- 1Department of Clinical and Experimental Medicine, University of Messina, Messina, Italy
- 2Sicilian Regional Pharmacovigilance Centre, University Hospital of Messina, Messina, Italy
Given the importance of inflammation at the onset of multiple sclerosis (MS), therapy is mainly based on the use of anti-inflammatory drugs including disease modifying therapies (DMTs). Considering the recent approval of some DMTs, pharmacovigilance becomes a fundamental tool for the acquisition of new safety data. The aim of the study was to analyze adverse drug reactions (ADRs) related to the use of drugs approved for MS. All national publicly-available aggregated ADR reports recorded from 2002 to 2020 into the Reports of Adverse Reactions of Medicines (RAM) system and all complete Sicilian data reported into the Italian spontaneous reporting system (SRS) database having as suspected drugs interferon β-1a (IFN β-1a), interferon β-1b (IFN β-1b), peginterferon β-1a (PEG-IFN β-1a), glatiramer acetate (GA), natalizumab (NTZ), fingolimod (FNG), teriflunomide (TRF), dimethyl fumarate (DMF), alemtuzumab (Alem), ocrelizumab (OCZ), or cladribine (Cladr), were collected. Descriptive analyses of national, publicly-available aggregated data and full-access regional data were performed to assess demographic characteristics and drug-related variables followed by a more in-depth analysis of all Sicilian ADRs with a case-by-case assessment and a disproportionality analysis of unexpected ADRs. A total of 13,880 national reports have been collected from 2002 to 2020: they were mainly not serious ADRs (67.9% vs. 26.1%) and related to females (71.7% vs. 26.3%) in the age group 18–65 years (76.5%). The most reported ADRs were general and administration site conditions (n = 6,565; 47.3%), followed by nervous (n = 3,090; 22.3%), skin (n = 2,763; 19.9%) and blood disorders (n = 2,180; 15.7%). Some unexpected Sicilian ADRs were shown, including dyslipidemia for FNG (n = 10; ROR 28.5, CI 14.3–59.6), NTZ (n = 5; 10.3, 4.1–25.8), and IFN β-1a (n = 4; 8.7, 3.1–24.1), abortion and alopecia for NTZ (n = 9; 208.1, 73.4–590.1; n = 3; 4.9, 1.5–15.7), and vitamin D deficiency for GA (n = 3; 121.2, 30.9–475.3). Moreover, breast cancer with DMF (n = 4, 62.8, 20.5–191.9) and hypothyroidism with Cladr (n = 3; 89.2, 25.9–307.5) were also unexpected. The reporting of drugs-related ADRs in MS were mostly reported in the literature, but some unknown ADRs were also found. However, further studies are necessary to increase the awareness about the safety profiles of new drugs on the market.
Introduction
Multiple sclerosis (MS) is chronic inflammatory disease with demyelination and axonal damage on the central nervous system (CNS), and autoimmune disease with a multifactorial etiology (Yamout and Alroughani, 2018). The disease is estimated to affect approximately 2.8 million people worldwide and it is the first cause of non-traumatic neurological disability in young people (The Multiple Sclerosis International Federation (MSIF), 2020; Yamout and Alroughani, 2018). Its prevalence is higher in females, especially in the age group 20–40 years (Filippi et al., 2018; Trojano et al., 2019). The autoimmune response of MS is initially mediated by T lymphocytes reactive to myelin proteins with a consequent infiltration of CD4+ T cells in the “acute demyelination plaques” (Compston and Coles, 2008; Filippi et al., 2018; Patsopoulos et al., 2019). At a later stage, B cells produce autoantibodies located in both oligoclonal bands and plaques (Arrambide et al., 2018). Moreover, monocytes and macrophages, as well as dendritic cells, play a key role in the immunopathogenesis of MS (Nuyts et al., 2013; Ma et al., 2019).
Given the importance of the inflammation at the onset of MS, the therapy is mainly based on the use of anti-inflammatory drugs. The introduction of disease modifying therapies (DMTs) has radically changed the treatment of MS for their ability to prevent and/or reduce the frequency of relapses as well as delay the progression of the disease (Giovannoni, 2018; Tintore et al., 2019). The first therapeutic strategy is characterized by immunostimulants including interferon β-1a (IFN β-1a), interferon β-1b (IFN β-1b), peginterferon β-1a (PEG-IFN β-1a), and glatiramer acetate (GA), followed by the recent introduction of immunosuppressants such as natalizumab (NTZ), fingolimod (FNG), teriflunomide (TRF), dimethyl fumarate (DMF), alemtuzumab (Alem), ocrelizumab (OCZ), and cladribine (Cladr). Of the First-line drugs, including IFNs and GA, are mostly associated with injection site reactions and flu-like symptoms (La Mantia et al., 2016). Of the new first-line oral therapies, DMF can cause flushing, gastrointestinal disturbances, and lymphopenia and TRF can cause diarrhea, nausea, headache, hepatotoxicity, alopecia, and elevated blood pressure (Pardo and Jones, 2017). All other previously cited drugs are used as second-line therapy and are characterized by a higher effectiveness, but an increased risk of adverse drug reactions (ADRs) (Gitto, 2017; Pardo and Jones, 2017). Moreover, FNG and Alem are related to cardiotoxicity (Faissner and Gold, 2018; Ahrabian et al., 2020), while NTZ to progressive multifocal leukoencephalopathy (PML) (Vermeer et al., 2015; Clerico et al., 2017; Butzkueven et al., 2020). Cladr appears to be better tolerated with mild to moderate ADRs predominantly concerning lymphopenia (Guarnera et al., 2017; Jacobs et al., 2018; Scott, 2019; Leist et al., 2020). Furthermore, pre-marketing studies showed an increased risk of neoplasms only with FNG (Guarnera et al., 2017; Lebrun and Rocher, 2018; Alping et al., 2020).
Considering that several DMTs have been recently approved, pharmacovigilance is a fundamental tool for the acquisition of new safety data to improve knowledge of the risk/benefit ratio of these drugs. In the last few years, several safety alerts on possible risks associated with DMTs have been issued by regulatory agencies, such as the onset of PML with Cladr (European Medicines Agency, 2017); the onset of basal cell cancer, lymphoma, and PML with FNG (European Medicines Agency, 2015); the contraindication of FNG in pregnancy due to the risk of congenital malformations (European Medicines Agency, 2019a); restrictions on the use of Alem for rare, but serious, side effects including fatal cases related to myocardial ischemia, myocardial infarction, cerebral hemorrhage, dissection of the cervico-cephalic arteries, pulmonary alveolar hemorrhage, and thrombocytopenia (European Medicines Agency, 2019b). The main objective of this study was to evaluate the characteristics of ADRs with drugs approved for MS through 1) the analysis of national open data from Reports of Adverse Reactions of Medicines (report Reazioni Avverse dei Medicinali, RAM) giving the global evaluation of ADRs in Italy, and 2) focusing on complete data from the regional Sicilian database allowing case-by-case causality assessment of ADRs followed by a disproportionality analysis for all unexpectedly identified ADRs not already reported in the summary of product characteristics (SmPCs) available by the European Medicines Agency (EMA) for each considered drug.
Materials and Methods
Design of the Study
This was a retrospective observational study in which data were collected through the Italian SRS database called Rete Nazionale di Farmacovigilanza (RNF), managed by the Italian Medicines Agency (AIFA). The RNF was established in 2001 with the aim of collecting all suspected ADR reports from drugs and vaccines sent by all Italian regions. As of July 2017, AIFA has organized a publicly available, online system RAM, which allows access to data relative to ADR reports, uploaded into the RNF since 2002, in aggregated form at a national level. The RAM system is an official website that provides public access to the Italian spontaneous reports of suspected ADRs. Only subsets of data from national spontaneous reports are publicly available, taking into account the need to comply with the European Union and Italian Data Protection Policies. Conversely, Regional Pharmacovigilance Centers have full access to the all spontaneous reporting data of their region in the RNF database, including Sicily. Drugs registered in RNF follow the Anatomical Therapeutical Chemical (ATC) classification, while suspected ADRs are grouped according to the Medical Dictionary for Regulatory Activities (MedDRA®).
