
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Pharmacol. , 26 April 2022
Sec. Inflammation Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.859951
Retinal vein occlusion (RVO) is one of the most common retinal vascular diseases. The pathogenesis of RVO is multifactorial and involves a complex interplay among a variety of vascular and inflammatory mediators. Many cytokines, chemokines, growth factors, and cell adhesion molecules have been reported to be implicated. Treatments for RVO are directed at the management of underlying risk factors and vision-threatening complications, including macula edema (ME) and neovascularization. Intravitreal anti-VEGF agents are currently considered as the first-line treatment for ME secondary to RVO (RVO-ME), but a substantial proportion of patients responded insufficiently to anti-VEGF agents. Since RVO-ME refractory to anti-VEGF agents generally responds to corticosteroids and its visual outcome is negatively correlated to disease duration, prediction of treatment response at baseline in RVO-ME may significantly improve both cost-effectiveness and visual prognosis. Several bioactive molecules in the aqueous humor were found to be associated with disease status in RVO. This review aims to present a comprehensive review of intraocular biomolecules reported in RVO, including VEGF, IL-6, IL-8, MCP-1, sICAM-1, IL-12, IL-13, sVEGFR-1, sVEGFR-2, PDGF-AA, etc., highlighting their association with disease severity and/or phenotype, and their potential roles in prognostic prediction and treatment selection. Some of these molecules may serve as biomarkers for aqueous humor-based companion diagnostics for the treatment of RVO in the future.
Retinal vein occlusion (RVO) is one of the most common retinal vascular diseases (Branch Vein Occlusion Study Group, 1986; The Central Vein Occlusion Study, 1993). It is caused by partial or complete occlusion of venous blood flow, which leads to an increase in venous pressure with subsequent leakage of the retinal microvasculature proximal to the occlusion site (Christoffersen and Larsen, 1999). Blockage of the main retinal vein is called central retinal vein occlusion (CRVO), and of a smaller vein is called branch retinal vein occlusion (BRVO). The estimated 15-year cumulative incidence of RVO was reported to be 2.3% in the population, with BRVO and CRVO representing 1.8 and 0.5%, respectively (Klein et al., 2008). In a meta-analysis that pooled data from the United States, Europe, Asia, and Australia, about 16.4 million people were affected by RVO worldwide in 2008 (Rogers et al., 2010). The pathogenesis of RVO is multifactorial and involves a complex interplay among a variety of vascular and inflammatory mediators. While vascular endothelial growth factor (VEGF), a potent mediator of both vascular permeability and inflammation, undoubtedly plays a central role in the pathological process of RVO, several cytokines, chemokines, growth factors, and cell adhesion molecules have been reported to be implicated (Noma et al., 2019; 2020).
Treatments for RVO are directed at the management of underlying risk factors and vision-threatening complications, including macula edema (ME) and neovascularization. Intravitreal anti-VEGF agents are currently considered as the first-line treatment for ME secondary to RVO (RVO-ME) (Schmidt-Erfurth et al., 2019), but a substantial proportion of patients responded insufficiently to anti-VEGF agents. Since RVO-ME refractory to anti-VEGF agents generally responds to corticosteroids and its visual outcome is negatively correlated to disease duration (Wallsh and Gallemore, 2021), prediction of treatment response at base line in RVO-ME may significantly improve both cost-effectiveness and visual prognosis.
A companion diagnostic is a set of diagnostic tests that predict the safety and/or effectiveness of a particular treatment and has been increasingly recognized as a means to improve the precision of treatments in cancer (Rosenbaum and Weisman, 2017). Several bioactive molecules in the aqueous humor were found to be associated with disease status in RVO, and thus may serve as biomarkers for treatment prediction. In fact, customized intravitreal injections based on aqueous humor cytokines were proved to be beneficial in an intractable RVO-ME patient (Modi et al., 2021), and “liquid biopsy”, a close concept of companion dialogistic, was proposed to dictate treatments in diabetic retinopathy (Vujosevic and Simó, 2017). This review aims to present a comprehensive review of intraocular biomolecules reported in RVO, highlighting their association with disease severity and/or phenotype and their potential roles in prognostic prediction and treatment selection. The most studied intraocular biomolecules are listed in Table 1 and Table 2, and the least studied biomolecules are listed in Table 3.

TABLE 2. Studies pertaining to intraocular biomolecules and treatments in RVOa.
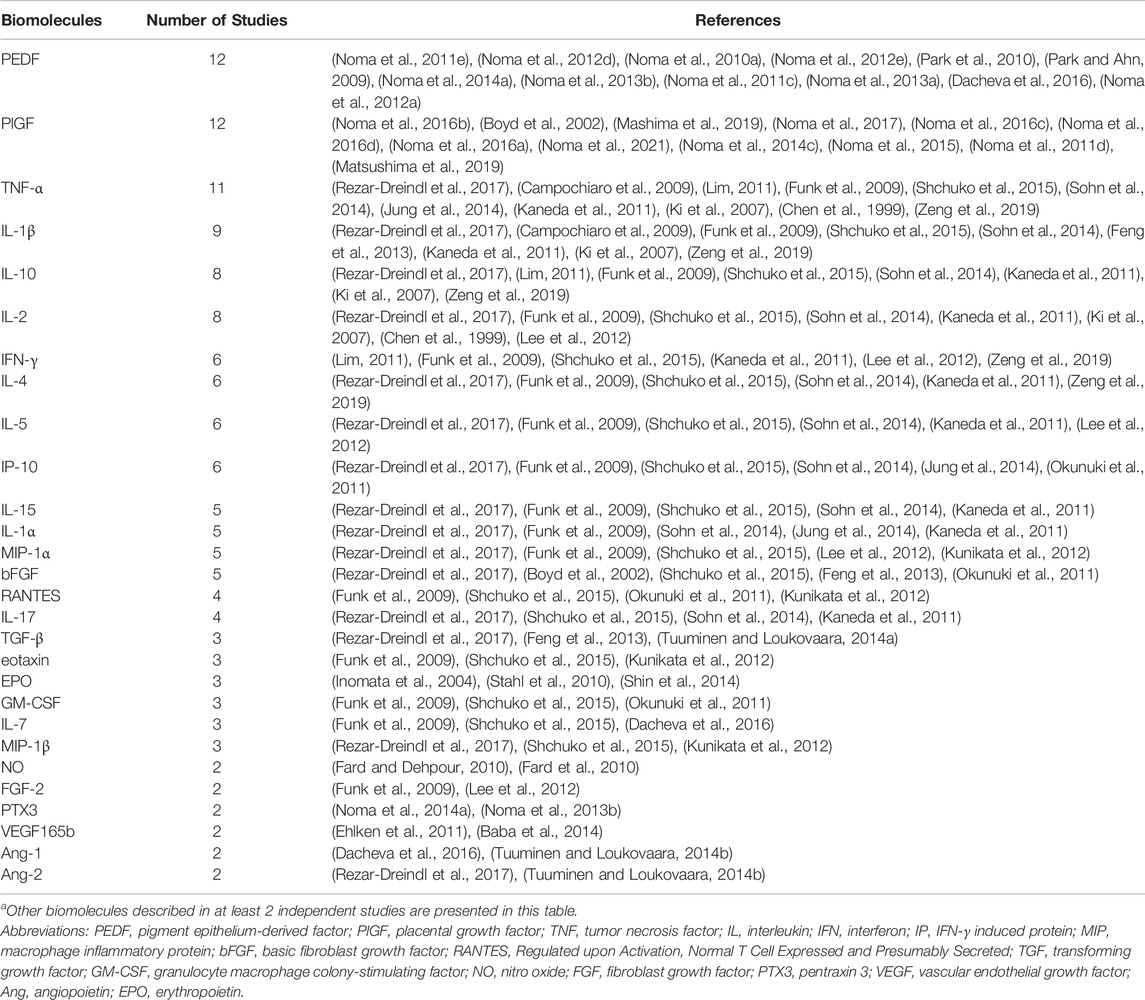
TABLE 3. Other intraocular biomolecules studied in RVOa.
In humans, the VEGF family includes VEGF-A (commonly referred to simply as VEGF), -B, -C, -D, and placental growth factor (PlGF). VEGF is an endothelial-cell-specific mitogen that promotes vascular permeability and angiogenesis (Keck et al., 1989). It is believed to be induced by the ischemic condition resulting from occlusion of retinal veins and plays an important role in RVO associated pathophysiological processes, including ME, the major cause of visual impairment, as well as neovascularization of the retina, optic disc, or the anterior segment, which may lead to vitreous hemorrhage or neovascular glaucoma (Chan et al., 2011). Intravitreal injection of anti-VEGF agents is, de facto, the most important treatment modality for RVO-ME. Intraocular VEGF levels are well demonstrated to be associated with disease severity in RVO from different aspects. VEGF concentrations in intraocular fluids were higher in CRVO than in BRVO (Campochiaro et al., 2009; Rezar-Dreindl et al., 2017), in ischemic than in nonischemic CRVO (Noma et al., 2008; Noma et al., 2009; 2010b; Noma et al., 2010c; Noma et al., 2011a; Noma et al., 2011e; Noma et al., 2012b), and in major BRVO than in macular BRVO (Lim, 2011; Noma et al., 2012d; Kim et al., 2016; Noma et al., 2016b). They were reported to be associated with severity of ME (Noma et al., 2005; Noma et al., 2006; Noma et al., 2008; Noma et al., 2009; Noma et al., 2010a; Noma et al., 2010b; Noma et al., 2010c; Noma et al., 2011a; Noma et al., 2011e), neovascularization of the iris (NVI) (Boyd et al., 2002; Noma et al., 2005; Noma et al., 2006; Noma et al., 2010a), serous retinal detachment (SRD) (Park et al., 2010; Noma et al., 2012e), electroretinogram parameters (Yasuda et al., 2011) and aqueous flare levels (Noma et al., 2013c; Noma et al., 2014b; Noma et al., 2017; Mashima et al., 2019). Intraocular VEGF level usually drop dramatically after an intravitreal anti-VEGF injection (Funk et al., 2009; Shchuko et al., 2015; Noma et al., 2016c; d; Matsushima et al., 2019) and parallel correlations between changes of aqueous VEGF concentration, visual acuity (VA), and optical coherence tomography (OCT) parameters after a single dose of intravitreal bevacizumab (IVB) (Noma et al., 2016c) or intravitreal ranibizumab (IVR) (Matsushima et al., 2019) injection were observed in RVO-ME patients.
Baseline intraocular VEGF levels may have value in predicting treatment response. Campochiaro et al. reported that baseline aqueous VEGF level was inversely correlated to VA improvement after 3 monthly IVR injections (Campochiaro et al., 2008). Similarly, Park, S.P. et al. detected higher baseline aqueous VEGF levels in patients who were unresponsive to a single IVB injection (Park and Ahn, 2009). However, in another study, Noma and others found that changes in aqueous VEGF after an IVB injection (1 month post-injection vs. baseline) were not associated with improvement of ME, although aqueous VEGF was suppressed to around the detection limit or lower in most patients (Noma et al., 2016d). The only study that measured aqueous VEGF at baseline and after corticosteroid treatment revealed that intravitreal dexamethasone implant (Ozurdex) has little effect on VEGF levels but causes a pan-suppression of aqueous inflammatory mediators (including interleukin (IL)-6, IL-8, monocyte chemoattractant protein -1 (MCP-1), soluble intercellular adhesion molecule-1 (sICAM-1), etc.) as well as angiopoietin (ANG)-2 levels (Rezar-Dreindl et al., 2017).
