
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol., 20 October 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.835991
This article is part of the Research TopicFrom Clinical Trials to Real-World Data Sciences: Evidence-Based Medicine for Value in HealthView all 36 articles
Background and objective: Pruritus is a common complication in patients with primary biliary cholangitis (PBC). The pathogenesis is not clear, and also the precise therapeutic measures remain alluring. In order to systematically evaluate the efficacy and safety of drug interventions in the treatment of pruritus associated with PBC, this systemic review and meta-analysis was conducted.
Methods: The randomized controlled trials (RCTs) on drug interventions in the treatment of pruritus associated with primary cholangitis were searched in the electronic databases of PubMed, EMBASE, Cochrane Library, Web of Science, and ClinicalTrials.gov. Two researchers independently screened the literature, extracted and integrated the data, and assessed the bias risk of the selected literature, according to the Cochrane handbook. Finally, the STATA 15.0 software was used for the meta-analysis.
Results: A total of 23 RCTs involving 2,194 patients were studied, that included 12 pharmacological interventions. In terms of itching relief, compared with placebo, UDCA, methotrexate and GSK2330672 had a definite effect in improving pruritus (pruritus remission rate before and after treatment, p < 0.05). In terms of serum indexes, compared with placebo group, UDCA, OCA, rifampicin, cyclosporine, NGM282, seladelpar and colchicine may improve blood alkaline phosphatase (ALP) (p < 0.05), but only rifampicin showed low heterogeneity. UDCA, bezafibrate, OCA, rifampicin, NGM282 and others may improve blood γ-glutamyl transpeptidase (γ-GGT) (p < 0.05), but due to the high heterogeneity and the limitation of research samples, a clear conclusion cannot be drawn. In terms of adverse events, except high (>15 mg/kg/day) and low doses (<13 mg/kg/day) of UDCA increased the incidence of adverse events, there were no risk of increasing the incidence of adverse events compared with placebo (p > 0.05), and a moderate dose of UDCA (13–15 mg/kg/day) and malotilate (1,500 mg/day) may also help in reducing the incidence of adverse events (p < 0.05).
Conclusion: UDCA, methotrexate and GSK2330672 may relieve itching in patients with PBC, but there is a lack of robust evidence to support their effect on ALP or γ-GGT. Due to the heterogeneity in the published studies, based on the present review, we cannot explicitly recommend any specific drug for the treatment of PBC-related pruritus.
Systematic Review Registration: link-https://osf.io/2g8ya, identifier 10.17605/OSF.IO/2G8YA
Primary biliary cholangitis (PBC), formerly known as primary biliary cirrhosis, is an autoimmune liver disease, which is predominantly seen in women. The interaction of specific anti-mitochondrial antibodies with specific autoantigens, accompanied by pathophysiological processes such as bile duct injury, cholestasis, liver fibrosis and even liver cirrhosis, are the main features of the pathological progress of PBC (Lee et al., 2019; Gulamhusein and Hirschfield, 2020). At present, the incidence of PBC is geographically varied, and the number is on the rise too. According to a survey, there are 118.75 cases of prostate cancer per million people in the Asia-Pacific region (Zeng et al., 2019), 218.1 cases per million people in North America, 145.9 cases per million people in Europe, and 189.0 cases per million in Victoria, Australia (J et al., 2020). It was reported to be 346.0 cases per million in Sweden (HU et al., 2019), 149.0 cases per million in Slovakia (Drazilova et al., 2020) and, 279.0 cases per million in Italy (Rajaobelina et al., 2019). Environmental and genetic factors play an important role in the occurrence of the disease (Carey et al., 2015). The levels of serum alkaline phosphatase (ALP) and γ-glutamyl transpeptidase (γ-GGT) are generally increased in patients with PBC.
Fatigue and pruritus are the most common symptoms in patients with PBC. Scratching, sleep deprivation, depression and, even suicidal thoughts caused by itching affect 20%–70% of patients (Beuers et al., 2014; Carey et al., 2015), seriously affecting their quality of life. The accumulation and deposition of bile acid and bile salt, the regulation of lysophosphatidic acid, the abnormality of endogenous opioid receptors and the effects of serotonin and substance P are considered to be the main pathophysiological mechanisms of cholangitis pruritus (Bray et al., 2018). Even so, the pathogenesis of cholestatic pruritus has not been well described because of its diversity and complexity (Tajiri and Shimizu, 2017; SP et al., 2019).
A cross-sectional study of 2194 PBC pruritus patients in the United Kingdom shows that there is a lack of adequate understanding, management and guidelines for the disease, and there is insufficient evidence on the recommended treatment (Hegade et al., 2019). There exist few evidence-based guidelines for treating PBC pruritus, but the limitation is that there are specific clinical conditions where these guidelines cannot be applied. Ursodeoxycholic acid (UDCA) and OCA are drugs approved by the U.S. Food and Drug Administration (FDA) for the treatment of PBC, which can improve the liver biochemical indexes, prolong survival time and delay the development of esophageal varices. However, UDCA cannot completely cure this disease, and its effectiveness in the treatment of cholangitis pruritus is still controversial (JS et al., 2012). Interestingly, another potential anti-PBC drug, OCA, was reported to increase the risk of itching (Trauner et al., 2019). The search for an effective treatment for PBC-related pruritus interventions has never stopped. Newer pharmacological interventions have been reported such as cholestyramine (TB et al., 1961), rifampicin (CN and SG, 1988), sertraline (MJ et al., 2007), ondansetron (JW et al., 2005), maralixibat (Mayo et al., 2019), ileal apical sodium bile acid transporter (ASBT) inhibitors such as GSK2330672 (Hegade et al., 2017). In order to determine the efficacy and safety of the available drugs for the treatment of PBC-associated pruritus, we conducted a systematic review and meta-analysis of pharmacological interventions in PBC-related pruritus.
We followed a predetermined protocol and the principles of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) for this systemic review and meta-analysis (DG et al., 2009).
To systematically evaluate the efficacy of interventions, we searched PubMed, EMBASE, Cochrane Library, Web of Science, and ClinicalTrials.gov from inception up to June 2021. The search strategy was implemented by an experienced medical librarian.
Strategies were selected using a combination of medical subject headings (MeSH) and text words, and search terms included “primary biliary cholangitis (cirrhosis),” “pruritus” or “pruritis” or “itching,” and “randomized controlled trial.” The language was limited to English, and the publication status was not restricted.
The inclusion criteria were as follows
(1) The study was a randomized controlled trial (RCT).
(2) The diagnosis of PBC was based on at least two of the following: the presence of anti-mitochondrial antibody (AMA), cholestasis with an elevation of ALP activity, histopathologic evidence of nonsuppurative cholangitis and destruction of small or medium-sized bile ducts (KD et al., 2019), and patients of primary biliary cholangitis with persistent pruritus (course of disease≥3 months).
(3) All pharmacological interventions related to the treatment of pruritus in PBC.
(4) Outcomes related to the efficacy and safety of pruritus in PBC.
(1) The interventions are not suitable (more than three interventions or the study interventions beyond our study).
(2) PBC associated with other underlying diseases.
(3) Unavailable data (there is no data we need in the study).
(4) Absence of a clear basic information about the study subjects.
(5) Duplicate publications.
(6) Studies with ambiguous diagnostic criteria.
Two reviewers (Chenyi Xu and Xuelian Lv) independently extracted basic information about the articles (article title, first author, year of publication, sample size, country or region), trial design (participants, interventions, time span of the trial, follow-up time), clinical efficacy and adverse events.
