
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 27 June 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.816995
This article is part of the Research Topic Neuropharmacology of Neurodegenerative diseases: Exploration of Pathways that involved in Neurodegenerative disorders View all 4 articles
Objectives: We conducted meta-analysis to demonstrate the efficacy and safety of ketamine on postoperative catheter-related bladder discomfort (CRBD).
Methods: A systematic search was performed through PubMed, Embase, and Cochrane Library to identify all randomized controlled trials that used ketamine in postoperative CRBD. This study was carried out by using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses. We used RevMan version 5.3.0. to analyze the data.
Results: Five RCTs involving 414 patients were included in the analysis. The incidence and severity of postoperative CRBD were assessed at 0, 1, 2, and 6 h. According to our results of meta-analysis, ketamine reduced the incidence of postoperative CRBD at 2 h (RR 0.39; 95% CI, 0.21–0.71; p = 0.002, I2 = 40%) and 6 h (RR 0.29; 95% CI, 0.16–0.50; p < 0.0001, I2 = 0%) significantly; however, there were no statistical differences at 0 h (RR 0.81; 95% CI, 0.35–1.88; p = 0.62, I2 = 96%) and 1 h (RR 0.57; 95% CI, 0.13–2.54; p = 0.46, I2 = 97%). In two studies, we compared the incidence of moderate-to-severe CRBD between groups according to the scaling system (none, mild, moderate, and severe), and data are presented as numbers. Patients in the ketamine group showed a significantly lower severity of CRBD than those in the placebo group at 1 h (RR 0.09; 95% CI, 0.03–0.31; p = 0.0001) and 2 h (RR 0.06; 95% CI, 0.01–0.44; p = 0.005). In contrast, there were no meaningful differences between the two groups in the severity of CRBD at 0 h (RR 0.18; p = 0.84) or 6 h (RR 0.20; 95% CI, 0.03–1.59; p = 0.13). There were no meaningful differences on the rate of adverse events between the ketamine group and control group, mainly including postoperative nausea and vomiting (RR 1.24; 95% CI, 0.89–1.72; p = 0.21), diplopia (RR 3.00; 95% CI, 0.48–18.67; p = 0.24), and hallucination (RR 3.00; 95% CI, 0.32–28.24; p = 0.34).
Conclusion: Our meta-analysis demonstrated that a sub-hypnotic dose of ketamine administration can reduce the incidence and severity of postoperative CRBD without causing evident side effects.
Indwelling urinary catheter during surgery is common in order to ensure postoperative bladder drainage. However, patients with a urinary catheter during surgery often complained about discomfort in the supra-pubic region or a burning sensation of the urethra and urinary frequency with or without urgency incontinence. These symptoms are described as postoperative catheter-related bladder discomfort (CRBD), which extremely reduces patient quality of life (Agarwal et al., 2005; Li et al., 2016).
CRBD is similar to symptoms of overactive bladder (OAB) (Binhas et al., 2011). Both CRBD and OAB are considered to be associated with involuntary contractions of the bladder smooth muscle mediated by muscarinic receptors directly (Anderson, 1993; Agarwal et al., 2005; Agarwal et al., 2006a). The muscarinic receptor antagonist ketamine has been proven successful for CRBD (Durieux, 1995; Hirota and Lambert, 1996). Ketamine, a phenylic dine derivative, is a general anesthetic drug. In recent years, there has been more interest in the use of ketamine as a perioperative analgesic (Adam et al., 2005; Loftus et al., 2010; Mueller and Golembiewski, 2011). Besides muscarinic receptors, ketamine also interacts with many other receptors, including N-methyl-d-aspartate (NMDA) receptors and opioid receptors (Kohrs and Durieux, 1998). The interaction mechanisms are variable and complex. Clinically, intravenous (IV) infusion is the most common route of ketamine administration. Although several studies have been published to verify the efficacy and safety of ketamine on postoperative CRBD, there is a lack of meta-analysis to identify the conclusion. Therefore, we conducted a meta-analysis of randomized controlled trials (RCTs).
