- 1Division of Epidemiology and Public Health, School of Medicine, University of Nottingham, Nottingham, United Kingdom
- 2The Nottingham Centre for Evidence-Based Healthcare: A JBI Centre of Excellence, Nottingham, United Kingdom
- 3Department of Family and Community Medicine, College of Medicine, King Faisal University, Alahsa, Saudi Arabia
- 4Sree Chitra Tirunal Institute for Medical Sciences and Technology, Thiruvananthapuram, India
- 5Centre for Pharmacognosy and Phytotherapy, School of Pharmacy, University College London, London, United Kingdom
- 6Institute of Applied Health Research, University of Birmingham, Birmingham, United Kingdom
- 7Department of Endocrinology, Metabolism and Diabetes, All India Institute of Medical Sciences, New Delhi, India
- 8Department of Kayachikitsa, J B Roy State Ayurvedic Medical College and Hospital, Kolkata, India
- 9Department of Non-communicable Disease Epidemiology, London School of Hygiene and Tropical Medicine, London, United Kingdom
Introduction: Many Ayurvedic medicines have the potential for managing type 2 diabetes mellitus (T2DM), with previous systematic reviews demonstrating effectiveness and safety for specific Ayurvedic medicines. However, many of the reviews need updating and none provide a comprehensive summary of all the Ayurvedic medicines evaluated for managing T2DM.
Objective: The objective of this systematic review was to evaluate and synthesize evidence on the effectiveness and safety of Ayurvedic medicines for managing T2DM.
Inclusion criteria: Published and unpublished RCTs assessing the effectiveness and safety of Ayurvedic medicines for managing T2DM in adults.
Methods: The JBI systematic review methodology was followed. A comprehensive search of sources (including 18 electronic databases) from inception to 16 January 2021 was made. No language restrictions were applied. Data synthesis was conducted using narrative synthesis and random effects meta-analyses, where appropriate. Pooled results are reported as mean differences (MD) with 95% confidence intervals (CI).
Results: Out of 32,519 records identified from the searches, 219 articles were included in the systematic review representing 199 RCTs (21,191 participants) of 98 Ayurvedic medicines. Overall, in the studies reviewed the methodology was not adequately reported, resulting in poorer methodological quality scoring. Glycated hemoglobin (HbA1c) was reduced using Aegle marmelos (L.) Corrêa (MD -1.6%; 95% CI −3 to −0.3), Boswellia serrata Roxb. (−0.5; −0.7 to −0.4), Gynostemma pentaphyllum (Thunb.) Makino (−1; −1.5 to −0.6), Momordica charantia L. (−0.3; −0.4 to −0.1), Nigella sativa L. (−0.4; −0.6 to −0.1), Plantago ovata Forssk. (−0.9; −1.4 to −0.3), Tinospora cordifolia (Willd.) Hook.f. and Thomson (−0.5; −0.6 to −0.5), Trigonella foenum-graecum L. (−0.6; −0.9 to −0.4), and Urtica dioica L. (−1.3; −2.4 to −0.2) compared to control. Similarly, fasting blood glucose (FBG) was reduced by 4–56 mg/dl for a range of Ayurvedic medicines. Very few studies assessed health-related quality of life (HRQoL). Adverse events were not reported in many studies, and if reported, these were mostly none to mild and predominately related to the gastrointestinal tract.
Conclusion: The current evidence suggests the benefit of a range of Ayurvedic medicines in improving glycemic control in T2DM patients. Given the limitations of the available evidence and to strengthen the evidence base, high-quality RCTs should be conducted and reported.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a complex disorder that has major health, social, and economic consequences. (European Medicines Agency, 2018; International Diabetes Federation, 2019) Chronic hyperglycemia is associated with macro- and micro-vascular complications and even death. (European Medicines Agency, 2018; International Diabetes Federation, 2019) Ayurveda is a dominant traditional medical system that has been used for thousands of years in many South Asian countries. (Sharma et al., 2007) In Ayurveda, the corresponding term for diabetes mellitus is madhumeha (madhu means ‘‘sweetness’’ and meha means ‘‘excessive urination’’). (Ministry of Ayush, 2016; Central Council for Research in Ayurvedic Sciences, 2017) Classical Ayurvedic texts, written in Sanskrit, have described this condition and its management in detail. (Ministry of Ayush, 2016; Central Council for Research in Ayurvedic Sciences, 2017) Briefly, a multi-pronged and individualized approach is used to manage the condition such as through lifestyle modification (including diet), Ayurvedic detoxifying and purifying therapies (e.g., Panchakarma), and Ayurvedic medicines (containing plant-, animal- or mineral-origin ingredients–single or in combination). It is hypothesized that many of these medicines work through pancreatic as well as extrapancreatic effects. (Ministry of Ayush, 2016; Central Council for Research in Ayurvedic Sciences, 2017) T2DM is one of the main diseases for which patients consult Ayurvedic practitioners and use Ayurvedic medicines, often continuously from the point of diagnosis. (Mehrotra et al., 2004; Kumar et al., 2006; Priya and Shweta, 2010; Chandra, 2011; Bhalerao et al., 2013; Chandra, 2013) Ayurveda is commonly used by patients as it fits with their health beliefs and culture; thus, its acceptability, satisfaction, and perceived relief are usually high, especially among rural, poor, older, and indigenous/minority populations. (Chacko, 2003; Bhalerao et al., 2013) Many T2DM patients prefer not to use Western medicines due to the associated side effects, cost, and mode of administration (e.g., injections). (Chandra, 2011; Bhalerao et al., 2013; Chandra, 2013)
Previous systematic reviews of clinical trials suggest beneficial effects of several Ayurvedic medicines on T2DM-related outcomes, including improvement in blood glucose, with no major safety issues. (Hardy et al., 2001; Shekelle et al., 2005; Sridharan et al., 2011) However, they are now outdated, and one was limited in scope in terms of the Ayurvedic medicines considered. The evidence base over the past 10 years has grown substantially, thereby highlighting the need to refocus and update the reviews to provide contemporary estimates of effect and safety for all the Ayurvedic medicines for the management of T2DM. Additionally, the review findings will be used to guide the development of a clinical guideline for managing T2DM using Ayurvedic medicines.
2 Review Questions
i) Are Ayurvedic medicines effective in controlling blood glucose levels in T2DM patients?
ii) Are Ayurvedic medicines effective in improving health-related quality of life (HRQoL) for T2DM patients?
iii) Are Ayurvedic medicines safe for use by T2DM patients?
3 Materials and Methods
The systematic review process adhered to the JBI Systematic Reviews of Effectiveness guidance and was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. (Moher et al., 2009; Tufanaru et al., 2017) This review was conducted according to a priori published protocol, (Chattopadhyay et al., 2020a) and registered with PROSPERO (CRD42018118285).
3.1 Inclusion Criteria
3.1.1 Participants
The systematic review included studies conducted among adults (≥18 years) with T2DM, irrespective of associated comorbidities (e.g., obesity, hypertension, dyslipidemia) or T2DM complications (such as macro- or micro-vascular). Both newly diagnosed T2DM/treatment naïve, as well as existing cases/on treatment, were eligible. Studies with mixed populations, e.g., adults and children, were included if the mean age of the participants was ≥18 years or the study findings were reported separately for adults. Studies that included participants with type 1 diabetes were excluded unless it was possible to extract the data on T2DM participants.
3.1.2 Interventions
Studies were included if they assessed any classical or proprietary Ayurvedic medicine (such as containing plant- or mineral-origin ingredients–single or in combination) in any form (e.g., tablets, capsules, powder, decoction) and administered for at least 8 weeks. Cross-checking of the eligibility of Ayurvedic medicine (to distinguish it from traditional Chinese and Western medicines) was performed by Ayurveda experts in the team via searching the Indian Medicinal Plants Database (http://medicinalplants.in), Encyclopedia on Indian Medicinal Plants (http://envis.frlht.org/implad), Traditional Knowledge Digital Library (http://www.tkdl.res.in), Ayurvedic Pharmacopoeia of India, and Ayurvedic Formulary of India. Studies on multi-modal interventions that included Ayurvedic medicine were included if it was possible to extract data relating to Ayurvedic medicine. To distinguish between Ayurvedic medicines and dietary ingredients, studies were excluded where medicines were used as dietary ingredients or foods. In addition, Ayurvedic detoxifying and purifying therapies (e.g., Panchakarma) were beyond the scope of this review.
3.1.3 Comparators
Studies comparing Ayurvedic medicines with no intervention, placebo (as defined by the study authors), non-pharmaceutical intervention (e.g., yoga), or pharmaceutical intervention (i.e., Western oral antidiabetic drug [OAD] or head-to-head comparison with another Ayurvedic medicine) were eligible for inclusion in the review. Co-intervention was allowed if all the eligible study arms received the same co-intervention. If a study had multiple treatment arms (multi-arms), the authors only included the arms that met the review inclusion criteria. Studies comparing two or more drug manufacturing processes, forms or timings of administration, doses, or Anupans (Ayurvedic medicines are usually taken with an Anupan, a carrier substance such as a liquid drink) of the same Ayurvedic medicine without any other comparator were excluded.
3.1.4 Outcomes
The following outcomes were included:
• Primary outcomes: blood glucose (i.e., glycated hemoglobin [HbA1c], fasting blood glucose [FBG]), HRQoL, and adverse events.
• Secondary outcomes: postprandial blood glucose (PPBG), fasting and stimulated insulin, fasting and stimulated C-peptide, insulin resistance (homeostasis model assessment of insulin resistance [HOMA-IR]), body weight, body mass index (BMI), waist circumference, systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate, and serum lipid (i.e., total cholesterol [TC], high-density lipoprotein cholesterol [HDL-C], low-density lipoprotein cholesterol [LDL-C], triglycerides [TG]).
The timing of outcome measurement had to be at least 8 weeks from randomization since this is the recommended minimum time for T2DM management studies. (Sridharan et al., 2011; European Medicines Agency, 2018) The management of complications of T2DM was beyond the scope of this review.
3.1.5 Types of Studies
The review included RCTs, of any design. Only the first phase of cross-over trials was included to avoid any carry-over of treatment effect.
3.2 Search Strategy
The authors searched for both published and unpublished studies via the following electronic databases and gray literature sources: MEDLINE (Ovid; from 1946), Embase (Ovid; from 1974), CINAHL (EBSCOhost; from 1937), PsycINFO (Ovid; from 1806), Web of Science (from 1900), Cochrane Central Register of Controlled Trials (CENTRAL; from 1996), Allied and Complementary Medicine Database (AMED; Ovid; from 1985), International Pharmaceutical Abstracts (Ovid; from 1970), Turning Research Into Practice (TRIP; from 1997), AYUSH Research Portal (a database of Ayurveda and other research articles; http://ayushportal.nic.in), Digital Helpline for Ayurveda Research Articles (DHARA; http://dharaonline.org), A Bibliography of Indian Medicine (ABIM; http://indianmedicine.eldoc.ub.rug.nl), CAM-QUEST (a database of complementary and alternative medicine research articles; https://www.cam-quest.org/en), Directory of Open Access Journals, EthOS, OpenGrey, and ProQuest Dissertations and Theses. The databases were searched on 16th January 2021. The search strategies are detailed in Supplementary Appendix S1, which were developed based on the previous systematic reviews and clinical guidelines on this topic (Hardy et al., 2001; Shekelle et al., 2005; Central Council for Research in Ayurvedic Sciences, 2011; Sridharan et al., 2011; National Institute for Health and Care Excellence, 2015; Ministry of Ayush, 2016; Ministry of Health and Family Welfare, 2016; Central Council for Research in Ayurvedic Sciences, 2017; Central Council for Research in Ayurvedic Sciences and Directorate General of Health Services, 2018) and in consultation with an experienced information specialist. A combination of search terms and index terms was used. No language restrictions were applied, and an external company was hired for professional translations. Similarly, no date restrictions were applied. Furthermore, Researches in Ayurveda and Ayurvedic Research Database (ARD; databases of Ayurveda-related dissertations and theses; seventh edition; 2001–2008; https://ayurvedahealthcare.info) was searched for additional studies. The reference list of relevant previous systematic reviews and included studies and the tables of content of 48 Ayurveda journals were screened till 16th January 2021 for additional studies. Relevant experts in the field were contacted (at least twice through email), including the Central Council for Research in Ayurvedic Sciences (Ministry of Ayush, India) and authors of the included studies (and manufacturers of the included Ayurvedic medicines), to locate additional studies.
3.3 Study Selection
Following the searches, the identified citations were collated and uploaded into EndNote v8.2 (Clarivate, Philadelphia, United States), and duplicate citations were removed. The remaining citations were uploaded into Rayyan [Qatar Computing Research Institute (Data Analytics), Doha, Qatar], and titles and abstracts were screened for eligibility by two independent reviewers. Studies identified as potentially eligible or those without an abstract had their full text retrieved. In case the full text of an article was not available even through the interlibrary loan service/British Library, the author and journal editor were approached (at least twice through email). Potentially eligible ongoing RCTs were contacted (at least twice through email) by the authors to access the study results. Full texts of the studies were assessed for eligibility by two independent reviewers. Any disagreements that arose between the two reviewers were resolved through discussion and in consultation with a third reviewer. Full texts of the studies that did not meet the inclusion criteria were excluded, and reasons for exclusion were recorded. In the case of multiple publications of the same study, the article having the most complete data was included. If partial data were provided in each article, then all such articles were included.
3.4 Assessment of Methodological Quality
Included studies were critically assessed using the standardized JBI critical appraisal checklist for RCTs independently by two reviews, assigning a score as met (yes), not met (no), or unclear. (Tufanaru et al., 2017) A third reviewer compared their work and highlighted the disagreements between the two reviewers. Any such disagreements were resolved through discussion between the three reviewers. All studies, regardless of their methodological quality, were included in the review.
3.5 Data Extraction
Two reviewers independently extracted data from the included studies, using a pre-developed and pre-tested data extraction tool. A third reviewer compared their work and highlighted the disagreements between the two reviewers. Any such disagreements were resolved through discussion between the three reviewers. Data extraction included details about the population, intervention, comparator, and outcomes. For biochemical, anthropometric, and physiological parameters and HRQoL data, the authors extracted the 8-week time point data. Where this time point was not reported or multiple time points were reported, data from the time point closest to 8 weeks were extracted. For adverse events, the authors extracted the end of study data. Intention-to-treat (ITT) data were preferred compared to per-protocol data. Post-intervention data were extracted in preference to change from baseline data (i.e., post-intervention score–baseline score). Percentage change from baseline was not extracted due to the sensitivity to changes in variance and failure to protect from baseline imbalances, thus leading to non-normally distributed outcome data. (Vickers, 2001) For each outcome, the data were converted into one standard unit of measurement. In multi-arm studies, where two or more drug manufacturing processes, forms or timings of administration, doses, or Anupans of the same Ayurvedic medicine were compared with another comparator, the two or more study arms were combined together before pooling with other studies. (Hedges and Olkin, 1985; Cohen, 1988) This was also performed for multi-arm studies when the interventions and comparators were provided with co-interventions (e.g., Ayurvedic medicine arm pooled with Ayurvedic medicine and exercise arm versus placebo arm pooled with placebo and exercise arm).
Multiple strategies were used to obtain the relevant missing data. The corresponding author of the included study was contacted by email (at least two times per author) to obtain the missing data. If the standard deviation (SD) was missing (in a small number of studies), SD was imputed from a similar study (in terms of intervention, comparator, sample size, and numerical outcome data). If only a median and interquartile range (IQR) was reported (in a small number of studies), the mean was assumed to be equal to the median and the SD was calculated using a standard formula (=IQR/1.35). (Higgins et al., 2019)
3.6 Data Synthesis
Initially, narrative syntheses were conducted to describe the studies. For each outcome, where at least two studies on an Ayurvedic medicine were included, random-effects meta-analyses were conducted to provide a weighted measure of treatment effect. For continuous outcomes, mean differences (MD) with 95% confidence intervals (CI) were reported where the same scale was used across studies. Where different scales were used across studies, standardized mean differences (SMD) with 95% CIs were reported. Where necessary, post-intervention data were pooled with change from baseline data and only for MDs but not SMDs. For the purpose of analysis, the following comparators were combined together: no medicine, no additional medicine, and placebo. For studies with more than one comparator group (e.g., mentioned above, OAD), the comparisons were included in separate meta-analysis models to avoid the issue of double-counting of the comparator group. Statistical heterogeneity was quantified using the I2 statistic. Analyses were conducted using STATA v16 for Windows (STATACorp, College Station, Texas, United States). The findings are interpreted from the clinical point of view.
3.7 Assessment of Publication Bias
Funnel plots were used to assess publication bias, where there were at least 10 studies included in the meta-analysis.
3.8 Sensitivity Analyses
Where a significant pooled association was found between the intervention and primary outcome, sensitivity analysis was performed to assess the robustness of the result by excluding studies that were not journal publications (i.e., not peer-reviewed). After excluding these studies, at least two eligible studies were needed for the sensitivity analysis.
3.9 Subgroup Analyses
Where a significant pooled association was found between the intervention and primary outcome, subgroup analysis was performed to explore the influence of the following factors on the result:
• Country: South Asia (i.e., Afghanistan, Bangladesh, Bhutan, India, Maldives, Nepal, Pakistan, or Sri Lanka) versus others.
• Comparator: no medicine or no additional medicine versus placebo.
For this purpose, at least two eligible studies per subgroup were needed.
4 Assessing Certainty in the Findings
The Grading of Recommendations, Assessment, Development and Evaluation (GRADE) method was used to assess the certainty of the findings (i.e., the primary outcomes). (Schünemann et al., 2013) Only those Ayurvedic medicines were included where the meta-analysis (at least two studies were needed) was possible on at least one primary outcome. Two reviewers were involved in the process, and the findings were initially ranked as high and were downgraded to moderate, low, or very low if there was evidence of the following: risk of bias, inconsistency of results, indirectness of evidence, imprecision, and/or publication bias. More specifically, in the risk of bias domain, the following were considered for downgrading: no allocation concealment (no to Q2 in the JBI critical appraisal checklist for RCTs), lack of blinding (no to Q4,5,6), attrition bias (no to Q9), and selective outcome reporting. If an issue out of these four was present in the majority of studies (i.e., >50%), then it was downgraded by one level. If more than one issue was present, then it was downgraded by two levels. In the inconsistency of results domain, if the statistical heterogeneity (i.e., I2 statistic) was 75%–89%, then it was downgraded by one level. If the I2 statistic was ≥90%, then it was downgraded by two levels. In the imprecision domain, if the total sample size was 100 to <400, then it was downgraded by one level. If the total sample size was <100, then it was downgraded by two levels. If the total sample size was ≥400 with wide CI and not earlier downgraded for inconsistency, then it was downgraded by one level. If publication bias was detected, then it was downgraded by one level. (See Summary of Findings table [Supplementary Material]).
5 Results
5.1 Inclusion of Studies
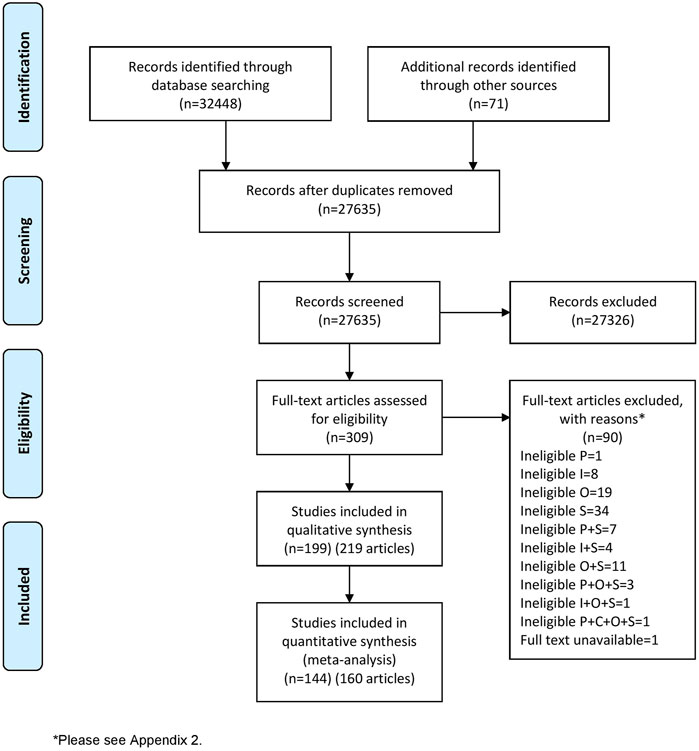
The literature search identified 32,519 records. After removing duplicate records, 27,635 titles and abstracts were screened. Full texts of 309 records were sought, and their eligibility for inclusion was assessed. 219 records were included in this systematic review representing 199 RCTs and including 21,191 participants (in the eligible study arms) (Figure 1). Data from 144 RCTs (160 records) were included in the meta-analyses. A further 28 ongoing RCTs were included, which are reported in Supplementary Appendix S2. The reasons for the exclusion of 90 records excluded at the full text screening stage are reported in Supplementary Appendix S3.
5.2 Characteristics of Included Studies
Characteristics of 199 included studies are reported in Supplementary Appendix S4. 82 studies (41%) were conducted in South Asia (one in Bangladesh, 72 in India, eight in Pakistan, and one in Sri Lanka), and 72 studies (36%) in Iran. 26 RCTs (13%) recruited newly diagnosed/treatment naïve T2DM patients, 146 (73%) recruited existing cases/on OAD, and 22 (11%) recruited both; it was unclear in the remaining five RCTs. The mean duration of T2DM in existing cases ranged from 2.5 to 12.8 years. 28 RCTs recruited participants with physical or mental health comorbidities or complications of T2DM. The sample size of the studies ranged from 12 to 6,114 (only eligible study arms were considered). The mean age of participants ranged from 37.5 to 65.4 years. The percentage of female participants ranged from 0% to 100%. At baseline, the mean HbA1c and FBG ranged from 6.4 to 11.8% and 118–360 mg/dl, respectively. 42 RCTs (21%) received commercial funding or other support; however, it was unclear in 86 RCTs (43%).
