- 1Department of Oncology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2Institute of Clinical Immunology, Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (EGFR-TKIs) significantly improve the prognosis of non-small cell lung cancer (NSCLC) with EGFR mutation-positive. Although third-generation EGFR-TKI osimertinib is demonstrated with superior efficacy compared with first-generation EGFR-TKIs, acquired resistance to EGFR-TKIs remains the bottleneck. The Chinese herbal medicine (CHM) Yiqi-Yangyin-Jiedu decoction (YYJD) has been shown to delay acquired resistance to first-generation EGFR-TKIs in the CATLA study, but there is no high-level evidence for its effect when combined with osimertinib. This trial aims to evaluate the efficacy and safety of YYJD combined with osimertinib as first-line treatment in EGFR mutation-positive advanced NSCLC.
Methods: This is a double-blind, multi-center, randomized controlled trial conducted in eight hospitals in China. A total of 314 participants will be randomly assigned to the osimertinib plus YYJD group (O+YYJD) or the osimertinib plus placebo group (O+placebo). Treatment will last until disease progression or death. Patients diagnosed with advanced NSCLC harboring EGFR Ex19del or L858R will be enrolled if they are ready to take osimertinib as first-line treatment, aged 18–74 years old, and provide signed informed consent. The primary outcome is progression-free survival (PFS). The secondary outcomes include a comparison of overall survival (OS), objective response rate (ORR), disease control rate (DCR), and quality of life (QoL). The analysis will be based on intention-to-treat and per-protocol subject analysis principles.
Discussion: The goal of this trial is to evaluate the efficacy and safety of YYJD when added to osimertinib as first-line treatment in EGFR mutation-positive advanced NSCLC.
Trial Registration: Chictr.org.cn, identifier ChiCTR1900026748
Introduction
Non-small cell lung cancer (NSCLC) is the leading cause of cancer death worldwide (Siegel et al., 2020). Epidermal growth factor receptor (EGFR) is the most common driver gene for NSCLC, and approximately 40% of NSCLC patients in Asian populations have EGFR mutations (Gelatti et al., 2019). The most common mutation types in EGFR include exon 19 deletion (Ex19del) and exon 21 Leu858Arg (L858R), often referred to as “classical” mutations (Gazdar, 2009), which account for about 85% of EGFR mutations (Harrison et al., 2020). In addition, 12%–34% of NSCLC patients with EGFR mutations have uncommon mutations (Yang et al., 2015; Castellanos et al., 2017; Robichaux et al., 2018), which include exon 20 insertion, exon 18 G791X, exon 20 S768I, and exon 21 L861Q mutations. However, evidence on the efficacy of treatment for patients with uncommon mutations is insufficient. First-generation epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs), such as gefitinib (Fukuoka et al., 2011) and erlotinib (Rosell et al., 2012), significantly improve the prognosis of advanced NSCLC patients harboring EGFR-TKI-sensitizing mutations, mainly EGFR Ex19del and L858R, when compared with traditional platinum-based chemotherapy as first-line treatment. Despite initial responses, most patients develop acquired resistance to first-generation EGFR-TKIs, and the disease will progress within 9–11 months (Fukuoka et al., 2011; Rosell et al., 2012). Third-generation EGFR-TKIs such as osimertinib have been shown to delay disease progression and prolong survival time compared to gefitinib (Soria et al., 2018; Ramalingam et al., 2020). Despite this, acquired resistance to osimertinib develops over a median of 18.9 months, so optimizing the effect of osimertinib is critical to the long-term survival of NSCLC patients.
Chinese herbal medicine (CHM) has been used in Chinese NSCLC patients treated with EGFR-TKIs for more than 10 years and has demonstrated its efficacy in delaying EGFR-TKI resistance and alleviating adverse effects in a number of clinical trials (Yang et al., 2018; Jiao et al., 2019; Tang et al., 2019). CHM contains several active compounds that interact with target proteins involved in EGFR-TKI resistance (Bing et al., 2018). Our previous multi-center, double-blind, placebo-controlled clinical trial (CATLA study) confirmed that the addition of CHM (Yiqi-Yangyin-Jiedu decoction, YYJD) to EGFR-TKI (gefitinib, erlotinib, or icotinib) significantly prolongs progression-free survival (PFS) and improves the quality of life (QoL) in NSCLC patients (Jiao et al., 2019). These trials mainly involve first-generation EGFR-TKIs. Osimertinib, as a third-generation EGFR-TKI, inhibits EGFR-sensitive mutations (Ex19del and L858R) like the first-generation EGFR-TKI. Given their similar mechanisms of action, it is reasonable to expect CHM to have clinical benefits for osimertinib as well.