A systematic search was performed through PubMed, Embase, and Cochrane Library to identify all studies published until October 2021. The following search terms were applied for the search: RCT, ketamine, and CRBD.
(a) Randomized controlled trials;
(b) The effect of ketamine on postoperative CRBD was studied, and the route of administration of ketamine is intravenous infusion;
(c) The placebo is saline;
(d) Full text and related data can be available;
(e) Articles published in English.
All authors independently browsed and read all searched articles, and the final list of included articles was decided through a consensus discussion.
Data extraction was performed independently by all authors. Disagreements were resolved by consensus. The following characteristics were included: the primary author, publication year, catheter size, types of surgery, a dose of ketamine, duration of operation, primary and secondary the outcome, and time point of ketamine administration, as shown in Table 1.
The abstracted data were calculated by using Rev Man version 5.3.0 (The Cochrane Collaboration, London, United Kingdom). Variables were pooled only if evaluated by ≧2 studies. The mean difference (MD) with 95% confidence intervals (CIs) was utilized to analyze the continuous data, and the risk ratios (RRs) with 95% CIs were applied to analyze the dichotomous data among the different groups. A fixed-effect model was used as there was no significant heterogeneity (p-value of x2 test no less than 0.10 and I2 not greater than 50%); otherwise, a random-effects model was used (p-value of x2 test less than 0.10 and I2 greater than 50%). A p value of less than 0.05 was considered statistically significant.
We used the Cochrane Risk of the bias assessment tool and assigned assessments of low, high, or unclear risk of bias (Higgins and Green, 2011). Figure 1 and Figure 2 demonstrated an overview of the risk of bias.
We obtained 18 relevant studies through a systematic search. After carefully reviewing the full text, 13 studies were excluded owing to the various reasons described in Figure 3. Figure 3 summarized the total flowchart. Finally, five RCTs (Agarwal et al., 2006b; Safavi et al., 2014; Shariat Moharari et al., 2014; Akça et al., 2016; Burimsittichai et al., 2016) involving 414 patients were included in the meta-analysis.
All patients in the included studies were treated with 16G or 18G Foley catheters. The patient characteristics in the two groups were similar with respect to age (MD 0.75, 95% CI -1.16 to 2.65, p = 0.44), weight (MD -0.24, 95% CI -4.02 to 3.53, p = 0.90), sex ratio (RR 0.99, 95% CI 0.90 to 1.09, p = 0.83), and duration of surgery (MD 2.27, 95% CI -3.03 to 7.56, p = 0.40). The dose of ketamine in the included studies was 0.25 mg/kg ((Agarwal et al., 2006b), (Safavi et al., 2014), and (Akça et al., 2016)) and 0.5 mg/kg ((Shariat Moharari et al., 2014) and (Burimsittichai et al., 2016)).
Three studies enrolling 214 participants (107 in the ketamine group and 107 in the control group) were used to analyze the impact of ketamine on the incidence of postoperative CRBD. Ketamine reduced the incidence of postoperative CRBD at 2 h (RR 0.39; 95% CI, 0.21–0.71; p = 0.002, I2 = 40%) and 6h (RR 0.29; 95% CI, 0.16–0.50; p < 0.0001, I2 = 0%) significantly; however, there were no statistical differences at 0 h (RR 0.81; 95% CI, 0.35–1.88; p = 0.62, I2 = 96%) and 1 h (RR 0.57; 95% CI, 0.13–2.54; p = 0.46, I2 = 97%) (Figure 4).
