- 1Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2School of Second Clinical Medicine, Guangzhou University of Chinese Medicine, Guangzhou, China
- 3TCM Regulating Metabolic Diseases Key Laboratory of Sichuan Province, Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
With the sharp change in our diet and lifestyle, the incidence of colorectal cancer (CRC) is increasing among young people and has become the second most common malignant tumor worldwide. Although the current treatment of CRC is getting updated rapidly, recurrence and metastasis are still inevitable. Therefore, new anticancer drugs are needed to break existing limitations. In recent years, Hedyotis diffusa Willd (HDW) extracts have been proved to demonstrate excellent anti-colorectal cancer effects and have been widely used in clinical practices. In this review, we aim to explore the advantages, potential signaling pathways, and representative active ingredients of HDW in the treatment of CRC from the perspective of molecular mechanism, in order to provide new ideas for the future treatment of CRC.
Introduction
Colorectal cancer (CRC) is one of the leading causes of cancer-related death in Western and developing countries, and it is the second most common cancer after lung cancer (Hemminki et al., 2021). Epidemiology results show that the global cases of CRC reached over 1.9 million in 2020 (Alyabsi et al., 2021). Global data indicate that although the incidence of CRC has declined significantly over the past few decades among people over the age of 50, an annual increase of about 1.8% has been observed in younger patients, and it is projected that the average incidence of CRC may increase by about 107.1% in patients aged 20–34 years by 2030 (Thanikachalam et al., 2019; Vidri et al., 2020). About 33% of CRC patients have distant metastasis at the time of diagnosis, although many advances in systematic approaches have occurred such as chemotherapy, targeted therapy, and immunotherapy, while about 86% of patients with advanced colorectal cancer still die within 5 years of diagnosis (Wrobel et al., 2019). These gloomy data present a challenging goal for clinicians and researchers, that is, to explore new anticancer drugs to overcome the limitations of existing therapies.
Hedyotis diffusa Willd (HDW) is a kind of Rubiaceae of Chinese herbal medicine, which is a famous Chinese herbal medicine with thousands of years of clinical practice history. HDW is an important ingredient of various anticancer formulations; it has been reported to inhibit tumor cell proliferation and metastasis and alleviate side effects after chemotherapy as well (Song et al., 2019). Early pharmacological studies have confirmed that HDW has medicinal properties like antitumor, anti-inflammatory, immunomodulatory, antioxidant, and other biological activities (Shen et al., 2016). As an antitumor herbal medicine, HDW and its extracts have been widely used in the treatment of CRC, breast cancer, prostate cancer, and so on (Wazir et al., 2021; Huang et al., 2021). In this review, we explore the advantages, potential signal pathways, and representative active ingredients of HDW in the treatment of CRC. To write this review, we tried to use the latest published articles in highly reputated journals.
Advantages of HDW
HDW is a widely used clinical herbal medicine. Meng et al. (2013) found 199 anticancer herbs by searching PubMed and SciFinder databases; they then ranked each herb by frequency of occurrence and found that HDW was in the top five; subsequently, MTS cell viability assay was used to confirm that HDW, and its extracts showed good anticancer cell proliferation activity. HDW is also a representative of many heat-clearing and detoxifying anticancer herbs, whose anticancer activity is second only to yew and equivalent to matrine, especially in the treatment of CRC highlighted more advantages (Song et al., 2019).
Although there are a variety of treatments for colorectal cancer, chemotherapy is still one of the main therapies for most patients. However, the resistance of cancer cells to chemotherapy drugs limits its long-term application, which is also the primary reason for clinical chemotherapy failure. Lai et al. (2017) demonstrated that HDW can inhibit the activity, migration, and invasion of drug-resistant colorectal cancer cells of HCT-8/5-FU, and reverse multiple drug resistance (MDR) of colorectal cancer cells. Compared with oxaliplatin alone, combined use of HDW can reduce toxicity, increase efficacy, reduce the incidence of bone marrow suppression after chemotherapy, enhance immune response, improve the quality of life of cancer patients, and prolong their survival (Chen et al., 2016; Ho, 2018). In addition, HDW also has multiple pharmacological effects such as antioxidant, anti-inflammatory, anti-fibroblast, and immune regulation, and it also has a certain blocking effect on the transformation of colitis cancer (DanQing et al., 2021).
The role of HDW in CRC is quite important. Next, we will make a systematic, comprehensive, and detailed review framework for the molecular mechanism of HDW and its extracts in the treatment of CRC.
Potential Signaling Pathways
To investigate the potential mechanism of HDW in inhibiting CRC cell growth, we will review the potential signaling pathways of HDW acting on CRC over the years, including the in vivo and in vitro experiments (Table 1).
Yan et al. (2017) suggested that the chloroform extract of HDW(CEHDW) may play an anticancer role by suppressing phosphorylation of PI3K/AKT and RAS/ERK signaling pathways: CEHDW could inhibit proliferation and promote apoptosis of the SW620 CRC cell lines, and in addition, it plays this role by decreasing the expression levels of B-cell lymphoma 2(Bcl-2), cyclin D1, cyclin-dependent kinase 4(CDK4), surviving and proliferating cell nuclear antigen (PCNA), and increasing the expression levels of Bcl-2-associated X (Bax) protein. CEHDW also inhibits the activation of protein kinase B (AKT) and extracellular signal-regulated kinase (ERK).
Multiple drug resistance (MDR) is one of the main causes of chemotherapy failure. Li et al. (2015); Li et al. (2018) proposed for the first time that the ethanol extract of HDW (EEHDW) may overcome drug resistance of HCT-8/5-FU cells by downregulating the expression of ABC subfamily G member 2 (ABCG2) and P-glycoprotein (P-gp), or via inhibiting the phosphorylation of the PI3K/AKT signaling pathway. It mainly suppresses the expression of PI3K and p-Akt key target genes; downregulates the expression of Bcl-2, cyclin D1, and CDK4; and upregulates the expression of Bax, p21, and phosphatase-tensin homolog (PTEN) to reduce the viability of cancer cells, inhibit cell colony formation, induce cell apoptosis and then reverse MDR.