The current study (CATLA-2) will determine whether the addition of CHM YYJD to third-generation EGFR-TKI osimertinib (O+YYJD) prolongs PFS compared with osimertinib plus placebo (O+placebo) in advanced NSCLC patients who have an activating EGFR mutation.
Methods and Analysis
Study Design
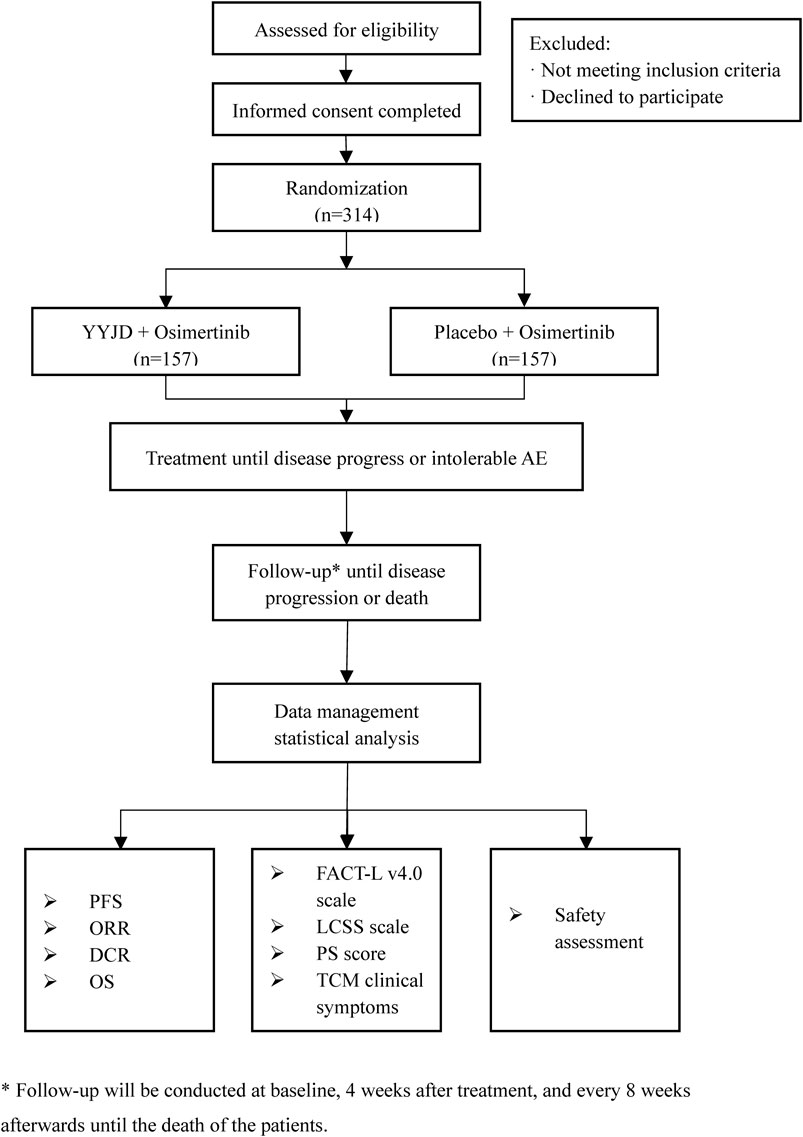
This is a multi-center, double-blind, randomized controlled trial, which will be conducted in Yueyang Hospital of Integrated Traditional Chinese and Western Medicine Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai Pulmonary Hospital Affiliated to Tongji University, Shanghai Chest Hospital Affiliated to Shanghai Jiaotong University, Guang’anmen Hospital of China Academy of Chinese Medical Sciences, First Affiliated Hospital of Guangzhou University of Traditional Chinese Medicine, First Affiliated Hospital of Tianjin University of Traditional Chinese Medicine, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, and Jiangsu Provincial Hospital of Traditional Chinese Medicine. The study aims to enroll 314 advanced NSCLC patients with EGFR mutation-positive. Patients will be randomized at a ratio of 1:1 to receive either osimertinib plus YYJD or osimertinib plus placebo. Follow-up will be conducted at baseline, 4 weeks after treatment, and every 8 weeks afterward until disease progression or death, which is evaluated according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. The study design is based on the Standard Protocol Items: Recommendations for Interventional Trials (SPIRIT) 2013 statement (Chan et al., 2013). The flow diagram of the study is shown in Figure 1.
Participants
Participants will be recruited through the outpatient and inpatient wards of the eight sites. Posters and digital media will be used for recruitment.
Inclusion Criteria
(1) Pathologically confirmed NSCLC (Health Commission of PRC and National Health Commission of the People’s Republic of China, 2019).
(2) Locally advanced or metastatic NSCLC that is not amenable to curative surgery or radiotherapy.
(3) The tumor has one of 2 common EGFR mutations known to be associated with EGFR-TKI sensitivity (Ex19del, L858R).
