- 1Department of Pharmacy, Zhongnan Hospital of Wuhan University, Wuhan, China
- 2Hospital Management Institute of Wuhan University, Zhongnan Hospital of Wuhan University, Wuhan, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
Background: Healthcare professionals’ knowledge and attitudes towards adverse drug reactions (ADRs) and ADR reporting play a significant role in pharmacovigilance. This study aims to investigate the gap between knowledge and practice in ADR reporting among hospital pharmacists.
Methods: This study is a multi-center, cross-sectional study based on a questionnaire survey. A semi-structured questionnaire was developed including knowledge, attitudes, and practices (KAP) towards ADR reporting. From October to November 2021, questionnaires were filled out on the internet by hospital pharmacists from a central province of China. The data analysis used a one-way ANOVA to analyze the differences between the pharmacist’s characteristics and knowledge and attitude, respectively. The ordinal logistic regression method was used to analyze the predictors of practice.
Results: A total of 1,026 valid questionnaires from 512 medical institutions were collected. It was found that 88.8% of participants have a clear understanding of the ADR definition, while 59.6% of them have misunderstandings about the reporting time of new and serious adverse reactions. Most pharmacists showed positive attitudes towards ADR reporting. Higher education background, higher professional title, attending training, and clinical pharmacist resulted in higher knowledge scores. Higher education background, shorter working years, attending training, and from non-tertiary hospital related to higher attitude scores. In terms of practice, age, hospital type, working years, training, and pharmacist type all have significant associations with practice scores. Pharmacists’ knowledge score and attitude score were significant predictors of practice score with OR being 1.19 (95% CI: 1.06, 1.33) and 1.04 (95% CI: 1.005, 1.07).
Conclusion: Although most hospital pharmacists showed positive attitudes towards ADR reporting, their knowledge and practice were still insufficient. Hospital pharmacists’ knowledge and attitude are associated with their practice towards ADR reporting. The training had a significant impact on the pharmacist’s knowledge, attitude, and practice.
Introduction
The World Health Organization (WHO) defines adverse drug reactions (ADRs) as harmful and unrelated to the purpose of medication when normal doses of drugs are used to prevent, diagnose, treat diseases or regulate physiological functions (Edwards and Aronson, 2000). ADR has a significant impact on the health of patients, and is a major problem that has led to an increase in global morbidity and mortality. It is estimated that about 5% of hospitalized patients are caused by ADR, and another 5% of hospitalized patients will experience ADR during hospitalization. In the European Union, ADR causes 197,000 deaths every year (Bouvy et al., 2015). In the United States, the total cost of hospitalization after adverse drug events in the intensive care unit (ICU) and non-ICU ward is estimated to be 19,685 dollars and 13,994 dollars, respectively (Cullen et al., 1997). Therefore, monitoring adverse drug reactions are critical for global health care.
In all countries, national pharmacovigilance systems rely primarily on spontaneous reporting, in which suspected adverse drug reactions are reported to a national coordinating center by health professionals, pharmaceut post-marketing ical producers, or individuals (Pal et al., 2013). Spontaneous reports of ADRs have some advantages for identifying potential safety signals, but they have apparent drawbacks, such as substantial underreporting, poor report quality, difficulty quantifying risk, and an unknown number of people who have been exposed (Alharf et al., 2018; Hazell and Shakir, 2006).
China has a nationwide ADR reporting and monitoring system, composed of four levels, which includes the National Center for ADR monitoring, 34 provincial ADR monitoring centers, and hundreds of municipal and county-level institutions (Li et al., 2018; Zhao et al., 2018). In China, the number of spontaneous ADR reports was 1.7 million in 2020, which equates to 1215 reporting per million people. With a population of 1.4 billion people, China is attempting to expand the number of spontaneous ADR reports (Song et al., 2022).
The majority of ADR reports in China come from healthcare professionals (85.4%) (NMPA, 2020). According to a survey in three provinces in China, pharmacists reported the largest proportion of ADRs (43.51%) among all sources during 2015–2017, however, the quality of ADR reporting by pharmacists was not promising, with only 11.5% of reports being of high quality (Chen et al., 2019). Another investigation showed that hospital pharmacists in a northern province of China have good knowledge and attitudes but poor practice towards ADR reporting (Su et al., 2010). To better understand the challenges pharmacists face in reporting ADRs and to offer suggestions for improving the rate and quality of ADR reporting, we conducted this survey on pharmacists’ knowledge, perceptions and practice of ADR reporting.
