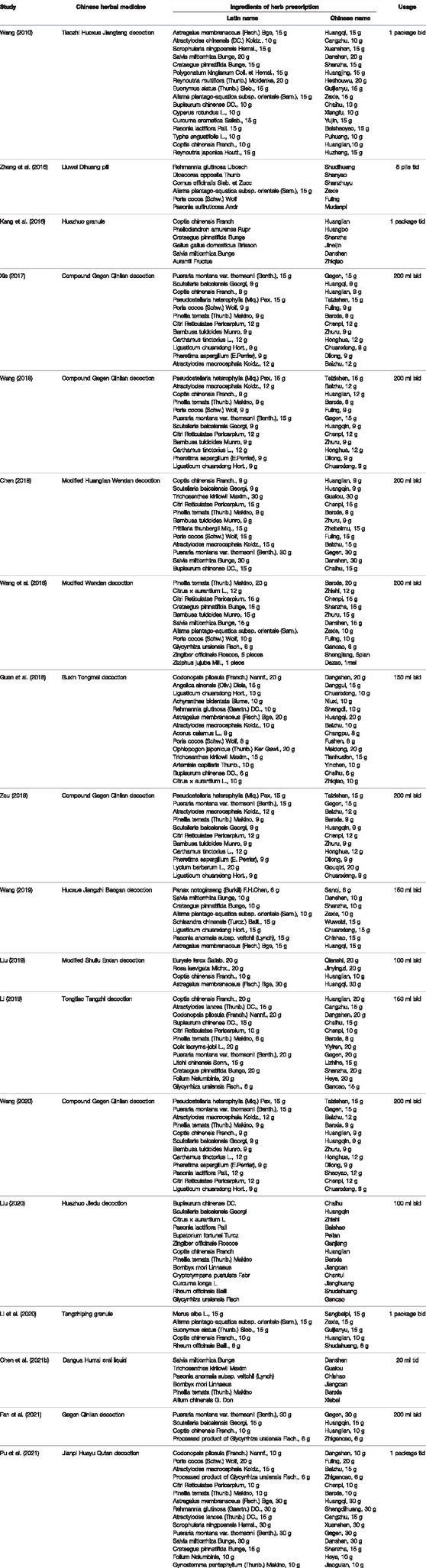
A total of 18 studies involving 1,463 patients were enrolled, 733 in treatment and 730 in control groups. All studies were RCTs conducted in China between 2010 and 2021. In these trials, the intervention of treatment groups was to add CHM to control groups, while there were two kinds of interventions in control groups: 7 studies (Wang, 2010; Kang et al., 2016; Zheng et al., 2016; Chen, 2018; Wang et al., 2018; Li, 2019; Li et al., 2020) used hypoglycemic drug, while 11 studies (Xia, 2017; Guan et al., 2018; Wang, 2018; Liu , 2019; Wang, 2019; Zou, 2019; Liu, 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) used the combination of hypoglycemic drug and hypolipidemic drug. In 18 RCTs, CHM in the treatment group was taken in the form of decoction in 14 studies (Wang, 2010; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Wang et al., 2018; Li, 2019; Liu, 2019; Wang, 2019; Zou, 2019; Liu, 2020; Wang, 2020; Fan et al., 2021; Pu et al., 2021), granules in 2 studies (Kang et al., 2016; Li et al., 2020), pills in 1 study (Zheng et al., 2016), and oral liquid in 1 study (Chen M. L. et al., 2021). The shortest and longest intervention durations were 2 (Xia, 2017; Chen, 2018; Wang, 2018; Wang, 2019; Fan et al., 2021) and 6 months, respectively (Kang et al., 2016; Liu, 2019; Pu et al., 2021). The characteristics of the 18 included studies are presented in Table 1 and the detailed components of CHM in Table 2.
3.3 Risk of Bias Assessment
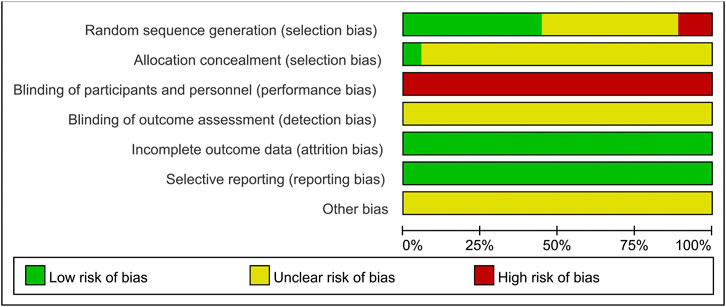
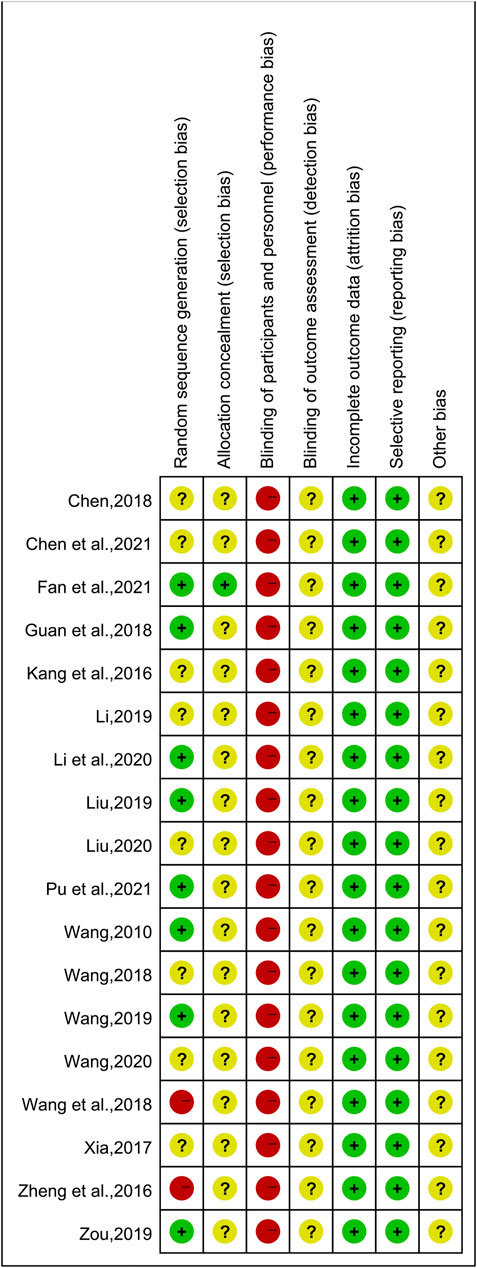
The results of the risk of bias assessment are shown in Figure 2 and Figure 3. Of the 18 included studies, 8 studies (Wang, 2010; Guan et al., 2018; Liu, 2019; Wang, 2019; Zou, 2019; Li et al., 2020; Fan et al., 2021; Pu et al., 2021) were classified as low risk of bias because they used the random number table for randomization. Two studies (Zheng et al., 2016; Wang et al., 2018) reported randomization according to the intervention, so they were at a high risk of bias. The other 8 studies (Kang et al., 2016; Xia, 2017; Chen, 2018; Wang, 2018; Li, 2019; Liu, 2020; Wang, 2020; Chen M. L. et al., 2021) claimed to use randomization but did not report details of randomization methodology and were therefore marked as “unclear risk.” Except for one study (Fan et al., 2021), the allocation concealment was unclear for the remaining studies. None of the experiments reported blinding of the participants or researchers. Thus, all studies were classified as having a high risk of bias in this aspect. However, these tests were considered to use objective outcome measures. With regards to other biases, none of the studies provided sufficient information that could be used in determining the presence of other significant risks of bias and thus assessed as “unclear risk.”
