
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 04 February 2022
Sec. Experimental Pharmacology and Drug Discovery
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.826771
This article is part of the Research Topic Nanomedicine in Infectious Diseases: Drug Delivery and Vaccines View all 9 articles
The p53 gene has the highest mutation frequency in tumors, and its inactivation can lead to malignant transformation, such as cell cycle arrest and apoptotic inhibition. Persistent high-risk human papillomavirus (HR-HPV) infection is the leading cause of cervical cancer. P53 was inactivated by HPV oncoprotein E6, promoting abnormal cell proliferation and carcinogenesis. To study the treatment of cervical intraepithelial neoplasia (CIN) and cervical cancer by restoring p53 expression and inactivating HPV oncoprotein, and to verify the effectiveness of nano drugs based on nucleic acid delivery in cancer treatment, we developed poly (beta-amino ester)537, to form biocompatible and degradable nanoparticles with plasmids (expressing p53 and targeting E7). In vitro and in vivo experiments show that nanoparticles have low toxicity and high transfection efficiency. Nanoparticles inhibited the growth of xenograft tumors and successfully reversed HPV transgenic mice’s cervical intraepithelial neoplasia. Our work suggests that the restoration of p53 expression and the inactivation of HPV16 E7 are essential for blocking the development of cervical cancer. This study provides new insights into the precise treatment of HPV-related cervical lesions.
P53, the most commonly mutated gene in human cancer, is essential for maintaining the stability of the human genome (Blandino and Di Agostino, 2018; Mendiratta et al., 2021). Its loss is a key event in the development of various tumors (Klco and Mullighan, 2021; Rudin et al., 2021). P53 plays an important role in protecting cells from malignant transformation by inducing cell cycle arrest or apoptosis (Zhou et al., 2019). In recent years, researchers have tried to reactivate inactivated p53. In spontaneous p53 mutant lymphoma and sarcoma models, the restored expression of wild-type p53 resulted in tumor growth arrest (Wang et al., 2011). Activating p53-dependent apoptosis in zebrafish inhibited angiogenesis (Zhou et al., 2016). Restoring p53 expression induced cycle arrest and apoptosis in non-small cell lung cancer (NSCLC) (Lu et al., 2017). Therefore, restoring p53 expression is a promising method in a variety of tumors.
Cervical cancer is the second most common cancer in less developed regions (Ferlay et al., 2015). Each year, 570,000 women are diagnosed with cervical cancer and 311,000 women die from this disease, which seriously affects women’s health worldwide (Bray et al., 2018; Arbyn et al., 2020). Persistent high-risk human papillomavirus (HR-HPV) infection is the leading cause of cervical cancer (Francis et al., 2010). Oncoproteins E6 and E7 are key factors in HPV-associated carcinogenesis. P53 was also inactivated by E6, promoting abnormal cell proliferation and carcinogenesis (Moody and Laimins, 2010; Taghizadeh et al., 2019). The HPV oncogenes E6 and E7 and their related host genes (such as p53) are key targets for prevention and treatment in the long process from HPV infection to CIN and ultimately cervical cancer (Pal and Kundu, 2019).
To date, surgical treatment is mainly used for CIN and early cervical cancer, however, it can’t solve the problem of virus infection and subsequent changes in key cell-signaling pathways. In recent years, an increasing number of gene-targeted therapies based on recombinant plasmids have been reported (Dominguez et al., 2016; Evers et al., 2016; Pahle and Walther, 2016; Zhang et al., 2016; Almeida et al., 2019). The CRISPR/Cas9 system is a powerful tool for the prevention and treatment of HPV infection due to its simple composition, convenient operation, high mutation efficiency, and low cost (Chen et al., 2019; Sharma et al., 2021; Song et al., 2021). Furthermore, it is necessary to develop a suitable vector to ensure the successful delivery of recombinant plasmids (Xiao et al., 2014; Xu et al., 2021). Clinical applications of viral vectors are limited due to safety issues (Zhou et al., 2017; Li et al., 2021; Ma et al., 2021). Non-viral vectors such as cationic liposomes (Wang et al., 2021), polyethyleneimine (PEI) (Radmanesh et al., 2021), and poly (β-amino ester) (PBAE) (Zhu et al., 2018; Zhang et al., 2021) are widely used due to their low toxicity and nonimmune characteristics. PBAEs have good biocompatibility and biodegradability. These materials can compress plasmids into nanosized composite particles, protect plasmids from nuclease degradation, and effectively deliver plasmids to cells (Tzeng et al., 2013; Guerrero-Cázares et al., 2014; Kim et al., 2016; Kaczmarek et al., 2021).
Therefore, to study the treatment of CIN and cervical cancer by restoring p53 expression and inactivating HPV oncoprotein, and to verify the effectiveness of nano drugs based on nucleic acid delivery in cancer treatment, we attempted to reverse cervical intraepithelial neoplasia by vaginal injection of nanoparticles composed of PBAE537 and plasmids (wild-type p53 expression and HPV16 E7 targeting), providing a new idea strategy for precise treatment of cervical cancer.
We purchased 1,5-pentanediol diacrylate (B5) from Aladdin (B136168, N184580, China), 3-amino-1-propanol (S3) from TCI (A0438, Japan), and 1-(3-aminopropyl)-4-methylpiperazine (E7) from ALFA Aesar (L04876, Ward Hill, MA). Branched poly(ethyleneimine) (water-free, 25 kDa, bPEI) was purchased from Sigma-Aldrich (408727, USA). Cationic liposome HP was purchased from Roche (6366236, USA). The overexpression (OE) p53 (PCDH-CMV-MCS-EF1-RFP-T2A-Puro) plasmids contained CMV promoter-expressing human p53 or mouse p53. The plasmids pDest-EGFP-N1 (plasmid 31796, GFP) and pAAV-CAG-RFP (plasmid 22910, RFP) were purchased from Addgene. The HPV16 E7-targeted CRISPR plasmid sequence was ATATTGTAATGGGCTCTGTC CGG. Plasmid DNA was prepared using an endotoxin-free plasmid extraction kit (Omega, USA) and stored at −80°C. PBAE537 was synthesized in a 2-step procedure (Figure 1) as follows: 1,5-pentanediol diacrylate (B5) was mixed with 3-amino-1-propanol (S3) and stirred on a magnetic stir plate at 90°C for 24 h with 2 ml of DMSO at a 1.2:1 M ratio. After stirring, 1-(3-aminopropyl)-4-methyl piperazine (E7) was added to the mixture (10-fold), and the mixture was stirred for 30 s, incubated at room temperature for 1 h, and precipitated in anhydrous diethyl ether to remove the solvent and monomers. The polymer was washed three times with ether and kept under a vacuum with desiccant for 48 h to remove the final traces of ether. The polymer poly (beta-amino ester) 537 was then divided into smaller volumes and stored at −20°C with desiccant until needed. For further use, the polymer was dissolved in DMSO at 100 mg/ml and stored at −20°C.
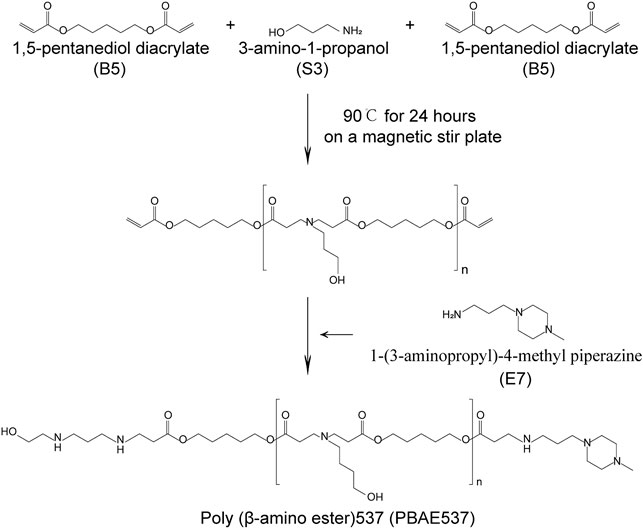
FIGURE 1. Synthesis of PBAE537. Synthesis of PBAE537. The PBAE nanomaterial PBAE537 was formed by mixing 1,5-pentanediol diacrylate (B5) with 3-amino-1-propanol (S3) and stirring at 90°C for 24 h on a magnetic stirring plate and adding 1-(3-aminopropyl)-4-methyl piperazine (E7).