5.3 Interventions
98 Ayurvedic medicines were included in the systematic review. The details of the interventions are provided in Supplementary Appendix S5. 56 Ayurvedic medicines were of single plant-origin (i.e., single herbs), two were of single mineral-origin (namely, Swarnamakshika Bhasma [ash obtained through incineration] and Yashad Bhasma), and Shilajit (a blackish-brown powder or an exudate from high mountain rocks). 31 Ayurvedic medicines contained ≥2 plant-origin ingredients, and one contained ≥2 mineral-origin ingredients (namely, Trivanga Bhasma). Seven Ayurvedic medicines were herbo-mineral formulations (namely, Hyponidd, Inolter, Madhumeha Nashini Gutika, Naga Bhasma and Nishamalaki combination, Nishamalaki and Shilajit combination, Salasaradi Kashaya, and Tejashiladi Vati). An oral route of administration was used for all of the Ayurvedic medicines, using a range of administration forms (including tablets, capsules, and powder) and timings of administration (ranging from one to four times daily). The daily doses of Ayurvedic medicines varied, depending on their type and form and timing of administration. Similarly, a range of Anupan of Ayurvedic medicines were reported. The treatment duration (and trial follow-up) ranged from 8 (i.e., based on the systematic review inclusion criteria) to 78 weeks (six RCTs reported 26 weeks, and one each reported 36, 39, 52, and 78 weeks).
5.4 Methodological Quality
Overall, in the studies reviewed the methodology was not adequately reported, resulting in poorer methodological quality scoring (Supplementary Appendix S6). The major issues in these studies were: 1) inadequately reporting the randomization process that was used to assign participants to study arms; 2) inadequately reporting the allocation concealment process; 3) inadequately reporting who was blinded to intervention assignment and how blinding was performed; 4) inadequate reporting of the placebo and on further interrogation some placebos were actually Ayurvedic medicines, and it was unclear if placebos were identical especially when other forms of administration like powder or liquid were used which are easy to differentiate taste and/or smell wise; 5) inadequately reporting whether the study arms were treated identically other than the intervention of interest and particularly, in existing cases/on OAD, it was not always clear whether OAD was continued for the rest of the trial; 6) inadequately reporting the ascertainment of outcomes (including adverse events); 7) errors/issues in the sample size calculation and reporting (e.g., not using the primary outcome or using an inappropriate outcome as primary for the sample size calculation, unclear minimum clinically important difference or using an inappropriate minimum clinically important difference for the sample size calculation; 8) errors/issues in data analysis and reporting (e.g., not performing ITT analysis or doing pre-post analysis of outcomes within study arms but no comparative analysis between study arms); and 9) in terms of the follow-up, not analyzing the differences between study arms, such as no analysis of the patterns of loss to follow-up and the impact of the loss to follow-up on results.
5.5 Effects of Interventions
Meta-analyses were conducted on 33 Ayurvedic medicines (32 single herbs and Shilajit). The majority of the Ayurvedic medicines were compared to no medicine or no additional medicine or placebo; however, two were compared to both no medicine or no additional medicine or placebo and OAD (Momordica charantia L. and Trigonella foenum-graecum L.) and two were compared to OAD (Enicostemma axillare (Lam.) Raynal and Pterocarpus marsupium Roxb.). (See ZIP file for forest and funnel plots [Supplementary Material]).
5.5.1 Aegle marmelos (L.) Corrêa
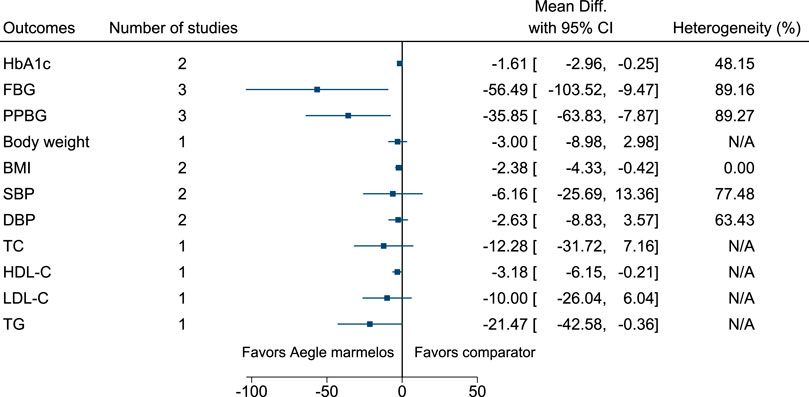
Aegle marmelos significantly reduced HbA1c (MD −1.6%; 95% CI −3 to −0.3), FBG (−56 mg/dl; −104 to −9), PPBG (−36 mg/dl; −64 to −8), and BMI (−2.4 kg/m2; −4.3 to −0.4) (see Figure 2). (Sankhla et al., 2009; Yaheya and Ismail, 2009; Sharma and Sharma, 2013; Nigam and Nambiar, 2019).
5.5.2 Allium sativum L.
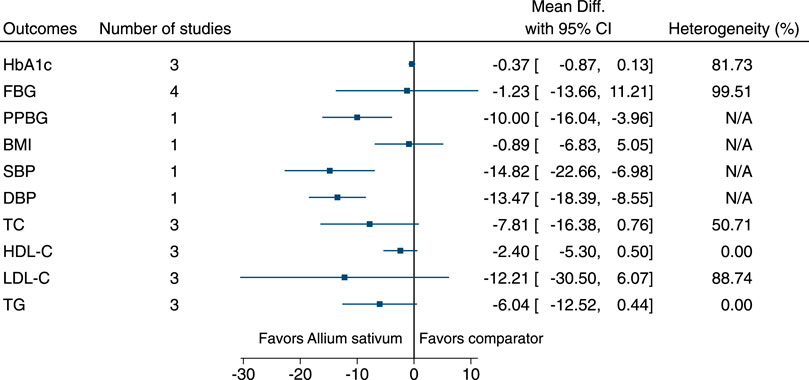
Allium sativum significantly reduced PPBG (−10 mg/dl; −16 to −4), SBP (−15 mmHg; −23 to −7), and DBP (−13 mmHg; −18 to −9) (see Figure 3). (Ashraf et al., 2005; Balasubramaniam et al., 2010; Ashraf et al., 2011a; Ashraf et al., 2011b; Kumar et al., 2013; Mansouri et al., 2018).
5.5.3 Aloe vera L.
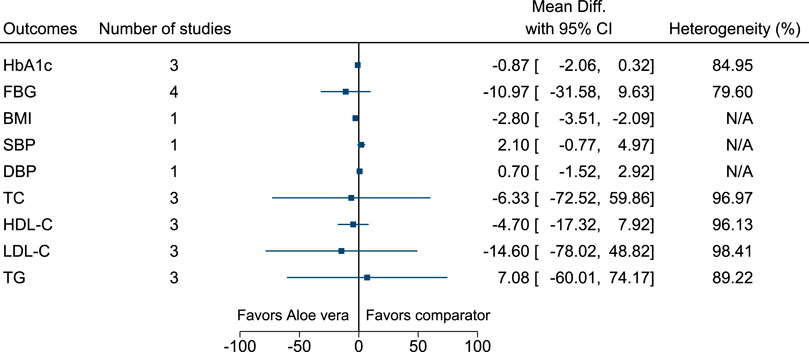
Aloe vera significantly reduced BMI (−2.8 kg/m2; −3.5 to −2.1) (see Figure 4). (Arora et al., 2009; Huseini et al., 2012a; Huseini et al., 2012b; Zarrintan et al., 2015; Maurya et al., 2017).
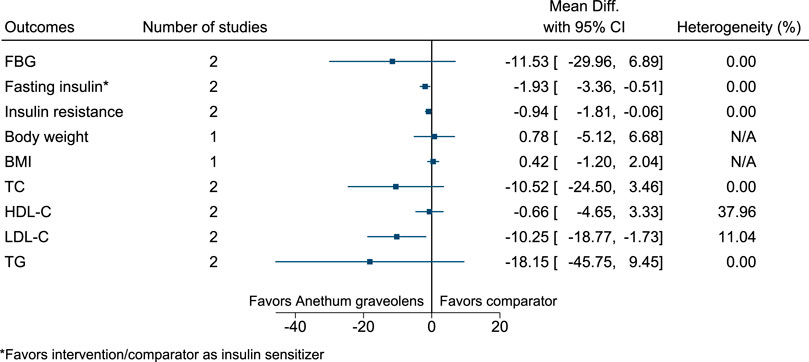
5.5.4 Anethum graveolens L.
Anethum graveolens significantly reduced fasting insulin (as insulin sensitizer; −2 mIU/L; −3 to −1), insulin resistance (−0.9; −1.8 to −0.1), and LDL-C (−10 mg/dl; −19 to −2) (see Figure 5). (Mobasseri et al., 2014; Haidari et al., 2020).
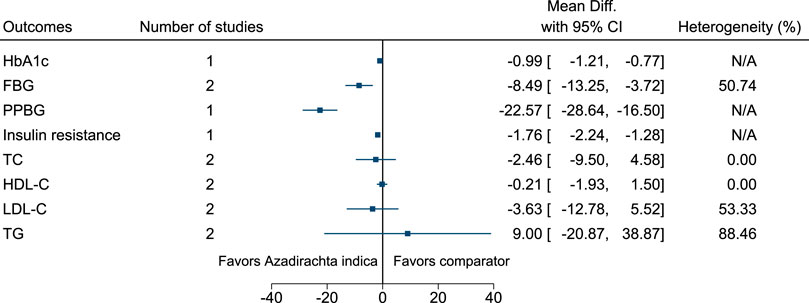
5.5.5 Azadirachta indica A.Juss.
Azadirachta indica significantly reduced HbA1c (−1%; −1.2 to −0.8), FBG (−8 mg/dl; −13 to −4), PPBG (−23 mg/dl; −29 to −17), and insulin resistance (−1.8; −2.2 to −1.3) (see Figure 6). (Balasubramaniam et al., 2010; Pingali et al., 2020).
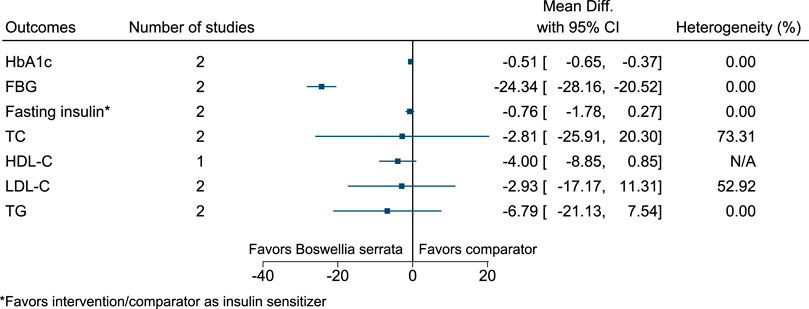
5.5.6 Boswellia serrata Roxb.
Boswellia serrata significantly reduced HbA1c (−0.5%; −0.7 to −0.4) and FBG (−24 mg/dl; −28 to −21) (see Figure 7). (Azadmehr et al., 2014; Mehrzadi et al., 2018).
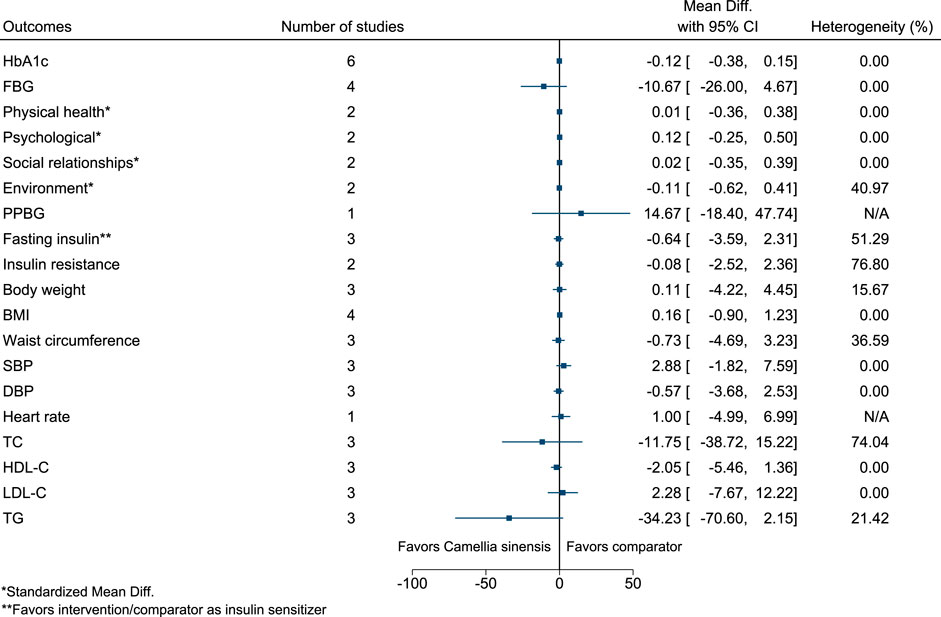
5.5.7 Camellia sinensis (L.) Kuntze
Figure 8 shows the summary forest plot for Camellia sinensis. (MacKenzie et al., 2007; Mirzaei et al., 2009; Hsu et al., 2011; Lasaite et al., 2014; Liu et al., 2014; Quezada-Fernández et al., 2019).
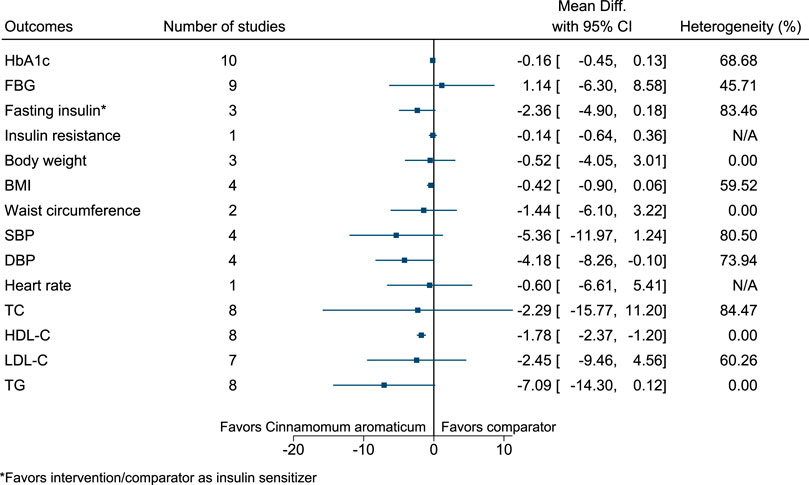
5.5.8 Cinnamomum aromaticum Nees
Cinnamomum aromaticum significantly increased HDL-C (2 mg/dl; 1–2) (see Figure 9). (Mang et al., 2006; Suppapitiporn et al., 2006; Blevins et al., 2007; Crawford, 2009; Akilen et al., 2010; Wainstein et al., 2011; Lu et al., 2012; Sharma et al., 2012; Hasanzade et al., 2013; Tangvarasittichai et al., 2015; Sengsuk et al., 2016).
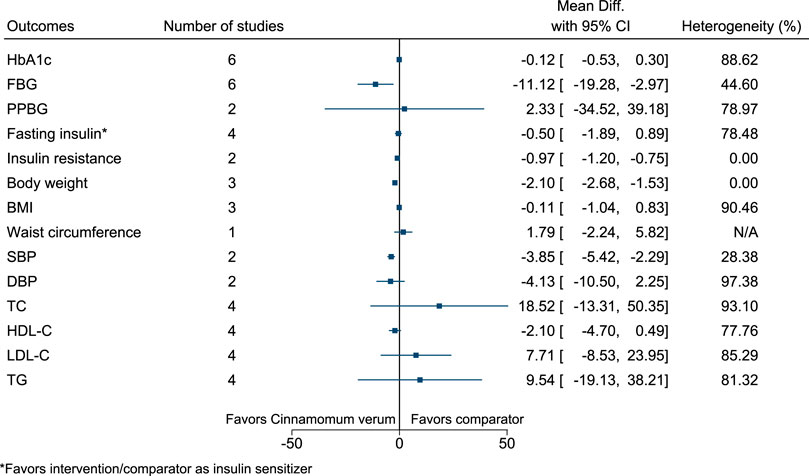
5.5.9 Cinnamomum verum J.Presl
Cinnamomum verum significantly reduced FBG (−11 mg/dl; −19 to −3), insulin resistance (−1; −1.2 to −0.8), body weight (−2.1 kg; −2.7 to −1.5), and SBP (−4 mmHg; −5 to −2) (see Figure 10). (Vafa et al., 2012; Zahmatkesh et al., 2012; Azimi et al., 2014; Azimi et al., 2016; Talaei et al., 2017a; Zare et al., 2019; Mirmiranpour et al., 2020).
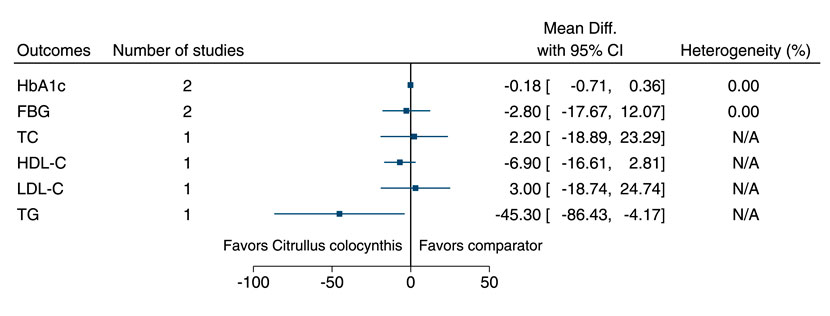
5.5.10 Citrullus colocynthis (L.) Schrad.
Citrullus colocynthis significantly reduced TG (−45 mg/dl; −86 to −4) (see Figure 11). (Huseini et al., 2009; Barghamdi et al., 2016).
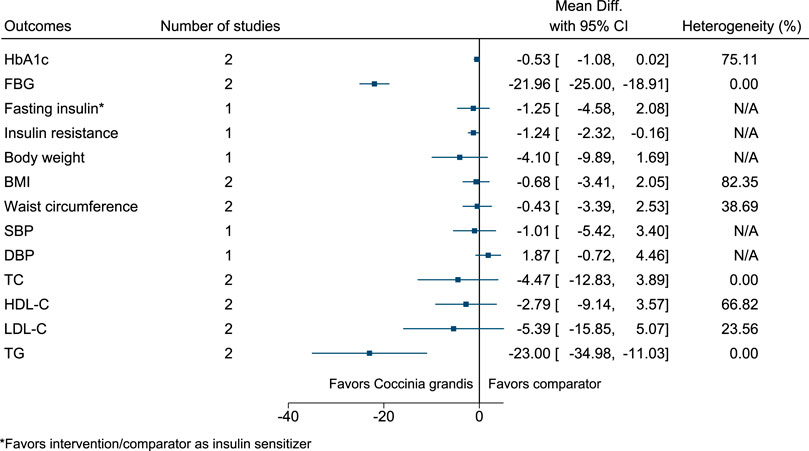
5.5.11 Coccinia grandis (L.) Voigt
Coccinia grandis significantly reduced FBG (−22 mg/dl; −25 to −19), insulin resistance (−1.2; −2.3 to −0.2), and TG (−23 mg/dl; −35 to −11) (see Figure 12). (Kuriyan et al., 2008; Kurpad and Raj, 2008; Wasana et al., 2021).
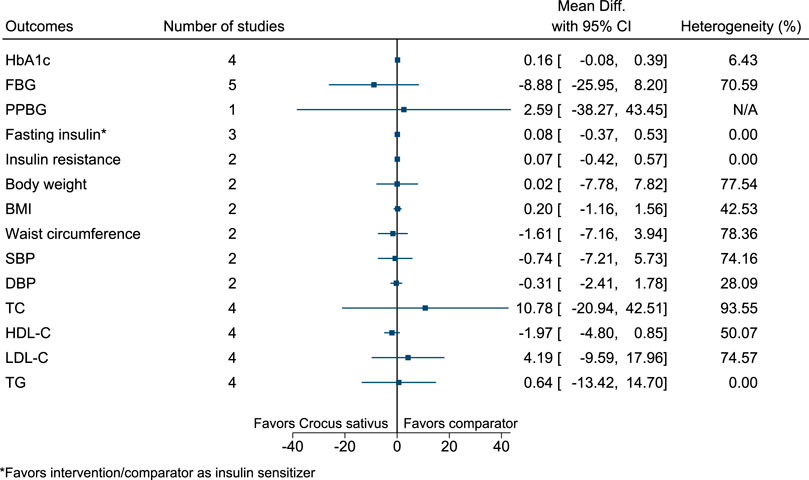
5.5.12 Crocus sativus L.
Figure 13 shows the summary forest plot for Crocus sativus. (Azimi et al., 2014; Azimi et al., 2016; Milajerdi et al., 2018; Ebrahimi et al., 2019; Moravej Aleali et al., 2019; Mobasseri et al., 2020).
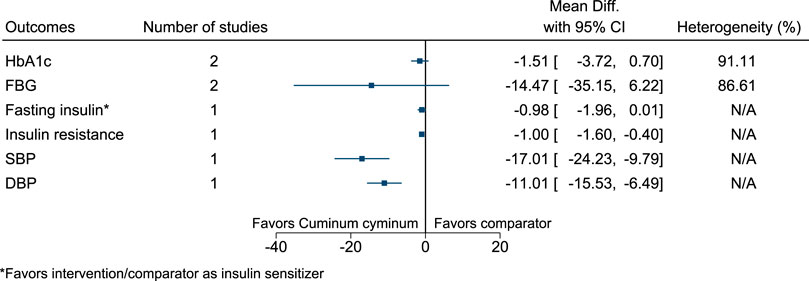
5.5.13 Cuminum cyminum L.
Cuminum cyminum significantly reduced insulin resistance (−1; −1.6 to −0.4), SBP (−17 mmHg; −24 to −10), and DBP (−11 mmHg; −16 to −6) (see Figure 14). (Jafari et al., 2017; Mansouri et al., 2018; Hendre et al., 2020).