Study Design and Methods
Participants and Setting
This is a multi center, cross-sectional study based on a questionnaire survey. The questionnaire was distributed to hospital pharmacists in QQ groups and WeChat groups in Hubei Province through the questionnaire collection software (Questionnaire Star), and a statement on the research project and consent form was distributed at the same time. From October to November 2020, a total of 1,128 people participated in the survey. The data was excluded based on the following criteria: I. Pharmacists who are not hospital pharmacists; II. Invalid. The questionnaire takes less than 1 min or more than 1 h to complete. In the end, a total of 1026 valid questionnaires were obtained, with an 89.7% effective rate. Participants were from 522 hospitals in Hubei Province. Participants’ responses are completely anonymous and voluntary.
Questionnaire Design
The self-administered questionnaire was composed of 25 mandatory single-choice items and one multiple-choice item, and it was developed based on scientific literature (Chatterjee et al., 2006; Al Dweik et al., 2017; Seid et al., 2018) and the practice experience of the authors. Two experts in pharmacovigilance reviewed the questionnaire draft and assessed its content validity. The questionnaire consists of five main parts: (i) Pharmacist characteristics: education, profession rank, and length of work experience, etc.; (ii) Knowledge part: definition of ADR, ADR reporting time, etc., we set multiple-choice questions, each question has a correct answer, and the correct answer receives 1 point, while the incorrect answer receives 0 point; (iii) Attitudes part: concerning and willingness about ADR reporting, this part was provided on a 5-level Likert scale (1 = “strongly disagree,” 2 = “disagree,” 3 = “neutral,” 4 = “agree,” and 5 = “strongly agree”) to indicate that they disagreed or agreed.; (iv) Practice part: covers two items based on the surveyors’ ADR reporting practice experience, we set up a yes or no question option, “yes” gets 2 points, “no” gets 1 point; (v) Part five: investigated the influencing factors for ADR reporting and was provided in selective form. Detailed explanations and correct answers were in Supplementary Material S1.
Data Processing
For describing demographic variables, descriptive statistics are used, using percentages or frequencies to demonstrate categorical variables. One-way ANOVA was used to explore the relations between pharmacists’ characteristics and knowledge and attitude scores, respectively, and ordinal logistic regression was used to analyze the correlation between knowledge, attitude and practice. The characteristic factors with p < 0.05 in the single factor analysis results were taken as covariates in the ordinal logistic regression. SPSS 22.0 was used for statistical analysis. p < 0.05 was considered statistically significant.
Results
Characteristics of Pharmacist
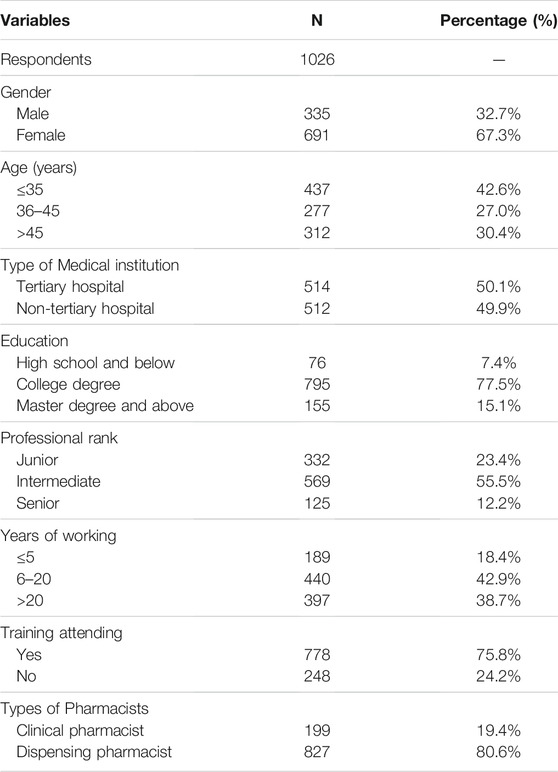
A total of 1,128 questionnaires were collected, and 1,026 of them were valid and included in the analysis. The effective rate was 89.1%. There were 335 (32.7%) males and 691 females (67.3%); 437 pharmacists (42.6%) were under the age of 35; 512 pharmacists (49.9%) were from non-tertiary medical institution; 950 pharmacists (92.6%) have undergraduate degrees; and 569 pharmacists (55.5%) have intermediate professional titles. 837 pharmacists (81.6%) had more than 6 years of work experience; 778 pharmacists (75.8%) had participated in ADR training before; and there were 199 clinical pharmacists (19.4%). The characteristics of pharmacists are showed in Table 1.
