- Department of Oral Medicine, Peking University School and Hospital of Stomatology, National Center of Stomatology, National Clinical Research Center for Oral Diseases, National Engineering Laboratory for Digital and Material Technology of Stomatology, Beijing, China
Background: Radiotherapy and chemotherapy-induced oral mucositis can affect cancer patients’ quality of life, even necessitate cancer therapy and influence prognosis. Chinese patent medicines (CPMs) have been widely used as complementary alternative medicines for the prevention and treatment of oral mucositis, and their efficacy and safety require further evaluation. Therefore, this study was conducted to provide references for clinical practice.
Methods: Ten databases were searched electronically and manually to identify randomized controlled trials (RCTs) from their inception to August 2021, concerning the prevention and treatment of radiotherapy and chemotherapy-induced oral mucositis with CPMs. The prevalence, pain level, and the severity of radiotherapy and chemotherapy-induced oral mucositis, as well as the effectiveness rate and adverse effects of CPMs, were set as the outcome criteria. The assessment criteria of the Cochrane Handbook were used to determine study quality and bias, and meta-analysis was conducted using Review Manager 5.4.1 software.
Results: A total of 2,312 cases from 27 RCTs were included. Most studies were considered to have a low or unclear risk of bias. More research is available on the use of CPMs in the prevention of radiotherapy and chemotherapy-induced oral mucositis than in its treatment. As for the prevention, it was proved that CPMs could significantly reduce the prevalence of radiotherapy and chemotherapy-induced oral mucositis, especially for the severe types, and decrease pain levels (p < 0.05). For treatment, CPMs could alleviate the symptoms, promote the healing of ulceration in radiotherapy and chemotherapy-induced oral mucositis, and thus improve the efficiency of clinical treatment (p < 0.05). The results of subgroup analyses were mainly consistent with the above results. The adverse effects of CPMs mainly included gastrointestinal reactions and bitter taste, and no serious adverse events were reported.
Conclusions: This systematic review and meta-analysis indicated CPMs might be effective for the prevention and treatment of radiotherapy and chemotherapy-induced oral mucositis through reducing the prevalence, decreasing the occurrence of severe types, alleviating the symptoms, and promoting the healing of ulceration. However, due to the limited number of eligible studies and the publication bias, more high-quality, double-blinded, placebo-controlled RCTs are still needed in future research.
Systematic Review Registration: [https://inplasy.com/], identifier [INPLASY2021100100].
1 Introduction
Oral mucositis is an inflammation of the mucosa with a burning or tingling sensation. It is a common toxicity associated with both chemotherapy and head and neck radiation used for the treatment of cancer (Scully et al., 2006; Lalla et al., 2014a). Although many therapeutic strategies such as systemic chemotherapy and radiotherapy have improved survival rates for tumors, these treatments also cause several side effects, including a high risk of developing oral mucositis (Lalla et al., 2014b). Mucositis can be divided by treatment modality of cancer patients: radiotherapy to head and neck (H&N) cancer-induced (RT-induced), radiotherapy and chemotherapy to H&N cancer-induced (RT-CT-induced), chemotherapy-induced (CT-induced), and hematopoietic stem cell transplantation–induced (HSCT-induced). Mucositis has been consistently reported to occur in at least 75% of these cancer patients (Scully et al., 2006). RT-induced oral mucositis occurs in almost all patients who are treated for cancers of H&N; Grade 3 and 4 oral mucositis occurs in 56% (Jones et al., 2006; Maria et al., 2017). About 40% of patients treated with standard chemotherapy develop CT-induced oral mucositis. The situation can be even worse for the combining of different chemotherapeutic drugs, and the likelihood of oral mucositis increases with the number of chemotherapy cycles (Naidu et al., 2004; Scully et al., 2006). Mucositis presents as erythema and ulceration of the oral mucosa and is pathologically characterized by atrophy of the squamous epithelium, vascular damage, inflammatory infiltration, and ulceration. It causes severe discomfort and impairs patients’ ability to swallow, eat, and talk. The pain of oral mucositis increases the use of opioid analgesics, impairs nutritional intake, affecting the quality of life (Elting et al., 2008; Jensen and Peterson, 2014). Severe mucositis can necessitate cancer therapy, causing interruptions and/or dose reductions in cancer treatment protocols, and even negatively influence prognosis (Peterson et al., 2015).
Numerous strategies have been recommended by the Multinational Association of Supportive Care in Cancer/International Society of Oral Oncology (MASCC/ISOO) for the prevention and treatment of radiotherapy and chemotherapy-induced oral mucositis. In the latest guideline conducted by the group in 2020, some natural agents, including herbal medicines, are included in the analysis. But due to the conflicting evidence, they are listed as having “no guideline possible” (Yarom et al., 2019; Yarom et al., 2020). Therefore, it is of great significance to assess their effects and thus lead to their appropriate clinical use. Chinese patent medicines (CPMs) are modern pharmaceutical preparations of traditional Chinese medicine (TCM) extracts, such as TCM pills, capsules, and injections, made mainly according to a standardized formulation of Chinese herbal medicines. The registration of CPMs is authorized by the China Food and Drug Administration (CDFA) and National Administration of Traditional Chinese Medicine (NATCM), with reference to laws such as Drug Administration Law of the People’s Republic of China and Provision for Drug Registration (Zhang, 2018). According to the TCM theory, CPMs with heat-clearing and detoxicating effects have become ideal options in the prevention and treatment of heat toxin syndrome, including redness, swelling, ulceration, and pain, which are also the main features of oral mucositis. In addition, unlike synthetic medicines, naturally originated CPMs are generally perceived to have a wider range of sources, fewer side effects, and multiple pharmacological targets (Lin et al., 2021). Therefore, CPMs are in wide clinical use to prevent or treat radiotherapy and chemotherapy-induced oral mucositis in China as important complementary alternative medicines (Zheng et al., 2011). However, as most articles relevant to CPMs are published in Chinese, their number and quality may be underestimated by clinicians and scientists outside China. Moreover, there has been no systematic review and meta-analysis evaluating the effectiveness of CPMs for radiotherapy and chemotherapy-induced oral mucositis currently. Therefore, with the inclusion of Chinese and English publications, a comprehensive assessment of the efficacy and safety of CPMs for the prevention and treatment of radiotherapy and chemotherapy-induced oral mucositis is conducted in this study to provide basic information for clinical practice.
2 Materials and Methods
2.1 Protocol and Registration
This study was conducted and presented according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (Page et al., 2021). The research protocol has been registered in Inplasy (https://inplasy.com/) (Registration NO: INPLASY2021100100).
2.2 Search Strategy
We selected all clinical trials in English or Chinese that focused on CPMs for the prevention and treatment of chemotherapy and radiotherapy-induced oral mucositis in the Pubmed, Web of Science, embase, CNKI, CBM, CQVIP, Wan-Fang, Ovid, Current Controlled Trials, and Cochrane Library. These databases were searched up to August 2021. The reference lists of the articles were also searched. The following search terms were used individually or combined: Chinese Patent Medicine, Oral Mucositis, Radiotherapy, Chemotherapy, as well as their synonyms. The searches were conducted by two independent investigators (X Fang and PR Zhou).
2.3 Inclusion and Exclusion Criteria
Studies that met all of the following five criteria were included in this review.
1) Participants: patients were diagnosed with cancer and received radiotherapy, chemotherapy, or both, with no restriction on the cancer location and nature.
2) Interventions: the treatment groups were treated with systemically or topically administered CPMs without the combination of Western medicines.
3) Control: the control groups were with no treatment, or treated with placebos and agents including vitamin preparations, normal saline, and antiseptic or anesthetic mouthwashes.
4) Outcome: the studies must have reported at least one of the following primary or secondary outcomes: the primary outcomes included the prevalence of oral mucositis, the level of pain, the grading of oral mucositis, and the effectiveness rate; the secondary outcomes were the cure days of oral mucositis and the adverse effects.
5) Types of studies: randomized controlled trials (RCTs).
The following types of studies were excluded.
1) Publication in a language other than English or Chinese.
2) Duplicate publications reporting the same groups of participants.