(4) Patients must have no treatment for locally advanced or metastatic NSCLC.
(5) Aged 18–74 years old.
(6) No major organ dysfunction: hemoglobin ≥120 g/L, absolute neutrophil count (ANC) ≥1.5 × 109/L, platelets ≥80 × 109/L, bilirubin ≤ 1.5ULN, alkaline phosphatase (AP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) ≤ 2.5 × ULN. INR ≤ 1.5, creatinine ≤ 1.5 × ULN.
(7) Provision of informed consent forms.
Exclusion Criteria
(1) Those with a history of other malignancies within 5 years;
(2) Those with symptomatic brain metastases;
(3) Those with congestive heart failure (> class II NYHA heart function), unstable (angina at rest), initial onset (starting within 3 months), angina pectoris, or myocardial infarction occurring within 6 months;
(4) Those with active infection (> Grade 2 adverse events according to CTCAE.5.0 version);
(5) Those with a history of uncontrollable mental illness.
Drop-Out Criteria
(1) Those who do not comply during the trial have an impact on the evaluation of effectiveness and safety;
(2) Those who withdraw themselves during the trial;
(3) Those who combine drugs, especially drugs that have a large effect on test drugs, affect effectiveness and safety evaluation;
(4) Those who withdraw from the trial, do not receive follow-up, or die before the end of the treatment course for other reasons;
(5) Those with incomplete information that affects the evaluation of effectiveness and safety;
The investigators will continue to evaluate the safety after they have been dropped out.
Voluntary Withdrawal of Participants
Participants are allowed to withdraw from the trial at any time, according to the informed consent form. Participants who do not formally withdraw from the trial but stop receiving intervention treatment or testing on their own, or are lost to follow-up, are considered to have withdrawn. The reasons for all participants who withdraw from the trial should be identified and documented to the extent possible.
Sample Size
The sample size was calculated by the statistician of the Clinical Evaluation Center of Shanghai University of Traditional Chinese Medicine by the Power Analysis and Sample Size (PASS) 14 software. PFS is the primary endpoint. According to our previous study, the median PFS of the control group is expected to be 18 months and that of the intervention group is expected to be 23 months, with a recruitment duration of 18 months and a total duration of 30 months. The two groups were allocated in a ratio of 1:1. Given that α = 0.05, β = 0.20, considering that the average monthly censored rate is 1%, the total sample size is 314 cases, 157 cases in each group.
Randomization
Eligible patients enrolled at each site will be randomly allocated to either the O+YYJD group or the O+placebo group at a 1:1 ratio through a dynamic random method. When patient gender (male vs. female), age (≥65 years old vs. <65 years old), and enrollment center is input as stratified factors, the software will automatically output the results of randomization. Personnel for drug administration will be able to obtain a random number and group allocation immediately in the form of a short message service and complete drug distribution according to the allocation.
Blinding
The randomization results and blinding codes will be kept strictly confidential. They will be concealed until interventions are all assigned and enrollment, follow-up, data collection, data cleaning, and analysis are completed. Participants and researchers, including paramedics, investigators, outcomes assessors, and statisticians, will be unaware of the allocation.
Interventions
Patients in the O+YYJD group will receive osimertinib and YYJD granule. Patients in the O+placebo group will receive osimertinib and placebo.
Yiqi-Yangyin-Jiedu Decoction
YYJD is produced into granules by Tianjiang Pharmaceutical Co., Ltd. (Jiangyin, Jiangsu Province, China) as previously reported (Wang et al., 2018) and the composition of YYJD is shown in Table 1. YYJD granules are taken with 150 ml of warm water twice a day after breakfast and lunch.
Placebo
Placebo is produced into granules by Tianjiang Pharmaceutical Co., Ltd. (Jiangyin, Jiangsu Province, China) with the most similar package, color, smell, and shape to YYJD but without medical ingredients. It is taken with 150 ml of warm water twice a day after breakfast and lunch.
Osimertinib
Osimertinib (TAGRISSO, AstraZeneca, United Kingdom) should be taken at 80 mg per day. Patients enrolled in this study should continue intervention until disease progression or intolerable adverse effects.
Outcomes
Computed tomography (CT) or magnetic resonance (MR) imaging will be used to assess the tumor at baseline, 4 weeks after treatment, and every 8 weeks afterward until disease progression or death. Objective response is evaluated according to RECIST 1.1, established by the National Cancer Institute (NCI), and divided into the following four situations: Complete Response (CR), Partial Response (PR), Stable Disease (SD), and Progressive Disease (PD).
Primary Outcome
PFS: measured with the date of the videography from a random assignment to the date of objective progression or death by the researcher.
Secondary Outcomes
(1) Overall survival (OS): calculated as the time from randomization to death due to any cause. For subjects who are lost to follow-up before death, the time of the last follow-up is used as the time of no death.
