- 1Department of Pharmacognosy, School of Pharmacy, Naval Medical University, Shanghai, China
- 2Department of Pharmacy, Fujian University of Traditional Chinese Medicine, Fuzhou, China
- 3Department of Medicine, Zhejiang Academy of Traditional Chinese Medicine, Hangzhou, China
Alzheimer’s disease (AD) and osteoporosis (OP) are progressive degenerative diseases caused by multiple factors, placing a huge burden on the world. Much evidence indicates that OP is a common complication in AD patients. In addition, there is also evidence to show that patients with OP have a higher risk of AD than those without OP. This suggests that the association between the two diseases may be due to a pathophysiological link rather than one disease causing the other. Several in vitro and in vivo studies have also proved their common pathogenesis. Based on the theory of traditional Chinese medicine, some classic and specific natural Chinese medicines are widely used to effectively treat AD and OP. Current evidence also shows that these treatments can ameliorate both brain damage and bone metabolism disorder and further alleviate AD complicated with OP. These valuable therapies might provide effective and safe alternatives to major pharmacological strategies.
Introduction
With the advent of an aging society, aging-related diseases have occupied the forefront of the disease spectrum in China. Alzheimer’s disease (AD) and osteoporosis (OP) are two common aging-related diseases in clinic, which have seriously threatened the health of middle-aged and elderly people. AD is a disease dominated by a series of central neurodegenerative conditions with hidden pathogenesis and unknown etiology (Hogh, 2017). It is clinically characterized by memory impairment, aphasia, impairment of visuospatial skills, executive dysfunction, and personality and behavior changes (Zhang and Zheng, 2019), which badly affect patients’ life. The pathologic hallmarks of AD are cerebral extracellular amyloid plaques and intracellular neurofibrillary tangles, but not all people with amyloid plaques or neurofibrillary tangles develop AD (Weller and Budson, 2018). However, the underlying pathophysiological mechanism is still elusive, especially for older adults. OP is a degenerative bone disease, which leads to a decrease in bone mineral density (BMD) and bone mass due to various reasons, and the degeneration of bone micro-structure increases bone brittleness, thus increasing the risk of fracture (Aspray and Hill, 2019). The pathogenesis of OP is considered to include the defect in bone micro-architecture, poor intrinsic material properties of bone, defective repair of micro-damage to the bone, and excessive bone remodeling (Armas and Recker, 2012). These points provide different treatment strategies for OP, as well as expanding the possibilities of new drug screening. In addition, clinical studies have found that patients diagnosed with AD are often accompanied by OP and hip fracture, and the BMD of patients with AD is often lower than that of the non-AD population (Chen and Lo, 2017), indicating that there is a correlation between the pathogenesis of AD and OP, and patients are prone to the symptoms of these two diseases.
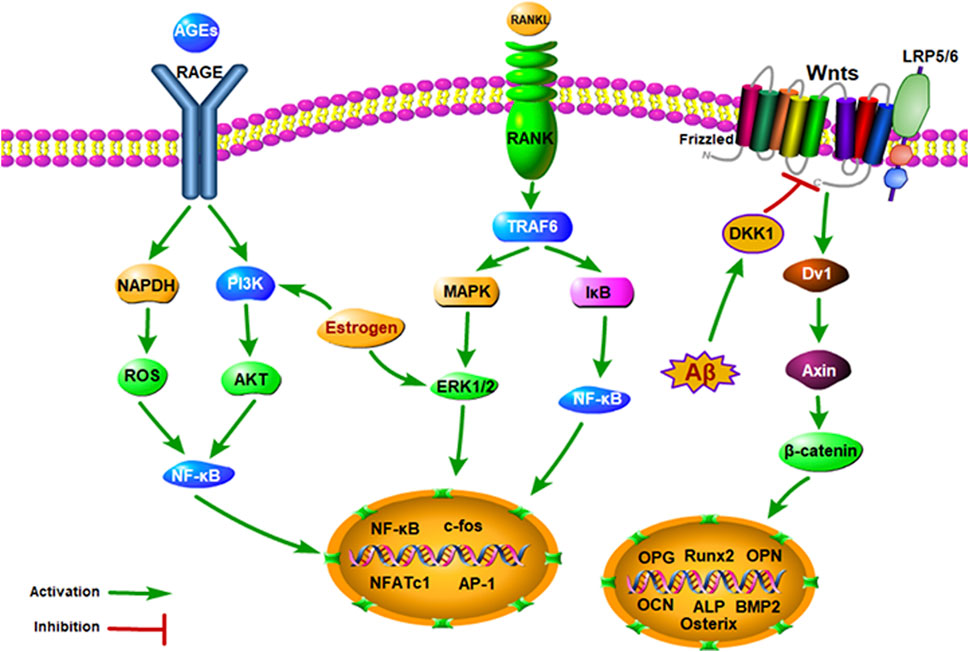
Traditional Chinese medicine (TCM) has become increasingly popular thanks to its fewer side effects and high effectiveness in treating diseases. TCM formula is usually composed of more than one kind of herb, and the purpose of the herbal formula is to improve the therapeutic effects or decrease the possible side effects of a single herb through the complex interactions among herbs. Meanwhile, TCM is rich in natural components, which can provide promising ideas for the screening and research of new drugs. The TCM formula has been widely used in effectively treating a variety of aging-related diseases, including AD and OP. Some are the most classical and specific drugs, such as Morinda officinalis How., Eucommia ulmoides Oliv., Lycium barbarum L., Atractylodes macrocephala Koidz., Salvia miltiorrhiza Bge., Rehmannia glutinosa Libosch., and Glycyrrhiza uralensis Fisch., Cistanche deserticola Ma., when applied to the treatment of dementia and bone loss, with beneficial effects on the growth and development of the nervous system and skeleton tissues (Figure 1) (Howes et al., 2017; Pei et al., 2020; Zhang ND et al., 2016; Wang T et al., 2017). Therefore, we review the link between AD and OP from the epidemiological perspective and the underlying molecular mechanism and introduce some natural Chinese medicines for treating AD complicated with OP to provide a reference for future clinical treatment.
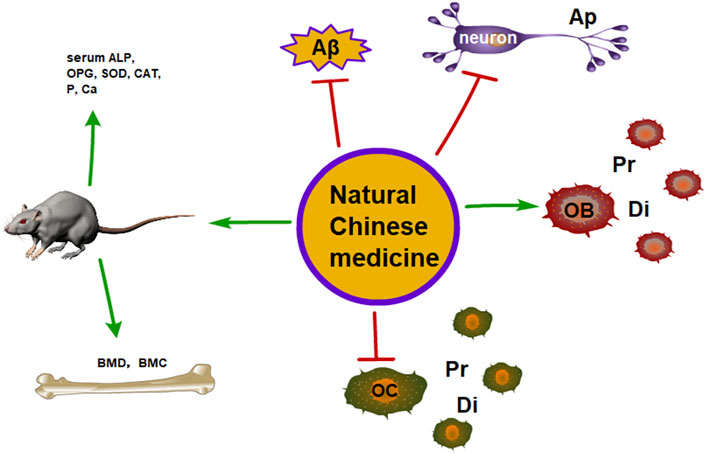
FIGURE 1. The therapeutic potential of the natural Chinese medicine for the treatment of AD and OP (Ap, apoptosis; Pr, proliferation; Di, differentiation).
Correlation Between Alzheimer’s Disease and Osteoporosis
The Epidemiology of AD and OP
AD is one of the most common diseases in elderly people. The World Health Organization (WHO) estimated that there was a strong link between age and the prevalence of AD, and the global prevalence of AD in the elderly (over 60) was as high as 5%–7% (Prince et al., 2013). In addition, AD is also related to sex, and recent studies have found that nearly two-thirds of AD patients are women (Kim et al., 2018). According to the Alzheimer’s Disease International (ADI) statistics, over 55 million people suffer from AD worldwide. This staggering figure grew rapidly and was predicted to reach 78 million by 2030 (Alzheimer’s Disease International, McGill University, 2021). In addition, as the number of AD patients continues to rise, the cost of AD treatment also rises. A report shows that the total cost of global AD treatment in 2020 was 305 billion US dollars (Wong, 2020). In China, the total cost of AD treatment in 2015 was 167.74 billion US dollars, and the total cost was expected to reach 507.49 billion US dollars in 2030 (Jia et al., 2018). These numbers reveal that AD has imposed a huge burden on the world economy. More precariously, according to a recent AD report in the United States, the number of AD patients and their cost have been increasing rapidly, despite an important breakthrough made in the prevention, mitigation, and treatment of AD, especially after the outbreak of COVID-19 (Alzheimer’s Disease International, McGill University, 2021).
As for osteoporosis, it was found in a survey that the incidence of fragility fractures associated with OP in 2017 was as many as 2.7 million people in European countries (Eric, 2020), and the incidence of osteoporosis in women is generally higher than that in men, especially in postmenopausal women (Lorentzon et al., 2021), who are consistent with AD. However, although bone mass loss in women is faster, the life expectancy of women is generally longer than that of men. A cohort study in Denmark found that the remaining life expectancy was 18.2 years for men with OP at the age of 50 and 7.5 years for men at the age of 75, while for women, the figure was 26.4 and 13.5 years, respectively (Abrahamsen et al., 2015). The prevalence rate of OP in China rose from 14.94% in 2008 to 27.96% in 2012–2015, and the prevalence rate increased with age (28.09% in 25–35 years old and 34.65% in people over 50 years old) (Chen et al., 2016). According to the results of China’s OP epidemiological survey in 2018, the prevalence rate of OP in people over 50 years old was 19.2%, as well as 32.0% in people over 65 years, suggesting its widespread features in middle-aged and elderly people in China (Osteoporosis and Bone Mineral Disease Branch of Chinese Medical Association, 2019). In addition, the lack of physical exercise, low vitamin D levels, smoking, drinking, and high caffeine intake can also reduce BMD, leading to OP. According to a report, the human BMD peaks at 35 years old, then begins to decline, and accelerates significantly after menopause. Hence, to prevent OP, young women, as well as men, should quit smoking, avoid excessive drinking, exercise regularly, and take in the right amount of calcium and vitamin D (Lamichhane, 2005).
In addition, the risk of OP in AD and the risk of AD in OP are two different aspects when considering the relationship between these two situations. A prospective, observational study found that lower BMD was associated with an increased risk of increasing AD (Tan et al., 2005). Dual-energy X-ray absorptiometry of the femoral neck was measured for BMD analysis in the study and the results indicated that the lowest quartile of femoral neck BMD was associated with a twice risk of AD in women but not in men, suggesting the possibility of a protective role of cumulative estrogen exposure. Compared with the general population, postmenopausal women with severe OP were found to have higher risks of AD and other dementia (Amouzougan et al., 2017). Estrogen has the potential to influence both brain aging and bone metabolism. The prevalence of AD is slightly higher in women than men, and postmenopausal women are predisposed to develop OP (Henderson, 2006; Depypere et al., 2016). AD is also considered a risk factor for OP. A population-based cohort study indicated that individuals with AD were at a higher risk of hip fracture (Wang et al., 2014). Another meta-analysis study also found that the AD population had a decreased level of hip BMD and approximately twice risk of hip fracture compared with healthy controls (Zhao et al., 2012). The circadian rhythm is usually poorly regulated, and daytime sleepiness is common in the AD population. This situation worsens as cognitive function exacerbates (Lee et al., 2007). The condition limits outdoor physical activities, which may, in turn, decrease sunlight exposure and subsequently cause vitamin D deficiency. Lack of physical activities and vitamin D deficiency may explain the higher risk of OP in the AD population (Nanes and Kallen, 2014). The relationship between AD and OP is further illustrated by the association between BMD values measured by dual-energy X-ray absorptiometry and cerebral volume measured by magnetic resonance imaging. BMD is decreased in the earliest clinical stages of AD and is connected with brain atrophy and memory loss (Loskutova et al., 2009). What is more, BMD and hypothalamic volume were demonstrated to have a positive relationship in the early AD group after controlling for age and gender (Loskutova et al., 2010).
In light of the above epidemiological statistics, it was clear that AD and OP have similarities in high morbidity and disability, strong correlation with age and gender, and high cost for treatment. Therefore, the common pathological mechanism is of great interest as it may direct the future study design for AD and OP prevention.
The Common Pathological Mechanism of AD and OP
Modern pharmacological studies indicate that there are many common pathological mechanisms between AD and OP, such as estrogen deficiency, Wnt signaling pathway inhibition, OPG/RANK/RANKL axis disorder, NF-κB signaling pathway activation, amyloid precursor protein cleavage, vitamin D deficiency, inflammation, parathyroid hormone (PTH) deficiency, calcitonin gene-related peptide (CGRP) expression, and autonomic nervous system (ANS) dysfunction (Figure 2).
Estrogen
Estrogen is a substance that promotes the maturation of the secondary sexual characteristics and sexual organs of the female. Estrogen receptors are distributed throughout the body, including the bones and brain. Therefore, estrogen has a wide range of important physiological effects. Estrogen can maintain the secondary sexual characteristics of women and have obvious effects on the growth and maturation of bones and the development of the brain (Patel et al., 2018). A cross-sectional study examined the BMD in women with varying degrees of cognitive impairment. It was found that cognitive impairment was related to low BMD, and cognitive impairment in postmenopausal women was significantly related to the decrease in BMD (Lee et al., 2012). This finding suggested that cognitive aging was multifactorial, and estrogen deficiency might be one of the crucial causes of cognitive impairment. By measuring the trabecular and cortical BMD of elderly women, Alice et al. found that reduced BMD showed a higher risk of cognitive decline (Laudisio et al., 2016). Similar to ovariectomized (OVX) rodents, it was found that low estrogen levels could also accelerate bone mass loss and lead to memory impairment (Eiken et al., 2014). In addition, estrogen therapy with continuous dependence can improve BMD and prevent osteoporotic fractures. Estrogen has a bone protective effect, which can promote bone formation by increasing osteoblasts (OB) and inhibiting high bone turnover so as to prevent bone loss (Stevenson, 2006). β-Amyloid (Aβ) deposition is the most important pathological feature of AD (Arbor et al., 2016). Estrogen has a negative regulatory effect on Aβ in the brain by activating Aβ degrading enzymes through estrogen receptors and finally promotes the degradation of Aβ (Liang et al., 2010). In OVX rats, Li et al. discovered that the expression levels of Aβ and its substrate synthetic amyloid precursor protein (APP) were significantly increased, and Aβ was mainly located in osteocyte membrane, plasma cells, and extracellular matrix, while APP was mainly located in the bone cell membrane (Li et al., 2014). Therefore, it is suggested that cognitive degradation and BMD reduction are both closely related to the lack of estrogen.