Li et al. (2019) constructed a human lymphatic endothelial cell (HLEC) model stimulated by vascular endothelial growth factor C (VEGF-C) and found that EEHDW regulates PI3K/AKT, ERK, and signal transducer and activator of transcription 3(STAT3) signaling pathways to inhibit VEGF-C-mediated lymphatic formation of HCT-116 and HCT-8 cell lines, thus blocking the migration of cancer cells and lymphangiogenesis. These signaling pathways are interrelated and can occur in parallel; important downregulated molecules in this process include cyclin D1, CDK4, MMP2, MMP9, and VEGFR-3.
In 2012, a CRC mice xenograft (CMX) model was used by some researchers to demonstrate the anticancer activity of EEHDW in vivo (Cai et al., 2012); they found EEHDW reduced tumor weight and volume in model mice. This may be attributed to EEHDW hobbling the phosphorylation of STAT3 signaling pathway. Feng et al. (2017) further confirmed that EEHDW has strong anti-colorectal cancer activity both in vivo and in vitro, and also built a CMX model and some human CRC cell lines (HCT-8, HT-29, HCT-116, and SW620 cells). The results show that EEHDW could regulate various inflammatory (IL-1β, IL-6, IL-4, IL-10, TNF-α) and angiogenic factors (COX-2, iNOS, eNOS, HIF-1α) and downregulate the expression of various oncogenes (Bcl-2, Bax, Pim-1, p53), thus affecting the proliferation and apoptosis of cancer cells and tumor angiogenesis. The changes in these key molecules suggest that EEHDW may play an important role in decreasing the activation of multiple signaling pathways, such as ERK1/2, AKT, STAT3, JNK, and p38.
Lin et al. (2015) studied the activity of EEHDW in a carcinogenic inflammatory environment and demonstrated that EEHDW treatment significantly reduced IL-6-induced STAT3 pathway phosphorylation and induced activation of pro-apoptotic factors Bax, caspase-9, and caspase-3, and downregulated Bcl-2, cyclin D1, and CDK4, thereby enhancing the local inflammatory environment and promoting tumor progression. In addition, Lin et al. (2011); Lin et al. (2015) also confirmed that EEHDW could prevent G1 to S progression of HT-29 cells and inhibit the expression levels of VEGF-A, to counteract tumor angiogenesis. In the early years, Lin et al. (2013) verified that EEHDW can reduce intra-tumor microvascular density (MVD) in a CMX model by inhibiting the expression of VEGF-A and VEGFR2, the target gene of the Sonic hedgehog (SHH) signaling pathway. Lin et al. (2010) also observed that EEHDW treatment could break the DNA, decrease the mitochondrial membrane potential, and increase the ratio of Bax/Bcl-2 of HT-29 cells, suggesting that EEHDW inhibited the growth of HT-29 cells via the mitochondrion-dependent pathway.
Lin et al. (2012) also believed that EEHDW could block the cell cycle G1 to S progression by decreasing the expression of cyclin D1, PCNA, and CDK4 but increasing the p21, which was positively correlated with the treatment time and concentration of EEHDW. Sun et al. (2016) showed that HDW may inhibit CRC stem cells; EEHDW can significantly reduce the expression of Lgr5, PCNA, ABCB1, survivin, β-catenin, and c-Myc in HT-29 SP cells and reduce the proportion of SP in HT-29 cells. These are associated with the inhibition of the Wnt/β-catenin signaling pathway and the expression of ABC transporters. Both Lai et al. (2017) and Chen et al. (2018) found that EEHDW inhibited metastasis of HCT-8/5-FU cells by regulating the transforming growth factor-β (TGF-β) signaling pathway, which showed inhibition of cell adhesion, migration, and invasion.
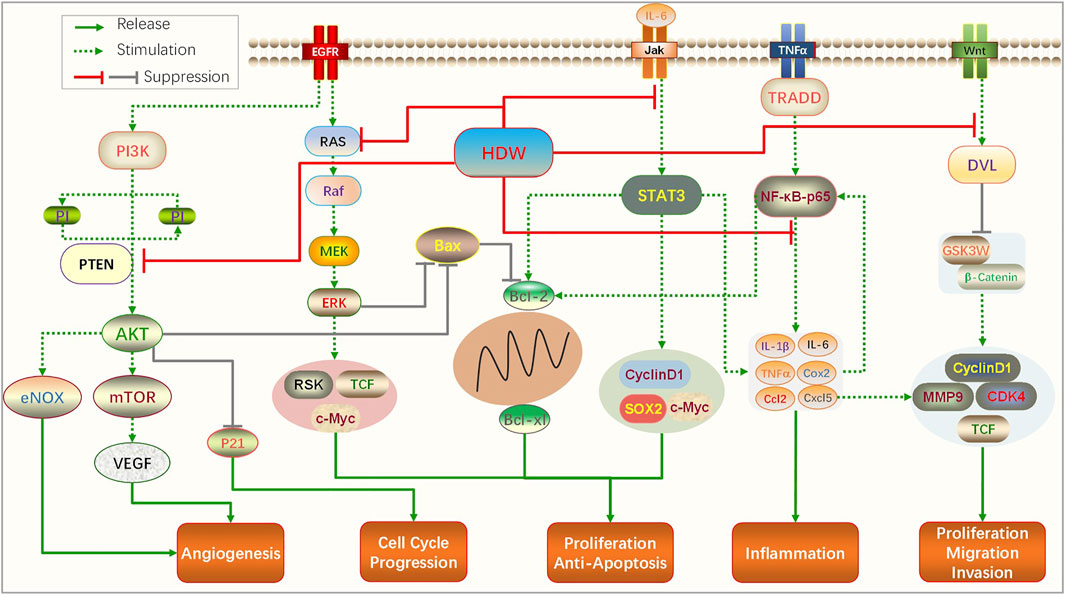
In summary, PI3K/AKT, RAS/ERK, STAT3, and cell cycle arrest are the most common signaling pathways for HDW extracts to intervene in CRC. Other signaling pathways include TGF-β, Wnt/-β-catenin, SHH, ABC, and mitochondrion-dependent pathways, which are closely related to the anticancer activity of HDW. It exerts anti-colorectal cancer activity mainly by promoting cells apoptosis; inhibiting cell proliferation, migration, and invasion; and suppressing tumor and lymphangiogenesis. It can also reverse the drug resistance of CRC cells. A network pharmacology research shows that the main targets of HDW therapy for CRC are AKT, PIK, TP53, BRAF, CDK2, and RAF, Gene Ontology (GO) analysis suggested that HDW may exert anticancer activity through regulating tumor-related pathways, cell motility and cell community, which is consistent with the main molecular mechanisms reviewed in this article (Liu X et al., 2018). The following is a schematic diagram of the main signaling pathways that HDW acts on CRC (Figure 1).