In other studies, baseline aqueous VEGF levels appeared less valuable than pro-inflammatory factors to predict ME recurrence. When Noma and others studied the correlation between aqueous factors and number of IVR injections needed to control ME recurrence during an observation period of 6 months, some pro-inflammatory cytokines (including IL-6, 8, etc.) but not VEGF were found to be correlated with the number of IVR injections (Noma et al., 2016a; Noma et al., 2021).
IL-6 is a key pro-inflammatory factor that can act on vascular endothelial cells and increase vascular permeability (Maruo et al., 1992; Alsaffar et al., 2018). Similar to VEGF, intraocular IL-6 was observed elevated in RVO eyes than normal controls (Campochiaro et al., 2009; Feng et al., 2013; Noma et al., 2014c; Jung et al., 2014; Sohn et al., 2014; Shchuko et al., 2015), and higher in major than macular BRVO (Lim, 2011; Noma et al., 2016b) and in ischemic than non-ischemic RVO (Noma et al., 2010b; Noma et al., 2010c; Funatsu et al., 2012; Koss et al., 2012; Noma et al., 2014a; Jung et al., 2014). It also positively correlated to central macular thickness (CMT) and/or nonperfusion area (NPA) (Noma et al., 2005; Noma et al., 2006; Noma et al., 2008; Noma et al., 2009, 2010b; Noma et al., 2010c; Kaneda et al., 2011; Noma et al., 2015), NVI (Chen et al., 1999; Ki et al., 2007), SRD (Noma et al., 2012b), and aqueous flare (Noma et al., 2013c; Noma et al., 2014b; Noma et al., 2017; Mashima et al., 2019). Indeed, significant correlations have been observed between VEGF and IL-6 in intraocular fluids of RVO (Noma et al., 2005; Noma et al., 2006; Ki et al., 2007; Noma et al., 2009; Noma et al., 2013c; Noma et al., 2015), and IL-6 was reported to be able to promote secretion of VEGF (Cohen et al., 1996; Noma et al., 2005).
Changes in intraocular IL-6 levels after intravitreal anti-VEGF treatment were conflicting. Funk et al. (2009) and Sohn, H.J. et al. (2014) reported that intraocular levels of cytokines and growth factors except VEGF were not significantly altered by IVB. However, Noma et al. showed a significant decrease in intraocular IL-6 level after the first IVB (Noma et al., 2016d), yet in another study of the same group, only borderline statistical significance was observed for change in IL-6 after 6 monthly IVB injections (Noma et al., 2016c). In studies with IVR, on the other hand, IL-6 levels were found to be significantly decreased after one dose of IVR (Mashima et al., 2019; Matsushima et al., 2019). The potential role of baseline IL-6 level for prediction of ME recurrence has been stated previously in the VEGF section, however, in another study by Campochiaro PA et al., no difference was observed in aqueous IL-6 levels between eyes with and without residual ME after two IVR injections (Campochiaro et al., 2009).
Decreases of intraocular IL-6 were also observed during intravitreal corticosteroid treatment, including intravitreal triamcinolone acetate (IVTA) (Sohn et al., 2014) and intravitreal dexamethasone implant (Querques et al., 2014; Rezar-Dreindl et al., 2017), and a decrease of aqueous IL-6 was found to be associated with improvement of ME (Rezar-Dreindl et al., 2017).
IL-8, also known as chemokine C-X-C motif ligand 8 (CXCL8), can be induced by injury and ischemia. It recruits neutrophils and other granulocytes and functions as a potent promoter of angiogenesis (Boisvert et al., 1998). IL-8 levels were found increased and positively correlated with the severity of ME and retinal ischemia in both BRVO (Noma et al., 2011d; Lee et al., 2012; Noma et al., 2014c) and CRVO (Noma et al., 2015), higher in CRVO than BRVO (Rezar-Dreindl et al., 2017), in major BRVO than macular BRVO (Lim, 2011; Noma et al., 2016b), and had a strong correlation with baseline aqueous flare value (Noma et al., 2017), NPA, CMT, as well as VA (Kaneda et al., 2011). Similar to IL-6, IL-8 was found to be able to stimulate the expression of VEGF in vascular endothelial cells (Martin et al., 2009). In addition to VEGF, correlations between intraocular IL-8 and MCP-1 levels were documented in different disease stages or scenarios, including at baseline (Noma et al., 2014c; 2015), during IVB treatment (Funk et al., 2009), post-treatment (Noma et al., 2016c), and in patients with insufficient efficacy (Shchuko et al., 2015).
Intraocular IL-8 level generally decrease along with absorption of ME in response to different treatment modalities including intravitreal anti-VEGF agents (Funk et al., 2009; Shchuko et al., 2015; Noma et al., 2016c; Noma et al., 2021), dexamethasone implant (Rezar-Dreindl et al., 2017) and vitrectomy (Okunuki et al., 2011). However, a single IVB, IVTA (Sohn et al., 2014; Noma et al., 2016d) or IVR (Matsushima et al., 2019) injection appeared insufficient to cause a statistically significant reduction of aqueous IL-8 level. Noma et al. revealed a significant reduction in aqueous IL-8 between the second and the third doses of IVB in both CRVO and BRVO eyes during a regimen of six monthly IVB injections, indicating a slow response of aqueous IL-8 downregulation (Noma et al., 2016c). Kotake et al. noted that two monthly injections of intravitreal aflibercept (IVA) significantly downregulated aqueous IL-8 but IVR did not, suggesting a stronger inhibitory effect of IVA than IVR on aqueous IL-8 (Kotake et al., 2019). Moreover, baseline IL-8 levels were found to be correlated with the number of IVR injections needed during a 6-month period with a “1 + PRN” regimen for BRVO (Noma et al., 2016a; Noma et al., 2021), which suggested the potential role of aqueous IL-8 as a predictor for ME recurrence.
MCP-1 is a chemotactic cytokine also known as chemokine C-C motif ligand 2 (CCL2). It plays a critical role in monocyte recruitment (Ajuebor et al., 1998; Schober and Zernecke, 2007), and may participate in microvascular endothelial injury (Ajuebor et al., 1998; Hodge et al., 2012), which leads to the breakdown of the inner blood-retinal barrier in pathologic conditions (Klaassen et al., 2013). MCP-1 level was observed higher in eyes affected by RVO than control (Noma et al., 2011d; Noma et al., 2013b; Jung et al., 2014; Noma et al., 2014c; 2015), CRVO than BRVO (Jung et al., 2014; Noma et al., 2015), ischemic than non-ischemic RVO (Noma et al., 2014a; Jung et al., 2014; Noma et al., 2015), and positively correlated to CMT (Noma et al., 2011d; Noma et al., 2013b; Noma et al., 2014a; Noma et al., 2014c; 2015), NPA (Kaneda et al., 2011; Noma et al., 2013b), SRD thickness (SRT) (Noma et al., 2014c; 2015) and aqueous flare value (Noma et al., 2014b; Noma et al., 2017; Mashima et al., 2019), although some studies failed to found a significant relevance (Kunikata et al., 2012; Sohn et al., 2014).
Research has revealed a complex interplay between MCP-1 and other cytokines, the most notable of which is its synergistic effect with VEGF. VEGF can bind to VEGF receptor (VEGFR)-2 and enhance the expression of MCP-1 (and IL-8, sICAM-1, etc.) through nuclear factor-kappa B (NF-kB) (Ledebur and Parks, 1995; Baldwin, 1996; Marumo et al., 1997), while MCP-1 can recruit eosinophils that have been identified as an important source of VEGF (Horiuchi and Weller, 1997). In addition, the correlations between MCP-1 and IL-6 and IL-8 have also been documented (Noma et al., 2011d; Noma et al., 2014c; 2015).
Intravitreal injection of anti-VEGF agents (Funk et al., 2009; Shchuko et al., 2015; Noma et al., 2016a; Noma et al., 2016c; d; Matsushima et al., 2019; Noma et al., 2021) and corticosteroids (Kunikata et al., 2012; Rezar-Dreindl et al., 2017) generally leads to a significant decrease in intraocular MCP-1. While a significant association was found between the changes of aqueous MCP-1 and VEGF during IVB treatment (Funk et al., 2009), no statistical correlations between the reduction of MCP-1 and improvement in vision or ME were found in studies using IVR (Matsushima et al., 2019) or IVB (Noma et al., 2016d). During IVR treatment, although higher intraocular levels of IL-8 and MCP-1 were detected in patients with insufficient efficacy (Shchuko et al., 2015), no statistical relationship was found between the baseline MCP-1 level and the number of injections needed in a follow-up period of 6 months (Noma et al., 2016a; Noma et al., 2021). These contradictory findings suggest that more studies are needed before MCP-1 can be considered as an ideal biomarker for treatment response prediction and/or disease monitoring during intravitreal anti-VEGF treatments.
Notably, in a study on intravitreal dexamethasone implants, statistically significant correlations between decreases of MCP- 1, sICAM-1, ANG-2, and improvement of ME were found in both BRVO and CRVO, and the rise of intraocular MCP-1 was detected earlier than the recurrence of ME, suggesting a potential role of MCP-1 in disease monitoring during intravitreal corticosteroid treatment (Rezar-Dreindl et al., 2017).
sICAM-1 is a circulating form of ICAM-1 and both of them have been reported to be involved in the inflammatory processes of many diseases (Witkowska and Borawska, 2004). sICAM-1 concentration in intraocular fluids were significantly elevated as compared to control (Noma et al., 2011a; Noma et al., 2011b; Noma et al., 2011d; 2012d; Noma et al., 2013b; Noma et al., 2014a; Noma et al., 2014b; Noma et al., 2014c; 2015; Noma et al., 2016b), and have been found associated with signs indicative disease severity, including degree of retinal vascular involvement (macular BRVO vs. major BRVO) (Noma et al., 2012d; Noma et al., 2016b), aqueous flare value (Noma et al., 2017; Mashima et al., 2019), degree of retinal ischemia (Noma et al., 2011a; Noma et al., 2011d; c; Noma et al., 2013c; Noma et al., 2014b; Noma et al., 2014c), CMT (Noma et al., 2013b; Noma et al., 2014c), and SRT (Noma et al., 2011d; Noma et al., 2014c).
Unlike MCP-1, intraocular sICAM-1 was not significantly suppressed by intravitreal anti-VEGF agents (Noma et al., 2016d; c; Mashima et al., 2019; Matsushima et al., 2019; Noma et al., 2021), and no significant correlations were found between changes in aqueous sICAM-1 level and improvements in visual acuity, ME (Noma et al., 2016d), or aqueous flare (Mashima et al., 2019). On the contrary, a significant decrease of aqueous sICAM-1 was observed after IVTA (Noma et al., 2013a).
