- 1Department of Oncology, No. 960 Hospital of PLA, Jinan, China
- 2Key Laboratory for Experimental Teratology of the Ministry of Education and Center for Experimental Nuclear Medicine, School of Basic Medical Sciences, Cheeloo College of Medicine, Shandong University, Jinan, China
- 3Department of Nuclear Medicine, No. 960 Hospital of PLA, Jinan, China
Fifteen dihydroartemisinin-isatin hybrids (5a-e and 6a-j) linked with three-carbon were designed, synthesized. The antiproliferative activity against lung cancer cell lines including drug-sensitive A549, doxorubicin-resistant A549 (A549/DOX) and cisplatin-resistant A549 (A549/DDP) lung cancer cell lines was tested. The cytotocivity towards normal lung epithelial BEAS-2B cell line was also investigated. From the structure-activity relationship (SAR), it was found that hydrogen bond donors (especially hydroxime and thiosemicarbazide) at C-3 position and electron-withdrawing groups (fluoro and chloro) at C-5 position of isatin moiety were beneficial for the activity. A significant part of them (half maximal inhibitory concentration/IC50: 5.72–55.52 μM) demonstrated considerable antiproliferative activity, and the activity was superior to that of dihydroartemisinin (IC50: 69.42–88.03 μM) and artemisinin (IC50: >100 μM). In particular, two hybrids 6a, e (IC50: 5.72–9.84 μM) were not inferior to doxorubicin (IC50: 4.06 μM) and cisplatin (IC50: 9.38 μM) against drug-sensitive A549 cells and were more potent than doxorubicin (IC50: 54.32 and 15.10 μM) and cisplatin (IC50: 19.74 and 66.89 μM) against multidrug-resistant A549/DOX and A549/DDP lung cancer cell lines. In addition, hybrids 6a, e (IC50: >100 μM) showed no toxicity towards BEAS-2B cells, proving their excellent selectivity profile. Furthermore, hybrid 6a also possessed good stability in mouse and human microsomes, as well as excellent pharmacokinetic properties. Accordingly, hybrid 6a could serve as a promising anti-lung cancer chemotherapeutic candidate for further preclinical evaluations.
Introduction
Lung cancer, caused by several environmental and genetic variables (Zhang et al., 2021; Yang et al., 2022), is the leading cause of cancer related deaths and is responsible for around 20% of all cancer deaths with an estimated 1.8 million new cases and 1.6 million deaths annually (Hirsch et al., 2017; Kramer and Annema, 2021). According to the histopathological characteristics, lung cancer is mainly divided into non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC), and NSCLC accounts for about 80–85% of lung cancers (Sławiński et al., 2020; Xie et al., 2021). Despite the advances in cancer diagnosis and therapy, the advent of chemotherapeutic resistance in lung cancer and serious side effects are the major obstacles for the effective treatment (Bade and Dela Cruz, 2020; Coakley and Popat, 2020). Therefore, it is imperative to develop novel anti-lung cancer agents.
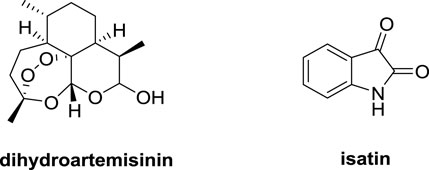
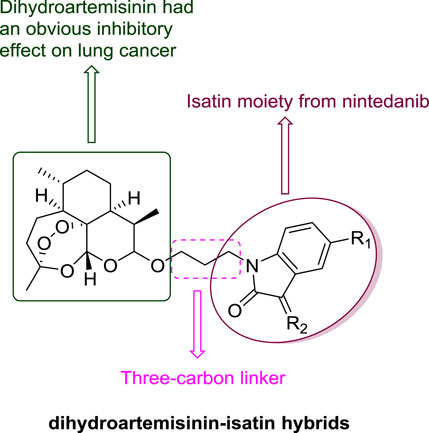
Dihydroartemisinin (DHA, Figure 1) has a unique sesquiterpene endoperoxide lactone moiety and could form highly reactive free radicals in the presence of ferrous ion (FeII). The concentration of FeII in cancer cells is 1,000 times higher than that in normal cells (Dai et al., 2017; Kiani et al., 2020). DHA could interact with FeII, resulting in an obvious inhibitory effect on lung cancer cells including lung squamous carcinoma, lung adenocarcinoma and large cells lung carcinoma without significant cytotoxicity to normal cells (Gao et al., 2020; Li et al., 2021). Isatin derivatives displayed promising anti-lung cancer activity, and the isatin-based nintedanib plus docetaxel has already been approved for treatment of patients with advanced NSCLC after first-line chemotherapy in Europe (Ding et al., 2020; Hou et al., 2020). Hence, DHA and isatin may provide useful templates for the development of novel anti-lung cancer agents.