All data of reports recorded from January 2002 through December 2020, with at least one of the following approved molecules for the treatment of MS and reported as suspected, were included in the analysis: immunostimulants (IFN β-1a, IFN β-1b, PEG-IFN β-1a, and GA) and immunosuppressants (NTZ, FNG, TRF, DMF, Alem, and OCZ). Moreover, only cases related to the branded name “Mavenclad®” for Cladr were included to avoid therapeutic bias with other available medications having Cladr as an active substance and approved for other indications. Daclizumab was not considered because it was withdrawn from the market in March 2018 and mitoxantrone was withdrawn due to its absence in regional data reports. In order to obtain an overview of ADRs at national level, data related to these drugs were retrieved from the RAM system. Subsequently, a collection of detailed ADR report information, available in the RNF, was carried out for Sicily. Before proceeding with the analysis, literature data and duplicates were excluded.
Statistical Analysis
Descriptive analyses of national, publicly-available aggregated data and full-access, regional data were performed to assess demographic characteristics and drug-related variables. Analyses were carried out by gender, age, seriousness, and outcome. An ADR was classified as serious when it was life-threatening or fatal, required hospitalization or prolongation of existing hospitalization, caused a persistent or significant disability/incapacity, a congenital anomaly/congenital defect, or was categorized as another serious medical condition based on clinical judgment or Eudravigilance Important Medical Event (IME) list. The ADRs were analyzed at the MedDRA® classification level of System Organ Class (SOC) and Preferred Term (PT) by ordering the individual ADRs in the SOC equivalent and grouping the PT synonyms of the same clinical condition under a single term (see Supplementary Table S1).
A case-by-case analysis of the reports recorded in Sicily was made by taking into account any concomitant drug, comorbidity and information related to the onset time (time to onset, TTO) and the resolution time (time-to- resolution, TTR) of the ADR. The TTO and TTR are expressed in days and were calculated considering the time elapsed between the beginning of the use of the suspected drug and the date of onset of ADR and the time elapsed between the onset of ADR and the resolution of the same ADR, respectively. Furthermore, the causal association between ADR and the suspected drug was evaluated using the Naranjo algorithm and, according to the algorithm score, each ADR was classified as: very likely (score ≥9), probable (scores 5–8), possible (scores 1–4) or doubtful (score ≤0) (Naranjo et al., 1981).
Absolute and relative frequencies with 95% confidence intervals (CI) were evaluated for categorical variables, while medians with interquartile intervals (IQR) were evaluated for continuous variables. Subsequently, a disproportionality analysis was performed for all unexpected ADRs of Sicilian data by checking the SmPCs available at the time of the study on the EMA website. The reporting odds ratio (ROR) and the CIs at 95% were calculated as a measure of disproportionality when the number of these cases was equal to or greater than three. Statistical analyses were conducted using the Statistical Package for the Social Science (SPSS) version 23.0 software for Windows (IBM Corp. SPSS Statistics).
Results
Analysis of National Aggregated Data
A total of 13,880 reports of suspected ADRs related to MS drugs have been collected from 2002 to 2020 into the RAM system with a gradual increase over the years, a peak in 2018 (n = 2,940, 21.2%), and a decrease in the last 2 years (Figure 1). From 2002 to 2006, approximately 90% of the reports had, as suspected drug, GA and IFN β-1a, while from 2008 to 2012 most reports were attributed to NTZ followed by immunostimulants. From 2013 to 2020, in addition to the high number related to IFN β-1a and GA, a gradual increase of reports was noticed with FNG, Alem, DMF, and TRF. More than half of the 13,880 cases were associated with not serious ADRs (not serious, 67.9% vs. serious, 26.1%). The highest number of serious ADRs was reported for FNG (n = 1,112; 41.9%), OCZ (n = 138; 40.6%), and NTZ (n = 496; 39.2%). Focusing on gender, a larger number of cases was registered in women (females, 71.7% vs. males, 26.3%), and 76.5% of ADRs was reported in the age group of 18–65 years (Table 1).
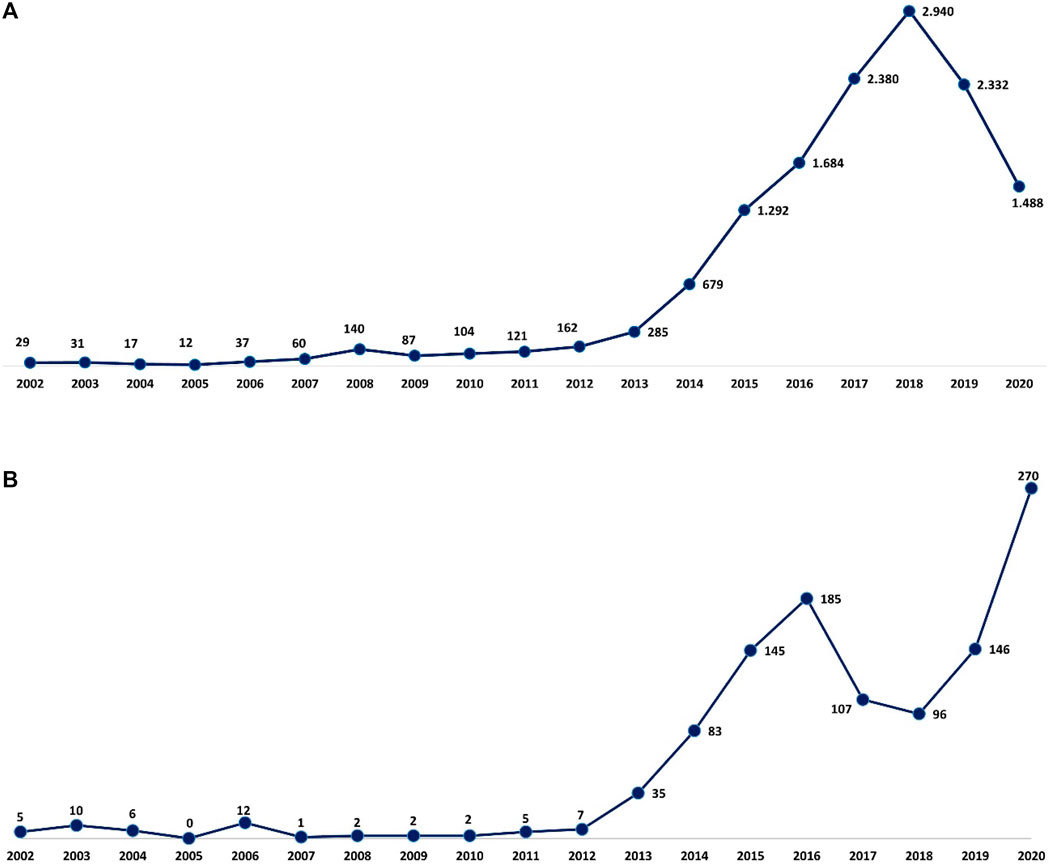
FIGURE 1. Italian (A) and Sicilian (B) trend of ADR reports related to drugs approved for MS over the years. ADR, adverse drug reaction; MS, multiple sclerosis.
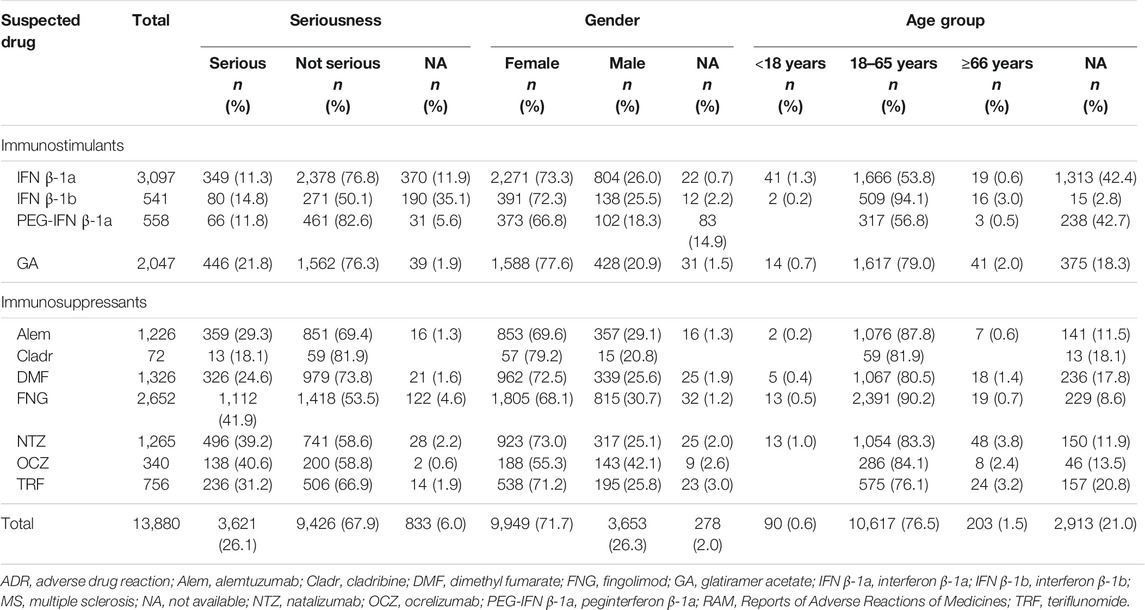
TABLE 1. Descriptions of Italian ADR reports related to drugs for MS collected into the RAM system from January 2002 to December 2020.
