- 1Department of Pharmacy, the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Suzhou, China
- 2Emergent Intensive Care Unit, the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Suzhou, China
- 3Intensive Care Unit, the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Suzhou, China
- 4Medical Laboratory, the Affiliated Suzhou Hospital of Nanjing Medical University, Suzhou Municipal Hospital, Suzhou, China
Background: Due to the lack of updated information on teicoplanin (TEI) for continuous renal replacement therapy (CRRT), no exact dosage regimen has been recommended. The aim of this study was to optimize the dosage regimen of TEI in renal dysfunction patients with or without CRRT, evaluate the influence factors of the eradication of Gram-positive bacteria, and evaluate the effect of CRRT on the clearance of TEI.
Methods: Patients with renal dysfunction receiving TEI treatment in the ICU were prospectively recruited and divided into CRRT and non-CRRT groups. Logistic regression analysis was used to screen the factors affecting the eradication of Gram-positive bacteria. The filtrate concentration of the CRRT group was measured at the time of TEI Cmin, and the filtration coefficient of TEI was calculated to evaluate the effect of CRRT on the clearance of TEI.
Results: A total of 106 patients were included, 40 cases in the CRRT group and 66 cases in the non-CRRT group. After giving high-loading doses of TEI, 75.8 and 70% of TEI Cmin in the non-CRRT and CRRT groups reached the range of 10–30 mg/L before the 3rd dose, respectively. The risk of G+ bacteria being uneradicated was higher while the APACHEⅡscore was higher than 22.5. The albumin level before the start of TEI administration and before the 6th–8th dose was lower than 32.8 g/L and 29.3 g/L, respectively, and Cmin before the 3rd dose and 6th–8th dose was lower than 13.2 mg/L and 17.1 mg/L, respectively, with the duration of TEI therapy shorter than 10.5 days. The correlation coefficient (r) was 0.6490 between Cmin before the 3rd dose and the albumin level (p < 0.001). The filtration coefficient of TEI was 10.7 ± 2.4% at Cmin and 11.1 ± 2.5% at Cmax. The GFR had no correlation with the filtration coefficient (r = −0.06204; r = −0.08059). The clearance of TEI in CRRT patients was negatively correlated with the albumin level (r = −0.6305, p = 0.0013).
Conclusion: The early stage of the albumin level can significantly affect the initial Cmin and clinical efficacy of TEI, and also had effect on the clearance of TEI by CRRT. The filtration coefficient of TEI was stable, even with a higher ultrafiltration rate.
Introduction
Severe acute kidney injury (AKI), especially caused or accompanied by sepsis, is associated with prolonged hospitalization, progression to chronic kidney disease (CKD), financial burden, and a high mortality rate. Many severe AKI patients need to be treated with continuous renal replacement therapy (CRRT) and antimicrobials simultaneously (Karkar and Ronco, 2020). Optimizing antimicrobial therapy in CRRT patients can be challenging because of unpredictable pathophysiological changes, different CRRT approaches, and residual renal functions which can alter the pharmacokinetics (PK) and pharmacodynamics (PD) of antimicrobials (Hoff et al., 2020). Data on drug clearance to guide antimicrobial dosing in CRRT patients are limited. To optimize antibiotic exposure and maximize effectiveness, it is important to individualize the antimicrobial regimen given to the patient and the method of CRRT utilization.
Teicoplanin (TEI) is a glycopeptide antibiotic which is effective against Gram-positive bacteria. Compared with vancomycin, TEI has similar clinical efficacy with advantages in renal toxicity and skin rashes (Kato-Hayashi et al., 2019; Kaur et al., 2021). It has a high protein binding rate of about 90% and long serum elimination half-life of about 50 h requiring the loading dose to quickly reach a stationary state serum concentration (Nakamura et al., 2015; Tang et al., 2020). Our previous studies indicated that the trough concentration (Cmin) of TEI is correlated with the clinical efficacy of TEI therapy (Tang et al., 2020; Ren et al., 2021). It has been recommended that the Cmin of TEI (at least 10 μg/ml is required for non-complicated MRSA infections) should be maintained at 15–30 μg/ml for most of the methicillin-resistant Staphylococcus aureus (MRSA) infections (Abdul-Aziz et al., 2020; Hanai et al., 2021). Our previous studies suggested that a high-loading and maintenance dosage regimen (6–12 mg/kg, q12 h × 3 doses and 6–12 mg/kg qd) is required to maintain the Cmin of TEI in its therapeutic range at the early stage of treatment, and no reduction in the loading dose is required for renal dysfunction patients (Tang et al., 2020; Ren et al., 2021). The maintenance dose is stratified by renal function. In addition to Cmin, studies about whether other factors affected the clinical efficacy of TEI treatment were limited.
TEI is a medium molecular agent mainly eliminated by the kidney and cannot be eliminated by hemodialysis (Papaioannou et al., 2002). However, the clearance of TEI had individual differences in the ultrafiltration rate (UFR), mode, and even in the filtrating function of CRRT; thus, the recommended dosage is controversial (Bellmann et al., 2010; Lim et al., 2019; Li et al., 2020; Oda et al., 2020). Considering the large molecular weight of TEI, CVVHD may not eliminate TEI efficiently. An early study indicated a loading dose of 800 mg and a maintenance dose of 400 mg q48-72 h under CVVHD (Li et al., 2020). CVVHDF could partly eliminate TEI according to the pharmacokinetic study; however, the TEI concentration varied greatly among CVVHDF patients (Bellmann et al., 2010; Lim et al., 2019; Oda et al., 2020). Current recommendations of patients receiving CRRT originate from the pharmacokinetic estimate of studies with a few patients and different modes of CRRT that are used today (Bellmann et al., 2010; Lim et al., 2019; Li et al., 2020; Oda et al., 2020). Insufficient current pharmacokinetic data limit evidence-based dose recommendations for impaired renal function (IRF) and CRRT patients. Due to a lack of updated information on TEI during CRRT on the CVVH mode, no exact dosage regimen was recommended for CRRT. Therefore, the objective of this prospective interventional study was to optimize the dosage regimen of TEI in renal dysfunction patients receiving CRRT (CVVH mode) or patients not receiving CRRT, and evaluate the factors influencing the eradication of Gram-positive bacteria. The PK parameters and filtration coefficient of TEI were also assessed in CRRT patients.
Materials and Methods
The protocol for this prospective study was approved by the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University (Approval No. IEC-C-008-A07-V1.0) and registered in the Chinese Clinical Trial Registration Center (registration number: ChiCTR-2000038259) prior to enrolling the patients into the intervention group. Information about the TEI treatments, therapeutic drug monitoring (TDM), and laboratory indicators was collected after written informed consent of the patients or their families was obtained.
Study Population
The inpatients with critically ill infections who received TEI therapy in the ICU of the Affiliated Suzhou Hospital of Nanjing Medical University from January 2019 to August 2021 were prospectively recruited. The following are the inclusion criteria: 1) the cultured pathogen of the infected site must be multiple-resistant Gram-positive bacteria, 2) patients should have been treated with TEI for anti-infection therapy, 3) renal dysfunction (Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, 2013) [creatinine clearance rate (CrCl) ≤ 60 ml/min/1.73 m2, according to the CKD-EPI equation] or CRRT for AKI, and 4) age >18 years. The following are the exclusion criteria: 1) urine volume >0.5 ml/kg/h in patients during CRRT, 2) patients receiving extracorporeal membrane oxygenation during CRRT, 3) patients who underwent CRRT for non-acute kidney injury, 4) patients with chronic renal failure and underwent regular hemodialysis, 5) CRRT converted to intermittent RRT before TEI treatment, 6) TEI treatment less than three days, or 7) the mode of CRRT was other than CVVH. Patients were divided into the following two groups according to whether they received CRRT: CRRT and non-CRRT groups.
