- 1Department of Medical Biotechnology, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
- 2Department of Systems Biotechnology, National Institute of Genetic Engineering and Biotechnology, Tehran, Iran
Aims: Sentrin-specific protease -2 (SENP2) is involved in deSUMOylation. Increased deSUMOylation in murine hearts by SENP2 upregulation resulted in cardiac dysfunction and congenital heart defects. Natural compounds via regulating cell proliferation and survival, induce cell cycle cessation, cell death, apoptosis, and producing reactive oxygen species and various enzyme systems cause disease prevention. Then, natural compounds can be suitable inhibitors and since SENP2 is a protein involved in heart disease, so our aim was inhibition of SENP2 by natural products for heart disease treatment. Material and methods: Molecular docking and molecular dynamics simulation of natural products i.e. Gallic acid (GA), Caffeic acid (CA), Thymoquinone (TQ), Betanin, Betanidin, Fisetin, and Ebselen were done to evaluate the SENP2 inhibitory effect of these natural products. The toxicity of compounds was also predicted. Results: The results showed that Betanin constituted a stable complex with SENP2 active site as it revealed low RMSD, high binding energy, and hydrogen bonds. Further, as compared to Ebselen, Betanin demonstrated low toxicity, formed a stable complex with SENP2 via four to seven hydrogen bonds, and constituted more stable MD plots. Therefore, depending upon the outcomes presented herein, Betanin significantly inhibited SENP2 and hence may be considered as a suitable natural compound for the treatment of heart failure. Further clinical trials must be conducted to validate its use as a potential SENP2 inhibitor.
1 Introduction
Heart failure after myocardial infarction is an increasing health obstacle worldwide (Virani et al., 2020) and is basically made by the low regenerative capacity of the adult human heart upon damage (Payan et al., 2020). Although new drugs and reperfusion therapy were developed, the unalterable loss of cardiomyocytes led to cardiac remodeling and, next, heart failure (Ongstad and Gourdie, 2016). Therefore, the identification of new targets to increase cardiac regeneration is a promising strategy to revert the development of heart failure after myocardial infarction. Small Ubiquitin-like Modifier (SUMO) modification is implicated in various cellular processes including protein trafficking, transcriptional regulation, protein stability, cell death, and survival (Seeler and Dejean, 2003). Various SUMOylated proteins preferably associate with particular complexes such as promyelocytic leukemia (PML) bodies and the nuclear pores (Melchior et al., 2003; Müller et al., 2004). SUMOylation (conjugation of SUMO) includes three steps: processing, conjugation, and transition. The transition process includes covalent conjugation of SUMO polypeptides to targets (Gill, 2004). SUMO modification plays a role in cardiac development and function (Wang and Schwartz, 2010; Wang, 2011). SUMO conjugates can be easily deconjugated by SENPs (sentrin-specific proteases) (Mukhopadhyay and Dasso, 2007). Cardiac transcription factors such as Nkx2.5, GATA4, and myocardin are SUMO targets that refer to the role of SUMOylation in cardiovascular development (Wang et al., 2004; Wang et al., 2007; Wang et al., 2008; Wang et al., 2011).
SENP2, a key member of the SENPs family, modulates embryonic development (Kang et al., 2010), fatty acid metabolism (Koo et al., 2015), atherosclerosis (Heo et al., 2015), and neurodegenerative diseases (Fu et al., 2014). This protein may be a SUMOylation suppressor. SENP2 upregulation in MCF7 breast cancer cells was led to reduced glycolysis, but SENP2 knockout in MEF cells resulted in enhanced glycolysis (Tang et al., 2013). SENP2 prevents keratinocyte migration by targeting NDR1 for deSUMOylation and inhibiting wound healing (Xiao et al., 2019). SUSP4 (mouse SENP2) overexpression repressed cell growth, but SUSP4 knockdown by RNA interference (RNAi) increased cell growth (Lee et al., 2006). Like SENP1, SENP2 is also complicated in the organization of gene expression programs in developmental procedures. The promoters of SENP1 and SENP2 possess response elements (REs) that bind to specific transcription factors (TFs). Both SENP1 and SENP2 can impress their transcription by deSUMOylation of transcription factors (Hickey et al., 2012).
The studies have also described a positive effect of SENP2 on the transcriptional activity of nuclear receptors such as androgen receptor (AR), progesterone receptor (PR), and ER-related receptor (ERR2) (Nait Achour et al., 2014). SUMOylation and deSUMOylation are reversible and dynamic processes which when interrupted result in abnormal organogenesis, i.e., development of cleft lip/palate (Alkuraya et al., 2006; Song et al., 2008).
Disturbed flow through SUMOylation of p53 and extracellular signal-regulated kinase 5 (ERK5) lead to atherosclerotic plaque constitution (Heo et al., 2013). Yong Kim et al. have been reported SENP2 upregulation with enhancing deSUMOylation in murine hearts results in cardiac dysfunction and congenital heart defects but SUM O 1 overexpression can improve cardiac structural formation in SENP2-Tg mice (Kim et al., 2012). Chen et al. also showed the loss of SENP2-mediated Akt deSUMOylation and increased Akt kinase activity decrease glycogen synthase kinase 3 beta (GSK3β) levels and subsequently promote cardiomyocyte proliferation and angiogenesis. SENP2 knockdown increased cardiomyocyte dedifferentiation and proliferation both in vitro and in vivo. SENP2 deficiency could also moderate cardiac remodeling and better cardiac function after myocardial infarction (Chen et al., 2021). After birth, overexpression of SENP2 alters cardiomyocyte division and causes congenital heart defects and cardiac dysfunction (Kim et al., 2012). Then due to its significant role in the development of many diseases especially heart failures, SENP2 can be an attractive target for drug discovery.
The in vivo and in vitro studies also demonstrated which natural compounds, especially phytochemicals, minerals, and vitamins, prevent cancer. More than 3,000 plant species have been reported in modern medicine (Millimouno et al., 2014). Natural compounds have many anti-cancerous and anti-turmeric properties such as anti-oxidative antiangiogenic, antiproliferative, and apoptotic effects (Alemi et al., 2013; Rashid et al., 2019; Ahmadi et al., 2020). Toona sinensis (leaf extract) had anti-cancer effects on prostate cancer cells and also caused apoptosis (Chen et al., 2009).Chin Leow et al. reported that curcumin has great therapeutic potential for the treatment of osteosarcoma (Leow et al., 2010). Curcumin has been reported to control autophagy, leading to the inhibition of several types of cancer cell proliferation (for example, chronic myeloid leukemia, malignant glioma, and oesophageal cancer cells) (Jia et al., 2009; Aoki et al., 2007; O'Sullivan-Coyne et al., 2009). Oridonin has been found to show significant anti-proliferative activity, especially inhibiting tumor growth, thus resulting in cancer cell death of melanoma and cervical carcinoma cells (Abelson, 1990; Cui et al., 2006).
Since enhanced deSUMOylation in murine hearts through overexpression of SENP2 led to congenital heart defects and cardiac dysfunction (Kim et al., 2012), we require inhibitor(s) which efficiently bind to SENP2, inhibit SENP2, and are used for heart failure treatment. Among the treatment methods for heart diseases, we can name Cardiac Hospitalization Atherosclerosis Management Program (CHAMP) (Fonarow et al., 2001) and cell therapy (Wollert and Drexler, 2010). Natural products are inexpensive with lower side effects compared with these treatment methods.
According to our previous studies on these secondary metabolites and observation of their anti-inflammatory and antioxidant effects in various cancer cell lines and the nervous system cells on SENP1 protein (Amiraslani et al., 2012; Alemi et al., 2013; Esmaeilzadeh et al., 2013; Rashid et al., 2019; Ahmadi et al., 2020; Taghvaei et al., 2021a; Taghvaei et al., 2021b) and considering that SENP2 protein is one of the factors involved in heart diseases, we suggested these compounds may also affect SENP2 and inhibition of SENP2 can be performed by these compounds to heart failure treatment (Kim et al., 2012; Chen et al., 2021).
Then, our aim was the selection of compounds with more affinity, among natural compounds, for decreasing SENP2 expression for the treatment of heart disease. Our study suggests natural products can be applied as starting points in the development of highly potent SENP2 inhibitors for therapeutic and biological targets. For this purpose, molecular docking was used for the measurement of affinity of natural products including Gallic acid (GA), Caffeic acid (CA), Thymoquinone (TQ), Betanin, Betanidin, Fisetin, and Ebselen as a control (Bernstock et al., 2018) to SENP2. Molecular dynamics (MD) simulation was also used to verify molecular docking and essential dynamic analysis was applied to more surveys. The toxicity of compounds was also predicted. Computational tools, such as computational ADME/Tox properties, ligand-based VS., and MD have extreme importance in pharmaceutical research and industry, to select molecules with therapeutic potential (Ciemny et al., 2018). This is an in silico study to inhibit SENP2 by natural products for use in heart failure treatment. By providing inhibition of SENP2, cardiac function can be improved. This study also introduces SENP2 as a therapeutic target.
2 Material and Methods
In this process, we selected a suitable protein structure based on the resolution and the amino acids of the binding site. In order to the binding of the chemical compounds to SENP2 after the determination of the active site, molecular docking was carried out. The compounds were also molecular docked by Lipinski’s Rule of Five and various modes consisting of ADMET (Hodgson, 2001) and TOPCAT (Taylor, 2005). Then, in order to find a compound bonded with higher efficiency to the active site of SENP2, the MD simulation and essential dynamic analysis were applied. Lastly, the toxicity of compounds was predicted.
2.1 Molecular Docking
2.1.1 Protein and Ligand Preparation
PDB ID: 1TH0 for SENP2 study was extracted from the RCSB PDB database (https://www.rcsb.org) (Rose et al., 2016). The ligand structure was also obtained through http://zinc.docking.org/(Irwin et al., 2012). Before initiating docking, protein and ligand structure were prepared.
Molecular docking of SENP2 was performed with the compounds GA, CA, TQ, Betanin, Betanidin, Fisetin, and Ebselen as control compounds (Supplementary Table S1) using AutoDock4 software (Morris et al., 2009). Molecular docking in the position: x center = -11.518 -y center = 15.863 -z center = 82.218 was performed by considering amino acids of the active site consisting of Leu411, Asn412, His474, Lys476, Val477, His478, Trp479, Met497, Gln542, Trp410Gly545, Ser546, Asp547, Ser548, and Gly549 which were used by other researches (Kumar et al., 2014). Active site verification was performed by the PockDrug server (Hussein et al., 2015).
