- 1School of Public Health, Wuhan University, Wuhan, China
- 2Global Health Institute, Wuhan University, Wuhan, China
- 3Xi’an Jiao Tong Liverpool University, Suzhou, China
Objectives: The purpose of this study was to quantitatively evaluate the impacts of the”4 + 7” pilot policy on purchase volume, purchase expenditures, and daily cost and to find the changes in the use of SSRIs.
Methods: Data was collected covering 31 months, before, during, and after the “4 + 7” pilot policy was implemented in Shenzhen. Interrupted time-series (ITS) analysis was used to examine whether there had been a significant effect with the onset of the “4 + 7” pilot policy in March 2019.
Findings: The daily cost of policy-related drugs had a substantial drop of 2.93 yuan under the “4 + 7” pilot policy. The result has shown a 76.70% increase in volume and a 3.39% decrease in the expenditure on policy-related drugs. This study found that the “4 + 7” pilot policy increased the proportion of purchasing winning drugs, with an increment of 85.60 percent. After the implementation of the “4 + 7” pilot policy, policy-related drugs decreased by 443.55thousand Chinese yuan. The study indicated that volume of winning products significantly increased as shown in the regression with a level coefficient (β2) of -224.17 (p < 0.001) and trend coefficient (β3) of 15.74 (p < 0.001). The result revealed that both volume and expenditures on branded products showed a significant decrease in the regression in the post-intervention period (level coefficient of volume: β2 = -57.65, p < 0.01, trend coefficient of volume: β3 = -3.44, p < 0.01; level coefficient of expenditure: β2 = -712.98, p < 0.01, trend coefficient of expenditure: β3 = -40.10, p < 0.01).
Conclusion: The volume-based procurement has successfully led to price reductions and improved the affordability of medicines, especially for those with chronic diseases. The volume-based procurement has demonstrated initial success in reshaping the composition of the Chinese pharmaceutical market in favor of generics with high quality and low prices.
Introduction
Global drug costs are growing rapidly and are set to exceed $1.5 trillion by 2023 (Science HD, 2019). Growing pharmaceutical spending remains a persistent challenge in many countries all over the world. Large sections of the global population can’t afford pharmaceutical spending (Cameron et al., 2009; Babar et al., 2019; Rodwin, 2021; San-Juan-Rodriguez et al., 2021). Notably, the problem is more severe in low-middle-income countries. Pharmaceutical expenditure in lower-middle-income countries can be up to 70% of total health expenditure, compared with 17% in higher-income countries (Papanicolas et al., 2018; Parente, 2018). China’s economic burden on pharmaceuticals has increased steadily over the last decade. Pharmaceutical spending doubled from 2009 to 2017, reaching up to 34% of total health care expenditure in 2017 (China National Health Development Research Center, 2018). The percentage of pharmaceutical expenses in total health care expenditure in China was much higher than in some developed countries such as the United States (12.6%) and Australia (13.8%), as well as most Asian countries such as Japan (17.8%) and South Korea (19.3%) (OECD, 2021). China’s relatively high proportion of drug costs has induced an increasing financial burden on patients. Thus, the Chinese government is currently exploring strategies to contain rapidly growing pharmaceutical spending.
Pharmaceutical expenditures depend on drug prices and drug volume, which were corresponding to the supply and demand sides of drugs respectively (Han et al., 2015). Price reduction strategies were direct measures to reduce pharmaceutical expenditures, which were associated with the supply side of drugs (Hakonsen et al., 2009; Lee et al., 2015; Rodwin, 2020; Yousefi et al., 2020). In China, to lower the prices of procured drugs, the Volume-Based Drug Centralized Procurement National Pilot Policy was officially launched in March 2019, aiming to reduce intermediates and marketing costs, promote marketing mode adjustment, and purify industry ecology. Four municipalities (Beijing, Tianjin, Shanghai, and Chongqing) and seven sub-provincial cities (Shenyang, Dalian, Xiamen, Guangzhou, Shenzhen, Chengdu, and Xi’an) were chosen as pilot cities. Therefore, the Volume-Based Drug Centralized Procurement Pilot Policy is also called the “4 + 7” pilot policy.
In the “4 + 7″ pilot policy, the purchase volume was pre-defined by centralizing the purchase volume from the public medical institutions in 11 selected cities. The Volume-Based Drug Centralized Procurement aimed to achieve lower prices through large-volume procurement, to implement the so-called “volume for price” strategy. It also can be seen as group purchasing which had bargaining power in the drug purchasing process (Noto et al., 2017). The drug supply enterprises reduced drug prices to obtain a larger market. Only one company would win the bidding for each policy-related drug, and the purchasing cycle was 12 months. The drug will be purchased with a bidding price until the purchase cycle expired. The policy-related drugs include branded drugs, generic drugs, and corresponding reference preparations. The quality of policy-related drugs was ensured by Generic Quality Consistency Evaluation (GQCE) approval. The prices of policy-related drugs were chopped, and price cuts ranged from 25 to 96% (Yuan et al., 2021). The massive price cuts dramatically impacted overall drug expenditures.
Depression is characterized by marked and lasting depressed mood and sadness, slow thinking, loss of interest or pleasure, decreased willpower, low self-worth, feelings of tiredness, and poor concentration (Guajardo et al., 2013; Lim et al., 2018). Depression is the leading cause of suicide in China (Phillips et al., 2002; Cheng et al., 2020). In the most severe form of depression, it can lead to suicide and increased risk of mortality (Yang et al., 2013). Furthermore, patients experiencing depression may endure periodic irritation, anxiety, emotional disorders, and/or other mental agonies (Jantaratnotai et al., 2017); therefore, taking the antidepressant for the patients is a relatively long even lasting process. As one of the most common mental disorders, depression is characterized by a significant and continuous low mood state and seriously affects the patients’ learning ability as well as life and social functions (WHO, 2017).
In China, with the rapid economic development, the accelerated pace of modern life, and the increasingly fierce social competition, life pressure is also increasing, and depression has become a common public health problem. With 50 million depression patients in China, the DALYs have increased by 36.5% in the past 30 years (Ren et al., 2020). If appropriate treatment is not applied, patients may develop a disability, premature death, and severe aftermath to their families from depression. However, in a national cross-sectional epidemiological survey from 157 representative points in 31 provinces across China, only 0.5% of participants with depressive disorders were treated adequately (Yu et al., 2021), indicating that only a few people have received adequate treatments. If the treatments of depression are not effective and standardized, it will result in a huge social and economic burden. A previous survey estimated the economic consequences of depressive disorders in China, conducted in five cities (Beijing, Changsha, Chengdu, Shanghai, and Suzhou) which represented the four broad geographic areas in China (North, Central, Southwest, and East Coast regions). As per the result of this survey, the proportion of medication costs in outpatients was 74.01% (Hu et al., 2007). To a certain extent, reducing the burden of drug costs can improve the compliance of depressive disorder patients. In China, national programs are needed to remove barriers to accessibility, availability, and affordability of medication treatment for depression (Yu et al., 2021).
After the “4 + 7″ pilot policy, more patients could receive drug treatments. Considering the incidence of depression in the Chinese population and the economic burden of the disease, this study limits the research scenario to Serotonin-Specific Reuptake Inhibitors (SSRIs) drugs, which are the most recommended treatments for depression according to the second version of the Chinese Guideline for Prevention and Treatment of Depression (Ya-June 2018).
The purpose of this study was to quantitatively evaluate the impacts of the “4 + 7” pilot policy on purchase volume, purchase expenditures, and daily cost and to find the changes in the use of SSRIs. Firstly, this study aimed to verify whether the “4 + 7” pilot policy would lead to a decrease in the overall expenditure of SSRIs. Second, the research team tried to conduct a subgroup analysis for policy-related drugs and alternative drugs, winning drugs and non-winning drugs, generic drugs, and branded drugs. Last but not least, our team members examined the trend of the volumes of and the expenditures on SSRIs and their subgroups.
Materials and methods
Data sources
Data on products purchased between June 2017 and December 2019 were extracted from the Drug Trading Platform of Shenzhen—Shenzhen Group Purchasing Organization (Shenzhen GPO). The team was able to compare the changes in volume, expenditure, and daily costs of drugs for depression treatment after a national-level interference. The project collected monthly drug purchase orders from June 2017 to December 2019 in each medical institution. Each drug purchase order included the code of drug, generic name, dosage form, strengths, procurement unit, price per unit, net quantity of contents, pharmaceutical manufacturer, medical institution, purchase date, purchase volume, purchase expenditures, etc.