In our study, we calculated CrCl according to the CKD-EPI equation, taking into account Scr, gender, and age, as follows, where Scr is the level of serum creatinine from the first day of TEI therapy:
Female: Serum creatinine µmol/l (mg/dl) ≤ 62 (≤ 0.7):
Female: Serum creatinine µmol/l (mg/dl) > 62 (> 0.7):
Male: Serum creatinine µmol/l (mg/dl) ≤ 80 (≤ 0.9):
Male: Serum creatinine µmol/l (mg/dl) > 80 (> 0.9):
Drug Administration
The dosage regimens of TEI in enrolled patients were as follows: 1) loading dose: each patient was given 8–10 mg/kg q12 h × 3 doses; 2) initial maintenance dose: (a) in the non-CRRT group, 6–8 mg/kg qd was given for patients with CrCl at 30–60 ml/min/1.73 m2, qd; 6–8 mg/kg qd for patients with CrCl at 10–29 ml/min/1.73 m2, and (b) in the CRRT group, 6–8 mg/kg, qd; 3) adjusted maintenance dose: clinical pharmacists advised physicians to adjust the dosage regimen when the initial Cmin was out of the target range. The maintenance dose was adjusted individually according to TDM and clinical efficacy evaluation of TEI.
Determination Methods of Plasma Concentration of TEI
The steady-state plasma Cmin of TEI was determined before the 3rd dose of the loading dose. The sampling time was half an hour before the administration. Again, the Cmin needs to be determined 72 h after adjusting the administration regimen or the 6th–8th dose of the maintenance dosage regimen. The target Cmin range of TEI is 10–30 mg/L.
We took a 4-ml venous blood sample in a tube containing the EDTA anticoagulant and centrifuged it immediately at 12,000 r/min for 10 min. The supernatant was taken out and stored at −80°C. The plasma concentration of TEI was determined by using HPLC. The chromatographic separation was performed by using Waters Symmetry C18 column (250 × 4.6 mm, 5 µm) (Waters, USA). Mobile phase was 0.01 mol/L NaH2PO4 (pH = 3.3) and acetonitrile (75: 25) under a flow rate of 1.0 ml/min. The detection wavelength was 215 nm or 240 nm, the column compartment was kept at 35°C, and the injection volume was 0.020 ml.
Recording Indicators
Patients’ data were obtained from the inpatients’ medical database. The main analysis indicators of each enrolled patient were recorded as follows: 1) demographic data: gender, age, weight, the APACHE Ⅱ score at the start of TEI treatment, and lactic acid in the blood gas during TEI treatment; 2) infection diagnosis, other clinical diagnoses, and the infection site; 3) the initial and reviewed pathogenic culture, drug susceptibility, and imaging examination; 4) the treatment of TEI, including the beginning time, loading dosage, and initial and adjusted maintenance dose regimens; 5) steady-state plasma Cmin and ultrafiltrate concentrations after the loading dose and initial or adjusted regimens and the PK parameters of TEI in CRRT patients; 6) changes in biochemical and inflammatory indexes before and after TEI medication; and 7) TEI-related adverse reactions, hospitalization time, and 28-day mortality.
Efficacy Evaluation
According to the guiding principles of Clinical Trial Technology of antibacterial agents (2014 edition) (Writing Group of Guidance for Clinical Trials of Antibacterial Drugs, 2014), the judgment on efficacy evaluation includes clinical efficacy and microbiological efficacy. Clinical efficacy was evaluated after 7–14 days of medication. Clinical efficacy was evaluated in terms of clinical cure and clinical failure. Clinical cure is defined as the complete resolution or significant improvement of pretreatment infection signs and symptoms such that no additional antimicrobial therapy is needed. Clinical failure is defined as no apparent response or an incomplete response that needs additional antibiotic therapy for infections. The microbiological efficacy was evaluated as eradication, presumed eradication, no eradication, and presumed no eradication. Microbiological success was defined as eradication or presumed eradication of Gram-positive bacteria (Writing Group of Guidance for Clinical Trials of Anti-bacterial Drugs, 2014). The primary outcomes were clinical cure and microbiological success rates at the end of treatment (EOT).
Influence Factors of the Eradication of Gram-Positive Bacteria
Univariate and multivariate logistic regression analyses were used to select the covariables of the regression model. We established the regression model for predicting the factors influencing the eradication of Gram-positive bacteria. In univariate analysis, the χ2 test or the Fisher exact test was used to compare the categorical variables. Continuous variables were compared by using the Mann–Whitney U test. Covariates with p values <0.1 were included in the multivariate logistic regression analysis (backward procedure, based on the p-value of the predictor removed) (Hammitt et al., 2019). Multivariate logistic regression analysis was used to screen for independent risk factors. SPSS software automatically screened out the independent variables when the optimal balance was reached between the fitting degrees of the prediction model.
Independent risk factor variables were used to establish regression equations and calculate prediction probabilities. The receiver operating characteristic (ROC) curves of multivariate factors were drawn; the area under the curve (AUC), cutoff point, Youden index, sensitivity, and specificity were used to evaluate their predictive value for predicting the factors influencing the eradication of Gram-positive bacteria.
CRRT Parameters
All CRRT patients had selected femoral venipuncture catheterization as the vascular access, and the catheter used was an ARROW double-lumen central venous catheter. The blood purification equipment used was the Prisma Flex apparatus (Gambro/Baxter, Lakewood, co) (Baxter, USA). All CRRT filters used were polymethyl methacrylate (PMMA) membrane blood filters (CH-1.0) (Toray Medical, Japan). The CRRT mode was CVVH, with a blood flow of 180–200 ml/min, replacement fluid volume of 2–3 L/h, ultrafiltration volume of 100–300 ml/h, pre-dilution of 60–90%, subsequent dilution of 10–40%, and ultrafiltration rate of 30–45%.
Effect of CRRT on Clearance of TEI
The blood and ultrafiltrate samples were taken on Day 2 or Days 6–8 of TEI therapy. One hour (Cmax) and 24 h after the start of infusion (Cmin), additional pre- and post-hemofilter blood and 10 ml ultrafiltrate samples from the ultrafiltrate outlet of the hemofilter were taken. The ultrafiltrate concentrations were also determined by using HPLC. This assay has been validated for ultrafiltrate samples using a spiked ultrafiltrate (1.0–30.0 μg/ml). The lower limit of quantification in the ultrafiltrate was 0.1 μg/ml. The precision of the assay was <9% (coefficient of variation).
The calculation formulas of the CRRT ultrafiltration rate (UFR) (ml/kgh) and TEI filtration coefficient for the first 48 h of CRRT during TEI treatment, according to the literature reports, were as follows (Naorungroj et al., 2021):
where Qp is the plasma flow rate (ml/min), BFR is the blood flow rate (ml/min), HCT is the hematocrit, Qpre is the flow rate of pre-replacement fluid pump (ml/h), Qpost is the flow rate of post-replacement fluid pump (ml/h), Quf is the water removal (ml/h), Wt is the body weight (kg) before medication, Sc is the TEI filtration coefficient, Cuf is the TEI concentration in ultrafiltrate (mg/L), and Cs is the blood concentration of TEI at the same time (mg/L).
The PK parameters were calculated by using a non-compartmental model and Kinetica-2000 (InnaPhase Corporation, France). The area under the concentration–time curve in 24 h (AUC0–24) was computed using the log-linear method or the trapezoidal method. Since the replacement fluid was applied simultaneously in pre-dilution and post-dilution modes, the hemofilter clearance CLHF was determined as follows (Bellmann et al., 2010):
where CLHF pre-dilution is the hemofilter clearance obtained by using the pre-dilution mode, CLHF post-dilution is the hemofilter clearance obtained by using the post-dilution mode, UFR is the ultrafiltration rate, CUF is the TEI concentration in the ultrafiltrate, CS is the TEI serum concentration, BFR is the blood flow rate through the hemofilter, and SuR is the flow rate of the substitution solution.