Molecular docking of the ligands with SENP2 was done by AutoDock4 software packages (Morris et al., 2009). Polar hydrogen atoms were incorporated, non-polar hydrogens were merged, and Gasteiger charges were added. The docking operation was fulfilled in a grid box consisting of 60 × 60 × 60 (x, y, z) points at the center with 100 runs and the grid resolution of 0.375 Å to cover the SENP2 active site. Other parameters were set to default amounts (Taghvaei et al., 2021a; Taghvaei et al., 2021b). The best pose was selected for MD simulation. LigPlot software was used to analyze the docking and MD simulation results obtained from AutoDock4.
2.2 Molecular Dynamics Simulation
2.2.1 Molecular Dynamics Simulation and Binding Free Energy Prediction
PRODRG (Schüttelkopf and Van Aalten, 2004) was applied for topology generation of ligand for the GROMOS force field. MD simulation of docking complexes was done by the genuine union tool of GROMACS (Van Der Spoel et al., 2005). In the MD simulation process, each one of the complexes immersed in a dodecahedron-modeled box (x, y, and z) with 238.58 nm3 and with 1nm of space between the protein periphery and the box edges. We used SPC/E water molecules in order to solvate the system. System neutralization was applied by the addition of five chloride ions for all compounds, Ebselen, and free-SENP2. To instability prevention in MD simulation, the solvated system was subjected to 1,000 cycles minimization. All MD simulations were performed by the GROMACS 4.6.5 package (Abraham et al., 2014) by the GROMOS53a6 force field (Oostenbrink et al., 2005). Before the MD simulation run, the temperature of the crystal structure was attained to 300 K and then equilibrated during 100 ps at the conditions of constant volume and temperature (NVT). Afterward, the system was altered to the constant pressure and temperature (NPT) and equilibrated for 100 ps. The non-bonded cut-off was set at 10 Å and for every 5 steps, the non-bonded pair list has been updated. All MD simulations were performed with the PME parallel version (Guckel, 1999) in the GROMACS suit. LINKS mode was used to constrain all hydrogen bonds and motion equation integration (Essmann et al., 1995) and structural snapshots were flushed every 500 steps (Van Der Spoel et al., 2005). A 50 ns MD simulation of SENP2 in 25 ×106 steps alone (free-SENP2), and in the presence of natural compounds were carried out as mentioned. The computation and analysis of the average hydrogen bonds number between receptor and ligand were performed by g_hbond. The cutoff radius between the acceptor and the donor was 0.35 nm (Taghvaei et al., 2021b).
2.2.2 Molecular Mechanics–Poisson Boltzmann Surface Area
MM-PBSA estimates the free energies and the scoring function in the computational drug design which is excessively applied to the survey of bio-molecular interactions (Kumari et al., 2014; Taghvaei et al., 2021a). The MM-PBSA mode was made in the GROMACS plan was used to compute the difference of the free energies (ΔG) among ligand configurations and free-SENP2.
2.2.3 Data Analysis and Visualization Software
Our 2D images were created using Discovery Studio (Studio, 2008) and 3D images were made using PyMOL (Seeliger and de Groot, 2010).
2.2.4 Analysis of Molecular Dynamics Trajectories
The generated results were analyzed using, g_rms, g_rmsf, g_gyrate, g_sasa, g_hbond, g_mindist, and do_dssp, etc. All plots and figures were prepared using GRACE software (http://plasma-gate.weizmann.ac.il/Grace/).
2.3 Essential Dynamics
Essential dynamics, known as Principal Component Analysis (PCA), can show the collective atomic motion of free-SENP2, GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin by the GROMACS tool. The principal component analysis was computed using g_covar and g_anaeig built-in functions of the GROMACS package. PCA is a standard protocol for the characterization of eigenvectors and the projection across the first PC1 and PC2 (Amadei et al., 1993; Udhaya Kumar et al., 2020; Taghvaei et al., 2021c).
2.4 Investigation of Toxicity by in Silico Method
After molecular docking, MD simulation, and essential dynamic analysis were done. It is necessary to investigate the toxicity of these compounds. Because when a chemical compound is used as an oral drug, it first enters the stomach. The drug must be resistant to the vicinity of gastric acid and must be able to enter intestinal cells after passing through the stomach. The drug must be able to enter the bloodstream through the intestinal wall and go through the blood vessels to the liver. In the liver, a drug must show resistance to metabolism, until it eventually enters the bloodstream and reaches its target. Thus, the drug that both computational and laboratory effects had verified as best on the target protein cannot be used as an oral drug, because it may be changed during this complex route and may not actively achieve its goal. On the other hand, about 20 years ago, before the introduction of computational methods, about 50% of potential therapeutic compounds failed before entering the clinical stage. As a result, a successful drug is not necessarily the best inhibitor of its target, because a drug can be introduced as a successful drug which has the necessary criteria from absorption, distribution, metabolism, excretion, and toxicity (ADMET). Knowing these can increase the speed of drug design.
The process of designing and building a potential therapeutic combination is very costly and time-consuming. As a result, the use of different computational methods to reduce the failure of these candidate compounds is very important and the use of ADMET methods has reduced the failure rate to less than 8% (Merlot, 2010; Cheng et al., 2012). Various software have been designed for this process. Some isfree such as Toxtree, SARpy, T.E.S.T, and CAESAR, and some are commercially available such as ADMET predictor, ACD/TOX suite, TOPKAT, and Derek Nexus. They each have their own advantages over the others, which makes their choice conditional on the type of study (Bakhtyari et al., 2013).
2.4.1 Lipinski Rule of Five
Lipinski Rule of Five consists of molecular weight <500D, hydrogen bond acceptors less than 10, hydrogen bond donors less than 5, logP<5, and Molar refractivity between 40-130 (Lipinski, 2000).
2.4.2 Absorption, Distribution, Metabolism, Excretion, and Toxicity
The human body is constantly exposed to several compounds over time. During the development, several defense barriers have been created to inactivate them. These defenses include the family of cytochrome isoenzymes (CYP) 450 present in the liver, the active return of drug compounds using P-gp (Permeability-glycoprotein), the blood-brain barrier, and the kidneys. Because the range of chemical compounds is so wide, it is almost impossible to examine each of them in the laboratory. Further investigation is assisted by QSAR and QSTR methods. The basis of these methods is based on the principle that compounds with similar structures will have similar functions. So, if we select several known compounds with specific properties as a training series, we can predict the properties of similar compounds with unknown properties (Cheng et al., 2012). In the ADMET study according to the training series, a series of properties of compounds obtained, and the properties of new compounds are measured based on them.
2.4.3 TOPKAT
As explained, the ADMET method is based on a training series. But there is a problem: that the small size of the series which can make the results somewhat erroneous. To solve this problem, a database called NTP (The National Toxicology Program) was introduced in which the effects of different chemical compounds at different concentrations were measured in vitro for a period of 2 years on mice and rats. The TOPKAT model is a tool for using this data as training series, using QSTR methods, and finding similar structures in the newly introduced structure to predict the toxic properties of new compounds. Because these training series contain a larger number of studied compounds that have been examined on living organisms for at least 2 years then, TOPCAT has more reliable results than ADMET. In the case of common indicators that are referred to in both ADMET and TOPKAT methods, the decision criteria are based on TOPKAT results.
3 Results
3.1 Molecular Docking Study
Molecular docking results demonstrate an exact and preferred orientation of natural products in the active pocket of the protein (Naqvi et al., 2018). The active site used was verified with a drug probability of 62%. The lowest binding free energy was computed with AutoDock4 and the compounds according to lowest binding energy included CA, GA, Betanin, Fisetin, Ebselen, Betanidin, and TQ, as shown in Table 1. CA, GA, Betanin, and Fisetin had better binding free energy than Ebselen. Energy items were the torsional and internal energy of the ligand, hydrogen bonds, intermolecular forces, electrostatic energy, van der Waals energy, and desolvation energy. Compounds bound deeper into the active pocket and perhaps diminished the accessibility of SENP2 which may be responsible for controlling its biological function.
Hydrogen bonds and hydrophobic bonds between compounds and SENP2 were displayed in Table 2. LigPlot images were also displayed in Supplementary Figure S1. We observed GA and Betanidin constituted the most hydrogen bonds with the active site of SENP2. Ebselen and CA were also showed the most hydrophobic bonds with the active site of SENP2, as shown by Table 2.
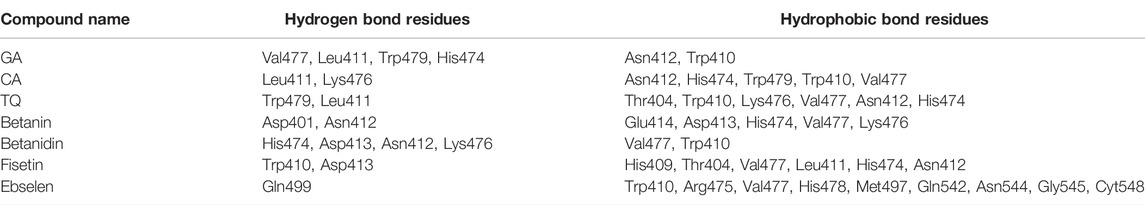
TABLE 2. Amino acid residues contributed in molecular docking including hydrogen bond and hydrophobic bond.
3.2 Molecular Dynamics Simulation Method
MD simulation is a strong computational procedure to display the flexibilities of molecules (Syed et al., 2018). Structural information of the binding mechanism of natural products with the SENP2 was obtained through MD simulation.
3.2.1 Free Energy Calculation
The binding energy for GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin were -179. 57, -195.599, -4.247, -328.872, -465.85, -72.844, and -89.984 kJ/mol which Betanin and Betanidin showed stronger binding compared with Ebselen, Table 3.

TABLE 3. Binding energy of MD simulation for compounds: GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin.
3.2.2 Average Potential Energy
The average potential energy was monitored to determine the equilibration of the systems before the MD analysis. At a constant temperature (300 K), the average potential energy for free-SENP2 was estimated to be -647,631 kJ/mol. Average potential energy for GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin, were -642,154 kJ/mol, -655,213 kJ/mol, -646,049 kJ/mol, -656,986 kJ/mol, 656,179 kJ/mol, -653,727 kJ/mol, and -654,855 kJ/mol, respectively, Table 4. The average potential energy of Betanin and Betanidin were better than others even than Ebselen.
