- 1West China School of Pharmacy, Sichuan University, Chengdu, China
- 2Department of Pharmacy, West China Second University Hospital, Sichuan University, Chengdu, China
- 3Evidence-based Pharmacy Center, West China Second University Hospital, Sichuan University, Chengdu, China
- 4Key Laboratory of Birth Defects and Related Diseases of Women and Children, Ministry of Education, Chengdu, China
- 5West China School of Medicine, Sichuan University, Chengdu, China
- 6Academic Division of Child Health, Derbyshire Children’s Hospital, University of Nottingham, Derby, United Kingdom
- 7Department of Pediatrics, West China Second University Hospital, Sichuan University, Chengdu, China
- 8Laboratory of Molecular Translational Medicine, Center for Translational Medicine, Sichuan University, Chengdu, China
- 9National Drug Clinical Trial Institute, West China Second University Hospital, Sichuan University, Chengdu, China
Background: Essential medicines for children are those medicines that satisfy the priority health care needs of children. Access to essential medicines for children is a big challenge, particularly in low- and middle-income countries. Our study aimed to assess the accessibility of essential medicines for children in public sector in Sichuan Province of China, based on availability, affordability, and price.
Methods: We adopted the modified World Health Organization/Health Action International (WHO/HAI) standardization methodology to measure the availability, affordability, and prices of 30 essential medicines for children in 20 public hospitals in nine regions of the Sichuan Province, China. Availability was expressed as the percentage of public medicine outlets that stocked surveyed medicines on the day of data collection, and prices were expressed as median price ratio (MPR). Affordability was assessed as the number of Sichuan Province’s daily wages required for the lowest-paid government unskilled worker (11.03 USD per day) to purchase one standard treatment of an acute disease or treatment for chronic disease for a month.
Results: The mean availability of originator brands (OBs) and lowest priced generics (LPGs) were 9.7 and 46.5% in public sector. MPRs of only 3 OBs could be calculated, ranging from 0.55 to 13.37. MPRs of 18 LPGs ranged from 0.07 to 25.05. Among them, 2 OBs and 11 LPGs were priced at more than 1.5 times their international reference prices (IRPs) in public sector, most of which were injections. Except for cefazolin injection and ceftriaxone injection, most LPGs were affordable for the treatment of childhood diseases in public sector, as they each cost one or less than the daily wage for the lowest-paid unskilled government worker.
Conclusions: Although the availability of LPGs for children was higher than OBs in public sector, the availability of children’s essential medicines was low in surveyed public sector in Sichuan Province, which was similar to previous studies in other provinces of China. The price of most medicines surveyed was higher than their IRPs in surveyed public sector, especially for some injections. The affordability of most surveyed LPGs was reasonable in surveyed public sector, except for ceftriaxone injection and cefazolin injection.
Introduction
Access to health care is a fundamental human right, and the provision of affordable, high-quality, and appropriate medicines for children is a vital part of a well-functioning health system (Zhang et al., 2017). To improve access to essential medicines for children and ensure that children’s basic medication needs can be met, the WHO has developed and revised seven editions of Essential Medicine Lists For Children since 2007 (World Health Organization, 2019). WHO also launched the “Make Medicines Child Size” campaign and “Better Medicines for Children” initiative in 2009 to ensure that children’s medicines were in appropriate formulations, safe and effective (World Health Organization, 2017; Finney, 2019).
Nowadays, a systematic review has identified 18 multicentre studies evaluating the accessibility of essential medicines for children (Chen et al., 2021). The majority of studies used the WHO/HAI standardized methodology (World Health Organization and Health Action International, 2008), which was jointly developed by WHO and HAI in 2000 for standardizing studies on the accessibility of medicines. This ensured the quality of studies, and made the study results in different regions comparable. The WHO/HAI standardized methodology estimates the accessibility of medicines by investigating medicine availability, price, and affordability. The results have shown that the availability of essential medicines for children is generally low in low-income and middle-income countries, the median price ratios of originator brands was higher than that of lowest-priced generics, and the most lowest-priced generics had better affordability (Chen et al., 2021).
In China, there is no essential medicine list for children, and the accessibility of essential medicines for children is still low and urgently needs to be improved (Wang et al., 2017). Moreover, the scope of medical insurance coverage for children is limited. Generally, hospitalization expenses for children can be reimbursed. But there are differences in the proportion of medical insurance payments in different levels of hospitals. Only a portion of medical expenses and the drugs are included in the Basic Medical Insurance, Work Injury Insurance and Maternity Insurance Drug List. Similarly, outpatient expenses for children at designated medical facilities are also reimbursed, but there is a limit to the total reimbursement of outpatient expenses.
At present, there is no study on the accessibility of essential medicines for children in Sichuan Province, China. The relevant evaluation data needs to be enriched. Therefore, the WHO/HAI standardized methodology is used to investigate the availability, price, and affordability of essential medicines for children in public sector in Sichuan Province, China.
Materials and Methods
Study Design
This study was a cross-sectional study on the accessibility of children’s essential medicines conducted by using the WHO/HAI standardized methodology in Sichuan Province, China (World Health Organization and Health Action International, 2008).
