
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 01 April 2022
Sec. Ethnopharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.839668
Background: Anorexia is a common obstacle to adequate nutrition in childhood, a critical period for physical growth. East Asian traditional medicine treatment modalities including herbal medicine (HM) a re considered an attractive therapeutic option, especially in East Asian countries. The purpose of this systematic review was to comprehensively examine the efficacy and safety of HM for anorexia in children.
Methods: A total of 12 electronic databases from their inception date to June 2021 were searched for randomized controlled trials (RCTs) assessing the efficacy of HM for the treatment of anorexia in children. The primary outcome was an improvement in anorexia clinical symptoms after treatment. In this meta-analysis, continuous and binary outcomes were assessed, and the data are presented as the mean difference or standardized mean difference and risk ratio (RR) with their 95% confidence intervals (CIs). The risk of bias and quality of evidence were assessed using the Cochrane Collaboration’s risk of bias tool and Grading of Recommendations, Assessment, Development, and Evaluations tool.
Results: A total of 205 RCTs were included. A comparison of HM with placebo revealed that the total effective rate based on anorexia symptom improvement was significantly higher in the HM group (RR 1.58, 95% CI 1.34, 1.85). In comparison with controls, HM as monotherapy or adjunctive therapy to dietary supplements or conventional medications led to significant improvements in anorexia symptoms, body measurements, levels of blood biomarkers related to gastrointestinal function, and nutrition indices, with a lower recurrence rate of anorexia. No serious adverse events related to HM were reported. The risk of bias of the included studies was generally unclear, and the quality of evidence was generally low to moderate.
Conclusion: Our study showed that HM could improve clinical symptoms, some anthropometric outcomes, and some biological markers related to appetite and growth in children with anorexia. However, considering the high risk of bias of the included studies and the heterogeneity of the HMs used, future research should focus on the use of standardized HMs and the implementation of methodologically robust clinical trials.
Systematic Review Registration: https://www.crd.york.ac.uk/prosperodisplay_record.php?ID=CRD42021274376, identifier CRD42021274376
Childhood is a critical period for physical growth, and proper nutrition during this period is crucial to ensure normal growth (Martins et al., 2011; Hurley et al., 2016). Anorexia, a disorder with a decreased desire to eat, is a common obstacle to adequate nutrition in children. In the Chatoor Diagnostic Criteria for Feeding Disorder, infantile anorexia can be defined when a child refuses to eat adequate amounts of food for at least 1 month (not caused by an underlying medical illness or traumatic event), does not express hunger and lacks interest in food but shows strong interest in exploration and interaction with caregivers, and shows reduced growth (Levine et al., 2011). According to some studies, poor appetite rather than insufficient food may be a contributing factor to low energy intake by infants and children (Brown et al., 1995; Mukatay et al., 2010; Carter et al., 2019). Therefore, an inappropriately decreased appetite in childhood is considered to have important public health implications.
Anorexia is common in children; according to an observational study of 504 children under 4 years of age in Korea, the prevalence of anorexia was 27% (Liu et al., 2016). Moreover, among these participants, anorexia was closely associated with a low rate and short duration of breastfeeding, starting on solid food at an inappropriate time, less interest in food during mealtime, and an unbalanced diet (Liu et al., 2016). Likewise, according to a questionnaire survey and a case-control study conducted in China, the main risk factors for this condition in children were the late addition of supplementary food, a high frequency of taking snacks and/or drinks, and eating while playing (Liu et al., 2016). Nutritional intervention (Naila et al., 2021), cyproheptadine (Najib et al., 2014; Sant’Anna et al., 2014; Kim et al., 2021), and folic acid (Hatamizadeh et al., 2007; Riahi et al., 2018) can be used to increase appetite and subsequent food intake in this population. In addition, East Asian traditional medicine (EATM) treatment modalities are considered an attractive therapeutic option, especially in East Asian countries.
In Korea, Korean medicine (KM) hospitals are the most common medical institutions where parents of children with anorexia receive counseling for anorexia (68%) in addition to pediatrics (20%) and internal medicine (3%) (Yoon et al., 2003). Around 10% of outpatients in the department of pediatrics in KM hospitals are presented with eating problems, including anorexia (Choi et al., 2010). In China, EATM is widely used for the treatment of anorexia in children, and a recent systematic review reported that pediatric tuina, which is a modality of traditional Chinese medicine, is a safe and effective treatment for improving the symptoms of anorexia in children under 14 years of age (Liang et al., 2020).
