- 1School of Acupuncture Moxibustion and Tuina, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 2Department of Medical Cosmetology, Affiliated Hospital of Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 3School of Chinese Classics, Chengdu University of Traditional Chinese Medicine, Chengdu, China
Ischemic heart disease has a high mortality, and the recommended therapy is reperfusion. Nevertheless, the restoration of blood flow to ischemic tissue leads to further damage, namely, myocardial ischemia/reperfusion injury (MIRI). Apoptosis is an essential pathogenic factor in MIRI, and ginsenosides are effective in inhibiting apoptosis and alleviating MIRI. Here, we reviewed published studies on the anti-apoptotic effects of ginsenosides and their mechanisms of action in improving MIRI. Each ginsenoside can regulate multiple pathways to protect the myocardium. Overall, the involved apoptotic pathways include the death receptor signaling pathway, mitochondria signaling pathway, PI3K/Akt signaling pathway, NF-κB signaling pathway, and MAPK signaling pathway. Ginsenosides, with diverse chemical structures, regulate different apoptotic pathways to relieve MIRI. Summarizing the effects and mechanisms of ginsenosides contributes to further mechanism research studies and structure–function relationship research studies, which can help the development of new drugs. Therefore, we expect that this review will highlight the importance of ginsenosides in improving MIRI via anti-apoptosis and provide references and suggestions for further research in this field.
Introduction
Ischemic heart disease (IHD) is characterized by insufficient blood flow to the cardiac tissue (Querio et al., 2021). In 2019, “The top 10 causes of death” presented that IHD was the world’s biggest killer, responsible for 16% of total deaths worldwide (WHO, 2020). Myocardial blood flow blockage causes inflammatory reactions, energy metabolism disorders, micrangium damage, oxidative stress, calcium overload, and arrhythmia (Hao et al., 2021; Vilela and Fontes-Carvalho, 2021). Reperfusion therapy is the standard therapy for IHD; nevertheless, the restoration of blood flow to ischemic areas aggravates myocardial damage, namely, myocardial ischemia/reperfusion injury (MIRI) (Neri et al., 2017; Li et al., 2019). Evidence indicated that the death rate of acute myocardial infarction (AMI) patients treated with optimal reperfusion therapy was approximately 7% (Hausenloy and Yellon, 2016). MIRI involves multiple regulatory mechanisms, such as cell death, oxidative stress response, and mitochondrial dysfunction (Neri et al., 2015; Dong et al., 2019; Bugger and Pfeil, 2020). Apoptosis, which is a programmed cell death, is the critical factor in the development of MIRI (Charununtakorn et al., 2016). Apoptosis causes myocardial infarction, damages cardiac systolic/diastolic dysfunction and electrophysiological performance (Li CY. et al., 2020; Cai et al., 2020), and even leads to irreversible damage (Liu et al., 2015). Previous studies indicated that inhibiting apoptotic pathways could effectively alleviate MIRI (Zhai et al., 2017; Cai et al., 2020; Liao et al., 2021). Regulating apoptosis is a promising therapeutic strategy.
Ginsenosides are triterpenoid saponins, which are deemed as the main bioactive components of Panax ginseng (Sabouri-Rad et al., 2017). Panax means “all healing” in Greek (Im and Nah, 2013), and Panax ginseng has effects in improving arrhythmia, decreasing the myocardial ischemic area, suppressing oxidative stress response, enhancing immune regulation, and inhibiting apoptosis (Sun et al., 2016; Wang H. et al., 2021). Ginsenosides have positive effects on MIRI via regulating oxidative stress, inflammation, calcium overload, and cell deaths (Fan et al., 2020; Wang et al., 2020). Previous evidence indicated that ginsenosides could improve myocardial cell (MC) apoptosis to promote cardiac functions and reduce infarct size in MIRI (Zhang et al., 2016; Li CY. et al., 2020). Ginsenosides are classified into three types: protopanaxadiol (PPD) type, oleanolic acid type, and protopanaxatriol (PPT) type (Sun et al., 2016). Ginsenosides inhibit MC apoptosis via different apoptotic pathways, owing to their distinct chemical structures (Kim et al., 2015).
In this review, PubMed, Embase, Web of Science, and China National Knowledge Infrastructure (CNKI) were searched from inception to 21 September 2021 by using the following terms: ginsenoside, myocardial reperfusion injury, etc. This research included and reviewed research studies addressing the anti-apoptosis effects of ginsenosides on MIRI to provide references and suggestions for further research in this field.
Myocardial Cell Apoptosis in Myocardial Ischemia/Reperfusion Injury
Myocardial ischemia (MI) is a complex pathological condition resulting from initial restriction of blood supply to the heart (Korshunova et al., 2021), which causes tissue hypoxia (Eltzschig and Eckle, 2011) and impediment of re-synthesis of energy sources (e.g., ATP) (Gunata and Parlakpinar, 2021). The lack of ATP reduces the activity of sodium–potassium pumps on the membrane, leading to calcium overload (Zhang et al., 2020). Calcium overload induces arrhythmias (Tribulova et al., 2016; Sugiyama et al., 2021), mitochondrial dysfunction (Tian and Zhang, 2021), and MC apoptosis (Gao et al., 2021). The recommended therapy of MI is reperfusion, namely, restoration of blood flow to ischemic areas (Jneid et al., 2017). Nevertheless, it also causes further myocardial damage (Virani et al., 2020; Ren et al., 2021). MIRI is characterized by metabolic disturbance, cardiac dysfunction, inflammatory reaction, and cell death (apoptosis, autophagy, necrocytosis, pyroptosis, ferroptosis) (Moens et al., 2005; Eltzschig and Eckle, 2011; Hwang et al., 2021; Lv et al., 2021; Peng et al., 2021).
