
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 05 April 2022
Sec. Neuropharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.811017
Purpose: A novel once-daily divalproex-extended release (ER) dose formulation has been developed; this formulation prolongs the therapeutic serum levels of the drug, compared with the twice-daily conventional divalproex-delayed release (DR) formulation. This study aimed to systematically examine and compare the efficacy, safety, and retention rates of the ER divalproex (VPA-ER) and conventional DR divalproex (VPA-DR) formulations.
Methods: Randomized control trials (RCTs) reporting the efficacy, adverse events (AEs), and medication compliance of ER and DR divalproex were searched in online databases, including PubMed, Embase, and Cochrane Library databases, by searching MeSH words and term words. Observational studies with potential biases were excluded. The meta-analysis was performed using Stata 16.0 software.
Findings: Thirteen RCTs, involving 1,028 participants, were included in this meta-analysis. Efficacy, AEs, and drug retention rates were the main study outcomes. According to our study, VPA-ER presented clinically significant benefits compared with the placebo in the population with bipolar disorder (BD) (39.5% versus 27.2%, p < 0.001). A similar efficacy of VPA-ER and VPA-DR in controlling seizures was observed in epilepsy patients (87.4% versus 86.5%, p = 0.769). A significantly lower incidence of AEs was reported in the VPA-ER group than in the placebo group (26.8% versus 34.8%, p = 0.003). By contrast, there was no evidence of difference in safety between VPA-ER and VPA-DR (29.4% versus 30.5%, p = 0.750). In addition, the drug retention rate was significantly lower in the VPA-ER group than in the placebo group (76.0% versus 82.7%, p = 0.020), especially in migraine patients (p = 0.022) and in patients who were treated for fewer than 4 weeks (p = 0.018).
Implications: The efficacy of VPA-ER was significantly superior to that of the placebo treatment, which provided efficacy similar to that of conventional VPA-DR. VPA-ER is well tolerated with a low rate of AEs compared to the placebo. In addition, the acceptable medicine compliance of VPA-ER was conducive to the long-term maintenance treatment of chronic diseases. Although we analyzed open labels and crossover design RCTs, large-scale multicenter studies on the efficacy and medicine compliance of new ER formulations with less AEs are required to validate our conclusion.
Valproic acid and its derivatives, including valproate sodium (sodium valproate), divalproex, and divalproex sodium, are all known as valproate (VPA). VPA is a weak sodium channel blocker that produces weak inhibitors of enzymes to inactivate gamma-aminobutyric acid (GABA)-like aminobutyric aminotransferase (Cipriani et al., 2013). In addition, VPA can regulate serotonergic and glutamatergic transmission, energy metabolism, neuronal membrane lipid synthesis, and neurotrophic and neuroplastic effects (Winterer and Hermann, 2000; Schloesser et al., 2008).
VPA is the most commonly applied first-generation broad-spectrum antiepileptic drug (AED) used to treat generalized and focal epilepsies in children and adults and has been approved by the Food and Drug Administration (FDA). Divalproex has been approved for use in the United States since 1983 (Sommerville et al., 2003). It has a broad-spectrum medicine used in the treatment of bipolar depression and rapid cycling, psychotic symptoms, impulsive aggression, post-traumatic stress disorder (PTSD), neuropathic pain, and the prophylaxis of migraine headaches (Ross, 2000; Davis et al., 2004; Adamou et al., 2007; Bialer and Yagen, 2007; Soares-Weiser et al., 2007; Wang et al., 2016). Epilepsy, bipolar disorder (BD), and migraine are three common chronic diseases that require long-term therapy, depending on the optimal regimen, dose, and patient compliance (Genton, 2005). Many AEDs, including VPA, have short half-lives and must be administered several times daily, with large fluctuations in peak-to-trough plasma concentrations (Dutta and Zhang, 2004), resulting in poor pharmacokinetic (PK) properties, adverse events (AEs), and unsatisfactory adherence (Leppik and Hovinga, 2013).
VPA has been approved in several formulations, including the original delayed-release tablet (e.g., Depakote), enteric-coated particles, sprinkle capsules, sustained-release tablets (e.g., Depakine Chrono), and a more recently approved extended-release tablet (e.g., Depakote ER). In the present study, VPA-ER and VPA-DR were defined as extended-release and delayed-release formulations of VPA, respectively. In ER formulations, the dosing interval is usually extended to minimize the dosing frequency (Leppik and Hovinga, 2013). In addition, they can potentially minimize spikes in the maximum plasma concentrations (Cmax) at a steady state and maintain a relatively constant plasma drug concentration. Moreover, they can minimize concentration-related AEs (Bialer, 2007). Once-daily VPA-ER is characterized by a hydrophilic polymer matrix controlled-release tablet system that allows the slow release of drugs in the stomach, small intestine, and large intestine for 18–24 h (Brandt and May, 2018). Compared with the standard twice-daily DR formulation, once-daily VPA-ER significantly stabilizes serum levels without marked peak-to-trough fluctuations and reduces the dosing frequency and the possibility of dosing flexibility, which improves patient compliance, satisfaction, and ultimately the quality of life (Davis et al., 2004; Genton, 2005).
Although once-daily formulations are more convenient than multiple doses per day, potential subtherapeutic concentrations following delayed or missed doses should be considered. Once-daily formulations are unable to improve therapeutic coverage because they cannot pharmacokinetically maintain the effective drug concentration in biological fluids and tissues. A missed dose of once-daily formulation usually has a greater influence on treatment because of the higher dose than that of the multiple-dose formulation. Therefore, the risk of breakthrough seizure is higher in once-daily AED administration than that in twice-daily administration (Dutta and Zhang, 2004; Ahmad et al., 2005). VPA-ER is limited by a high daily dose owing to its low bioavailability. A mean increase in the daily dose of divalproex ER at 12% (8–20%) can achieve an equivalent plasma exposure level to that of divalproex DR (Dutta and Zhang, 2004; Dutta et al., 2006).
Currently, ER formulations are preferred for the treatment of EP and prevention of migraine owing to better compliance, convenience, and consistent plasma concentration over time. To our knowledge, comparative conversion studies of VPA-ER and conventional VPA-DR are lacking. Small-sample studies analyzing the safety and efficacy of VPA-ER in different populations remain controversial. Therefore, this study aimed to investigate the efficacy, safety, and retention rate of VPA-ER and VPA-DR using meta-analysis.