The most reported ADRs were related to general and administration site conditions (n = 6,565; 47.3%), followed by nervous (n = 3,090; 22.3%), skin (n = 2,763; 19.9%), blood disorders (n = 2,180; 15.7%), investigations (n = 2,023; 14.6%), infections (n = 1,980; 14.3%), and gastrointestinal disorders (n = 1,914; 13.8%). General and administration site conditions were predominantly related to immunostimulants (GA: n = 1,918; 93.7%, IFN β-1b: n = 449; 83%, PEG-IFN β-1a: n = 453; 81.2%, and IFN β-1a: n = 2,302; 74.3%); PEG-IFN β-1a and IFN β-1b were mostly reported for pyrexia (n = 77; 13.8% and n = 65; 12%, respectively), while GA was reported for administration site pain (n = 313; 15.3%). Moreover, skin disorders, including rash (n = 333; 27.2%), were mostly described for Alem. Almost half of Cladr-related ADRs belonged to blood disorders (n = 30; 41.7%), especially lymphopenia (n = 21; 29.2%), while FNG-related reports mainly showed alteration of liver enzymes (n = 1,059; 39.9%) and bradycardia (n = 475; 17.9%). A higher number of ADRs related to gastrointestinal disorders, particularly abdominal pain (n = 198; 14.9%), nausea (n = 77; 5.8%), and diarrhea (n = 98; 7.4%) were reported for DMF and TRF. Furthermore, a greater number of nervous disorders were associated with NTZ (n = 430; 34%), Cladr (n = 24; 33.3%), and Alem (n = 404; 33%). Concerning infections, OCZ and Cladr had a higher number of related ADRs (n = 109; 32.1% and n = 21; 29.2%, respectively). The onset of respiratory disorders, especially dyspnea, was mainly associated with OCZ and GA (n = 73; 21.5% and n = 321; 15.7%, respectively) while the onset of neoplasms and metabolic disorders was mostly reported for FNG (n = 306; 11.5%; n = 233; 8.8%, respectively) (Table 2).
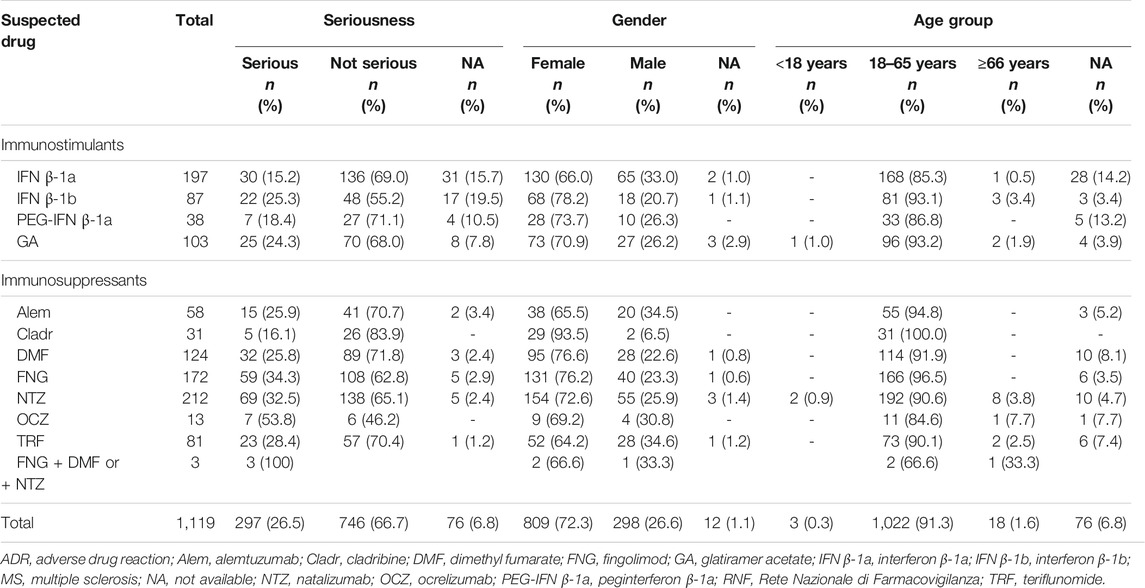
TABLE 2. Descriptions of Sicilian ADR reports related to drugs for MS collected into the RNF from January 2002 to December 2020.
Analysis of Regional Data
Concerning Sicilian data, 1,119 reports were reported of which 3 reports had 2 MS drugs described as suspected and, specifically, 2 cases of fingolimod + dimethyl fumarate and 1 case of fingolimod + natalizumab used in a different period. Different reporting over the years was observed, contrary to Italian reports, and a gradual increase was noticed until 2016, with a decrease in 2017 and 2018 and then another increase in the last 2 years with a peak in 2020 (n = 270; 24.2%) (Figure 1). As reported in national data, a higher prevalence of not serious ADRs was noticed for all DMTs except for OCZ (serious, 53.8% vs. not serious, 46.2%). Moreover, a higher number of reports was associated with females, and the 18–65 age group was found to have the most DMT cases (Table 3).
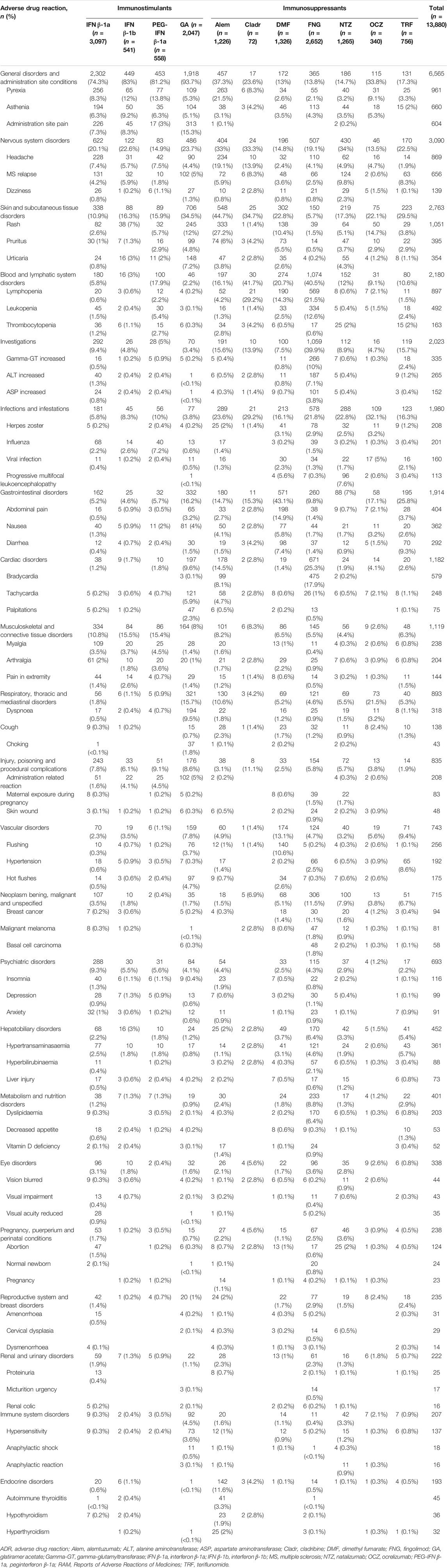
TABLE 3. Descriptions of main ADRs associated with drugs for MS treatment and reported into the RAM system.
Considering all reports with only one DMT reported as suspected, the median TTO of ADRs was almost equal for all drugs (from 182 to 322 days) excluding Alem which was related to a median (Q1-Q3) TTO of 27 (3–366) days and IFN β-1b with a median (Q1-Q3) TTO of 1,578 (709–3,094) days (Figure 2). Analyzing Sicilian data, the reported ADRs were substantially equal to national reports, even if other relevant ADRs were the onset of lymphopenia also with FNG and DMF (n = 49; 28.5% and n = 32; 25.8%, respectively), liver enzymes alteration and the onset of PML with NTZ (n = 28; 13.2%; n = 12; 5.7%, respectively) (Table 4).