In two studies (Agarwal et al., 2006b; Shariat Moharari et al., 2014) (82 in the ketamine group and 82 in the control group), we compared the incidence of moderate-to-severe CRBD between groups according to the scaling system (none, mild, moderate, and severe), and data are presented as numbers. Patients in the ketamine group showed a significantly lower severity of CRBD than those in the placebo group at 1 h (RR 0.09; 95% CI, 0.03–0.31; p = 0.0001) and 2 h (RR 0.06; 95% CI, 0.01–0.44; p = 0.005). In contrast, there were no meaningful differences between the two groups in the severity of CRBD at 0 h (RR 0.18; p = 0.84) or 6 h (RR 0.20; 95% CI, 0.03–1.59; p = 0.13) (Figure 5).
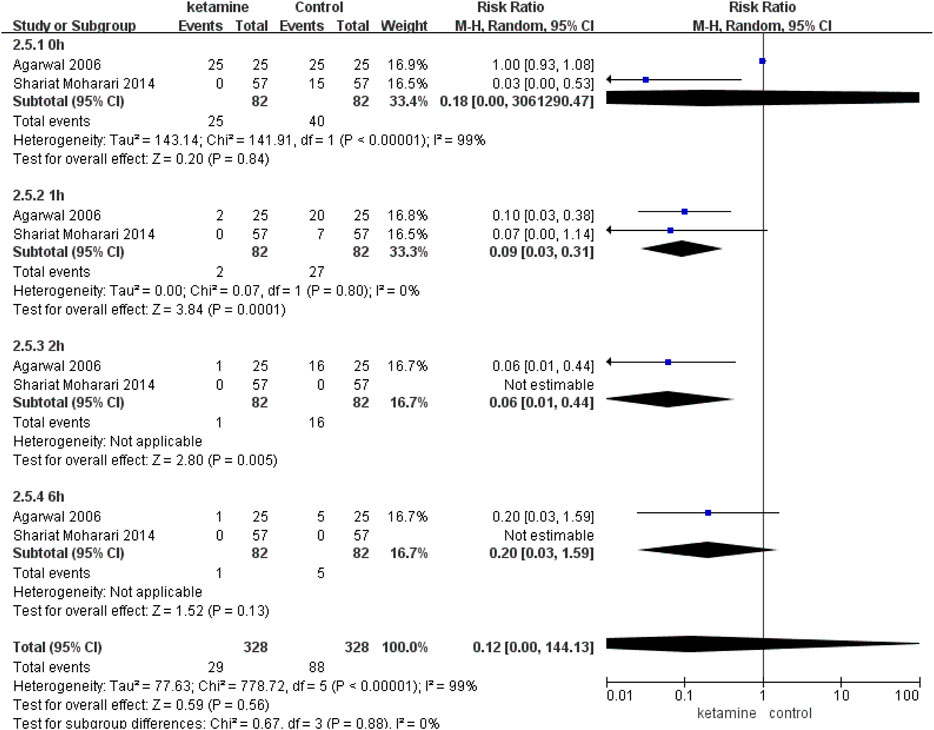
FIGURE 5. Incidence of moderate-to-severe catheter-related bladder discomfort in ketamine vs. placebo.
There were no meaningful differences on the rate of adverse events between the ketamine and control groups, mainly including postoperative nausea and vomiting (PONV) (RR 1.24; 95% CI, 0.89–1.72; p = 0.21), diplopia (RR 3.00; 95% CI, 0.48–18.67; p = 0.24), and hallucination (RR 3.00; 95% CI, 0.32–28.24; p = 0.34) (Figure 6).
Urinary catheterization is a necessary procedure in most surgeries. CRBD is secondary to an indwelling urinary catheter, which is characterized by the symptoms of urinary frequency and urgency in the overactive bladder and an urge to void or discomfort at the supra-pubic region (Hu et al., 2016). The incidence of CRBD has been reported to range from 47 to 90% (Binhas et al., 2011). CRBD is common in surgeries requiring postoperative catheterization, especially urologic surgery. Postoperative CRBD may increase postoperative complications, prolong hospital stay, and reduce the quality of recovery.