Cell death occurs widely during pathological processes in multiple diseases and is one of the leading causes of death (Wang F. et al., 2021). Apoptosis is a type of programmed cell death, characterized by cell shrinkage, chromatin condensation, and nuclear shrinkage (Hotchkiss et al., 2009). Apoptosis pathways include the death receptor apoptosis pathway, mitochondria apoptosis pathway, endoplasmic reticulum (ER) pathway, PI3K/Akt signaling pathway, NF-κB signaling pathway, and mitogen-activated protein kinase (MAPK) signaling pathway (Hotchkiss et al., 2009; Lai et al., 2021; Zhu and Zhou, 2021). Death receptors are activated by their ligands, namely, FasL, TNF-α, and TRAIL (Zhang et al., 2019a). Death-inducing signaling complex formed by receptors and ligands can activate caspase-8, and activated caspase-8 further up-regulates caspase-3, caspase-6, and caspase-7, resulting in apoptosis (Jeremias et al., 2000; Hotchkiss et al., 2009; Zhang et al., 2019a). MIRI increases mitochondrial permeability and caspase-9 expression level to activate caspase-3, caspase-6, and caspase-7 (Gottlieb and Engler, 1999; Hotchkiss et al., 2009). The mitochondria pathway is regulated by Bax and Bcl-2, which dissociates cytochrome C (cyt-C) and further activates caspase proteins (Hamacher-Brady et al., 2006; Hotchkiss et al., 2009). NF-κB can be transported to the nucleus by binding to IκB, thus causing apoptosis (Hayden and Ghosh, 2004). And NF-κB has function of inhibiting anti-apoptotic protein Bcl-2 (Neamatallah et al., 2018). MIRI up-regulates the levels of reactive oxygen species (ROS) and ER stress and further leads to intracellular calcium overload and apoptosis (Chaudhari et al., 2014; Lai et al., 2020; Song et al., 2020; Shi et al., 2021). ROS activates ER stress and JNK to increase the content of ROS (Chaudhari et al., 2014; Son et al., 2014; Lu et al., 2017; Chu et al., 2019). Increased ROS activates the PI3K/Akt signaling pathway, and the PI3K/Akt signaling pathway can regulate Nrf2 and eNOS to affect apoptosis (Gao et al., 2002; Syamsunarno et al., 2021; Zheng et al., 2021). MAPK contains JNK, p38, and ERK (Chang and Karin, 2001). JNK, p38, and ERK affect cell apoptosis via regulating c-Jun, MAPKAP-2, and Nrf2 (Davis, 2000; Ai et al., 2015; Zhang et al., 2019a). Figure 1 shows the apoptosis pathways in MIRI.
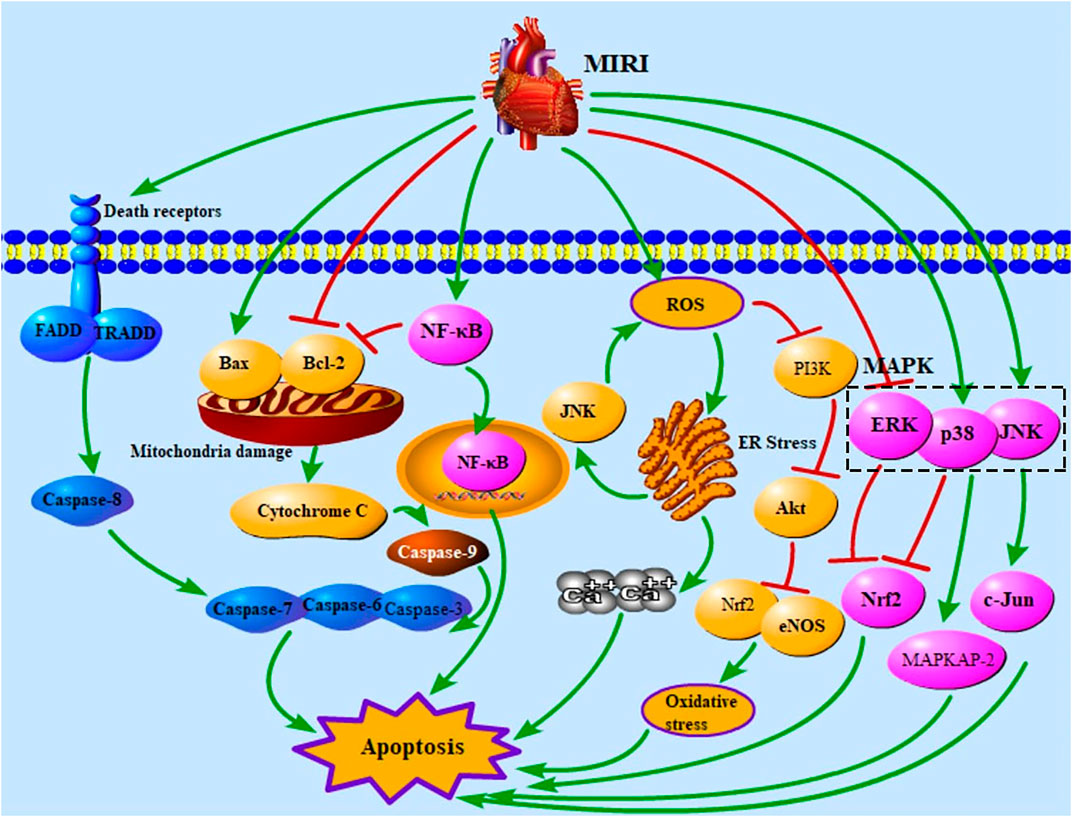
FIGURE 1. Apoptotic pathways in MIRI. MIRI, myocardial ischemia/reperfusion injury; FADD, Fas-associated death domain protein; TRADD, TNFR1-associated death domain protein; NF-κB, nuclear factor of kappaB; ROS, reactive oxygen species; ER, endoplasmic reticulum; PI3K, phosphatidylinositol-3-kinase; MAPK, mitogen-activated protein kinase; ERK, extracellular signal–regulated kinase; Nrf2, nuclear factor E2–related factor 2; eNOS, endothelial nitric oxide synthase; c-Jun, c-Jun N-terminal kinase; MAPKAP-2, MAPK-activated protein kinase-2.
Effects and Mechanisms of Ginsenosides on Myocardial Cell Apoptosis in Myocardial Ischemia/Reperfusion Injury
Panax ginseng, a medicinal plant, belongs to the Araliaceae family and has a long history of usage (Geng et al., 2010). The main active ingredients of Panax ginseng are ginsenosides (Wang H. et al., 2021), which inhibit oxidative stress, enhance immune regulation, promote physiological functions (Chen S. et al., 2019; Wang H. et al., 2021), and are adopted to improve IHD, depression, diabetes, etc. (Zhang JH. et al., 2021; Wang Q. et al., 2021; Jiang et al., 2021). Ginsenosides Rb1, Rb2, Rb3, Rd, Re, Rg1, Rg2, Rg3, Rh1, Rh2, Rh3, Rk3, and Rc were proved to alleviate MIRI. The chemical structures of ginsenosides determine their pharmacological effects, especially hydroxyl groups and sugar moieties (Kim et al., 2015). Based on the differences in the parent ring structure, ginsenosides are divided into PPD (Ra1/2/3, Rb1/2/3, Rc, Rd, Rg3, Rh2, F2, compound K), oleanolic acid (Rh3, Ro, Ri), and PPT (Re, Rf, Rg1/2, Rh1, F1) types (Sun et al., 2016). The parent ring structure of PPD type ginsenosides contains two hydroxyl groups at C-3 and C-12, and their sugar moieties attach to β-OH at C-3 and/or C-20 (Bai et al., 2014; Kim et al., 2015). The oleanolic acid type ginsenosides are comprised of a pentacyclic structure with the aglycone oleanolic acid (Choi, 2008; Kim et al., 2015). The parent ring structure of PPT type ginsenosides contains three hydroxyl groups at C-3, C-6, and C-12, and sugar moieties attach to β-OH at C-20 and/or α-OH at C-6 (Bai et al., 2014; Kim et al., 2015). It was demonstrated that the three types of ginsenosides were effective in inhibiting MC apoptosis and regulating different apoptotic pathways to relieve MIRI. According to the included studies, PPD type ginsenosides can trigger the death receptor–mediated signaling pathway; PPD and PPT type ginsenosides both regulate PI3K/Akt–mediated and NF-κB–mediated signaling pathways; furthermore, all three types of ginsenosides can affect mitochondria- and MAPK-mediated signaling pathways. The chemical structures of the ginsenosides included in this study are shown in Figure 2. The mechanisms of ginsenosides on MC apoptosis in MIRI are summarized in Supplementary Table S1.