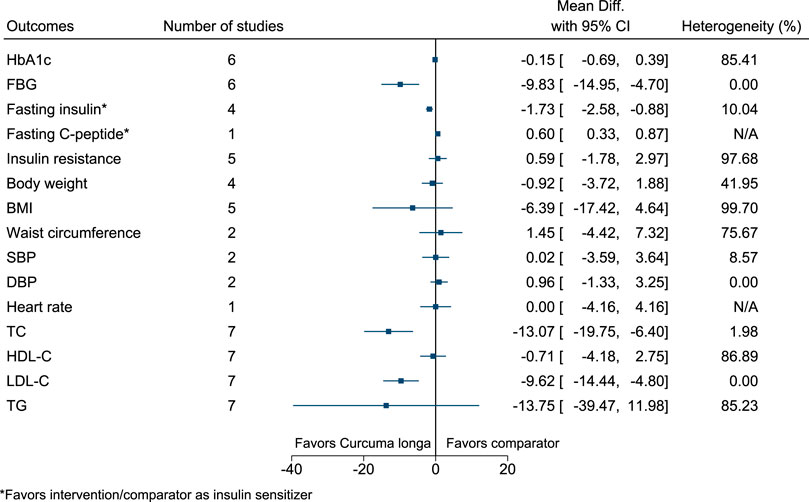
5.5.14 Curcuma longa L.
Curcuma longa significantly reduced FBG (−10 mg/dl; −15 to −5), fasting insulin (as insulin sensitizer; −2 mIU/L; −3 to −1), TC (−13 mg/dl; −20 to −6), and LDL-C (−10 mg/dl; −14 to −5), and increased fasting C-peptide (as insulin sensitizer; 0.6 ng/ml; 0.33–0.87) (see Figure 15). (Usharani et al., 2008; Na et al., 2013; Chuengsamarn et al., 2014; Panahi et al., 2017; Panahi et al., 2018; Adab et al., 2019; Adibian et al., 2019; Srinivasan et al., 2019; de Sousa et al., 2020).
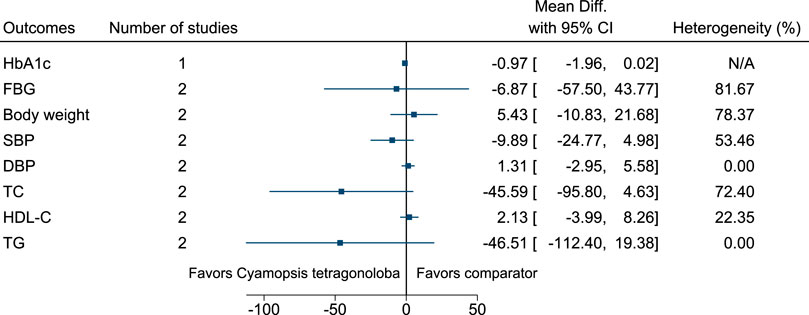
5.5.15 Cyamopsis tetragonoloba (L.) Taub.
Figure 16 shows the summary forest plot for Cyamopsis tetragonoloba. (Uusitupa et al., 1984; Uusitupa et al., 1989).
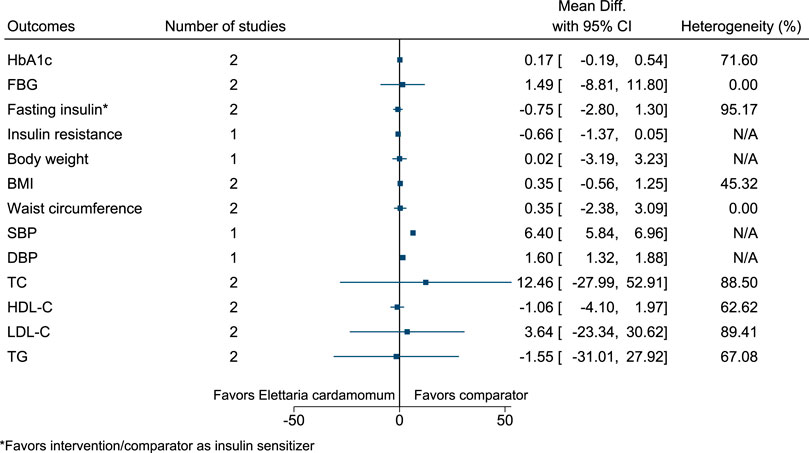
5.5.16 Elettaria cardamomum (L.) Maton
Elettaria cardamomum significantly increased SBP (6 mmHg; 6–7) and DBP (2 mmHg; 1–2) (see Figure 17). (Azimi et al., 2014; Azimi et al., 2016; Aghasi et al., 2019).
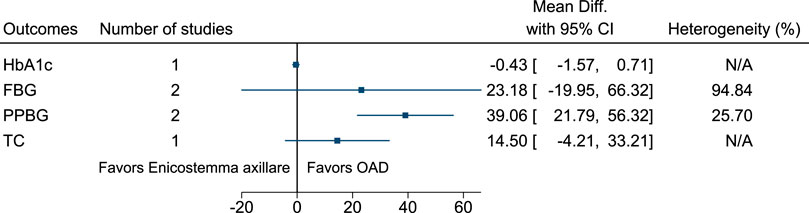
5.5.17 Enicostemma axillare (Lam.) Raynal (versus OAD)
Compared to Enicostemma axillare, OAD significantly reduced PPBG (−39 mg/dl; −56 to −22) (see Figure 18). (Kumar et al., 2014; Shankarrao et al., 2017).
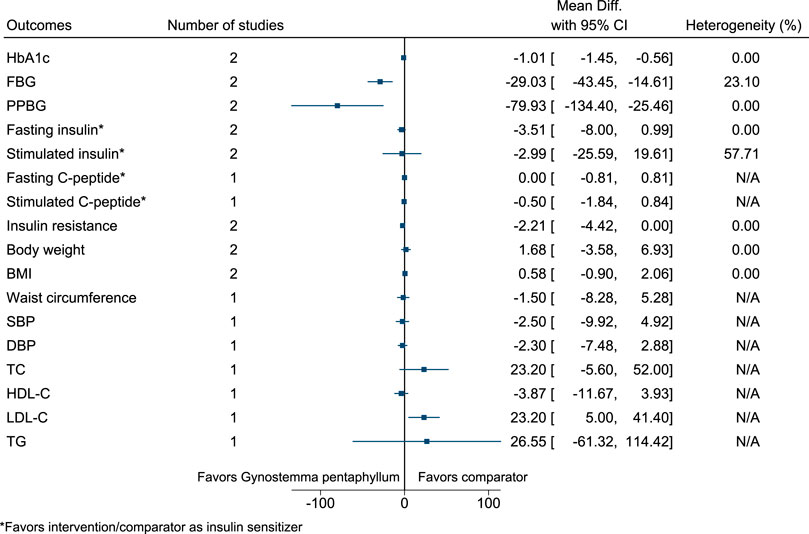
5.5.18 Gynostemma pentaphyllum (Thunb.) Makino
Gynostemma pentaphyllum significantly reduced HbA1c (−1%; −1.5 to −0.6), FBG (−29 mg/dl; −43 to −15), and PPBG (−80 mg/dl; −134 to −25), and increased LDL-C (23 mg/dl; 5–41) (see Figure 19). (Huyen et al., 2010; Huyen et al., 2012).
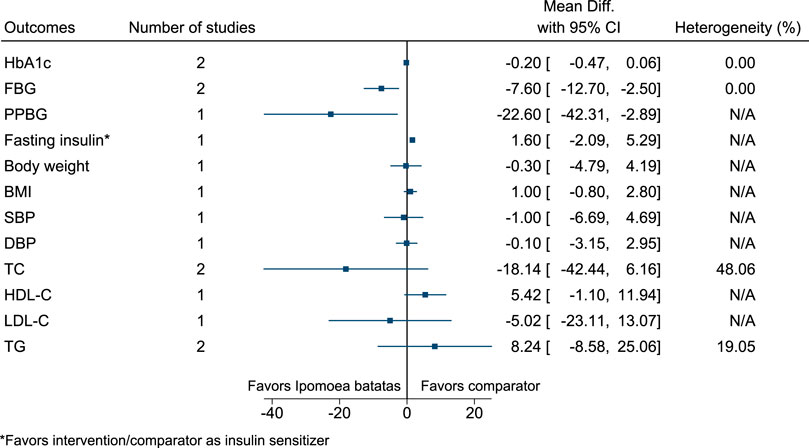
5.5.19 Ipomoea batatas (L.) Lam.
Ipomoea batatas significantly reduced FBG (−8 mg/dl; −13 to −3) and PPBG (−23 mg/dl; −42 to −3) (see Figure 20). (Ludvik et al., 2004; Ludvik et al., 2008).
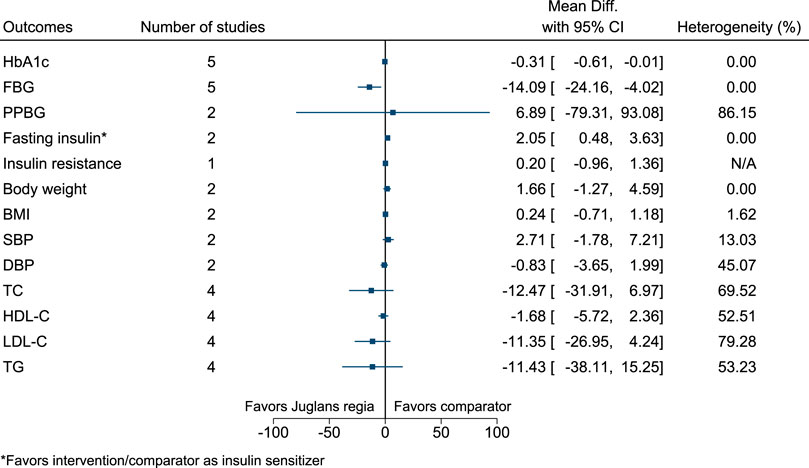
5.5.20 Juglans regia L.
Juglans regia significantly reduced FBG (−14 mg/dl; −24 to −4) (see Figure 21). (Hosseini et al., 2014a; Hosseini et al., 2014b; Zibaeenezhad et al., 2016; Abdoli et al., 2017; Zibaeenezhad et al., 2017; Rabiei et al., 2018).
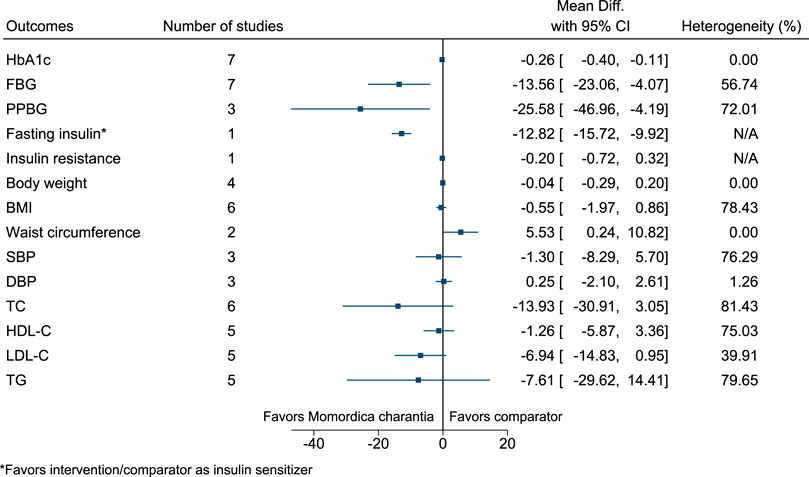
5.5.21 Momordica charantia L.
Momordica charantia significantly reduced HbA1c (−0.3%; −0.4 to −0.1), FBG (−14 mg/dl; −23 to −4), PPBG (−26 mg/dl; −47 to −4), and fasting insulin (as insulin sensitizer; −13 mIU/L; −16 to −10) (see Figure 22). (Dans et al., 2007; Zänker et al., 2012; Trakoon-osot et al., 2013; Suthar et al., 2016a; Cortez-Navarrete et al., 2018; Kumari et al., 2018; Amini et al., 2020; Kim et al., 2020) In the country subgroup analysis for HbA1c and FBG, no statistically significant difference was found between subgroups (p = 0.25 and p = 0.14, respectively).
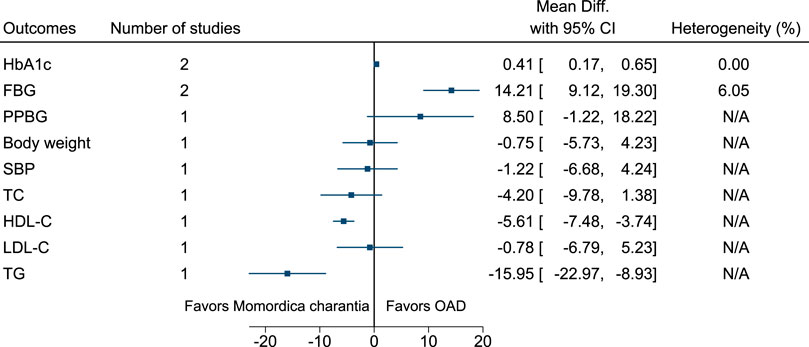
5.5.22 Momordica charantia L. (versus OAD)
Compared to Momordica charantia, OAD significantly reduced HbA1c (−0.4%; −0.7 to −0.2) and FBG (−14 mg/dl; −19 to −9). Compared to OAD, Momordica charantia significantly increased HDL-C (6 mg/dl; 4–7) and reduced TG (−16 mg/dl; −23 to −9) (see Figure 23). (Inayat U Rahman et al., 2015; Suthar et al., 2016b).
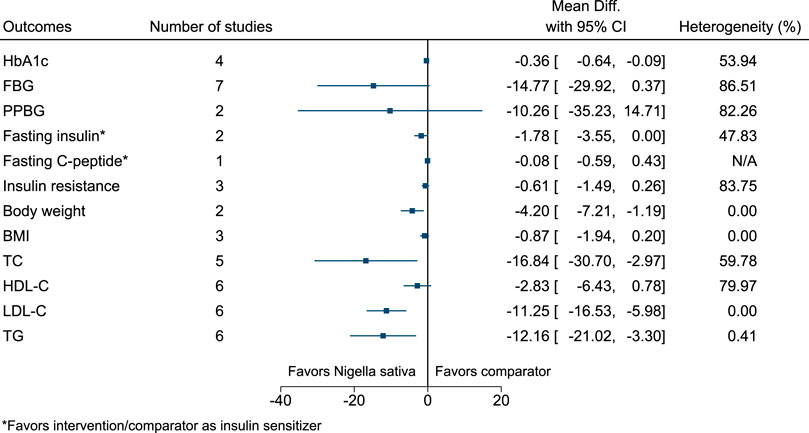
5.5.23 Nigella sativa L.
Nigella sativa significantly reduced HbA1c (−0.4%; −0.6 to −0.1), body weight (−4.2 kg; −7.2 to −1.2), TC (−17 mg/dl; −31 to −3), LDL-C (−11 mg/dl; −17 to −6), and TG (−12 mg/dl; −21 to −3) (see Figure 24). (Najmi et al., 2012; Hosseini et al., 2013; Hadi et al., 2015; Heshmati et al., 2015; Kaatabi et al., 2015; Kooshki et al., 2020; Jangjo-Borazjani et al., 2021).
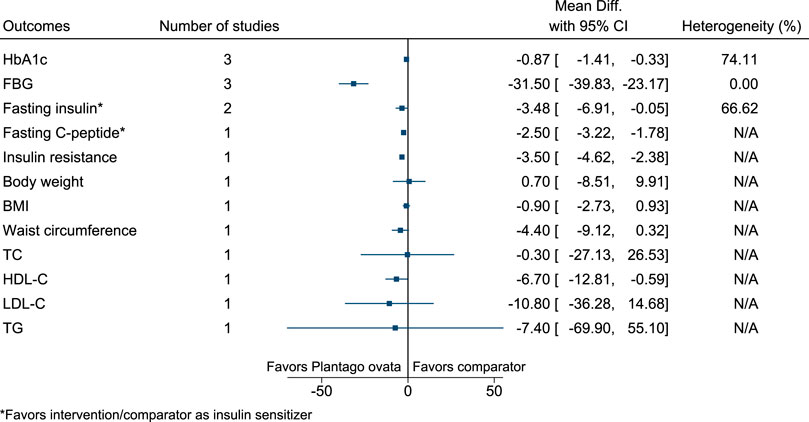
5.5.24 Plantago ovata Forssk.
Plantago ovata significantly reduced HbA1c (−0.9%; −1.4 to −0.3), FBG (−32 mg/dl; −40 to −23), fasting C-peptide (as insulin sensitizer; −2.5 ng/ml; −3.22 to −1.78), and insulin resistance (−3.5; −4.6 to −2.4), and increased HDL-C (7 mg/dl; 1–13) (see Figure 25). (Ziai et al., 2005; Feinglos et al., 2013; Abutair et al., 2016).
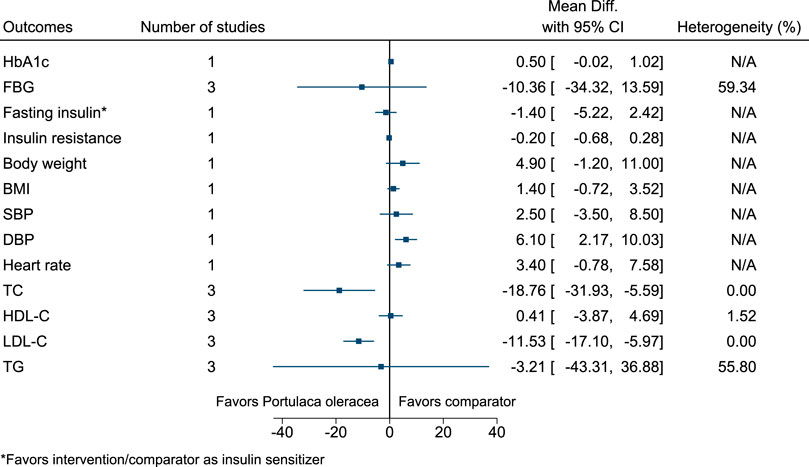
5.5.25 Portulaca oleracea L.
Portulaca oleracea significantly reduced TC (−19 mg/dl; −32 to −6) and LDL-C (−12 mg/dl; −17 to −6), and increased DBP (6 mmHg; 2–10) (see Figure 26). (Farzanegi, 2014; Dehghan et al., 2016; Wainstein et al., 2016).
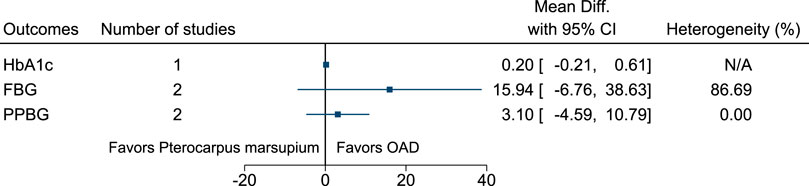
5.5.26 Pterocarpus marsupium Roxb. (versus OAD)
Figure 27 shows the summary forest plot for Pterocarpus marsupium (versus OAD). (Hariharan et al., 2005; Maurya et al., 2017).
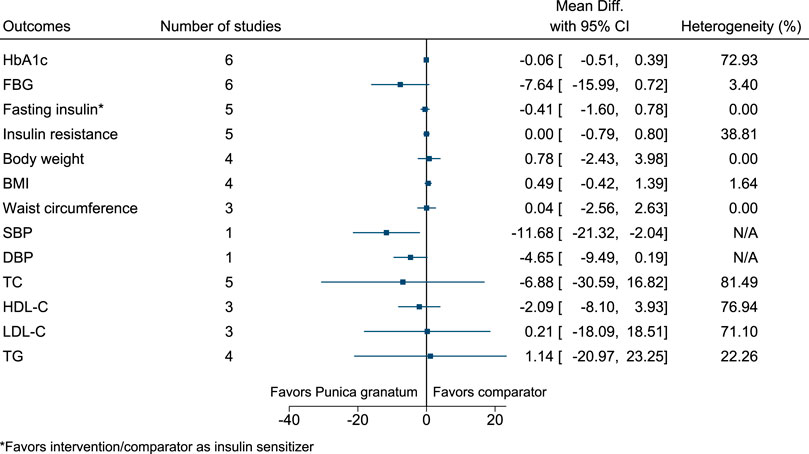
5.5.27 Punica granatum L.
Punica granatum significantly reduced SBP (−12 mmHg; −21 to −2) (see Figure 28). (Babaeian et al., 2013; Sohrab et al., 2014; Sohrab et al., 2015; Faghihimani et al., 2016; Khajebishak et al., 2019a; Khajebishak et al., 2019b; Grabež et al., 2020; Hashemi et al., 2020).
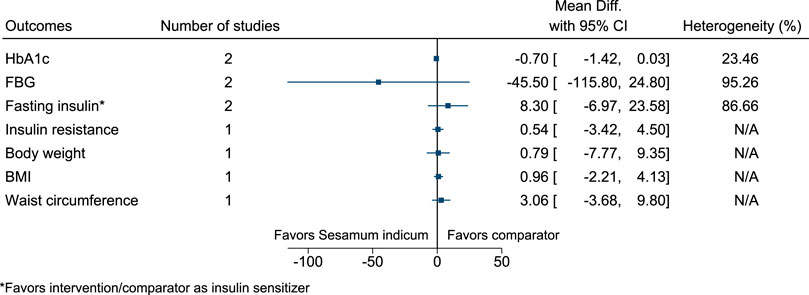
5.5.28 Sesamum indicum L.
Figure 29 shows the summary forest plot for Sesamum indicum. (Mohammad Shahi et al., 2017; Aslam et al., 2018).
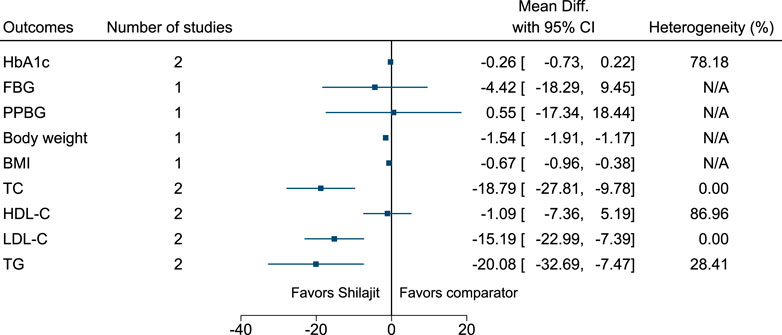
5.5.29 Shilajit
Shilajit significantly reduced body weight (−1.5 kg; −1.9 to −1.2), BMI (−0.7 kg/m2; −1 to −0.4), TC (−19 mg/dl; −28 to −10), LDL-C (−15 mg/dl; −23 to −7), and TG (−20 mg/dl; −33 to −7) (see Figure 30). (Narasimha Raju and Sharma, 2016; Niranjan et al., 2016).