3) Case Reports, Reviews, Workshop Summaries, and Observational Studies
2.4 Data Collection and Quality Assessment
The literature search, data collection, and the assessment of the risk of bias were carried out by two independent researchers (PR Zhou and X Fang) using the criteria from the Cochrane Reviewers’ Handbook 6.0 as follows (Higgins et al., 2019). 1) Search results from the ten databases were imported into the reference management software EndNote X9. 2) Duplicated and irrelevant studies were removed by screening the title and abstract. 3) Full papers were obtained for all possibly relevant trials. 4) Studies meeting the inclusion criteria were identified. 5) The following data were collected: title, article source, year of publication, author(s), study size, sample size, methodological details, cancer type, mucositis type (i.e., related to RT, RT-CT, CT, or HSCT), the intervention of the treatment and control groups, outcome measures, and adverse effects. Disagreements were resolved by discussion, and the consensus was achieved through additional reviewers (H Hua).
The risk of bias was evaluated by Review Manager 5.4.1 software according to seven domains: random sequence generation (selection bias), allocation concealment (selection bias), masking of participants and personnel (performance bias), masking of outcome assessment (detection bias), incomplete outcome data (attrition bias), selective reporting (reporting bias), and other bias.
2.5 Data Synthesis and Statistical Analysis
\Statistical analyses were conducted using Review Manager 5.4.1 software. Subgroup analysis was undertaken for systemically and topically used CPMs. The estimate of the intervention effect was expressed as the odds ratios (OR) together with the 95% confidence interval (CI) and plotted on a forest plot. The I2 test was performed to evaluate the heterogeneity of the studies and ranged from 0 to 100%; I2 values of 25 and 50% were used as cutoffs for modest and high heterogeneity (Ioannidis, 2008). If no significant heterogeneity was found, a fixed-effects model was used to calculate the combined OR. Otherwise, a random-effects model was used. A p value <0.05 was regarded as statistically significant. Funnel plots were used to evaluate underlying publication bias.
3 Result
3.1 Study Selection and Characteristics of the Included Studies
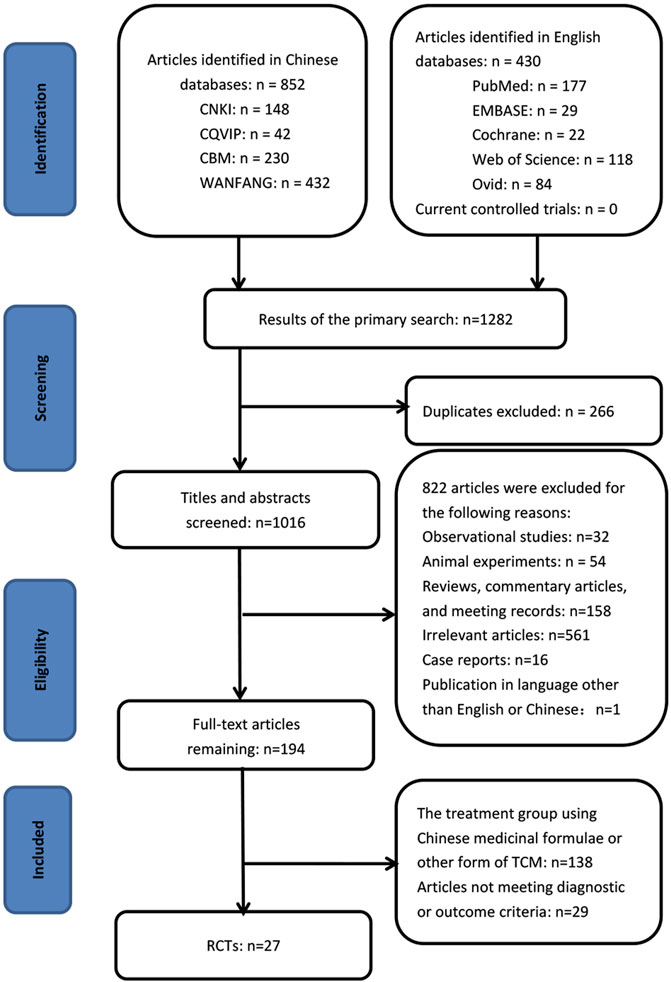
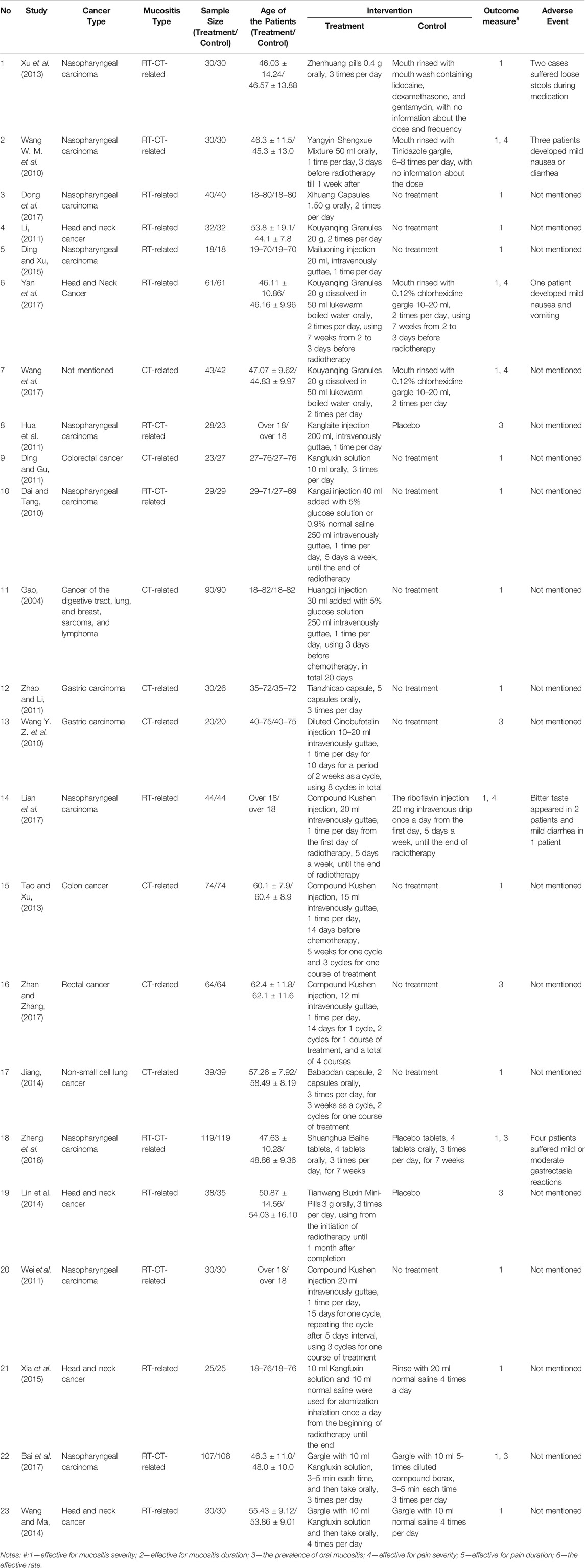
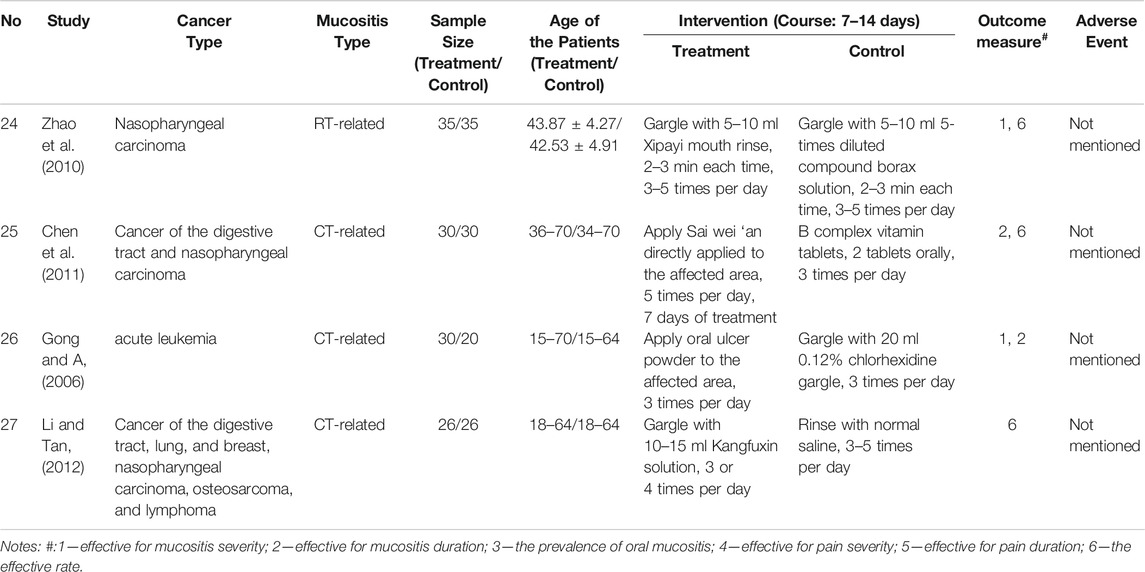
By searching the ten electronic databases, we initially identified 1,282 publications. Of these, 266 studies were excluded as duplicates, 561 were irrelevant articles, 260 were not RCTs or controlled clinical trials, and one was in a language other than English or Chinese. After we read the full papers of the remaining 194 articles, 138 were excluded because the treatment groups used Chinese medicinal formulas or other forms of Traditional Chinese Medicines rather than the standardized CPM, and 29 were excluded because they did not meet the diagnostic or outcome criteria. Finally, 27 trials were included in the final review: two studies were published in English, and 25 studies were in Chinese (Figure 1). The characteristics of the included 27 studies are shown in Tables 1, 2.
