- 1Department of Thyroid and Breast Surgery, Tongji Hospital, Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
- 2Department of Gastrointestinal Surgery, Harbin Medical University Cancer Hospital, Harbin Medical University, Harbin, China
Objective: This study aimed to investigate the prognostic value of the gastric immune prognostic index (GIPI) in gastric cancer patients treated with programmed death 1/programmed death-ligand 1 (PD-1/PD-L1) inhibitors.
Methods: This study was conducted to elucidate the role of GIPI using the data from 146 gastric cancer patients treated with PD-1/PD-L1 inhibitors between August 2016 and December 2020 in Harbin Medical University Cancer Hospital. The GIPI calculation was based on dNLR and LDH. Patients were categorized into three groups: 1) GIPI good (LDH ≤250 U/L and dNLR ≤3); 2) GIPI intermediate (LDH >250 U/L and NLR >3); 3) GIPI poor (LDH >250 U/L and dNLR >3). The correlations between GIPI and clinicopathologic characteristics were determined by the Chi-square test or the Fisher’s exact test. The Kaplan–Meier analysis and log-rank test were used to calculate and compare progression-free survival (PFS) and overall survival (OS). The univariate and multivariate Cox proportional hazards regression model was used to detect prognostic and predictive factors of PFS and OS.
Results: 146 patients treated with PD-1/PD-L1 inhibitors were included in this study, of which, 72.6% were GIPI good, 23.3% were GIPI intermediate, and 4.1% were GIPI poor. The GIPI was associated with the common blood parameters, including neutrophils and lymphocytes. The multivariate analysis showed that platelet, TNM stage, and treatment were the independent prognostic factors for PFS and OS. Patients with GIPI intermediate/poor were associated with shorter PFS (median: 24.63 vs. 32.50 months; p = 0.078) and OS (median: 28.37 months vs. not reached; p = 0.033) than those with GIPI good. GIPI intermediate/poor was correlated with shorter PFS and OS than GIPI good, especially in subgroups of patients with ICI treatment and patients with PD-1/PD-L1 positive status.
Conclusions: The GIPI correlated with poor outcomes for PD-1/PD-L1 expression status and may be useful for identifying gastric cancer patients who are unlikely to benefit from treatment.
Introduction
Gastric cancer, the sixth leading cause of cancer-related morbidity and the third leading cause of cancer-related mortality, is one of the commonest gastrointestinal tumors in the world (Sung et al., 2021). Although the incidence rate of gastric cancer has been declining gradually in recent decades, the affected population is always rising worldwide, especially in eastern countries, such as Korea, Japan, Mongolia, and China (Ito et al., 2021). A report indicated that the median survival time of patients with gastric cancer in China during two decades (1980–2000) was 33, 39, and 49 months in 1980, 1990, and 2000s, respectively (Zhang et al., 2011). Despite advances in surgical techniques, chemotherapy, and radiotherapy, the prognosis of gastric cancer has not been significantly improved. Moreover, the death of gastric cancer in China accounts for about 50% of gastric cancer deaths worldwide, with an age-standardized 5-year survival rate of approximately 20% (Zheng et al., 2014; Chen et al., 2016).
Immunotherapy, principally represented by programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors, has been approved for the treatment of locally advanced, recurrent, metastatic gastric cancer all over the world since September 2017 (Fashoyin-Aje et al., 2019). PD-1 and PD-L1 were momentous immune checkpoint components that essentially regulate the function of tumor-infiltrating lymphocytes and tumor cells. And, PD-1 can negatively regulate the activity of T cells via interacting with its ligands PD-L1 expressing on immune cells and tumor cells at some steps of the immune response. The ATTRACTION 02 trial has reported that the median overall survival (OS) was longer in the nivolumab group than in the placebo group. Furthermore, the 12-month OS rate was higher with nivolumab than with placebo in patients with advanced gastric cancer, demonstrating that nivolumab might be a new treatment option for these patients (Kang et al., 2017). The KEYNOTE-061 trial reported that the median OS was 9.1 months with pembrolizumab and 8.3 months with paclitaxel. The trial also reported that the median progression-free survival (PFS) was 1.5 months with pembrolizumab and 4.1 months with paclitaxel, demonstrating that pembrolizumab did not significantly improve OS compared to paclitaxel as second-line therapy for advanced gastric cancer with PD-L1 CPS ≥1 (Shitara et al., 2018). However, even in PD-1/PD-L1 positive populations, the benefits of immunotherapy do not apply to the whole population. This makes the identification of biomarkers in gastric cancer patients likely to respond to immune checkpoint inhibitors (ICIs) therapy—a key step in selecting candidate populations.
The inflammatory process is considered the immune resistance mechanism of cancer patients, promoting cancer growth and metastasis and activating carcinogenic signaling pathways (Gonzalez et al., 2018; McKelvey et al., 2018). In addition, peripheral inflammatory status is related to clinical outcomes in cancer patients. A plethora of routine blood parameters have been studied as potential inflammatory biomarkers in cancer patients, such as neutrophil count, monocyte count, platelet count, lymphocyte count, which are related to the prognosis of several cancer types (Feng et al., 2018; Yakovlev and Klyushin, 2018; Oh et al., 2019; Silvestre-Roig et al., 2019). Novel potential biomarkers, such as neutrophil to lymphocyte ratio, derived neutrophil to lymphocyte ratio [dNLR, absolute neutrophil count/(white blood cell count-absolute neutrophil count)], monocyte to lymphocyte ratio, and platelet to lymphocyte ratio, have been investigated to reflect patients’ immune and inflammatory status in different malignant tumors (Cupp et al., 2020; Gui et al., 2020; Hong et al., 2020; Jakubowska et al., 2020). These ratios, with simple and strong repeatability, are easy to obtain from peripheral blood routine examination.
The prognostic and predictive value of novel inflammatory biomarkers for ICIs is unknown in most tumor types. Recently, Mezquita and colleagues have developed a lung immune prognostic index (LIPI) based on advanced non-small cell lung cancer patients who received ICIs, especially PD-1/PD-L1 inhibitors (Mezquita et al., 2018). The composite index was based on dNLR >3 and LDH > upper limit of normal (ULN) (Mezquita et al., 2018) and characterized into three risk groups: 1) good: dNLR ≤3 and LDH ≤ upper limit of normal (ULN); 2) intermediate: dNLR >3 or LDH > ULN; 3) poor: dNLR >3 and LDH > ULN. The authors also observed that the LIPI was related to the clinical outcome with ICI-treated immunotherapy but not cytotoxic chemotherapy (CCT). This might help doctors determine which patients can benefit from treatment. However, the correlation of gastric immune prognostic index (GIPI) with PD-1/PD-L1 inhibitors outcomes has not been studied in gastric cancer patients. Therefore, we performed an exploratory retrospective analysis to investigate the prognostic value of GIPI in gastric cancer patients treated with PD-1/PD-L1 inhibitors.