Setting
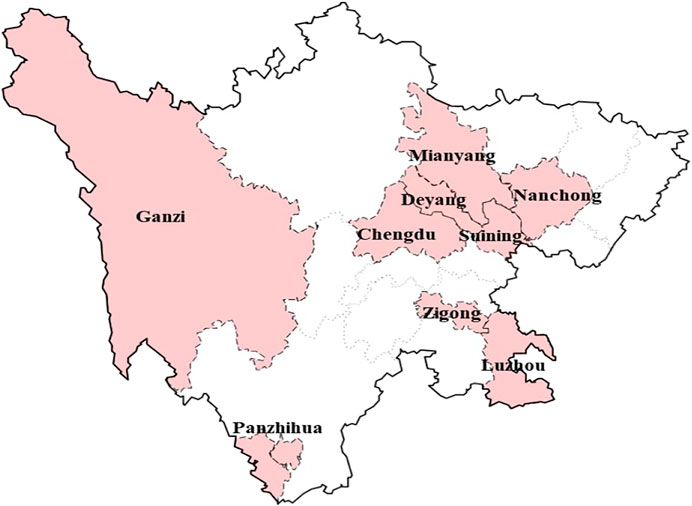
Sichuan Province is located in southwestern China, with 21 cities and a population of 91 million. Chengdu, the capital city of Sichuan Province, was chosen as the major urban center. Considering geographical position and level of economic development (Gross Domestic Product), 9 representative cities were selected as survey areas for data collection by stratified sampling: Chengdu, Mianyang, Deyang, Nanchong, Luzhou, Zigong, Suining, Panzhihua, and Ganzi. The selected cities can be reached within 1 day of travel from the capital city (Figure 1).
Participants
We designed a sampling frame for the public sector facilities. Convenience sampling was used in our study, where subjects were selected by their convenient accessibility to the researchers. Considering the hospital level and distribution of specialist children’s hospitals, we finally selected 10 specialist children’s hospitals in Chengdu, Mianyang, Luzhou, and 10 general hospitals with pediatrics outpatient departments in Chengdu, Mianyang, Deyang, Nanchong, Luzhou, Zigong, Suining, Panzhihua, and Ganzi. Because not each of the cities studied has a specialist children’s hospital.
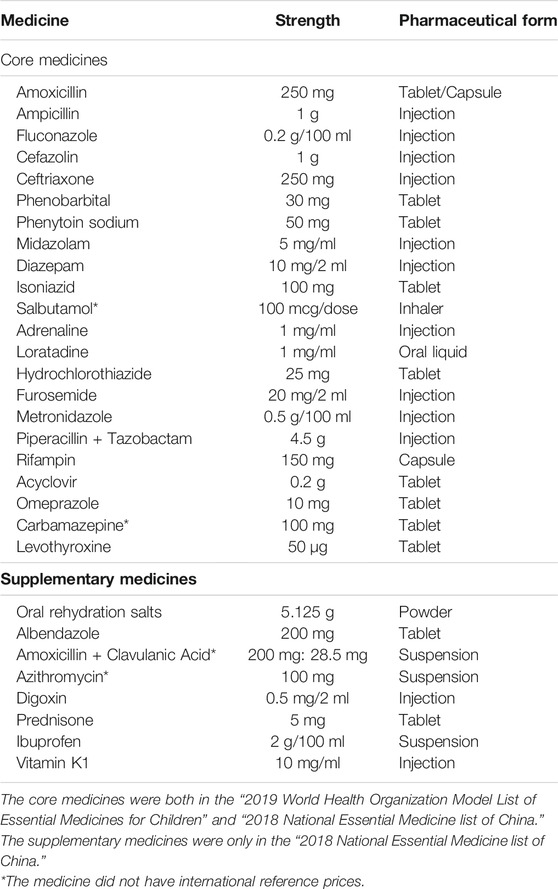
According to the requirements of the WHO/HAI guide book, core and supplementary medicines need to be determined in the survey following local medical burden, treatment resource, and prognosis management (World Health Organization and Health Action International, 2008). A total of 30 medicines were surveyed, all of which were registered in China (National Health Commission of China, 2018) (Table 1). Among them, 22 medicines were identified as core medicines referred from “2019 World Health Organization Model List of Essential Medicines for Children” and “2018 National Essential Medicine list of China” (National Health Commission of China, 2018; World Health Organization, 2019). These core medicines all have international reference prices (IRPs). Eight medicines were identified as supplementary medicines from the “2018 National Essential Medicine list of China.” The medicines were selected based on the local children’s disease burden and needs, literature reviews, and were assessed by pediatric clinical pharmacists.
For each medicine, price and availability data were collected for two products: the originator brand (OB) and the lowest price generic (LPG) equivalent. The OB product was defined as a product marketed by the originator pharmaceutical company. The LPG was defined as the same efficacy product sold under the generic name with the lowest unit price at each public medicine outlet at the time of data collection in the survey.
Variables
The WHO/HAI methodology recommended assessing the accessibility of medicines by three indicators, including availability, price and affordability.
Availability of medicines was expressed as the percentage (%) of pharmacies in which the individual medicines were available on the day of the survey.
Price was expressed by the median price ratios (MPR). The MPR was the ratio of the median medicine unit (each tablet, capsule, and milliliter, etc.) price to IRP (World Health Organization and Health Action International, 2008). The IRPs were the medians of recent procurement prices offered for medicines by not-for-profit suppliers. For instance, MPR was 2.0 meant that the local medicine price was 2 times the IRP. If the retail price of medicines in public sectors did not exceed 1.5 times the IRP, namely MPR ≤ 1.50, which was considered reasonable (Wang et al., 2017; Hailu and Mohammed, 2020; Abrha et al., 2018).