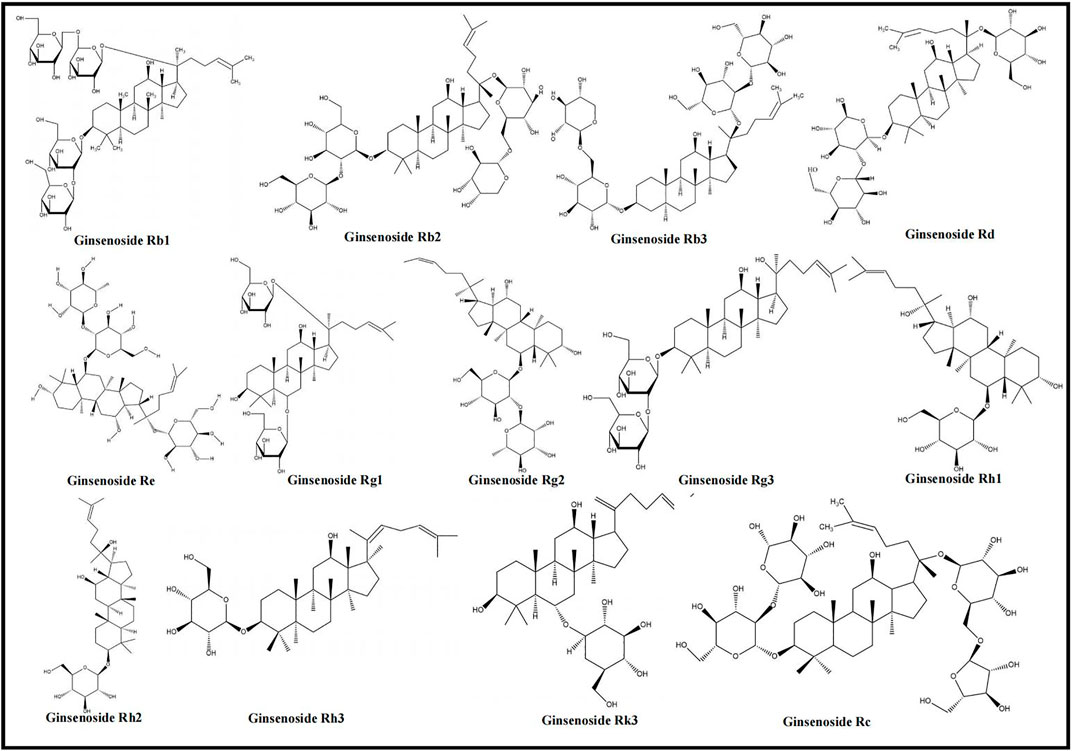
FIGURE 2. Chemical structures of ginsenosides (ginsenosides Rb1, Rb2, Rb3, Rd, Re, Rg1, Rg2, Rg3, Rh1, Rh2, Rh3, Rk3, and Rc).
Death Receptor–Mediated Signaling Pathway
The death receptor–mediated signaling pathway is the extrinsic pathway of apoptosis, induced by the binding of death receptors and their death ligands (Fas/FasL, TRAIL/TRAILR1, TRAIL/TRAILR2, TNF/TNFR1) (Galluzzi et al., 2012; Lee et al., 2012). Published studies addressing the death receptor–mediated MC apoptotic pathway of ginsenosides focus on the Fas/FasL signaling pathway. Fas/FasL binds to the FADD and transmits the apoptotic signal to procaspase-8, resulting in the formation of death-induced signal complex (DISC), which leads to caspase hydrolysis (Lee et al., 2012; Wang and Su, 2018). The combination of ginsenosides Rb3 and Rb2/Rb3 effectively regulates FasL and FADD to decrease the levels of caspase-8 and caspase-3 (Liu, 2014), while ginsenoside Rb1 improves MC apoptosis via the down-regulation of caspase-8 and caspase-3 (Ai et al., 2015). Moreover, caspase-8 can trigger the mitochondria-mediated signaling pathway by cleaving Bid (Chae et al., 2007). Bid decreases the level of Bcl-2 and increases the level of Bax to increase the release of cyt-C, and the levels of caspase-9 and caspase-3 (Chen et al., 2001; Pasdois et al., 2011; Kim et al., 2021). Ai et al. reported that ginsenoside Rb1 down-regulated caspase-8, bid, caspase-9, caspase-3, and cyt-C (Ai et al., 2015). The death receptor–mediated signaling pathway of ginsenosides in relieving MC apoptosis is shown in Figure 3.
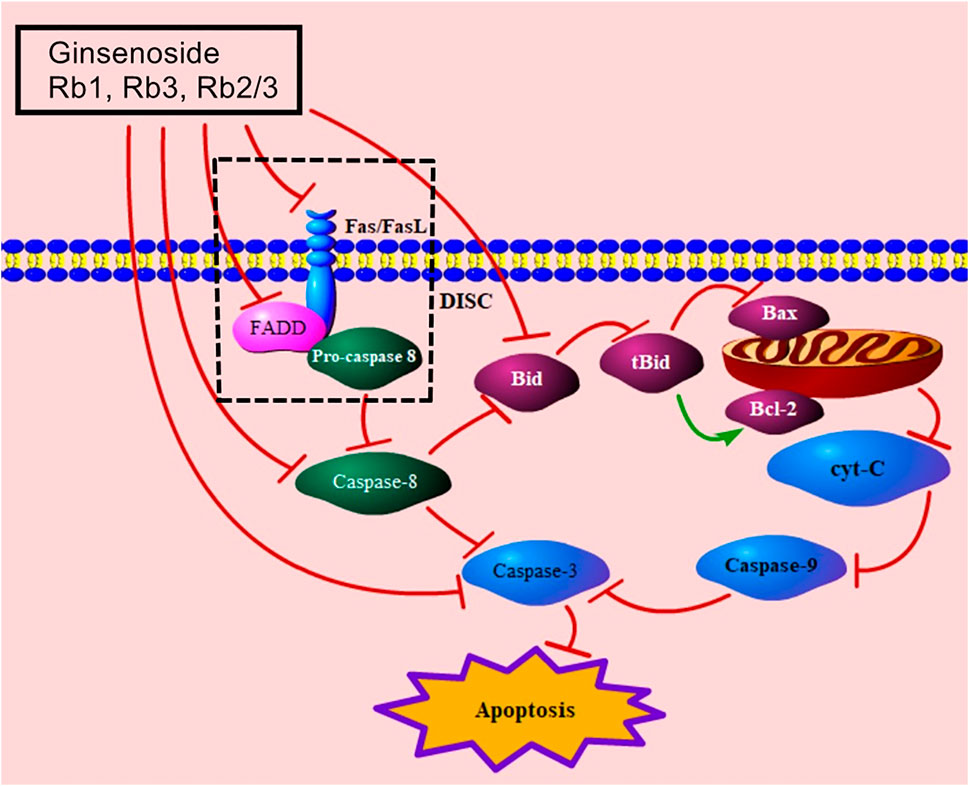
FIGURE 3. Death receptor–mediated signaling pathway of ginsenoside in relieving MC apoptosis. FasL, Fas ligand; FADD, Fas-associated death domain protein; DISC, death-induced signal complex; tBid, truncated Bid; cyt-C, cytochrome C.