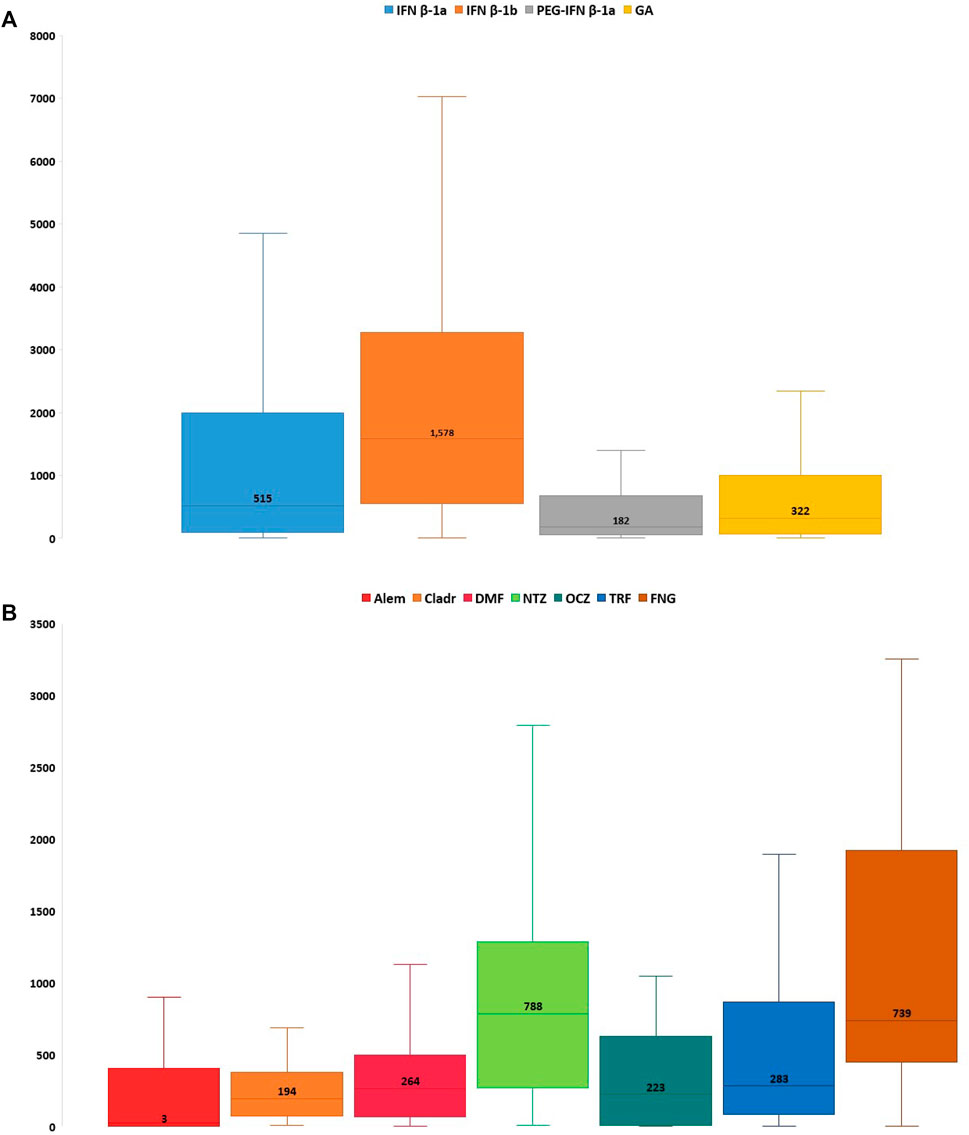
FIGURE 2. Time to onset of Sicilian ADRs related to immunostimulants (A) and immunosuppressants (B) approved for MS. ADR, adverse drug reaction; Alem, alemtuzumab; Cladr, cladribine; DMF, dimethyl fumarate; FNG, fingolimod; GA, glatiramer acetate; IFN β-1a, interferon β-1a; IFN β-1b, interferon β-1b; MS, multiple sclerosis; NTZ, natalizumab; OCZ, ocrelizumab; PEG-IFN β-1a, peginterferon β-1a; TRF, teriflunomide.
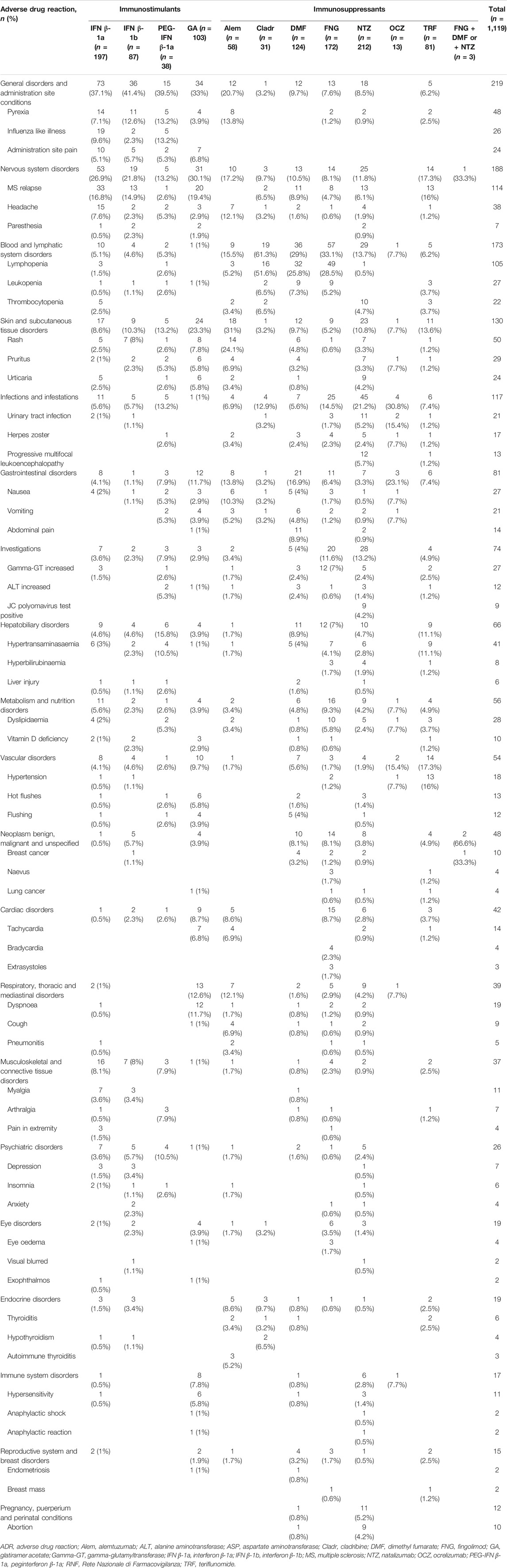
TABLE 4. Descriptions of main Sicilian ADRs associated with drugs for MS treatment and reported into the RNF.
The case-by-case assessment of Sicilian ADRs related to life-threatening events, persistent or significant disabilities and death was reported in Table 5. Among these reports, the majority concerned life threatening events (n = 11; 10.8%), followed by death and persistent or significant disabilities (n = 6; 0.5% and n = 2; 0.2%, respectively) with a median TTO of 240 (98.5–774) days. The analyzed reports concerned almost equally female and male patients (10 cases vs. 9 cases) and the median age of subjects was 46 (39–52.5) years. Most reported outcomes for life–threatening ADRs were fully recovered and not yet recovered and were especially related to the onset of breast cancer and skin disorders. Cases of death showed a completed suicide with IFN β-1b and the use of concomitant duloxetine for major depression, a cardiac arrest with FNG and cannabinoids, a cardiorespiratory arrest with Alem, a septic shock under the use of Alem, and a COVID-19 infection with FNG. Reports with a persistent or significant disability were related to a hepatic enzyme increase and to testicular infarction that led to a recovery with sequelae (Table 5).