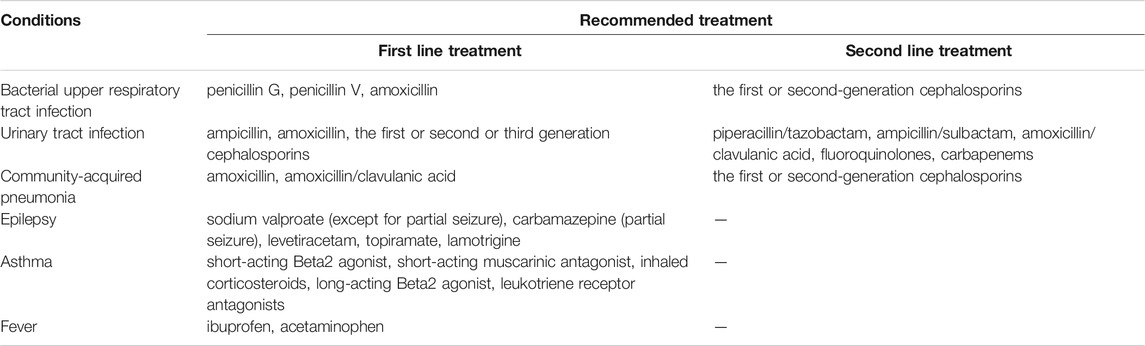
Affordability was assessed by comparing the daily wage of lowest-paid unskilled government workers and medicines expenses in the standard treatment of childhood diseases (a full course of therapy for acute diseases or the cost of a 30-days supply of medicines for chronic diseases). Medicines were considered affordable if the medicines cost less than the daily wage (Sado and Sufa, 2016). According to child disease burden from WHO (World Health Organization, 2019), the sixth national health service survey on child healthcare in China, and pediatric experts’ opinions, six common conditions in childhood were chosen to assess the affordability, including bacterial upper respiratory tract infection, urinary tract infection, community-acquired pneumonia, epilepsy, asthma, fever. The medicines recommended for these conditions were listed in Table 2 (Working Group on Revision of Guidelines for the Clinical Application of Antimicrobial Drugs, 2015; Respiratory Group of Pediatrics Branch of Chinese Medical Association, 2016; Luo et al.,2016). The dosage and duration of medicine treatment was the standard treatment recommended by (Working Group on Revision of Guidelines for the Clinical Application of Antimicrobial Drugs, 2015), guideline (Luo et al., 2016) or China National Formulary (Children’s Edition) (China National Formulary Editorial Board, 2013). Sichuan Province’s daily wage of the lowest-paid government unskilled employee was 71.26 CNY (approximately 11.03 USD in 7 May 2021) (The People’s Government of Sichuan Province, 2018). Besides, we assumed the child was 5 years old with a weight of 20.00 kg (National Health Commission of the People’s Republic of China, 2016).
Data Sources
We sent a special standardized electronic data collection format to each hospital pharmacy to collect information on availability and price. To verify the standardized data collection form and ensure data accuracy and reliability, we conducted a pilot study and trained the person who filled out the data collection form. We checked the completed data collection forms and verified the data integrity as the forms were submitted. Then, two well-trained researchers independently entered the survey data into the standardized WHO/HAI Excel Workbook (World Health Organization and Health Action International, 2008) using a double-entry technique. All data on the availability and prices of medicines in public sector was collected from 1 July 2021 to 10 July 2021.
Statistical Methods