3.2.3 Structural Deviations and Compactness
The binding of a ligand to the protein can generally induce significant conformational alterations in the structure. The root mean square deviation (RMSD) parameter was computed to observe whether the structure of a protein is stable and near the experimental structure (Taghvaei et al., 2021a). The average RMSD value for free-SENP2, GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin were found to be 0.21, 0.23, 0.24, 0.17, 0.21, 0.23, 0.20, and 0.21 nm, respectively. We observed TQ, Fisetin, and Betanin have lower RMSD than others and are very close to free-SENP2 and Ebselen. The RMSD plot proposed that the binding of all these compounds were significantly stabilized the structure of SENP2 and were resulted in fewer structural deviations from its native conformation and were equilibrated throughout the 50 ns MD simulation (Figure 1).
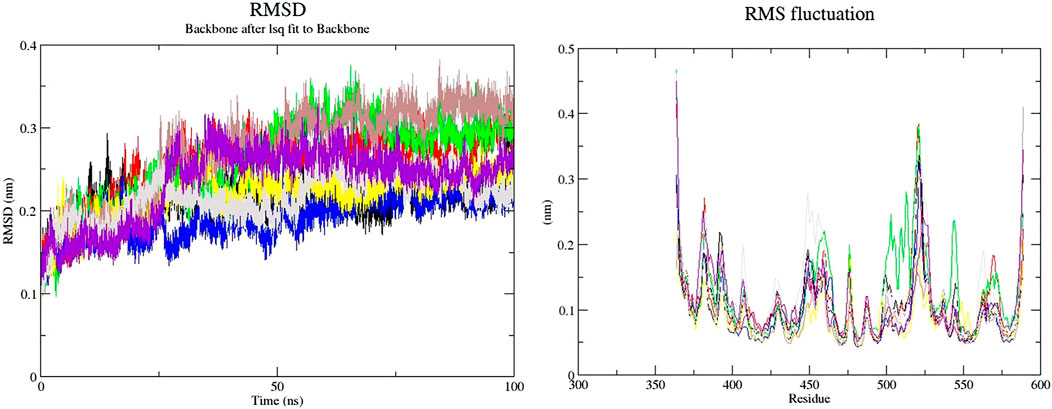
FIGURE 1. RMSD and RMSF plots of free-SENP2 and SENP2 complexes (black) free-SENP2, red) GA, green) CA, blue) TQ, yellow) Betanin, brown) Betanidin, gray) Ebselen, and purpule) Fisetin).
To compute the average fluctuation of residues, the root mean square fluctuation (RMSF) of the SENP2 upon ligands binding was drawn as a function of residue number (Figure 1). The RMSF plot demonstrated residual fluctuations present at various regions of SENP2, the especially active sites. We observed Betanidin to have lower fluctuations.
Rg is an indicator of the level of structure compaction, i.e. the polypeptide is unfolded or folded (Taghvaei et al., 2021b; Taghvaei et al., 2021c). The radius of gyration (Rg) is a parameter that is usually calculated to gain insights into the stability of the protein in terms of alteration in the volume of protein. A protein with higher Rg values has a flexible packing. The average Rg values for free-SENP2, GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin were found to be 1.81, 1.82, 1.84, 1.81, 1.82, 1.81, 1.81, and 1.82 nm, respectively (Figure 2). These differences in the Rg values were not remarkable and SENP2-compounds complexes were stable.
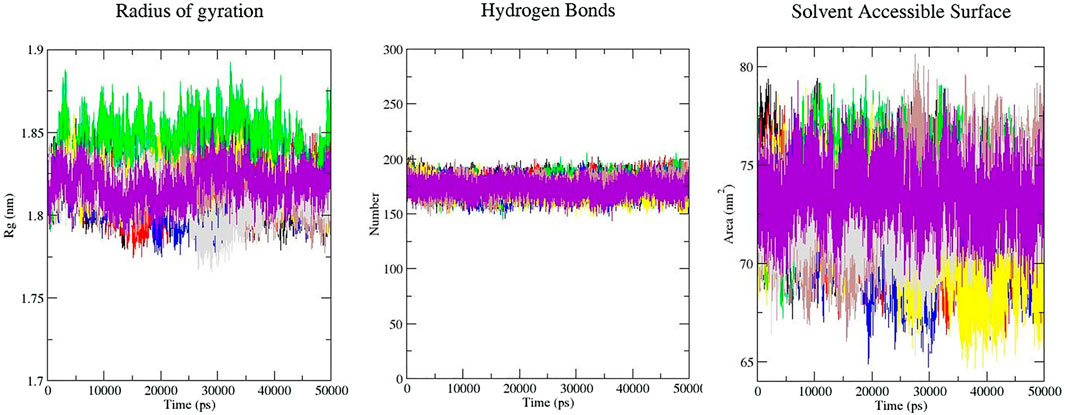
FIGURE 2. Rg, intramolecular hydrogen bonds, and SASA plots of SENP2 complexes, black) free-SENP2, red) GA, green) CA, blue) TQ, yellow) Betanin, brown) Betanidin, gray) Ebselen, and purple) Fisetin.
3.2.4 Solvent Accessible Surface Area
Calculation of SASA (Solvent Accessible Surface Area) supplies the conformational changes in protein upon ligand binding. Estimation of SASA provides information about the conformational changes in protein upon ligand binding (Taghvaei et al., 2021b). The average SASA values for free-SENP2 and SENP2-compounds were also monitored in 50 ns MD simulations. The average SASA values for free-SENP2, GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin were found to be 73, 72, 74, 72, 71, 73, 72, and 74 nm2, respectively. There were negligible differences, (Figure 2).
3.2.5 Hydrogen Bonds Analysis
The molecular identification between a protein and ligand is related to the hydrogen bonding pattern which supplies a specificity and directionality of interaction (Taghvaei et al., 2021a). We have computed hydrogen bonds pairing (within 0.35 nm) between SENP2 and ligands in the solvent condition. An average number of intramolecular hydrogen bonds were measured. We observed that the average number of intramolecular hydrogen bonds of free-SENP2, GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin were 177, 176, 176, 174, 172, 175, 174, and 174 hydrogen bonds, respectively, (Figure 2). We observed GA, CA, and Thymoquinone have more hydrogen bonds and potent binding. Of course, the differences were not significant.
It has been found that GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin bind to the active pocket of SENP2 with 4-5, 4–8, 2–3, 4–7, 4–7, 1, and two to three intermolecular hydrogen bonds, respectively (Figure 3).
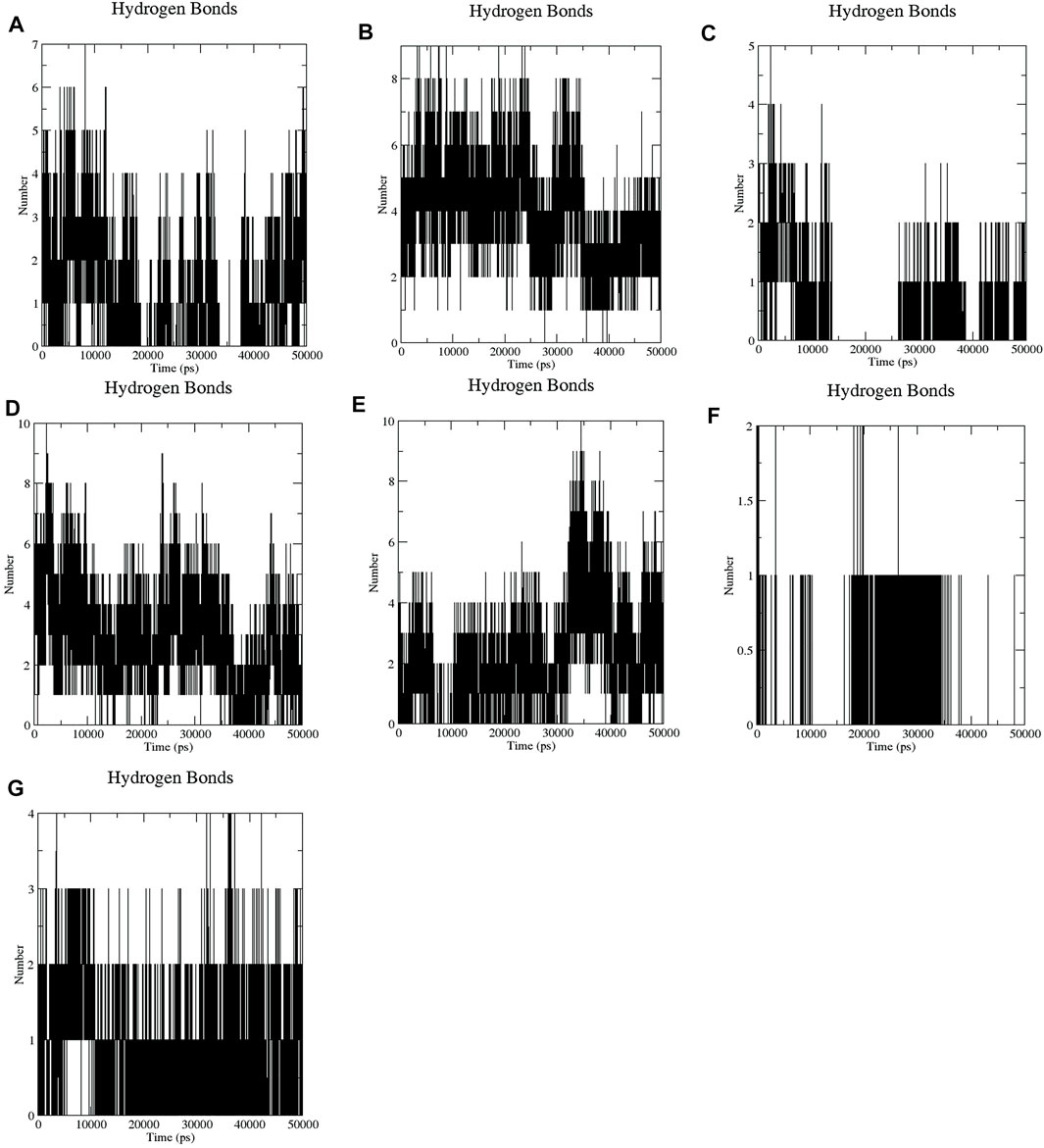
FIGURE 3. Intermolecular hydrogen bond plots of compounds (A) GA, (B) CA, (C) TQ, (D) Betanin, (E) Betanidin, (F) Ebselen, and (G) Fisetin.
3.2.6 Distance Between SENP2 and Compounds
Distance between SENP2 and ligands were obtained using the embedded packages within GROMACS. Distance between GA, CA, TQ, Betanin, Betanidin, Ebselen, Fisetin and SENP2 were 0.19, 0.15, 0.36, 0.17, 0.18, 0.22, and 0.18 nm, respectively, see Figure 4. CA and Betanin represented the lowest distance with SENP2 protein.