3.4 Outcomes
3.4.1 Effect of Chinese Herbal Medicine on Lipid Indices
3.4.1.1 Triglyceride
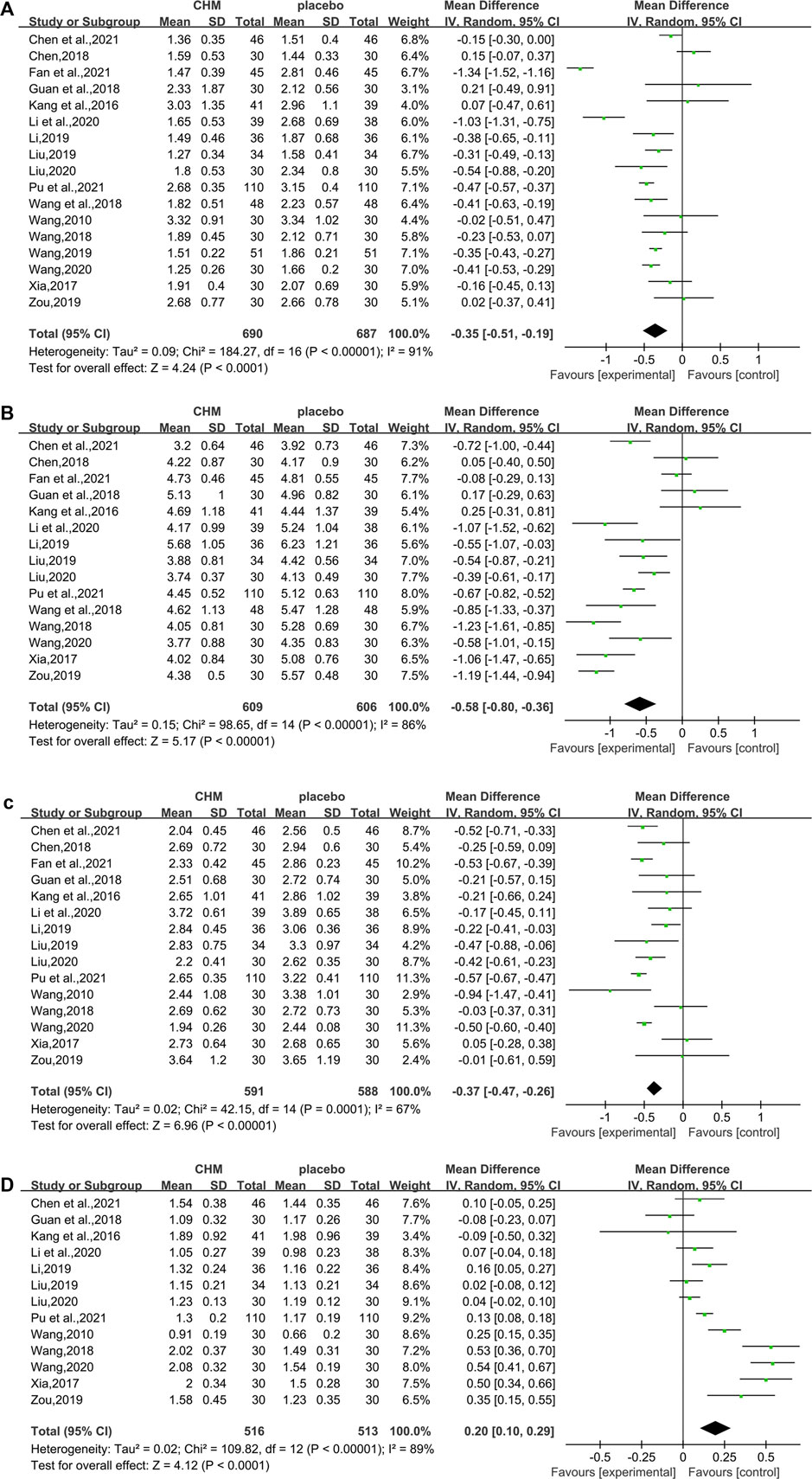
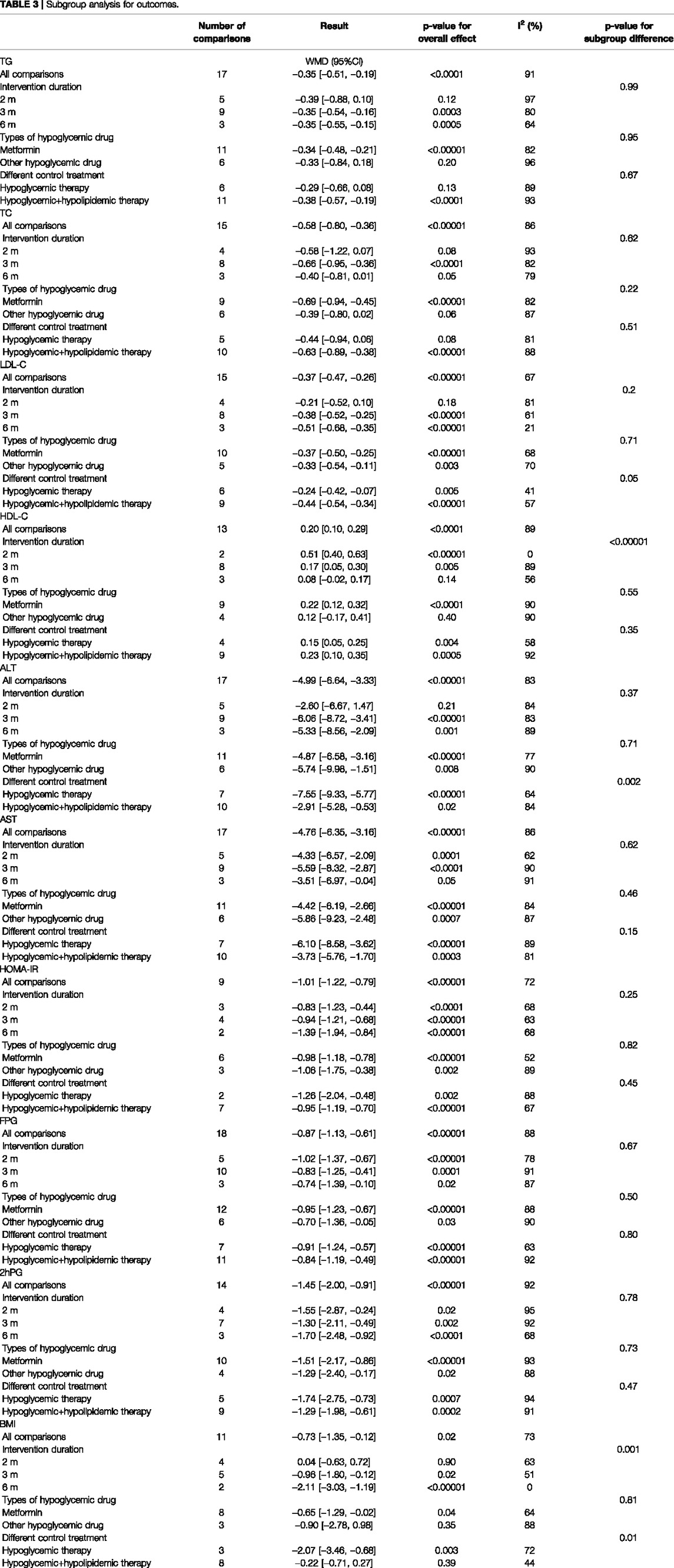
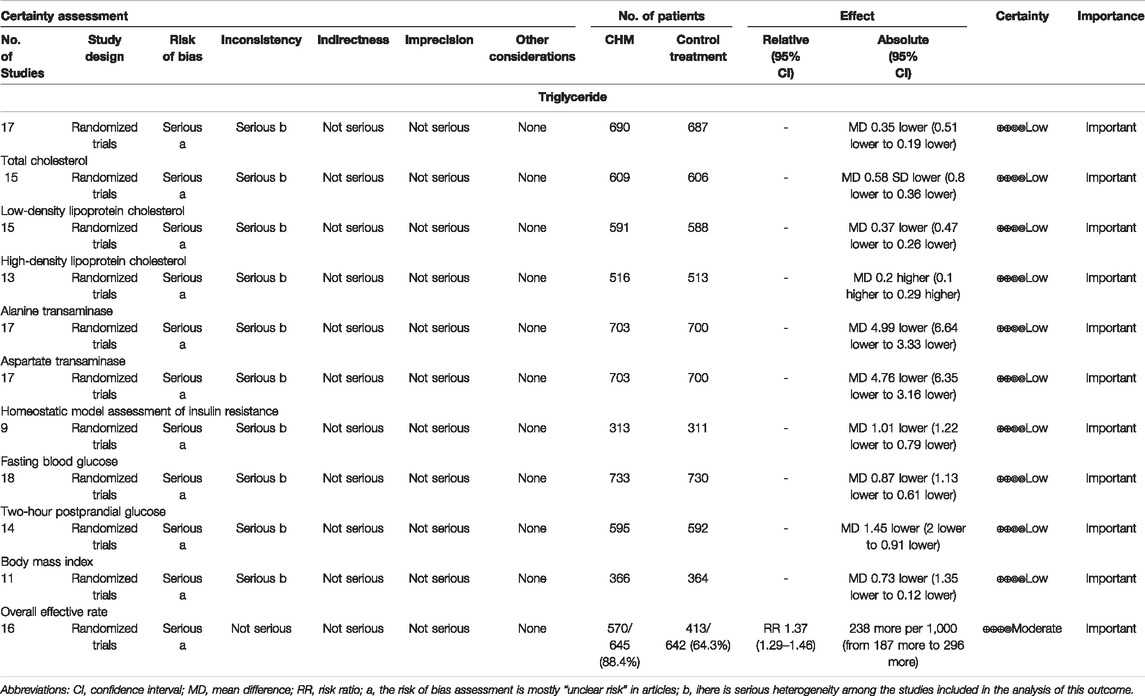
In total, 17 studies (1,377 subjects) (Wang, 2010; Kang et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Wang et al., 2018; Li, 2019; Liu, 2019; Wang, 2019; Zou, 2019; Li et al., 2020; Liu, 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) evaluated TG levels. Overall analyses suggested CHM combined with WM might reduce TG in T2DM with NAFLD patients (WMD = −0.35.95%CI [−0.51, −0.19],p < 0.0001,I2 = 91%, random effects model; Figure 4). With regards to subgroup analysis, there was no significant difference between different intervention durations, different types of hypoglycemic drugs, and different control treatments (p for interaction = 0.99, 0.95, and 0.67, respectively) (Table 3, Supplementary Figure S1).