(2) Objective response rate (ORR): calculated based on the effective rate of CR+PR patients.
(3) Disease control rate (DCR): calculated based on the effective rate of CR+PR+SD patients.
(4) Physical condition: assessed following the ECOG PS standard at the same time point of image evaluation.
(5) QoL: evaluated with Functional Assessment of Cancer Therapy-Lung (FACT-L) questionnaire, Lung Cancer Symptom Scale (LCSS) and TCM syndrome score at the same time point of image evaluation. TCM syndrome score will be evaluated based on the grading scales of lung cancer symptoms required in the Guiding Principles of Clinical Research of New Chinese Medicine Treating Primary Bronchial Lung Cancer (2002 Edition) issued by the National Medical Products Administration of China.
(6) Safety assessments: Participants will be asked and all adverse events (AEs) during treatment will be recorded at each visit, and all AEs reported will be analyzed. Blood, urine, and stool routine, liver function, and kidney function are tested for adverse reactions at the same time point of image evaluation and evaluated according to common terminology criteria for adverse events (CTCAE) version 5.0 (https://ctep.cancer.gov).
Participant Timeline
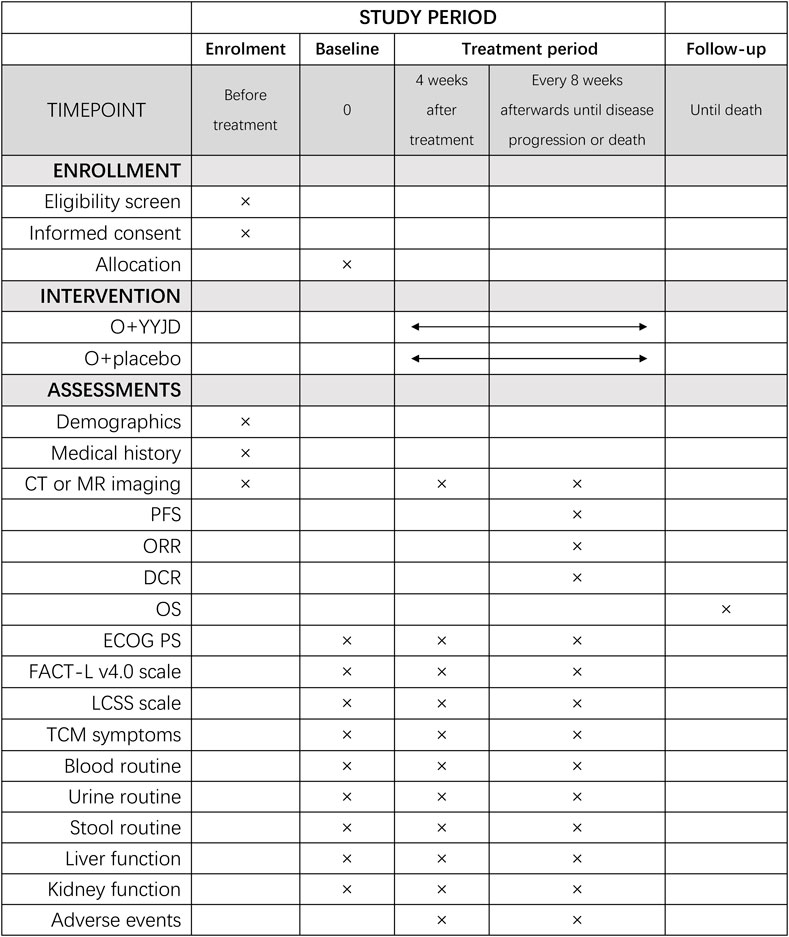
The schedule of enrolment, interventions, and assessments is as shown in Figure 2.
Data Collection and Management
The data will be collected with the use of paper case report forms (CRF) by researchers from each center. Participants will be given a separate space to fill out the QoL form for privacy concerns. The data management specialist of each center will use the electronic data capture (EDC) system for data entry and management. The system will verify the entered data, and the questionable data will be confirmed or corrected by researchers after re-checking. The researchers responsible for data analysis can access the data only after the patient recruitment is completed. The sub-center will conduct a self-inspection every month, the main center will conduct an inspection of the sub-center every 3 months, and the competent unit of the study will conduct a project verification once every 6 months. Relevant researchers will need to reply and rectify any problems found within 2 weeks.
Quality Control
All researchers will receive unified training so that they have a full understanding of clinical trials before the start of the trial. In order to improve patients’ compliance with osimertinib, we will provide detailed information on the benefits of using osimertinib as first-line treatment compared with first-generation EGFR-TKIs and inform patients that the first-line treatment of osimertinib for advanced non-small cell lung cancer with EGFR mutation-positive (Ex19del or L858R) can be reimbursed by medical insurance in China. This makes the financial burden caused by osimertinib relatively small and valuable.