Herbal medicine (HM) is one of the representative EATM therapies and widely used for the treatment of various diseases. Recently, modern biomedical research methodologies have been used to elucidate the therapeutic effects and underlying mechanisms of HM. The evidence suggests that some HMs can be used to improve anorexia. For example, standardized HMs such as Rikkunshito may improve anorexia by acting as a ghrelin potentiator (Fujitsuka and Uezono, 2014). Therefore, HM can be an attractive medical option in terms of appetite control. Although it is actively used to improve anorexia in children mainly in East Asian countries, the effect and safety of this intervention in children with anorexia have not been systematically and critically reviewed. Therefore, the purpose of this systematic review was to comprehensively examine the efficacy and safety of HM for anorexia in children.
We registered the protocol of this systematic review with PROSPERO (Registration number: CRD42021274376) and reported the study according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses 2020 statement (Page et al., 2021).
Only parallel group randomized controlled trials (RCTs) assessing the efficacy of HM for the treatment of anorexia in children were included. We excluded cross-over studies to reduce the risk of potential bias. There were no limitations regarding the publication language of the study. We included not only studies published in journals but also grey literature including degree thesis.
Studies involving children with anorexia were included. Studies involving children with anorexia with known organic causes were excluded. In addition, we excluded studies that did not clearly describe the diagnostic criteria for anorexia. There were no limitations regarding the sex, race, and nationality of the participants.
For treatment interventions, studies involving oral HM based on the EATM theory for the treatment of anorexia in children were included. However, studies in which the individual herb composition was not specified were excluded. For control interventions, studies with placebo HM, no treatment, and active controls such as dietary supplements or conventional medications were included. However, studies using other EATM therapies such as acupuncture as the control were excluded. Studies involving HM combined with other therapies as treatment interventions were included if the other therapies were used equally in both the treatment and the control groups.
The primary outcome was an improvement in the clinical symptoms of anorexia after treatment, measured using such as the total effective rate (TER). For assessment with the TER, participants were classified according to the degree of improvement in anorexia symptoms as “cured” (N1), “markedly improved” (N2), “improved” (N3), or “non-responder” after treatment. The TER was calculated using the following formula: TER = (N1 + N2 + N3)/total sample size. The secondary outcomes included 1) body measurements such as body weight and body mass index (BMI), 2) gastric emptying time, 3) levels of blood biomarkers related to gastrointestinal function, such as ghrelin and gastrin, 4) nutrition indices, such as levels of serum trace elements, 5) recurrence rate, and 6) incidence of adverse events (AEs) during the treatment period.
We comprehensively searched the following 12 English, Korean, Chinese, and Japanese electronic databases from their inception date to 3 June 2021: Medline (via PubMed), EMBASE (via Elsevier), Cochrane Central Register of Controlled Trials, Allied and Complementary Medicine Database (via EBSCO), Oriental Medicine Advanced Searching Integrated System, Korean Studies Information Service System, Korean Medical Database, ScienceON, China National Knowledge Infrastructure, Wanfang Data, Chongqing VIP, and CiNii. We additionally reviewed the reference lists of the relevant studies, Google Scholar, and trial registries such as ClinicalTrials.gov to include any potentially relevant studies. We set the search strategy as comprehensively as possible following consultation with specialists in pediatrics and systematic review experts. The detailed search strategies for each database and search results are presented in Supplementary File S1.
All studies searched from databases and identified from other sources were imported into EndNote 20 (Clarivate Analytics, Philadelphia, PA, United States). After removing duplicates, the titles and abstracts of the articles were reviewed for first inclusion. For the first included studies, the full-texts were retrieved and reviewed for final inclusion.
For the final included studies, we extracted the following information using a standardized, pilot-tested Excel form: basic information (first author’s name and country, publication year, study setting, or funding source), sample size, details of participants, treatment and control intervention, details of the HM used, outcome measures, results, and information for the assessment of the risk of bias.
Study selection and data extraction were independently conducted by two researchers (BL and CYK), and any disagreement was resolved by discussions with other researchers (SHL and GTC). If the data were ambiguous or insufficient, we contacted the authors of the included studies via e-mail if possible.
We evaluated the methodological quality of the included studies using the Cochrane Collaboration’s risk of bias tool including items of random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessors, completeness of outcome data, selective reporting, and other biases (Higgins et al., 2011). Specifically, for the assessment of other biases, the statistical homogeneity of baseline clinical characteristics such as mean age or sex between the treatment and control groups was determined. Each item was assessed as “low risk”, “unclear risk”, or “high risk”. Two researchers (BL and CYK) independently conducted the risk of bias assessment, and a consensus was reached through discussions with other researchers (SHL and GTC) if there were disagreements.