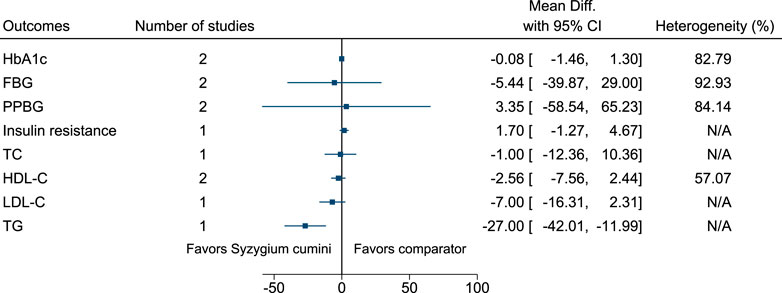
5.5.30 Syzygium cumini (L.) Skeels
Syzygium cumini significantly reduced TG (−27 mg/dl; −42 to −12) (see Figure 31). (Sahana et al., 2010; Sidana et al., 2016; Sidana et al., 2017).
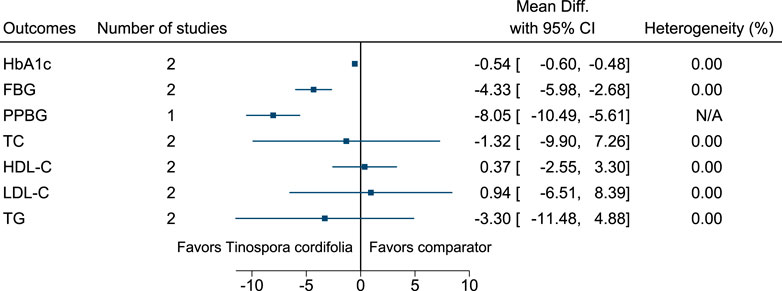
5.5.31 Tinospora cordifolia (Willd.) Hook.f. & Thomson
Tinospora cordifolia significantly reduced HbA1c (−0.5%; −0.6 to −0.5), FBG (−4 mg/dl; −6 to −3), and PPBG (−8 mg/dl; −10 to −6) (see Figure 32). (Balasubramaniam et al., 2010; Mishra et al., 2015; Roy et al., 2015).
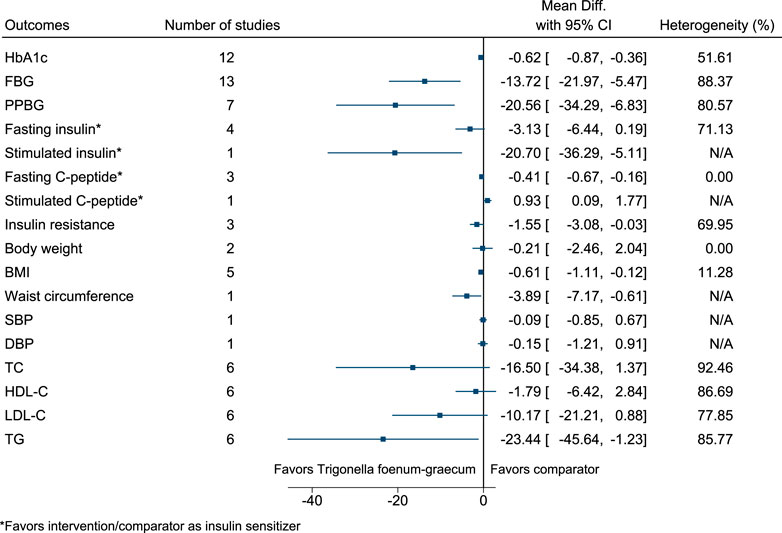
5.5.32 Trigonella foenum-graecum L.
Trigonella foenum-graecum significantly reduced HbA1c (−0.6%; −0.9 to −0.4), FBG (−14 mg/dl; −22 to −5), PPBG (−21 mg/dl; −34 to −7), stimulated insulin (as insulin sensitizer; −21 mIU/L; −36 to −5), fasting C-peptide (as insulin sensitizer; −0.41 ng/ml; −0.67 to −0.16), BMI (−0.6 kg/m2; −1.1 to −0.1), waist circumference (−4 cm; −7 to −1), and TG (−23 mg/dl; −46 to −1), and increased stimulated C-peptide (as insulin sensitizer; 0.93 ng/ml; 0.09–1.77) (see Figure 33). (Gupta et al., 2001; Lu et al., 2008; Yaheya and Ismail, 2009; Ansari et al., 2011; Rafraf et al., 2014; Suchitra and Parthasarathy, 2015; Kaur et al., 2016; Singh et al., 2016; Verma et al., 2016; Ranade and Mudgalkar, 2017; Gholaman and Gholami, 2018; Kandhare et al., 2018; Hassani et al., 2019; Hota et al., 2019; Rashid et al., 2019; Hadi et al., 2020) Publication bias was detected in the funnel plot for HbA1c but not for FBG. In the funnel plot for HbA1c, there was an absence of smaller sized studies showing a larger positive effect, which could imply the effect may be larger than that reported from the meta-analysis. In the sensitivity analysis, Trigonella foenum-graecum significantly reduced HbA1c and FBG even after excluding a publication that was not peer-reviewed. In the country subgroup analysis for HbA1c, no statistically significant difference was found between subgroups (p = 0.44). However, for FBG, a statistically significant difference was found between subgroups (p < 0.001). In the comparator subgroup analysis for HbA1c and FBG, a statistically significant difference was found between subgroups (p = 0.04 and p < 0.001, respectively).
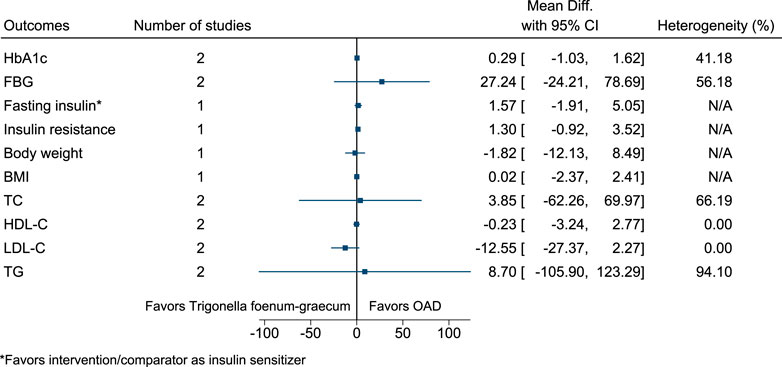
5.5.33 Trigonella foenum-graecum L. (versus OAD)
Figure 34 shows the summary forest plot for Trigonella foenum-graecum (versus OAD). (Singh et al., 2016; Najdi et al., 2019).
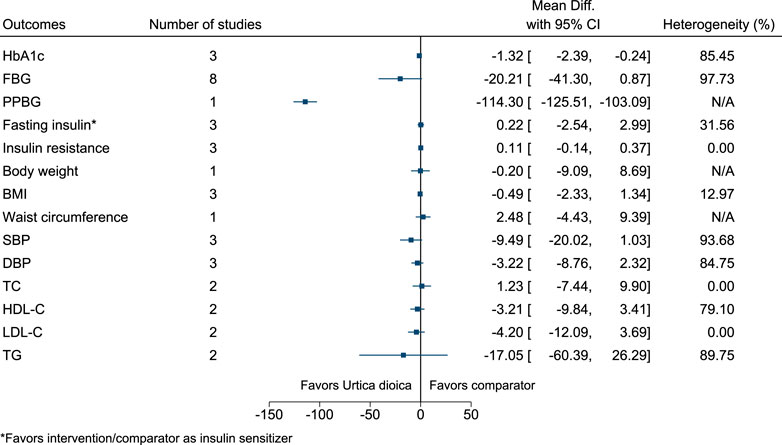
5.5.34 Urtica dioica L.
Urtica dioica significantly reduced HbA1c (−1.3%; −2.4 to −0.2) and PPBG (−114 mg/dl; −126 to −103) (see Figure 35). (Namazi et al., 2011; Esfanjani et al., 2012a; Esfanjani et al., 2012b; Kianbakht et al., 2013; Khajeh-Mehrizi et al., 2014; Dabagh and Nikbakht, 2016; Hassani et al., 2016; Dadvar et al., 2017; Ghalavand et al., 2017; Korani et al., 2017; Mohammadnia and Hassani, 2017).
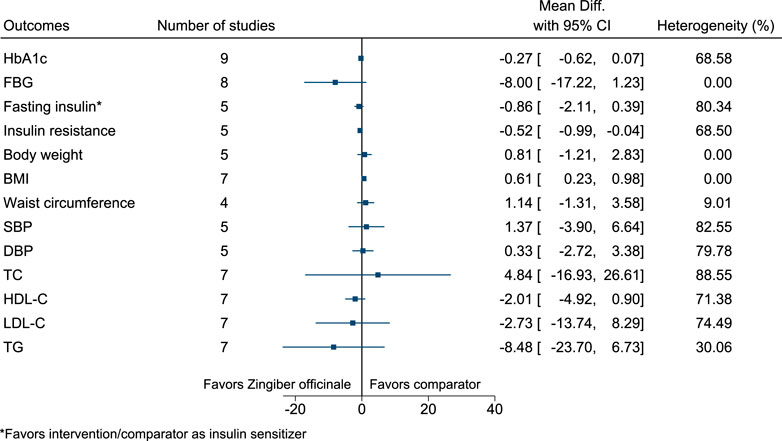
5.5.35 Zingiber officinale Roscoe
Zingiber officinale significantly increased BMI (0.6 kg/m2; 0.2–1) (see Figure 36). (Mahluji et al., 2013; Arablou et al., 2014a; Arablou et al., 2014b; Azimi et al., 2014; Mozaffari-Khosravi et al., 2014; Shidfar et al., 2015; Azimi et al., 2016; Arzati et al., 2017; Talaei et al., 2017b; Mohammadi et al., 2017; Talaei et al., 2018; Zarezadeh et al., 2018; Mohammadi and Avandi, 2019; Carvalho et al., 2020; Gholinezhad et al., 2020).
The following 65 Ayurvedic medicines, administered either as a single medicine or in combination with other Ayurvedic medicines, could not be included in any meta-analyses due to being assessed in single studies: Abelmoschus esculentus (L.) Moench, Acacia Senegal (L.) Willd., Acalypha indica L., Allium cepa L., Asanadi Ghana Vati, AYUBES, Berberis aristata DC, BGR-34, Bilvadi Churna, Capparis spinosa L., CardiPro, Cichorium intybus L., Cogent db, Convolvulus prostratus Forssk., Darvyadi Kwatha, DCBT 2345, Diabetea tea, Eclipta prostrata (L.) L., Emblica officinalis Gaertn., combination of Emblica officinalis and Withania somnifera (L.) Dunal, Herbal combination, Hibiscus sabdariffa L., Hyponidd, Inolter, Kalpit, Khadira-Kramuka Kashaya Ghanavati, Kiratadi Churna, Linum usitatissimum L., Lodhradi Kashaya Ghana Vati, Madhumeha Nashini Gutika, Madhumehari Vati, Mamajjaka Ghana Vati, Mangifera indica L., Mehagni, Murraya koenigii (L.) Spreng., Musa sapientum O.Kuntze, Mustadi Kwatha Ghana Vati, combination of Naga Bhasma and Nishamalaki, combination of Nigella sativa and Trigonella foenum-graecum, Nisha Katakadi Kashaya, Nishamalaki, combination of Nishamalaki and Hordeum vulgare L., combination of Nishamalaki and Shilajit, Ocimum tenuiflorum L., Pancreas tonic, Phyllanthus amarus Schumach. & Thonn., Polyherbal formulation, Salasaradi Kashaya, Swarnamakshika Bhasma, Talapotaka Churna, Tejashiladi Vati, Terminalia chebula Retz., combination of Tinospora cordifolia and Azadirachta indica, Tribulus terrestris L., combination of Trigonella foenum-graecum and Ocimum tenuiflorum, Trikatu Gutika, Triticum aestivum L., Trivanga Bhasma, Vernonia cinerea (L.) Less., Vidangadi Yoga, Vijaysaradi Ghana Vati, Withania coagulans (Stocks) Dunal, Withania somnifera, Yashad Bhasma, and Ziziphus mauritiana Lam. (See Supplementary Appendix S7 for 89 comparisons of Ayurvedic medicines which could not be included in meta-analyses).
5.6 Safety of Interventions
Supplementary Appendix S8 reports the adverse events and dropouts/withdrawals/discontinued interventions due to adverse events in the included studies. Adverse events were not reported in many studies. If reported, these were mostly none to mild and predominately related to the gastrointestinal tract. However, in the majority of cases, the relationship between the intervention and adverse events was not provided.
6 Discussions
Beneficial effects of several Ayurvedic medicines on T2DM-related outcomes, including blood glucose, were found. The reduction in HbA1c of at least 0.3% or 0.4% is considered to be clinically meaningful, (US Food and Drug Administration, 2008) and a number of Ayurvedic medicines were found to bring such reduction such as Aegle marmelos (L.) Corrêa, Boswellia serrata Roxb., Gynostemma pentaphyllum (Thunb.) Makino, Momordica charantia L., Nigella sativa L., Plantago ovata Forssk., Tinospora cordifolia (Willd.) Hook.f. & Thomson, Trigonella foenum-graecum L., and Urtica dioica L. compared to control. Similarly, FBG was reduced by 4–56 mg/dl for a range of Ayurvedic medicines. However, the majority of studies did not assess HRQoL, an important patient-reported outcome. Adverse events were not reported in many studies. If reported, these were mostly none to mild and predominantly related to the gastrointestinal tract. The findings are consistent with several systematic reviews conducted on single herbs as well as Ayurveda as a whole system. (Hardy et al., 2001; Yeh et al., 2003; Shekelle et al., 2005; Nahas and Moher, 2009; Shojaii et al., 2011; Sridharan et al., 2011; Suksomboon et al., 2011; Akilen et al., 2012; Allen et al., 2013; Gibb et al., 2015; Namazi et al., 2019; Peter et al., 2019; Jalali et al., 2020; Jamali et al., 2020) It should be noted that the majority of these Ayurvedic medicines are already in use in many countries, and many are used as dietary ingredients such as spices or foods. In many countries, Ayurvedic medicines are available over-the-counter (which includes online shopping) and are considered as dietary supplements. (Chattopadhyay et al., 2020b) Ayurveda is now recognized in 17 countries, including in and beyond South Asia. (Press Information Bureau, 2021) The integration of Ayurveda and Western medicine has been done in India. (Priya and Shweta, 2010) Many single herbs included in this review are not restricted to Ayurveda but are also used in other traditional therapies around the world such as Iranian traditional medicine and traditional Chinese medicine. Similarly, many traditional therapies use multi-ingredient medicines such as Unani (Graeco-Arabic), Siddha (from Southern part of India), traditional Chinese medicine, and Russian traditional medicine. (Chattopadhyay and Bochenek, 2008; Li et al., 2014; Shikov et al., 2021).
The Cochrane systematic review was conducted a decade ago and focused on multiherbal formulations and Ayurveda as a whole system and excluded single herbs and their extracts. (Sridharan et al., 2011) In this review, classical and proprietary Ayurvedic medicines in any form were included (containing plant- as well as mineral-origin ingredients–single or in combination). Many Ayurveda experts and Ayurvedic practitioners may view the inclusion of herb extracts and proprietary Ayurvedic medicines in this review as a deviation from the classical style of management. However, in reality, many Ayurvedic practitioners prescribe, and many people consume these types of medicines. Similarly, Ayurveda experts and Ayurvedic practitioners may view the exclusion of Ayurvedic detoxifying and purifying therapies (e.g., Panchakarma) in this review as a deviation from the classical style of management. However, considering the feasibility and practicality of the review work, these were beyond the scope of this review. The focus of this review was on Ayurvedic medicines, as these are commonly prescribed and consumed. Having said that, the future review work should consider synthesizing evidence on the effectiveness and safety of such complex interventions.
Overall, the methodology was not adequately reported in the studies, and this resulted in poor methodological quality scoring. The assessment of methodological quality is subjective to a large extent, and the reviewers were strict. For example, other systematic reviewers might be satisfied if the differences between study arms in terms of their follow-up are described. However, the reviewers went a step further and were expecting these to be analyzed. The strictness is one of the reasons for poor methodological quality scoring. In addition, if the funding statement was provided, it was mostly brief and difficult to determine the level of support received from pharmaceutical companies. For example, it was not always clear if a pharmaceutical company provided the trial medicines for free or these were purchased. Therefore, it was difficult to determine the funding bias i.e., the tendency of a study to support the interests of the study’s financial sponsor.
This systematic review has several strengths and weaknesses. To the best of our knowledge, this was the first comprehensive systematic review on any traditional medicine including Ayurveda, and which included a wide range of classical and proprietary Ayurvedic medicines in any form (containing plant- as well as mineral-origin ingredients–single or in combination). A large number of sources and databases were searched, without any date or language restrictions. An extensively robust methodology was followed to conduct this review. Although the information provided in the studies was at times confusing, the reviewers tried their best to extract the correct information. Two independent reviewers were involved throughout the process, and a third reviewer cross-checked everything. The kappa statistic was within the acceptable range i.e., 0.67 and 0.57 for the title and abstract screening and full text screening, respectively. A multi-disciplinary team was involved in the review, with expertise in Ayurveda, medicinal plants, diabetes, systematic reviewing, and statistics. The initial plan was to perform a range of sensitivity and subgroup analyses. However, many of these could not be performed. For example, complete information on commercial funding or other support was needed to correctly conduct the sensitivity analysis by excluding commercially funded studies. However, it was unclear in 43% of RCTs. Some of the issues were outside the scope of this review, and the evidence should be synthesized in future reviews to decide the optimal option. For example, comparison of two or more drug manufacturing processes, forms or timings of administration, doses, and Anupans of the same Ayurvedic medicine. Similarly, many other factors, such as patients’ age, sex, ethnicity, lifestyle (e.g., diet and physical activity), chronicity and severity of T2DM, and comorbidities, can influence the outcomes. However, due to limited data for some comparison, we were not able to conduct separate subgroup analyses to explore the potential influence of these factors. Apart from the issues highlighted in this review, there are some basic issues which were beyond its scope and need addressing as well. For example, standardization and quality control of Ayurvedic medicines. (Chattopadhyay and Bochenek, 2008)
7 Conclusion
The current evidence suggests the benefit of a range of Ayurvedic medicines in improving glycemic control in T2DM patients. This evidence base and more specifically the Summary of Findings table will be used to develop a clinical guideline for managing T2DM by Ayurvedic practitioners. Given the limitations of the available evidence and to strengthen the evidence base, high-quality RCTs should be conducted and reported.
8 Recommendations for Practice
There is a need to develop, evaluate, and implement need-sensitive, evidence-based interventions to manage T2DM among different population groups. (Chattopadhyay and Leonardi-Bee, 2021) Based on the best available evidence as found in this systematic review and more specifically the Summary of Findings table, a clinical guideline for managing T2DM by Ayurvedic practitioners will now be systematically developed. The next steps will be guided by the GRADE approach, the United Kingdom’s National Institute for Health and Care Excellence (NICE) manual for developing guidelines and the Appraisal of Guidelines for Research and Evaluation (AGREE) II instrument. (Brouwers et al., 2010; Schünemann et al., 2013; National Institute for Health and Care Excellence, 2014) Clinical guidelines for managing T2DM by Ayurvedic practitioners exist. (Central Council for Research in Ayurvedic Sciences, 2011; Ministry of Ayush, 2016; Ministry of Health and Family Welfare, 2016; Central Council for Research in Ayurvedic Sciences, 2017; Central Council for Research in Ayurvedic Sciences and Directorate General of Health Services, 2018) However, their quality is questionable due to several factors, including whether the best available evidence was considered. Low-quality clinical guidelines can lead to the use of ineffective interventions, inefficient use of scarce resources, and most importantly, harm to patients. (Institute of Medicine, 2011) The goal is to deter the usage of Ayurvedic medicines of no, minimal, or questionable value and promote the usage of effective and safe Ayurvedic medicines.
9 Recommendations for Research
In terms of recommendation for research, the future RCTs must address the following issues to strengthen the evidence base: 1) true randomization should be used to assign participants to study arms; 2) allocation concealment should be done to conceal the allocation to study arms; 3) adequate blinding should be done after considering who will be blinded and how blinding will be performed; 4) if placebo is used then it should be identical to the intervention, not only look and texture wise but also taste and smell wise, so that it is hard to differentiate from the intervention; 5) study arms should be treated identically other than the intervention of interest; 6) outcomes should be measured in the same way for study arms; 7) the sample size should be calculated based on appropriate components, such as primary outcome and minimum clinically important difference, and the trial should be adequately powered; 8) data should be analysed properly (including ITT analysis and post-intervention difference in outcomes between study arms); and 9) differences between study arms in terms of their follow-up should be described as well as analyzed. It should be noted that comprehensive and transparent reporting of the trial methods, funding, and results is important after designing and conducting a high-quality RCT. Clinical trial registration and prior publication of the trial protocol are also important. In order to update the clinical guideline in the future, robust RCTs should be conducted not only on those Ayurvedic medicines that were included in meta-analyses but also on those on which meta-analysis was not possible. It would be beneficial if the comparison is also made with the standard treatment (i.e., OAD). T2DM is a chronic condition, and so, long-term studies are needed to determine the effectiveness and safety of these Ayurvedic medicines, especially in terms of preventing macro- or micro-vascular complications of T2DM and death. In addition, a sufficiently detailed description of the botanical and phytochemical aspects of the study material should be provided.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
KC conceptualized and designed the systematic review with the help of JP, MH, SL, SG, NT, TB, SK, and JL-B. KC, HW, JK, GN, AA, BK, and JL-B conducted the systematic review. KC wrote the first draft of the manuscript. HW, JK, GN, AA, BK, JP, MH, SL, SG, NT, TB, SK, and JL-B contributed significantly to the revision of the manuscript. All authors read and approved the final manuscript.
