
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Pharmacol., 06 April 2022
Sec. Pharmacology of Anti-Cancer Drugs
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.838247
This article is part of the Research TopicNew Insights into The Mechanisms of Resistance to Anti-Cancer DrugsView all 10 articles
 Yue Zeng1
Yue Zeng1 Yuanqing Feng2
Yuanqing Feng2 Guihua Fu2
Guihua Fu2 Junlan Jiang3
Junlan Jiang3 Xiaohan Liu1
Xiaohan Liu1 Yue Pan1
Yue Pan1 Chunhong Hu1,4
Chunhong Hu1,4 Xianling Liu1
Xianling Liu1 Fang Wu1,4,5,6*
Fang Wu1,4,5,6*The acquired resistance of epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) is inevitable and heterogeneous. The strategies to overcome acquired resistance are significant. For patients with secondary T790M-positive after early generation EGFR-TKIs, osimertinib is the standard second-line therapy. In patients resistant to prior early generation EGFR-TKIs, the acquired T790M mutation overlaps with other driver gene resistance, such as HER2-and MET amplification, accounting for 4–8%. The efficacy of osimertinib is unclear in patients with concurrent multiple driver gene resistance. We here report a patient who acquired EGFR T790M, STRN-ALK fusion, and EGFR amplification after gefitinib progression and subsequent MET amplification acquired from osimertinib. The other patient acquired EGFR T790M and MET amplification post-dacomitinib and acquired CCDC6-RET fusion after osimertinib treatment. Besides, subsequent new bypass activations were the possible resistance mechanisms to second-line osimertinib. Both patients had progression-free survival (PFS) less than 4 months and limited benefits from osimertinib second-line therapy. The T790M accompanying driver gene resistance will be a new subtype after EGFR-TKIs progression, needing effective treatment options.
The resistance mechanisms to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) are inevitable and heterogeneous, which restricts the clinical benefits (Piper-Vallillo et al., 2020). The most frequent acquired resistance mechanism of early generation EGFR-TKIs is secondary T790M mutation, and osimertinib is the standard second-line therapy (Mok et al., 2017; Westover et al., 2018). Some oncogene alterations like ALK, ROS1, and RET are increasingly observed in resistance mechanisms (Piper-Vallillo et al., 2020). Concurrent driver gene alteration as a resistance mechanism occurs in lung adenocarcinoma with a low incidence rate. However, no good consensus was formulated when EGFR T790M mutation was combined with other driver gene resistance. Herein, we reported two cases that developed T790M-positive and driver gene resistance after receiving EGFR-TKIs, which provide valuable suggestions for clinical decisions. Further, extensive literature reviews were made to summarize the reported cases with T790M mutation and driver gene resistance in NSCLC patients and to analyze the prognosis and efficacy of osimertinib.
Case 1 reports a 75-year-old non-smoking woman suffering from cough and pain in her upper limbs and back. Computed tomography (CT) identified a nodule in the left superior lung and metastasis in the liver in October 2018 (Figure 1B). Single-photon emission computed tomography bone imaging showed multiple bone metastases. The pathology was confirmed as lung adenocarcinoma and ALK Ventana immunohistochemistry (D5F3) was negative (Figure 1C). Besides, genotype by amplification-refractory mutation system polymerase chain reaction (RT-PCR) revealed an EGFR 19 exon in-frame deletion (19del) (Supplementary Table S1). She started on gefitinib at 250 mg daily in December 2018 (Figure 1A). Partial response was obtained in the patient with liver metastasis disappearing. The disease progressed with lung lesion enlargement and bone metastases after 12 months. Plasma-based next-generation sequencing (NGS, 168-gene panel, Burning Rock, Guangzhou) in December 2019 showed an EGFR 19del, EGFR T790M, STRN-ALK fusion, TP53 mutation, and EGFR amplification. Then she began osimertinib at 80 mg daily, which controlled her lung mass within 2 months. Alectinib was combined with osimertinib since plasma-based NGS showed that the STRN-ALK frequency was increasing. The STRN-ALK frequency disappeared after 3 weeks of treatment. However, new liver and right iliopsoas metastases were identified after 7 weeks of combination treatment. The CT-guided needle biopsy of the right iliopsoas lesion revealed metastatic lung adenocarcinoma, and tissue-based NGS showed an EGFR 19del, MET amplification, and HER3 mutation (425 gene-panel, Geneseeq Technology Inc., Nanjing). Crizotinib combined with osimertinib was administrated. Due to gastrointestinal toxicity, crizotinib was reduced to 250 mg daily. The patient showed remission of the right iliopsoas mass after 2 months of treatment, but her clinical conditions worsened, and she eventually died 1 week later.

