- 1Graduate School, Guangxi University of Chinese Medicine, Nanning, China
- 2Department of Nephrology, The First Affiliated Hospital of Guangxi University of Chinese Medicine, Nanning, China
- 3Graduate School, Hunan University of Chinese Medicine, Changsha, China
- 4Department of Nephrology, Guangxi International Zhuang Medicine Hospital Affiliated to Guangxi University of Chinese Medicine, Nanning, China
Exosomes are small extracellular vesicles and play an essential role in the mediation of intercellular communication both in health and disease. Traditional Chinese medicine (TCM) has historically been used to maintain human health and treat various diseases up till today. The interplay between exosomes and TCM has attracted researchers’ growing attention. By integrating the available evidence, TCM formulas and compounds isolated from TCM as exosome modulators have beneficial effects on multiple disorders, such as tumors, kidney diseases, and hepatic disease, which may associate with inhibiting cells proliferation, anti-inflammation, anti-oxidation, and attenuating fibrosis. Exosomes, a natural delivery system, are essential in delivering compounds isolated from TCM to target cells or tissues. Moreover, exosomes may be the potential biomarkers for TCM syndromes, providing strategies for TCM treatment. These findings may provide a novel insight into TCM from exosomes and serve as evidence for better understanding and development of TCM.
Introduction
Intercellular communication is an essential hallmark of multicellular organisms to exchange information, which can be mediated via direct cell-to-cell contact or transfer of secreted molecules (Lee et al., 2012). Exosomes are small extracellular vesicles with a diameter of 30–150 nm involved in complex intercellular communication, which present in various biological fluids, such as blood, saliva, and urine (Andaloussi et al., 2013; Zabeo et al., 2017; Kalluri and LeBleu, 2020). Exosomes can be generated in the endolysosomal and multivesicular body compartments by most cells and secreted into the extracellular environment (Raposo and Stoorvogel, 2013). The process of exosome generation is associated with plasma membrane invagination, early-sorting and late-sorting endosome formation, multivesicular bodies construction, and outer membrane with the cellular plasma membrane fusion (Kalluri and LeBleu, 2020). Exosomes carry multiple intracellular signals, such as nucleic acids, proteins, lipids, and metabolites, for information and material exchange between cells, thus influencing their phenotype and cellular processes (Pegtel and Gould, 2019). Recipient cells uptake exosomes through cellular recognition, internalization, and cellular response (McKelvey et al., 2015; Mathieu et al., 2019).
Several studies demonstrated the pivotal role of exosomes in the diagnosis and treatment of multiple diseases, such as tumor diseases (Nam et al., 2020), metabolic disorders (Isaac et al., 2021), cardiovascular dysfunction (Barile et al., 2017), neurological injury (Zhang et al., 2019), and kidney disease (Jin et al., 2021). Notably, Exosomes, a bright star in drug delivery, have attracted researchers’ attention. Exosomes deliver nucleic acids, proteins, and small molecule drugs to target cells or tissues (Ferreira et al., 2022). Moreover, exosomes also enhance blood-brain barrier penetration (Liang L. et al., 2021). Collectively, exosomes hold significant promise with regard to biomarkers, therapies, and specific targeting.
Recently, exosome has become a hot spot in traditional Chinese medicine (TCM). The interplay between exosomes and TCM draws public attention increasingly. Growing evidence showed that TCM formulas, Chinese medicine monomers, or compounds isolated from TCM exhibit their effects on various diseases via modulating exosomes (Wei et al., 2016; Zheng et al., 2018; Liu et al., 2020; Wang X. J. et al., 2021). Moreover, exosomes may be the potential delivery carriers for compounds isolated from TCM (Dong M. et al., 2021a). TCM syndromes are closely associated with exosomes (He et al., 2021). These results demonstrate that exosomes may be a novel insight into TCM. The focus of this review is the interplay between exosomes and TCM.
Search strategy
We systematically searched the literature in electronic databases, including PubMed, Embase, Cochrane Library, Web of Science, and Clinical Trials.gov, from inception to 30 November 2021. Medical subject heading terms and free words were as search terms, including “exosome”, “extracellular vesicles”, “medicine, Chinese traditional”, “traditional medicine Chinese”, “Chinese herbal medicine”, etc. We also manually screened the reference list of eligible studies in case of missing appropriate studies. The detailed search strategies are described in Supplementary File S1. Articles were limited to English publications. Studies related to exosomes and TCM in vivo and/or in vitro were included. Reviews and conference abstracts were excluded.
A total of 1,179 citations from different databases were imported to the software Endnote X9. Literature was independently screened by two reviewers (CM and JZ) according to the inclusion and exclusion criteria. The full texts of eligible articles were further screened by the reviewers. Disagreements were resolved through discussion and consultation with a third investigator (GDH). Ultimately, 24 studies were included to further analysis, published from 2016 to 2021. The flow diagram is shown in Figure 1. Data, including the author’s name, year of publication, type of studies, characteristics of exosomes, effects, potential mechanisms, etc., were compiled in Tables 1–4.
Traditional chinese medicine
TCM, an essential part of the traditional Chinese culture, is one of the oldest healing systems with a long history of more than 2,000 years, gaining more and more attention due to its unique theoretical bases and wealth of experience. TCM is characterized by the concept of organic wholeness and treatment based on syndrome differentiation, which differs virtually from the bases of western medicine. The theories of TCM consist of different parts, including an integral whole, two opposing principles (Yin and Yang), three essential substances (Chi, blood, and essence), a four-seasons theory (spring, summer, autumn, and winter), five elements (wood, fire, earth, metal, and water). Humans are an integral whole based on the theories of TCM, including five internal organs (heart, liver, spleen, lungs, and kidneys), six hollow organs (gallbladder, stomach, large intestine, small intestine, bladder, and san jiao), and primary and collateral channels that links all of them together.
According to the TCM theories, the state of Yin and Yang keeps a dynamic balance in healthy persons. Disease syndromes will occur if the balanced state is disturbed by the cause of external evils, such as six evil (wind, cold, fire, dryness, dampness, summer heat) or endogenous factors, e.g., seven emotions stimulating (happiness, anger, worry, anxiety, sadness, fear, and terror) (Chan and Ng, 2020). Notably, four diagnostic methods (inspection, listening and smelling, inquiry, as well as pulse-taking and palpation) are indispensable steps in diagnosing TCM syndrome before taking therapeutic measures, namely “Pattern Differentiation and Treatment” (Tang et al., 2008). With respect to treatment, TCMs, including Chinese herbal medicine, natural products, acupuncture, moxibustion, acupoint application, or physical exercise, e.g., Tai Chi, Qigong, Baduanjin, are used to adjust yin-yang disharmony and boost the individual’s endogenous healing ability. Encouragingly, the effects of TCM on complicated miscellaneous diseases have been gradually revealed in recent years. For example, Tu’ Youyou won the 2015 Nobel Prize for discovering Artemisinin extracted from the Chinese herbal medicine Artemisia annua L. (Tu 2016). Artemisinin is essential for antimalarial treatment and brings light to malaria patients (Lyu H. N. et al., 2021). Although arsenic trioxide has been considered a poison since ancient times, it has significant effects on acute promyelocytic leukemia (Wang et al., 2020). Currently, as one of the strategies for the COVID-19 remedy, TCM has made meaningful and lasting contributions to treating COVID-19 (Lyu M. et al., 2021). Compared to western medicine alone, the combination of TCM and western medicine significantly improved clinical symptoms and reduced clinical deterioration and mortality in COVID-19 patients, indicating that TCM plays an irreplaceable role in the battle against COVID-19 (Huang et al., 2021; Zhao Z. et al., 2021).
Novel insight into understanding the connotation of TCM theory based on exosomes
TCM has established an abundant theoretical system. However, many theories of TCM cannot be accepted and interpreted by modern medicine due to the difference in origin and development background. With modern science and technology development, researchers commit themselves to seek TCM theory’s connotation and material basis. Cui et al. considered that the material basis of TCM theory should be characterized by uniqueness, specificity, and verifiability, which are indispensable (Cui et al., 2009). Uniqueness refers to the substance derived from a unique secretory source; specificity means that the substance plays an essential role in the physiological and pathological process of the secretory source and targeted goal; and verifiability can reflect the biological functions of the substance on the secretory source and targeted goal (Cui et al., 2009). The characteristics of exosomes, such as secretory source cells, powerful targeting, biological function, and biocompatibility, are consistent with the requirements of the material basis of TCM theory, indicating that exosomes may be the optimist material basis for understanding the connotation of TCM theory. For example, the TCM theory “kidney governs the bones and engenders marrow” can be explained by exosomes. Li et al. found that exosome-derived from HK-2 cells targeted osteoblasts and promoted their proliferation (Li et al., 2017), which provided a reference for further research on other TCM theories. TCM theories provide a hypothesis to study the interplay between exosomes and TCM, and exosomes offer a novel insight into the connotation and scientificity of TCM theories.
Exosomes modulators from TCM formulas
Since exosomes involve in the pathogenesis of various diseases (Aghabozorgi et al., 2019; Jia et al., 2021; Jing et al., 2021; Luo and Yi, 2021), investigating potential pharmaceutical agents with exosome-modulating ability has become a new field of drug. TCM formulas attract growing concern ascribe to their potential therapeutic in modulating exosomes.
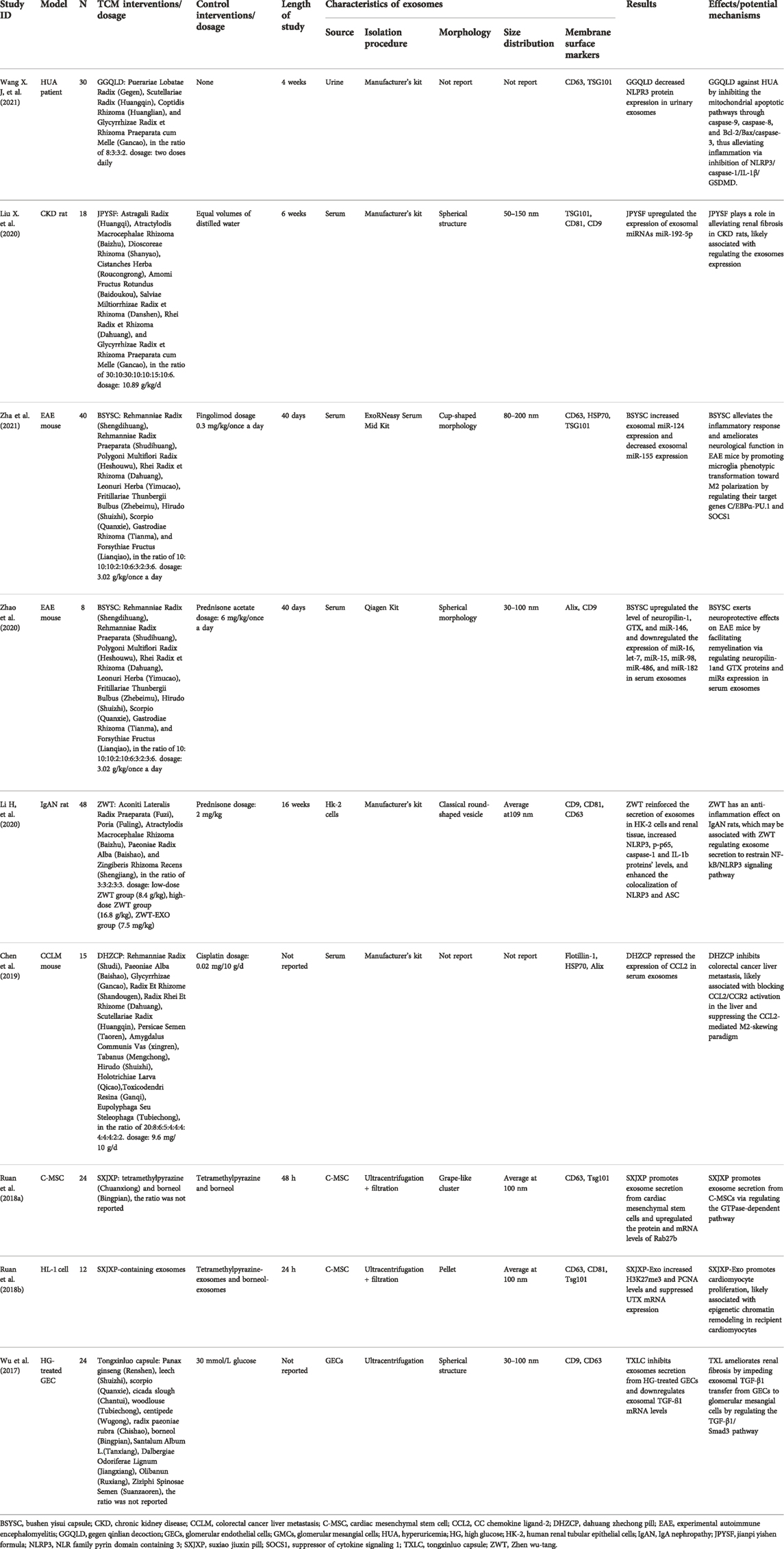
TCM formula refers to a group of two or more medicines appropriately combined according to syndrome differentiation and treatment principle, which has significant effects due to an enormously complex chemical cocktail (Bu et al., 2020). TCM formulas have been widely used in the treatment of multiple diseases. However, its pharmacological mechanism has not been fully illustrated. More and more researchers sought to explore the role and underlying mechanism of TCM formulas on various diseases from the perspective of exosomes. Exosomes modulators from TCM formulas on various diseases are discussed as follows (Table 1).