Clinical efficacy measures included pruritus scores; 0–10 numerical rating scale (NRS), quality of life scale for primary biliary cirrhosis (cholangitis) (PBC-40), and 5-D itch scale (or the pruritus relief rate before and after treatment). Secondary outcomes were laboratory parameters such as the changes in serum alkaline phosphatase (ALP) and gamma-glutamyl transpeptidase (γ–GGT). We considered both iatrogenic and non-iatrogenic adverse events, and carried out a quantitative analysis of these events.
Two investigators evaluated each of the included RCTs and recorded the following six items, as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins and Green, 2008): the methods of blinding, the generation of information, data, distribution of randomized control sequences, selective reports and other possible problems. The risk of biases was marked as high, uncertain, or low.
STATA15.0 (STATA statistical software: Release 15.0 College Station, TX: Stata Corp LP) was used to analyze the data. Successive mean differences in pruritus scores were reported as standardized mean differences (SMD), and binary variables used risk ratios (RR) or odds ratio (OR), providing a 95% confidence interval (CI) for each effect. Heterogeneity was evaluated by I2, p < 0.05 was considered to be statistically significant. The studies with high heterogeneity (p ≤ 0.10 and I2 ≥ 50%) were analyzed by random effect model, the studies with low heterogeneity (p > 0.10 and I2 < 50%) were analyzed by fixed effect model. The stability of the results was evaluated by subgroup analysis and sensitivity analysis. If enough studies were included in meta-analysis (n ≥ 10), funnel chart analysis was used for evaluate publication bias.
A total of 468 records were retrieved from the electronic database (PubMed n = 90, EMBASE n = 80, Cochrane Library n = 151, Web of Science n = 106, ClinicalTrials.gov n = 41), and 332 records were excluded after duplicates removed. After browsing the titles and abstracts, 41 articles were excluded based on article type (review n = 28, meta-analysis n = 12, protocol n = 1). After that, 54 articles were assessed as full text and 31 were excluded for the following reasons: not RCTs (n = 9), improper intervention (n = 7), data duplication (n = 1), unavailable data (n = 11), non-English literature (n = 1), absence of a clear basic information about the study subjects (n = 2). Finally, 23 studies were obtained. The details are shown in the flowchart (Figure 1). Of the 23 studies included, eight were RCTs studies of UDCA (Poupon et al., 1990; Oka et al., 1991; Poupon et al., 1991; Battezzati et al., 1993; Lindor et al., 1994; Heathcote et al., 1995; Vuoristo et al., 1995; Parés et al., 2000). The remaining 15 RCTs contained three studies of obeticholic acid in PBC (Hirschfield et al., 2015; Nevens et al., 2016; Kowdley et al., 2018), two studies of bezafibrate in PBC (Kanda et al., 2003; Corpechot et al., 2018), and two studies of rifampicin (Bachs et al., 1989; Podesta et al., 1991), and one RCT each evaluating the ileal bile acid transporter inhibitor GSK2330672 (Hegade et al., 2017), FGF19 analog NGM282 (Mayo et al., 2018), selective PPAR-δ agonist seladelpar (MBX-8025) (Jones et al., 2017), cyclosporine (Wiesner et al., 1990), colchicine (Almasio et al., 2000), methotrexate (Hendrickse et al., 1999), maralixibat (Mayo et al., 2019) and malotilate (Listed, 1993).
The 23 RCTs included 2,194 patients, 92% of the study population was women, and the average age was 54.9 years. The studies included in our systematic review are shown in Table 1.
Of all the 23, 11 studies mentioned the randomization techniques (including random sampling with computer (Battezzati et al., 1993; Hendrickse et al., 1999; Hirschfield et al., 2015; Hegade et al., 2017; Jones et al., 2017; Corpechot et al., 2018; Kowdley et al., 2018; Mayo et al., 2019), or interactive voice/web response system (Mayo et al., 2018), or random numbers (Listed, 1993; Heathcote et al., 1995)), while two studies were considered “high risk” because the random methods were inappropriate (Podesta et al., 1991; Vuoristo et al., 1995). Twelve studies followed allocation concealment (including third-party random allocation (Poupon et al., 1990; Battezzati et al., 1993; Lindor et al., 1994; Jones et al., 2017; Corpechot et al., 2018; Mayo et al., 2018; Mayo et al., 2019), sequence number (Listed, 1993; Heathcote et al., 1995; Hendrickse et al., 1999; Hegade et al., 2017), or envelope (Parés et al., 2000)). Of all the included studies, two followed single-blind (Oka et al., 1991; Heathcote et al., 1995), and the rest followed double-blind designs. The data evaluator was blinded in five studies. All the 23 studies reported complete data and there was no risk of other biases. The Cochrane deviation risk assessment tool was used for risk assessment, and the results are shown in Figures 2A,B.
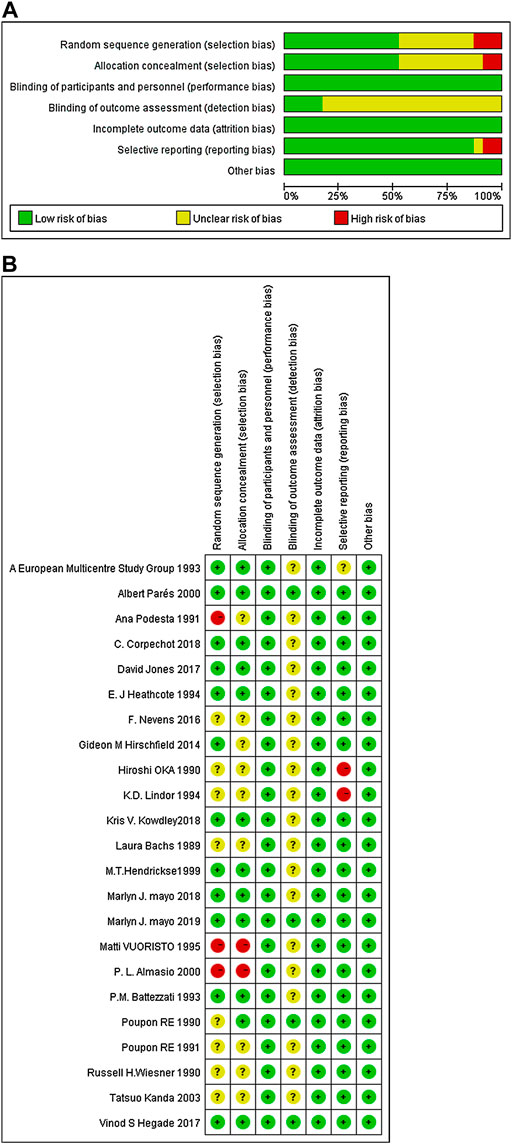
FIGURE 2. The quality assessment of included trials based on the Cochrane risk assessment tool. (A) Each risk of bias item presented as percentages across all included studies; (B) Each risk of bias item for each included study.
Most trials reported only the number of patients with pruritus pre-treatment and post-treatment, so we considered the relief rate as the primary outcome. Among the eight studies (Poupon et al., 1990; Oka et al., 1991; Poupon et al., 1991; Battezzati et al., 1993; Lindor et al., 1994; Heathcote et al., 1995; Vuoristo et al., 1995; Parés et al., 2000), of UDCA seven documented itching. The results showed that UDCA combined with or without cholestyramine, compared to placebo (RR = 1.85, 95%CI (1.40, 2.45), p < 0.001, I2 = 0.0%), had a significant difference in relieving pruritus (Figure 3A). Among these, two studies recorded the itching score in detail (Battezzati et al., 1993; Parés et al., 2000). Compared with placebo, UDCA combined with cholestyramine significantly reduced the itching score (SMD = −1.78, 95% CI (−2.26, −1.29), p < 0.001, I2 = 64%), but had a high heterogeneity (p = 0.095, I2 = 64.0%) (Figure 3B).