Hybrids synthesized by fusing or conjugating different active pharmacophores together are capable to modulate multiple targets, consequently enhancing the efficacy, overcoming drug resistance and reducing side effects (Nepali et al., 2014; Sharma et al., 2014; Mishra and Singh, 2016; Choudhary et al., 2018; Hou et al., 2021). Since both DHA and isatin hold potent anti-lung cancer activity, there is a high possibility that hybridization of DHA with isatin may provide novel anti-lung cancer candidates with high activity and efficacy.
Compared with other linkers like thiazole linker, alkyl linkers are more flexible, probably making the hybrids with alkyl linkers much easier to bind with different targets. To evaluate the influence of the length of the alkyl linker between dihydroartemisinin and isatin, various of dihydroartemisinin-isatin hybrids with two-four-carbon linkers were designed and synthesized by our group. In this paper, we only designed and synthezed a series of dihydroartemisinin-isatin hybrids with three-carbon linker (Figure 2). Afterwards, the antiproliferative activity against drug-sensitive (A549), and drug-resistant [doxorubicin-resistant A549 (A549/DOX) and cisplatin-resistant A549 (A549/DDP)] lung cancer cell lines was evaluated. The cytotocivity towards normal lung epithelial cell line (BEAS-2B) was also investigated. Moreover, the liver stability and pharmacokinetic properties of the representative hybrids were also tested to search for the potential anti-lung cancer candidates for further studies. The biological activity and the pharmacokinetic properties of the hybrids with two carbon or four carbon linker are still under investigation, and the anticancer characteristics of the hybrids with different length of linkers will be reported in the near future.
Results and Discussion
Preparetion of the Desired Hybrids
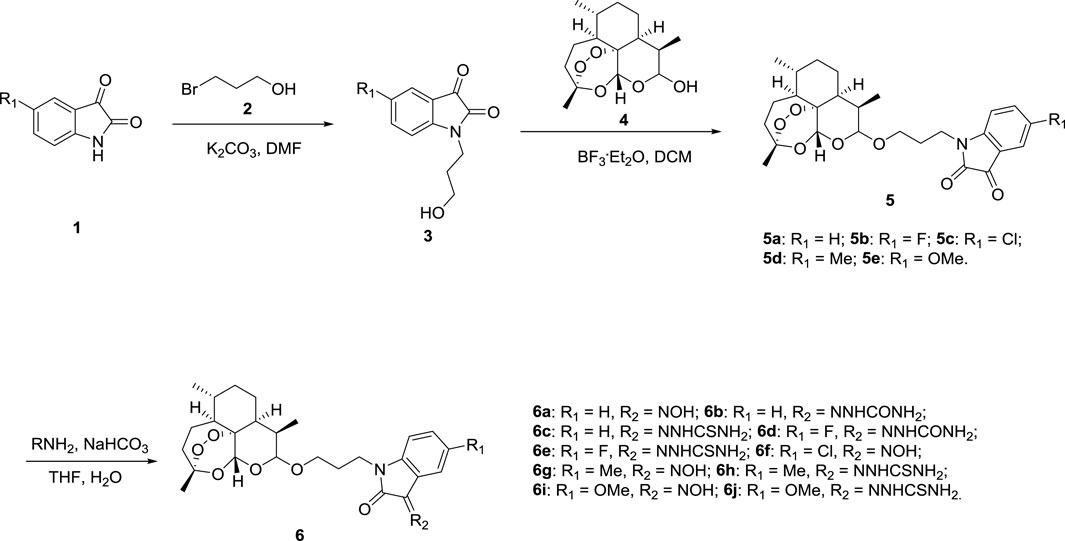
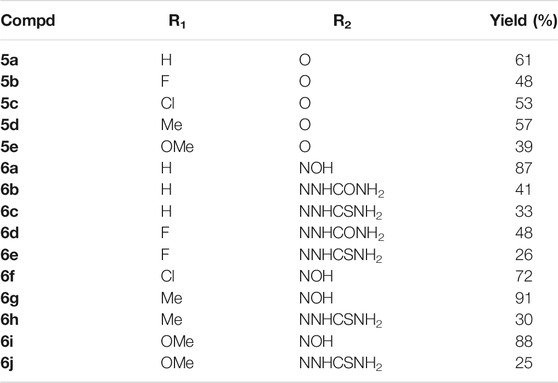
Scheme 1 describes the synthetic route of desired dihydroartemisinin-isatin hybrids 5a-e and 6a-j. (5-substituted) isatins 1 alkylating with 3-bromopropanol 2, using potassium carbonate (K2CO3) as base yielded intermediates 3. Intermediates 3 then reacted with dihydroartemisinin 4 in presence of boron trifluoride diethyl etherate (BF3. OEt2), giving the desired dihydroartemisinin-isatin hybrids 5a-e. Finally, the reation of hybrids 5a-e with hydroxylamine/semicarbazide/thiosemicarbazide