3.4.1.2 Total Cholesterol
In total, 15 studies (1,215 patients) (Kang et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Wang et al., 2018; Li, 2019; Liu, 2019; Zou, 2019; Li et al., 2020; Liu, 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) mentioned TC as a biomarker. A significant difference was found between the two groups (WMD = −0.58. 95%CI [−0.80, −0.36], p < 0.00001, I2 = 86%, random effects model; Figure 4). Subgroup analyses were carried out according to different intervention durations and types of hypoglycemic drugs, and control treatments showed no significant difference in intervention effect between groups (p for interaction = 0.62, 0.22, and 0.51, respectively), and significant heterogeneity was seen (Table 3, Supplementary Figure S1).
3.4.1.3 Low-Density Lipoprotein Cholesterol
In total, 15 studies (Wang, 2010; Kang et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Li, 2019; Liu, 2019; Zou, 2019; Li et al., 2020; Liu, 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) involving 1,179 T2DM patients with NAFLD demonstrated a therapeutic effect of adding CHM to conventional therapy on LDL-C. The results showed a significant lowering effect of CHM plus WM treatment on LDL-C levels (WMD = −0.37, 95%CI [−0.47, −0.26], p < 0.00001, I2 = 67%, random effects model; Figure 4). Subgroup analysis showed no significant difference between subgroups of different intervention durations (p = 0.20), different types of hypoglycemic drugs (p = 0.71), and different control treatments (p = 0.05) (Table 3, Supplementary Figure S1).
3.4.1.4 High Density Liptein Cholesterol
The HDL-C was assessed in 13 studies (1,029 patients) (Wang, 2010; Kang et al., 2016; Xia, 2017; Guan et al., 2018; Wang, 2018; Li, 2019; Liu, 2019; Zou, 2019; Li et al., 2020; Liu, 2020; Wang, 2020; Chen M. L. et al., 2021; Pu et al., 2021). The significant differences between groups were found, which revealed that the combination of CHM and WM provided superior benefit (WMD = 0.20, 95%CI [0.10, 0.29], p < 0.0001, I2 = 89%, random effects model; Figure 4). Subgroup analysis showed that there was a significant difference in different intervention durations (p < 0.00001), and no significant difference between different types of hypoglycemic drugs (p = 0.55) and control treatment (p = 0.35) was observed (Table 3, Supplementary Figure S1).
3.4.2 Effect of Chinese Herbal Medicine on Liver Functions
3.4.2.1 Alanine Transaminase
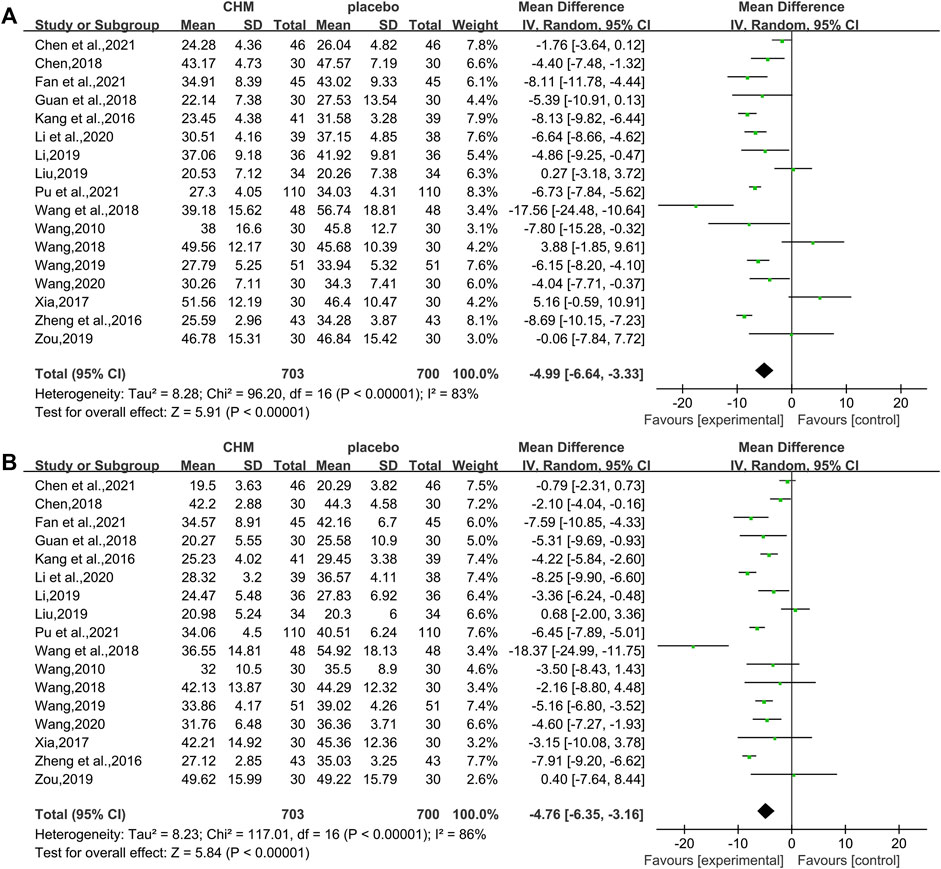
In total, 17 studies (1,403 patients) (Wang, 2010; Kang et al., 2016; Zheng et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Wang et al., 2018; Li, 2019; Liu , 2019; Wang, 2019; Zou, 2019; Li et al., 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) mentioned ALT as a biomarker. Results from the meta-analyses indicated that CHM plus WM showed more benefits in reducing ALT levels than WM alone (WMD = −4.99, 95%CI [−6.64, −3.33], p < 0.00001, I2 = 83%, random effects model; Figure 5). Results of subgroup analyses indicated a significant difference in different control treatments (p = 0.002), while no significant difference was observed between subgroups of different intervention durations (p = 0.37) and different types of hypoglycemic drugs (p = 0.71) (Table 3, Supplementary Figure S1).
3.4.2.2 Aspartate Transaminase
Of the 18 studies, 17 studies (1,403 patients) (Wang, 2010; Kang et al., 2016; Zheng et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Wang et al., 2018; Li, 2019; liu, 2019; Wang, 2019; Zou, 2019; Li et al., 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) reported the effectiveness of CHM on AST when compared with WM alone. Noteworthy lowering on AST was observed after treatments (WMD = −4.76, 95%CI [−6.35, −3.16], p < 0.00001, I2 = 86%, random effects model; Figure 5). Subgroup analyses by different intervention durations, types of hypoglycemic drugs, and control treatments showed no significant difference in effect size (p for interaction = 0.62, 0.46, and 0.15 respectively). (Table 3, Supplementary Figure S1).