Representative Antitumor Constituents
Modern technologies have helped us identify 58 kinds of antitumor active components in HDW (Han et al., 2020). We will review several representative chemical constituents.
Anthraquinones
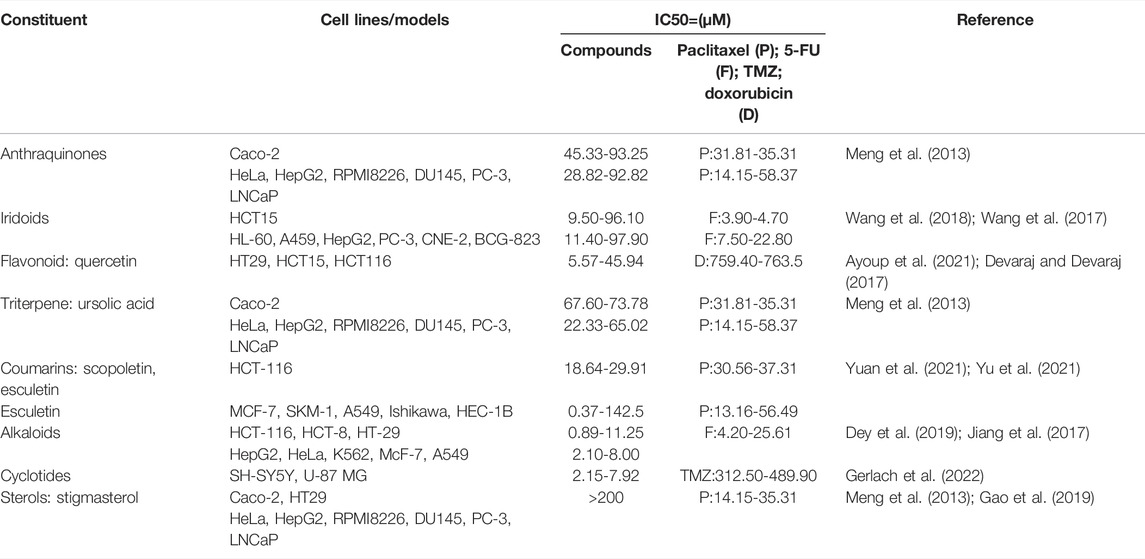
Anthraquinones are one of the main anticancer components of HDW, and their anticancer activity is similar to that of paclitaxel. Meng et al. (2013) evaluated the effects of 10 active components of HDW on seven cancer cell lines and peripheral blood mononuclear cells (PBMCs). They found that anthraquinones inhibit cancer cell vitality in a dose-dependent manner within a certain concentration range, especially 2-hydroxymethyl-1-hydroxyanthraquinone (IC50 = 45.33 µM) could significantly inhibit the proliferation of the colorectal cancer cell line Caco-2, followed by 2-methyl-3-methoxyanthraquinone, 2-hydroxymethylanthraquinone, and 2-hydroxy-3-methylanthraquinone (IC50 = 93–155 µM). Meanwhile, anthraquinones have almost no effect on PBMCs. Li et al. (2016) confirmed that the active component of the 1, 3-dihydroxy-2-methylanthraquinone fraction from HDW with a much high inhibitory rate up to 48.9 ± 3.3% against HepG2 carcinoma cells at 125 µMol/L, mainly mediating by death receptor and mitochondrial apoptosis pathways. 2-Hydroxymethylanthraquinone found in HDW has been demonstrated to significantly reduce LPS-induced acute lung injury and suppress the level of inflammatory factors by regulating the TLR4/NF-κB pathway, thereby inhibiting inflammatory cancer transformation (Tan et al., 2018).
Iridoids
Iridoids are also one of the main components of HDW in their anticancer activities and are widely found in plants (Wang et al., 2020). Wang et al. (2017) isolated nine iridoids (1–3, 5–10) from HDW and measured the cytotoxic effects of all the compounds on various human tumor cell lines in vitro. Iridoid glycosides of Shecaoiridoidside C (compound 3) were found to be highly cytotoxic to HCT15 (human colon cancer cells) and other tumor cells (IC50 = 9.6–62.2 µM), and compounds 1, 7, 9(IC50 = 37.6–86.6 µM, 34.2–71.3 µM, 78.3–97.9 µM) also showed certain cytotoxicity to HCT15, A459, and HepG2 cells. In 2018, Wang et al. (2018) further confirmed that compounds 1 and 2 (IC50 = 9.5–28.2 µM, 15.8–26.2 µM) obvious cytotoxicity to HCT15 and all tumor cells. In addition, compound 8 (IC50 = 16.5–40.4 µM) also showed a significant inhibitory effect on HCT15, CNE-2, HL-60, A459, and HepG2 cancer cells.
Flavonoids
Flavonoids are a class of polyphenols with a wide range of biological activities, their anticancer and antioxidant effects have been the focus of research for many years. Epidemiological studies have confirmed that dietary intake of flavonoids can reduce the risk of cancer (Maleki et al., 2019; Selvakumar et al., 2020). Badar et al. (2021) found that flavonoids can target the PI3K/Akt/mTOR signaling pathway in the treatment of cancer. It could also modulate and regulate reactive oxygen species (ROS) of colorectal cancer HCT15, HCT116, and SW480 cell lines to activate caspases, thus o stimulating cell apoptosis (Kopustinskiene et al., 2020). Li Y. L et al. (2020) also suggested that the flavonoids from HDW may inhibit the upstream of the H2O2-induced pathway by lowering ROS and increasing the levels of Trx1 and TrxR1, thereby blocking the ASK1/P38 MAPK signaling pathway and reversing cellular malignant transformation. It is believed that flavonoids have the therapeutic potential of epigenetic regulation of cancer pathogenesis (Khan et al., 2021). Quercetin is a representative compound of flavonoids; it has many preventive effects in colorectal cancer, such as promoting apoptosis and antioxidant and inhibiting angiogenesis, and it is very sensitive to HCT116 cells (IC50 = 5.57–45.94 µM) (Ayoup et al., 2021).