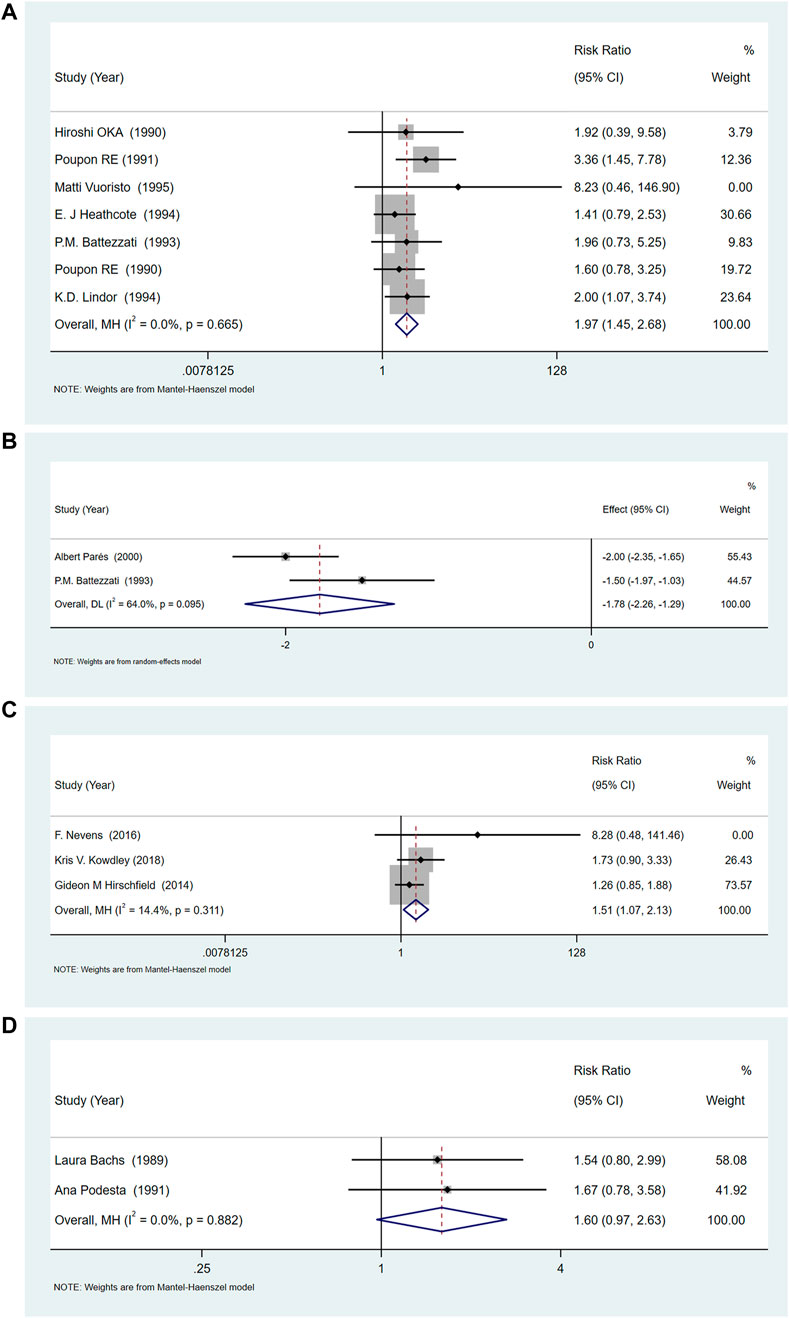
FIGURE 3. (A)The effect of UDCA on pruritus relief compared with placebo. (B) Two studies of UDCA in pruritus scores compared with placebo. (C) OCA increases the risk of pruritus compared with placebo. (D) The effect of Rifampicin on the rate of pruritus relief compared with placebo.
Three studies (Hirschfield et al., 2015; Nevens et al., 2016; Kowdley et al., 2018) compared the relief of pruritus after OCA treatment, and the heterogeneity among studies was low (p = 0.311, I2 = 14.4%). Meta-analysis showed that OCA increased the incidence of pruritus, compared with placebo (RR = 1.511, 95%CI (1.07, 2.12), p = 0.018) (Figure 3C).
Two studies (Bachs et al., 1989; Podesta et al., 1991) compared the relief of pruritus after rifampicin treatment, and the heterogeneity between the studies was low (p = 0.882, I2 = 0.0%). The results of meta-analysis showed that rifampicin had no significant effect on itching compared with placebo or control drugs [RR = 1.595, 95%CI (0.97, 2.63), p = 0.067] (Figure 3D).
One study (Wiesner et al., 1990) reported the incidence of pruritus in patients with PBC, before and after treatment, with cyclosporine and placebo. The results showed that cyclosporine did not significantly reduce the incidence of pruritus compared with placebo [RR = 0.726, 95%CI (0.51, 1.03), P = 0.072]
One study (Mayo et al., 2018) reported a comparison between NGM282 and placebo. The results suggested that NGM282 had no significant effect on 5-D itch score [SMD = −0.429, 95%CI (−1.06, 0.20), p = 0.179] and VAS score [SMD = 0.335, 95%CI (−0.29, 0.96), p = 0.293] in patients with PBC.
One study (Listed, 1993) reported a comparison between malotilate and placebo, which suggested that malotilate did not significantly reduce the incidence of pruritus [RR = 1.083, 95%CI (0.43, 2.73), p = 0.865].
One study (Jones et al., 2017) reported the comparison of seladelpar and placebo after treatment. The results suggested that seladelpar did not significantly reduce pruritus in patients with PBC. The 5-D itch score [SMD = 0.000, 95%CI (−0.79, 0.79), p = 1.000] and VAS score [SMD = 0.138, 95%CI(−0.65, 0.92), p = 0.731] showed no significant alteration.
One study (Mayo et al., 2019) reported a comparison between maralixibat and placebo. The results suggested that maralixibat did not significantly reduce the pruritus 5-D itch score in patients with PBC [SMD = −0.09, 95%CI (−0.59, 0.41), p = 0.725].
A study (Hendrickse et al., 1999) on methotrexate showed that, compared with placebo, it significantly reduced pruritus scores [SMD = 1.000, 95%CI (−1.54, −0.46), p = 0.000].
In a comparative study (Vuoristo et al., 1995) between colchicine plus UDCA and UDCA, it was found that colchicine had no significant effect on reducing the incidence of pruritus [RR = 0.964, 95%CI (0.42, 2.24), p = 0.931].
Another study (Hegade et al., 2017) on GSK2330672 used different scoring systems to evaluate the change in pruritus score before and after treatment. The percentage changes from baseline itch scores were -57% [95% CI (−73, −42), p < 0.0001] in NRS, −31% (−42∼-20, p < 0.0001) in PBC-40 itching, and −35% (−45∼−25, p < 0.0001) in 5-D itch score.
One study (Parés et al., 2000) of UDCA could not be analyzed because of the lack of data. Indicators changes before and after treatment for all included studies are listed in Table 2.
