- Department of Pharmacy, Faculty of Science, University of Rajshahi, Rajshahi, Bangladesh
Inflammatory diseases are considered major threats to human health worldwide. In Bangladesh, a number of medicinal plants have been used in traditional medicine from time immemorial in the treatment of diverse diseases, including inflammatory disorders. This assignment aims at providing the status of the medicinal plants of Bangladesh which are traditionally used in the management of inflammatory disorders and are investigated for their anti-inflammatory prospects using different preclinical studies and future research directions. The information of medicinal plants assembled in this review was obtained from a literature search of electronic databases such as Google Scholar, PubMed, Scopus, Web of Science and ScienceDirect up to December, 2020 from publications on plants investigated for their anti-inflammatory activities, in which the place of plant sample collection was identified as Bangladesh. Keywords for primary searches were “anti-inflammatory,” “Bangladeshi,” and “medicinal plants.” Criteria followed to include plant species were plants that showed significant anti-inflammatory activities in 1) two or more sets of experiments in a single report, 2) same or different sets of experiments in two or more reports, and, 3) plants which are traditionally used in the treatment of inflammation and inflammatory disorders. In this study, 48 species of medicinal plants have been reviewed which have been used in traditional healing practices to manage inflammatory disorders in Bangladesh. The mechanistic pathways of the in vivo and in vitro study models used for the evaluation of anti-inflammatory properties of plant samples have been discussed. Selected plants were described in further detail for their habitat, anti-inflammatory studies conducted in countries other than Bangladesh, and anti-inflammatory active constituents isolated from these plants if any. Medicinal plants of Bangladesh have immense significance for anti-inflammatory activity and have potential to contribute toward the discovery and development of novel therapeutic approaches to combat diseases associated with inflammation. However, the plants reviewed in this article had chiefly undergone preliminary screening and require substantial investigations including identification of active molecules, understanding the mechanism of action, and evaluation for safety and efficacy to be followed by the formulation of safe and effective drug products.
Introduction
Plants are the most abundant suppliers of safe and successful remedies from time immemorial to present either to humans or to other animals. It is estimated that more than 90% of traditional medicine recipes comprise medicinal plants (Sofowora et al., 2013) which are used to treat a wide array of acute and chronic diseases ranging from common cold to complex cancerous phases throughout the world (Issa et al., 2006). According to the World Health Organization, the use of traditional and complementary medicine is increasing rapidly in most of the countries (World Health Organization, 2013). Medicinal plants constitute 25% of all modern medicines (Birhanu, 2013), and the annual market value of these plants has surpassed $100 billion globally (Sofowora et al., 2013).
Plants are the reservoir of important bioactive molecules classified as phenolics, alkaloids, carotenoids, organosulfur compounds, etc. on the basis of their chemical nature, and these molecules are reclassified as antioxidants, analgesics, cardioactive, anticancerous, immunity potentiating, detoxifying, neuropharmacological agents, etc. on the basis of their pharmacological action (Ugboko et al., 2020). Thus, plants with values in traditional medicine integrated with scientific evidences have provided the opportunity to discover thousands of therapeutically potential drugs (Harvey, 2000).
Traditional Background of Bangladeshi Medicinal Plants
Bangladesh is a small country regarding her land area, but the fertile soil and favorable climate have enriched the country with highly biodiverse plants. The traditional healing practices of Bangladesh have been exercised from time immemorial and are profoundly embedded within the local communities (Ocvirk et al., 2013; Haque et al., 2018). A myriad of medicinal plants grows all over Bangladesh out of which about 1,000 are conservatively considered to have therapeutic usefulness by traditional healers (Mollik et al., 2010). The formulations of Ayurvedic, Unani, and Homeopathic systems of this country have been developed by exploring these natural resources (Rahman et al., 2001; Ghani, 2003). Even at present, traditional medicine is an integral part of the country’s overall healthcare system (Haque et al., 2014; Jahan et al., 2019). The usage of medicinal plants in the form of extract, decoction, juice, powder, paste, etc. in such traditional practices possesses the same long history to manage or cure diverse diseases (Akber et al., 2011; Rahmatullah et al., 2011a). The major reasons people of Bangladesh rely on these medicinal plants are 1) little or no access to modern medical assistance, 2) availability and cost-effectiveness of medicinal plants, and, 3) trust in the healing power of these natural gifts that has been built up with time, observations, and experiences. In addition, more than 30 tribes constitute 2% of the total population of Bangladesh, and the tribal healers are their principal health care providers who again rely on medicinal plants for treatment of different diseases (Rahmatullah et al., 2011b; 2012).
The increase in prescription rate and popularity of herbal medicine indicates the shift of the global trend from synthetic drugs toward the medicines of natural origin which has also been considered a promising future medicine (Ahmad Khan and Ahmad, 2019). Similarly, in Bangladesh, the manufacturing of herbal medicines has been increased, and the demand for medicinal plants is also increasing (Dulla and Jahan, 2017). But these plants are yet to undergo detailed scientific investigations for chemical constituents and bioactivities to evaluate their pharmacological properties (Rahman et al., 2001), for instance, anti-inflammatory potential.
Inflammation, Inflammatory Disorders, and Available Anti-Inflammatory Medication
The term “inflammation” is described as a prompt and strictly controlled physiological process (Barton, 2008) triggered by harmful foreign stimuli as well as infected and injured host tissue (Medzhitov, 2008; 2010). The foreign stimuli include pathogenic microbes, toxic chemicals, allergens, mechanical and thermal factors, etc. (Ambriz-Pérez et al., 2016; Arulselvan et al., 2016; Attiq et al., 2018). If not properly coordinated, this natural beneficial physiologic action persists instead of being resolved and evolves pathological consequences, leading to the development and progression of numerous human diseases which encompass asthma, rheumatoid arthritis, inflammatory bowel disease, cancer, atherosclerosis, type 2 diabetes, obesity, and neurodegenerative disorders, for example, Alzheimer’s disease, Parkinson’s disease, and multiple sclerosis (Nathan, 2002; Glass et al., 2010; Medzhitov, 2010; Fürst and Zündorf, 2014). These inflammatory diseases are the major health issues around the globe, causing an increase in the rate of morbidity and mortality every year (Gou et al., 2017).
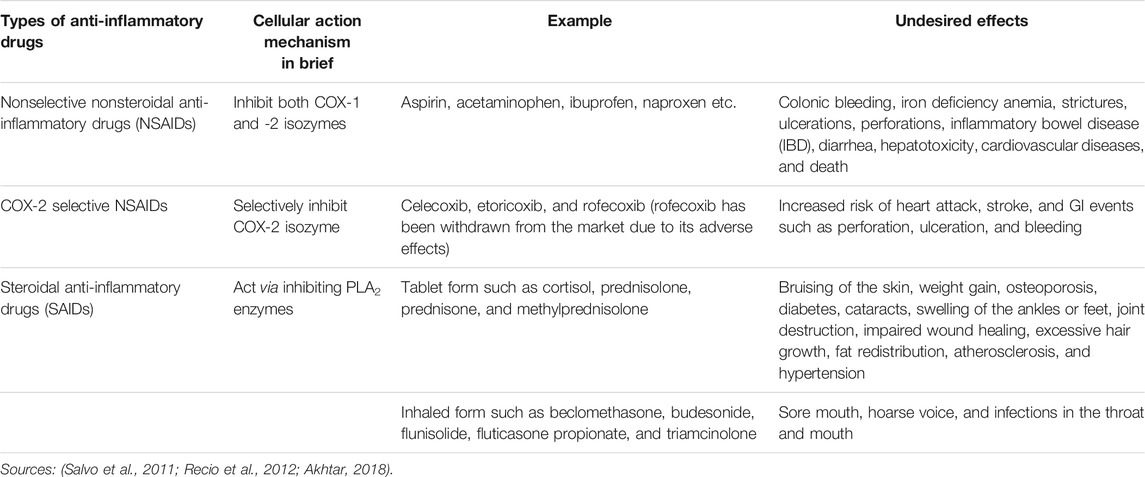
Developing an efficacious anti-inflammatory drug product with a higher margin of safety has always been a challenge. The currently prescribed common anti-inflammatory drugs can be divided into three classes 1) nonselective non-steroidal anti-inflammatory drugs (NSAIDs), 2) cyclooxygenase 2 (COX-2) selective NSAIDs, and, 3) steroidal anti-inflammatory drugs (SAIDs) (Recio et al., 2012). NSAIDs act by retarding the biosynthesis of prostanoids from arachidonic acid (AA) by inhibiting cyclooxygenase (COX) enzymes. These COX enzymes can exist as the constitutive COX-1 and the inducible COX-2 isoforms. Though responsible to mediate inflammation, the ubiquitous COX-1 isoform mainly performs physiologic functions associated with homeostasis as well as protection of cells and tissues accompanying the endothelium, monocytes, gastrointestinal epithelial cells, and platelets. On the other hand, COX-2 is mostly induced by cytokines expressed in the vascular endothelium, rheumatoid synovial endothelial cells, monocytes, and macrophages and plays the key role in inducing pain and inflammation (Vane and Botting, 1998; Smyth et al., 2009; Brune and Patrignani, 2015). The nonselective NSAIDs inhibit both COX-1 and -2, and thus besides providing therapeutic actions, their use can result in a number of undesired side effects (Table 1) (Davies, 1995). The selective COX-2 inhibitors were considered to be therapeutically superior to the conventional nonselective NSAIDs since they have little or no effect on COX-1 isozymes (Everts et al., 2000; Jackson and Hawkey, 2000), but in later studies, these drugs have also been found to cause cardiovascular events such as myocardial infarction, stroke, and heart failure as well as gastrointestinal (GI) complications (Table 1) (Salvo et al., 2011). The other class, that is the steroids, is potent anti-inflammatory agents which can inhibit phospholipase A2 (PLA2) enzymes required to release AA from phospholipids. AA liberates eicosanoids, for instance, prostaglandins, thromboxanes, leukotriene, etc. and platelet-activating factor (PAF) which are the principal inflammatory mediators (Balsinde et al., 2002; Ericson-Neilsen and Kaye, 2014). Adverse reactions associated with high dose or long-term use of small dose of steroid tablets and with inhaled steroids are summarized in Table 1 along with the adverse reactions of NSAIDs and COX-2 selective NSAIDs.
Anti-Inflammatory Compounds of Plant Origin
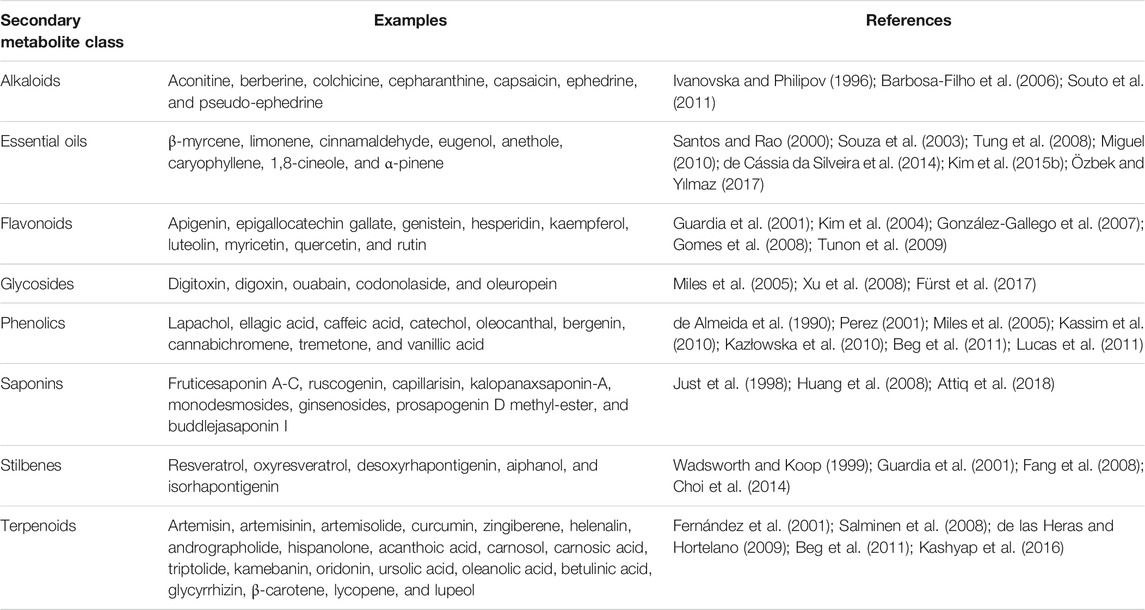
In recent years, research interest on screening of medicinal plants to discover potential anti-inflammatory agents has been intensified (Bellik et al., 2012; 2013). A variety of plant-derived natural products have been shown to exhibit significant anti-inflammatory activity by inhibiting important proinflammatory mediators (Figure 1) and have made way from preclinical studies to clinical trial (Kim et al., 2009; Fürst and Zündorf, 2014). For example, quercetin and kaempferol inhibit inducible nitric oxide synthase (iNOS) expression and signal transducer and activator of transcription-1 (STAT-1) and nuclear factor-κB (NF-κB) activation (Hämäläinen et al., 2007); resveratrol inhibits COX-2 expression followed by modulation of NF-κB and activator protein-1 (AP-1) pathways (Kundu et al., 2006); curcumin, the principal curcuminoid of turmeric (Curcuma longa L., Zingiberaceae), acts via inhibiting COX-1 and -2, lipoxygenase (LOX), NF-κB, and mitogen-activated protein kinase (MAPK) and also downregulates tumor necrosis factor-α (TNF-α) and interleukin-1β and 6 (IL-1β, and -6) secretion (Hong et al., 2004; Kim et al., 2005; Shah et al., 2010). Table 2 summarizes some of the known anti-inflammatory molecules of plant origin.
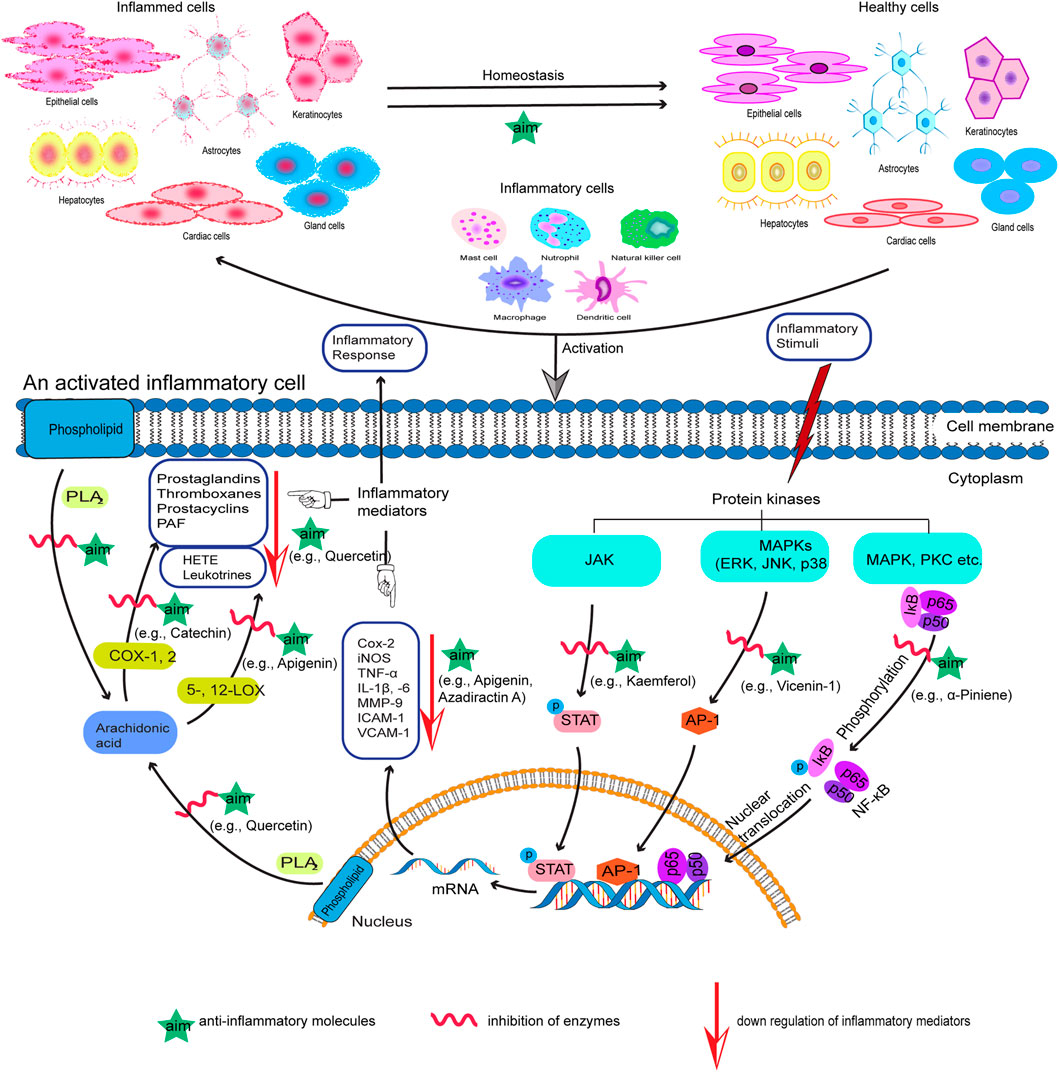
FIGURE 1. Proposed action mechanism of anti-inflammatory molecules (aim) of plant origin with examples. The specific pathways shown represent only a few of the plenty of diverse pathways involved in the inflammatory process.
Apu et al. (2012) published a brief review on anti-inflammatory medicinal plants of Bangladesh in 2012, where they enlisted 36 plants. Another report briefly reviewed 15 analgesic and anti-inflammatory medicinal plants of Bangladesh in addition to cognitive enhancer plants (Uddin and Islam, 2020). In the present study, 48 plants have been chosen on the basis of some inclusion and exclusion criteria. These plants are reviewed for their traditional uses and anti-inflammatory activity in various in vivo and in vitro experimental models, and toxicity test results have also been summarized in Supplementary Table S1 if conducted in the cited research(s). More insights have been given into selected plants, highlighting their identified anti-inflammatory molecules. The compelling motive behind the current study is to represent the scope and possibilities of medicinal plants of Bangladesh with traditional values supported by scientific evidences in the treatment of inflammation and inflammatory disorders.
Methods
The plant species included in this review (Supplementary Table S1) were based on the reports available from January, 2001 to December, 2020 by searching electronic databases such as Google Scholar, PubMed, Scopus, Web of Science, and ScienceDirect. Keywords used were primarily “anti-inflammatory,” “Bangladeshi,” and “medicinal plants.” A secondary search was conducted similarly with keywords “membrane stabilizing activity,” “Bangladeshi,” and “medicinal plants” since membrane stabilization is a well-documented mechanism of anti-inflammatory action, and the in vitro membrane stabilizing assay is established as a valid model to study the anti-inflammatory activity of extracts or molecules (Shinde et al., 1999; Thangaraj, 2016). In these published reports, the plants or their parts were collected from different areas of Bangladesh, and the experimental works were conducted in laboratories of Bangladesh and also in abroad as found in few studies.
The common name, local name, and traditional uses of plants included in Supplementary Table S1 were extracted mainly from the books of Professor Abdul Ghani and Sarder Nasir Uddin and from few other references. The database http://www.worldfloraonline.org/ (previously www.theplantlist.org) was followed for the accepted Latin name of each plant. Criteria followed to include plant species were plants that showed significant anti-inflammatory activities in 1) two or more sets of experiments in a single report, 2) same or different sets of experiments in two or more reports, and 3) plants which are traditionally used in the treatment of inflammation and inflammatory disorders. On the other hand, exclusion criteria include 1) reports not meeting the inclusion criteria, 2) plants reported with insufficient data or no data of doses of the sample or extract and/or positive control, and 3) plants showing anti-inflammatory activity in a single set of experiments in a single report. This review is, therefore, not exhaustive for all the medicinal plants of Bangladesh reported to have anti-inflammatory activity. The toxicity test results summarized in Supplementary Table S1 have been extracted from the same report reviewed for anti-inflammatory activity of plants if toxicity had been tested in the study.