Funding
This systematic review was part of a project ‘‘Introduction of a clinical guideline to manage type-2 diabetes by Ayurvedic practitioners in Nepal: intervention development and feasibility study,’’ funded by a grant from the United Kingdom’s Department of Health and Social Care; Foreign, Commonwealth and Development Office; Medical Research Council; and Wellcome Trust Joint Global Health Trials (MR/T003537/1). The funding agencies had no role in designing the study or in writing the paper.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor AS and reviewer ONP declared a past co-authorship with the author MH.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Douglas Grindlay, information specialist at the University of Nottingham (United Kingdom), for contributing to the search strategies.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.821810/full#supplementary-material
References
Abdoli, M., Dabaghian, F. H., Goushegir, A., Shirazi, M. T., Nakhjavani, M., Shojaii, A., et al. (2017). Anti-hyperglycemic Effect of Aqueous Extract of Juglans regia L. Leaf (Walnut Leaf) on Type 2 Diabetic Patients: A Randomized Controlled Trial. Adv. Integr. Med. 4 (3), 98–102. doi:10.1016/j.aimed.2017.10.001
Abutair, A. S., Naser, I. A., and Hamed, A. T. (2016). Soluble Fibers from Psyllium Improve Glycemic Response and Body Weight Among Diabetes Type 2 Patients (Randomized Control Trial). Nutr. J. 15 (1), 86. doi:10.1186/s12937-016-0207-4
Adab, Z., Eghtesadi, S., Vafa, M. R., Heydari, I., Shojaii, A., Haqqani, H., et al. (2019). Effect of Turmeric on Glycemic Status, Lipid Profile, Hs-CRP, and Total Antioxidant Capacity in Hyperlipidemic Type 2 Diabetes Mellitus Patients. Phytother. Res. 33 (4), 1173–1181. doi:10.1002/ptr.6312
Adibian, M., Hodaei, H., Nikpayam, O., Sohrab, G., Hekmatdoost, A., and Hedayati, M. (2019). The Effects of Curcumin Supplementation on High-Sensitivity C-Reactive Protein, Serum Adiponectin, and Lipid Profile in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytother. Res. 33 (5), 1374–1383. doi:10.1002/ptr.6328
Agarwal, V. (2013). A Clinical Study on the Effectiveness of DM II Herbal Compound (Kalpit) in the Management of Obese Diabetics. Glob. J. Res. Med. Plants Indigen. Med. 2 (1), 40–51.
Aghasi, M., Koohdani, F., Qorbani, M., Nasli-Esfahani, E., Ghazi-Zahedi, S., Khoshamal, H., et al. (2019). Beneficial Effects of Green Cardamom on Serum SIRT1, Glycemic Indices and Triglyceride Levels in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo Controlled Clinical Trial. J. Sci. Food Agric. 99 (8), 3933–3940. doi:10.1002/jsfa.9617
Agrawal, R. P., Sharma, A., Dua, A. S., Chandershekhar, , Kochar, D. K., and Kothari, R. P. (2002). A Randomized Placebo Controlled Trial of Inolter (Herbal Product) in the Treatment of Type 2 Diabetes. J. Assoc. Physicians India 50, 391–393.
Akilen, R., Tsiami, A., Devendra, D., and Robinson, N. (2010). Glycated Haemoglobin and Blood Pressure-Lowering Effect of Cinnamon in Multi-Ethnic Type 2 Diabetic Patients in the UK: A Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Diabet. Med. 27 (10), 1159–1167. doi:10.1111/j.1464-5491.2010.03079.x
Akilen, R., Tsiami, A., Devendra, D., and Robinson, N. (2012). Cinnamon in Glycaemic Control: Systematic Review and Meta Analysis. Clin. Nutr. 31 (5), 609–615. doi:10.1016/j.clnu.2012.04.003
Allen, R. W., Schwartzman, E., Baker, W. L., Coleman, C. I., and Phung, O. J. (2013). Cinnamon Use in Type 2 Diabetes: An Updated Systematic Review and Meta-Analysis. Ann. Fam. Med. 11 (5), 452–459. doi:10.1370/afm.1517
Amini, M., Abdi, A., and Abbassi-Daloii, A. (2020). Effects of Moderate-Intensity Exercise with Momordica charantia L. Consumption on Serum Reverse Cholesterol Transport Elements and Lipid Profile in Men with Type 2 Diabetes. Feyz 24 (4), 374–386.
Ansari, R., and Ansari, S. (2011). “Chapter 20: Effectiveness of Fenugreek for Lowering Hemoglobin (HbA1c) in Patients with Self-Management of Type 2 Diabetes: A Randomized, Controlled Trial,” in Medical Complications of Type 2 Diabetes. Editor C. Croniger (London: IntechOpen).
Arablou, T., Aryaeian, N., Valizadeh, M., Sharifi, F., Hosseini, A., and Djalali, M. (2014). The Effect of Ginger Consumption on Glycemic Status, Lipid Profile and Some Inflammatory Markers in Patients with Type 2 Diabetes Mellitus. Int. J. Food Sci. Nutr. 65 (4), 515–520. doi:10.3109/09637486.2014.880671
Arablou, T., Aryaeian, N., Valizadeh, M., Hosseini, A. F., and Djalali, M. (2014). The Effect of Ginger Consumption on Some Cardiovascular Risk Factors in Patients with Type 2 Diabetes Mellitus. Razi J. Med. Sci. 21 (118), 1–12.
Arora, D., Goyal, M., and Agarwal, R. P. (2009). Efficacy of Aloe vera Juice Consumption on Glycemic Response in Type-2 Diabetic Patients. J. Food Sci. Technol. 46 (2), 160–162.
Arzati, M. M., Honarvar, N. M., Saedisomeolia, A., Anvari, S., Effatpanah, M., Arzati, R. M., et al. (2017). The Effects of Ginger on Fasting Blood-Sugar, Haemoglobin A1c, and Lipid Profiles in Patients with Type 2 Diabetes. Int. J. Endocrinol. Metab. 15 (4), e57927.
Ashraf, R., Aamir, K., Shaikh, A. R., and Ahmed, T. (2005). Effects of Garlic on Dyslipidemia in Patients with Type 2 Diabetes Mellitus. J. Ayub Med. Coll. Abbottabad 17 (3), 60–64.
Ashraf, R., Khan, R. A., and Ashraf, I. (2011). Effects of Garlic on Blood Glucose Levels and HbA1c in Patients with Type 2 Diabetes Mellitus. J. Med. Plant Res. 5 (13), 2922–2928.
Ashraf, R., Khan, R. A., and Ashraf, I. (2011). Garlic (Allium sativum) Supplementation with Standard Antidiabetic Agent Provides Better Diabetic Control in Type 2 Diabetes Patients. Pak J. Pharm. Sci. 24 (4), 565–570.
Aslam, F., Iqbal, S., Nasir, M., and Anjum, A. A. (2018). White Sesame Seed Oil Mitigates Blood Glucose Level, Reduces Oxidative Stress, and Improves Biomarkers of Hepatic and Renal Function in Participants with Type 2 Diabetes Mellitus. J. Am. Coll. Nutr.. doi:10.1080/07315724.2018.1500183
Awasthi, H., Nath, R., Usman, K., Mani, D., Khattri, S., Nischal, A., et al. (2015). Effects of a Standardized Ayurvedic Formulation on Diabetes Control in Newly Diagnosed Type-2 Diabetics; A Randomized Active Controlled Clinical Study. Complement. Ther. Med. 23 (4), 555–561. doi:10.1016/j.ctim.2015.06.005
Azadmehr, A., Ziaee, A., Ghanei, L., Fallah Huseini, H., Hajiaghaee, R., Tavakoli-Far, B., et al. (2014). A Randomized Clinical Trial Study: Anti-Oxidant, Anti-Hyperglycemic and Anti-Hyperlipidemic Effects of Olibanum Gum in Type 2 Diabetic Patients. Iran J. Pharm. Res. 13 (3), 1003–1009.
Azimi, P., Ghiasvand, R., Feizi, A., Hariri, M., and Abbasi, B. (2014). Effects of Cinnamon, Cardamom, Saffron, and Ginger Consumption on Markers of Glycemic Control, Lipid Profile, Oxidative Stress, and Inflammation in Type 2 Diabetes Patients. Rev. Diabet Stud. 11 (3-4), 258–266. doi:10.1900/RDS.2014.11.258
Azimi, P., Ghiasvand, R., Feizi, A., Hosseinzadeh, J., Bahreynian, M., Hariri, M., et al. (2016). Effect of Cinnamon, Cardamom, Saffron and Ginger Consumption on Blood Pressure and a Marker of Endothelial Function in Patients with Type 2 Diabetes Mellitus: A Randomized Controlled Clinical Trial. Blood Press. 25 (3), 133–140. doi:10.3109/08037051.2015.1111020
Babaeian, S., Ebrahimi-Mameghani, M., Niafar, M., and Sanaii, S. (2013). The Effect of Unsweetened Pomegranate Juice on Insulin Resistance, High Sensitivity C-Reactive Protein and Obesity Among Type 2 Diabetes Patients. J. Ardabil Univ. Med. Sci. 13 (1), 7–15.
Babiker, R., Elmusharaf, K., Keogh, M. B., Banaga, A. S. I., and Saeed, A. M. (2017). Metabolic Effects of Gum Arabic (Acacia senegal) in Patients with Type 2 Diabetes Mellitus (T2DM): Randomized, Placebo Controlled Double Blind Trial. Funct. Foods Health Dis. 7 (3), 219–231.
Babiker, R., Elmusharaf, K., Keogh, M. B., and Saeed, A. M. (2018). Effect of Gum Arabic (Acacia senegal) Supplementation on Visceral Adiposity Index (VAI) and Blood Pressure in Patients with Type 2 Diabetes Mellitus as Indicators of Cardiovascular Disease (CVD): A Randomized and Placebo-Controlled Clinical Trial. Lipids Health Dis. 17 (1), 56. doi:10.1186/s12944-018-0711-y
Balasubramaniam, D., Mitra, A., and Mahadevappa, M. (2010). Antidiabetic and Hypolipidaemic Effects of Few Common Plants Extract in Type 2 Diabetic Patients at Bengal. Int. J. Diabetes Metab. 18, 59–65.
Barghamdi, B., Ghorat, F., Asadollahi, K., Sayehmiri, K., Peyghambari, R., and Abangah, G. (2016). Therapeutic Effects of Citrullus colocynthis Fruit in Patients with Type II Diabetes: A Clinical Trial Study. J. Pharm. Bioallied Sci. 8 (2), 130–134. doi:10.4103/0975-7406.171702
Barre, D. E., Mizier-Barre, K. A., Griscti, O., and Hafez, K. (2008). High Dose Flaxseed Oil Supplementation May Affect Fasting Blood Serum Glucose Management in Human Type 2 Diabetics. J. Oleo Sci. 57 (5), 269–273. doi:10.5650/jos.57.269
Bhagat, A. (2017). Pharmaceutical Standardization of Tejashiladi Vati and its Clinical Efficacy in Madhumeha (Type-2 Diabetes Mellitus) [Dissertation on the Internet]. Jamnagar: Gujarat Ayurved University.
Bhalerao, M. S., Bolshete, P. M., Swar, B. D., Bangera, T. A., Kolhe, V. R., Tambe, M. J., et al. (2013). Use of and Satisfaction with Complementary and Alternative Medicine in Four Chronic Diseases: A Cross-Sectional Study from India. Natl. Med. J. India 26 (2), 75–78.
Bhat, A. (2012). A Comparative Clinical Study on Madhumeha W.S.R. To Non Insulin Dependent Diabetes Mellitus [Dissertation on the Internet]. Bengaluru: Rajiv Gandhi University of Health Sciences.
Bhawana, S., and Kumar, G. D. (2015). A Comparative Clinical Evaluation of the Efficacy of Madhumeha Nashini Gutika and Darvyadi Kwath in Madhumeha W.S.R. To Diabetes Mellitus. Int. J. Ayur Pharma Res. 3 (8), 11–18.
Bin Sayeed, M. S., Mostofa, A. G., Ferdous, F. M., and Islam, M. S. (2013). A Randomized, Placebo-Controlled, Crossover Study of an Herbal Preparation Containing Vernonia cinerea in the Treatment of Type 2 Diabetes. J. Altern. Complement. Med. 19 (9), 767–771. doi:10.1089/acm.2012.0063
Blevins, S. M., Leyva, M. J., Brown, J., Wright, J., Scofield, R. H., and Aston, C. E. (2007). Effect of Cinnamon on Glucose and Lipid Levels in Non Insulin-dependent Type 2 Diabetes. Diabetes Care 30 (9), 2236–2237. doi:10.2337/dc07-0098
Bramhankar, R. B., Reddy, K. R. C., Singh, O. P., and Nille, G. (2017). Therapeutic Evaluation of Lodhradi Kashaya Ghanvati in Type II Diabetes Mellitus. Asian J. Pharmaceutics 11 (2), S251–S259.
Brouwers, M. C., Kho, M. E., Browman, G. P., Burgers, J. S., Cluzeau, F., Feder, G., et al. (2010). AGREE II: Advancing Guideline Development, Reporting and Evaluation in Health Care. CMAJ 182, E839–E842. doi:10.1503/cmaj.090449
Carvalho, G. C. N., Lira-Neto, J. C. G., Araújo, M. F. M., Freitas, R. W. J. F., Zanetti, M. L., and Damasceno, M. M. C. (2020). Effectiveness of Ginger in Reducing Metabolic Levels in People with Diabetes: A Randomized Clinical Trial. Rev. Lat Am. Enfermagem 28, e3369. doi:10.1590/1518-8345.3870.3369
Central Council for Research in Ayurvedic Sciences (2011). Ayurvedic Management of Select Geriatric Disease Conditions. New Delhi: CCRAS.
Central Council for Research in Ayurvedic Sciences (2017). Guidelines for Prevention and Management of Diabetes. New Delhi: CCRAS.
Central Council for Research in Ayurvedic Sciences and Directorate General of Health Services, (2018). Integration of Ayush (Ayurveda) with National Program for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS): Guidelines and Training Manual. New Delhi: CCRAS and DGHS.
Chacko, E. (2003). Culture and Therapy: Complementary Strategies for the Treatment of Type-2 Diabetes in an Urban Setting in Kerala, India. Soc. Sci. Med. 56 (5), 1087–1098. doi:10.1016/s0277-9536(02)00105-3
Chandra, K., Jain, V., Jabin, A., Dwivedi, S., Joshi, S., Ahmad, S., et al. (2020). Effect of Cichorium intybus Seeds Supplementation on the Markers of Glycemic Control, Oxidative Stress, Inflammation, and Lipid Profile in Type 2 Diabetes Mellitus: A Randomized, Double-Blind Placebo Study. Phytother Res. 34 (7), 1609–1618. doi:10.1002/ptr.6624
Chandra, S. (2011). Status of Indian Medicine and Folk Healing: With a Focus on Benefits that the Systems Have Given to the Public. Part I. New Delhi: Department of Ayush.
Chandra, S. (2013). Status of Indian Medicine and Folk Healing: With a Focus on Integration of Ayush Medical Systems in Health Care Delivery. Part II. New Delhi: Department of Ayush.
Chattopadhyay, K., and Bochenek, T. (2008). The Position of Drugs Used in Traditional Medicine within the Indian Healthcare System. Scientific J. Healthc. Public Health Manag. VI (1), 59–64.
Chattopadhyay, K., and Leonardi-Bee, J. (2021). Type 2 Diabetes Mellitus: Answering the Call for Need-Sensitive, Evidence-Based Interventions. JBI Evid. Synth. 19 (5), 909–910. doi:10.11124/jbies-21-00113
Chattopadhyay, K., Panniyammakal, J., Biswas, T. K., Heinrich, M., Lewis, S. A., Greenfield, S. M., et al. (2020). Effectiveness and Safety of Ayurvedic Medicines in Type 2 Diabetes Mellitus Management: A Systematic Review Protocol. JBI Evid. Synth. 18 (11), 2380–2389. doi:10.11124/JBISRIR-D-19-00350
Chattopadhyay, K. (2020). “Chapter 1: Globalisation of Ayurveda: Importance of Scientific Evidence Base,” in Herbal Medicine in India: Indigenous Knowledge, Practice, Innovation and its Value. Editors S. Sen, and R. Chakraborty (Singapore: Springer).
Chuengsamarn, S., Rattanamongkolgul, S., Phonrat, B., Tungtrongchitr, R., and Jirawatnotai, S. (2014). Reduction of Atherogenic Risk in Patients with Type 2 Diabetes by Curcuminoid Extract: A Randomized Controlled Trial. J. Nutr. Biochem. 25 (2), 144–150. doi:10.1016/j.jnutbio.2013.09.013
Cohen, J. (1988). Statistical Power Analysis for the Behavioral Sciences. 2nd ed.. Hillsdale, NJ: Lawrence Erlbaum Associates.
Cortez-Navarrete, M., Martínez-Abundis, E., Pérez-Rubio, K. G., González-Ortiz, M., and Méndez-Del Villar, M. (2018). Momordica charantia Administration Improves Insulin Secretion in Type 2 Diabetes Mellitus. J. Med. Food 21 (7), 672–677. doi:10.1089/jmf.2017.0114
Crawford, P. (2009). Effectiveness of Cinnamon for Lowering Hemoglobin A1C in Patients with Type 2 Diabetes: A Randomized, Controlled Trial. J. Am. Board Fam. Med. 22 (5), 507–512. doi:10.3122/jabfm.2009.05.080093
Dabagh, S., and Nikbakht, M. (2016). Glycemic Control by Exercise and Urtica dioica Supplements in Men with Type 2 Diabetes. Jundishapur J. Chronic Dis. Care 5 (1), e31745. doi:10.17795/jjcdc-31745
Dadvar, N., Ghalavand, A., Zakerkish, M., Hojat, S., Alijani, E., and Mahmoodkhanikooshkaki, R. (2017). The Effect of Aerobic Training and Urtica dioica on Lipid Profile and Fasting Blood Glucose in Middle Age Female with Type II Diabetes. Jundishapur Sci. Med. J. 15 (6), 507–516.
Dans, A. M., Villarruz, M. V., Jimeno, C. A., Javelosa, M. A., Chua, J., Bautista, R., et al. (2007). The Effect of Momordica charantia Capsule Preparation on Glycemic Control in Type 2 Diabetes Mellitus Needs Further Studies. J. Clin. Epidemiol. 60 (6), 554–559. doi:10.1016/j.jclinepi.2006.07.009
de Sousa, D. F., Araújo, M. F. M., de Mello, V. D., Damasceno, M. M. C., and Freitas, R. W. J. F. (2020). Cost-effectiveness of Passion Fruit Albedo Versus Turmeric in the Glycemic and Lipaemic Control of People with Type 2 Diabetes: Randomized Clinical Trial. J. Am. Coll. Nutr., 1–10. doi:10.1080/07315724.2020.1823909
Dehghan, F., Soori, R., Gholami, K., Abolmaesoomi, M., Yusof, A., Muniandy, S., et al. (2016). Purslane (Portulaca oleracea) Seed Consumption and Aerobic Training Improves Biomarkers Associated with Atherosclerosis in Women with Type 2 Diabetes (T2D). Sci. Rep. 6, 37819. doi:10.1038/srep37819
Desale, V. (2018). Comparative Pharmaceutico-Pharmacological Profile of Trinshati and Shashti Puta Naga Bhasma and Their Efficacy in the Management of Madhumeha (Type–II DM) [dissertation on the Internet]. Jamnagar: Gujarat Ayurved University.
Deshpande, S., and Jadhav, K. (2018). Efficacy and Safety of Vidangadi Yoga (Ayurvedic Polyherbal Medicine) in Type 2 Diabetes Mellitus: A Randomized Controlled Clinical Study. Clin. Trials Degener Dis. 3 (4), 123–129. doi:10.4103/2542-3975.248011
Ebrahimi, F., Sahebkar, A., Aryaeian, N., Pahlavani, N., Fallah, S., Moradi, N., et al. (2019). Effects of Saffron Supplementation on Inflammation and Metabolic Responses in Type 2 Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Diabetes Metab. Syndr. Obes. 12, 2107–2115. doi:10.2147/DMSO.S216666
El-Sayed, M. I. (2011). Effects of Portulaca oleracea L. Seeds in Treatment of Type-2 Diabetes Mellitus Patients as Adjunctive and Alternative Therapy. J. Ethnopharmacol 137 (1), 643–651. doi:10.1016/j.jep.2011.06.020
Esfanjani, A. T., Namazi, N., and Bahrami, A. (2012). Effect of Hydro-Alcoholic Nettle Extract on Lipid Profiles and Blood Pressure in Type 2 Diabetes Patients. Iranian J. Endocrinol. Metab. 13 (5), 449–458.
Esfanjani, A. T., Namazi, N., Bahrami, A., and Ehteshami, M. (2012). Effect of Hydroalcoholic Extract of Nettle (Urtica dioica) on Glycemic index and Insulin Resistance index in Type 2 Diabetic Patients. Iranian J. Endocrinol. Metab. 13 (6), 561–568.
European Medicines Agency, (2018). Guideline on Clinical Investigation of Medicinal Products in the Treatment or Prevention of Diabetes Mellitus. London: EMA.
Faghihimani, Z., Mirmiran, P., Sohrab, G., Iraj, B., and Faghihimani, E. (2016). Effects of Pomegranate Seed Oil on Metabolic State of Patients with Type 2 Diabetes Mellitus. Int. J. Prev. Med. 7, 124. doi:10.4103/2008-7802.194883
Farzanegi, P. (2014). Impact of the Synchronization of Portulaca oleracea and Aerobic Training on Levels of MMP2 and MMP9 and TIMP1 in Diabetic Women Type II. Res. Mol. Med. (Rmm) 2 (2), 34–39. doi:10.18869/acadpub.rmm.2.2.34
Fatima, N., Usharani, P., and Muralidhar, N. (2012). Randomized Double Blind Placebo Controlled Study to Evaluate the Effect of CardiPro on Endothelial Dysfunction and Biomarkers in Patients with Type 2 Diabetes Mellitus. Innovative J. Med. Health Sci. 2 (6), 122–128.
Feinglos, M. N., Gibb, R. D., Ramsey, D. L., Surwit, R. S., and McRorie, J. W. (2013). Psyllium Improves Glycemic Control in Patients with Type-2 Diabetes Mellitus. Bioactive Carbohydrates and Dietary Fibre 1 (2), 156–161. doi:10.1016/j.bcdf.2013.02.003
Ghalavand, A., Motamedi, P., Delaramnasab, M., and Khodadoust, M. (2017). The Effect of Interval Training and Nettle Supplement on Glycemic Control and Blood Pressure in Men WithType 2 Diabetes. Int. J. Basic Sci. Med. 2 (1), 33–40. doi:10.15171/ijbsm.2017.08
Gholaman, M., and Gholami, M. A. (2018). Effect of Eight Weeks' Endurance Training along with Fenugreek Ingestion on Lipid Profile, Body Composition, Insulin Resistance and VO2max in Obese Women's with Type 2 Diabetes. J. Med. Plants 17 (65), 83–92.