Pharmacist’s Knowledge of ADR
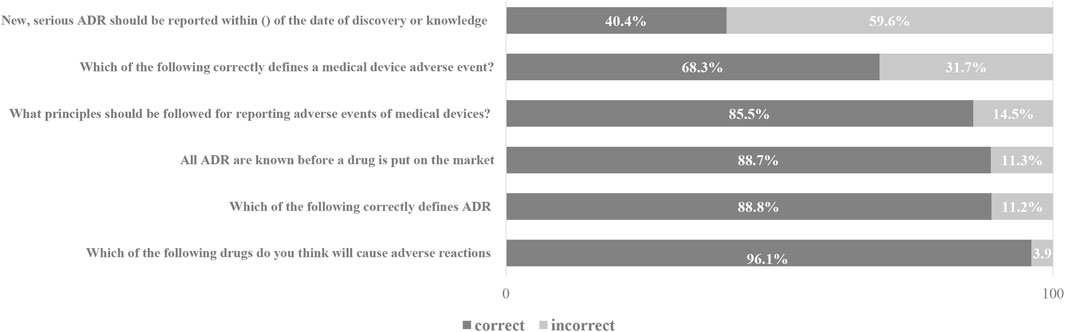
The results of pharmacists’ knowledge of ADR reporting showed that 88.8% of people had a clear understanding of the definition of adverse drug reactions, while 59.6% had misunderstandings about the reporting time of new and serious adverse reactions. At the same time, 31.7% of pharmacists have cognitive errors in the definition of medical device adverse events, and 14.5% have cognitive errors in the reporting principles of medical device adverse events. The pharmacist’s knowledge scores on ADR reporting are shown in Figure 1.
Pharmacist’s Attitude Towards ADR
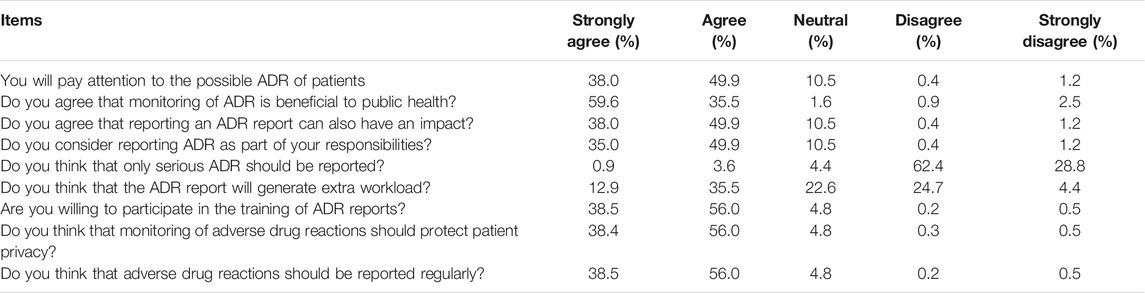
The results of the pharmacist’s attitude towards ADR reporting indicate that the majority of pharmacists have a positive attitude towards ADR reporting. 95.1% agreed that adverse drug reaction monitoring is beneficial to public health, 84.9% believed that adverse drug reaction reporting was part of their responsibilities, 91.2% disagreed that only serious adverse drug reactions were reported, and 94.5% were willing to participate in adverse drug reaction reporting training. Regarding the issue of whether the adverse drug reaction report will generate additional workload, 48.4% of people believe that ADR reporting will generate additional workload. The results are shown in Table 2.
Pharmacist’s Practice of ADR Reporting
According to the results of ADR reporting, 70.9% of pharmacists had encountered adverse drug reactions, of which 67.3% had reported adverse drug reactions.