3.4.3 Effect of Chinese Herbal Medicine on Insulin and Glycemic Indices
3.4.3.1 Homeostatic Model Assessment of Insulin Resistance
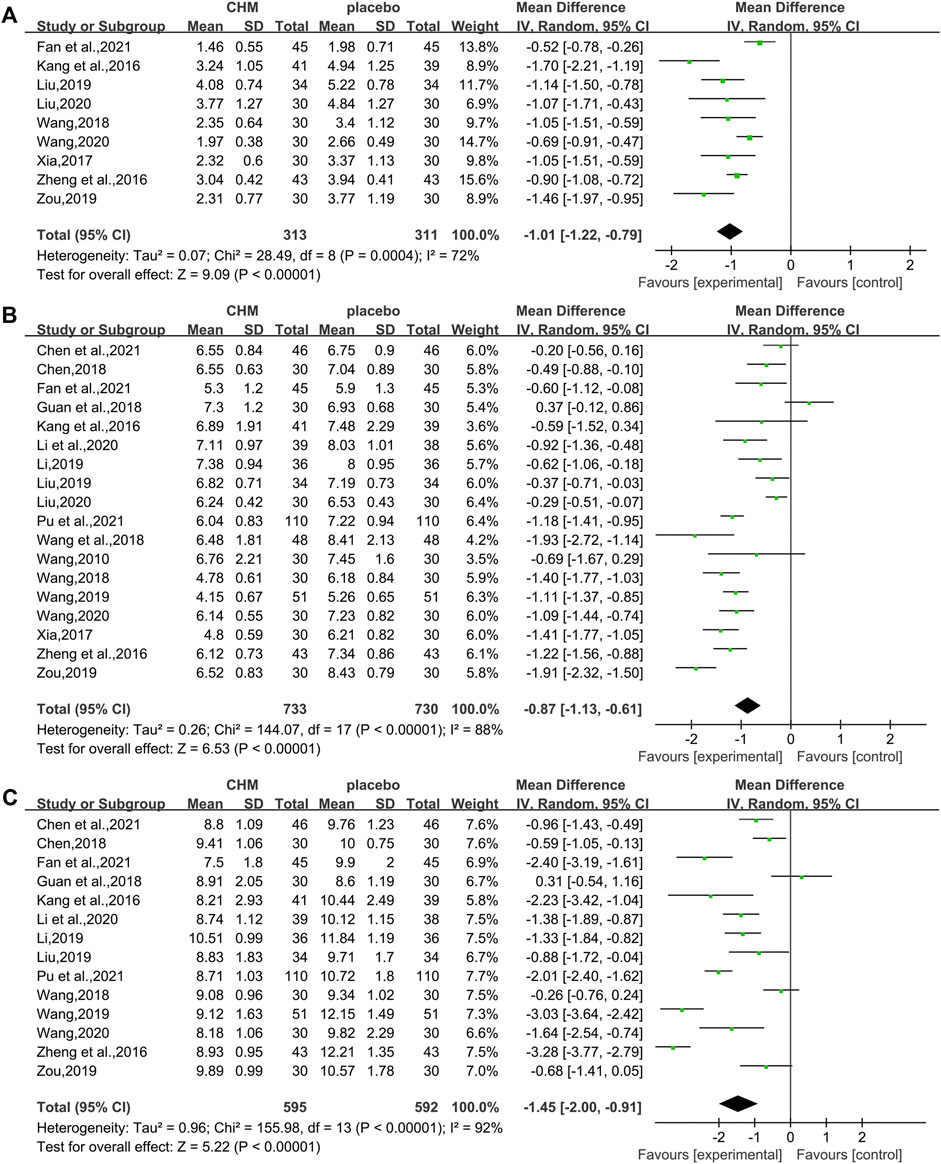
The HOMA-IR was assessed in 9 studies (624 patients) (Kang et al., 2016; Zheng et al., 2016; Xia, 2017; Wang, 2018; Liu , 2019; Zou, 2019; Liu, 2020; Wang, 2020; Fan et al., 2021). The analysis exhibited a significant difference between two groups (WMD = −1.01, 95%CI [−1.22, −0.79], p < 0.00001, I2 = 72%, random effects model; Figure 6). Obviously, CHM combined with WM was significantly superior to WM alone in improving HOMA-IR. With regards to subgroup analysis, no significant difference was found in different intervention durations (p = 0.25), different types of hypoglycemic drugs (p = 0.82), and different control treatments (p = 0.45) (Table 3, Supplementary Figure S1).
3.4.3.2 Fasting Blood Glucose
All the 18 studies (1,463 patients) (Wang, 2010; Kang et al., 2016; Zheng et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Wang et al., 2018; Li, 2019; Liu, 2019; Wang, 2019; Zou, 2019; Li et al., 2020; Liu, 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) mentioned the changes in FBG. Overall analyses revealed that a combination of CHM plus WM showed more effective in reducing FBG levels than WM alone (WMD = −0.87, 95%CI [−1.13, −0.61], p < 0.00001, I2 = 88%, random effects model; Figure 6). As to subgroup analyses, we observed no significant difference between subgroups of different intervention durations, types of hypoglycemic drugs, and control treatments (p for interaction = 0.67, 0.50 and 0.80 respectively). (Table 3, Supplementary Figure S1).
3.4.3.3 Two-Hour Postprandial Glucose
In total, 14 studies (Kang et al., 2016; Zheng et al., 2016; Chen, 2018; Guan et al., 2018; Wang, 2018; Li, 2019; Liu , 2019; Wang, 2019; Zou, 2019; Li et al., 2020; Wang, 2020; Chen M. L. et al., 2021; Fan et al., 2021; Pu et al., 2021) reported 2hPG level, with a total of 1.187 patients. A noteworthy reduction of 2hPG was observed by CHM plus WM compared with WM (WMD = −1.45.95%CI [−2.00, −0.91], p < 0.00001, I2 = 92%, random effects model; Figure 6). Results of subgroup analyses revealed that there was no significant difference in different intervention durations (p = 0.78), different types of hypoglycemic drugs (p = 0.73), and different control treatments (p = 0.47) (Table 3, Supplementary Figure S1).
3.4.4 Effect of CHM on the Body Mass Index
In total, 11 RCTs (730 patients) (Wang, 2010; Kang et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Liu , 2019; Wang, 2019; Zou, 2019; Liu, 2020; Wang, 2020) were evaluated for the effectiveness of CHM on BMI. Compared with WM, CHM combined with WM could reduce the BMI level (WMD = −0.73.95%CI [−1.35, −0.12], p = 0.02, I2 = 73%, random effects model; Figure 7). Subgroup analyses showed there was a significant difference in different intervention durations (p = 0.001) and different control treatments (p = 0.01), while no significant difference was detected in different types of hypoglycemic drugs (p = 0.81). (Table 3, Supplementary Figure S1).
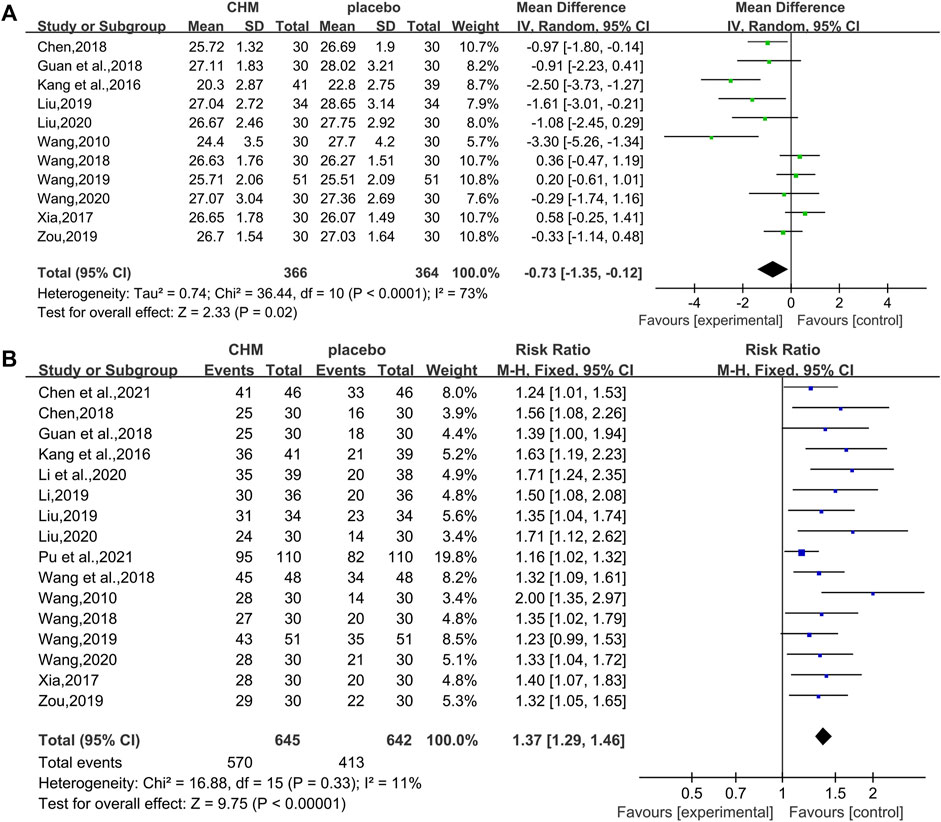
FIGURE 7. Forest plot for the body mass index and overall effective rate. (A) BMI and (B) overall effective rate.