Compared with placebo, UDCA, OCA and rifampicin could reduce serum ALP levels, UDCA [SMD = -2.91, 95%CI (−4.37, −1.44), p = 0.000], OCA [SMD = −5.56, 95%CI (−9.82, −1.30), p = 0.011], rifampicin [SMD = −0.53, 95%CI (−1.02, −0.04), p = 0.033]. However, the change brought about by bezafibrate [SMD = −4.98, 95%CI (−12.09, 2.16), p = 0.172] was not statistically significant. But the ALP results showed a high degree of heterogeneity in UDCA (p = 0.000, I2 = 98.7%), OCA (p < 0.01, I2 = 97.5%), and bezafibrate (p < 0.01, I2 = 98.8%) (Figures 4A–C). However, the heterogeneity of the results of rifampicin was low (p = 0.704, I2 = 0.0%) (Figure 4D). We conducted sensitivity analysis (Supplementary Material S1) and subgroup analysis (according to UDCA dose, low: <13 mg/kg/d, medium: 13–15 mg/kg/d, high:>15 mg/kg/d) (Supplementary Material S2), study area (Asia, Europe, America) (Supplementary Material S3), and if cholestyramine was used as a combination (Supplementary Material S4).
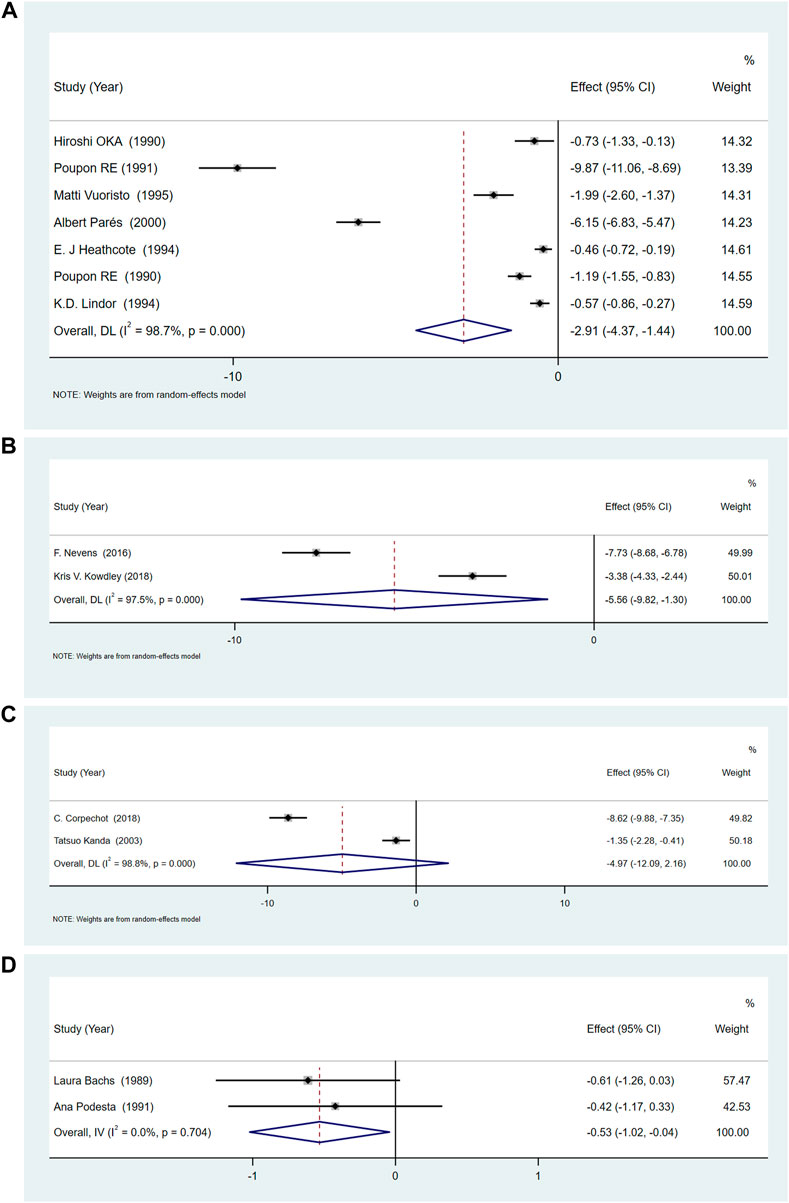
FIGURE 4. (A)The effect of UDCA in serum ALP. (B) The effect of OCA on serum ALP. (C) The effect of Bezafibrate on serum ALP. (D) The effect of Rifampicin on serum ALP.
A study (Wiesner et al., 1990) comparing cyclosporine with placebo found that, cyclosporine was superior to placebo in reducing ALP [SMD = −5.36, 95%CI (−6.98, −3.74), p = 0.000].
One study (Mayo et al., 2018) reported a comparison between NGM282 and placebo. The results suggested that NGM282 could significantly reduce the level of ALP in patients with PBC [SMD = −1.205, 95%CI (−1.98, −0.44), p = 0.002].
One study (Listed, 1993) reported a comparison between malotilate and placebo. The results showed no significant difference in the reduction of ALP between the two [SMD = −0.236, 95%CI (−0.63, 0.16), p = 0.238].
One study (Jones et al., 2017) reported a comparison between seladelpar and placebo, which suggested that seladelpar significantly reduced ALP [SMD = −2.224, 95%CI(−3.24, −1.21], p = 0.000).
One study (Mayo et al., 2019) on comparison between maralixibat and placebo concluded that maralixibat did not significantly reduce serum ALP [SMD = −0.183, 95%CI (−0.77, 0.40), p = 0.540].
A study (Vuoristo et al., 1995) on colchicine plus UDCA against UDCA alone showed that, compared with UDCA, colchicine plus UDCA significantly reduced ALP [SMD = −0.183, 95%CI (−0.77, 0.40), p = 0.540]. The other studies on methotrexate (Listed, 1993), colchicine (Almasio et al., 2000)and GSK2330672 (Hegade et al., 2017) did not report any change of ALP level after drug treatment.
Blood γ-GGT levels were reported in 5 RCTs of UDCA (Poupon et al., 1990; Oka et al., 1991; Poupon et al., 1991; Vuoristo et al., 1995; Parés et al., 2000)and two of bezafibrate (Kanda et al., 2003; Corpechot et al., 2018). The results showed that UDCA and bezafibrate could reduce serum γ-GGT levels (UDCA [SMD = −2.18, 95%CI (−2.43, −1.93), p = 0.000], bezafibrate [SMD = −2.65, 95%CI (−4.34, 0.96), p = 0.002]) (Figures 5A,B). However, there was obvious heterogeneity for UDCA (p < 0.01, I2 = 98.9%) and bezafibrate (p < 0.01, I2 = 88.8%). Therefore, we conducted a subgroup analysis based on the dose of UDCA (Supplementary Material S5), study area (Supplementary Material S6) and whether cholestyramine was combined with UDCA (Supplementary Material S7), to evaluate its effect on serum γ-GGT. Sensitivity analysis showed that the results were consistent (Supplementary Material S8).
A study (Hirschfield et al., 2015) on the effect of OCA in serum γ-GGT showed that OCA reduced serum γ-GGT than placebo [SMD = −2.935, 95%CI (−3.59, −2.28), p = 0.000].
One study (Bachs et al., 1989) reported a comparison between rifampicin and placebo. The results suggested that the former could significantly reduce the level of serum γ-GGT [SMD = −1.123, 95%CI (−1.80, −0.44), p = 0.001].
One study (Mayo et al., 2018) reported a comparison between NGM282 and placebo. The results suggested that NGM282 can reduce the level of γ-GGT [SMD = −0.738, 95%CI (−1.47, −0.01), p = 0.047] in patients with PBC.