PBAE537 (2 μg/L) was diluted with 25 mM sodium acetate (pH 5) into 200 ng of plasmid at mass ratios of 5:1, 10:1, 20:1, 40:1, 60:1, 80:1, and 100:1. PBAE537 and plasmid were mixed gently in 20 µL of NaAc for 30 s and incubated at room temperature for at least 20 min. The particle size and zeta potential of the nanoparticle (NP) were measured by laser light scattering (DB-525 Zeta PALS; Brookhaven Instruments, Holtsville, NY, USA).
Nanoparticles composed of PBAE537/GFP expressing plasmid (hereinafter referred to as the GFP) were deposited on porous carbon film carbon-coated copper mesh and characterized by TEM (JEM-1230, Japan).
The cervical cancer cell lines SiHa, HeLa, CaSki, MS751, C33A, and HEK293 were purchased from ATCC and passaged in our laboratory. The S12 cell line is an immortalized human cervical keratinocyte cell line, and it was a generous gift from Professor Kenneth Raj (Health Protection Agency) with permission from the original owner, Professor Margaret Stanley (Bechtold et al., 2003). SiHa, HeLa, MS751, C33A, and HEK293 cells were cultured in DMEM supplemented with 10% fetal bovine serum (FBS). CaSki cells were grown in RPMI-1640 supplemented with 10% FBS. S12 cells were maintained in a 1:1 mixture of DMEM/F12 (Gibco) and Ham’s F12 (Gibco) medium supplemented with 10% FBS, 24.3 mg/ml adenine, 0.5 mg/ml hydrocortisone, 8.4 ng/ml cholera toxin, 5 mg/ml insulin, and 10 ng/ml epidermal growth factor (EGF). All cells were cultured in a humidified incubator with 5% CO2 at 37°C.
C57BL/6 female mice were purchased from Beijing HFK Bioscience, and K14-HPV16 transgenic mice were provided by the National Cancer Institute (NCI) Mouse Repository (Frederick, MD, USA). All mice were housed at the SPF animal laboratory in the Experimental Animal Center, Tongji Medical College, Huazhong University of Science and Technology (HUST, Wuhan, China) and managed by regulations of Chinese law. The research was approved by the Experimental Animal Ethics Committee of Tongji Medical College of Huazhong University of Science and Technology.
PBAE537 solution (diluted with 25 mM sodium acetate at pH 5) was added to the green fluorescent protein (GFP) plasmid at mass ratios of 20:1, 40:1, 60:1, and 80:1. SiHa, HeLa, CaSki, and S12 cells were inoculated in 96-well plates with the same number of cells per cell line in each well. Each well was transfected with 100 ng GFP. bPEI was diluted with PBS to 1 mg/mL as a positive control. The cells were treated with nanoparticles for 4 h and then cultured in a complete medium. Cell viability was defined as the metabolic activity retained in each well after transfection, measured at various time points using a Cell Counting Kit-8 (CCK-8, Dojindo) according to the manufacturer’s instructions.
One hundred microliters of nanoparticles (PBAE537/GFP 60:1, 100 µg plasmid) were injected into the thigh muscles of C57BL/6 mice once a day for 3 days. The same volume of bPEI/GFP (bPEI/GFP 3:1, 100 μg) solution was used as a positive control. Thigh muscles and other organs were collected on the 4th and 7th days after the initial injection. PBAE537/GFP or bPEI/GFP (20 µL NPs containing 10 μg GFP) solution was pipetted into the vaginas of C57BL/6 mice once a day for 20 days. The cervix and other organs were then collected and fixed with paraformaldehyde. Toxicity was evaluated by hematoxylin-eosin (H&E) staining.
All cells were inoculated in sterile 6-well plates at a density of 40–70% at transfection. The nanoparticles formed by PBAE537 and GFP at different mass ratios (20:1, 40:1, 60:1, 80:1) were dissolved in 100 μL of 25 mM pH 5 sodium acetate and incubated at room temperature for 20 min before being added to plate wells containing 900 μL of serum-free medium. After incubation for 4 h, the nanoparticles were replaced with a complete medium. The bPEI/GFP complex (mass ratio 3:1, 2 μg GFP) was mixed in PBS for 20 min and then added to the cells.
Four-week-old C57BL/6 mice were injected with 20 μL of nanoparticles into the vagina once a day for 3 days. The vaginas were rinsed with PBS 3 times before each administration to remove vaginal mucus. Three days after administration, the mice were euthanized, and the cervix was separated and frozen into 7 μm sections using Thermo Fisher Scientific.
SiHa cells (5×106) were injected subcutaneously into 4-week-old nude mice. When the tumor grew to nearly 35 mm3, the mice were randomly assigned to four groups. We administered intra-tumoral injections of 100 μL of NPs coated with 60 μg of plasmid (PBAE537/plasmid 60:1) every 4 days. Subcutaneous tumors were collected after the mice were euthanized, and the tumor size was calculated using the following formula: L × W (Blandino and Di Agostino, 2018) × 0.5. All experimental protocols were approved by the Institutional Animal Care and Use Committee of HUST, and the study was carried out in strict accordance with the Guidelines for the Welfare of Animals in Experimental Neoplasia.
Six-week-old K14-HPV16 transgenic female mice were randomly divided into four groups for vaginal administration. After anesthesia with 4% chloral hydrate, the vagina was irrigated 3 times with PBS. PBAE537 and 10 μg plasmid with a final volume of 20 μL (PBAE537/plasmid 60:1) were injected into the mouse vaginas once a day for 20 days. The drugged mice were placed in the supine position for at least 20 min while anesthetized to prevent the drug from flowing out of the vagina due to pressure. After 20 days of treatment, the mice were euthanized, and the cervix was dissected and fixed with 4% paraformaldehyde. The cervical tissues of the mice were paraffin-embedded, sectionalized, and stained with HE and IHC staining.
Paraffin-embedded sections (5 μm) were stained with H and E and immunohistochemical (IHC) staining. The slides were incubated overnight at 4°C with mouse anti-HPV16 E7 (1:50, orb10573, Biorbyt), rabbit anti-P16 (1:100, A0262, Abclonal), rabbit anti-RB1 (1:100, 10048-2-Ig, Proteintech), rabbit anti-Ki67 (1:100, ab16667, Abcam), rabbit anti-CDK2 (1:400, ab6538, Abcam), rabbit anti-E2F1 (1:200,12171-1-Ap, Proteintech), rabbit anti-TP53 (1:150, A16989, Abclonal), rabbit anti-14-3-3 Sigma (1:50, A1026, Abclonal) and rabbit anti-P21 (1:150, A11454, Abclonal) primary antibodies. Diaminobenzidine (DAB) was used for antibody detection. Images were photographed from three randomly selected fields using cellSens Dimension (version 1.8.1, Olympus). Staining was assigned a score using a semiquantitative six category grading system: 0, no staining; 1,1%to 10% staining; 2, 11% to 25% staining; 3, 26% to 50% staining; 4, 51% to 75% staining; and 5, >75% staining. Staining intensity was assigned a score using a semiquantitative four-category grading system: 0, no staining; 1, weak staining; 2, moderate staining; and 3, strong staining. Every core was assessed individually and the mean of three readings was calculated for every slide. The staining score was determined separately by two experts under the same conditions.
Cells were lysed on ice for 30 min in lysis buffer containing 150 mmol/L NaCl, 1% sodium deoxycholate, 50 mmol/L Tris, 1% Triton X-100, 0.1% SDS, and a protease inhibitor cocktail. The primary antibodies used were rabbit anti-GAPDH (1:1,000, AM1020a, Abgent) and mouse anti-Cas9 (1:200, 14,697, CST).
All quantitative data are expressed as the mean ± SEM of at least three parallel measurements. One-way ANOVA was used for statistical analysis, and GraphPad Prism software was used for p < 0.05.