Gholinezhad, H., Bazyar, H., Rashidi, H., Salehi, P., Haghighi-Zadeh, M. H., and Zare Javid, A. (2020). Using Ginger Supplement in Adjunct with Non-surgical Periodontal Therapy Improves Metabolic and Periodontal Parameters in Patients with Type 2 Diabetes Mellitus and Chronic Periodontitis: A Double-Blind, Placebo-Controlled Trial. J. Herbal Med. 20, 100315. doi:10.1016/j.hermed.2019.100315
Gibb, R. D., McRorie, J. W., Russell, D. A., Hasselblad, V., and D'Alessio, D. A. (2015). Psyllium Fiber Improves Glycemic Control Proportional to Loss of Glycemic Control: A Meta-Analysis of Data in Euglycemic Subjects, Patients at Risk of Type 2 Diabetes Mellitus, and Patients Being Treated for Type 2 Diabetes Mellitus. Am. J. Clin. Nutr. 102 (6), 1604–1614. doi:10.3945/ajcn.115.106989
Godatwar, P. V. (2019). A Randomized, multi-center, Double Blind, Placebo Controlled, Prospective Clinical Study to Evaluate the Efficacy and Safety of AYUBES Capsule as an Add-On Therapy to Oral Hypoglycemic Agents (OHA) in Type 2 Diabetic Patients. CTRI/2017/09/009765. India: Clinical Trials Registry.
Gopalakrishna, R. N., Bannimath, G., and Huded, S. P. (2017). Herb-drug Interaction: Effect of Poly-Herbal Formulation on Glibenclamide Therapy in Patients with Type-2 Diabetes Mellitus. Pharm. Methods 8 (1), 62–70.
Grabež, M., Škrbić, R., Stojiljković, M. P., Rudić-Grujić, V., Paunović, M., Arsić, A., et al. (2020). Beneficial Effects of Pomegranate Peel Extract on Plasma Lipid Profile, Fatty Acids Levels and Blood Pressure in Patients with Diabetes Mellitus Type-2: A Randomized, Double-Blind, Placebo-Controlled Study. J. Funct. Foods 64, 103692.
Gupta, A., Gupta, R., and Lal, B. (2001). Effect of Trigonella foenum-graecum (Fenugreek) Seeds on Glycaemic Control and Insulin Resistance in Type 2 Diabetes Mellitus: A Double Blind Placebo Controlled Study. J. Assoc. Physicians India 49, 1057–1061.
Gupta, V., Keshari, B. B., Tiwari, S. K., and Murthy, K. H. H. V. S. S. N. (2016). A Comparative Study of Shilajatu and Asanadi Ghana Vati in the Management of Madhumeha W.S.R. To Type-2 Diabetes Mellitus. Ayu 37 (2), 120–124. doi:10.4103/ayu.AYU_211_15
Gupta, B. P., Sharma, I., Kohli, N., Sharma, S., Rathi, A., and Sharma, A. K. (2018). Preliminary Clinical Assessment and Non-Toxicity Evaluation of an Ayurvedic Formulation BGR-34 in NIDDM. J. Tradit Complement. Med. 8 (4), 506–514. doi:10.1016/j.jtcme.2017.11.004
Hadi, S., Mirmiran, P., Hosseinpour-Niazi, S., Hedayati, M., and Azizi, F. (2015). Effect of Nigella sativa Oil Extract on Lipid Profiles in Type 2 Diabetic Patients: A Randomized, Double Blind, Placebo-Controlled Clinical Trial. Iranian J. Endocrinol. Metab. 16 (6), 411–418.
Hadi, A., Arab, A., Hajianfar, H., Talaei, B., Miraghajani, M., Babajafari, S., et al. (2020). The Effect of Fenugreek Seed Supplementation on Serum Irisin Levels, Blood Pressure, and Liver and Kidney Function in Patients with Type 2 Diabetes Mellitus: A Parallel Randomized Clinical Trialffect of Fenugreek Seed Supplementation on Serum Irisin Levels, Blood Pressure, and Liver and Kidney Function in Patients with Type 2 Diabetes Mellitus: A Parallel Randomized Clinical Trial. Complement. Ther. Med. 49, 102315. doi:10.1016/j.ctim.2020.102315
Haidari, F., Zakerkish, M., Borazjani, F., Ahmadi Angali, K., and Amoochi Foroushani, G. (2020). The Effects of Anethum graveolens (Dill) Powder Supplementation on Clinical and Metabolic Status in Patients with Type 2 Diabetes. Trials 21 (1), 483. doi:10.1186/s13063-020-04401-3
Hardy, M. L., Coulter, I., Venuturupalli, S., Roth, E. A., Favreau, J., Morton, S. C., et al. (2001). Ayurvedic Interventions for Diabetes Mellitus: A Systematic Review. Evid. Rep. Technol. Assess. (Summ) 41, 2p.
Hariharan, R. S., Venkataraman, S., Sunitha, P., Rajalakshmi, S., Samal, K. C., Routray, B. M., et al. (2005). Efficacy of Vijayasar (Pterocarpus marsupium) in the Treatment of Newly Diagnosed Patients with Type 2 Diabetes Mellitus: A Flexible Dose Double-Blind Multicenter Randomized Controlled Trial. Diabetologia Croatica 34 (1), 13–20.
Hasanzade, F., Toliat, M., Emami, S. A., and Emamimoghaadam, Z. (2013). The Effect of Cinnamon on Glucose of Type II Diabetes Patients. J. Tradit Complement. Med. 3 (3), 171–174. doi:10.4103/2225-4110.114900
Hashemi, M. S., Namiranian, N., Tavahen, H., Dehghanpour, A., Rad, M. H., Jam-Ashkezari, S., et al. (2020). Efficacy of Pomegranate Seed Powder on Glucose and Lipid Metabolism in Patients with Type 2 Diabetes: A Prospective Randomized Double-Blind Placebo-Controlled Clinical Trial. Complement. Med. Res.. doi:10.1159/000510986
Hashemzadeh, A. A., Nasoohi, N., Raygan, F., Aghadavod, E., Akbari, E., Taghizadeh, M., et al. (2017). Flaxseed Oil Supplementation Improve Gene Expression Levels of PPAR-γ, LP(a), IL-1 and TNF-α in Type 2 Diabetic Patients with Coronary Heart Disease. Lipids 52 (11), 907–915. doi:10.1007/s11745-017-4295-5
Hassani, A., Ebrahimi, M., and Ramezanpoor, M. R. (2016). Survey on the Effect of Eight Weeks of Regular Aerobic Exercise with Consumption of Nettle Extract on Blood Glucose and Insulin Resistance index Among Women with Type II Diabetes. J. Knowledge Health 10 (4), 57–64.
Hassani, S. S., Fallahi, A., Esmaeili, S. S., and Gholami Fesharaki, M. (2019). The Effect of Combined Therapy with Fenugreek and Nutrition Training Based on Iranian Traditional Medicine on FBS, HgA1c, BMI, and Waist Circumference in Type 2 Diabetic Patients: A Randomized Double Blinded Clinical Trial. J. Adv. Med. Biomed. Res. 27 (120), 37–42. doi:10.30699/jambs.27.120.37
Hemalatha, V., and Vigasini, N. (2018). Effect of Supplementation of Withania coagulans Fruit on the Fasting Plasma Glucose Levels of Type 2 Diabetic Subjects. Asian J. Pharm. Clin. Res. 11 (12), 448–451.
Hendre, A. S., Sontakke, A. V., Phatak, R. S., Patil, S. R., and Jadhav, S. T. (2020). Role of Cumin in Management of Type 2 Diabetes Mellitus with Respect to its Antidiabetic and Antioxidant Property. Ijrps 11 (3), 4157–4161. doi:10.26452/ijrps.v11i3.2622
Heshmati, J., Namazi, N., Memarzadeh, M. R., Taghizadeh, M., and Kolahdooz, F. (2015). Nigella sativa Oil Affects Glucose Metabolism and Lipid Concentrations in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Food Res. Int. 70, 87–93. doi:10.1016/j.foodres.2015.01.030
Higgins, J. P. T., Li, T., and Deeks, J. J. (2019). “Chapter 6: Choosing Effect Measures and Computing Estimates of Effect,” in Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0. Editors J. P. T. Higgins, J. Thomas, J. Chandler, M. Cumpston, T. Li, M. J. Page, and V. A. Welch (Cochrane).
Hosseini, M. S., Mirkarimi, S. A., Amini, M., Mohtashami, R., Kianbakht, S., and Huseini, H. F. (2013). Effects of Nigella sativa L. Seed Oil in Type II Diabetic Patients: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Med. Plants 12 (47), 93–99.
Hosseini, S., Huseini, H. F., Larijani, B., Mohammad, K., Najmizadeh, A., Nourijelyani, K., et al. (2014). The Hypoglycemic Effect of Juglans regia Leaves Aqueous Extract in Diabetic Patients: A First Human Trial. Daru 22 (1), 19. doi:10.1186/2008-2231-22-19
Hosseini, S., Jamshidi, L., Mehrzadi, S., Mohammad, K., Najmizadeh, A. R., Alimoradi, H., et al. (2014). Effects of Juglans regia L. Leaf Extract on Hyperglycemia and Lipid Profiles in Type Two Diabetic Patients: A Randomized Double-Blind, Placebo-Controlled Clinical Trial. J. Ethnopharmacol. 152 (3), 451–456. doi:10.1016/j.jep.2014.01.012
Hota, D., Maiti, R., Padhy, B. M., Dey, A., Patro, B. K., Kumar, R., et al. (2019). Clinical Evaluation of Fenugreek Seed Extract, a Nutraceutical in Patients with Type-2 Diabetes: An Add-On Study [Report on the Internet]. Bhubaneswar: All India Institute of Medical Sciences.
Hsia, S. H., Bazargan, M., and Davidson, M. B. (2004). Effect of Pancreas Tonic (An Ayurvedic Herbal Supplement) in Type 2 Diabetes Mellitus. Metabolism 53 (9), 1166–1173. doi:10.1016/j.metabol.2004.04.007
Hsu, C. H., Liao, Y. L., Lin, S. C., Tsai, T. H., Huang, C. J., and Chou, P. (2011). Does Supplementation with Green Tea Extract Improve Insulin Resistance in Obese Type 2 Diabetics? A Randomized, Double-Blind, and Placebo-Controlled Clinical Trial. Altern. Med. Rev. 16 (2), 157–163.
Huseini, H. F., Darvishzadeh, F., Heshmat, R., Jafariazar, Z., Raza, M., and Larijani, B. (2009). The Clinical Investigation of Citrullus colocynthis (L.) Schrad Fruit in Treatment of Type II Diabetic Patients: A Randomized, Double Blind, Placebo-Controlled Clinical Trial. Phytother. Res. 23 (8), 1186–1189. doi:10.1002/ptr.2754
Huseini, H. F., Kianbakht, S., Hajiaghaee, R., and Dabaghian, F. H. (2012). Anti-Hyperglycemic and Anti-Hypercholesterolemic Effects of Aloe vera Leaf Gel in Hyperlipidemic Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Planta Med. 78 (4), 311–316. doi:10.1055/s-0031-1280474
Huseini, H. F., Kianbakht, S., Hajiaghaee, R., Afkhami-Ardekani, M., Bonakdaran, A., and Dabaghian, F. H. (2012). Aloe vera Leaf Gel in Treatment of Advanced Type 2 Diabetes Mellitus Needing Insulin Therapy: A Randomized Double-Blind Placebo-Controlled Clinical Trial. J. Med. Plants 11 (43), 19–27.
Huseini, H. F., Hasani-Rnjbar, S., Nayebi, N., Heshmat, R., Sigaroodi, F. K., Ahvazi, M., et al. (2013). Capparis spinosa L. (Caper) Fruit Extract in Treatment of Type 2 Diabetic Patients: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Complement. Ther. Med. 21 (5), 447–452. doi:10.1016/j.ctim.2013.07.003
Huyen, V. T., Phan, D. V., Thang, P., Hoa, N. K., and Ostenson, C. G. (2010). Antidiabetic Effect of Gynostemma pentaphyllum Tea in Randomly Assigned Type 2 Diabetic Patients. Horm. Metab. Res. 42 (5), 353–357. doi:10.1055/s-0030-1248298
Huyen, V. T., Phan, D. V., Thang, P., Ky, P. T., Hoa, N. K., and Ostenson, C. G. (2012). Antidiabetic Effects of Add-On Gynostemma pentaphyllum Extract Therapy with Sulfonylureas in Type 2 Diabetic Patients. Evid. Based Complement. Alternat Med. 2012, 452313. doi:10.1155/2012/452313
Inayat U Rahman, I. U., Khan, R. U., Khalil Ur Rahman, K. U., and Bashir, M. (2015). Lower Hypoglycemic but Higher Antiatherogenic Effects of Bitter Melon Than Glibenclamide in Type 2 Diabetic Patients. Nutr. J. 14, 13. doi:10.1186/1475-2891-14-13
Institute of Medicine (2011). Clinical Practice Guidelines We Can Trust. Washington DC: The National Academies Press.
Jafari, S., Sattari, R., and Ghavamzadeh, S. (2017). Evaluation the Effect of 50 and 100 Mg Doses of Cuminum cyminum Essential Oil on Glycemic Indices, Insulin Resistance and Serum Inflammatory Factors on Patients with Diabetes Type II: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Tradit. Complement. Med. 7 (3), 332–338. doi:10.1016/j.jtcme.2016.08.004
Jalali, R., Mahmoodi, M., Moosavian, S. P., Ferns, G. A., and Sohrabi, Z. (2020). Cinnamon Supplementation Improves Blood Pressure in Type 2 Diabetic Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Clin. Diabetology 9 (4), 259–266. doi:10.5603/dk.2020.0021
Jamali, N., Jalali, M., Saffari-Chaleshtori, J., Samare-Najaf, M., and Samareh, A. (2020). Effect of Cinnamon Supplementation on Blood Pressure and Anthropometric Parameters in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Clinical Trials. Diabetes Metab. Syndr. 14 (2), 119–125. doi:10.1016/j.dsx.2020.01.009
Jangjo-Borazjani, S., Dastgheib, M., Kiyamarsi, E., Jamshidi, R., Rahmati-Ahmadabad, S., Helalizadeh, M., et al. (2021). Effects of Resistance Training and Nigella sativa on Type 2 Diabetes: Implications for Metabolic Markers, Low-Grade Inflammation and Liver Enzyme Production. Arch. Physiol. Biochem., 1–9. doi:10.1080/13813455.2021.1886117
Kaatabi, H., Bamosa, A. O., Badar, A., Al-Elq, A., Abou-Hozaifa, B., Lebda, F., et al. (2015). Nigella sativa Improves Glycemic Control and Ameliorates Oxidative Stress in Patients with Type 2 Diabetes Mellitus: Placebo Controlled Participant Blinded Clinical Trial. PLoS One 10 (2), e0113486. doi:10.1371/journal.pone.0113486
Kandhare, A. D., Rais, N., Moulick, N., Deshpande, A., Thakurdesai, P., and Bhaskaran, S. (2018). Efficacy and Safety of Herbal Formulation Rich in Standardized Fenugreek Seed Extract as Add-On Supplementation in Patients with Type 2 Diabetes Mellitus on Sulfonylurea Therapy: A 12-week, Randomized, Double-Blind, Placebo-Controlled, Multi-center Study. Phcogn Mag. 14 (57), S393–S402. doi:10.4103/pm.pm_260_18
Kataria, D. K. (2017). A Comparative Study of Mamajjaka Ghanvati and Trikatu Gutika in the Management of Madhumeha (Type-2 Diabetes Mellitus) [dissertation on the Internet]. Jamnagar: Gujarat Ayurved University.
Kaur, M., Singh, N., Sharma, G., and Singh, D. (2016). To Study the Efficacy and Tolerability of Fenugreek Seed Powder as Add-On Therapy with Metformin in Patients of Type-2 Diabetes Mellitus. Int. J. Basic Clin. Pharmacol. 5 (2), 378–383. doi:10.18203/2319-2003.ijbcp20160748
Khajebishak, Y., Payahoo, L., Alivand, M., Hamishehkar, H., Mobasseri, M., Ebrahimzadeh, V., et al. (2019). Effect of Pomegranate Seed Oil Supplementation on the GLUT-4 Gene Expression and Glycemic Control in Obese People with Type 2 Diabetes: A Randomized Controlled Clinical Trial. J. Cel Physiol 234 (11), 19621–19628. doi:10.1002/jcp.28561
Khajebishak, Y., Payahoo, L., Hamishehkar, H., Alivand, M., Alipour, M., Solhi, M., et al. (2019). Effect of Pomegranate Seed Oil on the Expression of PPAR-γ and Pro-inflammatory Biomarkers in Obese Type 2 Diabetic Patients. Nutr. Food Sci. 49 (5), 854–865. doi:10.1108/nfs-10-2018-0298
Khajeh-Mehrizi, R., Mozaffari-Khosravi, H., Ghadiri-Anari, A., and Dehghani, A. (2014). The Effect of Urtica dioica Extract on Glycemic Control and Insulin Resistance Indices in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Iranian J. Diabetes Obes. 6 (4), 149–155.
Kianbakht, S., Khalighi-Sigaroodi, F., and Dabaghian, F. H. (2013). Improved Glycemic Control in Patients with Advanced Type 2 Diabetes Mellitus Taking Urtica dioica Leaf Extract: A Randomized Double-Blind Placebo-Controlled Clinical Trial. Clin. Lab. 59 (9-10), 1071–1076. doi:10.7754/clin.lab.2012.121019
Kim, S. K., Jung, J., Jung, J. H., Yoon, N., Kang, S. S., Roh, G. S., et al. (2020). Hypoglycemic Efficacy and Safety of Momordica charantia (Bitter Melon) in Patients with Type 2 Diabetes Mellitus. Complement. Ther. Med. 52, 102524. doi:10.1016/j.ctim.2020.102524
Kooshki, A., Tofighiyan, T., Rastgoo, N., Rakhshani, M. H., and Miri, M. (2020). Effect of Nigella sativa Oil Supplement on Risk Factors for Cardiovascular Diseases in Patients with Type 2 Diabetes Mellitus. Phytother Res. 34 (10), 2706–2711. doi:10.1002/ptr.6707
Korani, B., Mirzapour, A., Moghadamnia, A. A., Khafri, S., Neamati, N., and Parsian, H. (2017). The Effect of Urtica dioica Hydro-Alcoholic Extract on Glycemic Index and AMP-Activated Protein Kinase Levels in Diabetic Patients: A Randomized Single-Blind Clinical Trial. Iran Red Crescent Med. J. 19 (3), e40572.
Kumar, D., Bajaj, S., and Mehrotra, R. (2006). Knowledge, Attitude and Practice of Complementary and Alternative Medicines for Diabetes. Public Health 120 (8), 705–711. doi:10.1016/j.puhe.2006.04.010
Kumar, R., Chhatwal, S., Arora, S., Sharma, S., Singh, J., Singh, N., et al. (2013). Antihyperglycemic, Antihyperlipidemic, Anti-inflammatory and Adenosine Deaminase-Lowering Effects of Garlic in Patients with Type 2 Diabetes Mellitus with Obesity. Diabetes Metab. Syndr. Obes. 6, 49–56. doi:10.2147/DMSO.S38888
Kumar, S., Singh, G., Pandey, A. K., and Singh, R. H. (2014). A Clinical Study on the Naimittika Rasayana Effect of Silajatu and Mamajjaka in Type-2 Diabetes Mellitus. Ayu 35 (4), 404–410. doi:10.4103/0974-8520.159000
Kumari, M. W. S. J., Dave, H. H., and Kumar, B. (2016). A Critical Analysis of the Basic Principles of Stress Related Diabetes Mellitus and the Role of Counseling and Medhya Rasayana in its Management. J. Ayurveda 10 (4), 4–12.
Kumari, S., Dash, I., and Behera, K. K. (2018). Therapeutic Effect of Momordica charantia on Blood Glucose, Lipid Profile and Oxidative Stress in Type 2 Diabetes Mellitus Patients: A Randomised Controlled Trial. J. Clin. Diagn. Res. 12 (9), BC21–5. doi:10.7860/jcdr/2018/36354.12036
Kuriyan, R., Rajendran, R., Bantwal, G., and Kurpad, A. V. (2008). Effect of Supplementation of Coccinia cordifolia Extract on Newly Detected Diabetic Patients. Diabetes Care 31 (2), 216–220. doi:10.2337/dc07-1591
Kurpad, A. V., and Raj, R. (2008). The Effect of Ivy Gourd (Coccinia cordifolia) Extract on Diabetic Patients. Bull. Nutr. Found. India 29 (1), 1–5.
Kushwaha, V., and Kar, A. (2017). Clinical Study on Ayurvedic Herbal Drug (Mustadi Kwatha Ghanavati) Therapy in Patients with Type 2 Diabetes. Indian J. Traditional Knowledge 16 (Suppl. l), S40–S46.