Study setting
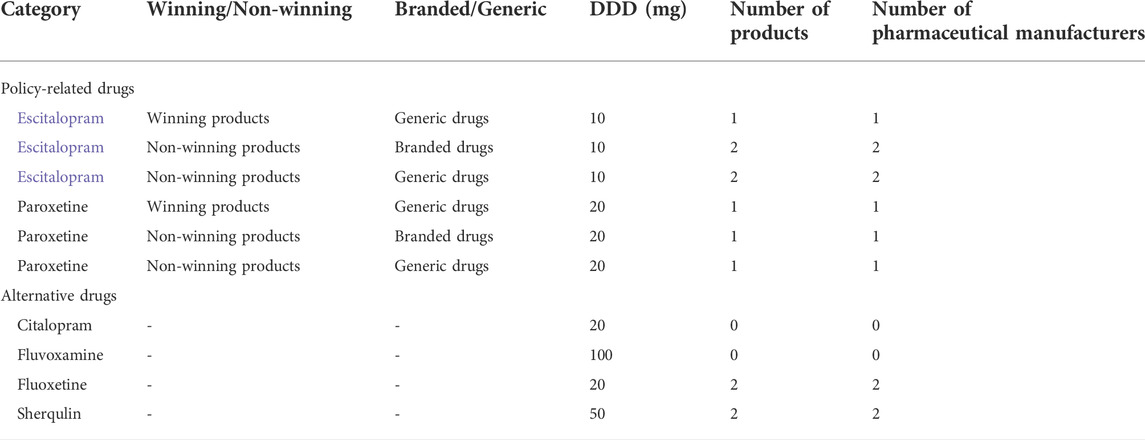
As the first of China’s Special Economic Zones, Shenzhen has undergone unprecedented economic development and social change, which has also led to tremendous changes in disease epidemiology (Gong et al., 2012). Consequently, the prevalence of depressive disorders in Shenzhen was the highest in China (Searle et al., 2019). Shenzhen started to investigate group purchasing strategies for certain types of drugs such as before and became one of 11 pilot cities to carry out drug volume-based purchasing. In this study, the team analyzed the SSRIs class which included Escitalopram, Paroxetine, Citalopram, Fluvoxamine, Fluoxetine, and Sherqulin. Escitalopram and Paroxetine were policy-related drugs. Citalopram, Fluvoxamine, Fluoxetine, and Sherqulin were alternative drugs. Then the research team divided policy-related drugs (including Escitalopram and Paroxetine) into two groups (winning products and non-winning products) based on whether the drugs won the bid in the “4 + 7” pilot policy. Only one company would win the bidding for each policy-related drug. Policy-related drugs were also divided into two subgroups based on whether the drugs were branded drugs or generic drugs. In this article, “product” hereafter designates a distinctive strength of a drug produced by a particular pharmaceutical manufacturer under the same generic name or the same strengths produced by different pharmaceutical manufacturers under the same generic name.
Variables and measurements
The primary measure is aimed at purchase volume, purchase expenditures, and daily costs of drugs. The defined daily dose (DDD) is the average daily dose of a particular drug set for use in adults for the treatment of a primary indication. The DDD of Escitalopram, Paroxetine, Citalopram, Fluvoxamine, Fluoxetine, and Sherqulin were 10, 20, 20, 100, 20, and 50 mg respectively. DDDs were standard measurements to calculate and compare drug purchase volume. According to the WHO Collaborating Centre for Drug Statistics Methodology (WHO, 2021), DDDs were calculated by the formula:
In this formula, the net quantity of contents expresses the numerical count of one specific drug in a marketed inner retail container (usually interchangeably immediate container in China). The net quantity of contents is the number of units of preparation contained in the smallest sales packaging unit. The strength is the amount of drug in the dosage form or a unit of the dosage form (e.g. 10 mg capsule, 20 mg/5 ml suspension). Thus, this formula can conduct calculations (in this case, additions or subtractions) between different products with different net quantities of contents and/or different strengths of drugs with a standardized and unified measurement that multiplied “net quantity of contents” and “strengths”, divided by “Defined Daily Doses,” and finally timed by the total purchased quantity. Each drug purchase order can be measured by DDDs. Then we convert the purchase volume of drugs to DDDs which was a standardized and unified consumption unit for this situation (Wessling and Boethius, 1990; Rodriguez and Vega, 2010). DDDs used as the drug utilization index comparable across regions, countries, and stages, allowing for long-term monitoring and continuous evaluation of drug utilization (Natsch et al., 1998).
The purchase expenditures were calculated by the amount of drug purchase orders in Chinese yuan (CNY). The daily costs of drugs were measured by Defined Daily Dose cost (DDD), a standard measure of the procurement cost of each product (Guan et al., 2018). In this study, DDDc was calculated by the ratio of expenditures and DDDs.
Statistical analysis
Two types of analysis were applied in this study: descriptive analysis and interrupted time-series analysis (ITSA). Descriptive analysis was used to present differences in DDDs, expenditures, and DDDc of SSRIs between before and after implementation of the “4 + 7” pilot, as the policy was effective in March 2019.
The effect of the “4 + 7” pilot policy was evaluated by interrupted time-series (ITSA) with segmented regressions. ITS was the best and most commonly used approach for evaluating the longitudinal effects on interventions occurring at a fixed point of time, e.g. the date on which the policy was implemented (Xiao et al., 2021). Many researchers considered ITS analysis as the most practical quasi-experimental design to evaluate the effects of interventions (Zhao et al., 2021). The model this study uses a linear trend in the outcome within each segment. The specification of the linear regression model to be analyzed is as the following equation:
In this model, Yit is the independent outcome variable (DDDs, expenditures, or DDDc). β0 reflects the baseline level of the outcome, which is a constant. β1 represents the change in the baseline trend that is independent of the intervention, which is the structural trend. β2 captures the change in the level of the outcome, representing SSRIs use after the intervention; and β3 estimates the change in trend in SSRIs use after the intervention. Some previous studies revealed a seasonal trend of depression (Yang et al., 2010; Ayers et al., 2013; Soreni et al., 2019). In this study, the seasonality effect was considered by β4 and β5.
Pre-requisite tests were conducted, for example, unit roots, white noise test, and autocorrelation (Phillips and Perron, 1988; Goodhart et al., 1993). Autocorrelation may lead to underestimated standard errors and overestimated significance of the effects of an intervention. Durbin–Watson statistic was performed to ensure that models adequately corrected for first-order autocorrelation. Values of the Durbin–Watson statistic close to 2.0 indicated the absence of serial autocorrelation (Bohnert et al., 2018). The specific estimation method in the ITS analysis was Newey-West standard errors for coefficients estimated. If autocorrelation is detected, a generalized least squares estimator, such as the Prais–Winsten method, was used to estimate the regression (Wagner et al., 2002; Lagarde, 2012). Data management and analysis were performed using Stata 16.0 (Stata Corporation, College Station, TX, United States). Statistical significance was noted when p-values were less than 0.05.
Results
Descriptive analysis of changes in volume, expenditures, and DDDc
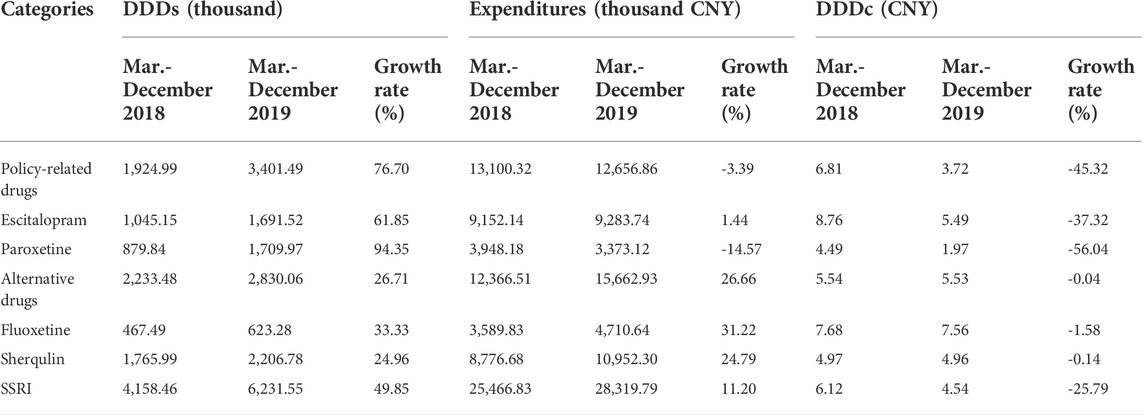
Six SSRIs (Escitalopram, Paroxetine, Citalopram, Fluvoxamine, Fluoxetine, Sherqulin) were included in this study where Escitalopram and Paroxetine were “4 + 7″ policy-related drugs and their alternatives were Citalopram, Fluvoxamine, Fluoxetine and Sherqulin (Table 1). The applied method of analysis was descriptive statistics, which was designed to compare the measures from two periods, which were 10 same selected months (March to December) from 2018 to 2019, since the intervention of the policy was launched in March 2019, and this study sought to conduct an unbiased comparison between pre-and post-intervention of the policy. Descriptive statistics also calculated the growth rate between the two periods. In Table 2, the DDDs indicated that the purchase volume of Escitalopram and Paroxetine increased by 76.70% after the intervention of the “4 + 7” policy; meanwhile, the expenditures and DDDc of Escitalopram and Paroxetine drugs decreased by 3.39%, 45.32% respectively. The DDDs and expenditures on alternative drugs increased by 26.71%, and 26.66% respectively. The DDDs and expenditures of SSRIs increased by 49.85%, and 11.20% respectively. The DDDc of SSRIs decreased by 25.79%. As shown in Supplementary Figures S1, 2 , the DDDs and expenditures of SSRIs increased after the policy intervention. At the same time, the DDDc of SSRIs and policy-related drugs both were decreased (SupplementaryFigure S3).