3.2 Risk of Bias Assessment
The risk of bias was assessed according to the Cochrane quality assessment criteria. The assessment is presented graphically by study (Figure 2) or by domain over all of the studies (Figure 3). All twenty-seven studies were RCTs. For random sequence generation, thirteen studies (Wang W. M. et al., 2010; Chen et al., 2011; Wei et al., 2011; Xu et al., 2013; Jiang, 2014; Lin et al., 2014; Bai et al., 2017; Dong et al., 2017; Lian et al., 2017; Wang et al., 2017; Yan et al., 2017; Zhan and Zhang, 2017; Zheng et al., 2018) were rated as low risk bias; For allocation concealment, three studies (Hua et al., 2011; Bai et al., 2017; Zheng et al., 2018) were rated as low risk bias; For blinding of participants and personnel, four studies (Hua et al., 2011; Lin et al., 2014; Bai et al., 2017; Zheng et al., 2018) were rated as low risk bias; For blinding of outcome assessment, four studies (Hua et al., 2011; Lin et al., 2014; Bai et al., 2017; Zheng et al., 2018) were rated as low risk bias; For imcomplete outcome data, only one study (Zhao et al., 2010) was rated as having an unclear risk of bias; For selective supporting and other bias, one (Zheng et al., 2018) and three studies (Jiang, 2014; Lin et al., 2014; Zheng et al., 2018) were rated as having an low risk of bias, respectively.
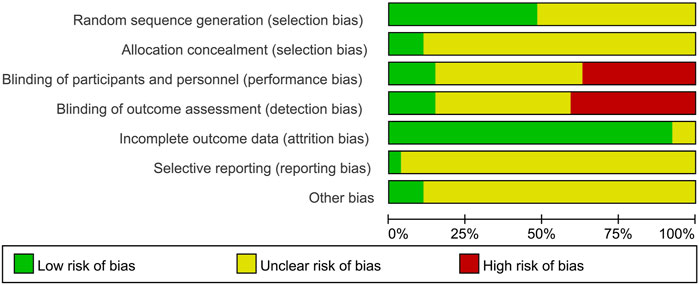
FIGURE 2. Risk of bias: review authors’ judgments about each risk of bias presented as percentages across all included studies.
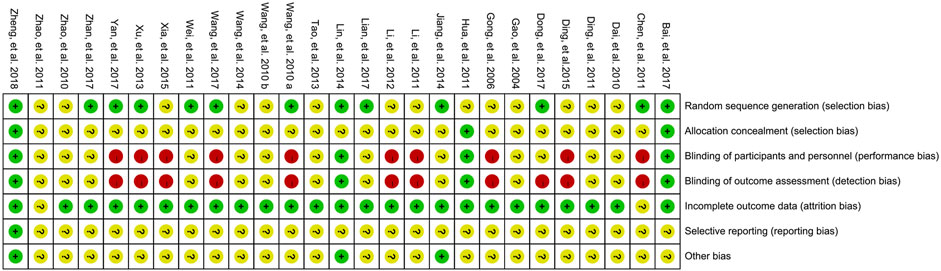
FIGURE 3. Risk of bias summary: review authors’ judgments about each risk of bias for each included study (green, low risk of bias; yellow, unclear risk of bias; red, high risk of bias).
3.3 Effectiveness of CPMs for the prevention of radiotherapy and chemotherapy-induced oral mucositis
We examined 23 studies (Gao, 2004; Dai and Tang, 2010; Wang W. M. et al., 2010; Wang Y. Z. et al., 2010; Ding and Gu, 2011; Hua et al., 2011; Li, 2011; Wei et al., 2011; Zhao and Li, 2011; Tao and Xu, 2013; Xu et al., 2013; Jiang, 2014; Lin et al., 2014; Wang and Ma, 2014; Ding and Xu, 2015; Xia et al., 2015; Bai et al., 2017; Dong et al., 2017; Lian et al., 2017; Wang et al., 2017; Yan et al., 2017; Zhan and Zhang, 2017; Zheng et al., 2018) of the effectiveness of CPMs in the prevention of radiotherapy and chemotherapy-induced oral mucositis, namely, Zhenhuang pills, Yangyin Shengxue Mixture, Xihuang capsules, Kouyanqing Granules, Mailuoning injection, Kanglaite injection, Kangfuxin solution, Kangai injection, Huangqi injection, Tianzhicao capsules, Cinobufotalin injection, Compound Kushen injection, Babao capsules, Shuanghua Baihe tablets, and Tianwang Buxin Mini-Pills. The comparators were drug-free controls, placebos, or agents including vitamin preparations, normal saline, and mouthwashes. The prevalences, moderate and severe pain, as well as Grade 3 and 4 radiotherapy and chemotherapy-induced oral mucositis, were pooled and meta-analyzed.
3.3.1 CPMs Reduced the Prevalence of Radiotherapy and Chemotherapy-Induced Oral Mucositis
Fourteen trials (Gao, 2004; Wang Y. Z et al., 2010; Ding and Gu, 2011; Wei et al., 2011; Zhao and Li, 2011; Tao and Xu, 2013; Xu et al., 2013; Jiang, 2014; Lin et al., 2014; Ding and Xu, 2015; Xia et al., 2015; Bai et al., 2017; Zhan and Zhang, 2017; Zheng et al., 2018) comparing the prevalence of radiotherapy and chemotherapy-induced oral mucositis were demonstrated in the forest plot. Due to fairly low heterogeneity among the trials (I2 = 18%), a fixed-effects model was chosen for the meta-analysis. The results showed that CPMs were superior to controls in reducing the prevalence of radiotherapy and chemotherapy-induced oral mucositis (pooled OR = 0.36, 95% CI = 0.27–0.49, p < 0.00001; Figure 4).
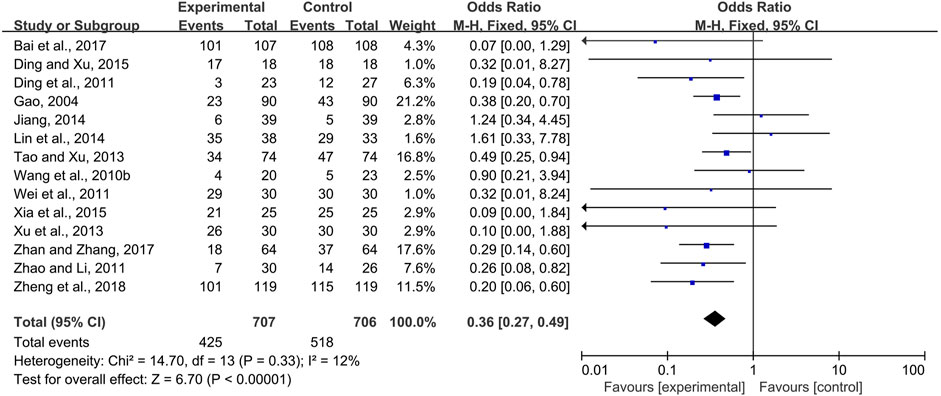
FIGURE 4. The efficacy of CPMs for the prevalence of radiotherapy and chemotherapy-induced oral mucositis.