Triterpenes
Triterpenes are essential for human health; they could suppress nuclear factor kappa B (NF-κB), STAT3, nuclear factor erythroid-2-related factor 2 (Nrf2), and other key signaling pathways to activate the antioxidant and anti-inflammatory ability, cell cycle regulation, and epigenetic to prevent tumor development (Li S et al., 2020). Four triterpenes have been isolated from HDW, namely, ursolic acid, oleanolic acid, isoarborinol, and arborinone (Chen et al., 2016). Studies have shown that ursolic acid has strong anti-inflammatory activity, via interfering with various biological processes such as free radical scavenging, pro-apoptotic and antiapoptotic protein expression, and G1/G2 cell cycle arrest, leading to apoptosis of cancer cells and inhibition of cell proliferation and angiogenesis (Yin et al., 2018; Alam et al., 2021). Meng et al. (2013) found that ursolic acid (IC50 = 71 µM) had the strongest inhibitory effect on Caco-2 cells among compounds isolated from HDW, and it also had a strong inhibitory effect on Hep G2, DU145, PC-3, LNCaP, and HeLa cancer cells (IC50 = 22.33–65.02 µM), which was close to the activity of paclitaxel. However, oleanolic acid has a less toxic effect on cancer cells than ursolic acid (IC50 = 65.18–198.10 µM).
Coumarins
The total coumarins of HDW, including scopoletin and esculetin, showed significant antiproliferative activity. Jiang et al. (2017) identified that HDW contains two kinds of coumarins, with a total content of 87.4% coumarins, which can activate caspases and inhibit PI3K/Akt pathway proteins, thus inducing SkM-1 cell apoptosis in a dose-dependent manner (IC50 = 104.48 μg/ml, 100.66 μg/ml). Yu et al. (2021) synthesized a new class of scopoletin derivatives and found that compound 18e exhibited antiproliferative activity against different cancer cells, especially MCF-7 cells (IC50 = 0.37 µM). A study proved that scopoletin could inhibit the proliferation of HCT-116 and A549 cells by reducing the level of RAS-Raf-MEK-ERK and PI3K/AKT pathways (IC50 = 32 μg/ml, 16 μg/ml) (Yuan et al., 2021). In addition, esculetin can target hnRNPa1 and downregulate the expression of Bcl-xl and Xiap, resulting in cell apoptosis and restricted proliferation of Ishikawa (IC50 = 95 µM) and HEC-1B (IC50 = 142.5 µM) (Jiang et al., 2021).
Alkaloids
Alkaloids can inhibit the proliferation of colorectal cancer cells by interfering with the cell cycle, which shows certain anticancer potential (Khan et al., 2022). Modern pharmacological studies show that steroidal alkaloids have anticancer, anti-inflammatory, bactericidal, analgesic, and other biological activities and showed strong cytotoxicity to HCT-116 (IC50 = 3.8 µM) and HepG2, HeLa, K562, McF-7, and A549 cells (IC50 = 2.1–8.0 µM); the application of this compound is promising (Dey et al., 2019; Jiang et al., 2016).
Sterols
Sterols are also common chemical constituents of HDW. Interestingly, the reduction of squalene epoxidase caused by sterol accumulation can activate the β-catenin oncogenic pathway and inhibit the p53 tumor suppressor pathway, leading to the progression of CRC (Jun et al., 2021). Gao et al. (2019) also observed that sterol regulatory element-binding protein-1 is overexpressed in HT29 cells, promoting the vascular endothelial generation, activating the NF-κB-P65 pathway, and thus causing uncontrolled proliferation of cancer cells. Meng et al. (2013) confirmed that stigmasterol showed very low anticancer activity (IC50 > 200 µM). This indicates that among many active ingredients, sterols may play a neutralizing or even opposite role.
Cyclotides
The unique ring structure of cyclotides shows great promise in the treatment of cancer as it is stable and difficult to be enzymatically hydrolyzed (Mehta et al., 2020). Gerlach et al. (2022) found that multiple cyclotides (CyO2, CyO13) were cytotoxic to SH-SY5Y and U-87 MG cells (IC50 = 2.15–7.92 µM), and combined application could enhance the efficacy of temozolomide (TMZ).
The representative antitumor constituents isolated from the HDW are organized and listed in Table 2, and their 3D structure is shown in Figure 2.
Conclusion and Discussion
Hedyotis diffusa Willd is a representative of heat-clearing and detoxifying herbs with strong anticancer activity and is widely used in clinical adjuvant therapy for postoperative patients with colorectal cancer. In many anticancer TCM formulations, its frequency is as high as 5.1% (Chao et al., 2014). In this study, we covered almost all relevant studies on the anti-colorectal cancer effects of HDW and its extracts in vitro and in vivo and summarized its advantages, potential signaling pathways, and representative active ingredients in the treatment of CRC as well. We found that the ethanol extract of HDW (EEHDW) had the best anticancer activity, and its anticancer ability was dose- and time-dependent, with a general study concentration of 0.5–2 mg/ml. HDW is less toxic to normal cells than chemotherapy, and it can also reverse MDR in colorectal cancer cells.
HDW exerts anti-colorectal cancer activity through multiple pathways and targets. PI3K/AKT, RAS/ERK, STAT3, NF-κB, Wnt/β-catenin, and cell cycle arrest are the most common signaling pathways for its intervention in colorectal cancer. The activation of the PI3K/AKT pathway is a very classic molecular event in the development process of colorectal cancer. Studies have found it plays an important role in regulating cell autophagy, inhibiting epithelial–mesenchymal transition (EMT), and promoting the G1/S-phase cell cycle (Duan et al., 2018; Wei et al., 2019). The RAS/ERK pathway also plays a vital role in the invasion and proliferation of CRC, and cyclin D1 secreted after its activation can cause the uncontrolled proliferation of colorectal cells (Zhu et al., 2019). Although these pathways are involved in the regulation of different oncogenic mechanisms, they contain common upstream and downstream effector factors and are linked at multiple levels. For example, the interaction of Wnt/β-catenin and PI3K/AKT/mTORC1 signaling pathways is one of the mechanisms of drug resistance in CRC patients (Prossomariti et al., 2020), and oncogene doublecortin-like kinase-1 (DCLK1) can activate NF-κBp65 and induce EMT through the PI3K/Akt/Iκα pathway (Liu W et al., 2018).
