- 1The Center for Data Science in Health and Medicine, Business School, Qingdao University, Qingdao, China
- 2Department of Ophthalmology, The Second People’s Hospital of Guilin, Guilin, China
Oral cancer (OC) is one of the most pernicious cancers with increasing incidence and mortality worldwide. Surgery is the primary approach for the treatment of early-stage OC, which reduces the quality of life of the patients. Therefore, there is an urgent need to discover novel treatments for OC. Targeting ferroptosis to induce cell death through the modulation of lipid oxidation has been used as a new approach to treat many cancers. Glutathione (GSH) is a coenzyme factor of GSH peroxidase 4, and it carries potential applicability in treating OC. By using network pharmacology and molecular docking followed by systematic bioinformatic analysis, we aimed to study GSH-targeting ferroptosis to treat OC. We identified 14 core molecular targets, namely, EGFR, PTGS2, HIF1A, VEGFA, TFRC, SLC2A1, CAV1, CDKN2A, SLC3A2, IFNG, NOX4, DDIT4, CA9, and DUSP1, involved in ferroptosis that were targeted by GSH for OC treatment. Functional characterization of these molecular targets showed their importance in the control of cell apoptosis, cell proliferation, and immune responses through various kinase activities such as the mitogen-activated protein kinase activity (e.g., ERK1 and ERK2 cascades) and modulation of TOR signaling (e.g., the HIF-1 signaling pathway). Molecular docking further revealed the direct binding of GSH with EGFR, PTGS2, and HIF1A proteins. These findings provide a novel insight into the targets of GSH in ferroptosis as well as possible molecular mechanisms involved, suggesting the possible use of GSH as a combined therapy for treating OC.
Introduction
Oral cancer (OC) is a type of malignant tumor that occurs in the oral cavity; it includes tongue cancer, gum cancer, palate cancer, oropharyngeal cancer, and lip cancer (Wong and Wiesenfeld 2018). OC is one of the most pernicious cancers reported in the world, accounting for around 2% of all new cases (Sung et al., 2021). In recent decades, the incidence and mortality of OC have shown markedly increasing trends (Chen et al., 2016). Potential risk factors for OC include smoking, drinking, poor oral hygiene, malnutrition, environmental impact, genetic factors, and infectious diseases (Shield et al., 2017). Tumor formation in OC results from abnormal activation patterns of proto- and anti-oncogenes and epigenetic modification (Osan et al., 2021). Early clinical detection of OC may be difficult to achieve because of insidious conditions and anatomical characteristics prior to initial treatment (Dhanuthai et al., 2018). Clinically, surgery is prioritized for early-stage OC with better treatment effects (D'Souza and Kumar 2020). However, chemotherapy is a palliative regimen, and its efficacy is not yet satisfactory for patients with advanced OC (Mohan et al., 2021). Therefore, markers for early detection of oral malignancies and alternative treatment agents are necessary. Ferroptosis, a type of programmed cell death dependent on iron and activated by lipid peroxidation, is closely related to cancer development (Nie et al., 2022). Initiation and induction of ferroptosis can cause abnormal function of mitochondria and peroxidation-based lipotoxicity, resulting in regulation of tumor formation (Wu et al., 2020). It is reported that malignant tumor cells contain high levels of iron elements for anarchic cell proliferation and tumor growth (Tang et al., 2021a). Therefore, targeting ferroptosis may be a promising approach for OC treatment. Glutathione (GSH) is a bioactive substance involved in cellular metabolism that physiologically functions to protect the body against lesions induced by reducing agents (Forman et al., 2009). GSH is a coenzyme factor of GSH peroxidase 4 (GPx4), an essential reaction substrate for the degradation of lipid peroxides. As a key enzyme regulating ferroptosis, GPx4 can inhibit the occurrence of ferroptosis by catalyzing the reduction of lipid peroxides (Xuan et al., 2021). Patients with OC have markedly low levels of superoxide dismutase, GSH peroxidase, and GSH transferase (Sushma et al., 2021). An in vitro study demonstrated that enhanced intracellular GSH activity induced by natural compounds might have anti-oral cancer action by modulating oxidative stress, autophagy, and cell death (Contant et al., 2021). Although the physiological function of GSH in OC and underlying molecular mechanisms are well-reported, pharmacological mechanisms of GSH against OC remain unclear, especially ferroptosis-associated signaling pathways. Recently, network pharmacology-based discovery of individual compounds that act against dysregulated disorders, including malignant cancer (Li et al., 2021a), has been demonstrated (Li et al., 2020). Using a network pharmacology screening approach, our previous study demonstrated the core targets and therapeutic mechanisms of the GSH action against the cleft lip (Li et al., 2021b). In this study, available bioinformatic data of GSH were processed and studied for the potential efficacy against OC via multi-step network pharmacology and molecular docking approach, revealing ferroptosis-associated biotargets and signaling mechanisms.
Material and Methods
Identification of Common Oral Cancer-, Ferroptosis-, and Glutathione-Associated Genes
The Cancer Genome Atlas (TCGA) database (https://portal.gdc.cancer.gov/) was used to determine OC-associated genes. Using the limma package of R in the Bioconductor software, genes with FDR <0.05 and |log2 fold change| > 1 were considered as OC-associated genes (Ritchie et al., 2015). The FerrDb database was then used to search for ferroptosis-associated genes (Zhou and Bao 2020). For GSH-associated genes, the chemical structure of GSH, C (CC(=O)NC(CS)C (=O)NCC(=O)O)C(C(=O)O)N, was obtained from the traditional Chinese medicine system pharmacology database (Ru et al., 2014) and used to determine the pharmacological targets of GSH using various online tools and databases including SwissTargetPrediction (Daina et al., 2019), SuperPred (Nickel et al., 2014), TargetNet (Yao et al., 2016), Batman (Liu et al., 2016), DrugBank (Wishart et al., 2018), and BindingDB (Liu et al., 2007). The target genes were subjected to UniProt for human database correction. Subsequently, all targets of OC, ferroptosis, and GSH were overlapped to obtain the common targets.