Descriptive analysis of the details of the participants, treatment and control interventions, and outcomes of the included studies was conducted. In particular, the meta-analysis was performed using Review Manager software (version 5.4; Cochrane, London, United Kingdom) if two or more studies used the same type of treatment and control intervention with the same outcome measures. Continuous and binary outcomes were assessed, and the data are presented as the mean difference (MD) or standardized mean difference (SMD) and risk ratio (RR) with their 95% confidence intervals (CIs). Heterogeneity between the studies was assessed using both the χ2 test and the I2 statistic; specifically, 50% ≤ I2 < 75% and I2 ≥ 75% were considered to indicate substantial and considerable heterogeneity, respectively. A random-effects model was used if the included studies have significant heterogeneity (I2 ≥ 50%), whereas a fixed-effects model was used if the heterogeneity is not significant or if the number of studies included in the meta-analysis is small because estimates of inter-study variance have poor accuracy (Borenstein et al., 2010). Subgroup analysis was performed depending on whether the participants’ pattern identification was specified. For sensitivity analysis to confirm the robustness of the meta-analysis, we excluded 1) studies with a high risk of bias and 2) outliers that are numerically distant from the rest of the data. If there were sufficient studies (≥10 studies) included in each meta-analysis, we assessed the evidence of publication bias by Egger’s test using Stata/MP software version 16.1 (StataCorp LLC, College Station, TX, United States).
We graded the quality of evidence of the main findings using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) tool (Balshem et al., 2011). Using GRADEpro (https://gradepro.org/), the risk of bias, inconsistency, indirectness, imprecision, and publication bias were evaluated for the main findings, and the final quality of evidence for each finding was presented as “very low”, “low”, “moderate”, or “high”. A researcher (BL) assessed the quality of evidence, and another researcher (SHL) reviewed the results. Any discrepancies were resolved through discussions with other researchers (CYK and GTC).
A total of 7,103 articles were identified from 12 electronic databases, and there were no additional records identified from the trial registry, Google Scholar, and reference lists of relevant articles. After removing 394 duplicate records using EndNote 20, 6,709 records were screened based on the titles and abstracts, and 425 eligible reports were further examined for retrieval. Among them, it was not possible to secure full-texts for 4 of them, and a full-text review was conducted for the remaining 421 articles. After removing 216 articles for the following reasons: 66 studies were not RCTs, 2 studies did not involve anorexia without organic disease, 68 studies did not have diagnostic criteria for anorexia, 7 studies were not about HM only, 13 studies did not have details on the herb composition, 44 studies were a comparison between HMs, 9 studies used other EATM interventions, 6 studies only had the abstract without raw data, and 1 study contained duplicate data (Supplementary File S2), finally, a total of 205 studies were included (Figure 1 and Supplementary File S3).
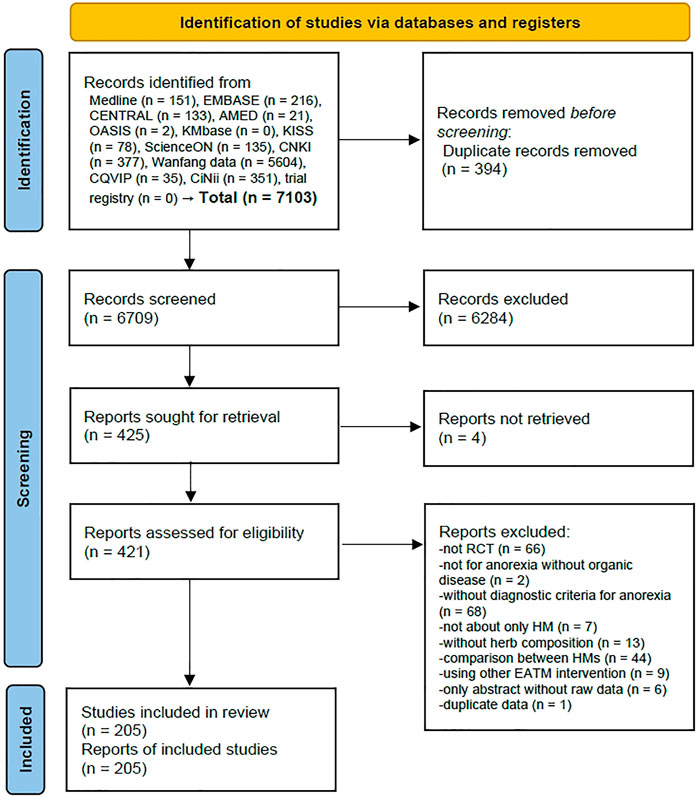
FIGURE 1. PRISMA 2020 flow diagram. No studies were identified from other sources (Google scholar and citation searching). Abbreviations: AMED, Allied and Complementary Medicine Database; CENTRAL, Cochrane Central Register of Controlled Trials; CNKI, China National Knowledge Infrastructure; CQVIP, Chongqing VIP; EATM, East Asian traditional medicine; HM, herbal medicine; KISS, Koreanstudies Information Service System; KMbase, Korean Medical Database; OASIS, Oriental Medicine Advanced Searching Integrated System; RCT, randomized controlled trial.