Wnt Signaling Pathway
Wnt protein is a secretory morphogen needed for the basic development of many different tissues, which participates in axonal guidance, neuroblastoma migration, and neuronal differentiation in the brain (Steinhart and Angers, 2018). The classical Wnt pathway activates transcriptional cofactor β-catenin, which is the main driving factor of bone formation and the mediator of synaptogenesis and neurogenesis in the brain (Ross et al., 2018). Studies have shown that the cellular signal cascade of the Wnt signal pathway is closely related to bone loss and neuropathology. Christine et al. found that the expression of osteogenic genes in female and male htau mice was significantly lower than that in normal mice, indicating that their bone remodeling was impaired. Furthermore, genes in the Wnt/β-catenin signal were changed in the bone and brain of the htau mice, suggesting that Wnt pathway was inhibited (Dengler-Crish et al., 2018). Dickkopf1 (DKK1) is an antagonist of the Wnt signaling pathway, which can bind to the Wnt co-receptor of the low-density lipoprotein-associated receptor protein (LRP5/6). DKK1 inhibits bone formation by competing with Wnt to bind with LRP5/6 and block Wnt/β-catenin (Macdonald et al., 2009). By studying the relationship between serum DKK1 and BMD, Joseph et al. found that the concentration of serum DKK1 in the osteoporosis mice was significantly higher than that in normal mice, suggesting a negative correlation between DKK1 and bone mass (Butler et al., 2011). As for the mechanism, on the one hand, blocking of DKK1 can promote the differentiation of osteoblasts (OB) and bone formation (Yaccoby et al., 2007), and on the other hand, DKK1 can also promote the maturation and differentiation of osteoclasts (OC) via upregulating osteoprotegerin (OPG)/receptor activator of nuclear factor-κB ligand (RANKL) pathway (Fujita and Janz, 2007). What is more, the inhibitory effect of DKK1 is related to the neurotoxicity of Aβ and tau protein hyperphosphorylation. In the brain of transgenic AD mice, it was found that Aβ deposition could increase the expression and secretion of DKK1 and indirectly lead to neuronal apoptosis. Activation of the Wnt pathway can reduce Aβ precursor protein lyase, while overexpression of DKK1 can inhibit tau protein hyperphosphorylation caused by the Wnt signaling pathway (Scali et al., 2006). These results suggest that overexpression of DKK1 will antagonize the Wnt signaling pathway, which thereby becomes a common risk factor for AD and OP.
OPG/RANK/RANKL and NF-κB Signaling Pathway
OB cell lineage secretes OPG, which is the only negative regulator of OC. In vitro, OPG can inhibit the differentiation of OC precursor cells and the formation of mature OC cells and induce OC apoptosis. The receptor activator of nuclear factor (RANK) is the only target receptor for RANKL to stimulate OC secretion and maturation (Li et al., 2016). It specifically binds with RANKL to activate the differentiation of osteoclast precursor cells and mediate bone resorption. Moreover, RANKL is a cytokine synthesized by OB and stromal cells, which can stimulate the formation of OB cells (Hsu et al., 1999). In in vitro experiments, it was shown that RANKL could make osteoclast precursor cells differentiate into OC, activate mature OC in a dose-dependent manner, finally prolong its survival time, and improve bone resorption capacity. OPG and RANK have competitive binding with RANKL, and OPG has a stronger competitive binding ability to RANKL than soluble RANK. On the one hand, OPG can promote OC apoptosis and terminate bone resorption by inhibiting the differentiation and maturation of OC. On the other hand, OPG can promote bone formation by nearly contacting OB. In vivo experiments have confirmed that OPG knockout mice had severe osteoporosis, while overexpression of OPG led to severe osteopetrosis (Bucay et al., 1998). Meanwhile, RANK is a signal complex with multiple downstream pathways. The binding of RANK and RANKL leads to the recruitment of tumor necrosis factor associated factor 6 (TRAF6) and rapidly activates the nuclear factor-κB (NF-κB) signaling pathway, thus initiating OC-specific gene transcription (van Dam et al., 2019). Long-term exposure to an inflammatory environment can significantly activate NF-κB and inhibit the function of OB. The activation of the NF-κB pathway is also related to neurofibrillary tangles (NFTs) and extracellular Aβ deposition. The increase in inflammatory factors in the AD brain will promote the degradation of APP to produce Aβ. Then, Aβ activates NF-κB that mediates the expression of inflammatory factors, which act on APP again to produce Aβ. This cascade reaction is critical in the pathogenesis of AD (June 2011). It was indicated that the inhibition of NF-κB could significantly decrease the inflammatory response caused by Aβ and reduce the damage of inflammatory factors to neuronal cells, as well as the deposition of NFTs and Aβ. Li et al. found that Aβ had no effect on RANKL-mediated OC production in rat bone marrow mononuclear cells, but it could promote bone resorption. At the molecular level, Aβ can improve the activity of NF-κB, activate the phosphorylation of extracellular signal-regulated kinase (ERK), and stimulate calcium ion oscillations, thus causing the upregulation of nuclear factor expression in OC-activated T cells (Li et al., 2016). All the above studies suggest that NF-κB can not only regulate the proliferation, differentiation, and apoptosis of OB and OC through OPG/RANK/RANKL axis system but also mediate inflammatory factors on APP to produce Aβ. This indicates that the activation of the NF-κB and OPG/RANK/RANKL signaling pathway can lead to AD and OP.
Amyloid Precursor Protein
Amyloid precursor protein (APP) is a membrane intrinsic protein expressed in various tissues and concentrated in the synapse of neurons. In vivo, APP is cleaved by β-site amyloid precursor protein cleavage enzyme (BACE1) to produce soluble APP (sAPP)β, which can bind to death receptor 6 to initiate apoptosis, and further trigger axonal pruning and neuronal death. After being cleaved by the BACE1 enzyme, γ secretase can cleave the remaining fragments of APP to produce the Aβ peptide (Corbett and Hooper, 2018; Guo et al., 2021). When Aβ transport disorders occur through the blood–brain barrier, Aβ oligomers are formed and deposited, resulting in neurotoxicity and neurological dysfunction (Magzoub, 2020). As for in vitro experiment, it was found that APP and Aβ could activate OC and promote osteoclastic bone resorption. In the biopsy of bone tissues, Li et al. (2014) found that Aβ42 and APP in OP patients and OVX rats were significantly higher in mRNA and protein expression, compared with the control group, and were negatively correlated with BMD. Tg257 mice are medical Swedish APP gene mutant mice with a two-way regulatory effect on the activation of OC. Cui et al. (2011) found that Aβ oligomers and receptors for advanced glycation end products (RAGE) promote OC production in Tg257 mouse models under 4 months of age. In addition, extracellular Aβ deposition is closely related to the NF-κB signal pathway. Aβ could promote the activity of NF-κB and activate ERK phosphorylation, then upregulate the expression of nuclear factor of activated T cells cytoplasmic 1 (NFATc1) and promote the OC activity (Li et al., 2016). For AD patients, the increase in inflammatory factors in the brain promotes the degradation of APP to form Aβ. Subsequently, Aβ activates the NF-κB pathway, mediates the expression of inflammatory factors, and interacts with APP degradation to produce Aβ. This cascade reaction is crucial in the pathogenesis of AD (Li et al., 2016). Therefore, it is suggested that APP/Aβ is a common characteristic of AD and OP in the regulation of bone remodeling in AD patients.
Vitamin D
Vitamin D deficiency is one of the risk factors inducing OP (Nanes and Kallen, 2014). Besides, although less well recognized, vitamin D deficiency is attracting more attention as a risk factor for AD. Observational studies have found that lower levels of plasma 25-hydroxy vitamin D are significantly related to increased risks of developing all-cause dementia and AD (Littlejohns et al., 2014). A current meta-analysis found a significant positive relationship between vitamin D deficiency and the risk of AD. It is especially noteworthy that moderate vitamin D deficiency was more strongly connected with the risk of AD compared with severe deficiency (Chai et al., 2019). Moreover, the connection was also investigated between AD and four previously identified single nucleotide polymorphisms related to vitamin D deficiency (Wang et al., 2010). It was found that an encoded vitamin D binding protein (DBP) played an important role in ameliorating AD (Mokry et al., 2016) via directly inhibiting Aβ aggregation and oligomerization, as well as improving Aβ-related neuronal injury and protecting against memory impairment in mice (Moon et al., 2013). On the other hand, vitamin D plays a critical role in regulating skeletal homeostasis both indirectly and directly. It was reported that vitamin D deficiency could lead to rickets in infants or children and induce OP in adults (Goltzman, 2018). As for the mechanism, the major effect of vitamin D is to regulate Ca2+ and Pi metabolism, promote intestinal Ca2+ and Pi absorption, induce bone calcification, and further protect against OP. These results provide converging evidence that vitamin D is a promising therapeutic agent for the treatment of AD and OP.
Inflammation
Recent studies found that inflammatory bone diseases were a potential risk factor for AD. A reduced prevalence of AD was described in rheumatoid arthritis patients who were long-term users of nonsteroidal anti-inflammatory agents (NSAIDs). The epidemiological studies also demonstrated that NSAIDs use is a protective factor for AD onset (Mcgeer et al., 1990). Furthermore, an increased risk of cognitive impairment in the population with midlife rheumatoid arthritis was confirmed based on 21 years of follow-up concerning the connection between rheumatoid arthritis and AD in several case-control, hospitals, and register-based studies (Wallin et al., 2012). Besides, Kamer et al. (2008) has discovered that periodontitis induces systemic inflammation, which stimulates the production of Aβ and tau protein in the brain, leading to AD neuropathology. Interleukin-1β (IL-1β) is a key inducer of the pathogenesis and tissues damage observed in cases of inflammatory bone diseases, including periodontitis and rheumatoid arthritis (Zwerina et al., 2007). IL-1β impairs the migration of OB (Hengartner et al., 2013) and upregulates the RANKL expression induced by osteocytes (Kulkarni et al., 2012). The result of the systematic review and meta-analysis indicated that the peripheral levels of IL-1β were significantly elevated in AD patients compared with controls (Ng et al., 2018). Lipopolysaccharide-induced chronic systemic inflammation in mice resulted in prolonged IL-1β production and microglial activation in the brain (Puntener et al., 2012). Additionally, chronic elevation of IL-1β also appeared in these mice, and a rising expression of hippocampal APP and its proteolytic fragments were discovered, resulting in significant memory impairment in old age (Krstic et al., 2012). These results further suggest that inflammation is a vital risk factor for the development of AD and OP.
Others
In addition to the above factors, there are some other common risk factors associated with AD and OP, such as PTH deficiency, CGRP, and ANS dysfunction. PTH is a key regulator of calcium homeostasis that has the potential to enhance bone regeneration in large bone defects, and its mechanism lies in its anabolic effect on bone. Daily injections of PTH are an effective treatment for OP approved by the US Food and Drug Administration (FDA) that results in increases in both BMD and bone volume (Wojda and Donahue, 2018). The connections between PTH and AD have been reported in a few prospective and case-control studies. In a prospective cohort study, high PTH concentrations were significantly and positively associated with risks of vascular dementia (Hagstrom et al., 2014). Another prospective study discovered that elevated PTH concentrations were connected with a significantly increasing risk of all-cause dementia during the 1-year and 5-year follow-up (Bjorkman et al., 2010). CGRP is a 37 amino acid regulatory neuropeptide resulting from the different merging of the CGRP gene, which has various physiological effects. However, this peptide affects inducing neuroinflammation in neurologic disorders (Malhotra, 2016). CGRP inhibition was also involved in the infiltration of macrophages and the expression of inflammatory mediators such as IL-1β and TNF-α (Singh et al., 2017). As for in vivo study, it was discovered that genetic depletion of calcitonin receptors in AD mice could ameliorate mice spatial memory and hippocampal synaptic plasticity, which was connected with a significant reduction in certain characteristic brain markers that were indicative of AD pathology (Patel et al., 2021). Peripheral release of CGRP contributed to the relief of arthritic pain; nevertheless, CGRP would transform the normal to persistent joint synovitis, and the expression of CGRP receptors would upregulate the following arthritic induction (Walsh et al., 2015). Besides, ANS dysfunction is also a key pathogenic factor of AD and OP. In summary, the CGRP antagonists, PTH, and agents for regulating ANS might be a potential therapeutic target to prevent persistent inflammation and attenuate the pathological cascade of AD and OP.
Natural Chinese Medicine
Morindae Officinalis Radix (Morinda officinalis How., Ba-Ji-Tian, MO)
Morinda officinalis How. is a traditional natural herb in China and northeast Asia, which contains many bioactive ingredients, such as iridoid glycosides, anthraquinones, and oligosaccharides. MO is known to be beneficial to the liver, kidney, and heart channels and has the action of tonifying kidney-yang, enhancing muscles and bones, and improving blood circulation. MO is widely used in TCM to treat different diseases associated with kidney-yang deficiency, such as male impotence, spermatorrhea and premature ejaculation, female infertility, and skeletal atrophy (Cai et al., 2017). Recent studies have found that MO can strengthen sexual and reproductive functions and ameliorate AD via regulating the microbiota-gut-brain axis, in evidence of improving memory and learning abilities (Chen et al., 2017).
According to traditional theories of Chinese medicine, cerebral diseases can be prevented by invigorating the kidneys. Therefore, MO is widely used to treat cerebral diseases, such as AD. A recent study revealed that oligosaccharides isolated from MO could significantly enhance the learning and memory abilities of rats with dementia and alleviate dementia symptoms in behavioral experiments. Subsequent in vivo experiments found that the oligosaccharides of MO could also increase the superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), and acetylcholine (Ach) activities; decrease the malondialdehyde (MDA), acetylcholinesterase (AChE), neuronal apoptosis; and inhibit the expression of tau and Aβ1-42 (Chen et al., 2017). Specifically, oligosaccharide monomers, such as Bajijiasu and nystose, exhibit various biological activities, including neuroprotective, antidementia, antiosteoporosis, and antidepressant effects (Zhang et al., 2018). MO aqueous extracts could also ameliorate learning and memory impairment in AD mice. After the administration of MO aqueous extracts for 4 weeks, it was observed that MO significantly improved the learning and memory ability in a water maze test, significantly reduced the activity of aldose reductase, decreased the serum advanced glycosylation end products (AGEs) and receptor for advanced glycosylation end products (RAGEs), and then decreased the damage to brain cells (Ye et al., 2015). In D-galactose and Aβ25-35-induced AD rats, different doses of MO also significantly increased the antioxidant enzyme activities (SOD, GSH-Px, and CAT), neurotransmitter levels (acetylcholine, gamma-aminobutyric, and dopamine), energy metabolism (Na+/K+-ATPsae), and relative synaptophysin expression levels (Deng et al., 2020). Furthermore, there was a significant decrease in MDA levels and relative expression levels of APP, tau, and caspase-3 in AD rats after treatment with MO.