Protein Network Involved in Glutathione Action Against Oral Cancer Through Ferroptosis
The common targets were subjected to a protein–protein interaction (PPI) network analysis by using the STRING (Version 11.0) database (Szklarczyk et al., 2019). The network analyzer in Cytoscape v3.7.2 was set under median or maximum degrees of freedom; the core targets were obtained under the upper limit of the screening range with a maximum degree value of the topology data, and the lower limit was twice the median degree of freedom (Shannon et al., 2003).
Gene Ontology and Pathway Enrichment Analysis of CA028 Action Against Oral Cancer
The common targets were subjected to Bioconductor packages in the R-language software for Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. Then, the Cytoscape software was used to visualize the biological processes and signaling pathways involved in the GSH action against OC through ferroptosis (Shannon et al., 2003).
Molecular Docking Analysis
The binding of GSH to its core targets including the epidermal growth factor receptor (EGFR), prostaglandin-endoperoxide synthase 2 (PTGS2), and hypoxia inducible factor 1 subunit alpha (HIF1A) was studied by molecular docking. The protein structures of EGFR, PTGS2, and HIF1A were obtained from the PDB database. Then, MGLTools 1.5.6 of AutoDock Vina and the ChemBio3D Draw tool were used to conduct the docking analysis (Morris et al., 2009; Trott and Olson 2010). The docking parameter setting was assessed according to the root mean square deviation (RMSD) of the ligand molecule. RMSD ≤ 4Å was the permissive threshold for the conformation of the ligand molecule.
Results
Identification of Glutathione Targets for Treating Oral Cancer Through Ferroptosis
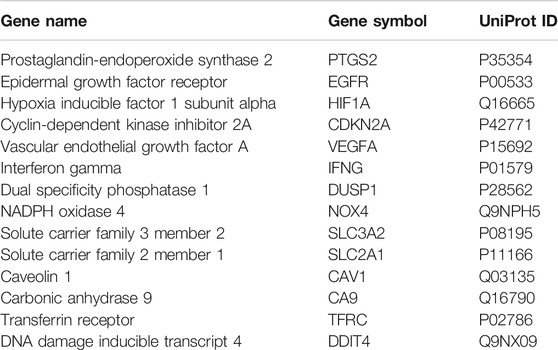
By searching the FerrDb and GeneCards databases, 259 ferroptosis-associated targets were identified (Figure 1A). Additionally, using TCGA database, the comparison of gene sequences from 32 normal adults and 330 patients with OC identified 4,237 differentially expressed genes (DEGs) (Figure 1A). Among these, 53 DEGs including 38 upregulated genes and 15 downregulated genes overlapped with ferroptosis-associated targets (Figure 1B). Furthermore, 6639 GSH-associated target genes were identified by searching different databases, and 44 of them were found to be shared with OC- and ferroptosis-associated targets (Figure 1C). Then, the common targets were subjected to the STRING analysis to delineate the PPI involved in the GSH action against OC through ferroptosis (Figure 1D). By using the Cytoscape tool, 14 core targets, namely, PTGS2, EGFR, HIF1A, cyclin-dependent kinase inhibitor 2A (CDKN2A), vascular endothelial growth factor A (VEGFA), interferon gamma (IFNG), dual specificity phosphatase 1 (DUSP1), NADPH oxidase 4 (NOX4), solute carrier family 3 member 2 (SLC3A2), solute carrier family 2 member 1 (SLC2A1), caveolin 1 (CAV1), carbonic anhydrase 9 (CA9), transferrin receptor (TFRC), and DNA damage-inducible transcript 4 (DDIT4) were obtained. The median degree of freedom was 5.37, whereas the maximum degree of freedom was 19 (Figure 1E and Table 1).
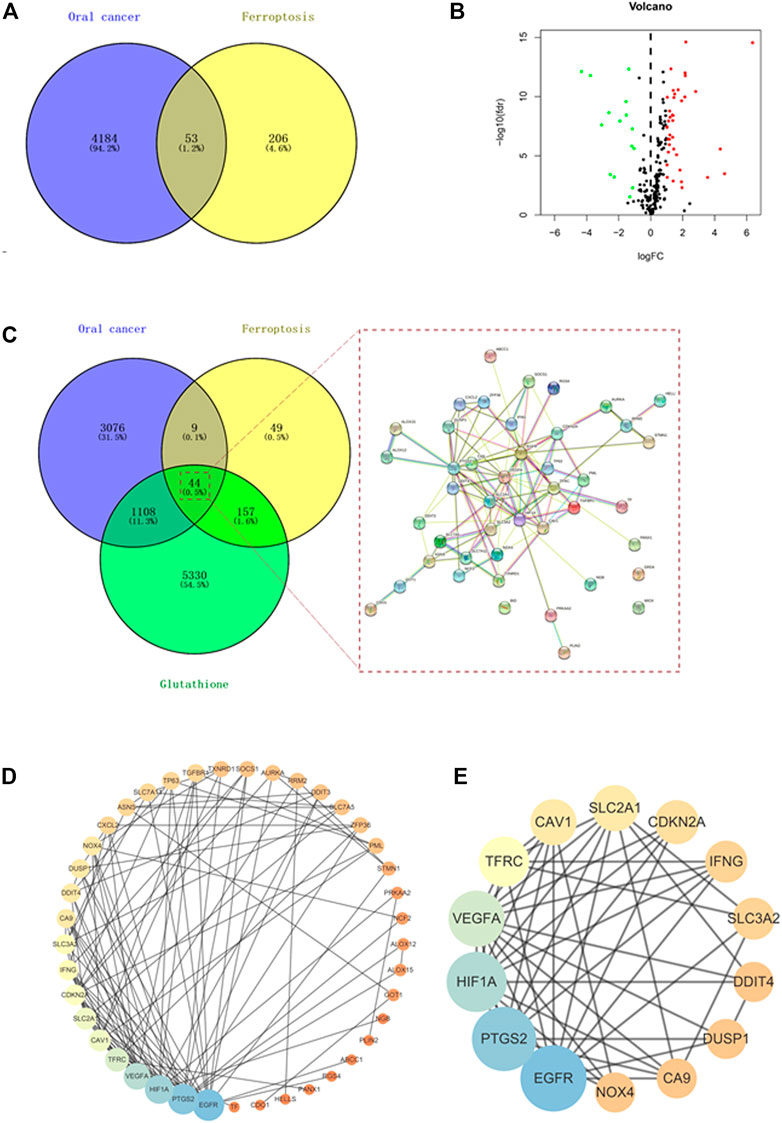
FIGURE 1. Network pharmacology identified the anti-oral cancer genes involved in targeting ferroptosis by glutathione (GSH). (A) Venn diagram showing the interacting genes associated with oral cancer (OC) and ferroptosis. (B) Volcano plot showing the differential expression of ferroptosis-associated genes in OC. The genes with FDR <0.05 and |log2 fold change| > 1 were considered as differentially expressed genes. Green dots represent downregulated genes, and red dots represent upregulated genes. (C) Venn diagram showing the interacting genes associated with OC, ferroptosis, and GSH. (D) Protein–protein interaction (PPI) of OC-, ferroptosis-, and GSH-associated genes. (E) Cytoscape analysis identified 14 core targets of GSH for treating OC by targeting ferroptosis.