Data were cleared and analyzed by using MS Excel Workbook provided by World Health Organization and Health Action International, 2008 part I. The median availability was calculated for OBs and LPGs for the overall basket of medicines surveyed within public sector. For medicine prices, we used the International Reference Price (IRP) in 2015 (International Drug Price Indicator Guide, 2015) published by Management Sciences for Health to calculate MPR of each medicine. MPR was only calculated when the medicine was available at a minimum of four public medicine outlets (Sun et al., 2018). The exchange rate used to calculate MPRs was 1 CNY = 0.15 USD in 7 May 2021, which was provided by the State Administration of Foreign Exchange (State Administration of Foreign Exchange, 2021).
Results
Ten specialist children’s hospitals and 10 general hospitals participated in the survey and returned the questionnaire.
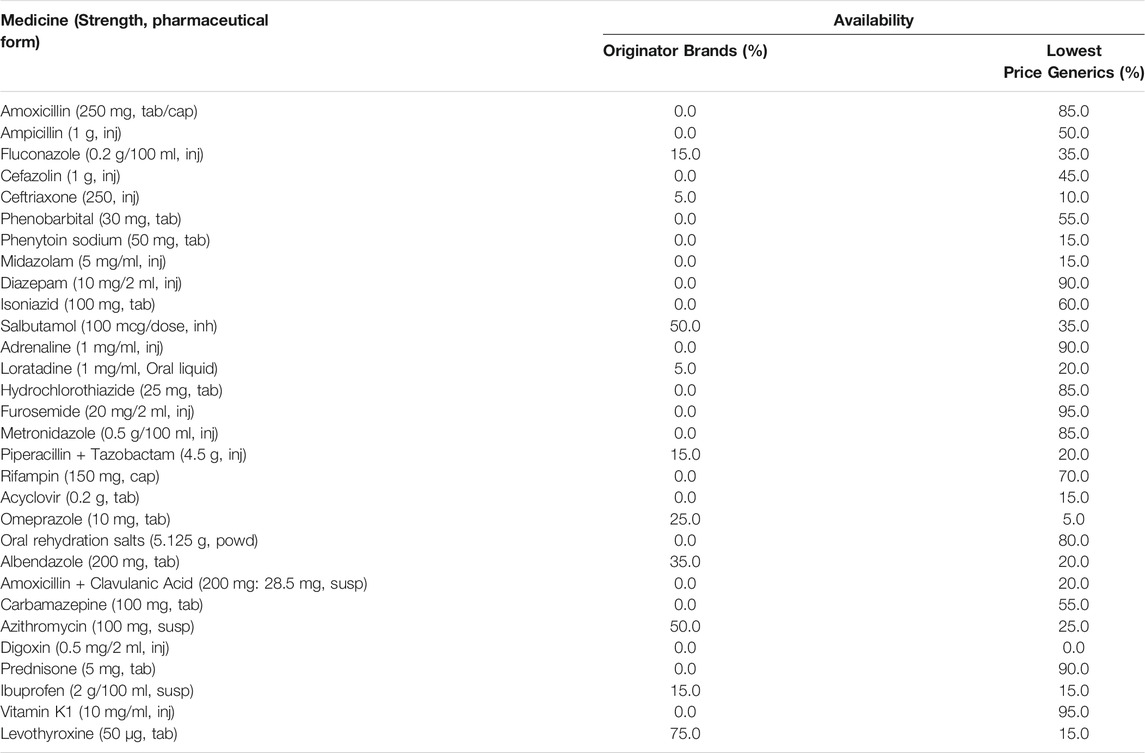
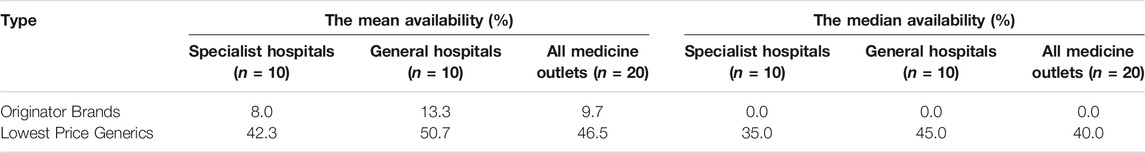
Among 30 OBs, 10 OBs were assessed for availability. Availability of 7 OBs were less than 50.0%, 3 OBs were between 50.0 and 80.0%, and no OB was found in more than 80.0% of public medicine outlets. Among 30 LPGs, 29 LPGs were assessed for availability. Availability of 15 LPGs were less than 50.0%, 5 LPGs were between 50.0 and 80.0%, and 9 LPGs were found in more than 80.0% of public medicine outlets. Neither the OB nor the LPG of digoxin injection was not found in any public medicine outlet (Table 3). The mean availabilities of OBs and LPGs were 9.7% (SD 18.9%) and 46.5% (SD 32.5%) in public sector. The median availability of all OBs and all LPGs were 0.0 and 40.0%. Though the availability of LPGs was higher than OBs, the availability of OBs and LPGs were both low. The availability of LPGs seemed to be no different in specialist hospitals and general hospitals (Table 4). No hospital had all the medicines or had none of the medicines available. The hospital having the most surveyed medicines was a general hospital and 25 medicines were available. The hospital having the least surveyed medicines was a specialist hospital and 6 medicines were available.
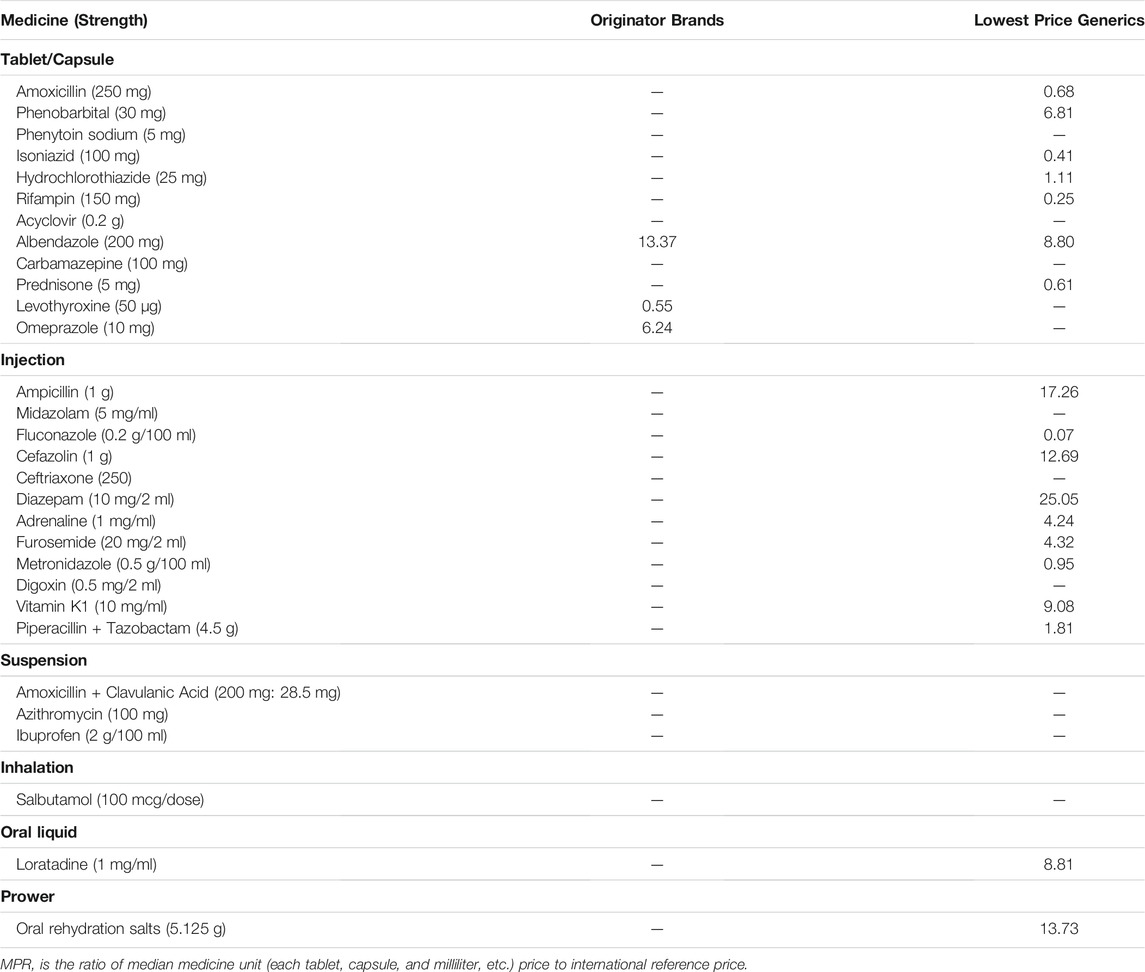
MPRs of only 3 OBs could be calculated, ranging from 0.55 to 13.37. MPRs of 18 LPGs could be calculated, ranged from 0.07 to 25.05. Among them, 2 of 3 OBs’ MPRs and 11 of 18 LPGs’ MPRs were >1.50, namely, those medicines’ prices were considered irrational. Among LPGs, the median of MPRs for injection was 4.32 and was much higher than the tablet/capsule whose median of MPRs was 0.68. The number of injections and tablets/capsules whose MPR was >1.50 were 7 and 2, respectively, (Table 5).