Statistical Analysis
All statistical analyses were performed using the Statistical Package for Social Sciences, version 22 (IBM, USA), and GraphPad Prism version 6 (GraphPad company, USA). The categorical variables were expressed as case numbers (n) and proportions (%). Pearson’s chi-square test was used to compare the categorical variables. All the continuous variables were checked for normality using the Shapiro–Wilk test. Continuous variables in accordance with the normal distribution were summarized as χ ± SD; the independent sample t-test was used to analyze these continuous data. The continuous variables were summarized as medians and interquartile ranges; the Mann–Whitney U test was used to analyze continuous data when these data were not normally distributed. The linear regression analysis was used to compare the correlation between two continuous variables. Two-tailed p < 0.05 were considered statistically significant.
Results
Patient Characteristics
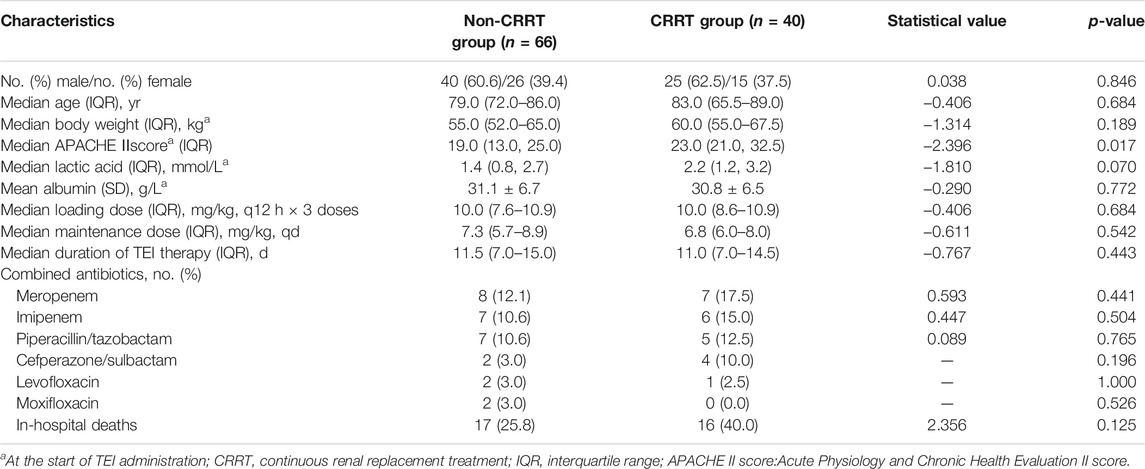
A total of 106 cases of renal dysfunction patients who received anti-infective treatment with TEI were enrolled in this study, including 40 cases in the CRRT group and 66 cases in the non-CRRT group. The APACHEⅡscore was higher in the CRRT group and had statistical differences when compared with the non-CRRT group (p = 0.017). There were no statistical differences in other variables between the two groups (p > 0.05), as shown in Table 1.
Comparison of TEI Cmin
There were 16 cases in the non-CRRT group that had the initial Cmin out of the target range, and 11 cases had their dosage regimen of TEI adjusted. In the CRRT group, 12 cases had the initial Cmin out of the target range, and 8 cases (86.9%) had the dosage regimen of TEI adjusted. A total of 76 trough concentrations were redetermined after adjusting the dosage regimen or the 6th–8th dose of the maintenance dosage regimen, 46 concentrations in the non-CRRT group, and 30 concentrations in the CRRT group. The rates of TEI Cmin before the 3rd dose and the 6th–8th dose in the range of 10–30 mg/L were 75.8 and 89.1% in the IRF group, and 70.0 and 90.0% in the CRRT group, as shown in Figures 1C,D. The rate of Cmin in the range of 10–30 mg/L was much higher before the 6th–8th dose than the 3rd dose in the CRRT group (90.0 vs. 70.0%, p = 0.044). The rates of Cmin in the range of 15–30 mg/L before the 6th–8th dose were 71.7 and 70% in the non-CRRT and CRRT groups.
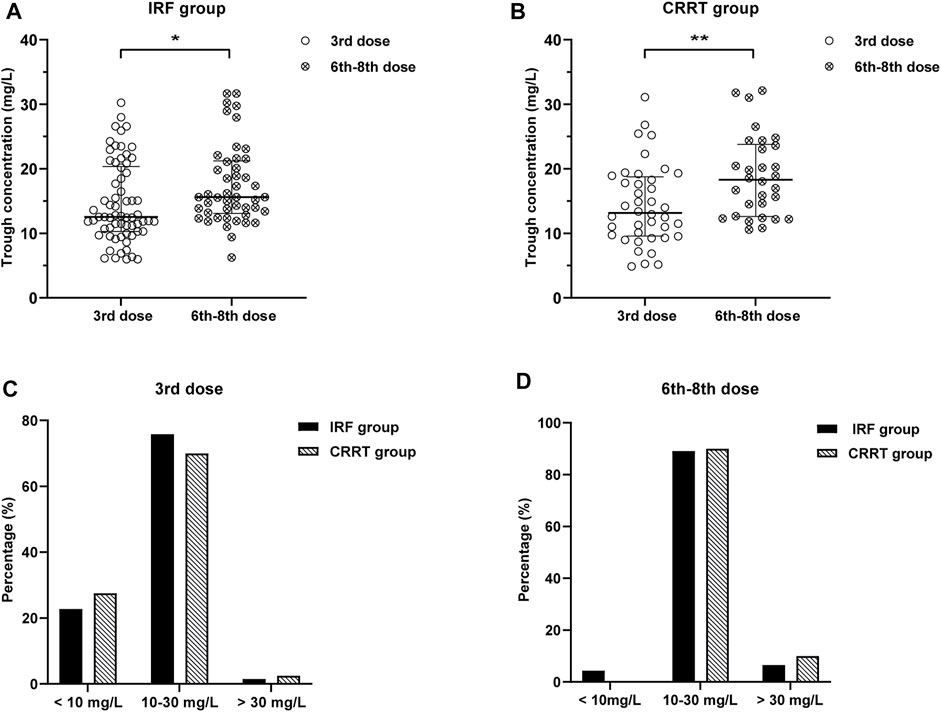
FIGURE 1. (A,B) Distribution of TEI Cmin before the 3rd dose and the 6th–8th dose in the IRF and CRRT groups. Cmin before the 6th–8th dose was much higher than that before the 3rd dose in the IRF and CRRT groups [16.2 (13.4–22.1) mg/L vs. 12.9 (10.7–20.8) mg/L, p = 0.028; 20.3 (10.8–24.8) mg/L vs. 12.5 (9.4–18.7) mg/L, p = 0.004]. (C,D) Rates of TEI Cmin before the 3rd dose and the 6th–8th dose in the range of 10–30 mg/L were 75.8 and 89.1% in the IRF group, and 70.0 and 90.0% in the CRRT group, respectively.
The distribution of TEI Cmin before the 3rd and the 6th–8th dose in the non-CRRT and CRRT groups is shown in Figures 1A,B. Cmin before the 6th–8th dose were much higher than that before the 3rd dose in the non-CRRT and CRRT groups [16.2 (13.4–22.1) mg/L vs. 12.9 (10.7–20.8) mg/L, p = 0.028; 20.3 (10.8–24.8) mg/L vs. 12.5 (9.4–18.7) mg/L, p = 0.004].