Statistical Analysis
Data analysis will follow the trial’s statistical analysis plan. All data will be processed by statistical analysis with SAS 9.4 software analysis. Two-tailed p values <0.05 are considered statistically significant. The analysis will follow intention-to-treat, full analysis set, and per-protocol subject principles for the evaluation of primary and secondary outcomes. The safety data set that includes all subjects who received at least one treatment after randomization will be used to evaluate safety and tolerance. Missing data will be processed with the multiple imputation method.
The baseline characteristics will be reported according to treatment groups. PFS and OS will be illustrated by the Kaplan–Meier survival curve and compared between groups using the log-rank test. The secondary outcomes will be summarized with frequency, mean, standard deviation, median, and range. At each time point, comparisons between the experimental group and the placebo group will be conducted using the Group t-test or Wilcoxon rank-sum test (for measurement data) and the rank-sum test and CMH test (for count data). Fisher’s exact test will be used to compare tumor response rates between the arms. For PFS, an adjusted Cox regression model will be used to estimate the adjusted HRs for differences between the treatment arms with the selected prognostic factors, including the center, EGFR mutation type, age, sex, EGFR-TKI drugs, smoking status, and ECOG PS.
Safety will be documented in adverse event forms and presented with descriptive statistics for each group. The frequency difference of adverse events between groups will be assessed by the chi-square test or Fisher’s exact test. For different AE severities, a rank-sum test will be performed to analyze the independent ordered multiple category data between the two groups.
Dissemination Plans
The results will be published in a paper after the completion of the study.
Trial Status
Protocol version: v2.0 was finished on 27th July 2020. Participant recruitment started in January 2021 and is expected to end in December 2022. Until 23rd December 2021, a total of 58 participants were enrolled in the study.
Discussion
EGFR-TKIs are the standard first-line treatment for patients with locally advanced or metastatic EGFR-mutated NSCLC (Hanna et al., 2017; Planchard et al., 2018; Wu et al., 2019). Although the emergence of EGFR-TKIs has brought a revolutionary breakthrough in the treatment of lung cancer, the disease still progresses after 9–11 months of treatment with EGFR-TKIs (Fukuoka et al., 2011; Rosell et al., 2012). Patients with EGFR-mutated NSCLC who are initially sensitive to EGFR-TKI therapy will inevitably experience treatment failure due to secondary drug resistance, and more than 50% of them have the T790M mutation (John et al., 2018; Wang et al., 2018). The emergence of the third-generation EGFR-TKI, osimertinib, has solved this problem. The FLAURA study shows that the median PFS of patients treated with osimertinib is as high as 18 months, and its first-line treatment significantly prolongs OS to 38.6 months (Soria et al., 2018; Ramalingam et al., 2020). Therefore, osimertinib is the first choice for first-line treatment in EGFR mutation-positive advanced NSCLC. However, resistance and toxicities of osimertinib have become new challenges. Osimertinib resistance mechanisms include EGFR C797S mutation and MET gene amplification (Kim et al., 2015; Planchard et al., 2015). The toxic and side effects of osimertinib significantly affect the quality of life of patients with NSCLC and even lead to the cessation of treatment (Khozin et al., 2017).
As a complementary and alternative medicine, TCM is widely used in cancer treatment in China, which has shown the effects of reducing chemotherapy toxicity, enhancing tumor treatment response, improving quality of life, and prolonging survival (Zhang et al., 2018). In the CATLA study, we found that the combination of EGFR-TKIs (gefitinib, erlotinib, or icotinib) and CHM YYJD as first- and second-line therapy significantly prolonged PFS and ORR versus EGFR-TKIs alone. The median PFS was prolonged by 5 months as first-line treatment. At the same time, the side effects of EGFR-TKIs, including fatigue, loss of appetite, diarrhea, pruritus, and skin rash, were reduced (Jiao et al., 2019). Nowadays, the advantage of the third-generation EGFR-TKI, osimertinib, over the first-generation EGFR-TKI is that it overcomes the T790M resistance mutation and has better clinical efficacy in the treatment of advanced NSCLC patients with EGFR mutation-positive. It is reasonable to speculate that YYJD may also have the effect of reducing toxicity and increasing the efficiency of osimertinib.