The DDDs and expenditures of Escitalopram increased by 61.85%, and 1.44%, respectively; the DDDc of Escitalopram decreased by 37.32%. The DDDs of Paroxetine increased by 94.35%; the expenditures and DDDc of Paroxetine decreased by 14.57%, and 56.04%, respectively. The DDDs of Fluoxetine and Sherqulin increased by 33.33%, and 24.96% respectively.
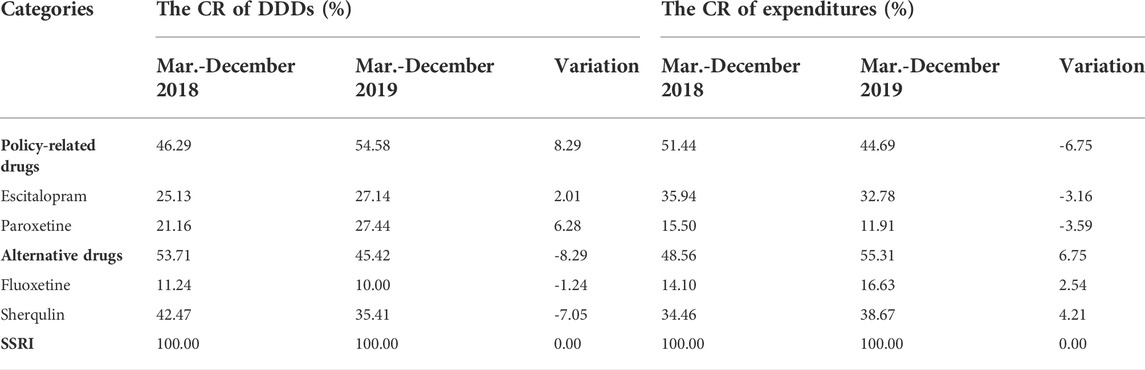
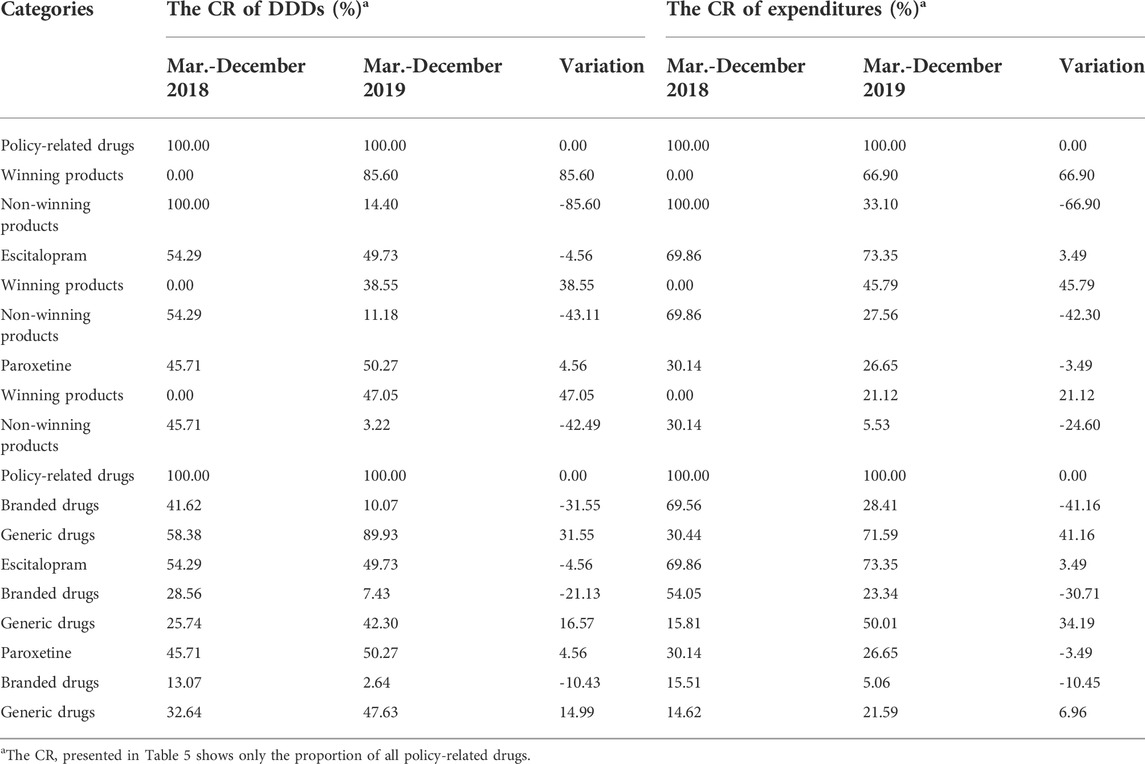
The constituent ratio (CR) of DDDs and expenditures of SSRIs between pre-and post-intervention periods are listed in Table 3. Before implementation of the policy, 25.13% of Escitalopram and 21.16% of Paroxetine comprised SSRIs measured in DDDs, while DDDs of both Escitalopram and Paroxetine increased after the policy was implemented. Especially, DDDs of Paroxetine increased by 6.28 percent. DDDs of policy-related drugs increased by 8.29 percent in the post-intervention period, and expenditures on policy-related drugs dropped by 6.75 percent. Moreover, the DDDs of Sherqulin decreased by 7.05 percentage points, while expenditures raised by 4.21 percentage points with the interference of the “4 + 7” policy.
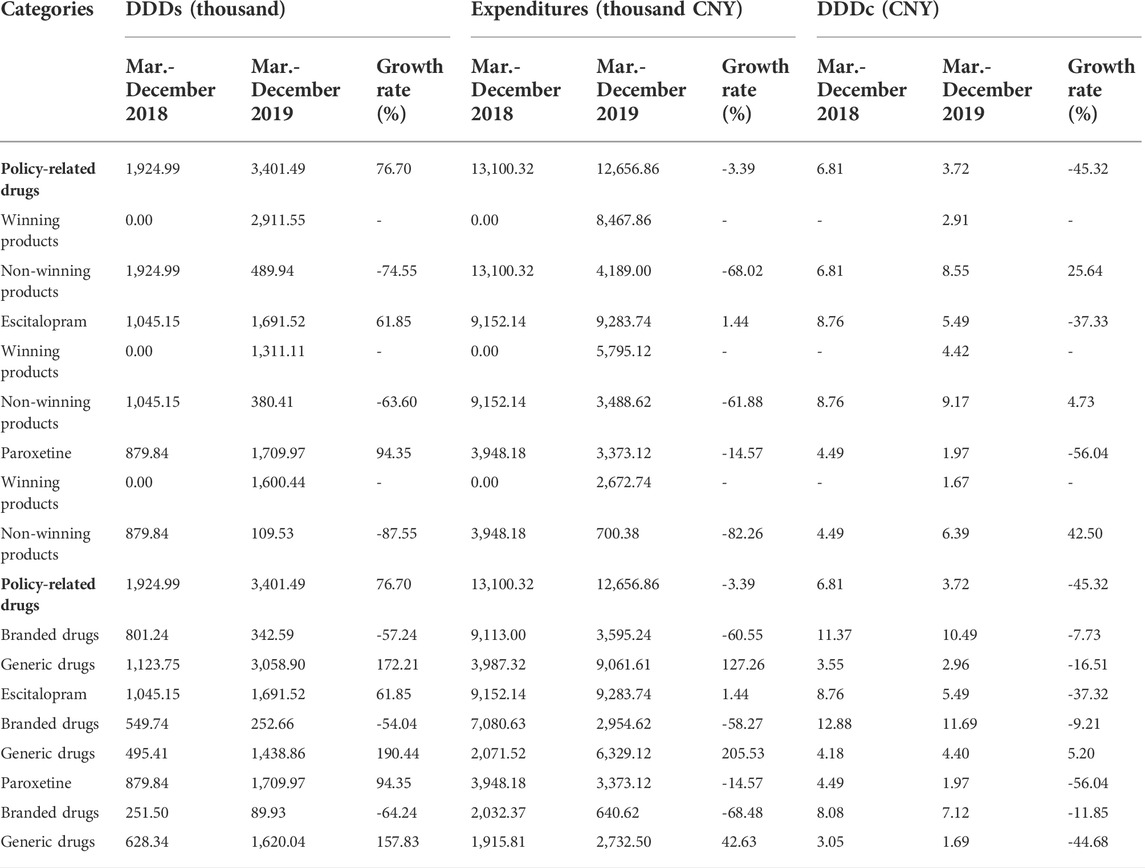
As displayed in Table 4, the DDDs and expenditures of all products in the non-winning group decreased by 74.55%, and 68.02%, respectively. The DDDc of policy-related products in the non-winning group increased by 25.64%. Table 4 proved that the DDDs of Escitalopram in the winning group increased from 0 to 1311.11 thousand. The DDDc of Entecavir, as a winning product, was 4.42 CNY. The DDDs of Paroxetine, another winning product, increased from 0 to 1600.44 thousand, and the DDDc of it was 1.67 CNY. Supplementary Figures S4, 5 revealed that the DDDs and expenditures of winning products were increased which both were 0 before the implementation of the “4 + 7” policy. Supplementary Figure S6 revealed that the DDDc of policy-related drugs decreased.