Result and Discussion
This study represents 48 medicinal plants of Bangladesh from 47 genera belonging to 29 families which have traditional values in the treatment of inflammatory disorders along with other medicinal uses. Inflammatory diseases such as arthritis, asthma, tumor, etc. have been managed conservatively using different parts or products of these plants (Supplementary Table S1). Acanthus ilicifolius L., Acmella paniculata (Wall. ex DC.) R.K.Jansen, Aegiceras corniculatum (L.) Blanco, Ageratum conyzoides (L.) L., Alangium salviifolium (L.f.) Wangerin, Alocasia macrorrhizos (L.) G.Don, Argyreia argentea (Roxb.) Sweet, Azadirachta indica A. Juss., Cyanthillium cinereum (L.) H.Rob., C. patulum (Dryand. ex Dryand.) H.Rob., Glycosmis pentaphylla (Retz.) DC., Heliotropium indicum L., Lantana camara L., Mangifera indica L., Manilkara zapota (L.) P.Royen, Mussaenda roxburghii Hook.f., Oroxylum indicum (L.) Kurz, Phrynium imbricatum Roxb., Phyllodium pulchellum (L.) Desv., Piper retrofractum Vahl, Steudnera colocasiifolia K.Koch, Swietenia mahagoni (L.) Jacq., Thunbergia grandiflora (Roxb. ex Rottl.) Roxb., Toona ciliata M.Roem., Vigna unguiculata (L.) Walp., Vitex negundo L., and Withania somnifera (L.) Dunal have values in the treatment of arthritis. Clerodendrum infortunatum L., Coccinia grandis (L.) Voigt, Eclipta prostrata (L.) L., Euphorbia hirta L., Flemingia stricta Roxb., P. retrofractum, and Terminalia arjuna (Roxb. ex DC.) Wight & Arn. have been used as a traditional remedy for asthma. Examples of plants credited for antitumor properties include Butea monosperma (Lam.) Taub., Gynura nepalensis DC., Leea macrophylla Roxb. ex Hornem., Mallotus repandus (Willd.) Müll.Arg., Microcos paniculata L., Premna esculenta Roxb., and Typhonium trilobatum (L.) Schott. Furthermore, Aglaia cucullata (Roxb.) Pellegr., M. zapota, and Urena sinuata L. are known to be efficacious against inflammation besides other traditional uses. These plants were reported to possess significant anti-inflammatory activities which were evaluated using various in vivo and in vitro experimental models. The mechanistic pathways of these models have been taken into consideration for discussion, which provides better understanding of the action mechanism of plants reviewed in this study.
Common Experimental Methods to Evaluate Anti-Inflammatory Activity of Natural Products
Numerous biochemical mediators work jointly to commence and continue the inflammatory cascade. Crude extracts and/or pure compounds derived from plants target these mediators and have paved the way for the development of new therapeutic approaches. In vitro methods are mainly based on the inhibition of such activated mediators. The potent biochemical mediators include, enzymes (PLA2, COX-1, COX-2, 5-LOX, 12-LOX, 15-LOX, MMP-2, MMP-9, inosine monophosphate dehydrogenase, and β-hexosaminidase); free radicals (ROS, RNS, and SOD); prostaglandins (PGE2, TXA2; hydroxyleicosatetraenoic acid [HETE]); leukotrienes (LTB4, LTC4); cluster of differentiation molecules (CD-2, CD-11a, CD-11b, CD-18, and CD-49d), etc. (Venkatesha et al., 2011; Asante-Kwatia et al., 2020). Other important biochemical targets include proinflammatory cytokines (TNF-α, INF-γ, IL-1, IL-6, and IL-1β) and chemokines (IL-8, ICAM-1, and VCAM-1). In addition to these, a number of transcription factors including nuclear factor (NF)-κB, mitogen-activated protein kinases (MAPKs), extracellular signal–regulated kinase (ERK), c-Jun-N-terminal kinase (JNK), signal transducer and activator of transcription-1 (STAT-1), p38 kinases, and AP-1 have been used as molecular targets of inflammation (Luster et al., 2005; Venkatesha et al., 2011). Red blood cell (RBC) membrane stabilization and protein denaturation assays have also been reported in a vast number of studies.
On the other hand, commonly used in vivo methods are carrageenan-induced paw edema, croton oil or TPA (12-O-tetradecanoylphorbol-13-acetate)-induced acute inflammation, xylene-induced ear edema, cotton pellet–induced granuloma, Freunds’ complete adjuvant (FCA)–induced arthritis, in vivo xanthine oxidase assay, LPS-induced peritonitis mouse model, acetic acid–induced vascular permeability assay or measuring writhing reflexes, UV erythema, pleurisy test, etc. (Ferrari et al., 2016; Asante-Kwatia et al., 2020).
Preclinical Models Used to Investigate Plants Included in this Review
In Vivo Studies
In this report, carrageenan-induced paw edema is found to be an extensively used in vivo model used in 28 investigations. In other studies, paw edema is induced using formalin, egg albumin, and also by exogenous administration of histamine and serotonin. Ear edema models are induced using xylene and croton oil. Cotton pellet–implanted granuloma has been exercised in 12 studies. Other in vivo studies include acetic acid–induced writhing test.
Paw Edema Model
Carrageenan-induced paw edema is found to be the most extensively used in vivo test procedure to screen medicinal plants for their anti-inflammatory activities by Bangladeshi researchers (Supplementary Table S1). This seaweed-derived sulfated polysaccharide is injected to experimental animals such as mouse or rat to induce acute and local inflammation. Paw edema induced by carrageenan is considered to be a highly sensitive, reproducible, and well-established model to investigate anti-inflammatory drugs. This model is characterized by biphasic response. Paw inflammation develops resulting in the synthesis of histamine, serotonin, and bradykinin in the first phase (0–1 h) followed by production of COX-2–mediated prostaglandins and cytokines such as IL-1β, IL-6, IL-10, and TNF-α in the second phase (2–3 h). These mediators can be generated in situ either by cellular infiltration or at the site of the local inflammatory insult. The second phase is sensitive to both steroidal and nonsteroidal anti-inflammatory drugs (Posadas et al., 2004; Dzoyem et al., 2017).
Histamine, serotonin, formalin, and egg albumin are injected to obtain paw inflammation in other sets of studies included in this review.
Histamine is a potent inflammatory mediator and is injected to rat or mice paw to induce acute inflammation. This vasoactive amine elicits the release of neuropeptides and prostaglandins from endothelial cells, contributes to neutrophil recruitments, and causes vasodilatation and increased vascular permeability leading to pain and inflammation (Yong et al., 2013b).
Serotonin is another vasoactive amine and inflammatory mediator which increases vascular permeability and can produce acute paw edema via direct injection (Yong et al., 2013a).
Formalin-induced paw edema is also a common method to screen medicinal plants for anti-inflammatory activity and is described as a biphasic response in studies. Neurogenic pain develops in the initial phase, and the second phase involves development of inflammatory responses generated by the release of mediators such as histamine, serotonin, bradykinin, prostaglandin, cytokines (IL-1β, IL-6, TNF-α, etc.), and nitric oxide (NO). Formalin-induced paw edema can be modeled for both acute and chronic type of inflammatory assays (John and Shobana, 2012; Arzi et al., 2015).
Paw edema induced by egg albumin is an acute and triphasic phenomenon as described by Dzoyem et al. (2017), where the first phase is mediated by histamine and serotonin and then the bradykinin-mediated second phase is followed by a third phase mediated by cyclooxygenase to produce bradykinin proteases and prostanoids (Dzoyem et al., 2017).
Usually the changes in thickness, volume, weight, and histology of edematous paw induced by any of the abovementioned methods are examined, and by comparison among the control and treated groups, the anti-inflammatory activities of the plant samples are determined (Kim et al., 2006).
Ear Edema Model
Xylene-induced ear edema is another in vivo model exercised to determine anti-inflammatory activity of plants. Topical administration of xylene causes cutaneous ear inflammation which is acute in nature and is characterized by vasodilatation, cellular infiltration, and edema formation (Lu et al., 2006). Ear edema can be induced by applying croton oil to the ear of rats or mice. This acute inflammatory action is also marked by synthesis of prostaglandins, migration of neutrophils, increased vascular permeability, and development of edema (Luo et al., 2014). In such models, the rats or mice are killed, and circular sections of ears are collected to examine the changes in weight, thickness, and histology profile, which are then compared among the control and treated groups to evaluate the anti-inflammatory activities of the plant samples.
Cotton Pellet–Induced Granuloma Model
Cotton pellet–inserted granuloma in rodents is described as a chronic model which is the proliferative phase of inflammation. This pharmacological procedure consists of three phases, namely, 1) transudative, 2) exudative, and, 3) proliferative (Swingle and Shideman, 1972). The granuloma is actually a highly vascularized tissue which is formed at the site of the subcutaneously implanted cotton pellet due to migration of monocytes, proliferation of fibroblasts, increased vascular permeability, and accumulation of fluid and proteinaceous components (Dzoyem et al., 2017). Anti-inflammatory substances can inhibit the growth of granuloma tissue via interfering with the phases of granuloma formation.
Acetic Acid–Induced Writhing Test
Acetic acid induced writhing is explained as a model of visceral inflammatory pain, generated by releasing endogenous mediators which can trigger nociceptors and are sensitive to nonsteroidal anti-inflammatory drugs and drugs that act on the central nervous system (Bighetti et al., 1999; Jones et al., 2005). Acetic acid is injected into the peritoneal cavity of the experimental animal which causes release of proinflammatory cytokines such as TNF-α, IL-1β, and IL-8 by peritoneal macrophages and mast cells (Ribeiro et al., 2000). As a result, acute inflammatory response arises in the peritoneal area of the experimental animals, and then the animals react with characteristic writhing (Dzoyem et al., 2017). Substances with anti-inflammatory property can inhibit the number of writhes over a time course.
In Vitro Studies
In vitro assays minimize the ethical issues associated with the use of animals in the early phases of drug discovery (Williams et al., 2008). They are mostly cell-based and protein-based and are usually rapid, easy to conduct, and cost-effective, and most importantly, they help understand the molecular mechanism of bioactive compounds to render anti-inflammatory activity (Dzoyem et al., 2017).
RBC membrane stabilization assay is the most extensively used screening tool which has been found to be exercised in 36 studies in this report. Other in vitro experiments include protein denaturation, protease inhibition, and direct estimation of lipoxygenase (LOX) inhibition by the plant samples using the LOX inhibition assay.
RBC Membrane Stabilization Method
In RBC membrane stabilization assay, the red blood cells (erythrocytes) are exposed to various injurious stimuli which can be thermal (heat), mechanical (hypotonic solution), or chemical (methyl salicylate, phenyl hydrazine, etc.) to induce hemolysis. The RBC membrane has a structure similar to that of the lysosome which is a membrane-bound cellular organelle and can release enzymes (PLA2, protease, etc.) capable of inducing inflammatory process. Stabilization of the lysosomal membrane prevents the release of those inflammatory mediators. Therefore, plant extracts or compounds effective in protecting the rupture of the erythrocyte membrane are expected to inhibit the release of inflammatory mediators from the lysosome by stabilizing the lysosomal membrane and hence considered to possess anti-inflammatory activity (Umapathy et al., 2010; Anosike et al., 2012).
Protein Anti-Denaturation Assay
Denaturation of protein has also served as an in vitro pharmacological method to screen anti-inflammatory activity of plant extracts. Bovine serum albumin (BSA) and egg albumin are commonly used proteins for this purpose. The protein loses its secondary and tertiary structures when exposed to heat or substances such as strong acid or base, concentrated salt solution, or organic solvent. Biologically active proteins usually lose their biological activity upon denaturation (Nirmal and Panichayupakaranant, 2015). As explained by Williams et al. (2008), the extracts or molecules which exhibit anti-denaturation property at a very low concentration (ng/ml) should be selected for further drug development processes.
Protease Inhibition Assay
Proteases (also called proteinase) are enzymes that catalyze proteolysis, that is, the hydrolysis of peptide bonds, leading to breakdown of proteins (López-Otín and Overall, 2002). Activation of these enzymes can cause tissue damage associated with inflammatory disorders and can be prevented with drugs having protease inhibitory activity (Kumar et al., 2013). Examples of proteases involved in inflammatory reactions include human neutrophil elastase, MMPs (MMP-2 and -9), trypsin etc. (Benedek et al., 2007; Aziz et al., 2015) and are utilized by the researchers to study the in vitro anti-inflammatory potential of plants.
Toxicological Aspect
Toxicology is defined as a branch of science that deals with poisons (Hodgson, 2004), and as stated by Macht (1938), “every drug that is worth anything as a medicinal agent is also a poison.” Toxicity arises due to the interaction between cellular macromolecules and poisons or toxicants (Jothy et al., 2011). Evaluation of toxicity of such agents is, therefore, also crucial besides evaluating their pharmacological properties. To assure the safe medicinal use of plants or plant products, estimation of toxicity is a must. Numerous laboratory procedures have been developed for this purpose.
It can be noticed in the present study that toxicity studies have not been conducted with almost half of the plants (Supplementary Table S1) at the same time when the anti-inflammatory activities are being evaluated. Out of 48 plants, 25 have undergone preliminary assessment of toxicity by at least one of the cited works. The acute toxicity text and brine shrimp (Artemia salina) lethality test were found to be the primarily used assay methods to assess the toxicity of plant extracts.
The acute toxicity test is an initial screening step to assess and evaluate toxic properties of substances. LD50 which is the dose of the test sample that leads to 50% lethality in the tested group of animals can be determined using this assay (Akhila et al., 2007). Multiple graded doses or a considerably higher single dose than that of the plant extract under investigation were commonly administered to rats or mice to make an estimation of the margin of safety using the acute toxicity test (Supplementary Table S1). Another assay, brine shrimp lethality test, is appraised to be the most convenient method to monitor toxicity of the medicinal plants. This is a rapid and simple predictive tool for toxic potential of plant extracts in humans (Hamidi et al., 2014). Brine shrimp is commonly used as the test organism. LC50 which is the concentration of the test sample that leads to 50% lethality in the nauplii is determined usually by using the graph of mean percentage mortality vs. the log of concentration (Syahmi et al., 2010). The Allium cepa toxicity test is also a common test used by researchers to investigate the toxicity of various substances. This is a sensitive in vivo test method and is used to determine cytotoxic and genotoxic effect of different substances. This test can serve as an indicator of toxicity of the tested samples since it shows good correlation with tests in other living systems. An inhibition of root growth or mitotic index values in the treated onion roots indicates cytotoxic effects, and the chromosomal aberration in the treated onion root tip meristems indicates genotoxic effects of the materials or plant extracts under investigation (Adegbite and Sanyaolu, 2009).
Overview on Selected Anti-Inflammatory Plants
Each of the plant included in the present work is useful in traditional medicine to manage inflammation or inflammatory diseases since that was one of the inclusion criteria. However, for ease of discussion, the following nine plants have been selected (Figure 2), which have demonstrated remarkable anti-inflammatory activity in three or more individual reports (Supplementary Table S1). The habitat, similar studies conducted with the species other than the Bangladeshi one, and anti-inflammatory molecules reported from these plants have been overviewed.

FIGURE 2. Plants with anti-inflammatory activity, namely, A. indica, C. infortunatum, C. asiaticum, G. pentaphylla, H. indicum, M. paniculata, P. retrofractum, S. villosa, and T. trilobatum were obtained from Wikimedia Commons under GNU free documentation license (http://en.wikipedia.org/wiki/GNU_Free_Documentation_License), whereas P. reticulatus was obtained from the web page “Flora of Bangladesh,” from the Survey of Vascular Flora of Chittagong and the Chittagong Hill Tracts Project, Bangladesh National Herbarium (http://bnh-flora.gov.bd), Ministry of Environment & Forest, People’s Republic of Bangladesh.
Azadirachta indica A. Juss.
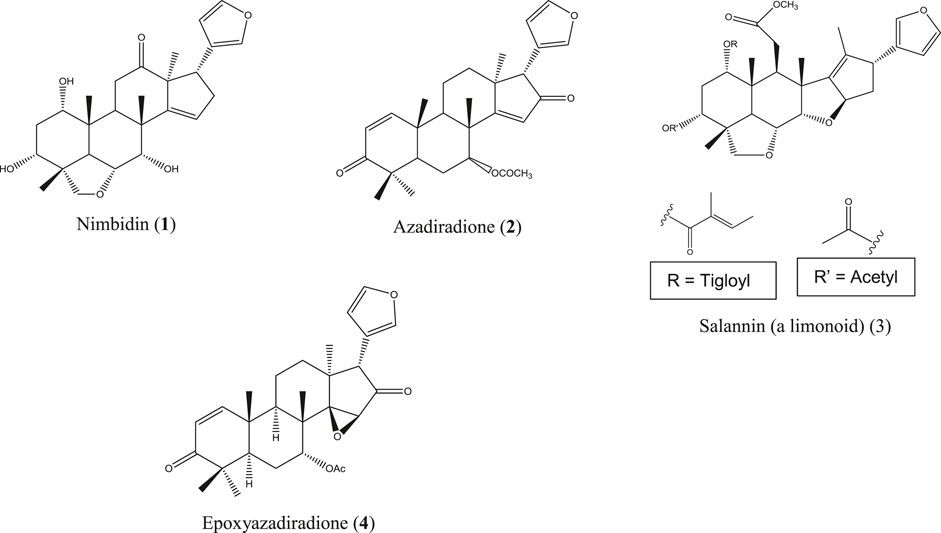
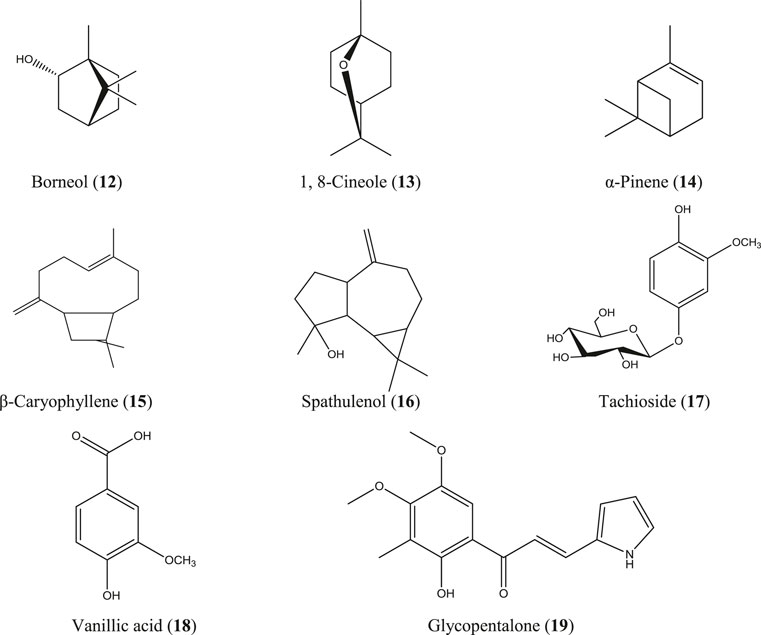
A. indica is locally known as neem and is commonly found all over Bangladesh and in India, Pakistan, and Nepal. This herb is used in traditional healing practices, including Ayurvedic, Unani, and Homeopathic systems, to a great extent (Alzohairy, 2016). Though originated from Asia, neem is now cultivated worldwide. Medicinal values, most importantly the anti-inflammatory action of neem extracts and neem compounds, have been reported in a number of studies. Indian neem leaf extract and neem seed oil have inhibited cotton pellet–induced granuloma and carrageenan-induced paw edema, respectively, in rats (Chattopadhyay, 1998; Naik et al., 2014). The Nigerian variety of neem showed pronounced anti-inflammatory activity by reducing carrageenan-induced rat paw edema (Okpanyi and Ezeukwu, 1981). Polysaccharide fractions from the neem seed tegument of Brazil exhibited potent anti-inflammatory activity in acute inflammatory test models (Pereira et al., 2012). However, in another Brazilian study, the ethanolic neem fruit extract did not reduce abdominal edema in the carrageenan-induced inflammatory model of zebrafish and was found to be nontoxic in zebra fish as well as the A. salina lethality test (Batista et al., 2018). The neem fruit extract azadirachtin A, purchased in China, markedly reduced the levels of TNF-α, IL-6, IL-1b, TLR4, and NF-κB followed by inhibition of tissue inflammation (He et al., 2020). In a study, conducted in Luxembourg, a strong effect of the neem extract has been observed on proinflammatory cell signaling via modulation of the NF-κB pathway (Schumacher et al., 2011). The neem compounds which have significantly attenuated inflammation and related diseases are nimbidin (1) (Pillai and Santhakumari, 1981; Kaur et al., 2004), azadiradione (2) (Ilango et al., 2013), salannin (3), epoxyazadiradione (4) (Alam et al., 2012) (Figure 3), and a series of other limonoids (Akihisa et al., 2011; Islas et al., 2020).
Clerodendrum infortunatum L.