Infection Sites and Distribution of Pathogens
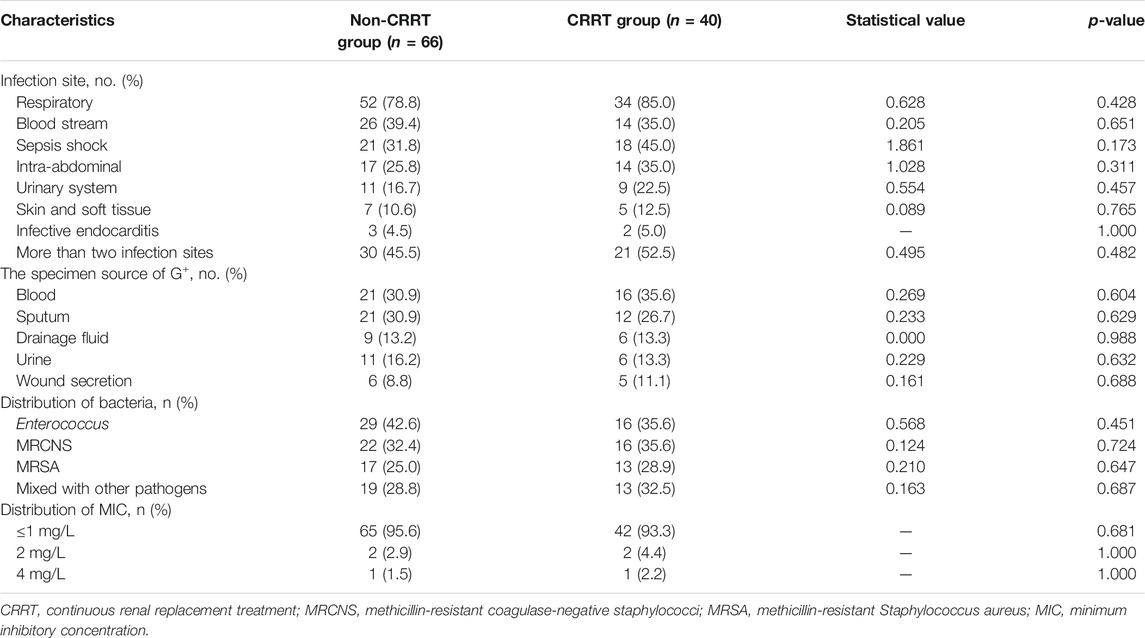
The respiratory system was the most common site of infection in the two groups, followed by bloodstream and intra-abdominal infections, and there was no statistical difference in the distribution of infected sites between the two groups (Table 2). Blood and sputum were the main culture specimens of Gram-positive bacteria. All Gram-positive bacteria were resistant to penicillin and methicillin. Enterococcus faecium was the most common pathogen in the target treatment of the two groups, followed by methicillin-resistant coagulase-negative staphylococci (MRCNS) and MRSA. There were no statistical differences in pathogen distribution and MIC values between the two groups, as shown in Table 3.
Clinical Outcomes in Patients Cured of Gram-Positive Bacteria Infection
Clinical outcome indicators are shown in Table 3. After TEI treatment, 46 cases were clinically cured in the non-CRRT group, 27 cases were clinically cured in the CRRT group, and there were no statistical differences (72.7 vs. 67.5%, p = 0.566). The eradication rates of Gram-positive bacteria were 81.8% (48 cases confirmed eradication and 6 cases presumed) in the non-CRRT group, 77.5% (27 cases confirmed eradication and 4 cases presumed) in the CRRT group, and had no statistical differences (81.8 vs. 77.5%, p = 0.589). Gram-positive bacteria were not eradicated in 21 cases totally. There were 4 cases that developed acute kidney injury (AKI) and one case of liver injury in the non-CRRT group after TEI treatment. Two cases of liver injury occurred in the CRRT group, and there were no statistical differences in the rate of adverse events (7.6 vs. 5.0%, p = 0.909). The 28-day hospital mortality rate also had no statistical significance in the CRRT and non-CRRT groups (22.5 vs. 15.2%, p = 0.339).
Influence Factors of the Eradication of Gram-Positive Bacteria
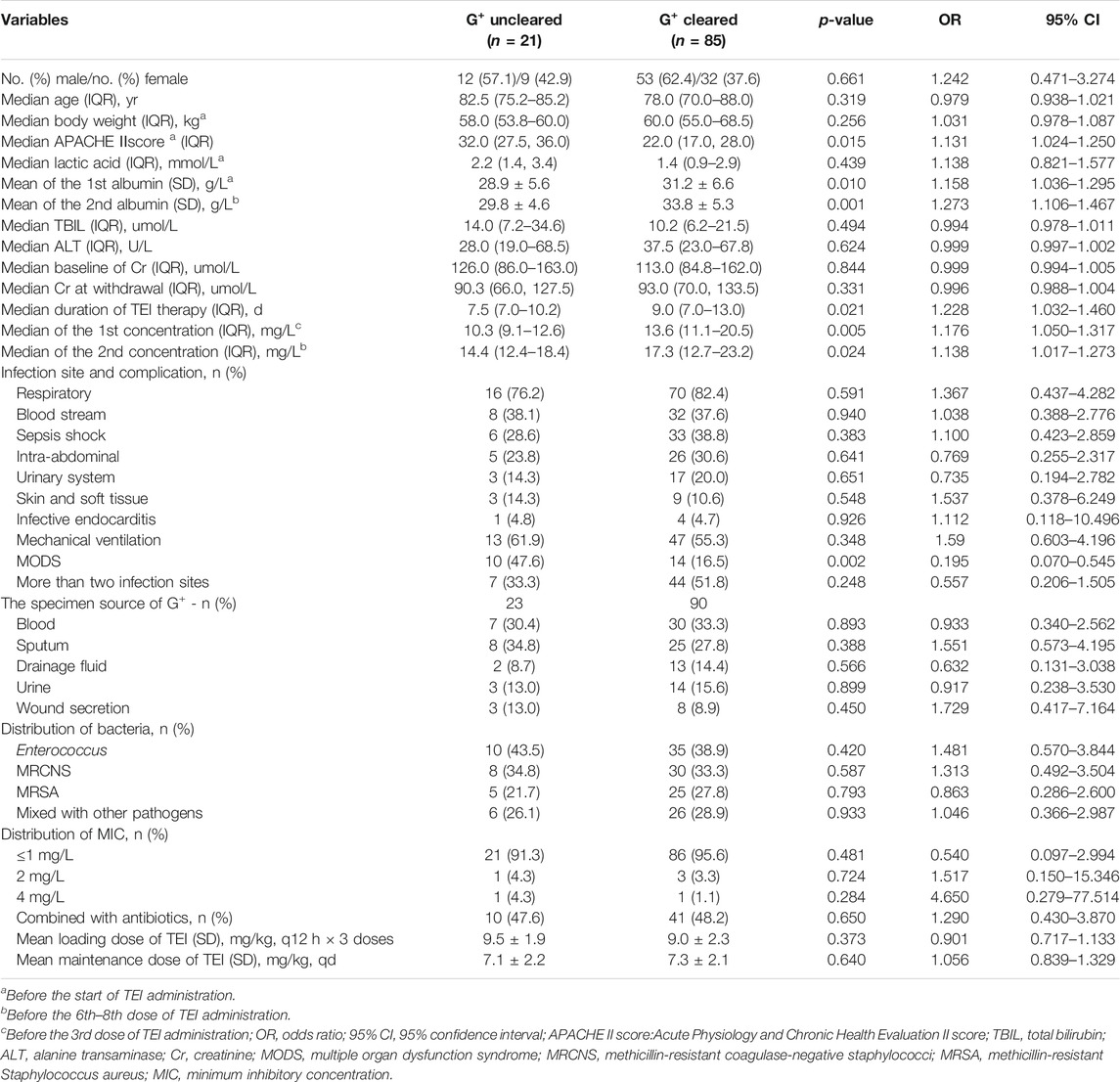
The total 106 cases were divided into G+ uneradicated (21 cases) and G+ eradicated groups (85 cases) according to whether the Gram-positive bacteria were eradicated. The univariate analysis indicated that the APACHEⅡscore, lower albumin levels before the start of TEI administration and before the 6th–8th dose, lower Cmin before the 3rd dose and the 6th–8th dose, the shorter duration of TEI therapy, and more of MODS were related with uneradicated G+ bacteria, and had statistically significant differences (p < 0.05) (Table 3).