Mitochondria-Mediated Signaling Pathway
Mitochondria play a significant role in adjusting metabolism, generating ROS, and guaranteeing cell activity (Zhang et al., 2019a; Zhang X. et al., 2021). Bcl-2 inhibits apoptosis, whereas Bax promotes it, causing damage to the membrane structure and potential of mitochondria (Wang Q. et al., 2016). When the balance between Bcl-2 and Bax is disrupted, the mitochondrial membrane potential is reduced and the permeability of mitochondrial membrane is increased (Wang G. et al., 2021). Damaged mitochondria release cyt-C, which then increases the levels of caspase-9 and caspase-3 (Zhang et al., 2019a; Pu et al., 2013); increased caspase-3 up-regulates ADP-ribose polymerase (PARP), leading to apoptosis (Aggeli et al., 2021; Toit et al., 2020). Previous studies indicated that ginsenoside Rb1, Rb2, Rb3, Rb2/Rb3 combination, Rd, Re, Rg1, Rg2, Rg3, Rh3, Rk3, Rc all relieved MC apoptosis via regulating Bax, Bcl-2, cyt-C, caspase-9, caspase-3, and PARP (Supplementary Table S1). Decreased SIRT1 can reduce Akt to trigger the mitochondria-mediated signaling pathway by regulating JNK, Nrf2, and Bax (Ai et al., 2015; Pai et al., 2021). Nrf2, an important signaling molecule involved in cardioprotection (Zhu et al., 2008), regulates HO-1, which also has a cardioprotective effect (Liu et al., 2007; Zhu et al., 2008). The mitochondria-mediated signaling pathway, induced by ginsenosides Rb1, Rk3, and Rg3, is associated with the regulation of Akt, Nrf2, and HO-1 (Sun, 2013; Ai et al., 2015; Li L. et al., 2020). Ginsenosides Rb2 and Rg2 also up-regulate SIRT1 to trigger the mitochondria-mediated apoptotic pathway (Fu et al., 2018; Xue et al., 2020). Ginsenoside Rb3 was found to be effective in regulating Nrf2 (Sun et al., 2019). Additionally, previous studies showed that Rb2/Rb3 combination, Rd and Rg1 reduced mitochondria damage via increment of Akt (Wang et al., 2013; Liu, 2014; Qin et al., 2018). Dephosphorylated Drp1 is recruited to the mitochondrial outer membrane to cause damage to mitochondria (Yang, 2013). Ginsenoside Rb1 can inhibit the mRNA level of Drp1 (Yang, 2013). The mitochondria-mediated signaling pathway of ginsenosides in improving MIRI is presented in Figure 4.
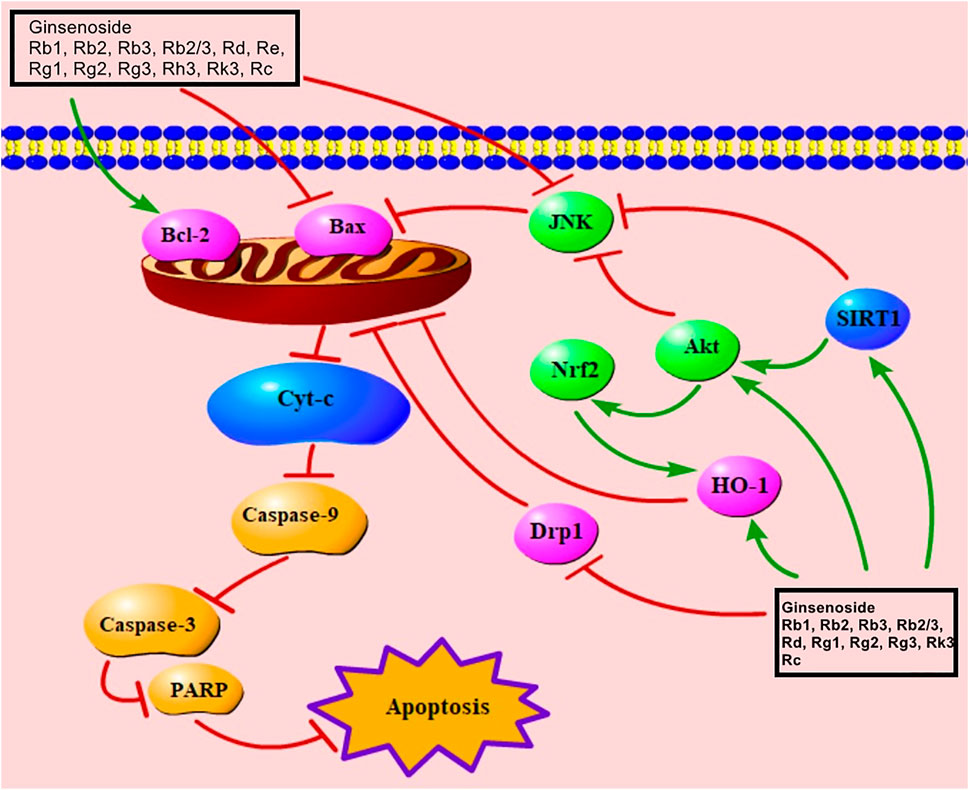
FIGURE 4. Mitochondria-mediated signaling pathway of ginsenoside in relieving MC apoptosis. Cyt-C, cytochrome C; PARP, poly(ADP-ribose) polymerase; Nrf2, nuclear factor E2–related factor 2; SIRT1, sirtuin 1; Drp1, dynamin-related protein 1; HO-1, heme oxygenase-1.