Baseline sICAM-1 levels may have predictive value for disease recurrence. It was reported to be associated with aqueous flare values at first recurrence (Noma et al., 2017) as well as the number of IVR injections needed during a period of 6 months (Noma et al., 2016a; Noma et al., 2021).
IL-12 is a key pro-inflammatory cytokine that drives the induction of naive CD4+ T lymphocytes into Th1 cells and activation of other immune cells such as neutral killer cells (Trinchieri, 1995). IL-13 is an inducer of Th2-type cytokines and plays an important role in the pathogenesis of allergy, cancer, and tissue fibrosis (Karam et al., 2011; Van Dyken and Locksley, 2013). Intraocular IL-12 and IL-13 were generally reported to be elevated in RVO (Noma et al., 2011d; Kaneda et al., 2011; Noma et al., 2014c, 2015; Shchuko et al., 2015; Noma et al., 2016b), although in one study they were not significantly different between BRVO and cataract eyes (Lee et al., 2012), and another study showed that IL-12 was even significantly lower in CRVO than cataract eyes (Rezar-Dreindl et al., 2017).
Correlations between intraocular levels of IL-12 and IL-13 in RVO eyes have been observed (Noma et al., 2011d; Noma et al., 2014c; 2015). While they were demonstrated to be negatively correlated to retinal ischemia, CMT and SRT in several studies (Noma et al., 2011d; Noma et al., 2014c), they were reported to not be significantly correlated with aqueous flare value (Noma et al., 2017; Mashima et al., 2019) and were not higher in major BRVO than macular BRVO (Noma et al., 2016b).
In BRVO, intraocular IL-12 and IL-13 levels were reported to not have significantly changed after IVB (Noma et al., 2016d) or IVR injections (Noma et al., 2021). However, in a study that observed the kinetics of multiple cytokines during a regimen of 6 monthly IVB injections, aqueous IL-13 was significantly suppressed after 3 consecutive IVB injections in BRVO but remained unchanged during the regimen in CRVO; and aqueous IL-12 remained changed in BRVO but significantly increased after 3 IVB injections in CRVO (Noma et al., 2016c). They proposed a protective anti-inflammatory effect of IL-12 and a pathogenetic pro-inflammatory role of IL-13 in RVO. The different responses of aqueous IL-12 and IL-13 levels after repeated IVB injections between CRVO and BRVO could be explained by the different extent of ocular damage involved between these two RVO subtypes (Noma et al., 2016c).
It is uncertain whether intraocular IL-12 or IL-13 levels have predictive value for treatment response. Kaneda et al. (2011) revealed a significant association between IL-12 level and refractoriness to IVB in BRVO, and Shchuko et al. (2015) presented a higher level of IL-13 in RVO patients with insufficient response to IVR. However, Noma et al. found no correlation between intraocular IL-12 or IL-13 levels and the number of IVR injections needed during a 6-month follow up period with a 1 + PRN regimen (Noma et al., 2016a).
Soluble VEGF receptors (sVEGFR)-1 and sVEGFR-2 are soluble forms of VEGF receptors (Ebos et al., 2008). sVEGFR-1, a receptor for VEGF, VEGF-B and PlGF, is a pro-inflammatory factor (Clauss et al., 1996; Kiba et al., 2003; Murakami et al., 2006), and sVEGFR-2 was reported to have anti-angiogenic activity (Maynard et al., 2003; Jacobi et al., 2004; Ebos et al., 2008) and promote vascular maturation by mediating the interaction between endothelial cells and mural cells (Lorquet et al., 2010).
sVEGFR-1 appeared to be a promising candidate biomarker for RVO. Activation of sVEGFR-1 by its ligands leads to the production of pro-inflammatory and pro-angiogenic mediators by macrophages and microglia in the retina (Ziche et al., 1997; Crespo-Garcia et al., 2017; Uemura et al., 2021). sVEGFR-1 was reported higher in intraocular fluids of RVO than control (Noma et al., 2011d; Noma et al., 2014c; 2015; Noma et al., 2016b), higher in ischemic than non-ischemic CRVO (Noma et al., 2015), and significantly decreased in response to anti-VEGF treatments (Noma et al., 2016d; c; Matsushima et al., 2019; Noma et al., 2021). In addition, intraocular sVEGFR-1 level significantly correlated with flare value at both baselines and recurrences (Noma et al., 2017; Mashima et al., 2019), and correlated with the number of injections needed during a 6-month “1 + PRN” IVR regimen (Noma et al., 2016a; Noma et al., 2021).
The intraocular level of sVEGFR-2 was also reported to be elevated in RVO (Noma et al., 2011b; Noma et al., 2011d; Noma and Mimura, 2013; Noma et al., 2013b; Noma et al., 2014b; Noma et al., 2014c; Noma et al., 2016b), however, its relationship with disease severity and refractoriness was less conclusive. Intraocular sVEGFR-2 was observed to be correlated with ME and SRT (Noma et al., 2011d; Noma et al., 2013b; Noma et al., 2014b; Noma et al., 2014c), but not with aqueous flare value (Noma et al., 2017; Mashima et al., 2019) and ischemic status in CRVO (Noma et al., 2015). In addition, no significant difference between intraocular sVEGFR-2 levels at baseline and 1 month after IVB (Noma et al., 2016d; c) was observed, and the correlations between baseline aqueous sVEGFR-2 level and the number of IVR injections needed within 6 months of a “1 + PRN” IVR regimen were conflicting in two studies (Noma et al., 2016a; Noma et al., 2021).
Platelet-derived growth factor (PDGF) is a growth factor that regulates the migration of mesenchymal cells (Hossain et al., 1998; Mamer et al., 2017) and has been reported to have a role in ocular neovascularization induced by hypoxia (Benjamin et al., 1998; Clapp et al., 2009). Intraocular PDGF-AA (an isoform of PDGF) level was reported higher in RVO than control (Noma et al., 2011d; Lee et al., 2012; Noma et al., 2014c, 2015; Jung et al., 2014; Noma et al., 2016b), in CRVO than BRVO (Jung et al., 2014), in major BRVO than macular BRVO (Lim, 2011; Noma et al., 2016b), and positively correlated to retinal ischemia, ME, and SRT in BRVO (Noma et al., 2011d; Noma et al., 2014c). However, exceptional results were derived in some studies that showed intraocular PDGF-AA was not elevated in RVO as compared to normal control (Lim, 2011; Sohn et al., 2014; Rezar-Dreindl et al., 2017). PDGF-AA was significantly correlated to aqueous flare values at baseline, in one study (Mashima et al., 2019) but not in another (Noma et al., 2017).
The aqueous humor level of PDGF-AA decreased significantly over time after multiple IVB (Noma et al., 2016c) or IVR injections (Noma et al., 2021). Notably, baseline aqueous PDGF-AA level was associated with the number of IVR injections needed in a period of 6 months during a “1 + PRN” regimen (Noma et al., 2016a; Noma et al., 2021), suggesting the potential role of aqueous PDGF-AA in the prediction of ME recurrence.
In addition to the biomolecules mentioned above, many other molecules have also been studied in RVO (Table 3), among which the following were measured in more studies than others, and thus are discussed briefly below, including pigment epithelium-derived factor (PEDF), PlGF, tumor necrosis factor (TNF)-α, erythropoietin (EPO), pentraxin 3 (PTX3), and nitric oxide (NO).
PEDF is a cytokine that has anti-angiogenic properties (Rychli et al., 2009) and was observed to antagonize the effect of VEGF in retinal neovascularization (Mori et al., 2001; Duh et al., 2002). Intraocular PEDF level was lower in both BRVO and CRVO as compared to control and was observed negatively correlated to retinal thickness (Noma et al., 2010a).
PlGF belongs to one of the VEGF subfamilies and is also a pro-angiogenic factor (Shibuya, 2008). In RVO, aqueous PlGF was reported positively correlated CMT, aqueous flare value and severity of ischemia (Noma et al., 2011d; Noma et al., 2017).
TNF-α is a key inflammatory cytokine which has pleiotropic effects on various cells and plays an important role in inflammation, cell proliferation and apoptosis. It can increase the permeability of vascular endothelium (Chen and Goeddel, 2002) and may participate in the pathogenesis of ocular inflammation, edema, and neovascularization (Rodrigues et al., 2009). TNF-α was increased in the intraocular fluids of RVO with higher levels found in ischemic compared to non-ischemic RVO (Jung et al., 2014; Zeng et al., 2019).
EPO, a hormone produced in kidney and fetal liver that regulates erythropoiesis, has pleiotropic functions including antioxidant, angiogenic, and neuroprotective activities (Junk et al., 2002; Watanabe et al., 2005). Upregulation of vitreous EPO was observed in both BRVO and CRVO, with higher levels detected in more ischemic subjects (Inomata et al., 2004; Stahl et al., 2010; Shin et al., 2014). These findings are explained by the fact that EPO production is primarily stimulated by hypoxia (Inomata et al., 2004; Weidemann and Johnson, 2009).
PTX3, a member of the acute response protein family Pentraxin, is associated with vascular injury (Peri et al., 2000; Suzuki et al., 2008), and has been proposed as a prognostic biomarker of myocardial infarction and heart failure (Latini et al., 2004; Suzuki et al., 2008). Interestingly, intraocular PTX3 was reported significantly increased in RVO patients with more profound changes observed in ischemic subtype (Noma et al., 2013b; Noma et al., 2014a), and reduced in response to dexamethasone implant (Campochiaro et al., 2015).
NO is a free radical gas molecule synthesized by nitric oxide synthase (NOS). It has a vasodilatory effect, which increases blood flow and is beneficial for vascular occlusive conditions; however, it can be neurotoxic when generated in excess (Dawson et al., 1994; Donati et al., 1998; Sennlaub et al., 2002). Aqueous humor NO levels were found significantly higher in RVO than control (Fard and Dehpour, 2010; Fard et al., 2010), but further studies are needed to unravel its role in disease pathogenesis of RVO.
While evidence is accumulating that PEDF, PlGF, TNF-α, EPO, PTX3, and NO may be involved in the pathogenesis of RVO and may be associated with disease severity, their roles as predictive factors for treatment response are less well studied.
Previous studies have investigated a wide range of biomolecules in the aqueous or vitreous of RVO eyes with various clinical characteristics or at different clinical stages and have generally delineated the complex network of pathogenetic mechanisms. Impaired blood drainage and increased venous pressure following RVO result in ischemia/hypoxia and capillary leakage/damage, which upregulate pro-angiogenic factors (VEGF, PlGF, VEGFR, EPO, PDGF, ANG, etc.) and cause inflammation. Retinal edema develops as a result of reduced venous drainage and increased capillary leakage/permeability, which is augmented by a variety of pro-angiogenic and inflammatory mediators (Figure 1).