The affordability of standard treatments for six different health conditions was calculated. Due to the low availability of OBs, we finally included seven LPGs. Amoxicillin capsule, carbamazepine tablet, salbutamol inhaler, ibuprofen suspension treatment cost less than the daily wage, which meant these medicines were affordable. Amoxicillin/clavulanic acid suspension treatment cost for community-acquired pneumonia was 1.41, which was slightly over the daily wage. But the ceftriaxone injection treatment cost far more than the daily wage and was unaffordable in the treatment of urinary tract infection. The cefazolin injection treatment also cost over the daily wage and was unaffordable in the treatment of urinary tract infection and community-acquired pneumonia. Excepting ceftriaxone injection and cefazolin injection, the cost of most LPGs in public sectors was between 0.02 and 1.41 the daily wage, which indicated that most LPGs were modestly affordable in Sichuan Province(Table 6).
Discussion
This study assessed the availability, price, and affordability of essential medicines for children by using the standardized WHO/HAI methodology in public sector in the Sichuan Province of China. The mean availability of originator brands (OBs) and lowest priced generics (LPGs) were 9.7 and 46.5% in public sector. MPRs of only 3 OBs could be calculated, ranging from 0.55 to 13.37. MPRs of 18 LPGs ranged from 0.07 to 25.05. Among them, 2 OBs and 11 LPGs were priced at more than 1.5 times their IRP in public sector, most of which were injections. Except for cefazolin injection and ceftriaxone injection, most LPGs were affordable for the treatment of childhood diseases in public sector, as they each cost one or less than the daily wage for the lowest-paid unskilled government worker.
There were several limitations in this study. The availability of medicine was collected and assessed on the survey day, which could not truly reflect the monthly or annual mean medicine supply. Moreover, this study was conducted only in public sector, not in private sector. The results might underestimate the accessibility of essential medicines for children, especially for OBs. The previous studies showed the availability in the private sector was higher than that in public sector (Wang et al., 2017; Sun et al., 2018). And with the continuous deepening of medical system reform and the development of Internet technology in China, the “Internet + medical” model has emerged (NHCPRC and NATCM, 2018; NHCPRC, 2020). Sichuan Province actively has supported and promoted the construction of prescription circulation platforms to provide patients with the entire chain of Internet pharmacy services, including drug delivery (HCSP and SPATCM, 2020; PAGP, 2020). Patients with the electronic prescription can independently purchase medicines in hospitals, online private pharmacies, or the third-party agency delivery services. For patients with stable chronic diseases, they may be inclined to obtain medicines at nearby physical or online private pharmacies (Wang et al., 2020). This “Internet + medical” model could increase availability of medicine in private sector. In addition, for the convenience of calculations, we selected 5-year-old children and their average weight to estimate the affordability of essential medicines for children. Thus, the medicine affordability might be overestimated, especially for older and heavier children, because they might need to take larger doses of medicines during treatment.
The previous similar studies were conducted in other provinces of China (Wang et al., 2017; Sun et al., 2018; Wei et al., 2019; Li et al., 2018; Dai, et al., 2020; Wang et al., 2020) and other low-income and middle-income countries (Anson et al., 2012; Swain et al., 2015; Sado and Sufa, 2016), which had similar results. The results showed that the availability of essential medicines for children in public sector was low. The median availability of existing lowest price generic medicines and their original products was less than 50% in public sector. Although the availability of LPGs was higher than OBs, the availability of LPGs also was low, the median was 40%. Compared with the studies conducted by Wang et al. (2017) in China’s Shaanxi Province, Sun et al. (2018) in China’s Jiangsu Province, and Wei et al. (2019) in eastern China, our findings showed lower availability of OBs and slightly higher availability of LPGs in public sector. In the survey of Jiangsu Province published in 2018, the mean availability of OBs and LPGs were 7.5 and 34.2% in the public hospitals (Sun et al., 2018).