Materials and Methods
Patients’ Selection
Institutional review board approval was acquired to review medical records at Harbin Medical University Cancer Hospital. All patient data accessed complied with relevant data protection and privacy regulations. All processes performed in the study were conducted in accordance with the standards of the institutional research committee and with the declaration of Goodyear et al. (2007) as well as its later amendments or comparable ethical standards. Informed consent was waived by the Ethics Committee of Harbin Medical University Cancer Hospital due to the retrospective nature of this study. Between August 2016 and December 2020, 146 patients with gastric cancer treated with PD-1/PD-L1 inhibitors at Harbin Medical University Cancer Hospital were included. We collected and searched the clinical data by electronic medical records. The inclusion criteria were as follows: 1) patients who were diagnosed with gastric cancer; 2) patients receiving PD-1/PD-L1 inhibitors or chemotherapy; and 3) Eastern Cooperative Oncology Group performance status: 0–2. The exclusion criteria were as follows: 1) absence of pretreatment blood test results; 2) autoimmune disease or systemic immunosuppression; and 3) absence of efficacy assessment.
Calculation of Gastric Immune Prognostic Index
The GIPI, which comprises two factors, was based on dNLR and serum LDH levels. Information on complete blood cell counts with differential counts and LDH levels within 7 days before treatment was extracted. The cutoff value of LDH was determined based on ULN (250 IU/L). The cutoff value of dNLR was set at >3, as reported by Mezquita et al. (2018). Patients were categorized into three groups: 1) GIPI good (LDH ≤250 U/L and dNLR ≤3); 2) GIPI intermediate (LDH >250 U/L and NLR >3); and 3) GIPI poor (LDH >250 U/L and dNLR >3).
Immunohistochemistry for Programmed Death 1/Programmed Death-Ligand 1
The gastric cancer tissues were fixed with methanol, embedded in paraffin, sectioned, and performed immunohistochemical analyses. PD-1/PD-L1 expression was analyzed on tumor cells using immunohistochemistry, according to the instructions of the manufacturer. The expression of at least 1% was considered positive (Zayac and Almhanna, 2020).
Follow-Up
All enrolled patients were routinely followed-up by telephone, inpatient, and outpatient. Follow-up assessments included laboratory tests, physical examination, multi-slice CT, gastroscopy, and some other examinations as it fits. PFS was calculated from the date of the first immunotherapy administration to the date of disease progression or death due to any cause. OS was calculated from the date of the first immunotherapy administration to the date of death from any cause. The date of the last follow-up in this study was November 2021.
Statistical Analysis
The clinical characteristics of the patients were presented as absolute values and percentages (%). Discrete variables were compared using the Chi-square test or Fishers exact test, and the Student’s t-test was used for continuous variables. The Kaplan-Meier curves were used to evaluate OS and PFS, and the differences were evaluated by a log-rank test. The univariate and multivariate Cox proportional hazards regression model was used to evaluate the independent prognostic factors. The hazard ratio with its 95% confidence interval was estimated using the univariate and multivariate Cox proportional hazards regression model. All p values were from two-sided tests and were considered statistically significant at two-tailed p < 0.05. All statistical analyses were performed using the R (version 3.6.0; Vienna, Austria. URL: http://www.R-project.org/), SPSS software (version 17.0; SPSS Inc., Chicago, IL, United States), and GraphPad Prism software (version 8.0; GraphPad Inc., La Jolla, CA, United States).
Results
Patient Characteristics
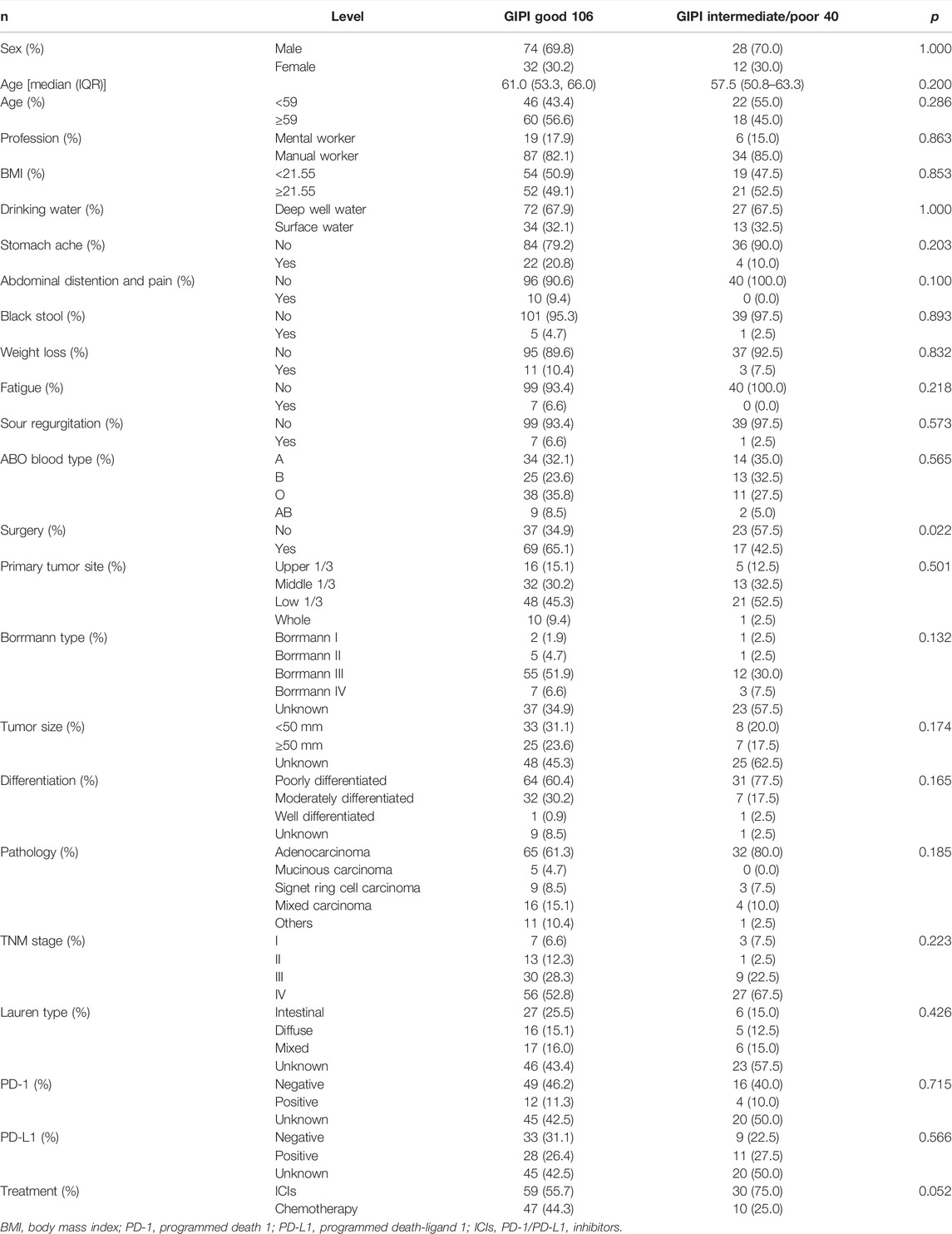
According to the GIPI, 106 (72.6%), 34 (23.3%), and 6 (4.1%) patients were allocated to the GIPI good, GIPI intermediate, and GIPI poor groups, respectively. Due to the small number of patients in the poor GIPI group, all patients were divided into two groups: GIPI good group with 106 (72.6%) patients and GIPI intermediate/poor group with 40 (37.4%) patients. The clinical characteristics of the enrolled patients are summarized in Table 1. There were 102 males and 44 females diagnosed with gastric cancer in the study population. The median age was 59 years (range: 34–82 years). The median BMI was 21.55 (range: 15.15–34.21). In the light of ABO blood type, A type was 48 cases (32.9%), B type was 38 cases (26.0%), O type was 49 cases (33.6%), and AB type was 11 cases (7.5%). Based on the 8th edition of the TNM classification, 10 (6.8%), 14 (9.6%), 39 (26.7%), and 83 (56.8%) gastric cancer patients were classified as stage I, II, III, and IV, respectively. GIPI was associated with surgery (p = 0.022). The detailed information is shown in Table 1.