The risk factors for the G+ uneradicated group were estimated by the step-wise multivariate linear regression analysis (backward procedure, based on the p-value of the predictor removed) and the identification of the cutoff values with ROC curve analyses. Variables with p < 0.1 in the univariate results were estimated in the multivariate analysis. The significant independent factors for the G+ uneradicated group were the APACHEⅡscore, lower albumin levels before TEI treatment, lower Cmin before the 3rd dose, and more of MODS (p < 0.05) (Table 4). After the introduction and elimination of the above independent variables, the logistic regression equation was finally established:
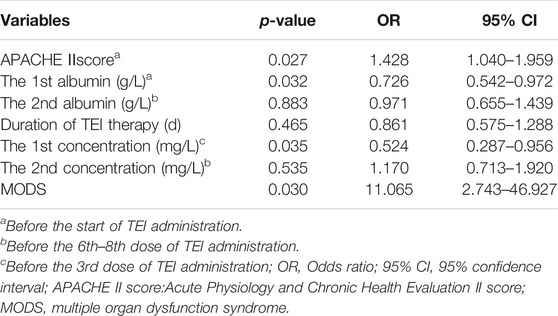
TABLE 4. Results of risk factors on clearance of Gram-positive bacteria screened by multivariate logistic regression analysis.
For each independent risk factor of continuous variables, we calculated the specificity and sensitivity of the resulting logistic regression model by constructing ROC curves and calculating the AUC statistic to estimate the model’s ability to identify uneradicated G+ bacteria. The ROC curves of the APACHEⅡscore, the albumin level, Cmin, the course of TEI treatment, and the predicted probability were drawn (Figure 2). The area under the ROC curve, cutoff point, Youden index, sensitivity, specificity, and predicted probability are shown in Table 5. An APACHEⅡscore of more than 22.5, the albumin levels before the start of TEI administration and before the 6th–8th dose lower than 32.8 g/L and 29.3 g/L, Cmin before the 3rd dose and the 6th–8th dose lower than 13.2 mg/L and 17.1 mg/L, and the duration of TEI therapy being shorter than 10.5 days had more risk of uneradicated G+ bacteria. The area under the ROC curve of the predicted probability was 0.954, which was higher than the other six factors, the Youden index was 0.798, and the sensitivity and specificity were 87.5 and 92.3%, respectively.
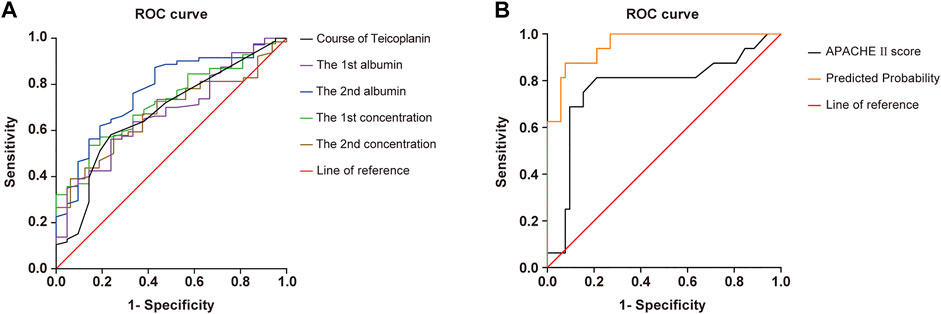
FIGURE 2. ROC curve of predicted probability, APACHE Ⅱscore at admission, course of TEI, the 1st albumin, the 2nd albumin, the 1st concentration, and the 2nd concentration, which were independent risk factors, and combined predictor on clearance of Gram-positive bacteria. The predicted probability of the APACHE Ⅱscore (AUC = 0.772), the course of TEI (AUC = 0.646), the 1st albumin (AUC = 0.758), the 2nd albumin (AUC = 0.764), the 1st concentration (AUC = 0.710), and the 2nd concentration (AUC = 0.666) had better sensitivity (87.5%) and specificity (92.3%), Youden index of 0.798, as well as the maximal AUC (AUC = 0.954).
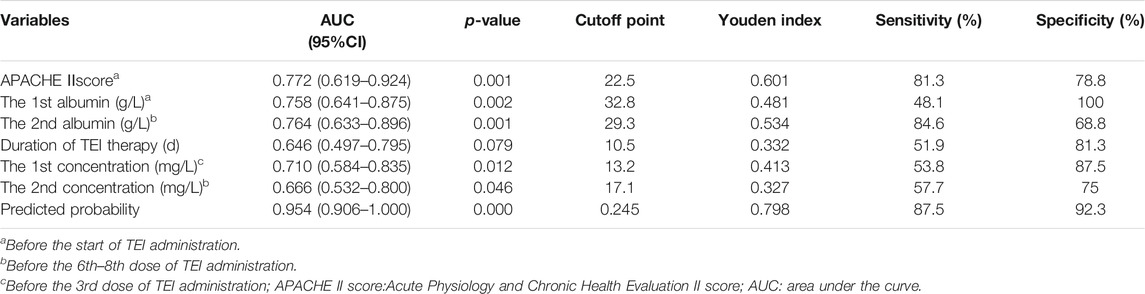
TABLE 5. Area under the curve and cutoff values of receiver operating characteristic curve for predicting effects on clearance of Gram-positive bacteria.
Relationship Between Albumin Levels and TEI Cmin
The linear regression analysis was used to assess the correlation between the albumin level and Cmin of TEI. The correlation coefficient (r) was 0.6490 between Cmin before the 3rd dose and the albumin level before the TEI administration (p < 0.001). The correlation of the albumin level with Cmin before the 3rd dose was much better than that of the albumin level with Cmin before the 6th–8th dose (Figures 3A,B). The Cmin before the 3rd dose in the albumin <35 g/L group was much lower than that in the albumin ≥35 g/L group [12.0 (9.7–15.3) vs. 21.5 (16.7–25.6), p = 0.000]. The Cmin before the 6th–8th dose in the albumin <35 g/L group was also lower than that in the albumin ≥35 g/L group [15.0 (12.3–19.9) vs. 20.0 (16.9–23.8), p = 0.011].

FIGURE 3. The linear regression analysis was used to compare the correlation between the albumin level and Cmin of TEI. (A) The correlation coefficient (r) was 0.6490 between the Cmin before the 3rd dose and the albumin level before the start of TEI administration (p < 0.001). (B) The correlation coefficient (r) was 0.2444 between the Cmin before the 6th–8th dose and the albumin level before the 6th–8th dose (p = 0.0445). (C) The Cmin before the 3rd dose was much lower in the albumin <35 g/L group than in the albumin ≥35 g/L group [12.0 (9.7–15.3) vs. 21.5 (16.7–25.6), p = 0.000]. (D) The Cmin before the 6th–8th dose was also lower in the albumin <35 g/L group than in the albumin ≥35 g/L group [15.0 (12.3–19.9) vs. 20.0 (16.9–23.8), p = 0.011].