This study did not require ethical approval since no subjects were recruited. According to PRISMA (Liberati et al., 2009) principles and MOOSE (Stroup et al., 2000) guidelines, the search strategy, selection criteria, data extraction, quality assessment, and statistical analysis were predesigned based on Cochrane Review Methods. We registered this study using INPLASY 2021110090, and the DOI number is 10.37766/inplasy 2021.11.0090 (https://inplasy.com/inplasy-2021-11-0090/).
Two researchers were independently responsible for searching eligible RCTs in online databases, including PubMed (published from 1983 to October 2021), Embase (published from 1982 to October 2021), and Cochrane Library (published from 2001 to October 2021) without language limitations. Relevant RCTs were searched through a combination of MeSH words and term words, including “Valproic acid”[MeSH], “divalpro*,” “Valpro*,” “Depak?e,” “Semisodium Valproate,” “Modified-release,” “Delayed-release,” “Extended-release,” “sustained-release,” “prolonged-release,” “controlled-release,” “treatment [MeSH],” “Therapeutics [Mesh],” “therap*,” and “Treatment*” (Supplementary Materials—additional file for full electronic search). References in each eligible study were also manually reviewed to avoid missing data.
Inclusion criteria were given as follows: 1) randomized controlled blind/double-blind, crossover, open-label trials reporting the efficacy, safety, and medication compliance (e.g., reduction in epileptic seizure frequency) of VPA-ER and the control were included. 2) Patients receiving VPA-ER without disease limitation.
The exclusion criteria were as follows: 1) observational studies, cohort studies, reviews, case reports, letters, communication, editorials, lectures, and conference abstracts; 2) unclearly defined divalproex ER; and 3) studies with missing or repeated data.
Two independent investigators (ZCQ and YW) individually screened the literature and extracted and evaluated the data. Any disagreement was resolved by discussion with a third reviewer (LHY or SHB). Planned data extraction included the following items: authors, year of publication, study design, study population (e.g., age, sex, sample size, and disease type), intervention measures (e.g., type of VPA, dose, mode of administration, and duration), comparison details, clinical outcome indicators (e.g., efficacy and retention rate), AEs, study methodology (e.g., method of randomization and blinding), and other characteristics.
Two reviewers (ZCQ and HYL) independently reviewed the quality of the included literature using Cochrane Handbooks (http://community.cochrane.org/handbook), including the following seven aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of assessors, incomplete outcome data, and selective outcome reporting.
Stata 16.0 software (STATA Corporation, College Station, TX) was used to perform the statistical analyses. Dichotomous variables were analyzed using the Mantel–Haenszel method and were expressed as risk ratios (RRs) with 95% confidence intervals (CIs), which examined the effectiveness, incidence of AEs, and drug retention rate between different groups.
The Q test and chi-square test were used to estimate statistical heterogeneity using the p value and I2 statistic. p < 0.05 and I2 > 50% were the criteria used to classify the data as heterogeneous, and data were analyzed using a random-effects model; otherwise, we used a fixed-effects model in the meta-analysis (p > 0.05, I2 ≤ 50%). Statistical significance was defined as a two-tailed p-value < 0.05.
For subgroup analysis and investigation of heterogeneity, we performed the following predefined subgroup analyses if sufficient data were available: different types of diseases, periods of treatment, and other outcome measures.
Had sufficient data been available, we would have examined the robustness of the meta-analysis by conducting a sensitivity analysis. Finally, both Begg and Egger tests were performed to identify publication bias.
A total of 672 records were initially obtained following an online search; after excluding duplications, 476 remained (Figure 1). We excluded non-RCTs and identified 34 human clinical trials. Following a review of the full text, a total of 13 eligible RCTs, involving 1,028 subjects, were finally selected for the following meta-analysis.
Notably, one study (Apostol et al., 2008) had a parallel design with three separate studies with different doses of the same drug (Depakote ER: 250 mg/d, 500 mg/d, 1,000 mg/d), compared with the placebo, to examine the effects of dose on clinical efficacy and safety. Consequently, we defined them as three trials. In addition, there were three types of administration schedules employed in one study (Hirschfeld et al., 2010): ChVbid, ChVom, and ChVoe, given the influence of these modes on AEs and efficacy. Consequently, we also considered this study as three trials. At last, 17 studies were recruited in the present study.
A total of 1,028 patients receiving VPA-ER were analyzed, including EP patients in four RCTs (Thibault et al., 2002; Sommerville et al., 2003; Kernitsky et al., 2005; Herranz et al., 2006), BD patients in six (Bowden et al., 2006; Ghaemi et al., 2007; Wagner et al., 2009; Hirschfeld et al., 2010; McElroy et al., 2010; Muzina et al., 2011), and MA patients in three (Jensen et al., 1994; Freitag et al., 2002; Apostol et al., 2008). Most RCTs were multicenter, and patients were mainly from the United States. The sample size of each trial ranged from 16 to 377, with ages ranging from 5 to 73 years, and that of VPA-ER-treated patients with a mean dose ranging from to 250–2,961 mg/day. The treatment period ranged from 11 days to 16 months (Table 1).
The quality of the 13 included RCTs was assessed and is depicted in Figure 2.
Therapeutic efficacy was assessed in patients with various diseases. In patients with epilepsy, at least a 50% reduction in the seizure frequency or complete freedom from seizures was achieved. The clinical effectiveness rate in the VPA-ER group was 87.4%, while that of the VPA-DR group was 86.5%. However, the pooled estimated data suggested that patients treated with VPA-ER had no statistical difference compared to those treated with VPA-DA (RR, 1.081; 95% CI, 0.644–1.814; p = 0.769) (Figure 3A).