All included articles were published in Chinese in China from 1990 to 2021, and the study settings were all hospitals. Overall, 3 studies compared HM with placebo, 1 study compared HM with non-treatment, 135 studies compared HM with active control, and 72 studies compared HM plus active control with active control alone. As active controls for the control group, dietary supplements (e.g., zinc gluconate, multi-enzyme, and probiotics) and conventional medications (e.g., domperidone) were used. A total of 101 studies included only participants with anorexia corresponding to a specific pattern identification or mentioned the participants’ pattern identification. Most of the pattern identifications were related to the spleen-stomach. Among them, spleen-stomach qi deficiency was the most common in 28 studies, followed by spleen failing in transportation (22 studies), spleen deficiency with effulgent liver (18 studies), spleen-stomach disharmony (12 studies), and spleen-stomach yin deficiency (8 studies). A total of 23 studies reported that they received approval from the institutional review board before conducting the study, and 93 studies reported that informed consent was obtained from the participants. A total of 21 studies reported the funding sources for their research, and all of them received funding from the government or province (Supplementary File S3).
Various HMs were used in the included studies; among them, Xingpi Yanger granule was the most commonly used in 16 studies. In addition, there was a high frequency of using Shenling Baizhu powder (11 studies), Xiaoer Yanshi decoction (9 studies), and Yigong powder (8 studies). For the HM dosage form, most were used in the decoction form (130 studies), followed by the granule (57 studies) and pill (8 studies) forms. Analysis of the individual herbs constituting the basic HMs revealed that Crataegi Fructus was the most frequently used in 122 studies. Additionally, Atractylodis Rhizoma Alba (121 studies), Poria Sclerotium (113 studies), Citri Unshius Pericarpium (109 studies), Glycyrrhizae Radix (108 studies), and Hordei Fructus Germinatus (100 studies) were widely used herbs. In 50 studies, additional herbs were used in addition to basic HMs according to specific symptoms or pattern identifications. The HM administration period varied from 5 days to 3 months, among which 4 weeks (64 studies) and 2 weeks (42 studies) were the most common. Follow-up after HM administration was performed in 26 studies. The follow-up period varied widely from 1 week to 1 year, of which 6 months were the most frequent in 7 studies (Supplementary File S4).
A total of 57 studies appropriately generated random sequence numbers using randomization methods such as random number tables, and the risk of selection bias was evaluated to be low. The remaining studies did not report the random number generation method; thus, the risk was evaluated as unclear. In the case of performance bias, 3 studies were evaluated as low risk as they used placebo HM in the control group. On the other hand, 9 studies were evaluated as having a high risk of performance bias because single blinding was performed or the blinding of participants and personnel was not possible as the HM was compared with non-treatment. No studies mentioned allocation concealment and blinding of outcome assessors. Although there were missing values, 7 studies did not clearly state the reasons for the missing values or did not perform intention-to-treat analysis; thus, the risk of attrition bias was evaluated as high. A total of 17 studies reported that TER was calculated based on changes in body weight; however, raw data before and after treatment on body weight were not reported. Particularly, in some studies (Yuan, 2010; Yu, 2014), when body weight increased by 0.25 kg or more, it was judged as “markedly improved”, when it increased by less than 0.25 kg, it was judged as “improved”, and when there was no clear improvement, it was defined as “non-responder”. However, pre- and post-treatment analysis data for body weight were not presented, and only the calculated TER value was presented. Therefore, the risk of reporting bias was evaluated as high. In the other biases domain, 11 studies were evaluated as having an unclear risk because they did not mention the baseline homogeneity of the treatment group and the control group. In one study, the total number of the control group and the total number of men and women did not match; thus, the related risk was evaluated as high (Supplementary File S5).
Three studies compared HM with placebo. In the HM group of all studies, Xiaoer Piweile granule was administered for 4 weeks to anorexic patients with an identified pattern identification indicating spleen failing in transportation. The meta-analysis showed that the TER based on anorexia symptom improvement was significantly higher in the HM group (3 studies, RR 1.58, 95% CI 1.34, 1.85, I2 = 77%). A study (Lin, 2012) reported that there was no difference in the body weight and serum hemoglobin levels between the two groups (p > 0.05, all). There was also no difference in the incidence of AEs between the two groups (2 studies, RR 0.66, 95% CI 0.25, 1.75) (Table 1).