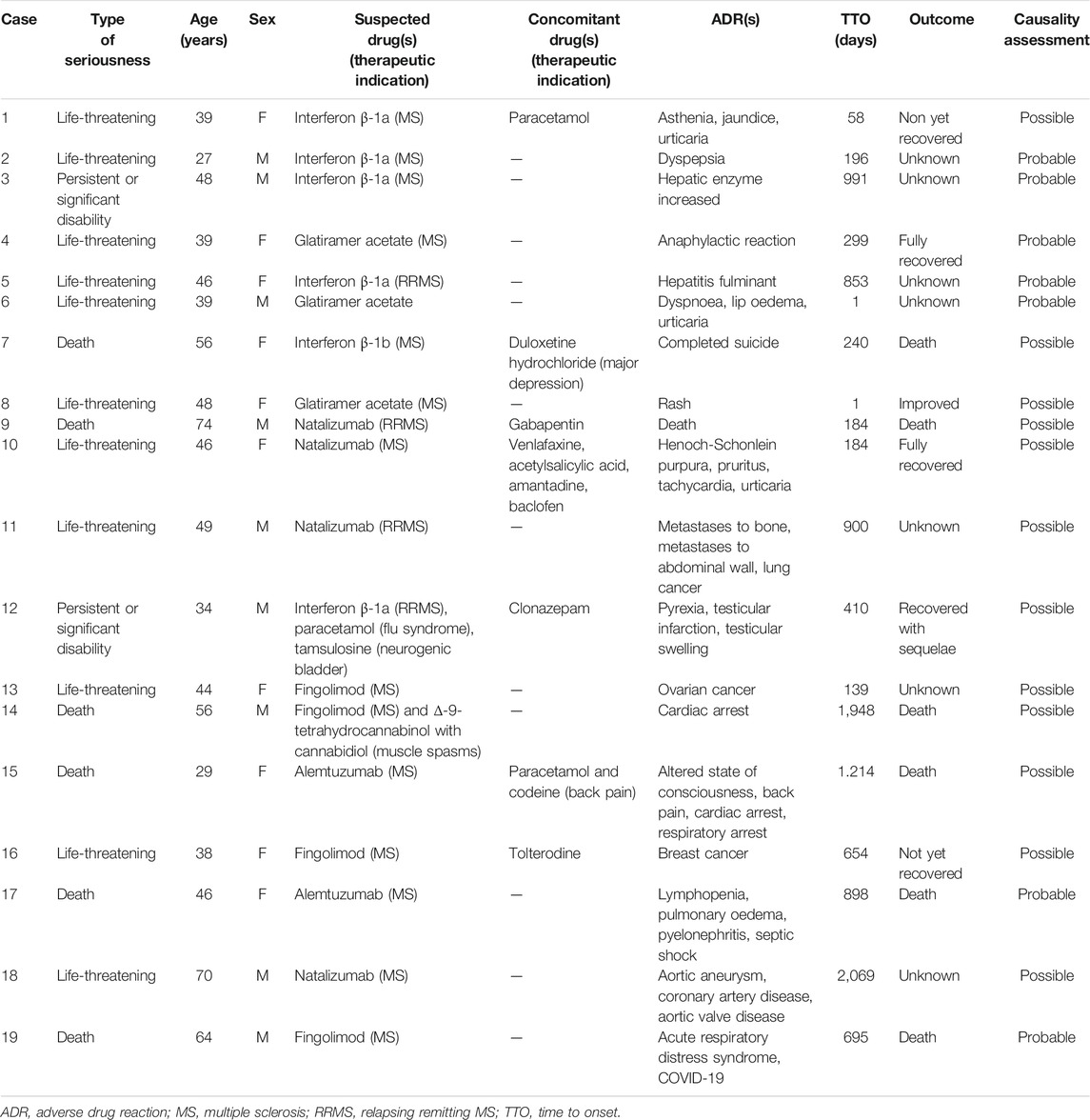
TABLE 5. Sicilian ADR reports related to drugs for MS with life-threatening events, persistent or significant disabilities and death.
The disproportionality analysis resulted in some unexpected ADRs including dyslipidemia which was mainly reported for FNG (n = 10; ROR 28.5, CI 14.3–59.6), NTZ (n = 5; 10.3, 4.1–25.8), and IFN β-1a (n = 4; 8.7, 3.1–24.1), and abortion reported for NTZ (n = 9; 208.1, 73.4–590.1). Moreover, some cases of unexpected malignancies including lung cancer and prostate cancer were shown for DMF, FNG, NTZ, and GA (all with n = 1); in particular, breast cancer was mostly reported for DMF [n = 5, of which one in association with FNG; 78.5 (28.1–219.3)], FNG (n = 3, of which one in association with DMF; 30.7 (8.9–106.4), and NTZ (both n = 2). Vitamin D deficiency was unknown for GA (n = 3; 121.2, 30.9–475.3), but also for IFN β-1a, IFN β-1b (both n = 2), FNG, and TRF (both n = 1). The analyzed reports also listed unexpected alopecia for NTZ (n = 3; 4.9, 1.5–15.7) and GA (n = 2). Some thyroid disorders were also unknown for Cladr, TRF, DMF, and included especially hypothyroidism with Cladr (n = 3; 89.2, 25.9–307.5) and thyroiditis with TRF (n = 2), Cladr and DMF (both n = 1).
Discussion
This study aimed to describe the frequency of ADRs collected in the Italian SRS database, focusing on Sicilian ADR reports associated with the use of DMTs for the treatment of MS. A different reporting trend at the national and regional level was shown in our study: with a gradual increase of reporting observed in Italy from 2014 to 2018, while MS drugs-related ADR reports peaked in Sicily between 2016 and 2020. This data are certainly influenced by several observational studies carried out at national or regional level in different periods during the last few years for evaluating the safety of MS drugs including the study BREMSO—BIIT 0114 for IFN β-1a, the study LEMTRADA PASS OBS13434 for Alem, the study FTY720D for FNG and some active pharmacovigilance projects, resulting in several solicited ADR reports in addition to the spontaneous studies in specific Italian regions. Most national and regional ADR reports concerned women (F/M ratio: 2.6) and adult patients. This probably reflects the prevalence of MS, which mostly occurs in patients aged 20–40 years and affects women three times more often than men (Filippi et al., 2018). The ADRs resolved completely or improved in over 40% of cases. Almost 25% of the reports concerned serious ADRs, but analyzing the reporting of individual drugs, a higher frequency of serious ADRs was reported for immunomodulators, including FNG, OCZ, and NTZ as shown in some studies (Hart and Bainbridge, 2016; Li et al., 2020).
With regard to each single type of ADR, our study confirmed well-known MS drug-related safety issues such as injection site reactions with immunostimulants, suggesting patient non-compliance, and the need to more closely monitor the subcutaneous administration for a greater therapeutic adherence (Stewart and Tran, 2012; Aviv et al., 2018; Maurelli et al., 2018). Moreover, a higher occurrence of reports with known skin reactions was shown for Alem (Vukusic et al., 2019), while a higher frequency of lymphopenia and infections was observed for Cladr (Leist et al., 2020). Lymphopenia is a long-known ADR linked to the mechanism of action of Cladr that is preferentially activated inside the B and T lymphocytes interfering with the synthesis of DNA: Cladr is an analogue of deoxyadenosine and, for its resistance to adenosine deaminase (ADA)-mediated deamination, it consequently accumulates in the lymphocytes and determines the formation of the cytotoxic triphosphorylated Cladr which causes ADA deficiency and leads to the development of severe lymphopenia (Baker et al., 2019). Lymphopenia could be associated with the higher onset of infections in patients treated with Cladr due to the immunodeficiency and for this reason, it would be advisable to check the immunological status of patients (Warny et al., 2018). Concerning FNG, reports showed a higher frequency of alteration of liver enzymes and cardiac arrhythmia as observed in the recent study of phase III FREEDOMS II (Calabresi et al., 2014): cardiac side effects with FNG are probably related to transient agonism to sphingosine-1-phosphate receptors on cardiomyocytes (Forrest et al., 2004). According to other results, OCZ was mainly associated with the onset of infections and respiratory disorders, the latter probably attributable to post-infusion reactions (Syed, 2018; Theriault and Solomon, 2020). In agreement with literature, Sicilian data showed a higher onset of PML with NTZ and DMF (Boffa et al., 2020), confirmed by antibody positivity to the JC virus. Activated B cells which host the JC virus probably promote viral replication and neurotropism via genetic transformation with increased risk of PML (Major, 2010).