In TCM, it is considered that kidney essence deficiency plays a vital role in the development of OP, and MO is commonly used to treat bone atrophy and OP. The aqueous extract of MO has been confirmed to be effective in the treatment of postmenopausal OP, as evidenced by a clinical trial involving 50 patients (Long et al., 2013). In modern pharmacology research, it was also discovered that MO ethanol extract had protective effects on ovariectomy-induced bone loss, as evidenced by the increased tibia bone mineral content (BMC) and BMD, improved phosphorus (Pi), calcium (Ca2+), and OPG levels (Li et al., 2009). As for in vitro studies, the aqueous extracts of MO root could increase the expression of core-banding factorα 1 (Cbfα1) in bone marrow stromal cells (BMSCs) and promote the differentiation from BMSCs to OB (Wang et al., 2004). MO polysaccharide is also shown to have antiosteoporosis activities. It was discovered that MO polysaccharides could increase the BMD and BMC and decrease the cytokine levels in the serum interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α) of OVX rats after 30 days of oral administration (Mengyong et al., 2008). MO anthraquinone could also increase the mRNA expression of RANKL, alkaline phosphatase (ALP), and OPG, thereby promoting osteoblastic bone formation (Bao et al., 2011).
Taken together, these findings suggest that MO and its bioactive compounds may play an important role in the treatment and prevention of AD and OP. MO appears to provide a potentially effective treatment for AD and OP by the alleviation of learning and memory impairment and the attenuation of bone loss and trabecular microstructural degradation. Therefore, MO is a good choice for screening potential drugs to treat AD and OP.
Eucommiae Cortex (Eucommia ulmoides Oliv., Du Zhong, EU)
Eucommia ulmoides Oliv. is an important economic plant, and its dried bark, leaves, stem, and even staminate flower are widely used in TCM. EU enjoys extensive pharmacological properties as an antioxidant, anti-inflammatory, antimicrobial, anticancer, cardioprotective, and neuroprotective agent that has been applied for the treatment of cardiovascular disease, sexual dysfunction cancer, neurological disease, rheumatoid arthritis, and osteoarthritis (He et al., 2014). The bioactive compounds of EU include lignans, iridoids, phenolics, steroids, and flavonoids, most of which have been proved to possess the ability to protect against AD and OP.
The aqueous extract of EU exhibits significant neuroprotective effects in several experimental models, thus proving to be a potential therapeutic drug in the treatment of neurodegenerative diseases, such as AD. In vivo studies showed that the aqueous extract of EU bark exerted a neuroprotective effect on Aβ25–35-induced mice and ameliorated learning and memory impairment (Kwon et al., 2011). The aqueous extract of EU significantly improved Aβ25–35-induced memory deficits in the Y-maze test and increased step-through latency time with Aβ25–35-induced learning and memory deficits in the passive avoidance test. In addition, the aqueous extract of EU also significantly inhibited AChE activity in the hippocampus and frontal cortex at 20 mg/kg concentrations and suppressed AChE activity in a dose-dependent manner, with the degrees of inhibition being 27.3% and 25.3%, respectively (Kwon et al., 2011). Moreover, aucubin in the EU appeared to ameliorate damage induced by lithium-pilocarpine in the hippocampus with status epileptics (SE), as well as reducing the number of apoptotic neurons and increasing the number of survival neurons by inducing autophagy and inhibiting necroptosis (Wang J et al., 2017). Additionally, the macranthoin G from EU effectively enhanced PC12 cells viability and increased the antioxidant enzymes, such as CAT, SOD, GSH, and GSH-Px. It also reduced intracellular reactive oxygen species (ROS), MDA content, caspase-3 activation, and PC12 cell apoptosis in in vitro experiments (Hu et al., 2014). These findings all revealed that EU had potential therapeutic effects on neurodegenerative diseases, such as AD.
In a rodent model of acetate-induced bone loss, EU cortex extract (EUCE) attenuated the loss of BMD of the rat’s lumbar spine and femur and restored serum Ca2+, Pi, ALP, osteocalcin, and RANKL to normal levels (Xiong and Zhao, 2016). Furthermore, anti-osteoclastic activity was indicated by the effect of EUCE on regulating the serum OPG/RANKL ratio to normal and significantly increased the serum Ca2+, Pi, ALP, and osteocalcin (Qi et al., 2019). Total lignans (TL) extracted from EU-cortex could increase BMD, biomechanical properties, and microarchitecture and inhibit the bone loss and bone turnover rate due to estrogen deficiency-induced osteoporosis in a rodent model. In vitro experiments also showed that TL not only increased the proliferation and differentiation of OB but also improved the formation of bone nodules (Zhang et al., 2014). The leaves and seeds of EU may also be of potential benefit for the treatment of osteoporosis. In a combined estrogen deficiency-induced osteoporosis and obesity rodent model, EU leaf extract decreased rats’ body weight and body mass index (BMI) and increased the BMD and bone strength, which appeared to be beneficial for improving bone metabolism and restoring bone loss (Zhao et al., 2020).
Taken together, it is demonstrated that EU is good medicine to treat neurodegenerative diseases and prevent bone loss for many parts of EU, including the cortex, leaf, and seed and possesses potential therapeutic benefits for the treatment of AD and OP as depicted above.
Lycii Fructus (Lycium barbarum L., Gou Qi Zi, LR)
Lycium barbarum L., as a traditional Chinese medicinal herb and food supplement, has been used in China and other Asian countries for more than 2000 years. These berries have also become increasingly popular in western countries as an anti-aging and antioxidant product (Jin et al., 2013). The TCM theory thinks that LR can tonify the kidney, benefit essence, and strengthen the activity of the waist and knee by balancing the “yin” and “yang” in the body. LR has pharmacological properties as an anti-aging, antioxidant, anticancer, antifatigue, anti-osteoporosis, and neuroprotective agent exhibiting a wide array of therapeutic effects on aging, fatigue, cancer, stroke, AD, and OP.
Aβ is the major component of senile plaques and is considered the main biomarker of AD. Studies have found that Aβ develops its toxicity to neurons and other brain tissues by increasing the N-methyl-D-aspartate receptor- (NMDAR-) mediated Ca2+ influx and subsequently generating ROS (Pinheiro and Faustino, 2019). LR can protect against neuronal injury induced by Aβ peptides and promote neurogenesis. The LR polysaccharide (LRP) also shows a beneficial effect on Aβ-induced caspase-3 activation and neuronal cell death (Ho et al., 2007). The alkaline extract of LR (containing LRP) could also protect neurons via elevating the pro-survival AKT pathway (Ho et al., 2007). The water extract of LR played its neuronal beneficial effects by inhibiting the apoptotic pathway and associated c-Jun N-terminal kinase (JNK)/double-stranded RNA-dependent protein kinase (PKP) pathway (Yu et al., 2007). Much evidence indicated that the over-activation of the glutamatergic NMDARs leads to postsynaptic Ca2+ overload and excitotoxicity, resulting in disturbed neuroplasticity and neuronal cell death. Ho et al. (2009) found that LRP could antagonize glutamate-induced toxicity in primary cultures of cortical neurons, with a similar function as memantine (this non-competitive NMDAR antagonist was approved by US and European Food and Drug Administrations to be widely used in clinical trials to treat AD). LRP can also inhibit caspase-3 activation, decrease the generation of ROS, and exert an antioxidant effect. In an amyloid precursor protein/presenilin 1 (APP/PS1) double-transgenic mice model, LR water extracts strongly ameliorated the learning and memory impairments of mice after 2-week administration, which was demonstrated by the Morris water maze test (Zhang et al., 2013). These findings confirmed the protective effects of LR on AD, and the mechanism might be related to the clearance of Aβ.
LR has also shown a potent anti-OP effect in in vivo and in vitro experiments. After oral administration with 1 and 100 mg/kg LR extracts for 6 weeks, OVX rats exhibited an enhancement of BMD and BMC, decreased serum osteocalcin levels, and a recovery of the calcium levels (Kim et al., 2017). In addition, LRP could reverse palmitate-induced apoptosis in MC3T3-E1 cells by inhibiting the activation of caspase-12 and the phosphorylation level of JNK (Jing and Jia, 2018). In addition, the extract of LR cortex could also prevent BMD loss in OVX mice, improve cellular proliferation and differentiation, and increase ALP activity and mRNA expression of OB marker runt-related transcription factor 2 (Runx2) in in vitro experiment (Park et al., 2014). Taken together, the role of LR in promoting OB proliferation and differentiation is beyond doubt.
Collectively, as a common dual-purpose product for medicine and food, LR has the potential to develop into a drug for the prevention and treatment of AD complicated with OP for its effects on clearing Aβ and promoting OB bone formation.
Atractylodis Macrocephalae Rhizoma (Atractylodes macrocephala Koidz., Bai Zhu, AM)
Atractylodes macrocephala Koidz., a traditional medicinal plant, is widely used as a tonic agent in China and other Asian countries. In the TCM theory, AM is considered to enter the stomach and spleen channels that can tonify “Qi” and clear damp and diuresis. AM enjoys diverse pharmacological activities, including improving gastrointestinal function, exerting anti-inflammatory, anti-oxidative, anti-aging, anti-osteoporotic, and neuroprotective activities (Zhu et al., 2018).
AM extract was found to have protective activity against AD. In the experiment of the rat passive avoidance test model, it was found to significantly prolong the step-through latency, reduce the error frequency, and decrease the AChE content after the administration of AM ethanol extract (0.5–2 mg/kg) for 15 days (Hong and Yu, 2015). This demonstrated that AM might improve memory impairment and prevent AD in aging rats, and the underlying mechanism might be associated with decreased AChE activity in the hippocampus. In a water maze experiment of AD rats induced by Aβ1-40, a supplement with AM biatractylolide (0.1–1.0 mg/kg) for 10 days led to a remarkably reduced swimming time and error frequency and reduced cholinesterase (ChE) content in rats, indicating that AM biatractylolide could improve the memory and learning ability of AD rats (Feng et al., 2009). In addition, AM atractylenolide also has a significant neuroprotective activity. It was discovered that the administration of AM atractylenolide could significantly enhance the cell viability in PC12 cells induced by hypoxia, calcium overloading, and excitatory amino acids (Luo and Sun, 2012). Likewise, in PC12 cells, it was found that the supplement with atractylenolide III (30 μg/ml) significantly enhanced the cell activity and suppressed apoptosis (Wang, 2012). These findings indicate that AM has a potent neuroprotective activity, especially in protecting against AD.
It was discovered that intraperitoneal injection of RANKL could rapidly reduce trabecular bone loss by stimulating OC function and differentiation rather than affecting OB formation. As for the in vitro experiment, AM ethanol extracts could attenuate RANKL-induced activation of the NF-κB signaling pathway and then inhibit the differentiation of OC in RANKL-induced osteoclastogenesis from OC precursors. Similar to in vitro results, AM also has protective effects on RANKL-induced bone loss in mice. AM inhibits RANKL-induced c-Fos and NFATc1 expression in OC precursors, thereby inhibiting OC differentiation (Ha et al., 2013). Moreover, in the mesenchymal stem cells (MSCs), the treatment with atractylenolides I and III (both are 1–300 μg/ml) can significantly improve the expression of specific chondrogenic markers and promote chondrogenic differentiation (Li X et al., 2012). Nevertheless, the anti-OP effect of other active components in AM needs to be evaluated.
Therefore, evidence suggests that AM can be used as an effective therapeutic agent for the treatment of AD and OP. Further research is needed to isolate and characterize the bioactive constituents of AM and determine the molecular mechanisms and signaling pathways that produce therapeutic effects.
Salviae Miltiorrhizae Radix Et Rhizoma (Salvia miltiorrhiza Bge., Dan Shen, SM)
Salvia miltiorrhiza Bge. is one of the most famous TCM herbs, which has been used to treat various diseases for centuries. Its traditional efficacy is HuoXue QuYu (activate blood circulation and remove stasis), Tongjing ZhiTong (regulate menstruation and relieve pain), QingXin ChuFan (tranquilize the mind and eliminate annoyance), LiangXue XiaoYong (reduce blood flow and eliminate carbuncle). Modern pharmacological studies demonstrate that SM has diverse pharmacological activities, such as anti-oxidation, anti-inflammation, neuroprotection, anti-apoptosis, and antiosteoporosis (Sreenivasmurthy et al., 2017; Chen et al., 2019; He et al., 2019).
Accumulated evidence indicates that many ingredients of SM, such as salvianolic acids A, salvianolic acids B, cryptotanshinone tanshinone I, and tanshinone IIA, have effects on reducing cell apoptosis, inhibiting Aβ aggregation, enhancing cholinergic, and further exerting neuroprotective effects. Studies have suggested that SM extracts could significantly improve the learning and memory abilities of AD rats after being supplemented with SM for 23 consecutive days. It protected against Aβ25-35 induced apoptosis by downregulating APP and PS1 levels (Liu M et al., 2015). Other studies showed that AM root extracts could enhance the neurogenesis of neural stem cells and progenitor cells (Zhang XZ et al., 2016). Several reports also showed that the chemical components in SM had anti-AD effects. It was discovered that salvianolic acids A and salvianolic acids B could prevent Aβ aggregation, delay AD paralysis in C. elegans, and reduce ROS production to protect against Aβ induced neurotoxicity (Yuen et al., 2021). Moreover, salvianolic acid B could also improve the cholinergic damage and ameliorate Aβ-induced cognitive impairment in the AD mice model (Lee YW et al., 2013). Other research suggested that tanshinone IIA could prevent spatial learning and memory deficits in APP/PS1 mice and attenuate Aβ accumulation and neuronal loss (Ding et al., 2020).
In TCM, OP is called “bone atrophy,” and its pathogenesis is related to the liver and kidney. Furthermore, SM plays an effective role in nourishing the liver and kidney. In vivo studies showed that SM could attenuate the unbalanced levels of serum ALP, OPG, and RANKL in OVX rats after 14 weeks of treatment. The decreased bone strength and BMD were inhibited, and the impaired bone micro-structures were improved (Liu H et al., 2018). Similarly, SM extracts could prevent trabecular bone loss and improve the serum level of OPG in OVX mice (Park et al., 2017). These results indicate that SM has an excellent anti-bone loss effect. In addition, other active components of SM, such as salvianolic acid, also have strong bone anabolism activities. It was discovered that salvianolic acid B could enhance cancellous bone formation, improve cancellous thickness, and prevent bone loss in glucocorticoid-induced OP rats. In vitro experiment showed that salvianolic acid B could stimulate BMSCs differentiation to OB and increase OB activity via increasing the secretion of ALP and osteocalcin, improving Runx2 mRNA expression, and decreasing DKK1 mRNA expression (Cui et al., 2012). Moreover, it was found that salvianolic acid A could improve fracture callus formation and micro-architecture with an accelerated mineralization rate in callus on a prednisone-induced delayed fracture union mouse model (Liu Y et al., 2018).