hydrochlorides using sodium bicarbonate (NaHCO3) as base provided the desired dihydroartemisinin-isatin hybrids 6a-j. The chemical structures and yields of the desired hybrids were listed in Table 1. The structure of the desired dihydroartemisinin-isatin hybrids 5a-e and 6a-j were determined by HRMS, 1H NMR and 13C NMR. All target compounds should be a mixture of α- and β-configuration in dihydroartemisinin skeleton, and for hybrids 6a-j, E- and Z-configuration should be existed at C-3 position of isatin moiety. Taking hybrid 6a for an example, the 1H NMR (600 Hz, DMSO-d6) was as follows: δ 0.86–0.99 (m, 7H, H-15, H-16 and H-5a), 1.26–1.57 (m, 7H, H-5a2, H-7a2, H-6, H-14 and H-8a), 1.64–1.90 (m, 3H, H-7a1 and H-8), 1.95–2.06 (m, 3H, -CH2- linker and H-5a1), 2.25–2.66 (m, 3H, H-9, H-4a1 and H-4a2), 3.45–4.12 (m, 4H, -OCH2- linker and -NCH2- linker), 4.95 (d, J = 2.0 Hz, 1H, H-10), 5.47 (s, 1H, H-12), 6.88 (d, J = 4.0 Hz, 1H, isatin-H), 7.09 (t, J = 4.0 Hz, 1H, isatin-H), 7.36 (t, J = 4.0 Hz, 1H, isatin-H), 8.12 (d, J = 4.0 Hz, 1H), 11.04 (brs, 1H, NOH).
Antiproliferative Activity and Cytotoxicity Study in vitro
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to determined the antiproliferative activity of dihydroartemisinin-isatin hybrids 5a-e and 6a-j with three-carbon linker against A549, multidrug-resistant A549/DOX and A549/DDP lung cancer cell lines as well as cytotoxicity towards normal lung epithelial cell line (BEAS-2B). The inhibitory effects were expressed in terms of half maximal inhibitory concentration (IC50) values. It can be seen from Table 2 that nine desired dihydroartemisinin-isatin hybrids (5c, 6a, 6c, 6e-j, IC50: 5.72–99.57 µM) showed decent antiproliferative activity against A549, A549/DOX and A549/DDP lung cancer cell lines. These nine hybrids were much more active than artemisinin (IC50: >100 µM), and seven of them (6a, 6c, 6e-g, 6i-j) disaplayed superior inhibition to the parent drugs DHA (IC50: 69.42–88.03 µM). The SAR revealed that introducing hydroxime and thiosemicarbazide into C-3 position of isatin moiety were beneficial for the activity, while carbonyl and semicarbazide had negative influence on the activity. Electron-withdrawing groups such as fluoro and chloro at C-5 position of isatin moiety could improve the activity to some extent, while electron-donating groups such as methyl and methoxy led to a significant loss of activity.