Some unexpected ADRs based on SmPCs of MS drugs were retrieved, for which ROR values were significant. Based on available scientific literature, some of these associations could require further investigations. Reports related to FNG, NTZ, and IFN β-1a had mostly ADRs involving metabolic disorders, especially dyslipidemia, even if a retrospective observational study suggests that routine lipid profile monitoring is unnecessary during FNG treatment in MS patients without pre-existing cardiovascular comorbidities (Rauma et al., 2020). A recent study has shown the correlation between MS-related systemic inflammation and the onset of mild dyslipidemia, especially in males in the early stages of the disease (Rádiková et al., 2020). Moreover, dyslipidemia can influence, as a cardiovascular risk factor, the course of the disease, leading to a greater risk of progression to disability (Kowalec et al., 2017). However, treatment with statins showed mixed evidence (Tettey et al., 2014); in particular, that statins do not appear to have an effect on IFN β-1a-induced dyslipidemia (Rudick et al., 2009). Another potential safety signal concerned spontaneous abortion in pregnant women treated with NTZ. Animal studies have shown reproductive toxicity, but clinical data from clinical trials, prospective pregnancy registry, post-marketing cases, and available literature do not suggest an effect of NTZ exposure on pregnancy outcomes (Rommer and Zettl, 2018).
Malignancies mainly involved cases of skin tumors, including malignant melanoma and basal cell carcinoma, and lymphoma as has been demonstrated in some studies (Carbone et al., 2020; Lebrun-Frenay et al., 2020). The other types of tumors were unexpected. The risk of cancer associated with MS therapies has been debated by the scientific community, especially with FNG, NTZ, and other immunomodulatory drugs (Lebrun and Rocher, 2018). No difference in the incidence rates of the type of malignancies were observed between patients treated with NTZ and those treated with placebo. However, an effect of NTZ on malignant tumors cannot be ruled out. It is conceivable that the α4β1 integrin block could interfere with the activation of antigen-specific T lymphocytes and their ability to migrate to tumor sites (Förster et al., 2019). Moreover, in a post-marketing study, DMF, NTZ, and FNG were significantly related to a greater reporting of cancer (Dolladille et al., 2021). In this regard, only DMF had a positive association with the onset of breast cancer (Dolladille et al., 2021). However, DMF, especially at high concentrations, blocks nuclear factor κB (NFκB) activity in breast cancer, indicating an anticancer activity (Kastrati et al., 2016; Saidu et al., 2019). It has recently been noted that vitamin D deficiency is associated with an increased risk of MS (Sintzel et al., 2018), but there is no data to support immunostimulants, FNG, and TRF-induced hypovitaminosis. Concerning thyroid disorders, a recent review showed that approximately 6.44% of MS patients developed autoimmune thyroid disease, making it one of the most common comorbidity in MS, especially in patients treated with Alem (Marrie et al., 2015; Faissner and Gold, 2018).
Limits and Strengths
This study is the first overview of ADRs related to the drugs approved for the treatment of MS based on a SRS database. SRS is the most used method to carry out post-marketing surveillance and to research ADRs not occurring during the preclinical and clinical studies (Barbieri et al., 2021a). Indeed, the post-marketing phase allows to analyze the onset of ADRs related to the use of DMTs in a real-world context, considering all risk factors probably associated with each individual event, such as comorbidities and polytherapy. The analysis of ROR does not allow to quantify the true risk of potential safety signals, especially for the limited number of cases; it only recommends a statistically significant disproportionality of detailed drug–ADR pairs, which should be further examined for signal validation (Raschi et al., 2019). Therefore, it is of primary importance that the identification of new and unexpected ADRs, which arise during the use of DMTs in patients affected by MS, help the clinician choose the best treatment. Nevertheless, this study has some limitations: the existence of under- or over-reporting of suspected ADRs and missing data are typical problems of SRS database; comorbidities, exam values, information on treatment and diagnostic tests are not always registered (Palleria et al., 2013). Furthermore, the lack of the total number of drug-exposed patients make it difficult to establish any meaningful conclusion around head to-head comparison and about the incidence of MS therapy-related ADRs (Pal et al., 2013). Moreover, a detailed description of ADR reports is available only for regional data reported into the Italian SRS database and related to Sicily. The aggregated data in the RAM system prevent the calculation of ROR at national level (Barbieri et al., 2021b). However, SRS helps to increase the knowledge of safety profiles in order to prevent some ADRs related to the use of drugs used in the treatment of MS, especially for those recently approved.
Conclusion
The reporting of ADRs recorded for drugs used for the treatment of MS in Sicily has been relevant in recent years, thanks to some regional observational studies. Most of the ADRs highlighted in this study were already reported in the literature, but some unknown ADRs were also found. Disproportionality analysis showed potential risk of malignancies for FNG, NTZ, and DMF; moreover, FNG, NTZ, and IFN β-1a were mostly reported for dyslipidemia while abortion was unexpected in pregnant women treated with NTZ. These potential signals are not easy to evaluate, given the many factors that could be associated with their onset. Considering the rare and long-term ADRs that may arise in patients chronically treated for MS, pharmacovigilance activities are essential. However, further studies are necessary to increase the awareness about the safety profiles of new drugs entering the market, such as OCZ and Cladr, which currently have a current lower number of reports.
Data Availability Statement
The datasets generated for this study will not be made publicly available. National dataset in aggregated form is available online, while the access to the regional in single, non-aggregated dataset requires the approval of the Italian Medicines Agency, Further inquiries can be directed to MB, bWJhcmJpZXJpQHVuaW1lLml0.
Ethics Statement
The study was approved by the Ethics Committee of Messina (protocol number 67/2021; approved on 14 September 2021).
Author Contributions
All authors listed have sufficiently contributed to the entirecontent of the manuscript and have given their consent for publication. Project coordination: ES. Acquisition of data: PMC and ES. Analysis and interpretation of data: MAB, EES, GC, and AB. Clinical evaluation of data: VR and ES. Wrote the paper: MAB, EES, and PMC. Critical revision: PMC, VR, and ES. Final approval of the version to be published: MAB, EES, GC, AB, PMC, VR, and ES.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.808370/full#supplementary-material
References
Ahrabian, D., Neill, L., Bell, R., and Leary, S. M. (2020). Acute Cardiotoxicity Associated with Alemtuzumab Infusion for Multiple Sclerosis. Mult. Scler. 26, 735–737. doi:10.1177/1352458519855725
Alping, P., Askling, J., Burman, J., Fink, K., Fogdell-Hahn, A., Gunnarsson, M., et al. (2020). Cancer Risk for Fingolimod, Natalizumab, and Rituximab in Multiple Sclerosis Patients. Ann. Neurol. 87, 688–699. doi:10.1002/ana.25701
Arrambide, G., Tintore, M., Espejo, C., Auger, C., Castillo, M., Río, J., et al. (2018). The Value of Oligoclonal Bands in the Multiple Sclerosis Diagnostic Criteria. Brain 141, 1075–1084. doi:10.1093/brain/awy006
Aviv, B., Yaron, Z., Anat, A., and Sharon, B. (2018). Patterns of Local Site Reactions to Subcutaneous Glatiramer Acetate Treatment of Multiple Sclerosis: a Clinicopathological Study. Int. J. Clin. Exp. Pathol. 11, 3126–3133.