A study (Mayo et al., 2019) on the effect of maralixibat on serum γ-GGT, compared with placebo, showed no significant difference between the two drugs [SMD = −0.068, 95% CI (−0.65, 0.52), p = 0.819].
The other two studies of OCA (Nevens et al., 2016; Kowdley et al., 2018), three of UDCA (Battezzati et al., 1993; Lindor et al., 1994; Heathcote et al., 1995), one of rifampicin (Podesta et al., 1991), one of cyclosporine (Wiesner et al., 1990), one of malotilate (Listed, 1993), one of seladelpar (Jones et al., 2017), one of methotrexate (Hendrickse et al., 1999), one of colchicine (Almasio et al., 2000) and one of GSK2330672 (Hegade et al., 2017) did not report any change of serum γ-GGT level after treatment.
Compared with placebo, the incidences of adverse events with UDCA were lower [OR = 0.61, 95%CI (0.42, 0.89), p = 0.011], and there was no significant difference in OCA (OR = 1.03, 95%CI (0.61, 1.75), p = 0.901) and bezafibrate (OR = 0.99, 95%CI (0.56, 1.74), p = 0.967). The results showed that the heterogeneity was low, (for UDCA: p = 0.195 and I2 = 32.0%, for OCA: p = 0.892 and I2 = 0.0%, and for bezafibrate: p = 0.504, I2 = 0.0%) (Figures 6A–C).
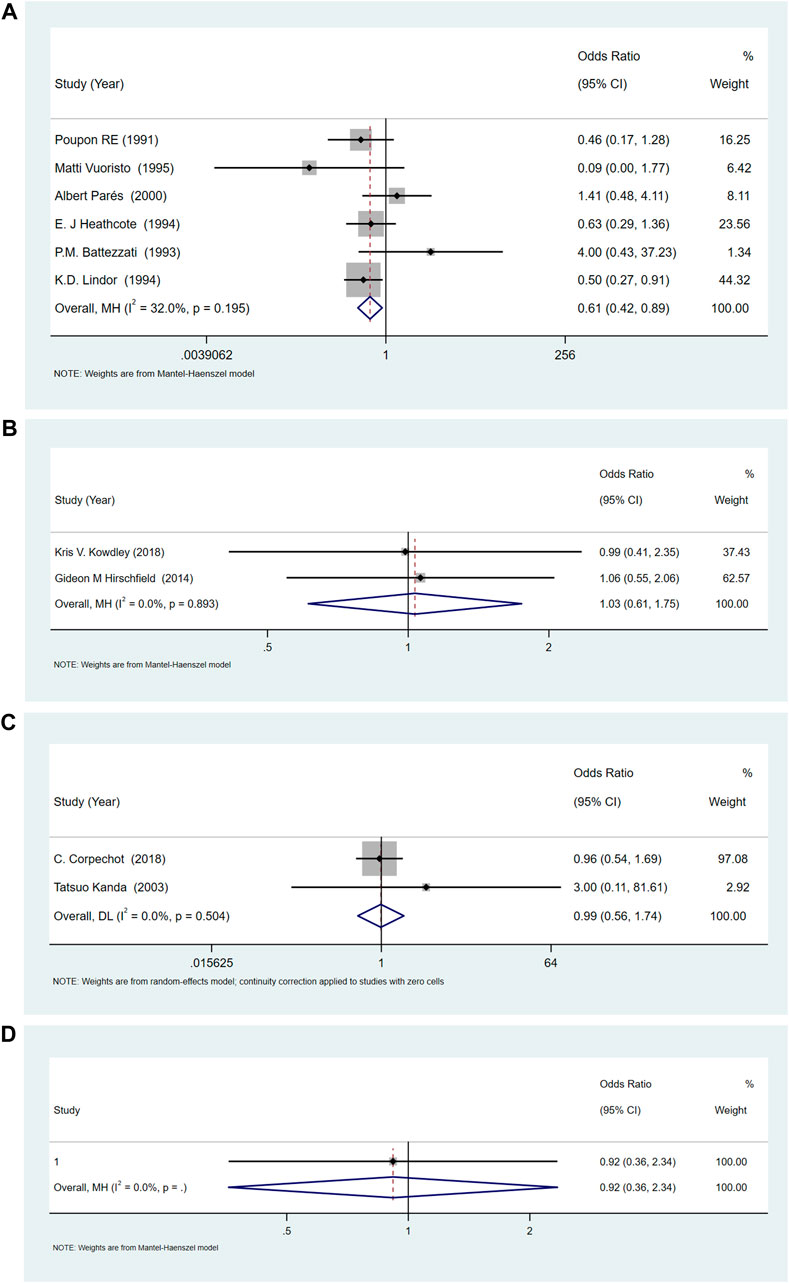
FIGURE 6. (A)The adverse events for UDCA. (B) The adverse events for OCA. (C) The adverse events for Bezafibrate. (D) The adverse events for NGM282.
Sensitivity analysis of UDCA indicated that the results were consistent (Supplementary Material S9). Subgroup analysis based on UDCA dose (Supplementary Material S10), study area (Supplementary Material S11), year of publication (Supplementary Material S12) and whether UDCA was combined with cholestyramine (Supplementary Material S13), showed that the occurrence of adverse events was dose-dependent. Both high (>15 mg/kg/day) and low doses (<13 mg/kg/day) of UDCA increased the incidence of adverse events, while the middle dose (13–15 mg/kg/day) of UDCA did not increase the incidence of adverse events.
A study (Mayo et al., 2018) on the comparison of adverse reactions between NGM282 and placebo showed no significant difference [OR = 0.917, 95%CI (0.36, 2.34), p = 0.856].
One study (Wiesner et al., 1990) reported no significant difference in the incidence of adverse events when cyclosporine and placebo were compared [OR = 1.579, 95%CI (0.44, 5.62), p = 0.481].
A study (Listed, 1993) compared malotilate with placebo, showed that malotilate was superior than placebo in reducing adverse events [OR = 6.125, 95%CI (1.31, 28.52), p = 0.021].
Two separate studies (Jones et al., 2017; Mayo et al., 2019) reported no significant difference in the reduction of adverse events between seladelpar (MBX-8025) and placebo groups [OR = 1.820, 95%CI (0.59, 5.62), p = 0.298], and between Maralixibat and placebo [OR = 1.558, 95%CI (0.59, 4.13), p = 0.372].
Similarly, studies (Hendrickse et al., 1999; Almasio et al., 2000; Hegade et al., 2017) reported no significant differences for the adverse events between methotrexate and placebo [OR = 1.105, 95%CI (0.50, 2.46), p = 0.806], colchicine and placebo [OR = 0.522, 95%CI (0.18, 1.53), p = 0.236], and GSK2330672 and placebo [OR = 1.000, 95%CI (0.41, 2.47), p = 1.000].
Two studies (Bachs et al., 1989; Podesta et al., 1991) reported no significant difference in the reduction of adverse events between rifampicin and placebo [OR = 0.286, 95%CI (0.03, 2.99), p = 0.296].