The Effect of CRRT on Clearance of TEI
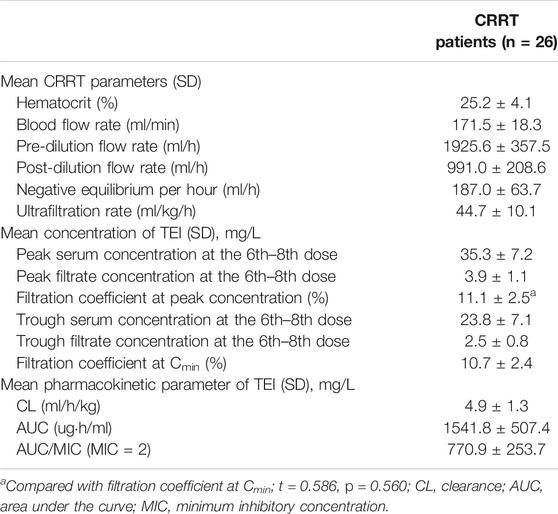
There were 26 patients in the CRRT (CVVH) group who had their Cmax, Cmin, and filtrate concentrations determined at the same time, as shown in Table 6. The ultrafiltration rate was 44.7 ± 10.1 ml/kgh. The filtration coefficient at the peak concentration was 11.1 ± 2.5 mg/L, and it had no statistical differences when compared with that at Cmin (p = 0.560). The linear regression analysis was used to compare the correlation between GFR and filtration coefficient at the 6th–8th dose of TEI Cmin or Cmax. It was shown that the GFR had no correlation with the filtration coefficient (r = −0.06204, p = 0.7585; r = −0.08059, p = 0.7017) (Figures 4A,B). However, the clearance of TEI in CRRT patients was positively correlated with the maintenance dose of TEI (r = −0.4479, p = 0.0321) and negatively correlated with the albumin level (r = −0.6305, p = 0.0013) (Figures 4C,D).
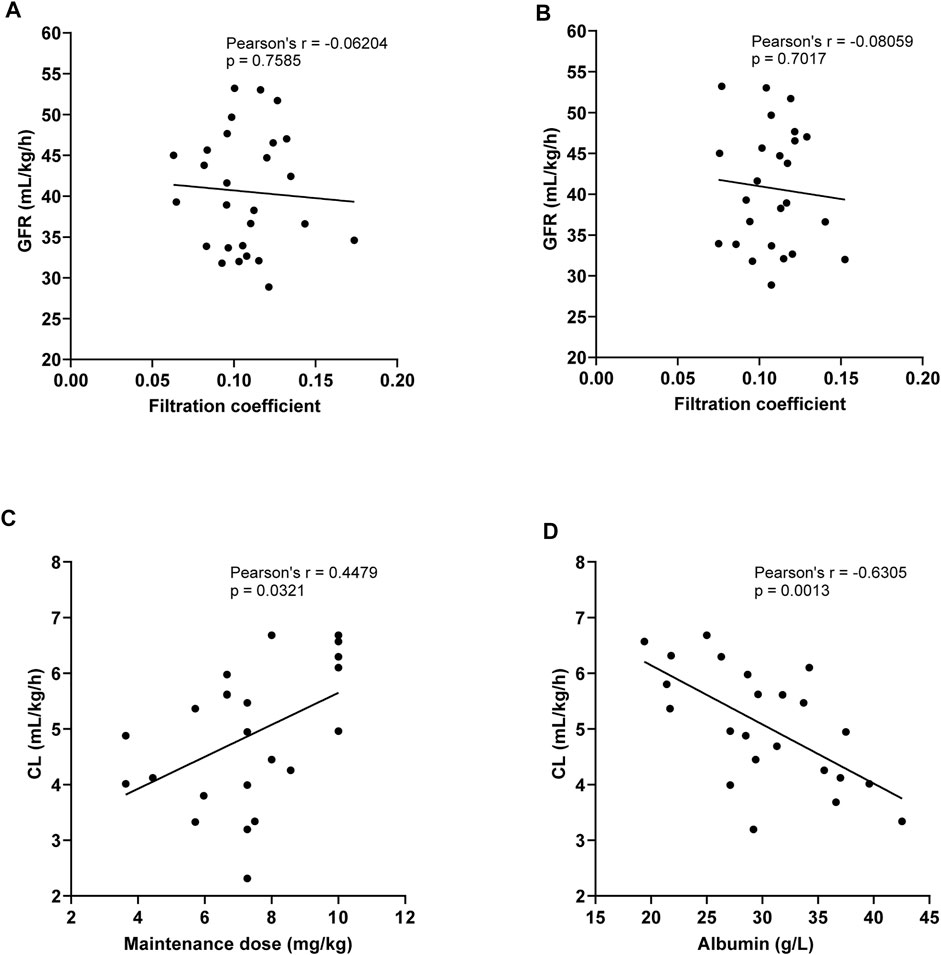
FIGURE 4. (A,B) Linear regression analysis was used to compare the correlation between the GFR and filtration coefficient at the 6th–8th dose of TEI Cmin (A) or Cmax (B). The GFR had no correlation with the filtration coefficient (r = −0.06204, p = 0.7585; r = −0.08059, p = 0.7017). (C) The clearance of TEI in CRRT patients was positively correlated with the maintenance dose of TEI (r = −0.4479, p = 0.0321). (D) The clearance of TEI in CRRT patients was negatively correlated with the albumin level (r = −0.6305, p = 0.0013).
We also evaluated the correlation of the lactic acid level and APACHE Ⅱscore with Cmin of TEI; it showed that the lactic acid level and APACHE Ⅱ score had no correlation with Cmin of TEI (r = 0.051, p = 0.690; r = 0.034, p = 0.843).
Discussion
Some studies suggested that patients with renal dysfunction need a high-loading dose of TEI to achieve a target Cmin in early stages of treatment (Nakamura et al., 2015; Ueda et al., 2016). Ueda et al. (2012) reported that only 20.3% of patients with renal dysfunction achieve the target Cmin of 15 μg/ml by employing the conventional TEI-loading regimen. In the next study, Ueda T proposed a high-loading dose (10 mg/kg, q12 h × 2 doses) regimen in patients with renal dysfunction. These results indicate that employing target therapeutic PK is necessary for improved clinical efficacy (Ueda et al., 2016). However, even a higher loading dose regimen (12 mg/kg, q12 h × 4 doses) of TEI in patients with renal dysfunction resulted in a greater rate of Cmin (more than 30 mg/L) and higher incidence of adverse effects (Nakamura et al., 2015). In our study, a high-loading dose (8–10 mg/kg, q12 h × 3 doses) of TEI achieved Cmin within 24 h of the first administration to 10–30 mg/L in more than 70% of the patients, indicating that the therapeutic concentration of TEI can be reached quickly with this loading dosage regimen.
Also, more than 70% of the Cmin reached 15–30 mg/L before the 6th–8th dose in our study. The Cmin before the 6th–8th dose was much higher than that before the 3rd dose, indicating that TEI was accumulated in renal dysfunction patients, which needed reducing the maintenance dose. In two studies, the maintenance dose of TEI was recommended to be adjusted individually according to the TDM results of renal dysfunction patients (Nakamura et al., 2015; Ueda et al., 2016). For patients with CVVHDF and the high-flux CVVH mode of CRRT partly eliminating TEI with high variability, the optimized maintenance dose was not recommended for adult patients with renal dysfunction and CRRT (Bellmann et al., 2010; Lim et al., 2019; Oda et al., 2020). In our study, the initial maintenance dose of CRRT (CVVH mode) was designed according to CrCl in 30–60 ml/min/1.73 m2, and the TDM results indicated the maintenance dosage regimen was appropriate.
Some studies indicated that the albumin level, body weight, and renal function have significant effects on free TEI plasma concentrations and PK parameters (Yano et al., 2007; Brink et al., 2015; Emoto et al., 2021). Hypoalbuminemia significantly reduced concentrations of free TEI; however, it had no effect on total TEI concentrations (Brink et al., 2015). In our study, the albumin level was significantly correlated with Cmin of total TEI, especially better with the initial Cmin. The Cmin was much lower in the albumin <35 g/L group than in the albumin ≥35 g/L group. These results indicated that increasing the level of albumin can significantly increase the initial Cmin of TEI.