FIGURE 1. (A): Timer shaft of gene testing and treatment measurement of case 1; (B): Computed tomographic images of primary lung lesion, liver, and iliopsoas mass metastases; (C): ALK Ventana immunohistochemistry (D5F3) as negative.
A 72-years woman was diagnosed with lung adenocarcinoma by percutaneous left lung biopsy in August 2019. The patient had multiple bone metastases with clinical disease stage Ⅳ. The gene testing showed an EGFR 19del by plasma-based ARMS-PCR (EGFR gene mutation assay). She started dacomitinib at 45 mg daily in August 2019. The tumor shrank with a duration of response (DOR) of 19 months (Figure 2). The patient had progression on the right supraclavicular and cervical lymph nodes. The ultrasound-guided percutaneous biopsy of the right supraclavicular lymph node showed metastatic lung adenocarcinoma. Besides, tissue- and plasma-based NGS (425 gene-panel, Geneseeq Technology Inc., Nanjing) revealed an EGFR 19del, T790M mutation in both, and MET amplification (CN:4.7) in plasma. Second-line osimertinib was administrated in May 2021. The patient’s lesion shrank in the first month of osimertinib therapy. However, the supraclavicular lymph nodes enlarged and new brain metastases appeared in September 2021 with progression-free survival (PFS) of less than 4 months. The plasma-based NGS (437 gene-panel, Geneseeq Technology Inc., Nanjing) revealed an EGFR 19del, T790M mutation, MET amplification (CN:14.2), and CCDC6-RET fusion (Supplementary Table S2, Figure 3). The patient received radiotherapy for brain and subsequent osimertinib and crizotinib combination therapy in October 2021. She showed mild gastrointestinal toxicity of nausea and vomiting to the combination. The brain metastatic lesion was controlled but the primary lung lesion progressed after a 1-month combination. The patient had a poor performance status and severe symptoms of dyspnea.
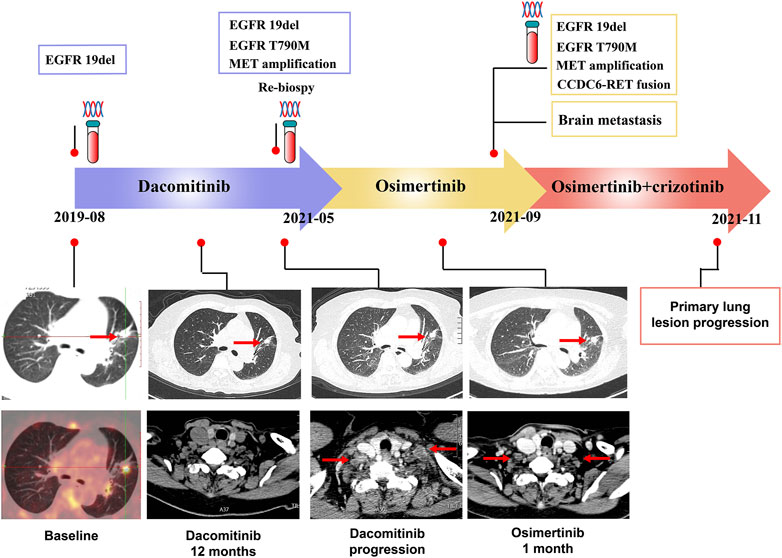
FIGURE 2. Timer shaft of gene testing and treatments measurement, and the computed tomographic images of primary lung lesion and lymph nodes metastases of case 2.