Glutathione Targeted Ferroptosis to Control Stress Responses and Cell Apoptosis of Oral Cancer
GO enrichment analysis of the 14 core targets highlighted the biological processes related to stress responses such as oxidative stress and hypoxic stress (Figure 2A). Besides, altered angiogenesis through vasoconstriction and vasculogenesis was observed (Figure 2A). Furthermore, this could lead to the control of cell apoptosis, cell proliferation, and immune responses through many kinase activities and pathways, such as the mitogen-activated protein kinase (MAPK) activity, the extracellular signal-regulated kinase (ERK)1 and ERK2 cascades, and TOR signaling (Figure 2A). Molecular functional analysis of GO showed the involvement of the core targets in many binding activities such as enzyme binding, protein binding, protein kinase binding, glycoprotein binding, double-stranded RNA binding, and protein heterodimerization activity (Figure 2B). GO analysis also highlighted the contribution of core targets to different cellular components such as the endosome, cytoplasm membrane, Golgi membrane, and extracellular space (Figure 2B). KEGG pathway analysis revealed the control of cell signaling pathways such as the HIF-1 signaling pathway by GSH targets (Figure 2C). In addition, many cancer-related signaling pathways, including mRNAs in cancer, bladder cancer, proteoglycans in cancer, central carbon metabolism in cancer, pancreatic cancer, renal cell carcinoma, and non-small cell lung cancer, were highlighted in our analysis (Figure 2C). Taken together, our results show the importance of GSH against ferroptosis in OC through the regulation of many cancer-related biological processes and signaling pathways (Figure 2D).
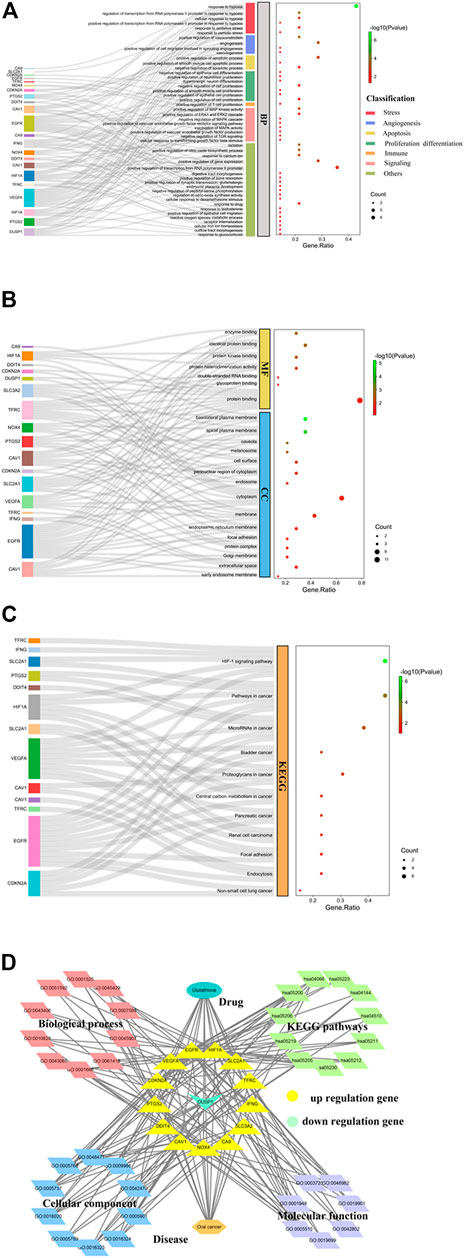
FIGURE 2. Functional characterization of glutathione (GSH)-targeting ferroptosis genes in oral cancer (OC). (A) Gene Ontology (GO) enrichment analysis highlighting the biological roles of GSH-targeting ferroptosis genes in stress responses, angiogenesis, apoptosis, cell proliferation, cell differentiation, immune responses, and cell signaling for treating OC. (B) GO enrichment analysis highlighting the molecular functions and cellular components of GSH-targeting ferroptosis genes for treating OC. (C) Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis showed the importance of target genes of GSH action in cell signaling pathways involved in OC. The size of the dots represents the number of genes, whereas the color of the dots represents the significance of the terms. (D) Complex network analysis using Cytoscape to visualize the biological processes and signaling pathways involved in the mechanism of GSH action against OC through ferroptosis.