<On the other hand, for those policy-related generic products, Table 4 indicated that the DDDs and the expenditures of them increased by 172.21%, and 127.26%, respectively, while the DDDc of all policy-related generic products decreased by 16.51%. Moreover, DDDs of generics of Escitalopram increased by 190.44%; meanwhile, expenditures and DDDc of those generics of Escitalopram also increased by 205.53 and 5.20%, respectively.
Supplementary Figures S7, 8 revealed that the DDDs and expenditures of branded drugs were obviously decreased after the implementation of the “4 + 7” policy. Supplementary Figure S9 revealed that the DDDc of policy-related drugs decreased.
Table 5 demonstrated the CR changes of DDDs, expenditures, and DDDc of winning and non-winning products, branded and generic products between pre-and post-intervention periods. Before the implementation of the “4 + 7” pilot policy, in public hospitals, the market share was 0 for some specific products of Escitalopram and Paroxetine in the winning group but after the implementation of the policy, these products of Escitalopram and Paroxetine achieved significant growth with market shares of 38.55 and 47.05%, respectively. The market share of policy-related generic products was 58.38% before intervention. Finally, the market share of Escitalopram and Paroxetine generic products in SSRIs measured by DDDs increased by 16.57 and 14.99%, respectively.
ITS analysis of changes in DDDs, expenditures, and DDDc
Table 6 represented the results of the segmented linear analysis with ITS. The DDDc of SSRIs dropped by 1.56 yuan (p < 0.001) with the “4 + 7” pilot policy.
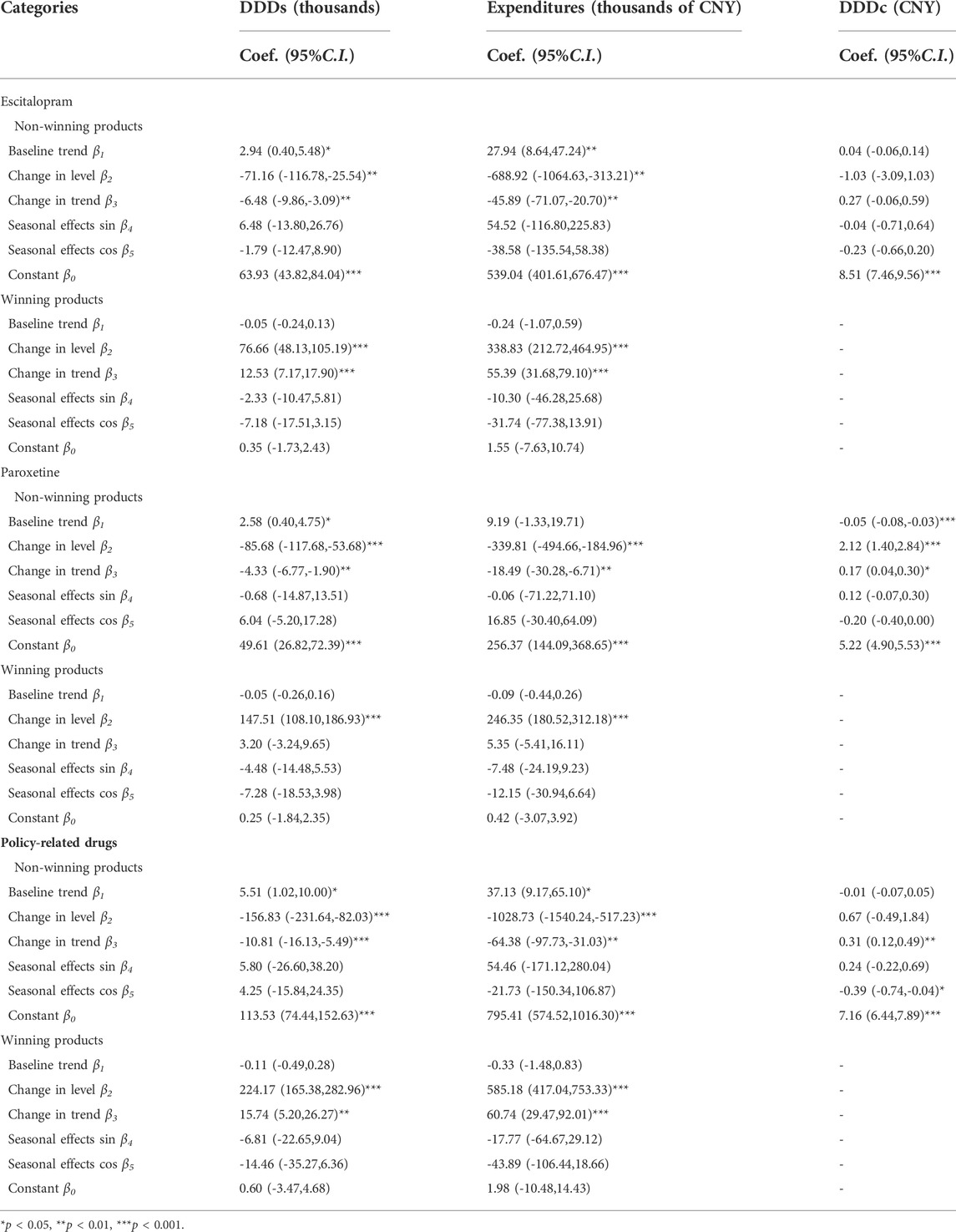
The DDDs of policy-related drugs has a baseline trend increased before the “4 + 7” pilot policy by 5.41 thousand (p < 0.05). The expenditures on policy-related drugs (β1 = 36.81, p < 0.05) represented an increasing baseline trend. And the expenditures on policy-related drugs showed decreasing in level with statistical significance (β3 = -443.55, p < 0.05). Moreover, the DDDc of those drugs had a substantial drop of 2.93 CNY (p < 0.001) after implementing the policy, but the change in trend after the intervention had only decreased by 0.02 CNY with no statistical significance.
The trend coefficient indicated that DDDs of Escitalopram increased after the implementation of the policy (β3 = 6.06, p < 0.05). The expenditures of Escitalopram with level coefficient: β2 = -350.09 (p<0.05), on the other hand, decreased. DDDc of Escitalopram showed a positive relation to expenditure, which also dropped by 3.22 CNY(p < 0.001). Similarly, DDDs of Paroxetine increased by the influence of the policy with a level coefficient: β2 = 61.84 and p < 0.01. DDDc of Paroxetine dropped by 1.90 CNY (p < 0.001) after the policy was launched.
The model for alternative drugs suggested that over the period studied, the baseline trend was a 4.86 thousand increase in the DDDs per month (p < 0.05). The analysis showed a change in the baseline trend of expenditures of a 28.90 thousand CNY increase. And Fluoxetine had a similar change in the baseline trend.
Overall, for SSRIs, the post-intervention period presented an increase in DDDs, which level coefficient (β2) equaled 31.17, and trend coefficient (β3) equaled 12.77, but the p-value both were more than 0.05. The expenditures demonstrated a decreasing trend with no statistical significance (level coefficient: β2 = -655.02, p > 0.05). After the intervention, there was a significant decline in DDDc since the level coefficient was equaled to -1.56 (p < 0.001).
Table 7 indicated that DDDs of non-winning products significantly decreased as shown in the regression with a level coefficient (β2) of -156.83 (p < 0.001) and trend coefficient (β3) of -10.81 (p < 0.001). As well as the expenditures on non-winning products they also had a significant decrease with a level coefficient (β2) of -1028.73 (p < 0.001) and trend coefficient (β3) of -64.38 (p < 0.001). Furthermore, DDDc had an increase of 0.31 CNY (p < 0.01) after the implementation of the policy. Table 7 also indicated that DDDs of non-winning products in Escitalopram and Paroxetine significantly decreased as shown in the regression with level coefficient and trend coefficient (p < 0.01).