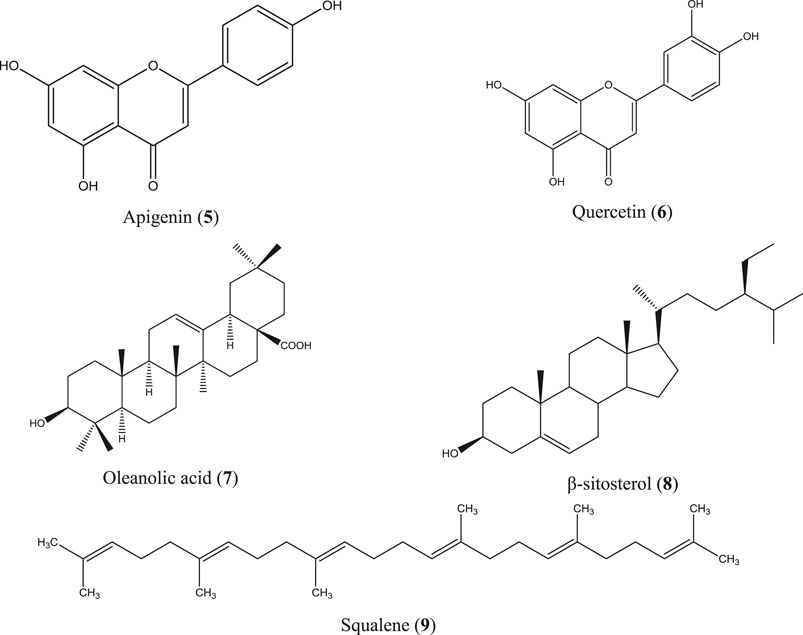
C. infortunatum is a shrub and is widely distributed throughout Bangladesh, India, Sri Lanka, Thailand, and Malaysia, and is used in the indigenous systems of medicine, including Ayurveda, Unani, and Homeopathy (Ahmed et al., 2007; Kekuda et al., 2019). Ethanol extract of leaf and aqueous acetone extract of the root bark prepared from the Indian species of this plant have shown anti-inflammatory activity by inhibiting paw edema in rats (Chandrashekar and Rao, 2013; Helen et al., 2018), whereas hydroethanolic extract of the leaf, stem, and root of C. infortunatum dose-dependently inhibited NO production in LPS-stimulated macrophage and showed no sign of mortality in the acute toxicity study (Dutta et al., 2018). In another study, both leaf and root extracts were found safe in the in vivo experimental model (Nandi et al., 2017). Several compounds have been identified from C. infortunatum with important medicinal values (Nandi and Mawkhlieng Lyndem, 2016), the major being 3-deoxy-d-mannoic lactone, glycerin, and xylitol as analyzed using the gas chromatography coupled with mass spectroscopy (GC-MS) technique (Ghosh et al., 2015) along with viscosene and several flavonoid glycosides (Uddin et al., 2020). Anti-inflammatory moieties reported from this plant include apigenin (5) (Sinha et al., 1981), quercetin (6) (Gupta and Gupta, 2012), oleanolic acid (7) (Sannigrahi et al., 2012), β-sitosterol (8) (Gupta and Singh, 2012; Paniagua-Pérez et al., 2017), and squalene (9) (Figure 4) (Choudhury et al., 2009).
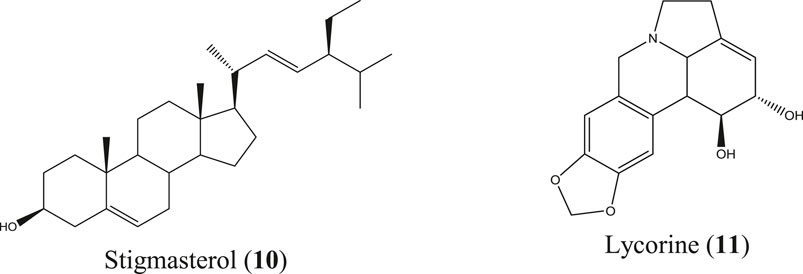
Crinum asiaticum L.
C. asiaticum is an evergreen herb found in Bangladesh, India, Sri Lanka, Myanmar, Thailand, Malaysia, China, and Japan. This herb is randomly inhabited in the hilly areas of Bangladesh, especially the Chittagong Hill Tracts, and has widely been used in traditional and Ayurvedic systems of medicines (Rahman et al., 2013; Sharma et al., 2020). The Malaysian species of this plant caused significant reduction of mice paw edema (Samud et al., 1999) and prevented new blood vessel formation from the aortic ring explants, exhibiting potential anticancer activity known to be influenced by inflammation (Yusoff et al., 2017). The list of compounds isolated from this plant is also long and includes lycorine, crinamin, stigmasterol, cycloartenol, etc. (World Health Organization, 1998), among which stigmasterol (10) (Al-Attas et al., 2015) and lycorine (11) (Figure 5) (Çitoğlu et al., 1998; Saltan Çitoğlu et al., 2012) are reported to be anti-inflammatory molecules. Crinamin, a protein isolated from the latex of C. asiaticum leaf exhibited anti-inflammatory activity by reducing carrageenan-induced paw edema and cotton pellet–induced granuloma in rats and also showed protein anti-denaturation and RBC membrane stabilization activity (Rai et al., 2018).
Glycosmis pentaphylla (Retz.) DC.
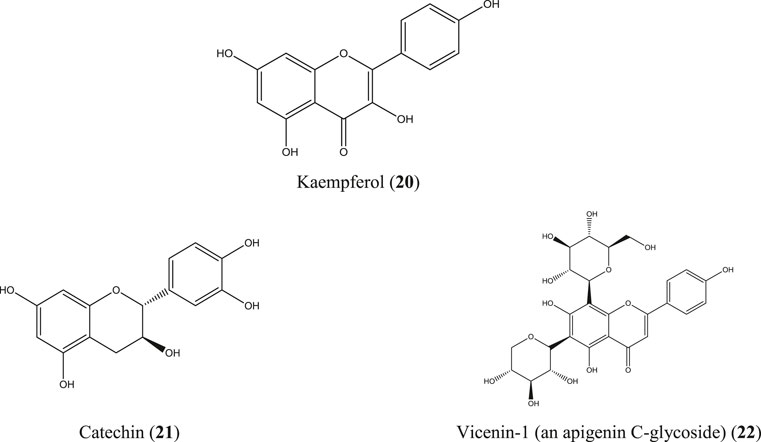
G. pentaphylla is a shrub or small tree that grows at low altitudes of Bangladesh, India, Malaysia, southern China, and the Philippine Islands (Hossain S. M. et al., 2012). Root extract from the same Indian plant inhibited carrageenan- and egg albumin–induced paw edema, xylene-induced ear edema, and formalin-induced arthritic inflammation in rats (Rao and Raju, 2009; Arora and Arora, 2016). The essential oils obtained from the bark, leaf, and seed of G. pentaphylla are enriched with numerous bioactive constituents (Ahmed et al., 2000), and some of them have well-documented anti-inflammatory activity such as borneol (12) (Zou et al., 2017), 1, 8-cineole (13) (Santos and Rao, 2000), α-pinene (14) (Kim DS. et al., 2015; Özbek and Yılmaz, 2017), caryophyllene (15), caryophyllene oxide (Tung et al., 2008), and spathulenol (16) (Figure 6) (do Nascimento et al., 2018). In studies in China, a number of prenylated sulfur-containing amides and a phenolic glycoside (tachioside) (17) (Figure 6) isolated from this plant also exhibited anti-inflammatory activity via downregulation of nitric oxide (NO) production in LPS-stimulated RAW 264.7 macrophages (Tian et al., 2014; Nian et al., 2020). Numerous other compounds have been isolated from this plant, including anti-inflammatory stigmasterol (10) (Figure 5), vanillic acid (18) (Siddiqui et al., 2019; Soma et al., 2019; Chokchaisiri et al., 2020; Ziadlou et al., 2020), and glycopentalone (19) (Figure 6) (Sreejith and Asha, 2015; Sasidharan and Vasumathi, 2017).
Heliotropium indicum L.
H. indicum is a perennial herb indigenous to Bangladesh, India, Sri Lanka, Nepal, and Thailand and also grows in some parts of Africa (Samira et al., 2016). The Indian H. indicum has been found to attenuate carrageenan-, egg white–, and formalin-induced paw edema and cotton pellet–inserted granuloma in rats (Srinivas et al., 2000; Betanabhatla et al., 2007; Ramamurthy et al., 2009; Shalini and Shaik, 2010), whereas the Thai and Ghanaian species have been reported to inhibit proinflammatory mediators in LPS-stimulated RAW 264.7 macrophages (Chunthorng-Orn et al., 2016; Kyei et al., 2016). Analyses on volatile oil composition of this plant have been carried out where phenylacetaldehyde and phytol were found to be the major constituents, and a number of pyrrolizidine alkaloids have also been reported from H. indicum (Birecka et al., 1984; Machan et al., 2006; Ogunbinu et al., 2009). In another GC-MS analysis of essential oil, it was revealed that this plant is rich in phenyl derivatives, where methyl salicylate is the major constituent (Joshi, 2020), and interestingly, a small concentration of methyl salicylate is used in topical preparations for the treatment of mild aches and pains of arthritis (Martin et al., 2004).
Microcos paniculata L.
M. paniculata is a shrub native to southern China, Southeast Asia, and South Asia. In Bangladesh, this plant is common at the Sylhet and Chittagong divisions (Al-Amin Sarker et al., 2016; Jiang and Liu, 2019). Besides numerous traditional uses, a diverse range of compounds have been identified from different parts of the plant which include some well-documented anti-inflammatory molecules such as apigenin (5), quercetin (6) (Figure 4), kaempferol (20), catechin (21), epicatechin (Perez, 2001; García-Mediavilla et al., 2007; Hämäläinen et al., 2007; Kim SH. et al., 2015; Lesjak et al., 2018), and apigenin C-glycosides (e.g., vicenin-1) (22) (Li et al., 2018) (Figure 7). A total flavonoid glycoside fraction obtained from Chinese M. paniculata demonstrated potential anti-inflammatory activity by reducing xylene-induced mice ear edema and suppressing proinflammatory cytokines (IL-6, IL-1β, and TNF-α) in LPS-stimulated RAW 264.7 macrophages. In the same study, ten flavone glycosides have been characterized from the total flavonoid glycoside fraction (Yuan et al., 2019).
Piper retrofractum Vahl
P. retrofractum is a flowering vine native to South and Southeast Asia including Bangladesh, India, Malaysia, Indonesia, Singapore, and Sri Lanka. This plant is used as a culinary herb in some regions of Bangladesh and is commonly known as Choi or Chui jhal (Islam MT. et al., 2020). Methanolic leaf extract of the same Indian species has been investigated using chronic anti-inflammatory study models, including carrageenan- and dextran-induced paw edema and an acute model of cotton pellet–induced granuloma (Vaghasiya et al., 2007). P. retrofractum fruit extract is an ingredient of a Thai traditional medicine “Benjakul” which has demonstrated strong anti-inflammatory activity in in vitro and in vivo test systems (Kuropakornpong et al., 2020). In another Thai study, the ethanolic fruit extract of P. retrofractum inhibited ethyl phenylpropiolate–induced ear edema and carrageenan-induced paw edema in rats (Sireeratawong et al., 2012). Chabamides, piperine, piplartine, etc. have been reported from this plant (Islam MT. et al., 2020). Essential oil composition of the fruit oils of P. retrofractum has also been analyzed, which contains 5.2% of β-caryophyllene (15) (Figure 6) (Tewtrakul et al., 2000).
Sterculia villosa Roxb.
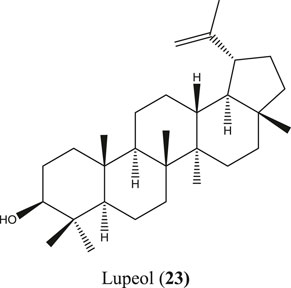
S. villosa is a deciduous small to large tree found in tropical and subtropical countries, including Bangladesh, India, Sri Lanka, and southern China (Hossain M. K. et al., 2012; Hossain et al., 2016). This plant has received special importance for its anti-inflammatory ethnomedicinal value besides many other traditional uses (Namsa et al., 2009; Thabet et al., 2018). Along with different bioactive molecules such as chrysoeriol, chrysoeriol 7-O-β-D-glucoside, diosmetin, etc., anti-inflammatory lupeol (23) (Figure 8) has also been reported from S. villosa (Seetharaman, 1990; Das et al., 2017).
Typhonium trilobatum (L.) Schott
T. trilobatum or Bengal arum is a perennial herb native to Asia, including Bangladesh, India, and to South Pacific. This is a soft plant that grows in the damp and moist places in Chittagong, Sylhet, and other areas of Bangladesh (Ghani, 2003; Haldar et al., 2011). This plant has plenty of traditional uses and has been evaluated in several studies for its anti-inflammatory action (Supplementary Table S1), but individual phytoconstituents from this plant have not been documented properly yet (Manna et al., 2016).
In addition to the abovementioned nine species, the following species have been found to be studied tremendously in recent years for anti-inflammatory activities and been reviewed here in brief.
Ageratum conyzoides (L.) L.
A. conyzoides is an annual herb with a long history of uses in traditional medicine in the tropical and subtropical countries (Kotta et al., 2020). This plant is very common in West Africa, South America, and some parts of Asia, including Bangladesh (Okunade, 2002; Hassan et al., 2012). Crude extract, organic fractions, and isolated compounds (5′- ethoxynobiletin, 1,2-benzopyrone and eupalestin) from the Brazilian variety of this plant inhibited carrageenan-induced pleurisy in mice by reducing the concentration of myeloperoxidase (MPO), adenosine deaminase (ADA), and NO, and by inhibiting several proinflammatory mediators (e.g., IL-17A, IL-6, TNF-α, and IFN-γ). The isolated compounds also reduced phosphorylation of NF-κB and MAPK (Vigil de Mello et al., 2016). In another study conducted in Brazil, the ethanol extract of the standardized polymethoxyflavone extract of this plant exhibited anti-inflammatory activity in mice (Faqueti et al., 2016), whereas Indonesian A. conyzoides leaf extract decreased TNF-α and MMP-9 levels in monosodium iodoacetate–induced osteoarthritis in rats, and anti-inflammatory quercetin (6) was detected in the extract (Bahtiar et al., 2017).
Eclipta prostrata (L.) L.
E. prostrata is an annual herb and a common weed native of Asia but is now widely distributed in tropical and sub-tropical areas over the world (Chung et al., 2017; Feng et al., 2019; Yu et al., 2020). In Asia, this plant grows in Bangladesh, India, Indonesia, Cambodia, Malaysia, Nepal, Pakistan, the Philippines, Sri Lanka, Thailand, Vietnam, China, Japan, and Korea (Uddin et al., 2015). The Korean variety of E. prostrata ethanolic extract prepared conventionally and using ultrasound as well as wedenolactone (a compound isolated from this plant) exhibited anti-inflammatory activity by reducing IL-6, TNF-α, and PGE2 levels (Lee, 2017). In another investigation, a series of flavonoid, flavonoid derivative, triterpenoid, and triterpenoid glycosides were isolated from this plant which reduced the NO level in LPS-stimulated RAW 264.7 cells in varying degrees, where 7-O-methylorobol-4′-O-β-D-glucopyranoside was found to be the most potent compound and reported as an inhibitor of IκB phosphorylation (Le et al., 2020). The aqueous seed extract of the Sri Lankan variety of this plant dose-dependently inhibited denaturation of egg albumin (Kannadas et al., 2020).
Lawsonia inermis L.
L. inermis commonly known as henna has plenty of traditional uses (Supplementary Table S1). This evergreen shrub is mainly cultivated for cosmetic purposes and also for its use in traditional medicine all over the world, but the plant is native to tropical and subtropical countries such as Bangladesh, India, Sri Lanka, and the Middle East (Ghani, 2003; Sharma et al., 2016). Flavonoids isolated from the Indian species of this plant inhibited carrageenan-induced rat paw edema (Manivannan et al., 2015), whereas the flower extract from the Tunisian species inhibited 5-LOX (Chaibi et al., 2015). The ethanol extract from the leaves of this plant reduced formalin-induced (Humaish, 2017) and carrageenan-induced (Vijayaraj and Kumaran, 2018) rat paw edema in studies conducted in Iraq and India. respectively. The Nigerian L. inermis leaf extract prepared with n-butanol and ethylacetate reduced carrageenan-induced foot edema in chicks (Chuku et al., 2020). Additionally, a topical formulation prepared from Iranian henna leaves improved contact dermatitis in patients using lower limb prosthetics (Niazi et al., 2020).
Manilkara zapota (L.) P.Royen
M. zapota is an evergreen tree thought to originate from Mexico, Central America, and West Indies and is cultivated throughout Bangladesh and India (Ganguly et al., 2013; Yong and Abdul Shukkoor, 2020). The leaf extract of Indian M. zapota inhibited hemolysis of the RBC membrane (Sravani et al., 2015) and inhibited PLA2 and 5-LOX, and carrageenan-induced rat paw edema in in vitro and in vivo experimental models, respectively (Konuku et al., 2017). Apigenin-7-O-β-D-glucuronide methyl ester isolated from Ethiopian M. zapota leaves downregulated COX-2 mRNA expression in MCF-7 breast cancer cells (Kamalakararao et al., 2018b) and also inhibited NO and PGE2 production in LPS-induced RAW 264.7 macrophages (Kamalakararao et al., 2018a). The fruit extract prepared from the Thai variety exhibited anti-inflammatory potential by inhibiting the TNF-α level in LPS-activated human PMBC cells (Leelarungrayub et al., 2019). Again, several prenylated coumarins identified from Chinese M. zapota fruit suppressed NO production in LPS-activated RAW 264.7 cells (Liu et al., 2019).
Withania somnifera (L.) Dunal
W. somnifera is an evergreen woody shrub commonly known as winter cherry or Indian ginseng distributed in tropical and subtropical regions of the world which include Bangladesh, India, Pakistan, Afghanistan, Sri Lanka, China, Egypt, Morocco, Jordan, Congo, and South Africa. This plant is extensively used in Unani and Ayurvedic systems of medicine (Shahriar et al., 2014; Dar et al., 2015; Dar and Ahmad, 2020). Several studies conducted with the Indian species of this plant exhibited significant anti-inflammatory activities. For example, root extract of W. somnifera exhibited acute and chronic anti-inflammatory activity in carrageenan-induced rat paw edema and Freund’s adjuvant–induced arthritis models, respectively (Giri, 2016), as well as in collagen-induced arthritic rats (Khan et al., 2019). Leaf extract demonstrated anti-neuroinflammatory activity by attenuating TNF-α, IL-1β, IL-6, RNS, and ROS production via the inhibition of NF-κB, AP-1, JNK, and MAPK pathways (Gupta and Kaur, 2016; 2018). Fatty acids from seeds inhibited the release of proinflammatory cytokines (IL-6 and TNF-α) and reduced NF-κB expression and were found to reduce edema besides repairing psoriatic lesions in the TPA-induced psoriatic mouse model. In the same study, the fatty acid components were identified as linoleic acid, oleic acid, palmitic acid, stearic acid, 11,14,17-eicosatrienoic acid, and nervonic acid along with other unidentified components (Balkrishna et al., 2020).
The brief analyses of the abovementioned plants again signify the values of Bangladeshi medicinal plants to be explored for novel anti-inflammatory molecules. However, the reported anti-inflammatory activities of the plants investigated in Bangladesh are primarily based on the anti-inflammatory activity of their crude organic extracts or partitionates. Therefore, a great detail is yet to be unveiled of these plants regarding the purification and characterization of their chemical constituents, which requires different separation techniques and spectroscopic analyses to pinpoint the anti-inflammatory molecules.
According to literature, a huge number of Bangladeshi medicinal plants are used in traditional medicines to combat inflammation and inflammatory disorders. However, due to the set criteria of plant selection, a limited number of plants have been summarized in the present study. The plants reviewed here are useful in traditional medicine to manage inflammation or inflammatory diseases such as arthritis, asthma, hepatitis, psoriasis, bronchitis, and tumor. The anti-inflammatory activities of these plants have been evaluated scientifically using different assay models. The plant samples were administered usually in graded doses of the extract or as a single dose of each of different organic fractions. Thus, in case of graded doses, plant extracts were found to inhibit inflammation in a dose-dependent manner, and in case of different organic fractions, a single dose of different samples demonstrated varying degrees of activity. The significance of activity was assessed in comparison with positive controls. Acetyl salicylic acid, diclofenac sodium, indomethacin, ibuprofen, phenylbutazone, hydrocortisone, etc. were commonly used as positive control drugs for these experiments.
Selected plants, namely, A. indica, C. infortunatum, C. asiaticum, G. pentaphylla, H. indicum, M. paniculata, P. retrofractum, S. villosa, and T. trilobatum were focused in further detail. In addition, A. conyzoides, E. prostrata, L. inermis, M. zapota, and W. somnifera have been reviewed briefly. Most of these plants have been found to be studied for the same purpose in other Asian countries, including India, Sri Lanka, Thailand, Malaysia, China, Korea, and Indonesia along with countries such as Brazil, Nigeria, Ghana, Ethiopia, Iraq, Iran, and Luxembourg where these plants have been proved again to have potential for anti-inflammatory activities. A number of well-documented anti-inflammatory molecules, for example, apigenin (5), quercetin (6), 1,8-cineole (13), α-pinene (14), β-caryophyllene (15), kaempferol (20), catechin (21), and apigenin C-glycosides (e.g., vicenin-1) (22) have been characterized from these plants.
Although attributed for diverse beneficial effects with a rich source of therapeutic agents’ plants have toxicological properties as well. The acute toxicity test has been conducted by many of the researchers to determine dose ranges for subsequent pharmacological assays and to observe changes in behavior that is signs such as restlessness, respiratory distress, general irritation, coma, convulsion, locomotor ataxia, diarrhea, and weight loss elicited by the plant samples under investigation. The brine shrimp lethality test is also used by the investigators to estimate toxicity of plant extracts. LC50 values of the extracts have been determined using this assay. Higher LC50 values indicate higher margin of safety, whereas samples with lower LC50 values are considered to be toxic and often recommended to be evaluated for anticancer properties. However, crude extract from plants usually is a combination of few or many biologically active constituents. Thus, the compound(s) demonstrating useful pharmacological activity may or may not be responsible for the elicited toxicity. Therefore, identifying the active constituent and evaluation of toxicity of that active constituent is highly crucial for these traditionally important anti-inflammatory plants of Bangladesh.