Direct Binding of Glutathione to Oral Cancer- and Ferroptosis-Associated Genes
Molecular docking analysis was conducted to investigate the possible direct binding of GSH to its target proteins. The results showed that GSH had a high binding affinity to EGFR, PTGS2, and HIF1A (Figure 3). The protein structures of EGFR, PTGS2, and HIF1A were obtained from the PDB database. Furthermore, the AutoDock Vina program was used to determine the binding affinity between these proteins and GSH. The results demonstrated that GSH formed hydrogen bonds with amino acid residues CYS-797 (1.9 Å), MET-793 (2.5 Å), and LYS-716 (2.5 Å) of EGFR (PDB ID: 5UGC) of EGFR, and the free binding energy was −5.1 kcal/mol. For PTGS2 protein (PDB ID: 5IKR), GSH formed hydrogen bonds with amino acid residues TYR-355 (2.6 Å), ARG-120 (2.3 Å), and VAL-523 (2.4 Å) of PTGS2 (Figure 3B), and the free binding energy was -5.12 kcal/mol. Similar bonds were observed between GSH and amino acid residues ARG-238 (2.5 Å) and GLN-239 (1.8 Å) of HIF1A (PDB ID: 1H2M) (Figure 3C), with a free binding energy of −5.32 kcal/mol. Collectively, our data suggested direct binding of GSH to its target proteins EGFR, PTGS2, and HIF1A.
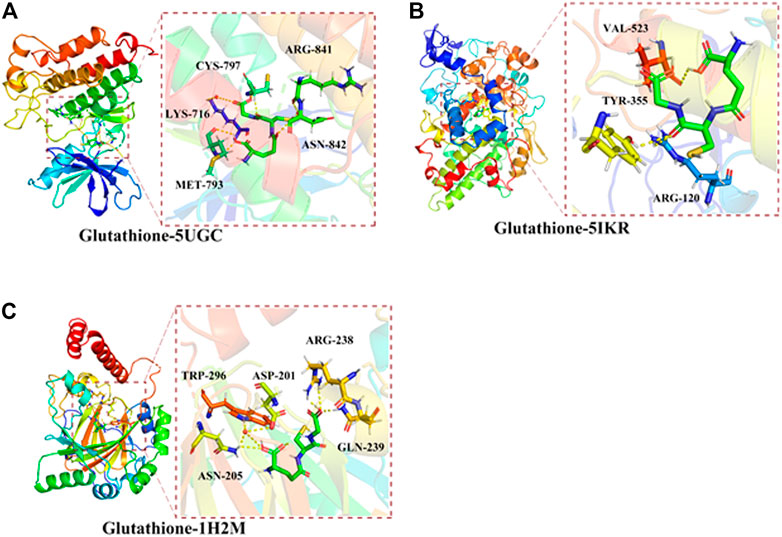
FIGURE 3. Direct binding of glutathione (GSH) to its targets against oral cancer through ferroptosis. Molecular docking analysis predicted the preferred orientation of hydrogen bonds between GSH and amino acid residues (A) CYS-797, MET-793, and LYS-716 of EGFR (PDB ID:5UGC) (B) TYR-355, ARG-120, and VAL-523 of PTGS2 (PDB ID: 5IKR) (C) ARG-238 and GLN-239 of HIF1A (PDB ID: 1H2M).
Discussion
In the present study, we investigated the possible use of GSH in treating OC by targeting ferroptosis. Ferroptosis, a newly discovered type of cell death mediated by iron-dependent lipid peroxidation (Chen S. et al., 2021), is reported to be a promising approach in cancer therapy (Tang et al., 2021b). Oral squamous cell carcinoma was shown to have a ferroptosis-specific gene-expression signature, suggesting ferroptosis regulation may be clinically relevant for combination therapies (Gu et al., 2021). We hypothesized that GSH can be a potential drug for combination therapies of OC as it is a key player in cellular oxidation–reduction homeostasis and is closely associated with ferroptosis in various cancers (Elakkad et al., 2021; Liu et al., 2021; Xu et al., 2021).
By using network pharmacology, we identified 14 core targets of GSH action against OC by targeting ferroptosis. Bioinformatic analysis of these 14 targets suggested the role of GSH in cell apoptosis, cell proliferation, and immune responses through the regulation of MAPK, ERK, and TOR signaling pathways. MAPK was found to be involved in ferroptosis regulation in many human body functions (Nguyen et al., 2020). Activation of MAPK signaling induced ferroptosis in human pancreatic islet-cell clusters (Li and Leung 2020). In contrast, inhibition of MAPK signaling suppressed inflammation and oxidative stress of acute respiratory distress syndrome caused by ferroptosis (Wang et al., 2022). In other studies, MAPK signaling was found to control ferroptosis in various cancers including nasopharyngeal carcinoma, hepatocellular carcinoma, osteosarcoma, and non-small cell lung cancer through redox balance (Poursaitidis et al., 2017; Lv et al., 2020; Chang et al., 2021; He et al., 2021). More importantly, MAPK signaling was linked to many other cell signaling pathways mediating the carcinogenicity of OC. For instance, MAPK signaling regulated the viability of OC via the response gene FOS as a subunit of the AP-1 complex (Greer et al., 2022). Also, inhibition of the MAPK pathway diminished invasion and the migration ability in OC through the regulation of matrix metalloproteinase-2 activity (Pramanik and Mishra 2022). Besides the MAPK signaling, our results highlighted the involvement of ERK signaling in ferroptosis targeted by GSH. ERK signaling is associated with ferroptosis in many cancers, including multiple myeloma (Chen J. et al., 2021), endometrial carcinoma (Qin et al., 2021), hepatocellular carcinoma (Fei et al., 2021), and pancreatic cancer (Ye et al., 2020). Specifically, the ERK signaling pathway has been linked with ferroptosis and associated with the prognosis of OC (Chen R. et al., 2021). For TOR signaling, only limited studies have demonstrated its direct link to ferroptosis. A study by Ni et al. demonstrated that inhibition of mTOR overcame anticancer drug resistance by promoting ferroptosis in lung cancer cells (Ni et al., 2021). Another study on xenograft mouse models suggested that the combination of mTORC1 inhibition with ferroptosis induction resulted in tumor regression in PI3K-mutated breast cancer and PTEN-defective prostate cancer (Yi et al., 2020). Therefore, the use of GSH to target ferroptosis could be a promising combination therapy for treating OC.