A study (Lian et al., 2020) compared HM with non-treatment; in the HM group, Tiaozhong decoction was administered for 4 weeks to anorexic patients with an identified pattern identification indicating spleen deficiency with effulgent liver. Lian et al. (Lian et al., 2020) reported that the TER and serum leptin level was significantly higher in the HM group than in the non-treatment group (p < 0.05, all).
A total of 135 studies compared HM with active control, and the meta-analysis revealed that the TER (130 studies, RR 1.25, 95% CI 1.22, 1.28, I2 = 54%), food intake (3 studies, MD 7.23 g/day, 95% CI 5.24, 9.22, I2 = 96%), body weight (11 studies, MD 0.89 kg, 95% CI 0.23, 1.54, I2 = 79%), and BMI (2 studies, MD 2.03, 95% CI 1.57, 2.49, I2 = 0%) were significantly higher in the HM group after treatment. In addition, both the levels of blood biomarkers related to gastrointestinal function (leptin, gastrin, motilin, neuropeptide Y (NPY), ghrelin, and orexin) and nutrition indices (serum Zn, Fe, and Ca, hemoglobin, and hair Zn) were significantly improved in the HM group compared with the active control group. The recurrence rate of anorexia (8 studies, RR 0.15, 95% CI 0.09, 0.25, I2 = 2%) and the incidence of AEs (29 studies, RR 0.34, 95% CI 0.14, 0.80, I2 = 0%) were significantly lower in the HM group. In subgroup analysis based on whether the pattern identification of the participants was specified, the TER, food intake, BMI, levels of gastrin, NPY, ghrelin, serum Fe, and hemoglobin, and recurrence rate showed a consistent HM effect regardless of pattern identification; however, the rest of the outcome indicators did not show a similar effect (Table 1). An evaluation of the risk of publication bias by Egger’s test showed that there was no risk of publication bias in the body weight (p = 0.104), serum leptin (p = 0.057), and incidence of AEs (p = 0.851); however, there was a risk of publication bias in the TER (p < 0.001) and serum Zn (p = 0.006).
A total of 72 studies compared HM plus active control with active control alone, and the symptoms of anorexia measured according to the TER (70 studies, RR 1.22, 95% CI 1.18, 1.25, I2 = 53%), appetite recovery time (4 studies, MD −2.09 days, 95% CI -2.44, -1.75, I2 = 83%), and food intake (2 studies, MD 37.96 g/day, 95% CI 23.13, 52.78, I2 = 0%) and all body measurement indicators including body weight (13 studies, MD 1.34 kg, 95% CI 1.03, 1.66, I2 = 58%), BMI (5 studies, MD 0.89, 95% CI 0.64, 1.13, I2 = 87%), and subcutaneous fat thickness (3 studies, MD 0.09 cm, 95% CI 0.05, 0.13, I2 = 0%) were significantly improved in the HM group compared with the control group. Additionally, both the levels of blood biomarkers related to gastrointestinal function (leptin, gastrin, motilin, NPY, and ghrelin) and nutrition indices (serum Zn, Fe, and Ca and hemoglobin) were significantly improved in the HM group. The recurrence rate of anorexia was significantly lower in the HM group (5 studies, RR 0.33, 95% CI 0.23, 0.48, I2 = 0%), and the incidence of AEs was not significantly different between the two groups (29 studies, RR 0.58, 95% CI 0.27, 1.24, I2 = 8%). In subgroup analysis based on whether the pattern identification of the participants was specified, all results except the BMI and NPY level consistently showed the significant effect of HM (Table 1). An evaluation of the risk of publication bias by Egger’s test showed there was no risk of publication bias in the body weight (p = 0.05), serum Zn (p = 0.812), and incidence of AEs (p = 0.779); however, there was a risk of publication bias in the TER (p < 0.001).
When HM and placebo were compared, the quality of evidence for the main outcome was moderate to high, and imprecision caused by a wide CI was the reason for the downgrade. When HM and active control or HM plus active control and active control alone were compared, the quality of evidence for the main outcome was very low to moderate. In particular, the risk of bias, inconsistency, imprecision, and publication bias of meta-analysis results were the reasons for the downgrade (Table 1).
This systematic review provides the most comprehensive review of HMs for anorexia in children. Based on the results of meta-analysis, there was some clinical evidence showing that HM as monotherapy or adjuvant therapy for children with anorexia could improve the clinical symptoms of anorexia (assessed by TER, appetite recovery time, and food intake), some anthropometric outcomes (including body weight and BMI), and some biological markers related to appetite and growth (including leptin, gastrin, motilin, NPY, ghrelin, orexin, and serum Zn, Fe, and Ca). HM was also found to significantly reduce the recurrence rate of anorexia and incidence of AEs in a head-to-head comparison with active controls such as dietary supplements or conventional medications in this population. However, the methodological quality of the included studies (evaluated as the risk of bias) was not optimal, and information for evaluating the risk of bias in selection bias, performance bias, and detection bias was not well described. Overall, the quality of evidence (evaluated using the GRADE approach) for the findings of the meta-analysis ranged from very low to moderate, with most cases evaluated as moderate.