Concluding Remarks
This review indicates that the medicinal plants of Bangladesh have immense significance for anti-inflammatory activity and have the potential to contribute toward the discovery and development of novel therapeutic approaches to combat inflammatory disorders which are the leading cause of innumerable human diseases all over the globe. Though possessing traditional significance to manage inflammatory diseases, these plants chiefly underwent preliminary screening for anti-inflammatory effects and require intensive research studies, including identification of active constituents, understanding the mechanism of action precisely, evaluation for safety and efficacy, and formulation of safe and effective drug products.
These early-stage screenings of Bangladeshi medicinal plants were mostly carried out for academic purposes, and there was hardly any industrial support behind these projects. Proper industrial collaboration can forge the aforementioned stages of exploration and can thereby contribute to extend the anti-inflammatory therapeutic armory.
Author Contributions
The author confirms being the sole contributor of this study and has approved it for publication.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
I sincerely acknowledge Professor Gerald Muench, Western Sydney University, Australia who is the inspiration for my research in the field of inflammation. I also thank Md. Rafiqur Rahman for helping me with the facilities of Central Library, Rajshahi University, Bangladesh.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.809324/full#supplementary-material
References
Adegbite, A. E., and Sanyaolu, E. B. (2009). Cytotoxicity Testing of Aqueous Extract of Bitter Leaf (Vernonia Amygdalina Del.) Using the Allium cepa Chromosome Aberration Assay. Scientific Res. Essays 4 (11), 311–1314. doi:10.5897/SRE.9000315
Ahmad Khan, M. S., and Ahmad, I. (2019). “Herbal Medicine,” in New Look to Phytomedicine. Editors M. S. Ahmad Khan, I. Ahmad, and D. Chattopadhyay (Academic Press), 3–13. doi:10.1016/b978-0-12-814619-4.00001-x
Ahmed, F., Shahid, I. Z., Biswas, U. K., Roy, B. A., Das, A. K., and Choudhuri, M. S. K. (2007). Anti-inflammatory, Antinociceptive, and Neuropharmacological Activities ofClerodendron Viscosum. Pharm. Biol. 45 (7), 587–593. doi:10.1080/13880200701501342
Ahmed, R., Choudhury, S., Vajczikova, I., and Leclercq, P. A. (2000). Essential Oils ofGlycosmis pentaphylla(Cor.). A New Report from Assam, India. J. Essent. Oil Res. 12 (4), 471–474. doi:10.1080/10412905.2000.9699568
Akber, M., Seraj, S., Nahar, N., Islam, F., Ahsan, S., Ferdausi, D., et al. (2011). A Survey of Medicinal Plants Used by the Traditional Medicinal Practitioners of Khulna City, Bangladesh. American-Eurasian J. Sustainable Agric. 5 (2), 177–195.
Akhila, J. S., Shyamjith, D., and Alwar, M. C. (2007). Acute Toxicity Studies and Determination of Median Lethal Dose. Curr. Sci. 93 (7), 917–920. Availableat: http://www.jstor.org/stable/24099255.
Akhtar, M. A. (2018). Australian Native Plants - A Source of Novel Anti-inflammatoty Compounds [Online]. Sydney, NSW, Australia: Western Sydney University. Availableat: https://core.ac.uk/download/pdf/226479947.pdf (Accessed January 12, 2021).
Akihisa, T., Takahashi, A., Kikuchi, T., Takagi, M., Watanabe, K., Fukatsu, M., et al. (2011). The Melanogenesis-Inhibitory, Anti-inflammatory, and Chemopreventive Effects of Limonoids in N-Hexane Extract of Azadirachta indica A. Juss. (Neem) Seeds. J. Oleo Sci. 60 (2), 53–59. doi:10.5650/jos.60.53
Aktar, A., Hassan, S. M. H., Parvin, T., Akhlas, M. B., Khatun, F., Islam, M. T., et al. (2019). Further Phytochemical Screening; Non-clinical Evaluation of Toxic and Anti- Inflammatory Effects of Crude Aqueous Extract of Gynura Nepalensis. Pharmacologyonline 1, 136–153.
Aktar, F., Kuddus, M. R., Kabir, S., Rashid, M. A., and Chakma, K. (2013). Membrane Stabilizing and Cytotoxic Activities of Different Kupchan Partitionates of Oroxylum Indicum (L.) Vent. Leaf and Bark Extracts. Dhaka Univ. J. Pharm. Sci. 12 (2), 181–183. doi:10.3329/dujps.v12i2.17625
Akter, M., Hira, T. E., Mian, M. Y., Ahmed, I., Rahman, M. M., and Rahman, S. M. A. (2014). Cytotoxic, Thrombolytic and Membrane Stabilizing Activities of Swietenia Mahagoni (L.) Jacq. Bark Extract. J. SUB 5 (1), 32–38.
Al Mahmud, Z., Emran, T. B., Qais, N., Bachar, S. C., Sarker, M., and Uddin, M. M. (2015). Evaluation of Analgesic, Anti-inflammatory, Thrombolytic and Hepatoprotective Activities of Roots of Premna Esculenta (Roxb). J. Basic Clin. Physiol. Pharmacol. 27, 63–70. doi:10.1515/jbcpp-2015-0056
Al-Amin Sarker, M., Banik, S., Saddam Hussain, M., Ghosh, A., and S. Hossain, M. M. (2016). In-vitro and In-Vivo Pharmacological Activities with Phytochemical Evaluation of Methanolic Extract of Microcos Paniculata Stem Barks. Cdth 11 (2), 142–149. doi:10.2174/1574885511666160520154529
Al-Attas, A. A., El-Shaer, N. S., Mohamed, G. A., Ibrahim, S. R., and Esmat, A. (2015). Anti-inflammatory Sesquiterpenes from Costus Speciosus Rhizomes. J. Ethnopharmacol 176, 365–374. doi:10.1016/j.jep.2015.11.026
Alam, A., Haldar, S., Thulasiram, H. V., Kumar, R., Goyal, M., Iqbal, M. S., et al. (2012). Novel Anti-inflammatory Activity of Epoxyazadiradione against Macrophage Migration Inhibitory Factor: Inhibition of Tautomerase and Proinflammatory Activities of Macrophage Migration Inhibitory Factor. J. Biol. Chem. 287 (29), 24844–24861. doi:10.1074/jbc.M112.341321
Alam, M. A., Rahman, M. M., Subhan, N., Majumder, M. M., Hasan, S. M. R., Akter, R., et al. (2009a). Antioxidant Potential of the Ethanol Extract of the Leaves of Vitex Negundo L. Turkish J. Pharm. Sci. 6 (1), 11–20.
Alam, M. A., Subhan, N., Awal, M. A., Alam, M. S., Sarder, M., Nahar, L., et al. (2009b). Antinociceptive and Anti-inflammatory Properties ofRuellia Tuberosa. Pharm. Biol. 47 (3), 209–214. doi:10.1080/13880200802434575
Alam, M. N., Biozid, M. S., Islam, M. R., Rahman, M. M., Chowdhury, A. I., and Mazumdar, M. M. U. (2015). In-vitro Comparative Study of Anti-inflammatory and Anti-arthritic Effects of the Methanol Extract of Cissus Pentagona Roxb. And Thunbergia Grandiflora Roxb. Leaf. Pharma Innovation J. 4 (4), 39–42.
Ali, K., Ashraf, A., and Nath Biswas, N. (2012). Analgesic, Anti-inflammatory and Anti-diarrheal Activities of Ethanolic Leaf Extract of Typhonium Trilobatum L. Schott. Asian Pac. J. Trop. Biomed. 2 (9), 722–726. doi:10.1016/S2221-1691(12)60217-2
Alzohairy, M. A. (2016). Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid. Based Complement. Alternat Med. 2016, 7382506. doi:10.1155/2016/7382506
Ambriz-Perez, D. L., Leyva-Lepez, N., Gutierrez-Grijalva, E. P., and Heredia, J. B. (2016). Phenolic Compounds: Natural Alternative in Inflammation Treatment. A Review. Cogent Food Agric. 2 (1), 1131412. doi:10.1080/23311932.2015.1131412
Anosike, C. A., Obidoa, O., and Ezeanyika, L. U. (2012). Membrane Stabilization as a Mechanism of the Anti-inflammatory Activity of Methanol Extract of Garden Egg (Solanum Aethiopicum). Daru 20 (1), 76. doi:10.1186/2008-2231-20-76
Ansari, P., Azam, S., Reyad-ul-ferdous, M., Hossain, A., Azad, T., Goswami, S., et al. (2015). Potential Investigation of Anti-inflammatory Activity and Phytochemical Investigations of Ethanolic Extract of Glycosmis Pentaphylla Leaves. Am. J. Biomed. Res. 3 (1), 6–8. doi:10.12691/ajbr-3-1-2
Anwar, R., Sultana, S. R., Hossen, S., and Chowdhury, M. R. H. (2018). Ethnopharmacological Investigation in Sterculia Villosa to Determine Anti-diabetic, Anti-inflammatory, Antioxidant, Thrombolytic, and Cytotoxic Effect. J. Pharmacognosy Phytochemistry 7 (2), 3203–3211.
Apu, A. S., Bhuyan, S. H., Prova, S. S., and Muhit, M. A. (2012). Anti-Inflammatory Activity of Medicinal Plants Native to Bangladesh: A Review. J. Appl. Pharm. Sci. 2 (2), 7–10.
Arefin, S. M. A., Islam, M. A., Rashid, S. T. B., Rayhan, M. A., Hossen, M. M., Alam, M. J., et al. (2015). Analgesic, Anti-inflammatory, Anticonvulsant and CNS Effect of the Ethanolic Extract of Butea Monosperma Roots. Jahangirnagar Univ. J. Biol. Sci. 4 (1), 9–18. doi:10.3329/jujbs.v4i1.27781
Arora, N., and Arora, P. (2016). Anti-inflammatory Activity of Methanolic Extract of Roots of Glycosmis Pentaphylla. Asian J. Pharm. Res. Development 4 (2), 1–4. doi:10.18203/2349-2902.isj20164471
Arulselvan, P., Fard, M. T., Tan, W. S., Gothai, S., Fakurazi, S., Norhaizan, M. E., et al. (2016). Role of Antioxidants and Natural Products in Inflammation. Oxid Med. Cel Longev 2016, 5276130. doi:10.1155/2016/5276130
Arzi, A., Olapour, S., Yaghooti, H., and Sistani Karampour, N. (2015). Effect of Royal Jelly on Formalin Induced-Inflammation in Rat Hind Paw. Jundishapur J. Nat. Pharm. Prod. 10 (1), e22466. doi:10.17795/jjnpp-22466
Asante-Kwatia, Evelyn., Abraham Yeboah, Mensah., and Baidoo, M. F. (2020). “Analgesic and Anti-inflammatory Effect of Ghanaian Medicinal Plants,” in Medicinal Plants - Use in Prevention and Treatment of Diseases. Editor B. A. R. Hassan (IntechOpen, 1–22. doi:10.5772/intechopen.90154
Attiq, A., Jalil, J., Husain, K., and Ahmad, W. (2018). Raging the War against Inflammation with Natural Products. Front. Pharmacol. 9, 976. doi:10.3389/fphar.2018.00976
Aziz, M. A. (2015). Qualitative Phytochemical Screening and Evaluation of Anti-inflammatory, Analgesic and Antipyretic Activities of Microcos Paniculata Barks and Fruits. J. Integr. Med. 13 (3), 173–184. doi:10.1016/S2095-4964(15)60179-0
Aziz, M. A., Akter, M. I., Sajon, S. R., Rahman, S. M. M., and Rana, M. S. (2018). Anti-inflammatory and Anti-pyretic Activities of the Hydro-Methanol and Petroleum-Benzene Extracts of Microcos Paniculata Barks. Pharmacologyonline 2, 23–30.
Aziz, M. A., Rahman, S., Islam, T., Islam, T., Alam, A. S., Uddin, N., et al. (2015). Anti-inflammatory, Anthelmintic & Antidiabetic Activity of Aqueous Extract of Microcos Paniculata Fruits. Pharmacologyonline 1, 121–125.
Bahtiar, A., Nurazizah, M., Roselina, T., Tambunan, A. P., and Arsianti, A. (2017). Ethanolic Extracts of Babandotan Leaves (Ageratum Conyzoides L.) Prevents Inflammation and Proteoglycan Degradation by Inhibiting TNF-α and MMP-9 on Osteoarthritis Rats Induced by Monosodium Iodoacetate. Asian Pac. J. Trop. Med. 10 (3), 270–277. doi:10.1016/j.apjtm.2017.03.006
Balkrishna, A., Nain, P., Chauhan, A., Sharma, N., Gupta, A., Ranjan, R., et al. (2020). Super Critical Fluid Extracted Fatty Acids from Withania Somnifera Seeds Repair Psoriasis-like Skin Lesions and Attenuate Pro-inflammatory Cytokines (TNF-α and IL-6) Release. Biomolecules 10(2), 185. doi: doi:doi:10.3390/biom10020185
Balsinde, J., Winstead, M. V., and Dennis, E. A. (2002). Phospholipase A(2) Regulation of Arachidonic Acid Mobilization. FEBS Lett. 531 (1), 2–6. doi:10.1016/S0014-5793(02)03413-0
Barbosa-Filho, J. M., Piuvezam, M. R., Moura, M. D., Silva, M. S., Lima, K. V. B., da-Cunha, E. V. L., et al. (2006). Anti-inflammatory Activity of Alkaloids: a Twenty-century Review. Rev. Bras. Farmacogn. 16 (1), 109–139. doi:10.1590/S0102-695X2006000100020
Barton, G. M. (2008). A Calculated Response: Control of Inflammation by the Innate Immune System. J. Clin. Invest. 118 (2), 413–420. doi:10.1172/JCI34431
Bashar, M., Ibrahim, M., Sultana, I., Hossain, M. I., Tasneem, Z., Kuddus, M. R., et al. (2014). Preliminary Phytochemical Screenings and Antipyretic, Analgesic and Anti-inflammatory Activities of Methanol Extract of Vernonia Cinerea Less. (Fam: Asteraceae). Ejmp 4 (10), 1178–1185. doi:10.9734/EJMP/2014/10050
Batista, F. L. A., Lima, L. M. G., Abrante, I. A., de Araújo, J. I. F., Batista, F. L. A., Abrante, I. A., et al. (2018). Antinociceptive Activity of Ethanolic Extract of Azadirachta indica A. Juss (Neem, Meliaceae) Fruit through Opioid, Glutamatergic and Acid-Sensitive Ion Pathways in Adult Zebrafish (Danio rerio). Biomed. Pharmacother. 108, 408–416. doi:10.1016/j.biopha.2018.08.160
Beg, S., Swain, S., Hasan, H., Barkat, M. A., and Hussain, M. S. (2011). Systematic Review of Herbals as Potential Anti-inflammatory Agents: Recent Advances, Current Clinical Status and Future Perspectives. Pharmacogn Rev. 5 (10), 120–137. doi:10.4103/0973-7847.91102
Begum, F., Begum, Z., Uddin, M., Haider, A., and Barman, R. (2012). Effects of Methanol Extract of Piper Chaba Stem Bark on Acute Inflammation in Rats. Faridpur Med. Coll. J. 7 (1), 26–28. doi:10.3329/fmcj.v7i1.10294
Begum, F., Uddin, K., Sultana, S., Ferdous, A. H., and Begum, Z. A. (2008). Effects of Methanol Extract of Piper Chaba Stem Bark on Chronic Inflamation in Rats. Ibrahim Med. Coll. J. 2 (2), 37–39. doi:10.3329/imcj.v2i2.2934
Begum, M. M., Islam, A., Begum, R., Uddin, M. S., Rahman, M. S., Alam, S., et al. (2019). Ethnopharmacological Inspections of Organic Extract of Oroxylum Indicum in Rat Models: A Promising Natural Gift. Evid. Based Complement. Alternat Med. 2019, 1562038. doi:10.1155/2019/1562038
Bellik, Y., Boukraâ, L., Alzahrani, H. A., Bakhotmah, B. A., Abdellah, F., Hammoudi, S. M., et al. (2013). Molecular Mechanism Underlying Anti-inflammatory and Anti-allergic Activities of Phytochemicals: An Update. Molecules 18 (1), 322–353. doi:10.3390/molecules18010322
Bellik, Y., Hammoudi, S. M., Abdellah, F., Iguer-Ouada, M., and Boukraâ, L. (2012). Phytochemicals to Prevent Inflammation and Allergy. Recent Pat Inflamm. Allergy Drug Discov. 6 (2), 147–158. doi:10.2174/187221312800166886
Benedek, B., Kopp, B., and Melzig, M. F. (2007). Achillea millefolium L. s.L. -- Is the Anti-inflammatory Activity Mediated by Protease Inhibition? J. Ethnopharmacol 113 (2), 312–317. doi:10.1016/j.jep.2007.06.014
Betanabhatla, K. S., Sajni, R., Karthik, R., Raamamurthy, J., Christina, A. J. M., and Sasikumar, S. (2007). Anti-inflammatory and Anti-nociceptive Activities of Heliotropium Indicum Linn. In Experimental Animal Models. Pharmacologyonline 3, 438–445.
Bighetti, E. J., Hiruma-Lima, C. A., Gracioso, J. S., and Brito, A. R. (1999). Anti-inflammatory and Antinociceptive Effects in Rodents of the Essential Oil of Croton Cajucara Benth. J. Pharm. Pharmacol. 51 (12), 1447–1453. doi:10.1211/0022357991777100
Bin Emran, T., Uddin, M. M. N., Rahman, M. A., Uddin, M., and Islam, M. (2015). Phytochemical, Antimicrobial, Cytotoxic, Analgesic and Anti-inflammatory Properties of Azadirachta indica: A Therapeutic Study. J. Bioanal. Biomed. 01007, 1–7. doi:10.4172/1948-593X.S12-007
Biozid, M. S., Rahman, M. M., Alam, M. N., Sayeed, M. A., Chowdhury, A. I., Faruk, M., et al. (2015). In-vitro Comparative Study of Anti-inflammatory and Anti-arthritic Effects of Flemingia Stricta Roxb. And Nymphaea Nouchali Leaf. Int. J. Pharm. Pharm. Sci. 7 (8), 49–52. Availableat: https://innovareacademics.in/journals/index.php/ijpps/article/view/6552.
Birecka, H., DiNolfo, T. E., Martin, W. B., and Frohlich, M. W. (1984). Polyamines and Leaf Senescence in Pyrrolizidine Alkaloid-Bearing Heliotropium Plants. Phytochemistry 23 (5), 991–997. doi:10.1016/S0031-9422(00)82598-4
Birhanu, Z. (2013). Traditional Use of Medicinal Plants by the Ethnic Groups of Gondar Zuria District, north-western Ethiopia. J. Nat. Remedies 8. doi:10.18311/jnr/2013/117
Biswas, R., Rahman, S. M. M., Didarul Islam, K. M., Billah, M. M., Aunjum, A., Nurunnabi, T. R., et al. (2019). Antioxidant, Anti-inflammatory, and Anticoagulation Properties of Aegiceras corniculatum and Acanthus ilicifolius. Pbr 5 (3), 35–44. doi:10.18502/pbr.v5i3.2117
Brune, K., and Patrignani, P. (2015). New Insights into the Use of Currently Available Non-steroidal Anti-inflammatory Drugs. J. Pain Res. 8, 105–118. doi:10.2147/JPR.S75160
Chaibi, R., Romdhane, M., Ferchichi, A., and Bouajila, J. (2015). Assessment of Antioxidant, Anti-inflammatory, Anti-cholinesterase and Cytotoxic Activities of Henna (Lawsonia Inermis) Flowers. J. Nat. Prod. 8, 85–92.
Chattopadhyay, R. R. (1998). Possible Biochemical Mode of Anti-inflammatory Action of Azadirachta indica A. Juss. In Rats. Indian J. Exp. Biol. 36 (4), 418–420. Availableat: http://europepmc.org/abstract/MED/9717455.
Chaudhuri, T., Dutta, S., Chakraborty, A., Kar, P., Dey, P., and Sen, A. (2018). Stimulation of Murine Immune Response by Clerodendrum Infortunatum. Phcog Mag. 14 (57), 417–429. doi:10.4103/pm.pm_549_17
Choi, R. J., Chun, J., Khan, S., and Kim, Y. S. (2014). Desoxyrhapontigenin, a Potent Anti-inflammatory Phytochemical, Inhibits LPS-Induced Inflammatory Responses via Suppressing NF-Κb and MAPK Pathways in RAW 264.7 Cells. Int. Immunopharmacol 18 (1), 182–190. doi:10.1016/j.intimp.2013.11.022
Chokchaisiri, S., Apiratikul, N., and Rukachaisirikul, T. (2020). A New Ent-Abietane Lactone from Glycosmis Pentaphylla. Nat. Prod. Res. 34 (21), 3019–3026. doi:10.1080/14786419.2018.1540477
Choudhury, M. D., Paul, S. B., Choudhury, S., Choudhury, S., and Choudhury, P. P. N. (2009). Isolation, Characterization and Bio-Activity Screening of Compound from Clerodendrum Viscosum Vent. Assam Univ. J. Sci. Technol. Biol. Sci. 4 (1), 29–34.