Blood Parameters
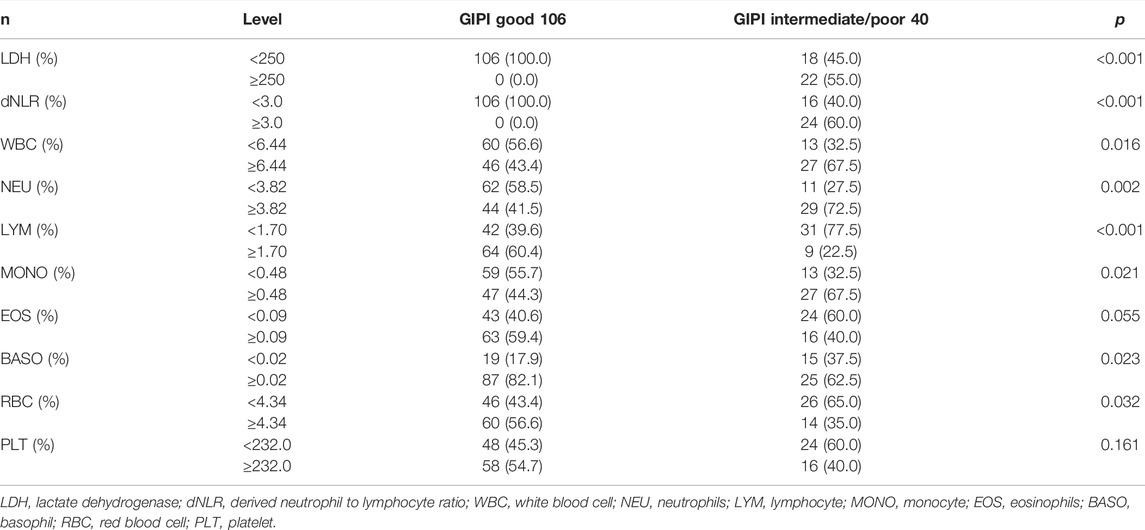
The median of the white blood cell (WBC), neutrophil (NEU), lymphocyte (LYM), monocyte (MONO), eosinophils (EOS), basophil (BASO), red blood cell (RBC), and platelet (PLT) counts were 6.44 × 109/L, 3.82 × 109/L, 1.70 × 109/L, 0.48 × 109/L, 0.09 × 109/L, 0.02 × 109/L, 4.34 × 1012/L, and 232 × 109/L, respectively. GIPI was associated with LDH (p < 0.001), dNLR (p < 0.001), WBC (p = 0.016), NEU (p = 0.002), LYM (p < 0.001), MONO (p = 0.021), BASO (p = 0.023), and RBC (p = 0.032), respectively. The detailed information is shown in Table 2.
Univariate and Multivariate Analysis for Progression-Free Survival and Overall Survival
The univariate analysis showed that PLT, GIPI, LDH, radical resection, surgery, TNM stage, Lauren type, treatment, PD-1, and PD-L1 were associated with the prognosis of patients with gastric cancer for PFS. However, the multivariate analysis indicated that PLT, TNM stage, and treatment were the independent prognostic factors for PFS (Table 3). Furthermore, the univariate analysis indicated that PLT, GIPI, LDH, radical resection, surgery, Borrmann type, Lauren type, treatment, PD-1, and PD-L1 were associated with the prognosis of patients with gastric cancer for OS. Nevertheless, the multivariate analysis showed that PLT, TNM stage, and treatment were the independent prognostic factors for OS (Table 3).
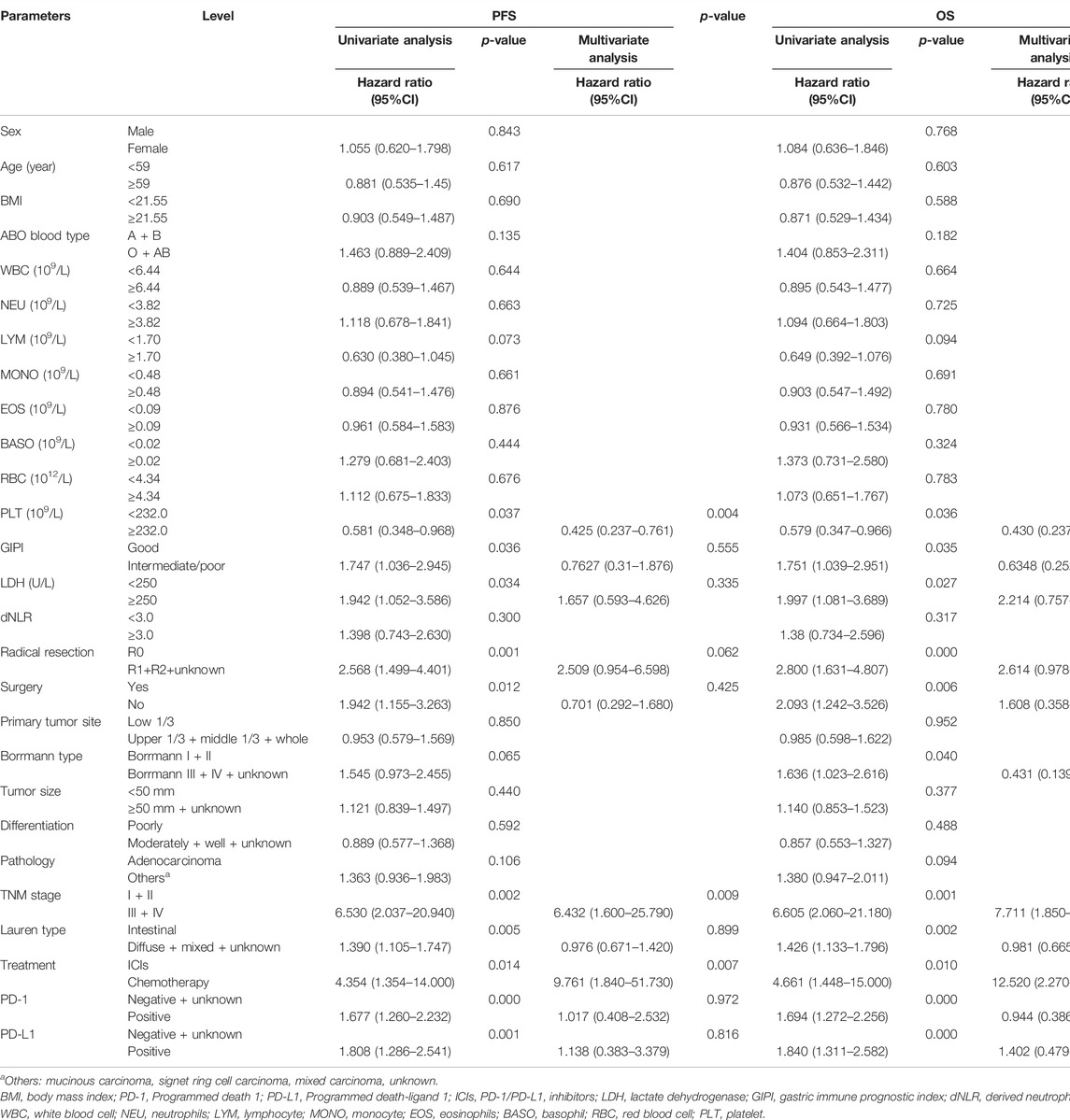
TABLE 3. Univariate and multivariate Cox hazard analysis of biomarkers for progression-free survival (PFS) and overall survival (OS).