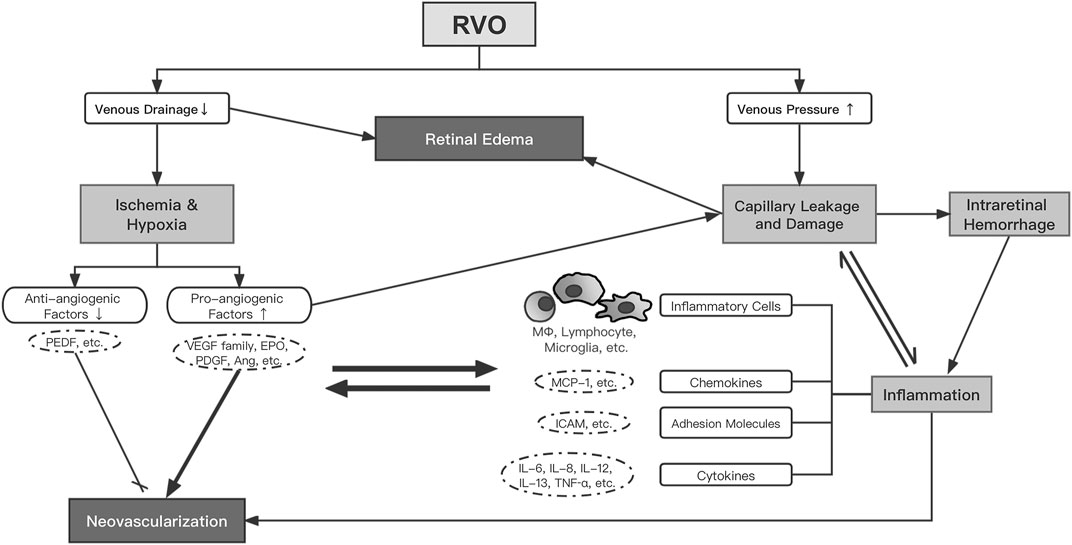
FIGURE 1. The proposed network of pathogenesis in RVO. Abbreviations: RVO, retinal vein occlusion; VEGF, vascular endothelial growth factor; EPO, erythropoietin; PDGF, platelet-derived growth factor; Ang, angiopoietin; PEDF, pigment epithelium-derived factor; MΦ, macrophage; ICAM, intercellular adhesion molecule; IL, interleukin; MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor.
Of note are the pleiotropy of the cytokines and the complex synergistic cross-talk among them. VEGF, the major pro-angiogenic factor, has strong stimulative effects on migration and proliferation of endothelial cells, but it also impedes pericyte recruitment by disrupting PDGF-related pathways via VEGF-R2-mediated signaling (Ferrara et al., 2003; Greenberg et al., 2008), which impairs vessel maturation and increases vascular leakage. VEGF also enhances vascular permeability by altering endothelial cell tight junctions (Vinores et al., 1999), in which NO-related signals may be involved (Lakshminarayanan et al., 2000). In addition, VEGF interacts with inflammatory mediators through NF-kB associated pathways, including IL-6, IL-8, IL-12, TNF-α, and MCP-1 (Kulms and Schwarz, 2006). These inflammatory mediators may affect vascular permeability through VEGF-dependent (Martin et al., 2009) and -independent mechanisms, such as IL-8, which can directly downregulate tight junctions (Yu et al., 2013). The sources of these cytokines are multiple, involving not only inflammatory cells but also a variety of ocular resident cells. For example, IL-6 was found to be secreted by Müller cells (Liu et al., 2015), endothelial cells (Akira et al., 1993) and retinal pigment epithelial (RPE) cells (Elner et al., 1992).
The synergism between VEGF and inflammation in RVO pathogenesis is also in line with the fact that both anti-VEGF treatments and corticosteroids are effective for the majority of RVO-ME patients. Anti-VEGF agents suppress intraocular VEGF to a sub-physiological level, resulting in a variety of anti-inflammatory effects, whereas intravitreal corticosteroids cause pan-suppression of inflammatory mediators by affecting VEGF-related downstream signals (Edelman et al., 2005; McAllister et al., 2009) and turning off activated inflammatory genes (Barnes, 2006). The varying results on intraocular biomolecule changes after intravitreal anti-VEGF or corticosteroid treatments among studies may be due to the complexity of disease pathogenesis, inter-individual heterogeneity of disease phenotype, differences in study design, and biomolecular analysis methods.
Current studies have demonstrated that intraocular levels of pro-angiogenic and inflammatory mediators can reflect disease phenotype, severity, treatment response, and/or refractoriness, providing the basis for using these molecules as candidates for companion diagnostic biomarkers. Based on the current review, a panel of biomolecules, which includes VEGF, IL-6, IL-8, MCP-1, sVEGFR-1, sICAM-1, and PDGF-AA, may be valuable to assess the involved mechanisms and/or disease severity at baseline; VEGF might be valuable to predict the response of the first intravitreal anti-VEGF treatment; IL-6, IL-8, MCP-1, sICAM-1, and PDGF-AA may provide valuable information on refractoriness or middle-term (6 months) requirements of anti-VEGF injections. On the other hand, however, there is a long way ahead to configure a preliminary panel of biomarkers for intravitreal corticosteroids as well as for anti-VEGF agents.
Aqueous humor appeared to be adequate as a source of intraocular biomarkers. In a meta-analysis involving 116 studies, authors revealed that significant differences in levels of most intraocular cytokines between RVO and control can be observed in both the vitreous and aqueous humor, including VEGF, IL-6, IL-8, and MCP-1 (Minaker et al., 2020). The current techniques of aqueous humor collection using small gauge syringes, however, are not safe and convenient enough to be performed routinely in an outpatient clinic. The novel disposable aqueous humor collector developed by our group, which facilitates one-handed anterior chamber paracentesis and accurate aqueous sampling, may overcome these limitations in the future (Qu et al., 2020). The most advanced multiplex immunoassay platforms, which allow simultaneous analysis of multiple biomarkers with a minimum sample volume requirement, have laid the technical basis for the development of aqueous humor-based companion diagnostics for ocular diseases.
Despite the significant advances achieved to date, more effort is needed to narrow down the range of possible biomarkers, to develop test kits specifically for particular companion diagnostic purposes, and to validate these kits in well-designed clinical trials. Ultimately, for widespread acceptance in the ophthalmic community and patients, a non-invasive assay of these biomarkers would be most useful.
CZ, YC and BW contributed to the conception and design of the study. CZ and BW searched the database and carefully reviewed all included publications. BW wrote the first draft of the manuscript. YC, XZ, HC and AK critically reviewed the manuscript and provided valuable revisions to the manuscript. All authors read and approved the submitted version.
This work was supported by the Non-profit Central Research Institute Fund of the Chinese Academy of Medical Sciences (2018PT32029).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Ajuebor, M. N., Flower, R. J., Hannon, R., Christie, M., Bowers, K., Verity, A., et al. (1998). Endogenous Monocyte Chemoattractant Protein-1 Recruits Monocytes in the Zymosan Peritonitis Model. J. Leukoc. Biol. 63 (1), 108–116. doi:10.1002/jlb.63.1.108
Akira, S., Taga, T., and Kishimoto, T. (1993). Interleukin-6 in Biology and Medicine. Adv. Immunol. 54, 1–78. doi:10.1016/s0065-2776(08)60532-5
Alsaffar, H., Martino, N., Garrett, J. P., and Adam, A. P. (2018). Interleukin-6 Promotes a Sustained Loss of Endothelial Barrier Function via Janus Kinase-Mediated STAT3 Phosphorylation and De Novo Protein Synthesis. Am. J. Physiol. Cel Physiol 314 (5), C589–C602. doi:10.1152/ajpcell.00235.2017
Baba, T., Bikbova, G., Kitahashi, M., Yokouchi, H., Oshitari, T., and Yamamoto, S. (2014). Level of Vascular Endothelial Growth Factor 165b in Human Aqueous Humor. Curr. Eye Res. 39 (8), 830–836. doi:10.3109/02713683.2013.877935
Baldwin, A. S. (1996). “THE NF-Κb and IκB PROTEINS: New Discoveries and Insights,” in Annual Review of Immunology. Editor W. E. Paul, 14, 649–681. doi:10.1146/annurev.immunol.14.1.649Annu. Rev. Immunol.
Barnes, P. J. (2006). How Corticosteroids Control Inflammation: Quintiles Prize Lecture 2005. Br. J. Pharmacol. 148 (3), 245–254. doi:10.1038/sj.bjp.0706736
Benjamin, L. E., Hemo, I., and Keshet, E. (1998). A Plasticity Window for Blood Vessel Remodelling Is Defined by Pericyte Coverage of the Preformed Endothelial Network and Is Regulated by PDGF-B and VEGF. Development 125 (9), 1591–1598. doi:10.1242/dev.125.9.1591
Bertelmann, T., Sekundo, W., Strodthoff, S., Witteborn, M. C., Stief, T., Irle, S., et al. (2014). Intravitreal Functional Plasminogen in Eyes with branch Retinal Vein Occlusion. Ophthalmic Res. 52 (2), 74–80. doi:10.1159/000362340
Boisvert, W. A., Santiago, R., Curtiss, L. K., and Terkeltaub, R. A. (1998). A Leukocyte Homologue of the IL-8 Receptor CXCR-2 Mediates the Accumulation of Macrophages in Atherosclerotic Lesions of LDL Receptor-Deficient Mice. J. Clin. Invest. 101 (2), 353–363. doi:10.1172/jci1195
Boyd, S. R., Zachary, I., Chakravarthy, U., Allen, G. J., Wisdom, G. B., Cree, I. A., et al. (2002). Correlation of Increased Vascular Endothelial Growth Factor with Neovascularization and Permeability in Ischemic central Vein Occlusion. Arch. Ophthalmol. 120 (12), 1644–1650. doi:10.1001/archopht.120.12.1644
Branch Vein Occlusion Study Group (1986). Argon Laser Scatter Photocoagulation for Prevention of Neovascularization and Vitreous Hemorrhage in branch Vein Occlusion. A Randomized Clinical Trial. Branch Vein Occlusion Study Group. Arch. Ophthalmol. 104 (1), 34–41. doi:10.1001/archopht.1986.01050130044017
Campochiaro, P. A., Choy, D. F., Do, D. V., Hafiz, G., Shah, S. M., Nguyen, Q. D., et al. (2009). Monitoring Ocular Drug Therapy by Analysis of Aqueous Samples. Ophthalmology 116 (11), 2158–2164. doi:10.1016/j.ophtha.2009.04.038
Campochiaro, P. A., Hafiz, G., Mir, T. A., Scott, A. W., Sophie, R., Shah, S. M., et al. (2015). Pro-Permeability Factors after Dexamethasone Implant in Retinal Vein Occlusion; the Ozurdex for Retinal Vein Occlusion (ORVO) Study. Am. J. Ophthalmol. 160 (2), 313–e19. e319. doi:10.1016/j.ajo.2015.04.025
Campochiaro, P. A., Hafiz, G., Shah, S. M., Nguyen, Q. D., Ying, H., Do, D. V., et al. (2008). Ranibizumab for Macular Edema Due to Retinal Vein Occlusions: Implication of VEGF as a Critical Stimulator. Mol. Ther. 16 (4), 791–799. doi:10.1038/mt.2008.10
Chan, C. K., Ip, M. S., Vanveldhuisen, P. C., Oden, N. L., Scott, I. U., Tolentino, M. J., et al. (2011). SCORE Study Report #11: Incidences of Neovascular Events in Eyes with Retinal Vein Occlusion. Ophthalmology 118 (7), 1364–1372. doi:10.1016/j.ophtha.2010.11.020
Chen, G., and Goeddel, D. V. (2002). TNF-R1 Signaling: A Beautiful Pathway. Science 296 (5573), 1634–1635. doi:10.1126/science.1071924
Chen, K. H., Wu, C. C., Roy, S., Lee, S. M., and Liu, J. H. (1999). Increased Interleukin-6 in Aqueous Humor of Neovascular Glaucoma. Invest. Ophthalmol. Vis. Sci. 40 (11), 2627–2632.