Many studies reported high rates of off-label in different pediatric patients, which increased the risk of medicine use in children (Kimland and Odlind, 2012; Gore et al., 2017). The low availability of medicines for children and the lack of medicines with child-friendly pharmaceutical form and dose definitely deteriorated this phenomenon further. In 2018–2019, in response to the lack of standardized management of shortage medicines in China’s medical institutions, the National Health Commission of China has developed Guidelines for the Management of Shortage Medicines in Public Medical Institutions and Technical Guide for the Classification, Categorization and Alternative Use of Shortage Medicines in Medical Institutions. According to the guidelines, when medicines are not available in the public medical institutions, the following actions are needed: the pharmacists need to confirm the information of shortage medicines, including but not limited to the reasons for the medicine shortage, the main affected patient population, the procurement history and actual use of shortage medicines, estimated duration of shortage, availability of shortage medicines in other hospitals. If the medicines are available in other medical institutions, the pharmacists can initiate a purchase path. The patient can then use the medicines after purchase according to the medicine procurement process of the institution. Otherwise, if the medicines are unavailable in other medical institutions, pharmacists should classify the shortage medicines according to necessity and urgency, and carry out evidence-based selection of alternative medicines. If the alternative medicines are in the institution’s medicine supply list, it can be used directly, otherwise, the Pharmaceutical Administration and Therapeutics Committee (Group) may initiate emergency procurement according to relevant regulations of the institution, and timely notify the information of alternative medicines in the hospital.
These reasons may explain the poor availability of medicines for children. Because of low-profit margins, Chinese pharmaceutical manufacturers lack the motivation to produce children’s medicines (Jiang, 2021). Children’s medicines generally need to be made into child-friendly pharmaceutical form, such as syrups, granules, patches, and suppositories, etc. The production process of these pharmaceutical form is more complicated because these pharmaceutical form require higher medicine stability, which increases the costs and reduces profits. Although there are more than 4,000 pharmaceutical manufacturers in China, only approximately 5.0% of the pharmaceutical manufacturers are willing to produce children’s essential medicines, and just 0.2% of pharmaceutical enterprises are specialized in producing children’s medicines (Sun et al., 2018). Moreover, the centralized bidding procurement and distribution system for medicines had been gradually established in China (Wang and Ge, 2009). In 2018, to improve the drug procurement and pricing mechanisms, China conducted a pilot program of the centralized drug procurement in 11 cities. In 2019, the volume-based purchasing pilot program, using a competitive bidding process to purchase high-quality generic drugs for which branded drug substitutes were available, further expanded the implementation. The objective of volume-based purchasing policy was to allow substitution of branded drug products with generic drug products, which was intended to promote cost savings. Therefore, the availability of OBs medicines could be affected. And the National Health Commission of China included the use proportion of volume-based purchasing drugs in the performance assessment of tertiary public medical institutions, which might lead tertiary public medical institutions to give priority to use generic drug products. Therefore, the reasons might explain the poor availability of medicines for children, especially for OBs. Usually, only the pharmaceutical firms that offer the lowest prices could win bids, which probably leads to shrinking revenues from medicine sales. To win the bid, some pharmaceutical companies have to decrease the medicine price (Jiao, 2013), but there are few price controls on active pharmaceutical ingredients (APIs). The high cost of APIs and low profits are the important reason for the shortage of medicines. In 2018, the “Early warning of medicines that are easy to be shortage” of the Liaoning Province in China, reported that 81 medicines could not be supplied in time, and the main reason was the increase in the price of APIs. Moreover, the cross-regional centralized drug procurement expanded the scope of the suppliers’ distribution area, which is undoubtedly a greater challenge for distributors, and it is another important reason (Liu and Mao, 2021). Besides, in public sector, inadequate funding, lack of incentives for maintaining stocks, and inability to forecast clinical needs accurately (Cameron et al., 2009) could be also the reasons for the low availability of medicines for children.
Compared with other previous studies (Wang et al., 2017; Sun et al., 2018), a slightly different result of this study was showed that the median of MPRs for LPGs was 4.28, which meant the price of essential medicine for children might be unreasonable in public sector. The results might be related to the selected survey medicines, because we found the median of MPRs for injection was 4.32 and was much higher than tablet/capsule whose median of MPRs was 0.68. Compared with the findings of the adult’s medicine price survey that had been conducted in Sichuan Province (Yang et al., 2015), the price of children’s LPGs seemed to be higher than adult’s medicines. In general, the prices of essential medicines for children were higher than IPRs, especially for many injections.
We evaluated the affordability of medicines for six common childhood conditions. Most LPGs were affordable in public sector, namely, the standard treatments cost less than 1 day’s wage. This finding was similar to the previous study in China (Wang et al., 2017; Sun et al., 2018). But our study found ceftriaxone injection and cefazolin injection treatment cost over 10 days’ wage and were unaffordable, no matter in urinary tract infection, or community-acquired pneumonia standard treatments. This might be related to the higher unit price of these injections.
Relevant measures should be taken to improve access to medicines for children. From a policy perspective, learn from other countries’ experience, and formulate relevant policies and suggestions to guarantee the accessibility of medicines for children in China. And the government should develop a list of national essential medicines for children, and take some measures and make some policies to mobilize pharmaceutical companies to produce children’s medicines. Moreover, investigate the pediatrician’s knowledge and use of essential medicines, to promote the use of essential medicines for children. In addition, to reduce the burden of children’s diseases, it is recommended that medicare should include more children’s medicines.
Conclusion
Although the availability of LPGs for children was higher than OBs in public sector, the availability of children’s essential medicines was still low in surveyed public sector in Sichuan Province, which was similar to previous studies in other provinces of China. The price of most medicines surveyed was higher than their IRPs in public sector, especially for some injections. The affordability of most surveyed LPGs was reasonable in public sector, except ceftriaxone injection and cefazolin injection.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
LLZ and IC contributed to the conception and design of the study. ZC and SL were involved in data collection, data analysis and writing the manuscript. LNZ, Z-jJ, GC and QY were involved in data collection. All authors gave their approval of the final version.