Differences Between Pharmacists’ Characteristics and KAP Towards ADR
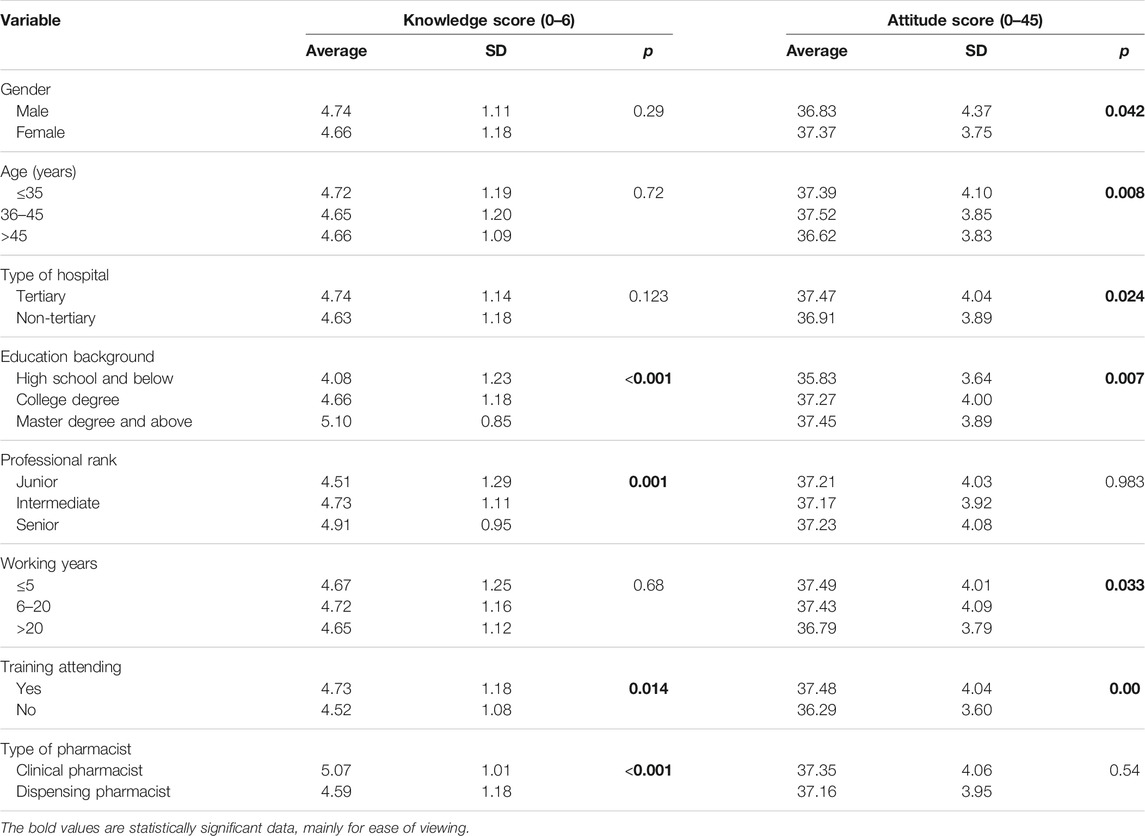
The study found that there was a significant difference between pharmacists’ characteristics and ADR knowledge scores. Among them, pharmacists’ education, professional title, whether they participate in training, and job types have significant differences in ADR cognitive scores (p < 0.05). Pharmacists with a high school degree or below, junior professional titles, pharmacists who have not participated in training, and dispensing pharmacists have relatively low knowledge scores. The knowledge scores of pharmacists in non-tertiary hospitals were lower than those in tertiary hospitals (p < 0.05). There is a significant difference between pharmacist characteristics and ADR attitude scores. Among them, male, younger than 35 years old, non-tertiary hospital, low educational background, less than 5 years of work experience, and pharmacists with no training have relatively lower scores (p < 0.05). The differences in knowledge and attitude of pharmacists are shown in Table 3.
The Predictors of Pharmacists’ Practice Toward ADR Reporting
The knowledge and attitude scores were used as predictors of pharmacists’ practice toward ADR reporting to explore the relationship within KAP (Table 4). A significant association was observed for both knowledge [OR (95% CI): 1.19 (1.06, 1.33), p = 0.002] and attitude score [OR (95% CI): 1.04 (1.005, 1.07), p = 0.023] with the practice score of pharmacists. The covariates of the model are gender, age, hospital grade, educational background, title, working years, whether to participate in training, and job type. Pharmacist characteristics were also predictors of practice scores. The results of the multivariate model revealed that the risks of having a higher practice score were 0.60 (95% CI: 0.39, 0.94) times higher among pharmacists belonging to the age group 36–45 years, when compared with pharmacists older than 45 years. Pharmacists from non-tertiary hospitals had 1.64 (95% CI: 1.24, 2.17) times greater risk of having higher scores in practice compared with pharmacists from tertiary hospitals. The risks of having a higher practice score were 2.98 (95% CI: 1.59, 5.59) times higher among pharmacists belonging to the working year group ≤5 compared with ≥20 years. Pharmacists with training experience had 1.75 (95% CI: 1.30, 2.35) times higher practice scores compared with pharmacists with no-training experience. Dispensing pharmacists have 0.22 (95% CI: 0.14, 0.35) times higher practice scores than clinical pharmacists.