In addition to network pharmacology, we applied molecular docking to investigate the possible direct binding of GSH to its target proteins involved in ferroptosis. Our results showed a strong binding affinity of GSH to EGFR, PTGS2, and HIFIA.
EGFR, a receptor tyrosine kinase, is a major regulator of cancer development (Ni et al., 2021). It coordinates with mTOR and MAPK signaling to regulate ferroptosis in cancer. For example, EGFR was associated with the mTOR pathway to mediate ferroptosis and apoptosis of ovarian cancer (Li et al., 2022). In addition, the EGFR tyrosine kinase inhibitor lapatinib regulated mTOR to promote ferroptosis in lung cancer cells (Ni et al., 2021). Another study of non-small cell lung cancer demonstrated that the blockade of EGFR or MAPK signaling protected the lung cancer cells from ferroptosis (Poursaitidis et al., 2017). Furthermore, it has been shown that ferroptosis can be targeted to treat EGFR-mutant lung cancer (Zhang et al., 2021). Additionally, driving EGFR could induce ferroptosis in hepatocellular carcinoma and glioblastoma (Kadioglu et al., 2021; Sun et al., 2021). PTGS2 is responsible for the prostanoid biosynthesis involved in inflammation and mitogenesis (Gómez-Valenzuela et al., 2021). It was reported that upregulation of PTGS2 induces the ferroptosis of colorectal cancer cells (Zhao and Chen 2021); thus, PTGS2 is considered as a marker of ferroptosis. The PTGS2 expression was associated with increased lipid peroxidation in gastric cancer cells (Guan et al., 2020). In another study, the PTGS2 expression modulated esophageal squamous cell carcinoma radiosensitivity by inhibiting ferroptosis (Feng et al., 2021). In addition, HIF1A was associated with increased tumor immunity and aggressive phenotypes in human cancers (Chen et al., 2020). Ferroptosis-related genes associated with the overall survival in patients with diffuse large B-cell lymphoma have also been reported (Chen H. et al., 2021).
In conclusion, we identified the targets of GSH in ferroptosis associated with OC, providing new mechanistic insights that may be clinically relevant for combination therapies of OC. However, the findings of the present study are mainly based on network pharmacology and bioinformatics analysis; therefore, further preclinical investigation is needed to verify the results.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author Contributions
CH and LZ contributed to the conception and design of the manuscript. CH and LZ contributed to the acquisition, analysis, and interpretation of data in this manuscript. CH and LZ drafted this manuscript. CH and LZ revised this manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Chang, W. T., Bow, Y. D., Fu, P. J., Li, C. Y., Wu, C. Y., Chang, Y. H., et al. (2021). A Marine Terpenoid, Heteronemin, Induces Both the Apoptosis and Ferroptosis of Hepatocellular Carcinoma Cells and Involves the ROS and MAPK Pathways. Oxid Med. Cel Longev 2021, 7689045. PMID: 33488943; PMCID: PMC7803406. doi:10.1155/2021/7689045
Chen, B., Li, L., Li, M., and Wang, X. (2020). HIF1A Expression Correlates with Increased Tumor Immune and Stromal Signatures and Aggressive Phenotypes in Human Cancers. Cel Oncol (Dordr) 43 (5), 877–888. Epub 2020 Jun 1. PMID: 32488852. doi:10.1007/s13402-020-00534-4
Chen, H., He, Y., Pan, T., Zeng, R., Li, Y., Chen, S., et al. (2021). Ferroptosis-Related Gene Signature: A New Method for Personalized Risk Assessment in Patients with Diffuse Large B-Cell Lymphoma. Pharmgenomics Pers Med. 14, 609–619. PMID: 34079336; PMCID: PMC8165657. doi:10.2147/PGPM.S309846
Chen, J., Zaal, E. A., Berkers, C. R., Ruijtenbeek, R., Garssen, J., and Redegeld, F. A. (2021). Omega-3 Fatty Acids DHA and EPA Reduce Bortezomib Resistance in Multiple Myeloma Cells by Promoting Glutathione Degradation. Cells 10 (9), 2287. PMID: 34571936; PMCID: PMC8465636. doi:10.3390/cells10092287
Chen, R., Cao, J., Jiang, W., Wang, S., and Cheng, J. (2021). Upregulated Expression of CYBRD1 Predicts Poor Prognosis of Patients with Ovarian Cancer. J. Oncol. 2021, 7548406. PMID: 34594380; PMCID: PMC8478559. doi:10.1155/2021/7548406
Chen, S., Zhu, J. Y., Zang, X., and Zhai, Y. Z. (2021). The Emerging Role of Ferroptosis in Liver Diseases. Front Cel Dev Biol 9, 801365. doi:10.3389/fcell.2021.801365
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer Statistics in China, 2015. CA Cancer J. Clin. 66, 115–132. doi:10.3322/caac.21338
Contant, C., Rouabhia, M., Loubaki, L., Chandad, F., and Semlali, A. (2021). Anethole Induces Anti-oral Cancer Activity by Triggering Apoptosis, Autophagy and Oxidative Stress and by Modulation of Multiple Signaling Pathways. Sci. Rep. 11, 13087. doi:10.1038/s41598-021-92456-w