Christoffersen, N. L., and Larsen, M. (1999). Pathophysiology and Hemodynamics of branch Retinal Vein Occlusion. Ophthalmology 106 (11), 2054–2062. doi:10.1016/s0161-6420(99)90483-9
Clapp, C., Thebault, S., Jeziorski, M. C., and Martínez De La Escalera, G. (2009). Peptide Hormone Regulation of Angiogenesis. Physiol. Rev. 89 (4), 1177–1215. doi:10.1152/physrev.00024.2009
Clauss, M., Weich, H., Breier, G., Knies, U., Röckl, W., Waltenberger, J., et al. (1996). The Vascular Endothelial Growth Factor Receptor Flt-1 Mediates Biological Activities. Implications for a Functional Role of Placenta Growth Factor in Monocyte Activation and Chemotaxis. J. Biol. Chem. 271 (30), 17629–17634. doi:10.1074/jbc.271.30.17629
Cohen, T., Nahari, D., Cerem, L. W., Neufeld, G., and Levi, B. Z. (1996). Interleukin 6 Induces the Expression of Vascular Endothelial Growth Factor. J. Biol. Chem. 271 (2), 736–741. doi:10.1074/jbc.271.2.736
Crespo-Garcia, S., Corkhill, C., Roubeix, C., Davids, A. M., Kociok, N., Strauss, O., et al. (2017). Inhibition of Placenta Growth Factor Reduces Subretinal Mononuclear Phagocyte Accumulation in Choroidal Neovascularization. Invest. Ophthalmol. Vis. Sci. 58 (12), 4997–5006. doi:10.1167/iovs.16-21283
Dacheva, I., Ullmer, C., Ceglowska, K., Nogoceke, E., Hartmann, G., Müller, S., et al. (2016). Lysophosphatidic Acids and Autotaxin in Retinal Vein Occlusion. Retina 36 (12), 2311–2318. (Philadelphia, Pa.). doi:10.1097/IAE.0000000000001112
Dawson, V. L., Brahmbhatt, H. P., Mong, J. A., and Dawson, T. M. (1994). Expression of Inducible Nitric Oxide Synthase Causes Delayed Neurotoxicity in Primary Mixed Neuronal-Glial Cortical Cultures. Neuropharmacology 33 (11), 1425–1430. doi:10.1016/0028-3908(94)90045-0
Donati, G., Pournaras, C. J., and Tsacopoulos, M. (1998). Effect of Nitroprusside on Arteriolar Constriction after Retinal branch Vein Occlusion. Invest. Ophthalmol. Vis. Sci. 39 (10), 1910–1917.
Duh, E. J., Yang, H. S., Suzuma, I., Miyagi, M., Youngman, E., Mori, K., et al. (2002). Pigment Epithelium-Derived Factor Suppresses Ischemia-Induced Retinal Neovascularization and VEGF-Induced Migration and Growth. Invest. Ophthalmol. Vis. Sci. 43 (3), 821–829.
Ebos, J. M., Lee, C. R., Bogdanovic, E., Alami, J., Van Slyke, P., Francia, G., et al. (2008). Vascular Endothelial Growth Factor-Mediated Decrease in Plasma Soluble Vascular Endothelial Growth Factor Receptor-2 Levels as a Surrogate Biomarker for Tumor Growth. Cancer Res. 68 (2), 521–529. doi:10.1158/0008-5472.Can-07-3217
Edelman, J. L., Lutz, D., and Castro, M. R. (2005). Corticosteroids Inhibit VEGF-Induced Vascular Leakage in a Rabbit Model of Blood-Retinal and Blood-Aqueous Barrier Breakdown. Exp. Eye Res. 80 (2), 249–258. doi:10.1016/j.exer.2004.09.013
Ehlken, C., Rennel, E. S., Michels, D., Grundel, B., Pielen, A., Junker, B., et al. (2011). Levels of VEGF but Not VEGF(165b) Are Increased in the Vitreous of Patients with Retinal Vein Occlusion. Am. J. Ophthalmol. 152 (2), 298–e1. e291. doi:10.1016/j.ajo.2011.01.040
Elner, V. M., Scales, W., Elner, S. G., Danforth, J., Kunkel, S. L., and Strieter, R. M. (1992). Interleukin-6 (IL-6) Gene Expression and Secretion by Cytokine-Stimulated Human Retinal Pigment Epithelial Cells. Exp. Eye Res. 54 (3), 361–368. doi:10.1016/0014-4835(92)90048-w
Fard, M. A., and Dehpour, A. R. (2010). Aqueous Humor Nitric Oxide Levels in Patients with branch Retinal Vein Occlusion. Jpn. J. Ophthalmol. 54 (1), 107–109. doi:10.1007/s10384-009-0758-4
Fard, M. A., Lashey, A., and Dehpour, A. R. (2010). Aqueous Humor Nitric Oxide in Patients with central Retinal Vein Occlusion. Nitric Oxide 23 (4), 332–334. doi:10.1016/j.niox.2010.09.006
Feng, J., Zhao, T., Zhang, Y., Ma, Y., and Jiang, Y. (2013). Differences in Aqueous Concentrations of Cytokines in Macular Edema Secondary to branch and central Retinal Vein Occlusion. PloS one 8 (7), e68149. doi:10.1371/journal.pone.0068149
Ferrara, N., Gerber, H. P., and LeCouter, J. (2003). The Biology of VEGF and its Receptors. Nat. Med. 9 (6), 669–676. doi:10.1038/nm0603-669
Fonollosa, A., Garcia-Arumi, J., Santos, E., Macia, C., Fernandez, P., Segura, R. M., et al. (2010). Vitreous Levels of Interleukine-8 and Monocyte Chemoattractant Protein-1 in Macular Oedema with branch Retinal Vein Occlusion. Eye (Lond) 24 (7), 1284–1290. doi:10.1038/eye.2009.340
Funatsu, H., Noma, H., Mimura, T., and Eguchi, S. (2012). Vitreous Inflammatory Factors and Macular Oedema. Br. J. Ophthalmol. 96 (2), 302–304. doi:10.1136/bjo.2010.181222
Funk, M., Kriechbaum, K., Prager, F., Benesch, T., Georgopoulos, M., Zlabinger, G. J., et al. (2009). Intraocular Concentrations of Growth Factors and Cytokines in Retinal Vein Occlusion and the Effect of Therapy with Bevacizumab. Invest. Ophthalmol. Vis. Sci. 50 (3), 1025–1032. doi:10.1167/iovs.08-2510
Greenberg, J. I., Shields, D. J., Barillas, S. G., Acevedo, L. M., Murphy, E., Huang, J., et al. (2008). A Role for VEGF as a Negative Regulator of Pericyte Function and Vessel Maturation. Nature 456 (7223), 809–813. doi:10.1038/nature07424
Hodge, D. L., Reynolds, D., Cerbán, F. M., Correa, S. G., Baez, N. S., Young, H. A., et al. (2012). MCP-1/CCR2 Interactions Direct Migration of Peripheral B and T Lymphocytes to the Thymus during Acute Infectious/inflammatory Processes. Eur. J. Immunol. 42 (10), 2644–2654. doi:10.1002/eji.201242408
Horiuchi, T., and Weller, P. F. (1997). Expression of Vascular Endothelial Growth Factor by Human Eosinophils: Upregulation by Granulocyte Macrophage colony-stimulating Factor and Interleukin-5. Am. J. Respir. Cel Mol Biol 17 (1), 70–77. doi:10.1165/ajrcmb.17.1.2796
Hossain, M. Z., Ao, P., and Boynton, A. L. (1998). Rapid Disruption of gap Junctional Communication and Phosphorylation of Connexin43 by Platelet-Derived Growth Factor in T51B Rat Liver Epithelial Cells Expressing Platelet-Derived Growth Factor Receptor. J. Cel Physiol 174 (1), 66–77. doi:10.1002/(SICI)1097-4652(199801)174:1<66::AID-JCP8>3.0.CO;2-E
Inomata, Y., Hirata, A., Takahashi, E., Kawaji, T., Fukushima, M., and Tanihara, H. (2004). Elevated Erythropoietin in Vitreous with Ischemic Retinal Diseases. Neuroreport 15 (5), 877–879. doi:10.1097/00001756-200404090-00029
Jacobi, J., Tam, B. Y., Wu, G., Hoffman, J., Cooke, J. P., and Kuo, C. J. (2004). Adenoviral Gene Transfer with Soluble Vascular Endothelial Growth Factor Receptors Impairs Angiogenesis and Perfusion in a Murine Model of Hindlimb Ischemia. Circulation 110 (16), 2424–2429. doi:10.1161/01.Cir.0000145142.85645.Ea
Jung, S. H., Kim, K. A., Sohn, S. W., and Yang, S. J. (2014). Association of Aqueous Humor Cytokines with the Development of Retinal Ischemia and Recurrent Macular Edema in Retinal Vein Occlusion. Invest. Ophthalmol. Vis. Sci. 55 (4), 2290–2296. doi:10.1167/iovs.13-13587
Junk, A. K., Mammis, A., Savitz, S. I., Singh, M., Roth, S., Malhotra, S., et al. (2002). Erythropoietin Administration Protects Retinal Neurons from Acute Ischemia-Reperfusion Injury. Proc. Natl. Acad. Sci. U S A. 99 (16), 10659–10664. doi:10.1073/pnas.152321399
Kaneda, S., Miyazaki, D., Sasaki, S., Yakura, K., Terasaka, Y., Miyake, K., et al. (2011). Multivariate Analyses of Inflammatory Cytokines in Eyes with branch Retinal Vein Occlusion: Relationships to Bevacizumab Treatment. Invest. Ophthalmol. Vis. Sci. 52 (6), 2982–2988. doi:10.1167/iovs.10-6299
Karam, M. C., Al-Kouba, J. E., Bazzi, S. I., Smith, C. B., and Leung, L. (2011). Interleukin-13 Reduces Hyperalgesia and the Level of Interleukin-1β in BALB/c Mice Infected with Leishmania Major with an Up-Regulation of Interleukin-6. J. Neuroimmunol 234 (1-2), 49–54. doi:10.1016/j.jneuroim.2011.02.003
Keck, P. J., Hauser, S. D., Krivi, G., Sanzo, K., Warren, T., Feder, J., et al. (1989). Vascular Permeability Factor, an Endothelial Cell Mitogen Related to PDGF. Science 246 (4935), 1309–1312. doi:10.1126/science.2479987
Ki-I, Y., Arimura, N., Noda, Y., Yamakiri, K., Doi, N., Hashiguchi, T., et al. (2007). Stromal-derived Factor-1 and Inflammatory Cytokines in Retinal Vein Occlusion. Curr. Eye Res. 32 (12), 1065–1072. doi:10.1080/02713680701758727
Kiba, A., Sagara, H., Hara, T., and Shibuya, M. (2003). VEGFR-2-specific Ligand VEGF-E Induces Non-edematous Hyper-Vascularization in Mice. Biochem. Biophys. Res. Commun. 301 (2), 371–377. doi:10.1016/s0006-291x(02)03033-4
Kim, J. H., Shin, J. P., Kim, I. T., and Park, D. H. (2016). Aqueous Angiopoietin-like 4 Levels Correlate with Nonperfusion Area and Macular Edema in Branch Retinal Vein Occlusion. Invest. Ophthalmol. Vis. Sci. 57 (1), 6–11. doi:10.1167/iovs.15-18304
Klaassen, I., Van Noorden, C. J., and Schlingemann, R. O. (2013). Molecular Basis of the Inner Blood-Retinal Barrier and its Breakdown in Diabetic Macular Edema and Other Pathological Conditions. Prog. Retin. Eye Res. 34, 19–48. doi:10.1016/j.preteyeres.2013.02.001
Klein, R., Moss, S. E., Meuer, S. M., and Klein, B. E. (2008). The 15-year Cumulative Incidence of Retinal Vein Occlusion: the Beaver Dam Eye Study. Arch. Ophthalmol. 126 (4), 513–518. doi:10.1001/archopht.126.4.513
Koss, M. J., Pfister, M., Rothweiler, F., Michaelis, M., Cinatl, J., Schubert, R., et al. (2012). Comparison of Cytokine Levels from Undiluted Vitreous of Untreated Patients with Retinal Vein Occlusion. Acta Ophthalmol. 90 (2), e98–e103. doi:10.1111/j.1755-3768.2011.02292.x
Kotake, O., Noma, H., Yasuda, K., Motohashi, R., Goto, H., and Shimura, M. (2019). Comparing Cytokine Kinetics between Ranibizumab and Aflibercept in Central Retinal Vein Occlusion with Macular Edema. Ophthalmic Res. 61 (4), 210–217. doi:10.1159/000488494
Kulms, D., and Schwarz, T. (2006). NF-kappaB and Cytokines. Vitam Horm. 74, 283–300. doi:10.1016/s0083-6729(06)74011-0
Kunikata, H., Shimura, M., Nakazawa, T., Sonoda, K. H., Yoshimura, T., Ishibashi, T., et al. (2012). Chemokines in Aqueous Humour before and after Intravitreal Triamcinolone Acetonide in Eyes with Macular Oedema Associated with branch Retinal Vein Occlusion. Acta Ophthalmol. 90 (2), 162–167. doi:10.1111/j.1755-3768.2010.01892.x
Lakshminarayanan, S., Antonetti, D. A., Gardner, T. W., and Tarbell, J. M. (2000). Effect of VEGF on Retinal Microvascular Endothelial Hydraulic Conductivity: The Role of NO. Invest. Ophthalmol. Vis. Sci. 41 (13), 4256–4261.