Poly β-amino ester 537 (PBAE537) was synthesized from 1,5-pentanediol diacrylate (B5), 3-amino-1-propanol (S3) and 1-(3-amino-propyl)-4-methyl piperazine (E7) as monomers. The polymer was named PBAE537 according to the three monomers of the main chain (B), side chain (S), and end cap (E). The synthetic scheme of PBAE537 is shown in Figure 1. Nanoparticles were prepared at different mass ratios of PBAE537 to GFP (5:1, 10:1, 20:1, 40:1, 80:1, and 100:1), and the plasmid encapsulation ability of PBAE537 was determined by agar gel electrophoresis. When the mass ratio of PBAE537 to GFP reached 40:1, most of the plasmids were encapsulated by PBAE537, and only a few plasmids migrated. When the mass ratio was increased to 60:1, the plasmid was almost completely encapsulated by PBAE537 and remained in the spot pores of the agarose gel without migration (Figure 2A). The dynamic light scattering particle size range shows that the NPs and different mass ratios were 146.1–354.5 nm, and the electric potential range was 14.5–30.6 mV (Figures 2B,C). The NP size was the minimum when the mass ratio was 40:1, suggesting that the interaction between PBAE537 and the GFP plasmid was closest to this mass ratio. Transmission electron microscopy (TEM) showed that the shape of NPs composed of PBAE537 and GFP was spherical and uniform (Supplementary Figures S1A,B). These results indicate that our synthesized material PBAE537 can wrap nucleic acid to form nanoparticles.
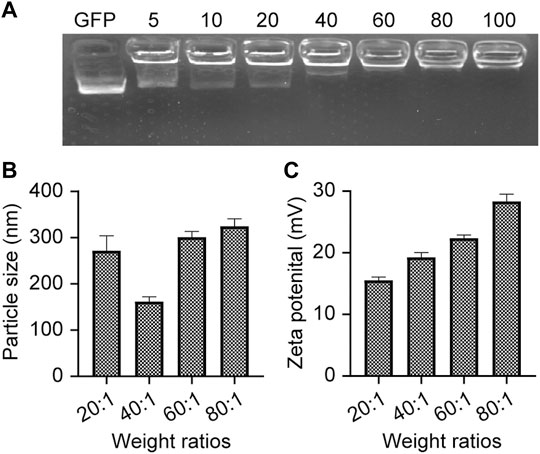
FIGURE 2. Characterization of NPs. (A) Agarose gel (2%) electrophoresis of NPs composed of PBAE537 and GFP at different mass ratios. (B) Measurement of particle sizes of NPs with different mass ratios (PBAE537:GFP) and (C) zeta potentials by dynamic light scattering. The data represent the mean ± SEM (n = 3 per group).
The efficacy of gene therapy depends on the uptake rate of nanoparticles by targeted cells and the expression rate of delivered genes. In vitro, nanoparticles formed by GFP plasmids and PBAE537 were transfected into seven cell lines (SiHa, HeLa, S12, CaSki, MS751, C33A, and HEK293). Transfection efficiency was evaluated by fluorescence microscopy and flow cytometry (Figures 3A,B, Supplementary Figure S5). The transfection efficiency of nanoparticles with the same mass ratio was different in each cell line. In general, the transfection efficiency was highest when the mass ratio of PBAE537 to GFP was 60:1. In some cell lines, such as SiHa, HeLa, S12, and MS751, the efficiency was even higher than that of bPEI (a cationic polymer with transfection ability) and the commercial transfection reagent HP. Transmission electron microscopy imaging of HEK293 cells transfected with nanoparticles revealed the presence of nanoparticles in cytoplasmic vesicles, indicating successful uptake of our nanoparticles into the cells (Supplementary Figure S2).
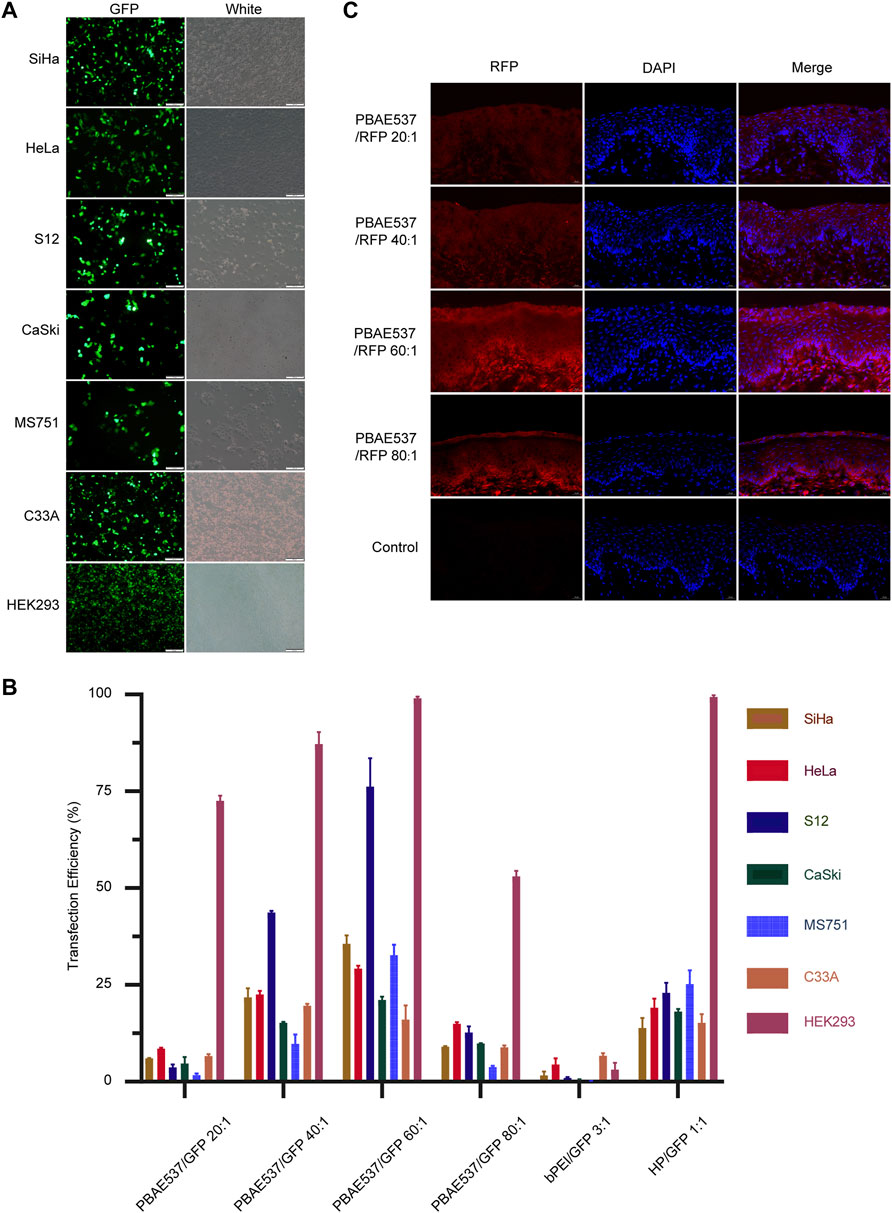
FIGURE 3. Efficiency of NPs taken up by cell lines and the mouse cervix. (A) Representative images of green fluorescent protein expression in SiHa, HeLa, S12, CaSki, MS751, C33A, and HEK293 cells after 72 h of nanoparticle (PBAE537/GFP 60:1) treatment. (B) Flow cytometry was used to determine the efficiency of nanoparticles transfected with PBAE537/GFP at different weight ratios (20:1, 40:1, 60:1, and, 80:1) and HP/GFP (1:1) for the expression of the green fluorescent protein. bPEI is a cationic polymer with transfection ability used as a control. (C) Representative images of red fluorescent protein expression in the cervix of the C57BL/6 mice treated with nanoparticles composed of different weight ratios of PBAE537 to RFP (10 μg RFP, once daily, for 3 days) compared with the control cervix treated with sodium acetate alone without nanoparticles. Scale, 20 μm.
In vivo, nanoparticles composed of PBAE537 and red fluorescent protein (RFP) plasmid were injected vaginally into female C57BL/6 mice, and the uptake of the nanoparticles in vivo was assessed by the intensity of red fluorescence (Figure 3C). To explore the experimental conditions to achieve the highest transfection efficiency in vivo, we adjusted the following conditions: the mass ratio of PBAE537 to GFP, the dose of transfected plasmid, and the detection time point after treatment (Supplementary Figure S3). The results showed that the transfection efficiency was the highest when the mass ratio was 60:1, the plasmid dose was 10 μg every 3 days, and detection occurred on the sixth day after treatment. The expression of fluorescent protein suggested that PBAE537 can efficiently deliver nucleic acid in vivo and in vitro.
To evaluate the toxicity of nanoparticles, we transfected SiHa, HeLa, CaSki, and S12 cells with nanoparticles at PBAE537/GFP mass ratios of 20:1, 40:1, 60:1, and 80:1. Cell viability was detected by CCK-8 assays at 24, 48, and 72 h after transfection (Figures 4A–D). When the mass ratio of PBAE537 to GFP was as high as 80:1, growth inhibition was most significant but less than that in the positive control group (bPEI/GFP 3:1). The PBAE537 showed low cytotoxicity in vitro.
