- 1Dipartimento Scienze della Salute della Donna, Del Bambino e di Sanità Pubblica, Fondazione Policlinico Universitario A. Gemelli IRCCS, Rome, Italy
- 2Dipartimento Scienze della Salute della Donna, Del Bambino e di Sanità Pubblica, Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica Del Sacro Cuore, Rome, Italy
- 3Department of Anesthesiology and Intensive Care Medicine, Fondazione Policlinico Universitario A. Gemelli IRCCS, Roma, Italy
- 4Arthritis Center, Reumatologia, Dipartimento di Scienze Cliniche Internistiche, Anestesiologiche e Cardiovascolari, Sapienza Università di Roma, Roma, Italy
Few data are available evaluating obstetrical outcome when thyroiditis coexist with autoimmune diseases. Objectives of our study were: 1) To assess the prevalence of thyroiditis in pregnant women with autoimmune diseases; 2) To evaluate the effects on pregnancy outcome when different autoimmune diseases are associated with thyroiditis. Two groups of pregnant women were analysed: a study group of pregnant women with autoimmune diseases (n = 268) versus a control group of pregnant women (n = 1,150). In both groups the research for thyroid antibodies, anti-thyroid peroxidase antibodies and anti-thyroglobulin antibodies, was performed. The positivity had a prevalence of 17.54% in women with autoimmune diseases (n = 47) versus 5.57% in the control group (n = 64) (p-value < 0.00001). Only major rheumatic diseases (MRD) were analysed for pregnancy outcome (week of delivery, birth weight and birth weight percentile): systemic lupus erythematosus (SLE) n = 36, antiphospholipid syndrome (APS) n = 44 and connective tissue diseases (CTD) n = 23. MRD were divided according to positive or negative results for thyroid antibodies. Thyroiditis in CDT patients showed a detrimental effect on pregnancy outcome, in terms of earlier week of delivery: 37.86 ± 0.90 (mean ± SD) in CTD with thyroiditis versus 38.56 ± 0.73 (mean ± SD) in CTD without thyroiditis (p-value = 0.03) and lower birth weight: 2,790.71 g ± 257.17 SD in CTD with thyroiditis versus 3,019.33 g ± 305.48 g in CTD without thyroiditis (p-value < 0.05). In SLE and APS thyroiditis did not appear to influence pregnancy outcome. However, we suggest investigating anti-thyroid antibodies in all autoimmune diseases with special attention to pregnant women with thyroiditis and CTD.
Introduction
During pregnancy, the maternal thyroid gland faces several metabolic, hemodynamic, and immunologic changes (Gaberšček and Zaletel, 2011). In particular, the presence of thyroid antibodies against thyroglobulin (anti-TG), thyroid peroxidase (anti-TPO), or thyrotropin receptor autoantigens (anti-TR) are common pregnancy-related diseases. The prevalence of thyroid peroxidase antibodies is increased almost 10-fold in women compared with men, it increases with age, and it has been reported in 2.7–10% of pregnant women (Pop et al., 2003; Casey et al., 2007; Negro et al., 2011; El Baba and Azar, 2012). Therefore, it is significant to track pregnant women with hypothyroidism, who consider pregnancy, furthermore treatment has demonstrated to improve implantation rate and live birth rate in sub fertile women (Myneni et al., 2021). A useful algorithm was recently proposed by expert opinions for the assessment and management of thyroid diseases in the pre-conception period or early pregnancy (Anandappa et al., 2020). The screening for thyroid diseases is highly recommended in case of women with autoimmune conditions (Anandappa et al., 2020).
In fact, autoimmune thyroid disorders are frequently associated with other organ and non-organ-specific autoimmune diseases, especially in major rheumatic diseases (MRD), such as systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS) and connective tissue diseases (CTD) (Tagoe, 2015).
Many reports in the last decades documented the deleterious impact that thyroid disease has on pregnancy and postpartum period in terms of spontaneous abortion, prematurity, gestational diabetes, low birth weight (Saki et al., 2014) or large birth weight and placental weight (Tagoe, 2015), increased perinatal mortality (Männistö et al., 2009) preterm delivery (Abbassi-Ghanavati et al., 2010; Haddow et al., 2010; Stagnaro-Green et al., 2011; Korevaar et al., 2019) and postpartum thyroiditis. Particularly, thyroid antibody positivity in euthyroid women have been associated with miscarriage and preterm delivery (Stagnaro-Green, 2009).