Survival Outcomes With Derived Neutrophil to Lymphocyte Ratio, Lactate Dehydrogenase, and Gastric Immune Prognostic Index
Patients with high dNLR were associated with shorter PFS (median: 26.20 vs. 27.00 months; p = 0.441) and OS (median: 39.07 vs. 42.67 months; p = 0.315) than those with high dNLR (Figures 1A,B). Patients with high LDH were associated with shorter PFS (median: 12.30 vs. 28.23 months; p = 0.054) and OS (median: 18.37 vs. 42.67 months; p = 0.024) than those with low LDH (Figures 1C,D). Patients with GIPI intermediate/poor were associated with shorter PFS (median: 24.63 vs. 32.50 months; p = 0.078) and OS (median: 28.37 months vs. not reached; p = 0.033) than those with GIPI good (Figures 1E,F).
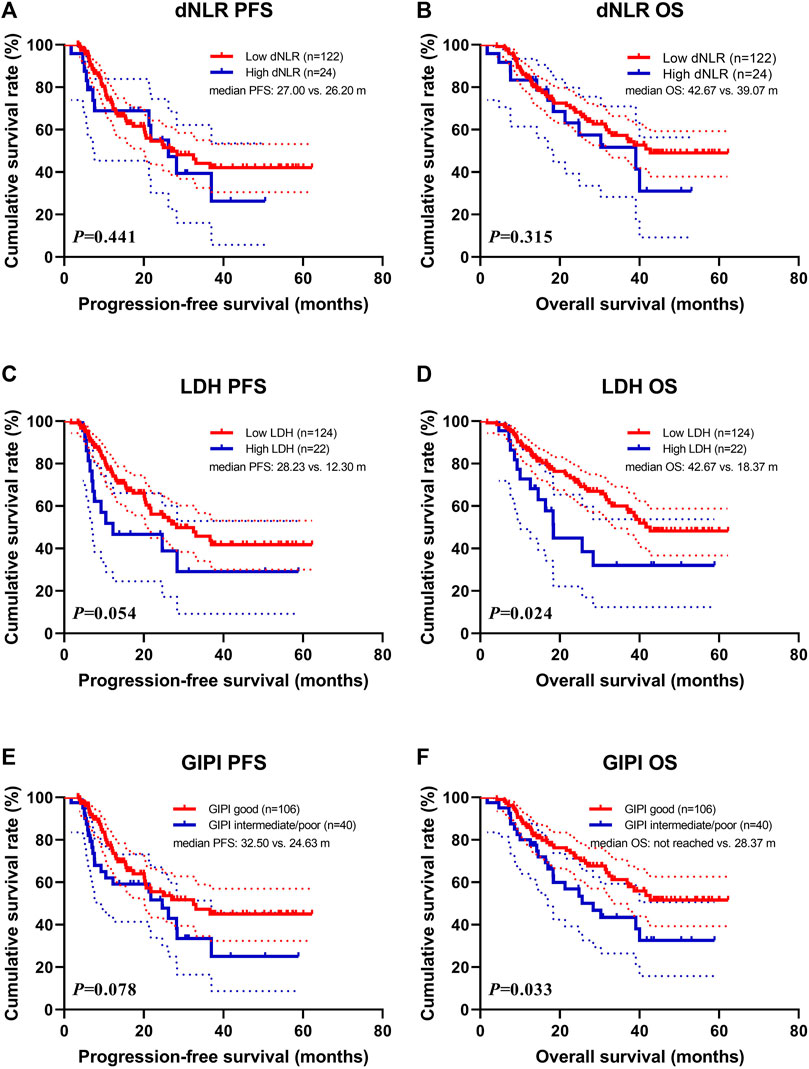
FIGURE 1. Survival according to dNLR, LDH, and GIPI groups for (A) progression-free survival (PFS) by dNLR; (B) overall survival (OS) by dNLR; (C) PFS by LDH; (D) OS by LDH; (E) PFS by GIPI; (F) OS by GIPI.
Treatment (Immune Checkpoint Inhibitors and Chemotherapy)
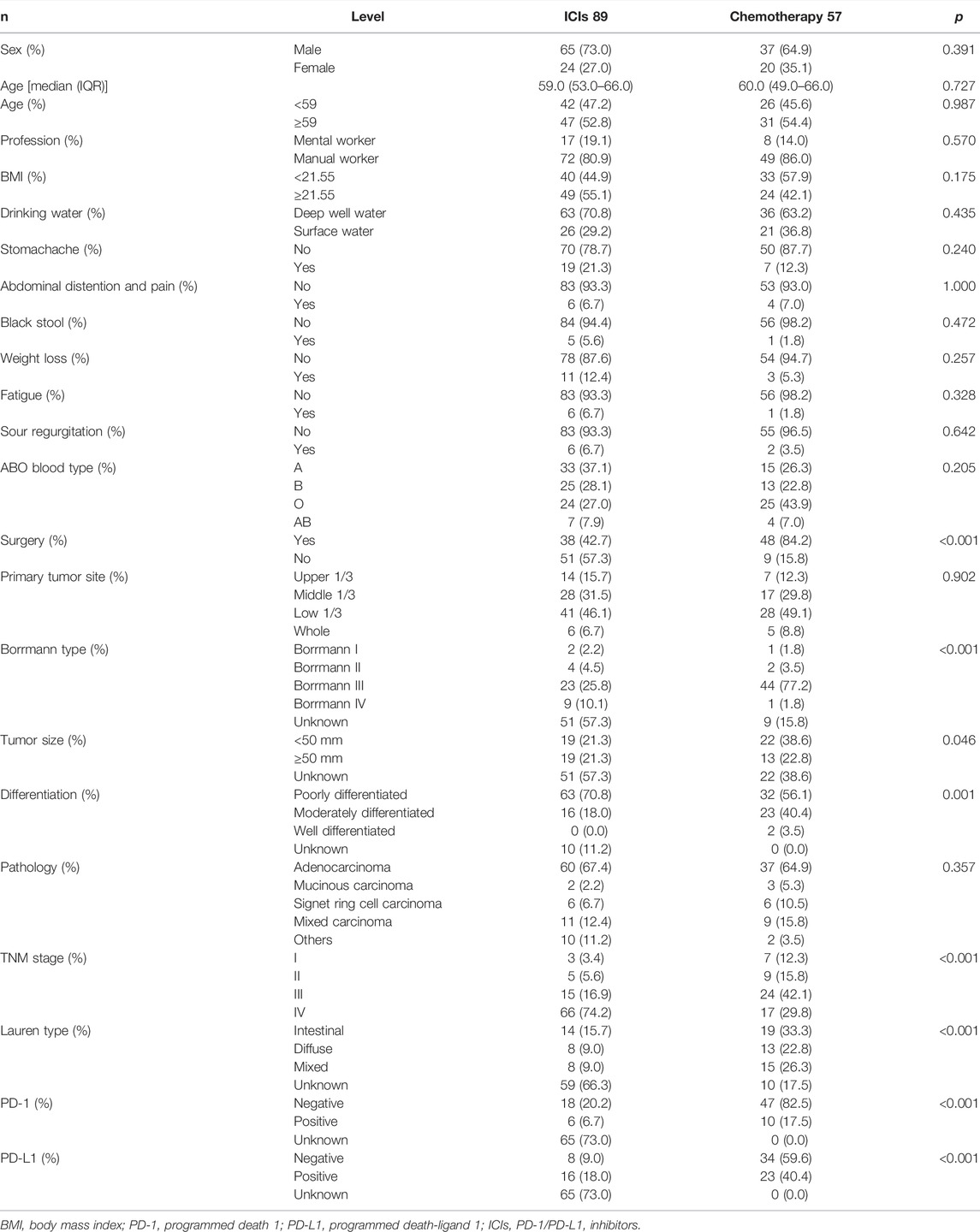
In this study, 89 patients received PD-1/PD-L1 inhibitors treatment (named ICIs group), and 57 patients received chemotherapy (including targeted therapy) treatment (named chemotherapy group). Baseline demographics and disease characteristics are shown in Table 4. Between the two groups, statistically significant differences were found in surgery (p < 0.001), Borrmann type (p < 0.001), tumor size (p = 0.046), differentiation (p = 0.001), TNM stage (p < 0.001), Lauren type (p < 0.001), PD-1 (p < 0.001), and PD-L1 (p < 0.001). Among the blood parameters, statistically significant differences were found in dNLR (p = 0.026), WBC (p = 0.042), NEU (p = 0.018), and MONO (p = 0.012) (Table 5).