Chowdhury, M., Sultana, M. I., Tasneem, M. Z., and Jubair, A. A. b. (2015). Phytochemical and Pharmacological Screening of Glycosmis Pentaphylla (Retz) A.DC. (Fam. Rutaceae). Int. J. Scientific Eng. Res. 6 (10), 928–934.
Chowdury, M., Alam, M., Chowdhury, S., Biozid, M., Faruk, M., Mazumdar, M., et al. (2015). Evaluation of Ex-Vivo Anti-arthritic, Anti-inflammatory, Anti-cancerous and Thrombolytic Activities of Mussaenda Roxburghii Leaf. Ejmp 10 (4), 1–7. doi:10.9734/EJMP/2015/20483
Chuku, L. C., Chinaka, N. C., and Damilola, D. (2020). Phytochemical Screening and Anti-inflammatory Properties of Henna Leaves (Lawsonia Inermis). Ejmp 31 (18), 23–28. doi:10.9734/EJMP/2020/v31i1830340
Chung, I. M., Rajakumar, G., Lee, J. H., Kim, S. H., and Thiruvengadam, M. (2017). Ethnopharmacological Uses, Phytochemistry, Biological Activities, and Biotechnological Applications of Eclipta Prostrata. Appl. Microbiol. Biotechnol. 101 (13), 5247–5257. doi:10.1007/s00253-017-8363-9
Chunthorng-Orn, J., Dechayont, B., Phuaklee, P., Prajuabjinda, O., Juckmeta, T., and Itharat, A. (2016). Cytotoxic, Anti-inflammatory and Antioxidant Activities of Heliotropium Indicum Extracts. J. Med. Assoc. Thai 99, S102–S109.
Çitoğlu, G., Tanker, M., and Gümüşel, B. (1998). Anti-inflammatory Effects of Lycorine and Haemanthidine. Phytotherapy Res. 12, 205–206. doi:10.1002/(sici)1099-1573(199805)12:3<205::Aid-ptr203>3.0.Co;2-7
Dar, N. J., Hamid, A., and Ahmad, M. (2015). Pharmacologic Overview of Withania Somnifera, the Indian Ginseng. Cell Mol Life Sci 72 (23), 4445–4460. doi:10.1007/s00018-015-2012-1
Dar, N. J., and MuzamilAhmad, M. (2020). Neurodegenerative Diseases and Withania Somnifera (L.): An Update. J. Ethnopharmacol 256, 112769. doi:10.1016/j.jep.2020.112769
Das, A., Jawed, J. J., Das, M. C., Sandhu, P., De, U. C., Dinda, B., et al. (2017). Antileishmanial and Immunomodulatory Activities of Lupeol, a Triterpene Compound Isolated from Sterculia Villosa. Int. J. Antimicrob. Agents 50 (4), 512–522. doi:10.1016/j.ijantimicag.2017.04.022
Das, A. K., Shahid, I. Z., Choudhuri, M. S. K., Shilpi, J., and Ahmed, F. (2005). Anti-inflammatory, Antinociceptive and Diuretic Activities of Amoora Cucullata Roxb. Oriental Pharm. Exp. Med. 5 (1), 37–42. doi:10.3742/opem.2005.5.1.037
Das, N., Goshwami, D., Hasan, M. S., Mahmud, Z. A., Raihan, S. Z., and Sultan, M. Z. (2015). Evaluation of Antinociceptive, Anti-inflammatory and Anxiolytic Activities of Methanolic Extract of Terminalia Citrina Leaves. Asian Pac. J. Trop. Dis. 5, S137–S141. doi:10.1016/S2222-1808(15)60875-1
Das, N., Hasan, M. S., Raihan, S. Z., and Sultan, M. Z. (2016). Antinociceptive, Anti-inflammatory and Hypoglycemic Activities of Terminalia Citrina Leaves. Bangla Pharma J. 19 (1), 25–31. doi:10.3329/bpj.v19i1.29232
Davies, N. M. (1995). Toxicity of Nonsteroidal Anti-inflammatory Drugs in the Large Intestine. Dis. Colon Rectum 38 (12), 1311–1321. doi:10.1007/BF02049158
de Almeida, E. R., da Silva Filho, A. A., Dos Santos, E. R., and Correia Lopes, C. A. (1990). Antiinflammatory Action of Lapachol. J. Ethnopharmacology 29 (2), 239–241. doi:10.1016/0378-8741(90)90061-w
de Cássia da Silveira E Sá, R., Andrade, L. N., dos Reis Barreto de Oliveira, R., and de Sousa, D. P. (2014). A Review on Anti-inflammatory Activity of Phenylpropanoids Found in Essential Oils. Molecules 19 (2), 1459–1480. doi:10.3390/molecules19021459
de las Heras, B., and Hortelano, S. (2009). Molecular Basis of the Anti-inflammatory Effects of Terpenoids. Inflamm. Allergy Drug Targets 8 (1), 28–39. doi:10.2174/187152809787582534
Dina, T. A., Taslima, M. A., Ahmed, N. U., and Uddin, M. N. (2010). Analgesic and Anti-inflammatory Properties of Argyreia Argentea Methanol Extract in Animal Model. J. Taibah Univ. Sci. 3, 1–7. doi:10.1016/S1658-3655(12)60014-4
do Nascimento, K. F., Moreira, F. M. F., Alencar Santos, J., Kassuya, C. A. L., Croda, J. H. R., Cardoso, C. A. L., et al. (2018). Antioxidant, Anti-inflammatory, Antiproliferative and Antimycobacterial Activities of the Essential Oil of Psidium Guineense Sw. And Spathulenol. J. Ethnopharmacol 210, 351–358. doi:10.1016/j.jep.2017.08.030
Dulla, O., and Jahan, F. I. (2017). Ethnopharmacological Survey on Traditional Medicinal Plants at Kalaroa Upazila, Satkhira District, Khulna Division, Bangladesh. J. Intercult Ethnopharmacol 6 (3), 316–325. doi:10.5455/jice.20170719010256
Dzoyem, J. P., McGaw, L. J., Kuete, V., and Bakowsky, U. (2017). “Anti-inflammatory and Anti-nociceptive Activities of African Medicinal Spices and Vegetables,” in Medicinal Spices and Vegetables from Africa. Editor V. Kuete (Academic Press), 239–270. doi:10.1016/b978-0-12-809286-6.00009-1
Emran, T., E-Mowla, T., Ahmed, S., Zahan, S., Rakib, A., Hasan, M., et al. (2018). Sedative, Anxiolytic, Antinociceptive, Anti-inflammatory and Antipyretic Effects of a Chloroform Extract from the Leaves of Urena Sinuata in Rodents. Jalsi 16 (3), 1–19. doi:10.9734/JALSI/2018/39073
Ericson-Neilsen, W., and Kaye, A. D. (2014). Steroids: Pharmacology, Complications, and Practice Delivery Issues. Ochsner J. 14 (2), 203–207. Availableat: http://www.ochsnerjournal.org/content/ochjnl/14/2/203.full.pdf.
Everts, B., Währborg, P., and Hedner, T. (2000). COX-2-Specific Inhibitors-Tthe Emergence of a New Class of Analgesic and Anti-inflammatory Drugs. Clin. Rheumatol. 19 (5), 331–343. doi:10.1007/s100670070024
Fang, S. C., Hsu, C. L., and Yen, G. C. (2008). Anti-inflammatory Effects of Phenolic Compounds Isolated from the Fruits of Artocarpus Heterophyllus. J. Agric. Food Chem. 56 (12), 4463–4468. doi:10.1021/jf800444g
Faqueti, L. G., Brieudes, V., Halabalaki, M., Skaltsounis, A. L., Nascimento, L. F., Barros, W. M., et al. (2016). Antinociceptive and Anti-inflammatory Activities of Standardized Extract of Polymethoxyflavones from Ageratum Conyzoides. J. Ethnopharmacol 194, 369–377. doi:10.1016/j.jep.2016.09.025
Faruq, A. A., Ibrahim, M., Mahmood, A., Chowdhury, M. M. U., Rashid, R. B., Kuddus, M. R., et al. (2014). Pharmacological and Phytochemical Screenings of Ethanol Extract of Leea Macrophylla Roxb. Innov. Pharmaceuticals Pharmacother. 2 (1), 321–327.
Feng, L., Zhai, Y. Y., Xu, J., Yao, W. F., Cao, Y. D., Cheng, F. F., et al. (2019). A Review on Traditional Uses, Phytochemistry and Pharmacology of Eclipta Prostrata (L.) L. J. Ethnopharmacol 245, 112109. doi:10.1016/j.jep.2019.112109
Fernández, M. A., de las Heras, B., Garcia, M. D., Sáenz, M. T., and Villar, A. (2010). New Insights into the Mechanism of Action of the Anti-inflammatory Triterpene Lupeol. J. Pharm. Pharmacol. 53 (11), 1533–1539. doi:10.1211/0022357011777909
Ferrari, F. C., Lemos Lima, Rde. C., Schimith Ferraz Filha, Z., Barros, C. H., de Paula Michel Araújo, M. C., and Antunes Saúde-Guimarães, D. (2016). Effects of Pimenta Pseudocaryophyllus Extracts on Gout: Anti-inflammatory Activity and Anti-hyperuricemic Effect through Xantine Oxidase and Uricosuric Action. J. Ethnopharmacol 180, 37–42. doi:10.1016/j.jep.2016.01.007
Fürst, R., Zündorf, I., and Dingermann, T. (2017). New Knowledge about Old Drugs: The Anti-inflammatory Properties of Cardiac Glycosides. Planta Med. 83 (12-13), 977–984. doi:10.1055/s-0043-105390
Fürst, R., and Zündorf, I. (2014). Plant-derived Anti-inflammatory Compounds: Hopes and Disappointments Regarding the Translation of Preclinical Knowledge into Clinical Progress. Mediators Inflamm. 2014, 1–9. doi:10.1155/2014/146832
Ganguly, A., Mahmud, Z. A., Uddin, M. M. N., and Rahman, S. A. (2013). In-vivo Anti-inflammatory and Anti-pyretic Activities of Manilkara Zapota Leaves in Albino Wistar Rats. Asian Pac. J. Trop. Dis. 3 (4), 301–307. doi:10.1016/S2222-1808(13)60073-0
García-Mediavilla, V., Crespo, I., Collado, P. S., Esteller, A., Sánchez-Campos, S., Tuñón, M. J., et al. (2007). The Anti-inflammatory Flavones Quercetin and Kaempferol Cause Inhibition of Inducible Nitric Oxide Synthase, Cyclooxygenase-2 and Reactive C-Protein, and Down-Regulation of the Nuclear Factor kappaB Pathway in Chang Liver Cells. Eur. J. Pharmacol. 557 (2), 221–229. doi:10.1016/j.ejphar.2006.11.014
Ghani, A. (2003). Medicinal Plants of Bangladesh with Chemical Constituents and Uses. Dhaka, Bangladesh: The Asiatic Society of Bangladesh.
Ghosh, G., Panda, P., Rath, M., Pal, A., Sharma, T., and Das, D. (2015). GC-MS Analysis of Bioactive Compounds in the Methanol Extract of Clerodendrum Viscosum Leaves. Pharmacognosy Res. 7 (1), 110–113. doi:10.4103/0974-8490.147223
Giri, K. R. (2016). Comparative Study of Anti-inflammatory Activity of Withania Somnifera (Ashwagandha) with Hydrocortisone in Experimental Animals (Albino Rats). J. Med. Plants Stud. 4 (1), 78–83.
Glass, C. K., Saijo, K., Winner, B., Marchetto, M. C., and Gage, F. H. (2010). Mechanisms Underlying Inflammation in Neurodegeneration. Cell 140 (6), 918–934. doi:10.1016/j.cell.2010.02.016
Gomes, A., Fernandes, E., Lima, J. L., Mira, L., and Corvo, M. L. (2008). Molecular Mechanisms of Anti-inflammatory Activity Mediated by Flavonoids. Curr. Med. Chem. 15 (16), 1586–1605. doi:10.2174/092986708784911579
González-Gallego, J., Sánchez-Campos, S., and Tuñón, M. J. (2007). Anti-inflammatory Properties of Dietary Flavonoids. Nutrición Hospitalaria 22 (3), 287–293.
Gou, K. J., Zeng, R., Dong, Y., Hu, Q. Q., Hu, H. W., Maffucci, K. G., et al. (2017). Anti-inflammatory and Analgesic Effects of Polygonum Orientale L. Extracts. Front. Pharmacol. 8 (562), 562. doi:10.3389/fphar.2017.00562
Guardia, T., Rotelli, A. E., Juarez, A. O., and Pelzer, L. E. (2001). Anti-inflammatory Properties of Plant Flavonoids. Effects of Rutin, Quercetin and Hesperidin on Adjuvant Arthritis in Rat. Farmaco 56 (9), 683–687. doi:10.1016/S0014-827X(01)01111-9
Guha, G., Rajkumar, V., Ashok Kumar, R., and Mathew, L. (2011). Therapeutic Potential of Polar and Non-polar Extracts of Cyanthillium cinereum In Vitro. Evid. Based Complement. Alternat Med. 2011, 784826. doi:10.1093/ecam/nep155
Gupta, M., and Kaur, G. (2016). Aqueous Extract from the Withania Somnifera Leaves as a Potential Anti-neuroinflammatory Agent: a Mechanistic Study. J. Neuroinflammation 13 (1), 193. doi:10.1186/s12974-016-0650-3
Gupta, M., and Kaur, G. (2018). Withania Somnifera as a Potential Anxiolytic and Anti-inflammatory Candidate against Systemic Lipopolysaccharide-Induced Neuroinflammation. Neuromolecular Med. 20 (3), 343–362. doi:10.1007/s12017-018-8497-7
Gupta, R., and Singh, H. (2012). Detection and Quantitation of SS-Sitosterol in Clerodendrum Infortunatum and Alternanthera Sessilis by HPTLC. Phcog Commn. 2 (1), 31–36. doi:10.5530/pc.2012.1.6
Gupta, S., and Gupta, R. (2012). Detection and Quantification of Quercetin in Roots, Leaves and Flowers of Clerodendrum Infortunatum L. Asian Pac. J. Trop. Dis. 2, S940–S943. doi:10.1016/S2222-1808(12)60296-5
Haldar, K. M., Ghosh, P., and Chandra, G. (2011). Evaluation of Target Specific Larvicidal Activity of the Leaf Extract of Typhonium Trilobatum against Culex quinquefasciatus Say. Asian Pac. J. Trop. Biomed. 1 (2Suppl. ment), S199–S203. doi:10.1016/S2221-1691(11)60156-1
Hämäläinen, M., Nieminen, R., Vuorela, P., Heinonen, M., and Moilanen, E. (2007). Anti-inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-kappaB Activations, whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit Only NF-kappaB Activation along with Their Inhibitory Effect on iNOS Expression and NO Production in Activated Macrophages. Mediators Inflamm. 2007, 45673. doi:10.1155/2007/45673
Hamidi, М. R., Jovanova, B., and Panovska, Т. K. (2014). Toxicоlogical Evaluation of the Plant Products Using Brine Shrimp (Artemia salina L.) Model. Macedonian Pharm. Bull. 60 (1), 9–18. doi:10.33320/maced.pharm.bull.2014.60.01.002
Hamiduzzaman, M., Nur, F., and Bhuiyan, M. I. (2017). Evaluation of Anti-inflammatory Activity through Assaying the Stability of Erythrocytes' Membrane by Different Organic Extracts of Heliotropium Indicum (Boraginacea). J. Nat. Product. Plant Resour. 7 (2), 74–78.
Haque, M. A., Louis, V. R., Phalkey, R., and Sauerborn, R. (2014). Use of Traditional Medicines to Cope with Climate-Sensitive Diseases in a Resource Poor Setting in Bangladesh. BMC Public Health 14 (1), 202. doi:10.1186/1471-2458-14-202
Haque, M. I., Chowdhury, A. B. M. A., Shahjahan, M., and Harun, M. G. D. (2018). Traditional Healing Practices in Rural Bangladesh: a Qualitative Investigation. BMC Complement. Altern. Med. 18 (1), 62. doi:10.1186/s12906-018-2129-5
Harvey, A. (2000). Strategies for Discovering Drugs from Previously Unexplored Natural Products. Drug Discov. Today 5 (7), 294–300. doi:10.1016/S1359-6446(00)01511-7
Hasan, M. M., Uddin, N., Hasan, M. R., Islam, A. F., Hossain, M. M., Rahman, A. B., et al. (2014). Analgesic and Anti-inflammatory Activities of Leaf Extract of Mallotus Repandus (Willd.) Muell. Arg. Biomed. Res. Int. 2014, 539807. doi:10.1155/2014/539807
Hasan, M. R., Uddin, N., Sana, T., Hossain, M. M., Mondal, M., Kanta, I. J., et al. (2018). Analgesic and Anti-inflammatory Activities of Methanolic Extract of Mallotus Repandus Stem in Animal Models. Orient Pharm. Exp. Med. 18 (2), 139–147. doi:10.1007/s13596-018-0312-3
Hassan, M., Khan, S., Shaikat, A., Hossain, M., Islam, S., Hoque, M., et al. (2013). Analgesic and Anti-inflammatory Effects of Ethanol Extracted Leaves of Selected Medicinal Plants in Animal Model. Vet. World 6 (2), 68–71. doi:10.5455/vetworld.2013.68-71
Hassan, M. M., Daula, A. F. M. S., Jahan, I. A., Nimmi, I., Adnan, M., and Hossain, H. (2012). Anti-inflammatory Activity, Total Flavonoids and Tannin Content from the Ethanolic Extract of Ageratum Conyzoides Linn. Leaf. Int. J. Pharm. Phytopharmacological Res. 1 (5), 234–241.
He, J. B., Fang, M. J., Ma, X. Y., Li, W. J., and Lin, D. S. (2020). Angiogenic and Anti-inflammatory Properties of Azadirachtin A Improve Random Skin Flap Survival in Rats. Exp. Biol. Med. (Maywood) 245 (18), 1672–1682. doi:10.1177/1535370220951896
Helen, L. R., Jayesh, K., Vysakh, A., and Latha, M. (2018). Polyphenolic Content Correlates with Anti-inflammatory Activity of Root Bark Extract from Clerodendrum Infortunatum L. And Inhibit Carrageenan Induced Paw Edema. J. Pharmacognosy Phytochemistry 7 (2), 2032–2014.
Hira, A., Dey, S. K., Howlader, M. S., Ahmed, A., Hossain, H., and Jahan, I. A. (2013). Anti-inflammatory and Antioxidant Activities of Ethanolic Extract of Aerial Parts of Vernonia Patula (Dryand.) Merr. Asian Pac. J. Trop. Biomed. 3 (10), 798–805. doi:10.1016/S2221-1691(13)60158-6
Hodgson, E. (2004). A Textbook of Modern Toxicology. Hoboken, New Jersey, United States of America: John Wiley & Sons.
Hong, J., Bose, M., Ju, J., Ryu, J. H., Chen, X., Sang, S., et al. (2004). Modulation of Arachidonic Acid Metabolism by Curcumin and Related Beta-Diketone Derivatives: Effects on Cytosolic Phospholipase A(2), Cyclooxygenases and 5-lipoxygenase. Carcinogenesis 25 (9), 1671–1679. doi:10.1093/carcin/bgh165
Hoque, T., Sikder, M. A. A., Kaisar, M. A., Chowdhury, A. A., and Rashid, M. A. (2011). Biological Screenings of Two Araceous Plants Growing in Bangladesh. Dhaka Univ. J. Pharm. Sci. 10 (2), 131–135. doi:10.3329/dujps.v10i2.11794
Hossain, H., Akbar, P. N., Rahman, S. E., Khan, T. A., Rahman, M. M., and Jahan, I. A. (2014a). In Vivo anti-inflammatory and In Vitro Antioxidant Activities of Toona Ciliata Leaves Native to Bangladesh. Glob. J. Med. Res. (B) 14 (7), 17–26.
Hossain, M. F., Talukder, B., Rana, M. N., Tasnim, R., Nipun, T. S., Uddin, S. M., et al. (2016). In Vivo sedative Activity of Methanolic Extract of Stericulia Villosa Roxb. Leaves. BMC Complement. Altern. Med. 16 (1), 398. doi:10.1186/s12906-016-1374-8
Hossain, M. H., Jahan, F., JahanHowlader, M. S. I. H., Dey, S. K., Hira, A. H., Ahmed, A. A., et al. (2012a). Evaluation of Anti-inflammatory Activity and Total Flavonoids Content of Manilkara Zapota (Linn.) Bark. Int. J. Pharm. Phytopharmacological Res. 2 (1), 35–39. Availableat: https://eijppr.com/uP6NLQj.