FIGURE 4. Min distance between SENP2 and compounds (A) GA, (B) CA, (C) TQ, (D) Betanin, (E) Betanidin, (F) Ebselen, and (G) Fisetin.
3.2.7 Secondary Structure Changes
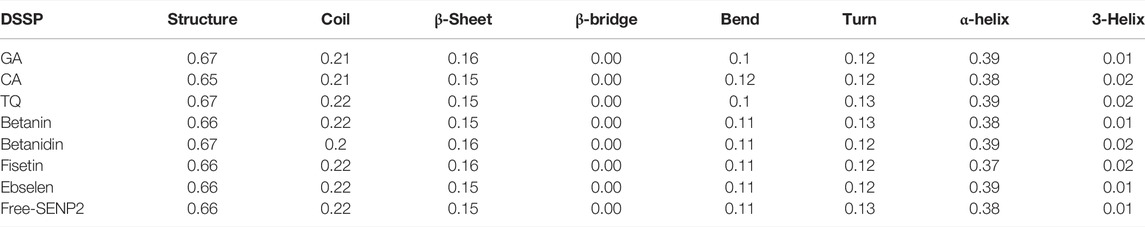
The aim of this analysis is the measurement of the alteration in the secondary structure of SENP2 upon binding to the compounds as a function of time. (Table 5). We did not observe any changes for Betanin. After Betanin, Ebselen showed the lowest changes including a decrease in bend and an increase in α-helix. Secondary structure changes for TQ included a decrease in bend, and an increase in α-helix and 3-helix. The most changes occurred in CA, GA, and Betanidin. Secondary structure changes for Fisetin also included an increase in β-sheet and 3-helix and a decrease in turn and α-helix. Although minor changes were seen in the secondary structure, the Betanin did not show any alteration (Figure 5).
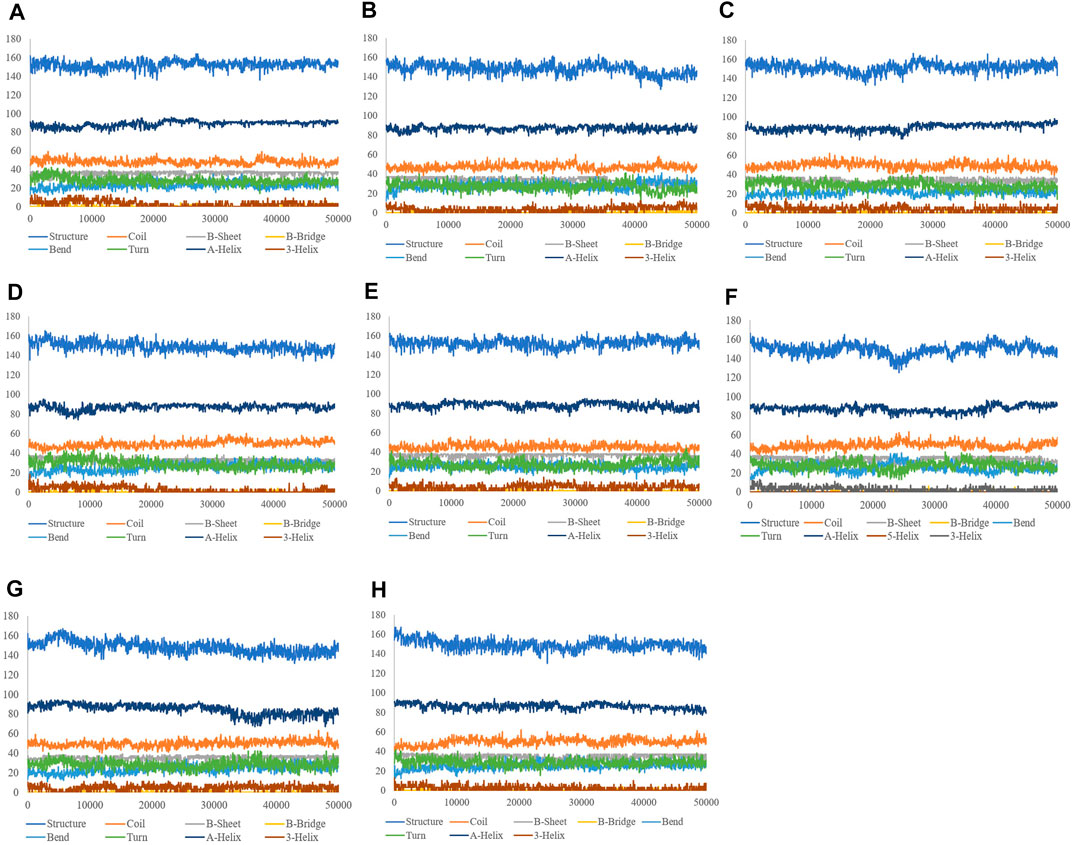
FIGURE 5. Secondary structure plots of compounds (A) GA, (B) CA, (C) TQ, (D) Betanin, (E) Betanidin, (F) Ebselen, (G) Fisetin, and (H) free-SENP2.
3.2.8 Data Visualization
After molecular dynamics simulation, the interaction between SENP2 and ligands by LigPlot. As we were displayed in Figure 5, Betanin constitutes bond with Thr404, Trp408, His409, Trp410, Leu411, Asn412, His474, Val477, Trp479, Gly545, and Ser546. Betanidin constitutes a bond with Trp410, Arg475, Lys476, Val477, His478, Met497, Gln499, Gln542, Gly545, and Ser548. Ebselen also binds to Trp410, Leu411, Asn412, Ile416, Val477, His478, Trp479, Met497, Gln542, Gly545, Ser546, Asp547, Ser548, and Gly549, Met550, Asn544, and Arg576. The binding of these compounds is with the active site of SENP2, see Figure 6 and Supplementary Figure S2. The 3D structures have also been displayed in Figure 7.
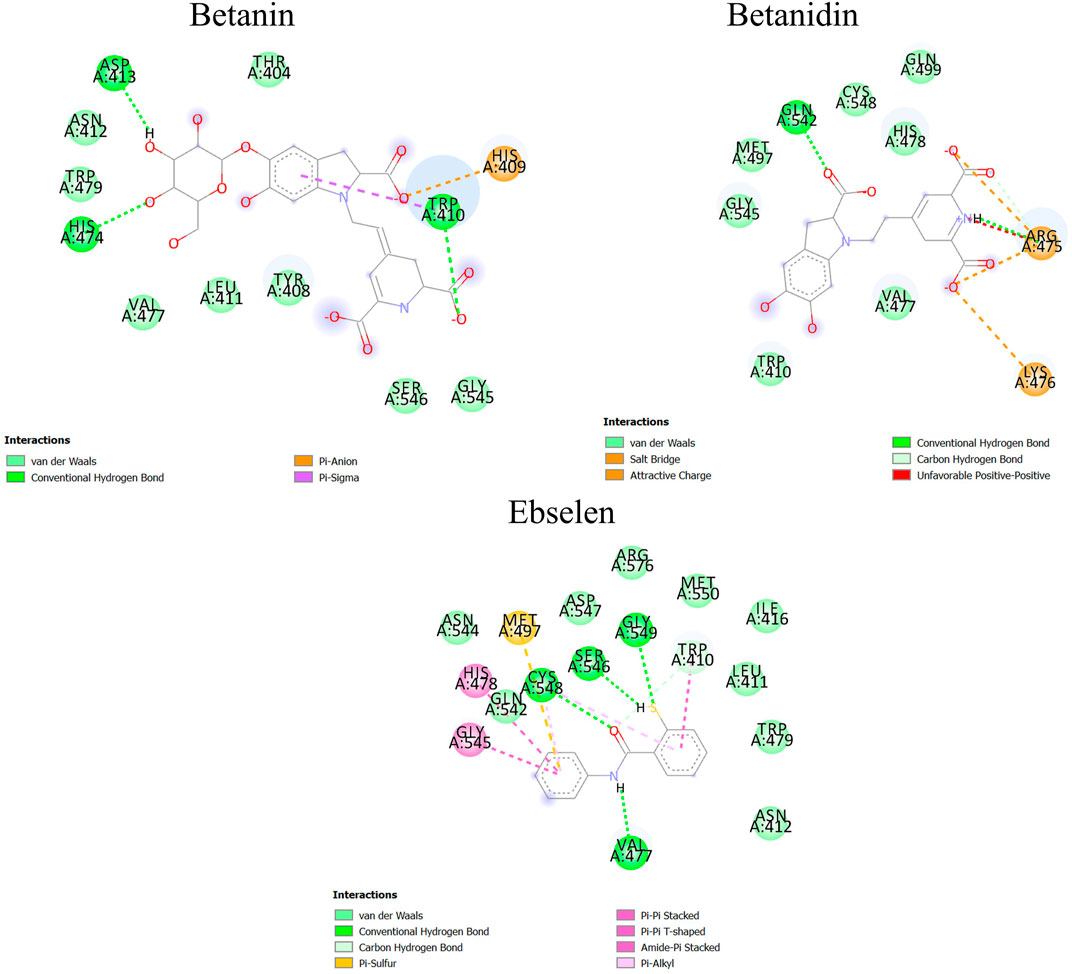
FIGURE 6. Binding between Betanin, Betanidin, and Ebselen with SENP2 by Discovery Studio after molecular dynamics simulation (2D structures).
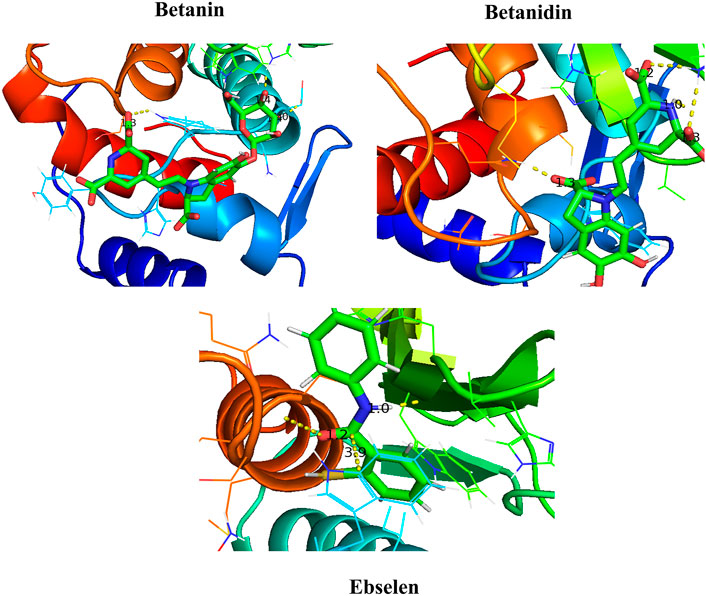
FIGURE 7. Binding between Betanin, Betanidin, and Ebselen with SENP2 by PyMOL after molecular dynamics simulation (3D structures).