In conclusion, these results indicate that both AM extracts and AM active components have significant anti-AD and anti-OP potentials by attenuating Aβ accumulation and regulating OPG and Wnt pathways. However, the underlying mechanism and clinical application are not very clear, so more research is needed.
Rehmanniae Radix (Rehmannia glutinosa Libosch., Di Huang, RR)
Rehmannia glutinosa Libosch. has been widely used in the form of decoction pieces containing dry Radix Rehmanniae and Prepared Radix Rehmanniae in clinic. RR has various pharmacological actions, such as lowering blood pressure, anti-hyperglycemia anti-inflammation, neuroprotection, improving renal function, and antiosteoporosis (Zhang ND et al., 2016). Therefore, the clinical application of RR is to treat different diseases such as renal failure, hypertension, AD, and OP.
Although RR is not indicated in the current pharmacopeia for symptoms associated with AD, it is one of the most frequently reported ingredients of multiple TCM formulas indicated for memory impairment associated with aging (May et al., 2016). In in vivo screening system using the Drosophila model of AD, the RR extract was found to have neuroprotective activities against Aβ neurotoxicity (Liu QF et al., 2015). Catalpol is one of the most active compositions of RR and has a great potential for treating AD. In AD mice, catalpol could significantly increase the activity of SOD, GSH-Px, and CAT and reduce the levels of soluble Aβ40 and Aβ42 in the cerebral cortex of the hippocampus, thus inhibiting the formation of senile plaques. Morris water maze test revealed that the time spent in the target quadrant and average number of hidden platforms could be increased after the administration of catalpol, indicating its effects on improving the learning and memory impairment of AD mice (Huang et al., 2016). Liu et al. (Liu C et al. 2018) found that the treatment of endothelial (bEND.3) cells with catalpol could prevent endothelial damage, reduce blood–brain barrier hyperpermeability induced by fibrillar Aβ1-42, and enhance soluble Aβ clearance, indicating that catalpol had a protective effect on Aβ1-42-induced blood–brain barrier leakage. In aged rats with Aβ induced nerve injury, catalpol promoted nerve recovery and improved the expression of synaptic proteins, which was important in synaptic plasticity and neuronal development (Xia et al., 2017). Wang et al. (2018) also discovered that catalpol could upregulate α-secretase expression, promote α-cleavage of APP processing, and decrease Aβ formation via ERK/cAMP response element-binding protein (CREB) signaling pathway in Swedish mutant APP overexpressed N2a (SweAPP N2a) cells.
In TCM, RR has the effects on promoting blood circulation, tonifying the kidney, and regulating the liver, which are closely related to the pathogenesis of OP. Studies have discovered that water extracts of RR (300 mg/kg) could significantly improve BMD in the lumbar and femurs and markedly reduce the serum ALP level in OVX rats after an 8-week treatment (Lim and Kim, 2013). Some active components in RR, such as acteoside and catalpol, also showed a potent anti-osteoporosis effect. Acteoside could reduce ROS production and inhibit OC formation and bone resorption by macrophages via downregulating early RANKL signaling pathways and inhibiting the expression of TNF-α, NFATc1, and c-Fos (Lee SY et al., 2013). In addition, oral administration with acteoside could also inhibit the alteration of OP biochemical markers and bone loss in OVX rats (Lee SY et al., 2013). Similarly, Gong et al. (2019) found that acteoside, echinacoside, and catalpol could improve the proliferation and differentiation of osteoblastic MC3T3-E1 cells and increase bone morphogenetic proteins 2 (BMP2), Runx2, and osterix to prompt bone formation. As for in vivo study, RR extract could increase ALP activity, decrease osteocalcin (OCN) levels, and improve BMD and bone microarchitecture in diabetic rats. In addition, RR water extract could increase the serum OPG level; reduce the serum of ALP, RANKL, and tartrate-resistant acid phosphatase (TRAP) in OVX rats; and increase cortical bone and epiphyseal thickness to preserve BMD and mechanical strength by regulating Wnt/β-catenin signaling pathway (Liu et al., 2019).
Altogether, RR is considered a well-tolerated herbal medicine for AD and OP with potential neurogenic and bone-protective activities in vivo and in vitro. Its mechanism is mainly related to Aβ clearance, ERK/CREB pathway regulation, and Wnt/β-catenin activation.
Glycyrrhizae Radix Et Rhizoma (Glycyrrhiza uralensis Fisch., Gan Cao, GR)
Glycyrrhiza uralensis Fisch. is the dried root and rhizomes of Glycyrrhiza uralensis Fisch., Glycyrrhiza inflata Bat., or Glycyrrhiza glabra L. and has traditionally been used in the treatment of cough, influenza, and detoxification all over the world for hundreds of years. Its clinical application has attracted extensive attention worldwide (Ji et al., 2016). Recent studies have shown that GR has antioxidant, anti-AD, anti-inflammatory, and bone protective activities.
Licochalcone B is an active compound in GR and has neuroprotective activity through inhibiting Aβ42 self-aggregation, reducing ROS generation, and preventing H2O2-induced SH-SY5Y cell death (Cao et al., 2020). In lipopolysaccharide-treated C57BL/6 mice, oral intake of GR extract (150 mg/kg) led to a significant reduction in spatial cognitive and memory impairment, as evidenced by the T-maze novel object recognition test (Cho et al., 2018). In another study, it was discovered that GR extract and its bio-activated compound semilicoisoflavone B could decrease the secretion of Aβ through regulating BACE1 transcription factors, as well as activating receptor-γ (PPARγ) expression and inhibiting the phosphorylation of signal transducer and activator of transcription 3 (STAT3) (Gu et al., 2018). Glycyrrhizic acid, another main bioactive compound of GR, has a neuroprotective effect on scopolamine-induced cognitive impairment. The Y-maze test found that glycyrrhizic acid could significantly improve the mice cognitive impairment induced by scopolamine, decrease AChE activity, and increase SOD and CAT activity. The mechanism was via increasing phosphorylation of JNK and ERK protein and improving the mitogen-activated protein kinase (MAPK) pathway (Ban et al., 2020). Additionally, some active components in GR also showed potent anti-AD effects. The coumarin glycyrol in GR could significantly suppress the butyrylcholinesterase (BChE) and AChE, and another compound, liquiritigenin, effectively inhibited monoamine oxidase B (MAO-B) and monoamine oxidase A (MAO-A). These results indicate that GR can be considered a promising herb for the treatment of AD with multi-targeting activities (Jeong et al., 2020).
In the theory of TCM, GR has a strong nutritious function and is usually used as a life enhancer. Previous studies have found that the extract of GR and its active ingredients have estrogenic-like activities (Tamir et al., 2001). Di et al. confirmed that the GR extracts notably protected BMD loss in OVX rats by measuring the BMD of the total tibia and proximal tibia. This protective effect did not affect changes in histology and weight of the uterine, indicating the absence of a uterus-focused effect of GR extract (Galanis et al., 2019). Liquiritigenin is an active flavonoid extracted from GR. It was discovered that liquiritigenin could inhibit the formation of OP phenotype in glucocorticoid-induced adult zebrafish model via inhibiting OC activation in scales (Carnovali et al., 2020). Another active ingredient, 18β-glycyrrhetinic acid (18β-GA), could also inhibit osteoclastogenesis and RANKL-mediated OC differentiation at an early stage in vitro by suppressing RANK expression in preosteoclasts and blocking the binding of TNF receptor-associated factor 6 (TRAF6) and RANK, thereby leading to the inhibition of MAPK and NF-κB signaling pathways. Furthermore, 18β-GA could reduce the serum tartrate-resistant acid phosphatase 5b (TRAP5b), TNF-α, and interleukin-6 (IL-6) and increase bone matrix mineralization, which indicated that 18β-GA decreased bone loss in OVX mice via suppressing osteoclastogenesis (Chen et al., 2018). In addition, studies have found that ethyl acetate extract from GR could also play a similar role as estrogen and promote the proliferation of human BMSCs (Azizsoltani et al., 2018). These results indicate that GR may be a potential candidate for the prevention of OP.
These findings indicate that GR appears to provide beneficial therapeutic effects for AD and OP, including attenuating cognitive impairment and bone loss. Hence, as a potent life enhancer, GR has the potential to be developed into a drug for the prevention and treatment of AD complicated with OP.
Cistanches Herba (Cistanche deserticola Ma., Rou Cong Rong, CH)
Cistanche deserticola Ma., a desert living plant known as desert ginseng, is of a high medicinal value. It is characterized as the major material of tonifying kidney “yang” in the TCM theory and has been widely used to ameliorate forgetfulness, improve the strength of reproduction, and develop fertility function. Modern pharmacological studies reveal that CH has neuroprotective, immunomodulatory, anti-viral, anti-inflammatory, anti-tumor, anti-bacterial, anti-oxidative, and bone-formation activities. Therefore, CH is widely used in Chinese medicine prescriptions for treating various diseases, such as dementia, aging, and OP (Jiang et al., 2016; Fu et al., 2018; Fan et al., 2019).
Previous studies confirmed that CH extracts or its components exerted a potential neuroprotective effect against AD. Zhou et al. (2019) found that the CH extract had antiapoptotic and antioxidant stress effects via inhibiting Aβ deposition and tau protein hyperphosphorylation and promoting synapse protection. Chao et al. (2019) also discovered that the CH extract protected against AD by inhibiting Aβ peptide aggregation and deposition. In a clinical trial, AD patients were treated with CH capsules for 48 weeks. The results showed that the levels of TNF-α, total-tau, and IL-1β were significantly reduced, and volume changes of the hippocampus slowed down, indicating that CH could ameliorate cognitive and independent living ability through inhibiting the expression of TNF-α, total-tau, and IL-1β in the cerebrospinal fluid of AD patients (Li et al., 2015). Acteoside is one of the active phenylethanoid glycosides in CH and is known to have neuroprotective and antioxidant effects. In the AD mice model of senescence induced by a combination of AlCl3 and D-galactose, it was discovered that acteoside could shorten the latency of step down, reduce the number of errors, and decrease the activity of nitric oxide synthase and caspase-3 (Peng et al., 2015). Similarly, CH decoction could also improve the mice’s spatial learning and memory in the Morris water maze test by decreasing monoamine oxidase and increasing dopamine in the brain (Wang D et al., 2017).
In OVX rats, it was found that the BMD and BMC were significantly improved after treatment with CH extracts, and serum ALP and SOD CAT GSH-Px were also markedly reduced (Liang et al., 2011), suggesting that CH could reverse the bone loss and prevent postmenopausal osteoporosis. Further investigation indicated that the molecular mechanism that CH reduced bone degeneration and regulated involved bone metabolism genes, including Smad1, Smad5, TGF-β-inducible early gene 1 (TIEG1), and transforming growth factor-β1 (TGF-β1) (Liang et al., 2013). In vitro experiments also demonstrated that CH extracts could enhance the BMP2, osteopontin (OPN), and ALP expression and induce the OB differentiation, maturation, and bone mineralization by regulating ERK, JNK, NF-κB, and p38 (MAPK) pathways (Li TM et al., 2012). Additionally, CH polysaccharide enhanced the expression of antioxidant enzymes and reduced the RANKL-induced ROS overproduction to decrease the OC differentiation and inhibit bone resorption (Song et al., 2018).
All of these studies demonstrate the efficacy and therapeutic potential of CH in the treatment of neurodegenerative damage and bone loss. Further study is necessary to extensively characterize the bioactive effects of CH that may render better therapeutic strategies for the treatment of AD and OP.
Discussion
In summary, with the increasingly aging population worldwide, dementia and osteoporotic fracture have become a major health and social issues. The side effects of existing clinical drugs have prompted researchers to study natural therapeutic compounds for their effectiveness and safety in the treatment of AD and OP, with fewer adverse side effects.
OP and hip fracture are commonly observed complications seen in patients with AD. Although the mechanisms underlying this association remain poorly understood, emerging evidence supports the view that AD risk genes may also be a risk factor for osteoporosis and that AD and OP may share conserved oxidative stress-driven pathogenic mechanisms (Weller and Schatzker, 2004). In addition, the pathogenesis of AD and OP is complicated in terms of occurrence, development, and progression, including estrogen deficiency; Aβ deposition; and the dysregulation of Wnt, RANKL, and NF-κB signaling pathways. Thus, the combination of multi-component and multi-target targeted therapy may be of great significance.
TCM herbs may contain effective components for the treatment of AD and OP, and this review summarizes current evidence of their potential bio-pharmacological effects and possible mechanisms. A summary of natural herbs against AD and OP in vivo and in vitro is shown in Table 1 and Table 2, summarizing the change in related indicators of each drug in in vivo and in vitro experiments.