Table 7 indicated that DDDs of winning products significantly increased as shown in the regression with a level coefficient (β2) of -224.17 (p < 0.001) and trend coefficient (β3) of 15.74 (p < 0.001). As well to the expenditures of winning products they also had a significant increase with a level coefficient (β2) of 585.18 (p < 0.001) and trend coefficient (β3) of 60.74 (p < 0.001). The winning products in Escitalopram and Paroxetine The winning products in Escitalopram showed similar changes. The winning products in Paroxetine only showed an increase in level.
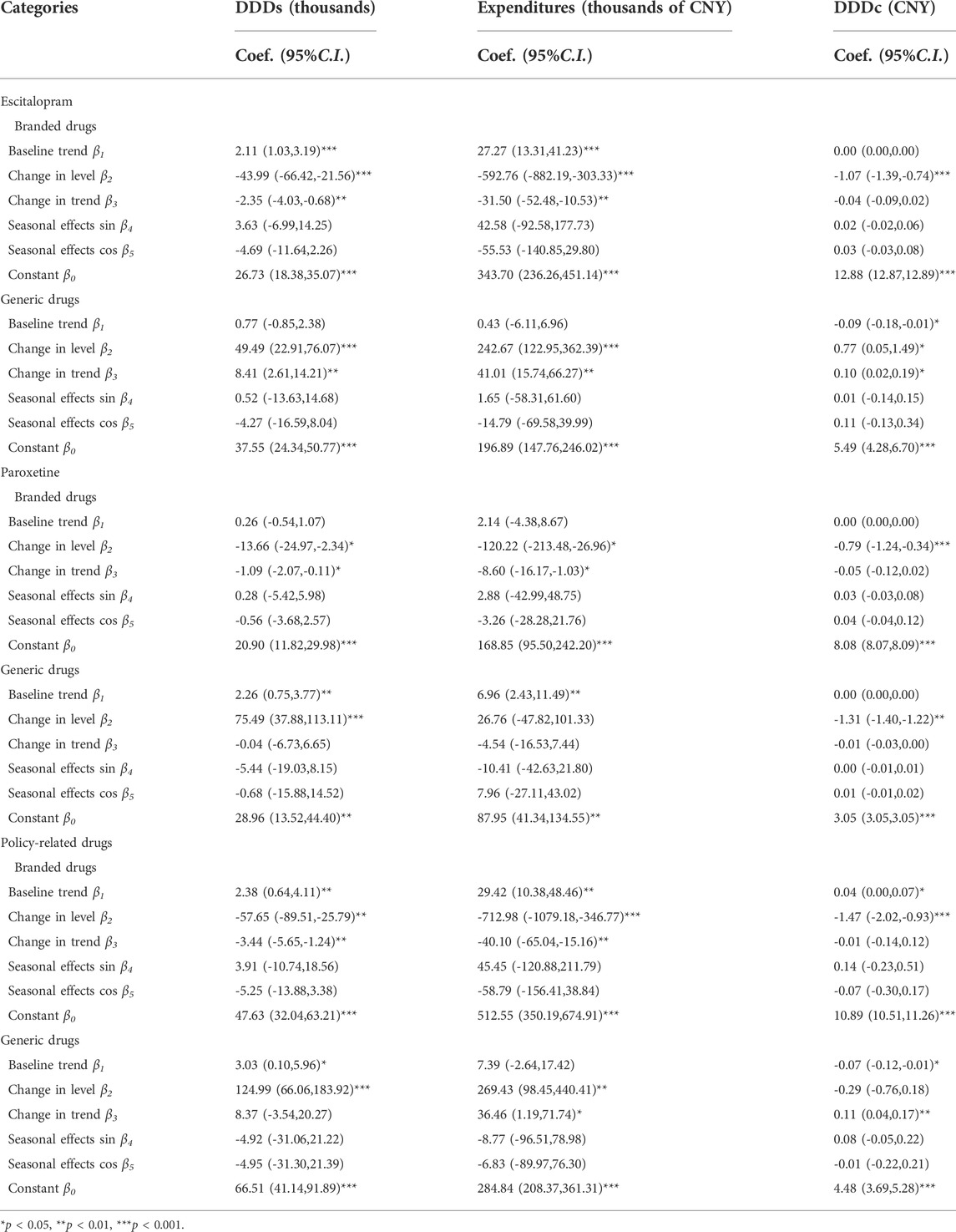
Table 8 demonstrated that both DDDs and expenditures on branded products showed a significant decrease in the regression in the post-intervention period (level coefficient of DDDs: β2 = -57.65, p < 0.01, trend coefficient of DDDs: β3 = -3.44, p < 0.01; level coefficient of expenditure: β2 = -712.98, p < 0.01, trend coefficient of expenditure: β3 = -40.10, p < 0.01). The DDD of branded products shrunk as shown in Table 8 (level coefficient: β2 = -1.47, p < 0.001). The branded drugs in Escitalopram and Paroxetine have similar changes.
Table 8 demonstrated that both DDDs and expenditures of generic products showed a significant increase in the regression in the post-intervention period (level coefficient of DDDs: β2 = 124.99, p < 0.001; level coefficient of expenditure: β2 = 269.43, p < 0.01, trend coefficient of expenditure: β3 = -40.10, p < 0.01). But the DDDc of generic products increased as shown in Table 8 (trend coefficient: β3 = 0.11, p < 0.01). The generic drugs in Escitalopram have similar changes.
Discussion
The “4 + 7” pilot policy has shown the initial success of lowering prices by government-oriented group purchases. Thus, this study analyzed the effect of the “4 + 7” pilot policy on the daily cost of SSRIs in Shenzhen. For example, the DDD of SSRIs decreased with the “4 + 7” pilot policy by 1.56 yuan (p < 0.001). The DDD of policy-related drugs had an immediate drop of 2.93 yuan (95%CI -3.68 to -2.19, p < 0.001) under the “4 + 7” pilot policy. The “4 + 7” pilot policy was designed to achieve lower prices through competitive bidding processes between accredited generic drug manufacturers.
The reduced price of drugs may improve the accessibility of drugs. A previous survey revealed that the proportion of medication costs in outpatient costs was 74.01%, conducted in five cities (Beijing, Changsha, Chengdu, Shanghai, and Suzhou) (Hu et al., 2007). The result revealed that the proportion of medication costs in outpatient costs was 86.14%, which surveyed 652 outpatients with depression in Shanghai (Zhou Xuedong et al., 2008). Reducing the burden of drug costs may improve the compliance of depressive disorder patients. Medicine’s price control measures were used to increase medicine’s affordability (Rawson, 2020). This study revealed that the “4 + 7” pilot policy led to an increase in the total volume of SSRIs, as well as each of the four study medications. The DDDs of SSRIs increased by 49.85%. The result has shown a 76.70% increase in the purchased volume of policy-related drugs and a 3.39% decrease in the expenditure on policy-related drugs. Over the first 9 months of implementation, this study found that the “4 + 7” pilot policy increased the proportion of purchasing policy-related drugs. It was consistent with the result of the study conducted in one of 11 pilot cities Dalian (Sheng Liang-Liang and Hu, 2019).
New Zealand controlled pharmaceutical expenditures by a combination of strong negotiation, bundling agreements, tendering sole supply, and contracts. Then it resulted in immediate savings on pharmaceutical expenditures with up to 90 percent on some drugs, despite a 50% increase in volumes (Lybecker, 2013). After the implementation of the “4 + 7” pilot policy, policy-related drugs decreased by 443.55 thousand CNY. The “4 + 7” pilot policy led to significant savings and improvement in the efficient resource allocation of the healthcare system, which was consistent with previous studies (Qi et al., 2020; Yang et al., 2021).
But the policy effects were smaller observed in SSRIs, including price reductions, cost-saving, unleashing medication demand, and improving accessibility. A previous study revealed that the largest reduction in spending occurred on drugs for the treatment of cardiovascular diseases in the “4 + 7” pilot policy (Chen et al., 2021). Another study in Shenzhen revealed that the post-intervention period witnessed a significant increase in the regression level for nucleos(t)ide analogs DDDs (level coefficient: β2 = 631.87, p < 0.05). The expenditures (trend coefficient: β3 = 392.24, p < 0.05) and DDDc (level coefficient: β2 = −6.17, p < 0.001; trend coefficient: β3 = −0.21, p < 0.05) of NAs showed decreasing trend in the post-intervention period (Wen et al., 2021). It may be due to SSRIs having less market competition because of fewer generic drug manufacturers, as well as less willingness for healthcare providers to clinical conversion in patients taking antidepressant medication for a long time (Yang et al., 2021). The volume and expenditures of alternative drugs increased after the “4 + 7” pilot policy. It was a side effect of pharmaceutical policies (Kwon et al., 2013; Kwon et al., 2019; Chen et al., 2020; Yang et al., 2021). In this study, the volume and expenditures of alternative drugs didn’t show statistic significant changes in the interrupted time-series analysis. Most of depression patients got prescription based on a doctor’s diagnosis. We need monitor the using of SSRIs for a long term to ensure rational use of drugs.