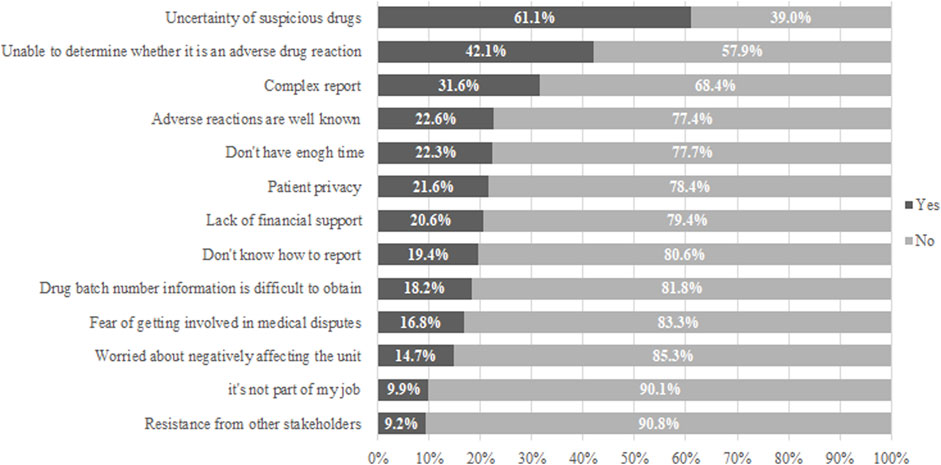
Factors Affecting Pharmacists’ Reporting of ADRs
Our study also investigated the factors affecting pharmacists’ ADR reporting (Figure 2). The investigation’s findings indicated that the first three main factors affecting pharmacists’ ADR reporting were the uncertainty about the suspected drug, the inability to determine whether it was an adverse drug reaction, and the report’s complexity. At the same time, 24.7% of people don’t know how to report.
Discussion
The Importance of Hospital Pharmacists in the ADR Reporting Process
Drugs are the most common treatment for diseases, so it is necessary to pay attention to the rational use of drugs. If the safety of the drug is not considered properly, it may lead to consequences ranging from lifelong disability to death. Similarly, if a drug-related ADR is reported, the safety of the drug can be improved (Babar and Jamshed, 2008). This study aims to evaluate and compare the differences in knowledge, attitude, and practice of ADR reporting among pharmacists with different characteristics. Since many serious ADRs occur in hospitals or lead to hospitalization, pharmacists in medical institutions play an important role in ADR reporting (Lazarou et al., 1998). At the same time, most new drugs will first be used in hospitals. Therefore, the research on the knowledge, attitude, and practice of hospital pharmacists in the ADR reporting process is particularly important.
Hospital Pharmacists’ Knowledge, Attitude and Practice Characteristics
In this study, we found that 11.2% of pharmacists are still unclear about the basic definition of ADR, which is similar to the results of previous research reports (Su et al., 2010). 59.6% of pharmacists have misunderstandings about the reporting time of new and serious adverse reactions. It reflects that pharmacist still lacks basic knowledge about ADR reports. Among them, pharmacists with low academic qualifications, low professional titles, and untrained pharmacists have relatively little basic knowledge related to ADR reporting. It reflects that pharmacist still lacks knowledge about ADR reporting. Among them, educational backgrounds, professional titles, and whether they have participated in the training are related to the basic knowledge related to ADR reporting. Highly educated and trained pharmacists are more familiar with the basic knowledge related to ADR reporting. In addition, the survey showed that 40% of the pharmacists had poor knowledge about medical device adverse events. This indicates that pharmacists need to be properly trained for ADR reporting so that the quality of ADR reporting can be improved.
Pharmacists’ attitude is considered the key to reporting ADR, so a positive attitude may encourage timely reporting of ADR. In the current study, pharmacists have a very positive attitude towards reporting ADR. Most pharmacists agree that ADR reporting is part of their responsibilities, which is consistent with the results of other similar studies (Hallit et al., 2019; Kopciuch et al., 2019; Al-Mutairi et al., 2021). Although most pharmacists have no significant differences in their attitudes towards ADR reporting, male pharmacists or under 35 years of age have slightly lower positive attitudes towards ADR reporting. It may be related to the new employee’s relatively short length of service. Since pharmacists who have worked for a long time have been exposed to more adverse events at work, they have a better understanding of the harm that adverse events can cause to patients. And get more training and assessment related to ADR reporting in the workplace. Therefore, they pay more attention to the harmfulness of ADRs and their attitude towards ADR reporting are more positive. The positive attitude among pharmacists who have participated in ADR training is higher. Most of the participants expressed interest in ADR report training, indicating that they believe it is important to learn more about ADR reporting. In fact, it is also possible that the participants are unwilling to present their problems due to the deviation of social expectations. Because of the importance of pharmacists’ knowledge and positive attitude towards ADR reporting, ADR administrative centers at all levels needs to strengthen training and education in the field of ADR.