Although either thyroid or rheumatic autoimmune disorders have been associated with pregnancy complications, there are scarce data in literature on the effect of the association between the two disorders on pregnancy outcome.
The pathogenic mechanisms of this coexistence are not completely defined, but genetics, epigenetics, immune defects, hormonal and environmental factors may play pivotal roles in poly-autoimmunity.
The objectives of our study were: 1) To assess the prevalence of autoimmune thyroiditis in pregnant women affected by autoimmune diseases in comparison to that of control pregnant women; 2) To evaluate the effects on pregnancy outcome when different autoimmune diseases are associated with thyroiditis in terms of gestational week at delivery, birth weight and birth weight percentile.
Methods
A retrospective observational study was performed, analysing two groups of pregnant women, consisted by a study group of pregnant women with autoimmune diseases (n = 268) versus a control group of control pregnant women (n = 1,150).
The study group comprised 268 women with a confirmed diagnosis of autoimmune disease that were followed in our clinic throughout pregnancy and delivered in our centre from January 2010 to September 2020 (Fondazione Policlinico Universitario A. Gemelli—IRCCS). On the other hand, the control group comprised 1,150 pregnant women that consecutively delivered in our centre from September 2018 to December 2018 that included both physiological and complicated pregnancy (Figure 1).
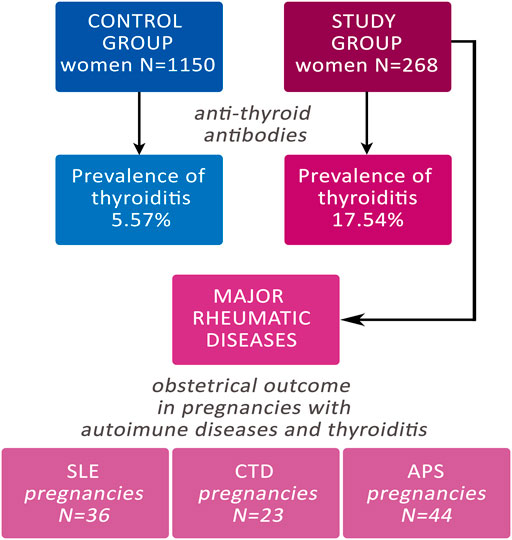
FIGURE 1. Flow-chart of the study. CTD: Connective Tissue Diseases; SLE: Systemic Erythematosus Lupus; APS: Anti-Phospholipid Syndrome.
Both in the study group and the control group, the research for thyroid antibodies, anti-TPO and anti-TG, was performed during the pregnancy, despite TSH value. In fact, the research involved either euthyroid pregnant women or women with altered thyroid function. In our series of cases, therapy of hypothyroidism was given/adjusted when necessary.
Patients with autoimmune diseases were 268 with an overall amount of 294 pregnancies, because some patients had more than one pregnancy in the described time interval. The study group was very heterogeneous, represented pregnancies with APS, SLE, CTD, arthritis, celiac disease, inflammatory bowel disease, Sjogren syndrome and other autoimmune diseases. Some patients had one or more autoimmune diseases.
The analysis was subsequently focused on pregnancies of patients suffering for major rheumatic diseases (MRD): SLE, APS and CTD. To compare the pregnancy outcome in the two groups (study group versus control group), multiple pregnancies and intrauterine fetal deaths were not included in the analysis. Therefore, pregnancies with MRD eligible for the analysis were: SLE n = 36, APS n = 44, and CTD n = 23. The three different disorders considered in MRD were divided according to the positive or negative results for thyroid antibodies.
Furthermore, the obstetric outcome was analysed in terms of week of delivery, neonatal birth weight, and neonatal birth weight percentile for understanding the eventual detrimental role of thyroid antibodies in pregnant women having associated other autoimmune diseases.
For the evaluation of neonatal birth weight percentile an Italian population-based study was employed (Ferrazzani et al., 2017).