The volume-based procurement policy is aimed at reducing pharmaceutical expenditures by creating economies of scale and improving purchasing power (Seidman and Atun, 2017). On the one hand, pharmaceutical companies offered lower prices in exchange for a larger volume of purchases, given the result of winning drugs replacing the non-winning drugs. Winning products were given priority to use, which resulted in putting winning products in the place of non-winning products (Jialing et al., 2021). This study found that the “4 + 7” pilot policy increased the proportion of purchasing winning drugs, with an increment of 85.60 percent. The volume of non-winning products had a significant decrease (β2 = -156.83, p < 0.001; β3 = -10.81, p < 0.001). The volume of non-winning products experienced attenuation following the entry of winning products, and both Escitalopram and Paroxetine decreased. Because all public medical institutions (including public hospitals and government-run primary healthcare centers) in the “4 + 7″ pilot cities need to give priority to using drugs which won the bidding.
Volume-based procurement policy potentially reshaped the market share of pharmaceuticals by substituting branded with generic drugs (World Health Organization, 2007; Waning et al., 2009; Lybecker, 2013). The proportion of branded drugs decreased by 31.55 percent. Only one company would win the bidding for each policy-related drug in the “4 + 7” pilot policy. And the company won the bidding in the “4 + 7” pilot policy for Escitalopram and Paroxetine both were generic products. All public hospitals and primary healthcare centers in the “4 + 7″ pilot cities gave priority to using drugs that won the bidding. In this way, the decrease mainly occurred in branded drugs. The promotion of generic drugs using was a commonly used strategy, which could improve medicine’s affordability and accessibility. Most of the Association of Southeast Asian Nations (ASEAN) countries also applied generic medicine promotion, which can enhance the use of much cheaper generic medicines (You et al., 2019). The result revealed that the volume of branded drugs both showed a significant decrease in the regression level and trend in the post-intervention period (β2 = -57.65, p < 0.01; β3 = -3.44, p < 0.01). The volume-based procurement policy accredited generics in place of off-patent branded drugs, which also resulted in lower SSRIs total drug purchasing costs. It was consistent with previous studies (Dylst et al., 2015; Wouters et al., 2017). The volume-based procurement policy relieves the overall drug burden on patients (Son, 2021). It also accomplished the goal of controlling drug costs (Nunes et al., 2020). For example, generic substitution was compulsory in Greece (Wouters et al., 2017). Policymakers usually require generic prescribing and substitution to achieve significant savings in the United States. They also streamline the generic drug approval process for this purpose.
The implementation of the “4 + 7” pilot policy, improves the quality of medicines because the generic drugs winning the bid got generic quality consistency evaluation approval (Lijun, 2019). Generic drugs which did not get generic quality consistency evaluation approval would be out of the market very soon. Some small pharmaceutical companies could not take part in the bidding or lost the bid. Then they may stop manufacturing and exit the market (Hu et al., 2015). The volume-based procurement policy drove small drug manufacturers with inferior research and production capacity out of business.
However, it is unclear whether the lowest price for a drug will always be the best value, and it is an issue that many purchasers must consider (van Valen et al., 2018). In the “4 + 7” pilot policy, sole supply may cause drug shortages (Zhang, 2019). The researchers also found that later rounds of volume-based procurement have to change the number of pharmaceuticals in the bidding rules. Considering all other factors will lead to the timely, reliable delivery of safe, high-quality products, and ultimately result in lower prices from increased competition. Big data analytics might help set reasonable cap prices and monitor the real-world data and evidence to support price negotiations for procurement. To deliver safe and cost-effective medicines, it was necessary to systematically evaluate the effectiveness, safety and economics are necessary (Li et al., 2018). With the moving toward value-based medical, health decision-making pays more attention to Health Technology Assessment. In volume-based procurement. Health Technology Assessment could serve as an effective tool based on real-world data.
More rounds of volume-based procurement have been rapidly carried out in the country, and assessing long-term trends in volume and expenditure is significant. Evaluation of the effect of policy could guide policymakers, healthcare providers, and patients to better understand the reform and adapt accordingly. And it is still important to evaluate the policy effects on special disease categories by assessing further data from more rounds of volume-based procurements.
The main strength of this study was using ITS quantitative analysis of the impact of the “4 + 7″ pilot policy. It may be a valuable reference for policy effect evaluation. It offered suggestions for policy promotion.
Limitations
The present study had some limitations that should be borne in mind when interpreting the results. First, one of the limitations was the lack of inclusion of drugstores, as one of the main stakeholders of the pharmaceutical industry in the study. The reason was only public medical institutions were included in the purchasing alliance in the “4 + 7″ pilot policy. Second, this study only used 9 months of time series data post-intervention. In exploring the long-term trend of the “4 + 7″ pilot policy, it would be better if this study could get access to all pilot cities as research objects and full purchasing cycle as research time points. The purchasing cycle was 12 months. The drug will be purchased with a bidding price until the purchase cycle expired. We didn’t get purchasing records for the other 3 months during purchasing cycle at present. But the trend would same as the result of this study.
Conclusion
The volume-based procurement has successfully led to price reductions and improved the affordability of medicines, especially for those with chronic diseases. The volume-based procurement has demonstrated initial success in reshaping the composition of the Chinese pharmaceutical market in favor of generics with high quality and low prices. Future studies are needed to investigate the long-term impact of the volume-based procurement policy on various outcomes, such as patient outcomes, drug utilization, and changes in the pharmaceutical industry.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material further inquiries can be directed to the corresponding authors.
Author contributions
Author Contributions: Conceptualization, ZM, DC, and XW; Formal analysis, XW; Funding acquisition, ZM and DC; Investigation, XW, ZW, LX, JL, XG, XC, and YY; Methodology, XW and YY; Project administration, ZM, DC, and XW; Supervision, ZM and DC; Writing—original draft, XW and ZW; Writing—review and editing, XW, ZW, LX, JL, XG, XC, and YY; All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the School of Public Health and the Global Health Institute of Wuhan University, China.
Acknowledgments
The project administration and supervision were the School of Public Health and the Global Health Institute of Wuhan University. We would like to express our great appreciation to the collaborating agencies, including Shenzhen Municipal Medical Security Bureau. We also like to thank all the teachers and students who took part in the research design and the field investigation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.829660/full#supplementary-material
Abbreviations
CNY, Chinese yuan renminbi; OECD, Organization for Economic Co-operation and Development; DDD, Defined Daily Dose DDDc, Defined Daily Dose cost; ITS, Interrupted time-series.
References
Ayers, J. W., Althouse, B. M., Allem, J. P., Rosenquist, J. N., and Ford, D. E. (2013). Seasonality in seeking mental health information on google. Am. J. Prev. Med. 44 (5), 520–525. doi:10.1016/j.amepre.2013.01.012
Babar, Z. U. D., Ramzan, S., El-Dahiyat, F., Tachmazidis, I., Adebisi, A., and Hasan, S. S. (2019). The availability, pricing, and affordability of essential diabetes medicines in 17 low-middle-and high-income countries. Front. Pharmacol. 10, 1375. doi:10.3389/fphar.2019.01375
Bohnert, A. S. B., Guy, G. P., and Losby, J. L. (2018). Opioid prescribing in the United States before and after the centers for disease control and prevention's 2016 opioid guideline. Ann. Intern. Med. 169 (6), 367–375. doi:10.7326/m18-1243
Cameron, A., Ewen, M., Ross-Degnan, D., Ball, D., and Laing, R. (2009). Medicine prices, availability, and affordability in 36 developing and middle-income countries: A secondary analysis. Lancet 373 (9664), 240–249. doi:10.1016/S0140-6736(08)61762-6
Chen, L., Yang, Y., Luo, M., Hu, B. R., Yin, S. C., and Mao, Z. F. (2020). The impacts of national centralized drug procurement policy on drug utilization and drug expenditures: The case of shenzhen, China. Int. J. Environ. Res. Public Health 17 (24), E9415. doi:10.3390/ijerph17249415
Chen, Y., Ji, X., Xiao, H., Unger, J. M., Cai, Y., Mao, Z., et al. (2021). Impact of the pilot volume-based drug purchasing policy in China: Interrupted time-series analysis with controls. Front. Pharmacol. 12, 804237. doi:10.3389/fphar.2021.804237
Cheng, Y., Zhang, X. M., Ye, S. Y., Jin, H. M., and Yang, X. H. (2020). Suicide in Chinese graduate students: A review from 2000 to 2019. Front. Psychiatry 11, 579745. doi:10.3389/fpsyt.2020.579745
China National Health Development Research Center (2018). Abstract of China national health accounts. National Health Commission, China Health Statistical Yearbook 2018.