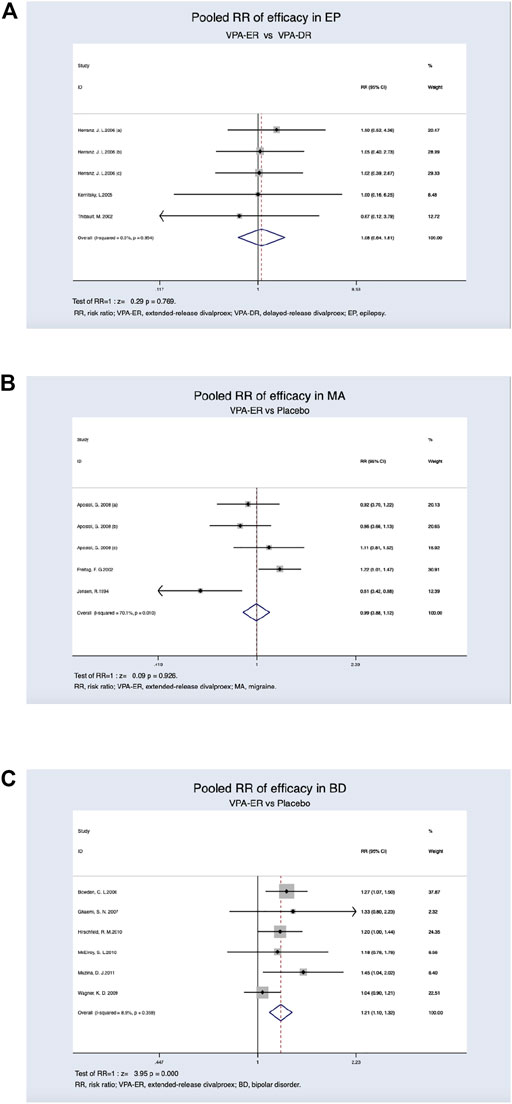
FIGURE 3. (A) Pooled RR of efficacy between VPA-ER with VPA-DR in EP. (B) Pooled RR of efficacy between VPA-ER with placebo in MA. (C) Pooled RR of efficacy between VPA-ER with placebo in BD.
Patients with migraine achieved at least 50% reduction in the migraine headache rate, and this was deemed to be effective, and the response rates were 39.8% for VPA-ER and 40.2% for placebo. We evaluated the RR of the effective rate of VPA-ER compared with that of placebo, but it was shown that VPA-ER did not have an efficacy larger than that of placebo (RR, 0.994; 95% CI, 0.885–1.118; p = 0.926) (Figure 3B).
In patients with BD, at least 50% improvement from baseline in the Mania Rating Scale (MRS) scores or Young Mania Rating Scale (YMRS) scores was deemed to be valid data for analysis. Pooled estimated data suggested that patients treated with VPA-ER exhibited a substantially higher response rate than those treated with placebo (RR, 1.207 95% CI, 1.099–1.325; p < 0.001) (Figure 3C), and significantly effective rate in the VPA-ER group was 39.5% and in the placebo group was 27.2%. The results of the pooled analysis are summarized in detail in Table 2.
AEs were reported in 14 RCTs, and three only described them rather than reporting the number or incidence (Kernitsky et al., 2005; McElroy et al., 2010; Muzina et al., 2011); these were therefore excluded from the assessment of the safety analysis.
Regardless of age and dosage, a lower incidence of AEs was detected in patients treated with the ER formulation (26.8%) than in those treated with placebo (34.8%), and this difference was found to be statistically significant (RR, 0.893; 95% CI, 0.834–0.956; p = 0.001) (Figure 4A). Adverse reaction rates were similar in patients receiving VPA-ER and VPA-DR formulations (29.4% versus 30.5%). However, the differences were not statistically significant (RR, 0.982; 95% CI, 0.877–1.099; p = 0.750) (Figure 4B).
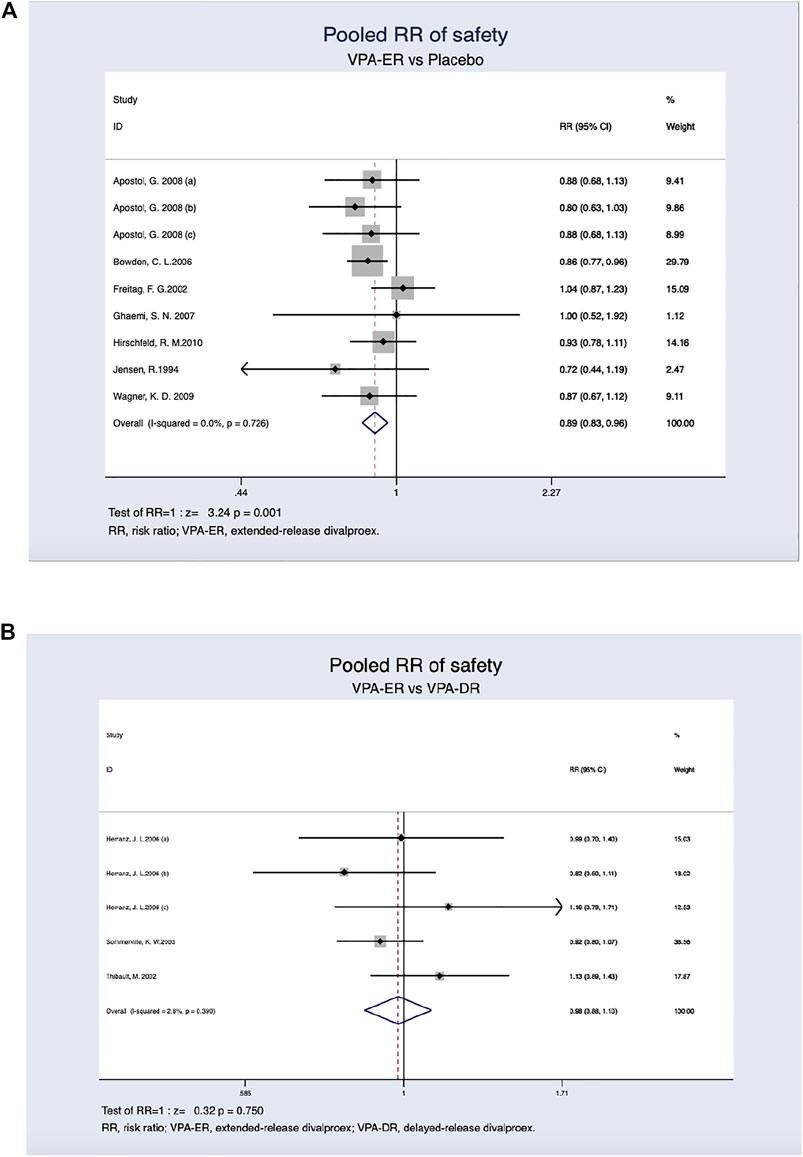
FIGURE 4. (A) Pooled RR of safety between VPA-ER with placebo. (B) Pooled RR of safety between VPA-ER with VPA-DR.