3.3 Essential Dynamics
The dynamics of GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin were obtained through a principal component analysis (PCA) (Kumar et al., 2019). The projection of trajectories of GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin during the molecular dynamic’s simulation in the phase space along the first two principal components (PC1, PC2) at 300 K were plotted in Figure 7. It predicts the large-scale collective motions for GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin. PCA analysis showed that these compounds change the structural dynamics of SENP2. Figure 8 plot clearly shows that TQ, Betanin, Ebselen, and Fisetin had fewer movements and occupied less space in phase space while free-SENP2 occupied more space which verifies the overall increased stability of TQ, Betanin, Ebselen, and Fisetin in the binding to SENP2. It is comparable to compounds MB_241, MB_250, and MB_266 in the study of Ahmad et al. for SARS-COV-2 inhibition (Ahamad et al., 2021). The PCA analysis results agreed with the results from MDS.
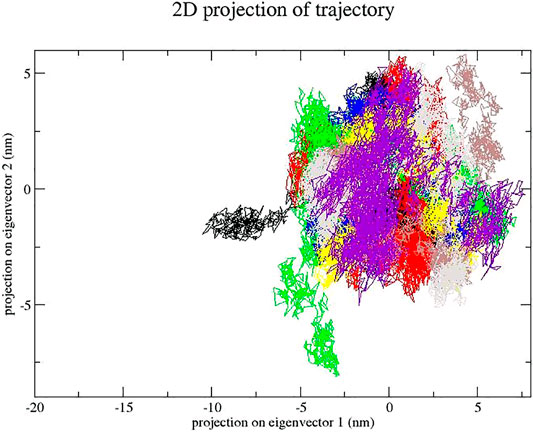
FIGURE 8. Principal component analysis. Projection of the motion for compounds (red) GA, green) CA, blue) TQ, yellow) Betanin, brown) Betanidin, gray) Ebselen, purple) Fisetin, and black) free-SENP2. in phase space along the PC1 and PC2.
3.4 Investigation of Toxicity by in Silico Method
Druglikness is a qualitative meaning used in the design of a drug indicating how a substance is “druglike”. The drug-likeness properties of these compounds were gained using Lipinski’s Rule of Five, admetSAR, and TOPKAT. All of the compounds obeyed Lipinski’s Rule of Five. The results of the drug-likeness by ADMET and TOPKAT are presented in Supplementary Table S2, S3. The chemical properties of the identified compounds require to the determination of pharmacokinetic properties evaluated in terms of absorption, distribution, metabolism (how they interact with cytochromes), excretion (excretion of the kidney), and toxicity. AMES carcinogenicity was safe for all of the compounds and they were not a carcinogen and not a mutagen. The results of ADMET also showed TQ, Ebselen, and Fisetin able to cross the blood-brain barrier and the intestinal wall. So, these can be used for brain tumors and can be used orally. All the compounds were permeable to CaCO2 except Betanin and Betanidin. All compounds could be localized in the mitochondria except CA which could be localized in the nucleus. Only GA and CA cross the intestinal wall, but Betanin and Betanidin did not cross the intestinal wall and blood-brain barrier.
Other indicators that were evaluated at this stage were the ability to bind and suppress glycoproteins which are actively involved in the removal of xenobiotics from the cell. The ideal druglike compounds are compounds that do not bind to glycoproteins and therefore do not leave the cell. In this case, GA, CA, TQ, and Ebselen were not substrates for glycoproteins, but Betanidin, Betanin, and Fisetin were substrates for glycoproteins. The point to be considered is that from these selected compounds, ideal ones are neither glycoprotein substrates nor inhibitors. Because, these glycoproteins have other roles that by inhibiting them, these roles can be inhibited and the normal function of the cell is likely disturbed. Thus, GA, Betanidin, CA, Fisetin, Betanin, and Ebselen were not glycoprotein inhibitors, but TQ inhibits P-glycoprotein. All compounds were not an inhibitor of P-glycoprotein. Another indicator that has been measured is the possibility of metabolizing by cytochrome 450 and inhibiting this complex of metabolic proteins. The compound which cannot be metabolized can accumulate in the body and can lead to unwanted side effects. The compounds which can be metabolized by these proteins were selected. GA, CA, Fisetin, and TQ were not CYP450 substrates but Betanidin, Betanin, and Ebselen were CYP450 3A4 substrates. On the other hand, GA, TQ, CA, Betanidin, and Betanin were not inhibitors of CYP450, but Fisetin was an inhibitor of CYP450 1A2, and Ebselen was an inhibitor of CYP450 1A2, CYP450 2C9, CYP450 2D6, CYP450 2C19, and CYP450 3A4. Fish Toxicity (FHMT), Tetrahymena Pyriformis Toxicity (TPT), and Honey Bee Toxicity (HBT) were high for all compounds while, Honey Bee Toxicity (HBT) was low for Betanin, Betanidin, and Ebselen. Also, all the compounds represented weak inhibition potential of the human ether-a-go-go-related gene (hERG), which its expression has a significant role in the repolarization of the cardiac action potential (Sanguinetti et al., 1995).
The ADMET method is based on QSAR and QSTR methods, the number of properties proposed through statistical calculations, and the similarity between the studied compounds and compounds with approved properties. While in TOPKAT method, calculations are based on laboratory examination of a series of basic compounds in 2 years that their similar structures are used in other chemical structures. The results of these experiments predict the similar structures of the structure under study. As a result, differences in the studies between the two methods can be expected and, in such cases, a conclusion is made based on the TOPKAT results.
On the other hand, to evaluate the toxicity of the identified compounds based on the QSTR model, TOPKAT (Discovery Studio 2.5, Biovia, San Diego, CA, United States) was applied. This model is based on repetitive statistical methods with high credit ratings and is highly developed. In this model, the toxic effects of these compounds based on their chemical structure are predicted. The numeric values of TOPKAT software are divided into two categories. The first group of numbers contains from 0.0 to 1.0. These numbers are related to endpoint investigations which represent the probable calculated values for each of the compounds. The values from 0.0 to 0.3 represent the negative response of the compounds in the laboratory tests. While values between 0.7 and 1.0 represent a positive response in these experiments, the values between 0.3 and 0.7 indicate an intermediate state. The second group of numbers related to the amounts consumed by these compounds with a concentration value greater than 1.0. The results are presented in Supplementary Table S3. TOPKAT results showed all compounds are safe for the AMES mutagenicity test except Fisetin, CA, Betanin, and Ebselen. Also, in NTP Carcinogenicity tests, GA, CA, and Betanin in NTP Carcinogenicity Call (Male Rat) (v3.2) test were not carcinogenic, but Fisetin and Ebselen were carcinogenic. GA, Betanidin, TQ, and Betanin were not carcinogenic in NTP Carcinogenicity Call (Female Rat) (v3.2) test, but Fisetin and Ebselen were carcinogenic. GA, Betanidin, TQ, CA, Betanin, and Ebselen were not carcinogenic in NTP Carcinogenicity Call (Male Mouse) (v3.2) test, but Fisetin was carcinogenic. GA, TQ, CA, Fisetin, Betanin, and Ebselen were not carcinogenic in NTP Carcinogenicity Call (Female Mouse) test (v3.2) but Betanidin was carcinogenic. FDA Carcinogenicity tests emphasize contact frequency and long-term effects faced by the compounds under investigation. GA, TQ, Fisetin, and CA were not carcinogenic in the FDA Carcinogenicity Male Rat Non vs. Carc (v3.1) test. Only Betanidin and Ebselen were not carcinogenic in the FDA Carcinogenicity Male Rat Single vs. Mult (v3.1) test. GA and CA were not carcinogenic in the FDA Carcinogenicity Female Rat Non vs. Carc (v3.1) test. GA, Betanidin, CA, Fisetin, Betanin, and Ebselen were not carcinogenic in the FDA Carcinogenicity Female Rat Single vs. Mult (v3.1) test. GA, Betanidin, Fisetin, TQ, and Ebselen were not carcinogenic in the FDA Carcinogenicity Male Mouse Non vs. Carc (v3.1) test. GA, Betanidin, TQ, CA, Fisetin, Betanin, and Ebselen were not carcinogenic in the FDA Carcinogenicity Male Mouse Single vs. Mult (v3.1) test. Only GA, Fisetin, and TQ were not carcinogenic in FDA Carcinogenicity Female Mouse Non vs. Carc (v3.1) test and Only GA, Fisetin, and Ebselen were not carcinogenic in FDA Carcinogenicity Female Mouse Single vs. Mult (v3.1) test.
Developmental Toxicity Potential indicates mutagenic characteristics during development that can restrict their use in the pregnancy. Only GA, TQ, and CA were safe. Skin Irritation test (v6.1) showed only GA, Fisetin, Betanin, and Ebselen do not irritate the skin. Skin Sensitization examination revealed all of the compounds that cause skin allergies in Sensitization NEG v SENS (v6.1) test and GA, TQ, CA, Betanin, and Ebselen through Skin Sensitization MLD/MOD v SEV (v6.1) test do not cause skin allergies. Ocular Irritancy test also showed GA, Betanidin, CA, Fisetin, Betanin, and Ebselen do not cause ocular irritation in Ocular Irritancy SEV vs. MOD (v5.1) test. In Ocular Irritancy MLD vs. NON (v5.1) test also GA, Fisetin, and Betanin did not cause ocular irritation and in the Ocular Irritancy, SEV/MOD vs. MLD/NON (v5.1) test only Fisetin and Ebselen did not result in ocular irritation. In the Aerobic Biodegradability test, Fisetin, Betanidin, TQ, and Ebselen were resistant to the effects of biodegradation. We concluded using TOPKAT and ADMET properties, GA, CA, and Betanin had the lowest toxicity and side effects.
4 Discussion
SUMO activation and deconjugation from targets are carried out by SENPs. It is difficult to study this posttranslational modification because of the lack of reagents to obstruct the removal of SUMO from targets (Albrow et al., 2011). SENP1 and SENP2 have equal substrate specificity (Cheng et al., 2007). SENP2 is a nuclear envelope-related protease and when upregulated, it possesses activity like SENP1 (Cheng et al., 2004). Cobenas-Potts et al. found SENP1 and SENP2 are situated to change spatial and temporal regulation of SUMOylation via unequaled assemblies with kinetochores, centrosomes, and spindle microtubules. SENP1 or SENP2 play roles in temporal and spatial regulation of SUMOylation in mitosis and alteration of their expression cause to failings in chromosome congression in prometaphase or sister chromatid separation at metaphase. SENP2 upregulation induces a defect in chromosomal congression and pertains to its accurate kinetochore pointing (Cubeñas-Potts et al., 2013). In addition to, SENP2 upregulation in murine hearts induced cardiac dysfunction and congenital heart defects (Kim et al., 2012). Inhibition of Akt deSUMOylation by SENP2 can raise cardiomyocyte proliferation and angiogenesis to improve cardiac function after myocardial infarction (Chen et al., 2021).