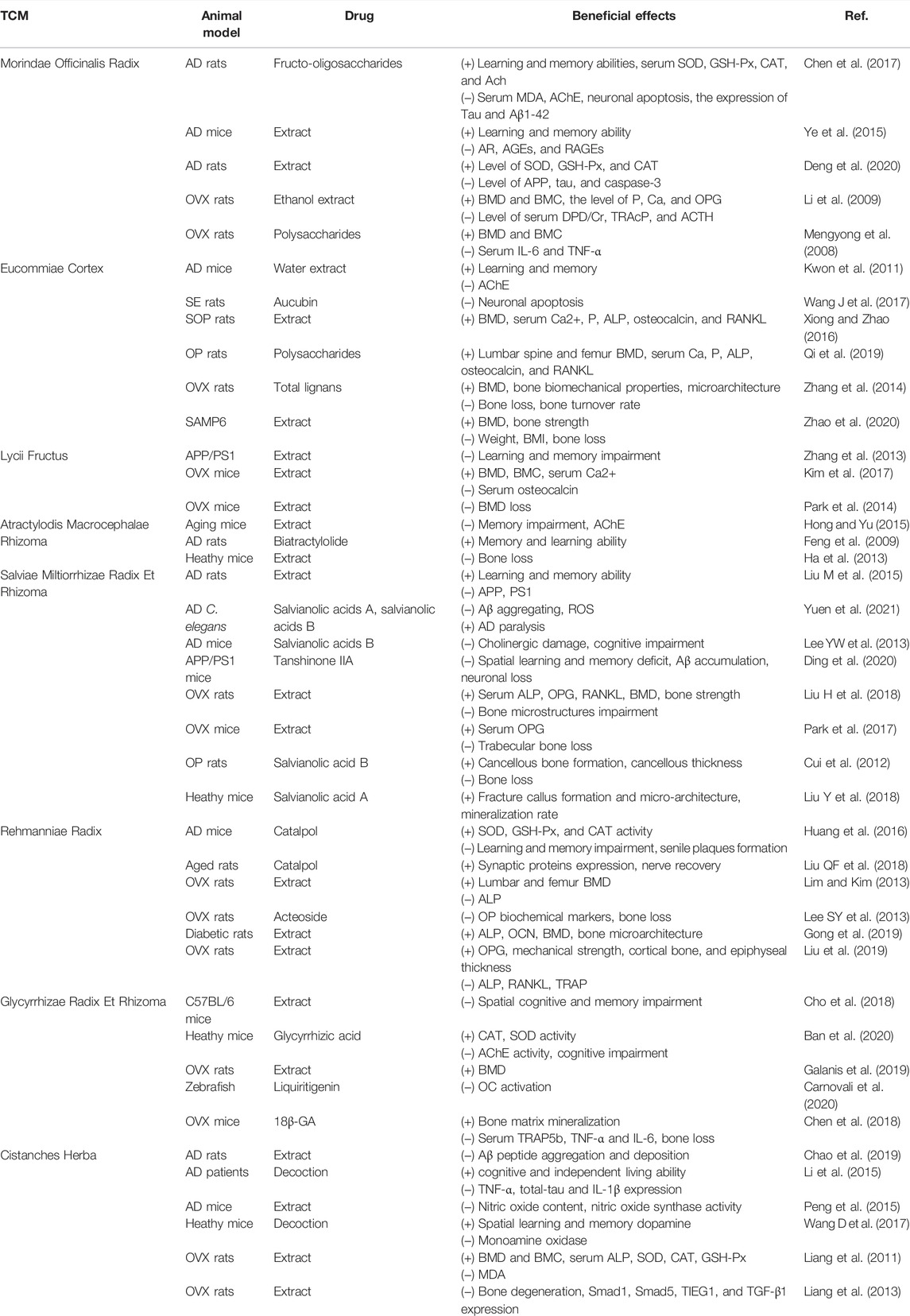
TABLE 1. Summary of in vivo studies for the anti-Alzheimer’s disease and antiosteoporotic effects of natural Chinese medicine.
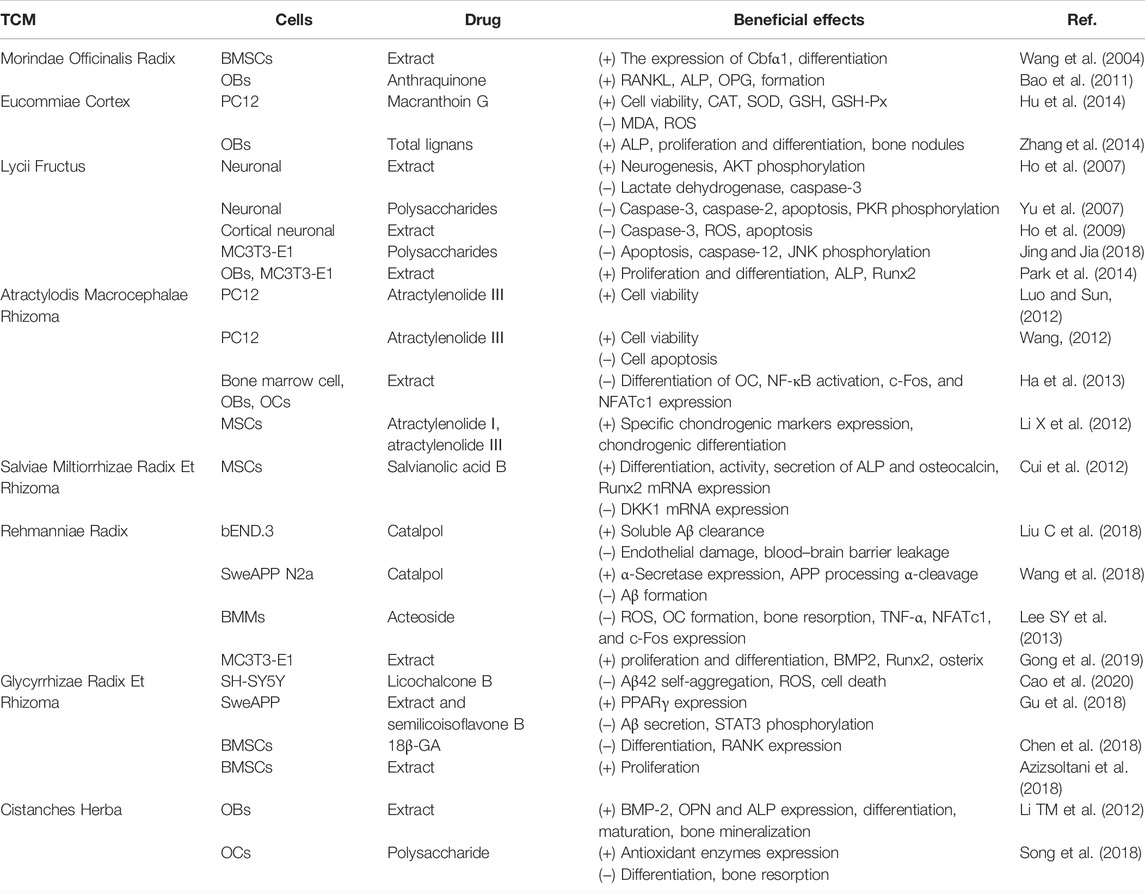
TABLE 2. Summary of in vitro studies for the anti-Alzheimer’s disease and antiosteoporotic effects of natural Chinese medicine.
According to the clinical experience and the TCM theory, Chinese herbs are divided into different categories with special functions. Because of their effects on tonifying the kidney, some of them are classic and specific drugs for improving the impairment of the brain and bone. In addition, these herbs can not only treat diseases but also have a certain preventive effect. However, the development of AD and OP remains very complex in elderly people and postmenopausal women. The mechanism of natural Chinese herbs for the treatment of AD and OP has not been fully clarified. Therefore, further study on the isolation and characterization of bioactive components against AD and OP from classic and specific drugs is necessary to widely profile components for pharmacological uses, especially their efficacy, safety, and potential interaction with other medications. The research to determine the special and targeted cellular and molecular mechanisms of natural Chinese medicine components should develop their potential application in the treatment of AD and OP as an effective and safe alternative to the major treatment strategy or in combination with current pharmacological therapy. Besides, few high-quality clinical research studies have presented against the AD and OP effects of components with known structures. Because there maybe unknown chemical interactions between various drugs and non-specific components in traditional formulas, the clinical drug research results still have deficiencies and limitations. Therefore, more high-quality clinical research studies of TCM are required in the future to explore the anabolic and anti-metabolic effects.
Conclusion
As common age-related diseases, AD and OP have similarities in pathological characteristics and pathogenesis. Recent in vivo and in vitro findings indicate that TCM herbs may well be effective for the treatment of AD complicated with OP. Further study is required to assure the efficacy, safety, and specificity of the components in TCM in order to tap its therapeutic potential. Therefore, more high-quality clinical studies of these natural drugs are required to provide more powerful evidence for candidate drugs so that they can be used as beneficial and safer against AD and OP applications.
Author Contributions
All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (82004015, 82174079) and the Project of Science and Technology Commission of Shanghai Municipality (21S21902600).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Aβ, β-amyloid; AD, Alzheimer’s disease; AGEs, advance glycosylation end products; APP, amyloid precursor protein; AR, aldose reductase; BACE1, β-site amyloid precursor protein cleavage enzyme 1; BMD, bone mineral density; BMI, body mass index; BMP2, bone morphogenetic proteins 2; BMSCs, bone marrow stromal cells; CAT, catalase; Cbfα1, core-banding factor α-1; ChE, cholinesterase; DKK1, Dickkopf1; ERK, extracellular signal-regulated kinase; GSH-Px, glutathione peroxidase; IL-6, interleukin-6; JNK, c-Jun N-terminal kinase; MAO, monoamine oxidase; MDA, malondialdehyde; MSCs, mesenchymal stem cells; NFATc1, nuclear factor of activated T cells cytoplasmic 1; NF-κB, nuclear factor-κB; NFTs, neurofibrillary tangles; NMDAR, N-methyl-D-aspartate receptor; OB, osteoblasts; OC, osteoclasts; OCN, osteocalcin; OP, osteoporosis; OPG, osteoprotegerin; OPN, osteopontin; PPARγ, activate receptor-γ; RAGEs, receptor for advanced glycosylation end products; RANK, receptor activator of nuclear factor; RANKL, receptor for activator nuclear factor-κB ligand; ROS, reactive oxygen species; Runx2, runt-related transcription factor 2; STAT3, signal transducer and activator of transcription 3; TCM, traditional Chinese medicine; TL, total lignans; TNF-α, tumor necrosis factor-α; TRAF6, tumor necrosis factor associated factor 6.
References
Abrahamsen, B., Osmond, C., and Cooper, C. (2015). Life Expectancy in Patients Treated for Osteoporosis: Observational Cohort Study Using National Danish Prescription Data. J. Bone Miner. Res. 30 (9), 1553–1559. doi:10.1002/jbmr.2478
Amouzougan, A., Lafaie, L., Marotte, H., Dẻnariẻ, D., Collet, P., Pallot-Prades, B., et al. (2017). High Prevalence of Dementia in Women with Osteoporosis. Jt. Bone Spine 84 (5), 611–614. doi:10.1016/j.jbspin.2016.08.002
Arbor, S. C., Lafontaine, M., and Cumbay, M. (2016). Amyloid-beta Alzheimer Targets - Protein Processing, Lipid Rafts, and Amyloid-Beta Pores. Yale J. Biol. Med. 89 (1), 5–21.
Armas, L. A., and Recker, R. R. (2012). Pathophysiology of Osteoporosis: New Mechanistic Insights. Endocrinol. Metab. Clin. North. Am. 41 (3), 475–486. doi:10.1016/j.ecl.2012.04.006
Aspray, T. J., and Hill, T. R. (2019). Osteoporosis and the Ageing Skeleton. Subcell Biochem. 91, 453–476. doi:10.1007/978-981-13-3681-2_16
Alzheimer’s Disease International, McGill University (2021). Available at: https://www.alzint.org/resource/world-alzheimer-report-2021/ (Accessed September 21, 2021).
Osteoporosis and Bone Mineral Disease Branch of Chinese Medical Association (2019). Epidemiological Survey of Osteoporosis in China and “Healthy Bones” Special Action Results Released. Chin. J. Osteoporos. Bone Mineral Res. 12 (04), 317–318.
Azizsoltani, A., Piri, K., Behzad, S., Soleimani, M., Nekouei, M., Mahmoudi, Z., et al. (2018). Ethyl Acetate Extract of Licorice Root (Glycyrrhiza Glabra) Enhances Proliferation and Osteogenic Differentiation of Human Bone Marrow Mesenchymal Stem Cells. Iran. J. Pharm. Res. 17 (3), 1057–1067.