As a standard clinical practice guideline for the diagnosis and management of children with anorexia has not been developed, it is clinically meaningful to analyze the efficacy and safety of HM, which is widely used in Asian countries (Yoon et al., 2003; Choi et al., 2010). Therefore, this review was conducted to summarize the available evidence. The findings of this review highlight the heterogeneity of the HMs used and the lack of high-quality trials, along with the potential of HM therapies in the management of children with anorexia. Specifically, to optimize the management of this condition with HM, the HM prescription strategy should be standardized, and more robust clinical trials should be conducted.
Pattern identification is a unique diagnostic system of EATM, and HM tends to rely on pattern identification rather than a diagnosed disease (Li and Xu, 2015). In other words, even in patients with different diagnosed diseases, if they have the same identified pattern identification, the same or at least a similar HM will likely be prescribed. On the other hand, in patients with the same diagnosed disease, if they have different identified pattern identifications, different HMs will likely be prescribed. Therefore, the standardization of pattern identification can help overcome the barriers of using heterogeneous HMs, thus promoting precision medicine (Li and Xu, 2015). Standardized pattern identification clusters heterogeneous HMs, and this clustering can lead to the standardization of the HM prescription strategy. Around half of the included studies in this systematic review included only participants with anorexia corresponding to a specific pattern identification or mentioned the participants’ pattern identification. In addition, most of them were related to the spleen-stomach. We conducted subgroup analysis based on whether the pattern identification of the participants was specified to investigate statistical and clinical heterogeneity and to ensure that the results are consistent. Although the results of subgroup analysis for the various outcome measures were not consistent and statistical heterogeneity was often unresolved in our study, the authors identified some specific pattern identifications frequently observed in children with anorexia, including spleen-stomach qi deficiency and spleen failing in transportation. It is important to explore the standardization of pattern identification for this condition in further studies such as expert consensus and large prospective cohort studies.
This review identified specific individual herbs that were used frequently, including Crataegi Fructus, Atractylodis Rhizoma Alba, Poria Sclerotium, Citri Unshius Pericarpium, Glycyrrhizae Radix, and Hordei Fructus Germinatus. Among them, the most frequently used herbs including Crataegii Fructus, Atractylodis Rhizoma Alba, Citri Unshius Pericarpium, and Hordei Fructus Germiniatus are effective in promoting digestion and invigorating the stomach. Furthermore, Poria Sclerotium and Glycyrrhizae Radix are the component herbs of Rikkunshito, a well-known herbal ghrelin potentiator (Fujitsuka and Uezono, 2014). However, there are limited studies on the appetite-stimulating mechanisms of these herbs. Considering the potentially beneficial properties of the complex components of the herbs (Wang et al., 2012), the nutritional, psychological, and physiological effects of these herbs for the treatment of anorexia in children should be comprehensively reviewed. The findings could help standardize and optimize HM therapies for this condition in the future.
Although we provide the most comprehensive systematic review on anorexia in children for the first time, some limitations should be considered. First, among the included studies, only a few studies compared HM with placebo. In most of the included studies, the risk of performance bias was evaluated to be unclear; thus, the possibility that the appetite-promoting effect of HM may be exaggerated cannot be excluded. In future controlled clinical trials, it is important to minimize the possibility of performance bias, specifically by comparing the effects of HM with those of placebo. Second, all included studies were conducted in China with Chinese subjects. Therefore, the results of this review are not only less generalizable but also vulnerable to the placebo effect because individuals in China may be more likely than those in Western countries to be familiar with EATM treatment modalities such as HM. To overcome the generalization issue, future studies should include other countries such as Korea, Taiwan, and Japan, which have incorporated HM therapies in the national medical system. In addition, it would be ideal to evaluate the participants’ familiarity and expectations for HM in trials and consider them in the interpretation of the results. Third, the diagnostic criteria for anorexia in children used in the included studies were all developed locally in China, and there was no internationally accepted diagnostic standard, which restricts the generalizability of the results for future studies. Therefore, it is recommended that future studies include internationally accepted diagnostic criteria for anorexia, such as infantile anorexia in the Chatoor Diagnostic Criteria for Feeding Disorder (Levine et al., 2011). Fourth, despite our efforts to interpret the heterogeneity through a subgroup analysis, considering the significant clinical heterogeneity among the included studies such as the age of the participants and the HMs used, caution is required when interpreting the meta-analysis results. Finally, the pre-planned sensitivity analysis could not be performed in this review because the included studies had an unclear risk of bias in most domains. This finding highlights the need for better quality clinical trials in the future.