Patients with PD-1/PD-L1 inhibitors treatment were associated with shorter PFS (median: 20.60 months vs. not reached; p = 0.0004) and OS (median: 30.27 months vs. not reached; p = 0.0001) than those with chemotherapy treatment (Figures 2A,B). In the ICIs group, patients with GIPI intermediate/poor were associated with shorter PFS (median: 20.43 vs. 21.77 months; p = 0.483) and OS (median: 24.83 vs. 32.40 months; p = 0.206) than those with GIPI good (Figures 2C,D). In the chemotherapy group, patients with GIPI intermediate/poor were associated with shorter PFS (median: not reached vs. not reached; p = 0.492) and OS (median: not reached vs. not reached; p = 0.319) than those with GIPI good (Figures 2E,F).
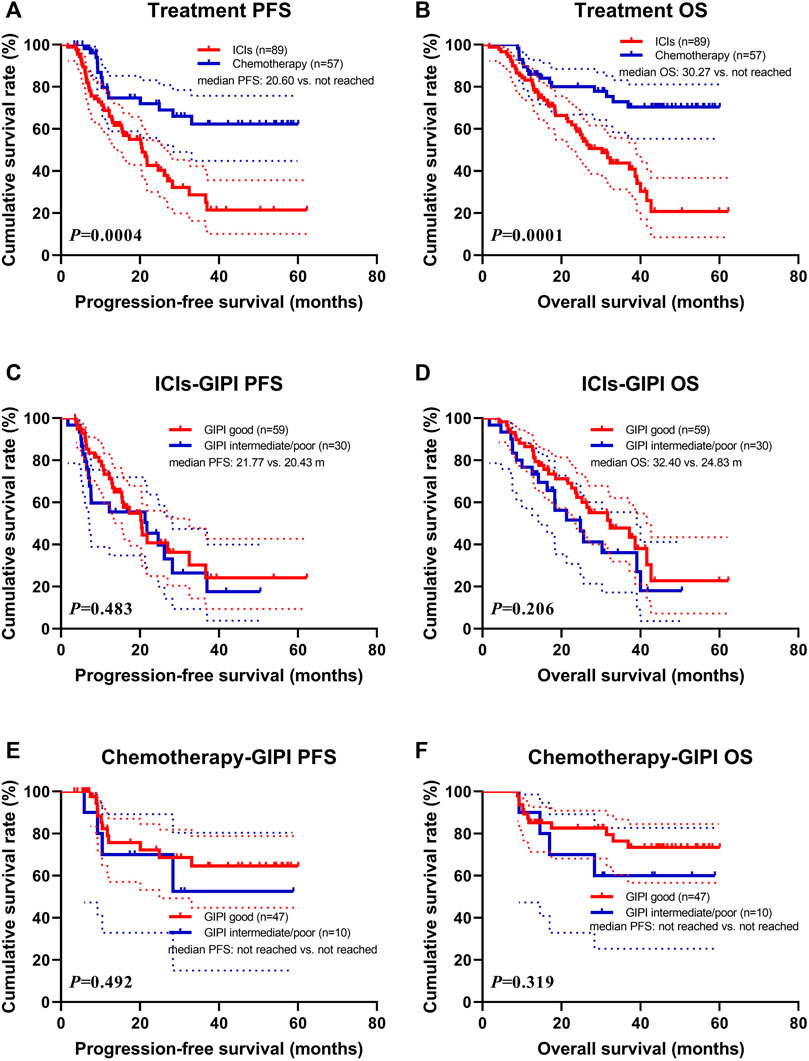
FIGURE 2. Survival according to ICIs and GIPI groups for (A) progression-free survival (PFS) by treatment; (B) overall survival (OS) by treatment; (C) PFS by ICIs; (D) OS by ICIs; (E) PFS by chemotherapy; (F) OS by chemotherapy.
Programmed Death 1/Programmed Death-Ligand 1 Only Positive Expression
Data for PD-1/PD-L1 expression were analyzed on tumor cells using immunohistochemistry, according to standard practice. Expression of at least 1% was considered positive (Zayac and Almhanna, 2020). PD-1 status was positive in 16 patients (11.0%), negative in 65 (44.5%) and unknown in 65 (44.5%). PD-L1 status was positive in 39 patients (26.7%), negative in 42 (28.8%) and unknown in 65 (44.5%). Overall, PD-1/PD-L1 status was positive in 43 patients (29.5%), negative in 38 (26.0%) and unknown in 65 (44.5%). The high rate of missing PD-L1 status was because it was not mandatory for ICI prescription. According to the PD-1/PD-L1 positive status for 43 patients, 31 patients were GIPI good and 12 patients were GIPI intermediate/poor. According to the subgroup analysis, patients with GIPI intermediate/poor were associated with shorter PFS (median: 10.47 months vs. not reached; p = 0.001) and OS (median: 14.57 months vs. not reached; p = 0.0001) than those with GIPI good (Figures 3A,B).
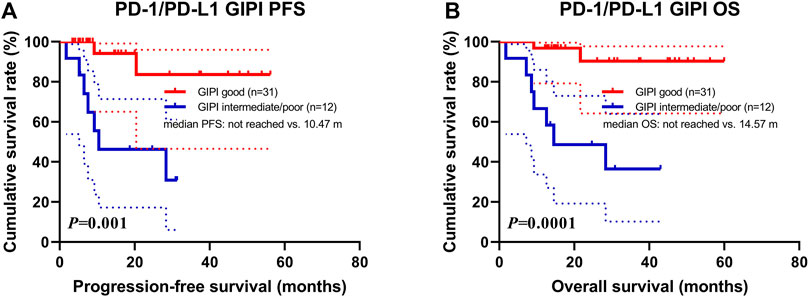
FIGURE 3. Survival according to PD-1/PD-L1 positive expression groups for (A) progression-free survival (PFS) and (B) overall survival (OS).