Up to 170 compounds have been isolated from HDW, these anthraquinones, iridoids, flavonoids, triterpenes, coumarins, alkaloids, and cyclotides are the main components of anticancer activity. Interestingly, the compound sterols could activate the Wnt/β-catenin pathway, which may play a neutralizing or even opposite role in many compounds. The IC50 of these constituents in colorectal cancer cell lines were close to chemical drugs such as paclitaxel and 5-FU and had no inhibitory effect on normal cell lines (IC50 > 200 µM). They upregulate pro-apoptotic proteins and downregulate antiapoptotic proteins by acting on these key signaling pathways, promote cell apoptosis, inhibit cell proliferation, and inhibit the formation of tumor blood vessels and lymphatics (Figure 3).
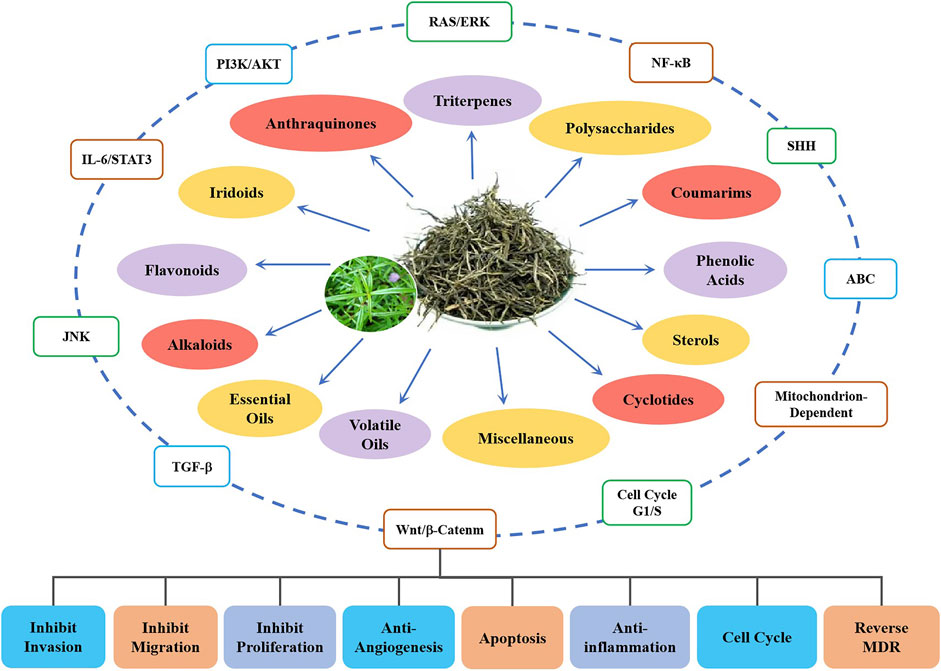
FIGURE 3. Schematic diagram of anti-colorectal cancer components from HDW and their related mechanisms.
Modern research technology has provided much evidence for the anti-colorectal cancer effect and molecular mechanism of HDW, which has provided a scientific basis for its wide clinical application, and it is good news for colorectal cancer patients. However, most of the research objects are model mice or cancer cells, which are different from human physiological and pathological environments. On the other hand, traditional Chinese medicine prescription is a combination of several herbs, with the structure of monarch, minister, assistant, and guide. These studies only involve one HDW or one of its components, which obviously cannot fully reflect the anticancer thought of TCM. Moreover, many studies have shown that the combination of multiple components or extracts has more advantages in the intervention of tumor-related signaling pathways than a single herbal. Therefore, the clinical practice of HDW and its extract in the treatment of CRC needs to be further verified, and more suitable options should be explored in future studies.
Author Contributions
WZH is responsible for collecting and organizing the data and writing the review. YB is responsible for perfecting graphics and tables, as well as reviewing the full text. In the process of revising the manuscript. YFM is responsible for the critical framework resetting, methodology designing and overall writing quality control. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This research is supported by the National Nature Science Foundation of China(No.8274315), with the project title of “Tongxieyaofang, Effect in Delaying Colon Cancer Progression through Inhitibion of Stress-induced TSC22D3 Over-expression and Recovering of Th1/Tc1 Immune Responses from the Perspective of ‘Simultaneous Regulation of Form, Qi, and Spirit’”.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alam, M., Ali, S., Ahmed, S., Elasbali, A. M., Adnan, M., Islam, A., et al. (2021). Therapeutic Potential of Ursolic Acid in Cancer and Diabetic Neuropathy Diseases. Int. J. Mol. Sci. 22 (22), 12162. doi:10.3390/ijms222212162
Alyabsi, M., Algarni, M., and Alshammari, K. (2021). Trends in Colorectal Cancer Incidence Rates in Saudi Arabia (2001-2016) Using Saudi National Registry: Early- versus Late-Onset Disease. Front. Oncol. 11, 730689. doi:10.3389/fonc.2021.730689
Ayoup, M. S., Abu-Serie, M. M., Awad, L. F., Teleb, M., Ragab, H. M., and Amer, A. (2021). Halting Colorectal Cancer Metastasis via Novel Dual Nanomolar MMP-9/MAO-A Quinoxaline-Based Inhibitors; Design, Synthesis, and Evaluation. Eur. J. Med. Chem. 222, 113558. doi:10.1016/j.ejmech.2021.113558
Badar Ul Islam, B., Khan, M. S., Husain, F. M., Rehman, M. T., Zughaibi, T. A., Abuzenadah, A. M., et al. (2021). mTOR Targeted Cancer Chemoprevention by Flavonoids. Curr. Med. Chem. 28 (39), 8068–8082. doi:10.2174/0929867327666201109122025
Cai, Q., Lin, J., Wei, L., Zhang, L., Wang, L., Zhan, Y., et al. (2012). Hedyotis Diffusa Willd Inhibits Colorectal Cancer Growth In Vivo via Inhibition of STAT3 Signaling Pathway. Int. J. Mol. Sci. 13 (5), 6117–6128. doi:10.3390/ijms13056117
Chao, T. H., Fu, P. K., Chang, C. H., Chang, S. N., Chiahung Mao, F., and Lin, C. H. (2014). Prescription Patterns of Chinese Herbal Products for post-surgery colon Cancer Patients in Taiwan. J. Ethnopharmacol 155 (1), 702–708. doi:10.1016/j.jep.2014.06.012
Chen, R., He, J., Tong, X., Tang, L., and Liu, M. (2016). The Hedyotis Diffusa Willd. (Rubiaceae): A Review on Phytochemistry, Pharmacology, Quality Control and Pharmacokinetics. Molecules 21 (6), 710. doi:10.3390/molecules21060710