In this study, we examined GA, CA, TQ, Betanin, Betanidin, Ebselen, and Fisetin. Docking and simulation results showed that among these ligands, Betanin is the most stable compound in the binding to SENP2. Betanin showed the lowest RMSD value (0.21 nm). Betanin also constituted four to seven hydrogen bonds and had the highest potential energy (-656,986 kJ/mol) even than Ebselen as inhibitor of SENP2. Betanin also represented the highest MD binding energy (-328.872 kJ/mol) after Betanidin. Betanin with hydrogen bonds and hydrophobic bonds also formed a powerful complex with the active site of SENP2. Betanin also showed a low distance with SENP2 even than Ebselen. This in silico finding is consistent with the results of in vivo and in vitro studies. Betanin presents in Beta vulgaris. Betanin is a principal pigment and an active phytochemical of beetroot (Gliszczyńska-Świgło et al., 2006) that has the properties of anti-proliferative, antioxidant, free radical-scavenging, anti-inflammatory, and pro-apoptotic. Betanin through the activation of Nrf2 may induce the expression of phase II detoxifying enzymes in human non-tumor liver cells. Betanin with the activation of Nrf2 led to increased GSTM, GSTP, GSTT, and NQO1 mRNA and protein levels and GST and NQO1 activities in the THLE-2 cells. Betanin has significantly enhanced the mRNA and protein levels of p53 in the THLE-2 cells (Krajka-Kuźniak et al., 2013). Betanin induced apoptosis and inhibited carcinogenesis in esophageal (Lechner et al., 2010), myeloid leukemia (Sreekanth et al., 2007), skin, and lung (Kapadia et al., 1996). The different scientists reported the interaction of molecular docking of various Betalain compounds including Betanin against NS2/NS3 protease (ul Qamar et al., 2014), and Betanidin against Nrf2 activator (Gacesa et al., 2015). In another study, the interaction of Betalain to LOX11 and COX22 was reported (Vidal et al., 2014). These studies are representative of the anti-virus, anti-oxidative and anti-inflammatory properties of Betalain.
In the previous studies, we showed GA can inhibit SENP1 and can be explored as a drug for cancer treatment (Taghvaei et al., 2021b) and Bethanidine with SENP1 inhibition can be a suitable candidate against cardiovascular diseases (Taghvaei et al., 2021a). Kumar et al. introduced 1, 2, 5-Oxadiazoles as SENP2 inhibitors (Kumar et al., 2014). Kumar et al docked Namiki-shoji small molecule library with ∼4 million commercially available compounds. We also docked 1, 2, 5-Oxadiazoles with IC50 < 10 µM which were displayed in Table 6. We observed our compounds have the lowest binding energy very close to 1, 2, 5-Oxadiazoles (Kumar et al., 2014).
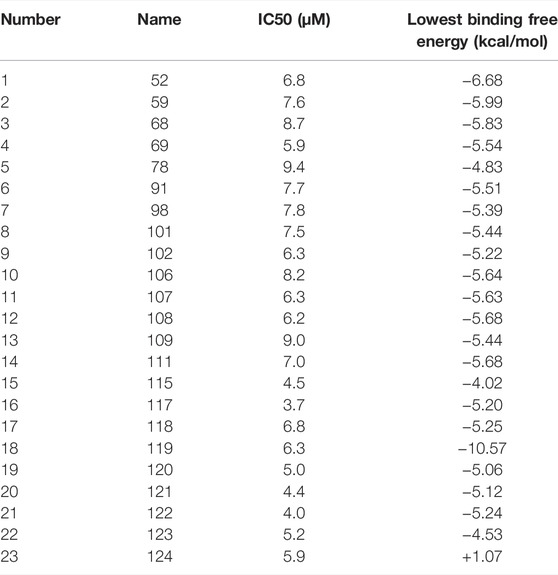
TABLE 6. molecular docking of 1, 2, 5-Oxadiazoles (from virtual screening of Namiki-shoji small molecule library with SENP2, Kumar, et al.) with IC50 < 10µM with SENP2.
We hope Betanin is useful in the treatment of various and complex diseases. Using natural compounds, side effects can be diminished and the cost of drugs can be decreased. Our results propose that SENP2 may be an attractive target for the design of new targets for cardiac regeneration in the future. The method used to identify and introduce higher binding power compounds to SENP2 as a potential therapeutic target is an effective way of identifying novel inhibitor compounds.
It is expected that this study will demonstrate the potential of Betanin for use in laboratory studies against SENP2 in heart defects and cardiac dysfunction which initially studies have demonstrated the potentiality of this drug-like compound.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
ST wrote the manuscript and analyzed the data. FS and ZM conducted the research. ST conceived or designed the studies. ZM participated edited the manuscript. All authors read andapproved the final manuscript.
Funding
This work was supported by the National Institutes of Genetic Engineering and Biotechnology.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.817990/full#supplementary-material
Footnotes
1Lipoxygenase1.
2Cyclooxygenase2.
References
Abelson, P. H. (1990). Medicine from Plants. Science 247 (4942), 513–514. doi:10.1126/science.2300807
Abraham, M. J., Van Der Spoel, D., Lindahl, E., and Hess, B. (2014). “The GROMACS Development Team” in GROMACS User Manual Version 5.0. KTH (Sweden: Royal Institute of Technology, Solna, Sweden and Uppsala University).
Ahamad, S., Kanipakam, H., Birla, S., Ali, M. S., and Gupta, D. (2021). Screening Malaria-Box Compounds to Identify Potential Inhibitors against SARS-CoV-2 Mpro, Using Molecular Docking and Dynamics Simulation Studies. Eur. J. Pharmacol. 890, 173664. doi:10.1016/j.ejphar.2020.173664
Ahmadi, H., Nayeri, Z., Minuchehr, Z., Sabouni, F., and Mohammadi, M. (2020). Betanin Purification from Red Beetroots and Evaluation of its Anti-oxidant and Anti-inflammatory Activity on LPS-Activated Microglial Cells. PloS one 15 (5), e0233088. doi:10.1371/journal.pone.0233088
Albrow, V. E., Ponder, E. L., Fasci, D., Békés, M., Deu, E., Salvesen, G. S., et al. (2011). Development of Small Molecule Inhibitors and Probes of Human SUMO Deconjugating Proteases. Chem. Biol. 18 (6), 722–732. doi:10.1016/j.chembiol.2011.05.008
Alemi, M., Sabouni, F., Sanjarian, F., Haghbeen, K., and Ansari, S. (2013). Anti-inflammatory Effect of Seeds and Callus of Nigella Sativa L. Extracts on Mix Glial Cells with Regard to Their Thymoquinone Content. Aaps Pharmscitech 14 (1), 160–167. doi:10.1208/s12249-012-9899-8
Alkuraya, F. S., Saadi, I., Lund, J. J., Turbe-Doan, A., Morton, C. C., and Maas, R. L. (2006). SUMO1 Haploinsufficiency Leads to Cleft Lip and Palate. Science 313 (5794), 1751. doi:10.1126/science.1128406
Amadei, A., Linssen, A. B., and Berendsen, H. J. (1993). Essential Dynamics of Proteins. Proteins 17 (4), 412–425. doi:10.1002/prot.340170408
Amiraslani, B., Sabouni, F., Abbasi, S., Nazem, H., and Sabet, M. (2012). Recognition of Betaine as an Inhibitor of Lipopolysaccharide-Induced Nitric Oxide Production in Activated Microglial Cells. Iran Biomed. J. 16 (2), 84–89. doi:10.6091/ibj.1012.2012
Aoki, H., Takada, Y., Kondo, S., Sawaya, R., Aggarwal, B. B., and Kondo, Y. (2007). Evidence that Curcumin Suppresses the Growth of Malignant Gliomas In Vitro and In Vivo through Induction of Autophagy: Role of Akt and Extracellular Signal-Regulated Kinase Signaling Pathways. Mol. Pharmacol. 72 (1), 29–39. doi:10.1124/mol.106.033167
Bakhtyari, N. G., Raitano, G., Benfenati, E., Martin, T., and Young, D. (2013). Comparison of In Silico Models for Prediction of Mutagenicity. J. Environ. Sci. Health C Environ. Carcinog Ecotoxicol Rev. 31 (1), 45–66. doi:10.1080/10590501.2013.763576
Bernstock, J. D., Ye, D., Smith, J. A., Lee, Y. J., Gessler, F. A., Yasgar, A., et al. (2018). Quantitative High-Throughput Screening Identifies Cytoprotective Molecules that Enhance SUMO Conjugation via the Inhibition of SUMO-specific Protease (SENP)2. FASEB J. 32 (3), 1677–1691. doi:10.1096/fj.201700711R
Chen, H. M., Wu, Y. C., Chia, Y. C., Chang, F. R., Hsu, H. K., Hsieh, Y. C., et al. (2009). Gallic Acid, a Major Component of Toona Sinensis Leaf Extracts, Contains a ROS-Mediated Anti-cancer Activity in Human Prostate Cancer Cells. Cancer Lett. 286 (2), 161–171. doi:10.1016/j.canlet.2009.05.040
Chen, Y., Xu, T., Li, M., Li, C., Ma, Y., Chen, G., et al. (2021). Inhibition of SENP2-Mediated Akt deSUMOylation Promotes Cardiac Regeneration via Activating Akt Pathway. Clin. Sci. (Lond) 135 (6), 811–828. doi:10.1042/CS20201408
Cheng, F., Li, W., Zhou, Y., Shen, J., Wu, Z., Liu, G., et al. (2012). admetSAR: A Comprehensive Source and Free Tool for Assessment of Chemical ADMET Properties. ACS Publications.