Cmin of TEI had a significant correlation with clinical efficacy. Initial Cmin ≥ 15 μg/ml was an independent factor for increasing clinical efficacy. Achieving Cmin ≥ 15 mg/L with dosage adjustment after TDM in patients with initial Cmin <15 mg/L had no significant improvement in clinical efficacy (Ueda et al., 2016). Kim et al. (2019) performed a retrospective study of TEI TDM, focusing on the dose–serum concentration relationship and clinical outcomes, indicating that a high dose regimen and high mean Cmin over 10 days were significantly associated with treatment efficacy. However, higher Cmin was associated with adverse events during treatment. Routine TDM can be helpful in optimizing TEI administration. However, the reports about other factors affecting clinical efficacy were limited. In our study, the APACHE Ⅱscore, the albumin level, Cmin, the duration of TEI therapy, and MODS had effects on the eradication of Gram-positive bacteria. The APACHE Ⅱscore was designed as a mortality prediction tool, and for classifying patients by disease severity, some studies indicated that the APACHE Ⅱscore was significantly correlated with the efficacy of tigecycline (Fan et al., 2020), similar to our study. The albumin level and Cmin at the early stage of TEI treatment had better correlation with the clearance of Gram-positive bacteria than that before the 6th–8th dose. These results indicated that a high level of Cmin and albumin at the early stage of TEI treatment could significantly improve clinical efficacy. The regression model had a high prediction value for predicting clinical efficacy.
TEI elimination by CRRT depends on the technique and the protocol applied. Lim et al. (2019) assessed the effect of CVVHDF on the pharmacokinetics of TEI as maintenance therapy. The results showed that CL of TEI in the CVVHDF mode was 5.8 ± 4.2 ml/h/kg, which was higher than that of CVVH and the CVVHD mode (Bellmann et al., 2010). The size of the unbound TEI fraction and trough concentrations correlates inversely with the plasma albumin level (Yano et al., 2007; Brink et al., 2015; Ueda et al., 2020). However, no studies have confirmed whether hypoalbuminemia enhanced hemofilter clearance. In our study, the CL of TEI in the CVVH mode was 4.9 ± 1.3 ml/h/kg, which was lower than the literature reportings on the CVVHDF mode (Bellmann et al., 2010). The CL of TEI in the CVVH mode was positively correlated with the maintenance dose of TEI and negatively correlated with the albumin level. These results indicated that hypoalbuminemia also enhances the clearance of TEI in CRRT patients. The filtration coefficient of TEI was about 10% or higher, indicating that the molecular weight of TEI is large, and the rate of clearance by CRRT is limited. CRRT can promote the elimination of toxins (serum creatinine, lactic acid, and urea nitrogen) from the body (Wang et al., 2021), which partly explains the reason for no correlation between lactic acid and Cmin of TEI.
Our study has several limitations that need to be considered. First, the number of patients was small, although previous studies on TEI in CRRT had even smaller numbers of patients. Second, urine TEI concentration was not measured, and the effect of residual renal function on TEI clearance was not adequately assessed. Third, we missed Cmax and Cmin for 14 cases of CRRT patients due to the following conditions: CRRT was stopped, changed the CVVH to another mode, and switched to IRRT.
Conclusion
The therapeutic concentration range of TEI can be reached quickly with a high-loading dosage regimen in renal dysfunction and CRRT patients. The albumin level in the early stages can significantly affect the initial Cmin, clinical efficacy of TEI, and the clearance of TEI by CRRT. The amelioration of hypoproteinemia could reduce the variation of TEI PK/PD parameters. The filtration coefficient of TEI was stable at about 10%, even at a higher ultrafiltration rate. The loading dose and individualized maintenance dosage regimen in our study are appropriate and can be used as a reference to TEI treatment in renal dysfunction and CRRT (CVVH mode) patients.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the Affiliated Suzhou Hospital of Nanjing Medical University (Approval No. IEC-C-008-A07-V1.0). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
LT, LuS, and JiL conceived and designed the experiments. XW, XX, ZZ, and JiL collected the information about the patients. HX, XW, and YY contributed to the methodology. LT, LuS, CZ, and HX contributed to the data analysis. QZ and XL contributed to the supervision. ZZ contributed to the project administration. LiS, JhL, JS, and QZ contributed to the validation. LT, LD, CZ, and XL drafted the manuscript. All authors read and approved the manuscript.
Funding
This work was supported by the Jiangsu Provincial Pharmaceutical Association Project for Hospital Pharmaceutical Research (grant numbers Q2019071, A201914, A201816, and A201915); Suzhou Science and Technology Development Planning Projects (grant numbers SYSD2019186, SYSD2018243, SYSD2019189, SKJYD2021171, and SKJYD2021172); Suzhou Ke Jiao Xing Wei Youth Science and Technology Projects (KJXW2018024); and the Suzhou Pharmaceutical Association Hospital Pharmacy Research Project (Syhky201805). Collaborative Innovation Project of GuSu School of Nanjing Medical University (GSKY 20210236). Suzhou special technical project for diagnosis and treatment of key clicnial diseases (LCZX202112).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank all the patients and their families for participating in this study. We also thank all ICU physicians and nurses who participated in this study.
References
Abdul-Aziz, M. H., Alffenaar, J. C., Bassetti, M., Bracht, H., Dimopoulos, G., Marriott, D., et al. (2020). Antimicrobial Therapeutic Drug Monitoring in Critically Ill Adult Patients: a Position Paper. Intensive Care Med. 46 (6), 1127–1153. doi:10.1007/s00134-020-06050-1
Bellmann, R., Falkensammer, G., Seger, C., Weiler, S., Kountchev, J., and Joannidis, M. (2010). Teicoplanin Pharmacokinetics in Critically Ill Patients on Continuous Veno-Venous Hemofiltration. Int. J. Clin. Pharmacol. Ther. 48 (4), 243–249.
Brink, A. J., Richards, G. A., Lautenbach, E. E., Rapeport, N., Schillack, V., van Niekerk, L., et al. (2015). Albumin Concentration Significantly Impacts on Free Teicoplanin Plasma Concentrations in Non-critically Ill Patients with Chronic Bone Sepsis. Int. J. Antimicrob. Agents 45 (6), 647–651. doi:10.1016/j.ijantimicag.2015.01.015
Emoto, C., Johnson, T. N., Yamada, T., Yamazaki, H., and Fukuda, T. (2021). Teicoplanin Physiologically Based Pharmacokinetic Modeling Offers a Quantitative Assessment of a Theoretical Influence of Serum Albumin and Renal Function on its Disposition. Eur. J. Clin. Pharmacol. 77 (8), 1157–1168. doi:10.1007/s00228-021-03098-w
Fan, G., Jin, L., Bai, H., Jiang, K., Xie, J., and Dong, Y. (2020). Safety and Efficacy of Tigecycline in Intensive Care Unit Patients Based on Therapeutic Drug Monitoring. Ther. Drug Monit. 42 (6), 835–840. doi:10.1097/FTD.0000000000000784
Hammitt, L. L., Etyang, A. O., Morpeth, S. C., Ojal, J., Mutuku, A., Mturi, N., et al. (2019). Effect of Ten-Valent Pneumococcal Conjugate Vaccine on Invasive Pneumococcal Disease and Nasopharyngeal Carriage in Kenya: a Longitudinal Surveillance Study. Lancet 393 (10186), 2146–2154. doi:10.1016/s0140-6736(18)33005-8
Hanai, Y., Takahashi, Y., Niwa, T., Mayumi, T., Hamada, Y., Kimura, T., et al. (2021). Optimal Trough Concentration of Teicoplanin for the Treatment of Methicillin-Resistant Staphylococcus aureus Infection: A Systematic Review and Meta-Analysis. J. Clin. Pharm. Ther. 46 (3), 622–632. doi:10.1111/jcpt.13366
Hoff, B. M., Maker, J. H., Dager, W. E., and Heintz, B. H. (2020). Antibiotic Dosing for Critically Ill Adult Patients Receiving Intermittent Hemodialysis, Prolonged Intermittent Renal Replacement Therapy, and Continuous Renal Replacement Therapy: An Update. Ann. Pharmacother. 54 (1), 43–55. doi:10.1177/1060028019865873
Karkar, A., and Ronco, C. (2020). Prescription of CRRT: a Pathway to Optimize Therapy. Ann. Intensive Care 10 (1), 32. doi:10.1186/s13613-020-0648-y
Kato-Hayashi, H., Niwa, T., Ohata, K., Harada, S., Matsumoto, T., Kitagawa, J., et al. (2019). Comparative Efficacy and Safety of Vancomycin versus Teicoplanin in Febrile Neutropenic Patients Receiving Hematopoietic Stem Cell Transplantation. J. Clin. Pharm. Ther. 44 (6), 888–894. doi:10.1111/jcpt.13011
Kaur, J., Mir, T., Dixit, P., Uddin, M., Kadari, S., Lee, Y., et al. (2021). The Use of Vancomycin versus Teicoplanin in Treating Febrile Neutropenia: A Meta-Analysis and Systematic Review. Cureus 13 (5), e15269. doi:10.7759/cureus.15269
Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group, (2013). KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. Suppl. 3, 1–150.