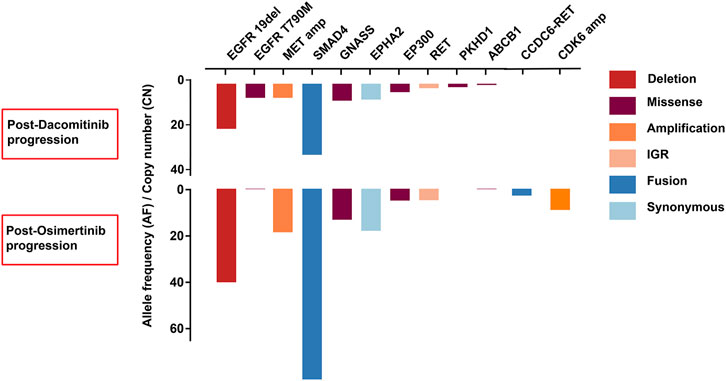
FIGURE 3. The comparison of resistance mechanisms of genetic alterations between dacomitinib progression and osimertini progression in case 2.
The mechanisms of resistance were heterogeneous and the remaining unknown mechanisms need to be discovered. The acquired secondary EGFR T790M mutation could be overcome by second-line osimertinib with a median PFS of 10.1 months in the AURA3 study (Mok et al., 2017; Westover et al., 2018). The driver gene acquired resistance, such as MET, ALK, and RET alterations, are increasingly reported (Zeng et al., 2022). It is unclear if osimertinib will exert its efficacy in patients with concurrent T790M-positive and driver gene resistance.
For the first time, we reported cases harboring concurrent alterations of EGFR T790M with ALK fusion and MET amplification after EGFR-TKIs. Both patients had a PFS of less than 4 months under osimertinib second-line therapy. The presented cases showed concurrent EGFR T790M and that driver gene resistance could not benefit from osimertinib monotherapy. Besides, the two patients developed MET amplification and RET fusions after osimertinib, which indicated that subsequent new bypass activations were possible resistance mechanisms to second-line osimertinib in patients with EGFR T790M and driver gene resistance.
Further, we conducted extensive literature reviews to identify the incidence rate and prognosis of concurrent driver gene resistance. EGFR secondary T790M, accompanying ALK, ROS1, MET, RET, HER2, KRAS, or NTRK gene alterations, were included. The incidence of T790M mutation overlapping with driver gene mutation, such as HER2-and MET amplification, was 4–8% after prior EGFR-TKIs treatment (Yu et al., 2013; Gou et al., 2016; Fu et al., 2021). Wang and coworkers reported a T790M lative allele frequency less than 20% was more likely concurrent driver gene resistance, like MET and HER2-amplification in EGFR-TKIs progression patients, who had a lower objective response rate and disease control rate to osimertinib (Wang et al., 2020). Besides, the reported cases of concurrent EGFR T790M and driver gene resistance are summarized in Table 1. The patients with T790M and KRAS G12V mutation coexistence did not respond to osimertinib therapy (Xiang et al., 2015; Fu et al., 2021). The patients harboring acquired EGFR T790M, HER2 mutation, and HER2 amplification from first-generation EGFR-TKIs did not respond to osimertinib and the combination of trastuzumab with a frustrating PFS (Meedendorp et al., 2018; Zhang et al., 2018; Ralki et al., 2019). The statistics also presented the poor efficacy of osimertinib on T790M and driver gene resistance.