Lasaite, L., Spadiene, A., Savickiene, N., Skesters, A., and Silova, A. (2014). The Effect of Ginkgo biloba and Camellia sinensis Extracts on Psychological State and Glycemic Control in Patients with Type 2 Diabetes Mellitus. Nat. Prod. Commun. 9 (9), 1345–1350. doi:10.1177/1934578x1400900931
Li, S., Fan, T. P., Jia, W., Lu, A., and Zhang, W. (2014). Network Pharmacology in Traditional Chinese Medicine. Evid. Based Complement. Alternat Med. 2014, 138460. doi:10.1155/2014/138460
Liu, C. Y., Huang, C. J., Huang, L. H., Chen, I. J., Chiu, J. P., and Hsu, C. H. (2014). Effects of Green Tea Extract on Insulin Resistance and Glucagon-like Peptide 1 in Patients with Type 2 Diabetes and Lipid Abnormalities: A Randomized, Double-Blinded, and Placebo-Controlled Trial. PLoS One 9 (3), e91163. doi:10.1371/journal.pone.0091163
Lu, F. R., Shen, L., Qin, Y., Gao, L., Li, H., and Dai, Y. (2008). Clinical Observation on Trigonella foenum-graecum L. Total Saponins in Combination with Sulfonylureas in the Treatment of Type 2 Diabetes Mellitus. Chin. J. Integr. Med. 14 (1), 56–60. doi:10.1007/s11655-007-9005-3
Lu, T., Sheng, H., Wu, J., Cheng, Y., Zhu, J., and Chen, Y. (2012). Cinnamon Extract Improves Fasting Blood Glucose and Glycosylated Hemoglobin Level in Chinese Patients with Type 2 Diabetes. Nutr. Res. 32 (6), 408–412. doi:10.1016/j.nutres.2012.05.003
Ludvik, B., Neuffer, B., and Pacini, G. (2004). Efficacy of Ipomoea batatas (Caiapo) on Diabetes Control in Type 2 Diabetic Subjects Treated with Diet. Diabetes Care 27 (2), 436–440. doi:10.2337/diacare.27.2.436
Ludvik, B., Hanefeld, M., and Pacini, G. (2008). Improved Metabolic Control by Ipomoea batatas (Caiapo) is Associated with Increased Adiponectin and Decreased Fibrinogen Levels in Type 2 Diabetic Subjects. Diabetes Obes. Metab. 10 (7), 586–592. doi:10.1111/j.1463-1326.2007.00752.x
MacKenzie, T., Leary, L., and Brooks, W. B. (2007). The Effect of an Extract of Green and Black Tea on Glucose Control in Adults with Type 2 Diabetes Mellitus: Double-Blind Randomized Study. Metabolism 56 (10), 1340–1344. doi:10.1016/j.metabol.2007.05.018
Mahluji, S., Attari, V. E., Mobasseri, M., Payahoo, L., Ostadrahimi, A., and Golzari, S. E. (2013). Effects of Ginger (Zingiber officinale) on Plasma Glucose Level, HbA1c and Insulin Sensitivity in Type 2 Diabetic Patients. Int. J. Food Sci. Nutr. 64 (6), 682–686. doi:10.3109/09637486.2013.775223
Mahmoud, F., Al-Ozairi, E., Haines, D., Novotny, L., Dashti, A., Ibrahim, B., et al. (2016). Effect of Diabetea Tea™ Consumption on Inflammatory Cytokines and Metabolic Biomarkers in Type 2 Diabetes Patients. J. Ethnopharmacol. 194, 1069–1077. doi:10.1016/j.jep.2016.10.073
Mang, B., Wolters, M., Schmitt, B., Kelb, K., Lichtinghagen, R., Stichtenoth, D. O., et al. (2006). Effects of a Cinnamon Extract on Plasma Glucose, HbA, and Serum Lipids in Diabetes Mellitus Type 2. Eur. J. Clin. Invest. 36 (5), 340–344. doi:10.1111/j.1365-2362.2006.01629.x
Mansouri, A., Vahed, A. S., Shahdadi, H., Dashtban, F., and Arbabisarjou, A. (2018). The Effect of Garlic and Cumin on Blood Pressure and Glycosylated Hemoglobin in Patients with Type 2 Diabetes. Bali Med. J. 7 (1), 156–160. doi:10.15562/bmj.v7i1.849
Maurya, A. K., Jain, A., Pathak, A., Chaudhary, P., and Rajdan, N. (2017). Efficacy of Vijaysar, Aloe vera Alone and Their Combination in the Treatment of Newly Diagnosed Cases of Type 2 Diabetes Mellitus: A Randomized Single Blind Prospective Study. Int. J. Basic Clin. Pharmacol. 6 (4), 962–967. doi:10.18203/2319-2003.ijbcp20171112
Mehrotra, R., Bajaj, S., and Kumar, D. (2004). Use of Complementary and Alternative Medicine by Patients with Diabetes Mellitus. Natl. Med. J. India 17 (5), 243–245.
Mehrzadi, S., Tavakolifar, B., Huseini, H. F., Mosavat, S. H., and Heydari, M. (2018). The Effects of Boswellia serrata Gum Resin on the Blood Glucose and Lipid Profile of Diabetic Patients: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Evid. Based Integr. Med. 23, 2515690X18772728–7. doi:10.1177/2515690X18772728
Memon, A. R., Memon, A. R., Shah, S. S., Khand, F., and Shaikh, I. A. (2010). Effect of Combination of Nigella sativa and Trigonella foenum-graecum Seeds with Glibenclamide on Blood Sugar Levels in Type-2 Diabetes Mellitus Patients. ISRA Med. J. 2 (2), 46–51.
Memon, A. R., Memon, A. R., Shah, S. S., Khand, F., Shaikh, I. A., and Khushk, I. A. (2010). Effect of Combination Therapy of Nigella sativa and Trigonella foenum-graecum on Body Mass index in Type-2 Diabetes Mellitus Patients. ISRA Med. J. 2 (2), 10–15.
Memon, A. R., Shah, S. S., Memon, A. R., and Naqvi, S. H. R. (2012). Effect of Combination of Nigella sativa and Trigonella foenum-graecum with Glibenclamide on Serum Triglyceride, HDL and Creatinine Levels in Type-2 Diabetes Mellitus Patients. Pakistan J. Pharmacol. 29 (1), 1–6.
Milajerdi, A., Jazayeri, S., Hashemzadeh, N., Shirzadi, E., Derakhshan, Z., Djazayeri, A., et al. (2018). The Effect of Saffron (Crocus sativus L.) Hydroalcoholic Extract on Metabolic Control in Type 2 Diabetes Mellitus: A Triple-Blinded Randomized Clinical Trial. J. Res. Med. Sci. 23, 16. doi:10.4103/jrms.JRMS_286_17
Ministry of Ayush, (2016). Protocol for Prevention and Control of Diabetes through Ayurveda. New Delhi: Ministry of Ayush.
Ministry of Health and Family Welfare (2016). Madhumeha (Diabetes mellitus). [Internet]. New Delhi: MoHFW. [Cited 2019 Jul 5]. Available at: https://www.nhp.gov.in/Madhumeha-(Diabetes-mellitus)_mtl.
Mirmiranpour, H., Huseini, H. F., Derakhshanian, H., Khodaii, Z., and Tavakoli-far, B. (2020). Effects of Probiotic, Cinnamon, and Synbiotic Supplementation on Glycemic Control and Antioxidant Status in People with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Study. J. Diabetes Metab. Disord. 19 (1), 53–60. doi:10.1007/s40200-019-00474-3
Mirzaei, K., Hossein-Nezhad, A., Karimi, M., Hosseinzadeh-Attar, M. J., Jafari, N., Najmafshar, A., et al. (2009). Effect of Green Tea Extract on Bone Turnover Markers in Type 2 Diabetic Patients: A Double-Blind, Placebo-Controlled Clinical Trial Study. Daru 17 (Suppl. 1), 38–44.
Mishra, S., Verma, N., Bhattacharya, S., Usman, K., Himanshu, D., Singh, P., et al. (2015). Effect of Tinospora cordifolia as an Add-on Therapy on the Blood Glucose Levels of Patients with Type 2 Diabetes. Int. J. Basic Clin. Pharmacol. 4 (3), 537–541. doi:10.18203/2319-2003.ijbcp20150035
Mitra, M., and Bhattacharya, D. (2006). Dose-dependent Effects of Fenugreek Composite in Diabetes with Dislipidaemia. Internet J. Food Saf. 8, 49–55.
Mobasseri, M., Payahoo, L., Ostadrahimi, A., Bishak, Y. K., Jafarabadi, M. A., and Mahluji, S. (2014). Anethum graveolens Supplementation Improves Insulin Sensitivity and Lipid Abnormality in Type 2 Diabetic Patients. Pharm. Sci. 20 (2), 40–45.
Mobasseri, M., Ostadrahimi, A., Tajaddini, A., Asghari, S., Barati, M., Akbarzadeh, M., et al. (2020). Effects of Saffron Supplementation on Glycemia and Inflammation in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind, Placebo-Controlled Clinical Trial Study. Diabetes Metab. Syndr. 14 (4), 527–534. doi:10.1016/j.dsx.2020.04.031
Mohammad Shahi, M., Zakerzadeh, M., Zakerkish, M., Zarei, M., and Saki, A. (2017). Effect of Sesamin Supplementation on Glycemic Status, Inflammatory Markers, and Adiponectin Levels in Patients with Type 2 Diabetes Mellitus. J. Diet. Suppl. 14 (1), 65–75. doi:10.1080/19390211.2016.1204404
Mohammadi, H., and Avandi, S. M. (2019). Effect of Eight Weeks Resistance Training with Ginger Supplementation on Malondialdehyde and Body Compostion index in Type 2 Diabetes Patients. Koomesh 21 (1), 73–82.
Mohammadi, H., Avandi, S. M., Jamshidi, M., and Gooya, M. (2017). Effect of Eight Weeks Resistance Training and Ginger Supplementation on Glycosylated Hemoglobin index in Type 2 Diabetes Patients. Koomesh 19 (4), 852–860.
Mohammadnia, Z., and Hassani, A. (2017). Effects of Eight Weeks Aerobic Exercise with Consumption of Nettle Extract on Hs-CRP in Inactive Middle-Aged Women with Type II Diabetes. Koomesh 19 (1), 56–63.
Mohan, V., Poongothai, S., Deepa, R., Subramanian, S. L., Nalini, K., and Murali, P. M. (2001). Efficacy of DCBT 2345: An Ayurvedic Compound in Treatment of Type 2 Diabetic Patients with Secondary Failure to Oral Drugs: Randomized Double Blind Placebo Control Study. Int. J. Diab Dev. Countries 21 (4), 176–183.
Moher, D., Liberati, A., Tetzlaff, J., and Altman, D. G., PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ 339, b2535. doi:10.1136/bmj.b2535
Moradi, A., Tarrahi, M. J., Ghasempour, S., Shafiepour, M., Clark, C. C. T., and Safavi, S. M. (2020). The Effect of Okra (Abelmoschus esculentus) on Lipid Profiles and Glycemic Indices in Type 2 Diabetic Adults: Randomized Double Blinded Trials. Phytother Res. 34 (12), 3325–3332. doi:10.1002/ptr.6782
Moravej Aleali, A., Amani, R., Shahbazian, H., Namjooyan, F., Latifi, S. M., and Cheraghian, B. (2019). The Effect of Hydroalcoholic Saffron (Crocus sativus L.) Extract on Fasting Plasma Glucose, HbA1c, Lipid Profile, Liver, and Renal Function Tests in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Clinical Trial. Phytother Res. 33 (6), 1648–1657. doi:10.1002/ptr.6351
Moustafa, H. A. M., El Wakeel, L. M., Halawa, M. R., Sabri, N. A., El-Bahy, A. Z., and Singab, A. N. (2019). Effect of Nigella sativa Oil versus Metformin on Glycemic Control and Biochemical Parameters of Newly Diagnosed Type 2 Diabetes Mellitus Patients. Endocrine 65 (2), 286–294. doi:10.1007/s12020-019-01963-4
Mozaffari-Khosravi, H., Talaei, B., Jalali, B. A., Najarzadeh, A., and Mozayan, M. R. (2014). The Effect of Ginger Powder Supplementation on Insulin Resistance and Glycemic Indices in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Complement. Ther. Med. 22 (1), 9–16. doi:10.1016/j.ctim.2013.12.017
Na, L. X., Li, Y., Pan, H. Z., Zhou, X. L., Sun, D. J., Meng, M., et al. (2013). Curcuminoids Exert Glucose-Lowering Effect in Type 2 Diabetes by Decreasing Serum Free Fatty Acids: A Double-Blind, Placebo-Controlled Trial. Mol. Nutr. Food Res. 57 (9), 1569–1577. doi:10.1002/mnfr.201200131
Nahas, R., and Moher, M. (2009). Complementary and Alternative Medicine for the Treatment of Type 2 Diabetes. Can. Fam. Physician 55 (6), 591–596.
Najdi, R. A., Hagras, M. M., Kamel, F. O., and Magadmi, R. M. (2019). A Randomized Controlled Clinical Trial Evaluating the Effect of Trigonella foenum-graecum (Fenugreek) versus Glibenclamide in Patients with Diabetes. Afr. Health Sci. 19 (1), 1594–1601. doi:10.4314/ahs.v19i1.34
Najmi, A., Nasiruddin, M., Khan, R. A., and Haque, S. F. (2012). Therapeutic Effect of Nigella sativa in Patients of Poor Glycemic Control. Asian J. Pharm. Clin. Res. 5 (3), 224–228.
Namazi, N., Esfanjani, A. T., Heshmati, J., and Bahrami, A. (2011). The Effect of Hydro Alcoholic Nettle (Urtica dioica) Extracts on Insulin Sensitivity and Some Inflammatory Indicators in Patients with Type 2 Diabetes: A Randomized Double-Blind Control Trial. Pak J. Biol. Sci. 14 (15), 775–779. doi:10.3923/pjbs.2011.775.779
Namazi, N., Khodamoradi, K., Khamechi, S. P., Heshmati, J., Ayati, M. H., and Larijani, B. (2019). The Impact of Cinnamon on Anthropometric Indices and Glycemic Status in Patients with Type 2 Diabetes: A Systematic Review and Meta-Analysis of Clinical Trials. Complement. Ther. Med. 43, 92–101. doi:10.1016/j.ctim.2019.01.002
Narasimha Raju, K. V., and Sharma, R. S. (2016). A Comparative Clinical Study of Vamana and Virechana with and without Shilajit Yoga in Management of Madhumeha W.S.R. To Type-2 Diabetes Mellitus. Ayushdhara 3 (4), 749–763.
National Institute for Health and Care Excellence (2014). Developing NICE Guidelines: The Manual. London: NICE.
National Institute for Health and Care Excellence (2015). Type 2 Diabetes in Adults: Management. UK: NICE.
Nigam, V., and Nambiar, V. S. (2019). Aegle marmelos Leaf Juice as a Complementary Therapy to Control Type 2 Diabetes - Randomised Controlled Trial in Gujarat, India. Adv. Integr. Med. 6 (1), 11–22. doi:10.1016/j.aimed.2018.03.002
Nille, G. C., Reddy, K. R. C., and Tripathi, J. S. (2018). Clinical Evaluation of Talapotaka Churna: A Polyherbal Ayurvedic Formulation in Type 2 Diabetes Mellitus. Indian J. Traditional Knowledge 17 (1), 168–175.
Niranjan, K., Ramakanth, G. S. H., Fatima, N., and Usharani, P. (2016). Evaluation of the Effect of Purified Aqueous Extract of Shilajit in Modifying Cardiovascular Risk with Special Reference to Endothelial Dysfunction in Patients with Type 2 Diabetes Mellitus. Int. J. Ayur Pharma Res. 4 (4), 1–7.
Paliwal, M. (2018). The Critical Exposition of Nidana-Dosha-Dushya of Prameha Roga as Described in Brihattrayi and Clinical Study of Khadira-Kramuka Kashaya Ghanavati in Cases of Kshaudrameha. CTRI/2018/03/012823. (India: Clinical Trials Registry).
Panahi, Y., Khalili, N., Sahebi, E., Namazi, S., Reiner, Ž., Majeed, M., et al. (2017). Curcuminoids Modify Lipid Profile in Type 2 Diabetes Mellitus: A Randomized Controlled Trial. Complement. Ther. Med. 33, 1–5. doi:10.1016/j.ctim.2017.05.006
Panahi, Y., Khalili, N., Sahebi, E., Namazi, S., Simental-Mendía, L. E., Majeed, M., et al. (2018). Effects of Curcuminoids Plus Piperine on Glycemic, Hepatic and Inflammatory Biomarkers in Patients with Type 2 Diabetes Mellitus: A Randomized Double-Blind Placebo-Controlled Trial. Drug Res. (Stuttg) 68 (7), 403–409. doi:10.1055/s-0044-101752
Patel, D. V., Chandola, H., Baghel, M. S., and Joshi, J. R. (2012). Clinical Efficacy of Shankhapushpi and a Herbo-Mineral Compound in Type-II Diabetes. Ayu 33 (2), 230–237. doi:10.4103/0974-8520.105243
Peter, E. L., Kasali, F. M., Deyno, S., Mtewa, A., Nagendrappa, P. B., Tolo, C. U., et al. (2019). Momordica charantia L. Lowers Elevated Glycaemia in Type 2 Diabetes Mellitus Patients: Systematic Review and Meta-Analysis. J. Ethnopharmacol 231, 311–324. doi:10.1016/j.jep.2018.10.033
Pingali, U., Ali, M. A., Gundagani, S., and Nutalapati, C. (2020). Evaluation of the Effect of an Aqueous Extract of Azadirachta indica (Neem) Leaves and Twigs on Glycemic Control, Endothelial Dysfunction and Systemic Inflammation in Subjects with Type 2 Diabetes Mellitus - A Randomized, Double-Blind, Placebo-Controlled Clinical Study. Diabetes Metab. Syndr. Obes. 13, 4401–4412. doi:10.2147/DMSO.S274378
Poongothai, S., Karkuzhali, K., Sharadha, J., Deepa, R., and Mohan, V. (2002). Evaluation of Safety and Efficacy of Hyponidd, an Ayurvedic Compound: A Double Blind, Placebo Controlled Study in Type 2 Diabetic Patients with Secondary Failure to Oral Drugs. Int. J. Diab Dev. Countries 22, 19–24.
Press Information Bureau, (2021). Promoting Ayush Practice in the International Sphere. [Internet]. New Delhi: Government of India. [Cited 2022 Feb 20]. Available at: https://pib.gov.in/Pressreleaseshare.aspx?PRID=1696430.
Priya, R., and Shweta, A. S. (2010). Status and Role of Ayush and Local Health Traditions under the National Rural Health Mission. New Delhi: National Health Systems Resource Centre.
Quamri, M. A., Begum, S., Siddiqui, M. A., and Alam, M. A. (2017). Efficacy of Kanduri (Coccinia indica) in Diabetes Associated Dyslipidemia: A Randomized Single Blind Standard Controlled Study. Int. J. Unani Integr. Med. 1 (1), 1–4.
Quezada-Fernández, P., Trujillo-Quiros, J., Pascoe-González, S., Trujillo-Rangel, W. A., Cardona-Müller, D., Ramos-Becerra, C. G., et al. (2019). Effect of Green Tea Extract on Arterial Stiffness, Lipid Profile and sRAGE in Patients with Type 2 Diabetes Mellitus: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. J. Food Sci. Nutr. 70 (8), 977–985. doi:10.1080/09637486.2019.1589430
Rabiei, K., Ebrahimzadeh, M. A., Saeedi, M., Bahar, A., Akha, O., and Kashi, Z. (2018). Effects of a Hydroalcoholic Extract of Juglans regia (Walnut) Leaves on Blood Glucose and Major Cardiovascular Risk Factors in Type 2 Diabetic Patients: A Double-Blind, Placebo-Controlled Clinical Trial. BMC Complement. Altern. Med. 18 (1), 206. doi:10.1186/s12906-018-2268-8
Rafraf, M., Malekiyan, M., Asghari-Jafarabadi, M., and Aliasgarzadeh, A. (2014). Effect of Fenugreek Seeds on Serum Metabolic Factors and Adiponectin Levels in Type 2 Diabetic Patients. Int. J. Vitam Nutr. Res. 84 (3-4), 196–205. doi:10.1024/0300-9831/a000206
Ranade, M., and Mudgalkar, N. (2017). A Simple Dietary Addition of Fenugreek Seed Leads to the Reduction in Blood Glucose Levels: A Parallel Group, Randomized Single-Blind Trial. Ayu 38 (1-2), 24–27. doi:10.4103/ayu.AYU_209_15
Rashid, R., Ahmad, H., Ahmed, Z., Rashid, F., and Khalid, N. (2019). Clinical Investigation to Modulate the Effect of Fenugreek Polysaccharides on Type-2 Diabetes. Bioactive Carbohydrates and Dietary Fibre 19, 100194. doi:10.1016/j.bcdf.2019.100194
Ricklefs-Johnson, K., Johnston, C. S., and Sweazea, K. L. (2017). Ground Flaxseed Increased Nitric Oxide Levels in Adults with Type 2 Diabetes: A Randomized Comparative Effectiveness Study of Supplemental Flaxseed and Psyllium Fiber. Obes. Med. 5, 16–24. doi:10.1016/j.obmed.2017.01.002
Roy, K., Shah, R., and Iyer, U. (2015). Tinospora cordifolia Stem Supplementation in Diabetic Dyslipidemia: An Open Labelled Randomized Controlled Trial. Funct. Foods Health Dis. 5 (7), 265–274. doi:10.31989/ffhd.v5i8.208
Sahana, D. A., Shivprakash, G., Baliga, R., Adhikari, P., and Jyothi, G. (2010). Effect of Eugenia jambolana on Plasma Glucose, Insulin Sensitivity and HDL-C Levels: Preliminary Results of a Randomized Clinical Trial. J. Pharm. Res. 3 (6), 1268–1270.
Samagandi, K., Sharma, J., Kumar, S., and Tripathy, T. B. (2012). A Clinical Study on the Efficacy of Wheat Grass Juice in Prameha. Int. Res. J. Pharm. 3 (4), 194–199.
Samani, N. B., Jokar, A., Soveid, M., Heydari, M., and Mosavat, S. H. (2016). Efficacy of the Hydroalcoholic Extract of Tribulus terrestris on the Serum Glucose and Lipid Profile of Women with Diabetes Mellitus: A Double-Blind Randomized Placebo-Controlled Clinical Trial. J. Evid. Based Complement. Altern. Med. 21 (4), NP91–7. doi:10.1177/2156587216650775
Sankhla, A., Sharma, S., and Sharma, N. (2009). Hypoglycemic Effect of Bael Patra (Aegle marmelos) in NIDDM Patients. J. Dairying, Foods HS 28 (3/4), 233–236.
Sarbini, D., Huriyati, E., Sadewa, H., and Wahyuningsih, M. S. H. (2019). The Effect of Rosella (Hibiscus sabdariffa Linn) on Insulin Resistance in Patients with Type 2 Diabetes Mellitus: A Randomized Clinical Trial. Int. Summit Sci. Tech. Humanity, 572–585.
Sazia, S. S., Shankar, P., Nath, R., Sachan, A. K., and Dixit, R. K. (2015). Effect of Metformin vs. Eclipta alba on Blood Glucose Level in Diabetic Patients. Int. J. Pharmacognosy Phytochem. Res. 7 (2), 215–218.
Sazia, S. S., Shankar, P., Nath, R., Sachan, A. K., and Dixit, R. K. (2015). Effects of Eclipta alba and Diabetic Diet with Life Style Modifications on Blood Glucose Levels in Diabetic Patient. Int. J. Res. Ayurveda Pharm. 6 (3), 375–378.