The bladder expresses many muscarinic receptors (a majority of M2 muscarinic receptor subtype and a few of M3 receptors). Activation of the M2 receptor causes the contraction of the detrusor smooth muscles; whereas selective M3 receptor inactivation results in M2-mediated contraction of the detrusor muscle (YamanishiChapple et al., 2001). Muscarinic receptor antagonists were investigated to treat CRBD. Currently, many antimuscarinic reagents, including ketamine, have been used successfully for the treatment of CRBD (Andersson, 1999; Agarwal et al., 2007; Tauzin-Fin et al., 2007; Bala et al., 2012; Ryu et al., 2013; Zhang et al., 2014).
Ketamine is well-received because of its unique properties such as protection of the upper respiratory tract reflex, without significant respiratory inhibition, and its effective analgesic effect (Mion and Villevieille, 2013; Persson, 2013). Ketamine, a dissociative anesthetic, has an analgesic effect in sub-anesthetic doses. Ketamine is a complex medication with two isomers, R (-)-ketamine and S (+)- ketamine (Sinner and Graf, 2008). Ketamine could interact with many receptors, including opioid receptors, NMDA receptors, and muscarinic receptors (Kohrs and Durieux, 1998). The activation of the NMDA receptor leads to an influx of Ca2+ closely involved in the development of central sensitization of dorsal horn neurons (Quibell et al., 2011), which plays an important role in pain sensation. Ketamine acts by reducing the frequency and opening of the Ca2+ channel and also prevents Ca2+ influx by antagonizing the NMDA receptor noncompetitively (Orser et al., 1997; Fan et al., 2012).
The bioavailability of oral ketamine is poor. Due to the extensive first-pass elimination effect, only 17% of an oral dose is absorbed (Clements et al., 1982). At present, there are no FDA-approved nonparenteral preparations for oral administration. Intravenous injection is the main medication administration in postoperative pain. IV ketamine gets an advantage because it takes effect quickly (within 30 s and the maximum effect occurs in about 1 min).
Ketamine is dose-dependent. According to the United States Food and Drug Administration (FDA) prescribing information, the average dose is 2 mg/kg as induction of anesthesia. However, the anesthetic dose is considered undesirable because it may produce prolonged emergence and unpleasant side effects. Ketamine is recommended for use in sub-anesthetic doses to provide adequate analgesia (Slogoff et al., 1974). The Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management recommends lower doses (0.1–0.5 mg/kg per hour) in acute pain therapy to achieve an adequate balance of analgesia and adverse effects (Schwenk et al., 2018). The common sub-anesthetic dose of IV ketamine used in clinical practice is 0.25 and 0.5 mg/kg.
At present, a sub-hypnotic dose of ketamine has been used in pain management. The pharmacokinetics of ketamine has been widely reported. It was reported that the mean serum half-life of ketamine was about 2–3 h (Reves et al., 2010; Clements and Nimmo, 1981; Idvall et al., 1979; Wieber et al., 1975). In our meta-analysis, five RCTs involving 414 patients were included. The incidence and severity of CRBD after surgery were assessed at 0, 1, 2, and 6 h. According to our results, ketamine IV administration could reduce the incidence of postoperative CRBD significantly at 2 and 6 h and reduce the severity of CRBD at 1 and 2 h compared with the control group. Our results are consistent with the pharmacokinetics of ketamine. In the early stage after surgery, ketamine can reduce the severity of CRBD because the maximum plasma concentration can be reached rapidly. With prolonged postoperative observation time, the incidence of CRBD is also reduced.
The probable side effects reported for ketamine were PONV, sedation, diplopia, hallucination, unpleasant dreams, and respiratory depression. We analyzed PONV, hallucination, and diplopia according to the data of the included RCTs. The results showed ketamine was safe without evident side effects. It is worth mentioning that sedation is a more important side effect for sub-anesthetic dose of ketamine. However, although four articles (Agarwal et al., 2006b; Shariat Moharari et al., 2014; Akça et al., 2016; Burimsittichai et al., 2016) in our meta-analysis showed information on the side effects of sedation, the data were still insufficient and the sedation scales used in the enrolled literature were different. We finally obtained the data on moderate and severe sedation in two studies ((Shariat Moharari et al., 2014) and (Burimsittichai et al., 2016)). The results showed statistically significant differences in terms of moderate and severe sedation (RR 7.67; 95% CI, 1.42–41.39; p = 0.02). However, because there are only two included articles, the conclusion needs to be further verified in the future.