Results
In the study group of women with autoimmune diseases 47 out of 268 resulted positive for thyroiditis having positive the research for anti-TPO, anti-TG or both antibodies, showing a prevalence of 17.54%. Out of 1,150 women of control group, 64 resulted positive for thyroid antibodies, with a positivity rate of 5.57%. The prevalence of thyroiditis was statistically significant increased in the study group (p < 0.00001) (Table 1).
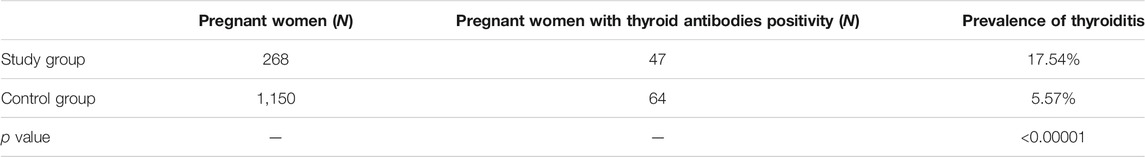
TABLE 1. Prevalence of thyroiditis in the Study group of pregnant women with autoimmune diseases and in the Control group of pregnant women.
In the study group the most common autoimmune disease associated with thyroiditis was celiac disease with a rate of 53% of thyroiditis. APS showed a rate of 35%, CTD a rate of 25%, and SLE had a rate of 24%.
The analysis of pregnancy outcome in the MRD group, according to the presence of autoimmune antibodies, revealed the following findings. Autoimmune thyroiditis in patients having CTD showed a detrimental effect on pregnancy outcome, in terms of earlier week of delivery: 37.86 ± 0.90 (mean ± SD) in CTD with thyroiditis versus 38.56 ± 0.73 (mean ± SD) in CTD without thyroiditis (p value = 0.03) and lower birth weight: 2,790.71 g ± 257.17 SD in CTD with thyroiditis versus 3,019.33 g ± 305.48 g in CTD without thyroiditis (p value < 0.05), and lower birth weight percentile, although the decrease did not reach a statistically significant difference.
No statistically significant difference in pregnancy outcome was reported in the other two groups (SLE and APS) when thyroiditis was associated (Table 2).
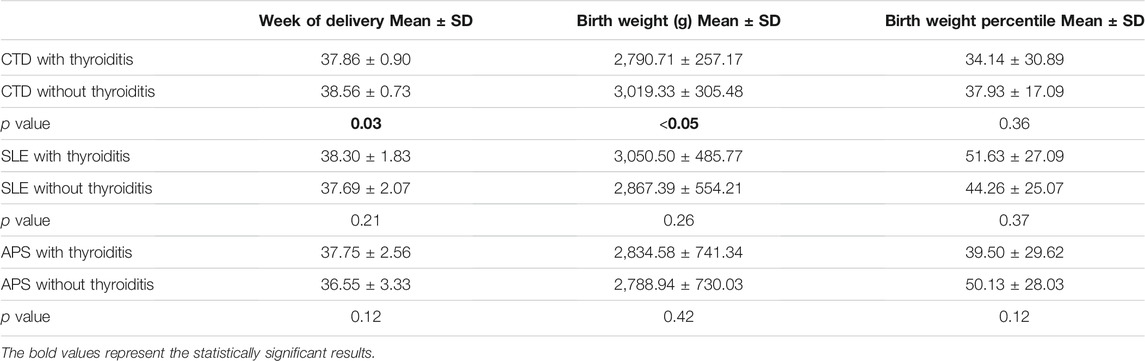
TABLE 2. Obstetrical outcome in pregnancies with thyroiditis associated with MRD (Major Rheumatic Diseases). CTD: Connective Tissue Diseases; SLE: Systemic Erythematosus Lupus; APS: Anti-Phospholipid Syndrome.
Discussion
Autoimmune diseases could be considered a family of disorders that often coexist in the same subject. The isolated thyroiditis per se could impair the pregnancy outcome and fecundity in childbearing women. Up to date, only few data are available evaluating the obstetrical outcome when thyroiditis coexists with autoimmune diseases.