Discussion
Although the accuracy of gastric cancer treatment has been significantly improved in recent years, gastric cancer is still challenging (Smyth et al., 2020). ICIs, such as PD-1/PD-L1 inhibitors, have emerged as a promising treatment approach with curable potential and durable survival. However, many patients with gastric cancer receiving PD-1 or PD-L1 inhibitor treatment do not experience survival benefits due to substantial heterogeneity (Akin Telli et al., 2020; Kawazoe et al., 2021). Biomarkers, including PD-1, PD-L1, CTC, and TMB, have limited predictive accuracy due to the unavailability of tumor tissue and molecular or microscopic analyses (Lianidou et al., 2015; Yi et al., 2018; Ritterhouse, 2019). Systemic inflammatory status has been found to be related to the survival and prognosis of patients with different types of cancer (McMillan, 2009; Diakos et al., 2014). Although the inflammatory markers have been observed in patients treated with surgery, chemotherapy, or targeted therapy, the effect of systemic inflammatory status on immunotherapy benefit is not well known (Clarke et al., 2011; Li et al., 2020; Ravindranathan et al., 2021). Hence, it is important to look for biomarkers that can predict treatment outcomes.
The LIPI, based on dNLR and LDH, was first developed by Mezquita and colleagues and is supposed to be related to ICIs outcomes in patients with non-small cell lung cancer (Mezquita et al., 2018). The prognostic relationship between higher LIPI scores and poorer outcomes has also been confirmed in lung cancer. Most notably, the LIPI is an ideal biomarker because it is non-invasive, cost-effective, and can easily be obtained from serum. More recently, in a monocentric retrospective cohort of 720 advanced melanoma patients treated with ipilimumab, dNLR ≥3 was associated with a negative effect on survival and may help in risk-group stratification and disease-management strategies (Ferrucci et al., 2016). LDH is a classic inflammatory marker in cancer patients and has been found to be related to shorter survival when increased from 1 to 2.5 ULN (Van Wilpe et al., 2020). Diem and colleagues reported that LDH was a useful biomarker at baseline and during treatment to predict objective response in 66 consecutive patients with advanced or metastatic melanoma treated with nivolumab or pembrolizumab. It was significantly associated with shorter OS, reflecting the potential value of monitoring these markers (Diem et al., 2016). Castello and colleagues have reported immune-metabolic prognostic index (IMPI) at the first restaging, combining both inflammatory and metabolic biomarkers, was correlated with PFS and OS. IMPI can be a potentially valuable tool for identifying NSCLC patients who are likely to benefit from ICI (Castello et al., 2020).
To our knowledge, this is the first study to investigate the relationship between GIPI and survival outcomes of gastric cancer patients undergoing PD-1/PD-L1 inhibitors treatment. Our results indicated that patients in GIPI good group treated with PD-1/PD-L1 inhibitors have improved PFS and OS compared with those in the GIPI intermediate/poor group. The prognostic value of this biomarker was also consistent with that of previous studies for patients who received ICIs therapy in advanced hepatocellular carcinoma (Chen et al., 2020), advanced small cell lung cancer (Li et al., 2021), esophageal squamous cell carcinoma (Feng et al., 2021). Furthermore, a dNLR >3 and LDH >250 U/L were associated with shorter PFS and OS. Based on the univariate analysis, our findings also showed that GIPI was related to PFS and OS in gastric cancer patients who received PD-1/PD-L1 inhibitors therapy. However, the multivariate analysis indicated that GIPI was not the potential independent prognostic factor for PFS and OS. Nevertheless, considering the retrospective nature of this study, the negative results of PFS and OS should be interpreted with caution as they may have been influenced by multiple factors, including the enrolled patients and tumor type. In contrast, the difference in PFS and OS between GIPI good group, and GIPI intermediate/poor group is more convincing. Simultaneously, we also analyzed the difference by a treatment that was significant. Furthermore, we conducted a subgroup analysis by ICIs or chemotherapy, and the results showed that the patients with GIPI intermediate/poor were associated with shorter PFS and OS. We also performed the PD-1/PD-L1 expression status by GIPI, and the results indicated patients with GIPI intermediate/poor were associated with shorter PFS and OS.
There are several plausible mechanisms to evaluate the relationship between GIPI and the prognosis of gastric cancer. Neutrophils can be influenced and manipulated, including the differentiation process and the development of different phenotypes and functional polarization states (Shaul and Fridlender, 2019; McFarlane et al., 2021). In the proinflammatory state, this will induce “emergency granulopoiesis” that rapidly increases the production of neutrophils, thereby releasing immature or poorly differentiated neutrophils related to tumor progression (Zhang et al., 2020). The increase in LDH level is the product of tumor glycolytic activity and tumor necrosis caused by hypoxia, and the latter is related to the high tumor burden (Van Wilpe et al., 2020). The LDH levels are inversely associated with response to checkpoint inhibitors and glycolysis inhibitors (Laganá et al., 2019; Ke et al., 2021).
This study had several limitations. First, our exploratory evaluation was retrospective, and the data was from a single-center study conducted on a small number of gastric cancer patients. As a result, the sample size of the GIPI poor group was too small. Hence, we divided the study subjects into two groups (GIPI good and GIPI intermediate/poor) rather than three groups (GIPI good, GIPI intermediate, and GIPI poor). In addition, some confounding factors and selective bias could not be avoided. Second, most of the enrolled patients received PD-1/PD-L1 inhibitors as their second-line treatment or beyond. The degree of baseline inflammation may be affected by previous treatment. More so, due to the bias of drug selection, the results should be interpreted with caution. Despite these limitations, a unique aspect of this study was the combined model of three baseline peripheral blood markers for the outcome of PD-1/PD-L1 inhibitors. Finally, GIPI is a nonspecific tumor marker; hence, the need to further verify the correlation between GIPI and cancer prognosis in a prospective study.
Conclusion
In conclusion, based on the GIPI intermediate/poor group, combining dNLR >3 and LDH > ULN was related to poor outcomes. The GIPI may be useful for identifying gastric cancer patients who are unlikely to benefit from treatment. The GIPI is also related to the PD-1 or PD-L1 expression, and poor baseline GIPI correlated with poor outcomes for PD-1 or PD-L1 expression status.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Harbin Medical University Cancer Hospital. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Writing-original draft and writing-review and editing: LC and RZ; data curation and investigation: HSu, RH, and HP; methodology and supervision: YZ and LZ; resources, funding acquisition, and project administration: YX, XL, and HSo.
Funding
This study was supported by the Department of Education of Heilongjiang Province (No: 12541458) and the Clinical Research Foundation of the Wu Jieping Medical Foundation (grant no. 320.6750.13105 and no. 320.6750.18278).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
dNLR, derived neutrophil to lymphocyte ratio; LDH, lactate dehydrogenase; GIPI, gastric immune prognostic index; ICIs, immune checkpoint inhibitors; ULN, upper limit of normal; PD-1, programmed death 1; PD-L1, programmed death-ligand 1; OS, overall survival; PFS, progression-free survival; LIPI, lung immune prognostic index; WBC, white blood cell; NEU, neutrophils; LYM, lymphocyte; MONO, monocyte; EOS, eosinophils; BASO, basophil; RBC, red blood cell; PLT, Platelet.