Gegen Qinlian decoction
Gegen Qinlian decoction (GGQLD), a classic TCM formula documented in <Shang Han Lun>, has been widely used to treat intestinal and metabolic diseases for hundreds of years in China (Lu et al., 2021). Wang et al. initiated a clinical trial to explore the anti-inflammatory effects of GGQLD on ameliorating hyperuricemia (HUA) patients (Wang Y. et al., 2021). Compared with before treatment, GGQLD significantly lowered serum uricemia levels and promoted urine uric acid excretion. Besides, GGQLD downregulated the NLR family pyrin domain containing 3 (NLRP3) expression in urinary exosomes and peripheral blood mononuclear cells. Furthermore, the protection of GGQLD against HUA was verified in Oxonic acid-treated rat models and UA-induced proximal tubular epithelial cells (PTECs). As a result, GGQLD significantly alleviated the expression of NLRP3, IL-1β, caspase-1/3/8, Gasdermin D (GSDMD), and Bcl-2 in vivo and in vitro. The results indicated that GGQLD exhibits an anti-inflammatory effect on HUA by inhibiting the NLRP3/caspase-1/IL-1β/GSDMD pathways and the mitochondrial apoptotic pathways. However, the study has not reported whether GGQLD affects the expression of exosomes in serum or plasma in HUA. Further studies are warranted to explore the molecular mechanisms of GGQLD against HUA via exosomes.
Jianpi Yishen formula
Jianpi Yishen formula (JPYSF), a TCM formula with notable renoprotective, antioxidative, anti-inflammation, and antiapoptosis effects, has been widely used to treat chronic kidney diseases (CKD) for decades (Liu et al., 2018; Zhou F. et al., 2021). Liu et al. conducted a study to investigate whether the underlying mechanisms of JPYSF on CKD are related to serum exosomal miRNAs (Liu et al., 2020). In the CKD rat model, the levels of exosomal miR-192-5p, miR-194-5p, miR-802-5p, and miR-143-3p were markedly downregulated, while JPYSF treatment significantly reversed these expressions. Further identified by real-time PCR showed that only miR-192-5p was significantly restored in the JPYSF treatment group, and its ROC curve for the diagnosis of CKD was 77.8% after JPYSF treatment, indicating that exosomal miR-192-5p was the most potential target for CKD for JPYSF treatment and CKD diagnosis. miR-192 is one of the most abundant miRNAs in the kidney and contributes to alleviating renal fibrosis by inhibiting TGF-β/Smad3 signaling (Chung et al., 2010). Furthermore, miR-192-5p suppresses the mRNA of the β1 subunit of the Na+/K + -ATPase, exerting effects on renal handling of fluid balance on a high-salt diet (Mladinov et al., 2013). Nevertheless, the role and mechanism of miR-192-5p in JPYSF-treated CKD have not been clarified. Whether JPYSF plays a role in alleviating renal fibrosis via targeting miR-192-5p to inhibit the activation of TGF-β/Smad3 signaling and JPYSF keeps renal handling of fluid balance by targeting miR-192-5p to modulate Na+/K + -ATPase through in CKD warrants further exploration.
Bushen Yisui capsule
Bushen Yisui capsule (BSYSC), a Chinese compound medicine comprising ten herbs, has significant effects on anti-neuroinflammatory and neuroprotective in multiple sclerosis and its animal model of experimental autoimmune encephalomyelitis (EAE) (Fang and Zhang, 2017; Zha et al., 2021). Zhao et al. conducted an experimental study to explore the potential roles of BSYSC in promoting remyelination via regulating exosomes in EAE mice (Zhao et al., 2020). Compared with normal mice, EAE mice had a significant increase in neuropilin-1 and GTX expressions in serum exosomes, while BSYSC could reverse the phenomenon in EAE mice. Besides, the serum exosomal miR-146 level in EAE mice treated with BSYSC markedly upregulated, while the expression of miR-16, let-7, miR-15, miR-98, miR-486, and miR-182 decreased. The disequilibrium of Neuropilin-1 and GTX led to remyelination failure (Boyd et al., 2013), indicating that BSYSC facilitates remyelination via regulating neuropilin-1and GTX proteins and miRs expression in serum exosomes, thus exerting neuroprotective effects. However, the mechanism of BSYSC regulating the expression of neuropilin-1and GTX proteins and miRs in serum exosomes and whether BSYSC plays a direct or indirect role in the regulation of remyelination via exosomes has not been elucidated, warranting further exploration.
Another experimental study was conducted by Zha et al. to investigate the potential roles of BSYSC on microglial polarization in EAE mice. Compared with EAE mice, serum exosomal miR-155 expression was markedly increased, accompanied by downregulating exosomal miR-124 expression in EAE mice treated with BSYSC. Furthermore, the levels of C/EBPα and PU.1 were downregulated after BSYSC treatment, while the suppressor of cytokine signaling 1 (SOCS1) expression was upregulated. C/EBPα and PU.1 are downstream target genes of miR-124. It has been shown that miR-124 alleviates EAE by skewing microglial polarization from the M1 phenotype to the M2 phenotype via targeting C/EBPα-PU.1 (Ponomarev et al., 2011). SOCS1 is a target gene of miR-155 and plays a critical role in promoting differentiation from the M1 to M2 (Whyte et al., 2011; Zhang et al., 2020). The findings indicated that BSYSC alleviates the inflammatory response and ameliorates neurological function in EAE through promoting microglia phenotypic transformation toward M2 polarization to modulate the expression of pro/anti-inflammatory factors, which may be associated with regulating the expression of miR-124 and miR-155 (Zha et al., 2021). However, the role of exosomal miR-124 and miR-155 in BSYSC-mediated microglia M2 polarization in EAE remains unclear, needing further studies to illuminate. Moreover, whether the effects of BSYSC-mediated microglia M2 polarization in vitro also requires to be verified in cell experiments.
Zhenwu tang
Zhenwu Tang (ZWT) is a well-known prescription from Treatise on Febrile Diseases written by Zhongjing Zhang. It has been widely used in Parkinson’s and various kidney diseases, such as IgA nephropathy (IgAN), membranous nephropathy, and diabetic nephropathy (Cai et al., 2010; Li et al., 2011; Liu B. et al., 2019; Li J. et al., 2020). Li et al. implemented a study to investigate the potential mechanisms of ZWT on IgAN from the perspective of exosomes (Li J. et al., 2020). The rats were injected with exosomes isolated from HK-2 cells treated with 10% ZWT by tail vein as the ZWT-EXO group. Compared with the model group, CD63 fluorescence was notably intensified in the ZWT group, and the levels of CD63, CD81, and CD9 in the renal tissues were also dramatically upregulated in the ZWT group, indicating that ZWT reinforces the secretion of exosomes in IgAN rats. In addition, the protein levels of NLRP3, p-p65, caspase-1, and IL-1b were increased, and the colocalization of NLRP3 and ASC was highly expressed in the kidney tissues of IgAN rats, while these expressions were markedly reversed after following ZWT-EXO and ZWT treatment. Nuclear factor-kappa B (NF-κB) is an early responding regulator related to the expression of numerous proteins involved in inflammation (Wang et al., 2018). The transcription NF-kB promotes NLRP3 activation, which recruits ASC and caspase-1 proteins and leads to the release of IL-1b and IL-18, thus inciting phlogistic response (Ma et al., 2019). ZWT has an anti-inflammation effect on IgAN, which may be associated with ZWT regulating exosome secretion to restrain NF-kB/NLRP3 signaling pathway, thereby attenuating renal dysfunction. The cargoes in exosomes include proteins, miRNAs, long noncoding RNAs, mRNAs, and lipids; however, this study did not identify which one was regulated by ZWT. And whether ZWT inhibits the NF-kB/NLRP3 signaling pathway via regulating its downstream or upstream target genes in IgAN also needs further exploration.
Dahuang zhechong pill
Dahuang Zhechong Pill (DHZCP) is a famous and classical formula from “Synopsis of Prescriptions of the Golden Chamber.” DHZCP has been widely used in the treatment of atherosclerosis, and gynecological disease, especially in hepatic diseases in China (Ji et al., 2007; Yuan 2009; Gong et al., 2020). To investigate whether DHZCP suppresses the metastasis of colorectal cancer, Chen et al. established a colorectal cancer liver metastasis model (Chen et al., 2019). Compared with the model group, DHZCP dramatically decreased serum exosomal CC chemokine ligand-2 (CCL2) level and its receptor CCR2. Exosomal CCL2 triggered macrophage recruitment and transformed the M1/M2 paradigm to a M2 phenotype in the liver; luckily, DHZCP treatment curbed the condition. CCL2/CCR2 is a prime factor in triggering fibrosis and promoting tumor metastasis by inducing macrophage accumulation (Fei et al., 2021; Klueh et al., 2016). M2 macrophages play critical roles in fibrosis, collagen synthesis, tissue remodeling, and pro-tumor processes (Mantovani et al., 2013; Braga et al., 2015; Shapouri-Moghaddam et al., 2018). The macrophage polarization states accelerate tumor growth, especially by skewing macrophages toward the M2 phenotype (Ricketts et al., 2021). The results indicated that DHZCP inhibits colorectal cancer liver metastasis, likely associated with blocking CCL2/CCR2 activation in the liver and suppressing the CCL2-mediated M2-skewing paradigm. However, further studies should focus on the mechanisms of DHZCP regulating CCL2/CCR2.
Suxiao Jiuxin Pill
Suxiao Jiuxin pill (SXJXP), one of the Chinese patent medicines included in the Chinese Pharmacopeia, is well-known for notable cardioprotective effects (Ren et al., 2018). Ruan et al. studied the impact of SJP on exosome secretion in cardiac mesenchymal stem cells (C-MSCs) treated with SXJXP (Ruan et al., 2018a; Ruan et al., 2018b). SXJXP increased exosomes secretion from C-MSCs and upregulated the protein and mRNA levels of Rab27b; however, Rab27b knockdown inhibited exosome secretion. Rab27b is a small GTPase in the Rab family and controls exosome secretion (Ostrowski et al., 2010), indicating that SXJXP promotes exosome secretion from C-MSCs via regulating the GTPase-dependent pathway. Results of another study showed that SXJXP-Exo increased histone-3-lysine 27 trimethylation (H3K27me3) and proliferating cell nuclear antigen (PCNA) levels, accompanied by suppressing UTX mRNA expression in the HL-1 cells, a mouse cardiomyocyte line. H3K27me3, a critical epigenetic chromatin marker, promotes cell proliferation (Hansen et al., 2008). UTX is a demethylase of H3K27me3. The H3K27me3 demethylation is essential for the induction of direct cardiac reprogramming (Dal-Pra et al., 2017). Therefore, SXJXP-Exo promotes cardiomyocyte proliferation, likely associated with epigenetic chromatin remodeling in recipient cardiomyocytes.
TongXinLuo capsule
Tongxinluo Capsule (TXLC) possesses a variety of pharmacological effects, including antihypertensive, improving ventricular remodeling, and antioxidant, protecting podocytes, which has beneficial effects on angina pectoris and CKD (Li et al., 2021; Mao et al., 2015). Wu et al. conducted an experimental study to investigate the role of TXLC on exosomes secretion from high glucose (HG)-treated glomerular endothelial cells (GECs) (Wu et al., 2017). Wu found that TXLC repressed the expression of TGF-β1-containing exosomes secretion from HG-Treated GECs. TGF-β1/Smad3 signaling has been considered a significant pathway in glomerular mesangial cells (GMCs) activation and renal fibrosis (Yu et al., 2022). Exosomes secretion from HG-Treated GECs dramatically increased the deposition and secretion of Col-IV and FN and upregulated TGF-β1 and phospho-Smad3 in GMCs. Luckily, TXLC curbed these phenomena. The findings suggested that TXL ameliorates renal fibrosis by impeding exosomal TGF-β1 transfer from GECs to GMCs by regulating the TGF-β1/Smad3 pathway. However, whether TXLC has beneficial effects on patients with diabetic kidney disease via the regulation of exosomes remains elusive.
Exosomes modulators from compounds isolated from TCM
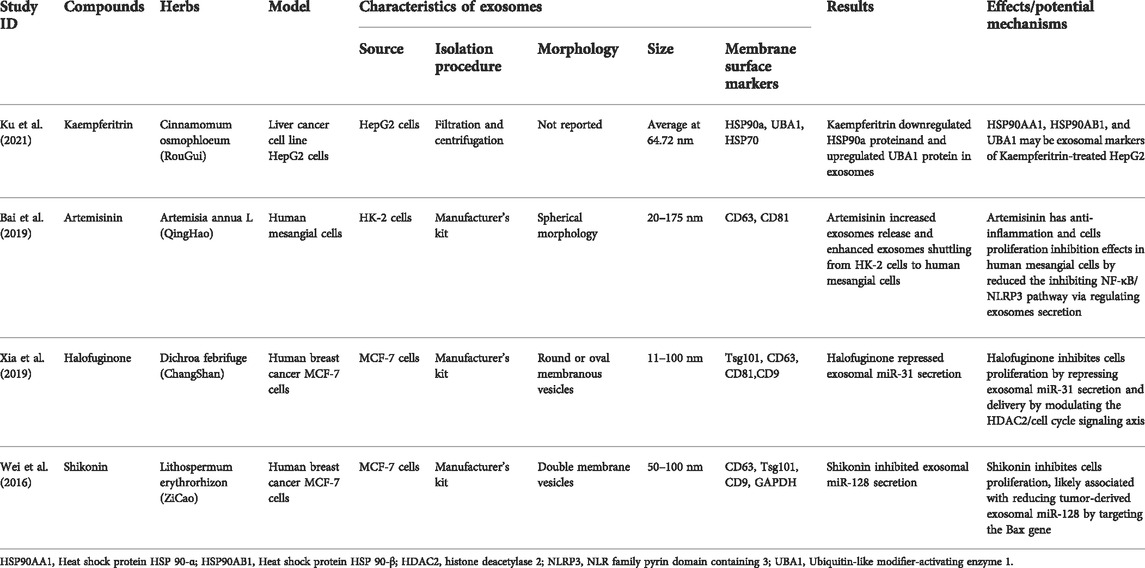
Natural products are compounds derived from various natural sources, such as plants, animals, and micro-organisms, which play a vital role in human health (Bernardini et al., 2018). It has been reported that natural products derived from TCM, such as Artemisinin, have significant effects on various diseases by modulating exosomes (Bai et al., 2019; Qiu et al., 2022). Compounds isolated from TCM are typically categorized as alkaloids, flavonoids, saccharides, saponins, terpenoids, and polyphenols based on their chemical property. Researchers have discovered the modulators of exosomes from compounds isolated from TCM and sought to clarify their mechanisms. The information on exosome modulators from compounds isolated from TCM is compiled in Table 2.