Qi and Yin deficiency syndrome is one of the most common deficiency syndromes in TCM, manifesting as a pathological state in which both qi and yin deficiency coexist. The typical symptoms are cough, less sputum, fatigue, shortness of breath, dry mouth with less drinking, spontaneous sweating, reddish tongue, or tongue with teeth imprints, thready and weak pulse (Zheng, 2002). Meanwhile, the types of TCM syndromes under EGFR mutations in lung adenocarcinoma are dominated by Qi and Yin deficiency syndrome, and treatment should pay attention to tonifying Qi and nourishing Yin (Wang and Xu, 2017). YYJD is a CHM compound based on TCM theory that mainly exerts the effects of tonifying Qi, nourishing Yin, and detoxifying. In the subgroup analysis of the CATLA study, we found that YYJD had a better clinical benefit for advanced NSCLC patients with EGFR-sensitive mutations, extending median PFS by 16.26 months (Jiao et al., 2019). In addition, most trials have shown that the combination of EGFR-TKIs with oral CHM that tonifies Qi and/or nourishes Yin significantly delays acquired resistance while increasing the ORR of EGFR-TKIs (Lu et al., 2021). Therefore, in the design of this study, the intervention was set as YYJD with greater clinical benefit in order to minimize the impact of TCM syndrome differentiation on outcomes.
In summary, this study is a large-sample multicenter randomized controlled trial of osimertinib combined with YYJD as first-line treatment in EGFR mutation-positive advanced NSCLC. If effective, it will provide a high-quality evidence-based basis for the efficacy and safety of osimertinib combined with YYJD and provide a treatment strategy integrating Chinese and Western medicine that can be widely and successfully applied in the clinic.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Yueyang Hospital of Integrated Traditional Chinese and Western Medicine, Shanghai University of Traditional Chinese Medicine (No. 2020-176). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
JY and YL planned the study protocol. YL drafted the manuscript, and JY revised the manuscript. YG and LX were responsible for the concept and design of this study. LX was responsible for obtaining ethics approval and the acquisition of funding. YG, LX, YL, JY, LJ, LB, WY, LS, ZW, and JS recruited and screened eligible patients. YL, JY, WY, LS, and JS collected and assembly of data. LJ and LB were responsible for the data analysis and interpretation. All authors have read and approved the final manuscript.
Funding
This work is supported by grants from the State Administration of Traditional Chinese Medicine of the People’s Republic of China (No. 2019XZZX-ZL004), Shanghai Hospital Development Center (No. SHDC2020CR1052B), Shanghai Municipal Health Commission (No. ZXYXZ-201901), Shanghai University of Traditional Chinese Medicine (No. 2019LK026). The funders played no role in the study design, data collection, analysis, management, and interpretation of data, or writing of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bing, Z., Cheng, Z., Shi, D., Liu, X., Tian, J., Yao, X., et al. (2018). Investigate the Mechanisms of Chinese Medicine Fuzhengkangai towards EGFR Mutation-Positive Lung Adenocarcinomas by Network Pharmacology. BMC Complement. Altern. Med. 18, 293. doi:10.1186/s12906-018-2347-x
Castellanos, E., Feld, E., and Horn, L. (2017). Driven by Mutations: The Predictive Value of Mutation Subtype in EGFR -Mutated Non–small Cell Lung Cancer. J. Thorac. Oncol. 12, 612–623. doi:10.1016/j.jtho.2016.12.014
Chan, A.-W., Tetzlaff, J. M., Altman, D. G., Laupacis, A., Gøtzsche, P. C., Krleža-Jerić, K., et al. (2013). SPIRIT 2013 Statement: Defining Standard Protocol Items for Clinical Trials. Ann. Intern. Med. 158, 200. doi:10.7326/0003-4819-158-3-201302050-00583
Fukuoka, M., Wu, Y-L., Thongprasert, S., Sunpaweravong, P., Leong, S-S., Sriuranpong, V., et al. (2011). Biomarker Analyses and Final Overall Survival Results from a Phase III, Randomized, Open-Label, First-Line Study of Gefitinib versus Carboplatin/paclitaxel in Clinically Selected Patients with Advanced Non-small-cell Lung Cancer in Asia (IPASS). J. Clin. Oncol. 29, 2866–2874. doi:10.1200/JCO.2010.33.4235
Gazdar, A. F. (2009). Activating and Resistance Mutations of EGFR in Non-small-cell Lung Cancer: Role in Clinical Response to EGFR Tyrosine Kinase Inhibitors. Oncogene 28, S24–S31. doi:10.1038/onc.2009.198