Cheng, J., Kang, X., Zhang, S., and Yeh, E. T. (2007). SUMO-specific Protease 1 Is Essential for Stabilization of HIF1alpha during Hypoxia. Cell 131 (3), 584–595. doi:10.1016/j.cell.2007.08.045
Cheng, J., Wang, D., Wang, Z., and Yeh, E. T. (2004). SENP1 Enhances Androgen Receptor-dependent Transcription through Desumoylation of Histone Deacetylase 1. Mol. Cel Biol 24 (13), 6021–6028. doi:10.1128/MCB.24.13.6021-6028.2004
Ciemny, M., Kurcinski, M., Kamel, K., Kolinski, A., Alam, N., Schueler-Furman, O., et al. (2018). Protein-peptide Docking: Opportunities and Challenges. Drug Discov. Today 23 (8), 1530–1537. doi:10.1016/j.drudis.2018.05.006
Cubeñas-Potts, C., Goeres, J. D., and Matunis, M. J. (2013). SENP1 and SENP2 Affect Spatial and Temporal Control of Sumoylation in Mitosis. Mol. Biol. Cel 24 (22), 3483–3495. doi:10.1091/mbc.E13-05-0230
Cui, Q., Tashiro, S., Onodera, S., and Ikejima, T. (2006). Augmentation of Oridonin-Induced Apoptosis Observed with Reduced Autophagy. J. Pharmacol. Sci. 101 (3), 230–239. doi:10.1254/jphs.fpj06003x
E. L. Ongstad, and R. G. Gourdie (Editors) (2016). “Can Heart Function Lost to Disease Be Regenerated by Therapeutic Targeting of Cardiac Scar Tissue?,” Seminars in Cell & Developmental Biology (Elsevier).
Esmaeilzadeh, E., Gardaneh, M., Gharib, E., and Sabouni, F. (2013). Shikonin Protects Dopaminergic Cell Line PC12 against 6-Hydroxydopamine-Mediated Neurotoxicity via Both Glutathione-dependent and Independent Pathways and by Inhibiting Apoptosis. Neurochem. Res. 38 (8), 1590–1604. doi:10.1007/s11064-013-1061-9
Essmann, U., Perera, L., Berkowitz, M. L., Darden, T., Lee, H., and Pedersen, L. G. (1995). A Smooth Particle Mesh Ewald Method. J. Chem. Phys. 103 (19), 8577–8593. doi:10.1063/1.470117
Fonarow, G. C., Gawlinski, A., Moughrabi, S., and Tillisch, J. H. (2001). Improved Treatment of Coronary Heart Disease by Implementation of a Cardiac Hospitalization Atherosclerosis Management Program (CHAMP). Am. J. Cardiol. 87 (7), 819–822. doi:10.1016/s0002-9149(00)01519-8
Fu, J., Yu, H. M., Chiu, S. Y., Mirando, A. J., Maruyama, E. O., Cheng, J. G., et al. (2014). Disruption of SUMO-specific Protease 2 Induces Mitochondria Mediated Neurodegeneration. Plos Genet. 10 (10), e1004579. doi:10.1371/journal.pgen.1004579
Gacesa, R., Dunlap, W. C., and Long, P. F. (2015). Bioinformatics Analyses Provide Insight into Distant Homology of the Keap1-Nrf2 Pathway. Free Radic. Biol. Med. 88, 373–380. doi:10.1016/j.freeradbiomed.2015.06.015
Gill, G. (2004). SUMO and Ubiquitin in the Nucleus: Different Functions, Similar Mechanisms? Genes Dev. 18 (17), 2046–2059. doi:10.1101/gad.1214604
Gliszczyńska-Świgło, A., Szymusiak, H., and Betanin, M. P. (2006). The Main Pigment of Red Beet: Molecular Origin of its Exceptionally High Free Radical-Scavenging Activity. Food additives and contaminants 23 (11), 1079–1087.
Guckel, E. K. (1999). Large Scale Simulations of Particulate Systems Using the PME Method. University of Illinois at Urbana-Champaign.
Heo, K. S., Chang, E., Le, N. T., Cushman, H., Yeh, E. T., Fujiwara, K., et al. (2013). De-SUMOylation Enzyme of sentrin/SUMO-specific Protease 2 Regulates Disturbed Flow-Induced SUMOylation of ERK5 and P53 that Leads to Endothelial Dysfunction and Atherosclerosis. Circ. Res. 112 (6), 911–923. doi:10.1161/CIRCRESAHA.111.300179
Heo, K. S., Le, N. T., Cushman, H. J., Giancursio, C. J., Chang, E., Woo, C. H., et al. (2015). Disturbed Flow-Activated p90RSK Kinase Accelerates Atherosclerosis by Inhibiting SENP2 Function. J. Clin. Invest. 125 (3), 1299–1310. doi:10.1172/JCI76453
Hickey, C. M., Wilson, N. R., and Hochstrasser, M. (2012). Function and Regulation of SUMO Proteases. Nat. Rev. Mol. Cel Biol 13 (12), 755–766. doi:10.1038/nrm3478
Hodgson, J. (2001). ADMET--turning Chemicals into Drugs. Nat. Biotechnol. 19 (8), 722–726. doi:10.1038/90761
Hussein, H. A., Borrel, A., Geneix, C., Petitjean, M., Regad, L., and Camproux, A. C. (2015). PockDrug-Server: a New Web Server for Predicting Pocket Druggability on Holo and Apo Proteins. Nucleic Acids Res. 43 (W1), W436–W442. doi:10.1093/nar/gkv462
Irwin, J. J., Sterling, T., Mysinger, M. M., Bolstad, E. S., and Coleman, R. G. (2012). ZINC: a Free Tool to Discover Chemistry for Biology. J. Chem. Inf. Model. 52 (7), 1757–1768. doi:10.1021/ci3001277
Jia, Y. L., Li, J., Qin, Z. H., and Liang, Z. Q. (2009). Autophagic and Apoptotic Mechanisms of Curcumin-Induced Death in K562 Cells. J. Asian Nat. Prod. Res. 11 (11), 918–928. doi:10.1080/10286020903264077
Kang, X., Qi, Y., Zuo, Y., Wang, Q., Zou, Y., Schwartz, R. J., et al. (2010). SUMO-specific Protease 2 Is Essential for Suppression of Polycomb Group Protein-Mediated Gene Silencing during Embryonic Development. Mol. Cel 38 (2), 191–201. doi:10.1016/j.molcel.2010.03.005
Kapadia, G. J., Tokuda, H., Konoshima, T., and Nishino, H. (1996). Chemoprevention of Lung and Skin Cancer by Beta Vulgaris (Beet) Root Extract. Cancer Lett. 100 (1-2), 211–214. doi:10.1016/0304-3835(95)04087-0
Kim, E. Y., Chen, L., Ma, Y., Yu, W., Chang, J., Moskowitz, I. P., et al. (2012). Enhanced Desumoylation in Murine Hearts by Overexpressed SENP2 Leads to Congenital Heart Defects and Cardiac Dysfunction. J. Mol. Cel Cardiol 52 (3), 638–649. doi:10.1016/j.yjmcc.2011.11.011
Koo, Y. D., Choi, J. W., Kim, M., Chae, S., Ahn, B. Y., Kim, M., et al. (2015). SUMO-specific Protease 2 (SENP2) Is an Important Regulator of Fatty Acid Metabolism in Skeletal Muscle. Diabetes 64 (7), 2420–2431. doi:10.2337/db15-0115
Krajka-Kuźniak, V., Paluszczak, J., Szaefer, H., and Baer-Dubowska, W. (2013). Betanin, a Beetroot Component, Induces Nuclear Factor Erythroid-2-Related Factor 2-mediated Expression of Detoxifying/antioxidant Enzymes in Human Liver Cell Lines. Br. J. Nutr. 110 (12), 2138–2149. doi:10.1017/S0007114513001645
Kumar, A., Ito, A., Takemoto, M., Yoshida, M., and Zhang, K. Y. (2014). Identification of 1,2,5-oxadiazoles as a New Class of SENP2 Inhibitors Using Structure Based Virtual Screening. J. Chem. Inf. Model. 54 (3), 870–880. doi:10.1021/ci4007134
Kumar, R., Bansal, A., Shukla, R., Raj Singh, T., Wasudeo Ramteke, P., Singh, S., et al. (2019). In Silico screening of Deleterious Single Nucleotide Polymorphisms (SNPs) and Molecular Dynamics Simulation of Disease Associated Mutations in Gene Responsible for Oculocutaneous Albinism Type 6 (OCA 6) Disorder. J. Biomol. Struct. Dyn. 37 (13), 3513–3523. doi:10.1080/07391102.2018.1520649
Kumari, R., Kumar, R., Consortium, O. S. D. D., and Lynn, A. (2014). g_mmpbsa--a GROMACS Tool for High-Throughput MM-PBSA Calculations. J. Chem. Inf. Model. 54 (7), 1951–1962. doi:10.1021/ci500020m
Lechner, J. F., Wang, L. S., Rocha, C. M., Larue, B., Henry, C., McIntyre, C. M., et al. (2010). Drinking Water with Red Beetroot Food Color Antagonizes Esophageal Carcinogenesis in N-Nitrosomethylbenzylamine-Treated Rats. J. Med. Food 13 (3), 733–739. doi:10.1089/jmf.2008.0280
Lee, M. H., Lee, S. W., Lee, E. J., Choi, S. J., Chung, S. S., Lee, J. I., et al. (2006). SUMO-specific Protease SUSP4 Positively Regulates P53 by Promoting Mdm2 Self-Ubiquitination. Nat. Cel Biol 8 (12), 1424–1431. doi:10.1038/ncb1512
Leow, P. C., Tian, Q., Ong, Z. Y., Yang, Z., and Ee, P. L. (2010). Antitumor Activity of Natural Compounds, Curcumin and PKF118-310, as Wnt/β-Catenin Antagonists against Human Osteosarcoma Cells. Invest. New Drugs 28 (6), 766–782. doi:10.1007/s10637-009-9311-z
Lipinski, C. A. (2000). Drug-like Properties and the Causes of Poor Solubility and Poor Permeability. J. Pharmacol. Toxicol. Methods 44 (1), 235–249. doi:10.1016/s1056-8719(00)00107-6
M. B. Taylor (Editor) (2005). TOPCAT & STIL: Starlink table/VOTable Processing Software (Astronomical data analysis software and systems XIV).