Miscarriage and preterm delivery are the most common obstetric complications in pregnant women with isolated thyroiditis, also in presence of a normal thyroid hormone status (Thangaratinam et al., 2011). It is still unclear whether thyroid antibodies exert a direct pathogenetic effect at the fetal-maternal interface or they represent an epiphenomenon of other autoimmune disorders underlying.
Concerning the impact on pregnancy outcome when thyroiditis is associated with autoimmune disorders, the results of the various studies are conflicting and non-conclusive (De Carolis et al., 2004; Stagnaro-Green et al., 2011; Beneventi et al., 2016; Cellini et al., 2020).
Our study is one of the few reports evaluating the impact of thyroid antibody positivity on the pregnancy outcome in women affected by autoimmune diseases. Two major considerations can be done: the first one is that the prevalence of thyroiditis was significantly increased in the autoimmune disease pregnant women (17.54%) respect to that of the control group (5.57%); the second one is that the coexistence of thyroiditis with autoimmune diseases negatively impaired the pregnancy outcome in a specific group of rheumatic disorders such as CTD.
Regarding the prevalence of thyroid antibodies in MRD pregnancies, our findings confirm prior reports. In fact, the prevalence of thyroiditis in pregnant patients with SLE was of 24% in our study, very similar to the findings by Stagnaro et al. (23.8%). These authors described also a very high rate of preterm delivery (67%) in women who had thyroid disease, compared with the SLE women that were thyroid disease free (18%) (Stagnaro-Green et al., 2011).
In women with primary APS and history of recurrent spontaneous abortions, it was described a prevalence of 27% of thyroid antibody positivity (De Carolis et al., 2004). Further, anti-thyroid positivity was often associated with either reduced fecundity or with poor pregnancy outcome (De Carolis et al., 2004). In our study, we confirm similar findings (35%) in pregnant women with APS, while we were not able to demonstrate an impact on pregnancy outcome in case of anti-thyroid antibody positivity.
Moreover, we observed in CTD patients a prevalence of 24% of thyroiditis, although in another study the overall prevalence of either anti-TPO or anti-TG detection was up to 62.3% among CTD patients while it was 8% among controls (Beneventi et al., 2016). Furthermore, these authors suggested that thyroid antibody positivity could increase the risk of adverse pregnancy outcome, in terms of spontaneous abortion, fetal growth restriction, preeclampsia, and preterm delivery (Beneventi et al., 2016).
Surprisingly, in our study, the presence of anti-TPO and anti-TG antibodies was related with an increased risk of adverse pregnancy outcome, in terms of a lower week of delivery and a lower birth weight only in patients with CTD. Probably, in cases with SLE and APS the results were compromised by the small sample size. Other weaknesses of our analysis were: the retrospective design of the study; the lack of informations about replacement treatment; and the absence of cases with miscarriages, because we recruited women after the earlier weeks of pregnancy.
Future larger studies need to clarify the role of thyroid antibodies in the different autoimmune diseases, and the eventual role of thyroid hormone replacement on pregnancy outcome.
Finally, it is well known the EULAR recommendations (Andreoli et al., 2017) are to investigate the thyroid diseases at pre-conception counselling as well as in pregnancy in SLE and APS patients.
In our opinion, it is important to extend the prenatal screening for thyroid antibodies to all women affected by autoimmune diseases, paying special attention to the patients with CDT. On the other hand, it could be a field of interest to do the screening for autoimmune diseases in women in childbearing age with thyroiditis, particularly in presence of new specific symptoms or rheumatic clinical manifestations.
Defining the co-morbidities in high-risk pregnancies, as are women with autoimmune diseases, could better stratify the risk profile of the patient and could lead the clinicians to a tailored management of pregnancy.
Data Availability Statement
The datasets presented in this article are not readily available due to the patients privacy. Requests to access the datasets should be directed to ZnJhcml6em85MkBnbWFpbC5jb20=.