3.4.5 Overall Effective Rate and Adverse Effects of CHM
3.4.5.1 Overall Effective Rate
In total, 16 studies (1,287 patients) (Wang, 2010; Kang et al., 2016; Xia, 2017; Chen, 2018; Guan et al., 2018; Wang, 2018; Wang et al., 2018; Li, 2019; Liu , 2019; Wang, 2019; Zou, 2019; Li et al., 2020; , 2020; Wang, 2020; Chen M. L. et al., 2021; Pu et al., 2021) reported overall effective rate. The combined effects suggested a significant improving effect of CHM plus WM on the overall effective rate in T2DM with NAFLD patients when compared with WM alone (RR = 1.37.95%CI [1.29, 1.46], p < 0.00001, I2 = 11%, fixed effects model; Figure 7).
3.4.5.2 Adverse Effects
A total of 13 RCTs reported adverse effects. Among them, 6 literatures (Chen, 2018; Guan et al., 2018; Li, 2019; liu, 2019; Liu , 2020; Chen M. L. et al., 2021) indicated that no adverse events occurred during the treatment period, while 7 literatures (Kang et al., 2016; Xia, 2017; Wang, 2018; Wang et al., 2018; Zou, 2019; Li et al., 2020; Wang, 2020) reported that general adverse events occurred. The most common adverse effect was abdominal fullness (Kang et al., 2016; Xia, 2017; Wang, 2018; Zou, 2019; Li et al., 2020; Wang, 2020) followed by diarrhea (Xia, 2017; Wang, 2018; Zou, 2019; Li et al., 2020; Wang, 2020). Adverse effects such as nausea, anorexia, and dizziness have also been reported in these studies. However, all adverse effects resolved (or disappeared) after symptomatic treatment.
3.4.6 Publication Bias
Funnel plots and Egger’s test were performed to evaluate publication bias. The funnel plot (Figures 8C,I,J) revealed a slight asymmetry and Egger’s test indicated possible publication bias in LDL-C (t = 2.75, p = 0.016),BMI(t = −3.04, p = 0.014), and overall effective rate (t = 8.02, p < 0.001) (Supplementary Figure S2). Figures 8A, B, D–H show inverted and symmetrical funnels. The results of Egger’s test were as follows: TG (t = 0.54, p = 0.599), TC (t = 0.12, p = 0.909), HDL-C (t = 1.32, p = 0.213), ALT (t = 1.56, p = 0.139), AST (t = 0.44, p = 0.666), FBG (t = −0.22, p = 0.830), and 2hPG (t = 0.23, p = 0.821) (Supplementary Figure S2), indicating that these outcomes had no significant publication bias.
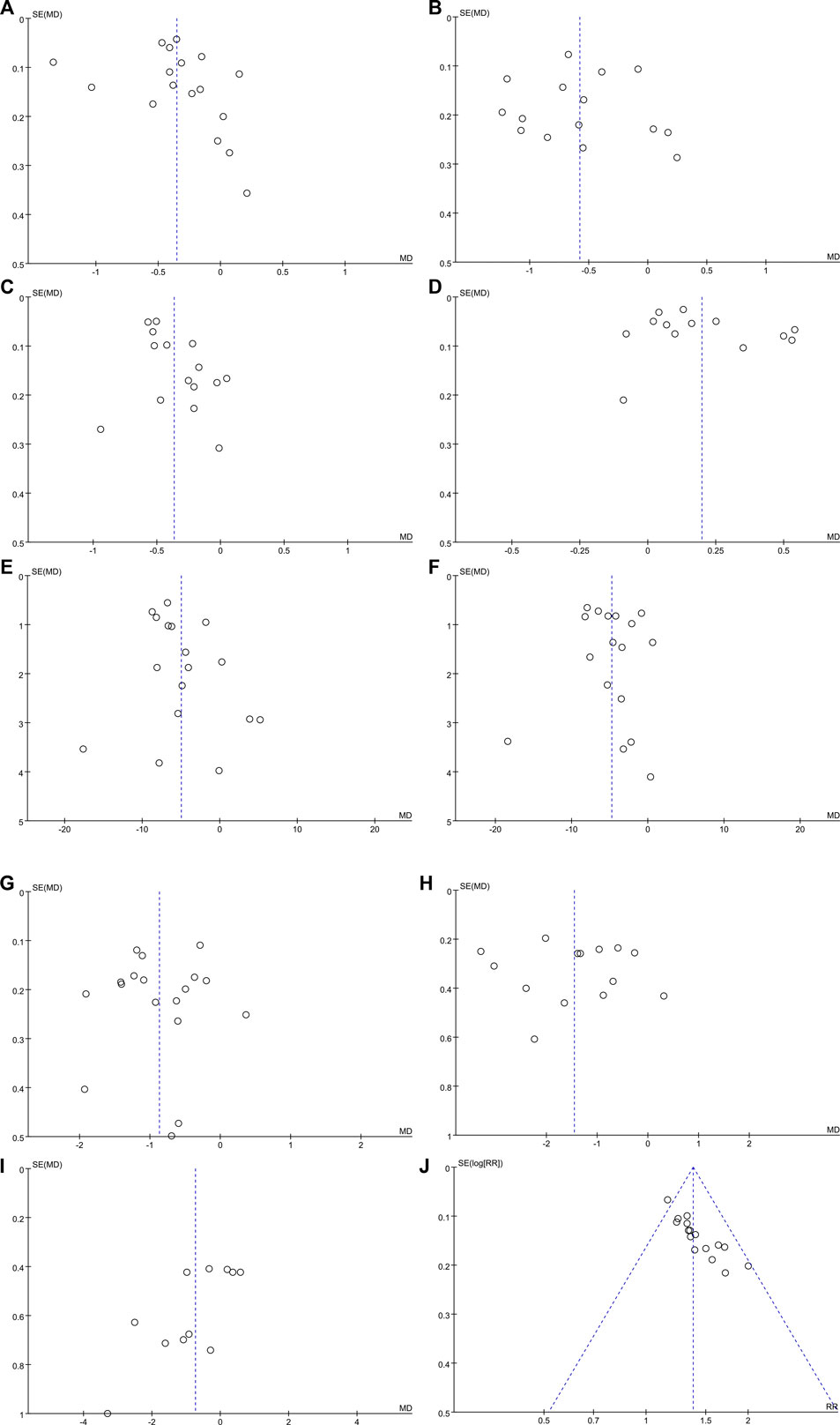
FIGURE 8. Funnel plots for assessing publication bias. (A) TG; (B) TC; (C) LDL-C; (D) HDL-C; (E) ALT; (F) AST; (G) FBG; (H) 2hPG; (I) BMI; and (J) overall effective rate.
3.4.7 Sensitivity Analysis
After excluding each study, we found no significant changes in the results, and all the results showed good agreement. For further validation, we used STATA v14.0 and performed sensitivity analysis of TG, ALT, FBG, and overall effective rate; the results were considered robust (Supplementary Figure S3).