Kim, S. H., Kang, C. I., Huh, K., Cho, S. Y., Chung, D. R., Lee, S. Y., et al. (2019). Evaluating the Optimal Dose of Teicoplanin with Therapeutic Drug Monitoring: Not Too High for Adverse Event, Not Too Low for Treatment Efficacy. Eur. J. Clin. Microbiol. Infect. Dis. 38 (11), 2113–2120. doi:10.1007/s10096-019-03652-6
Li, L., Li, X., Xia, Y., Chu, Y., Zhong, H., Li, J., et al. (2020). Recommendation of Antimicrobial Dosing Optimization during Continuous Renal Replacement Therapy. Front. Pharmacol. 11, 786. doi:10.3389/fphar.2020.00786
Lim, S. K., Lee, S. A., Kim, C. W., Kang, E., Choi, Y. H., and Park, I. (2019). High Variability of Teicoplanin Concentration in Patients with Continuous Venovenous Hemodiafiltration. Hemodial Int. 23 (1), 69–76. doi:10.1111/hdi.12704
Nakamura, A., Takasu, O., Sakai, Y., Sakamoto, T., Yamashita, N., Mori, S., et al. (2015). Development of a Teicoplanin Loading Regimen that Rapidly Achieves Target Serum Concentrations in Critically Ill Patients with Severe Infections. J. Infect. Chemother. 21 (6), 449–455. doi:10.1016/j.jiac.2015.02.002
Naorungroj, T., Neto, A. S., Zwakman-Hessels, L., Yanase, F., Eastwood, G., Murugan, R., et al. (2021). Early Net Ultrafiltration Rate and Mortality in Critically Ill Patients Receiving Continuous Renal Replacement Therapy. Nephrol. Dial. Transpl. 36 (6), 1112–1119. doi:10.1093/ndt/gfaa032
Oda, K., Jono, H., Sagishima, K., and Saito, H. (2020). Augmented Teicoplanin Clearance through Probable Adsorption onto a Polymethyl Methacrylate (PMMA) Hemofilter during Continuous Venovenous Hemodiafiltration. J. Infect. Chemother. 26 (9), 992–994. doi:10.1016/j.jiac.2020.04.001
Papaioannou, M. G., Marinaki, S., Pappas, M., Stamatiadis, D., Giamarellos-Bourboulis, E. J., Giamarellou, H., et al. (2002). Pharmacokinetics of Teicoplanin in Patients Undergoing Chronic Haemodialysis. Int. J. Antimicrob. Agents 19, 233–236. doi:10.1016/s0924-8579(02)00005-5
Ren, X., Tang, L., Xu, X., Ruan, J., Ding, Q., and Zhuang, Z. (2021). Therapeutic Drug Monitoring and Clinical Efficacy of Teicoplanin in Critical Ill Infection Patients. Chin. J. Crit. Care 41, 688–692.
Tang, L., Shi, L., Xue, H., Zhuang, Z., Yuan, Y., Qian, C., et al. (2020). Effects of Augmented Renal Clearance on Blood Trough Concentration of Patients Receiving High- Dose Regimen of Teicoplanin. China Pharm. 31, 2650–2655. doi:10.6039/j.issn.1001-0408.2020.21.16
Ueda, T., Takesue, Y., Nakajima, K., Ichiki, K., Doita, A., Wada, Y., et al. (2016). Enhanced Loading Regimen of Teicoplanin Is Necessary to Achieve Therapeutic Pharmacokinetics Levels for the Improvement of Clinical Outcomes in Patients with Renal Dysfunction. Eur. J. Clin. Microbiol. Infect. Dis. 35 (9), 1501–1509. doi:10.1007/s10096-016-2691-z
Ueda, T., Takesue, Y., Nakajima, K., Ichiki, K., Ishikawa, K., Takai, Y., et al. (2020). Clinical Efficacy and Safety in Patients Treated with Teicoplanin with a Target Trough Concentration of 20 μg/mL Using a Regimen of 12 mg/kg for Five Doses within the Initial 3 days. BMC Pharmacol. Toxicol. 21 (1), 50. doi:10.1186/s40360-020-00424-3
Wang, J., Wu, Z., Wen, Q., and Wang, X. (2021). Effects of CRRT on Renal Function and Toxin Clearance in Patients with Sepsis: a Case-Control Study. J. Int. Med. Res. 49 (9), 3000605211042981. doi:10.1177/03000605211042981
Writing Group of Guidance for Clinical Trials of Anti-bacterial Drugs (2014). Guidance for Clinical Trials of Anti-bacterial Drugs. Chin. J. Clin. Pharmacol. 30 (9), 844–856.
Keywords: teicoplanin, renal dysfunction, continuous renal replacement therapy, therapeutic drug monitoring, high loading dose, albumin, filtration coefficient
Citation: Shi L, Zhuang Z, Duan L, Zhu C, Xue H, Wang X, Xu X, Yuan Y, Shi L, Li J, Sun J, Liu X, Zhou Q, Lu J and Tang L (2022) Dose Optimization of Teicoplanin for Critically Ill Patients With Renal Dysfunction and Continuous Renal Replacement Therapy: Experience From a Prospective Interventional Study. Front. Pharmacol. 13:817401. doi: 10.3389/fphar.2022.817401
Received: 19 November 2021; Accepted: 26 January 2022;
Published: 08 March 2022.
Edited by:
Dario Cattaneo, Luigi Sacco Hospital, ItalyReviewed by:
Iain MacPhee, St George’s, University of London, United KingdomMuhammad Usman, University of Veterinary and Animal Sciences, Pakistan
Copyright © 2022 Shi, Zhuang, Duan, Zhu, Xue, Wang, Xu, Yuan, Shi, Li, Sun, Liu, Zhou, Lu and Tang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lian Tang, dGFuZ2xpYW43MTZAYWxpeXVuLmNvbQ==; Jian Lu, MzgyMTM5Nzc3QHFxLmNvbQ==; Qin Zhou, emhvdXl4MTIyM0B5ZWFoLm5ldA==
†These authors have contributed equally to this work
 Lu Shi
Lu Shi Zhiwei Zhuang2†
Zhiwei Zhuang2† Lian Tang
Lian Tang