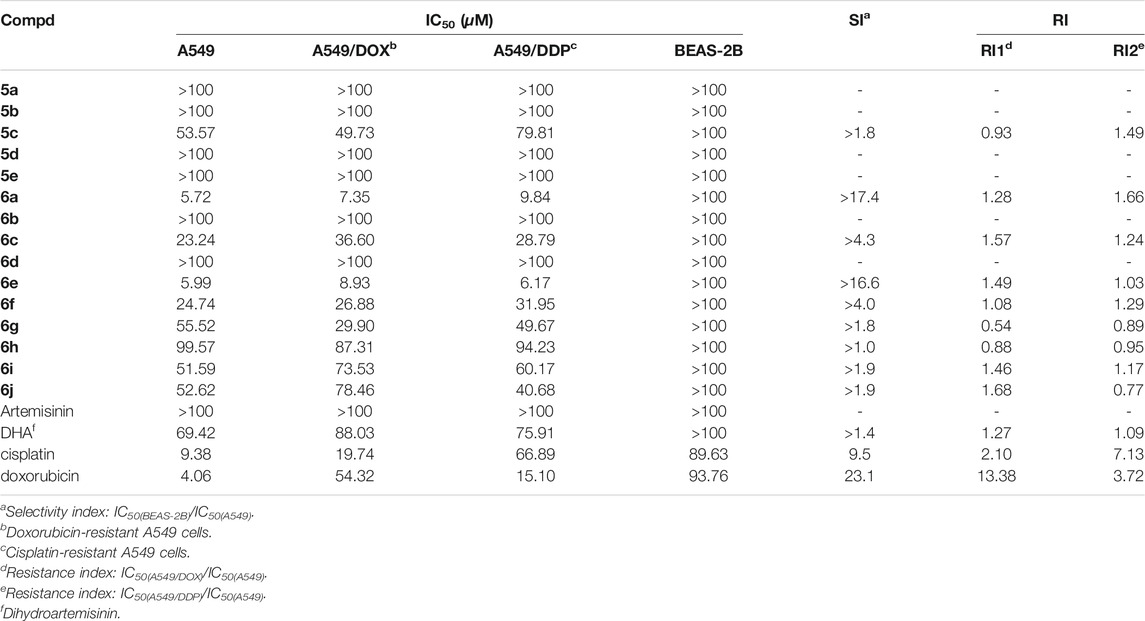
TABLE 2. In vitro antiproliferative activities, cytotoxicity of dihydroartemisinin-isatin hybrids 5a-e and 6a-j.
The cytotoxic results showed that all the desired hybrids (IC50: >100 μM) were non-cytotoxic towards normal lung epithelial cell line (BEAS-2B), and nine of them (5c, 6a, 6c, 6e-j) had the selectivity index (SI: IC50(BEAS-2B)/IC50(A549)) values > 1.0. In addition, the desired hybrids possessed the activity in the same level against both drug-sensitive and multidrug-resistant A549 lung cancer cell lines, and the drug resistance index (RI: IC50(MDR A549)/IC50(A549)) values were 0.54–1.68, demonstrating their potential to overcome drug resistance.
In particular, hybrids 6a, e (IC50: 5.72–9.84 μM) showed pronounced activity against A549, A549/DOX and A549/DDP lung cancer cell lines. The activity of hybrids 6a, e was not inferior to that of cisplatin (IC50: 9.38 μM) and doxorubicin (IC50: 4.06 μM) against A549 cells. These two hybrids were 1.5–10.8 times superior to cisplatin (IC50: 19.74 and 66.89 μM) and doxorubicin (IC50: 54.32 and 15.10 μM) against multidrug-resistant A549/DOX and A549/DDP lung cancer cell lines. Moreover, the SI index values of hybrids 6a, e were >16.6, revealing that both of them possessed high specifity.
The metabolic stability of hybrids 6a, e determined in mouse and human microsomes (Table 3) illustrated that both hybrids 6a, e (liver microsomes: 51-70%) exhibited good microsomal stability, and hybrid 6a (liver microsomes: 70 and 62%) was more stable than 6e (liver microsomes: 54 and 51%) in both mouse and human microsomes.