4 Discussion
4.1 Summary of the Main Results
For this study, a total of 783 relevant articles were retrieved, and 18 articles were included in the meta-analysis after screening. The main findings of our meta-analysis showed that when compared with WM therapy alone, a combination of CHM and WM therapy was effective for improvement of lipid and glucose metabolism, liver function, insulin resistance, and body mass. The high level of heterogeneity could be attributable to different interventions. The subgroup analysis was performed based on different intervention durations and different types of WM to explain or reduce the degree of associated heterogeneity and obtain a more reliable conclusion. Subgroup analyses showed that intervention duration was primarily responsible for the positive changes in HDL-C and BMI. A few studies did not report adverse effects. The methodological quality of the included studies was poor by the risk assessment of bias. Sensitivity analysis indicated that the results were robust.
4.2 Quality of Evidence
In this study, we used GRADEpro to assess the quality of evidence. The assessment showed that the evidence of all the results was of low quality, except that the evidence quality of the overall effective rate was moderate (Table 4). The decreased certainty of the evidence was mainly attributed to the low methodological quality and the high level of heterogeneity among the studies. Therefore, the results of this study should be applied cautiously to clinical practice, and more high-quality RCTs are needed to evaluate efficacy.
4.3 Frequency Distribution Analysis of Chinese Herb Medicines
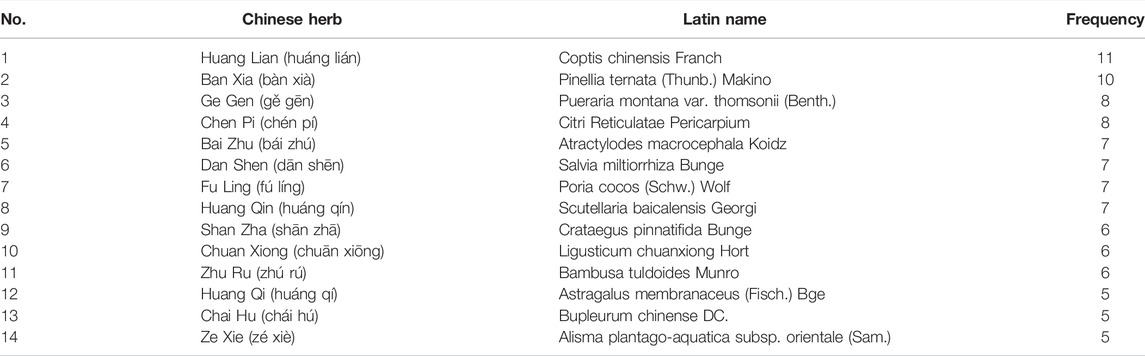
A total of 70 single CHMs were recorded, sorted by frequency of occurrence from high to low, thereafter listed the CHMs with a frequency of more than 5 times (Table 5). The top four were Coptis chinensis Franch (huáng lián), Pinellia ternata (Thunb.) Makino (bàn xià), Pueraria montana var. thomsonii (Benth.) (gě gēn), and Citri Reticulatae Pericarpium (chén pí).
For Coptis chinensis Franch., which has the highest frequency of occurrence, pharmacological studies have shown that berberine is the main active ingredient, which has definite effects of reducing hepatocyte lipid accumulation, anti-inflammation, and anti-fibrosis (Li et al., 2021), lowering blood glucose and improving insulin resistance (Chen Y. et al., 2021), and providing typical multi-target and multi-system pharmacological effects on T2DM with NAFLD. Berberine can regulate hepatic metabolism by protecting the intestinal mucosal epithelial barrier, thereby regulating the microenvironment of the intestinal microbiota and changing microbiota-derived metabolites such as short-chain fatty acids and secondary bile acids (Betrapally et al., 2017; Sun et al., 2017; Tian et al., 2019). Berberine can also promote GLP-1 secretion to improve glucose metabolism by up-regulating the related expression of proglucagon and prohormone convertase mRNA (Yu et al., 2010). With regards to Pueraria montana var. thomsonii (Benth.), its main active ingredients are isoflavones, including puerarin and daidzin. Studies have shown that puerarin can repair liver injury and reduce dyslipidemia caused by liver fat deposition by inhibiting IκBα/NF-κB p65 signaling axis activity (Hu et al., 2021). Furthermore, it can improve insulin resistance and oxidative stress by affecting insulin receptor signaling pathways and adjusting the structure of the intestinal microbiome (Zhang H. M. et al., 2019).
4.4 Strengths and Limitations for Research
This study was conducted in strict accordance with the method of systematic review, and we interpreted the results cautiously to avoid confusion while ensuring accuracy. We found that combined CHM therapy might improve lipid and glucose metabolism, liver function and insulin resistance, and reduce body weight as well as increase overall effective rate, better than conventional therapy. Therefore, the results may provide new treatment opportunities, new ideas, and new directions for the study of T2DM with NAFLD.
However, this review has a few limitations that warrant discussion. For example, due to the incomplete nature of information provided by most articles as well as the flawed study design, the overall methodological quality of the included studies is poor, which may lead to overestimation of efficacy. Therefore results should be interpreted cautiously.
Second, while we performed the subgroup analysis, the source of heterogeneity could not be determined completely. Heterogeneity could have emerged due to the different composition and dose of CHM used in interventions and different dosage forms of CHM (such as decoction, tablets, granules, and pills). In addition, since some studies did not report adverse effects in the aftermath of CHM treatment, the safety associated with CHM remains unclear, and further studies are needed to confirm it. Finally, T2DM with NAFLD as a metabolic disease, lifestyle intervention (such as physical exercise and diet) and pharmacological treatment are the important therapy modalities and have a great impact on values of the biomarkers evaluated. In this study, lifestyle intervention and pharmacological treatment were only briefly statistically analyzed in Table 1, so subsequent studies can further explore the effects of different physical exercises, diets, and pharmacological treatments on the basis of this study, for example, physical exercise, to analyze whether physical exercise is combined, and the specific method and duration of physical exercise. This allows a more detailed and in-depth discussion of T2DM with NAFLD. In the meantime, it is necessary and important to compare whether the effect of two herbs differ on T2DM with NAFLD and T2DM without NAFLD. This is also a significant direction in our subsequent study.
5 Conclusion
In summary, the CHM in combination with WM seems to be more beneficial in T2DM with NAFLD patients in improving lipid and glucose metabolism, liver function, and insulin resistance as well as improving overall efficiency and reducing body weight. Given the poor quality of reports from these studies and uncertain evidence, these findings should be interpreted cautiously. Future RCTs with larger samples and higher quality should be conducted to provide more accurate and complete data to support and validate the clinical efficacy and safety of CHM in the treatment of T2DM with NAFLD.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author Contributions
SP and YL conceived and designed the study. LL, ZX, XYZ, and SY conducted this meta-analysis. XL and HW drafted the manuscript. XGZ and CX revised this article. YL supervised all aspects of this study. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by grants from the Science and Technology Project of Sichuan Province (2019YFS0196), the Sichuan Provincial Scientific and Technological Innovation Seedling Project (2022036), the Sichuan Provincial Administration of Traditional Chinese Medicine (2021MS095 and 2021MS504), and the Science and Technology Development Fund of Hospital of Chengdu University of Traditional Chinese Medicine (21HL04).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.863839/full#supplementary-material
Abbreviations
2hPG, two-hour postprandial glucose; ALT, alanine transaminase; AST, aspartate transaminase; BMI, body mass index; CHM: Chinese herbal medicine; CIs, confidence intervals; CNKI, China National Knowledge Infrastructure; FBG, fasting blood glucose; GRADE, grading of recommendations assessment, development, and evaluation; HDL-C, high-density lipoprotein cholesterol; IR, insulin resistance; HOMA-IR, homeostatic model assessment of insulin resistance; LDL-C: low-density lipoprotein cholesterol; NAFLD, nonalcoholic fatty liver disease; PRISMA-P, Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols; RCTs, randomized controlled trials; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG: triglyceride; RR, risk ratio; TCM, traditional Chinese medicine; WM: Western medicine; WMDs, weighted mean differences.
References
Betrapally, N. S., Gillevet, P. M., and Bajaj, J. S. (2017). Gut Microbiome and Liver Disease. Transl. Res. 179, 49–59. doi:10.1016/j.trsl.2016.07.005
Bril, F., and Cusi, K. (2017). Management of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes: A Call to Action. Diabetes care 40 (3), 419–430. doi:10.2337/dc16-1787
Buzzetti, E., Pinzani, M., and Tsochatzis, E. A. (2016). The Multiple-Hit Pathogenesis of Non-alcoholic Fatty Liver Disease (NAFLD). Metabolism 65 (8), 1038–1048. doi:10.1016/j.metabol.2015.12.012
Chen, M., Zhou, Y. P., and Liu, Z. H. (2016). Research Progress of Type 2 Diabetes Combined with Non-alcoholic Fatty Liver Disease in Traditional Chinese Medicine. J. Gansu Univ. TCM 33 (01), 86–89.