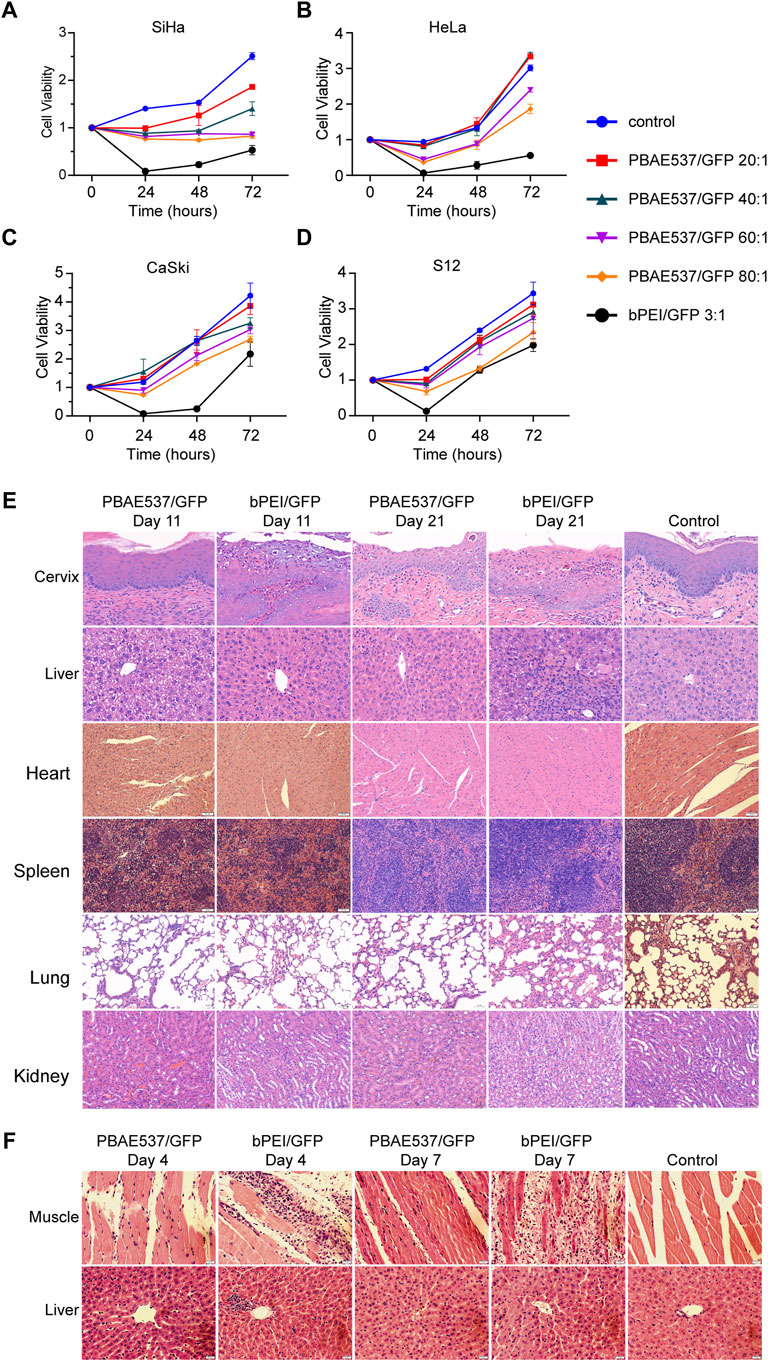
FIGURE 4. Toxicity analysis of nanoparticles in cell lines and mouse organs. (A–D) The toxicity of PBAE537/GFP nanoparticles with different mass ratios (20:1, 40:1, 60:1, and 80:1) was detected in (A) SiHa, (B) HeLa, (C) S12, and (D) CaSki cells. bPEI/GFP (3:1) was used as a positive control. The time points of cytotoxicity were 24, 48, and 72 h after transfection. Each time point represents the mean ± SEM (n = 4). (E) HE staining of paraffin sections of cervical and liver tissues of mice that received continuous vaginal injections of PBAE537/GFP (60:1) and bPEI GFP (3:1) for 10 and 20 days. Mice were injected intravaginally with 10 μL nanoparticles containing 10 μg plasmids. Vaginal treatment was performed once daily for 10 or 20 days. Significant damage was observed only in the bPEI group, but less in the PBAE537 group. (F) Nanoparticles composed of PBAE537/GFP (60:1) and bPEI/GFP (3:1) were injected into the thigh muscles of mice for 3 consecutive days. Representative images of HE staining in paraffin sections of thigh muscle tissue and liver tissue of mice at 4 and 7 days after the initial injection. One hundred microliters of plasmids containing 100 μg nanoparticles were injected into the thigh muscles of mice every day. Scale, 20 μm.
To further detect the toxicity of nanoparticles in vivo, we reviewed the literature (Anderson et al., 2004) and conducted maximum dose toxicity tests with 200 and 300 μg plasmids for vaginal administration and intra-tumoral administration, respectively. Nanoparticles consisting of PBAE537 or bPEI coated with 100 μg of GFP were injected into the vaginas of C57BL/6 mice daily for 20 days, and toxicity was detected on the 11th and 21st days after initiation of vaginal administration. Compared with the untreated control group, the bPEI/GFP treatment group exhibited significant increases in the number of inflammatory necroses in the cervix and pyknosis of the liver nucleus, while no similar changes were observed in the nanoparticle (PBAE546/GFP 60:1)-treated group (Figure 4E). Low toxicity was observed in the heart, spleen, lungs, or kidneys. Nanoparticles consisting of PBAE537 or bPEI coated with 100 μg GFP were injected daily into the thigh muscles of C57BL/6 mice for 3 days, and toxicity was detected on the 4th and 7th days after initiation injection. In the bPEI/GFP treatment group, local injection caused large-scale necrotic inflammatory infiltration of muscular tissue, while no similar changes were observed in the 60:1 group treated with PBAE546/GFP (Figure 4F). Low toxicity was observed in other important organs (Supplementary Figure S4). The above data indicate our nanoparticles’ safety and low toxicity, which are necessary characteristics for pharmaceutical use.
Based on the clear understanding of the toxicity of PBAE537, we further studied the therapeutic effect of PBAE537/therapeutic plasmid in vivo and in vitro. As shown in Figures 5A−D, targeting HPV16 E7 decreased the viability of HPV16-positive SiHa and CaSki cells but did not significantly inhibit the growth of HPV18-positive HeLa and HPV-negative C33A cells. The p53 overexpression group and the group with the combination targeting HPV16 E7 and over-expressing p53 showed significant growth inhibition in all four cell lines. To observe the inhibitory effect of NPs on tumor growth in vivo, SiHa cells were inoculated subcutaneously into nude mice. When xenograft tumors grew to about 35 mm (Klco and Mullighan, 2021), NPs were used for intra-tumoral injection. The grouping of the experiment was the same as that of the in vitro experiment, including the single target of HPV16 E7, p53, and double target of both. Compared with the control group, SiHa subcutaneous tumor growth and volume were significantly reduced in the three groups of targeted therapy mice (Figures 5E,F). Growth inhibition of cervical cancer cell lines and subcutaneous tumors confirmed the therapeutic effect of our PBAE537 nanoparticles.