D'Souza, W., and Kumar, A. (2020). microRNAs in Oral Cancer: Moving from Bench to Bed as Next Generation Medicine. Oral Oncol. 111, 104916. doi:10.1016/j.oraloncology.2020.104916
Daina, A., Michielin, O., and Zoete, V. (2019). SwissTargetPrediction: Updated Data and New Features for Efficient Prediction of Protein Targets of Small Molecules. Nucleic Acids Res. 47, W357–W364. doi:10.1093/nar/gkz382
Dhanuthai, K., Rojanawatsirivej, S., Thosaporn, W., Kintarak, S., Subarnbhesaj, A., Darling, M., et al. (2018). Oral Cancer: A Multicenter Study. Med. Oral Patol Oral Cir Bucal 23, e23–29. doi:10.4317/medoral.21999
Elakkad, Y. E., Mohamed, S. N. S., and Abuelezz, N. Z. (2021). Potentiating the Cytotoxic Activity of a Novel Simvastatin-Loaded Cubosome against Breast Cancer Cells: Insights on Dual Cell Death via Ferroptosis and Apoptosis. Breast Cancer (Dove Med. Press. 13, 675–689. PMID: 34934357; PMCID: PMC8684378. doi:10.2147/BCTT.S336712
Fei, Z., Lijuan, Y., Jing, Z., Xi, Y., Yuefen, P., and Shuwen, H. (2021). Molecular Characteristics Associated with Ferroptosis in Hepatocellular Carcinoma Progression. Hum. Cel 34 (1), 177–186. Epub 2020 Sep 16. PMID: 32936424. doi:10.1007/s13577-020-00431-w
Feng, L., Zhao, K., Sun, L., Yin, X., Zhang, J., Liu, C., et al. (2021). SLC7A11 Regulated by NRF2 Modulates Esophageal Squamous Cell Carcinoma Radiosensitivity by Inhibiting Ferroptosis. J. Transl Med. 19 (1), 367. PMID: 34446045; PMCID: PMC8393811. doi:10.1186/s12967-021-03042-7
Forman, H. J., Zhang, H., and Rinna, A. (2009). Glutathione: Overview of its Protective Roles, Measurement, and Biosynthesis. Mol. Aspects Med. 30, 1–12. doi:10.1016/j.mam.2008.08.006
Gómez-Valenzuela, F., Escobar, E., Pérez-Tomás, R., and Montecinos, V. P. (2021). The Inflammatory Profile of the Tumor Microenvironment, Orchestrated by Cyclooxygenase-2, Promotes Epithelial-Mesenchymal Transition. Front. Oncol. 11, 686792. PMID: 34178680; PMCID: PMC8222670. doi:10.3389/fonc.2021.686792
Greer, P. F. C., Rich, A., and Coates, D. E. (2022). Effects of Galectin-1 Inhibitor OTX008 on Oral Squamous Cell Carcinoma Cells In Vitro and the Role of AP-1 and the MAPK/ERK Pathway. Arch. Oral Biol. 134, 105335. Epub ahead of print. PMID: 34891102. doi:10.1016/j.archoralbio.2021.105335
Gu, W., Kim, M., Wang, L., Yang, Z., Nakajima, T., and Tsushima, Y. (2021). Multi-omics Analysis of Ferroptosis Regulation Patterns and Characterization of Tumor Microenvironment in Patients with Oral Squamous Cell Carcinoma. Int. J. Biol. Sci. 17 (13), 3476–3492. PMID: 34512160; PMCID: PMC8416738. doi:10.7150/ijbs.61441
Guan, Z., Chen, J., Li, X., and Dong, N. (2020). Tanshinone IIA Induces Ferroptosis in Gastric Cancer Cells through P53-Mediated SLC7A11 Down-Regulation. Biosci. Rep. 40 (8), BSR20201807. PMID: 32776119; PMCID: PMC7953492. doi:10.1042/BSR20201807
He, X., Yao, Q., Fan, D., Duan, L., You, Y., Liang, W., et al. (2021). Cephalosporin Antibiotics Specifically and Selectively Target Nasopharyngeal Carcinoma through HMOX1-Induced Ferroptosis. Life Sci. 277, 119457. Epub 2021 Apr 5. PMID: 33831425. doi:10.1016/j.lfs.2021.119457
Kadioglu, O., Saeed, M. E. M., Mahmoud, N., Azawi, S., Mrasek, K., Liehr, T., et al. (2021). Identification of Novel Drug Resistance Mechanisms by Genomic and Transcriptomic Profiling of Glioblastoma Cells with Mutation-Activated EGFR. Life Sci. 284, 119601. Epub 2021 May 13. PMID: 33991550. doi:10.1016/j.lfs.2021.119601
Li, H. W., Liu, M. B., Jiang, X., Song, T., Feng, S. X., Wu, J. Y., et al. (2022). GALNT14 Regulates Ferroptosis and Apoptosis of Ovarian Cancer through the EGFR/mTOR Pathway. Future Oncol. 18 (2), 149–161. Epub 2021 Oct 13. PMID: 34643088. doi:10.2217/fon-2021-0883
Li, R., Guo, C., Li, Y., Liang, X., Yang, L., and Huang, W. (2020). Therapeutic Target and Molecular Mechanism of Vitamin C-Treated Pneumonia: a Systematic Study of Network Pharmacology. Food Funct. 11, 4765–4772. doi:10.1039/d0fo00421a
Li, R., Li, Y., Liang, X., Yang, L., Su, M., and Lai, K. P. (2021a). Network Pharmacology and Bioinformatics Analyses Identify Intersection Genes of Niacin and COVID-19 as Potential Therapeutic Targets. Brief Bioinform 22, 1279–1290. doi:10.1093/bib/bbaa300
Li, R., Huang, C., Ho, J. C. H., Leung, C. C. T., Kong, R. Y. C., Li, Y., et al. (2021b). The Use of Glutathione to Reduce Oxidative Stress Status and its Potential for Modifying the Extracellular Matrix Organization in Cleft Lip. Free Radic. Biol. Med. 164, 130–138. doi:10.1016/j.freeradbiomed.2020.12.455