Latini, R., Maggioni, A. P., Peri, G., Gonzini, L., Lucci, D., Mocarelli, P., et al. (2004). Prognostic Significance of the Long Pentraxin PTX3 in Acute Myocardial Infarction. Circulation 110 (16), 2349–2354. doi:10.1161/01.Cir.0000145167.30987.2e
Ledebur, H. C., and Parks, T. P. (1995). Transcriptional Regulation of the Intercellular Adhesion Molecule-1 Gene by Inflammatory Cytokines in Human Endothelial Cells. Essential Roles of a Variant NF-Kappa B Site and P65 Homodimers. J. Biol. Chem. 270 (2), 933–943. doi:10.1074/jbc.270.2.933
Lee, W. J., Kang, M. H., Seong, M., and Cho, H. Y. (2012). Comparison of Aqueous Concentrations of Angiogenic and Inflammatory Cytokines in Diabetic Macular Oedema and Macular Oedema Due to branch Retinal Vein Occlusion. Br. J. Ophthalmol. 96 (11), 1426–1430. doi:10.1136/bjophthalmol-2012-301913
Lim, J. W. (2011). Intravitreal Bevacizumab and Cytokine Levels in Major and Macular branch Retinal Vein Occlusion. Ophthalmologica 225 (3), 150–154. doi:10.1159/000322364
Liu, X., Ye, F., Xiong, H., Hu, D. N., Limb, G. A., Xie, T., et al. (2015). IL-1β Induces IL-6 Production in Retinal Müller Cells Predominantly through the Activation of P38 MAPK/NF-κB Signaling Pathway. Exp. Cel Res 331 (1), 223–231. doi:10.1016/j.yexcr.2014.08.040
Lorquet, S., Berndt, S., Blacher, S., Gengoux, E., Peulen, O., Maquoi, E., et al. (2010). Soluble Forms of VEGF Receptor-1 and -2 Promote Vascular Maturation via Mural Cell Recruitment. FASEB J. 24 (10), 3782–3795. doi:10.1096/fj.09-149070
Machalinska, A., Mozolewska-Piotrowska, K., Czepita, M., Spoz, W., Dzieciolowska, M., Kubasik-Kladna, K., et al. (2016). Aqueous Levels of VEGF Correlate with Retinal Non-perfusion Areas in Patients with Diabetic Macular Edema and Macular Edema Secondary to central Retinal Vein Occlusion. Klin Oczna 117 (4), 225–229.
Mamer, S. B., Chen, S., Weddell, J. C., Palasz, A., Wittenkeller, A., Kumar, M., et al. (2017). Discovery of High-Affinity PDGF-VEGFR Interactions: Redefining RTK Dynamics. Sci. Rep. 7, 16439. doi:10.1038/s41598-017-16610-z
Martin, D., Galisteo, R., and Gutkind, J. S. (2009). CXCL8/IL8 Stimulates Vascular Endothelial Growth Factor (VEGF) Expression and the Autocrine Activation of VEGFR2 in Endothelial Cells by Activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) Complex. J. Biol. Chem. 284 (10), 6038–6042. doi:10.1074/jbc.C800207200
Marumo, T., Schini-Kerth, V. B., Fisslthaler, B., and Busse, R. (1997). Platelet-derived Growth Factor-Stimulated Superoxide Anion Production Modulates Activation of Transcription Factor NF-kappaB and Expression of Monocyte Chemoattractant Protein 1 in Human Aortic Smooth Muscle Cells. Circulation 96 (7), 2361–2367. doi:10.1161/01.cir.96.7.2361
Maruo, N., Morita, I., Shirao, M., and Murota, S. (1992). IL-6 Increases Endothelial Permeability In Vitro. Endocrinology 131 (2), 710–714. doi:10.1210/endo.131.2.1639018
Mashima, A., Noma, H., Yasuda, K., Goto, H., and Shimura, M. (2019). Anti-vascular Endothelial Growth Factor Agent Reduces Inflammation in Macular Edema with central Retinal Vein Occlusion. J. Inflamm. (Lond) 16, 9. doi:10.1186/s12950-019-0214-2
Matsushima, R., Noma, H., Yasuda, K., Goto, H., and Shimura, M. (2019). Role of Cytokines in Ranibizumab Therapy for Macular Edema in Patients with Central Retinal Vein Occlusion. J. Ocul. Pharmacol. Ther. 35 (7), 407–412. doi:10.1089/jop.2019.0011
Maynard, S. E., Min, J. Y., Merchan, J., Lim, K. H., Li, J., Mondal, S., et al. (2003). Excess Placental Soluble Fms-like Tyrosine Kinase 1 (sFlt1) May Contribute to Endothelial Dysfunction, Hypertension, and Proteinuria in Preeclampsia. J. Clin. Invest. 111 (5), 649–658. doi:10.1172/JCI17189
McAllister, I. L., Vijayasekaran, S., Chen, S. D., and Yu, D. Y. (2009). Effect of Triamcinolone Acetonide on Vascular Endothelial Growth Factor and Occludin Levels in branch Retinal Vein Occlusion. Am. J. Ophthalmol. 147 (5), 838–846. doi:10.1016/j.ajo.2008.12.006
Minaker, S. A., Mason, R. H., Bamakrid, M., Lee, Y., and Muni, R. H. (2020). Changes in Aqueous and Vitreous Inflammatory Cytokine Levels in Retinal Vein Occlusion: A Systematic Review and Meta-Analysis. Los Angeles, CA: SAGE Publications.