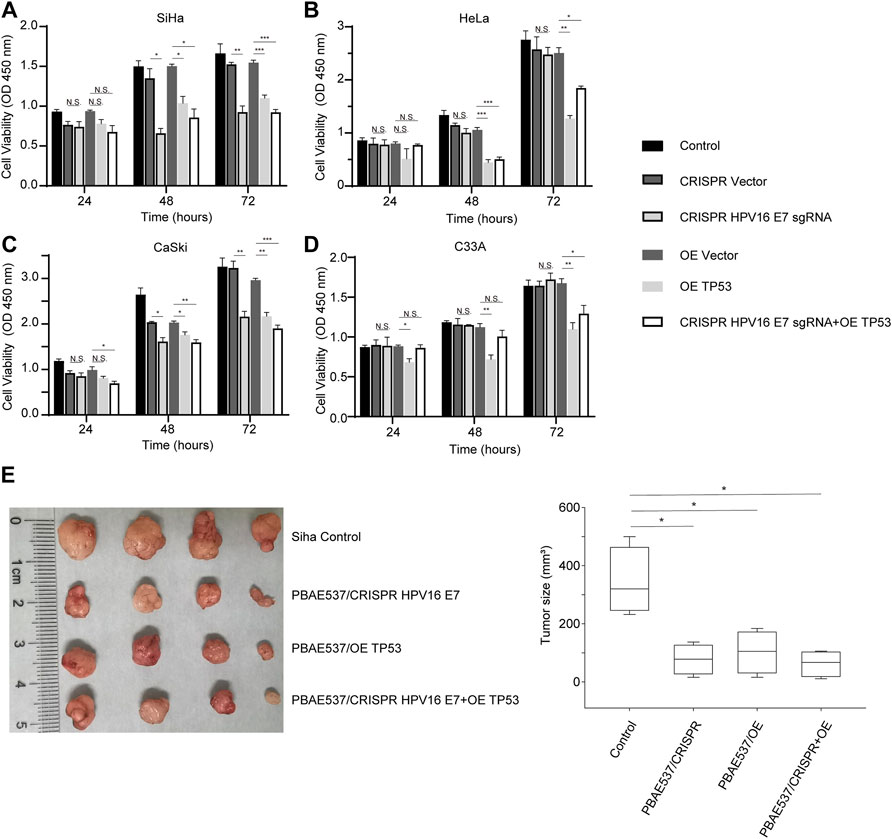
FIGURE 5. Growth inhibition of cervical cancer cells by PBAE537–therapeutic plasmid polyplex NPs in vitro and in vivo. (A–C) The CCK-8 method was used to detect the cell viability of (A) SiHa, (B) HeLa, (C) S12, and (D) CaSki cells at 24, 48, and 72 h after treatment with nanoparticles (PBAE537/plasmid 60:1) composed of PBAE537 and three targeted plasmids (HPV16 E7 inactivation group, p53 overexpression group, and 1:1 mixture of the two plasmids group). (E, F) SiHa cells were injected subcutaneously into the right hind limb of BALB/c-nu mice. When the transplanted tumor grew to approximately 35 mm3, NPs consisting of PBAE537 and the CRISPR/Cas9 plasmid targeting HPV16 E7 and p53 were injected intratumorally (60:1). NPs were injected as a volume of 100 µL containing 60 μg of plasmid once every 4 days. (E) The subcutaneously formed tumors were photographed, and (F) the estimated sizes were measured after treatment with NPs. One-way ANOVA was used for statistical analysis, *: p < 0.05, **: p < 0.01, ***: p < 0.001, N.S.: no significant difference.
Encouraged by the above results, we next performed cervical in situ therapy with nanoparticles in HPV16 transgenic mice (Medler et al., 2018), a cervical precancerous lesion model. After 20 days of continuous treatment, the malignant phenotype of the cervical epithelium in the mice treated with the three targeted nanoparticles was significantly reversed. The proliferation of epithelial cells was inhibited, the basal cells were arranged neatly, and the nuclear volume was reduced. The cervical epithelial cells of the untreated control mice were hyperplastic. Immunohistochemistry was used to detect the expression levels of HPV16 E7 and p53 and related proteins. The expression of the target molecule HPV16 E7 and the HR-HPV substitute marker P16 was significantly reduced in all three targeted administration groups, while the expression of the tumor suppressor gene RB, which interacts with the E7 oncoprotein, was restored. The levels of p53 and its downstream molecules P21 and 14-3-3 Sigma, which are responsible for cell cycle regulation, were increased to a certain extent in both targeted activation groups. The expression of the proliferation-related protein Ki67, cell cycle-related protein CDK2, transcription factor E2F1, and apoptosis suppressor gene Bcl2 was significantly inhibited (Figure 6). These results suggest that our nanoparticles targeting HPV16 E7 or supplementation with wild-type p53 can effectively reduce the expression of oncoproteins, restore the expression of suppressed tumor suppressor factors, and affect the related signaling pathways, thereby reversing the malignant phenotype of cervical epithelium in HPV16 transgenic mice.
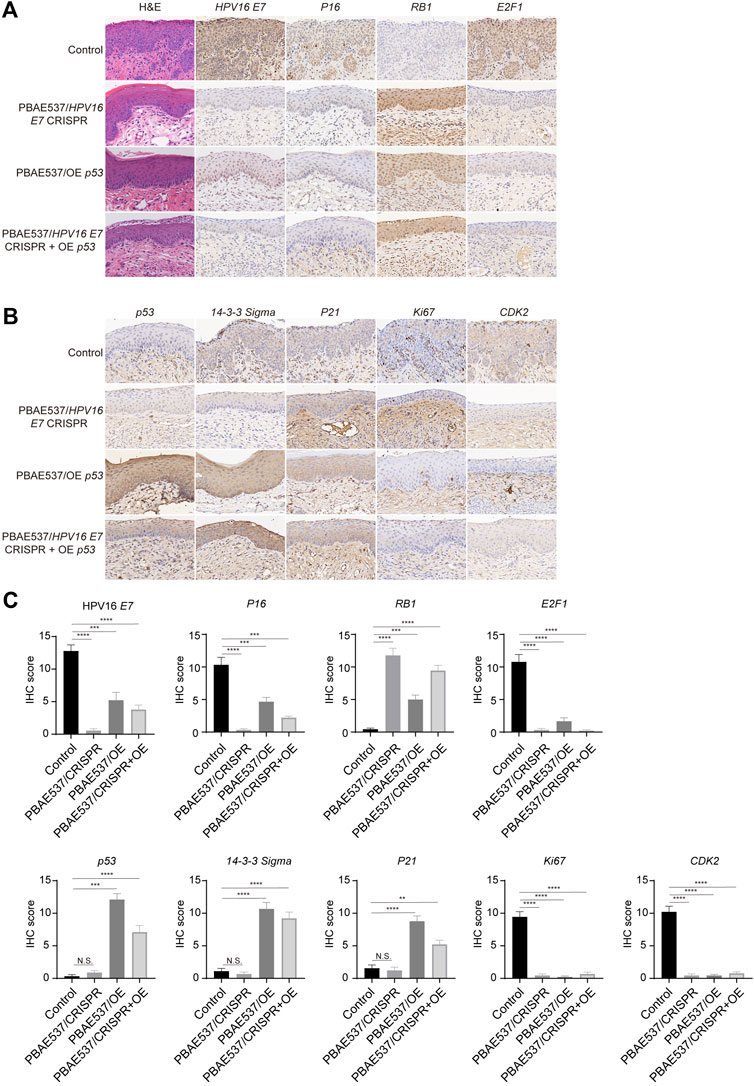
FIGURE 6. Therapeutic effects of nanoparticles composed of PBAE537 and therapeutic plasmids targeting HPV16 E7 and p53 K14-HPV16 transgenic mice. (A, B) Representative images of H and E and IHC staining and (C) comparison of the cervical epithelium between untreated HPV16 transgenic mice (control group) and three other targeted groups treated with nanoparticles composed of PBAE537 and plasmids (60:1, 10 μg of plasmids per day for 20 days continuously. IHC staining indicators included HPV16 E7, P16, RB1, E2F1, p53, 14-3-3 Sigma, P21, Ki67, and CDK2. Scale bars, 20 μm. The data represent the mean ± SEM (n = 3). One-way ANOVA was used for statistical analysis, *: p < 0.05, **: p < 0.01, ***: p < 0.001, ****: p < 0.0001, N.S.: no significant difference.
In this study, we developed the nanomaterial PBAE537, which showed low biotoxicity, degradability, and high transfection efficiency (Figures 1, 2). In addition to the size of the nanoparticles, the electric potential of the nanoparticles and the characteristics of the transfected cells also affect the transfection efficiency. These all affect the ability of nanoparticles to encapsulate and release plasmids. We believe that PBAEs are suitable for vaginal usage because the acidic environment of the vagina is conducive to the stability of PBAEs (Li et al., 2020). Vaginal usage can significantly reduce the amount of PBAEs in the blood and thus significantly reduce side effects such as hemolysis. Toxicity is the greatest challenge in nanomaterial therapy, so we chose to fully evaluate the toxicity of PBAE537 by muscular and vaginal delivery forms. The effects on other organs can be observed after nanoparticles enter the circulatory system by muscle administration. Vaginal delivery has the advantage of mimicking what occurs in clinical trials, although a small fraction of the drug may flow out of the vagina. PBAE537 showed very low biotoxicity (Figure 4), which is very promising for the further clinical transformation of drugs. In addition, PBAEs are highly plastic, and higher efficacy and fewer side effects can be achieved by optimizing their structure (Liu et al., 2019). The delivery efficiency of PBA537 was satisfactory (Figure 3). These optimizations deserve further study.