PI3K/Akt-Mediated Signaling Pathway
The PI3K/Akt signaling pathway is an important pathway by which ginsenosides improve apoptosis. The activation of this pathway promotes angiogenesis, alleviates tissue hypoxia, suppresses cell damage, and improves MC apoptosis (Wang M. et al., 2021; Zhang J. et al., 2021; Cao et al., 2021). Evidence showed that ginsenoside Rb1, Rb3, Rb2/Rb3 combination, Rd, Rg1, Rg2, Rg3, Rh1, Rh2, Rk3 activated the PI3K/Akt-mediated signaling pathway (Supplementary Table S1). Activated PI3K can phosphorylate Akt to regulate Nrf2, JNK, eNOS, and NF-κB for decreasing the number of apoptotic cells (Liu SX. et al., 2012; Ai et al., 2015; Liu et al., 2017; Luan et al., 2019; Fu Y. et al., 2021). When MIRI occurs, Nrf2 is released from Keap1 to the nucleus and activates HO-1 to alleviate apoptosis (Mann et al., 2007). Moreover, Nrf2 nuclear export is regulated by the MAPK-mediated signaling pathway (Mann et al., 2007). Ginsenosides Rb1 and Rk3 both increase Nrf2 by down-regulating JNK, ERK, and p38 MAPK (Sun, 2013; Ai et al., 2015). As mentioned above, the PI3K/Akt signaling pathway can decrease JNK to trigger the mitochondria-mediated signaling pathway for inhibiting apoptosis. A previous study presented that ginsenosides Rg2, Rg3, Rh1, and Rh2 up-regulated Akt and down-regulated JNK to inhibit apoptosis (Feng et al., 2017). Phosphorylated eNOS increases NO production and improves MC apoptosis (He et al., 2016), and ginsenosides Rb1, Rg1, and Rg3 can induce the phosphorylation of eNOS (Wang, 2008; Wang et al., 2015; Qin et al., 2018). In addition, ginsenosides Rb3 and Rg1 both up-regulate Akt to decrease NF-κB (Li, 2014; Ma et al., 2014). The PI3K/AKT/NF-κB ginsenoside pathway is considered an important mediator of cell survival and immune responses (Peng et al., 2013). This PI3K/Akt-mediated signaling pathway is presented in Figure 5.
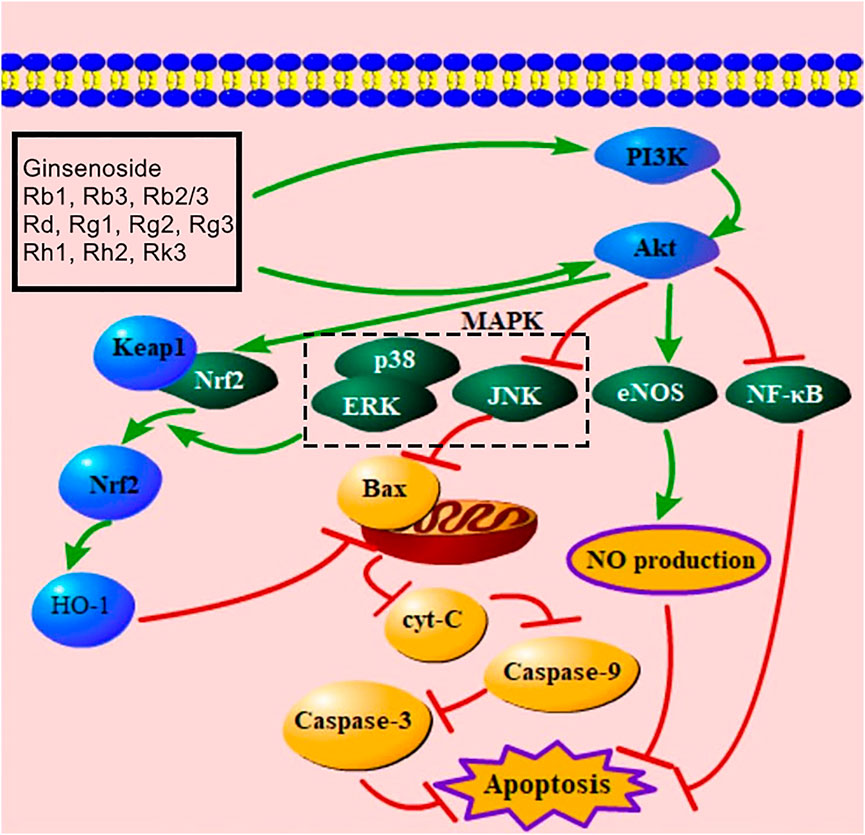
FIGURE 5. PI3K/Akt-mediated signaling pathway of ginsenoside in relieving MC apoptosis. PI3K, phosphatidylinositol-3-kinase; MAPK, mitogen-activated protein kinase; Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor E2–related factor 2; HO-1, heme oxygenase-1; ERK, extracellular signal–regulated kinase; Cyt-C, cytochrome C; eNOS, endothelial nitric oxide synthase; NF-κB, nuclear factor of kappaB; NO, nitric oxide.
NF-κB–Mediated Signaling Pathway
NF-κB belongs to a family of related transcription factors and participates in the regulation of immune responses, proinflammatory cytokines’ control, and cell death (Hussen et al., 2021; Hall et al., 2006). The Rel homology domain of NF-κB binds to IκB, and the complex of NF-κB/IκB inhibits the transport of NF-κB to the nucleus, thus inducing apoptosis (Hayden and Ghosh, 2004). Meanwhile, IκB is phosphorylated by IKK (Hayden and Ghosh, 2008). Recent studies have stated that ginsenosides Rb1, Rb3, Re, Rg1, and Rg3 can down-regulate IKKα, IκBα, and NF-κB, thus relieving MC apoptosis via inhibiting the NF-κB–mediated signaling pathway (Supplementary Table S1). Moreover, NF-κB can down-regulate Bcl-2 to trigger the mitochondria-mediated signaling pathway (Neamatallah et al., 2018; Duan et al., 2021). Figure 6 presents the NF-κB–mediated signaling pathway of ginsenoside in relieving MC apoptosis.
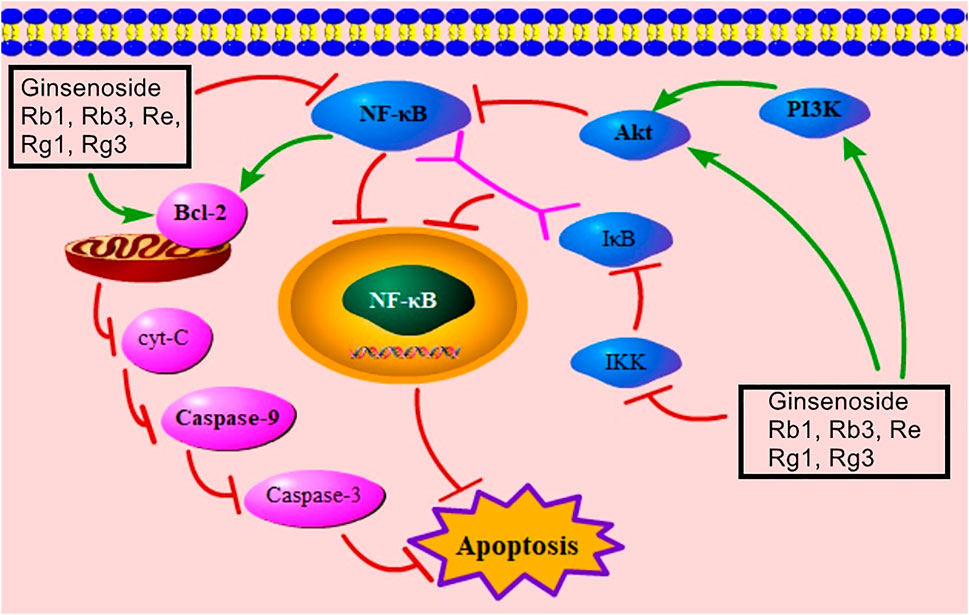
FIGURE 6. NF-κB–mediated signaling pathway of ginsenoside in relieving MC apoptosis. NF-κB, nuclear factor of kappaB; cyt-C, cytochrome C; PI3K, phosphatidylinositol-3-kinase; IκB, inhibitor of NF-κB; IKK, IκB kinase.