Hossain, M. K., Prodhan, M. A., Even, A. S. M. I. H., Morshed, H., and Hossain, M. M. (2012b). Anti-inflammatory and Antidiabetic Activity of Ethanolic Extracts of Sterculia Villosa Barks on Albino Wistar Rats. J. App. Pharm. Sci. 2 (8), 96–100. doi:10.7324/JAPS.2012.2815
Hossain, M. M., Ahamed, S. K., Dewan, S. M., Hassan, M. M., Istiaq, A., Islam, M. S., et al. (2014b). In Vivo antipyretic, Antiemetic, In Vitro Membrane Stabilization, Antimicrobial, and Cytotoxic Activities of Different Extracts from Spilanthes Paniculata Leaves. Biol. Res. 47, 45. doi:10.1186/0717-6287-47-45
Hossain, M. M., Kabir, M. S. H., Hasanat, A., Kabir, M. I., Chowdhury, T. A., and Kibria, A. S. M. G. (2015). Investigation of In Vitro Anti-arthritic and Membrane Stabilizing Activity of Ethanol Extracts of Three Bangladeshi Plants. Pharma Innovation J. 4 (1), 76–80.
Hossain, M. S., Alam, M. B., Chowdhury, N. S., Asadujjama, M., Zahan, R., Islam, M. M., et al. (2011). Antioxidant, Analgesic and Anti-inflammatory Activities of the Herb Eclipta Prostrata. J. Pharmacol. Toxicol. 6 (5), 468–480. doi:10.3923/jpt.2011.468.480
Hossain, S. M., Islam, F., Sharmin, T., Sheikh, H., Hasan, A. R., and Rashid, M. A. (2012c). In Vitro antioxidant, Membrane Stabilizing and Thrombolytic Activities of Glycosmis Arborea. Bangla Pharma J. 15 (2), 141–143. doi:10.3329/bpj.v15i2.12579
Huang, Y. L., Kou, J. P., Ma, L., Song, J. X., and Yu, B. Y. (2008). Possible Mechanism of the Anti-inflammatory Activity of Ruscogenin: Role of Intercellular Adhesion Molecule-1 and Nuclear Factor-kappaB. J. Pharmacol. Sci. 108 (2), 198–205. doi:10.1254/jphs.08083fp
Humaish, H. H. (2017). Study Comparison Analgesic, Antipyretic and Anti-inflammatory Activity of Aqueous and Alcoholic Leaves Extract of Lawsonia Inermis L. (Henna) with Ketoprofen in Male Albino Rats. Kufa J. Vet. Med. Sci. 8 (2), 88–100.
Ilango, K., Maharajan, G., and Narasimhan, S. (2013). Anti-nociceptive and Anti-inflammatory Activities of Azadirachta indica Fruit Skin Extract and its Isolated Constituent Azadiradione. Nat. Prod. Res. 27 (16), 1463–1467. doi:10.1080/14786419.2012.717288
Imam, H., Mahbub, N. U., Khan, M. F., Hana, H. K., and Sarker, M. M. (2013). Alpha Amylase Enzyme Inhibitory and Anti-inflammatory Effect of Lawsonia Inermis. Pak J. Biol. Sci. 16 (23), 1796–1800. doi:10.3923/pjbs.2013.1796.1800
Islam, M. E., Islam, K. M. D., Billah, M. M., Biswas, R., Sohrab, M. H., and Rahman, S. M. M. (2020a). Antioxidant and Anti-inflammatory Activity of Heritiera Fomes (Buch.-Ham), a Mangrove Plant of the Sundarbans. Adv. Tradit Med. (Adtm) 20 (2), 189–197. doi:10.1007/s13596-019-00401-0
Islam, M., Mannan, M., Kabir, M., Islam, A., and Olival, K. (2010). Analgesic, Anti-inflammatory and Antimicrobial Effects of Ethanol Extracts of Mango Leaves. J. Bangladesh Agric. Univ. 8 (2), 239–244. doi:10.3329/jbau.v8i2.7932
Islam, M. T., Hasan, J., Snigdha, H. M. S. H., Ali, E. S., Sharifi-Rad, J., Martorell, M., et al. (2020b). Chemical Profile, Traditional Uses, and Biological Activities of Piper Chaba Hunter: A Review. J. Ethnopharmacol 257, 112853. doi:10.1016/j.jep.2020.112853
Islas, J. F., Acosta, E., G-Buentello, Z., Delgado-Gallegos, J. L., Moreno-Treviño, M. G., Escalante, B., et al. (2020). An Overview of Neem (Azadirachta indica) and its Potential Impact on Health. J. Funct. Foods 74, 104171. doi:10.1016/j.jff.2020.104171
Issa, A. Y., Volate, S. R., and Wargovich, M. J. (2006). The Role of Phytochemicals in Inhibition of Cancer and Inflammation: New Directions and Perspectives. J. Food Compost. Anal. 19 (5), 405–419. doi:10.1016/j.jfca.2006.02.009
Ivanovska, N., and Philipov, S. (1996). Study on the Anti-inflammatory Action of Berberis Vulgaris Root Extract, Alkaloid Fractions and Pure Alkaloids. Int. J. Immunopharmacol 18 (10), 553–561. doi:10.1016/S0192-0561(96)00047-1
Jackson, L. M., and Hawkey, C. J. (2000). COX-2 Selective Nonsteroidal Anti-inflammatory Drugs: Do They Really Offer Any Advantages? Drugs 59 (6), 1207–1216. doi:10.2165/00003495-200059060-00001
Jahan, F. I., Sultana, S., Brishti, S. A., and Dulla, O. (2019). Ethnopharmacological Survey on Traditional Medicinal Plants at Keraniganj, Dhaka, Bangladesh. Orient Pharm. Exp. Med. 19 (3), 331–339. doi:10.1007/s13596-019-00362-4
Jiang, Y. Q., and Liu, E. H. (2019). Microcos Paniculata: A Review on its Botany, Traditional Uses, Phytochemistry and Pharmacology. Chin. J. Nat. Med. 17 (8), 561–574. doi:10.1016/S1875-5364(19)30058-5
John, N. A. A., and Shobana, G. (2012). Anti-inflammatory Activity of Talinum Fruticosum L. On Formalin Induced Paw Edema in Albino Rats. J. Appl. Pharm. Sci. 2 (1), 123–127.
Jones, C. K., Peters, S. C., and Shannon, H. E. (2005). Efficacy of Duloxetine, a Potent and Balanced Serotonergic and Noradrenergic Reuptake Inhibitor, in Inflammatory and Acute Pain Models in Rodents. J. Pharmacol. Exp. Ther. 312 (2), 726–732. doi:10.1124/jpet.104.075960
Joshi, R. K. (2020). GC-MS Analysis of Volatile Organic Constituents of Traditionally Used Medicinal Plants from the Western Ghats of India: Blumea Lanceolaria (Roxb.) Druce., Heliotropium Indicum L. And Triumfetta Rhomboidea Jacq. J. Mex. Chem. Soc. 64, 74–82. doi:10.29356/jmcs.v64i2.1093
Jothy, S. L., Zakaria, Z., Chen, Y., Lau, Y. L., Latha, L. Y., and Sasidharan, S. (2011). Acute Oral Toxicity of Methanolic Seed Extract of Cassia Fistula in Mice. Molecules 16 (6), 5268–5282. doi:10.3390/molecules16065268
Just, M. J., Recio, M. C., Giner, R. M., Cuéllar, M. J., Máñez, S., Bilia, A. R., et al. (1998). Anti-inflammatory Activity of Unusual Lupane Saponins from Bupleurum Fruticescens. Planta Med. 64 (5), 404–407. doi:10.1055/s-2006-957469
Kader, M. A., Parvin, S., Chowduri, M. A. U., and Haque, M. E. (2014). Antibacterial, Antifungal and Insecticidal Activities of Ruellia Tuberosa (L.) Root Extract. J. Bio-sci. 20 (0), 91–97. doi:10.3329/jbs.v20i0.17720
Kamalakararao, K., Gopalakrishnan, V. K., Hagos, Z., Rao, G. D., Padal, S. B., and Chaithanya, K. K. (2018a). Anti-inflammatory Activity of Bioactive Flavonoid Apigenin-7-O-β-D-Glucuronide Methyl Ester from Ethyl Acetate Leaf Extract of Manilkara Zapota on Lipopolysaccharide-Induced Pro-inflammatory Mediators Nitric Oxide (NO), Prostaglandin E2 (PGE2) in Raw 264.7 Cells. Drug Invention Today 10 (4), 531–535.
Kamalakararao, K., Rao, D., Muthulingam, M., Gopalakrishnan, V. K., Hagos, Z., Palleti, J., et al. (2018b). Effect of Isolated Bioactive Flavonoid Apigenin-7-O-β- D-Glucuronide Methyl Ester on Cyclooxygenase-2 Gene Expression in the Breast Cancer MCF-7 Cell Lines. Drug Invention Today 10 (4), 3552–3555.
Kannadas, S., Pathirana, R., and Ratnasooriya, W. (2020). In-vitro Investigation of Anti-inflammatory Activity and Evaluation of Phytochemical Profile of Eclipta Prostrata (L.). J. Pharmacognosy Phytochemistry 9 (2), 831–835.
Kashyap, D., Sharma, A., Tuli, H. S., Punia, S., and Sharma, A. K. (2016). Ursolic Acid and Oleanolic Acid: Pentacyclic Terpenoids with Promising Anti-inflammatory Activities. Recent Pat Inflamm. Allergy Drug Discov. 10 (1), 21–33. doi:10.2174/1872213x10666160711143904
Kassim, M., Achoui, M., Mustafa, M. R., Mohd, M. A., and Yusoff, K. M. (2010). Ellagic Acid, Phenolic Acids, and Flavonoids in Malaysian Honey Extracts Demonstrate In Vitro Anti-inflammatory Activity. Nutr. Res. 30 (9), 650–659. doi:10.1016/j.nutres.2010.08.008
Kaur, G., Sarwar Alam, M., and Athar, M. (2004). Nimbidin Suppresses Functions of Macrophages and Neutrophils: Relevance to its Antiinflammatory Mechanismsflammatory Mechanisms. Phytother Res. 18 (5), 419–424. doi:10.1002/ptr.1474
Kawsar, M. H., Sikder, M. A. A., Rana, M. S., Nimmi, I., and Rashid, M. A. (2011). Studies of Thrombolytic, Antioxidant and Cytotoxic Properties of Two Asteraceous Plants of Bangladesh. Bangladesh Pharm. J. 14 (2), 103–106.
Kazłowska, K., Hsu, T., Hou, C. C., Yang, W. C., and Tsai, G. J. (2010). Anti-inflammatory Properties of Phenolic Compounds and Crude Extract from Porphyra Dentata. J. Ethnopharmacol 128 (1), 123–130. doi:10.1016/j.jep.2009.12.037
Khan, M. A., Ahmed, R. S., Chandra, N., Arora, V. K., and Ali, A. (2019). In Vivo, Extract from Withania Somnifera Root Ameliorates Arthritis via Regulation of Key Immune Mediators of Inflammation in Experimental Model of Arthritis. Antiinflamm Antiallergy Agents Med. Chem. 18 (1), 55–70. doi:10.2174/1871523017666181116092934
Khan, M. S. S., Syeed, S. H., Uddin, M. H., Akter, L., Ullah, M. A., Jahan, S., et al. (2013). Screening and Evaluation of Antioxidant, Antimicrobial, Cytotoxic, Thrombolytic and Membrane Stabilizing Properties of the Methanolic Extract and Solvent-Solvent Partitioning Effect of Vitex Negundo Bark. Asian Pac. J. Trop. Dis. 3 (5), 393–400. doi:10.1016/S2222-1808(13)60090-0
Khatry, N., Kundu, J., Bachar, S. C., Uddin, M. N., and Kundu, J. K. (2005). Studies on Antinociceptive, Antiinflammatory and Diuretic Activities of Methanol Extract of the Aerial Parts of Clerodendron Viscosum Vent. Dhaka Univ. J. Pharm. Sci. 5 (1-2), 63–66. doi:10.3329/dujps.v5i1.231
Kim, D. S., Lee, H. J., Jeon, Y. D., Han, Y. H., Kee, J. Y., Kim, H. J., et al. (2015a). Alpha-Pinene Exhibits Anti-inflammatory Activity through the Suppression of MAPKs and the NF-Κb Pathway in Mouse Peritoneal Macrophages. Am. J. Chin. Med. 43 (04), 731–742. doi:10.1142/s0192415x15500457
Kim, G. Y., Kim, K. H., Lee, S. H., Yoon, M. S., Lee, H. J., Moon, D. O., et al. (2005). Curcumin Inhibits Immunostimulatory Function of Dendritic Cells: MAPKs and Translocation of NF-Kappa B as Potential Targets. J. Immunol. 174 (12), 8116–8124. doi:10.4049/jimmunol.174.12.8116
Kim, H. D., Cho, H. R., Moon, S. B., Shin, H. D., Yang, K. J., Jang, H. J., et al. (2006). Effect of Exopolymers from Aureobasidium Pullulans on Formalin-Induced Chronic Paw Inflammation in Mice. J. Microbiol. Biotechnol. 12 (16), 1954–1060.
Kim, H. P., Son, K. H., Chang, H. W., and Kang, S. S. (2004). Anti-inflammatory Plant Flavonoids and Cellular Action Mechanisms. J. Pharmacol. Sci. 96 (3), 229–245. doi:10.1254/jphs.crj04003x
Kim, S. H., Park, J. G., Lee, J., Yang, W. S., Park, G. W., Kim, H. G., et al. (2015b). The Dietary Flavonoid Kaempferol Mediates Anti-inflammatory Responses via the Src, Syk, IRAK1, and IRAK4 Molecular Targets. Mediators Inflamm. 2015, 904142. doi:10.1155/2015/904142
Kim, Y. S., Young, M. R., Bobe, G., Colburn, N. H., and Milner, J. A. (2009). Bioactive Food Components, Inflammatory Targets, and Cancer Prevention. Cancer Prev. Res. (Phila) 2 (3), 200–208. doi:10.1158/1940-6207.Capr-08-0141
Konuku, K., Karri, K. C., Gopalakrishnan, V. K., Hagos, Z., Kebede, H., Naidu, T. K., et al. (2017). Anti-inflammatory Activity of Manilkara Zapota Leaf Extract. Int. J. Curr. Pharm. Sci. 9 (4), 130–134. doi:10.22159/ijcpr.2017v9i4.20977
Kotta, J. C., Lestari, A. B. S., Candrasari, D. S., and Hariono, M. (2020). Medicinal Effect, In Silico Bioactivity Prediction, and Pharmaceutical Formulation of Ageratum Conyzoides L.: A Review. Scientifica (Cairo) 2020, 6420909. doi:10.1155/2020/6420909
Kumar, A. D. N., Bevara, G. B., Laxmikoteswramma, K., and Malla, R. R. (2013). Antioxidant, Cytoprotective and Anti-inflammatory Activities of Stem Bark Extract of Semecarpus Anacardium. Asian J. Pharm. Clin. Res. 6, 213–219.
Kundu, J. K., Shin, Y. K., and Surh, Y. J. (2006). Resveratrol Modulates Phorbol Ester-Induced Pro-inflammatory Signal Transduction Pathways in Mouse Skin In Vivo: NF-kappaB and AP-1 as Prime Targets. Biochem. Pharmacol. 72 (11), 1506–1515. doi:10.1016/j.bcp.2006.08.005
Kuropakornpong, P., Itharat, A., Panthong, S., Sireeratawong, S., and Ooraikul, B. (2020). In Vitro and In Vivo Anti-inflammatory Activities of Benjakul: A Potential Medicinal Product from Thai Traditional Medicine. Evid. Based Complement. Alternat Med. 2020, 9760948. doi:10.1155/2020/9760948
Kyei, S., Koffuor, G. A., Ramkissoon, P., Ameyaw, E. O., and Asiamah, E. A. (2016). Anti-inflammatory Effect of Heliotropium Indicum Linn on Lipopolysaccharide-Induced Uveitis in New Zealand white Rabbits. Int. J. Ophthalmol. 9 (4), 528–535. doi:10.18240/ijo.2016.04.08
Laboni, F. R., Sultana, T., Kamal, S., Karim, S., Das, S., Harun-Or-Rashid, M., et al. (2017). Biological Investigations of the Ethanol Extract of the Aerial Part (Leaf) of Coccinia Grandis L. J. Pharmacognosy Phytochemistry 6 (2), 134–138.
Le, D. D., Nguyen, D. H., Ma, E. S., Lee, J. H., Min, B. S., Choi, J. S., et al. (2021). PTP1B Inhibitory and Anti-inflammatory Properties of Constituents from Eclipta Prostrata L. Biol. Pharm. Bull. 44, 298–304. doi:10.1248/bpb.b20-00994
Lee, H. (2017). Enhancement of Skin Anti-inflammatory Activities of Eclipta Prostrata L. From the Ultrasonic Extraction Process. Appl. Sci. 7 (12), 1227. doi:10.3390/app7121227
Leelarungrayub, J., Sriboonreung, T., Pothasak, Y., Kaju, J., and Puntumetakul, R. (2019). Anti-oxidant and Anti-inflammatory Activities of Manilkara Zapota (Sapodilla) In Vitro and Efficiency in Healthy Elderly Persons. Biomed. J. Scientific Tech. Res. 15 (2), 11294–11308. doi:10.26717/bjstr.2019.15.002684
Lesjak, M., Beara, I., Simin, N., Pintać, D., Majkić, T., Bekvalac, K., et al. (2018). Antioxidant and Anti-inflammatory Activities of Quercetin and its Derivatives. J. Funct. Foods 40, 68–75. doi:10.1016/j.jff.2017.10.047
Li, K., He, Z., Wang, X., Pineda, M., Chen, R., Liu, H., et al. (2018). Apigenin C-Glycosides of Microcos Paniculata Protects Lipopolysaccharide Induced Apoptosis and Inflammation in Acute Lung Injury through TLR4 Signaling Pathway. Free Radic. Biol. Med. 124, 163–175. doi:10.1016/j.freeradbiomed.2018.06.009
Liu, Y. P., Yan, G., Guo, J. M., Liu, Y. Y., Li, Y. J., Zhao, Y. Y., et al. (2019). Prenylated Coumarins from the Fruits of Manilkara Zapota with Potential Anti-inflammatory Effects and Anti-HIV Activities. J. Agric. Food Chem. 67 (43), 11942–11947. doi:10.1021/acs.jafc.9b04326
López-Otín, C., and Overall, C. M. (2002). Protease Degradomics: A New challenge for Proteomics. Nat. Rev. Mol. Cel Biol. 3 (7), 509–519. doi:10.1038/nrm858
Lu, H. M., Liang, Y. Z., Yi, L. Z., and Wu, X. J. (2006). Anti-inflammatory Effect of Houttuynia Cordata Injection. J. Ethnopharmacol 104 (1), 245–249. doi:10.1016/j.jep.2005.09.012
Lucas, L., Russell, A., and Keast, R. (2011). Molecular Mechanisms of Inflammation. Anti-inflammatory Benefits of virgin Olive Oil and the Phenolic Compound Oleocanthal. Curr. Pharm. Des. 17 (8), 754–768. doi:10.2174/138161211795428911
Luo, H., Lv, X. D., Wang, G. E., Li, Y. F., Kurihara, H., and He, R. R. (2014). Anti-inflammatory Effects of Anthocyanins-Rich Extract from Bilberry (Vaccinium Myrtillus L.) on croton Oil-Induced Ear Edema and Propionibacterium Acnes Plus LPS-Induced Liver Damage in Mice. Int. J. Food Sci. Nutr. 65 (5), 594–601. doi:10.3109/09637486.2014.886184
Luster, A. D., Alon, R., and von Andrian, U. H. (2005). Immune Cell Migration in Inflammation: Present and Future Therapeutic Targets. Nat. Immunol. 6 (12), 1182–1190. doi:10.1038/ni1275
Machan, T., Korth, J., Liawruangrath, B., Liawruangrath, S., and Pyne, S. G. (2006). Composition and Antituberculosis Activity of the Volatile Oil ofHeliotropium Indicum Linn. Growing in Phitsanulok, Thailand. Flavour Fragr. J. 21 (2), 265–267. doi:10.1002/ffj.1577
Mahabub-Uz-Zaman, M., Ahmed, N. U., Akter, R., Ahmed, K., Aziz, M. S. I., and Ahmed, M. S. (2009). Studies on Anti-inflammatory, Antinociceptive and Antipyretic Activities of Ethanol Extract of Azadirachta indica Leaves. Bangladesh J. Scientific Ind. Res. 44 (2), 199–206.