Dylst, P., Vulto, A., and Simoens, S. (2015). Societal value of generic medicines beyond cost-saving through reduced prices. Expert Rev. pharmacoecon. Outcomes Res. 15 (4), 701–711. doi:10.1586/14737167.2015.1017565
Gong, P., Liang, S., Carlton, E. J., Jiang, Q. W., Wu, J. Y., Wang, L., et al. (2012). Urbanisation and health in China. Lancet 379 (9818), 843–852. doi:10.1016/s0140-6736(11)61878-3
Goodhart, C. A. E., McMahon, P. C., and Ngama, Y. L. (1993). Testing for unit roots with very high-frequency spot exchange-rate data. J. Macroecon. 15 (3), 423–438. doi:10.1016/0164-0704(93)90002-4
Guajardo, V. D., Souza, B. P., Henriques, S. G., Lucia, M. C., and Health, R. F. J. B. P. (2013). Loss of interest, depressed mood and impact on the quality of life: Cross-sectional survey. BMC Public Health 11 (1), 826. doi:10.1186/1471-2458-11-826
Guan, X. D., Tian, Y., Ross-Degnan, D., Man, C. X., and Shi, L. W. (2018). Interrupted time-series analysis of the impact of generic market entry of antineoplastic products in China. BMJ Open 8, e022328. doi:10.1136/bmjopen-2018-022328
Hakonsen, H., Horn, A. M., and Toverud, E. L. (2009). Price control as a strategy for pharmaceutical cost containment-What has been achieved in Norway in the period 1994-2004? Health Policy 90 (2-3), 277–285. doi:10.1016/j.healthpol.2008.09.018
Han, E., Chae, S. M., Kim, N. S., and Park, S. (2015). Effects of pharmaceutical cost containment policies on doctors' prescribing behavior: Focus on antibiotics. Health Policy 119 (9), 1245–1254. doi:10.1016/j.healthpol.2015.05.005
Hu, S., Zhang, Y., He, J., Du, L., Xu, M., Xie, C., et al. (2015). A case study of pharmaceutical pricing in China: Setting the price for off-patent originators. Appl. Health Econ. Health Policy 13 (1), S13–S20. doi:10.1007/s40258-014-0150-5
Hu, T. W., He, Y. L., Zhang, M. Y., and Chen, N. S. (2007). Economic costs of depression in China. Soc. Psychiatry Psychiatr. Epidemiol. 42 (2), 110–116. doi:10.1007/s00127-006-0151-2
Hunsberger, S., Albert, P. S., Follmann, D. A., and Suh, E. (2002). Parametric and semiparametric approaches to testing for seasonal trend in serial count data. Biostatistics 3 (2), 289–298. doi:10.1093/biostatistics/3.2.289
Jantaratnotai, N., Mosikanon, K., Lee, Y., and McIntyre, R. S. (2017). The interface of depression and obesity. Obes. Res. Clin. Pract. 11 (1), 1–10. doi:10.1016/j.orcp.2016.07.003
Jialing, L., Jian, W., Yan, Q., and Zhu, J. (2021). Analysis of utilization of statins in the Shanghai pudong new area people’s hospital based on “4+7” quantified purchasing. Pharm. Care Res. 21 (01), 68–71. doi:10.5428/pcar20210116
Kwon, H. Y., Bae, S., Choi, S. E., Park, S., Lee, E. K., Park, S., et al. (2019). Easy cuts, easy rebound: Drug expenditures with massive price cuts in Korea. Health Policy 123 (4), 388–392. doi:10.1016/j.healthpol.2018.11.002
Kwon, H. Y., Hong, J. M., Godman, B., and Yang, B. M. (2013). Price cuts and drug spending in South Korea: The case of antihyperlipidemic agents. Health Policy 112 (3), 217–226. doi:10.1016/j.healthpol.2013.08.011
Lagarde, M. (2012). How to do (or not to do). Assessing the impact of a policy change with routine longitudinal data. Health Policy Plan. 27 (1), 76–83. doi:10.1093/heapol/czr004
Lee, I. H., Bloor, K., Hewitt, C., and Maynard, A. (2015). International experience in controlling pharmaceutical expenditure: Influencing patients and providers and regulating industry - a systematic review. J. Health Serv. Res. Policy 20 (1), 52–59. doi:10.1177/1355819614545675
Li, H., Liu, G. G., Wu, J., Wu, J. H., Dong, C. H., and Hu, S. L. (2018). Recent pricing negotiations on innovative medicines pilot in China: Experiences, implications, and suggestions. Value Health Reg. Issues 15, 133–137. doi:10.1016/j.vhri.2018.01.009
Lijun, M. (2019). Analysis on the pilot operation of drug centralized procurement and use in shenyang. China Health Insur. 7 (08), 28–31. doi:10.19546/j.issn.1674-3830.2019.8.007
Lim, G. Y., Tam, W. W., Lu, Y. X., Ho, C. S., Zhang, M. W., and Ho, R. C. (2018). Prevalence of depression in the community from 30 countries between 1994 and 2014. Sci. Rep. 8, 2861. doi:10.1038/s41598-018-21243-x
Lybecker,, Kristina M. (2013). The bulk purchase of pharmaceuticals: The experiences of the United States, europe, and New Zealand. Studies in Health Policy. Fraser Institute.
Natsch, S., Hekster, Y. A., de Jong, R., Heerdink, E. R., Herings, R. M. C., and van der Meer, J. W. M. (1998). Application of the ATC/DDD methodology to monitor antibiotic drug use. Eur. J. Clin. Microbiol. Infect. Dis. 17 (1), 20–24. doi:10.1007/bf01584358
Noto, K., Kojo, T., and Innami, I. (2017). Does scale of public hospitals affect bargaining power? Evidence from Japan. Int. J. Health Policy Manag. 6 (12), 695–700. doi:10.15171/ijhpm.2017.29
Nunes, A. M., Ferreira, D. C., de Matos, A., and Juliao, R. M. (2020). The Portuguese generic medicines market: What's next? Health Policy 124 (4), 397–403. doi:10.1016/j.healthpol.2020.02.014
OECD (2021). Pharmaceutical spending (indicator) [Online]. Available at: https://www.oecd-ilibrary.org/content/data/998febf6-en [Accessed May 1st 2021].
Papanicolas, I., Woskie, L. R., and Jha, A. K. (2018). Health care spending in the United States and other high-income countries. Jama-Journal Am. Med. Assoc. 319 (10), 1024–1039. doi:10.1001/jama.2018.1150
Parente, S. T. (2018). Factors contributing to higher health care spending in the United States compared with other high-income countries. Jama-Journal Am. Med. Assoc. 319 (10), 988–990. doi:10.1001/jama.2018.1149
Phillips, M. R., Li, X. Y., and Zhang, Y. P. (2002). Suicide rates in China, 1995-99. Lancet 359 (9309), 835–840. doi:10.1016/s0140-6736(02)07954-0
Phillips, P. C. B., and Perron, P. (1988). Testing for a unit-root in time-series regression. Biometrika 75 (2), 335–346. doi:10.1093/biomet/75.2.335
Qi, Y., Yannan, Z., Hongyan, Z., and Wei, G. (2020). Effects of “4+7” procurement with target quantity on the using of antidepressants in a hospital. Chin. J. Hosp. Pharm. 40 (13), 1479–1483. doi:10.13286/j.1001-5213.2020.13.16
Rawson, N. S. B. (2020). National pharmacare in Canada: Equality or equity, accessibility or affordability comment on "universal pharmacare in Canada: A prescription for equity in healthcare". Int. J. Health Policy Manag. 9 (12), 524–527. doi:10.15171/ijhpm.2019.146
Ren, X. W., Yu, S. C., Dong, W. L., Yin, P., Xu, X. H., and Zhou, M. G. (2020). Burden of depression in China, 1990-2017: Findings from the global burden of disease study 2017. J. Affect. Disord. 268, 95–101. doi:10.1016/j.jad.2020.03.011
Rodriguez, J. A. B., and Vega, O. A. G. (2010). Useffulness of cost per defined daily dose (DDD) to identify problematic drugs in medium- and high-level complexity hospitals from Colombia. Value Health 13 (3), A93. doi:10.1016/S1098-3015(10)72445-1
Rodwin, M. A. (2021). How the United Kingdom controls pharmaceutical prices and spending: Learning from its experience. Int. J. Health Serv. 51 (2), 229–237. doi:10.1177/0020731421997094
Rodwin, M. A. (2020). Pharmaceutical price and spending controls in France: Lessons for the United States. Int. J. Health Serv. 50 (2), 156–165. doi:10.1177/0020731419897580
San-Juan-Rodriguez, A., Gellad, W. F., Shrank, W. H., Good, C. B., and Hernandez, I. (2021). A decade of increases in medicare Part B pharmaceutical spending: What are the drivers? J. Manag. Care Spec. Pharm. 27 (5), 565–573. doi:10.18553/jmcp.2021.27.5.565
Science HD (2019). “The global use of medicine in 2019 and outlook to 2023,” in Forecasts and areas to watch (Institute T. I., The IQVIA Institute).