Systemic and topical use of CPMs were further analyzed separately. The results of meta-analysis using fixed-effects models (I2 = 12 and 0%, respectively) showed that, whether used systemically or topically, CPMs were superior to controls in reducing the prevalence of radiotherapy and chemotherapy-induced oral mucositis (pooled OR = 0.39, 95% CI = 0.29–0.52, p < 0.00001; pooled OR = 0.08, 95% CI = 0.01–0.64, p = 0.02; respectively) (Figures 5, 6).
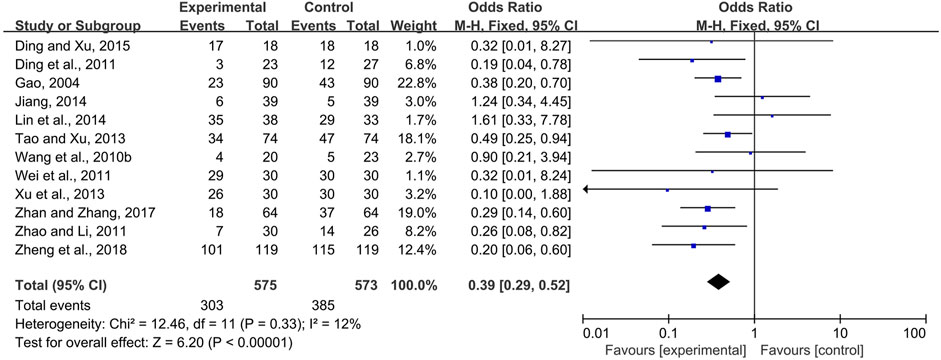
FIGURE 5. The efficacy of systemically used CPMs for the prevalence of radiotherapy and chemotherapy-induced oral mucositis.
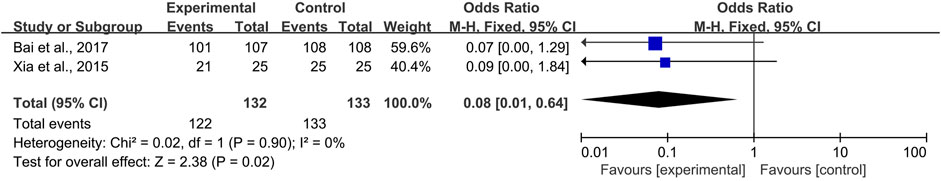
FIGURE 6. The efficacy of topically used CPMs for the prevalence of radiotherapy and chemotherapy-induced oral mucositis.
3.3.2 CPMs Decreased the Pain Level in Radiotherapy and Chemotherapy-Induced Oral Mucositis
Two trials of systemic use (Wang W. M. et al., 2010; Lian et al., 2017) and one trial (Bai et al.,2017) of topical use compared the pain level of the CPM groups and control groups. Moderate and severe pain scored above four on the 0–10 numerical rating scale or visual analog scale. Due to heterogeneity among the trials (I2 = 70%), a random-effects model was chosen for the meta-analysis. The results showed that CPMs were superior to controls in reducing the prevalence of moderate and severe pain (pooled OR = 0.40, 95% CI = 0.17–0.95, p = 0.04; Figure 7). An analysis of systemic and topical subgroups was not conducted because of the limited number of studies.
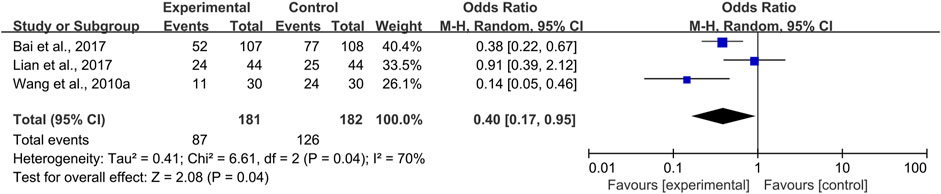
FIGURE 7. The efficacy of CPMs for the prevalence of moderate and severe pain in radiotherapy and chemotherapy-induced oral mucositis.
3.3.3 CPMs Reduced the Severity of Radiotherapy and Chemotherapy-Induced Oral Mucositis
Eighteen trials (Gao, 2004; Dai and Tang, 2010; Wang W. M. et al., 2010; Ding and Gu, 2011; Hua et al., 2011; Li, 2011; Wei et al., 2011; Zhao and Li, 2011; Tao and Xu, 2013; Xu et al., 2013; Lin et al., 2014; Wang and Ma, 2014; Ding and Xu, 2015; Xia et al., 2015; Bai et al., 2017; Dong et al., 2017; Lian et al., 2017; Yan et al., 2017) compared the prevalence of severe radiotherapy and chemotherapy-induced oral mucositis in the experimental and control groups, i.e., Grade 3 and 4 radiotherapy and chemotherapy-induced oral mucositis according to the criteria of the World Health Organization or toxicity criteria of the Radiation Therapy Oncology Group (RTOG) (Miller et al., 1981; Cox et al., 1995). Due to modest heterogeneity among the trials (I2 = 43%), a fixed-effects model was chosen for the meta-analysis. The results showed that CPMs were superior to controls in reducing the severity of radiotherapy and chemotherapy-induced oral mucositis (pooled OR = 0.31, 95% CI = 0.24–0.40, p < 0.00001; Figure 8).
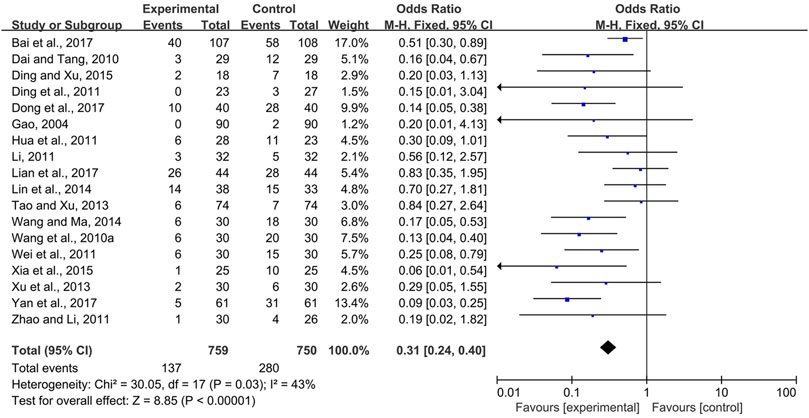
FIGURE 8. The efficacy of CPMs for reducing the severity of radiotherapy and chemotherapy-induced oral mucositis.
The effectiveness of systemic and topical use of CPMs was further analyzed separately. The results of meta-analysis using a fixed-effects model and a random-effects model (I2 = 40 and 66%, respectively) showed that, whether used systemically or topically, CPMs were superior to controls in reducing the severity of radiotherapy and chemotherapy-induced oral mucositis (pooled OR = 0.29, 95% CI = 0.21–0.40, p < 0.00001; pooled OR = 0.24, 95% CI = 0.08–0.74, p = 0.01 respectively) (Figures 9, 10).
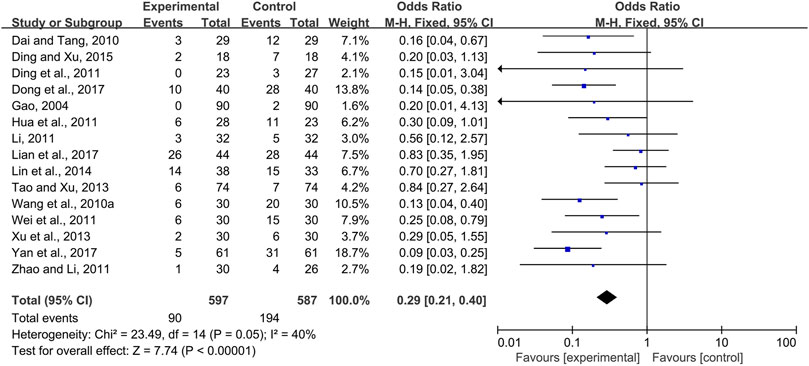
FIGURE 9. The efficacy of systemically used CPMs for the prevalence of severe radiotherapy and chemotherapy-induced oral mucositis.