Modi, A., Sharma, K., Sudhakar, N. P., and Yadav, N. K. (2021). Aqueous Humor Cytokines and Therapeutic Customization in Nonresponding Macular Edema Secondary to Retinal Vein Occlusion. Retin. Cases Brief. Rep. 15 (2), 127–130. doi:10.1097/icb.0000000000000768
Mori, K., Duh, E., Gehlbach, P., Ando, A., Takahashi, K., Pearlman, J., et al. (2001). Pigment Epithelium-Derived Factor Inhibits Retinal and Choroidal Neovascularization. J. Cel Physiol 188 (2), 253–263. doi:10.1002/jcp.1114
Murakami, M., Iwai, S., Hiratsuka, S., Yamauchi, M., Nakamura, K., Iwakura, Y., et al. (2006). Signaling of Vascular Endothelial Growth Factor Receptor-1 Tyrosine Kinase Promotes Rheumatoid Arthritis through Activation of Monocytes/macrophages. Blood 108 (6), 1849–1856. doi:10.1182/blood-2006-04-016030
Noma, H., Funatsu, H., Harino, S., Mimura, T., Eguchi, S., and Hori, S. (2011a). Vitreous Inflammatory Factors in Macular Edema with central Retinal Vein Occlusion. Jpn. J. Ophthalmol. 55 (3), 248–255. doi:10.1007/s10384-011-0016-4
Noma, H., Funatsu, H., and Mimura, T. (2013a). Changes of Inflammatory Factors after Intravitreal Triamcinolone Acetonide for Macular Edema with central Retinal Vein Occlusion. J. Ocul. Pharmacol. Ther. 29 (3), 363–365. doi:10.1089/jop.2011.0222
Noma, H., Funatsu, H., Mimura, T., Eguchi, S., and Hori, S. (2011b). Soluble Vascular Endothelial Growth Factor Receptor-2 and Inflammatory Factors in Macular Edema with branch Retinal Vein Occlusion. Am. J. Ophthalmol. 152 (4), 669–e1. e661. doi:10.1016/j.ajo.2011.04.006
Noma, H., Funatsu, H., Mimura, T., Eguchi, S., Shimada, K., and Hori, S. (2011e). Vitreous Levels of Pigment Epithelium-Derived Factor and Vascular Endothelial Growth Factor in Macular Edema with central Retinal Vein Occlusion. Curr. Eye Res. 36 (3), 256–263. doi:10.3109/02713683.2010.513090
Noma, H., Funatsu, H., Mimura, T., Eguchi, S., and Shimada, K. (2012d). Inflammatory Factors in Major and Macular branch Retinal Vein Occlusion. Ophthalmologica 227 (3), 146–152. doi:10.1159/000335047
Noma, H., Funatsu, H., Mimura, T., Eguchi, S., and Shimada, K. (2011c). Influence of Vitreous Factors after Vitrectomy for Macular Edema in Patients with central Retinal Vein Occlusion. Int. Ophthalmol. 31 (5), 393–402. doi:10.1007/s10792-011-9480-6
Noma, H., Funatsu, H., Mimura, T., Eguchi, S., and Shimada, K. (2011d). Role of Soluble Vascular Endothelial Growth Factor Receptor-2 in Macular Oedema with central Retinal Vein Occlusion. Br. J. Ophthalmol. 95 (6), 788–792. doi:10.1136/bjo.2010.192468
Noma, H., Funatsu, H., Mimura, T., and Eguchi, S. (2012c). Vascular Endothelial Growth Factor Receptor-2 in Macular Oedema with Retinal Vein Occlusion. Ophthalmic Res. 48 (1), 56–58. doi:10.1159/000336020
Noma, H., Funatsu, H., Mimura, T., Harino, S., Eguchi, S., and Hori, S. (2010a). Pigment Epithelium-Derived Factor and Vascular Endothelial Growth Factor in branch Retinal Vein Occlusion with Macular Edema. Graefes Arch. Clin. Exp. Ophthalmol. 248 (11), 1559–1565. doi:10.1007/s00417-010-1486-7
Noma, H., Funatsu, H., Mimura, T., Harino, S., and Hori, S. (2010b). Aqueous Humor Levels of Vasoactive Molecules Correlate with Vitreous Levels and Macular Edema in central Retinal Vein Occlusion. Eur. J. Ophthalmol. 20 (2), 402–409. doi:10.1177/112067211002000222
Noma, H., Funatsu, H., Mimura, T., Harino, S., and Hori, S. (2009). Vitreous Levels of Interleukin-6 and Vascular Endothelial Growth Factor in Macular Edema with central Retinal Vein Occlusion. Ophthalmology 116 (1), 87–93. doi:10.1016/j.ophtha.2008.09.034
Noma, H., Funatsu, H., Mimura, T., Harino, S., Sone, T., and Hori, S. (2010c). Increase of Vascular Endothelial Growth Factor and Interleukin-6 in the Aqueous Humour of Patients with Macular Oedema and central Retinal Vein Occlusion. Acta Ophthalmol. 88 (6), 646–651. doi:10.1111/j.1755-3768.2009.01524.x
Noma, H., Funatsu, H., and Mimura, T. (2012a). Pigment Epithelium-Derived Factor Is Related to Macular Microcirculation in Patients with Macular Edema and branch Retinal Vein Occlusion. Int. Ophthalmol. 32 (5), 485–489. doi:10.1007/s10792-012-9584-7
Noma, H., Funatsu, H., Mimura, T., Tatsugawa, M., Shimada, K., and Eguchi, S. (2012e). Vitreous Inflammatory Factors and Serous Macular Detachment in branch Retinal Vein Occlusion. Retina 32 (1), 86–91. (Philadelphia, Pa.). doi:10.1097/IAE.0b013e31821801de
Noma, H., Funatsu, H., and Mimura, T. (2012b). Vascular Endothelial Growth Factor and Interleukin-6 Are Correlated with Serous Retinal Detachment in central Retinal Vein Occlusion. Curr. Eye Res. 37 (1), 62–67. doi:10.3109/02713683.2011.614370
Noma, H., Funatsu, H., Sakata, K., Mimura, T., and Hori, S. (2010d). Association between Macular Microcirculation and Soluble Intercellular Adhesion Molecule-1 in Patients with Macular Edema and Retinal Vein Occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 248 (10), 1515–1518. doi:10.1007/s00417-010-1350-9
Noma, H., Funatsu, H., Yamasaki, M., Tsukamoto, H., Mimura, T., Sone, T., et al. (2008). Aqueous Humour Levels of Cytokines Are Correlated to Vitreous Levels and Severity of Macular Oedema in branch Retinal Vein Occlusion. Eye (Lond) 22 (1), 42–48. doi:10.1038/sj.eye.6702498
Noma, H., Funatsu, H., Yamasaki, M., Tsukamoto, H., Mimura, T., Sone, T., et al. (2005). Pathogenesis of Macular Edema with branch Retinal Vein Occlusion and Intraocular Levels of Vascular Endothelial Growth Factor and Interleukin-6. Am. J. Ophthalmol. 140 (2), 256–261. doi:10.1016/j.ajo.2005.03.003
Noma, H., and Mimura, T. (2013). Aqueous Soluble Vascular Endothelial Growth Factor Receptor-2 in Macular Edema with branch Retinal Vein Occlusion. Curr. Eye Res. 38 (12), 1288–1290. doi:10.3109/02713683.2013.821135
Noma, H., Mimura, T., and Eguchi, S. (2013b). Association of Inflammatory Factors with Macular Edema in branch Retinal Vein Occlusion. JAMA Ophthalmol. 131 (2), 160–165. doi:10.1001/2013.jamaophthalmol.228
Noma, H., Mimura, T., Masahara, H., and Shimada, K. (2014a). Pentraxin 3 and Other Inflammatory Factors in central Retinal Vein Occlusion and Macular Edema. Retina 34 (2), 352–359. (Philadelphia, Pa.). doi:10.1097/IAE.0b013e3182993d74
Noma, H., Mimura, T., and Shimada, K. (2014b). Role of Inflammation in Previously Untreated Macular Edema with branch Retinal Vein Occlusion. BMC Ophthalmol. 14, 67. doi:10.1186/1471-2415-14-67
Noma, H., Mimura, T., Tatsugawa, M., and Shimada, K. (2013c). Aqueous Flare and Inflammatory Factors in Macular Edema with central Retinal Vein Occlusion: a Case Series. BMC Ophthalmol. 13, 78. doi:10.1186/1471-2415-13-78
Noma, H., Mimura, T., Yasuda, K., Nakagawa, H., Motohashi, R., Kotake, O., et al. (2016a). Cytokines and Recurrence of Macular Edema after Intravitreal Ranibizumab in Patients with Branch Retinal Vein Occlusion. Ophthalmologica 236 (4), 228–234. doi:10.1159/000451062
Noma, H., Mimura, T., Yasuda, K., Nakagawa, H., Motohashi, R., Kotake, O., et al. (2016b). Intravitreal Ranibizumab and Aqueous Humor Factors/Cytokines in Major and Macular Branch Retinal Vein Occlusion. Ophthalmologica 235 (4), 203–207. doi:10.1159/000444923
Noma, H., Mimura, T., Yasuda, K., and Shimura, M. (2016c). Cytokine Kinetics after Monthly Intravitreal Bevacizumab for Retinal Vein Occlusion Associated with Macular Oedema. Ophthalmic Res. 56 (4), 207–214. doi:10.1159/000445211
Noma, H., Mimura, T., Yasuda, K., and Shimura, M. (2017). Functional-morphological Parameters, Aqueous Flare and Cytokines in Macular Oedema with branch Retinal Vein Occlusion after Ranibizumab. Br. J. Ophthalmol. 101 (2), 180–185. doi:10.1136/bjophthalmol-2015-307989
Noma, H., Mimura, T., Yasuda, K., and Shimura, M. (2016d). Possible Molecular Basis of Bevacizumab Therapy for Macular Edema in Branch Retinal Vein Occlusion. Retina 36 (9), 1718–1725. (Philadelphia, Pa.). doi:10.1097/IAE.0000000000000983
Noma, H., Mimura, T., Yasuda, K., and Shimura, M. (2015). Role of Soluble Vascular Endothelial Growth Factor Receptor Signaling and Other Factors or Cytokines in central Retinal Vein Occlusion with Macular Edema. Invest. Ophthalmol. Vis. Sci. 56 (2), 1122–1128. doi:10.1167/iovs.14-15789
Noma, H., Mimura, T., Yasuda, K., and Shimura, M. (2014c). Role of Soluble Vascular Endothelial Growth Factor Receptors-1 and -2, Their Ligands, and Other Factors in branch Retinal Vein Occlusion with Macular Edema. Invest. Ophthalmol. Vis. Sci. 55 (6), 3878–3885. doi:10.1167/iovs.14-13961
Noma, H., Minamoto, A., Funatsu, H., Tsukamoto, H., Nakano, K., Yamashita, H., et al. (2006). Intravitreal Levels of Vascular Endothelial Growth Factor and Interleukin-6 Are Correlated with Macular Edema in branch Retinal Vein Occlusion. Graefes Arch. Clin. Exp. Ophthalmol. 244 (3), 309–315. doi:10.1007/s00417-004-1087-4
Noma, H., Yasuda, K., and Shimura, M. (2021). Change of Cytokines after Intravitreal Ranibizumab in Patients with Recurrent branch Retinal Vein Occlusion and Macular Edema. Eur. J. Ophthalmol. 31 (1), 204–210. doi:10.1177/1120672119885054
Noma, H., Yasuda, K., and Shimura, M. (2020). Cytokines and Pathogenesis of Central Retinal Vein Occlusion. J. Clin. Med. 9 (11), 3457. doi:10.3390/jcm9113457
Noma, H., Yasuda, K., and Shimura, M. (2019). Cytokines and the Pathogenesis of Macular Edema in Branch Retinal Vein Occlusion. J. Ophthalmol. 2019, 5185128. doi:10.1155/2019/5185128
Okunuki, Y., Usui, Y., Katai, N., Kezuka, T., Takeuchi, M., Goto, H., et al. (2011). Relation of Intraocular Concentrations of Inflammatory Factors and Improvement of Macular Edema after Vitrectomy in branch Retinal Vein Occlusion. Am. J. Ophthalmol. 151 (4), 610–e1. e611. doi:10.1016/j.ajo.2010.09.030