Li, X. Y., and Leung, P. S. (2020). Erastin-induced Ferroptosis Is a Regulator for the Growth and Function of Human Pancreatic Islet-like Cell Clusters. Cell Regen 9 (1), 16. PMID: 32893325; PMCID: PMC7475162. doi:10.1186/s13619-020-00055-3
Liu, J., Wen, Q., Zhou, B., Yuan, C., Du, S., Li, L., et al. (2021). "Clickable" ZIF-8 for Cell-type-specific Delivery of Functional Proteins. ACS Chem. Biol. 17, 32–38. Epub ahead of print. PMID: 34936351. doi:10.1021/acschembio.1c00872
Liu, T., Lin, Y., Wen, X., Jorissen, R. N., and Gilson, M. K. (2007). BindingDB: a Web-Accessible Database of Experimentally Determined Protein-Ligand Binding Affinities. Nucleic Acids Res. 35 (Database issue), D198–D201. doi:10.1093/nar/gkl999
Liu, Z., Guo, F., Wang, Y., Li, C., Zhang, X., Li, H., et al. (2016). BATMAN-TCM: a Bioinformatics Analysis Tool for Molecular mechANism of Traditional Chinese Medicine. Sci. Rep. 6, 21146. doi:10.1038/srep21146
Lv, H., Zhen, C., Liu, J., and Shang, P. (2020). β-Phenethyl Isothiocyanate Induces Cell Death in Human Osteosarcoma through Altering Iron Metabolism, Disturbing the Redox Balance, and Activating the MAPK Signaling Pathway. Oxid Med. Cel Longev 2020, 5021983. PMID: 32322335; PMCID: PMC7160723. doi:10.1155/2020/5021983
Mohan, S., Popli, G., and Aggarwal, K. (2021). Oral Oncology and Reconstructive Surgery Fellowship Training Programs in India-A Trainee Perspective. South. Asian J. Cancer 10, 267–268. doi:10.1055/s-0041-1731582
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., et al. (2009). AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 30 (16), 2785–2791. doi:10.1002/jcc.21256
Nguyen, T. H. P., Mahalakshmi, B., and Velmurugan, B. K. (2020). Functional Role of Ferroptosis on Cancers, Activation and Deactivation by Various Therapeutic Candidates-An Update. Chem. Biol. Interact 317, 108930. Epub 2019 Dec 19. PMID: 31866335. doi:10.1016/j.cbi.2019.108930
Ni, J., Chen, K., Zhang, J., and Zhang, X. (2021). Inhibition of GPX4 or mTOR Overcomes Resistance to Lapatinib via Promoting Ferroptosis in NSCLC Cells. Biochem. Biophys. Res. Commun. 567, 154–160. Epub 2021 Jun 20. PMID: 34157442. doi:10.1016/j.bbrc.2021.06.051
Nickel, J., Gohlke, B. O., Erehman, J., Banerjee, P., Rong, W. W., Goede, A., et al. (2014). SuperPred: Update on Drug Classification and Target Prediction. Nucleic Acids Res. 42, W26–W31. doi:10.1093/nar/gku477
Nie, Q., Hu, Y., Yu, X., Li, X., and Fang, X. (2022). Induction and Application of Ferroptosis in Cancer Therapy. Cancer Cel Int 22, 12. doi:10.1186/s12935-021-02366-0
Osan, C., Chira, S., Nutu, A. M., Braicu, C., Baciut, M., Korban, S. S., et al. (2021). The Connection between MicroRNAs and Oral Cancer Pathogenesis: Emerging Biomarkers in Oral Cancer Management. Genes (Basel) 12, 1989. doi:10.3390/genes12121989
Poursaitidis, I., Wang, X., Crighton, T., Labuschagne, C., Mason, D., Cramer, S. L., et al. (2017). Oncogene-Selective Sensitivity to Synchronous Cell Death Following Modulation of the Amino Acid Nutrient Cystine. Cell Rep 18 (11), 2547–2556. PMID: 28297659; PMCID: PMC5368412. doi:10.1016/j.celrep.2017.02.054
Pramanik, K. K., and Mishra, R. (2022). ERK-mediated Upregulation of Matrix Metalloproteinase-2 Promotes the Invasiveness in Human Oral Squamous Cell Carcinoma (OSCC). Exp. Cel Res 411 (1), 112984. Epub ahead of print. PMID: 34951997. doi:10.1016/j.yexcr.2021.112984
Qin, J., Shao, X., Wu, L., and Du, H. (2021). Identification of the Ferroptosis-Associated Gene Signature to Predict the Prognostic Status of Endometrial Carcinoma Patients. Comput. Math. Methods Med. 2021, 9954370. PMID: 34531924; PMCID: PMC8440105. doi:10.1155/2021/9954370
Ritchie, M. E., Phipson, B., Wu, D., Hu, Y., Law, C. W., Shi, W., et al. (2015). Limma powers Differential Expression Analyses for RNA-Sequencing and Microarray Studies. Nucleic Acids Res. 43 (7), e47. doi:10.1093/nar/gkv007
Ru, J., Li, P., Wang, J., Zhou, W., Li, B., Huang, C., et al. (2014). TCMSP: a Database of Systems Pharmacology for Drug Discovery from Herbal Medicines. J. Cheminform 6, 13. doi:10.1186/1758-2946-6-13
Shannon, P., Markiel, A., Ozier, O., Baliga, N. S., Wang, J. T., Ramage, D., et al. (2003). Cytoscape: a Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 13, 2498–2504. doi:10.1101/gr.1239303
Shield, K. D., Ferlay, J., Jemal, A., Sankaranarayanan, R., Chaturvedi, A. K., Bray, F., et al. (2017). The Global Incidence of Lip, Oral Cavity, and Pharyngeal Cancers by Subsite in 2012. CA Cancer J. Clin. 67, 51–64. doi:10.3322/caac.21384
Sun, J., Zhou, C., Zhao, Y., Zhang, X., Chen, W., Zhou, Q., et al. (2021). Quiescin Sulfhydryl Oxidase 1 Promotes Sorafenib-Induced Ferroptosis in Hepatocellular Carcinoma by Driving EGFR Endosomal Trafficking and Inhibiting NRF2 Activation. Redox Biol. 41, 101942. Epub 2021 Mar 13. PMID: 33770521; PMCID: PMC8024711. doi:10.1016/j.redox.2021.101942