According to the findings, there was a low to moderate quality of evidence showing that HM as monotherapy or adjuvant therapy for children with anorexia could improve clinical symptoms of anorexia, anthropometric outcomes, and biological markers related to appetite and growth. However, the methodological quality of the included studies was generally poor, and there was heterogeneity in the HMs used. Therefore, future high-quality clinical trials using a placebo as the control and research focusing on the standardization of HM prescription for children with anorexia should be performed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
The study was conceptualized by BL and GC. The study search, study screening, data extraction, and quality assessment were conducted by BL and C-YK. The manuscript was drafted BL and C-YK and revised by SL and GC. All authors have read and approved the final manuscript.
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant Number: HF21C0096).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.839668/full#supplementary-material
Balshem, H., Helfand, M., Schünemann, H. J., Oxman, A. D., Kunz, R., Brozek, J., et al. (2011). GRADE Guidelines: 3. Rating the Quality of Evidence. J. Clin. Epidemiol. 64 (4), 401–406. doi:10.1016/j.jclinepi.2010.07.015
Borenstein, M., Hedges, L. V., Higgins, J. P., and Rothstein, H. R. (2010). A Basic Introduction to Fixed-Effect and Random-Effects Models for Meta-Analysis. Res. Synth. Methods 1 (2), 97–111. doi:10.1002/jrsm.12
Brown, K. H., Peerson, J. M., Lopez de Romaña, G., de Kanashiro, H. C., and Black, R. E. (1995). Validity and Epidemiology of Reported Poor Appetite Among Peruvian Infants from a Low-Income, Periurban Community. Am. J. Clin. Nutr. 61 (1), 26–32. doi:10.1093/ajcn/61.1.26
Carter, L. E., Klatchuk, N., Sherman, K., Thomsen, P., Mazurak, V. C., and Brunetwood, M. K. (2019). Barriers to Oral Food Intake for Children Admitted to Hospital. Can. J. Diet. Pract. Res. 80 (4), 195–199. doi:10.3148/cjdpr-2019-012
Choi, M.-H., Kim, D.-G., and Lee, J.-Y. (2010). A Study of the Chief Complaint of Pediatric Outpatients in the Kyung Hee oriental Medicine Hospital. J. Pediatr. Korean Med. 24 (3), 121–137. doi:10.7778/jpkm.2016.30.1.045
Fujitsuka, N., and Uezono, Y. (2014). Rikkunshito, a Ghrelin Potentiator, Ameliorates Anorexia-Cachexia Syndrome. Front. Pharmacol. 5, 271. doi:10.3389/fphar.2014.00271
Hatamizadeh, N., Eftekhar, H., Shafaghi, B., and Mohammad, K. (2007). Effects of Folic Acid on Preschool Children's Appetite: Randomized Triple-Blind Clinical Trial. Pediatr. Int. 49 (5), 558–563. doi:10.1111/j.1442-200X.2007.02421.x
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. Bmj 343, d5928. doi:10.1136/bmj.d5928
Hurley, K. M., Yousafzai, A. K., and Lopez-Boo, F. (2016). Early Child Development and Nutrition: A Review of the Benefits and Challenges of Implementing Integrated Interventions. Adv. Nutr. 7 (2), 357–363. doi:10.3945/an.115.010363
Kim, S. Y., Yun, J. M., Lee, J. W., Cho, Y. G., Cho, K. H., Park, Y. G., et al. (2021). Efficacy and Tolerability of Cyproheptadine in Poor Appetite: A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study. Clin. Ther. 43 (10), 1757–1772. doi:10.1016/j.clinthera.2021.08.001
Levine, A., Bachar, L., Tsangen, Z., Mizrachi, A., Levy, A., Dalal, I., et al. (2011). Screening Criteria for Diagnosis of Infantile Feeding Disorders as a Cause of Poor Feeding or Food Refusal. J. Pediatr. Gastroenterol. Nutr. 52 (5), 563–568. doi:10.1097/MPG.0b013e3181ff72d2
Li, J. G., and Xu, H. (2015). Incorporating Pattern Identification of Chinese Medicine into Precision Medicine: An Integrative Model for Individualized Medicine. Chin. J. Integr. Med. 21 (11), 807–810. doi:10.1007/s11655-015-2058-9
Lian, J., Wang, H., Zhan, L., and Shao, Z. (2020). Effects of Tiaozhong Yin (调中饮) on Clinical Efficacy of Anorexia in Children with Asthenia of the Spleen and Effulgence of the Liver Syndrome and Electrogastrogram,serum Zinc and Vasoactive Intestinal Peptide. J. Pediatr. Tradit. Chin. Med. 16 (1), 42–46. doi:10.16840/j.issn1673-4297.2020.01.13
Liang, S. B., Lai, B. Y., Cao, H. J., Cai, Q. H., Bai, X., Li, J., et al. (2020). Pediatric Tuina for the Treatment of Anorexia in Children under 14 years: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Complement. Ther. Med. 51, 102411. doi:10.1016/j.ctim.2020.102411
Lin, L. M. (2012). The Pediatric Xiaoerpiweilekeli Granule in Treating Infantile Anorexia Spleen Fails to Clinical Research. Wuhan, China: Hubei University of Chinese Medicine.