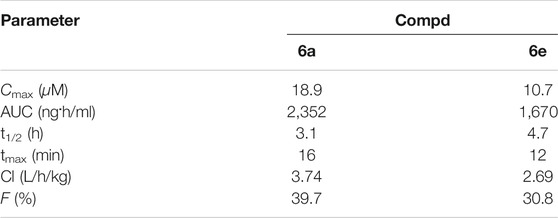
CD-1 mice model was administered with single intravenous 4) dose of 30 mg/kg to test the pharmacokinetic properties of hybrid 6a, e. Hybrids 6a, e showed the maximum plasma concentrations (Cmax) of 18.9 and 10.7 μM, area under curve (AUC) of 2,352 and 1,670 ng.h/ml, half-lives (t1/2) of 3.1 and 4.7 h, peak time of 16 and 12 min, clearance rates (Cl) of 3.74 and 2.69 L/h/kg, and bioavailability of 39.7 and 30.8% (Table 4).
Conclusion
In this study, we designed and synthesized fifteen novel dihydroartemisinin-isatin hybrids 5a-e and 6a-j with three-carbon linker. Their antiproliferative activity against both drug-sensitive and multidrug-resistant lung cancer cell lines was also evaluated. Among them, hybrids 6a, e (IC50: 5.72–9.84 μM) were highly potent against the three tested lung cancer cell lines, and the activity was comparable or superior to that of cisplatin (IC50: 9.38–66.89 μM) and doxorubicin (IC50: 4.06–54.32 μM). These two hybrids (IC50: >100 μM) also demonstrated non-cytotoxicity towards normal lung epithelial cell line (BEAS-2B), and the SI index values of these two hybrids were >16.6, demonstrating their excellent safety profile. The RI values of hybrids 6a, e were 1.03–1.66, indicating that these two hybrids could overcome drug resistance. In addition, hybrid 6a also exhibited good stability in mouse and human microsomes and excellent pharmacokinetic properties. From these results, hybrid 6a could be considered as a promising anti-lung cancer chemotherapeutic candidate for further evaluations.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the Animal Care and Use Committee of Shandong University with the corresponding ethical approval code (LL-201602040, 2016-2022).
Author Contributions
FG designed the experiment; MD and GZ performed the experiment and wrote the draft. FG, ML and CZ corrected the manuscript. All authors approved the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.834317/full#supplementary-material
References
Bade, B. C., and Dela Cruz, C. S. (2020). Lung Cancer 2020. Clin. Chest Med. 41 (1), 1–24. doi:10.1016/j.ccm.2019.10.001
Choudhary, S., Singh, P. K., Verma, H., Singh, H., and Silakari, O. (2018). Success Stories of Natural Product-Based Hybrid Molecules for Multi-Factorial Diseases. Eur. J. Med. Chem. 151, 62–97. doi:10.1016/j.ejmech.2018.03.057
Coakley, M., and Popat, S. (2020). Management of Lung Cancer. Medicine 48 (4), 273–278. doi:10.1016/j.mpmed.2020.01.003
Dai, Y.-F., Zhou, W.-W., Meng, J., Du, X.-L., Sui, Y.-P., Dai, L., et al. (2017). The Pharmacological Activities and Mechanisms of Artemisinin and its Derivatives: A Systematic Review. Med. Chem. Res. 26, 867–880. doi:10.1007/s00044-016-1778-5
Ding, Z., Zhou, M., and Zeng, C. (2020). Recent Advances in Isatin Hybrids as Potential Anticancer Agents. Arch. Pharm. (Weinheim) 353 (3), e1900367. doi:10.1002/ardp.201900367
Gao, F., Sun, Z., Kong, F., and Xiao, J. (2020). Artemisinin-derived Hybrids and Their Anticancer Activity. Eur. J. Med. Chem. 188, 112044. doi:10.1016/j.ejmech.2020.112044
Hirsch, F. R., Scagliotti, G. V., Mulshine, J. L., Kwon, R., Curran, W. J., Wu, Y. L., et al. (2017). Lung Cancer: Current Therapies and New Targeted Treatments. Lancet 389 (10066), 299–311. doi:10.1016/S0140-6736(16)30958-8