Gelatti, A. C. Z., Drilon, A., and Santini, F. C. (2019). Optimizing the Sequencing of Tyrosine Kinase Inhibitors (TKIs) in Epidermal Growth Factor Receptor (EGFR) Mutation-Positive Non-small Cell Lung Cancer (NSCLC). Lung Cancer 137, 113–122. doi:10.1016/j.lungcan.2019.09.017
Hanna, N., Johnson, D., Temin, S., Baker, S., Brahmer, J., Ellis, P. M., et al. (2017). Systemic Therapy for Stage IV Non–small-cell Lung Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update. JCO 35, 3484–3515. doi:10.1200/JCO.2017.74.6065
Harrison, P. T., Vyse, S., and Huang, P. H. (2020). Rare Epidermal Growth Factor Receptor (EGFR) Mutations in Non-small Cell Lung Cancer. Semin. Cancer Biol. 61, 167–179. doi:10.1016/j.semcancer.2019.09.015
Health Commission of PRC, N. and National Health (2019). Commission of the People’s Republic of China (2019). Chinese Guidelines for Diagnosis and Treatment of Primary Lung Cancer 2018 (English Version). Chin. J. Cancer Res. 31, 1–28. doi:10.21147/j.issn.1000-9604.2019.01.01
Jiao, L., Xu, J., Sun, J., Chen, Z., Gong, Y., Bi, L., et al. (2019). Chinese Herbal Medicine Combined with EGFR-TKI in EGFR Mutation-Positive Advanced Pulmonary Adenocarcinoma (CATLA): A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial. Front. Pharmacol. 10, 732. doi:10.3389/fphar.2019.00732
John, T., Akamatsu, H., Delmonte, A., Su, W.-C., Lee, J. S., Chang, G.-C., et al. (2018). EGFR Mutation Analysis for Prospective Patient Selection in AURA3 Phase III Trial of Osimertinib versus Platinum-Pemetrexed in Patients with EGFR T790M-Positive Advanced Non-small-cell Lung Cancer. Lung Cancer 126, 133–138. doi:10.1016/j.lungcan.2018.10.027
Khozin, S., Weinstock, C., Blumenthal, G. M., Cheng, J., He, K., Zhuang, L., et al. (2017). Osimertinib for the Treatment of Metastatic EGFR T790M Mutation–Positive Non–small Cell Lung Cancer. Clin. Cancer Res. 23, 2131–2135. doi:10.1158/1078-0432.CCR-16-1773
Kim, T. M., Song, A., Kim, D.-W., Kim, S., Ahn, Y.-O., Keam, B., et al. (2015). Mechanisms of Acquired Resistance to AZD9291. J. Thorac. Oncol. 10, 1736–1744. doi:10.1097/JTO.0000000000000688
Lu, Y., Sun, C., Jiao, L., Liu, Y., Gong, Y., and Xu, L. (2021). Chinese Herbal Medicine Combined with First-Generation EGFR-TKIs in Treatment of Advanced Non-small Cell Lung Cancer with EGFR Sensitizing Mutation: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12, 698371. doi:10.3389/fphar.2021.698371
Planchard, D., Loriot, Y., André, F., Gobert, A., Auger, N., Lacroix, L., et al. (2015). EGFR-independent Mechanisms of Acquired Resistance to AZD9291 in EGFR T790M-Positive NSCLC Patients. Ann. Oncol. 26, 2073–2078. doi:10.1093/annonc/mdv319
Planchard, D., Popat, S., Kerr, K., Novello, S., Smit, E. F., Faivre-Finn, C., et al. (2018). Metastatic Non-small Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 29, iv192–iv237. doi:10.1093/annonc/mdy275
Ramalingam, S. S., Vansteenkiste, J. J., Planchard, D., Cho, B. C., and Gray, J. E. (2020). Yuichiro Ohe, et alOverall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N. Engl. J. Med. 382, 41–50. doi:10.1056/nejmoa1913662
Robichaux, J. P., Elamin, Y. Y., Tan, Z., Carter, B. W., Zhang, S., Liu, S., et al. (2018). Mechanisms and Clinical Activity of an EGFR and HER2 Exon 20–selective Kinase Inhibitor in Non–small Cell Lung Cancer. Nat. Med. 24, 638–646. doi:10.1038/s41591-018-0007-9
Rosell, R., Carcereny, E., Gervais, R., Vergnenegre, A., Massuti, B., Felip, E., et al. (2012). Erlotinib versus Standard Chemotherapy as First-Line Treatment for European Patients with Advanced EGFR Mutation-Positive Non-small-cell Lung Cancer (EURTAC): a Multicentre, Open-Label, Randomised Phase 3 Trial. Lancet Oncol. 13, 239–246. doi:10.1016/S1470-2045(11)70393-X
Siegel, R. L., Miller, K. D., and Jemal, A. (2020). Cancer Statistics, 2020. CA Cancer J. Clin. 70, 7–30. doi:10.3322/caac.21637
Soria, J. C., Ohe, Y., Vansteenkiste, J., Reungwetwattana, T., Chewaskulyong, B., Lee, K. H., et al. (2018). Osimertinib in Untreated EGFR -Mutated Advanced Non–small-cell Lung Cancer. N. Engl. J. Med. 378, 113–125. doi:10.1056/NEJMoa1713137
Tang, M., Wang, S., Zhao, B., Wang, W., Zhu, Y., Hu, L., et al. (2019). Traditional Chinese Medicine Prolongs Progression-free Survival and Enhances Therapeutic Effects in Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor (EGFR-TKI) Treated Non-small-cell Lung Cancer (NSCLC) Patients Harboring EGFR Mutations. Med. Sci. Monit. 25, 8430–8437. doi:10.12659/MSM.917251
Wang, J., and Xu, L. (2017). Study on the Relationship between Common Gene Mutations and TCM Syndrome in 102 Cases of Lung Adenocarcinoma. Chin. J. Basic Med. Tradit. Chin. Med. 23, 1724–1727.