In addition, we think urologists need to know about ketamine-related cystitis which is also a serious problem in the clinic. The United States Food and Drug Administration (FDA) prescribing information shows in individuals with a history of chronic ketamine use or abuse that there have been case reports of genitourinary pain that may be related to the ketamine treatment, not the underlying condition (Schwenk et al., 2018). Ketamine cystitis was first reported in a case series in 2007 and is characterized by severe dysuria, urgency, frequency, and gross hematuria (Shahani et al., 2007). The pathophysiology of ketamine cystitis remains unclear. It was reported that there is a dose and frequency response relationship between ketamine use and urinary symptoms, and 51% of patients had improved their symptoms after stopping ketamine usage (Winstock et al., 2012), therefore considering the cessation of ketamine if genitourinary pain continues in the setting of other genitourinary symptoms.
There are several limitations in our analysis: 1) the number of included studies was small. Therefore, subgroup analysis or sensitivity analysis could not be conducted further. In our opinion, the lack of industry interest in funding large and multicenter studies, as well as the ethical and practical concerns related to enrolling patients with acute pain conditions in controlled clinical trials, may be the main reasons for the insufficient number of clinical trials. However, we believe that our meta-analysis is meaningful and can provide new information for doctors in reducing postoperative CRBD. At the same time, we also hope there will be more RCTs with larger sample sizes and high quality for a better understanding of ketamine for the treatment of CRBD in the future; 2) significant heterogeneity exists in our analysis, which may introduce a bias; 3) type of surgery may result in potential bias. Only one study included in the meta-analysis was elective laparoscopic surgery, while the other four studies were all urology surgeries. We could not make a further subgroup analysis because of a small number of studies; 4) it is worth mentioning that the time point of ketamine administration in our analysis is inconsistent which may contribute to the high heterogeneity. In our study, we did not assess the dose–response effects and timing of ketamine administration, which can be a very important variable in outcome analysis.
In conclusion, we suggest that a sub-hypnotic dose of ketamine administration decreases the incidence and severity of postoperative CRBD. In addition, a sub-hypnotic dose of ketamine is safe for postoperative CRBD. More RCTs with larger sample sizes and high quality are needed for a better understanding of a sub-hypnotic dose of ketamine for the treatment of CRBD.
Our meta-analysis demonstrates that a sub-hypnotic dose of ketamine decreases the incidence and severity of postoperative CRBD without causing evident side effects. More experimental studies are needed to confirm the causality. Further research is worthwhile regarding the timing or dose of ketamine administration.
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding authors.
JW and YC designed the research, interpreted the data, and revised the manuscript. All authors performed the data extraction and data analysis. YL and QL drafted the manuscript. All authors read and approved the final manuscript.
This study was funded by the Yantai Science and Technology Bureau (2018SFGY117; 2019YD016) and the Joint Fund of the Shandong Natural Science Foundation (ZR2021LSW019).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CRBD: catheter-related bladder discomfort; OAB: overactive bladder; NMDA: N-methyl-d-aspartate; IV: intravenous; RCTs: randomized controlled trials; MD: mean difference; RR: risk ratio; CI: confidence interval; NG, not given in the article.