The rising incidence of pruritus in PBC and the lack of effective treatment methods were serious concerns. One study found that the incidence of pruritus can growing from 19% at the beginning to 80% in 10 years later (Prince et al., 2002). Pruritus in PBC was belong to cholestatic pruritus. Unlike histamine-related pruritus, cholestatic pruritus had no evidence-based guidelines because the underlying pathogenesis was unclear. Some studies had shown that itching symptoms often occured in female patients with PBC, accompanied by increasing levels of ALP and γ-GGT, but the mechanism behind it was still unknown. Existing studies had found that PBC-related pruritus was mainly related to bile acids, lysophosphatidic acid A (LPA), G-protein coupled bile acid receptor1 (GPBAR1), endogenous opioids, 5-hydroxytryptamine (5-HT), nitric oxide and substance P. But since the circulation level of these substances was not well correlated with the severity of pruritus, there may be complex interactions among multiple pruritus substances (Quarneti et al., 2015). Firstly, for the treatment of PBC-related pruritus, we need to rule out renal failure, psoriasis, idiopathic dermatitis and other diseases that can cause pruritus. Secondly, drugs treatment and non-drugs treatment measures should be adopted according to the severity of the disease. The first-line treatment drugs were mainly bile acid-binding resin (Gideon et al., 2017), such as colesevelam hydrochloride, which has better therapeutic effect than cholestyramine. Second-line therapeutic drugs such as rifampicin, opioid antagonists (naltrexone) and modulators of 5-HT receptor pathway (sertraline) can be used as supplements to first-line drugs. In addition, most of the non-drug treatments belong to invasive treatment strategies, and liver transplantation was the last choice for patients with intractable pruritus when all treatment strategies were ineffective. Unfortunately, due to lack of evidence-based evidence, the role of many drug interventions in the treatment of cholestatic pruritus, including PBC was uncertain. This result has also been confirmed in a recent related study (Dervout et al., 2022).
Moreover, drugs for PBC were indispensable in the treatment of pruritus. In recent years, newer target drugs were introduced, which mainly focus on reducing cholestasis and reduced bile acid toxicity, and are immunomodulatory in action and antifibrotic in nature (Shah and Kowdley, 2020). However, the efficacy of these drugs remains to be further evaluated. For example, OCA was a semisynthetic chenodeoxycholic acid analogue, which can inhibit bile acid synthesis and stimulate bile secretion to protect hepatocytes by activating farnesol X receptor. Amazingly, it was found that pruritus increased dose-dependently with OCA treatment (Gong et al., 2008; Trauner et al., 2019), and so it using in patients with PBC associated pruritus was limited. Systematic reviews and meta-analyses on UDCA showed that it improved the levels of serum ALP and γ-GGT, but there were no significant effects on pruritus, fatigue, or reduction in adverse events in PBC (Gong and Gluud, 2005). Similar results were obtained for methotrexate (Guo et al., 2015), fenofibrate (Zhang et al., 2015), and bezafibrate (Gong et al., 2007). Cyclosporin A may significantly improving pruritus, but it had significant side effects compared with placebo (Gerussi et al., 2021). However, we found that these studies did not consider pruritus as a primary outcome measure, and we need an effective and safe pharmacological intervention for pruritus associated with PBC was yet unmet.
Therefore, we conduct this systematic review and meta-analysis to find the effective and safety pharmacological intervention for managing pruritus in PBC.
This meta-analysis of 23 RCTs found that UDCA, methotrexate and GSK2330672 improved pruritus (comparing the pruritus relief rate before and after treatment). OCA may increase the risk of pruritus, hence it was not recommended for patients suffering from PBC pruritus. However, due to the limited number of studies, it was suggested that more RCTs be conducted to understand their role in improving the symptom of itching (Specific data are summarized in Table 2).
Serum ALP and γ-GGT were two important indicators for the diagnosis and prognosis of PBC[57], while these also serve as important markers to diagnose the existence of cholestasis. We analyzed the effects of drug intervention on ALP and γ-GGT. All the included studies reported that UDCA, OCA, rifampicin, cyclosporine, NGM282, seladelpar and colchicine may improve blood ALP. Further, UDCA, bezafibrate, OCA, rifampicin and NGM282 may improve blood γ-GGT. We found that rifampicin can significantly reduce the blood ALP level with low heterogeneity, while UDCA and OCA have high heterogeneity in reducing the level of ALP. Although UDCA and bezafibrate reduce the level of blood γ-GGT, they have high heterogeneity. Rifampicin and NGM282 were equally efficacious in reducing blood ALP and γ- GGT, which was an area worthy of further study.
The existing evidence showed that a medium dose of UDCA (13–15 mg/kg/day) and malotilate may have the benefit of reducing the incidence of adverse events (p < 0.05), and these studies showed low heterogeneity. It was also found that the other drugs that inculded did not significantly raising the incidence of adverse events compared with placebo (p > 0.05).
In this study, we attempted to evaluate the efficacy and safety of drug interventions in the treatment of PBC-associated pruritus. It was found that UDCA, methotrexate, and GSK2330672 may improve pruritus, but due to the existence of literature quality and heterogeneity of the included drugs, we cannot recommend some therapeutic drugs in line with clinical practice. Notably, with the continuous accumulation of high-quality clinical trial evidence of some emerging drugs such as bezafibrate and ileal apical bile acid transporter inhibitors (GSK2330672), perhaps in the near future, PBC-related pruritus will get more high-quality evidence and standardized treatment, and the rate of utilization of liver transplantation will become lower.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Conceptualization: CX and RY. Date extracted: CX and XL. Assessed evidence: XL and CX. Date analyzed: CX and XL. Writing-review and editing: CX and MD. Interpreted the results: SW.
This project is funded by the National Natural Science Foundation of China (No. 81774279). The sponsors are not involved in the design, execution, or writing the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.835991/full#supplementary-material
Almasio, P., Floreani, A., Chiaramonte, M., Provenzano, G., Battezzati, P., CrosignAni, A., et al. (2000). Multicentre randomized placebo-controlled trial of ursodeoxycholic acid with or without colchicine in symptomatic primary biliary cirrhosis. Aliment. Pharmacol. Ther. 14 (12), 1645–1652. doi:10.1046/j.1365-2036.2000.00869.x