We present separate analyses based on the types of AEs; however, most subgroups comprised only one study. The results shows that people treated with VPA-ER were more likely to have somnolence (RR, 2.110; 95% CI, 1.470–3.027; p < 0.001), dizziness (RR, 2.380; 95% CI, 1.395–4.061; p = 0.001), nausea (RR, 1.686; 95% CI, 1.255–2.267; p = 0.001), vomiting (RR, 2.233; 95% CI, 1.384–3.603; p = 0.001), abdominal pain (RR, 2.406; 95% CI, 1.292–4.277; p = 0.006), and dyspepsia (RR, 2.099; 95% CI, 1.432–3.075; p < 0.001) but were less likely to have constipation (RR, 0.360; 95% CI, 0.156–0.828; p = 0.016) (Table 3).
In addition, when VPA-ER was compared with VPA-DR, the incidences of fatigue/asthenia, headache, memory loss, tremor, weight increase, and hair alterations were lower in the VPA-ER group, and those of nausea, vomiting, appetite increase, constipation, hepatic transaminases increased, and pain were higher, although the differences were not statistically significant (Table 4).
Treatment retention rates in the VPA-ER and placebo groups were 76.0 and 82.7%, respectively. The ratio of all-cause dropout was significantly higher in patients treated with VPA-ER than in those treated with placebo (RR, 0.95; 95% CI, 0.90–0.99; p = 0.020) (Figure 5A). The reasons for treatment withdrawal in the VPA-ER group included AEs, non-compliance, suicidality, and others (Table 5).
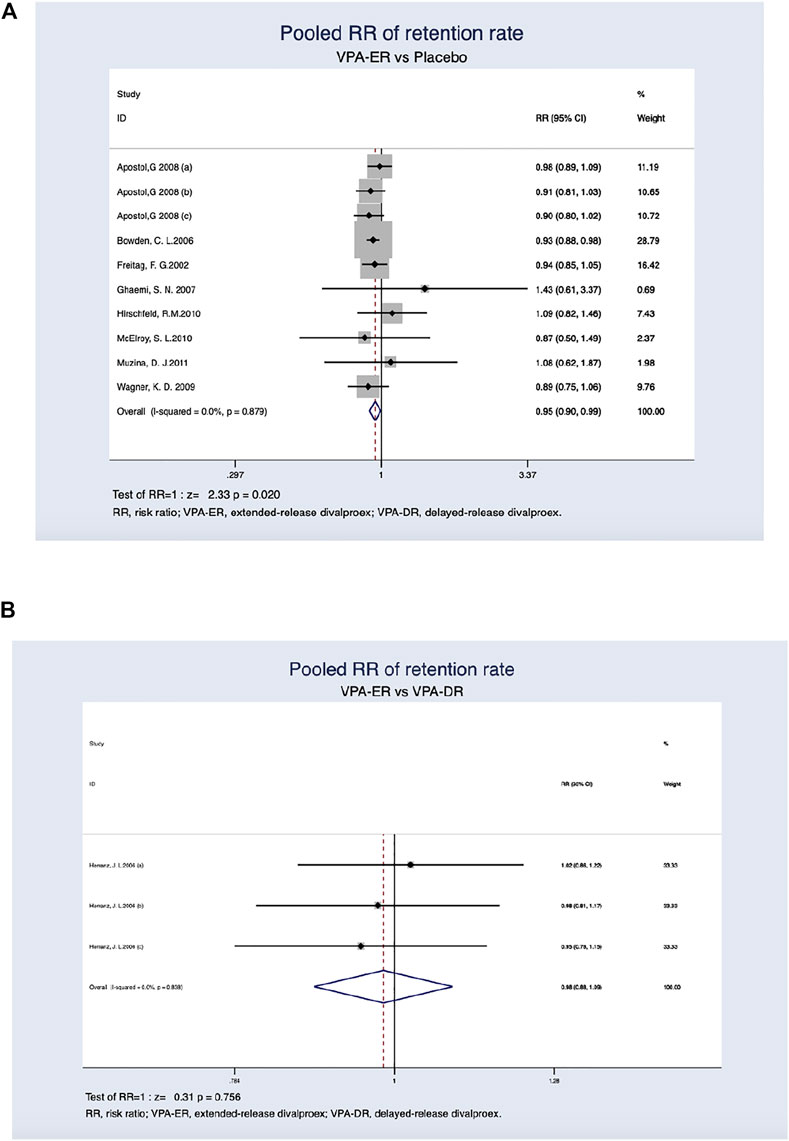
FIGURE 5. (A) Pooled RR of retention rate between VPA-ER with placebo. (B) Pooled RR of retention rate between VPA-ER with VPA-DR.
In comparison, a single article (Herranz et al., 2006) reported the overall drug retention rates between the VPA-ER and VPA-DR groups. Due to the small sample size and attrition, descriptive analyses were reported. According to a trial (Herranz et al., 2006), both groups had similar drug retention rates (82.0% in VPA-ER versus 83.3% in VPA-DR) (Figure 5B). Lack of efficacy was the main cause of dropout in the VPA-DR group (Table 6).
Despite the absence of statistical heterogeneity, we performed an additional subgroup analysis to determine whether there were significant subgroup differences (Table 7).
We investigated disease efficacy of three different treatment durations, for 4, 4–12, and >12 weeks. The curative efficacy of VPA-ER in treating BD was significantly better than that of the placebo group within 4 weeks (RR, 1.187; 95% CI, 1.072–1.314; p = 0.001) and 4–12 weeks (RR, 1.315; 95% CI, 1.039–1.664; p = 0.022) during the follow-up period. For migraines and epilepsy, the length of follow-up did not differ between the treatment and control groups.
Safety and AE analyses were subgrouped according to the disease field and duration. The incidence of AEs was significantly lower in patients with BD (RR, 0.882; 95%CI, 0.809–0.963; p = 0.005) than in those treated with placebo. However, epilepsy and migraine did not differ significantly between groups. When the duration of therapy was less than 4 weeks, the incidence of side effects was significantly lower in the VPA-ER group (RR, 0.870; 95% CI, 0.806–0.939; p < 0.001). Compared with VPA-DR treatment alone, no statistically significant differences were found between the duration points and disease.