Baker, D., Pryce, G., Herrod, S. S., and Schmierer, K. (2019). Potential Mechanisms of Action Related to the Efficacy and Safety of Cladribine. Mult. Scler. Relat. Disord. 30, 176–186. doi:10.1016/j.msard.2019.02.018
Barbieri, M. A., Cutroneo, P. M., Baratelli, C., Cicala, G., Battaglia, A., Santoro, V., et al. (2021a). Adverse Drug Reactions with Oral Anticoagulants: Data from Sicilian Spontaneous Reporting System Database. J. Clin. Pharm. Ther. 46, 1027–1040. doi:10.1111/jcpt.13391
Barbieri, M. A., Sorbara, E. E., Cicala, G., Santoro, V., Cutroneo, P. M., Franchina, T., et al. (2021b). Adverse Drug Reactions with HER2-Positive Breast Cancer Treatment: An Analysis from the Italian Pharmacovigilance Database. Drugs - Real World Outcomes. doi:10.1007/s40801-021-00278-z
Boffa, G., Bruschi, N., Cellerino, M., Lapucci, C., Novi, G., Sbragia, E., et al. (2020). Fingolimod and Dimethyl-Fumarate-Derived Lymphopenia Is Not Associated with Short-Term Treatment Response and Risk of Infections in a Real-Life MS Population. CNS Drugs 34, 425–432. doi:10.1007/s40263-020-00714-8
Butzkueven, H., Kappos, L., Wiendl, H., Trojano, M., Spelman, T., Chang, I., et al. (2020). Long-term Safety and Effectiveness of Natalizumab Treatment in Clinical Practice: 10 Years of Real-World Data from the Tysabri Observational Program (TOP). J. Neurol. Neurosurg. Psychiatry 91, 660–668. doi:10.1136/jnnp-2019-322326
Calabresi, P. A., Radue, E. W., Goodin, D., Jeffery, D., Rammohan, K. W., Reder, A. T., et al. (2014). Safety and Efficacy of Fingolimod in Patients with Relapsing-Remitting Multiple Sclerosis (FREEDOMS II): a Double-Blind, Randomised, Placebo-Controlled, Phase 3 Trial. Lancet Neurol. 13, 545–556. doi:10.1016/S1474-4422(14)70049-3
Carbone, M. L., Lacal, P. M., Messinese, S., De Giglio, L., Pozzilli, C., Persechino, S., et al. (2020). Multiple Sclerosis Treatment and Melanoma Development. Int. J. Mol. Sci. 21, 2950. doi:10.3390/ijms21082950
Clerico, M., Artusi, C. A., Liberto, A. D., Rolla, S., Bardina, V., Barbero, P., et al. (2017). Natalizumab in Multiple Sclerosis: Long-Term Management. Int. J. Mol. Sci. 18, 1–12. doi:10.3390/ijms18050940
Compston, A., and Coles, A. (2008). Multiple Sclerosis. Lancet 372, 1502–1517. doi:10.1016/S0140-6736(08)61620-7
Dolladille, C., Chrétien, B., Peyro-Saint-Paul, L., Alexandre, J., Dejardin, O., Fedrizzi, S., et al. (2021). Association between Disease-Modifying Therapies Prescribed to Persons with Multiple Sclerosis and Cancer: a WHO Pharmacovigilance Database Analysis. Neurotherapeutics 18, 1657–1664. doi:10.1007/s13311-021-01073-y
European Medicines Agency (2015). New Recommendations to Minimise Risks of the Rare Brain Infection PML and a Type of Skin Cancer with Gilenya. Available at: https://www.ema.europa.eu/en/documents/press-release/new-recommendations-minimise-risks-rare-brain-infection-pml-type-skin-cancer-gilenya_en.pdf.
European Medicines Agency (2017). PRAC Recommendations on Signals. Available at: https://www.ema.europa.eu/en/documents/prac-recommendation/prac-recommendations-signals-adopted-25-29-september-2017-prac-meeting_en.pdf.
European Medicines Agency (2019a). Updated Restrictions for Gilenya: Multiple Sclerosis Medicine Not to Be Used in Pregnancy. Available at: https://www.ema.europa.eu/en/documents/press-release/updated-restrictions-gilenya-multiple-sclerosis-medicine-not-be-used-pregnancy_en.pdf.
European Medicines Agency (2019b). Use of Multiple Sclerosis Medicine Lemtrada Restricted while EMA Review Is Ongoing. 12 Apr 2019. Available at: https://www.ema.europa.eu/en/documents/press-release/use-multiple-sclerosis-medicine-lemtrada-restricted-while-ema-review-ongoing_en.pdf.
Faissner, S., and Gold, R. (2018). Efficacy and Safety of the Newer Multiple Sclerosis Drugs Approved since 2010. CNS Drugs 32, 269–287. doi:10.1007/s40263-018-0488-6
Filippi, M., Bar-Or, A., Piehl, F., Preziosa, P., Solari, A., Vukusic, S., et al. (2018). Multiple Sclerosis. Nat. Rev. Dis. Primers 4, 1–27. doi:10.1038/s41572-018-0041-4
Forrest, M., Sun, S.-Y., Hajdu, R., Bergstrom, J., Card, D., Doherty, G., et al. (2004). Immune Cell Regulation and Cardiovascular Effects of Sphingosine 1-Phosphate Receptor Agonists in Rodents Are Mediated via Distinct Receptor Subtypes. J. Pharmacol. Exp. Ther. 309, 758–768. doi:10.1124/jpet.103.062828
Förster, M., Küry, P., Aktas, O., Warnke, C., Havla, J., Hohlfeld, R., et al. (2019). Managing Risks with Immune Therapies in Multiple Sclerosis. Drug Saf. 42, 633–647. doi:10.1007/s40264-018-0782-8
Giovannoni, G. (2018). Disease-modifying Treatments for Early and Advanced Multiple Sclerosis: a New Treatment Paradigm. Curr. Opin. Neurol. 31, 233–243. doi:10.1097/WCO.0000000000000561
Gitto, L. (2017). “Living with Multiple Sclerosis in Europe: Pharmacological Treatments, Cost of Illness, and Health-Related Quality of Life across Countries,” in MULTIPLE SCLEROSIS Perspectives In Treatment And Pathogenesis. Editors I. S. Zagon, and P. J. McLaughlin (Brisbane: Codon Publications), 17–37. doi:10.15586/codon.multiplesclerosis.2017.ch2
Guarnera, C., Bramanti, P., and Mazzon, E. (2017). Comparison of Efficacy and Safety of Oral Agents for the Treatment of Relapsing-Remitting Multiple Sclerosis. Drug Des. Devel. Ther. 11, 2193–2207. doi:10.2147/DDDT.S137572
Hart, F. M., and Bainbridge, J. (2016). Current and Emerging Treatment of Multiple Sclerosis. Am. J. Manag. Care 22, s159–70. doi:10.1177/1352458515591862
Jacobs, B. M., Ammoscato, F., Giovannoni, G., Baker, D., and Schmierer, K. (2018). Cladribine: Mechanisms and Mysteries in Multiple Sclerosis. J. Neurol. Neurosurg. Psychiatry 89, 1266–1271. doi:10.1136/jnnp-2017-317411
Kastrati, I., Siklos, M. I., Calderon-Gierszal, E. L., El-Shennawy, L., Georgieva, G., Thayer, E. N., et al. (2016). Dimethyl Fumarate Inhibits the Nuclear Factor κB Pathway in Breast Cancer Cells by Covalent Modification of P65 Protein. J. Biol. Chem. 291, 3639–3647. doi:10.1074/jbc.M115.679704
Kowalec, K., McKay, K. A., Patten, S. B., Fisk, J. D., Evans, C., Tremlett, H., et al. (2017). Comorbidity Increases the Risk of Relapse in Multiple Sclerosis: A Prospective Study. Neurology 89, 2455–2461. doi:10.1212/WNL.0000000000004716
La Mantia, L., Di Pietrantonj, C., Rovaris, M., Rigon, G., Frau, S., Berardo, F., et al. (20162016). Interferons-beta versus Glatiramer Acetate for Relapsing-Remitting Multiple Sclerosis. Cochrane Database Syst. Rev. 2016. doi:10.1002/14651858.CD009333.pub3
Lebrun, C., and Rocher, F. (2018). Cancer Risk in Patients with Multiple Sclerosis: Potential Impact of Disease-Modifying Drugs. CNS Drugs 32, 939–949. doi:10.1007/s40263-018-0564-y
Lebrun-Frenay, C., Berestjuk, I., Cohen, M., and Tartare-Deckert, S. (2020). Effects on Melanoma Cell Lines Suggest No Significant Risk of Melanoma under Cladribine Treatment. Neurol. Ther. 9, 599–604. doi:10.1007/s40120-020-00204-5
Leist, T., Cook, S., Comi, G., Montalban, X., Giovannoni, G., Nolting, A., et al. (2020). Long-term Safety Data from the Cladribine Tablets Clinical Development Program in Multiple Sclerosis. Mult. Scler. Relat. Disord. 46, 102572. doi:10.1016/j.msard.2020.102572
Li, H., Hu, F., Zhang, Y., and Li, K. (2020). Comparative Efficacy and Acceptability of Disease-Modifying Therapies in Patients with Relapsing-Remitting Multiple Sclerosis: a Systematic Review and Network Meta-Analysis. J. Neurol. 267, 3489–3498. doi:10.1007/s00415-019-09395-w