Ban, J. Y., Park, H. K., and Kim, S. K. (2020). Effect of Glycyrrhizic Acid on Scopolamine-Induced Cognitive Impairment in Mice. Int. Neurourol J. 24 (Suppl. 1), S48–S55. doi:10.5213/inj.2040154.077
Bao, L., Qin, L., Liu, L., Wu, Y., Han, T., Xue, L., et al. (2011). Anthraquinone Compounds from Morinda Officinalis Inhibit Osteoclastic Bone Resorption In Vitro. Chem. Biol. Interact 194 (2-3), 97–105. doi:10.1016/j.cbi.2011.08.013
Björkman, M. P., Sorva, A. J., and Tilvis, R. S. (2010). Does Elevated Parathyroid Hormone Concentration Predict Cognitive Decline in Older People? Aging Clin. Exp. Res. 22 (2), 164–169. doi:10.1007/BF03324791
Bucay, N., Sarosi, I., Dunstan, C. R., Morony, S., Tarpley, J., Capparelli, C., et al. (1998). Osteoprotegerin-Deficient Mice Develop Early Onset Osteoporosis and Arterial Calcification. Genes Dev. 12 (9), 1260–1268. doi:10.1101/gad.12.9.1260
Butler, J. S., Murray, D. W., Hurson, C. J., O'Brien, J., Doran, P. P., and O'Byrne, J. M. (2011). The Role of Dkk1 in Bone Mass Regulation: Correlating Serum Dkk1 Expression with Bone mineral Density. J. Orthop. Res. 29 (3), 414–418. doi:10.1002/jor.21260
Cai, H., Wang, Y., He, J., Cai, T., Wu, J., Fang, J., et al. (2017). Neuroprotective Effects of Bajijiasu against Cognitive Impairment Induced by Amyloid-β in APP/PS1 Mice. Oncotarget 8 (54), 92621–92634. doi:10.18632/oncotarget.21515
Cao, Y., Xu, W., Huang, Y., and Zeng, X. (2020). Licochalcone B, a Chalcone Derivative from Glycyrrhiza Inflata, as a Multifunctional Agent for the Treatment of Alzheimer's Disease. Nat. Prod. Res. 34 (5), 736–739. doi:10.1080/14786419.2018.1496429
Carnovali, M., Banfi, G., and Mariotti, M. (2020). Liquiritigenin Reduces Osteoclast Activity in Zebrafish Model of Glucocorticoid-Induced Osteoporosis. J. Pharmacol. Sci. 143 (4), 300–306. doi:10.1016/j.jphs.2020.06.001
Chai, B., Gao, F., Wu, R., Dong, T., Gu, C., Lin, Q., et al. (2019). Vitamin D Deficiency as a Risk Factor for Dementia and Alzheimer's Disease: an Updated Meta-Analysis. BMC Neurol. 19 (1), 284. doi:10.1186/s12883-019-1500-6
Chao, C. L., Huang, H. W., Huang, H. C., Chao, H. F., Yu, S. W., Su, M. H., et al. (2019). Inhibition of Amyloid Beta Aggregation and Deposition of Cistanche Tubulosa Aqueous Extract. Molecules 24 (4). doi:10.3390/molecules24040687
Chen, D., Yang, X., Yang, J., Lai, G., Yong, T., Tang, X., et al. (2017). Prebiotic Effect of Fructooligosaccharides from Morinda Officinalis on Alzheimer's Disease in Rodent Models by Targeting the Microbiota-Gut-Brain Axis. Front. Aging Neurosci. 9, 403. doi:10.3389/fnagi.2017.00403
Chen, P., Li, Z., and Hu, Y. (2016). Prevalence of Osteoporosis in China: a Meta-Analysis and Systematic Review. BMC Public Health 16 (1), 1039. doi:10.1186/s12889-016-3712-7
Chen, X., Yu, J., Zhong, B., Lu, J., Lu, J. J., Li, S., et al. (2019). Pharmacological Activities of Dihydrotanshinone I, a Natural Product from Salvia Miltiorrhiza Bunge. Pharmacol. Res. 145, 104254. doi:10.1016/j.phrs.2019.104254
Chen, X., Zhi, X., Yin, Z., Li, X., Qin, L., Qiu, Z., et al. (2018). 18β-Glycyrrhetinic Acid Inhibits Osteoclastogenesis In Vivo and In Vitro by Blocking RANKL-Mediated RANK-TRAF6 Interactions and NF-Κb and MAPK Signaling Pathways. Front. Pharmacol. 9, 647. doi:10.3389/fphar.2018.00647
Chen, Y. H., and Lo, R. Y. (2017). Alzheimer's Disease and Osteoporosis. Ci Ji Yi Xue Za Zhi 29 (3), 138–142. doi:10.4103/tcmj.tcmj_54_17
Cho, M. J., Kim, J. H., Park, C. H., Lee, A. Y., Shin, Y. S., Lee, J. H., et al. (2018). Comparison of the Effect of Three Licorice Varieties on Cognitive Improvement via an Amelioration of Neuroinflammation in Lipopolysaccharide-Induced Mice. Nutr. Res. Pract. 12 (3), 191–198. doi:10.4162/nrp.2018.12.3.191
Corbett, N. J., and Hooper, N. M. (2018). Soluble Amyloid Precursor Protein α: Friend or Foe? Adv. Exp. Med. Biol. 1112, 177–183. doi:10.1007/978-981-13-3065-0_13
Cui, L., Li, T., Liu, Y., Zhou, L., Li, P., Xu, B., et al. (2012). Salvianolic Acid B Prevents Bone Loss in Prednisone-Treated Rats through Stimulation of Osteogenesis and Bone Marrow Angiogenesis. PLoS One 7 (4), e34647. doi:10.1371/journal.pone.0034647
Cui, S., Xiong, F., Hong, Y., Jung, J. U., Li, X. S., Liu, J. Z., et al. (2011). APPswe/Aβ Regulation of Osteoclast Activation and RAGE Expression in an Age-dependent Manner. J. Bone Miner. Res. 26 (5), 1084–1098. doi:10.1002/jbmr.299
Deng, S., Lu, H., Chi, H., Wang, Y., Li, X., and Ye, H. (2020). Neuroprotective Effects of OMO within the Hippocampus and Cortex in a D-Galactose and Aβ 25-35-Induced Rat Model of Alzheimer's Disease. Evid. Based Complement. Alternat Med. 2020, 1067541. doi:10.1155/2020/1067541
Dengler-Crish, C. M., Ball, H. C., Lin, L., Novak, K. M., and Cooper, L. N. (2018). Evidence of Wnt/β-Catenin Alterations in Brain and Bone of A tauopathy Mouse Model of Alzheimer's Disease. Neurobiol. Aging 67, 148–158. doi:10.1016/j.neurobiolaging.2018.03.021
Depypere, H., Vierin, A., Weyers, S., and Sieben, A. (2016). Alzheimer's Disease, Apolipoprotein E and Hormone Replacement Therapy. Maturitas 94, 98–105. doi:10.1016/j.maturitas.2016.09.009
Ding, B., Lin, C., Liu, Q., He, Y., Ruganzu, J. B., Jin, H., et al. (2020). Tanshinone IIA Attenuates Neuroinflammation via Inhibiting RAGE/NF-κB Signaling Pathway In Vivo and In Vitro. J. Neuroinflammation 17 (1), 302. doi:10.1186/s12974-020-01981-4
Eiken, P., Vestergaard, P., and Jensen, J. E. (2014). Hormone Replacement Therapy as Primary Prevention? Ugeskr Laeger 176 (6).
Fan, X., Chen, Y., Li, L., Wang, Y., Zhang, Y., Lu, S., et al. (2019). Efficacy of Chinese Herb Cistanche Yishen Granules in Treatment of Tinnitus for Patients with Chronic Nephritis. J. Cel. Biochem. 120 (4), 5505–5509. doi:10.1002/jcb.27833
Feng, X., Wang, Z., Lin, Y., Zhou, Y., Liu, Y., and Yang, H. (2009). Effects of Biatractylolide on the AD Rats Induced by Aβ_(1-40). Chin. Pharmacol. Bull. 25, 949–951. doi:10.3321/j.issn:1001-1978.2009.07.029
Fu, Z., Fan, X., Wang, X., and Gao, X. (2018). Cistanches Herba: An Overview of its Chemistry, Pharmacology, and Pharmacokinetics Property. J. Ethnopharmacol. 219, 233–247. doi:10.1016/j.jep.2017.10.015
Fujita, K., and Janz, S. (2007). Attenuation of WNT Signaling by DKK-1 and -2 Regulates BMP2-Induced Osteoblast Differentiation and Expression of OPG, RANKL and M-CSF. Mol. Cancer 6, 71. doi:10.1186/1476-4598-6-71
Galanis, D., Soultanis, K., Lelovas, P., Zervas, A., Papadopoulos, P., Galanos, A., et al. (2019). Protective Effect of Glycyrrhiza Glabra Roots Extract on Bone mineral Density of Ovariectomized Rats. Biomedicine (Taipei) 9 (2), 8. doi:10.1051/bmdcn/2019090208
Goltzman, D. (2018). Functions of Vitamin D in Bone. Histochem. Cel Biol. 149 (4), 305–312. doi:10.1007/s00418-018-1648-y
Gong, W., Zhang, N., Cheng, G., Zhang, Q., He, Y., Shen, Y., et al. (2019). Rehmannia Glutinosa Libosch Extracts Prevent Bone Loss and Architectural Deterioration and Enhance Osteoblastic Bone Formation by Regulating the IGF-1/PI3K/mTOR Pathway in Streptozotocin-Induced Diabetic Rats. Int. J. Mol. Sci. 20 (16). doi:10.3390/ijms20163964
Gu, M. Y., Chun, Y. S., Zhao, D., Ryu, S. Y., and Yang, H. O. (2018). Glycyrrhiza Uralensis and Semilicoisoflavone B Reduce Aβ Secretion by Increasing PPARγ Expression and Inhibiting STAT3 Phosphorylation to Inhibit BACE1 Expression. Mol. Nutr. Food Res. 62 (6), e1700633. doi:10.1002/mnfr.201700633
Guo, Y., Wang, Q., Chen, S., and Xu, C. (2021). Functions of Amyloid Precursor Protein in Metabolic Diseases. Metabolism 115, 154454. doi:10.1016/j.metabol.2020.154454
Ha, H., An, H., Shim, K. S., Kim, T., Lee, K. J., Hwang, Y. H., et al. (2013). Ethanol Extract of Atractylodes Macrocephala Protects Bone Loss by Inhibiting Osteoclast Differentiation. Molecules 18 (7), 7376–7388. doi:10.3390/molecules18077376
Hagström, E., Kilander, L., Nylander, R., Larsson, E. M., Michaëlsson, K., Melhus, H., et al. (2014). Plasma Parathyroid Hormone Is Associated with Vascular Dementia and Cerebral Hyperintensities in Two Community-Based Cohorts. J. Clin. Endocrinol. Metab. 99 (11), 4181–4189. doi:10.1210/jc.2014-1736
He, J., Li, X., Wang, Z., Bennett, S., Chen, K., Xiao, Z., et al. (2019). Therapeutic Anabolic and Anticatabolic Benefits of Natural Chinese Medicines for the Treatment of Osteoporosis. Front. Pharmacol. 10, 1344. doi:10.3389/fphar.2019.01344
He, X., Wang, J., Li, M., Hao, D., Yang, Y., Zhang, C., et al. (2014). Eucommia Ulmoides Oliv.: Ethnopharmacology, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. J. Ethnopharmacol. 151 (1), 78–92. doi:10.1016/j.jep.2013.11.023
Henderson, V. W. (2006). Estrogen-containing Hormone Therapy and Alzheimer's Disease Risk: Understanding Discrepant Inferences from Observational and Experimental Research. Neuroscience 138 (3), 1031–1039. doi:10.1016/j.neuroscience.2005.06.017
Hengartner, N. E., Fiedler, J., Ignatius, A., and Brenner, R. E. (2013). IL-1β Inhibits Human Osteoblast Migration. Mol. Med. 19, 36–42. doi:10.2119/molmed.2012.00058
Ho, Y. S., Yu, M. S., Lai, C. S., So, K. F., Yuen, W. H., and Chang, R. C. (2007). Characterizing the Neuroprotective Effects of Alkaline Extract of Lycium Barbarum on Beta-Amyloid Peptide Neurotoxicity. Brain Res. 1158, 123–134. doi:10.1016/j.brainres.2007.04.075
Ho, Y. S., Yu, M. S., Yik, S. Y., So, K. F., Yuen, W. H., and Chang, R. C. (2009). Polysaccharides from Wolfberry Antagonizes Glutamate Excitotoxicity in Rat Cortical Neurons. Cell. Mol. Neurobiol. 29 (8), 1233–1244. doi:10.1007/s10571-009-9419-x
Hong, J. Z., and Yu, Y. X. (2015). Effect of Ethanol Extracts of Atractylodis Macrocephalae on Memeory Impairment in Aging Mice. Dalian, China: Dalian University.
Howes, M. R., Fang, R., and Houghton, P. J. (2017). Effect of Chinese Herbal Medicine on Alzheimer's Disease. Int. Rev. Neurobiol. 135, 29–56. doi:10.1016/bs.irn.2017.02.003
Hsu, H., Lacey, D. L., Dunstan, C. R., Solovyev, I., Colombero, A., Timms, E., et al. (1999). Tumor Necrosis Factor Receptor Family Member RANK Mediates Osteoclast Differentiation and Activation Induced by Osteoprotegerin Ligand. Proc. Natl. Acad. Sci. U S A. 96 (7), 3540–3545. doi:10.1073/pnas.96.7.3540
Hu, W., Wang, G., Li, P., Wang, Y., Si, C. L., He, J., et al. (2014). Neuroprotective Effects of Macranthoin G from Eucommia Ulmoides against Hydrogen Peroxide-Induced Apoptosis in PC12 Cells via Inhibiting NF-Κb Activation. Chem. Biol. Interact 224, 108–116. doi:10.1016/j.cbi.2014.10.011
Huang, J. Z., Wu, J., Xiang, S., Sheng, S., Jiang, Y., Yang, Z., et al. (2016). Catalpol Preserves Neural Function and Attenuates the Pathology of Alzheimer's Disease in Mice. Mol. Med. Rep. 13 (1), 491–496. doi:10.3892/mmr.2015.4496
Jeong, G. S., Kang, M. G., Lee, J. Y., Lee, S. R., Park, D., Cho, M., et al. (2020). Inhibition of Butyrylcholinesterase and Human Monoamine Oxidase-B by the Coumarin Glycyrol and Liquiritigenin Isolated from Glycyrrhiza Uralensis. Molecules 25 (17). doi:10.3390/molecules25173896
Ji, S., Li, Z., Song, W., Wang, Y., Liang, W., Li, K., et al. (2016). Bioactive Constituents of Glycyrrhiza Uralensis (Licorice): Discovery of the Effective Components of a Traditional Herbal Medicine. J. Nat. Prod. 79 (2), 281–292. doi:10.1021/acs.jnatprod.5b00877
Jia, J., Wei, C., Chen, S., Li, F., Tang, Y., Qin, W., et al. (2018). The Cost of Alzheimer's Disease in China and Re-estimation of Costs Worldwide. Alzheimers Dement 14 (4), 483–491. doi:10.1016/j.jalz.2017.12.006
Jiang, Z., Wang, J., Li, X., and Zhang, X. (2016). Echinacoside and Cistanche Tubulosa (Schenk) R. Wight Ameliorate Bisphenol A-Induced Testicular and Sperm Damage in Rats through Gonad axis Regulated Steroidogenic Enzymes. J. Ethnopharmacol. 193, 321–328. doi:10.1016/j.jep.2016.07.033
Jin, M., Huang, Q., Zhao, K., and Shang, P. (2013). Biological Activities and Potential Health Benefit Effects of Polysaccharides Isolated from Lycium Barbarum L. Int. J. Biol. Macromol. 54, 16–23. doi:10.1016/j.ijbiomac.2012.11.023
Jing, L., and Jia, X. W. (2018). Lycium Barbarum Polysaccharide Arbitrates Palmitate-Induced Apoptosis in MC3T3-E1 C-ells through D-ecreasing the A-ctivation of ERS-mediated A-poptosis P-athway. Mol. Med. Rep. 17 (2), 2415–2421. doi:10.3892/mmr.2017.8128
Jun, W. (2011). Experimental Study on the Pathogenesis, Diagnosis and Treatment of Alzheimer's Disease. Wuhan, China: Huazhong University of Science and Technology.