Bachs, L., Parés, A., Elena, M., Pares, A., and Rodes, J. (1989). Comparison of rifampicin with phenobarbitone for treatment of pruritus in biliary cirrhosis. Lancet 1 (8638), 574–576. doi:10.1016/s0140-6736(89)91608-5
Battezzati, P. M., Podda, M., Bianchi, F. B., NaccaRato, R., OrlandiF., , Surrenti, C., et al. (1993). Ursodeoxycholic acid for symptomatic primary biliary cirrhosis. Preliminary analysis of a double-blind multicenter trial. Italian Multicenter Group for the Study of UDCA in PBC. J. Hepatol. 17 (3), 332–338. doi:10.1016/S0168-8278(05)80214-4
Beuers, U., Kremer, A., Bolier, R., and Elferink, R. P. J. O. (2014). Pruritus in cholestasis: Facts and fiction. Hepatology 60 (1), 399–407. doi:10.1002/hep.26909
Bray, G., Heisel, W., Afshin, A., Jensen, M. D., Dietz, W. H., Long, M., et al. (2018). The science of obesity management: An endocrine society scientific statement. Endocr. Rev. 39 (2), 79–132. doi:10.1210/er.2017-00253
Carey, E. J., Ali, A. H., and Lindor, K. D. (2015). Primary biliary cirrhosis. Lancet 386 (10003), 1565–1575. doi:10.1016/s0140-6736(15)00154-3
Cn, G., and Sg, C. (1988). Treatment of pruritus in primary biliary cirrhosis with rifampin. Results of a double-blind, crossover, randomized trial. Gastroenterology 94 (2), 488–493. doi:10.1016/0016-5085(88)90442-8
Corpechot, C., Chazouillères, O., Rousseau, A., Le Gruyer, A., Habersetzer, F., Mathurin, P., et al. (2018). A placebo-controlled trial of bezafibrate in primary biliary cholangitis. N. Engl. J. Med. 378 (23), 2171–2181. doi:10.1056/NEJMoa1714519
Dervout, C., Boulais, N., Barnetche, T., Nousbaum, J. B., Brenaut, E., and Misery, L. (2022). Efficacy of treatments for cholestatic pruritus: A systemic review and meta-analysis. Acta Derm. Venereol. 102, adv00653. doi:10.2340/actadv.v102.310
Dg, A., J, T., Tetzlaff, J., Mulrow, C., Gotzsche, P. C., Ioannidis, J. P. A., et al. (2009). The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ Clin. Res. ed.) 339, b2700. doi:10.1136/bmj.b2700
Drazilova, S., Babinska, I., Gazda, J., Halanova, M., Janicko, M., Kucinsky, B., et al. (2020). Epidemiology and clinical course of primary biliary cholangitis in Eastern Slovakia. Int. J. Public Health 65 (5), 683–691. doi:10.1007/s00038-020-01391-6
Gerussi, A., Bernasconi, D., O'Donnell, S., Lammers, W. J., Van Buuren, H., Hirschfield, G., et al. (2021). Measurement of gamma glutamyl transferase to determine risk of liver transplantation or death in patients with primary biliary cholangitis. Clin. Gastroenterol. Hepatol. 19 (8), 1688–1697. e1614. doi:10.1016/j.cgh.2020.08.006
Gideon, H., Ulrich, B., Christophe, C., Pietro, I., David, J., Marco, M., et al. (2017). EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 67 (1), 145–172. doi:10.1016/j.jhep.2017.03.022
Gong, Y., Christensen, E., and Gluud, C. (2007). Cyclosporin A for primary biliary cirrhosis. Cochrane Database Syst. Rev. 3, CD005526. doi:10.1002/14651858.CD005526.pub2
Gong, Y., and Gluud, C. (2005). Methotrexate for primary biliary cirrhosis. Cochrane Database Syst. Rev. 3 (3), CD004385. doi:10.1002/14651858.CD004385.pub2
Gong, Y., Zhi, B. H., Christensen, E., and Gluud, C. (2008). Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst. Rev. 3 (3), CD000551. doi:10.1002/14651858.CD000551.pub2
Gulamhusein, A. F., and Hirschfield, G. M. (2020). Primary biliary cholangitis: Pathogenesis and therapeutic opportunities. Nat. Rev. Gastroenterol. Hepatol. 17 (2), 93–110. doi:10.1038/s41575-019-0226-7
Guo, C., Zhang, Y., Lei, H., Wang, F., Chen, K., Li, J., et al. (2015). Combination therapy of fenofibrate and ursodeoxycholic acid in patients with primary biliary cirrhosis who respond incompletely to UDCA monotherapy: A meta-analysis. Drug Des. devel. Ther. 9, 2757–2766. doi:10.2147/DDDT.S79837
Heathcote, E. J., Cauch-Duder, K., Walker, V., Bailey, R. J., Blendis, L. M., Ghent, C. M., et al. (1995). The Canadian multi-center double-blind randomized controlled trial of ursodeoxycholic acid in primary biliary cirrhosis. Hepatology 19, 1149–1156. doi:10.1016/0270-9139(95)90074-8
Hegade, V. S., Kendrick, S., Dobbins, R. L., Miller, S. R., Thompson, D., Richards, D., et al. (2017). Effect of ileal bile acid transporter inhibitor GSK2330672 on pruritus in primary biliary cholangitis: A double-blind, randomised, placebo-controlled, crossover, phase 2a study. Lancet 389 (10074), 1114–1123. doi:10.1016/S0140-6736(17)30319-7
Hegade, V. S., Mells, G. F., Fisher, H., Kendrick, S., DiBello, J., Gilchrist, K., et al. (2019). Pruritus is common and undertreated in patients with primary biliary cholangitis in the United Kingdom. Clin. Gastroenterol. Hepatol. 17 (7), 1379–1387. e1373. doi:10.1016/j.cgh.2018.12.007
Hendrickse, M. T., Rigney, E., Giaffer, M. H., Soomro, I., Triger, D. R., Underwood, J. C., et al. (1999). Low-dose methotrexate is ineffective in primary biliary cirrhosis: Long-term results of a placebo-controlled trial. Gastroenterology 117 (2), 400–407. doi:10.1053/gast.1999.0029900400
Higgins, J. P., and Green, S. (2008). Cochrane handbook for systematic reviews of interventions. Wiley-Blackwell. doi:10.1002/9780470712184.ch1
Hirschfield, G. M., Mason, A., Luketic, V., Lindor, K., Gordon, S. C., Mayo, M., et al. (2015). Efficacy of obeticholic acid in patients with primary biliary cirrhosis and inadequate response to ursodeoxycholic acid. Gastroenterology 148 (4), 751–761. doi:10.1053/j.gastro.2014.12.005
Hu, M., I, H., S, L., Soderdahl, F., Thuresson, M., Wahlin, S., et al. (2019). Incidence, prevalence, and outcome of primary biliary cholangitis in a nationwide Swedish population-based cohort. Sci. Rep. 9 (1), 11525. doi:10.1038/s41598-019-47890-2
J, F., I, v. d. M., S, S., Ng, J., Angus, P., Lubel, J., et al. (2020). Increasing prevalence of primary biliary cholangitis in Victoria, Australia. J. Gastroenterol. Hepatol. 35 (4), 673–679. doi:10.1111/jgh.14924
Jones, D., Boudes, P. F., Swain, M. G., Bowlus, C. L., Galambos, M. R., Bacon, B. R., et al. (2017). Seladelpar (MBX-8025), a selective PPAR-δ agonist, in patients with primary biliary cholangitis with an inadequate response to ursodeoxycholic acid: A double-blind, randomised, placebo-controlled, phase 2, proof-of-concept study. Lancet. Gastroenterol. Hepatol. 2 (10), 716–726. doi:10.1016/S2468-1253(17)30246-7
Js, R., Mn, K., Bjelakovic, G., and Gluud, C. (2012). Ursodeoxycholic acid for primary biliary cirrhosis. Cochrane Database Syst. Rev. 12, CD000551. doi:10.1002/14651858.CD000551.pub3
Jw, O. D., Sp, P., Ac, A., Haigh, C. G., Wilkinson, J. R., and Williams, R. (2005). A controlled trial of ondansetron in the pruritus of cholestasis. Aliment. Pharmacol. Ther. 21 (8), 1041–1045. doi:10.1111/j.1365-2036.2005.02430.x
Kanda, T., Yokosuka, O., Imazeki, F., and Saisho, H. (2003). Bezafibrate treatment: A new medical approach for PBC patients? J. Gastroenterol. 38 (6), 573–578. doi:10.1007/s00535-002-1102-7