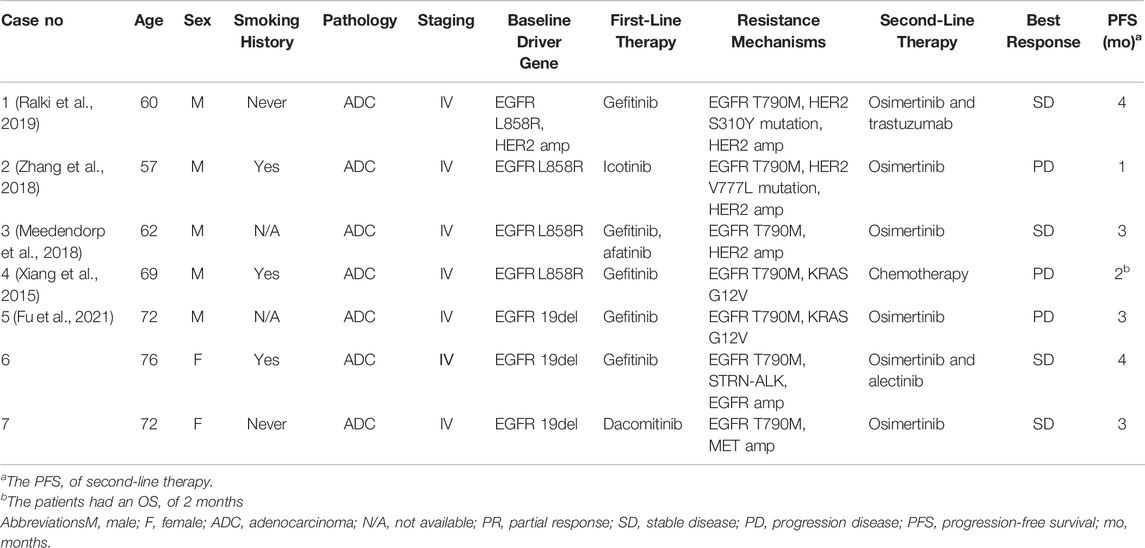
TABLE 1. Clinicopathological characteristics of concurrent EGFR-T790M and driver gene-resistant NSCLC patients.
EGFR-TKIs combined with bypass targeted therapy showed potent antitumor activity. Osimertinib plus savolitinib showed an acceptable risk-benefit in NSCLC patients with acquired MET amplification from pretreated EGFR-TKIs in a phase Ⅰb study (Sequist et al., 2020). crizotinib combined with osimertinib also demonstrated improved PFS in a real-world study (Liu et al., 2021). Patients with acquired ALK fusions resistant to EGFR-TKIs, such as EML4-ALK and STRN-ALK, may benefit from EGFR plus ALK inhibitors (Offin et al., 2018; Zhou et al., 2019). RET fusion appearance post-osimertinib progression responded to osimertinib and pralsetinib, the selective RET-TKI (Piotrowska et al., 2018). trastuzumab emtansine (T-DM1) and trastuzumab deruxtecan were available targeted agents with activity against the HER2 alterations, however, strategies against acquired HER2 alterations await more clinical evidence (Zeng et al., 2022).
Hence, strategies to rival multiple driver gene resistance are urgently needed. Firstly, the plasma assay of EGFR T790M single-point is insufficient to identify the resistance of EGFR-TKIs, since concurrent driver gene resistance impairs osimertinib’s efficacy. Osimertinib combined with bypass TKIs is a promising treatment for concurrent EGFR T790M and driver gene resistance. The circulating tumor DNA (ctDNA) genomic profile was an effective tool to observe the resistance mechanisms (Del Re et al., 2019). ctDNA monitoring may allow the timely combination of osimertinib and bypass inhibitors. When EGFR T790M is accompanied with ALK/MET/ROS1 resistance, osimertinib and crizotinib with AEs observation could be taken into consideration since crizotinib may prevent the activation of the other two mechanisms.
We firstly reported lung cancer patients harboring concurrent alterations of EGFR T790M with ALK fusion, MET amplification, and RET fusions as mechanisms of resistance after EGFR-TKIs. Both patients had poor outcomes from osimertinib. Additionally, subsequent new bypass activations were possible resistance mechanisms to second-line osimertinib. T790M accompanying driver gene resistance will be a new subtype after EGFR-TKI progression and needs effective treatment options.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the minor(s)' legal guardian/next of kin for the publication of any potentially identifiable images or data included in this article.