Kaempferitrin
Kaempferitrin, a potent flavonoid compound derived from the leaves of Cinnamomum osmophloeum, is well-known for its anti-diabetic, antioxidant, and anti-inflammatory properties (Li et al., 2013; Jiang et al., 2018; Wang et al., 2019). Ku et al. explored the effects of Kaempferitrin on HepG2 differentially expressed exosomes and extracellular vesicle sizes (Ku et al., 2021). The proteins expressions of Heat shock protein HSP 90-α (HSP90AA1), Heat shock protein HSP 90-β (HSP90AB1), and Ubiquitin-like modifier-activating enzyme 1(UBA1) were differentially expressed in exosomes from Kaempferitrin-treated HepG2 group and the control group, of which HSP90a was downregulated and UBA1 was upregulated. The extracellular vesicle size was more significant in HepG2 cells treated with Kaempferitrin than in the control group. The results suggested that HSP90AA1, HSP90AB1, and UBA1 may be exosomal markers of Kaempferitrin-treated HepG2. And the extracellular vesicle sizes may be regulated by kaempferitrin. Kaempferol, a molecule with a structure similar to kaempferitrin, plays a crucial role in activating the membrane-bound ATPase, which provides energy during membrane budding from the cytoplasm (Al-Numair et al., 2015). Whether Kaempferitrin regulates the exosomal size and membrane assembly by modulating ATPase activities requires further exploration. Besides, the role and potential mechanism of exosome secretion and size regulated by Kaempferitrin on disease pathogenesis remain unclear, which will be an interesting direction to research.
Artemisinin
Artemisinin, a sesquiterpene lactone isolated from Artemisia annua L. with outstanding antimalarial effect, has been regarded as the most effective and impactful antimalarial drug (Paddon et al., 2013). Except for its antimalarial effect, ART has exhibited effects on antioxidant, anti-inflammation, anti-fibrotic, and anti-tumor (Dolivo et al., 2021; Meng et al., 2021). Bai et al. conducted a study investigating whether Artemisinin could regulate inflammation factors by mediating exosome release in vivo (Bai et al., 2019). Artemisinin increased exosomes release in human renal tubular epithelial cells (HK-2) and enhanced exosomes shutting from HK-2 cells to human mesangial cells (HMCs). Mesangial cells are essential to maintain the normal function of the glomerulus, which play important roles in structural support and injury repair (Nowling 2022). The inflammatory states accelerate mesangial cells proliferation, a major contributor to glomerulosclerosis (Donegan et al., 2016). NF-κB, an upstream activator of the NLRP3 inflammasome, is known to regulate inflammatory processes in many diseases, including chronic kidney diseases (Lei et al., 2020). The protein expressions of IκB-α, p-p65, NLRP3, ASC, IL-1β, and caspase-1 were upregulated in HMCs, while these increased expressions were markedly curbed by Artemisinin. These findings suggested that ART has a positive anti-inflammation in HMCs, which may be associated with inhibiting NF-κB/NLRP3 pathway via regulating exosomes secretion, thereby ameliorating renal damage. However, the mechanisms of exosomes how to regulate the NF-κB/NLRP3 pathway have not been elucidated.
Halofuginone
Halofuginone, a minor alkaloid extracted from the Chinese traditional herb Dichroa febrifuge, has significant anti-tumor, anti-hypertrophic, and anti-fibrotic properties (Pines and Spector, 2015; Jain et al., 2021). Xia et al. investigated the mechanism of Halofuginone inhibiting MCF-7 cell growth in vitro from the perspective of exosomes (Xia et al., 2019). As shown in the results, Halofuginone inhibited MCF-7 cell growth via repressing exosome secretion. Further miRNA profiles analysis showed that miR-100, miR-222, miR-31, miR-200, miR-223, and miR-21 were more abundant in exosomes secretion from MCF-7 cells treated with Halofuginone than in MCF-7 cells. And the inhibition of MCF-7 cell proliferation was strengthened only in the miR-31 knockdown exosomes, whereas miR-31 overexpression could attenuate Halofuginone-inhibited MCF-7 cell proliferation. Importantly, the level of HDAC2 was reduced by pre-miRNA of MCF-7-derived exosomal miR-31. HDAC2 siRNA repressed the level of cell cycle components in the MCF-7 cell’s G1/S transition, thereby inhibiting the MCF-7 cell’s growth. These findings suggest that Halofuginone inhibits MCF-7 cell proliferation by repressing exosomal miR-31 secretion and delivery by modulating the HDAC2/cell cycle signaling axis, thus exerting an anti-tumor effect. However, it remains to investigate whether Halofuginone inhibits tumor progress by modulating exosomal miR-31 in vivo.
Shikonin
Shikonin, a naphthoquinone isolated from Lithospermum erythrorhizon, is well-known for its strong anti-tumor effects (Sun et al., 2022). It has been used to treat various cancers, such as colon, renal, and lung cancer (Pan et al., 2021; Shi G. et al., 2021; Tsai et al., 2021). To elucidate the roles and mechanisms of Shikonin on breast cancer, Wei et al. performed a study on MCF-7 cells with Shikonin treatment (Wei et al., 2016). Shikonin inhibited MCF-7 cell proliferation and exosome secretion from MCF-7 cells with a positive relationship. According to the results from analyzing miRNA profiles and qRT-PCR, exosomal miR-128 positively inhibited MCF-7 cell proliferation. Bax, a key regulator of the intrinsic or mitochondrial apoptosis pathway, has been reported as a target of miR-128 (Ji et al., 2013; Cosentino et al., 2022). Exosomal miR-128 significantly suppressed Bax expression in recipient MCF-7 cells. Therefore, Shikonin inhibits MCF-7 cell proliferation, which is likely associated with reducing tumor-derived exosomal miR-128 by targeting the Bax gene. However, the regulatory effects of Bax on the cell cycle modulation have not been clarified. Whether the mechanisms of Bax in MCF-7 cells proliferation is related to mitochondrial morphological changes or mitochondrial apoptosis pathway is also not well understood.
Exosomes as delivery vehicles for compounds isolated from TCM
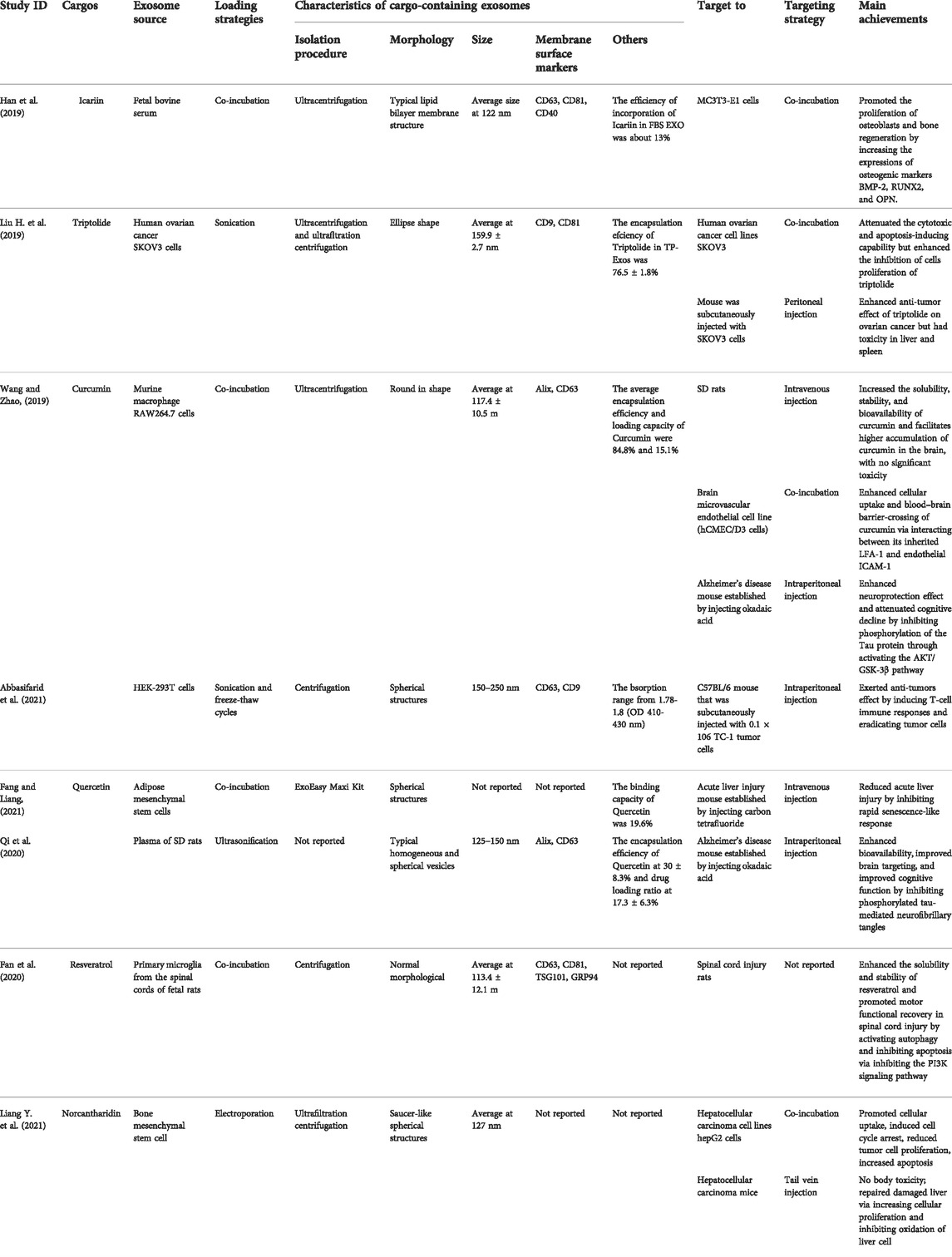
Chinese herbal medicine is composed of various compounds with multiple pharmacological effects. However, compounds isolated from TCM are confronted with obstacles in the application due to their poor water solubility, poor intestinal absorption, and low bioavailability, resulting in decreased therapeutic efficacy (Puglia et al., 2017). Thus, there is an urgent need for TCM compound-based drug delivery systems to overcome these constraints to maximize the performance of TCM in therapy. Exosomes are regarded as a natural delivery system due to their excellent biodistribution, biocompatibility, and low immunogenicity (Liao et al., 2019; Gutierrez-Millan et al., 2021). Recently, many studies have confirmed the feasibility of using exosomes for drug delivery in various diseases, exhibiting enhanced curative effects. For example, exosomes derived from dendritic cells delivered siRNA across the blood-brain barrier in wild-type mice, demonstrating the therapeutic potential of exosome-mediated siRNA delivery (Alvarez-Erviti et al., 2011). Doxorubicin-loaded exosomes showed great efficiency in breast cancer cells (Xie et al., 2021). Thus, exosomes as delivery vehicles for compounds isolated from TCM have become a hotspot in pharmaceutical research and therapeutics (Table 3).
Icariin
Icariin, a prenylated flavonoid, is one of the main components of Herba Epimedii (Li et al., 2015). It has been reported that Icariin possesses various biological effects, including osteoporosis prevention, ameliorating sexual dysfunction, modulation of the immune system, improvement of cardiovascular function, anti-inflammatory, antioxidant, and anti-depressant (Fang et al., 2017; Angeloni et al., 2019). Although icariin has significant efficacy on diverse diseases, such as osteoporosis, the main challenge remains its very low solubility, permeability, and poor bioavailability (Han et al., 2019).
Fetal bovine serum (FBS)-derived exosomes as natural nanoscale carriers have been studied to deliver Icariin (Exo- Ica). The results showed that Icariin-containing exosomes significantly increased the proliferation of MC3T3-E1 cells and the protein levels of osteogenic markers Bone morphogenetic protein-2 (BMP-2), Runt-related transcription factor 2 (RUNX2), and Osteopontin (OPN) compared to those treated with Icariin and FBS exosomes, indicating that Exo-Ica effectively promotes the proliferation of osteoblasts and bone regeneration (Dong M. et al., 2021a). However, the mechanisms of cellular uptake of Exo-Ica and Exo-Ica on promoting osteoblast proliferation remain unknown. Runx2 is an important transcriptional factor involved in osteogenic differentiation, and its deletion leads to a disorder of bone formation (Takahata et al., 2021). AMP-activated protein kinase (AMPK) regulates the differentiation of osteoblasts and bone formation (Li et al., 2018). It has been demonstrated that the activation of the AMPK/Runx2 pathway is an important mechanism underlying the facilitating effects of agents against osteogenic differentiation in MC3T3-E1 cells (Wang Q. et al., 2022). Thus, whether Exo-Ica enhances osteogenic differentiation in MC3T3-E1 cells by regulating the AMPK-α/Runx2 pathway may be an interesting direction in the next studies.
Triptolide
Triptolide, a natural diterpene triepoxide compound isolated from Tripterygium wilfordii Hook F, has promising multiple pharmacological activities, particularly anti-inflammatory, immunosuppressive and anti-tumor activities (Yuan et al., 2019; Gao et al., 2021). However, the application of TPL in the clinic is restricted due to its multiorgan toxicity and poor solubility (Hou et al., 2019).
Triptolide was loaded into exosomes derived from human ovarian cancer SKOV3 cells, and the effects of the triptolide-loaded exosomes (Exos-TP) delivery system on ovarian tumors was observed both in vitro and in vivo (Liu H. et al., 2019). The cytotoxicity and apoptosis-inducing capability of Exos-TP were weaker than that of free triptolide in SKOV3 cells, but the inhibitory effect of Exos-TP on cell proliferation was superior to free triptolide. Interestingly, Exos-TP significantly enhanced the inhibition of SKOV3 cells proliferation at 24 h but not at 48 h, likely associated with TP-Exo blocked cells in the S phase at 24 h and G0/G1 phase at 48 h. The effects of Exos-TP on proliferation and apoptosis of cells were confirmed in vivo, which was consistent with that in vitro. Besides, the tumor suppression of TP-Exo was significantly better than that of free triptolide in vivo. Although the pathological damage in the kidney, heart, lung, and ovary was not observed in all groups, the pathological damage in the liver was obvious in SKOV3 cells-derived exosomes group and TP-Exo group, and the spleen damage was in the free triptolide group and TP-Exo group, indicating that SKOV3 cells-derived exosomes in TP-Exo and triptolide in TP-Exo may be the cause of liver injury and spleen injury, respectively. Together, these findings suggested that TP-Exo can enhance the inhibitory effect of triptolide on tumor cell proliferation (Liu B. et al., 2019). However, the cytotoxicity of TP-Exo on liver and spleen injury needs to be further optimized to solve, and the underlying mechanisms of TP-Exo anti-tumor effects also require further study.