In the current study, there is a significant difference between pharmacist characteristics and ADR practice scores. The difference of education level mainly affects the score of pharmacists’ basic knowledge of adverse reactions. Among them, only pharmacists with education below senior high school have a downward trend in the score of reporting attitude towards adverse drug reactions. It indicates that the educational level of some pharmacists needs to be improved. Lower practice scores are found amount pharmacists who are over 45 years old, have less than 5 years of experience, or have not participated in ADR training. Explain that work experience and ADR training have a greater impact on the practice of ADR reporting. Interestingly, pharmacists’ practice scores in tertiary hospitals are low, which may be related to the work nature of pharmacists in tertiary hospitals. Most pharmacists in tertiary hospitals are not only engaged in pharmacotherapeutic work, but also conduct research and teach, which diverts their attention away from observing ADRs in patients. Clinical pharmacists in Chinese hospitals are typically the ones who handle ADR reporting and are better prepared than dispensing pharmacists to detect and report ADRs (Chen et al., 2015). That could explain why dispensing pharmacists have a lower ADR reporting practice score than clinical pharmacists.
The Relationship Between Pharmacist Characteristics and KAP
This study shows that both knowledge and attitude have a positive effect on the practice of pharmacists, and future improvement strategies can be carried out from the aspect of improving pharmacists’ knowledge and attitude towards ADR monitoring. As a strategy to improve the ADR reporting, it should be aimed at healthcare professionals’ level and the pharmacist level. In addition to encouraging pharmacists to report ADR, continuous professional development plans should be used to make up for their lack of knowledge and skills in discovering and reporting ADR. In this study, the main factors affecting pharmacists’ reports of adverse reactions are the uncertainty of suspected drugs, the inability to judge whether they belong to adverse drug reactions and the complexity of the report. There is evidence that providing continuing education to health professionals can help change their behavior and attitudes towards ADR reports (Gonzalez-Gonzalez et al., 2013; Pagotto et al., 2013). The purpose of such education should not only be limited to improving pharmacists’ knowledge of ADR but also be aimed at changing their attitudes and views on ADR report. The results of this study also show that pharmacists who have participated in ADR training have higher knowledge, attitude, and practice scores. As experts, pharmacists play an important role in ensuring drug safety by detecting and reporting ADRs (Hadi et al., 2017). In the past few decades, the role of pharmacists has changed worldwide, from dispensers to guardians of drug safety (Su et al., 2010; Oreagba et al., 2011; Hadi et al., 2017). Research evidence shows that hospital pharmacists can not only detect and report ADRs but also help prevent ADR-related occurrences. In addition, pharmacists who have a clinical background and work closely with prescribers and patients can better understand suspicious ADRs (Calvert, 1999; Schlienger et al., 1999). Therefore, training and education about ADR is particularly important.
Research Limitations
The current research still has certain limitations. This study as a whole only covers one province in the central region, and the conclusions are extrapolated to other regions and need further research to confirm. At the same time, the study relied on pharmacists’ self-assessment of their ADR knowledge, attitudes, and practice, which may be considered a social expectations deviation, because some participants may be unwilling to reveal practice flaws. Although we used anonymity to reduce this social expectation bias during the investigation, there may be some social expectation bias because the participants may be influenced by their hospital administrators. Therefore, the true knowledge, attitude and practice of pharmacists cannot be comprehensively summarized. Despite these limitations, we believe that our research results are reliable and may help healthcare professionals improve ADR reporting in the future.
Conclusion
Although most hospital pharmacists showed positive attitudes towards ADR reporting, their knowledge and practice were still insufficient. Hospital pharmacists’ knowledge and practice are associated with their practice towards ADR reporting. The training had a significant impact on the pharmacist’s knowledge, attitude and practice.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of Zhongnan Hospital of Wuhan University. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Concept and design: HC, LL, FS, and WH. Acquisition, analysis, or interpretation of data: HC, WH, YT, YL, SG, XW, WL, and QJ. Drafting of the manuscript: WH, YT, and HC. Critical revision of the manuscript: All authors. Statistical analysis: YT.