Author Contributions
SD and CG are co-senior authors. AB, SD, and FR contributed to conception and design of the study. AC, TA, and EG organized the database. FR and AB performed the statistical analysis. SD, FR, and CG wrote the first draft of the manuscript. AB, FR, CG, and SD wrote sections of the manuscript. All authors contributed to manuscript revision, read, and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abbassi-Ghanavati, M., Casey, B. M., Spong, C. Y., McIntire, D. D., Halvorson, L. M., and Cunningham, F. G. (2010). Pregnancy Outcomes in Women with Thyroid Peroxidase Antibodies. Obstet. Gynecol. 116 (2 Pt 1), 381–386. doi:10.1097/AOG.0b013e3181e904e5
Anandappa, S., Joshi, M., Polanski, L., and Carroll, P. V. (2020). Thyroid Disorders in Subfertility and Early Pregnancy. Ther. Adv. Endocrinol. Metab. 11, 2042018820945855. Oct 13. doi:10.1177/2042018820945855
Andreoli, L., Bertsias, G. K., Agmon-Levin, N., Brown, S., Cervera, R., Costedoat-Chalumeau, N., et al. (2017). EULAR Recommendations for Women's Health and the Management of Family Planning, Assisted Reproduction, Pregnancy and Menopause in Patients with Systemic Lupus Erythematosus And/or Antiphospholipid Syndrome. Ann. Rheum. Dis. 76 (3), 476–485. doi:10.1136/annrheumdis-2016-209770
Beneventi, F., Locatelli, E., Caporali, R., Alpini, C., Lovati, E., Ramoni, V., et al. (2016). Connective Tissue Diseases and Autoimmune Thyroid Disorders in the First Trimester of Pregnancy. J. Reprod. Immunol. 114, 32–37. doi:10.1016/j.jri.2016.02.004
Casey, B. M., Dashe, J. S., Spong, C. Y., McIntire, D. D., Leveno, K. J., and Cunningham, G. F. (2007). Perinatal Significance of Isolated Maternal Hypothyroxinemia Identified in the First Half of Pregnancy. Obstet. Gynecol. 109 (5), 1129–1135. doi:10.1097/01.AOG.0000262054.03531.24
Cellini, M., Santaguida, M. G., Stramazzo, I., Capriello, S., Brusca, N., Antonelli, A., et al. (2020). Recurrent Pregnancy Loss in Women with Hashimoto's Thyroiditis with Concurrent Non-endocrine Autoimmune Disorders. Thyroid 30 (3), 457–462. doi:10.1089/thy.2019.0456
De Carolis, C., Greco, E., Guarino, M. D., Perricone, C., Dal Lago, A., Giacomelli, R., et al. (2004). Anti-thyroid Antibodies and Antiphospholipid Syndrome: Evidence of Reduced Fecundity and of Poor Pregnancy Outcome in Recurrent Spontaneous Aborters. Am. J. Reprod. Immunol. 52 (4), 263–266. doi:10.1111/j.1600-0897.2004.00215.x
El Baba, K. A., and Azar, S. T. (2012). Thyroid Dysfunction in Pregnancy. Int. J. Gen. Med. 5, 227–230. doi:10.2147/IJGM.S27009
Ferrazzani, S., Degennaro, V. A., Di Stasio, E., Poppa, G., Moresi, S., Salvi, S., et al. (2017). Development of a New Fetal Growth Curve from a Large Sample of Italian Population. Minerva Pediatr. 69 (4), 245–250. doi:10.23736/S0026-4946.16.04258-4
Gaberšček, S., and Zaletel, K. (2011). Thyroid Physiology and Autoimmunity in Pregnancy and after Delivery. Expert Rev. Clin. Immunol. 7 (5), 697–707. quiz 707. doi:10.1586/eci.11.42
Haddow, J. E., Cleary-Goldman, J., McClain, M. R., Palomaki, G. E., Neveux, L. M., Lambert-Messerlian, G., et al. (2010). Thyroperoxidase and Thyroglobulin Antibodies in Early Pregnancy and Preterm Delivery. Obstet. Gynecol. 116 (1), 58–62. doi:10.1097/AOG.0b013e3181e10b30