Funding
This work was supported by Sichuan Province Science and Technology Plan Project (No.2020YFS0035), Subproject of Science and Technology Plan Project of Sichuan Province (No. 20ZDYF3101), National Natural Science Foundation for Young Scholars of China (No. 72004151).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrha, S., Tadesse, E., Atey, T. M., Molla, F., Melkam, W., Masresha, B., et al. (2018). Availability and Affordability of Priority Life-Saving Medicines for Under-five Children in Health Facilities of Tigray Region, Northern Ethiopia. BMC Pregnancy Childbirth 18 (1), 464. doi:10.1186/s12884-018-2109-2
Anson, A., Ramay, B., de Esparza, A. R., and Bero, L. (2012). Availability, Prices and Affordability of the World Health Organization's Essential Medicines for Children in Guatemala. Glob. Health 8 (1), 22. doi:10.1186/1744-8603-8-22
Cameron, A., Ewen, M., Ross-Degnan, D., Ball, D., and Laing, R. (2009). Medicine Prices, Availability, and Affordability in 36 Developing and Middle-Income Countries: A Secondary Analysis. Lancet 373 (9659), 240–249. doi:10.1016/S0140-6736(08)61762-6
Chen, Z., Li, S., Zeng, L., Liu, Y., Zhang, M., Choonara, I., et al. (2021). Accessibility of Medicines for Children: A Systematic Review. Front. Pharmacol. 12, 1–9. doi:10.3389/fphar.2021.691606
China National Formulary Editorial Board (2013). China National Formulary (Children's Edition) Chemical and Biological Products. People's Military Medical Publishing House.
Dai, Y., Li, Z. P., Xu, H., Zhu, L., Zhu, Y. Q., Cheng, H., et al. (2020). A Multicenter Survey of the Accessibility of Essential Medicines for Children in China. Zhonghua Er Ke Za Zhi 58 (4), 301–307. doi:10.3760/cma.j.cn11214010.3760/cma.j.cn112140-20190820-00527
Finney, E. (2019). Children's Medicines: A Situational Analysis. Available: http://www.who.int/childmedicines/progress/CM_analysis.pdf (Accessed May 27, 2021).
Gore, R., Chugh, P. K., Tripathi, C. D., Lhamo, Y., and Gautam, S. (2017). Pediatric Off-Label and Unlicensed Drug Use and its Implications. Curr. Clin. Pharmacol. 12 (1), 18–25. doi:10.2174/1574884712666170317161935
Hailu, A. D., and Mohammed, S. A. (2020). Availability, price, and Affordability of Who Priority Maternal and Child Health Medicine in Public Health Facilities of Dessie, north-east ethiopia. BMC Med. Inform. Decis. Mak 20 (1), 221. doi:10.1186/s12911-020-01247-2
HCSP and SPATCM (2020). Health Commission of Sichuan Province (HCSP), Sichuan Province Administration of Traditional Chinese Medicine (SPATCM). Notice on the Issuance of the "Sichuan Province Action Plan for Promoting "Internet + Medical" Convenient and Benefiting Services for the People (2019-2020)". http://hsa.gd.gov.cn/gkmlpt/content/2/2930/post_2930552.html#464 (Accessed December 16, 2021).
International Drug Price Indicator Guide (2015). International Medical Products Price Guide. Available: http://mshpriceguide.org/en/home/(Accessed May 28, 2021).
Jiang, Y. (2021). Policy Urgently Needs to Be Optimized, Children's Drug Predicament Still Needs to Be Solved. Economic Information. Available: https://xhpfmapi.zhongguowangshi.com/vh512/share/9802099?channel=weixin (Accessed March 3, 2021).
Jiao, X. H. (2013). Talking about the Problems and Countermeasures in the Centralized Bidding and Purchasing of Drugs. China J. Pharm. Econ. 1, 24–26.
Kimland, E., and Odlind, V. (2012). Off-label Drug Use in Pediatric Patients. CLIN. PHARMACOL. THER. 91 (5), 796–801. doi:10.1038/clpt.2012.26
Li, S. S., Xu, W., Du, W. W., and Fu, Y. B. (2018). Study on Availability of Pediatric Essential Medicines in China: Based on the Surveys in 19 Provinces. Chin. J. Health Pol. 11 (12), 12–18.
Liu, S. S., and Mao, N. Y. (2021). The Influence of Centralized Procurement Policy on Drug Supply Guarantee. Chin. Pharm. Aff. 35 (4), 380–385.
Luo, S. H., Shu, M., Wen, Y., Ding, J. J., Gong, Z. R., Zhang, P., et al. (2016). Evidence-based Guideline for the Diagnosis and Management of Several Problems in Acute Fever of Unknown Etiology in Children Aged 0 to 5 Years in China (Standard Version). Chin. J. Evidence-Based Pediatr. 11, 81–96. doi:10.3969/j.issn.1673-5501.2016.02.001
National Health Commission of China (2018). National Essential Medicines List. Available at: http://www.nhc.gov.cn/wjw/jbywml/201810/600865149f4740eb8ebe729c426fb5d7.shtml (Accessed May 28, 2021).
National Health Commission of the People's Republic of China (2016). The Fifth National Health Service Survey. Available: http://www.nhc.gov.cn/wjw/xwdt/201606/043e914f05cb49fbba44c435b0808476.shtml (Accessed july 17, 2021).
NHCPRC (2020). Notice on Issuing Opinions on Strengthening the Administration of Pharmaceutical Affairs in Medical Institutions to Promote Rational Use of Medicines. http://www.nhc.gov.cn/yzygj/s7659/202002/ea3b96d1ac094c47a1fc39cf00f3960e.shtml (Accessed December 16, 2021).