Funding
This research was supported by the Clinical Toxicology Grants from Chinese Society of Toxicology (Grant No. CST2020CT302).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are grateful to Dr. Jing Nie and Dr. Li Zhao who assisted in organizing the questionnaire survey. The authors would also like to thank all pharmacists who participated in the study.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.823944/full#supplementary-material
References
Al Dweik, R., Stacey, D., Kohen, D., and Yaya, S. (2017). Factors Affecting Patient Reporting of Adverse Drug Reactions: a Systematic Review. Br. J. Clin. Pharmacol. 83, 875–883. doi:10.1111/bcp.13159
Al-Mutairi, A., Alfayyad, I., Altannir, Y., and Al-Tannir, M. (2021). Medication Safety Knowledge, Attitude, and Practice Among Hospital Pharmacists in Tertiary Care Hospitals in Saudi Arabia: a Multi-center Study. Arch. Public Health 79, 130. doi:10.1186/s13690-021-00616-1
Alharf, A., Alqahtani, N., Saeed, G., Alshahrani, A., Alshahrani, M., Aljasser, N., et al. (2018). Saudi Vigilance Program: Challenges and Lessons Learned. Saudi Pharm. J. 26, 388–395. doi:10.1016/j.jsps.2018.01.002
Babar, Z. U., and Jamshed, S. (2008). Social Pharmacy Strengthening Clinical Pharmacy: Why Pharmaceutical Policy Research Is Needed in Pakistan? Pharm. World Sci. 30, 617–619. doi:10.1007/s11096-008-9246-z
Bouvy, J. C., De Bruin, M. L., and Koopmanschap, M. A. (2015). Epidemiology of Adverse Drug Reactions in Europe: a Review of Recent Observational Studies. Drug Saf. 38, 437–453. doi:10.1007/s40264-015-0281-0
Calvert, R. T. (1999). Clinical Pharmacy-Aa Hospital Perspective. Br. J. Clin. Pharmacol. 47, 231–238. doi:10.1046/j.1365-2125.1999.00845.x
Chatterjee, S., Lyle, N., and Ghosh, S. (2006). A Survey of the Knowledge, Attitude and Practice of Adverse Drug Reaction Reporting by Clinicians in Eastern India. Drug Saf. 29, 641–642. doi:10.2165/00002018-200629070-00009
Chen, B., Huang, J. J., Chen, H. F., and Xu, B. M. (2015). Clinical Pharmacy Service Practice in a Chinese Tertiary Hospital. Drug Metab. Pers Ther. 30, 215–230. doi:10.1515/dmpt-2015-0009
Chen, Y., Niu, R., Xiang, Y., Wang, N., Bai, J., and Feng, B. (2019). The Quality of Spontaneous Adverse Drug Reaction Reports in China: A Descriptive Study. Biol. Pharm. Bull. 42, 2083–2088. doi:10.1248/bpb.b19-00637
Cullen, D. J., Sweitzer, B. J., Bates, D. W., Burdick, E., Edmondson, A., and Leape, L. L. (1997). Preventable Adverse Drug Events in Hospitalized Patients: a Comparative Study of Intensive Care and General Care Units. Crit. Care Med. 25, 1289–1297. doi:10.1097/00003246-199708000-00014
Edwards, I. R., and Aronson, J. K. (2000). Adverse Drug Reactions: Definitions, Diagnosis, and Management. Lancet 356, 1255–1259. doi:10.1016/S0140-6736(00)02799-9
Gonzalez-Gonzalez, C., Lopez-Gonzalez, E., Herdeiro, M. T., and Figueiras, A. (2013). Strategies to Improve Adverse Drug Reaction Reporting: a Critical and Systematic Review. Drug Saf. 36, 317–328. doi:10.1007/s40264-013-0058-2
Hadi, M. A., Neoh, C. F., Zin, R. M., Elrggal, M. E., and Cheema, E. (2017). Pharmacovigilance: Pharmacists' Perspective on Spontaneous Adverse Drug Reaction Reporting. Integr. Pharm. Res. Pract. 6, 91–98. doi:10.2147/IPRP.S105881
Hallit, S., Hajj, A., Shuhaiber, P., Iskandar, K., Ramia, E., Sacre, H., et al. (2019). Medication Safety Knowledge, Attitude, and Practice Among Hospital Pharmacists in Lebanon. J. Eval. Clin. Pract. 25, 323–339. doi:10.1111/jep.13082
Hazell, L., and Shakir, S. A. (2006). Under-reporting of Adverse Drug Reactions : a Systematic Review. Drug Saf. 29, 385–396. doi:10.2165/00002018-200629050-00003
Kopciuch, D., Zaprutko, T., Paczkowska, A., Ratajczak, P., Zielińska-Tomczak, Ł., Kus, K., et al. (2019). Safety of Medicines-Pharmacists' Knowledge, Practice, and Attitudes toward Pharmacovigilance and Adverse Drug Reactions Reporting Process. Pharmacoepidemiol. Drug Saf. 28, 1543–1551. doi:10.1002/pds.4792
Lazarou, J., Pomeranz, B. H., and Corey, P. N. (1998). Incidence of Adverse Drug Reactions in Hospitalized Patients: a Meta-Analysis of Prospective Studies. JAMA 279, 1200–1205. doi:10.1001/jama.279.15.1200
Li, X., Li, H., Deng, J., Zhu, F., Liu, Y., Chen, W., et al. (2018). Active Pharmacovigilance in China: Recent Development and Future Perspectives. Eur. J. Clin. Pharmacol. 74, 863–871. doi:10.1007/s00228-018-2455-z
NMPA (2020). Annual Report for National Adverse Drug Reaction Monitoring. Available at https://www.nmpa.gov.cn/xxgk/fgwj/gzwj/gzwjyp/20210325170127199.html (Accessed Feb 27, 2022).