For retention rates, subgroup analysis was undertaken according to the treatment duration and disease type. We found significantly lower retention for the VPA-ER groups within 4 weeks of follow-up than the placebo groups (RR, 0.940; 95% CI, 0.897–0.990; p = 0.018) and migraine disease (RR, 0.936; 95% CI, 0.885–0.991; p = 0.022). In the remaining subgroups, there was no evidence of subgroup differences.
Sensitivity analysis was performed by eliminating one study at a time and calculating the pooled estimate of the remaining data. The pooled estimates of efficacy (Figure 6A), incidence of AEs (Figure 6B), and medication compliance (Figure 6C) were not significantly influenced by the elimination method, indicating robust results.
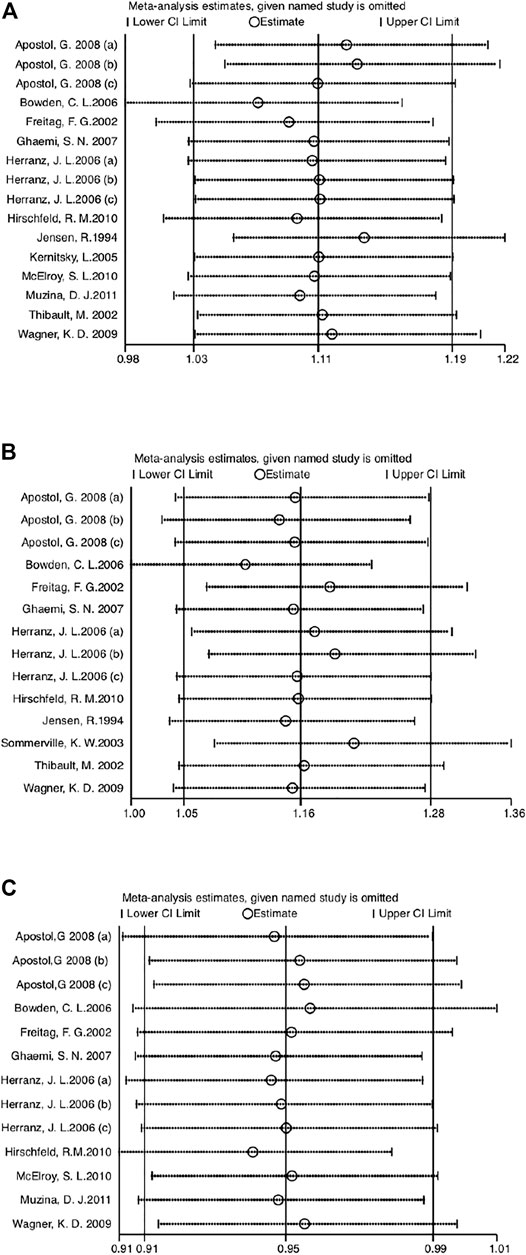
FIGURE 6. (A) Sensitivity analysis of efficacy compared VPA-ER with control groups. (B) Sensitivity analysis of safety compared VPA-ER with control groups. (C) Sensitivity analysis of retention rate compared VPA-ER with control groups.
Begg’s test (Figure 7) and Egger’s test (Figure 8) were conducted to assess potential publication bias, and no publication bias was examined.
This meta-analysis explored the efficacy, safety, and retention rates of patients treated with VPA-ER versus VPA-DR and placebo in 13 RCTs. The pooled analysis demonstrated that VPA-ER could slightly reduce the mean number of migraine days per month compared to placebo. Nevertheless, no significant difference was found in the percentage of patients with ≥50% response rate in headache frequency per month between the VPA-ER and placebo groups. The extended-release (ER) formulation of divalproex sodium has also been approved by the U.S. Food and Drug Administration for migraine prevention in patients >18 years. Several RCTs have revealed that VPA-ER significantly reduced the monthly headache frequency and improved the response rate in adult patients (Klapper, 1997; Freitag, 2003; Kaniecki, 2008; Linde et al., 2013). Jia et al. (2021) also made this conclusion, corroborating the results of our research.
Pooled estimates suggested that VPA-ER had a favorable efficacy, which reduced the baseline values of the rate of MRS scores or YMRS scores in BD patients by at least 50% compared to those in the placebo group (Klapper, 1997; Freitag, 2003; Kaniecki, 2008; Linde et al., 2013; Jia et al., 2021). This is in agreement with the study of Bond et al., which also suggested that divalproex is efficacious in the treatment of BD (Dutta and Zhang, 2004). In addition, VPA can prevent new episodes of BD in adults, as reported by Nestsiarovich et al. (2022). Valproate (VPA) has been used to treat epilepsy and bipolar disorder for many years. Our results also provide evidence that supports VPA-ER had a good efficacy for BD patients.
In the evaluation of clinical efficacy in patients with epilepsy, it has been suggested that VPA-ER treatment confers a higher risk of breakthrough owing to its lower bioavailability (Bialer, 1992; Dutta and Zhang, 2004; Verrotti et al., 2007). However, in our study, we demonstrated that the efficacy of once-daily VPA-ER was superior to that of conventional twice-daily VPA-DR, although the difference was not statistically significant. This is consistent with a previous study showing similar or better efficacy of divalproex ER on seizures and mood disorders in nine open-label trials (Smith et al., 2004).
Reducing the daily dosing frequency can result in a significant increase in treatment compliance (Sommerville et al., 2003). High treatment adherence can significantly improve therapeutic efficacy (Minirth and Neal, 2005). An observational study involving 359 epilepsy patients demonstrated that over 95% of patients administered once-daily evening dosing of valproate sustained-release minitablets had good acceptance (Stefan and Fraunberger, 2005). However, we draw the opposite conclusions from this study, which revealed a significantly inferior drug retention rate in patients treated with VPA-ER to those treated with VPA-DR and placebo, especially in patients with migraine who were treated for less than 4 weeks. While the VPA-ER did not differ with the VPA-DR with regard to the retention rate, and the value was slightly lower than that of VPA-DA, this result was contrary to what we would expect. We performed a detailed analysis of withdrawals and found that the main reasons for dropping out of VPA-ER and placebo included AEs, non-compliance, suicidality, or others and lack of efficacy for VPA-DR. According to Redden et al. (2011), divalproex sodium does not appear to increase the risk of suicide-related AEs, relative to the placebo. However, the U.S. FDA reported that all antiepileptic drugs were related to increased risks of suicidal ideation and behaviors, and clinicians should nonetheless remain vigilant in assessing suicidality (Klein et al., 2021). Overall, the retention rate of VPA-ER was considered acceptable.