MAPK-Mediated Signaling Pathway
MAPK is an important signal transducing enzyme that has effects on the regulation of gene expression, cell proliferation, and cell death (Chang and Karin, 2001). MAPK kinase (MAPKK) is activated by MAPK kinase kinase (MAPKKK) to reactivate MAPK (Junttila et al., 2008). MAPK includes JNK, p38, and ERK, which are activated by special MAPKK and have different functions (Chang and Karin, 2001). The activation of JNK and p38 mediates apoptosis (Junttila et al., 2008). JNK promotes apoptosis through regulating c-Jun, which is its most classical substrate (Davis, 2000). JNK also effectively regulates pro-apoptotic protein, Bax (Syeda et al., 2019). The p38 MAPK pathway is related to the regulation of inflammation, gene expression, and energetic metabolism (Bassi et al., 2008). p38 participates in the promotion of apoptosis via its substrates, such as MAPKAP-2, MSK-1, and GADD153 (Zhang et al., 2019a; Ashraf et al., 2014; Das et al., 2006). Inhibition of the p38 MAPK pathway up-regulates the levels of Nrf2 and HO-1 to increase antioxidative proteins and improve apoptosis (Chen et al., 2021). Evidence indicated that ginsenosides Rb1, Rb3, Rg2, Rg3, Rh1, Rh2, and Rk3 had a function of down-regulating JNK, and ginsenosides Rb1 and Rk3 decreased the level of p38 (Supplementary Table S1). ERK can proliferate cells and regulate cell growth, and activated ERK inhibits the formation of DISC to relieve death receptor–mediated signaling pathway–induced apoptosis (Meloche and Pouysségur, 2007; Holmström et al., 2000). Additionally, ERK can increase Nrf2 to alleviate mitochondria damage (Ai et al., 2015). Ginsenosides Rb1, Rg1, and Rk3 can up-regulate ERK (Supplementary Table S1). Notably, one study indicated that ginsenoside Rb1 down-regulated ERK to inhibit apoptosis (Ai et al., 2015). In this study, MIRI increases the level of ERK. And over-expressed ERK leads to reversible or permanent cell cycle arrest (Meloche and Pouysségur, 2007); thus, ginsenoside Rb1 may decrease over-expressed ERK induced by apoptosis to protect MCs. Figure 7 showed MAPK-mediated signaling pathway of ginsenoside in relieving MC apoptosis.
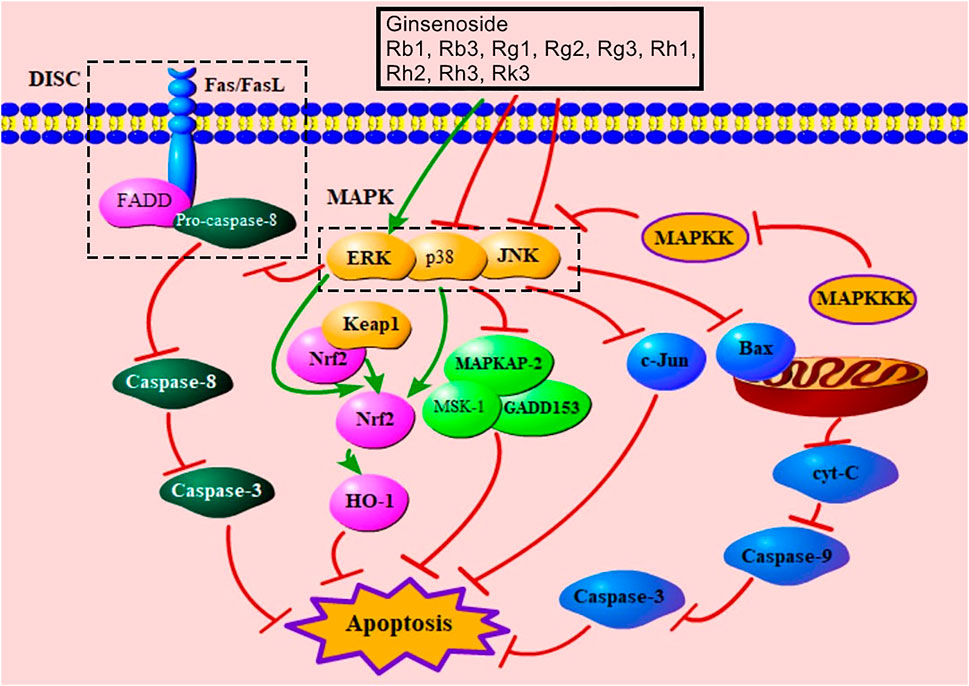
FIGURE 7. MAPK-mediated signaling pathway of ginsenoside in relieving MC apoptosis. DISC, death-induced signal complex; FasL, Fas ligand; FADD, Fas-associated death domain protein; MAPK, mitogen-activated protein kinase; ERK, extracellular signal–regulated kinase; Keap1, Kelch-like ECH-associated protein 1; Nrf2, nuclear factor E2–related factor 2; HO-1, heme oxygenase-1; MAPKAP-2, MAPK-activated protein kinase-2; MSK-1, mitogen- and stress-activated protein kinase; MAPKK, MAPK kinase; MAPKKK, MAPK kinase kinase; c-Jun, c-Jun N-terminal kinase; cyt-C, cytochrome C.
Other Pathways
In addition to the above-mentioned apoptotic signaling pathway, other pathways have been reported in previous studies. Li et al. reported that ginsenoside Rb1 improved MIRI by preserving PDH activity and inhibiting SDH activity (Li et al., 2017). Ginsenoside Rb1 also inhibits apoptosis by regulating microRNAs (miRNAs), namely, mir-208, mir-1, mir-29a, mir-21, and mir-320 (Yan et al., 2015; Yan et al., 2016). In 2012, Zhang et al. indicated that ginsenoside Rg1 increased ATP content and mTOR and decreased AMPKα, LC3B-1, and Beclin-1 to inhibit apoptosis and autophagy (Zhang et al., 2012). Moreover, ginsenoside Rg2 improves antioxidant enzyme activity (SOD, LDH, GXH-Px), and ginsenoside Rh3 increases SERCA (Zhou, 2009; Wang J. et al., 2016).