Oreagba, I. A., Ogunleye, O. J., and Olayemi, S. O. (2011). The Knowledge, Perceptions and Practice of Pharmacovigilance Amongst Community Pharmacists in Lagos State, South West Nigeria. Pharmacoepidemiol. Drug Saf. 20, 30–35. doi:10.1002/pds.2021
Pagotto, C., Varallo, F., and Mastroianni, P. (2013). Impact of Educational Interventions on Adverse Drug Events Reporting. Int. J. Technol. Assess. Health Care 29, 410–417. doi:10.1017/S0266462313000457
Pal, S. N., Duncombe, C., Falzon, D., and Olsson, S. (2013). WHO Strategy for Collecting Safety Data in Public Health Programmes: Complementing Spontaneous Reporting Systems. Drug Saf. 36, 75–81. doi:10.1007/s40264-012-0014-6
Schlienger, R. G., Lüscher, T. F., Schoenenberger, R. A., and Haefeli, W. E. (1999). Academic Detailing Improves Identification and Reporting of Adverse Drug Events. Pharm. World Sci. 21, 110–115. doi:10.1023/a:1008631926100
Seid, M. A., Kasahun, A. E., Mante, B. M., and Gebremariam, S. N. (2018). Healthcare Professionals' Knowledge, Attitude and Practice towards Adverse Drug Reaction (ADR) Reporting at the Health center Level in Ethiopia. Int. J. Clin. Pharm. 40, 895–902. doi:10.1007/s11096-018-0682-0
Song, H., Pei, X., Liu, Z., Shen, C., Sun, J., Liu, Y., et al. (2022). Pharmacovigilance in China: Evolution and Future Challenges. Br. J Clin. Pharma. doi:10.1111/bcp.15277
Su, C., Ji, H., and Su, Y. (2010). Hospital Pharmacists' Knowledge and Opinions Regarding Adverse Drug Reaction Reporting in Northern China. Pharmacoepidemiol. Drug Saf. 19, 217–222. doi:10.1002/pds.1792
Keywords: adverse drug reaction reporting, hospital pharmacists, knowledge, attitude, practice, China
Citation: Hu W, Tao Y, Lu Y, Gao S, Wang X, Li W, Jiang Q, Lin L, Sun F and Cheng H (2022) Knowledge, Attitude and Practice of Hospital Pharmacists in Central China Towards Adverse Drug Reaction Reporting: A Multicenter Cross‐Sectional Study. Front. Pharmacol. 13:823944. doi: 10.3389/fphar.2022.823944
Received: 28 November 2021; Accepted: 07 March 2022;
Published: 22 March 2022.
Edited by:
Joseph O. Fadare, Ekiti State University, NigeriaReviewed by:
Nejc Horvat, University of Ljubljana, SloveniaFathi M. Sherif, University of Tripoli, Libya
Copyright © 2022 Hu, Tao, Lu, Gao, Wang, Li, Jiang, Lin, Sun and Cheng. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Cheng, Y2hlbmdob25nQHpuaG9zcGl0YWwuY24=; Feng Sun, c3VuZmVuZ0Biam11LmVkdS5jbg==; Likai Lin, bGlubGlrYWlfMTk2M0AxNjMuY29t
†These authors have contributed equally to this work
 Wen Hu
Wen Hu Yun Tao2†
Yun Tao2† Yun Lu
Yun Lu Suyu Gao
Suyu Gao Feng Sun
Feng Sun Hong Cheng
Hong Cheng