We selected therapeutic targets. The E6 and E7 viral oncogenes encode oncoproteins that disrupt cell cycle regulation and enhance cell proliferation by inactivating the tumor suppressors p53 and RB, respectively (Longworth and Laimins, 2004; Strickland and Vande Pol, 2016; Ghanaat et al., 2021). P53 inactivation mediated by E6 or p53 mutations may be a key step in the development of cervical cancer (Scheffner et al., 1991; Park et al., 1994). Previous studies have used CRISPR/Cas viral vectors to specifically inactivate E6 or E7 in tumor cells, but these therapies are limited by the safety of viral vectors and the specificity of oncogene sequences of different HPV types. Their downstream tumor suppressors, such as p53, avoid the complexity of the single guide RNA design. Dysfunction of the tumor suppressor p53 is closely related to insensitivity to treatment and recurrence of many malignant tumors, including cervical cancer.
An increasing number of studies have shown that the recovery of p53 activity can induce cell cycle arrest and apoptosis, eliminate chemoradiotherapy resistance, and inhibit the growth of tumor cells (Einbond et al., 2021; Granados-López et al., 2021). Therefore, activating wild-type p53 and restoring p53 function seems to be an attractive therapeutic strategy (Merlin et al., 2021). Pharmacological activation of the p53 pathway has been reported for the treatment of skin cancer, neuroblastoma, prostate cancer, and other tumors (Loureiro et al., 2020; Patiño-Morales et al., 2020; Won and Seo, 2020; Zafar et al., 2021). Some studies directly restore p53 expression to achieve the purpose of tumor treatment (Blandino and Di Agostino, 2018; Kong et al., 2019; Hu et al., 2021). Reactivation of p53 has also been considered as a potential treatment in cervical cancer (Zhao et al., 2021). Thus, in this study, we chose to focus on the efficacy of restoring p53 expression and targeted knockout of E7 in a mouse model of cervical precancerous lesions. The CRISPR/Cas9 recombinant plasmid expressing wild-type p53 and the overexpression plasmid targeting HPV16 E7 and their mixtures were delivered by PBAE537.
Nucleic acid delivery by PBAE has been used in many kinds of tumors (Zhang et al., 2020; Qu et al., 2020; Niu et al., 2020; Kaczmarek et al., 2018). For example, it was reported that the transfection efficiency of PBAE in B16-F10 cells was 65% (Routkevitch et al., 2020). Our PBAE537 showed comparable transfection efficiency and low toxicity to previous studies, both in vitro and in vivo. In this study, growth inhibition of cervical cancer cell lines (SiHa (HPV16 positive), HeLa (HPV18 positive), CaSki (HPV16 positive, HPV18 positive), and C33A (HPV negative)) and subcutaneous tumors confirmed the therapeutic effect of our PBAE537 nanoparticles (Figure 5). Restoration of p53 expression in cervical vaginal epithelium of HPV transgenic mice reversed the phenotype of CIN (Figure 6). Restored p53 expression increases apoptosis of epithelial cells, and when apoptosis increases, E7 and Rb expression may be affected. We hypothesized that p53 overexpression may restore RB expression through some pathways. Previous reports have confirmed the cross-talk between p53 and Rb/E2F signaling mechanisms (Rajasekaran et al., 2017). We can see that E7 is still expressed in the basal region. The expression of E7 is derived from exogenous HPV and is relatively independent, so it remains expressed in the basal region even with increased apoptosis.
Our work suggests that the restoration of p53 expression and the inactivation of HPV16 E7 are essential for blocking the development of cervical cancer. Restoration of p53 activity can be extended to a variety of tumors, and the PBAE537 delivery vector can also be used for the treatment of other genes and tumors. Restoration of p53 expression can also be applied to other tumors caused by p53 inactivation, such as lung cancer, hematological malignancies, etc., becoming a promising broad-spectrum targeted anticancer drug.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
The animal study was reviewed and approved by Ethics Committee of Tongji Hospital.
HW and DZ designed and supervised the research together with JX. GL and JX provided technical support. JX, XM, JD and HS performed the experiments. JX and GL provided analysis and interpretation of data. The manuscript was drafted by JX and DZ. All authors critically reviewed the article and approved the final manuscript.
This work was supported by funds from National Natural Science Foundation of China (81830074, 81974412, 81772786, 81502253 and 82002763).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.826771/full#supplementary-material
Almeida, A. M., Queiroz, J. A., Sousa, F., and Sousa, Â. (2019). Cervical Cancer and HPV Infection: Ongoing Therapeutic Research to Counteract the Action of E6 and E7 Oncoproteins. Drug Discov. Today 24 (10), 2044–2057. doi:10.1016/j.drudis.2019.07.011
Anderson, D. G., Peng, W., Akinc, A., Hossain, N., Kohn, A., Padera, R., et al. (2004). A Polymer Library Approach to Suicide Gene Therapy for Cancer. Proc. Natl. Acad. Sci. U S A. 101 (45), 16028–16033. doi:10.1073/pnas.0407218101
Arbyn, M., Weiderpass, E., Bruni, L., de Sanjosé, S., Saraiya, M., Ferlay, J., et al. (2020). Estimates of Incidence and Mortality of Cervical Cancer in 2018: a Worldwide Analysis. Lancet Glob. Health 8 (2), e191–e203. doi:10.1016/S2214-109X(19)30482-6
Bechtold, V., Beard, P., and Raj, K. (2003). Human Papillomavirus Type 16 E2 Protein Has No Effect on Transcription from Episomal Viral DNA. J. Virol. 77 (3), 2021–2028. doi:10.1128/jvi.77.3.2021-2028.2003
Blandino, G., and Di Agostino, S. (2018). New Therapeutic Strategies to Treat Human Cancers Expressing Mutant P53 Proteins. J. Exp. Clin. Cancer Res. 37 (1), 30. doi:10.1186/s13046-018-0705-7
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Chen, M., Mao, A., Xu, M., Weng, Q., Mao, J., and Ji, J. (2019). CRISPR-Cas9 for Cancer Therapy: Opportunities and Challenges. Cancer Lett. 447, 48–55. doi:10.1016/j.canlet.2019.01.017
Dominguez, A. A., Lim, W. A., and Qi, L. S. (2016). Beyond Editing: Repurposing CRISPR-Cas9 for Precision Genome Regulation and Interrogation. Nat. Rev. Mol. Cel Biol 17 (1), 5–15. doi:10.1038/nrm.2015.2
Einbond, L. S., Zhou, J., Wu, H.-a., Mbazor, E., Song, G., Balick, M., et al. (2021). A Novel Cancer Preventative Botanical Mixture, TriCurin, Inhibits Viral Transcripts and the Growth of W12 Cervical Cells Harbouring Extrachromosomal or Integrated HPV16 DNA. Br. J. Cancer 124 (5), 901–913. doi:10.1038/s41416-020-01170-3