Curcumin
Curcumin, a polyphenol compound extracted from the rhizomes of Curcuma longa L. (Zingiberaceae) (Kharat et al., 2017), has diverse pharmacological effects, including anti-tumor, anti-inflammatory, and antioxidative activities (Memarzia et al., 2021). However, its clinical application has been limited due to its poor water solubility and stability, bioavailability, and poor brain targeting.
Compared with free curcumin, exosomes derived from murine macrophage RAW264.7 cells with encapsulated curcumin (Exos-Cur) significantly improved the solubility, stability, and bioavailability in vivo. Besides, Exos-Cur enhanced cellular uptake and blood-brain barrier penetration via interacting with the inherited LFA-1 and endothelial ICAM-1 in vitro (Wang et al., 2019). Exos-Cur have great potential in enhancing neuroprotection and attenuating cognitive decline effects in Alzheimer’s disease therapy, as a study on Alzheimer’s disease mice showed that Exos-Cur markedly relieved the symptoms of Alzheimer’s disease by inhibiting phosphorylation of the tau protein via activating the AKT/GSK-3β pathway (Wang et al., 2019). A recent study supported that HEK-293T cells-exosomes loaded with curcumin significantly increased the levels of total IgG, IgG2a, IgG2b, IFNγ, Granzyme B, and lymphocyte proliferation in the C57BL/6 mice model injected with TC-1 tumor cells (Abbasifarid et al., 2021). The results indicate that the induction of T-cell immune responses eradicates tumor cells, thereby exerting anti-tumor effects. However, the cellular uptake mechanism of the exosomal curcumin has not been illustrated.
Quercetin
Quercetin, a phenolic flavonol compound, can be extracted from multiple Chinese medicine herbs, including Mulberry leaves, Radix Bupleuri, licorice, Astragali Radix, and Panax notoginseng (Shi W. et al., 2021; Zhao J. et al., 2021; Chen et al., 2022; Tu et al., 2022). Accumulating studies have shown that quercetin has diverse pharmacological effects, including antioxidant, anti-aging, anti-fungal, anti-tumor, anti-inflammatory, anti-depressant, and hepatoprotective activities (Arabpour et al., 2021; Zou et al., 2021; Chen et al., 2022). However, it has not been fully harnessed in the clinic due to its low bioavailability.
A previous study has reported that exosomes derived from adipose mesenchymal stem cells (ASCs) show beneficial effects on liver diseases (Qu et al., 2017). Quercetin was encapsulated into ASCs-derived exosomes, and the effect of quercetin-loading exosomes on acute liver injury was studied (Fang and Liang, 2021). It was found that exosomal quercetin is more stable than free quercetin. Furthermore, the liver index, aging-related genes P16 and P21 levels, senescence-associated secretory phenotype markers IL-6, Ccl2, and Cxcl2 expressions were significantly reduced in the quercetin-laden exosomes group compared to the model group and the exosomes group, suggesting that quercetin-laden exosomes exhibit hepatoprotective effects on acute liver injury by inhibiting rapid senescence-like response and quercetin may enhance the therapeutic efficacy of ASCs-derived exosomes in liver disease (Fang and Liang, 2021). Nevertheless, whether the roles of quercetin in acute liver injury are enhanced by exosomes requires further research. Another study supported that exosomes loaded with quercetin not only significantly enhanced the bioavailability of quercetin and its accumulation in the brain region in SD rats but also improved cognitive function in Alzheimer’s disease mice by inhibiting the formation of insoluble neurofibrillary tangles by reducing cyclin-dependent kinase five mediated phosphorylation of tau protein (Qi et al., 2020), indicating exosomal quercetin as a potent inhibitor of tau protein aggregation in Alzheimer’s disease.
Resveratrol
Resveratrol, a nonflavonoid polyphenol phytoalexin, is broadly presented in grapes, giant knotweed, peanuts, etc. (Tian and Liu, 2020). Resveratrol is also enriched in the root of Polygonum cuspidatum, a well-known traditional Chinese medicine (Hu and Li, 2019). It is quite famous for various biological effects, including antioxidative, anti-tumor, anti-inflammation, anti-fibrosis, and neuroprotective activities (Parsamanesh et al., 2021; Uddin et al., 2021). Despite its potential for these effects on multiple diseases, the clinical usage of resveratrol is limited due to its pharmaceutical limitations, such as low bioavailability and poor bioavailability (Robertson et al., 2022).
Primary microglia-derived exosomes with encapsulated resveratrol (Exo-Res) have been shown to enhance the solubility and stability of resveratrol both in vivo and in vitro (Fan et al., 2020). A recent study supported that Exo-Res could increase muscle tension in hind limbs and improve foot functional movements in spinal cord injury rats. Furthermore, Exo-Res significantly inhibited apoptosis-related proteins caspase-3 and TUNEL expressions, accompanied by increasing autophagy-related proteins LC3B and Beclin-1 levels and p-PI3K expression in spinal cord injury rats (Fan et al., 2020). These findings suggested that Exo-Res has the potential for promoting motor functional recovery in spinal cord injury by activating autophagy and inhibiting apoptosis via the PI3K pathway. Nevertheless, the mechanism of autophagy that inhibits apoptosis has not been fully clarified. The release and targeting of resveratrol loaded in exosomes both in vivo and in vitro needs to be further studied.
Norcantharidin
Norcantharidin (NCTD), a derivative of Cantharidin isolated from the dried body of Mylabris phalerata Pallas, has various pharmacological activities, including anti-tumor, anti-inflammatory, and anti-fibrosis properties (Zhou et al., 2020). Norcantharidin has been used to treat lung, breast, bladder, hepatic carcinoma, and prostate cancers (Pan et al., 2020). However, the clinical usage of Norcantharidin is restricted due to its poor water solubility, low tumor-targeting efficiency, and short half-life (Chi et al., 2019).
Bone mesenchymal stem cell-derived exosomes (BMSC-Exos) were used as drug carriers to encase NCTD (BMSC-Exos-NCTD), and its potential therapeutic effects against hepatocellular carcinoma (HCC) were explored (Liang Y. et al., 2021). The drug release study showed the release of NCTD was continuous and slow in vitro after it was packaged into BMSC-Exos. Compared with free NCTD, BMSC-Exos-NCTD significantly facilitated cellular uptake, induced cell cycle arrest in the G2 phase, inhibited tumor cell proliferation, decreased cell migration and invasion, and induced apoptosis in HepG2 cells. Interestingly, BMSC-Exos also inhibited HepG2 cell proliferation but was weaker than that of BMSC-Exos-NCTD treatment. Besides, BMSC-Exos-NCTD showed more obvious tumor inhibition effects in vivo than NCTD treatment alone, with no obvious tissue damage in the liver and kidney. The fluorescence intensity of ROS in the NCTD group was enhanced but weakened in the BMSC-Exos-NCTD group in the normal liver cell line L02. Importantly, Cy5.5, a fluorescent probe used to label BMS-Exos, was only concentrated in the liver tissues, while BMS-Exos-Cy5.5 enriched in liver tissues and tumor areas, especially in tumor areas, indicating that BMSC-Exos exhibit a homing effect on the tumor sites of HCC mice(Liang L. et al., 2021). Collectively, BMSC-Exos can be used as safe and effective drug-delivery carriers for HCC therapy. BMSC-Exos-NCTD has beneficial effects on anti-tumor and repairing liver cells in HCC without obvious toxicity. The mechanism of BMSC-Exos-NCTD on hepatocyte repairing may be related to increase cellular proliferation and inhibit the oxidation of liver cells. However, the underlying mechanisms of BMSC-Exos and BMSC-Exos-NCTD on HCC therapy have not been fully explained, hoping to be explored in future studies.
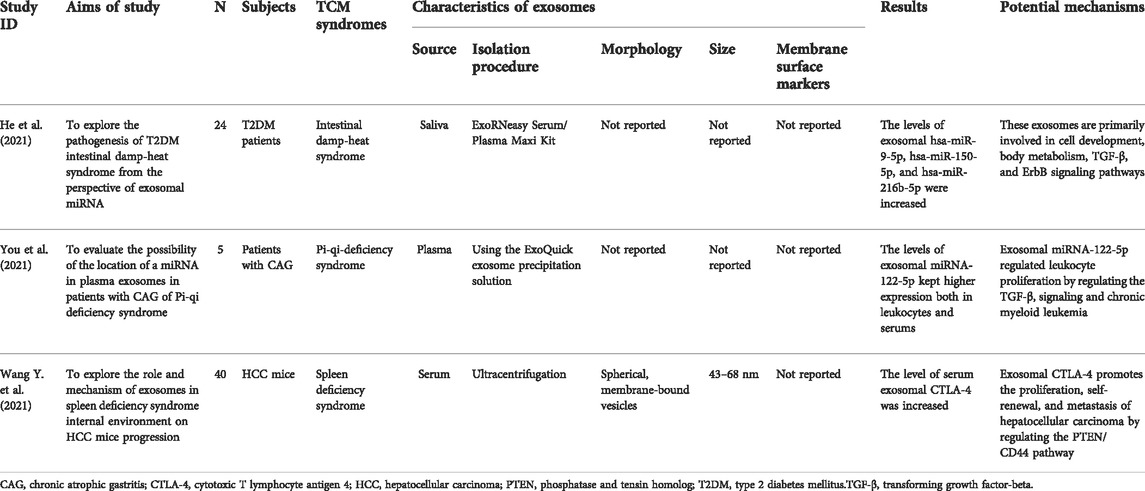
Interplay between exosomes and TCM syndromes
Studies associated with TCM syndromes and exosomes have attracted more and more researchers’ attention. TCM syndrome, also known as “zheng” in Chinese, is a vital part of TCM theory and an abstract generalization of the pathological changes of a disease at a certain phase, which reveals inherent pathological variations of signs and symptoms (Su et al., 2014). In the clinical practice of TCM, TCM syndrome is of great significance for identifying human body patterns and guiding TCM clinicians to conduct an individual diagnosis and treatment with TCM herbs. Although there are few studies on TCM syndromes and exosomes, exosomes may provide a novel insight into TCM syndromes. Studies associated with TCM syndromes and exosomes are presented in Table 4.
Intestinal damp-heat syndrome
He et al. initiated a controlled study to explore the pathogenesis of type 2 diabetes mellitus (T2DM) intestinal damp-heat syndrome (IDHS) from the perspective of exosomal miRNA, which enrolled 24 patients with IDHS of T2DM and 24 healthy people (He et al., 2021). According to the results of chIp-sequencing and qPCR verification, exosomal hsa-miR-9-5p, hsa-miR-150-5p, and hsa-miR-216b-5p were significantly increased in the IDHS patients compared with the healthy group. These exosomal miRNAs are mainly enriched in cell development, body metabolism, and TGF-β signaling pathways. miR-9-5p exhibits effects on angiogenesis, anti-inflammation, and antioxidation. miR-9-5p inhibitor inhibited insulin release and triggered oxidative stress in streptozocin-induced INS-l cells (Ma et al., 2021). Exosomal miR-150-5p secreted by renal tubular epithelial cells activated fibroblasts and aggravated renal fibrosis (Zhou X. et al., 2021). Silencing of miR-150-5p played a renoprotective role in diabetic kidney disease (Dong W. et al., 2021b). The activation of the TGF-β signaling pathway contributes to metabolic disorders and fibrosis (Hou et al., 2018; Ji et al., 2022). Collectively, we infer that these exosomes may involve in oxidative stress, metabolism, and fibrosis via these signaling pathways in the T2DM patients with IDHS. However, the study’s findings should be interpreted cautiously because of a limited number of patients, necessitating more large-scale, multi-center, and high-quality clinical investigations to verify. Besides, the effects and potential mechanisms of these exosomal miRNA on T2DM by regulating the pathways mentioned above in the internal environment of intestinal damp-heat remain unknown, needing further investigation in vivo.
Pi Qi deficiency syndrome
You et al. reported a study to investigate the miRNA-gene interactions underlying leukocyte functions and the potential serum biomarkers in patients with chronic atrophic gastritis (CAG) of Pi-qi deficiency syndrome (PQDS) (You et al., 2021). Ninety-nine differential miRNAs were found in the serums between the CAG patients with PQDS and healthy individuals. Among them, the hsa-miR-122-5p was the common differential miRNA in the leukocytes and serums of the CAG patients with PQDS. The hsa-miRNA-122-5p was loaded in human plasma exosomes and delivered to recipient cells throughout the body. Interestingly, exosomal miRNA-122-5p kept higher expression both in leukocytes and serums, indicating that the hsa-miR-122-5p may be a potential biomarker for CAG patients with PQDS. Furthermore, the target genes of the exosomal hsa-miR-122-5p were enrichment in the transforming growth factor-beta signaling and chronic myeloid leukemia, hinting it might have potential roles in the regulation of leukocyte proliferation in CAG patients with PQDS. However, the conclusions should be interpreted cautiously because only five patients were enrolled, wanting further studies with large-scale, multi-center, and high-quality to strengthen the findings. The roles and underlying mechanisms of exosomal miR-122-5p in CAG with PQDS also require exploration in subsequent studies.