NHCPRCNATCM (2018). Commission of the People’s Republic of China (NHCPRC), National Administration of Traditional Chinese Medicine (NATCM). Notice on the Issuance of 3 Documents Including Internet Diagnosis and Treatment Management Measures (Trial Version). http://www.gov.cn/gongbao/content/2019/content_5358684.htm 0561bbf0581f1.shtml (Accessed December 16, 2021).
Pharmaceutical Association of Guangdong Province (PAGP) (2020). Expert Consensus on Standardized Management of Prescription Circulation Platform of Internet Hospital. Pharm. Today 30 (11), 721–727.
Respiratory Group of Pediatrics Branch of Chinese Medical Association (2016). Guideline for the Diagnosis and Prevention of Bronchial Asthma in. Child. Chin. J. Pediatr. 54, 167–181. doi:10.3760/cma.j.issn.0578-1310.2016.03.003
Sado, E., and Sufa, A. (2016). Availability and Affordability of Essential Medicines for Children in the Western Part of Ethiopia: Implication for Access. BMC Pediatr. 16, 40. doi:10.1186/s12887-016-0572-3
State Administration of Foreign Exchange (2021). Conversion Rate Table of Various Currencies to U.S. Dollar. Available: http://www.safe.gov.cn/safe/2021/0630/19297.html (Accessed June 30, 2021).
Sun, X., Wei, J., Yao, Y., Chen, Q., You, D., Xu, X., et al. (2018). Availability, Prices and Affordability of Essential Medicines for Children: A Cross-Sectional Survey in Jiangsu Province, China. BMJ Open 8 (10), e023646. doi:10.1136/bmjopen-2018-023646
Swain, T. R., Rath, B., Dehury, S., Tarai, A., Das, P., Samal, R., et al. (2015). Pricing and Availability of Some Essential Child Specific Medicines in Odisha. Indian J. Pharmacol. 47 (5), 496–501. doi:10.4103/0253-7613.165197
The People's Government of Sichuan Province (2018). Notice on Adjusting the Provincial Minimum Wage Standard. Available: http://www.fsxzf.gov.cn/zcwj/-/articles/8912080.shtml (Accessed May 7, 2021).
Wang, Jing., Xiao, Guirong., Sun, Guorui., Huang, Zhen., and Hu, Ming. (2020). Evaluation of the Current Situation and Rationality of Electronic Prescription in Retail Pharmacies in Chengdu in the Context of “Internet + Drug Circulation”. Chin. J. Evidence-Based Med. 20 (11), 1274–1279.
Wang, L. J., and Ge, J. H. (2009). Background and Evolution of the Group Procurement Policy of Drugs for Hospitals. Chin. J. Health Pol. 2 (04), 8–13.
Wang, X., Fang, Y., Yang, S., Jiang, M., Yan, K., Wu, L., et al. (2017). Access to Paediatric Essential Medicines: A Survey of Prices, Availability, Affordability and Price Components in Shaanxi Province, China. PLoS One 9 (3), e90365. doi:10.1371/journal.pone.0090365X
Wei, G. X., Wang, X. L., Li, X., Li, L. Y., Chen, J., and Shi, L. W. (2019). A Survey on the Availability, price and Affordability of Essential Medicine for Children in Eastern China. Chin. J. Health Pol. 12 (10), 72–78. doi:10.3969/j.issn.1674-2982
Working Group on Revision of Guidelines for the Clinical Application of Antimicrobial Drugs (2015). Guidelines for the Clinical Application of Antibacterial Drugs: People's Health Publishing House. Available: http://www.nhc.gov.cn/yzygj/s3593/201508/c18e1014de6c45ed9f6f9d592b43db42.shtml (Accessed June 30, 2021).
World Health Organization (2019). 7th WHO Model List of Essential Medicines for Children. Available: https://www.who.int/medicines/publications/essentialmedicines/en/(Accessed May 23, 2021).
World Health Organization (2017). Better Medicines for Children: Country Implementation. Available: http://www.who.int/childmedicines/countries/en/(Accessed May 28, 2021).
World Health OrganizationHealth Action International (2008). Measuring Medicine Prices, Availability, Affordability and price Components. 2nd. Available: https://www.haiweb.org/medicineprices/manual/documents.html (Accessed May 7, 2021).
Yang, Y., Zhou, N. T., Hu, M., and Jiang, X. H. (2015). Survey on the Availability and price of Essential Medicines in Public Sectors in Sichuan Province Based on the WHO/HAI Standardized Methodology in Annual Meeting of the Pharmaceutical Affairs Management Professional Committee of the Chinese Pharmaceutical Association and "Promoting the Construction of the Rule of Law and Managing Drugs According to Law (Haikou, Hainan, China: Academic Seminar).
Keywords: children, medicine, accessibility, availability, price, affordability, cross-sectional study
Citation: Chen Z, Li S, Choonara I, Zeng L, Jia Z-j, Cheng G, Yu Q and Zhang L (2022) Accessibility of Essential Medicines for Children in Sichuan Province of China: A Cross-Sectional Study. Front. Pharmacol. 13:828152. doi: 10.3389/fphar.2022.828152
Received: 03 December 2021; Accepted: 26 January 2022;
Published: 16 February 2022.
Edited by:
Tais Freire Galvao, State University of Campinas, BrazilCopyright © 2022 Chen, Li, Choonara, Zeng, Jia, Cheng, Yu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qin Yu, OTA4OTI5OTM2QHFxLmNvbQ==; Lingli Zhang, emhhbmdsaW5nbGlAc2N1LmVkdS5jbg==
 Zhe Chen
Zhe Chen Siyu Li
Siyu Li Imti Choonara6
Imti Choonara6