Kamer, A. R., Craig, R. G., Dasanayake, A. P., Brys, M., Glodzik-Sobanska, L., and de Leon, M. J. (2008). Inflammation and Alzheimer's Disease: Possible Role of Periodontal Diseases. Alzheimers Dement 4 (4), 242–250. doi:10.1016/j.jalz.2007.08.004
Kim, M. H., Lee, J. E., Lee, J. S., and Yang, W. M. (2017). Improvement of Osteoporosis by Lycium Chinense Administration in Ovariectomized Mice. J. Chin. Med. Assoc. 80 (4), 222–226. doi:10.1016/j.jcma.2016.11.006
Kim, M. Y., Kim, K., Hong, C. H., Lee, S. Y., and Jung, Y. S. (2018). Sex Differences in Cardiovascular Risk Factors for Dementia. Biomol. Ther. (Seoul) 26 (6), 521–532. doi:10.4062/biomolther.2018.159
Krstic, D., Madhusudan, A., Doehner, J., Vogel, P., Notter, T., Imhof, C., et al. (2012). Systemic Immune Challenges Trigger and Drive Alzheimer-like Neuropathology in Mice. J. Neuroinflammation 9, 151. doi:10.1186/1742-2094-9-151
Kulkarni, R. N., Bakker, A. D., Everts, V., and Klein-Nulend, J. (2012). Mechanical Loading Prevents the Stimulating Effect of IL-1β on Osteocyte-Modulated Osteoclastogenesis. Biochem. Biophys. Res. Commun. 420 (1), 11–16. doi:10.1016/j.bbrc.2012.02.099
Kwon, S. H., Lee, H. K., Kim, J. A., Hong, S. I., Kim, S. Y., Jo, T. H., et al. (2011). Neuroprotective Effects of Eucommia Ulmoides Oliv. Bark on Amyloid Beta(25-35)-Induced Learning and Memory Impairments in Mice. Neurosci. Lett. 487 (1), 123–127. doi:10.1016/j.neulet.2010.10.042
Lamichhane, A. P. (2005). Osteoporosis-an Update. JNMA J. Nepal Med. Assoc. 44 (158), 60–66. doi:10.31729/jnma.404
Laudisio, A., Fontana, D. O., Rivera, C., Ruggiero, C., Bandinelli, S., Gemma, A., et al. (2016). Bone Mineral Density and Cognitive Decline in Elderly Women: Results from the InCHIANTI Study. Calcif Tissue Int. 98 (5), 479–488. doi:10.1007/s00223-015-0102-6
Lee, D. Y., Na, D. L., Seo, S. W., Chin, J., Lim, S. J., Choi, D., et al. (2012). Association between Cognitive Impairment and Bone mineral Density in Postmenopausal Women. Menopause 19 (6), 636–641. doi:10.1097/gme.0b013e31823dbec7
Lee, J. H., Bliwise, D. L., Ansari, F. P., Goldstein, F. C., Cellar, J. S., Lah, J. J., et al. (2007). Daytime Sleepiness and Functional Impairment in Alzheimer Disease. Am. J. Geriatr. Psychiatry 15 (7), 620–626. doi:10.1097/JGP.0b013e3180381521
Lee, S. Y., Lee, K. S., Yi, S. H., Kook, S. H., and Lee, J. C. (2013). Acteoside Suppresses RANKL-Mediated Osteoclastogenesis by Inhibiting C-Fos Induction and NF-Κb Pathway and Attenuating ROS Production. PLoS One 8 (12), e80873. doi:10.1371/journal.pone.0080873
Lee, Y. W., Kim, D. H., Jeon, S. J., Park, S. J., Kim, J. M., Jung, J. M., et al. (2013). Neuroprotective Effects of Salvianolic Acid B on an Aβ25-35 Peptide-Induced Mouse Model of Alzheimer's Disease. Eur. J. Pharmacol. 704 (1-3), 70–77. doi:10.1016/j.ejphar.2013.02.015
Li J, J., Wang, G. B., Feng, X., Zhang, J., and Fu, Q. (2016). Effect of Gallium Nitrate on the Expression of Osteoprotegerin and Receptor Activator of Nuclear factor-κB L-igand in O-steoblasts In V-ivo and In V-itro. Mol. Med. Rep. 13 (1), 769–777. doi:10.3892/mmr.2015.4588
Li, N., Qin, L. P., Han, T., Wu, Y. B., Zhang, Q. Y., and Zhang, H. (2009). Inhibitory Effects of morinda Officinalis Extract on Bone Loss in Ovariectomized Rats. Molecules 14 (6), 2049–2061. doi:10.3390/molecules14062049
Li, N., Wang, J., Ma, J., Gu, Z., Jiang, C., Yu, L., et al. (2015). Neuroprotective Effects of Cistanches Herba Therapy on Patients with Moderate Alzheimer's Disease. Evid. Based Complement. Alternat Med. 2015, 103985. doi:10.1155/2015/103985
Li, S., Liu, B., Zhang, L., and Rong, L. (2014). Amyloid Beta Peptide Is Elevated in Osteoporotic Bone Tissues and Enhances Osteoclast Function. Bone 61, 164–175. doi:10.1016/j.bone.2014.01.010
Li S, S., Yang, B., Teguh, D., Zhou, L., Xu, J., and Rong, L. (2016). Amyloid β Peptide Enhances RANKL-Induced Osteoclast Activation through NF-Κb, ERK, and Calcium Oscillation Signaling. Int. J. Mol. Sci. 17 (10). doi:10.3390/ijms17101683
Li, T. M., Huang, H. C., Su, C. M., Ho, T. Y., Wu, C. M., Chen, W. C., et al. (2012). Cistanche Deserticola Extract Increases Bone Formation in Osteoblasts. J. Pharm. Pharmacol. 64 (6), 897–907. doi:10.1111/j.2042-7158.2012.01483.x
Li, X., Wei, G., Wang, X., Liu, D. H., Deng, R. D., Li, H., et al. (2012). Targeting of the Sonic Hedgehog Pathway by Atractylenolides Promotes Chondrogenic Differentiation of Mesenchymal Stem Cells. Biol. Pharm. Bull. 35 (8), 1328–1335. doi:10.1248/bpb.b12-00265
Liang, H., Yu, F., Tong, Z., and Huang, Z. (2011). Effect of Cistanches Herba Aqueous Extract on Bone Loss in Ovariectomized Rat. Int. J. Mol. Sci. 12 (8), 5060–5069. doi:10.3390/ijms12085060
Liang, H. D., Yu, F., Tong, Z. H., Zhang, H. Q., and Liang, W. (2013). Cistanches Herba Aqueous Extract Affecting Serum BGP and TRAP and Bone Marrow Smad1 mRNA, Smad5 mRNA, TGF-Β1 mRNA and TIEG1 mRNA Expression Levels in Osteoporosis Disease. Mol. Biol. Rep. 40 (2), 757–763. doi:10.1007/s11033-012-2065-2
Liang, K., Yang, L., Yin, C., Xiao, Z., Zhang, J., Liu, Y., et al. (2010). Estrogen Stimulates Degradation of Beta-Amyloid Peptide by Up-Regulating Neprilysin. J. Biol. Chem. 285 (2), 935–942. doi:10.1074/jbc.M109.051664
Lim, D. W., and Kim, Y. T. (2013). Dried Root of Rehmannia Glutinosa Prevents Bone Loss in Ovariectomized Rats. Molecules 18 (5), 5804–5813. doi:10.3390/molecules18055804
Littlejohns, T. J., Henley, W. E., Lang, I. A., Annweiler, C., Beauchet, O., Chaves, P. H., et al. (2014). Vitamin D and the Risk of Dementia and Alzheimer Disease. Neurology 83 (10), 920–928. doi:10.1212/WNL.0000000000000755
Liu, C., Chen, K., Lu, Y., Fang, Z., and Yu, G. (2018). Catalpol Provides a Protective Effect on Fibrillary Aβ1-42 -induced Barrier Disruption in an In Vitro Model of the Blood-Brain Barrier. Phytother. Res. 32 (6), 1047–1055. doi:10.1002/ptr.6043
Liu, C., Wang, L., Zhu, R., Liu, H., Ma, R., Chen, B., et al. (2019). Correction to: Rehmanniae Radix Preparata Suppresses Bone Loss and Increases Bone Strength through Interfering with Canonical Wnt/β-Catenin Signaling Pathway in OVX Rats. Osteoporos. Int. 30 (7), 1537–1540. doi:10.1007/s00198-019-05028-0
Liu, H., Zhu, R., Wang, L., Liu, C., Ma, R., Qi, B., et al. (2018). Radix Salviae Miltiorrhizae Improves Bone Microstructure and Strength through Wnt/β-Catenin and Osteoprotegerin/receptor Activator for Nuclear Factor-Κb Ligand/cathepsin K Signaling in Ovariectomized Rats. Phytother. Res. 32 (12), 2487–2500. doi:10.1002/ptr.6188
Liu, M., Guo, H., Li, C., Wang, D., Wu, J., Wang, C., et al. (2015). Cognitive Improvement of Compound Danshen in an Aβ25-35 Peptide-Induced Rat Model of Alzheimer's Disease. BMC Complement. Altern. Med. 15, 382. doi:10.1186/s12906-015-0906-y
Liu, Q. F., Lee, J. H., Kim, Y. M., Lee, S., Hong, Y. K., Hwang, S., et al. (2015). In Vivo Screening of Traditional Medicinal Plants for Neuroprotective Activity against Aβ42 Cytotoxicity by Using Drosophila Models of Alzheimer's Disease. Biol. Pharm. Bull. 38 (12), 1891–1901. doi:10.1248/bpb.b15-00459
Liu, Y., Jia, Z., Akhter, M. P., Gao, X., Wang, X., Wang, X., et al. (2018). Bone-targeting Liposome Formulation of Salvianic Acid A Accelerates the Healing of Delayed Fracture Union in Mice. Nanomedicine 14 (7), 2271–2282. doi:10.1016/j.nano.2018.07.011
Long, H., Ye, W. M., and Mai, W. (2013). Effect of Medicinal Indianmulberry Root in the Treatment of Postmenopausal Osteoporosis. China Med. Pharm. 3 (14), 80–81.
Lorentzon, M., Johansson, H., Harvey, N. C., Liu, E., Vandenput, L., Mccloskey, E. V., et al. (2021). Osteoporosis and Fractures in Women: the burden of Disease. Climacteric 25, 4–10. doi:10.1080/13697137.2021.1951206
Loskutova, N., Honea, R. A., Brooks, W. M., and Burns, J. M. (2010). Reduced Limbic and Hypothalamic Volumes Correlate with Bone Density in Early Alzheimer's Disease. J. Alzheimers Dis. 20 (1), 313–322. doi:10.3233/JAD-2010-1364
Loskutova, N., Honea, R. A., Vidoni, E. D., Brooks, W. M., and Burns, J. M. (2009). Bone Density and Brain Atrophy in Early Alzheimer's Disease. J. Alzheimers Dis. 18 (4), 777–785. doi:10.3233/JAD-2009-1185
Luo, L., and Sun, Y. (2012). Effect of Atractylenolide Ⅲ on Neuronal Cell Injury. Guangzhou, China: Guangdong Pharmaceutical University.
Macdonald, B. T., Tamai, K., and He, X. (2009). Wnt/beta-catenin Signaling: Components, Mechanisms, and Diseases. Dev. Cel. 17 (1), 9–26. doi:10.1016/j.devcel.2009.06.016
Magzoub, M. (2020). Combating Proteins with Proteins: Engineering Cell-Penetrating Peptide Antagonists of Amyloid-β Aggregation and Associated Neurotoxicity. DNA Cel Biol 39 (6), 920–925. doi:10.1089/dna.2020.5604
Malhotra, R. (2016). Understanding Migraine: Potential Role of Neurogenic Inflammation. Ann. Indian Acad. Neurol. 19 (2), 175–182. doi:10.4103/0972-2327.182302
May, B. H., Feng, M., Zhou, I. W., Chang, S. Y., Lu, S. C., Zhang, A. L., et al. (2016). Memory Impairment, Dementia, and Alzheimer's Disease in Classical and Contemporary Traditional Chinese Medicine. J. Altern. Complement. Med. 22 (9), 695–705. doi:10.1089/acm.2016.0070
Mcgeer, P. L., Mcgeer, E., Rogers, J., and Sibley, J. (1990). Anti-inflammatory Drugs and Alzheimer Disease. Lancet 335 (8696), 1037. doi:10.1016/0140-6736(90)91101-f
Mengyong, Z., Caijiao, W., Husheng, Z., Xianwu, P., and Jianmin, F. (2008). Protective Effect of Polysaccharides from morinda Officinalis on Bone Loss in Ovariectomized Rats. Int. J. Biol. Macromol. 43 (3), 276–278. doi:10.1016/j.ijbiomac.2008.06.008
Mokry, L. E., Ross, S., Morris, J. A., Manousaki, D., Forgetta, V., and Richards, J. B. (2016). Genetically Decreased Vitamin D and Risk of Alzheimer Disease. Neurology 87 (24), 2567–2574. doi:10.1212/WNL.0000000000003430
Moon, M., Song, H., Hong, H. J., Nam, D. W., Cha, M.-Y., Oh, M. S., et al. (2013). Vitamin D-Binding Protein Interacts with Aβ and Suppresses Aβ-Mediated Pathology. Cell Death Differ 20 (4), 630–638. doi:10.1038/cdd.2012.161
Nanes, M. S., and Kallen, C. B. (2014). Osteoporosis. Semin. Nucl. Med. 44 (6), 439–450. doi:10.1053/j.semnuclmed.2014.06.006
Ng, A., Tam, W. W., Zhang, M. W., Ho, C. S., Husain, S. F., Mcintyre, R. S., et al. (2018). IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer's Disease: Systematic Review and Meta-Analysis. Sci. Rep. 8 (1), 12050. doi:10.1038/s41598-018-30487-6
Park, B., Song, H. S., Kwon, J. E., Cho, S. M., Jang, S. A., Kim, M. Y., et al. (2017). Effects of Salvia Miltiorrhiza Extract with Supplemental Liquefied Calcium on Osteoporosis in Calcium-Deficient Ovariectomized Mice. BMC Complement. Altern. Med. 17 (1), 545. doi:10.1186/s12906-017-2047-y
Park, E., Jin, H. S., Cho, D. Y., Kim, J., Kim, M. C., Choi, C. W., et al. (2014). The Effect of Lycii Radicis Cortex Extract on Bone Formation In Vitro and In Vivo. Molecules 19 (12), 19594–19609. doi:10.3390/molecules191219594
Patel, A., Kimura, R., Fu, W., Soudy, R., Mactavish, D., Westaway, D., et al. (2021). Genetic Depletion of Amylin/Calcitonin Receptors Improves Memory and Learning in Transgenic Alzheimer's Disease Mouse Models. Mol. Neurobiol. 58 (10), 5369–5382. doi:10.1007/s12035-021-02490-y
Patel, S., Homaei, A., Raju, A. B., and Meher, B. R. (2018). Estrogen: The Necessary Evil for Human Health, and Ways to Tame it. Biomed. Pharmacother. 102, 403–411. doi:10.1016/j.biopha.2018.03.078
Pei, H., Ma, L., Cao, Y., Wang, F., Li, Z., Liu, N., et al. (2020). Traditional Chinese Medicine for Alzheimer's Disease and Other Cognitive Impairment: A Review. Am. J. Chin. Med. 48 (3), 487–511. doi:10.1142/S0192415X20500251