Park, S. P., and Ahn, J. K. (2008). Changes of Aqueous Vascular Endothelial Growth Factor and Interleukin-6 after Intravitreal Triamcinolone for branch Retinal Vein Occlusion. Clin. Exp. Ophthalmol. 36 (9), 831–835. doi:10.1111/j.1442-9071.2009.01909.x
Park, S. P., and Ahn, J. K. (2009). Changes of Aqueous Vascular Endothelial Growth Factor and Pigment Epithelium-Derived Factor Following Intravitreal Bevacizumab for Macular Oedema Secondary to branch Retinal Vein Occlusion. Clin. Exp. Ophthalmol. 37 (5), 490–495. doi:10.1111/j.1442-9071.2009.02061.x
Park, S. P., Ahn, J. K., and Mun, G. H. (2010). Aqueous Vascular Endothelial Growth Factor Levels Are Associated with Serous Macular Detachment Secondary to branch Retinal Vein Occlusion. Retina 30 (2), 281–286. (Philadelphia, Pa.). doi:10.1097/IAE.0b013e3181b9f153
Peri, G., Introna, M., Corradi, D., Iacuitti, G., Signorini, S., Avanzini, F., et al. (2000). PTX3, A Prototypical Long Pentraxin, Is an Early Indicator of Acute Myocardial Infarction in Humans. Circulation 102 (6), 636–641. doi:10.1161/01.cir.102.6.636
Qu, Y., Chen, Y., and Zhao, C. (2020). Testing a Novel Disposable Aqueous Humor Collector: An Approach to Improve Safety, Accuracy, and Efficiency. Biopreserv Biobank 18 (5), 449–453. doi:10.1089/bio.2020.0039
Querques, G., Lattanzio, R., Querques, L., Triolo, G., Cascavilla, M. L., Cavallero, E., et al. (2014). Impact of Intravitreal Dexamethasone Implant (Ozurdex) on Macular Morphology and Function. Retina 34 (2), 330–341. (Philadelphia, Pa.). doi:10.1097/IAE.0b013e31829f7495
Rezar-Dreindl, S., Eibenberger, K., Pollreisz, A., Bühl, W., Georgopoulos, M., Krall, C., et al. (2017). Effect of Intravitreal Dexamethasone Implant on Intra-ocular Cytokines and Chemokines in Eyes with Retinal Vein Occlusion. Acta Ophthalmol. 95 (2), e119–e127. doi:10.1111/aos.13152
Rodrigues, E. B., Farah, M. E., Maia, M., Penha, F. M., Regatieri, C., Melo, G. B., et al. (2009). Therapeutic Monoclonal Antibodies in Ophthalmology. Prog. Retin. Eye Res. 28 (2), 117–144. doi:10.1016/j.preteyeres.2008.11.005
Rogers, S., McIntosh, R. L., Cheung, N., Lim, L., Wang, J. J., Mitchell, P., et al. (2010). The Prevalence of Retinal Vein Occlusion: Pooled Data from Population Studies from the United States, Europe, Asia, and Australia. Ophthalmology 117 (2), 313–e1. doi:10.1016/j.ophtha.2009.07.017
Rosenbaum, J. N., and Weisman, P. (2017). The Evolving Role of Companion Diagnostics for Breast Cancer in an Era of Next-Generation Omics. Am. J. Pathol. 187 (10), 2185–2198. doi:10.1016/j.ajpath.2017.04.021
Rychli, K., Huber, K., and Wojta, J. (2009). Pigment Epithelium-Derived Factor (PEDF) as a Therapeutic Target in Cardiovascular Disease. Expert Opin. Ther. Targets 13 (11), 1295–1302. doi:10.1517/14728220903241641
Schmidt-Erfurth, U., Garcia-Arumi, J., Gerendas, B. S., Midena, E., Sivaprasad, S., Tadayoni, R., et al. (2019). Guidelines for the Management of Retinal Vein Occlusion by the European Society of Retina Specialists (EURETINA). Ophthalmologica 242 (3), 123–162. doi:10.1159/000502041
Schober, A., and Zernecke, A. (2007). Chemokines in Vascular Remodeling. Thromb. Haemost. 97 (5), 730–737. doi:10.1160/th07-02-0085
Sennlaub, F., Courtois, Y., and Goureau, O. (2002). Inducible Nitric Oxide Synthase Mediates Retinal Apoptosis in Ischemic Proliferative Retinopathy. J. Neurosci. 22 (10), 3987–3993. doi:10.1523/jneurosci.22-10-03987.2002
Shchuko, A. G., Zlobin, I. V., Iureva, T. N., Ostanin, A. A., Chernykh, E. R., and Mikhalevich, I. M. (2015). Intraocular Cytokines in Retinal Vein Occlusion and its Relation to the Efficiency of Anti-vascular Endothelial Growth Factor Therapy. Indian J. Ophthalmol. 63 (12), 905–911. doi:10.4103/0301-4738.176031
Shibuya, M. (2008). Vascular Endothelial Growth Factor-dependent and -independent Regulation of Angiogenesis. BMB Rep. 41 (4), 278–286. doi:10.5483/BMBRep.2008.41.4.278
Shin, H. J., Kim, H. C., and Moon, J. W. (2014). Aqueous Levels of Erythropoietin in Acute Retinal Vein Occlusion with Macular Edema. Int. J. Ophthalmol. 7 (3), 501–506. doi:10.3980/j.issn.2222-3959.2014.03.21
Sin, B. H., Song, B. J., and Park, S. P. (2013). Aqueous Vascular Endothelial Growth Factor and Endothelin-1 Levels in branch Retinal Vein Occlusion Associated with normal Tension Glaucoma. J. Glaucoma 22 (2), 104–109. doi:10.1097/IJG.0b013e3182312047
Sohn, H. J., Han, D. H., Lee, D. Y., and Nam, D. H. (2014). Changes in Aqueous Cytokines after Intravitreal Triamcinolone versus Bevacizumab for Macular Oedema in branch Retinal Vein Occlusion. Acta Ophthalmol. 92 (3), e217–24. doi:10.1111/aos.12219
Stahl, A., Buchwald, A., Martin, G., Junker, B., Chen, J., Hansen, L. L., et al. (2010). Vitreal Levels of Erythropoietin Are Increased in Patients with Retinal Vein Occlusion and Correlate with Vitreal VEGF and the Extent of Macular Edema. Retina 30 (9), 1524–1529. doi:10.1097/IAE.0b013e3181d37539
Suzuki, S., Takeishi, Y., Niizeki, T., Koyama, Y., Kitahara, T., Sasaki, T., et al. (2008). Pentraxin 3, a New Marker for Vascular Inflammation, Predicts Adverse Clinical Outcomes in Patients with Heart Failure. Am. Heart J. 155 (1), 75–81. doi:10.1016/j.ahj.2007.08.013
The Central Vein Occlusion Study (1993). Baseline and Early Natural History Report. The Central Vein Occlusion Study. Arch. Ophthalmol. 111 (8), 1087–1095. doi:10.1001/archopht.1993.01090080083022
Trinchieri, G. (1995). Interleukin-12: a Proinflammatory Cytokine with Immunoregulatory Functions that Bridge Innate Resistance and Antigen-specific Adaptive Immunity. Annu. Rev. Immunol. 13, 251–276. doi:10.1146/annurev.iy.13.040195.001343
Tuuminen, R., and Loukovaara, S. (2014a). High Intravitreal TGF-Β1 and MMP-9 Levels in Eyes with Retinal Vein Occlusion. Eye (Lond) 28 (9), 1095–1099. doi:10.1038/eye.2014.137
Tuuminen, R., and Loukovaara, S. (2014b). Increased Intravitreal Angiopoietin-2 Levels in Patients with Retinal Vein Occlusion. Acta Ophthalmol. 92 (2), e164–5. doi:10.1111/aos.12223
Uemura, A., Fruttiger, M., D'Amore, P. A., De Falco, S., Joussen, A. M., Sennlaub, F., et al. (2021). VEGFR1 Signaling in Retinal Angiogenesis and Microinflammation. Prog. Retin. Eye Res. 84, 100954. doi:10.1016/j.preteyeres.2021.100954
Van Dyken, S. J., and Locksley, R. M. (2013). “Interleukin-4-and Interleukin-13-Mediated Alternatively Activated Macrophages: Roles in Homeostasis and Disease,” in Annual Review of Immunology. Editors D. R. Littman, and W. M. Yokoyama, 317–343. 31. doi:10.1146/annurev-immunol-032712-095906
Vinores, S. A., Derevjanik, N. L., Ozaki, H., Okamoto, N., and Campochiaro, P. A. (1999). Cellular Mechanisms of Blood-Retinal Barrier Dysfunction in Macular Edema. Doc Ophthalmol. 97 (3-4), 217–228. doi:10.1023/a:1002136712070
Vujosevic, S., and Simó, R. (2017). Local and Systemic Inflammatory Biomarkers of Diabetic Retinopathy: An Integrative Approach. Invest. Ophthalmol. Vis. Sci. 58 (6), Bio68–bio75. doi:10.1167/iovs.17-21769
Wallsh, J. O., and Gallemore, R. P. (2021). Anti-VEGF-Resistant Retinal Diseases: A Review of the Latest Treatment Options. Cells 10 (5), 1049. doi:10.3390/cells10051049
Watanabe, D., Suzuma, K., Matsui, S., Kurimoto, M., Kiryu, J., Kita, M., et al. (2005). Erythropoietin as a Retinal Angiogenic Factor in Proliferative Diabetic Retinopathy. N. Engl. J. Med. 353 (8), 782–792. doi:10.1056/NEJMoa041773
Weidemann, A., and Johnson, R. S. (2009). Nonrenal Regulation of EPO Synthesis. Kidney Int. 75 (7), 682–688. doi:10.1038/ki.2008.687
Witkowska, A. M., and Borawska, M. H. (2004). Soluble Intercellular Adhesion Molecule-1 (sICAM-1): an Overview. Eur. Cytokine Netw. 15 (2), 91–98.
Yasuda, S., Kachi, S., Kondo, M., Ushida, H., Uetani, R., Terui, T., et al. (2011). Significant Correlation between Electroretinogram Parameters and Ocular Vascular Endothelial Growth Factor Concentration in central Retinal Vein Occlusion Eyes. Invest. Ophthalmol. Vis. Sci. 52 (8), 5737–5742. doi:10.1167/iovs.10-6923
Yasuda, S., Kachi, S., Ueno, S., Ushida, H., Piao, C. H., Kondo, M., et al. (2014). Electroretinograms and Level of Aqueous Vascular Endothelial Growth Factor in Eyes with Hemicentral Retinal Vein Occlusion or branch Retinal Vein Occlusion. Jpn. J. Ophthalmol. 58 (3), 232–236. doi:10.1007/s10384-014-0316-6
Yu, H., Huang, X., Ma, Y., Gao, M., Wang, O., Gao, T., et al. (2013). Interleukin-8 Regulates Endothelial Permeability by Down-Regulation of Tight junction but Not Dependent on Integrins Induced Focal Adhesions. Int. J. Biol. Sci. 9 (9), 966–979. doi:10.7150/ijbs.6996
Zeng, Y., Cao, D., Yu, H., Zhuang, X., Yang, D., Hu, Y., et al. (2019). Comprehensive Analysis of Vitreous Chemokines Involved in Ischemic Retinal Vein Occlusion. Mol. Vis. 25, 756–765.
Keywords: biomarker, retinal vein occlusion, aqueous humor, companion diagnostic, cytokine
Citation: Wang B, Zhang X, Chen H, Koh A, Zhao C and Chen Y (2022) A Review of Intraocular Biomolecules in Retinal Vein Occlusion: Toward Potential Biomarkers for Companion Diagnostics. Front. Pharmacol. 13:859951. doi: 10.3389/fphar.2022.859951
Received: 24 January 2022; Accepted: 25 March 2022;
Published: 26 April 2022.
Edited by:
Stefania Tacconelli, University of Studies G.d'Annunzio Chieti and Pescara, ItalyReviewed by:
Dragos Serban, Carol Davila University of Medicine and Pharmacy, RomaniaCopyright © 2022 Wang, Zhang, Chen, Koh, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chan Zhao, emhhb2NoYW5AcHVtY2guY24=; Youxin Chen, Y2hlbnl4QHB1bWNoLmNu
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.