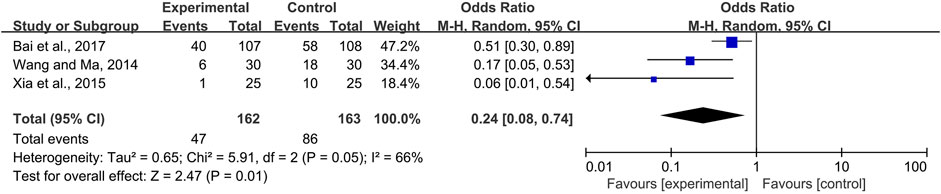
FIGURE 10. The efficacy of topically used CPMs for the prevalence of severe radiotherapy and chemotherapy-induced oral mucositis.
3.4 Effectiveness of CPMs for the treatment of radiotherapy and chemotherapy-induced oral mucositis
Four studies (Gong and A, 2006; Zhao et al., 2010; Chen et al., 2011; Li and Tan, 2012) compared the effects of CPMs, including Xipayi mouth rinse, Sai wei’an, oral ulcer powder, and Kangfuxin solution in the treatment of radiotherapy and chemotherapy-induced oral mucositis; the comparators were the compound borax solution, B complex vitamin tablets, 0.12% chlorhexidine gargle, and normal saline, respectively. Three trials (Zhao et al., 2010; Chen et al., 2011; Li and Tan, 2012) reported the effective rate of radiotherapy and chemotherapy-induced oral mucositis, which means the percentage of oral mucositis patients with pain and/or ulcer relief. Due to no heterogeneity among the trials (I2 = 0%), a fixed-effects model was chosen for the meta-analysis. The results showed that CPMs were superior to controls in increasing the effective rate (pooled OR = 9.25, 95% CI = 3.76–22.73, p < 0.00001; Figure 11).
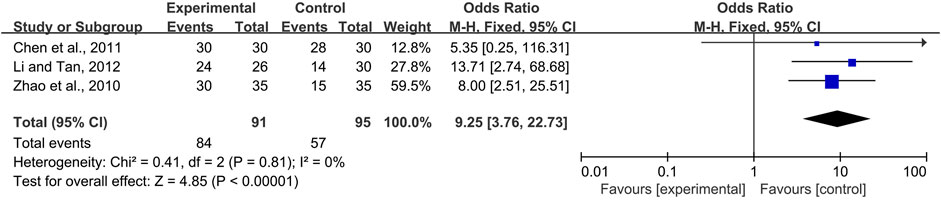
FIGURE 11. The efficacy of CPMs for the effective rate of radiotherapy and chemotherapy-induced oral mucositis.
Moreover, there was evidence from one trial (Gong and A, 2006) that oral ulcer powder was more effective in shortening the cure days of radiotherapy and chemotherapy-induced oral mucositis (p < 0.05) than chlorhexidine gargle. However, no difference between the two groups was found with regard to the average grade of radiotherapy and chemotherapy-induced oral mucositis after treatment (p > 0.05).
3.5 Safety Assessment
Five trials (Wang W. M. et al., 2010; Xu et al., 2013; Lian et al., 2017; Yan et al., 2017; Zheng et al., 2018) reported adverse effects. The CPMs included Zhenhuang pills, Yangyin Shengxue Mixture, Kouyanqing Granules, Compound Kushen injection, and Shuanghua Baihe tablets. The most frequently reported adverse effects were gastrointestinal reactions including diarrhea, loose stools, nausea, and vomiting, which involved Yangyin Shengxue Mixture, Kouyanqing Granules, Compound Kushen injection, Shuanghua Baihe tablets, and Zhenhuang pills (Wang W. M. et al., 2010; Lian et al., 2017; Yan et al., 2017; Zheng et al., 2018). Bitter taste was reported in two patients treated by Compound Kushen injection during medication (Lian et al., 2017). The adverse effects were generally mild and improved after the patients discontinued the medicine. No serious adverse events were reported.
3.6 Bias Analysis
Concerns about biases affecting the outcomes of this study existed due to the low quality of the RCTs reviewed for analysis. Moreover, because of the substantial heterogeneity among the included studies evaluating the prevalence of moderate and severe pain (I2 = 70%), it is inappropriate to confirm the above results directly. Therefore, the publication bias was assessed. The shape of the funnel plot showed no evidence of asymmetry (Figure 12).
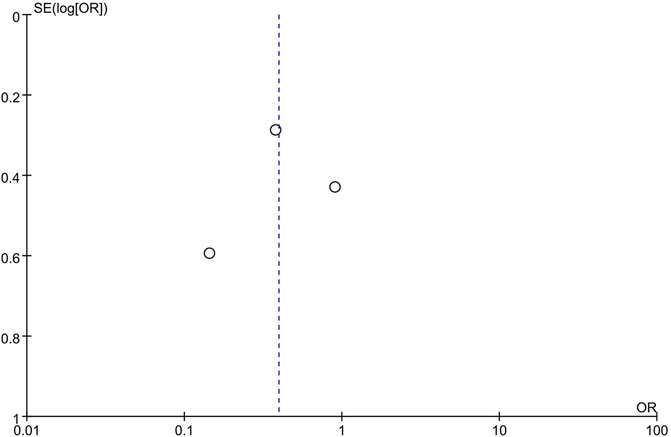
FIGURE 12. Funnel plot of studies evaluating the prevalence rate of moderate and severe pain between CPMs groups and control groups.
4 Discussion
In China, more and more patients receiving radiotherapy and chemotherapy choose CPMs to prevent or treat radiotherapy and chemotherapy-induced oral mucositis, but the application of CPMs is basically experience-based. Therefore, we tried to build its missing link to the evidence-based medical sciences in the present study. From the review, we found that CPMs were more frequently used in the prevention than the treatment of radiotherapy and chemotherapy-induced oral mucositis. That means the use of CPMs was usually begun before the onset of the disease, which may reduce the progression of the disease and thus had the effect of reducing the severity of radiotherapy and chemotherapy-induced oral mucositis. By meta-analysis, it was proved that CPMs could significantly reduce the prevalence, pain level, and severity of radiotherapy and chemotherapy-induced oral mucositis. As for the treatment, CPMs were effective in alleviating the symptoms, reducing the cure days, and thus improving the efficiency of clinical treatment for radiotherapy and chemotherapy-induced oral mucositis. We also found that the systemic administration of CPMs was more frequent than topical. Systemic CPMs could prevent and treat radiotherapy and chemotherapy-induced oral mucositis mainly by reducing the prevalence and severity of oral mucositis, and topical CPMs could play a role primarily through relieving the symptoms, promoting the healing of ulceration, and reducing the severity of oral mucositis.
According to the TCM theory, chemotherapy and radiotherapy belong to the “drug poison” and “evil poison” from the environment. The clinical feature of oral mucositis, including redness, swelling, ulceration, and pain, are the characteristics of TCM heat toxin syndrome ((Lin et al., 2021)). Heat clearing and detoxicating are classic TCM treatments for heat toxin syndrome (Jin et al., 2009). Therefore, CPMs with the above-mentioned effects have become our ideal option in the prevention and treatment of oral mucositis. The possible mechanisms of CPMs related to their main active components, such as matrine and cockroach extract, mainly attribute to the antioxidant, radical scavenging, anti-inflammatory, antimicrobial, and immunostimulatory properties. More specifically, matrine, as a primary component of Compound Kushen injection, could exert anti-inflammatory effects via lowering the expression of MyD88, NLRP3, and caspase-1, inhibiting the phosphorylation of I-κB α, and affecting the translocation of NF-κB from the cytoplasm to nucleus (Li et al., 2021). Cockroach extract, as the major component of Kangfuxin solution, could relieve the symptoms of oral mucositis by inhibiting the opening of the calcium-dependent potassium channel, promoting granulation tissue growth and angiogenesis, improving the phagocytic ability of macrophages and lymphocytes (Luo et al., 2016). Xihuang capsules, consisting of four ingredients extracted from natural herbs, including Bovis Calculus artifactus, Moschus, Olibanum, and Myrrha, were proved to reduce the levels of TNF-α and IL-6 detected by enzyme-linked immunosorbent assay (ELISA) in patients’ saliva (Dong et al., 2017). Moreover, Yangyin Shengxue Mixture, Kouyanqing Granules, Mailuoning injection, Kangfuxin solution, and Xipayi mouth rinse were also reported to have the effects of promoting microcirculation, reducing local vascular occlusion, and thus accelerating mucosal repair (Wang W. M. et al., 2010; Zhao et al., 2010; Ding and Gu, 2011; Li, 2011; Li and Tan, 2012; Wang and Ma, 2014; Ding and Xu, 2015; Xia et al., 2015; Bai et al., 2017; Wang et al., 2017; Yan et al., 2017). Therefore the mechanism of CPMs in the prevention and treatment of oral mucositis is not only in accordance with the TCM theory, but also related to the anti-inflammatory mechanism of its main active components, and the two complement and promote each other.