References
Akin Telli, T., Bregni, G., Camera, S., Deleporte, A., Hendlisz, A., and Sclafani, F. (2020). PD-1 and PD-L1 Inhibitors in Oesophago-Gastric Cancers. Cancer Lett. 469, 142–150. doi:10.1016/j.canlet.2019.10.036
Castello, A., Toschi, L., Rossi, S., Mazziotti, E., and Lopci, E. (2020). The Immune-Metabolic-Prognostic index and Clinical Outcomes in Patients with Non-small Cell Lung Carcinoma under Checkpoint Inhibitors. J. Cancer Res. Clin. Oncol. 146 (5), 1235–1243. doi:10.1007/s00432-020-03150-9
Chen, W., Zheng, R., Baade, P. D., Zhang, S., Zeng, H., Bray, F., et al. (2016). Cancer Statistics in China, 2015. CA Cancer J. Clin. 66 (2), 115–132. doi:10.3322/caac.21338
Chen, S., Huang, Z., Jia, W., Tao, H., Zhang, S., Ma, J., et al. (2020). Association of the Pretreatment Lung Immune Prognostic Index with Survival Outcomes in Advanced Hepatocellular Carcinoma Patients Treated with PD-1 Inhibitors. J. Hepatocell Carcinoma 7, 289–299. doi:10.2147/JHC.S277453
Clarke, S. J., Chua, W., Moore, M., Kao, S., Phan, V., Tan, C., et al. (2011). Use of Inflammatory Markers to Guide Cancer Treatment. Clin. Pharmacol. Ther. 90 (3), 475–478. doi:10.1038/clpt.2011.122
Cupp, M. A., Cariolou, M., Tzoulaki, I., Aune, D., Evangelou, E., and Berlanga-Taylor, A. J. (2020). Neutrophil to Lymphocyte Ratio and Cancer Prognosis: an Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies. BMC Med. 18 (1), 360. doi:10.1186/s12916-020-01817-1
Diakos, C. I., Charles, K. A., McMillan, D. C., and Clarke, S. J. (2014). Cancer-related Inflammation and Treatment Effectiveness. Lancet Oncol. 15 (11), e493–503. doi:10.1016/S1470-2045(14)70263-3
Diem, S., Kasenda, B., Spain, L., Martin-Liberal, J., Marconcini, R., Gore, M., et al. (2016). Serum Lactate Dehydrogenase as an Early Marker for Outcome in Patients Treated with Anti-PD-1 Therapy in Metastatic Melanoma. Br. J. Cancer 114 (3), 256–261. doi:10.1038/bjc.2015.467
Fashoyin-Aje, L., Donoghue, M., Chen, H., He, K., Veeraraghavan, J., Goldberg, K. B., et al. (2019). FDA Approval Summary: Pembrolizumab for Recurrent Locally Advanced or Metastatic Gastric or Gastroesophageal Junction Adenocarcinoma Expressing PD-L1. Oncologist 24 (1), 103–109. doi:10.1634/theoncologist.2018-0221
Feng, F., Zheng, G., Wang, Q., Liu, S., Liu, Z., Xu, G., et al. (2018). Low Lymphocyte Count and High Monocyte Count Predicts Poor Prognosis of Gastric Cancer. BMC Gastroenterol. 18 (1), 148. doi:10.1186/s12876-018-0877-9
Feng, J. F., Zhao, J. M., Chen, S., and Chen, Q. X. (2021). Prognostic Significance of the Lung Immune Prognostic Index in Patients with Resected Esophageal Squamous Cell Carcinoma. Cancer Manag. Res. 13, 2811–2819. doi:10.2147/CMAR.S298412
Ferrucci, P. F., Ascierto, P. A., Pigozzo, J., Del Vecchio, M., Maio, M., Antonini Cappellini, G. C., et al. (2016). Baseline Neutrophils and Derived Neutrophil-To-Lymphocyte Ratio: Prognostic Relevance in Metastatic Melanoma Patients Receiving Ipilimumab. Ann. Oncol. 27 (4), 732–738. doi:10.1093/annonc/mdw016
Gonzalez, H., Hagerling, C., and Werb, Z. (2018). Roles of the Immune System in Cancer: from Tumor Initiation to Metastatic Progression. Genes Dev. 32 (19-20), 1267–1284. doi:10.1101/gad.314617.118
Goodyear, M. D., Krleza-Jeric, K., and Lemmens, T. (2007). The Declaration of Helsinki. BMJ 335(7621), 624-5. doi:10.1136/bmj.39339.610000.BE
Gui, W., Wang, X., Luo, Y., and Wang, J. (2020). Platelet to Lymphocyte Ratio as a Prognostic Factor in Patients with Advanced Colorectal Cancer Undergoing Palliative Treatment. Ann. Palliat. Med. 9 (5), 3271–3277. doi:10.21037/apm-20-1389
Hong, H., Fang, X., Huang, H., Wang, Z., Lin, T., and Yao, H. (2020). The Derived Neutrophil-To-Lymphocyte Ratio Is an Independent Prognostic Factor in Patients with Angioimmunoblastic T-Cell Lymphoma. Br. J. Haematol. 189 (5), 908–912. doi:10.1111/bjh.16447
Ito, Y., Miyashiro, I., Ishikawa, T., Akazawa, K., Fukui, K., Katai, H., et al. (2021). Determinant Factors on Differences in Survival for Gastric Cancer between the United States and Japan Using Nationwide Databases. J. Epidemiol. 31 (4), 241–248. doi:10.2188/jea.JE20190351
Jakubowska, K., Koda, M., Grudzińska, M., Kańczuga-Koda, L., and Famulski, W. (2020). Monocyte-to-lymphocyte Ratio as a Prognostic Factor in Peripheral Whole Blood Samples of Colorectal Cancer Patients. World J. Gastroenterol. 26 (31), 4639–4655. doi:10.3748/wjg.v26.i31.4639
Kang, Y. K., Boku, N., Satoh, T., Ryu, M. H., Chao, Y., Kato, K., et al. (2017). Nivolumab in Patients with Advanced Gastric or Gastro-Oesophageal junction Cancer Refractory to, or Intolerant of, at Least Two Previous Chemotherapy Regimens (ONO-4538-12, ATTRACTION-2): a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet 390 (10111), 2461–2471. doi:10.1016/S0140-6736(17)31827-5
Kawazoe, A., Shitara, K., Boku, N., Yoshikawa, T., and Terashima, M. (2021). Current Status of Immunotherapy for Advanced Gastric Cancer. Jpn. J. Clin. Oncol. 51 (1), 20–27. doi:10.1093/jjco/hyaa202
Ke, L., Wang, L., Yu, J., and Meng, X. (2021). Prognostic Significance of SUVmax Combined with Lactate Dehydrogenase in Advanced Lung Cancer Patients Treated with Immune Checkpoint Inhibitor Plus Chemotherapy: A Retrospective Study. Front. Oncol. 11, 652312. doi:10.3389/fonc.2021.652312
Laganá, G., Barreca, D., Calderaro, A., and Bellocco, E. (2019). Lactate Dehydrogenase Inhibition: Biochemical Relevance and Therapeutical Potential. Curr. Med. Chem. 26 (18), 3242–3252. doi:10.2174/0929867324666170209103444