Spleen deficiency syndrome
You et al. (Wang Y. et al., 2021) conducted an experimental study to explore the role and mechanism of exosomes in spleen deficiency syndrome (SDS) internal environment on hepatocellular carcinoma (HCC) mice progression. It was found that the degree of malignancy of tumor tissue was higher in the HCC-SDS group than in the HCC group. Compared with HCC mice, the serum exosomal protein expressions of cytotoxic T lymphocyte antigen 4 (CTLA-4), programmed cell death protein 1 (PD-1), phosphatase and tensin homolog (PTEN), and AKT were higher in HCC-SDS mice. HepG2 cells treated with HCC-SDS mice serum had a stronger cell proliferation, and migration and invasion ability, while the CTLA-4 inhibitors could reverse these changes, indicating spleen deficiency may boost the occurrence and development of HCC via exosomal CTLA-4. It has been reported that the PTEN/CD44 involved tumor initiation, invasion, and metastasis (Luongo et al., 2019). The HCC-SDS group had significantly higher levels of CTLA-4 and CD44, and PTEN in the liver tissues compared with the HCC group. These results suggest that in the internal environment of spleen deficiency, exosomal CTLA-4 promotes the proliferation, self-renewal, and metastasis of HCC, likely associated with regulating the PTEN/CD44 pathway. However, the molecular mechanism of how CTLA-4 regulates the PTEN/CD44 pathway remains unclear. Although these findings may provide a new therapeutic target for patients with liver cancer, the conversion between animal experiments and clinical trials needs further exploration.
Challenges and future prospects
TCM is a treasure of Chinese traditional culture, with thousands of years in clinical practice and development, which has accumulated abundant clinical experience and systematic theories. Nowadays, the modernization of TCM and the rapid iteration of systems biology provide an unprecedented opportunity to understand the science of TCM better. To our knowledge, this is the first review that presents a novel insight into TCM from the perspective of exosomes. Exosomes are important in delivering compounds isolated from TCM to target cells or tissues. TCM formulas and compounds isolated from TCM have beneficial effects on multiple disorders by regulating exosomes. Moreover, exosomes may be the potential biomarkers for TCM syndromes, providing strategies for TCM treatment. The interaction between exosomes and TCM is shown in Figure 2.
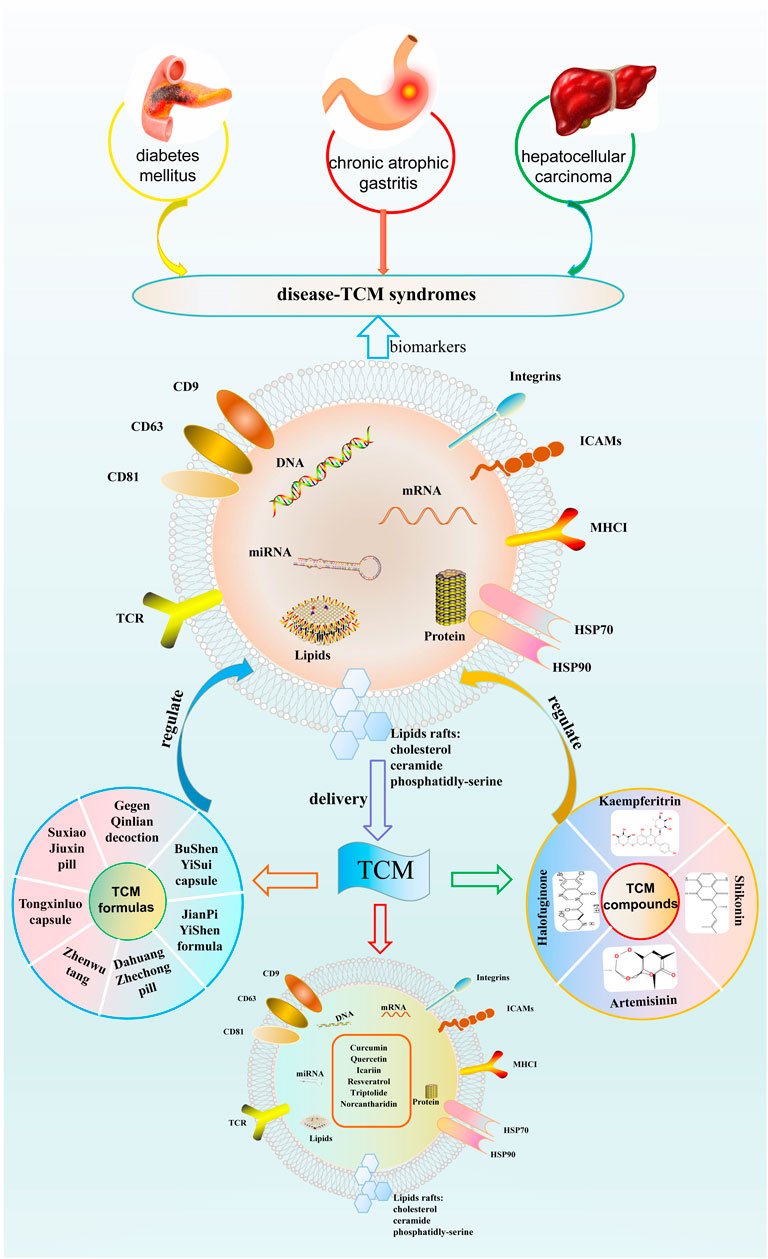
FIGURE 2. The interaction between exosomes and traditional Chinese medicine. TCM, traditional Chinese medicine.
Recently, the studies of the interplay between exosomes and TCM have increased gradually, indicating this research field is getting more and more attention. However, there are still several obstacles to studying exosomes and TCM.
Firstly, studies of the interplay between exosomes and TCM are preliminary. Most studies have not elucidated the underlying mechanisms of TCM formulas and compounds isolated from TCM against diseases via regulating exosomes. More thorough understanding and research are still required both in vivo and in vitro. Moreover, the findings of clinical studies should be interpreted cautiously due to a limited number of subjects and poor study design. Large-sample, multicentre, high-quality, and well-designed clinical studies should be registered to achieve convincing results.
Secondly, the compositions of TCM formulas or compounds isolated from TCM are quite complex, with hundreds if not thousands of constituents. The various components of TCM can affect organisms via many biological reactions; however, this diversity may contribute to different active ingredients in TCM being synergistic, enhancing, and antagonistic (Wang A. et al., 2022). It is challenging to identify the effects and mechanisms of TCMs on modulating exosomes, highlighting that exploring the pathways and targets of each component alone and in different combinations is necessary.
Thirdly, there are still challenges with exosomes in studying TCM. Exosomes, as delivery vehicles, carry multiple intracellular signals, such as nucleic acids and proteins, which may play roles in the pathogenesis and progression of diseases. For example, BMSC-Exos could inhibit HepG2 cell proliferation (Liang L. et al., 2021). But the functions and mechanisms of exosomes on illness remain unclear in many studies that evaluated the effects of compounds-containing exosomes against disease; hoping the exosomes group should be considered in further studies both in vivo and in vitro. The purification and isolation techniques of exosomes are focused on the Manufacturer’s kit and ultracentrifugation (Tables 1–4). However, the results of the Manufacturer’s kit are easily affected by the laboratory environment and extractive technique. Ultracentrifugation has low yields, which can even damage exosomes due to the centrifugal forces applied to the vesicles (Massey et al., 2021). Other isolation methods, such as ultrafiltration, microfluidics-based isolation, and immunoaffinity capture, may obtain higher quality results. Besides, co-incubation was the most common strategy for cargos incorporated into exosomes and cargos-containing exosomes’ cellular uptake by recipient cells (Table 3). Although co-incubation is perhaps the simplest method to incorporate therapeutic agents into exosomes or target recipient cells, it is affected by multiple factors, such as temperature and humidity (Massey et al., 2021). Electroporation, sonication, freeze-thaw cycling, and chemical transfection are also methods for therapeutic loading in exosomes. However, the advantages and disadvantages of these methods have not been fully clarified, which may be a direction worthy of exploring.
Moreover, TCM syndrome contains a group of clinical symptoms with complicated pathological mechanisms. However, the potential mechanisms of exosomes on diseases in the internal environment of TCM syndrome are not well understood. Besides, the studies that investigated exosomes as biomarkers for TCM syndromes only focused on the expression of exosomes, which barely further validated their sensitivity and specificity. It is necessary to find an appropriate breakthrough point on the potential mechanisms and establish reasonable diagnostic exosomal models of TCM syndromes based on multi-omics technologies, bioinformatics analysis, and artificial intelligence. Importantly, further validation in clinical trials with large-sample and multicentre is indispensable.
Conclusion
The review provides novel perspectives on the interplay between exosomes and TCM. TCM formulas and compounds isolated from TCM as exosome modulators have beneficial effects on multiple disorders, such as tumors, kidney diseases, and hepatic disease, which may involve cells proliferation inhibition, anti-inflammation, anti-oxidation, and attenuating fibrosis. Exosomes, a natural delivery system, play important roles in delivering compounds isolated from TCM to target cells or tissues. Moreover, exosomes may be the potential biomarkers for TCM syndromes, providing strategies for TCM treatment.
Author contributions
CM, JZ, JYL, HLW, and YC performed the literature collection and analysis; CM and JZ wrote the draft manuscript; GH revised the manuscript and contributed to the funding for supporting this review. All authors read and approved the final manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 81560808 and 81960913).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.844782/full#supplementary-material
References
Abbasifarid, E., Bolhassani, A., Irani, S., and Sotoodehnejadnematalahi, F. (2021). Synergistic Effects of Exosomal Crocin or Curcumin Compounds and HPV L1-E7 Polypeptide Vaccine Construct on Tumor Eradication in C57BL/6 Mouse Model. PLoS One 16 (10), e0258599. doi:10.1371/journal.pone.0258599
Aghabozorgi, A. S., Ahangari, N., Eftekhaari, T. E., Torbati, P. N., Bahiraee, A., Ebrahimi, R., et al. (2019). Circulating Exosomal miRNAs in Cardiovascular Disease Pathogenesis: New Emerging Hopes. J. Cell. Physiol. 234 (12), 21796–21809. doi:10.1002/jcp.28942
Al-Numair, K. S., Veeramani, C., Alsaif, M. A., and Chandramohan, G. (2015). Influence of Kaempferol, a Flavonoid Compound, on Membrane-Bound ATPases in Streptozotocin-Induced Diabetic Rats. Pharm. Biol. 53 (9), 1372–1378. doi:10.3109/13880209.2014.982301
Alvarez-Erviti, L., Seow, Y., Yin, H., Betts, C., Lakhal, S., and Wood, M. J. (2011). Delivery of siRNA to the Mouse Brain by Systemic Injection of Targeted Exosomes. Nat. Biotechnol. 29 (4), 341–345. doi:10.1038/nbt.1807
Andaloussi, S. E., Mäger, I., Breakefield, X. O., and Wood, M. J. (2013). Extracellular Vesicles: Biology and Emerging Therapeutic Opportunities. Nat. Rev. Drug Discov. 12 (5), 347–357. doi:10.1038/nrd3978
Angeloni, C., Barbalace, M. C., and Hrelia, S. (2019). Icariin and its Metabolites as Potential Protective Phytochemicals against Alzheimer's Disease. Front. Pharmacol. 10, 271. doi:10.3389/fphar.2019.00271
Arabpour, M., Saghazadeh, A., and Rezaei, N. (2021). Anti-inflammatory and M2 Macrophage Polarization-Promoting Effect of Mesenchymal Stem Cell-Derived Exosomes. Int. Immunopharmacol. 97, 107823. doi:10.1016/j.intimp.2021.107823
Bai, L., Li, J., Li, H., Song, J., Zhou, Y., Lu, R., et al. (2019). Renoprotective Effects of Artemisinin and Hydroxychloroquine Combination Therapy on IgA Nephropathy via Suppressing NF-Κb Signaling and NLRP3 Inflammasome Activation by Exosomes in Rats. Biochem. Pharmacol. 169, 113619. doi:10.1016/j.bcp.2019.08.021
Barile, L., Moccetti, T., Marbán, E., and Vassalli, G. (2017). Roles of Exosomes in Cardioprotection. Eur. Heart J. 38 (18), 1372–1379. doi:10.1093/eurheartj/ehw304
Bernardini, S., Tiezzi, A., Laghezza Masci, V., and Ovidi, E. (2018). Natural Products for Human Health: an Historical Overview of the Drug Discovery Approaches. Nat. Prod. Res. 32 (16), 1926–1950. doi:10.1080/14786419.2017.1356838
Boyd, A., Zhang, H., and Williams, A. (2013). Insufficient OPC Migration into Demyelinated Lesions Is a Cause of Poor Remyelination in MS and Mouse Models. Acta Neuropathol. 125 (6), 841–859. doi:10.1007/s00401-013-1112-y
Braga, T. T., Agudelo, J. S., and Camara, N. O. (2015). Macrophages during the Fibrotic Process: M2 as Friend and Foe. Front. Immunol. 6, 602. doi:10.3389/fimmu.2015.00602
Bu, L., Dai, O., Zhou, F., Liu, F., Chen, J. F., Peng, C., et al. (2020). Traditional Chinese Medicine Formulas, Extracts, and Compounds Promote Angiogenesis. Biomed. Pharmacother. 132, 110855. doi:10.1016/j.biopha.2020.110855
Cai, Y., Chen, J., Jiang, J., Cao, W., and He, L. (2010). Zhen-Wu-tang, a Blended Traditional Chinese Herbal Medicine, Ameliorates Proteinuria and Renal Damage of Streptozotocin-Induced Diabetic Nephropathy in Rats. J. Ethnopharmacol. 131 (1), 88–94. doi:10.1016/j.jep.2010.06.004
Chan, H. H. L., and Ng, T. (2020). Traditional Chinese Medicine (TCM) and Allergic Diseases. Curr. Allergy Asthma Rep. 20 (11), 67. doi:10.1007/s11882-020-00959-9
Chen, C., Yao, X., Xu, Y., Zhang, Q., Wang, H., Zhao, L., et al. (2019). Dahuang Zhechong Pill Suppresses Colorectal Cancer Liver Metastasis via Ameliorating Exosomal CCL2 Primed Pre-metastatic Niche. J. Ethnopharmacol. 238, 111878. doi:10.1016/j.jep.2019.111878
Chen, S., Tang, Y., Gao, Y., Nie, K., Wang, H., Su, H., et al. (2022). Antidepressant Potential of Quercetin and its Glycoside Derivatives: A Comprehensive Review and Update. Front. Pharmacol. 13, 865376. doi:10.3389/fphar.2022.865376
Chi, J., Jiang, Z., Chen, X., Peng, Y., Liu, W., Han, B., et al. (2019). Studies on Anti-hepatocarcinoma Effect, Pharmacokinetics and Tissue Distribution of Carboxymethyl Chitosan Based Norcantharidin Conjugates. Carbohydr. Polym. 226, 115297. doi:10.1016/j.carbpol.2019.115297
Chung, A. C., Huang, X. R., Meng, X., and Lan, H. Y. (2010). miR-192 Mediates TGF-beta/Smad3-Driven Renal Fibrosis. J. Am. Soc. Nephrol. 21 (8), 1317–1325. doi:10.1681/asn.2010020134
Cosentino, K., Hertlein, V., Jenner, A., Dellmann, T., Gojkovic, M., Peña-Blanco, A., et al. (2022). The Interplay between BAX and BAK Tunes Apoptotic Pore Growth to Control Mitochondrial-DNA-Mediated Inflammation. Mol. Cell 82 (5), 933–949.e9. doi:10.1016/j.molcel.2022.01.008
Cui, X. J., Jiang, J. C., Shi, Q., and Wang, Y. J. (2009). On the Material Basis of the Theory of BMP-7 as "kidney Main Bone. J. Basic Chin. Med. 15 (07), 515–516.