Searle, K., Blashki, G., Kakuma, R., Yang, H., Zhao, Y. L., and Minas, H. (2019). Current needs for the improved management of depressive disorder in community healthcare centres, shenzhen, China: A view from primary care medical leaders. Int. J. Ment. Health Syst. 13, 47. doi:10.1186/s13033-019-0300-0
Seidman, G., and Atun, R. (2017). Do changes to supply chains and procurement processes yield cost savings and improve availability of pharmaceuticals, vaccines or health products? A systematic review of evidence from low-income and middle income countries. BMJ Glob. Health 2, e000243. doi:10.1136/bmjgh-2016-000243
Sheng Liang-Liang, L. X.-H., and Hu, X.-Y. (2019). Application of nucleoside anti-hepatitis B drugs based on “4+7” quantified purchasing. China J. Pharm. Econ. 14 (07), 36–39. doi:10.12010/j.issn.1673-5846.2019.07.007
Son, K.-B. (2021). Understanding long-listed pharmaceutical products without competition in South Korea: Policy implications in managing generic entrants and pharmaceutical expenditures. Expert Rev. pharmacoecon. Outcomes Res. 22, 815–822. doi:10.1080/14737167.2021.1908890
Soreni, N., Cameron, D. H., Streiner, D. L., Rowa, K., and McCabe, R. E. (2019). Seasonality patterns of internet searches on mental health: Exploratory infodemiology study. Jmir Ment. Health 6 (4), e12974. doi:10.2196/12974
van Valen, M., Jamieson, D., Parvin, L., and Ramirez, C. L. (2018). Dispelling myths about drug procurement policy. Lancet. Glob. Health 6 (6), E609–E610. doi:10.1016/s2214-109x(18)30190-6
Wagner, A. K., Soumerai, S. B., Zhang, F., and Ross-Degnan, D. (2002). Segmented regression analysis of interrupted time series studies in medication use research. J. Clin. Pharm. Ther. 27 (4), 299–309. doi:10.1046/j.1365-2710.2002.00430.x
Waning, B., Kaplan, W., King, A. C., Lawrence, D. A., Leufkens, H. G., and Fox, M. P. (2009). Global strategies to reduce the price of antiretroviral medicines: Evidence from transactional databases. Bull. World Health Organ. 87 (7), 520–528. doi:10.2471/blt.08.058925
Wen, X. T., Yin, S. C., Cui, L. Y., Mao, L. N., Lin, Z. Y., Yaermaimaiti, Z., et al. (2021). The effects of the national centralized drug purchasing pilot program on nucleos(t)ide analogs in shenzhen city: An interrupted time series analysis. Front. Public Health 9, 718013. doi:10.3389/fpubh.2021.718013
Wessling, A., and Boethius, G. (1990). Measurement of drug-use in A defined population - evaluation of the defined daily dose (DDD) methodology. Eur. J. Clin. Pharmacol. 39 (3), 207–210. doi:10.1007/bf00315097
WHO (2021). ATC/DDD index [online]. Oslo, Norway: WHO Collaborating Centre for Drug Statistics Methodology. Available: https://www.whocc.no/atc_ddd_index/[Accessed 6th, May 2021].
WHO (2017). Depression and other common mental disorders: Global health estimates. Geneva: World Health Organization.:
World Health Organization (2007). Multi-country regional pooled procurement of medicines: Identifying key principles for enabling regional pooled procurement and a framework for inter-regional collaboration in the african, caribbean and pacific island countries. Geneva: WHO.
Wouters, O. J., Kanavos, P. G., and McKee, M. (2017). Comparing generic drug markets in europe and the United States: Prices, volumes, and spending. Milbank Q. 95 (3), 554–601. doi:10.1111/1468-0009.12279
Xiao, H., Augusto, O., and Wagenaar, B. H. (2021). Reflection on modern methods: A common error in the segmented regression parameterization of interrupted time-series analyses. Int. J. Epidemiol. 50 (3), 1011–1015. doi:10.1093/ije/dyaa148
Ya-Jun, N. (2018). Interpretation of drug therapy of Chinese guidelines for prevention and treatment of depression. Clin. Medicat. J. 16 (5), 3. doi:10.3969/j.issn.1672-3384.2018.05.002
Yang, A. C., Huang, N. E., Peng, C. K., and Tsai, S. J. (2010). Do seasons have an influence on the incidence of depression? The use of an internet search engine query data as a proxy of human affect. PLoS One 5, e13728. doi:10.1371/journal.pone.0013728
Yang, G. H., Wang, Y., Zeng, Y. X., Gao, G. F., Liang, X. F., Zhou, M. G., et al. (2013). Rapid health transition in China, 1990-2010: Findings from the global burden of disease study 2010. Lancet 381 (9882), 1987–2015. doi:10.1016/s0140-6736(13)61097-1
Yang, Y., Chen, L., Ke, X. F., Mao, Z. F., and Zheng, B. (2021). The impacts of Chinese drug volume-based procurement policy on the use of policy-related antibiotic drugs in shenzhen, 2018-2019: An interrupted time-series analysis. BMC Health Serv. Res. 21 (1), 668. doi:10.1186/s12913-021-06698-5
You, H. W., Tajuddin, N. S. A., and Anwar, Y. (2019). Measuring availability, prices and affordability of ischaemic heart disease medicines in bangi, selangor, Malaysia. Malays. J. Med. Sci. 26 (5), 113–121. doi:10.21315/mjms2019.26.5.10
Yousefi, N., Moghaddam, M. P., Afsharmanesh, G., and Peiravian, F. (2020). Evaluation of efficiency enhancement in Iran health insurance organization: A policy brief for pharmaceutical cost containment. Int. J. Health Plann. Manage. 35 (6), 1503–1511. doi:10.1002/hpm.3028
Yu, L. Y., Zhu, W. J., Zhu, X. P., Lu, Y., Yu, Z. W., and Dai, H. B. (2021). Anti-seizure medication prescription in adult outpatients with epilepsy in China, 2013-2018. Front. Neurology 12, 649589. doi:10.3389/fneur.2021.649589
Yuan, J., Lu, Z. K., Xiong, X., and Jiang, B. (2021). Lowering drug prices and enhancing pharmaceutical affordability: An analysis of the national volume-based procurement (NVBP) effect in China. BMJ Glob. Health 6 (9), e005519. doi:10.1136/bmjgh-2021-005519
Zhang, L., Xu, J., Gao, J., Chen, P., Yin, M., and Zhao, W. (2019). Decreased immunoglobulin G in brain regions of elder female APOE4-TR mice accompany with Aβ accumulation. Immun. Ageing. 16, 2. doi:10.1186/s12979-018-0142-7
Zhao, M. Y., Gillani, A. H., Ji, D., Feng, Z. T., Fang, Y., and Yang, C. J. (2021). Impact of the low-price medicine policy on medicine supply in China: An interrupted time-series analysis. Front. Pharmacol. 12, 621307. doi:10.3389/fphar.2021.621307
Keywords: the “4+7” pilot policy, volume-based procurement, price, expenditures, serotonin-specific reuptake inhibitors (SSRIs), antidepressants, interrupted time-series analysis (ITSA), quasi-experimental design and analysis
Citation: Wen X, Wang Z, Xu L, Luo J, Geng X, Chen X, Yang Y, Cui D and Mao Z (2022) The impacts of the “4+7” pilot policy on the volume, expenditures, and daily cost of Serotonin-Specific Reuptake Inhibitors (SSRIs) antidepressants: A quasi-experimental study. Front. Pharmacol. 13:829660. doi: 10.3389/fphar.2022.829660
Received: 06 December 2021; Accepted: 21 July 2022;
Published: 17 August 2022.
Edited by:
Hye-Young Kwon, Mokwon University, South KoreaReviewed by:
Chenxi Liu, Huazhong Univeristy of Science and Technology, ChinaSimão Pinho, São João University Hospital Center, Portugal
Kona Chowdhury, Gonoshathaya Samaj Vittik Medical College, Bangladesh
Copyright © 2022 Wen, Wang, Xu, Luo, Geng, Chen, Yang, Cui and Mao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaotong Wen, MjAyMDEwMzA1MDAxNEB3aHUuZWR1LmNu; Dan Cui, MDAwMDg3NjZAd2h1LmVkdS5jbg==; Zongfu Mao, emZtYW9Ad2h1LmVkdS5jbg==
†These authors have contributed equally to this work and share the first authorship.
 Xiaotong Wen
Xiaotong Wen Zhaolun Wang
Zhaolun Wang Luxinyi Xu1,2
Luxinyi Xu1,2 Ying Yang
Ying Yang