Adam, F., Chauvin, M., Du Manoir, B., Langlois, M., Sessler, D. I., and Fletcher, D. (2005). Small-dose Ketamine Infusion Improves Postoperative Analgesia and Rehabilitation after Total Knee Arthroplasty. Anesth. Analg. 100, 475–480. doi:10.1213/01.ANE.0000142117.82241.DC
Agarwal, A., Dhiraaj, S., Pawar, S., Kapoor, R., Gupta, D., and Singh, P. K. (2007). An Evaluation of the Efficacy of Gabapentin for Prevention of Catheter-Related Bladder Discomfort: a Prospective, Randomized, Placebo-Controlled, Double-Blind Study. Anesth. Analg. 105, 1454–contents. doi:10.1213/01.ane.0000281154.03887.2b
Agarwal, A., Dhiraaj, S., Singhal, V., Kapoor, R., and Tandon, M. (2006). Comparison of Efficacy of Oxybutynin and Tolterodine for Prevention of Catheter Related Bladder Discomfort: a Prospective, Randomized, Placebo-Controlled, Double-Blind Study. Br. J. Anaesth. 96, 377–380. doi:10.1093/bja/ael003
Agarwal, A., Gupta, D., Kumar, M., Dhiraaj, S., Tandon, M., and Singh, P. K. (2006). Ketamine for Treatment of Catheter Related Bladder Discomfort: a Prospective, Randomized, Placebo Controlled and Double Blind Study. Br. J. Anaesth. 96, 587–589. doi:10.1093/bja/ael048
Agarwal, A., Raza, M., Singhal, V., Dhiraaj, S., Kapoor, R., Srivastava, A., et al. (2005). The Efficacy of Tolterodine for Prevention of Catheter-Related Bladder Discomfort: A Prospective, Randomized, Placebo-Controlled, Double-Blind Study. Anesth. Analg. 101, 1065–contents. doi:10.1213/01.ane.0000167775.46192.e9
Akça, B., Aydoğan-Eren, E., Canbay, Ö., Karagöz, A. H., Üzümcügil, F., Ankay-Yilbaş, A., et al. (2016). Comparison of Efficacy of Prophylactic Ketamine and Dexmedetomidine on Postoperative Bladder Catheter-Related Discomfort. Saudi Med. J. 37, 55–59. doi:10.15537/smj.2016.1.14122
Anderson, K. E. (1993). Pharmacology of Lower Urinary Tract Smooth Muscles and Penile Erectile Tissues. Pharmacol. Rev. 45, 253–308.
Andersson, K. E. (1999). Advances in the Pharmacological Control of the Bladder. Exp. Physiol. 84 (1), 195–213. doi:10.1111/j.1469-445x.1999.tb00083.x
Bala, I., Bharti, N., Chaubey, V. K., and Mandal, A. K. (2012). Efficacy of Gabapentin for Prevention of Postoperative Catheter-Related Bladder Discomfort in Patients Undergoing Transurethral Resection of Bladder Tumor. Urology 79, 853–857. doi:10.1016/j.urology.2011.11.050
Binhas, M., Motamed, C., Hawajri, N., Yiou, R., and Marty, J. (2011). Predictors of Catheter-Related Bladder Discomfort in the Post-anaesthesia Care Unit. Ann. Fr. Anesth. Reanim. 30 (2), 122–125. doi:10.1016/j.annfar.2010.12.009
Burimsittichai, R., Limraksasin, P., Hurst, C. P., and Charuluxananan, S. (2016). Comparison of Intravenous Tramadol and Ketamine for Prevention of Catheter-Related Bladder Discomfort after Laparoscopic Surgery: a Randomized, Placebo-Controlled, Double-Blind Study. Asian Biomed. 10, 253–260.
Clements, J. A., Nimmo, W. S., and Grant, I. S. (1982). Bioavailability, Pharmacokinetics, and Analgesic Activity of Ketamine in Humans. J. Pharm. Sci. 71, 539–542. doi:10.1002/jps.2600710516
Clements, J. A., and Nimmo, W. S. (1981). Pharmacokinetics and Analgesic Effect of Ketamine in Man. Br. J. Anaesth. 53 (1), 27–30. doi:10.1093/bja/53.1.27
Durieux, M. E. (1995). Inhibition by Ketamine of Muscarinic Acetylcholine Receptor Function. Anesth. Analg. 81, 57–62. doi:10.1097/00000539-199507000-00012
Fan, W., Huang, F., Wu, Z., Zhu, X., Li, D., and He, H. (2012). The Role of Nitric Oxide in Orofacial Pain. Nitric Oxide 26, 32–37. doi:10.1016/j.niox.2011.11.003
Higgins, J. P. T.,, and Green, S. (Editors) (2011). Cochrane Handbook for Systematic Reviews of Interventions (The Cochrane collaboration). Available at: http://www.cochrane-handbook.org (Accessed Mar, 2011).