Dal-Pra, S., Hodgkinson, C. P., Mirotsou, M., Kirste, I., and Dzau, V. J. (2017). Demethylation of H3K27 Is Essential for the Induction of Direct Cardiac Reprogramming by miR Combo. Circ. Res. 120 (9), 1403–1413. doi:10.1161/circresaha.116.308741
Dolivo, D., Weathers, P., and Dominko, T. (2021). Artemisinin and Artemisinin Derivatives as Anti-fibrotic Therapeutics. Acta Pharm. Sin. B 11 (2), 322–339. doi:10.1016/j.apsb.2020.09.001
Donegan, D., Bale, L. K., and Conover, C. A. (2016). PAPP-A in Normal Human Mesangial Cells: Effect of Inflammation and Factors Related to Diabetic Nephropathy. J. Endocrinol. 231 (1), 71–80. doi:10.1530/joe-16-0205
Dong, M., Wu, S., Xu, H., Yu, X., Wang, L., Bai, H., et al. (2021a). FBS-derived Exosomes as a Natural Nano-Scale Carrier for Icariin Promote Osteoblast Proliferation. Front. Bioeng. Biotechnol. 9, 615920. doi:10.3389/fbioe.2021.615920
Dong, W., Zhang, H., Zhao, C., Luo, Y., and Chen, Y. (2021b). Silencing of miR-150-5p Ameliorates Diabetic Nephropathy by Targeting SIRT1/p53/AMPK Pathway. Front. Physiol. 12, 624989. doi:10.3389/fphys.2021.624989
Fan, Y., Li, Y., Huang, S., Xu, H., Li, H., and Liu, B. (2020). Resveratrol-primed Exosomes Strongly Promote the Recovery of Motor Function in SCI Rats by Activating Autophagy and Inhibiting Apoptosis via the PI3K Signaling Pathway. Neurosci. Lett. 736, 135262. doi:10.1016/j.neulet.2020.135262
Fang, J., and Liang, W. (2021). ASCs -derived Exosomes Loaded with Vitamin A and Quercetin Inhibit Rapid Senescence-like Response after Acute Liver Injury. Biochem. Biophys. Res. Commun. 572, 125–130. doi:10.1016/j.bbrc.2021.07.059
Fang, J., and Zhang, Y. (2017). Icariin, an Anti-atherosclerotic Drug from Chinese Medicinal Herb Horny Goat Weed. Front. Pharmacol. 8, 734. doi:10.3389/fphar.2017.00734
Fang, L., Wang, Y., Zheng, Q., Yang, T., Zhao, P., Zhao, H., et al. (2017). Effects of Bu Shen Yi Sui Capsule on NogoA/NgR and its Signaling Pathways RhoA/ROCK in Mice with Experimental Autoimmune Encephalomyelitis. BMC Complement. Altern. Med. 17 (1), 346. doi:10.1186/s12906-017-1847-4
Fei, L., Ren, X., Yu, H., and Zhan, Y. (2021). Targeting the CCL2/CCR2 axis in cancer immunotherapy: One stone, three birds?. Front. Immunol. 12, 771210. doi:10.3389/fimmu.2021.771210
Ferreira, D., Moreira, J. N., and Rodrigues, L. R. (2022). New Advances in Exosome-Based Targeted Drug Delivery Systems. Crit. Rev. Oncol. Hematol. 172, 103628. doi:10.1016/j.critrevonc.2022.103628
Gao, J., Zhang, Y., Liu, X., Wu, X., Huang, L., and Gao, W. (2021). Triptolide: Pharmacological Spectrum, Biosynthesis, Chemical Synthesis and Derivatives. Theranostics 11 (15), 7199–7221. doi:10.7150/thno.57745
Gong, Z., Lin, J., Zheng, J., Wei, L., Liu, L., Peng, Y., et al. (2020). Dahuang Zhechong Pill Attenuates CCl4-Induced Rat Liver Fibrosis via the PI3K-Akt Signaling Pathway. J. Cell. Biochem. 121 (2), 1431–1440. doi:10.1002/jcb.29378
Gutierrez-Millan, C., Calvo Díaz, C., Lanao, J. M., and Colino, C. I. (2021). Advances in Exosomes-Based Drug Delivery Systems. Macromol. Biosci. 21 (1), e2000269. doi:10.1002/mabi.202000269
Han, L. Y., Wu, Y. L., Zhu, C. Y., Wu, C. S., and Yang, C. R. (2019). Improved Pharmacokinetics of Icariin (ICA) within Formulation of PEG-PLLA/PDLA-PNIPAM Polymeric Micelles. Pharmaceutics 11 (2), 51. doi:10.3390/pharmaceutics11020051
Hansen, K. H., Bracken, A. P., Pasini, D., Dietrich, N., Gehani, S. S., Monrad, A., et al. (2008). A Model for Transmission of the H3K27me3 Epigenetic Mark. Nat. Cell Biol. 10 (11), 1291–1300. doi:10.1038/ncb1787
He, L., Bao, T., Yang, Y., Wang, H., Gu, C., Chen, J., et al. (2021). Exploring the Pathogenesis of Type 2 Diabetes Mellitus Intestinal Damp-Heat Syndrome and the Therapeutic Effect of Gegen Qinlian Decoction from the Perspective of Exosomal miRNA. J. Ethnopharmacol. 285, 114786. doi:10.1016/j.jep.2021.114786
Hou, B., Zhao, Y., Qiang, G., Yang, X., Xu, C., Chen, X., et al. (2018). Puerarin Mitigates Diabetic Hepatic Steatosis and Fibrosis by Inhibiting TGF-β Signaling Pathway Activation in Type 2 Diabetic Rats. Oxid. Med. Cell. Longev. 2018, 4545321. doi:10.1155/2018/4545321
Hou, W., Liu, B., and Xu, H. (2019). Triptolide: Medicinal Chemistry, Chemical Biology and Clinical Progress. Eur. J. Med. Chem. 176, 378–392. doi:10.1016/j.ejmech.2019.05.032
Hu, C., and Li, L. (2019). The Application of Resveratrol to Mesenchymal Stromal Cell-Based Regenerative Medicine. Stem Cell Res. Ther. 10 (1), 307. doi:10.1186/s13287-019-1412-9
Huang, K., Zhang, P., Zhang, Z., Youn, J. Y., Wang, C., Zhang, H., et al. (2021). Traditional Chinese Medicine (TCM) in the Treatment of COVID-19 and Other Viral Infections: Efficacies and Mechanisms. Pharmacol. Ther. 225, 107843. doi:10.1016/j.pharmthera.2021.107843
Isaac, R., Reis, F. C. G., Ying, W., and Olefsky, J. M. (2021). Exosomes as Mediators of Intercellular Crosstalk in Metabolism. Cell Metab. 33 (9), 1744–1762. doi:10.1016/j.cmet.2021.08.006
Jain, P. P., Zhao, T., Xiong, M., Song, S., Lai, N., Zheng, Q., et al. (2021). Halofuginone, a Promising Drug for Treatment of Pulmonary Hypertension. Br. J. Pharmacol. 178 (17), 3373–3394. doi:10.1111/bph.15442
Ji, S., Shao, G., Lv, X., Liu, Y., Fan, Y., Wu, A., et al. (2013). Downregulation of miRNA-128 Sensitises Breast Cancer Cell to Chemodrugs by Targeting Bax. Cell Biol. Int. 37 (7), 653–658. doi:10.1002/cbin.10100
Ji, T., Wang, J., Xu, Z., Cai, H. D., Su, S. L., Peng, X., et al. (2022). Combination of Mulberry Leaf Active Components Possessed Synergetic Effect on SD Rats with Diabetic Nephropathy by Mediating Metabolism, Wnt/β-Catenin and TGF-β/Smads Signaling Pathway. J. Ethnopharmacol. 292, 115026. doi:10.1016/j.jep.2022.115026
Ji, Y. Y., Liu, J. T., Wang, Z. D., Li, J. L., and Li, X. K. (2007). Study on Anti-atherosclerotic Mechanisms of Divided Functional Recipes of Dahuang Zhechong Pill in Rabbits. Zhongguo Zhong Yao Za Zhi 32 (11), 1077–1081.
Jia, X., Zhai, T., and Zhang, J. A. (2021). Circulating Exosome Involves in the Pathogenesis of Autoimmune Thyroid Diseases through Immunomodulatory Proteins. Front. Immunol. 12, 730089. doi:10.3389/fimmu.2021.730089
Jiang, W., Wang, R., Liu, D., Zuo, M., Zhao, C., Zhang, T., et al. (2018). Protective Effects of Kaempferitrin on Advanced Glycation End Products Induce Mesangial Cell Apoptosis and Oxidative Stress. Int. J. Mol. Sci. 19 (11), 3334. doi:10.3390/ijms19113334
Jin, C., Wu, P., Li, L., Xu, W., and Qian, H. (2021). Exosomes: Emerging Therapy Delivery Tools and Biomarkers for Kidney Diseases. Stem Cells Int. 2021, 7844455. doi:10.1155/2021/7844455
Jing, Z., Chen, K., and Gong, L. (2021). The Significance of Exosomes in Pathogenesis, Diagnosis, and Treatment of Esophageal Cancer. Int. J. Nanomedicine 16, 6115–6127. doi:10.2147/ijn.S321555
Kalluri, R., and LeBleu, V. S. (2020). The Biology, Function, and Biomedical Applications of Exosomes. Science 367 (6478), eaau6977. doi:10.1126/science.aau6977
Kharat, M., Du, Z., Zhang, G., and McClements, D. J. (2017). Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 65 (8), 1525–1532. doi:10.1021/acs.jafc.6b04815
Klueh, U., Czajkowski, C., Ludzinska, I., Qiao, Y., Frailey, J., and Kreutzer, D. L. (2016). Impact of CCL2 and CCR2 chemokine/receptor deficiencies on macrophage recruitment and continuous glucose monitoring in vivo. Biosens. Bioelectron. 86, 262–269. doi:10.1016/j.bios.2016.06.026
Ku, W. C., Sridharan, B., Chen, J. Y., Li, J. Y., Yang, S. Y., and Lee, M. J. (2021). Kaempferitrin-Treated HepG2 Differentially Expressed Exosomal Markers and Affect Extracellular Vesicle Sizes in the Secretome. Biomolecules 11 (2), 187. doi:10.3390/biom11020187
Lee, Y., El Andaloussi, S., and Wood, M. J. (2012). Exosomes and Microvesicles: Extracellular Vesicles for Genetic Information Transfer and Gene Therapy. Hum. Mol. Genet. 21 (R1), R125–R134. doi:10.1093/hmg/dds317
Lei, X., Li, S., Luo, C., Wang, Y., Liu, Y., Xu, Z., et al. (2020). Micheliolide Attenuates Lipopolysaccharide-Induced Inflammation by Modulating the mROS/NF-Κb/nlrp3 Axis in Renal Tubular Epithelial Cells. Mediat. Inflamm. 2020, 3934769. doi:10.1155/2020/3934769
Li, C., Li, Q., Mei, Q., and Lu, T. (2015). Pharmacological Effects and Pharmacokinetic Properties of Icariin, the Major Bioactive Component in Herba Epimedii. Life Sci. 126, 57–68. doi:10.1016/j.lfs.2015.01.006
Li, H., Lu, R., Pang, Y., Li, J., Cao, Y., Fu, H., et al. (2020). Zhen-Wu-Tang Protects IgA Nephropathy in Rats by Regulating Exosomes to Inhibit NF-Κb/nlrp3 Pathway. Front. Pharmacol. 11, 1080. doi:10.3389/fphar.2020.01080
Li, J., Cao, Y., Lu, R., Li, H., Pang, Y., Fu, H., et al. (2020). Integrated Fecal Microbiome and Serum Metabolomics Analysis Reveals Abnormal Changes in Rats with Immunoglobulin A Nephropathy and the Intervention Effect of Zhen Wu Tang. Front. Pharmacol. 11, 606689. doi:10.3389/fphar.2020.606689
Li, P., Xin, Q., Hui, J., Yuan, R., Wang, Y., Miao, Y., et al. (2021). Efficacy and safety of tongxinluo capsule as adjunctive treatment for unstable angina pectoris: A systematic review and meta-analysis of randomized controlled trials. Front. Pharmacol. 12, 742978. doi:10.3389/fphar.2021.742978
Li, R., Liang, T., Xu, L., Li, Y., Zhang, S., and Duan, X. (2013). Protective Effect of Cinnamon Polyphenols against STZ-Diabetic Mice Fed High-Sugar, High-Fat Diet and its Underlying Mechanism. Food Chem. Toxicol. 51, 419–425. doi:10.1016/j.fct.2012.10.024
Li, X. M., Xu, C. L., Deng, J. M., Li, L. F., Ma, S. P., and Qu, R. (2011). Protective Effect of Zhen-Wu-Tang (ZWT) through Keeping DA Stable and VMAT 2/DAT mRNA in Balance in Rats with Striatal Lesions Induced by MPTP. J. Ethnopharmacol. 134 (3), 768–774. doi:10.1016/j.jep.2011.01.040
Li, Y. M., Tan, X. N., Ma, R. L., Luo, J., Luo, Y., Lv, Y., et al. (2017). A New Interpretation of Viscera Related Theory in Traditional Chinese Medicine-Probe into the Relationship between Exosome and Viscera Related Theory. Hunan J. Traditional Chin. Med. 33 (02), 1–4. doi:10.16808/j.cnki.issn1003-7705.2017.02.001
Li, Y., Su, J., Sun, W., Cai, L., and Deng, Z. (2018). AMP-Activated Protein Kinase Stimulates Osteoblast Differentiation and Mineralization through Autophagy Induction. Int. J. Mol. Med. 41 (5), 2535–2544. doi:10.3892/ijmm.2018.3498