Peng, X. M., Gao, L., Huo, S. X., Liu, X. M., and Yan, M. (2015). The Mechanism of Memory Enhancement of Acteoside (Verbascoside) in the Senescent Mouse Model Induced by a Combination of D-Gal and AlCl3. Phytother. Res. 29 (8), 1137–1144. doi:10.1002/ptr.5358
Pinheiro, L., and Faustino, C. (2019). Therapeutic Strategies Targeting Amyloid-β in Alzheimer's Disease. Curr. Alzheimer Res. 16 (5), 418–452. doi:10.2174/1567205016666190321163438
Prince, M., Bryce, R., Albanese, E., Wimo, A., Ribeiro, W., and Ferri, C. P. (2013). The Global Prevalence of Dementia: a Systematic Review and Metaanalysis. Alzheimers Dement 9 (1), 63–e2. e2. doi:10.1016/j.jalz.2012.11.007
Püntener, U., Booth, S. G., Perry, V. H., and Teeling, J. L. (2012). Long-term Impact of Systemic Bacterial Infection on the Cerebral Vasculature and Microglia. J. Neuroinflammation 9, 146. doi:10.1186/1742-2094-9-146
Qi, S., Zheng, H., Chen, C., and Jiang, H. (2019). Du-Zhong (Eucommia Ulmoides Oliv.) Cortex Extract Alleviates Lead Acetate-Induced Bone Loss in Rats. Biol. Trace Elem. Res. 187 (1), 172–180. doi:10.1007/s12011-018-1362-6
Ross, R. D., Shah, R. C., Leurgans, S., Bottiglieri, T., Wilson, R. S., and Sumner, D. R. (2018). Circulating Dkk1 and TRAIL Are Associated with Cognitive Decline in Community-Dwelling, Older Adults with Cognitive Concerns. J. Gerontol. A. Biol. Sci. Med. Sci. 73 (12), 1688–1694. doi:10.1093/gerona/glx252
Scali, C., Caraci, F., Gianfriddo, M., Diodato, E., Roncarati, R., Pollio, G., et al. (2006). Inhibition of Wnt Signaling, Modulation of Tau Phosphorylation and Induction of Neuronal Cell Death by DKK1. Neurobiol. Dis. 24 (2), 254–265. doi:10.1016/j.nbd.2006.06.016
Singh, Y., Gupta, G., Shrivastava, B., Dahiya, R., Tiwari, J., Ashwathanarayana, M., et al. (2017). Calcitonin Gene-Related Peptide (CGRP): A Novel Target for Alzheimer's Disease. CNS Neurosci. Ther. 23 (6), 457–461. doi:10.1111/cns.12696
Song, D., Cao, Z., Liu, Z., Tickner, J., Qiu, H., Wang, C., et al. (2018). Cistanche Deserticola Polysaccharide Attenuates Osteoclastogenesis and Bone Resorption via Inhibiting RANKL Signaling and Reactive Oxygen Species Production. J. Cel. Physiol. 233 (12), 9674–9684. doi:10.1002/jcp.26882
Sreenivasmurthy, S. G., Liu, J. Y., Song, J. X., Yang, C. B., Malampati, S., Wang, Z. Y., et al. (2017). Neurogenic Traditional Chinese Medicine as a Promising Strategy for the Treatment of Alzheimer's Disease. Int. J. Mol. Sci. 18 (2). doi:10.3390/ijms18020272
Steinhart, Z., and Angers, S. (2018). Wnt Signaling in Development and Tissue Homeostasis. Development 145 (11). doi:10.1242/dev.146589
Stevenson, J. C. (2006). HRT, Osteoporosis and Regulatory Authorities Quis Custodiet Ipsos Custodes? Hum. Reprod. 21 (7), 1668–1671. doi:10.1093/humrep/del043
Tamir, S., Eizenberg, M., Somjen, D., Izrael, S., and Vaya, J. (2001). Estrogen-like Activity of Glabrene and Other Constituents Isolated from Licorice Root. J. Steroid Biochem. Mol. Biol. 78 (3), 291–298. doi:10.1016/s0960-0760(01)00093-0
Tan, Z. S., Seshadri, S., Beiser, A., Zhang, Y., Felson, D., Hannan, M. T., et al. (2005). Bone mineral Density and the Risk of Alzheimer Disease. Arch. Neurol. 62 (1), 107–111. doi:10.1001/archneur.62.1.107
van Dam, P. A., Verhoeven, Y., Trinh, X. B., Wouters, A., Lardon, F., Prenen, H., et al. (2019). RANK/RANKL Signaling Inhibition May Improve the Effectiveness of Checkpoint Blockade in Cancer Treatment. Crit. Rev. Oncol. Hematol. 133, 85–91. doi:10.1016/j.critrevonc.2018.10.011
Wallin, K., Solomon, A., Kåreholt, I., Tuomilehto, J., Soininen, H., and Kivipelto, M. (2012). Midlife Rheumatoid Arthritis Increases the Risk of Cognitive Impairment Two Decades Later: a Population-Based Study. J. Alzheimers Dis. 31 (3), 669–676. doi:10.3233/JAD-2012-111736
Walsh, D. A., Mapp, P. I., and Kelly, S. (2015). Calcitonin Gene-Related Peptide in the Joint: Contributions to Pain and Inflammation. Br. J. Clin. Pharmacol. 80 (5), 965–978. doi:10.1111/bcp.12669
Wang D, D., Wang, H., and Gu, L. (2017). The Antidepressant and Cognitive Improvement Activities of the Traditional Chinese Herb Cistanche. Evid. Based Complement. Alternat Med. 2017, 3925903. doi:10.1155/2017/3925903
Wang, H. K., Hung, C. M., Lin, S. H., Tai, Y. C., Lu, K., Liliang, P. C., et al. (2014). Increased Risk of Hip Fractures in Patients with Dementia: a Nationwide Population-Based Study. BMC Neurol. 14, 175. doi:10.1186/s12883-014-0175-2
Wang, H. M., Wang, L., and Li, N. (2004). Study the Influence of Morinda Officinalis How on the Differentiation From Marrow Stroma Cell to Osteoblast. Rehabil. Med. 14, 16–20.
Wang J, J., Li, Y., Huang, W. H., Zeng, X. C., Li, X. H., Li, J., et al. (2017). The Protective Effect of Aucubin from Eucommia Ulmoides against Status Epilepticus by Inducing Autophagy and Inhibiting Necroptosis. Am. J. Chin. Med. 45 (3), 557–573. doi:10.1142/S0192415X17500331
Wang T, T., Liu, Q., Tjhioe, W., Zhao, J., Lu, A., Zhang, G., et al. (2017). Therapeutic Potential and Outlook of Alternative Medicine for Osteoporosis. Curr. Drug Targets 18 (9), 1051–1068. doi:10.2174/1389450118666170321105425
Wang, T. J., Zhang, F., Richards, J. B., Kestenbaum, B., van Meurs, J. B., Berry, D., et al. (2010). Common Genetic Determinants of Vitamin D Insufficiency: a Genome-wide Association Study. Lancet 376 (9736), 180–188. doi:10.1016/S0140-6736(10)60588-0
Wang, X. Z. (2012). Extraction, the Structure Modification of Atractylenolide and Protective Effect on PC12 Cells. [Master Thesis]. Guangzhou, China: Guangzhou University of Chinese Medicine.
Wang, Z., Huang, X., Zhao, P., Zhao, L., and Wang, Z. Y. (2018). Catalpol Inhibits Amyloid-β Generation through Promoting α-Cleavage of APP in Swedish Mutant APP Overexpressed N2a Cells. Front. Aging Neurosci. 10, 66. doi:10.3389/fnagi.2018.00066
Weller, I., and Schatzker, J. (2004). Hip Fractures and Alzheimer's Disease in Elderly Institutionalized Canadians. Ann. Epidemiol. 14 (5), 319–324. doi:10.1016/j.annepidem.2003.08.005
Weller, J., and Budson, A. (2018). Current Understanding of Alzheimer's Disease Diagnosis and Treatment. F1000Res 7, 1. doi:10.12688/f1000research.14506.1
Wojda, S. J., and Donahue, S. W. (2018). Parathyroid Hormone for Bone Regeneration. J. Orthop. Res. 36 (10), 2586–2594. doi:10.1002/jor.24075
Wong, W. (2020). Economic burden of Alzheimer Disease and Managed Care Considerations. Am. J. Manag. Care 26 (8 Suppl. l), S177–S183. doi:10.37765/ajmc.2020.88482
Xia, Z., Wang, F., Zhou, S., Zhang, R., Wang, F., Huang, J. H., et al. (2017). Catalpol Protects Synaptic Proteins from Beta-Amyloid Induced Neuron Injury and Improves Cognitive Functions in Aged Rats. Oncotarget 8 (41), 69303–69315. doi:10.18632/oncotarget.17951
Xiong, W., and Zhao, L. (2016). Effect of Eucommia Ulmoides with Salt Water on Blood Biochemical Indexes in Senile Osteoporosis Rats. Lishizhen Med. Materia Med. Res.
Yaccoby, S., Ling, W., Zhan, F., Walker, R., Barlogie, B., and Shaughnessy, J. D. (2007). Antibody-based Inhibition of DKK1 Suppresses Tumor-Induced Bone Resorption and Multiple Myeloma Growth In Vivo. Blood 109 (5), 2106–2111. doi:10.1182/blood-2006-09-047712
Ye, Z. X., Xiao, L. Y., and Pan, J. Q. (2015). Effect of morinda Officinalis Extraction Learning and Memory Impairment in Mice. Guangzhou, China: Guangzhou Panyu sanatorium.
Yu, M. S., Lai, C. S., Ho, Y. S., Zee, S. Y., So, K. F., Yuen, W. H., et al. (2007). Characterization of the Effects of Anti-aging Medicine Fructus Lycii on Beta-Amyloid Peptide Neurotoxicity. Int. J. Mol. Med. 20 (2), 261–268. doi:10.3892/ijmm.20.2.261
Yuen, C. W., Murugaiyah, V., Najimudin, N., and Azzam, G. (2021). Danshen (Salvia Miltiorrhiza) Water Extract Shows Potential Neuroprotective Effects in Caenorhabditis elegans. J. Ethnopharmacol. 266, 113418. doi:10.1016/j.jep.2020.113418
Zhang, H., and Zheng, Y. (2019). β Amyloid Hypothesis in Alzheimer's Disease:Pathogenesis,Prevention,and Management. Zhongguo Yi Xue Ke Xue Yuan Xue Bao 41 (5), 702–708. doi:10.3881/j.issn.1000-503X.10875
Zhang, J. H., Xin, H. L., Xu, Y. M., Shen, Y., He, Y. Q., Hsien-Yeh, H., et al. (2018). Morinda Officinalis How. - A Comprehensive Review of Traditional Uses, Phytochemistry and Pharmacology. J. Ethnopharmacol. 213, 230–255. doi:10.1016/j.jep.2017.10.028
Zhang Nd, N. D., Han, T., Huang, B. K., Rahman, K., Jiang, Y. P., Xu, H. T., et al. (2016). Traditional Chinese Medicine Formulas for the Treatment of Osteoporosis: Implication for Antiosteoporotic Drug Discovery. J. Ethnopharmacol. 189, 61–80. doi:10.1016/j.jep.2016.05.025
Zhang, Q., Du, X., Xu, Y., Dang, L., Xiang, L., and Zhang, J. (2013). The Effects of Gouqi Extracts on Morris Maze Learning in the APP/PS1 Double Transgenic Mouse Model of Alzheimer's Disease. Exp. Ther. Med. 5 (5), 1528–1530. doi:10.3892/etm.2013.1006
Zhang, R., Pan, Y. L., Hu, S. J., Kong, X. H., Juan, W., and Mei, Q. B. (2014). Effects of Total Lignans from Eucommia Ulmoides Barks Prevent Bone Loss In Vivo and In Vitro. J. Ethnopharmacol. 155 (1), 104–112. doi:10.1016/j.jep.2014.04.031
Zhang Xz, X. Z., Qian, S. S., Zhang, Y. J., and Wang, R. Q. (2016). Salvia Miltiorrhiza: A Source for Anti-alzheimer's Disease Drugs. Pharm. Biol. 54 (1), 18–24. doi:10.3109/13880209.2015.1027408
Zhao, X., Wang, Y., Nie, Z., Han, L., Zhong, X., Yan, X., et al. (2020). Eucommia Ulmoides Leaf Extract Alters Gut Microbiota Composition, Enhances Short-Chain Fatty Acids Production, and Ameliorates Osteoporosis in the Senescence-Accelerated Mouse P6 (SAMP6) Model. Food Sci. Nutr. 8 (9), 4897–4906. doi:10.1002/fsn3.1779
Zhao, Y., Shen, L., and Ji, H. F. (2012). Alzheimer's Disease and Risk of Hip Fracture: a Meta-Analysis Study. ScientificWorldJournal 2012, 872173. doi:10.1100/2012/872173
Zhou, X. L., Xu, M. B., Jin, T. Y., Rong, P. Q., Zheng, G. Q., and Lin, Y. (2019). Preclinical Evidence and Possible Mechanisms of Extracts or Compounds from Cistanches for Alzheimer's Disease. Aging Dis. 10 (5), 1075–1093. doi:10.14336/AD.2018.0815-1
Zhu, B., Zhang, Q. L., Hua, J. W., Cheng, W. L., and Qin, L. P. (2018). The Traditional Uses, Phytochemistry, and Pharmacology of Atractylodes Macrocephala Koidz.: A Review. J. Ethnopharmacol 226, 143–167. doi:10.1016/j.jep.2018.08.023
Keywords: Alzheimer’s disease, osteoporosis, pathophysiological link, action mechanism, traditional Chinese medicine
Citation: Xu W, Jiang Y, Wang N, Bai H, Xu S, Xia T and Xin H (2022) Traditional Chinese Medicine as a Promising Strategy for the Treatment of Alzheimer’s Disease Complicated With Osteoporosis. Front. Pharmacol. 13:842101. doi: 10.3389/fphar.2022.842101
Received: 23 December 2021; Accepted: 21 March 2022;
Published: 01 June 2022.
Edited by:
Longhuo Wu, Gannan Medical University, ChinaReviewed by:
Young-Ji Shiao, National Research Institute of Chinese Medicine, TaiwanMinyi Chen, Universitätsklinikum Heidelberg, Germany
Rui Li, Shaanxi Provincial People’s Hospital, China
Copyright © 2022 Xu, Jiang, Wang, Bai, Xu, Xia and Xin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianshuang Xia, MTgzMDUxODM5MTFAMTYzLmNvbQ==; Hailiang Xin, aGFpbGlhbmd4aW5AMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
‡ORCID: Huanhuan Bai, orcid.org/0000-0002-9943-804X; Shengyan Xu, orcid.org/0000-0002-1694-6699; Tianshuang Xia, orcid.org/0000-0001-8396-307X
 Weifan Xu
Weifan Xu Yiping Jiang
Yiping Jiang Nani Wang
Nani Wang Huanhuan Bai1‡
Huanhuan Bai1‡ Hailiang Xin
Hailiang Xin