Hou, H., Qu, B., Su, C., Hou, G., and Gao, F. (2021). Design, Synthesis and Anti-lung Cancer Evaluation of 1, 2, 3-Triazole Tethered Dihydroartemisinin-Isatin Hybrids. Front. Pharmacol. 12, 801580. doi:10.3389/fphar.2021.801580
Hou, Y., Shang, C., Wang, H., and Yun, J. (2020). Isatin-azole Hybrids and Their Anticancer Activities. Arch. Pharm. (Weinheim) 353 (1), e1900272. doi:10.1002/ardp.201900272
Kiani, B. H., Kayani, W. K., Khayam, A. U., Dilshad, E., Ismail, H., and Mirza, B. (2020). Artemisinin and its Derivatives: a Promising Cancer Therapy. Mol. Biol. Rep. 47 (8), 6321–6336. doi:10.1007/s11033-020-05669-z
Kramer, T., and Annema, J. T. (2021). Advanced Bronchoscopic Techniques for the Diagnosis and Treatment of Peripheral Lung Cancer. Lung Cancer 161, 152–162. doi:10.1016/j.lungcan.2021.09.015
Li, Y., Luan, G., and Guo, P. (2021). The Inhibitory Effect of Dihydroartemisinin on Non-small Cells Lung Cancer. Pharmacol. Res. - Mod. Chin. Med. 1, 100006. doi:10.1016/j.prmcm.2021.100006
Mishra, S. S., and Singh, P. (2016). Hybrid Molecules: The Privileged Scaffolds for Various Pharmaceuticals. Eur. J. Med. Chem. 124, 500–536. doi:10.1016/j.ejmech.2016.08.039
Nepali, K., Sharma, S., Sharma, M., Bedi, P. M., and Dhar, K. L. (2014). Rational Approaches, Design Strategies, Structure Activity Relationship and Mechanistic Insights for Anticancer Hybrids. Eur. J. Med. Chem. 77, 422–487. doi:10.1016/j.ejmech.2014.03.018
Sharma, M., Sharma, S., Buddhiraja, A., Saxena, A. K., Nepali, K., and Bedi, P. M. S. (2014). Synthesis and Cytotoxicity Studies of 3,5-diaryl N-Acetyl Pyrazoline-Isatin Hybrids. Med. Chem. Res. 23, 4337–4344. doi:10.1007/s00044-014-1001-5
Sławiński, G., Wrona, A., Dabrowska-Kugacka, A., Raczak, G., and Lewicka, E. (2020). Immune Checkpoint Inhibitors and Cardiac Toxicity in Patients Treated for Non-small Lung Cancer: A Review. Int. J. Mol. Sci. 21 (19), e7195. doi:10.3390/ijms21197195
Xie, S., Wu, Z., Qi, Y., Wu, B., and Zhu, X. (2021). The Metastasizing Mechanisms of Lung Cancer: Recent Advances and Therapeutic Challenges. Biomed. Pharmacolther. 138, e111450. doi:10.1016/j.biopha.2021.111450
Yang, X., Zhang, T., Zhang, X., Chu, C., and Sang, S. (2022). Global burden of Lung Cancer Attributable to Ambient fine Particulate Matter Pollution in 204 Countries and Territories, 1990-2019. Environ. Res. 204, e112023. doi:10.1016/j.envres.2021.112023
Keywords: dihydroartemisinin, hybrid molecules, multidrug resistance, structure-activity relationship, anticancer
Citation: Dong M, Zheng G, Gao F, Li M and Zhong C (2022) Three-Carbon Linked Dihydroartemisinin-Isatin Hybrids: Design, Synthesis and Their Antiproliferative Anticancer Activity. Front. Pharmacol. 13:834317. doi: 10.3389/fphar.2022.834317
Received: 13 December 2021; Accepted: 06 January 2022;
Published: 26 January 2022.
Edited by:
Elias Georges, McGill University, CanadaReviewed by:
Sahil Sharma, Memorial Sloan Kettering Cancer Center, United StatesGuozheng Huang, Anhui University of Technology, China
Copyright © 2022 Dong, Zheng, Gao, Li and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng Gao, cmdnYW9mZW5nQHNkdS5lZHUuY24=; Min Li, bGltaW55aW5neGlhbmdAMTYzLmNvbQ==; Chen Zhong, emhvbmdjaGVuMDUwNEBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Min Dong1†
Min Dong1† Feng Gao
Feng Gao Min Li
Min Li