Chen, W., Jin, Y., Yang, H., Wei, L., and Lin, J. (2018). Hedyotis Diffusa Willd Reduces Migration and Invasion through Inhibition of TGF-β-Induced EMT in Colorectal Cancer Cells. Eur. J. Integr. Med. 23, 57–63. doi:10.1016/j.eujim.2018.09.008
DanQing, L., YuJie, G., ChengPeng, Z., HongZhi, D., Yi, H., BiSheng, H., et al. (2021). N-butanol Extract of Hedyotis Diffusa Protects Transgenic Caenorhabditis elegans from Aβ-Induced Toxicity. Phytother Res. 35 (2), 1048–1061. doi:10.1002/ptr.6871
Devaraj, H., and Devaraj, S. N. (2017). Differential Cytotoxic Activity of Quercetin on Colonic Cancer Cells Depends on ROS Generation through COX-2 Expression. Food Chem. Toxicol. 106 (Pt A), 92–106. doi:10.1016/j.fct.2017.05.006
Dey, P., Kundu, A., Chakraborty, H. J., Kar, B., Choi, W. S., Lee, B. M., et al. (2019). Therapeutic Value of Steroidal Alkaloids in Cancer: Current Trends and Future Perspectives. Int. J. Cancer 145 (7), 1731–1744. doi:10.1002/ijc.31965
Duan, S., Huang, W., Liu, X., Liu, X., Chen, N., Xu, Q., et al. (2018). IMPDH2 Promotes Colorectal Cancer Progression through Activation of the PI3K/AKT/mTOR and PI3K/AKT/FOXO1 Signaling Pathways. J. Exp. Clin. Cancer Res. 37 (1), 304. doi:10.1186/s13046-018-0980-3
Feng, J., Jin, Y., Peng, J., Wei, L., Cai, Q., Yan, Z., et al. (2017). Hedyotis Diffusa Willd Extract Suppresses Colorectal Cancer Growth through Multiple Cellular Pathways. Oncol. Lett. 14 (6), 8197–8205. doi:10.3892/ol.2017.7244
Gao, Y., Nan, X., Shi, X., Mu, X., Liu, B., Zhu, H., et al. (2019). SREBP1 Promotes the Invasion of Colorectal Cancer Accompanied Upregulation of MMP7 Expression and NF-Κb Pathway Activation. BMC Cancer 19 (1), 685. doi:10.1186/s12885-019-5904-x
Gerlach, S. L., Dunlop, R. A., Metcalf, J. S., Banack, S. A., and Cox, P. A. (2022). Cyclotides Chemosensitize Glioblastoma Cells to Temozolomide. J. Nat. Prod. 85 (1), 34–46. doi:10.1021/acs.jnatprod.1c00595
Han, X., Zhang, X., Wang, Q., Wang, L., and Yu, S. (2020). Antitumor Potential of Hedyotis Diffusa Willd: A Systematic Review of Bioactive Constituents and Underlying Molecular Mechanisms. Biomed. Pharmacother. 130, 110735. doi:10.1016/j.biopha.2020.110735
Hemminki, K., Försti, A., and Hemminki, A. (2021). Survival in colon and Rectal Cancers in Finland and Sweden through 50 Years. BMJ Open Gastroenterol. 8 (1), e000644. doi:10.1136/bmjgast-2021-000644
Ho, K. (2018). Hedyotis Diffusa and Panax Ginseng Combination: Better Anticancer Properties. Appl. Food Sci. 2, 15.
Huang, L., Xu, H., Wu, T., and Li, G. (2021). Hedyotis Diffusa Willd. Suppresses Hepatocellular Carcinoma via Downregulating AKT/mTOR Pathways. Evid. Based Complement. Alternat Med. 2021, 5210152. doi:10.1155/2021/5210152
Jiang, J., Wang, B., Li, J., Ye, B., Lin, S., Qian, W., et al. (2017). Total Coumarins of Hedyotis Diffusa Induces Apoptosis of Myelodysplastic Syndrome SKM-1 Cells by Activation of Caspases and Inhibition of PI3K/Akt Pathway Proteins. J. Ethnopharmacol 196, 253–260. doi:10.1016/j.jep.2016.12.012
Jiang, Q. W., Chen, M. W., Cheng, K. J., Yu, P. Z., Wei, X., and Shi, Z. (2016). Therapeutic Potential of Steroidal Alkaloids in Cancer and Other Diseases. Med. Res. Rev. 36 (1), 119–143. doi:10.1002/med.21346
Jiang, R., Su, G., Chen, X., Chen, S., Li, Q., Xie, B., et al. (2021). Esculetin Inhibits Endometrial Cancer Proliferation and Promotes Apoptosis via hnRNPA1 to Downregulate BCLXL and XIAP. Cancer Lett. 521, 308–321. doi:10.1016/j.canlet.2021.08.039
Jun, S. Y., Brown, A. J., Chua, N. K., Yoon, J. Y., Lee, J. J., Yang, J. O., et al. (2021). Reduction of Squalene Epoxidase by Cholesterol Accumulation Accelerates Colorectal Cancer Progression and Metastasis. Gastroenterology 160 (4), 1194–1207. doi:10.1053/j.gastro.2020.09.009
Khan, H., Alam, W., Alsharif, K. F., Aschner, M., Pervez, S., and Saso, L. (2022). Alkaloids and Colon Cancer: Molecular Mechanisms and Therapeutic Implications for Cell Cycle Arrest. Molecules 27 (3), 920. doi:10.3390/molecules27030920
Khan, H., Belwal, T., Efferth, T., Farooqi, A. A., Sanches-Silva, A., Vacca, R. A., et al. (2021). Targeting Epigenetics in Cancer: Therapeutic Potential of Flavonoids. Crit. Rev. Food Sci. Nutr. 61 (10), 1616–1639. doi:10.1080/10408398.2020.1763910
Kopustinskiene, D. M., Jakstas, V., Savickas, A., and Bernatoniene, J. (2020). Flavonoids as Anticancer Agents. Nutrients. 12 (2), 457. doi:10.3390/nu12020457
Lai, Z., Yan, Z., Chen, W., Peng, J., Feng, J., Li, Q., et al. (2017). Hedyotis Diffusa Willd Suppresses Metastasis in 5-fluorouracil-resistant Colorectal Cancer Cells by Regulating the TGF-β Signaling Pathway. Mol. Med. Rep. 16 (5), 7752–7758. doi:10.3892/mmr.2017.7500