Conclusion and Perspective
MIRI is functional and organic damage to the heart, which results from restoration of blood flow in ischemic areas (Wang K. et al., 2021). Through a number of studies addressing MIRI, the mechanisms of MIRI have not been fully revealed. Previous studies indicated that MC apoptosis was one of the fundamental pathogenic factors of MIRI, and the inhibition of MC apoptosis was effective in alleviating MIRI (Zhou et al., 2018; Xu et al., 2019; Fu D. et al., 2021). Ginsenosides can improve MIRI by relieving mitochondria damage, resisting oxidation, reducing inflammatory response, and inhibiting the generation of DISC (Wang and Roh, 2020; Shaukat et al., 2021). Ginsenosides can relieve MIRI via multiple signaling pathways, such as the death receptor signaling pathway, mitochondria signaling pathway, PI3K/Akt signaling pathway, NF-κB signaling pathway, and MAPK signaling pathway. The occurrence and development of MIRI is complex and multi-factor interacted; thus, it is vital to investigate multi-target therapy in future studies. Ginsenosides, which are regarded as undoubtedly low-toxicity drugs (Xu JF. et al., 2021), have favorable safety profiles (Mancuso and Santangelo, 2017). Toxicity studies showed that most ginsenosides have no oral toxicity, such as Re, Rg2, and Rh2 (Wang et al., 2006; Lu et al., 2012; Gou et al., 2020). Undeniably, in vitro studies indicated that ginsenosides Rb1, Rg1, and Re had embryotoxic and teratogenic effects (Chan et al., 2004; Liu et al., 2005; Liu et al., 2006). However, results from in vitro animal studies may not reflect the true conditions in humans; thus, previous studies suggested that these ginsenosides need to be used with caution in clinics during the first trimester of gestation, before more data in humans are available (Liu et al., 2006; Mancuso and Santangelo, 2017). Overall, the development and application of ginsenosides in improving MIRI are significant, and the toxicity data from in vivo studies and clinical studies are needed.
Currently, multiple studies have explored the anti-apoptotic mechanism of ginsenosides; however, problems still exist, and further studies are needed. Firstly, the research studies about the structure–function relationship of ginsenosides in inhibiting MIRI are still needed to be conducted. The hydroxyl groups and sugar moieties have influences on the pharmacological effects of ginsenosides, which can interact with membrane lipids (Kim et al., 2015). Thus, the research of structure–function relationship of ginsenosides in anti-MIRI can contribute to developing safe and effective drugs via chemical modification. Secondly, the current results are mainly generated by ex vivo experiments or animal experiments. Rare clinical evidence has showed that ginsenoside Rb has protective effects on MIRI in patients undergoing mitral valve surgery (Zhan et al., 1994). Existing studies are in infancy, and more clinical research studies are needed to be designed and conducted to supply further clinical evidence. Thirdly, evidence showed that pyroptosis occurred during the development of MIRI (Xu XN. et al., 2021; Ji et al., 2021), and pro-apoptotic caspase-3 can cleave GSDME to induce pyroptosis (Wang Y. et al., 2017). However, no study has confirmed the anti-pyroptosis effect of ginsenosides in improving MIRI. Thus, the mechanisms of ginsenosides need to be further explored.
Overall, this review of anti-apoptotic mechanisms of ginsenoside in MIRI presents pharmacological mechanisms and lays the foundation for further research studies, hoping to contribute to the development of undiscovered mechanism and new drugs.
Author Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81873239 and 81573885) and Xinglin Scholar Research Premotion Project of Chengdu University of TCM (Nos. YXRC2018014).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.806216/full#supplementary-material
References
Aggeli, I. K., Kapogiannatou, A., Paraskevopoulou, F., and Gaitanaki, C. (2021). Differential Response of Cardiac Aquaporins to Hyperosmotic Stress; Salutary Role of AQP1 against the Induced Apoptosis. Eur. Rev. Med. Pharmacol. Sci. 25, 313–325. doi:10.26355/eurrev_202101_24397
Ai, Q., Sun, G., Luo, Y., Dong, X., Hu, R., Meng, X., et al. (2015). Ginsenoside Rb1 Prevents Hypoxia-Reoxygenation-Induced Apoptosis in H9c2 Cardiomyocytes via an Estrogen Receptor-dependent Crosstalk Among the Akt, JNK, and ERK 1/2 Pathways Using a Label-free Quantitative Proteomics Analysis. RSC Adv. 5, 26346–26363. doi:10.1039/c5ra02432c
Ashraf, M. I., Ebner, M., Wallner, C., Haller, M., Khalid, S., Schwelberger, H., et al. (2014). A p38MAPK/MK2 Signaling Pathway Leading to Redox Stress, Cell Death and Ischemia/reperfusion Injury. Cell Commun Signal 12, 6. doi:10.1186/1478-811X-12-6
Bai, M., Mao, Q., Xu, J. D., Zhu, L. Y., Zhu, H., Wang, Q., et al. (2014). Advance in Saponins of Aerial Parts of Panax Species. Zhongguo Zhong Yao Za Zhi 39, 412–422.
Bao, J., Zhang, X., Lu, X., and Rao, M. (2010). Effect of Panax Notoginsenoside Rb1 and Rg1 on Cell Apoptosis of Hypoxia-Reoxygenation Injury in Angiotensin Ⅱ Induced Hypertrophied Neonatal Rat Myocytes. Gansu Med. J. 29, 121–124.
Bassi, R., Heads, R., Marber, M. S., and Clark, J. E. (2008). Targeting P38-MAPK in the Ischaemic Heart: Kill or Cure? Curr. Opin. Pharmacol. 8, 141–146. doi:10.1016/j.coph.2008.01.002
Bugger, H., and Pfeil, K. (2020). Mitochondrial ROS in Myocardial Ischemia Reperfusion and Remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 1866, 165768. doi:10.1016/j.bbadis.2020.165768
Cai, Y., Ying, F., Liu, H., Ge, L., Song, E., Wang, L., et al. (2020). Deletion of Rap1 Protects against Myocardial Ischemia/reperfusion Injury through Suppressing Cell Apoptosis via Activation of STAT3 Signaling. FASEB J. 34, 4482–4496. doi:10.1096/fj.201901592RR
Cao, L., Gao, Y., Zhu, J., Zhang, J., Dong, M., and Mao, Y. (2020). Protective Action of the Ginsenoside Rh3 in a Rat Myocardial Ischemia-Reperfusion Injury Model by Inhibition of Apoptosis Induced via P38 Mitogen-Activated Protein Kinase/caspase-3 Signaling. J. Int. Med. Res. 48, 300060520969090. doi:10.1177/0300060520969090
Cao, X. (2004). Experimental Studies on Protective Effects of Ginsenoside Re on Myocardium of Ischemia-Reperfusion Injury. Jilin (China): Jilin University. [Doctor's Thesis].