Consortium on Thyroid and Pregnancy—Study Group on Preterm Birth Korevaar, T. I. M., Korevaar, T. I. M., Derakhshan, A., Taylor, P. N., Meima, M., Chen, L., et al. (2019). Association of Thyroid Function Test Abnormalities and Thyroid Autoimmunity with Preterm Birth: A Systematic Review and Meta-analysisErratum in. JAMAJAMA 322322 (717), 6321718–6322641. 2019 Nov 5. doi:10.1001/jama.2019.10931
Männistö, T., Vääräsmäki, M., Pouta, A., Hartikainen, A.-L., Ruokonen, A., Surcel, H.-M., et al. (2009). Perinatal Outcome of Children Born to Mothers with Thyroid Dysfunction or Antibodies: a Prospective Population-Based Cohort Study. J. Clin. Endocrinol. Metab. 94 (3), 772–779. doi:10.1210/jc.2008-1520
Myneni, R., Chawla, H. V., Grewal, A. S., Vivekanandan, G., Ndakotsu, A., Abubacker, A. P., et al. (2021). Thyroxine Replacement for Subfertile Females with Subclinical Hypothyroidism and Autoimmune Thyroiditis: A Systematic Review. Cureus 13 (8), e16872. Aug 4. doi:10.7759/cureus.16872
Negro, R., Schwartz, A., Gismondi, R., Tinelli, A., Mangieri, T., and Stagnaro-Green, A. (2011). Thyroid Antibody Positivity in the First Trimester of Pregnancy Is Associated with Negative Pregnancy Outcomes. J. Clin. Endocrinol. Metab. 96 (6), E920–E924. doi:10.1210/jc.2011-0026
Pop, V. J., Brouwers, E. P., Vader, H. L., Vulsma, T., van Baar, A. L., and de Vijlder, J. J. (2003). Maternal Hypothyroxinaemia during Early Pregnancy and Subsequent Child Development: a 3-year Follow-Up Study. Clin. Endocrinol. (Oxf) 59 (3), 282–288. doi:10.1046/j.1365-2265.2003.01822.x
Saki, F., Dabbaghmanesh, M. H., Ghaemi, S. Z., Forouhari, S., Ranjbar Omrani, G., and Bakhshayeshkaram, M. (2014). Thyroid Function in Pregnancy and its Influences on Maternal and Fetal Outcomes. Int. J. Endocrinol. Metab. 12 (4), e19378. doi:10.5812/ijem.19378
Stagnaro-Green, A., Akhter, E., Yim, C., Davies, T. F., Magder, L., and Petri, M. (2011). Thyroid Disease in Pregnant Women with Systemic Lupus Erythematosus: Increased Preterm Delivery. Lupus 20 (7), 690–699. doi:10.1177/0961203310394894
Stagnaro-Green, A. (2009). Maternal Thyroid Disease and Preterm Delivery. J. Clin. Endocrinol. Metab. 94 (1), 21–25. doi:10.1210/jc.2008-1288
Tagoe, C. E. (2015). Rheumatic Symptoms in Autoimmune Thyroiditis. Curr. Rheumatol. Rep. 17 (2), 5. doi:10.1007/s11926-014-0479-7
Keywords: thyroiditis, pregnancy outcome, autoimmune diseases, connective tissue disease (CTD), major rheumatic diseases (MRD), systemic lupus erythematosus (SLE), antiphospholipid syndrome (APS)
Citation: Botta A, Rizzo F, Antonielli T, Ciliberti A, Garufi E, Lanzone A, Garufi C and De Carolis S (2022) The Detrimental Effect of Thyroiditis on Pregnancy Outcome of Patients Affected by Autoimmune Diseases: An Open Question. Front. Pharmacol. 13:827735. doi: 10.3389/fphar.2022.827735
Received: 02 December 2021; Accepted: 14 January 2022;
Published: 09 February 2022.
Edited by:
Laura Andreoli, University of Brescia, ItalyReviewed by:
Victoria Bitsadze, I. M. Sechenov First Moscow State Medical University, RussiaRoberta Erra, University of Milan, Italy
Copyright © 2022 Botta, Rizzo, Antonielli, Ciliberti, Garufi, Lanzone, Garufi and De Carolis. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesca Rizzo, ZnJhcml6em85MkBnbWFpbC5jb20=
 Angela Botta
Angela Botta Francesca Rizzo
Francesca Rizzo Tatiana Antonielli
Tatiana Antonielli Alessandra Ciliberti
Alessandra Ciliberti Ester Garufi3
Ester Garufi3 Antonio Lanzone
Antonio Lanzone Cristina Garufi
Cristina Garufi Sara De Carolis
Sara De Carolis