The courses of CPMs for the prevention of radiotherapy and chemotherapy-induced oral mucositis were basically the same as that of the chemotherapy or radiotherapy, and the courses for the treatment were about 1–2 weeks. Regarding safety evaluation, five trials reported the occurrence of adverse effects. The most frequently reported adverse effects were gastrointestinal reactions, which involved Zhenhuang pills, Yangyin Shengxue Mixture, Kouyanqing Granules, Compound Kushen injection, and Shuanghua Baihe tablets (Wang W. M. et al., 2010; Xu et al., 2013; Lian et al., 2017; Yan et al., 2017; Zheng et al., 2018). Specifically, Zhenhuang pills would cause loose stools; Yangyin Shengxue Mixture may lead to mild nausea or diarrhea; Kouyanqing granules could cause mild nausea and vomiting; Compound Kushen injection would sometimes induce mild diarrhea and bitter taste; Shuanghua Baihe tablets may bring about mild or moderate gastrectasia reactions. In the TCM theory, Chinese medicines are divided into four properties, including cold, hot, warm, and cool (Yang et al., 2013). Among the main active components of the above-mentioned CPMs, Rehmannia glutinosa, Asparagus, Sophora flavescens, Coptis chinensis, Bezoar, and Scutellaria baicalensis belong to cold or cool Chinese medicines, and these cold or cool components may cause gastrointestinal reactions such as loose stool, diarrhea, and vomiting due to the patients’ asthenia of spleen and stomach, and heavy dampness (Wang W. M. et al., 2010; Xu et al., 2013; Lian et al., 2017; Yan et al., 2017; Zheng et al., 2018). These adverse reactions are all closely related to the patients’ individual “cold” or “heat” constitution, gastrointestinal environment, and the underlying diseases. However, as the adverse effects of CPMs in the experimental groups were all mild and could disappear after stopping drugs, and fewer people were affected as the drug was discontinued, the statistical difference between the control and experimental groups was not clinically meaningful. Therefore, CPMs could still be considered safe in the prevention and treatment of radiotherapy and chemotherapy-induced oral mucositis.
Nevertheless, there are still some limitations in this study. First, as the study only included Chinese and English literature, and most articles relevant to CPMs are published in Chinese, it may lead to potential language bias and citation bias. Second, since most of the original literatures only showed the min-max age range without detailed age grouping, the patients’ age is a major confounding factor, and as the clinical data for each patient in the original RCT studies are inaccessible, it is impossible for this study to further perform subgroup analysis according to the patients’ age. Therefore, age grouping to reduce confounding bias in future studies was recommended. In addition, due to the application of CPMs depends on the identification of their effective chemical components, and the laboratory evidence was still lacking for most of the CPMs, more high-quality laboratory and animal experiments are still needed. Furthermore, due to the limited number of eligible studies and the publication bias, more high-quality, double-blinded, placebo-controlled RCTs are still needed to confirm the efficacy and safety of CPMs for the prevention and treatment of radiotherapy and chemotherapy-induced oral mucositis in future research.
5 Conclusion
This systematic review and meta-analysis indicated CPMs were more frequently used in the prevention than the treatment of radiotherapy and chemotherapy-induced oral mucositis. CPMs such as Compound Kushen injection, Kouyanqing Granules, and Shuanghua Baihe tablets may be effective for the prevention of radiotherapy and chemotherapy-induced oral mucositis through reducing the prevalence, relieving the pain level, and decreasing the occurrence of severe oral mucositis. As for the treatment, CPMs such as Xipayi mouth rinse, Sai wei’an, oral ulcer powder, and Kangfuxin solution may be superior in alleviating the symptoms, reducing the cure days than controls like normal saline, vitamin tablets, and antiseptic or anesthetic mouthwash. More high-quality, double-blinded, placebo-controlled RCTs are still warranted to verify and extend the present results to optimize CPMs for the prevention and treatment of radiotherapy and chemotherapy-induced oral mucositis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
HH and PZ designed the study. PZ and XF conducted the identification, selection, and evaluation of the studies. YX and PZ conducted the statistical analysis and result discussion. YX and PZ wrote the manuscript in consultation with HH. All authors contributed to the final article.
Funding
This research was supported by the Beijing Natural Science Foundation (grant number 7204330) and the Program for New Clinical Techniques and Therapies of Peking University School and Hospital of Stomatology (PKUSSNCT-21B13).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We gratefully acknowledge the assistance of Professor Elad Sharon for her constructive review comments during the revising process.
References
Bai, H. F., Jiang, Q. H., Zeng, W. Q., Li, B., Xiang, K. H., and Lang, J. Y. (2017). Effect of Kangfuxin Solution on the Prevention and Treatment of Radiotherapy-Induced Oral Mucositis in Patients with Nasopharyngeal Carcinoma. J. Canc. Control Treat. 30 (1), 43–48. doi:10.3969/j.issn.1674-0904.2017.01.009
Chen, J. Z., Liu, Y., Li, Y. J., and Li, X. F. (2011). 30 Cases of Chemotherapy-Induced Oral Mucositis Treated with Saiwei'an. Fujian J. TCM 42 (2), 40–41. doi:10.3969/j.issn.1000-338X.2011.02.023
Cox, J. D., Stetz, J., and Pajak, T. F. (1995). Toxicity Criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int. J. Radiat. Oncol. Biol. Phys. 31 (5), 1341–1346. doi:10.1016/0360-3016(95)00060-C
Dai, Y. H., and Tang, J. (2010). Clinical Observation of Kangai Injection on Treatment of Chemotherapy and Radiotherapy Induced Oral Mucositis in Patients with Advanced Nasopharyngeal Carcinoma. Med. Inf. 05 (11), 3299–3300. doi:10.3969/j.issn.1006-1959.2010.11.287
Ding, C., and Gu, X. X. (2011). The Effects of Kangfuxin Solution on Prevention and Treatment of 23 Cases of Oral Mucositis Caused by FORFIRI Chemotherapy. Jiangxi J. Traditional Chin. Med. 42 (8), 23–24. doi:10.3969/j.issn.1006-0979.2011.20.004
Ding, D., and Xu, J. L. (2015). Posterior Fossa Arteriovenous Malformations: Effect of Infratentorial Location on Outcomes after Intervention. Clin. Neurol. Neurosurg. 131 (3), 89–90. doi:10.1016/j.clineuro.2015.01.024
Dong, K. H., Xia, L., Liang, Y., Jin, G. H., and Zhu, L. Y. (2017). The protection of Xihuang Capsules against Acute Radiation-Induced Oral Mucositis and its Mechanisms. Chin. Traditional Patent Med. 39 (6), 1156–1160. doi:10.3969/j.issn.1001-1528.2017.06.009
Elting, L. S., Keefe, D. M., Sonis, S. T., Garden, A. S., Spijkervet, F. K., Barasch, A., et al. (2008). Patient-reported Measurements of Oral Mucositis in Head and Neck Cancer Patients Treated with Radiotherapy with or without Chemotherapy: Demonstration of Increased Frequency, Severity, Resistance to Palliation, and Impact on Quality of Life. Cancer 113 (10), 2704–2713. doi:10.1002/cncr.23898
Gao, Y. H. (2004). Clinical Analysis of Huangqi Injection in Treating Toxic and Side-Effect of Chemotherapy. J. Med. Forum 25 (3), 18–19. doi:10.3969/j.issn.1672-3422.2004.03.011
Gong, C. Q., and A, M. L. (2006). Clinical Observation of Mongolian Medicine on Treatment of Chemotherapy-Induced Oral Mucositis in Acute Leukemia Patients. Chin. J. Ethnomedicine Ethnopharmacy (2), 93–94. doi:10.3969/j.issn.1672-1721.2006.02.080
Higgins, J. P. T., Thomas, J., Chandler, J., Cumpston, M., Li, T., Page, M. J., et al. (2019). Cochrane Handbook for Systematic Reviews of Interventions. Version 6.0. Available at: www.training.cochrane.org/handbook (Accessed March 5th.