Liang, L., Zhao, L., Wang, Y., and Wang, Y. (2021). Treatment for Hepatocellular Carcinoma Is Enhanced when Norcantharidin Is Encapsulated in Exosomes Derived from Bone Marrow Mesenchymal Stem Cells. Mol. Pharm. 18 (3), 1003–1013. doi:10.1021/acs.molpharmaceut.0c00976
Liang, Y., Duan, L., Lu, J., and Xia, J. (2021). Engineering Exosomes for Targeted Drug Delivery. Theranostics 11 (7), 3183–3195. doi:10.7150/thno.52570
Liao, W., Du, Y., Zhang, C., Pan, F., Yao, Y., Zhang, T., et al. (2019). Exosomes: The Next Generation of Endogenous Nanomaterials for Advanced Drug Delivery and Therapy. Acta Biomater. 86, 1–14. doi:10.1016/j.actbio.2018.12.045
Liu, B., Lu, R., Li, H., Zhou, Y., Zhang, P., Bai, L., et al. (2019). Zhen-Wu-tang Ameliorates Membranous Nephropathy Rats through Inhibiting NF-Κb Pathway and NLRP3 Inflammasome. Phytomedicine. 59, 152913. doi:10.1016/j.phymed.2019.152913
Liu, H., Shen, M., Zhao, D., Ru, D., Duan, Y., Ding, C., et al. (2019). The Effect of Triptolide-Loaded Exosomes on the Proliferation and Apoptosis of Human Ovarian Cancer SKOV3 Cells. Biomed. Res. Int. 2019, 2595801. doi:10.1155/2019/2595801
Liu, X., Chen, J., Liu, X., Wang, D., Zheng, P., Qi, A., et al. (2018). Jian-Pi-Yi-Shen Formula Ameliorates Chronic Kidney Disease: Involvement of Mitochondrial Quality Control Network. BMC Complement. Altern. Med. 18 (1), 340. doi:10.1186/s12906-018-2395-2
Liu, X., Liu, S., Luo, D., Huang, S., Wang, F., Zhang, B., et al. (2020). Involvement of Circulating Exosomal MicroRNAs in Jian-Pi-Yi-Shen Formula Protection against Adenine-Induced Chronic Kidney Disease. Front. Pharmacol. 11, 622658. doi:10.3389/fphar.2020.622658
Lu, J. Z., Ye, D., and Ma, B. L. (2021). Constituents, Pharmacokinetics, and Pharmacology of Gegen-Qinlian Decoction. Front. Pharmacol. 12, 668418. doi:10.3389/fphar.2021.668418
Luo, H., and Yi, B. (2021). The Role of Exosomes in the Pathogenesis of Nasopharyngeal Carcinoma and the Involved Clinical Application. Int. J. Biol. Sci. 17 (9), 2147–2156. doi:10.7150/ijbs.59688
Luongo, F., Colonna, F., Calapà, F., Vitale, S., Fiori, M. E., and De Maria, R. (2019). PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers (Basel) 11 (8), 1076. doi:10.3390/cancers11081076
Lyu, H. N., Ma, N., Meng, Y., Zhang, X., Wong, Y. K., Xu, C., et al. (2021). Study towards Improving Artemisinin-Based Combination Therapies. Nat. Prod. Rep. 38 (7), 1243–1250. doi:10.1039/d0np00079e
Lyu, M., Fan, G., Xiao, G., Wang, T., Xu, D., Gao, J., et al. (2021). Traditional Chinese Medicine in COVID-19. Acta Pharm. Sin. B 11 (11), 3337–3363. doi:10.1016/j.apsb.2021.09.008
Ma, J., Wu, Y., and He, Y. (2021). Silencing circRNA LRP6 Down-Regulates PRMT1 to Improve the Streptozocin-Induced Pancreatic β-cell Injury and Insulin Secretion by Sponging miR-9-5p. J. Bioenerg. Biomembr. 53 (3), 333–342. doi:10.1007/s10863-021-09895-3
Ma, M., Pei, Y., Wang, X., Feng, J., Zhang, Y., and Gao, M. Q. (2019). LncRNA XIST Mediates Bovine Mammary Epithelial Cell Inflammatory Response via NF-Κb/nlrp3 Inflammasome Pathway. Cell Prolif. 52 (1), e12525. doi:10.1111/cpr.12525
Mantovani, A., Biswas, S. K., Galdiero, M. R., Sica, A., and Locati, M. (2013). Macrophage Plasticity and Polarization in Tissue Repair and Remodelling. J. Pathol. 229 (2), 176–185. doi:10.1002/path.4133
Mao, C., Fu, X. H., Yuan, J. Q., Yang, Z. Y., Chung, V. C., Qin, Y., et al. (2015). Tong-xin-luo capsule for patients with coronary heart disease after percutaneous coronary intervention. Cochrane Database Syst. Rev. 5, cd010237. doi:10.1002/14651858.CD010237.pub2
Massey, A. E., Malik, S., Sikander, M., Doxtater, K. A., Tripathi, M. K., Khan, S., et al. (2021). Clinical Implications of Exosomes: Targeted Drug Delivery for Cancer Treatment. Int. J. Mol. Sci. 22 (10), 5278. doi:10.3390/ijms22105278
Mathieu, M., Martin-Jaular, L., Lavieu, G., and Théry, C. (2019). Specificities of Secretion and Uptake of Exosomes and Other Extracellular Vesicles for Cell-To-Cell Communication. Nat. Cell Biol. 21 (1), 9–17. doi:10.1038/s41556-018-0250-9
McKelvey, K. J., Powell, K. L., Ashton, A. W., Morris, J. M., and McCracken, S. A. (2015). Exosomes: Mechanisms of Uptake. J. Circ. Biomark. 4, 7. doi:10.5772/61186
Memarzia, A., Khazdair, M. R., Behrouz, S., Gholamnezhad, Z., Jafarnezhad, M., Saadat, S., et al. (2021). Experimental and Clinical Reports on Anti-inflammatory, Antioxidant, and Immunomodulatory Effects of Curcuma Longa and Curcumin, an Updated and Comprehensive Review. Biofactors 47 (3), 311–350. doi:10.1002/biof.1716
Meng, Y., Ma, N., Lyu, H., Wong, Y. K., Zhang, X., Zhu, Y., et al. (2021). Recent Pharmacological Advances in the Repurposing of Artemisinin Drugs. Med. Res. Rev. 41 (6), 3156–3181. doi:10.1002/med.21837
Mladinov, D., Liu, Y., Mattson, D. L., and Liang, M. (2013). MicroRNAs Contribute to the Maintenance of Cell-type-specific Physiological Characteristics: miR-192 Targets Na+/K+-ATPase β1. Nucleic Acids Res. 41 (2), 1273–1283. doi:10.1093/nar/gks1228
Nam, G. H., Choi, Y., Kim, G. B., Kim, S., Kim, S. A., and Kim, I. S. (2020). Emerging Prospects of Exosomes for Cancer Treatment: From Conventional Therapy to Immunotherapy. Adv. Mat. 32 (51), e2002440. doi:10.1002/adma.202002440
Nowling, T. K. (2022). Mesangial Cells in Lupus Nephritis. Curr. Rheumatol. Rep. 23 (12), 83. doi:10.1007/s11926-021-01048-0
Ostrowski, M., Carmo, N. B., Krumeich, S., Fanget, I., Raposo, G., Savina, A., et al. (2010). Rab27a and Rab27b Control Different Steps of the Exosome Secretion Pathway. Nat. Cell Biol. 12 (1), 19–30. doi:10.1038/ncb2000
Paddon, C. J., Westfall, P. J., Pitera, D. J., Benjamin, K., Fisher, K., McPhee, D., et al. (2013). High-level Semi-synthetic Production of the Potent Antimalarial Artemisinin. Nature 496 (7446), 528–532. doi:10.1038/nature12051
Pan, J., Li, M., Yu, F., Zhu, F., Wang, L., Ning, D., et al. (2021). Up-Regulation of p53/miR-628-3p Pathway, a Novel Mechanism of Shikonin on Inhibiting Proliferation and Inducing Apoptosis of A549 and PC-9 Non-small Cell Lung Cancer Cell Lines. Front. Pharmacol. 12, 766165. doi:10.3389/fphar.2021.766165
Pan, M. S., Cao, J., and Fan, Y. Z. (2020). Insight into Norcantharidin, a Small-Molecule Synthetic Compound with Potential Multi-Target Anticancer Activities. Chin. Med. 15, 55. doi:10.1186/s13020-020-00338-6
Parsamanesh, N., Asghari, A., Sardari, S., Tasbandi, A., Jamialahmadi, T., Xu, S., et al. (2021). Resveratrol and Endothelial Function: A Literature Review. Pharmacol. Res. 170, 105725. doi:10.1016/j.phrs.2021.105725
Pegtel, D. M., and Gould, S. J. (2019). Exosomes. Annu. Rev. Biochem. 88, 487–514. doi:10.1146/annurev-biochem-013118-111902
Pines, M., and Spector, I. (2015). Halofuginone - the Multifaceted Molecule. Molecules 20 (1), 573–594. doi:10.3390/molecules20010573
Ponomarev, E. D., Veremeyko, T., Barteneva, N., Krichevsky, A. M., and Weiner, H. L. (2011). MicroRNA-124 Promotes Microglia Quiescence and Suppresses EAE by Deactivating Macrophages via the C/EBP-α-PU.1 Pathway. Nat. Med. 17 (1), 64–70. doi:10.1038/nm.2266
Puglia, C., Lauro, M. R., Tirendi, G. G., Fassari, G. E., Carbone, C., Bonina, F., et al. (2017). Modern Drug Delivery Strategies Applied to Natural Active Compounds. Expert Opin. Drug Deliv. 14 (6), 755–768. doi:10.1080/17425247.2017.1234452
Qi, Y., Guo, L., Jiang, Y., Shi, Y., Sui, H., and Zhao, L. (2020). Brain Delivery of Quercetin-Loaded Exosomes Improved Cognitive Function in AD Mice by Inhibiting Phosphorylated Tau-Mediated Neurofibrillary Tangles. Drug Deliv. 27 (1), 745–755. doi:10.1080/10717544.2020.1762262
Qiu, Y., Qiu, Y., Yao, G. M., Luo, C., and Zhang, C. (2022). Natural Product Therapies in Chronic Kidney Diseases: An Update. Nephrol. Ther. 18 (2), 75–79. doi:10.1016/j.nephro.2021.05.003
Qu, Y., Zhang, Q., Cai, X., Li, F., Ma, Z., Xu, M., et al. (2017). Exosomes Derived from miR-181-5p-Modified Adipose-Derived Mesenchymal Stem Cells Prevent Liver Fibrosis via Autophagy Activation. J. Cell. Mol. Med. 21 (10), 2491–2502. doi:10.1111/jcmm.13170
Raposo, G., and Stoorvogel, W. (2013). Extracellular Vesicles: Exosomes, Microvesicles, and Friends. J. Cell Biol. 200 (4), 373–383. doi:10.1083/jcb.201211138
Ren, L., Wang, J., Feng, L., Wang, S., and Li, J. (2018). Efficacy of suxiao jiuxin pill on coronary heart disease: A meta-analysis of randomized controlled trials. Evid Based Complement Alternat. Med. 2018, 9745804. doi:10.1155/2018/9745804
Ricketts, T. D., Prieto-Dominguez, N., Gowda, P. S., and Ubil, E. (2021). Mechanisms of Macrophage Plasticity in the Tumor Environment: Manipulating Activation State to Improve Outcomes. Front. Immunol. 12, 642285. doi:10.3389/fimmu.2021.642285
Robertson, I., Wai Hau, T., Sami, F., Sajid Ali, M., Badgujar, V., Murtuja, S., et al. (2022). The Science of Resveratrol, Formulation, Pharmacokinetic Barriers and its Chemotherapeutic Potential. Int. J. Pharm. 618, 121605. doi:10.1016/j.ijpharm.2022.121605
Ruan, X. F., Ju, C. W., Shen, Y., Liu, Y. T., Kim, I. M., Yu, H., et al. (2018a). Suxiao Jiuxin Pill Promotes Exosome Secretion from Mouse Cardiac Mesenchymal Stem Cells In Vitro. Acta Pharmacol. Sin. 39 (4), 569–578. doi:10.1038/aps.2018.19
Ruan, X. F., Li, Y. J., Ju, C. W., Shen, Y., Lei, W., Chen, C., et al. (2018b). Exosomes from Suxiao Jiuxin Pill-Treated Cardiac Mesenchymal Stem Cells Decrease H3K27 Demethylase UTX Expression in Mouse Cardiomyocytes In Vitro. Acta Pharmacol. Sin. 39 (4), 579–586. doi:10.1038/aps.2018.18
Shapouri-Moghaddam, A., Mohammadian, S., Vazini, H., Taghadosi, M., Esmaeili, S. A., Mardani, F., et al. (2018). Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 233 (9), 6425–6440. doi:10.1002/jcp.26429
Shi, G., Kang, Z., Liu, H., Ren, F., and Zhou, Y. (2021). The Effects of Quercetin Combined with Nucleopolyhedrovirus on the Growth and Immune Response in the Silkworm (Bombyx mori). Arch. Insect Biochem. Physiol. 108 (2), e21839. doi:10.1002/arch.21839
Shi, W., Men, L., Pi, X., Jiang, T., Peng, D., Huo, S., et al. (2021). Shikonin Suppresses Colon Cancer Cell Growth and Exerts Synergistic Effects by Regulating ADAM17 and the IL-6/STAT3 Signaling Pathway. Int. J. Oncol. 59 (6), 99. doi:10.3892/ijo.2021.5279