Ma, W. T., Gao, F., Gu, K., and Chen, D. K. (2019). The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 10, 1140. doi:10.3389/fimmu.2019.01140
Major, E. O. (2010). Progressive Multifocal Leukoencephalopathy in Patients on Immunomodulatory Therapies. Annu. Rev. Med. 61, 35–47. doi:10.1146/annurev.med.080708.082655
Marrie, R. A., Cohen, J., Stuve, O., Trojano, M., Sørensen, P. S., Reingold, S., et al. (2015). A Systematic Review of the Incidence and Prevalence of Comorbidity in Multiple Sclerosis: Overview. Mult. Scler. 21, 263–281. doi:10.1177/1352458514564491
Maurelli, M., Bergamaschi, R., Antonini, A., Fargnoli, M. C., Puma, E., Mallucci, G., et al. (2018). Interferon-beta Injection Site Reactions in Patients with Multiple Sclerosis. J. Dermatolog. Treat. 29, 831–834. doi:10.1080/09546634.2018.1467539
Naranjo, C. A., Busto, U., Sellers, E. M., Sandor, P., Ruiz, I., Roberts, E. A., et al. (1981). A Method for Estimating the Probability of Adverse Drug Reactions. Clin. Pharmacol. Ther. 30, 239–245. doi:10.1038/clpt.1981.154
Nuyts, A. H., Lee, W. P., Bashir-Dar, R., Berneman, Z. N., and Cools, N. (2013). Dendritic Cells in Multiple Sclerosis: Key Players in the Immunopathogenesis, Key Players for New Cellular Immunotherapies? Mult. Scler. 19, 995–1002. doi:10.1177/1352458512473189
Pal, S. N., Duncombe, C., Falzon, D., and Olsson, S. (2013). WHO Strategy for Collecting Safety Data in Public Health Programmes: Complementing Spontaneous Reporting Systems. Drug Saf. 36, 75–81. doi:10.1007/s40264-012-0014-6
Palleria, C., Leporini, C., Chimirri, S., Marrazzo, G., Sacchetta, S., Bruno, L., et al. (2013). Limitations and Obstacles of the Spontaneous Adverse Drugs Reactions Reporting: Two “Challenging” Case Reports. J. Pharmacol. Pharmacother. 4, S66–S72. doi:10.4103/0976-500X.120955
Pardo, G., and Jones, D. E. (2017). The Sequence of Disease-Modifying Therapies in Relapsing Multiple Sclerosis: Safety and Immunologic Considerations. J. Neurol. 264, 2351–2374. doi:10.1007/s00415-017-8594-9
Patsopoulos, N. A., Baranzini, S. E., Santaniello, A., Shoostari, P., Cotsapas, C., Wong, G., et al. (2019). Multiple Sclerosis Genomic Map Implicates Peripheral Immune Cells and Microglia in Susceptibility. Science 365, 365. doi:10.1126/science.aav7188
Rádiková, Ž., Penesová, A., Vlček, M., Havranová, A., Siváková, M., Šiarnik, P., et al. (2020). Lipoprotein Profiling in Early Multiple Sclerosis Patients: Effect of Chronic Inflammation? Lipids Health Dis. 19, 49. doi:10.1186/s12944-020-01221-x
Raschi, E., Moretti, U., Salvo, F., Pariente, A., Cosimo Antonazzo, I., De Ponti, F., et al. (2019). Evolving Roles of Spontaneous Reporting Systems to Assess and Monitor Drug Safety, Pharmacovigilance. doi:10.5772/intechopen.79986
Rauma, I., Huhtala, H., Soilu-Hänninen, M., and Kuusisto, H. (2020). Lipid Profile Alterations during Fingolimod Treatment in Multiple Sclerosis Patients. J. Neuroimmune Pharmacol. 15, 567–569. doi:10.1007/s11481-020-09937-4
Rommer, P. S., and Zettl, U. K. (2018). Managing the Side Effects of Multiple Sclerosis Therapy: Pharmacotherapy Options for Patients. Expert Opin. Pharmacother. 19, 483–498. doi:10.1080/14656566.2018.1446944
Rudick, R. A., Pace, A., Rani, M. R., Hyde, R., Panzara, M., Appachi, S., et al. (2009). Effect of Statins on Clinical and Molecular Responses to Intramuscular Interferon Beta-1a. Neurology 72, 1989–1993. doi:10.1212/WNL.0b013e3181a92b96
Saidu, N. E. B., Kavian, N., Leroy, K., Jacob, C., Nicco, C., Batteux, F., et al. (2019). Dimethyl Fumarate, a Two-Edged Drug: Current Status and Future Directions. Med. Res. Rev. 39, 1923–1952. doi:10.1002/med.21567
Scott, L. J. (2019). Teriflunomide: A Review in Relapsing-Remitting Multiple Sclerosis. Drugs 79, 875–886. doi:10.1007/s40265-019-01135-8
Sintzel, M. B., Rametta, M., and Reder, A. T. (2018). Vitamin D and Multiple Sclerosis: A Comprehensive Review. Neurol. Ther. 7, 59–85. doi:10.1007/s40120-017-0086-4
Stewart, T. M., and Tran, Z. V. (2012). Injectable Multiple Sclerosis Medications: A Patient Survey of Factors Associated with Injection-Site Reactions. Int. J. MS Care 14, 46–53. doi:10.7224/1537-2073-14.1.46
Syed, Y. Y. (2018). Ocrelizumab: A Review in Multiple Sclerosis. CNS Drugs 32, 883–890. doi:10.1007/s40263-018-0568-7
Tettey, P., Simpson, S., Taylor, B. V., and van der Mei, I. A. (2014). Vascular Comorbidities in the Onset and Progression of Multiple Sclerosis. J. Neurol. Sci. 347, 23–33. doi:10.1016/j.jns.2014.10.020
The Multiple Sclerosis International Federation (MSIF) (2020). Atlas of MS 3 Rd Edition. London, UK: Multiple Sclerosis International Federation, 1–37.
Theriault, M., and Solomon, A. J. (2020). Two Cases of Meningitis Associated with Ocrelizumab Therapy. Mult. Scler. Relat. Disord. 38, 101866. doi:10.1016/j.msard.2019.101866
Tintore, M., Vidal-Jordana, A., and Sastre-Garriga, J. (2019). Treatment of Multiple Sclerosis - success from Bench to Bedside. Nat. Rev. Neurol. 15, 53–58. doi:10.1038/s41582-018-0082-z
Trojano, M., Bergamaschi, R., Amato, M. P., Comi, G., Ghezzi, A., Lepore, V., et al. (2019). Correction to: The Italian Multiple Sclerosis Register. Neurol. Sci. 40, 907. doi:10.1007/s10072-019-03772-z
Vermeer, N. S., Straus, S. M., Mantel-Teeuwisse, A. K., Hidalgo-Simon, A., Egberts, A. C., Leufkens, H. G., et al. (2015). Drug-induced Progressive Multifocal Leukoencephalopathy: Lessons Learned from Contrasting Natalizumab and Rituximab. Clin. Pharmacol. Ther. 98, 542–550. doi:10.1002/cpt.207
Vukusic, S., Brassat, D., de Seze, J., Izquierdo, G., Lysandropoulos, A., Moll, W., et al. (2019). Single-arm Study to Assess Comprehensive Infusion Guidance for the Prevention and Management of the Infusion Associated Reactions (IARs) in Relapsing-Remitting Multiple Sclerosis (RRMS) Patients Treated with Alemtuzumab (EMERALD). Mult. Scler. Relat. Disord. 29, 7–14. doi:10.1016/j.msard.2019.01.019
Warny, M., Helby, J., Nordestgaard, B. G., Birgens, H., and Bojesen, S. E. (2018). Lymphopenia and Risk of Infection and Infection-Related Death in 98,344 Individuals from a Prospective Danish Population-Based Study. PLoS Med. 15, e1002685–22. doi:10.1371/journal.pmed.1002685
Keywords: multiple sclerosis, disease modifying therapies, injectable drugs, adverse drug reactions, pharmacovigilance
Citation: Barbieri MA, Sorbara EE, Battaglia A, Cicala G, Rizzo V, Spina E and Cutroneo PM (2022) Adverse Drug Reactions with Drugs Used in Multiple Sclerosis: An Analysis from the Italian Pharmacovigilance Database. Front. Pharmacol. 13:808370. doi: 10.3389/fphar.2022.808370
Received: 03 November 2021; Accepted: 10 January 2022;
Published: 23 February 2022.
Edited by:
Maryse Lapeyre-Mestre, Université de Toulouse, FranceReviewed by:
Sophie Nguyen, Centre Hospitalier Universitaire de Caen, FranceKlaus G. Petry, Institut National de la Santé et de la Recherche Médicale (INSERM), France
Copyright © 2022 Barbieri, Sorbara, Battaglia, Cicala, Rizzo, Spina and Cutroneo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Antonietta Barbieri, bWJhcmJpZXJpQHVuaW1lLml0
 Maria Antonietta Barbieri
Maria Antonietta Barbieri Emanuela Elisa Sorbara
Emanuela Elisa Sorbara Alessandro Battaglia1
Alessandro Battaglia1 Giuseppe Cicala
Giuseppe Cicala Vincenzo Rizzo
Vincenzo Rizzo Edoardo Spina
Edoardo Spina Paola Maria Cutroneo
Paola Maria Cutroneo