Hirota, K., and Lambert, D. G. (1996). Ketamine: Its Mechanism(s) of Action and Unusual Clinical Uses. Br. J. Anaesth. 77, 441–444. doi:10.1093/bja/77.4.441
Hu, B., Li, C., Pan, M., Zhong, M., Cao, Y., Zhang, N., et al. (2016). Strategies for the Prevention of Catheter-Related Bladder Discomfort: A PRISMA-Compliant Systematic Review and Meta-Analysis of Randomized Controlled Trials. Med. Baltim. 95 (37), e4859. doi:10.1097/MD.0000000000004859
Idvall, J., Ahlgren, I., Aronsen, K. F., and Stenberg, P. (1979). Ketamine Infusions: Pharmacokinetics and Clinical Effects. Br. J. Anaesth. 51, 1167–1173. doi:10.1093/bja/51.12.1167
Kohrs, R., and Durieux, M. E. (1998). Ketamine: Teaching an Old Drug New Tricks. Anesth. Analg. 87, 1186–1193. doi:10.1097/00000539-199811000-00039
Li, J.-y., Yi, M.-l., and Liao, R. (2016). Dorsal Penile Nerve Block with Ropivacaine-Reduced Postoperative Catheter-Related Bladder Discomfort in Male Patients after Emergence of General Anesthesia. Medicine 95, e3409. doi:10.1097/md.0000000000003409
Loftus, R. W., Yeager, M. P., Clark, J. A., Brown, J. R., Abdu, W. A., Sengupta, D. K., et al. (2010). Intraoperative Ketamine Reduces Perioperative Opiate Consumption in Opiate-dependent Patients with Chronic Back Pain Undergoing Back Surgery. Anesthesiology 113, 639–646. doi:10.1097/ALN.0b013e3181e90914
Mion, G., and Villevieille, T. (2013). Ketamine Pharmacology: an Update (Pharmacodynamics and Molecular Aspects, Recent Findings). CNS Neurosci. Ther. 19, 370–380. doi:10.1111/cns.12099
Mueller, M. F., and Golembiewski, J. (2011). The Changing Landscape of Perioperative Pain Management. J. Perianesth Nurs. 26, 290–293. doi:10.1016/j.jopan.2011.05.004
Orser, B. A., Pennefather, P. S., and MacDonald, J. F. (1997). Multiple Mechanisms of Ketamine Blockade of N-Methyl-D-Aspartate Receptors. Anesthesiology 86, 903–917. doi:10.1097/00000542-199704000-00021
Persson, J. (2013). Ketamine in Pain Management. CNS Neurosci. Ther. 19, 396–402. doi:10.1111/cns.12111
Quibell, R., Prommer, E. E., Mihalyo, M., Twycross, R., and Wilcock, A. (2011). Ketamine*. J. Pain Symptom Manag. 41, 640–649. doi:10.1016/j.jpainsymman.2011.01.001
Reves, J. G., Glass, P. S. A., Lubarsky, D. A., McEvoy, M. D., and Martinez-Ruiz, R. (2010). “Intravenous Anesthetics,” in Miller’s Anesthesia. Editor R. D Miller (Philadelphia: Churchill Livingstone), 719–768. doi:10.1016/b978-0-443-06959-8.00026-1
Ryu, J. H., Hwang, J. W., Lee, J. W., Seo, J. H., Park, H. P., Oh, A. Y., et al. (2013). Efficacy of Butylscopolamine for the Treatment of Catheter-Related Bladder Discomfort: A Prospective, Randomized, Placebo-Controlled, Double-Blind Study. Br. J. Anaesth. 111, 932–937. doi:10.1093/bja/aet249