Manivannan, R., Aeganathan, R., Aeganathan, R., and Prabakaran, K. (2015). Anti-microbial and Anti-inflammatory Flavonoid Constituents from the Leaves of Lawsonia Inermis. J. Phytopharmacol 4 (4), 212–216. doi:10.31254/phyto.2015.4404
Manna, K., Debnath, B., Das, M., and Marwein, S. (2016). A Comprehensive Review on Pharmacognostical Investigation and Pharmacology of Typhonium Trilobatum. Npj 6 (3), 172–178. doi:10.2174/2210315506666160810145157
Martin, D., Valdez, J., Boren, J., and Mayersohn, M. (2004). Dermal Absorption of Camphor, Menthol, and Methyl Salicylate in Humans. J. Clin. Pharmacol. 44 (10), 1151–1157. doi:10.1177/0091270004268409
Md, A. R., S M Azad, H., Nazim, U. A., and Md, S. I. (2013). Analgesic and Anti-inflammatory Effects of Crinum asiaticum Leaf Alcoholic Extract in Animal Models. Afr. J. Biotechnol. 12 (2), 212–218. doi:10.5897/AJB12.1431
Md. Atiar Rahman, M. A., Akter, N., Rashid, H., Ahmed, N. U., and Islam, M. S. (2012). Analgesic and Anti-inflammatory Effect of Whole Ageratum Conyzoides and Emilia Sonchifolia Alcoholic Extracts in Animal Models. Afr. J. Pharm. Pharmacol. 6 (20), 1469–1476. doi:10.5897/AJPP12.083
Medzhitov, R. (2010). Inflammation 2010: New Adventures of an Old Flame. Cell 140 (6), 771–776. doi:10.1016/j.cell.2010.03.006
Medzhitov, R. (2008). Origin and Physiological Roles of Inflammation. Nature 454 (7203), 428–435. doi:10.1038/nature07201
Miguel, M. G. (2010). Antioxidant and Anti-inflammatory Activities of Essential Oils: a Short Review. Molecules 15 (12), 9252–9287. doi:10.3390/molecules15129252
Miles, E. A., Zoubouli, P., and Calder, P. C. (2005). Differential Anti-inflammatory Effects of Phenolic Compounds from Extra virgin Olive Oil Identified in Human Whole Blood Cultures. Nutrition 21 (3), 389–394. doi:10.1016/j.nut.2004.06.031
Mollik, M. A. H., Hossan, M. S., Paul, A. K., Taufiq-Ur-Rahman, M., Jahan, R., and Rahmatullah, M. (2010). A Comparative Analysis of Medicinal Plants Used by Folk Medicinal Healers in Three Districts of Bangladesh and Inquiry as to Mode of Selection of Medicinal Plants. Ethnobot. Res. App. 8, 195–218. doi:10.17348/era.8.0.195-218
Morshed, M. A., Uddin, M., Hasan, T., Ahmed, T., Uddin, F., Zakaria, M., et al. (2011). Evaluation of Analgesic and Anti-inflammatory Effect of Terminalia Arjuna Ethanol Extract. Int. J. Pharm. Sci. Res. 2 (10), 2577–2585.
Mosaddek, A. S. M., and Rashid, M. M. U. (2008). A Comparative Study of the Anti-inflammatory Effect of Aqueous Extract of Neem Leaf and Dexamethasone. Bangladesh J. Pharmacol. 3, 44–47. doi:10.3329/bjp.v3i1.836
Mourin, N. A., Sharmin, T., Chowdhury, S. R., Islam, F., Rahman, M. S., and Rashid, M. A. (2013). Evaluation of Bioactivities of Heliotropium Indicum, a Medicinal Plant of Bangladesh. Pharma Innovation 2 (5), 217–221.
Naik, M., Agrawal, D., Behera, R., Bhattacharya, A., Dehury, S., and Kumar, S. (2014). Study of Anti-inflammatory Effect of Neem Seed Oil (Azadirachta indica) on Infected Albino Rats. J. Health Res. Rev. 1 (3), 66–69. doi:10.4103/2394-2010.153880
Namsa, N. D., Tag, H., Mandal, M., Kalita, P., and Das, A. K. (2009). An Ethnobotanical Study of Traditional Anti-inflammatory Plants Used by the Lohit Community of Arunachal Pradesh, India. J. Ethnopharmacol 125 (2), 234–245. doi:10.1016/j.jep.2009.07.004
Nandi, S., and Lyndem, L. M. (2016). Clerodendrum Viscosum: Traditional Uses, Pharmacological Activities and Phytochemical Constituents. Nat. Prod. Res. 30 (5), 497–506. doi:10.1080/14786419.2015.1025229
Nandi, S., Ukil, B., and Lyndem, L. M. (2017). Acute and Sub-acute Toxicological Evaluation of the Alcoholic Leaf and Root Extracts of Clerodendrum Infortunatum L. Nat. Prod. Res. 32 (17), 2062–2066. doi:10.1080/14786419.2017.1360879
Nathan, C. (2002). Points of Control in Inflammation. Nature 420 (6917), 846–852. doi:10.1038/nature01320
Nesa, L., Munira, S., Mollika, S., Islam, M., Choin, H., Chouduri, A. U., et al. (2014). Evaluation of Analgesic, Anti-inflammatory and CNS Depressant Activities of Methanolic Extract of Lawsonia Inermis Barks in Mice. Avicenna J. Phytomed 4 (4), 287–296. doi:10.9734/bjpr/2014/7845
Nian, H., Xiong, H., Zhong, F., Teng, H., Teng, H., Chen, Y., et al. (2020). Anti-inflammatory and Antiproliferative Prenylated sulphur-containing Amides from the Leaves of Glycosmis Pentaphylla. Fitoterapia 146, 104693. doi:10.1016/j.fitote.2020.104693
Niazi, M., Mehrabani, M., Namazi, M. R., Salmanpour, M., Heydari, M., Karami, M. M., et al. (2020). Efficacy of a Topical Formulation of Henna (Lawsonia Inermis L.) in Contact Dermatitis in Patients Using Prosthesis: A Double-Blind Randomized Placebo-Controlled Clinical Trial. Complement. Ther. Med. 49, 102316. doi:10.1016/j.ctim.2020.102316
Nirmal, N. P., and Panichayupakaranant, P. (2015). Antioxidant, Antibacterial, and Anti-inflammatory Activities of Standardized Brazilin-Rich Caesalpinia Sappan Extract. Pharm. Biol. 53 (9), 1339–1343. doi:10.3109/13880209.2014.982295
Noor, S., Rahman, S. M. A., Ahmed, Z., Das, A., and Hossain, M. M. (2013). Evaluation of Anti-inflammatory and Antidiabetic Activity of Ethanolic Extracts of Desmodium Pulchellum Benth. (Fabaceae) Barks on Albino Wistar Rats. J. Appl. Pharm. Sci. 3 (7), 48–51. doi:10.9734/bjpr/2013/3505
Ocvirk, S., Kistler, M., Khan, S., Talukder, S. H., and Hauner, H. (2013). Traditional Medicinal Plants Used for the Treatment of Diabetes in Rural and Urban Areas of Dhaka, Bangladesh--an Ethnobotanical Survey. J. Ethnobiol. Ethnomed 9 (1), 43. doi:10.1186/1746-4269-9-43
Ogunbinu, A. O., Flamini, G., Cioni, P. L., Adebayo, M. A., and Ogunwande, I. A. (2009). Constituents of Cajanus Cajan (L.) Millsp., Moringa Oleifera Lam., Heliotropium Indicum L. And Bidens Pilosa L. from Nigeria. Nat. Prod. Commun. 4 (4), 573–578. doi:10.1177/1934578x0900400427
Okpanyi, S. N., and Ezeukwu, G. C. (1981). Anti-inflammatory and Antipyretic Activities of Azadirachta indica. Planta Med. 41 (01), 34–39. doi:10.1055/s-2007-971670
Okunade, A. L. (2002). Ageratum Conyzoides L. (Asteraceae). Fitoterapia 73 (1), 1–16. doi:10.1016/s0367-326x(01)00364-1
Özbek, H., and Yılmaz, B. S. (2017). Anti-inflammatory and Hypoglycemic Activities of Alpha-Pinene. actapharm 55 (4), 7–14. doi:10.23893/1307-2080.APS.05522
Paniagua-Pérez, R., Flores-Mondragón, G., Reyes-Legorreta, C., Herrera-López, B., Cervantes-Hernández, I., Madrigal-Santillán, O., et al. (2016). Evaluation of the Anti-inflammatory Capacity of Beta-Sitosterol in Rodent Assays. Ajtcam 14 (1), 123–130. doi:10.21010/ajtcam.v14i1.13
Parvin, T., Akhlas, M. B., Khatun, F., Akter, A., Al Amin, M., Islam, M. T., et al. (2019). Phytochemical Screening and Evaluation of Pharmacological Activities of Aqueous Extract of Typhonium Trilobatum (L.) Schott. Orient Pharm. Exp. Med. 19 (4), 445–454. doi:10.1007/s13596-019-00382-0
Pereira, L. d. P., Silva, K. E. S. d., Silva, R. O. d., Assreuy, A. M. S., and Pereira, M. G. (2012). Anti-inflammatory Polysaccharides of Azadirachta indica Seed Tegument. Rev. Bras. Farmacogn. 22 (3), 617–622. doi:10.1590/S0102-695X2012005000031
Perez, R. M. (2001). Anti-inflammatory Activity of Compounds Isolated from Plants. ScientificWorldJournal 1, 713–784. doi:10.1100/tsw.2001.77
Pillai, N. R., and Santhakumari, G. (1981). Anti-arthritic and Anti-inflammatory Actions of Nimbidin. Planta Med. 43 (09), 59–63. doi:10.1055/s-2007-971474
Posadas, I., Bucci, M., Roviezzo, F., Rossi, A., Parente, L., Sautebin, L., et al. (2004). Carrageenan-induced Mouse Paw Oedema Is Biphasic, Age-Weight Dependent and Displays Differential Nitric Oxide Cyclooxygenase-2 Expression. Br. J. Pharmacol. 142 (2), 331–338. doi:10.1038/sj.bjp.0705650
Prashith Kekuda, T. R., Dhanya Shree, V. S., Saema Noorain, G. K., Sahana, B. K., and Raghavendra, H. L. (2019). Ethnobotanical Uses, Phytochemistry and Pharmacological Activities of Clerodendrum Infortunatum L. (Lamiaceae): A Review. J. Drug Deliv. Ther. 9 (2), 547–559. doi:10.22270/jddt.v9i2.2433
Qais, N., Mahmud, Z. A., Karim, M. R., and Bachar, S. C. (2011). Anti-nociceptive, Anti-inflammatory and Sedating Activities of Leaf Extracts of Premna Esculenta (Roxb). J. Pharm. Res. 4 (10), 3463–3465.
R, C., and Rao, S. N. (2013). Chronic Anti-inflammatory Activity of Ethanolic Extract of Leaves of Clerodendrum Viscosum by Carrageenin Induced Paw Oedema in Wistar Albino Rats. Bjpr 3 (4), 579–586. doi:10.7897/2277-4343.04227
Rahman, M. A., Sharmin, R., Uddin, M. N., Mahbub Uz, Z., Rana, S., and Ahmed, N. U. (2011a). Antinociceptive and Anti-inflammatory Effect of Crinum asiaticum Bulb Extract. Asian J. Pharm. Clin. Res. 4 (3), 34–37.
Rahman, M. A., Solaiman, M., Haque, M. E., and Das, A. K. (2011b). Analgesic and Anti-inflammatory Activities of Alocasia Indica (Roxb.) Schott. Orient Pharm. Exp. Med. 11 (3), 143–146. doi:10.1007/s13596-011-0027-1
Rahman, S., Akter, M., Hira, T. E., Sharmin, T., and Nayeen, M. J. (2014). Cytotoxic, Thrombolytic and Membrane Stabilizing Activities of Swietenia Mahagoni (L.) Jacq. Flower Extract. Ejmp 4 (10), 1232–1239. doi:10.9734/ejmp/2014/11047
Rahman, S., Hasnat, A., Hasan, C. M., Rashid, M. A., and Ilias, M. (2001). Pharmacological Evaluation of Bangladeshi Medicinal Plants - a Review. Pharm. Biol. 39 (1), 1–6. doi:10.1076/phbi.39.1.1.5939
Rahmatullah, M., Jahan, R., Azam, F. M., Hossan, S., Mollik, M. A., and Rahman, T. (2011b). Folk Medicinal Uses of Verbenaceae Family Plants in Bangladesh. Afr. J. Tradit Complement. Altern. Med. 8, 53–65. doi:10.4314/ajtcam.v8i5S.15
Rahmatullah, M., Khatun, Z., Hasan, A., Parvin, W., Moniruzzaman, M., Khatun, A., et al. (2012). Survey and Scientific Evaluation of Medicinal Plants Used by the Pahan and Teli Tribal Communities of Natore District, Bangladesh. Afr. J. Tradit Complement. Altern. Med. 9 (3), 366–373. doi:10.4314/ajtcam.v9i3.10
Rahmatullah, M., Azam, M. N. K., Rahman, M. M., Seraj, S., Mahal, M. J., Mou, S. M., et al. (2011a). A Survey of Medicinal Plants Used by Garo and Non-garo Traditional Medicinal Practitioners in Two Villages of Tangail District, Bangladesh. American-Eurasian J. Sustainable Agric. 5 (3), 350–357.
Rai, U., Rawal, A., and Singh, S. (2018). Evaluation of the Anti-inflammatory Effect of an Anti-platelet Agent Crinumin on Carrageenan-Induced Paw Oedema and Granuloma Tissue Formation in Rats. Inflammopharmacology 26 (3), 769–778. doi:10.1007/s10787-017-0411-7
Ramamurthy, V., Fathima, M. A., Govindaraju, G., and Raveendran, S. (2009). Anti-inflammatory Activity of Heliotropium Indicum Extract on Formaldehyde Induced Inflammation in Rats. J. Ecobiology 24 (4), 345–351. Availableat: http://www.palaniparamount.com.
Rao, B. G., and Raju, N. J. (2009). Investigation of Anti-inflammatory Activity of Glycosmis Pentaphylla (Rutaceae). Int. J. Chem. Sci. 7 (4), 2891–2899.
Razia, S., Kamrun, N., and Sitesh, C. B. (2018). In-vitro Membrane Stabilizing, Thrombolytic, Antioxidant and Antimicrobial Activities of Bangladeshi Origin Coccinia Indica (Cucurbitaceae). Afr. J. Pharm. Pharmacol. 12 (16), 188–192. doi:10.5897/AJPP2018.4913
Recio, M. C., Andujar, I., and Rios, J. L. J. (2012). Anti-inflammatory Agents from Plants: Progress and Potential. Curr. Med. Chem. 19 (14), 2088–2103. doi:10.2174/092986712800229069
Ribeiro, R. A., Vale, M. L., Thomazzi, S. M., Paschoalato, A. B., Poole, S., Ferreira, S. H., et al. (2000). Involvement of Resident Macrophages and Mast Cells in the Writhing Nociceptive Response Induced by Zymosan and Acetic Acid in Mice. Eur. J. Pharmacol. 387 (1), 111–118. doi:10.1016/S0014-2999(99)00790-6
Ripon, S. S., Mahmood, A., Chowdhury, M. M. U., and Islam, M. T. (2016). A Possible Cytoprotectivity Triggering Anti- Atherothrombosis Activity of Lantana Camera L. Methanol Extract. Asian J. Ethnopharmacology Med. Foods 2 (6), 1–5.
Sabikunnahar, J., Jalal, U., and Labu, Z. K. (2016). Naturally Growing Medicinal Plant Clerodendrum Viscosum in Bangladesh - Thrombolytic, Membrane Stabilizing and Anti-microbial Properties. Int. J. Pharm. 7 (1), 2678–2684.
Saddam Hus, M., Mazbah Udd, A. H. M., Azmerin, M., Tohidul Am, M., Hasan, M., Das Aka, T., et al. (2019). An In Vivo and In Vitro Evaluation of Anti-inflammatory Action of Seeds of Vigna Unguiculata Available in Bangladeshi Region. J. Appl. Sci. 19 (2), 62–67. doi:10.3923/jas.2019.62.67
Salminen, A., Lehtonen, M., Suuronen, T., Kaarniranta, K., and Huuskonen, J. (2008). Terpenoids: Natural Inhibitors of NF-kappaB Signaling with Anti-inflammatory and Anticancer Potential. Cel Mol Life Sci 65 (19), 2979–2999. doi:10.1007/s00018-008-8103-5
Saltan Çitoğlu, G., Bahadır Acıkara, Ö., Sever Yılmaz, B., and Özbek, H. (2012). Evaluation of Analgesic, Anti-inflammatory and Hepatoprotective Effects of Lycorine from Sternbergia Fisheriana (Herbert) Rupr. Fitoterapia 83 (1), 81–87. doi:10.1016/j.fitote.2011.09.008
Salvo, F., Fourrier-Réglat, A., Bazin, F., Robinson, P., Riera-Guardia, N., Haag, M., et al. (2011). Cardiovascular and Gastrointestinal Safety of NSAIDs: A Systematic Review of Meta-Analyses of Randomized Clinical Trials. Clin. Pharmacol. Ther. 89 (6), 855–866. doi:10.1038/clpt.2011.45
Samira, K., Md, K., Laboni, F. R., Julie, A. S., Jalal, U., and Labu, Z. K. (2016). Biological Investigations of Medicinal Plants of Heliotropium Indicum Indigenous to Bangladesh. J. Coast Life Med. 4 (11), 874–878. doi:10.12980/jclm.4.2016J6-175
Samud, A. M., Asmawi, M. Z., Sharma, J. N., and Yusof, A. P. (1999). Anti-inflammatory Activity of Crinum asiaticum Plant and its Effect on Bradykinin-Induced Contractions on Isolated Uterus. Immunopharmacology 43 (2), 311–316. doi:10.1016/S0162-3109(99)00132-0
Sannigrahi, S., Mazumder, U. K., Pal, D., and Mishra, S. L. (2012). Terpenoids of Methanol Extract of Clerodendrum Infortunatum Exhibit Anticancer Activity against Ehrlich's Ascites Carcinoma (EAC) in Mice. Pharm. Biol. 50 (3), 304–309. doi:10.3109/13880209.2011.604089
Santos, F. A., and Rao, V. S. (2000). Antiinflammatory and Antinociceptive Effects of 1,8-cineole a Terpenoid Oxide Present in many Plant Essential Oils. Phytother Res. 14 (4), 240–244. doi:10.1002/1099-1573(200006)14:4<240::aid-ptr573>3.0.co;2-x
Sasidharan, S. P., and Vasumathi, A. V. (2017). In Vitro pharmacological, In Vivo Toxicological and In Silico Molecular Docking Analysis of Glycopentalone, a Novel Compound from Glycosmis Pentaphylla (Retz.) Correa. Med. Chem. Res. 26 (8), 1697–1707. doi:10.1007/s00044-017-1880-3
Schumacher, M., Cerella, C., Reuter, S., Dicato, M., and Diederich, M. (2011). Anti-inflammatory, Pro-apoptotic, and Anti-proliferative Effects of a Methanolic Neem (Azadirachta indica) Leaf Extract Are Mediated via Modulation of the Nuclear Factor-Κb Pathway. Genes Nutr. 6 (2), 149–160. doi:10.1007/s12263-010-0194-6
Seetharaman, T. R. (1990). Flavonoids of Firmiana Simplex and Sterculia Villosa. Fitoterapia 61 (4), 373–374.
Shafa, F., Shahriar, M., Opo, F. A. D. M., Akhter, R., Hossain, M. M., and Choudhury, N. (2016). Characterization of Phytoconstituents, In Vitro Antioxidant Activity and Pharmacological Investigation of the Root Extract of Typhonium Trilobatum. Int. J. Pharm. Sci. Res. 7 (4), 1694–1704. doi:10.13040/IJPSR.0975-8232.7(4).1694-04
Shah, V. O., Ferguson, J. E., Hunsaker, L. A., Deck, L. M., and Vander Jagt, D. L. (2010). Natural Products Inhibit LPS-Induced Activation of Pro-inflammatory Cytokines in Peripheral Blood Mononuclear Cells. Nat. Prod. Res. 24 (12), 1177–1188. doi:10.1080/14786410903112680
Shahriar, M., Alam, F., and Uddin, M. M. N. (2014). Membrane Stabilizing and Thrombolytic Activity of Withania Somnifera Root. Am. J. Phytomedicine Clin. Ther. 2 (2), 252–256.
Shahriar, M., Sharmin, F. A., Islam, A. S. M., Dewan, I., and Kabir, S. (2012). Membrane Stabilizing and Anti-thrombolytic Activities of Four Medicinal Plants of Bangladesh. Exp. 4 (4), 265–270.
Shahriar, M., Tithi, N. A., Akhter, R., Kamal, S., Narjish, S. N., and Bhuiyan, M. A. (2015). Phytochemical and Pharmacological Investigation of the Crude Extract of Typhonium Trilobatum (L.) Schott. World J. Pharm. Res. 4 (2), 167–188.
Shalini, S., and Shaik, F. (2010). Study on the Anti-inflammatory Activity of Heliotropium Indicum. J. Innovative Trends Pharm. Sci. 1 (1), 43–46.