Chen, X. (2018). Clinical observation on Huanglian wendan decoction to interfere in the syndrome of phlegm and heat intermingling in type 2 diabetes with non-alcoholic fatty liver. Master’s thesis. China: Shandong University of Traditional Chinese Medicine.
Chen, C., Chen, J. X., Yu, R., Chen, Y. Z., Liu, C. J., Zhou, Z., et al. (2021a). Effects of Zuogui Jiangtang Qingzhi Recipe on Expressions of PI3K Signal Pathway of Liver Tissue in MKR Mice with Type 2 Diabetes Mellitus Complicated with Non-alcoholic Fatty Liver Disease. CJTCMP 36 (01), 106–111.
Chen, M. L., Wang, Z. T., Yang, L. Q., Li, L., and Heng, X. P. (2021b). Effect of Dangua Humai Oral Liquid on Vascular Endothelial Function and Oxidative Stress Indicators in Patients with Type 2 Diabetes Mellitus Complicated with Non-alcoholic Fatty Liver Disease. Eval. Analysis Drug-use Hosp. China 21 (08), 918–921.
Chen, Y., Li, Z. H., and Yue, R. S. (2021c). Exploration on Treatment of Type 2 Diabetes Based on Astrointestinal Heat Accumulation in Traditional Chinese Medicine. Lishizhen Med. Materia Medica Res. 32 (09), 2229–2230.
Eslam, M., Sanyal, A. J., and George, J. (2020). MAFLD: A Consensus-Driven Proposed Nomenclature for Metabolic Associated Fatty Liver Disease. Gastroenterology 158 (7), 1999–e1. e1991. doi:10.1053/j.gastro.2019.11.312
European Association for the Study of the Liver (EASL) (2016). EASL-EASD-EASO Clinical Practice Guidelines for the Management of Non-alcoholic Fatty Liver Disease. Obes. facts 9 (2), 65–90.
Fan, Y. F., Cao, L., Sun, H. P., Xu, J., Bao, W. T., and Zhu, X. Q. (2021). Clinical Study of Gegenqinlian Decoction in Type 2 Diabetes with Non-alcoholic Fatty Liver Disease. Chin. General Pract. 24 (36), 4587–4592.
Gao, J. F., Liu, M. M., Shen, Y. H., Feng, Z. F., Hu, C. P., Chen, J. F., et al. (2020). The Influence of Zicui Jiangtang Formula on Insulin Resistance of Rats with Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver Disease. Henan J. TCM 40 (11), 1679–1683.
Guan, J. T., Liu, B. Z., Hu, Y. H., Zhou, D. H., Shao, G. J., and Li, L. Y. (2018). Clinical Study on 30 Case of Type 2 Diabetes Mellitus Combined with Non-alcoholic Fatty Live Disease Treated by Integrative Chinese and Western Medicine. Jiangsu J. TCM 50 (1), 32–34.
Hazlehurst, J. M., Woods, C., Marjot, T., Cobbold, J. F., and Tomlinson, J. W. (2016). Non-alcoholic Fatty Liver Disease and Diabetes. Metabolism 65 (8), 1096–1108. doi:10.1016/j.metabol.2016.01.001
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, T. J., Thomas, J., Cumpston, M., and Chandler, J. (2019). Cochrane Handbook for Systematic Reviews of Interventions Version 6.0. Available at: https://training.cochrane.org/handbook.
Hu, B., Xu, Y., Ding, H., Fang, X. Q., and Wang, T. B. (2021). Puerarin Alleviates Nonalcoholic Fatty Liver Injury in Rats by Inhibiting IκBα/NF-Κb P65 Signal axis. J. Guangzhou Univ. TCM 38 (02), 366–372.
Hu, M., Phan, F., Bourron, O., Ferré, P., and Foufelle, F. (2017). Steatosis and NASH in Type 2 Diabetes. Biochimie 143, 37–41. doi:10.1016/j.biochi.2017.10.019
Hua, X., Sun, Y., Zhong, Y., Feng, W., Huang, H., Wang, W., et al. (2014). Low Serum Sex Hormone-Binding Globulin Is Associated with Nonalcoholic Fatty Liver Disease in Type 2 Diabetic Patients. Clin. Endocrinol. (Oxf) 80 (6), 877–883. doi:10.1111/cen.12360
Kang, X. D., Dang, X. J., Wang, Y. M., Yu, C. Z., and Yang, W. J. (2016). Clinical Effect of Huazhuo Granule in Treating Type 2 Diabetes Mellitus Combined with Nonalcoholic Fatty Liver (Phlegm-dampness Accumulating Spleen Type). Chin. J. Exp. Traditional Med. Formulae 22 (1), 171‐175.
Lee, B. W., Lee, Y. H., Park, C. Y., Rhee, E. J., Lee, W. Y., Kim, N. H., et al. (2020). Non-Alcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus: A Position Statement of the Fatty Liver Research Group of the Korean Diabetes Association. Diabetes Metab. J. 44 (3), 382–401. doi:10.4093/dmj.2020.0010
Li, L., Wang, Y. L., Qin, H. Y., Qu, Y. Y., Yang, T. S., Wang, Z. Y., et al. (2021). Research Progress of Berberine in Regulating Enterohepatic axis in the Treatment of Non-alcoholic Fatty Liver Disease. J. Chin. Herb. Med. 52 (05), 1501–1509.
Li, Q., Xie, B. X., Zhang, Y., Wu, R., and Gao, Y. B. (2020). Clinical Study on Tangzhiping in the Treatment of Type 2 Diabetes Mellitus with Phlegm-Blood Stasis Type Combined with Nonalcoholic Fatty Liver Disease. Hebei J. TCM 42 (3), 365–369.
Li, Y. (2019). China: Changchun University of Traditional Chinese Medicine. Master's thesis.Clinical Study on Tongtiao Tangzhi Fang Treatment of Type 2 Diabetes Mellitus with Nonalcoholic Fatty Liver Disease
Liu, J. (2019). China: China Academy of Chinese Medical Sciences. Docter’s thesis.Research on Diabetes and Non-alcoholic Fatty Liver Disease Wiht the Modified Shuilu Erxian Dan
Liu, X. Y. (2020). China: Tianjin University of Traditional Chinese Medicine. Master's thesis.Observation of Clinical Efficacy of Huazhuojiedu Prescription Combined with Western Medicine in the Treatment of Type 2 Diabetes Mellitus with Non-alcoholic Fatty disease(Turbidity Toxin inside Syndrome)
Lomonaco, R., Bril, F., Portillo-Sanchez, P., Ortiz-Lopez, C., Orsak, B., Biernacki, D., et al. (2016). Metabolic Impact of Nonalcoholic Steatohepatitis in Obese Patients with Type 2 Diabetes. Diabetes care 39 (4), 632–638. doi:10.2337/dc15-1876
Loria, P., Lonardo, A., and Anania, F. (2013). Liver and Diabetes. A Vicious Circle. Hepatol. Res. 43 (1), 51–64. doi:10.1111/j.1872-034X.2012.01031.x
Mantovani, A., Byrne, C. D., Bonora, E., and Targher, G. (2018). Nonalcoholic Fatty Liver Disease and Risk of Incident Type 2 Diabetes: A Meta-Analysis. Diabetes care 41 (2), 372–382. doi:10.2337/dc17-1902
Moher, D., Shamseer, L., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015 Statement. Syst. Rev. 4 (1), 1. doi:10.1186/2046-4053-4-1
Nguyen, V., and George, J. (2015). Nonalcoholic Fatty Liver Disease Management: Dietary and Lifestyle Modifications. Semin. Liver Dis. 35 (3), 318–337. doi:10.1055/s-0035-1562950
Portillo-Sanchez, P., Bril, F., Maximos, M., Lomonaco, R., Biernacki, D., Orsak, B., et al. (2015). High Prevalence of Nonalcoholic Fatty Liver Disease in Patients with Type 2 Diabetes Mellitus and Normal Plasma Aminotransferase Levels. J. Clin. Endocrinol. Metab. 100 (6), 2231–2238. doi:10.1210/jc.2015-1966
Pu, Y. L., Liu, F., Zhang, Y. H., and Li, Y. H. (2021). Clinical Study on Treatment of Type 2 Diabetes Combined with Non-alcoholic Fatty Liver Disease with Phlegm and Blood Stasis Mutual Stagnation Syndrome with Lipi Huatan Quyu Decoction. J. Basic Chin. Med. 27 (4), 613–616.