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Sushma, P. S., Jamil, K., Udaykumar, P., Aldakheel, F. M., Alduraywish, S. A., Alali, B. H., et al. (2021). Analysis of CCND1 Protein and Circulatory Antioxidant Enzyme Activity Association in Oral Squamous Cell Carcinoma. Saudi J. Biol. Sci. 28, 6987–6991. doi:10.1016/j.sjbs.2021.07.085
Szklarczyk, D., Gable, A. L., Lyon, D., Junge, A., Wyder, S., Huerta-Cepas, J., et al. (2019). STRING V11: Protein-Protein Association Networks with Increased Coverage, Supporting Functional Discovery in Genome-wide Experimental Datasets. Nucleic Acids Res. 47 (D1), D607–D613. doi:10.1093/nar/gky1131
Tang, Z., Huang, Z., Huang, Y., Chen, Y., Huang, M., Liu, H., et al. (2021). Ferroptosis: The Silver Lining of Cancer Therapy. Front. Cel Dev Biol 9, 765859. doi:10.3389/fcell.2021.765859
Tang, Z., Huang, Z., Huang, Y., Chen, Y., Huang, M., Liu, H., et al. (2021). Ferroptosis: The Silver Lining of Cancer Therapy. Front. Cel Dev Biol, 9. 765859PMC8667274. PMCID, PMID: 34912804. doi:10.3389/fcell.2021.765859
Trott, O., and Olson, A. J. (2010). AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 31 (2), 455–461. doi:10.1002/jcc.21334
Wang, X., Zhang, C., Zou, N., Chen, Q., Wang, C., Zhou, X., et al. (2022). Lipocalin-2 Silencing Suppresses Inflammation and Oxidative Stress of Acute Respiratory Distress Syndrome by Ferroptosis via Inhibition of MAPK/ERK Pathway in Neonatal Mice. Bioengineered 13 (1), 508–520. PMID: 34969358. doi:10.1080/21655979.2021.2009970
Wishart, D. S., Feunang, Y. D., Guo, A. C., Lo, E. J., Marcu, A., Grant, J. R., et al. (2018). DrugBank 5.0: a Major Update to the DrugBank Database for 2018. Nucleic Acids Res. 46, D1074–D1082. doi:10.1093/nar/gkx1037
Wong, T., and Wiesenfeld, D. (2018). Oral Cancer. Aust. Dent J. 63 Suppl 1, S91–S99. doi:10.1111/adj.12594
Wu, Y., Yu, C., Luo, M., Cen, C., Qiu, J., Zhang, S., et al. (2020). Ferroptosis in Cancer Treatment: Another Way to Rome. Front. Oncol. 10, 571127. doi:10.3389/fonc.2020.571127
Xu, X. L., Zhang, N. N., Shu, G. F., Liu, D., Qi, J., Jin, F. Y., et al. (2021). A Luminol-Based Self-Illuminating Nanocage as a Reactive Oxygen Species Amplifier to Enhance Deep Tumor Penetration and Synergistic Therapy. ACS Nano 15 (12), 19394–19408. Epub 2021 Nov 22. PMID: 34806870. doi:10.1021/acsnano.1c05891
Xuan, Z., Zhang, Y., Pan, Z., Zheng, X., and Huang, P. (2021). Natural Medicinal Ingredients Induce Tumor Ferroptosis and Related Mechanisms. J. Zhejiang Univ. (Med Sci. 50, 601–606. doi:10.3724/zdxbyxb-2021-0198
Yao, Z. J., Dong, J., Che, Y. J., Zhu, M. F., Wen, M., Wang, N. N., et al. (2016). TargetNet: a Web Service for Predicting Potential Drug-Target Interaction Profiling via Multi-Target SAR Models. J. Comput. Aided Mol. Des. 30, 413–424. doi:10.1007/s10822-016-9915-2
Ye, Z., Hu, Q., Zhuo, Q., Zhu, Y., Fan, G., Liu, M., et al. (2020). Abrogation of ARF6 Promotes RSL3-Induced Ferroptosis and Mitigates Gemcitabine Resistance in Pancreatic Cancer Cells. Am. J. Cancer Res. 10 (4), 1182–1193. PMID: 32368394; PMCID: PMC7191101.
Yi, J., Zhu, J., Wu, J., Thompson, C. B., and Jiang, X. (2020). Oncogenic Activation of PI3K-AKT-mTOR Signaling Suppresses Ferroptosis via SREBP-Mediated Lipogenesis. Proc. Natl. Acad. Sci. U S A. 117 (49), 31189–31197. Epub 2020 Nov 23. PMID: 33229547; PMCID: PMC7733797. doi:10.1073/pnas.2017152117
Zhang, T., Sun, B., Zhong, C., Xu, K., Wang, Z., Hofman, P., et al. (2021). Targeting Histone Deacetylase Enhances the Therapeutic Effect of Erastin-Induced Ferroptosis in EGFR-Activating Mutant Lung Adenocarcinoma. Transl Lung Cancer Res. 10 (4), 1857–1872. PMID: 34012798; PMCID: PMC8107764. doi:10.21037/tlcr-21-303
Zhao, X., and Chen, F. (2021). Propofol Induces the Ferroptosis of Colorectal Cancer Cells by Downregulating STAT3 Expression. Oncol. Lett. 22 (5), 767. Epub 2021 Sep 8. PMID: 34589146; PMCID: PMC8442167. doi:10.3892/ol.2021.13028
Keywords: oral cancer, GSH, ferroptosis, network pharmacology, targets
Citation: Huang C and Zhan L (2022) Network Pharmacology Identifies Therapeutic Targets and the Mechanisms of Glutathione Action in Ferroptosis Occurring in Oral Cancer. Front. Pharmacol. 13:851540. doi: 10.3389/fphar.2022.851540
Received: 10 January 2022; Accepted: 23 February 2022;
Published: 14 March 2022.
Edited by:
Jiaoti Huang, Duke University, United StatesCopyright © 2022 Huang and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chen Huang, Y2hlbmgwNjA4QDEyNi5jb20=
†These authors have contributed equally to this work.
 Chen Huang
Chen Huang Lei Zhan2†
Lei Zhan2†