Liu, W. X., Lang, J. F., and Zhang, Q. F. (2016). Risk Factors for Anorexia in Children. Zhongguo Dang Dai Er Ke Za Zhi 18 (11), 1119–1122. doi:10.7499/j.issn.1008-8830.2016.11.013
Martins, V. J., Toledo Florêncio, T. M., Grillo, L. P., do Carmo P Franco, M., Martins, P. A., Clemente, A. P., et al. (2011). Long-lasting Effects of Undernutrition. Int. J. Environ. Res. Public Health 8 (6), 1817–1846. doi:10.3390/ijerph8061817
Mukatay, A. W., Kalenga, P. M., Dramaix, M., Hennart, P., Schirvel, C., Kabamba, L. M., et al. (2010). Factors Associated with Malnutrition in Children Aged under Five Years in Lubumbashi (DRC). Sante Publique 22 (5), 541–550. doi:10.3917/spub.105.0541
Naila, N. N., Mahfuz, M., Hossain, M., Arndt, M., Walson, J. L., Nahar, B., et al. (2021). Improvement in Appetite Among Stunted Children Receiving Nutritional Intervention in Bangladesh: Results from a Community-Based Study. Eur. J. Clin. Nutr. 75 (9), 1359–1367. doi:10.1038/s41430-020-00843-9
Najib, K., Moghtaderi, M., Karamizadeh, Z., and Fallahzadeh, E. (2014). Beneficial Effect of Cyproheptadine on Body Mass index in Undernourished Children: a Randomized Controlled Trial. Iran J. Pediatr. 24 (6), 753–758.
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: an Updated Guideline for Reporting Systematic Reviews. Bmj 372, n71. doi:10.1136/bmj.n71
Riahi, F., Tashakori, A., and Vanani, G. S. (2018). Effects of Folic Acid on Appetite in Children with Attention Deficit Hyperactivity Disorder (ADHD) Treated with Methylphenidate: A Randomized Double-Blind Clinical Trial. Iran J. Med. Sci. 43 (1), 9–17.
Sant'Anna, A. M., Hammes, P. S., Porporino, M., Martel, C., Zygmuntowicz, C., and Ramsay, M. (2014). Use of Cyproheptadine in Young Children with Feeding Difficulties and Poor Growth in a Pediatric Feeding Program. J. Pediatr. Gastroenterol. Nutr. 59 (5), 674–678. doi:10.1097/mpg.0000000000000467
Wang, Y., Fan, X., Qu, H., Gao, X., and Cheng, Y. (2012). Strategies and Techniques for Multi-Component Drug Design from Medicinal Herbs and Traditional Chinese Medicine. Curr. Top. Med. Chem. 12 (12), 1356–1362. doi:10.2174/156802612801319034
Yoon, Y. H., Park, Y. B., Yang, E. S., Rho, Y. I., Kim, E. Y., Moon, K. R., et al. (2003). Eating Habits of Children under 4 Years with Poor-Feeding. Korean J. Pediatr. Gastroenterol. Nutr. 6 (2), 167–173. doi:10.5223/kjpgn.2003.6.2.167
Yu, J. (2014). Clinical Observation of Self-Made Anorexia Oral Liquid in Treating Infantile Anorexia. Nei Mongol J. Tradit. Chin. Med. 33 (34), 14.
Keywords: herbal medicine, anorexia, children, systematic review, meta-analysis
Citation: Lee B, Kwon C-Y, Lee SH and Chang GT (2022) Herbal Medicine for the Treatment of Anorexia in Children: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:839668. doi: 10.3389/fphar.2022.839668
Received: 20 December 2021; Accepted: 18 February 2022;
Published: 01 April 2022.
Edited by:
Francesca Borrelli, University of Naples Federico II, ItalyReviewed by:
Kristen Ward, University of Michigan, United StatesCopyright © 2022 Lee, Kwon, Lee and Chang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gyu Tae Chang, Z3RjaGFuZ0BraHUuYWMua3I=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.