Wang, Q., Jiao, L., Wang, S., Chen, P., Bi, L., Zhou, D., et al. (2018a). Maintenance Chemotherapy with Chinese Herb Medicine Formulas vs. With Placebo in Patients with Advanced Non-small Cell Lung Cancer after First-Line Chemotherapy: A Multicenter, Randomized, Double-Blind Trial. Front. Pharmacol. 9, 1233. doi:10.3389/fphar.2018.01233
Wang, Z.-F., Ren, S.-X., Li, W., and Gao, G.-H. (2018b). Frequency of the Acquired Resistant Mutation T790 M in Non-small Cell Lung Cancer Patients with Active Exon 19Del and Exon 21 L858R: a Systematic Review and Meta-Analysis. BMC Cancer 18, 148. doi:10.1186/s12885-018-4075-5
Wu, Y.-L., Planchard, D., Lu, S., Sun, H., Yamamoto, N., Kim, D.-W., et al. (2019). Pan-Asian Adapted Clinical Practice Guidelines for the Management of Patients with Metastatic Non-small-cell Lung Cancer: a CSCO–ESMO Initiative Endorsed by JSMO, KSMO, MOS, SSO and TOS. Ann. Oncol. 30, 171–210. doi:10.1093/annonc/mdy554
Yang, J. C.-H., Sequist, L. V., Geater, S. L., Tsai, C.-M., Mok, T. S. K., Schuler, M., et al. (2015). Clinical Activity of Afatinib in Patients with Advanced Non-small-cell Lung Cancer Harbouring Uncommon EGFR Mutations: a Combined post-hoc Analysis of LUX-Lung 2, LUX-Lung 3, and LUX-Lung 6. Lancet Oncol. 16, 830–838. doi:10.1016/S1470-2045(15)00026-1
Yang, X-B., Chai, X-H., Wu, W-Y., Long, S-Q., Deng, H., Pan, Z-Q., et al. (2018). Gefitinib Plus Fuzheng Kang’ai Formula in Patients with Advanced Non-small Cell Lung Cancer with Epidermal Growth Factor Receptor Mutation: A Randomized Controlled Trial. Chin. J. Integr. Med. 24, 734–740. doi:10.1007/s11655-017-2819-8
Zhang, X-W., Liu, W., Jiang, H-L., and Mao, B. (2018). Chinese Herbal Medicine for Advanced Non-small-cell Lung Cancer: A Systematic Review and Meta-Analysis. Am. J. Chin. Med. 46, 923–952. doi:10.1142/S0192415X18500490
Keywords: non-small cell lung cancer, epidermal growth factor receptor, osimertinib, Yiqi-Yangyin-Jiedu decoction, Chinese medicine
Citation: Yao J, Lu Y, Jiao L, Bi L, Yang W, Su L, Shi J, Wang Z, Gong Y and Xu L (2022) Chinese Herbal Medicine (Yiqi-Yangyin-Jiedu Decoction) Combined With Osimertinib as First-Line Treatment in EGFR Mutation-Positive Advanced Non-Small-Cell Lung Cancer (CATLA-2): A Study Protocol for a Double-Blind Randomized Controlled Trial. Front. Pharmacol. 13:840889. doi: 10.3389/fphar.2022.840889
Received: 21 December 2021; Accepted: 28 February 2022;
Published: 01 April 2022.
Edited by:
Jianbin Zhang, Zhejiang Provincial People’s Hospital, ChinaReviewed by:
In-Jae Oh, Chonnam National University Hwasun Hospital, South KoreaUmberto Malapelle, University of Naples Federico II, Italy
Copyright © 2022 Yao, Lu, Jiao, Bi, Yang, Su, Shi, Wang, Gong and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yabin Gong, Z29uZ3lhYmluQGhvdG1haWwuY29t; Ling Xu, eHVscTY3QGFsaXl1bi5jb20=
†These authors have contributed equally to this work.
 Jialin Yao
Jialin Yao Yan Lu
Yan Lu Lijing Jiao
Lijing Jiao Ling Bi
Ling Bi Wenxiao Yang
Wenxiao Yang Lingzi Su
Lingzi Su Jun Shi
Jun Shi Zhe Wang
Zhe Wang Yabin Gong
Yabin Gong Ling Xu
Ling Xu