Safavi, M., Honarmand, A., Atari, M., Chehrodi, S., and Amoushahi, M. (2014). An Evaluation of the Efficacy of Different Doses of Ketamine for Treatment of Catheter-Related Bladder Discomfort in Patients Underwent Urologic Surgery: A Prospective, Randomized, Placebo-Controlled, Double-Blind Study. Urol. Ann. 6 (1), 51–56. doi:10.4103/0974-7796.127030
Schwenk, E. S., Viscusi, E. R., Buvanendran, A., Hurley, R. W., Wasan, A. D., Narouze, S., et al. (2018). Consensus Guidelines on the Use of Intravenous Ketamine Infusions for Acute Pain Management from the American Society of Regional Anesthesia and Pain Medicine, the American Academy of Pain Medicine, and the American Society of Anesthesiologists. Reg. Anesth. Pain Med. 43 (5), 456–466. doi:10.1097/AAP.0000000000000806
Shahani, R., Streutker, C., Dickson, B., and Stewart, R. J. (2007). Ketamine-associated Ulcerative Cystitis: A New Clinical Entity. Urology 69 (5), 810–812. doi:10.1016/j.urology.2007.01.038
Shariat Moharari, R., Lajevardi, M., Khajavi, M., Najafi, A., Shariat Moharari, G., and Etezadi, F. (2014). Effects of Intra-operative Ketamine Administration on Postoperative Catheter-Related Bladder Discomfort: a Double-Blind Clinical Trial. Pain Pract. 14, 146–150. doi:10.1111/papr.12055
Slogoff, S., Allen, G. W., Wessels, J. V., and Cheney, D. H. (1974). Clinical Experience with Subanesthetic Ketamine. Anesth. Analg. 53, 354–358. doi:10.1213/00000539-197405000-00009
Tauzin-Fin, P., Sesay, M., Svartz, L., Krol-Houdek, M. C., and Maurette, P. (2007). Sublingual Oxybutynin Reduces Postoperative Pain Related to Indwelling Bladder Catheter after Radical Retropubic Prostatectomy. Br. J. Anaesth. 99, 572–575. doi:10.1093/bja/aem232
Winstock, A. R., Mitcheson, L., Gillatt, D. A., and Cottrell, A. M. (2012). The Prevalence and Natural History of Urinary Symptoms Among Recreational Ketamine Users. BJU Int. 110 (11), 1762–1766. doi:10.1111/j.1464-410X.2012.11028.x
YamanishiChapple, T. C. R., Chapple, C. R., and Chess-Williams, R. (2001). Which Muscarinic Receptor Is Important in the Bladder? World J. Urol. 19, 299–306. doi:10.1007/s003450100226
Keywords: ketamine, catheter-related bladder discomfort, randomized controlled trials, CRBD, meta-analysis
Citation: Lu Y, Li Q, Wang Y, Zhou Z, Zhang D, Bao Y, Wu J and Cui Y (2022) Meta-Analysis of the Efficacy and Safety of Ketamine on Postoperative Catheter-Related Bladder Discomfort. Front. Pharmacol. 13:816995. doi: 10.3389/fphar.2022.816995
Received: 17 November 2021; Accepted: 16 May 2022;
Published: 27 June 2022.
Edited by:
Philippe De Deurwaerdere, Université de Bordeaux, FranceReviewed by:
Lijia Chang, Chiba University, JapanCopyright © 2022 Lu, Li, Wang, Zhou, Zhang, Bao, Wu and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jitao Wu, bHl5XzU2MUAxNjMuY29t; Yuanshan Cui, ZG9jdG9yY3VpeXNAMTYzLmNvbQ==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.