Schünemann, H., Brożek, J., Guyatt, G., and Oxman, A. (Editors) (2013). Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach (The GRADE Working Group).
Sengsuk, C., Sanguanwong, S., Tangvarasittichai, O., and Tangvarasittichai, S. (2016). Effect of Cinnamon Supplementation on Glucose, Lipids Levels, Glomerular Filtration Rate, and Blood Pressure of Subjects with Type 2 Diabetes Mellitus. Diabetol. Int. 7 (2), 124–132. doi:10.1007/s13340-015-0218-y
Shankarrao, S. Y., Raut, A., Phartale, V. D., and Shinde, K. P. (2017). Controlled Clinical Evaluation of “Mamajjak Choorna” in Sthula Madhumeha Viz-A-Viz Type-2 Diabetes Mellitus. Int. J. Ayur Pharma Res. 5 (7), 26–31.
Sharma, P., and Sharma, S. (2013). A Randomised, Double-Blind, Placebo-Controlled Trial of Aegle marmelos Supplementation on Glycaemic Control and Blood Pressure Level in Type 2 Diabetes Mellitus. Aust. J. Herbal Med. 25 (3), 141–145.
Sharma, H., Chandola, H. M., Singh, G., and Basisht, G. (2007). Utilization of Ayurveda in Health Care: An Approach for Prevention, Health Promotion, and Treatment of Disease. Part 2-Ayurveda in Primary Health Care. J. Altern. Complement. Med. 13 (10), 1135–1150. doi:10.1089/acm.2007.7017-B
Sharma, P., Sharma, S., Agrawal, R. P., Agarwal, V., and Singhal, S. (2012). A Randomised Double Blind Placebo Control Trial of Cinnamon Supplementation on Glycemic Control and Lipid Profile in Type 2 Diabetes Mellitus. Aust. J. Herbal Med. 24 (1), 108–119.
Sharma, R., Sharma, B., Jindal, M., Gupta, A., Kunwar, R., Lata, S., et al. (2017). Evaluation of Hypolipidemic Effect of Stem Part of Berberis aristata in Type 2 Diabetes Mellitus Patients as Add on Therapy. Natl. J. Physiol. Pharm. Pharmacol. 7 (11), 1159–1169. doi:10.5455/njppp.2017.7.0517510062017
Sharma, R., Shahi, V. K., Khanduri, S., Goyal, A., Chaudhary, S., Rana, R. K., et al. (2019). Effect of Ayurveda Intervention, Lifestyle Modification and Yoga in Prediabetic and Type 2 Diabetes Under the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases and Stroke (NPCDCS)-AYUSH Integration Project. Ayu 40 (1), 8–15. doi:10.4103/ayu.AYU_105_19
Sharma, S. K. (2018). Clinical Study on Vijaysaradi Ghanvati and Madhumehari Vati in the Management of Madhumeha (Type 2 Diabetes): An Open Randomized Comparative Trial [Dissertation on the Internet]. Jamnagar: Gujarat Ayurved University.
Shekelle, P. G., Hardy, M., Morton, S. C., Coulter, I., Venuturupalli, S., Favreau, J., et al. (2005). Are Ayurvedic Herbs for Diabetes Effective? J. Fam. Pract. 54 (10), 876–886.
Shekhar, K. C., Achike, F. I., Kaur, G., Kumar, P., and Hashim, R. (2002). A Preliminary Evaluation of the Efficacy and Safety of Cogent Db (An Ayurvedic Drug) in the Glycemic Control of Patients with Type 2-diabetes. J. Altern. Complement. Med. 8 (4), 445–457. doi:10.1089/107555302760253649
Shidfar, F., Rajab, A., Rahideh, T., Khandouzi, N., Hosseini, S., and Shidfar, S. (2015). The Effect of Ginger (Zingiber officinale) on Glycemic Markers in Patients with Type 2 Diabetes. J. Complement. Integr. Med. 12 (2), 165–170. doi:10.1515/jcim-2014-0021
Shikov, A. N., Narkevich, I. A., Akamova, A. V., Nemyatykh, O. D., Flisyuk, E. V., Luzhanin, V. G., et al. (2021). Medical Species Used in Russia for the Management of Diabetes and Related Disorders. Front. Pharmacol. 12, 697411. doi:10.3389/fphar.2021.697411
Shojaii, A., Goushegir, A., Dabaghian, F. H., Abdollahi, M., and Huseini, H. F. (2011). Herbs and Herbal Preparations for Glycemic Control in Diabetes Mellitus (A Systematic Review). J. Med. Plant Res. 5 (16), 3846–3855.
Shokoohi, R., Kianbakht, S., Faramarzi, M., Rahmanian, M., Nabati, F., Mehrzadi, S., et al. (2017). Effects of an Herbal Combination on Glycemic Control and Lipid Profile in Diabetic Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. J. Evid. Based Complement. Altern. Med. 22 (4), 798–804. doi:10.1177/2156587217737683
Sidana, S., Singh, V., Meena, B., Beniwal, S., Chandra, S., Singh, K., et al. (2016). Effect of Syzygium cumini (Jamun) Seed Powder on Dyslipidemia: A Double Blind Randomized Control Trial. Int. J. Res. Med. Sci. 4 (7), 2603–2610. doi:10.18203/2320-6012.ijrms20161917
Sidana, S., Singh, V., Meena, B., Beniwal, S., Singh, K., Kumar, D., et al. (2017). Effect of Syzygium cumini (Jamun) Seed Powder on Glycemic Control: A Double-Blind Randomized Controlled Trial. J. Med. Soc. 31 (3), 185–189. doi:10.4103/jms.jms_62_16
Siddiqui, M. Y., Akhtar, M. W., Azmat, J., and Khalique, A. (2017). A Comparative Clinical Study of Unani Formulation (Maghz Tukhm-E-Jamun Wa Tukhm-E-Hayat) and Metformin in the Management of Ziabetus Shakari (Type 2 Diabetes Mellitus). Med. J. Islamic World Acad. Sci. 25 (2), 40–49. doi:10.5505/ias.2017.37888
Singh, A., Rai, J., and Mahajan, D. (2016). Comparative Evaluation of Glipizide and Fenugreek (Trigonella foenum-graecum) Seeds as Monotherapy and Combination Therapy on Glycaemic Control and Lipid Profile in Patients with Type 2 Diabetes Mellitus. Int. J. Basic Clin. Pharmacol. 5 (3), 942–950. doi:10.18203/2319-2003.ijbcp20161549
Sohrab, G., Nasrollahzadeh, J., Zand, H., Amiri, Z., Tohidi, M., and Kimiagar, M. (2014). Effects of Pomegranate Juice Consumption on Inflammatory Markers in Patients with Type 2 Diabetes: A Randomized, Placebo-Controlled Trial. J. Res. Med. Sci. 19 (3), 215–220.
Sohrab, G., Angoorani, P., Tohidi, M., Tabibi, H., Kimiagar, M., and Nasrollahzadeh, J. (2015). Pomegranate (Punica granatum) Juice Decreases Lipid Peroxidation, but has no Effect on Plasma Advanced Glycated End-Products in Adults with Type 2 Diabetes: A Randomized Double-Blind Clinical Trial. Food Nutr. Res. 59, 28551. doi:10.3402/fnr.v59.28551
Sridharan, K., Mohan, R., Ramaratnam, S., and Panneerselvam, D. (2011). Ayurvedic Treatments for Diabetes Mellitus. Cochrane Database Syst. Rev. 12, CD008288. doi:10.1002/14651858.CD008288.pub2
Srinivas, P., Sharma, S. K., Sharma, O. R., Srinivas, P., Subhose, V., Babu, G., et al. (2018). Clinical Evaluation of Nisha Katakadi Kashaya and Yashada Bhasma in the Management of Type-2 Diabetes Mellitus (Madhumeha): A Multicentre, Open Label Prospective Study. J. Res. Ayurvedic Sci. 2 (2), 108–121. doi:10.5005/jp-journals-10064-0046
Srinivasan, A., Selvarajan, S., Kamalanathan, S., Kadhiravan, T., Prasanna Lakshmi, N. C., and Adithan, S. (2019). Effect of Curcuma longa on Vascular Function in Native Tamilians with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Parallel Arm, Placebo-Controlled Trial. Phytother. Res. 33 (7), 1898–1911. doi:10.1002/ptr.6381
Suchitra, M. R., and Parthasarathy, S. (2015). Effect of Administration of Fenugreek Seeds on HbA1c Levels in Uncontrolled Diabetes Mellitus: A Randomized Controlled Trial. Int. J. Pharmtech. Res. 8 (2), 180–182.
Suksomboon, N., Poolsup, N., Boonkaew, S., and Suthisisang, C. C. (2011). Meta-analysis of the Effect of Herbal Supplement on Glycemic Control in Type 2 Diabetes. J. Ethnopharmacol. 137 (3), 1328–1333. doi:10.1016/j.jep.2011.07.059
Suppapitiporn, S., Kanpaksi, N., and Suppapitiporn, S. (2006). The Effect of Cinnamon Cassia Powder in Type 2 Diabetes Mellitus. J. Med. Assoc. Thai 89 (Suppl. 3), S200–S205.
Suthar, A. C., Deshmukh, A., Babu, V., Mohan, V. S., Chavan, M. V., Kumar, D., et al. (2016). Efficacy and Safety of Glycebal (PDM011011) Capsules as Adjuvant Therapy in Subjects with Type 2 Diabetes Mellitus: An Open Label, Randomized, Active Controlled, Phase II Trial. Clin. Diabetology 5 (3), 88–94. doi:10.5603/dk.2016.0015
Suthar, A. C., Pai, V. G., Kadam, Y., Tongaonkar, A., Kale, S., Deshpande, A. B., et al. (2016). Efficacy and Safety of PDM011011 Capsules as Compared to Metformin in Subjects with Type-2 Diabetes Mellitus: An Open-Label, Randomized, Active-Controlled, Multicentric, Phase III Study. J. Diabetes Mellitus 6 (1), 38–48. doi:10.4236/jdm.2016.61005
Talaei, B., Amouzegar, A., Sahranavard, S., Hedayati, M., Mirmiran, P., and Azizi, F. (2017). Effects of Cinnamon Consumption on Glycemic Indicators, Advanced Glycation End Products, and Antioxidant Status in Type 2 Diabetic Patients. Nutrients 9 (9), 991. doi:10.3390/nu9090991
Talaei, B., Mozaffari-Khosravi, H., and Bahreini, S. (2017). The Effect of Ginger on Blood Lipid and Lipoproteins in Patients with Type 2 Diabetes: A Double-Blind Randomized Clinical Controlled Trial. J. Nutr. Food Security 2 (1), 87–95.
Talaei, B., Mozaffari-Khosravi, H., and Bahreini, S. (2018). The Effect of Ginger Powder Supplementation on Blood Pressure of Patients with Type 2 Diabetes: A Double-Blind Randomized Clinical Controlled Trial. J. Nutr. Food Security 3 (2), 70–78.
Tangvarasittichai, S., Sanguanwong, S., Sengsuk, C., and Tangvarasittichai, O. (2015). Effect of Cinnamon Supplementation on Oxidative Stress, Inflammation and Insulin Resistance in Patients with Type 2 Diabetes Mellitus. Int. J. Toxicol. Pharmacol. Res. 7 (4), 158–164.
Taviad, K. P. (2016). Pharmaceutical Study of Swarnamakshika Bhasma and its Clinical Efficacy in Madhumeha (DM Type-II) [Dissertation on the Internet]. Jamnagar: Gujarat Ayurved University.
Trakoon-osot, W., Sotanaphun, U., Phanachet, P., Porasuphatana, S., Udomsubpayakul, U., and Komindr, S. (2013). Pilot Study: Hypoglycemic and Antiglycation Activities of Bitter Melon (Momordica charantia L.) in Type 2 Diabetic Patients. J. Pharm. Res. 6 (8), 859–864. doi:10.1016/j.jopr.2013.08.007
Tufanaru, C., Munn, Z., Aromataris, E., Campbell, J., and Hopp, L. (2017). “Chapter 3: Systematic Reviews of Effectiveness,” in JBI Reviewer’s Manual. Editors E. Aromataris, and Z. Munn (Adelaide: JBI).
US Food and Drug Administration, (2008). Guidance for Industry: Diabetes Mellitus: Developing Drugs and Therapeutic Biologics for Treatment and Prevention. US: FDA.
Usharani, P., Mateen, A. A., Naidu, M. U., Raju, Y. S., and Chandra, N. (2008). Effect of NCB-02, Atorvastatin and Placebo on Endothelial Function, Oxidative Stress and Inflammatory Markers in Patients with Type 2 Diabetes Mellitus: A Randomized, Parallel-Group, Placebo-Controlled, 8-week Study. Drugs R. D 9 (4), 243–250. doi:10.2165/00126839-200809040-00004
Usharani, P., Fatima, N., and Muralidhar, N. (2013). Effects of Phyllanthus emblica Extract on Endothelial Dysfunction and Biomarkers of Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Controlled Study. Diabetes Metab. Syndr. Obes. 6, 275–284. doi:10.2147/DMSO.S46341
Usharani, P., Fatima, N., Kumar, C. U., and Kishan, P. V. (2014). Evaluation of a Highly Standardized Withania somnifera Extract on Endothelial Dysfunction and Biomarkers of Oxidative Stress in Patients with Type 2 Diabetes Mellitus: A Randomized, Double Blind, Placebo Controlled Study. Int. J. Ayur Pharma Res. 2 (3), 22–32.
Usharani, P., Kishan, P. V., Fatima, N., and Kumar, C. U. (2014). A Comparative Study to Evaluate the Effect of Highly Standardised Aqueous Extracts of Phyllanthus emblica, Withania somnifera and Their Combination on Endothelial Dysfunction and Biomarkers in Patients with Type II Diabetes Mellitus. Int. J. Pharm. Sci. Res. 5 (7), 2687–2697.
Usharani, P., Sukumaran, D., and Nutalapati, C. (2020). Effect of an Aqueous Extract of Terminalia chebula on Endothelial Dysfunction, Systemic Inflammation, and Lipid Profile in Type 2 Diabetes Mellitus: A Randomized Double-Blind, Placebo-Controlled Clinical Study. Phytother. Res. 34 (12), 3226–3235.
Uusitupa, M., Tuomilehto, J., Karttunen, P., and Wolf, E. (1984). Long Term Effects of Guar Gum on Metabolic Control, Serum Cholesterol and Blood Pressure Levels in Type 2 (Non-insulin-dependent) Diabetic Patients with High Blood Pressure. Ann. Clin. Res. 16 (Suppl. 43), 126–131.
Uusitupa, M., Siitonen, O., Savolainen, K., Silvasti, M., Penttilä, I., and Parviainen, M. (1989). Metabolic and Nutritional Effects of Long-Term Use of Guar Gum in the Treatment of Noninsulin-dependent Diabetes of Poor Metabolic Control. Am. J. Clin. Nutr. 49 (2), 345–351. doi:10.1093/ajcn/49.2.345
Vafa, M., Mohammadi, F., Shidfar, F., Sormaghi, M. S., Heidari, I., Golestan, B., et al. (2012). Effects of Cinnamon Consumption on Glycemic Status, Lipid Profile and Body Composition in Type 2 Diabetic Patients. Int. J. Prev. Med. 3 (8), 531–536.
Verma, N., Usman, K., Patel, N., Jain, A., Dhakre, S., Swaroop, A., et al. (2016). A Multicenter Clinical Study to Determine the Efficacy of a Novel Fenugreek Seed (Trigonella foenum-graecum) Extract (Fenfuro™) in Patients with Type 2 Diabetes. Food Nutr. Res. 60 (1), 32382. doi:10.3402/fnr.v60.32382
Vickers, A. J. (2001). The Use of Percentage Change from Baseline as an Outcome in a Controlled Trial is Statistically Inefficient: A Simulation Study. BMC Med. Res. Methodol. 1, 6. doi:10.1186/1471-2288-1-6
Wainstein, J., Stern, N., Heller, S., and Boaz, M. (2011). Dietary Cinnamon Supplementation and Changes in Systolic Blood Pressure in Subjects with Type 2 Diabetes. J. Med. Food 14 (12), 1505–1510. doi:10.1089/jmf.2010.0300
Wainstein, J., Landau, Z., Bar Dayan, Y., Jakubowicz, D., Grothe, T., Perrinjaquet-Moccetti, T., et al. (2016). Purslane Extract and Glucose Homeostasis in Adults with Type 2 Diabetes: A Double-Blind, Placebo-Controlled Clinical Trial of Efficacy and Safety. J. Med. Food 19 (2), 133–140. doi:10.1089/jmf.2015.0090
Wasana, K. G. P., Attanayake, A. P., Weerarathna, T. P., and Jayatilaka, K. A. P. W. (2021). Efficacy and Safety of a Herbal Drug of Coccinia grandis (Linn.) Voigt in Patients with Type 2 Diabetes Mellitus: A Double Blind Randomized Placebo Controlled Clinical Trial. Phytomedicine 81, 153431. doi:10.1016/j.phymed.2020.153431
Yaheya, M., and Ismail, M. (2009). Clinical Evaluation of Antidiabetic Activity of Trigonella Seeds and Aegle marmelos Leaves. World Appl. Sci. J. 7 (10), 1231–1234.
Yazdanpanah, Z., Ghadiri-Anari, A., Mehrjardi, A. V., Dehghani, A., Zardini, H. Z., and Nadjarzadeh, A. (2017). Effect of Ziziphus jujube Fruit Infusion on Lipid Profiles, Glycaemic Index and Antioxidant Status in Type 2 Diabetic Patients: A Randomized Controlled Clinical Trial. Phytother Res. 31 (5), 755–762. doi:10.1002/ptr.5796
Yeh, G. Y., Eisenberg, D. M., Kaptchuk, T. J., and Phillips, R. S. (2003). Systematic Review of Herbs and Dietary Supplements for Glycemic Control in Diabetes. Diabetes Care 26 (4), 1277–1294. doi:10.2337/diacare.26.4.1277
Zahmatkesh, M., Huseini, H. F., Hajiaghaee, R., Heidari, M., Mehrafarin, A., and Tavakoli-far, B. (2012). The Effects of Cinnamomum zeylanicum J. Presl on Blood Glucose Level in Patients with Type 2 Diabetes, a Double-Blind Clinical Trial. J. Med. Plants 11 (41), 258–263.
Zänker, K. S., Mang, B., Wolters, M., and Hahn, A. (2012). Personalized Diabetes and Cancer Medicine: A Rationale for Anti-diabetic Nutrition (Bitter Melon) in a Supportive Setting. Curr. Cancer Ther. Rev. 8 (1), 66–77.
Zare, R., Nadjarzadeh, A., Zarshenas, M. M., Shams, M., and Heydari, M. (2019). Efficacy of Cinnamon in Patients with Type II Diabetes Mellitus: A Randomized Controlled Clinical Trial. Clin. Nutr. 38 (2), 549–556. doi:10.1016/j.clnu.2018.03.003
Zarezadeh, M., Saedisomeolia, A., Khorshidi, M., Kord Varkane, H., Makhdoomi Arzati, M., Abdollahi, M., et al. (2018). Asymmetric Dimethylarginine and Soluble Inter-cellular Adhesion Molecule-1 Serum Levels Alteration Following Ginger Supplementation in Patients with Type 2 Diabetes: A Randomized Double-Blind, Placebo-Controlled Clinical Trial. J. Complement. Integr. Med. 16 (2), 20180019. doi:10.1515/jcim-2018-0019
Zarrintan, A., Mobasseri, M., Zarrintan, A., and Ostadrahimi, A. (2015). Effects of Aloe vera Supplements on Blood Glucose Level and Lipid Profile Markers in Type 2 Diabetic Patients - a Randomized Clinical Trial. Pharm. Sci. 21 (2), 65–71. doi:10.15171/ps.2015.19
Ziai, S. A., Larijani, B., Akhoondzadeh, S., Fakhrzadeh, H., Dastpak, A., Bandarian, F., et al. (2005). Psyllium Decreased Serum Glucose and Glycosylated Hemoglobin Significantly in Diabetic Outpatients. J. Ethnopharmacol. 102 (2), 202–207. doi:10.1016/j.jep.2005.06.042
Zibaeenezhad, M., Aghasadeghi, K., Hakimi, H., Yarmohammadi, H., and Nikaein, F. (2016). The Effect of walnut Oil Consumption on Blood Sugar in Patients with Diabetes Mellitus Type 2. Int. J. Endocrinol. Metab. 14 (3), e34889. doi:10.5812/ijem.34889
Keywords: ayurveda, effectiveness, meta-analysis, safety, systematic review, type 2 diabetes mellitus
Citation: Chattopadhyay K, Wang H, Kaur J, Nalbant G, Almaqhawi A, Kundakci B, Panniyammakal J, Heinrich M, Lewis SA, Greenfield SM, Tandon N, Biswas TK, Kinra S and Leonardi-Bee J (2022) Effectiveness and Safety of Ayurvedic Medicines in Type 2 Diabetes Mellitus Management: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:821810. doi: 10.3389/fphar.2022.821810
Received: 24 November 2021; Accepted: 07 April 2022;
Published: 08 June 2022.
Edited by:
Alexander N. Shikov, Saint-Petersburg State Chemical Pharmaceutical Academy, RussiaReviewed by:
Olga Pozharitskaya, Murmansk Marine Biological Institute, RussiaAmir Hadi, Isfahan University of Medical Sciences, Iran
Mohd Helmy Mokhtar, National University of Malaysia, Malaysia
Copyright © 2022 Chattopadhyay, Wang, Kaur, Nalbant, Almaqhawi, Kundakci, Panniyammakal, Heinrich, Lewis, Greenfield, Tandon, Biswas, Kinra and Leonardi-Bee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kaushik Chattopadhyay, a2F1c2hpay5jaGF0dG9wYWRoeWF5QG5vdHRpbmdoYW0uYWMudWs=
 Kaushik Chattopadhyay
Kaushik Chattopadhyay Haiquan Wang
Haiquan Wang Jaspreet Kaur1
Jaspreet Kaur1 Gamze Nalbant
Gamze Nalbant Burak Kundakci
Burak Kundakci Jeemon Panniyammakal
Jeemon Panniyammakal Michael Heinrich
Michael Heinrich Sheila Margaret Greenfield
Sheila Margaret Greenfield Sanjay Kinra
Sanjay Kinra