Evers, B., Jastrzebski, K., Heijmans, J. P., Grernrum, W., Beijersbergen, R. L., and Bernards, R. (2016). CRISPR Knockout Screening Outperforms shRNA and CRISPRi in Identifying Essential Genes. Nat. Biotechnol. 34 (6), 631–633. doi:10.1038/nbt.3536
Ferlay, J., Soerjomataram, I., Dikshit, R., Eser, S., Mathers, C., Rebelo, M., et al. (2015). Cancer Incidence and Mortality Worldwide: Sources, Methods and Major Patterns in GLOBOCAN 2012. Int. J. Cancer 136 (5), E359–E386. doi:10.1002/ijc.29210
Francis, S. A., Nelson, J., Liverpool, J., Soogun, S., Mofammere, N., and Thorpe, R. J. (2010). Examining Attitudes and Knowledge about HPV and Cervical Cancer Risk Among Female Clinic Attendees in Johannesburg, South Africa. Vaccine 28 (50), 8026–8032. doi:10.1016/j.vaccine.2010.08.090
Ghanaat, M., Goradel, N. H., Arashkia, A., Ebrahimi, N., Ghorghanlu, S., Malekshahi, Z. V., et al. (2021). Virus against Virus: Strategies for Using Adenovirus Vectors in the Treatment of HPV-Induced Cervical Cancer. Acta Pharmacol. Sin 42, 1981–1990. doi:10.1038/s41401-021-00616-5
Granados-López, A. J., Manzanares-Acuña, E., López-Hernández, Y., Castañeda-Delgado, J. E., Fraire-Soto, I., Reyes-Estrada, C. A., et al. (2021). UVB Inhibits Proliferation, Cell Cycle and Induces Apoptosis via P53, E2F1 and Microtubules System in Cervical Cancer Cell Lines. Ijms 22 (10), 5197. doi:10.3390/ijms22105197
Guerrero-Cázares, H., Tzeng, S. Y., Young, N. P., Abutaleb, A. O., Quiñones-Hinojosa, A., and Green, J. J. (2014). Biodegradable Polymeric Nanoparticles Show High Efficacy and Specificity at DNA Delivery to Human Glioblastoma In Vitro and In Vivo. ACS Nano 8 (5), 5141–5153. doi:10.1021/nn501197v
Hu, J., Cao, J., Topatana, W., Juengpanich, S., Li, S., Zhang, B., et al. (2021). Targeting Mutant P53 for Cancer Therapy: Direct and Indirect Strategies. J. Hematol. Oncol. 14 (1), 157. doi:10.1186/s13045-021-01169-0
Kaczmarek, J. C., Kauffman, K. J., Fenton, O. S., Sadtler, K., Patel, A. K., Heartlein, M. W., et al. (2018). Optimization of a Degradable Polymer-Lipid Nanoparticle for Potent Systemic Delivery of mRNA to the Lung Endothelium and Immune Cells. Nano Lett. 18 (10), 6449–6454. doi:10.1021/acs.nanolett.8b02917
Kaczmarek, J. C., Patel, A. K., Rhym, L. H., Palmiero, U. C., Bhat, B., Heartlein, M. W., et al. (2021). Systemic Delivery of mRNA and DNA to the Lung Using Polymer-Lipid Nanoparticles. Biomaterials 275, 120966. doi:10.1016/j.biomaterials.2021.120966
Kim, J., Kang, Y., Tzeng, S. Y., and Green, J. J. (2016). Synthesis and Application of Poly(ethylene Glycol)-Co-Poly(β-Amino Ester) Copolymers for Small Cell Lung Cancer Gene Therapy. Acta Biomater. 41, 293–301. doi:10.1016/j.actbio.2016.05.040
Klco, J. M., and Mullighan, C. G. (2021). Advances in Germline Predisposition to Acute Leukaemias and Myeloid Neoplasms. Nat. Rev. Cancer 21 (2), 122–137. doi:10.1038/s41568-020-00315-z
Kong, N., Tao, W., Ling, X., Wang, J., Xiao, Y., Shi, S., et al. (2019). Synthetic mRNA Nanoparticle-Mediated Restoration of P53 Tumor Suppressor Sensitizes P53 -deficient Cancers to mTOR Inhibition. Sci. Transl. Med. 11 (523), 11. doi:10.1126/scitranslmed.aaw1565
Li, J., Røise, J. J., He, M., Das, R., and Murthy, N. (2021). Non-viral Strategies for Delivering Genome Editing Enzymes. Adv. Drug Deliv. Rev. 168, 99–117. doi:10.1016/j.addr.2020.09.004
Li, W., Sun, J., Zhang, X., Jia, L., Qiao, M., Zhao, X., et al. (2020). Synthesis and Characterization of pH-Responsive PEG-Poly(β-Amino Ester) Block Copolymer Micelles as Drug Carriers to Eliminate Cancer Stem Cells. Pharmaceutics 12 (2), 111. doi:10.3390/pharmaceutics12020111
Liu, Y., Li, Y., Keskin, D., and Shi, L. (2019). Poly(β-Amino Esters): Synthesis, Formulations, and Their Biomedical Applications. Adv. Healthc. Mater. 8 (2), e1801359. doi:10.1002/adhm.201801359
Longworth, M. S., and Laimins, L. A. (2004). Pathogenesis of Human Papillomaviruses in Differentiating Epithelia. Microbiol. Mol. Biol. Rev. 68 (2), 362–372. doi:10.1128/MMBR.68.2.362-372.2004
Loureiro, J. B., Abrantes, M., Oliveira, P. A., and Saraiva, L. (2020). P53 in Skin Cancer: From a Master Player to a Privileged Target for Prevention and Therapy. Biochim. Biophys. Acta Rev. Cancer 1874 (2), 188438. doi:10.1016/j.bbcan.2020.188438
Lu, W., Cheng, F., Yan, W., Li, X., Yao, X., Song, W., et al. (2017). Selective Targeting p53WT Lung Cancer Cells Harboring Homozygous P53 Arg72 by an Inhibitor of CypA. Oncogene 36 (33), 4719–4731. doi:10.1038/onc.2017.41
Ma, K., Mi, C.-L., Cao, X.-X., and Wang, T.-Y. (2021). Progress of Cationic Gene Delivery Reagents for Non-viral Vector. Appl. Microbiol. Biotechnol. 105 (2), 525–538. doi:10.1007/s00253-020-11028-6
Medler, T. R., Murugan, D., Horton, W., Kumar, S., Cotechini, T., Forsyth, A. M., et al. (2018). Complement C5a Fosters Squamous Carcinogenesis and Limits T Cell Response to Chemotherapy. Cancer Cell 34 (4), 561–e6. doi:10.1016/j.ccell.2018.09.003
Mendiratta, G., Ke, E., Aziz, M., Liarakos, D., Tong, M., and Stites, E. C. (2021). Cancer Gene Mutation Frequencies for the U.S. Population. Nat. Commun. 12 (1), 5961. doi:10.1038/s41467-021-26213-y
Merlin, J. P. J., Rupasinghe, H. P. V., Dellaire, G., and Murphy, K. (2021). Role of Dietary Antioxidants in P53-Mediated Cancer Chemoprevention and Tumor Suppression. Oxid Med. Cel Longev 2021, 9924328. doi:10.1155/2021/9924328
Moody, C. A., and Laimins, L. A. (2010). Human Papillomavirus Oncoproteins: Pathways to Transformation. Nat. Rev. Cancer 10 (8), 550–560. doi:10.1038/nrc2886
Niu, G., Jin, Z., Zhang, C., He, D., Gao, X., Zou, C., et al. (2020). An Effective Vaginal Gel to Deliver CRISPR/Cas9 System Encapsulated in Poly (β-Amino Ester) Nanoparticles for Vaginal Gene Therapy. EBioMedicine 58, 102897. doi:10.1016/j.ebiom.2020.102897
Pahle, J., and Walther, W. (2016). Vectors and Strategies for Nonviral Cancer Gene Therapy. Expert Opin. Biol. Ther. 16 (4), 443–461. doi:10.1517/14712598.2016.1134480
Pal, A., and Kundu, R. (2019). Human Papillomavirus E6 and E7: The Cervical Cancer Hallmarks and Targets for Therapy. Front. Microbiol. 10, 3116. doi:10.3389/fmicb.2019.03116
Park, D. J., Wilczynski, S. P., Paquette, R. L., Miller, C. W., and Koeffler, H. P. (1994). p53 Mutations in HPV-Negative Cervical Carcinoma. Oncogene 9 (1), 205–210.