Hua, Y., Chen, D. Y., Liu, J., and Dai, D. F. (2011). Preliminary Study on the Toxicity Reducing and Efficacy Enhancing Effect of Kanglaite in Chemoradiotherapy of Locally Advanced Nasopharyngeal Carcinoma. J. Qiqihar Univ. Med. 32 (19), 3125–3126. doi:10.3969/j.issn.1002-1256.2011.19.024
Ioannidis, J. P. (2008). Interpretation of Tests of Heterogeneity and Bias in Meta-Analysis. J. Eval. Clin. Pract. 14 (5), 951–957. doi:10.1111/j.1365-2753.2008.00986.x
Jensen, S. B., and Peterson, D. E. (2014). Oral Mucosal Injury Caused by Cancer Therapies: Current Management and New Frontiers in Research. J. Oral Pathol. Med. 43 (2), 81–90. doi:10.1111/jop.12135
Jiang, F. E. (2014). Clinical Study on Babao Capsule Combined with TP Regimen in the First-Line Treatment of Advanced Non-small Cell Lung Cancer. Shandong, China: Qingdao University.
Jin, T., Shen, M. H., Sun, Y. F., and Zhang, J. (2009). Observe the Effect of Shuizhongcao Decoction Treating Chemotherapy-Induced Oral Ulcer. Chin. Arch. Trad. Chin. Med. 27, 303–305. doi:10.13193/j.archtcm.2009.02.80.jint.013
Jones, J. A., Avritscher, E. B., Cooksley, C. D., Michelet, M., Bekele, B. N., and Elting, L. S. (2006). Epidemiology of Treatment-Associated Mucosal Injury after Treatment with Newer Regimens for Lymphoma, Breast, Lung, or Colorectal Cancer. Support. Care Cancer 14 (6), 505–515. doi:10.1007/s00520-006-0055-4
Lalla, R. V., Bowen, J., Barasch, A., Elting, L., Epstein, J., Keefe, D. M., et al. (2014a). MASCC/ISOO Clinical Practice Guidelines for the Management of Mucositis Secondary to Cancer Therapy. Cancer 120 (10), 1453–1461. doi:10.1002/cncr.28592
Lalla, R. V., Saunders, D. P., and Peterson, D. E. (2014b). Chemotherapy or Radiation-Induced Oral Mucositis. Dent. Clin. North. Am. 58 (2), 341–349. doi:10.1016/j.cden.2013.12.005
Li, L., and Tan, T. (2012). Effect of Kangfuxin Solution on Chemotherapy Induced Oral Mucositis. J. Mod. Med. Health 28 (1), 121. https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E5%BA%B7%E5%A4%8D%E6%96%B0%E6%B6%B2%E6%B2%BB%E7%96%97%E5%8C%96%E7%96%97%E5%BC%95%E8%B5%B7%E7%9A%84%E5%8F%A3%E8%85%94%E9%BB%8F%E8%86%9C%E7%82%8E%E7%9A%84%E7%96%97%E6%95%88%E8%A7%82%E5%AF%9F&btnG=.
Li, W. Q. (2011). Clinical Observation of Kouyanqing Granules on Treatment of Radiotherapy Induced Oral Mucositis in Patients with Head and Neck Cancer. Chin. J. Aesthet. Med. z2 (20), 140. doi:10.3969/j.issn.1008-6455.2011.z2.130
Li, X., Tang, Z., Wen, L., Jiang, C., and Feng, Q. (2021). 1Matrine: A Review of its Pharmacology, Pharmacokinetics, Toxicity, Clinical Application and Preparation Researches. J. Ethnopharmacol 269, 113682. doi:10.1016/j.jep.2020.113682
Lian, L. X., Kong, C. Q., Luo, J. C., and Niu, P. (2017). Efficacy of Riboflavin Sodium Phosphate Combined with Compound Kushen Injection of Radioactive Oral Mucosal Injury on Nasopharyngeal Carcinoma. J. Mod. Oncol. 25 (24), 3957–3961. doi:10.3969/j.issn.1672-4992.2017.24.009
Lin, K. S., Jen, Y. M., Chao, T. Y., Lin, Y. S., Wang, L. H., Lin, C. L., et al. (2014). Prevention of Acute Radiation-Associated Toxicity by Traditional Chinese Medicine Tianwang Buxin Mini-Pills in Patients with Head and Neck Cancer. J. Med. Sci. 34 (4), 152–160. doi:10.4103/1011-4564.139185
Lin, Z., Chen, J., and Han, S. (2021). The Efficacy of Heat-Clearing (Qingre) and Detoxifying (Jiedu) Traditional Chinese Medicine Gargle for Chemotherapy-Induced Oral Mucositis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 12, 627628. doi:10.3389/fphar.2021.627628
Luo, Y., Feng, M., Fan, Z., Zhu, X., Jin, F., Li, R., et al. (2016). Effect of Kangfuxin Solution on Chemo/Radiotherapy-Induced Mucositis in Nasopharyngeal Carcinoma Patients: A Multicenter, Prospective Randomized Phase III Clinical Study. Evid. Based Complement. Alternat Med. 2016, 8692343. doi:10.1155/2016/8692343
Maria, O. M., Eliopoulos, N., and Muanza, T. (2017). Radiation-Induced Oral Mucositis. Front. Oncol. 7, 89. doi:10.3389/fonc.2017.00089
Miller, A. B., Hoogstraten, B., Staquet, M., and Winkler, A. (1981). Reporting Results of Cancer Treatment. Cancer 47 (1), 207–214. doi:10.1002/1097-0142(19810101)47:1<207::aid-cncr2820470134>3.0.co;2-6
Naidu, M. U., Ramana, G. V., Rani, P. U., Mohan, I. K., Suman, A., Roy, P., et al. (2004). Chemotherapy-induced And/or Radiation Therapy-Induced Oral Mucositis-Ccomplicating the Treatment of Cancer. Neoplasia 6 (5), 423–431. doi:10.1593/neo.04169
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Plos Med. 18 (3), e1003583. doi:10.1371/journal.pmed.1003583
Peterson, D. E., Srivastava, R., and Lalla, R. V. (2015). Oral Mucosal Injury in Oncology Patients: Perspectives on Maturation of a Field. Oral Dis. 21 (2), 133–141. doi:10.1111/odi.12167
Scully, C., Sonis, S., and Diz, P. D. (2006). Oral Mucositis. Oral Dis. 12 (3), 229–241. doi:10.1111/j.1601-0825.2006.01258.x
Tao, C. H., and Xu, J. F. (2013). Clinical Observation of Compound Kushen Injection on Treatment of Chemotherapy-Induced Oral Mucositis in 74 Patients with Advanced Colon Carcinoma. Guiding J. Traditional Chin. Med. Pharm. 19 (11), 42–44. https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E5%A4%8D%E6%96%B9%E8%8B%A6%E5%8F%82%E6%B3%A8%E5%B0%84%E6%B6%B2%E8%81%94%E5%90%88%E5%8C%96%E7%96%97%E6%B2%BB%E7%96%97%E6%99%9A%E6%9C%9F%E7%BB%93%E8%82%A0%E7%99%8C74%E4%BE%8B%E4%B8%B4%E5%BA%8A%E8%A7%82%E5%AF%9F&btnG=.
Wang, S. M., and Ma, Z. M. (2014). Preventing and Treating 30 Cases of Radiation Stomatitis by Gargling with KangFu XinYe. West. J. Traditional Chin. Med. 27 (12), 66–67. doi:10.3969/j.issn.1004-6852.2014.12.026
Wang, W. M., Li, C. F., and Yao, R. J. (2010). Clinical Observation of Cinobufotalin Injection on Treatment of Chemotherapy-Induced Oral Mucositis in Patients with Advanced Gastric Carcinoma. Clin. J. Traditional Chin. Med. 22 (04), 36–37. https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E5%8D%8E%E8%9F%BE%E7%B4%A0%E6%B3%A8%E5%B0%84%E6%B6%B2%E8%81%94%E5%90%88%E5%8C%96%E7%96%97%E6%B2%BB%E7%96%97%E6%99%9A%E6%9C%9F%E8%83%83%E7%99%8C%E4%B8%B4%E5%BA%8A%E8%A7%82%E5%AF%9F+%E7%8E%8B%E4%B8%BA%E6%B0%91&btnG=.