Kd, L., Cl, B., J, B., Levy, C., and Mayo, M. (2019). Primary biliary cholangitis: 2018 practice guidance from the American association for the study of liver diseases. Hepatol. Baltim. Md.) 69 (1), 394–419. doi:10.1002/hep.30145
Kowdley, K., Luketic, V., Chapman, R., Hirschfield, G. M., Poupon, R., Schramm, C., et al. (2018). A randomized trial of obeticholic acid monotherapy in patients with primary biliary cholangitis. Hepatology 67 (5), 1890–1902. doi:10.1002/hep.29569
Lee, J. Y., Danford, C. J., Trivedi, H. D., Tapper, E. B., Patwardhan, V. R., and Bonder, A. (2019). Treatment of fatigue in primary biliary cholangitis: A systematic review and meta-analysis. Dig. Dis. Sci. 64 (8), 2338–2350. doi:10.1007/s10620-019-5457-5
Lindor, K. D., Dickson, E. R., Baldus, W. P., Jorgensen, R. A., Ludwig, J., Murtaugh, P. A., et al. (1994). Ursodeoxycholic acid in the treatment of primary biliary cirrhosis. Gastroenterology 106 (5), 1284–1290. doi:10.1016/0016-5085(94)90021-3
Listed, N. A. (1993). The results of a randomized double blind controlled trial evaluating malotilate in primary biliary cirrhosis. A European multicentre study group. J. Hepatol. 17 (2), 227–235. doi:10.1016/s0168-8278(05)80043-1
Mayo, M. J., Pockros, P. J., Jones, D., Bowlus, C. L., Levy, C., Patanwala, I., et al. (2019). A randomized, controlled, phase 2 study of maralixibat in the treatment of itching associated with primary biliary cholangitis. Hepatol. Commun. 3 (3), 365–381. doi:10.1002/hep4.1305
Mayo, M. J., Wigg, A. J., Leggett, B. A., Arnold, H., Thompson, A. J., Weltman, M., et al. (2018). NGM282 for treatment of patients with primary biliary cholangitis: A multicenter, randomized, double-blind, placebo-controlled trial. Hepatol. Commun. 2 (9), 1037–1050. doi:10.1002/hep4.1209
Mj, M., I, H., S, S., Jacobe, H., Getachew, Y., and Rush, A. J. (2007). Sertraline as a first-line treatment for cholestatic pruritus. Hepatology 45 (3), 666–674. doi:10.1002/hep.21553
Nevens, F., Andreone, P., Mazzella, G., Strasser, S. I., Bowlus, C., Invernizzi, P., et al. (2016). A placebo-controlled trial of obeticholic acid in primary biliary cholangitis. N. Engl. J. Med. 375 (7), 631–643. doi:10.1056/NEJMoa1509840
Oka, H., Toda, G., Ikeda, Y., HashimotoN., , Hasumura, Y., Kamimura, T., et al. (1991). A multi-center double-blind controlled trial of ursodeoxycholic acid for primary biliary cirrhosis. Gastroenterol. Jpn. 25 (6), 774–780. doi:10.1007/BF02779195
Parés, A., Caballería, L., Rodés, J., Bruguera, M., Rodrigo, L., García-Plaza, A., et al. (2000). Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: Results of a double-blind controlled multicentric trial. UDCA-cooperative group from the Spanish association for the study of the liver. J. Hepatol. 32 (4), 561–566. doi:10.1016/s0168-8278(00)80216-0
Podesta, A., Lopez, P., Terg, R., Villamil, F., Flores, D., Mastai, R., et al. (1991). Treatment of pruritus of primary biliary cirrhosis with rifampin. Dig. Dis. Sci. 36 (2), 216–220. doi:10.1007/BF01300759
Poupon, R. E., Balkau, B., Eschwège, E., and Poupon, R. (1991). A multicenter, controlled trial of ursodiol for the treatment of primary biliary cirrhosis. UDCA-PBC Study Group. N. Engl. J. Med. 324 (22), 1548–1554. doi:10.1056/NEJM199105303242204
Poupon, R. E., Eschwège, E., and Poupon, R. (1990). Ursodeoxycholic acid for the treatment of primary biliary cirrhosis: Interim analysis of a double-blind multicentre randomized trial. J. Hepatol. 11 (1), 16–21. doi:10.1016/0168-8278(90)90265-s
Prince, M., Chetwynd, A., Newman, W., Metcalf, J. V., and James, O. F. W. (2002). Survival and symptom progression in a geographically based cohort of patients with primary biliary cirrhosis: Follow-up for up to 28 years. Gastroenterology 123 (4), 1044–1051. doi:10.1053/gast.2002.36027
Quarneti, C., Muratori, P., Lalanne, C., Fabbri, A., Menichella, R., Granito, A., et al. (2015). Fatigue and pruritus at onset identify a more aggressive subset of primary biliary cirrhosis. Liver Int. 35 (2), 636–641. doi:10.1111/liv.12560
Rajaobelina, K., Dow, C., Romana Mancini, F., Dartois, L., Boutron-Ruault, M. C., Balkau, B., et al. (2019). Population attributable fractions of the main type 2 diabetes mellitus risk factors in women: Findings from the French E3N cohort. J. Diabetes 11 (3), 242–253. doi:10.1111/1753-0407.12839
Shah, R. A., and Kowdley, K. V. (2020). Current and potential treatments for primary biliary cholangitis. Lancet. Gastroenterol. Hepatol. 5 (3), 306–315. doi:10.1016/S2468-1253(19)30343-7
Sp, P., C, V., B, H., Meixiong, J., Dong, X., and Kwatra, S. G. (2019). Cholestatic pruritus: Emerging mechanisms and therapeutics. J. Am. Acad. Dermatol. 81 (6), 1371–1378. doi:10.1016/j.jaad.2019.04.035
Tajiri, K., and Shimizu, Y. (2017). Recent advances in the management of pruritus in chronic liver diseases. World J. Gastroenterol. 23 (19), 3418–3426. doi:10.3748/wjg.v23.i19.3418
Tb, V. I., Sa, H., Rs, C., and Tennent, D. M. (1961). The treatment of pruritus and hypercholesteremia of primary biliary cirrhosis with cholestyramine. N. Engl. J. Med. 265, 469–474. doi:10.1056/nejm196109072651004
Trauner, M., Nevens, F., Shiffman, M., Drenth, J. P. H., Bowlus, C. L., Vargas, V., et al. (2019). Long-term efficacy and safety of obeticholic acid for patients with primary biliary cholangitis: 3-year results of an international open-label extension study. Lancet. Gastroenterol. Hepatol. 4 (6), 445–453. doi:10.1016/s2468-1253(19)30094-9
Vuoristo, M., Farkkil, M., Karvonen, A. L., Leino, R., Lehtola, J., Makinen, J., et al. (1995). A placebo-controlled trial of primary biliary cirrhosis treatment with colchicine and ursodeoxycholic acid. Gastroenterology 108 (5), 1470–1478. doi:10.1016/0016-5085(95)90696-7
Wiesner, R. H., Ludwig, J., Lindor, K. D., Jorgensen, R. A., Baldus, W. P., Homburger, H. A., et al. (1990). A controlled trial of cyclosporine in the treatment of primary biliary cirrhosis. N. Engl. J. Med. 322 (20), 1419–1424. doi:10.1056/NEJM199005173222003
Zeng, N., Duan, W., Chen, S., Wu, S., Ma, H., Ou, X., et al. (2019). Epidemiology and clinical course of primary biliary cholangitis in the asia–pacific region: A systematic review and meta-analysis. Hepatol. Int. 13 (1333–1341), 788–799. doi:10.1007/s12072-019-09984-x
Keywords: primary biliary cholangitis, pruritus, pharmacological interventions, alkaline phosphatase, γ-glutamyl transpeptidase
Citation: Xu C, Yue R, Lv X, Wang S and Du M (2022) Efficacy and safety of pharmacological interventions for pruritus in primary biliary cholangitis: A systematic review and meta-analysis. Front. Pharmacol. 13:835991. doi: 10.3389/fphar.2022.835991
Received: 15 December 2021; Accepted: 27 September 2022;
Published: 20 October 2022.
Edited by:
Kevin Lu, University of South Carolina, United StatesReviewed by:
Laurent Misery, Université de Bretagne Occidentale, FranceCopyright © 2022 Xu, Yue, Lv, Wang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rensong Yue, eXVlcmVuc29uZzE2M0Bob3RtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.