Patiño-Morales, C. C., Soto-Reyes, E., Arechaga-Ocampo, E., Ortiz-Sánchez, E., Antonio-Véjar, V., Pedraza-Chaverri, J., et al. (2020). Curcumin Stabilizes P53 by Interaction with NAD(P)H:quinone Oxidoreductase 1 in Tumor-Derived Cell Lines. Redox Biol. 28, 101320. doi:10.1016/j.redox.2019.101320
Qu, M., Kim, H. J., Zhou, X., Wang, C., Jiang, X., Zhu, J., et al. (2020). Biodegradable Microneedle Patch for Transdermal Gene Delivery. Nanoscale 12 (32), 16724–16729. doi:10.1039/d0nr02759f
Radmanesh, F., Sadeghi Abandansari, H., Ghanian, M. H., Pahlavan, S., Varzideh, F., Yakhkeshi, S., et al. (2021). Hydrogel-mediated Delivery of microRNA-92a Inhibitor Polyplex Nanoparticles Induces Localized Angiogenesis. Angiogenesis 24 (3), 657–676. doi:10.1007/s10456-021-09778-6
Rajasekaran, N., Jung, H. S., Bae, S. H., Chelakkot, C., Hong, S., Choi, J. S., et al. (2017). Effect of HPV E6/E7 siRNA with Chemotherapeutic Agents on the Regulation of TP53/E2F Dynamic Behavior for Cell Fate Decisions. Neoplasia 19 (10), 735–749. doi:10.1016/j.neo.2017.07.005
Routkevitch, D., Sudhakar, D., Conge, M., Varanasi, M., Tzeng, S. Y., Wilson, D. R., et al. (2020). Efficiency of Cytosolic Delivery with Poly(β-Amino Ester) Nanoparticles Is Dependent on the Effective pKa of the Polymer. ACS Biomater. Sci. Eng. 6 (6), 3411–3421. doi:10.1021/acsbiomaterials.0c00271
Rudin, C. M., Brambilla, E., Faivre-Finn, C., and Sage, J. (2021). Small-cell Lung Cancer. Nat. Rev. Dis. Primers 7 (1), 3. doi:10.1038/s41572-020-00235-0
Scheffner, M., Münger, K., Byrne, J. C., and Howley, P. M. (1991). The State of the P53 and Retinoblastoma Genes in Human Cervical Carcinoma Cell Lines. Proc. Natl. Acad. Sci. U S A. 88 (13), 5523–5527. doi:10.1073/pnas.88.13.5523
Sharma, G., Sharma, A. R., Bhattacharya, M., Lee, S.-S., and Chakraborty, C. (2021). CRISPR-Cas9: A Preclinical and Clinical Perspective for the Treatment of Human Diseases. Mol. Ther. 29 (2), 571–586. doi:10.1016/j.ymthe.2020.09.028
Song, X., Liu, C., Wang, N., Huang, H., He, S., Gong, C., et al. (2021). Delivery of CRISPR/Cas Systems for Cancer Gene Therapy and Immunotherapy. Adv. Drug Deliv. Rev. 168, 158–180. doi:10.1016/j.addr.2020.04.010
Strickland, S. W., and Vande Pol, S. (2016). The Human Papillomavirus 16 E7 Oncoprotein Attenuates AKT Signaling to Promote Internal Ribosome Entry Site-dependent Translation and Expression of C-MYC. J. Virol. 90 (12), 5611–5621. doi:10.1128/JVI.00411-16
Taghizadeh, E., Jahangiri, S., Rostami, D., Taheri, F., Renani, P. G., Taghizadeh, H., et al. (2019). Roles of E6 and E7 Human Papillomavirus Proteins in Molecular Pathogenesis of Cervical Cancer. Curr. Protein Pept. Sci. 20 (9), 926–934. doi:10.2174/1389203720666190618101441
Tzeng, S. Y., Higgins, L. J., Pomper, M. G., and Green, J. J. (2013). Student Award winner in the Ph.D. Category for the 2013 Society for Biomaterials Annual Meeting and Exposition, April 10-13, 2013, Boston, Massachusetts : Biomaterial-Mediated Cancer-specific DNA Delivery to Liver Cell Cultures Using Synthetic Poly(beta-Amino Ester)s. J. Biomed. Mater. Res. A. 101 (7), 1837–1845. doi:10.1002/jbm.a.34616
Wang, D. Y., Yang, G., Mei, H. C., Ren, Y., Busscher, H. J., and Shi, L. (2021). Liposomes with Water as a pH‐Responsive Functionality for Targeting of Acidic Tumor and Infection Sites. Angew. Chem. Int. Ed. 60 (32), 17714–17719. doi:10.1002/anie.202106329
Wang, Y., Suh, Y. A., Fuller, M. Y., Jackson, J. G., Xiong, S., Terzian, T., et al. (2011). Restoring Expression of Wild-type P53 Suppresses Tumor Growth but Does Not Cause Tumor Regression in Mice with a P53 Missense Mutation. J. Clin. Invest. 121 (3), 893–904. doi:10.1172/JCI44504
Won, Y. S., and Seo, K. I. (2020). Sanggenol L Induces Apoptosis and Cell Cycle Arrest via Activation of P53 and Suppression of PI3K/Akt/mTOR Signaling in Human Prostate Cancer Cells. Nutrients 12 (2), 12. doi:10.3390/nu12020488
Xiao, B., Laroui, H., Viennois, E., Ayyadurai, S., Charania, M. A., Zhang, Y., et al. (2014). Nanoparticles with Surface Antibody against CD98 and Carrying CD98 Small Interfering RNA Reduce Colitis in Mice. Gastroenterology 146 (5), 1289–1300 e119. doi:10.1053/j.gastro.2014.01.056
Xu, C.-F., Chen, G.-J., Luo, Y.-L., Zhang, Y., Zhao, G., Lu, Z.-D., et al. (2021). Rational Designs of In Vivo CRISPR-Cas Delivery Systems. Adv. Drug Deliv. Rev. 168, 3–29. doi:10.1016/j.addr.2019.11.005
Zafar, A., Wang, W., Liu, G., Xian, W., McKeon, F., Zhou, J., et al. (2021). Targeting the P53-MDM2 Pathway for Neuroblastoma Therapy: Rays of hope. Cancer Lett. 496, 16–29. doi:10.1016/j.canlet.2020.09.023
Zhang, H., Li, J., Yuan, R., Li, Y., Zhang, Y., Hu, X., et al. (2021). Augment the Efficacy of Eradicating Metastatic Lesions and Tumor Proliferation in Breast Cancer by Honokiol-Loaded pH-Sensitive Targeted Lipid Nanoparticles. Colloids Surf. B Biointerfaces 207, 112008. doi:10.1016/j.colsurfb.2021.112008
Zhang, J., Ding, M., Xu, K., Mao, L., and Zheng, J. (2016). shRNA-Armed Conditionally Replicative Adenoviruses: a Promising Approach for Cancer Therapy. Oncotarget 7 (20), 29824–29834. doi:10.18632/oncotarget.8035
Zhang, L., Deng, S., Zhang, Y., Peng, Q., Li, H., Wang, P., et al. (2020). Homotypic Targeting Delivery of siRNA with Artificial Cancer Cells. Adv. Healthc. Mater. 9 (9), e1900772. doi:10.1002/adhm.201900772
Zhao, X., Sun, W., Ren, Y., and Lu, Z. (2021). Therapeutic Potential of P53 Reactivation in Cervical Cancer. Crit. Rev. Oncol. Hematol. 157, 103182. doi:10.1016/j.critrevonc.2020.103182
Zhou, T., Dong, Q., Shen, Y., Wu, W., Wu, H., Luo, X., et al. (2016). PEG-b-PCL Polymeric Nano-Micelle Inhibits Vascular Angiogenesis by Activating P53-dependent Apoptosis in Zebrafish. Int. J. Nanomedicine 11, 6517–6531. doi:10.2147/IJN.S112658
Zhou, Y., Niu, W., Luo, Y., Li, H., Xie, Y., Wang, H., et al. (2019). p53/Lactate Dehydrogenase A axis Negatively Regulates Aerobic Glycolysis and Tumor Progression in Breast Cancer Expressing Wild-type P53. Cancer Sci. 110 (3), 939–949. doi:10.1111/cas.13928
Zhou, Z., Liu, X., Zhu, D., Wang, Y., Zhang, Z., Zhou, X., et al. (2017). Nonviral Cancer Gene Therapy: Delivery cascade and Vector Nanoproperty Integration. Adv. Drug Deliv. Rev. 115, 115–154. doi:10.1016/j.addr.2017.07.021
Keywords: p53, human papillomavirus, cervical cancer, poly (beta-amino ester), nanoparticle
Citation: Xiong J, Li G, Mei X, Ding J, Shen H, Zhu D and Wang H (2022) Co-Delivery of p53 Restored and E7 Targeted Nucleic Acids by Poly (Beta-Amino Ester) Complex Nanoparticles for the Treatment of HPV Related Cervical Lesions. Front. Pharmacol. 13:826771. doi: 10.3389/fphar.2022.826771
Received: 01 December 2021; Accepted: 14 January 2022;
Published: 04 February 2022.
Edited by:
Srujan Marepally, Center for Stem Cell Research (CSCR), IndiaReviewed by:
Alfredo Cruz-Gregorio, National Autonomous University of Mexico, MexicoCopyright © 2022 Xiong, Li, Mei, Ding, Shen, Zhu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hui Shen, Mzg5MzU5NzE3QHFxLmNvbQ==; Da Zhu, MTUzOTM4ODAxQHFxLmNvbQ==; Hui Wang, aHVpdDcxQHNvaHUuY29t
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.