Shi, L. W., Ni, Q., Feng, L., and Zhang, Y. Y. (2019). Research Progress and Outlook of Traditional Chinese Medicine for Type 2 Diabetes Mellitus with Nonalcoholic Fatty Liver Disease. Liaoning J. TCM 46 (01), 208–212.
Sun, R., Yang, N., Kong, B., Cao, B., Feng, D., Yu, X., et al. (2017). Orally Administered Berberine Modulates Hepatic Lipid Metabolism by Altering Microbial Bile Acid Metabolism and the Intestinal FXR Signaling Pathway. Mol. Pharmacol. 91 (2), 110–122. doi:10.1124/mol.116.106617
Targher, G., Lonardo, A., and Byrne, C. D. (2018). Nonalcoholic Fatty Liver Disease and Chronic Vascular Complications of Diabetes Mellitus. Nat. Rev. Endocrinol. 14 (2), 99–114. doi:10.1038/nrendo.2017.173
Targher, G., Corey, K. E., Byrne, C. D., and Roden, M. (2021). The Complex Link between NAFLD and Type 2 Diabetes Mellitus - Mechanisms and Treatments. Nat. Rev. Gastroenterol. Hepatol. 18 (9), 599–612. doi:10.1038/s41575-021-00448-y
Tian, Y., Cai, J., Gui, W., Nichols, R. G., Koo, I., Zhang, J., et al. (2019). Berberine Directly Affects the Gut Microbiota to Promote Intestinal Farnesoid X Receptor Activation. Drug Metab. Dispos. 47 (2), 86–93. doi:10.1124/dmd.118.083691
Tilg, H., Moschen, A. R., and Roden, M. (2017). NAFLD and Diabetes Mellitus. Nat. Rev. Gastroenterol. Hepatol. 14 (1), 32–42. doi:10.1038/nrgastro.2016.147
Wang, H. H. (2020). China: Anhui University of Traditional Chinese Medicine. Master’s thesis.Clinical Evaluation of Gegen Qinlian Dispelling Turbidity Decoction in the Treatment of Type 2 Diabetes Mellitus Complicated with Non-alcoholic Fatty Liver Disease and its Correlation with the Levels of Omentin-1 and A-FABP
Wang, T. X. (2018). China: Anhui University of Traditional Chinese Medicine. Master’s thesis.Clinical Study on the Correlation between Visfatin,RBP4 and SFRP5 Levels in Patients with Type 2 Diabetes Mellitus Combined with Non-alcoholic Fatty Liver Disease
Wang, Y. B. (2010). Clinical Observation on 30 Cases of Type 2 Diabetes Combined with Non-alcoholic Fatty Liver Diseases. Henan J. TCM 30 (10), 974–976.
Wang, Y. (2019). Clinical Study on Huoxue Jiangzhi Baogan Tang Combined with Western Medicine for T2DM Complicated with NAFLD. J. New Chin. Med. 1 (10), 151–154.
Wang, Y. D., Song, Z. H., Chi, H. Y., Su, L. Q., Zhou, Y. P., and Wang, Q. (2018). Clinical Study on Treatment of Primary Type 2 Diabetes Mellitus with Non-alcoholic Fatty Liver Disease by Modified Wendan Decoction Combined with Western Medicine. Sichuan J. TCM 36 (6), 98–100.
Wei, Q., Xu, X., Guo, L., Li, J., and Li, L. (2021). Effect of SGLT2 Inhibitors on Type 2 Diabetes Mellitus with Non-alcoholic Fatty Liver Disease: A Meta-Analysis of Randomized Controlled Trials. Front. Endocrinol. (Lausanne) 12, 635556. doi:10.3389/fendo.2021.635556
Wong, V. W., Chan, W. K., Chitturi, S., Chawla, Y., Dan, Y. Y., Duseja, A., et al. (2018). Asia-Pacific Working Party on Non-alcoholic Fatty Liver Disease Guidelines 2017-Part 1: Definition, Risk Factors and Assessment. J. Gastroenterol. Hepatol. 33 (1), 70–85. doi:10.1111/jgh.13857
Wu, R., and Wei, J. P. (2019). Thinking and Method of Traditional Chinese Medicine Treatment on Type 2 Diabetes Complicated with Non-alcoholic Fatty Liver Disease. World Sci. Technology/Modernization Traditional Chin. Med. Materia Medica 21 (01), 59–64.
Xia, M. L. (2017).. China: Anhui University of Traditional Chinese Medicine. Master’s thesis.Clinical Study on the Correlation between FGF21,APN and TNF-α Levels in Patients with Type 2 Diabetes Mellitus Combined with Non-alcoholic Fatty Liver Disease
Younossi, Z., Anstee, Q. M., Marietti, M., Hardy, T., Henry, L., Eslam, M., et al. (2018). Global Burden of NAFLD and NASH: Trends, Predictions, Risk Factors and Prevention. Nat. Rev. Gastroenterol. Hepatol. 15 (1), 11–20. doi:10.1038/nrgastro.2017.109
Younossi, Z. M., Koenig, A. B., Abdelatif, D., Fazel, Y., Henry, L., and Wymer, M. (2016). Global Epidemiology of Nonalcoholic Fatty Liver Disease-Meta-Analytic Assessment of Prevalence, Incidence, and Outcomes. Hepatology 64 (1), 73–84. doi:10.1002/hep.28431
Yu, Y., Liu, L., Wang, X., Liu, X., Liu, X., Xie, L., et al. (2010). Modulation of Glucagon-like Peptide-1 Release by Berberine: In Vivo and In Vitro Studies. Biochem. Pharmacol. 79 (7), 1000–1006. doi:10.1016/j.bcp.2009.11.017
Zhang, H. M., Cao, S. J., Qiu, F., and Zhang, D. Q. (2019a). Research Progress of Pueraria Lobata and Puerarin in Treating Diabetes and Complications. J. Tianjin Univ. TCM 38 (06), 607–615.
Zhang, L. L., Chen, T. H., and Ye, L. L. (2019b). A Randomized Parallel Controlled Study of Jiawei Sini Decoction in the Treatment of Type 2 Diabetic with Nonalcoholic Fatty Liver Disease (Liver Depression and Qi Stagnation). J. Pract. Traditional Chin. Intern. Med. 33 (05), 13–17.
Zheng, Z. P., Tian, F., Zheng, Z. G., Zhang, G. Q., Guo, F., and Guo, X. H. (2016). Effect of Metformin Combined with Liuwei Dihuang Pill on Serum Illness-Related Molecule Contents in T2DM Patients Wiht NAFLD. J. Hainan Med. Univ. 22 (10), 972–975.
Zou, H. (2019). China: Anhui University of Traditional Chinese Medicine. Master’s thesis.Clinical Evaluation of Compound Gegen Qinlian Decoction in the Treatment of Type 2 Diabetes Mellitus Complicated with Non-alcoholic Fatty Liver Disease and its Correlation with the Levels of Chemerin and Resistin
Keywords: type 2 diabetes mellitus(T2DM), nonalcoholic fatty liver disease(NAFLD), Chinese herbal medicine (CHM), meta-analysis, systematic review
Citation: Peng S, Liu L, Xie Z, Zhang X, Xie C, Ye S, Zhang X, Liang X, Wang H and Liu Y (2022) Chinese Herbal Medicine for Type 2 Diabetes Mellitus With Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:863839. doi: 10.3389/fphar.2022.863839
Received: 27 January 2022; Accepted: 26 April 2022;
Published: 27 June 2022.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Ana Isabel Alvarez-Mercado, University of Granada, SpainMasahide Hamaguchi, Kyoto Prefectural University of Medicine, Japan
Copyright © 2022 Peng, Liu, Xie, Zhang, Xie, Ye, Zhang, Liang, Wang and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ya Liu, bGl1eWF5YTkxOEAxNjMuY29t
 Sihan Peng
Sihan Peng Lu Liu1,2
Lu Liu1,2 Chunguang Xie
Chunguang Xie Ya Liu
Ya Liu