In addition, the rate of adverse reactions in the VPA-ER group, especially in the treatment of migraine, was considerably lower than that in the placebo group. Somnolence, dizziness, nausea, vomiting, dyspepsia, and abdominal pain were the primary adverse effects of the VPA-ER treatment, although most AEs were mild or moderate and did not affect the treatment. In addition, we compared the AEs between the VPA-ER and VPA-DR groups. It has been reported that extended-release divalproex sodium can reduce the incidence of AEs associated with divalproex sodium (Stoner et al., 2004), and another retrospective study also reported fewer AEs in patients taking the divalproex ER formulation (Minirth and Neal, 2005). Here, we obtained a consistent finding that the incidence of AEs was slightly lower in VPA-ER than in VPA-DR. Patients receiving VPA-ER had fewer central nervous system adverse effects (i.e., fatigue/asthenia, headache, memory loss, and tremor), weight increase, and hair alterations. At present, a large number of pharmacokinetic studies have shown that the significantly decreased peak-trough fluctuation of plasma valproic acid levels and the stable serum concentrations of divalproex contribute to preventing concentration-dependent AEs (Ieiri et al., 1995; Kondo et al., 2002; Smith et al., 2004; Fagiolino et al., 2007), such as central AEs. Nevertheless, the specific reasons for this need to be further explored.
Some limitations of this meta-analysis should be noted. First, only five RCTs reported VPA-DR as a control, and the placebo was set as the control in the remaining RCTs. Second, some cross-design studies were used, which may have resulted in potential biases, although we performed a sensitivity analysis and publication bias assessment. Third, we did not analyze the drug concentration–effect relationship due to limited availability of data. In the future, we will further explore this based on daily doses in a larger sample over a longer period. Moreover, VPA should be compared to other VPA-ER formulations to identify the optimal formulation.
This study compared the efficacy, safety, and retention rates of VPA-ER and VPA-DR in various populations. We suggest that the daily dose of VPA-ER is superior in terms of therapeutic efficacy and inferior in terms of AEs, especially on BD, to that of placebo, which provides similar or improved efficacy in controlling seizures, without increasing the occurrence of AEs, compared to conventional VPA-DR formulations. Based on these results, the new ER formulation is recommended because of its better efficacy, medicine compliance, and lower dosing frequency. It can also be used as a substitute for conventional VPA-DR. However, due to its low bioavailability, conversion to divalproex ER requires an appropriate dose increase to avoid subtherapeutic-related problems, and further investigations regarding this aspect of VPA-ER are needed.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
CQZ and XYB conceived the study and finalized the search strategy. CQZ and YW primarily screened studies and extracted data with supervision from HBS and HYL. HBS led data analysis and contributed to the conception and design of the review article, critical review of the first draft, and subsequent revisions to the manuscript. CQZ drafted the manuscript. LG and JW took part in the revision and language improvement of the manuscript. All authors participated in the data interpretation and critically reviewed the manuscript. All authors read and approved the final manuscript.
This research was supported by Administration of Traditional Chinese Medicine of Sichuan Province, China (Grant no. 2016C001).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The authors greatly appreciate Jessye Zhang for her assistance with language revision.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.811017/full#supplementary-material
Adamou, M., Puchalska, S., Plummer, W., and Hale, A. S. (2007). Valproate in the Treatment of PTSD: Systematic Review and Meta Analysis. Curr. Med. Res. Opin. 23 (6), 1285–1291. doi:10.1185/030079907X188116
Ahmad, A. M., Douglas Boudinot, F., Barr, W. H., Reed, R. C., and Garnett, W. R. (2005). The Use of Monte Carlo Simulations to Study the Effect of Poor Compliance on the Steady State Concentrations of Valproic Acid Following Administration of Enteric-Coated and Extended Release Divalproex Sodium Formulations. Biopharm. Drug Dispos. 26 (9), 417–425. doi:10.1002/bdd.473
Apostol, G., Cady, R. K., Laforet, G. A., Robieson, W. Z., Olson, E., Abi-Saab, W. M., et al. (2008). Divalproex Extended-Release in Adolescent Migraine Prophylaxis: Results of a Randomized, Double-Blind, Placebo-Controlled Study. Headache 48 (7), 1012–1025. doi:10.1111/j.1526-4610.2008.01081.x
Bialer, M. (2007). Extended-release Formulations for the Treatment of Epilepsy. CNS Drugs 21 (9), 765–774. doi:10.2165/00023210-200721090-00005
Bialer, M. (1992). Pharmacokinetic Evaluation of Sustained Release Formulations of Antiepileptic Drugs. Clinical Implications. Clin. Pharmacokinet. 22 (1), 11–21. doi:10.2165/00003088-199222010-00002
Bialer, M., and Yagen, B. (2007). Valproic Acid: Second Generation. Neurotherapeutics 4 (1), 130–137. doi:10.1016/j.nurt.2006.11.007