Wang, X. L., Lin, H. S., and Dai, J. (2017). Effects of Kouyanqing Granules on the Relief and Comfort Degree of Patients with Stomatitis after Chemotherapy. Henan Traditional Chin. Med. 37 (12), 179–181. https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E5%8F%A3%E7%82%8E%E6%B8%85%E9%A2%97%E7%B2%92%E5%AF%B9%E5%8C%96%E7%96%97%E5%90%8E%E5%8F%A3%E8%85%94%E7%82%8E%E6%82%A3%E8%80%85%E5%8F%A3%E8%85%94%E7%96%BC%E7%97%9B%E5%8F%8A%E5%8F%A3%E8%85%94%E8%88%92%E9%80%82%E5%BA%A6%E7%9A%84%E5%BD%B1%E5%93%8D+%E7%8E%8B%E6%99%93%E7%90%B3&btnG=<=rW.
Wang, Y. Z., Feng, W., Wang, Z., Zhou, X., and Zheng, X. (2010). Clinical Observation on Yangyin Shengxue Mixture in Prevention and Treatment of Radiation Injury of Oral Mucosa in Nasopharyngeal Carcinoma Patients: A Report of 30 Cases. J. Traditional Chin. Med. 5 (01), 45–47. https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E5%85%BB%E9%98%B4%E7%94%9F%E8%A1%80%E5%90%88%E5%89%82%E9%98%B2%E6%B2%BB%E9%BC%BB%E5%92%BD%E7%99%8C%E6%82%A3%E8%80%85%E6%94%BE%E5%B0%84%E6%80%A7%E5%8F%A3%E8%85%94%E9%BB%8F%E8%86%9C%E6%8D%9F%E4%BC%A430%E4%BE%8B%E4%B8%B4%E5%BA%8A%E8%A7%82%E5%AF%9F+%E7%8E%8B%E8%B7%83%E7%8F%8D&btnG=.
Wei, R., Yang, D. Y., Jiang, W. Z., Dai, Y. Y., Wan, L. Y., and Yang, Z. (2011). Efficacy of Yanshu Injection (A Compound Chinese Traditional Medicine) Combined with Concurrent Radiochemotherapy in Patients with Stage III Nasopharyngeal Carcinoma. Zhonghua Zhong Liu Za Zhi 33 (5), 391–394. doi:10.3760/cma.j.issn.0253-3766.2011.05.020
Xia, Y. P., Zhang, . H., Ke, Y. M., and Hu, W. (2015). Kangfuxin for the Treatment of Oral Mucosa Reaction in Patients with Head and Neck Malignant Tumor Radiotherapy Curative Effect Observation. Med. Inf. 28 (38), 81. doi:10.3969/j.issn.1006-1959.2015.38.103
Xu, X. Y., Wan, Y. T., and Hong, Y. (2013). Clinical Study of Zhenhuang Pills Used to Prevent and Control of Radioactive Oral Cavity Mucositis in Locally Advanced Nasopharygeal Carcinoma Treatment. J. Jiangxi Univ. TCM 25 (6), 12–14. https://scholar.google.com/scholar?hl=zh-CN&as_sdt=0%2C5&q=%E7%8F%8D%E9%BB%84%E4%B8%B8%E9%98%B2%E6%B2%BB%E5%B1%80%E9%83%A8%E6%99%9A%E6%9C%9F%E9%BC%BB%E5%92%BD%E7%99%8C%E6%B2%BB%E7%96%97%E4%B8%AD%E6%94%BE%E5%B0%84%E6%80%A7%E5%8F%A3%E5%92%BD%E7%82%8E30%E4%BE%8B&btnG=.
Yan, Q. H., Lin, H. S., Zhuo, W. J., Fan, Y. M., and Dai, J. (2017). Effects of Kouyanqing Granules on Prevention and Treatment of Radiation-Induced Oral Mucositis Patients with Head and Neck Cancer after Radiotherapy. China Pharm. 28 (27), 3778–3781. doi:10.6039/j.issn.1001-0408.2017.27.09
Yang, X. M., Lin, D. Y., Lai, X. M., Chen, M. M., and Huang, L. Q. (2013). Mining Rules on Determination of Four Properties Based on Traditional Chinese Medicine Functional Combination. Zhongguo Zhong Yao Za Zhi 38 (10), 1624–1626. doi:10.4268/cjcmm20131036
Yarom, N., Hovan, A., Bossi, P., Ariyawardana, A., Jensen, S. B., Gobbo, M., et al. (2019). Systematic Review of Natural and Miscellaneous Agents for the Management of Oral Mucositis in Cancer Patients and Clinical Practice Guidelines—Part 1: Vitamins, Minerals, and Nutritional Supplements. Support. Care Cancer 27 (10), 3997–4010. doi:10.1007/s00520-019-04887-x
Yarom, N., Hovan, A., Hovan, A., Bossi, P., Ariyawardana, A., Jensen, S. B., et al. (2020). Systematic Review of Natural and Miscellaneous Agents, for the Management of Oral Mucositis in Cancer Patients and Clinical Practice Guidelines — Part 2: Honey, Herbal Compounds, Saliva Stimulants, Probiotics, and Miscellaneous Agents. Support. Care Cancer 28 (5), 2457–2472. doi:10.1007/s00520-019-05256-4
Zhan, H., and Zhang, H. X. (2017). Effects of Complex Sophorae Injection Combined with FOLFOX4 on the Levels of Th17 Cells, IL-17 and the Survival Rate in Patients with Rectal Cancer. Int. J. Traditional Chin. Med. 39 (5), 412–415. doi:10.3760/cma.j.issn.1673-4246.2017.05.007
Zhang, J. L. (2018). Chinese Patent Medicines. Beijing, China: China Traditional Chinese Medicine Publishing House, 5–10. 9787513244046.
Zhao, D., Zhou, X., and Wang, Z. M. (2010). Evaluation of Xipayi Mouth Rinse in the Treatment of Radiation Mucositis. Beijing J. Stomatology 10 (5), 53–54. doi:10.3969/j.issn.1006-673X.2010.05.01410.1167/10.7.1006
Zhao, S. P., and Li, X. H. (2011). Clinical Experience of First-Line Chemotherapy Combined Tianzhicao Capsule with the Treatment of Advanced Gastric Cancer. Chin. Med. Mod. Distance Edu. China 09 (5), 154–155. doi:10.3969/j.issn.1672-2779.2011.05.109
Zheng, B., Zhu, X., Liu, M., Yang, Z., Yang, L., Lang, J., et al. (2018). Randomized, Double-Blind, Placebo-Controlled Trial of Shuanghua Baihe Tablets to Prevent Oral Mucositis in Patients with Nasopharyngeal Cancer Undergoing Chemoradiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 100 (2), 418–426. doi:10.1016/j.ijrobp.2017.10.013
Keywords: Chinese patent medicine, oral mucositis, radiotherapy, chemotherapy, systematic review, meta-analysis
Citation: Xie Y, Fang X, Hua H and Zhou P (2022) Efficacy and Safety of Chinese Patent Medicine for the Prevention and Treatment of Radiotherapy and Chemotherapy-Induced Oral Mucositis: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:812085. doi: 10.3389/fphar.2022.812085
Received: 09 November 2021; Accepted: 07 March 2022;
Published: 28 March 2022.
Edited by:
Colin William Wright, University of Bradford, United KingdomReviewed by:
Gioacchino Calapai, University of Messina, ItalyBunleu Sungthong, Mahasarakham University, Thailand
Copyright © 2022 Xie, Fang, Hua and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Peiru Zhou, emhvdXBlaXJ1MTk4OUAxNjMuY29t; Hong Hua, aG9uZ2h1YTE5NjhAYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
 Yufei Xie
Yufei Xie Xin Fang†
Xin Fang† Hong Hua
Hong Hua Peiru Zhou
Peiru Zhou