YZ collected and analyzed data, and wrote the draft of the paper. FW conceptualized the study and revised the paper. YF, GF, and JJ provided data. XlL and CH provided guidance. YP and XhL collected data. All authors critically reviewed and revised the final manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We sincerely thank the multidisciplinary team (MDT) of thoracic oncology, the Second Xiangya Hospital, Central South University, for their guidance in clinical work.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.838247/full#supplementary-material
Del Re, M., Crucitta, S., Gianfilippo, G., Passaro, A., Petrini, I., Restante, G., et al. (2019). Understanding the Mechanisms of Resistance in EGFR-Positive NSCLC: From Tissue to Liquid Biopsy to Guide Treatment Strategy. Int. J. Mol. Sci. 20 (16). doi:10.3390/ijms20163951
Fu, Y., Wang, A., Zhou, J., Feng, W., Shi, M., Xu, X., et al. (2021). Advanced NSCLC Patients with EGFR T790M Harboring TP53 R273C or KRAS G12V Cannot Benefit from Osimertinib Based on a Clinical Multicentre Study by Tissue and Liquid Biopsy. Front. Oncol. 11, 621992. doi:10.3389/fonc.2021.621992
Gou, L. Y., Li, A. N., Yang, J. J., Zhang, X. C., Su, J., Yan, H. H., et al. (2016). The Coexistence of MET Over-expression and an EGFR T790M Mutation Is Related to Acquired Resistance to EGFR Tyrosine Kinase Inhibitors in Advanced Non-small Cell Lung Cancer. Oncotarget 7 (32), 51311–51319. doi:10.18632/oncotarget.9697
Liu, L., Qu, J., Heng, J., Zhou, C., Xiong, Y., Yang, H., et al. (2021). A Large Real-World Study on the Effectiveness of the Combined Inhibition of EGFR and MET in EGFR-Mutant Non-small-cell Lung Cancer after Development of EGFR-TKI Resistance. Front. Oncol. 11, 722039. doi:10.3389/fonc.2021.722039
Meedendorp, A. D., Ter Elst, A., 't Hart, N. A., Groen, H. J. M., Schuuring, E., and van der Wekken, A. J. (2018). Response to HER2 Inhibition in a Patient with Brain Metastasis with EGFR TKI Acquired Resistance and an HER2 Amplification. Front. Oncol. 8, 176. doi:10.3389/fonc.2018.00176
Mok, T. S., Wu, Y-L., Ahn, M-J., Garassino, M. C., Kim, H. R., Ramalingam, S. S., et al. (2017). Osimertinib or Platinum-Pemetrexed in EGFR T790M-Positive Lung Cancer. N. Engl. J. Med. 376 (7), 629–640. doi:10.1056/NEJMoa1612674
Offin, M., Somwar, R., Rekhtman, N., Benayed, R., Chang, J. C., Plodkowski, A., et al. (2018). Acquired ALK and RET Gene Fusions as Mechanisms of Resistance to Osimertinib in EGFR-Mutant Lung Cancers. JCO Precis Oncol. 2. doi:10.1200/po.18.00126
Piotrowska, Z., Isozaki, H., Lennerz, J. K., Gainor, J. F., Lennes, I. T., Zhu, V. W., et al. (2018). Landscape of Acquired Resistance to Osimertinib in EGFR-Mutant NSCLC and Clinical Validation of Combined EGFR and RET Inhibition with Osimertinib and BLU-667 for Acquired RET Fusion. Cancer Discov. 8 (12), 1529–1539. doi:10.1158/2159-8290.Cd-18-1022
Piper-Vallillo, A. J., Sequist, L. V., and Piotrowska, Z. (2020). Emerging Treatment Paradigms for EGFR-Mutant Lung Cancers Progressing on Osimertinib: A Review. Jco 38–2936. doi:10.1200/jco.19.03123