Su, S. B., Jia, W., Lu, A., and Li, S. (2014). Evidence-Based ZHENG: A Traditional Chinese Medicine Syndrome 2013. Evid. Based. Complement. Altern. Med. 2014, 484201. doi:10.1155/2014/484201
Sun, Q., Gong, T., Liu, M., Ren, S., Yang, H., Zeng, S., et al. (2022). Shikonin, a Naphthalene Ingredient: Therapeutic Actions, Pharmacokinetics, Toxicology, Clinical Trials and Pharmaceutical Researches. Phytomedicine. 94, 153805. doi:10.1016/j.phymed.2021.153805
Takahata, Y., Hagino, H., Kimura, A., Urushizaki, M., Kobayashi, S., Wakamori, K., et al. (2021). Smoc1 and Smoc2 Regulate Bone Formation as Downstream Molecules of Runx2. Commun. Biol. 4 (1), 1199. doi:10.1038/s42003-021-02717-7
Tang, J. L., Liu, B. Y., and Ma, K. W. (2008). Traditional Chinese Medicine. Lancet 372 (9654), 1938–1940. doi:10.1016/s0140-6736(08)61354-9
Tian, B., and Liu, J. (2020). Resveratrol: a Review of Plant Sources, Synthesis, Stability, Modification and Food Application. J. Sci. Food Agric. 100 (4), 1392–1404. doi:10.1002/jsfa.10152
Tsai, M. F., Chen, S. M., Ong, A. Z., Chung, Y. H., Chen, P. N., Hsieh, Y. H., et al. (2021). Shikonin Induced Program Cell Death through Generation of Reactive Oxygen Species in Renal Cancer Cells. Antioxidants (Basel) 10 (11), 1831. doi:10.3390/antiox10111831
Tu, W., Hong, Y., Huang, M., Chen, M., and Gan, H. (2022). Effect of Kaempferol on Hedgehog Signaling Pathway in Rats with -chronic Atrophic Gastritis - Based on Network Pharmacological Screening and Experimental Verification. Biomed. Pharmacother. 145, 112451. doi:10.1016/j.biopha.2021.112451
Tu, Y. (2016). Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 55 (35), 10210–10226. doi:10.1002/anie.201601967
Uddin, M. J., Farjana, M., Moni, A., Hossain, K. S., Hannan, M. A., and Ha, H. (2021). Prospective Pharmacological Potential of Resveratrol in Delaying Kidney Aging. Int. J. Mol. Sci. 22 (15), 8258. doi:10.3390/ijms22158258
Wang, A., Zhao, W., Yan, K., Huang, P., Zhang, H., Zhang, Z., et al. (2022). Mechanisms and Efficacy of Traditional Chinese Medicine in Heart Failure. Front. Pharmacol. 13, 810587. doi:10.3389/fphar.2022.810587
Wang, H., Sui, H., Zheng, Y., Jiang, Y., Shi, Y., Liang, J., et al. (2019). Curcumin-primed Exosomes Potently Ameliorate Cognitive Function in AD Mice by Inhibiting Hyperphosphorylation of the Tau Protein through the AKT/GSK-3β Pathway. Nanoscale 11 (15), 7481–7496. doi:10.1039/c9nr01255a
Wang, J., and Zhao, Q. (2019). Kaempferitrin Inhibits Proliferation, Induces Apoptosis, and Ameliorates Inflammation in Human Rheumatoid Arthritis Fibroblast-like Synoviocytes. Phytother. Res. 33 (6), 1726–1735. doi:10.1002/ptr.6364
Wang, Q. Q., Hua, H. Y., Naranmandura, H., and Zhu, H. H. (2020). Balance between the Toxicity and Anticancer Activity of Arsenic Trioxide in Treatment of Acute Promyelocytic Leukemia. Toxicol. Appl. Pharmacol. 409, 115299. doi:10.1016/j.taap.2020.115299
Wang, Q., Xie, X., Zhang, D., Mao, F., Wang, S., and Liao, Y. (2022). Saxagliptin Enhances Osteogenic Differentiation in MC3T3-E1 Cells, Dependent on the Activation of AMP-Activated Protein Kinase α (AMPKα)/runt-Related Transcription Factor-2 (Runx-2). Bioengineered 13 (1), 431–439. doi:10.1080/21655979.2021.2008667
Wang, Q., Zhou, X., Zhao, Y., Xiao, J., Lu, Y., Shi, Q., et al. (2018). Polyphyllin I Ameliorates Collagen-Induced Arthritis by Suppressing the Inflammation Response in Macrophages through the NF-Κb Pathway. Front. Immunol. 9, 2091. doi:10.3389/fimmu.2018.02091
Wang, X. J., Qi, Y. D., Guan, H. C., Lin, H. G., He, P. Q., Guan, K. W., et al. (2021). Gegen Qinlian Decoction Ameliorates Hyperuricemia-Induced Renal Tubular Injury via Blocking the Inflammatory Signaling Pathway. Front. Pharmacol. 12, 665398. doi:10.3389/fphar.2021.665398
Wang, Y., Li, P., Mao, S., Mo, Z., Cao, Z., Luo, J., et al. (2021). Exosome CTLA-4 Regulates PTEN/CD44 Signal Pathway in Spleen Deficiency Internal Environment to Promote Invasion and Metastasis of Hepatocellular Carcinoma. Front. Pharmacol. 12, 757194. doi:10.3389/fphar.2021.757194
Wei, Y., Li, M., Cui, S., Wang, D., Zhang, C. Y., Zen, K., et al. (2016). Shikonin Inhibits the Proliferation of Human Breast Cancer Cells by Reducing Tumor-Derived Exosomes. Molecules 21 (6), 777. doi:10.3390/molecules21060777
Whyte, C. S., Bishop, E. T., Rückerl, D., Gaspar-Pereira, S., Barker, R. N., Allen, J. E., et al. (2011). Suppressor of Cytokine Signaling (SOCS)1 Is a Key Determinant of Differential Macrophage Activation and Function. J. Leukoc. Biol. 90 (5), 845–854. doi:10.1189/jlb.1110644
Wu, X. M., Gao, Y. B., Xu, L. P., Zou, D. W., Zhu, Z. Y., Wang, X. L., et al. (2017). Tongxinluo Inhibits Renal Fibrosis in Diabetic Nephropathy: Involvement of the Suppression of Intercellular Transfer of TGF-[Formula: See Text]1-Containing Exosomes from GECs to GMCs. Am. J. Chin. Med. 45 (5), 1075–1092. doi:10.1142/s0192415x17500586
Xia, X., Wang, X., Zhang, S., Zheng, Y., Wang, L., Xu, Y., et al. (2019). miR-31 Shuttled by Halofuginone-Induced Exosomes Suppresses MFC-7 Cell Proliferation by Modulating the HDAC2/cell Cycle Signaling axis. J. Cell. Physiol. 234 (10), 18970–18984. doi:10.1002/jcp.28537
Xie, X., Lian, S., Zhou, Y., Li, B., Lu, Y., Yeung, I., et al. (2021). Tumor-derived Exosomes Can Specifically Prevent Cancer Metastatic Organotropism. J. Control. Release 331, 404–415. doi:10.1016/j.jconrel.2021.01.030
You, L., Zhang, S., Li, T., Sang, X., Li, K., Wang, W., et al. (2021). Integrated Analyses of miRNA and mRNA Profiles in Leukocytes and Serums in Traditional Chinese Medicine (TCM)-defined Pi-Qi-Deficiency Syndrome and Pi-Wei Damp-Heat Syndrome Resulting from Chronic Atrophic Gastritis. Chin. Med. 16 (1), 4. doi:10.1186/s13020-020-00416-9
Yu, X. Y., Sun, Q., Zhang, Y. M., Zou, L., and Zhao, Y. Y. (2022). TGF-β/Smad Signaling Pathway in Tubulointerstitial Fibrosis. Front. Pharmacol. 13, 860588. doi:10.3389/fphar.2022.860588
Yuan, H. X. (2009). Clinical Application of Dahuang Zhechong Pill in Gynecology. Zhong Xi Yi Jie He Xue Bao 7 (2), 168–170. doi:10.3736/jcim20090214
Yuan, K., Li, X., Lu, Q., Zhu, Q., Jiang, H., Wang, T., et al. (2019). Application and Mechanisms of Triptolide in the Treatment of Inflammatory Diseases-A Review. Front. Pharmacol. 10, 1469. doi:10.3389/fphar.2019.01469
Zabeo, D., Cvjetkovic, A., Lässer, C., Schorb, M., Lötvall, J., and Höög, J. L. (2017). Exosomes Purified from a Single Cell Type Have Diverse Morphology. J. Extracell. Vesicles 6 (1), 1329476. doi:10.1080/20013078.2017.1329476
Zha, Z., Gao, Y. F., Ji, J., Sun, Y. Q., Li, J. L., Qi, F., et al. (2021). Bu Shen Yi Sui Capsule Alleviates Neuroinflammation and Demyelination by Promoting Microglia toward M2 Polarization, Which Correlates with Changes in miR-124 and miR-155 in Experimental Autoimmune Encephalomyelitis. Oxid. Med. Cell. Longev. 2021, 5521503. doi:10.1155/2021/5521503
Zhang, W., Li, X., Tang, Y., Chen, C., Jing, R., and Liu, T. (2020). miR-155-5p Implicates in the Pathogenesis of Renal Fibrosis via Targeting SOCS1 and SOCS6. Oxid. Med. Cell. Longev. 2020, 6263921. doi:10.1155/2020/6263921
Zhang, Z. G., Buller, B., and Chopp, M. (2019). Exosomes - beyond Stem Cells for Restorative Therapy in Stroke and Neurological Injury. Nat. Rev. Neurol. 15 (4), 193–203. doi:10.1038/s41582-018-0126-4
Zhao, P. Y., Ji, J., Liu, X. H., Zhao, H., Xue, B., Jin, L. Y., et al. (2020). Bu-Shen-Yi-Sui Capsule, an Herbal Medicine Formula, Promotes Remyelination by Modulating the Molecular Signals via Exosomes in Mice with Experimental Autoimmune Encephalomyelitis. Oxid. Med. Cell. Longev. 2020, 7895293. doi:10.1155/2020/7895293
Zhao, Z., Li, Y., Zhou, L., Zhou, X., Xie, B., Zhang, W., et al. (2021). Prevention and Treatment of COVID-19 Using Traditional Chinese Medicine: A Review. Phytomedicine. 85, 153308. doi:10.1016/j.phymed.2020.153308
ZhaoJ., , Mo, C., Shi, W., Meng, L., and Ai, J. (2021). Network Pharmacology Combined with Bioinformatics to Investigate the Mechanisms and Molecular Targets of Astragalus Radix-Panax Notoginseng Herb Pair on Treating Diabetic Nephropathy. Evid. Based. Complement. Altern. Med. 2021, 9980981. doi:10.1155/2021/9980981
Zheng, J., Yu, L., Chen, W., Lu, X., and Fan, X. (2018). Circulating Exosomal microRNAs Reveal the Mechanism of Fructus Meliae Toosendan-Induced Liver Injury in Mice. Sci. Rep. 8 (1), 2832. doi:10.1038/s41598-018-21113-6
Zhou, F., Zou, X., Zhang, J., Wang, Z., Yang, Y., and Wang, D. (2021). Jian-Pi-Yi-Shen Formula Ameliorates Oxidative Stress, Inflammation, and Apoptosis by Activating the Nrf2 Signaling in 5/6 Nephrectomized Rats. Front. Pharmacol. 12, 630210. doi:10.3389/fphar.2021.630210
Zhou, J., Ren, Y., Tan, L., Song, X., Wang, M., Li, Y., et al. (2020). Norcantharidin: Research Advances in Pharmaceutical Activities and Derivatives in Recent Years. Biomed. Pharmacother. 131, 110755. doi:10.1016/j.biopha.2020.110755
ZhouX., , Zhao, S., Li, W., Ruan, Y., Yuan, R., Ning, J., et al. (2021). Tubular Cell-Derived Exosomal miR-150-5p Contributes to Renal Fibrosis Following Unilateral Ischemia-Reperfusion Injury by Activating Fibroblast In Vitro and In Vivo. Int. J. Biol. Sci. 17 (14), 4021–4033. doi:10.7150/ijbs.62478
Keywords: traditional Chinese medicine, exosome, modulator, delivery carrier, biomarker
Citation: Mo C, Zhao J, Liang J, Wang H, Chen Y and Huang G (2022) Exosomes: A novel insight into traditional Chinese medicine. Front. Pharmacol. 13:844782. doi: 10.3389/fphar.2022.844782
Received: 28 December 2021; Accepted: 22 July 2022;
Published: 29 August 2022.
Edited by:
James C. B. Li, BAGI Biosciences, Hong Kong SAR, ChinaReviewed by:
William Chi-Shing Tai, Hong Kong Polytechnic University, Hong Kong SAR, ChinaArmandodoriano Bianco, Sapienza University of Rome, Italy
Sandhya Bansal, St. Joseph’s Hospital and Medical Center, United States
Copyright © 2022 Mo, Zhao, Liang, Wang, Chen and Huang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guodong Huang, aHVhbmdub25nLjIwMDdAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
 Chao Mo
Chao Mo Jie Zhao
Jie Zhao Jingyan Liang1
Jingyan Liang1 Yu Chen
Yu Chen Guodong Huang
Guodong Huang