Li, H., Lai, Z., Yang, H., Peng, J., Chen, Y., and Lin, J. (2019). Hedyotis Diffusa Willd. Inhibits VEGF-C-mediated Lymphangiogenesis in Colorectal Cancer via Multiple Signaling Pathways. Oncol. Rep. 42 (3), 1225–1236. doi:10.3892/or.2019.7223
Li, Q., Lai, Z., Yan, Z., Peng, J., Jin, Y., Wei, L., et al. (2018). Hedyotis diffusa Willd Inhibits Proliferation and Induces Apoptosis of 5-FU Resistant Colorectal Cancer Cells by Regulating the PI3K/AKT Signaling Pathway. Mol. Med. Rep. 17 (1), 358–365. doi:10.3892/mmr.2017.7903
Li, Q., Wang, X., Shen, A., Zhang, Y., Chen, Y., Sferra, T. J., et al. (2015). Hedyotis Diffusa Willd Overcomes 5-fluorouracil Resistance in Human Colorectal Cancer HCT-8/5-FU Cells by Downregulating the Expression of P-Glycoprotein and ATP-Binding Casette Subfamily G Member 2. Exp. Ther. Med. 10 (5), 1845–1850. doi:10.3892/etm.2015.2762
Li, S., Kuo, H. D., Yin, R., Wu, R., Liu, X., Wang, L., et al. (2020). Epigenetics/epigenomics of Triterpenoids in Cancer Prevention and in Health. Biochem. Pharmacol. 175, 113890. doi:10.1016/j.bcp.2020.113890
Li, Y. L., Chen, X., Niu, S. Q., Zhou, H. Y., and Li, Q. S. (2020). Protective Antioxidant Effects of Amentoflavone and Total Flavonoids from Hedyotis Diffusa on H2 O2 -Induced HL-O2 Cells through ASK1/p38 MAPK Pathway. Chem. Biodivers 17 (7), e2000251. doi:10.1002/cbdv.202000251
Li, Y. L., Zhang, J., Min, D., Hongyan, Z., Lin, N., and Li, Q. S. (2016). Anticancer Effects of 1,3-Dihydroxy-2-Methylanthraquinone and the Ethyl Acetate Fraction of Hedyotis Diffusa Willd against HepG2 Carcinoma Cells Mediated via Apoptosis. PLoS One 11 (4), e0151502. doi:10.1371/journal.pone.0151502
Lin, J., Chen, Y., Wei, L., Chen, X., Xu, W., Hong, Z., et al. (2010). Hedyotis Diffusa Willd Extract Induces Apoptosis via Activation of the Mitochondrion-dependent Pathway in Human colon Carcinoma Cells. Int. J. Oncol. 37 (5), 1331–1338. doi:10.3892/ijo_00000785
Lin, J., Li, Q., Chen, H., Lin, H., Lai, Z., and Peng, J. (2015). Hedyotis Diffusa Willd. Extract Suppresses Proliferation and Induces Apoptosis via IL-6-inducible STAT3 Pathway Inactivation in Human Colorectal Cancer Cells. Oncol. Lett. 9 (4), 1962–1970. doi:10.3892/ol.2015.2956
Lin, J., Wei, L., Shen, A., Cai, Q., Xu, W., Li, H., et al. (2013). Hedyotis Diffusa Willd Extract Suppresses Sonic Hedgehog Signaling Leading to the Inhibition of Colorectal Cancer Angiogenesis. Int. J. Oncol. 42 (2), 651–656. doi:10.3892/ijo.2012.1753
Lin, J., Wei, L., Xu, W., Hong, Z., Liu, X., and Peng, J. (2011). Effect of Hedyotis Diffusa Willd Extract on Tumor Angiogenesis. Mol. Med. Rep. 4 (6), 1283–1288. doi:10.3892/mmr.2011.577
Lin, M., Lin, J., Wei, L., Xu, W., Hong, Z., Cai, Q., et al. (2012). Hedyotis Diffusa Willd Extract Inhibits HT-29 Cell Proliferation via Cell Cycle Arrest. Exp. Ther. Med. 4 (2), 307–310. doi:10.3892/etm.2012.599
Liu, W., Wang, S., Sun, Q., Yang, Z., Liu, M., and Tang, H. (2018). DCLK1 Promotes Epithelial-Mesenchymal Transition via the PI3K/Akt/NF-Κb Pathway in Colorectal Cancer. Int. J. Cancer 142 (10), 2068–2079. doi:10.1002/ijc.31232
Liu, X., Wu, J., Zhang, D., Wang, K., Duan, X., and Zhang, X. (2018). A Network Pharmacology Approach to Uncover the Multiple Mechanisms of Hedyotis Diffusa Willd. On Colorectal Cancer. Evid. Based Complement. Alternat Med. 2018, 6517034. doi:10.1155/2018/6517034
Maleki, S. J., Crespo, J. F., and Cabanillas, B. (2019). Anti-inflammatory Effects of Flavonoids. Food Chem. 299, 125124. doi:10.1016/j.foodchem.2019.125124
Mehta, L., Dhankhar, R., Gulati, P., Kapoor, R. K., Mohanty, A., and Kumar, S. (2020). Natural and Grafted Cyclotides in Cancer Therapy: An Insight. J. Pept. Sci. 26 (4-5), e3246. doi:10.1002/psc.3246
Meng, Q. X., Roubin, R. H., and Hanrahan, J. R. (2013). Ethnopharmacological and Bioactivity Guided Investigation of Five TCM Anticancer Herbs. J. Ethnopharmacol 148 (1), 229–238. doi:10.1016/j.jep.2013.04.014
Prossomariti, A., Piazzi, G., Alquati, C., and Ricciardiello, L. (2020). Are Wnt/β-Catenin and PI3K/AKT/mTORC1 Distinct Pathways in Colorectal Cancer? Cell Mol Gastroenterol Hepatol 10 (3), 491–506. doi:10.1016/j.jcmgh.2020.04.007
Selvakumar, P., Badgeley, A., Murphy, P., Anwar, H., Sharma, U., Lawrence, K., et al. (2020). Flavonoids and Other Polyphenols Act as Epigenetic Modifiers in Breast Cancer. Nutrients 12 (3), 761. doi:10.3390/nu12030761
Shen, H., Bai, Y., and Huo, Z. (2016). The Protective Effect of Hedyotis Diffusa on Collagen Induced Arthritis Rats. Int. J. Clin. Exp. Med. 9, 12880–12887.