Melchior, F., Schergaut, M., and Pichler, A. (2003). SUMO: Ligases, Isopeptidases and Nuclear Pores. Trends Biochem. Sci. 28 (11), 612–618. doi:10.1016/j.tibs.2003.09.002
Merlot, C. (2010). Computational Toxicology-Aa Tool for Early Safety Evaluation. Drug Discov. Today 15 (1-2), 16–22. doi:10.1016/j.drudis.2009.09.010
Millimouno, F. M., Dong, J., Yang, L., Li, J., and Li, X. (2014). Targeting Apoptosis Pathways in Cancer and Perspectives with Natural Compounds from Mother Nature. Cancer Prev. Res. (Phila) 7 (11), 1081–1107. doi:10.1158/1940-6207.CAPR-14-0136
Morris, G. M., Huey, R., Lindstrom, W., Sanner, M. F., Belew, R. K., Goodsell, D. S., et al. (2009). AutoDock4 and AutoDockTools4: Automated Docking with Selective Receptor Flexibility. J. Comput. Chem. 30 (16), 2785–2791. doi:10.1002/jcc.21256
Mukhopadhyay, D., and Dasso, M. (2007). Modification in Reverse: the SUMO Proteases. Trends Biochem. Sci. 32 (6), 286–295. doi:10.1016/j.tibs.2007.05.002
Müller, S., Ledl, A., and Schmidt, D. (2004). SUMO: a Regulator of Gene Expression and Genome Integrity. Oncogene 23 (11), 1998–2008. doi:10.1038/sj.onc.1207415
Nait Achour, T., Sentis, S., Teyssier, C., Philippat, A., Lucas, A., Corbo, L., et al. (2014). Transcriptional Repression of Estrogen Receptor α Signaling by SENP2 in Breast Cancer Cells. Mol. Endocrinol. 28 (2), 183–196. doi:10.1210/me.2013-1376
Naqvi, A. A. T., Mohammad, T., Hasan, G. M., and Hassan, M. I. (2018). Advancements in Docking and Molecular Dynamics Simulations towards Ligand-Receptor Interactions and Structure-Function Relationships. Curr. Top. Med. Chem. 18 (20), 1755–1768. doi:10.2174/1568026618666181025114157
O'Sullivan-Coyne, G., O'sullivan, G. C., O'Donovan, T. R., Piwocka, K., and McKenna, S. L. (2009). Curcumin Induces Apoptosis-independent Death in Oesophageal Cancer Cells. Br. J. Cancer 101 (9), 1585–1595. doi:10.1038/sj.bjc.6605308
Oostenbrink, C., Soares, T. A., Van der Vegt, N. F., and Van Gunsteren, W. F. (2005). Validation of the 53A6 GROMOS Force Field. Eur. Biophys. J. 34 (4), 273–284. doi:10.1007/s00249-004-0448-6
Payan, S. M., Hubert, F., and Rochais, F. (2020). Cardiomyocyte Proliferation, a Target for Cardiac Regeneration. Biochim. Biophys. Acta Mol. Cel Res 1867 (3), 118461. doi:10.1016/j.bbamcr.2019.03.008
Rashid, M., Sanjarin, F., and Sabouni, F. (2019). Thymoquinone Effects on Cell Viability, Apoptosis and VEGF-A Gene Expression Level in AGS(CRL-1739) Cell Line. Anticancer Agents Med. Chem. 19 (6), 820–826. doi:10.2174/1871520619666190206163504
Rose, P. W., Prlić, A., Altunkaya, A., Bi, C., Bradley, A. R., Christie, C. H., et al. (2016). The RCSB Protein Data Bank: Integrative View of Protein, Gene and 3D Structural Information. Nucleic acids research, gkw1000.
Sanguinetti, M. C., Jiang, C., Curran, M. E., and Keating, M. T. (1995). A Mechanistic Link between an Inherited and an Acquired Cardiac Arrhythmia: HERG Encodes the IKr Potassium Channel. Cell 81 (2), 299–307. doi:10.1016/0092-8674(95)90340-2
Schüttelkopf, A. W., and Van Aalten, D. M. (2004). PRODRG: a Tool for High-Throughput Crystallography of Protein-Ligand Complexes. Acta Crystallogr. D Biol. Crystallogr. 60 (8), 1355–1363. doi:10.1107/S0907444904011679
Seeler, J. S., and Dejean, A. (2003). Nuclear and Unclear Functions of SUMO. Nat. Rev. Mol. Cel Biol 4 (9), 690–699. doi:10.1038/nrm1200
Seeliger, D., and de Groot, B. L. (2010). Ligand Docking and Binding Site Analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 24 (5), 417–422. doi:10.1007/s10822-010-9352-6
Song, T., Li, G., Jing, G., Jiao, X., Shi, J., Zhang, B., et al. (2008). SUMO1 Polymorphisms Are Associated with Non-syndromic Cleft Lip with or without Cleft Palate. Biochem. Biophys. Res. Commun. 377 (4), 1265–1268. doi:10.1016/j.bbrc.2008.10.138
Sreekanth, D., Arunasree, M. K., Roy, K. R., Chandramohan Reddy, T., Reddy, G. V., and Reddanna, P. (2007). Betanin a Betacyanin Pigment Purified from Fruits of Opuntia Ficus-Indica Induces Apoptosis in Human Chronic Myeloid Leukemia Cell Line-K562. Phytomedicine 14 (11), 739–746. doi:10.1016/j.phymed.2007.03.017
Syed, S. B., Khan, F. I., Khan, S. H., Srivastava, S., Hasan, G. M., Lobb, K. A., et al. (2018). Mechanistic Insights into the Urea-Induced Denaturation of Kinase Domain of Human Integrin Linked Kinase. Int. J. Biol. Macromol 111, 208–218. doi:10.1016/j.ijbiomac.2017.12.164
Taghvaei, S., Minuchehr, Z., and Sabouni, F. (2021a). Computational Drug Repurposing of Bethanidine for SENP1 Inhibition in Cardiovascular Diseases Treatment. Life Sciences, 120122.
Taghvaei, S., Sabouni, F., Minuchehr, Z., and Taghvaei, A. (2021b). Identification of Novel Anti-cancer Agents, Applying In Silico Method for SENP1 Protease Inhibition. J. Biomol. Struct. Dyn., 1–15. doi:10.1080/07391102.2021.1880480
Taghvaei, S., Saremi, L., and Babaniamansour, S. (2021c). Computational Analysis of Gly482Ser Single-Nucleotide Polymorphism in PPARGC1A Gene Associated with CAD, NAFLD, T2DM, Obesity, Hypertension, and Metabolic Diseases. PPAR research, 2021.
Tang, S., Huang, G., Tong, X., Xu, L., Cai, R., Li, J., et al. (2013). Role of SUMO-specific Protease 2 in Reprogramming Cellular Glucose Metabolism. PloS one 8 (5), e63965. doi:10.1371/journal.pone.0063965
Udhaya Kumar, S., Thirumal Kumar, D., Bithia, R., Sankar, S., Magesh, R., Sidenna, M., et al. (2020). Analysis of Differentially Expressed Genes and Molecular Pathways in Familial Hypercholesterolemia Involved in Atherosclerosis: a Systematic and Bioinformatics Approach. Front. Genet. 11, 734. doi:10.3389/fgene.2020.00734
ul Qamar, M. T., Mumtaz, A., Ashfaq, U. A., Adeel, M. M., and Fatima, T. (2014). Potential of Plant Alkaloids as Dengue Ns3 Protease Inhibitors: Molecular Docking and Simulation Approach. Bangladesh J. Pharmacol. 9 (3), 262–267. doi:10.3329/bjp.v9i3.18555
Van Der Spoel, D., Lindahl, E., Hess, B., Groenhof, G., Mark, A. E., and Berendsen, H. J. (2005). GROMACS: Fast, Flexible, and Free. J. Comput. Chem. 26 (16), 1701–1718. doi:10.1002/jcc.20291
Vidal, P. J., López-Nicolás, J. M., Gandía-Herrero, F., and García-Carmona, F. (2014). Inactivation of Lipoxygenase and Cyclooxygenase by Natural Betalains and Semi-synthetic Analogues. Food Chem. 154, 246–254. doi:10.1016/j.foodchem.2014.01.014
Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2020). Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 141 (9), e139–e596. doi:10.1161/CIR.0000000000000757
Wang, J. (2011). Cardiac Function and Disease: Emerging Role of Small Ubiquitin-Related Modifier. Wiley Interdiscip. Rev. Syst. Biol. Med. 3 (4), 446–457. doi:10.1002/wsbm.130
Wang, J., Chen, L., Wen, S., Zhu, H., Yu, W., Moskowitz, I. P., et al. (2011). Defective Sumoylation Pathway Directs Congenital Heart Disease. Birth Defects Res. A. Clin. Mol. Teratol 91 (6), 468–476. doi:10.1002/bdra.20816
Wang, J., Feng, X. H., and Schwartz, R. J. (2004). SUMO-1 Modification Activated GATA4-dependent Cardiogenic Gene Activity. J. Biol. Chem. 279 (47), 49091–49098. doi:10.1074/jbc.M407494200
Wang, J., Li, A., Wang, Z., Feng, X., Olson, E. N., and Schwartz, R. J. (2007). Myocardin Sumoylation Transactivates Cardiogenic Genes in Pluripotent 10T1/2 Fibroblasts. Mol. Cel Biol 27 (2), 622–632. doi:10.1128/MCB.01160-06
Wang, J., and Schwartz, R. J. (2010). Sumoylation and Regulation of Cardiac Gene Expression. Circ. Res. 107 (1), 19–29. doi:10.1161/CIRCRESAHA.110.220491
Wang, J., Zhang, H., Iyer, D., Feng, X. H., and Schwartz, R. J. (2008). Regulation of Cardiac Specific nkx2.5 Gene Activity by Small Ubiquitin-like Modifier. J. Biol. Chem. 283 (34), 23235–23243. doi:10.1074/jbc.M709748200
Wollert, K. C., and Drexler, H. (2010). Cell Therapy for the Treatment of Coronary Heart Disease: a Critical Appraisal. Nat. Rev. Cardiol. 7 (4), 204–215. doi:10.1038/nrcardio.2010.1
Keywords: SENP2, natural compounds, betanin, heart failure, molecular docking, molecular dynamics simulation
Citation: Taghvaei S, Sabouni F and Minuchehr Z (2022) Identification of Natural Products as SENP2 Inhibitors for Targeted Therapy in Heart Failure. Front. Pharmacol. 13:817990. doi: 10.3389/fphar.2022.817990
Received: 20 November 2021; Accepted: 14 February 2022;
Published: 01 April 2022.
Edited by:
Abdur Rauf, University of Swabi, PakistanReviewed by:
Ahmed Olatunde, Abubakar Tafawa Balewa University, NigeriaDharmendra Kumar Yadav, Gachon University, South Korea
Anees Ahmed Khalil, University of Lahore, Pakistan
Copyright © 2022 Taghvaei, Sabouni and Minuchehr. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Farzaneh Sabouni, c2Fib3VuaUBuaWdlYi5hYy5pcg==; Zarrin Minuchehr, bWludWNoZWhyQG5pZ2ViLmFjLmly
 Somayye Taghvaei1
Somayye Taghvaei1 Farzaneh Sabouni
Farzaneh Sabouni