Bowden, C. L., Swann, A. C., Calabrese, J. R., Rubenfaer, L. M., Wozniak, P. J., Collins, M. A., et al. (2006). A Randomized, Placebo-Controlled, Multicenter Study of Divalproex Sodium Extended Release in the Treatment of Acute Mania. J. Clin. Psychiatry 67 (10), 1501–1510. doi:10.4088/jcp.v67n1003
Brandt, C., and May, T. W. (2018). Extended-release Drug Formulations for the Treatment of Epilepsy. Expert Opin. Pharmacother. 19 (8), 843–850. doi:10.1080/14656566.2018.1465561
Cipriani, A., Reid, K., Young, A. H., Macritchie, K., and Geddes, J. (2013). Valproic Acid, Valproate and Divalproex in the Maintenance Treatment of Bipolar Disorder. Cochrane Database Syst. Rev. 2013 (10), Cd003196. doi:10.1002/14651858.CD003196.pub2
Davis, L. L., Williams, R., and Cates, M. (2004). Divalproex Sodium in the Treatment of Adults with Bipolar Disorder. Expert Rev. Neurother. 4 (3), 349–362. doi:10.1586/14737175.4.3.349
Dutta, S., Reed, R. C., and O'Dea, R. F. (2006). Comparative Absorption Profiles of Divalproex Sodium Delayed-Release versus Extended-Release Tablets -- Clinical Implications. Ann. Pharmacother. 40 (4), 619–625. doi:10.1345/aph.1G617
Dutta, S., and Zhang, Y. (2004). Bioavailability of Divalproex Extended-Release Formulation Relative to the Divalproex Delayed-Release Formulation. Biopharm. Drug Dispos. 25 (8), 345–352. doi:10.1002/bdd.420
Fagiolino, P., Martín, O., González, N., and Malanga, A. (2007). Actual Bioavailability of Divalproex Sodium Extended-Release Tablets and its Clinical Implications. J. Epilepsy Clin. Neurophysiol. 13 (2), 75–78. doi:10.1590/s1676-26492007000200007
Freitag, F. G., Collins, S. D., Carlson, H. A., Goldstein, J., Saper, J., Silberstein, S., et al. (2002). A Randomized Trial of Divalproex Sodium Extended-Release Tablets in Migraine Prophylaxis. Neurology 58 (11), 1652–1659. doi:10.1212/wnl.58.11.1652
Freitag, F. G. (2003). Divalproex Sodium Extended-Release for the Prophylaxis of Migraine Headache. Expert Opin. Pharmacother. 4 (9), 1573–1578. doi:10.1517/14656566.4.9.1573
Genton, P. (2005). Progress in Pharmaceutical Development Presentation with Improved Pharmacokinetics: a New Formulation for Valproate. Acta Neurol. Scand. Suppl. 182, 26–32. doi:10.1111/j.1600-0404.2005.00524.x
Ghaemi, S. N., Gilmer, W. S., Goldberg, J. F., Zablotsky, B., Kemp, D. E., Kelley, M. E., et al. (2007). Divalproex in the Treatment of Acute Bipolar Depression: a Preliminary Double-Blind, Randomized, Placebo-Controlled Pilot Study. J. Clin. Psychiatry 68 (12), 1840–1844. doi:10.4088/jcp.v68n1203
Herranz, J. L., Arteaga, R., Adín, J., and Armijo, J. A. (2006). Conventional and Sustained-Release Valproate in Children with Newly Diagnosed Epilepsy: a Randomized and Crossover Study Comparing Clinical Effects, Patient Preference and Pharmacokinetics. Eur. J. Clin. Pharmacol. 62 (10), 805–815. doi:10.1007/s00228-006-0175-2
Hirschfeld, R. M., Bowden, C. L., Vigna, N. V., Wozniak, P., and Collins, M. (2010). A Randomized, Placebo-Controlled, Multicenter Study of Divalproex Sodium Extended-Release in the Acute Treatment of Mania. J. Clin. Psychiatry 71 (4), 426–432. doi:10.4088/JCP.08m04960yel
Ieiri, I., Morioka, T., Ichimiya, T., Nishio, S., Fukui, M., Ohtsubo, K., et al. (1995). Pharmacokinetic Study of Valproic Acid Sustained-Release Preparation in Patients Undergoing Brain Surgery. Ther. Drug Monit. 17 (1), 6–11. doi:10.1097/00007691-199502000-00002
Jensen, R., Brinck, T., and Olesen, J. (1994). Sodium Valproate Has a Prophylactic Effect in Migraine without Aura: A Triple-Blind, Placebo-Controlled Crossover Study. Neurology 44 (4), 647–651. doi:10.1212/wnl.44.4.647
Jia, G., Wang, X., Lv, H., Nonyane, M. S. C., Hou, H., Ma, L., et al. (2021). The Efficacy and Safety of Antiepileptics in the Prophylaxis of Pediatric Migraine: the Meta-Analysis of Randomized Controlled Trials. Transl. Pediatr. 10 (7), 1779–1791. doi:10.21037/tp-20-478
Kaniecki, R. (2008). Neuromodulators for Migraine Prevention. Headache 48 (4), 586–600. doi:10.1111/j.1526-4610.2007.01040.x
Kernitsky, L., O'Hara, K. A., Jiang, P., and Pellock, J. M. (2005). Extended-release Divalproex in Child and Adolescent Outpatients with Epilepsy. Epilepsia 46 (3), 440–443. doi:10.1111/j.0013-9580.2005.39804.x
Klapper, J. (1997). Divalproex Sodium in Migraine Prophylaxis: a Dose-Controlled Study. Cephalalgia 17 (2), 103–108. doi:10.1046/j.1468-2982.1997.1702103.x
Klein, P., Devinsky, O., French, J., Harden, C., Krauss, G. L., McCarter, R., et al. (2021). Suicidality Risk of Newer Antiseizure Medications: A Meta-Analysis. JAMA Neurol. 78 (9), 1118–1127. doi:10.1001/jamaneurol.2021.2480
Kondo, T., Tokinaga, N., Suzuki, A., Ono, S., Yabe, H., Kaneko, S., et al. (2002). Altered Pharmacokinetics and Metabolism of Valproate after Replacement of Conventional Valproate with the Slow-Release Formulation in Epileptic Patients. Pharmacol. Toxicol. 90 (3), 135–138. doi:10.1034/j.1600-0773.2002.900304.x
Leppik, I. E., and Hovinga, C. A. (2013). Extended-release Antiepileptic Drugs: a Comparison of Pharmacokinetic Parameters Relative to Original Immediate-Release Formulations. Epilepsia 54 (1), 28–35. doi:10.1111/epi.12043
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. Plos Med. 6 (10), e1000100–34. doi:10.1371/journal.pmed.1000100