Song, Y., Wang, H., Pan, Y., and Liu, T. (2019). Investigating the Multi-Target Pharmacological Mechanism of Hedyotis Diffusa Willd Acting on Prostate Cancer: A Network Pharmacology Approach. Biomolecules 9 (10), E591. doi:10.3390/biom9100591
Sun, G., Wei, L., Feng, J., Lin, J., and Peng, J. (2016). Inhibitory Effects of Hedyotis Diffusa Willd. On Colorectal Cancer Stem Cells. Oncol. Lett. 11 (6), 3875–3881. doi:10.3892/ol.2016.4431
Tan, J., Li, L., Shi, W., Sun, D., Xu, C., Miao, Y., et al. (2018). Protective Effect of 2-Hydroxymethyl Anthraquinone from Hedyotis Diffusa Willd in Lipopolysaccharide-Induced Acute Lung Injury Mediated by TLR4-NF-Κb Pathway. Inflammation 41 (6), 2136–2148. doi:10.1007/s10753-018-0857-9
Thanikachalam, K., and Khan, G. (2019). Colorectal Cancer and Nutrition. Nutrients 11 (1), 164. doi:10.3390/nu11010164
Vidri, R. J., and Fitzgerald, T. L. (2020). GSK-3: An Important Kinase in colon and Pancreatic Cancers. Biochim. Biophys. Acta Mol. Cel Res 1867 (4), 118626. doi:10.1016/j.bbamcr.2019.118626
Wang, C., Gong, X., Bo, A., Zhang, L., Zhang, M., Zang, E., et al. (2020). Iridoids: Research Advances in Their Phytochemistry, Biological Activities, and Pharmacokinetics. Molecules 25 (2), 287. doi:10.3390/molecules25020287
Wang, C., Xin, P., Wang, Y., Zhou, X., Wei, D., Deng, C., et al. (2018). Iridoids and Sfingolipids from Hedyotis Diffusa. Fitoterapia 124, 152–159. doi:10.1016/j.fitote.2017.11.004
Wang, C., Zhou, X., Wang, Y., Wei, D., Deng, C., Xu, X., et al. (2017). The Antitumor Constituents from Hedyotis Diffusa Willd. Molecules 22 (12), 2101. doi:10.3390/molecules22122101
Wazir, J., Ullah, R., Khongorzul, P., Hossain, M. A., Khan, M. W., Aktar, N., et al. (2021). The Effectiveness of Hedyotis Diffusa Willd Extract in a Mouse Model of Experimental Autoimmune Prostatitis. Andrologia 53 (1), e13913. doi:10.1111/and.13913
Wei, R., Xiao, Y., Song, Y., Yuan, H., Luo, J., and Xu, W. (2019). FAT4 Regulates the EMT and Autophagy in Colorectal Cancer Cells in Part via the PI3K-AKT Signaling axis. J. Exp. Clin. Cancer Res. 38 (1), 112. doi:10.1186/s13046-019-1043-0
Wrobel, P., and Ahmed, S. (2019). Current Status of Immunotherapy in Metastatic Colorectal Cancer. Int. J. Colorectal Dis. 34 (1), 13–25. doi:10.1007/s00384-018-3202-8
Yan, Z., Feng, J., Peng, J., Lai, Z., Zhang, L., Jin, Y., et al. (2017). Chloroform Extract of Hedyotis Diffusa Willd Inhibits Viability of Human Colorectal Cancer Cells via Suppression of AKT and ERK Signaling Pathways. Oncol. Lett. 14 (6), 7923–7930. doi:10.3892/ol.2017.7245
Yin, R., Li, T., Tian, J. X., Xi, P., and Liu, R. H. (2018). Ursolic Acid, a Potential Anticancer Compound for Breast Cancer Therapy. Crit. Rev. Food Sci. Nutr. 58 (4), 568–574. doi:10.1080/10408398.2016.1203755
Yu, N., Li, N., Wang, K., Deng, Q., Lei, Z., Sun, J., et al. (2021). Design, Synthesis and Biological Activity Evaluation of Novel Scopoletin-NO Donor Derivatives against MCF-7 Human Breast Cancer In Vitro and In Vivo. Eur. J. Med. Chem. 224, 113701. doi:10.1016/j.ejmech.2021.113701
Yuan, C., Wang, M. H., Wang, F., Chen, P. Y., Ke, X. G., Yu, B., et al. (2021). Network Pharmacology and Molecular Docking Reveal the Mechanism of Scopoletin against Non-small Cell Lung Cancer. Life Sci. 270, 119105. doi:10.1016/j.lfs.2021.119105
Keywords: colorectal cancer, Hedyotis diffusa Willd, advantages, signaling pathways, active ingredients, molecular mechanism
Citation: Wu Z, Yin B and You F (2022) Molecular Mechanism of Anti-Colorectal Cancer Effect of Hedyotis diffusa Willd and Its Extracts. Front. Pharmacol. 13:820474. doi: 10.3389/fphar.2022.820474
Received: 23 November 2021; Accepted: 06 April 2022;
Published: 02 June 2022.
Edited by:
Youhua Xu, Macau University of Science and Technology, Macao SAR, ChinaReviewed by:
Cui-Ting Peng, Sichuan University, ChinaSung-Gook Cho, Korea National University of Transportation, South Korea
Copyright © 2022 Wu, Yin and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bei Yin, MTc4MTczMTU5MTZAMTYzLmNvbQ==; Fengming You, eWZtZG9jQDE2My5jb20=
 Zihong Wu
Zihong Wu Bei Yin
Bei Yin Fengming You
Fengming You