Li, Z., Li, S., Ying, X., Zhang, L., Shan, F., Jia, Y., et al. (2020). The Clinical Value and Usage of Inflammatory and Nutritional Markers in Survival Prediction for Gastric Cancer Patients with Neoadjuvant Chemotherapy and D2 Lymphadenectomy. Gastric Cancer 23 (3), 540–549. doi:10.1007/s10120-019-01027-6
Li, L., Pi, C., Yan, X., Lu, J., Yang, X., Wang, C., et al. (2021). Prognostic Value of the Pretreatment Lung Immune Prognostic Index in Advanced Small Cell Lung Cancer Patients Treated with First-Line PD-1/PD-L1 Inhibitors Plus Chemotherapy. Front. Oncol. 11, 697865. doi:10.3389/fonc.2021.697865
Lianidou, E. S., Markou, A., and Strati, A. (2015). The Role of CTCs as Tumor Biomarkers. Adv. Exp. Med. Biol. 867, 341–367. doi:10.1007/978-94-017-7215-0_21
McFarlane, A. J., Fercoq, F., Coffelt, S. B., and Carlin, L. M. (2021). Neutrophil Dynamics in the Tumor Microenvironment. J. Clin. Invest. 131 (6), e143759. doi:10.1172/JCI143759
McKelvey, K. J., Hudson, A. L., Back, M., Eade, T., and Diakos, C. I. (2018). Radiation, Inflammation and the Immune Response in Cancer. Mamm. Genome 29 (11-12), 843–865. doi:10.1007/s00335-018-9777-0
McMillan, D. C. (2009). Systemic Inflammation, Nutritional Status and Survival in Patients with Cancer. Curr. Opin. Clin. Nutr. Metab. Care 12 (3), 223–226. doi:10.1097/MCO.0b013e32832a7902
Mezquita, L., Auclin, E., Ferrara, R., Charrier, M., Remon, J., Planchard, D., et al. (2018). Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients with Advanced Non-small Cell Lung Cancer. JAMA Oncol. 4 (3), 351–357. doi:10.1001/jamaoncol.2017.4771
Oh, S. E., Seo, J. E., An, J. Y., Lee, J. H., Sohn, T. S., Bae, J. M., et al. (2019). Prognostic Impact of Increased Perioperative Platelet Count in Gastric Cancer Patients. J. Surg. Res. 242, 296–303. doi:10.1016/j.jss.2019.04.052
Ravindranathan, D., Master, V. A., and Bilen, M. A. (2021). Inflammatory Markers in Cancer Immunotherapy. Biology (Basel) 10 (4), 325. doi:10.3390/biology10040325
Ritterhouse, L. L. (2019). Tumor Mutational burden. Cancer Cytopathol. 127 (12), 735–736. doi:10.1002/cncy.22174
Shaul, M. E., and Fridlender, Z. G. (2019). Tumour-associated Neutrophils in Patients with Cancer. Nat. Rev. Clin. Oncol. 16 (10), 601–620. doi:10.1038/s41571-019-0222-4
Shitara, K., Özgüroğlu, M., Bang, Y. J., Di Bartolomeo, M., Mandalà, M., Ryu, M. H., et al. (2018). Pembrolizumab versus Paclitaxel for Previously Treated, Advanced Gastric or Gastro-Oesophageal junction Cancer (KEYNOTE-061): a Randomised, Open-Label, Controlled, Phase 3 Trial. Lancet 392 (10142), 123–133. doi:10.1016/S0140-6736(18)31257-1
Silvestre-Roig, C., Fridlender, Z. G., Glogauer, M., and Scapini, P. (2019). Neutrophil Diversity in Health and Disease. Trends Immunol. 40 (7), 565–583. doi:10.1016/j.it.2019.04.012
Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C., and Lordick, F. (2020). Gastric Cancer. Lancet 396 (10251), 635–648. doi:10.1016/S0140-6736(20)31288-5
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 71 (3), 209–249. doi:10.3322/caac.21660
Van Wilpe, S., Koornstra, R., Den Brok, M., De Groot, J. W., Blank, C., De Vries, J., et al. (2020). Lactate Dehydrogenase: a Marker of Diminished Antitumor Immunity. Oncoimmunology 9 (1), 1731942. doi:10.1080/2162402X.2020.1731942
Yakovlev, P., and Klyushin, D. (2018). Lymphocyte Count in Peripheral Blood Is a Sensitive Tool in Pretreatment Assessment of Patients with Urological Cancer. Exp. Oncol. 40 (2), 119–123. doi:10.31768/2312-8852.2018.40(2):119-123
Yi, M., Jiao, D., Xu, H., Liu, Q., Zhao, W., Han, X., et al. (2018). Biomarkers for Predicting Efficacy of PD-1/PD-L1 Inhibitors. Mol. Cancer 17 (1), 129. doi:10.1186/s12943-018-0864-3
Zayac, A., and Almhanna, K. (2020). Esophageal, Gastric Cancer and Immunotherapy: Small Steps in the Right Direction? Transl. Gastroenterol. Hepatol. 5, 9. doi:10.21037/tgh.2019.09.05
Zhang, H., Sun, L. L., Meng, Y. L., Song, G. Y., Hu, J. J., Lu, P., et al. (2011). Survival Trends in Gastric Cancer Patients of Northeast China. World J. Gastroenterol. 17 (27), 3257–3262. doi:10.3748/wjg.v17.i27.3257
Zhang, B., Yu, Y., Hubert, S. M., Zhang, Y., Lu, J., Liu, S., et al. (2020). Prognostic Value of Pro-inflammatory Neutrophils and C-Reactive Protein in Cancer Patient with Coronavirus Disease 2019: A Multi-Center, Retrospective Study. Front. Pharmacol. 11, 576994. doi:10.3389/fphar.2020.576994
Keywords: gastric cancer, gastric immune prognostic index, immune checkpoint inhibitors, derived neutrophil to lymphocyte ratio, lactate dehydrogenase
Citation: Chen L, Zhao R, Sun H, Huang R, Pan H, Zuo Y, Zhang L, Xue Y, Li X and Song H (2022) The Prognostic Value of Gastric Immune Prognostic Index in Gastric Cancer Patients Treated With PD-1/PD-L1 Inhibitors. Front. Pharmacol. 13:833584. doi: 10.3389/fphar.2022.833584
Received: 13 December 2021; Accepted: 31 March 2022;
Published: 20 June 2022.
Edited by:
Maen Abdelrahim, Houston Methodist Research Institute, United StatesReviewed by:
Zhibo Zhang, The 78th Group Army Hospital of Chinese PLA, ChinaXiangyu Wang, Chinese Academy of Medical Sciences and Peking Union Medical College, China
Copyright © 2022 Chen, Zhao, Sun, Huang, Pan, Zuo, Zhang, Xue, Li and Song. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingrui Li, bGl4aW5ncnVpQHRqaC50am11LmVkdS5jbg==; Hongjiang Song, aG9uZ2ppYW5nc29uZzIwMTZAMTYzLmNvbQ==
†ORCID: Li Chen, orcid.org/0000-0002-6989-1177
‡These authors have contributed equally to this work
 Li Chen
Li Chen Ruihu Zhao2‡
Ruihu Zhao2‡ Xingrui Li
Xingrui Li Hongjiang Song
Hongjiang Song