Ralki, M., Maes, B., Pat, K., Wynants, J., and Cuppens, K. (2019). Triple Trouble: A Case of Multiple Resistance Mechanisms after First Generation EGFR-TKI in NSCLC. Case Rep. Oncol. 12 (2), 625–630. doi:10.1159/000502214
Sequist, L. V., Han, J. Y., Ahn, M. J., Cho, B. C., Yu, H., Kim, S. W., et al. (2020). Osimertinib Plus Savolitinib in Patients with EGFR Mutation-Positive, MET-Amplified, Non-small-cell Lung Cancer after Progression on EGFR Tyrosine Kinase Inhibitors: Interim Results from a Multicentre, Open-Label, Phase 1b Study. Lancet Oncol. 21 (3), 373–386. doi:10.1016/s1470-2045(19)30785-5
Wang, Y., He, Y., Tian, P., Wang, W., Wang, K., Chuai, S., et al. (2020). Low T790M Relative Allele Frequency Indicates Concurrent Resistance Mechanisms and Poor Responsiveness to Osimertinib. Transl Lung Cancer Res. 9 (5), 1952–1962. doi:10.21037/tlcr-20-915
Westover, D., Zugazagoitia, J., Cho, B. C., Lovly, C. M., and Paz-Ares, L. (2018). Mechanisms of Acquired Resistance to First- and Second-Generation EGFR Tyrosine Kinase Inhibitors. Ann. Oncol. 29 i10–i9. doi:10.1093/annonc/mdx703
Xiang, X., Yu, J., Lai, Y., He, W., Li, S., Wang, L., et al. (2015). L858R-positive Lung Adenocarcinoma with KRAS G12V, EGFR T790M and EGFR L858R Mutations: A Case Report. Oncol. Lett. 10 (3), 1293–1296. doi:10.3892/ol.2015.3435
Yu, H. A., Arcila, M. E., Rekhtman, N., Sima, C. S., Zakowski, M. F., Pao, W., et al. (2013). Analysis of Tumor Specimens at the Time of Acquired Resistance to EGFR-TKI Therapy in 155 Patients with EGFR-Mutant Lung Cancers. Clin. Cancer Res. 19 (8), 2240–2247. doi:10.1158/1078-0432.Ccr-12-2246
Zeng, Y., Yu, D., Tian, W., and Wu, F. (2022). Resistance Mechanisms to Osimertinib and Emerging Therapeutic Strategies in Nonsmall Cell Lung Cancer. Curr. Opin. Oncol. 34 (1), 54–65. doi:10.1097/CCO.0000000000000805
Zhang, P., Nie, X., Wang, B., and Li, L. (2018). Combined Therapy with Osimertinib and Afatinib in a Lung Adenocarcinoma Patient with EGFR T790M Mutation and Multiple HER2 Alterations after Resistance to Icotinib: A Case Report. Thorac. Cancer 9 (12), 1774–1777. doi:10.1111/1759-7714.12889
Keywords: non-small-cell lung cancer, EGFR-TKI acquired resistance, T790M, diver gene resistance, ALK fusion, MET amplification, RET fusion
Citation: Zeng Y, Feng Y, Fu G, Jiang J, Liu X, Pan Y, Hu C, Liu X and Wu F (2022) Acquired Concurrent EGFR T790M and Driver Gene Resistance From EGFR-TKIs Hampered Osimertinib Efficacy in Advanced Lung Adenocarcinoma: Case Reports. Front. Pharmacol. 13:838247. doi: 10.3389/fphar.2022.838247
Received: 17 December 2021; Accepted: 11 March 2022;
Published: 06 April 2022.
Edited by:
Francesco Bertoni, Institute of Oncology Research (IOR), SwitzerlandReviewed by:
Lizhen Huang, South China University of Technology, ChinaCopyright © 2022 Zeng, Feng, Fu, Jiang, Liu, Pan, Hu, Liu and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Wu, d3VmYW5nNDQ2MUBjc3UuZWR1LmNu, b3JjaWQub3JnLzAwMDAtMDAwMi02NjI3LTM0Mzc=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.