Sharma, B., Vasudeva, N., and Sharma, S. (2020). Phytopharmacological Review on Crinum asiaticum: A Potential Medicinal Herb. Npj 10 (4), 342–354. doi:10.2174/2210315509666190731142333
Sharma, R. K., Goel, A., and Bhatia, A. K. (2016). Lawsonia Inermis Linn: A Plant with Cosmetic and Medical Benefits. Int. J. Appl. Sci. Biotechnol. 4 (1), 15–20. doi:10.3126/ijasbt.v4i1.14728
Shinde, U. A., Phadke, A. S., Nair, A. M., Mungantiwar, A. A., Dikshit, V. J., and Saraf, M. N. (1999). Membrane Stabilizing Activity - a Possible Mechanism of Action for the Anti-inflammatory Activity of Cedrus Deodara wood Oil. Fitoterapia 70, 251–257. doi:10.1016/S0367-326X(99)00030-1
Siddiqui, S., Kamal, A., Khan, F., Jamali, K. S., and Saify, Z. S. (2019). Gallic and Vanillic Acid Suppress Inflammation and Promote Myelination in an In Vitro Mouse Model of Neurodegeneration. Mol. Biol. Rep. 46 (1), 997–1011. doi:10.1007/s11033-018-4557-1
Sikder, M. A. A., Rahman, M. A., Islam, M. R., Kaisar, M. A., Rahman, M. S., and Rashid, M. A. (2010). In Vitro antioxidant , Reducing Power, Free Radical Scavenging and Membrane Stabilizing Activities of Spilanthes Calva. Bangladesh Pharm. J. 13 (1), 63–67.
Sinha, N. K., Seth, K. K., Pandey, V. B., Dasgupta, B., and Shah, A. H. (1981). Flavonoids from the Flowers of Clerodendron Infortunatum. Planta Med. 42 (07), 296–298. doi:10.1055/s-2007-971645
Sireeratawong, S., Itharat, A., Lerdvuthisopon, N., Piyabhan, P., Khonsung, P., Boonraeng, S., et al. (2012). Anti-inflammatory, Analgesic, and Antipyretic Activities of the Ethanol Extract of Piper Interruptum Opiz. And Piper Chaba Linn. ISRN Pharmacol. 2012, 480265. doi:10.5402/2012/480265
Sm, M. R., Rana, S., Afm, A. I., Kumer, A., Hashem, A. K., Rahman, H., et al. (2019). Antithrombotic and Anti-inflammatory Activities of Leaf Methanolic Extract of Euphorbia Hirta Lin. Ijcam 12 (4), 154–162. doi:10.15406/ijcam.2019.12.00466
Smyth, E. M., Grosser, T., Wang, M., Yu, Y., and FitzGerald, G. A. (2009). Prostanoids in Health and Disease. J. Lipid Res. 50, S423–S428. doi:10.1194/jlr.R800094-JLR200
Sofowora, A., Ogunbodede, E., and Onayade, A. (2013). The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit Complement. Altern. Med. 10 (5), 210–229. doi:10.4314/ajtcam.v10i5.2
Soma, M. A., Khan, M. F., Tahia, F., Al-Mansur, M. A., Rahman, M. S., and Rashid, M. A. (2019). Cyclooxygenase-2 Inhibitory Compounds from the Leaves of Glycosmis Pentaphylla (Retz.) A. DC.: Chemical and In Silico Studies. Asian J. Chem. 31, 1260–1264. doi:10.14233/ajchem.2019.21913
Souto, A. L., Tavares, J. F., da Silva, M. S., Diniz, Mde. F., de Athayde-Filho, P. F., and Barbosa Filho, J. M. (2011). Anti-inflammatory Activity of Alkaloids: an Update from 2000 to 2010. Molecules 16 (10), 8515–8534. doi:10.3390/molecules16108515
Souza, M. C., Siani, A. C., Ramos, M. F., Menezes-de-Lima, O. J., Henriques, M. G., and Henriques, M. G. M. O. (2003). Evaluation of Anti-inflammatory Activity of Essential Oils from Two Asteraceae Species. Pharmazie 58 (8), 582–586. Availableat: https://www.ingentaconnect.com/content/govi/pharmaz/2003/00000058/00000008/art00014.
Sravani, D., Aarathi, K., Kumar, N. S. S., Krupanidhi, S., Ramu, D. V., and Venkateswarlu, T. C. (2015). In VitroAnti-Inflammatory Activity ofMangifera indicaandManilkara zapotaLeaf Extract. Rese. Jour. Pharm. Technol. 8 (11), 1477–1480. doi:10.5958/0974-360x.2015.00264.4
Sreejith, P. S., and Asha, V. V. (2015). Glycopentalone, a Novel Compound from Glycosmis Pentaphylla (Retz.) Correa with Potent Anti-hepatocellular Carcinoma Activity. J. Ethnopharmacol 172, 38–43. doi:10.1016/j.jep.2015.05.051
Srinivas, K., Rao, M. E. B., and Rao, S. S. (2000). Anti-inflammatory Activity of Heliotropium Indicum Linn. And Leucas Aspera Spreng. in Albino Rats. Indian J. Pharmacol. 32 (1), 37–38.
Sufian, M., Chowdhury, T., Mian, M., Mohiuddin, M., Koly, S., Uddin, M., et al. (2017). In Vitro study on Thrombolytic and Membrane Stabilizing Activities of Alternanthera Paronychioides and Vernonia Patula Leaves and Stems. Arrb 15 (3), 1–9. doi:10.9734/ARRB/2017/34776
Sukardiman, , and Ervina, M. (2020). The Recent Use of Swietenia Mahagoni (L.) Jacq. As Antidiabetes Type 2 Phytomedicine: A Systematic Review. Heliyon 6 (3), e03536. doi:10.1016/j.heliyon.2020.e03536
Sumi, F., Ansari, P., Sikder, B., AnaytullaZhumur, N. A., Zhumur, N., Mohamed, M., et al. (2015). Investigation of the Different Ethnopharmacological Activity of Fractional Root Extracts of Mussaenda Roxburghii in In Vitro Model. Ejmp 10 (2), 1–9. doi:10.9734/EJMP/2015/19315
Swingle, K. F., and Shideman, F. E. (1972). Phases of the Inflammatory Response to Subcutaneous Implantation of a Cotton Pellet and Their Modification by Certain Anti-inflammatory Agents. J. Pharmacol. Exp. Ther. 183 (1), 226–234. Availableat: http://jpet.aspetjournals.org/content/jpet/183/1/226.full.pdf.
Syahmi, A. R., Vijayarathna, S., Sasidharan, S., Latha, L. Y., Kwan, Y. P., Lau, Y. L., et al. (2010). Acute Oral Toxicity and Brine Shrimp Lethality of Elaeis Guineensis Jacq., (Oil palm Leaf) Methanol Extract. Molecules 15 (11), 8111–8121. doi:10.3390/molecules15118111
Tania, K. N., Islam, M. T., Mahmood, A., Ibrahim, M., Chowdhury, M. M. U., Kuddus, M. R., et al. (2013). Pharmacological and Phytochemical Screenings of Ethanol Extract of Sterculia Villosa Roxb. J. Biomed. Pharm. Res. 2 (1), 9–14.
Taufiq-Ur-Rahman, M., Shilpi, J. A., Ahmed, M., and Faiz Hossain, C. (2005). Preliminary Pharmacological Studies on Piper Chaba Stem Bark. J. Ethnopharmacol 99 (2), 203–209. doi:10.1016/j.jep.2005.01.055
Tewtrakul, S., Hase, K., Kadota, S., Namba, T., Komatsu, K., and Tanaka, K. (2000). Fruit Oil Composition of Piper Chaba Hunt., P. Longum L. And P. Nigrum L. J. Essent. Oil Res. 12 (5), 603–608. doi:10.1080/10412905.2000.9712168
Thabet, A. A., Youssef, F. S., El-Shazly, M., and Singab, A. N. B. (2018). Sterculia and Brachychiton: a Comprehensive Overview on Their Ethnopharmacology, Biological Activities, Phytochemistry and the Role of Their Gummy Exudates in Drug Delivery. J. Pharm. Pharmacol. 70 (4), 450–474. doi:10.1111/jphp.12876
Thangaraj, P. (2016). “Anti-inflammatory Activity,” in Pharmacological Assays of Plant-Based Natural Products. Progress in Drug Research (Cham: Springer). doi:10.1007/978-3-319-26811-8_16
Tian, E. L., Cui, Y. Y., Yang, G. Z., Mei, Z. N., and Chen, Y. (2014). Phenolic Glycosides from Glycosmis Pentaphylla. J. Asian Nat. Prod. Res. 16 (12), 1119–1125. doi:10.1080/10286020.2014.951924
Tung, Y. T., Chua, M. T., Wang, S. Y., and Chang, S. T. (2008). Anti-inflammation Activities of Essential Oil and its Constituents from Indigenous Cinnamon (Cinnamomum Osmophloeum) Twigs. Bioresour. Technol. 99 (9), 3908–3913. doi:10.1016/j.biortech.2007.07.050
Tuñón, M. J., García-Mediavilla, M. V., Sánchez-Campos, S., and González-Gallego, J. (2009). Potential of Flavonoids as Anti-inflammatory Agents: Modulation of Pro-inflammatory Gene Expression and Signal Transduction Pathways. Curr. Drug Metab. 10 (3), 256–271. doi:10.2174/138920009787846369
Uddin, J., Biswas, A. J., and Labu, Z. K. (2018). In Vitro assessment of Butea Monosperma (Lam.) Leaves: Thrombolytic, Membrane Stabilizing Potentials and Total Phenolic Content. Npj 8 (3), 239–246. doi:10.2174/2210315508666180410143634
Uddin, J., Julie, A. S., Ali, M. H., Islam, M. N., Khan, S. A., and Labu, Z. K. (2015). Antimicrobial, Thrombolytic, Membrane Stabilizing Activities and Total Flavonoid Content of Various Partitionates of Aerial Parts of Eclipta alba (L.) Hassk. Dhaka Univ. J. Pharm. Sci. 14 (2), 207–213. doi:10.3329/dujps.v14i2.28512
Uddin, M., Begum, J., Rahman, M. A., Ahmed, N., Akter, R., and Abdullah, A. M. (2010). Antinociceptive and Anti-inflammatory Properties of the Methanol Leaf Extract of Argyreia Argentea. J. Pharm. Sci. Res. 2 (8), 465–471.
Uddin, M. J., Çiçek, S. S., Willer, J., Shulha, O., Abdalla, M. A., Sönnichsen, F., et al. (2020). Phenylpropanoid and Flavonoid Glycosides from the Leaves of Clerodendrum Infortunatum (Lamiaceae). Biochem. Syst. Ecol. 92, 104131. doi:10.1016/j.bse.2020.104131
Uddin, M. S. (2019). Medicinal Plants of Bangladesh. Dhaka: MPB. Availableat: https://www.natureinfo.com.bd/mpb/ (Accessed July 25, 2021).
Uddin, M., Uddin, M. Z., Emran, T. B., Nath, A. K., Jenny, A., Duttaa, M., et al. (2012). Anti-inflammatory and Antioxidant Activity of Leaf Extract of Crinum asiaticum. J. Pharm. Res. 05 (12), 5553–5556.
Uddin, S. N. (2006). Traditional Uses of Ethnomedicinal Plants of the Chittagong hill Tracts. Dhaka, Bangladesh: Bangladesh National Herbarium.
Uddin, S. S., and Islam, M. S. (2020). Anti-inflammatory, Analgesic and Cognitive Enhancer Plants Present in Bangladesh: A Study Review. Univ. J. Pharm. Res. 5 (5). doi:10.22270/ujpr.v5i5.490
Ugboko, H. U., Nwinyi, O. C., Oranusi, S. U., Fatoki, T. H., and Omonhinmin, C. A. (2020). Antimicrobial Importance of Medicinal Plants in Nigeria. ScientificWorldJournal 2020, 7059323. doi:10.1155/2020/7059323
Ullah, H. M. A., Akter, L., Zaman, S., Juhara, F., Tareq, S. M., Bhattacharjee, R., et al. (2015). Anti-inflammatory and Analgesic Activities of Methanolic Seed Extract of Sterculia Villosa Roxb. Asian J. Pharm. Clin. Res. 8 (5), 285–289.
Umapathy, E. U., Ndebia, E., Meeme, A., Adam, B., Menziwa, P., Nkeh-Chungag, B., et al. (2010). An Experimental Evaluation of Albuca Setosa Aqueous Extract on Membrane Stabilization, Protein Denaturation and white Blood Cell Migration during Acute Inflammation. J. Med. Plants Res. 4 (9), 789–795.
Vaghasiya, Y., Nair, R., and Chanda, S. (2007). Investigation of Some Piper Species for Anti-bacterial and Anti-inflammatory Property. Int. J. Pharmacol. 3 (5), 400–405. doi:10.3923/ijp.2007.400.405
Vane, J. R., and Botting, R. M. (1998). Mechanism of Action of Nonsteroidal Anti-inflammatory Drugs. Am. J. Med. 104 (3Suppl. 1), 2S–22S. doi:10.1016/S0002-9343(97)00203-9
Venkatesha, S. H., Berman, B. M., and Moudgil, K. D. (2011). Herbal Medicinal Products Target Defined Biochemical and Molecular Mediators of Inflammatory Autoimmune Arthritis. Bioorg. Med. Chem. 19 (1), 21–29. doi:10.1016/j.bmc.2010.10.053
Vigil de Mello, S. V., da Rosa, J. S., Facchin, B. M., Luz, A. B., Vicente, G., Faqueti, L. G., et al. (2016). Beneficial Effect of Ageratum Conyzoides Linn (Asteraceae) upon Inflammatory Response Induced by Carrageenan into the Mice Pleural Cavity. J. Ethnopharmacol 194, 337–347. doi:10.1016/j.jep.2016.09.003
Vijayaraj, R., and Kumaran, N. S. (2018). Protective Effect of Lawsonia Inermis Linn. On Chronic Inflammation in Rats. Int. J. Green Pharm. 12 (3), S549–S554.
Wadsworth, T. L., and Koop, D. R. (1999). Effects of the Wine Polyphenolics Quercetin and Resveratrol on Pro-inflammatory Cytokine Expression in RAW 264.7 Macrophages. Biochem. Pharmacol. 57 (8), 941–949. doi:10.1016/s0006-2952(99)00002-7
Williams, L. A., O'Connar, A., Latore, L., Dennis, O., Ringer, S., Whittaker, J. A., et al. (2008). The In Vitro Anti-denaturation Effects Induced by Natural Products and Non-steroidal Compounds in Heat Treated (Immunogenic) Bovine Serum Albumin Is Proposed as a Screening Assay for the Detection of Anti-inflammatory Compounds, without the Use of Animals, in the Early Stages of the Drug Discovery Process. West. Indian Med. J. 57 (4), 327–331.
World Health Organization, (1998). Medicinal Plants in the South Pacific : Information on 102 Commonly Used Medicinal Plants in the South Pacific. Manila: WHO Regional Office for the Western Pacific.
World Health Organization, (2013). WHO Traditional Medicine Strategy: 2014-2023. Geneva: World Health Organization.
Xu, L. P., Wang, H., and Yuan, Z. (2008). Triterpenoid Saponins with Anti-inflammatory Activity from Codonopsis Lanceolata. Planta Med. 74 (11), 1412–1415. doi:10.1055/s-2008-1081318
Yesmin, S., Paul, A., Naz, T., Rahman, A. B. M. A., Akhter, S. F., Wahed, M. I. I., et al. (2020). Membrane Stabilization as a Mechanism of the Anti-inflammatory Activity of Ethanolic Root Extract of Choi (Piper Chaba). Clin. Phytosci 6 (1), 59. doi:10.1186/s40816-020-00207-7
Yong, K. Y., and Abdul Shukkoor, M. S. (2020). Manilkara Zapota: A Phytochemical and Pharmacological Review. Mater. Today Proc. 29, 30–33. doi:10.1016/j.matpr.2020.05.688
Yong, Y. K., Sulaiman, N., Hakim, M. N., Lian, G. E., Zakaria, Z. A., Othman, F., et al. (2013a). Suppressions of Serotonin-Induced Increased Vascular Permeability and Leukocyte Infiltration by Bixa Orellana Leaf Extract. Biomed. Res. Int. 2013, 463145. doi:10.1155/2013/463145
Yong, Y. K., Zakaria, Z. A., Kadir, A. A., Somchit, M. N., Ee Cheng Lian, G., and Ahmad, Z. (2013b). Chemical Constituents and Antihistamine Activity of Bixa Orellana Leaf Extract. BMC Complement. Altern. Med. 13, 32. doi:10.1186/1472-6882-13-32
Yu, S. J., Yu, J. H., Yu, Z. P., Yan, X., Zhang, J. S., Sun, J. Y., et al. (2020). Bioactive Terpenoid Constituents from Eclipta Prostrata. Phytochemistry 170, 112192. doi:10.1016/j.phytochem.2019.112192
Yuan, M., Zhang, C., He, Z., Liu, C., and Li, K. (2019). Anti-inflammatory Effect and Chemical Constituents of Microcos Paniculata Total Flavone Glycosides Fraction. IOP Conf. Ser. Earth Environ. Sci. 332, 032018. doi:10.1088/1755-1315/332/3/032018
Yusoff, S. a. M., Asmawi, M. Z., Abdul Majid, S., Asif, M., Basheer, M. K. A., Mohamed, S. K., et al. (2017). Anti-angiogenesis as a Possible Mechanism of Action for Anti-tumor (Potential Anti-cancer) Activity of Crinum asiaticum Leaf Methanol Extract. J. Angiotherapy 1 (1), E012–E017. doi:10.25163/angiotherapy.11000210419100517
Zahan, R., Nahar, L., and Nesa, M. L. (2013). Antinociceptive and Anti-inflammatory Activities of Flower (Alangium Salvifolium) Extract. Pak J. Biol. Sci. 16 (19), 1040–1045. doi:10.3923/pjbs.2013.1040.1045
Ziadlou, R., Barbero, A., Martin, I., Wang, X., Qin, L., Alini, M., et al. (2020). Anti-inflammatory and Chondroprotective Effects of Vanillic Acid and Epimedin C in Human Osteoarthritic Chondrocytes. Biomolecules 10 (6), 932. doi:10.3390/biom10060932
Zou, L., Zhang, Y., Li, W., Zhang, J., Wang, D., Fu, J., et al. (2017). Comparison of Chemical Profiles, Anti-inflammatory Activity, and UPLC-Q-TOF/MS-based Metabolomics in Endotoxic Fever Rats between Synthetic Borneol and Natural Borneol. Molecules 22 (9), 1446. doi:10.3390/molecules22091446
Glossary
ADA adenosine deaminase
AA arachidonic acid
AP-1 activator protein-1
BSA bovine serum albumin
CD cluster of differentiation
COX cyclooxygenase
ELISA enzyme-linked immunosorbent assay
ERK extracellular signal–regulated kinase
FCA Freund’s’ complete adjuvant
GC-MS gas chromatography–mass spectroscopy
GI gastrointestinal
HETE hydroxyleicosatetraenoic acid
IBD inflammatory bowel disease
ICAM-1 intercellular adhesion molecule-1
IκB inhibitory κB
IL interleukin
INF-γ interferon-γ
IP intraperitoneal
JAK Janus kinase
JNK c-Jun-N-terminal kinase
LOX lipoxygenase
LC50 lethal concentration
LD50 lethal dose
LTB4 leukotriene B4
LTC4 leukotriene C4
LPS lipopolysaccharide
MMP matrix metalloproteinase
MAPKs mitogen-activated protein kinases
MPO myeloperoxidase
NF-κB nuclear factor-κB
ng nanogram
NO nitric oxide
NSAIDs nonsteroidal anti-inflammatory drugs
PAF platelet-activating factor
PKC protein kinase C
PLA2 phospholipase A2
PBMCs peripheral blood mononuclear cells
PGE2 prostaglandin E2
P.O. per oral
RBC red blood cell
RNS reactive nitrogen species
ROS reactive oxygen species
SAIDs steroidal anti-inflammatory drugs
SOD superoxide dismutase
STAT signal transducer and activator of transcription
TNF-α tumor necrosis factor-α
TPA 12-O-tetradecanoylphorbol-13-acetate
TXA2 thromboxane A2
VCAM-1 vascular cell adhesion molecule-1
Keywords: Bangladeshi, traditional, inflammation, medicinal plants, preclinical study
Citation: Akhtar MA (2022) Anti-Inflammatory Medicinal Plants of Bangladesh—A Pharmacological Evaluation. Front. Pharmacol. 13:809324. doi: 10.3389/fphar.2022.809324
Received: 04 November 2021; Accepted: 01 February 2022;
Published: 24 March 2022.
Edited by:
Lucia Recinella, University of Studies G. d'Annunzio Chieti and Pescara, ItalyReviewed by:
Armando Caceres, Universidad de San Carlos de Guatemala, GuatemalaOscar Herrera-Calderon, Universidad Nacional Mayor de San Marcos, Peru
Copyright © 2022 Akhtar. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Most. Afia Akhtar, YWZpYS5ha2h0YXJAcnUuYWMuYmQ=, b3JjaWQub3JnLzAwMDAtMDAwMi02MjA1LTk0ODQ=
 Most. Afia Akhtar
Most. Afia Akhtar