Linde, M., Mulleners, W. M., Chronicle, E. P., and McCrory, D. C. (2013). Valproate (Valproic Acid or Sodium Valproate or a Combination of the Two) for the Prophylaxis of Episodic Migraine in Adults. Cochrane Database Syst. Rev. (6), Cd010611. doi:10.1002/14651858.CD010611
McElroy, S. L., Martens, B. E., Creech, R. S., Welge, J. A., Jefferson, L., Guerdjikova, A. I., et al. (2010). Randomized, Double-Blind, Placebo-Controlled Study of Divalproex Extended Release Loading Monotherapy in Ambulatory Bipolar Spectrum Disorder Patients with Moderate-To-Severe Hypomania or Mild Mania. J. Clin. Psychiatry 71 (5), 557–565. doi:10.4088/JCP.08m04854yel
Minirth, F. B., and Neal, V. (2005). Assessment of Patient Preference and Side Effects in Patients Switched from Divalproex Sodium Delayed Release to Divalproex Sodium Extended Release. J. Clin. Psychopharmacol. 25 (1), 99–101. doi:10.1097/01.jcp.0000150233.91995.6b
Muzina, D. J., Gao, K., Kemp, D. E., Khalife, S., Ganocy, S. J., Chan, P. K., et al. (2011). Acute Efficacy of Divalproex Sodium versus Placebo in Mood Stabilizer-Naive Bipolar I or II Depression: a Double-Blind, Randomized, Placebo-Controlled Trial. J. Clin. Psychiatry 72 (6), 813–819. doi:10.4088/JCP.09m05570gre
Nestsiarovich, A., Gaudiot, C. E. S., Baldessarini, R. J., Vieta, E., Zhu, Y., and Tohen, M. (2022). Preventing New Episodes of Bipolar Disorder in Adults: Systematic Review and Meta-Analysis of Randomized Controlled Trials. Eur Neuropsychopharmacol. 54, 75–89. doi:10.1016/j.euroneuro.2021.08.264
Redden, L., Pritchett, Y., Robieson, W., Kovacs, X., Garofalo, M., Tracy, K., et al. (2011). Suicidality and Divalproex Sodium: Analysis of Controlled Studies in Multiple Indications. Ann. Gen. Psychiatry 10 (1), 1. doi:10.1186/1744-859X-10-1
Ross, E. L. (2000). The Evolving Role of Antiepileptic Drugs in Treating Neuropathic Pain. Neurology 55 (5 Suppl. 1), S41–S48.
Schloesser, R. J., Huang, J., Klein, P. S., and Manji, H. K. (2008). Cellular Plasticity Cascades in the Pathophysiology and Treatment of Bipolar Disorder. Neuropsychopharmacology 33 (1), 110–133. doi:10.1038/sj.npp.1301575
Smith, M. C., Centorrino, F., Welge, J. A., and Collins, M. A. (2004). Clinical Comparison of Extended-Release Divalproex versus Delayed-Release Divalproex: Pooled Data Analyses from Nine Trials. Epilepsy Behav. 5 (5), 746–751. doi:10.1016/j.yebeh.2004.07.007
Soares-Weiser, K., Bravo Vergel, Y., Beynon, S., Dunn, G., Barbieri, M., Duffy, S., et al. (2007). A Systematic Review and Economic Model of the Clinical Effectiveness and Cost-Effectiveness of Interventions for Preventing Relapse in People with Bipolar Disorder. Health Technol. Assess. 11 (39iii-iv), iii–206. doi:10.3310/hta11390
Sommerville, K. W., Dutta, S., Biton, V., Zhang, Y., Cloyd, J. C., and Uthman, B. (2003). Bioavailability of a Divalproex Extended-Release Formulation versus the Conventional Divalproex Formulation in Adult Patients Receiving Enzyme-Inducing Antiepileptic Drugs. Clin. Drug Investig. 23 (10), 661–670. doi:10.2165/00044011-200323100-00005
Stefan, H., and Fraunberger, B. (2005). Valproate Sustained Release in the Treatment of Epilepsy. Fortschr Neurol. Psychiatr. 73 (11), 681–686. doi:10.1055/s-2004-830299
Stoner, S. C., Dubisar, B. M., Lea, J. W., Marken, P. A., Ramlatchman, L. V., and Reynolds, J. B. (2004). Extended-release Divalproex Sodium for Mood Stabilization. Pharmacotherapy 24 (9), 1147–1153. doi:10.1592/phco.24.13.1147.38088
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of Observational Studies in Epidemiology: a Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. Jama 283 (15), 2008–2012. doi:10.1001/jama.283.15.2008
Thibault, M., Blume, W. T., Saint-Hilaire, J. M., Zakhari, R., and Sommerville, K. W. (2002). Divalproex Extended-Release versus the Original Divalproex Tablet: Results of a Randomized, Crossover Study of Well-Controlled Epileptic Patients with Primary Generalized Seizures. Epilepsy Res. 50 (3), 243–249. doi:10.1016/s0920-1211(02)00048-7
Verrotti, A., Salladini, C., Di Marco, G., Pisciella, F., and Chiarelli, F. (2007). Extended-release Formulations in Epilepsy. J. Child. Neurol. 22 (4), 419–426. doi:10.1177/0883073807302760
Wagner, K. D., Redden, L., Kowatch, R. A., Wilens, T. E., Segal, S., Chang, K., et al. (2009). A Double-Blind, Randomized, Placebo-Controlled Trial of Divalproex Extended-Release in the Treatment of Bipolar Disorder in Children and Adolescents. J. Am. Acad. Child. Adolesc. Psychiatry 48 (5), 519–532. doi:10.1097/CHI.0b013e31819c55ec
Wang, Y., Xia, J., Helfer, B., Li, C., and Leucht, S. (2016). Valproate for Schizophrenia. Cochrane Database Syst. Rev. 11, CD004028. doi:10.1002/14651858.CD004028.pub4
Keywords: divalproex, extended release, efficacy, safety, meta-analysis
Citation: Zhang CQ, Li HY, Wan Y, Bai XY, Gan L, Wang J and Sun HB (2022) Efficacy, Safety, and Retention Rate of Extended-Release Divalproex Versus Conventional Delayed-Release Divalproex: A Meta-Analysis of Controlled Clinical Trials. Front. Pharmacol. 13:811017. doi: 10.3389/fphar.2022.811017
Received: 25 November 2021; Accepted: 23 February 2022;
Published: 05 April 2022.
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Masafumi Yoshimura, Kansai Medical University, JapanCopyright © 2022 Zhang, Li, Wan, Bai, Gan, Wang and Sun. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Bin Sun, c2hiMDAxMzY5eEAxNjMuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.