Cao, Y., Li, Q., Yang, Y., Ke, Z., Chen, S., Li, M., et al. (2021). Cardioprotective Effect of Stem-Leaf Saponins from Panax Notoginseng on Mice with Sleep Deprivation by Inhibiting Abnormal Autophagy through PI3K/Akt/mTOR Pathway. Front. Cardiovasc. Med. 8, 694219. doi:10.3389/fcvm.2021.694219
Chae, S. U., Ha, K. C., Piao, C. S., Chae, S. W., and Chae, H. J. (2007). Estrogen Attenuates Cardiac Ischemia-Reperfusion Injury via Inhibition of Calpain-Mediated Bid Cleavage. Arch. Pharm. Res. 30, 1225–1235. doi:10.1007/BF02980263
Chan, L. Y., Chiu, P. Y., and Lau, T. K. (2004). Embryotoxicity Study of Ginsenoside Rc and Re in In Vitro Rat Whole Embryo Culture. Reprod. Toxicol. 19, 131–134. doi:10.1016/j.reprotox.2004.06.001
Chang, L., and Karin, M. (2001). Mammalian MAP Kinase Signalling Cascades. Nature 410, 37–40. doi:10.1038/35065000
Charununtakorn, S. T., Shinlapawittayatorn, K., Chattipakorn, S. C., and Chattipakorn, N. (2016). Potential Roles of Humanin on Apoptosis in the Heart. Cardiovasc. Ther. 34, 107–114. doi:10.1111/1755-5922.12168
Chaudhari, N., Talwar, P., Parimisetty, A., Lefebvre d'Hellencourt, C., and Ravanan, P. (2014). A Molecular Web: Endoplasmic Reticulum Stress, Inflammation, and Oxidative Stress. Front Cel Neurosci 8, 213. doi:10.3389/fncel.2014.00213
Chen, L., Guo, Y., Qu, S., Li, K., Yang, T., Yang, Y., et al. (2021). The Protective Effects of Shengmai Formula against Myocardial Injury Induced by Ultrafine Particulate Matter Exposure and Myocardial Ischemia Are Mediated by the PI3K/AKT/p38 MAPK/Nrf2 Pathway. Front. Pharmacol. 12, 619311. doi:10.3389/fphar.2021.619311
Chen, M., He, H., Zhan, S., Krajewski, S., Reed, J. C., and Gottlieb, R. A. (2001). Bid Is Cleaved by Calpain to an Active Fragment In Vitro and during Myocardial Ischemia/reperfusion. J. Biol. Chem. 276, 30724–30728. doi:10.1074/jbc.M103701200
Chen, S., Li, X., Wang, Y., Mu, P., Chen, C., Huang, P., et al. (2019a). Ginsenoside Rb1 Attenuates Intestinal Ischemia/reperfusion-induced I-nflammation and O-xidative S-tress via A-ctivation of the PI3K/Akt/Nrf2 S-ignaling P-athway. Mol. Med. Rep. 19, 3633–3641. doi:10.3892/mmr.2019.10018
Chen, X., Wang, Q., Shao, M., Ma, L., Guo, D., Wu, Y., et al. (2019b). Ginsenoside Rb3 Regulates Energy Metabolism and Apoptosis in Cardiomyocytes via Activating PPARα Pathway. Biomed. Pharmacother. 120, 109487. doi:10.1016/j.biopha.2019.109487
Choi, K. T. (2008). Botanical Characteristics, Pharmacological Effects and Medicinal Components of Korean Panax Ginseng C A Meyer. Acta Pharmacol. Sin 29, 1109–1118. doi:10.1111/j.1745-7254.2008.00869.x
Chu, Q., Zhang, Y., Zhong, S., Gao, F., Chen, Y., Wang, B., et al. (2019). N-n-Butyl Haloperidol Iodide Ameliorates Oxidative Stress in Mitochondria Induced by Hypoxia/Reoxygenation through the Mitochondrial C-Jun N-Terminal Kinase/Sab/Src/Reactive Oxygen Species Pathway in H9c2 Cells. Oxid Med. Cel Longev 2019, 7417561. doi:10.1155/2019/7417561
Cui, Y. C., Pan, C. S., Yan, L., Li, L., Hu, B. H., Chang, X., et al. (2017). Ginsenoside Rb1 Protects against Ischemia/reperfusion-Induced Myocardial Injury via Energy Metabolism Regulation Mediated by RhoA Signaling Pathway. Sci. Rep. 7, 44579. doi:10.1038/srep44579
Das, S., Tosaki, A., Bagchi, D., Maulik, N., and Das, D. K. (2006). Potentiation of a Survival Signal in the Ischemic Heart by Resveratrol through P38 Mitogen-Activated Protein Kinase/mitogen- and Stress-Activated Protein Kinase 1/cAMP Response Element-Binding Protein Signaling. J. Pharmacol. Exp. Ther. 317, 980–988. doi:10.1124/jpet.105.095133
Davis, R. J. (2000). Signal Transduction by the JNK Group of MAP Kinases. Cell 103, 239–252. doi:10.1016/s0092-8674(00)00116-1
Dong, Y., Chen, H., Gao, J., Liu, Y., Li, J., and Wang, J. (2019). Molecular Machinery and Interplay of Apoptosis and Autophagy in Coronary Heart Disease. J. Mol. Cel Cardiol 136, 27–41. doi:10.1016/j.yjmcc.2019.09.001
Du Toit, E. F., Tai, W. S., Cox, A., O'Connor, D., Griffith, T. A., Helman, T., et al. (2020). Synergistic Effects of Low-Level Stress and a Western Diet on Metabolic Homeostasis, Mood, and Myocardial Ischemic Tolerance. Am. J. Physiol. Regul. Integr. Comp. Physiol. 319, R347–R357. doi:10.1152/ajpregu.00322.2019
Duan, H., Zhang, Q., Liu, J., Li, R., Wang, D., Peng, W., et al. (2021). Suppression of Apoptosis in Vascular Endothelial Cell, the Promising Way for Natural Medicines to Treat Atherosclerosis. Pharmacol. Res. 168, 105599. doi:10.1016/j.phrs.2021.105599
Eltzschig, H. K., and Eckle, T. (2011). Ischemia and Reperfusion-Ffrom Mechanism to Translation. Nat. Med. 17, 1391–1401. doi:10.1038/nm.2507
Fan, H., Xiaoling, S., Yaliu, S., Mingming, L., Xiansheng, M., and Li, F. (2015). Tissue Distribution of Ginsenoside Rg3 and its Metabolites in the Body of Rats. Pharmacol. Clin. Chin. Materia Med. 31, 16–19.
Fan, W., Huang, Y., Zheng, H., Li, S., Li, Z., Yuan, L., et al. (2020). Ginsenosides for the Treatment of Metabolic Syndrome and Cardiovascular Diseases: Pharmacology and Mechanisms. Biomed. Pharmacother. 132, 110915. doi:10.1016/j.biopha.2020.110915
Feng, R., Liu, J., Wang, Z., Zhang, J., Cates, C., Rousselle, T., et al. (2017). The Structure-Activity Relationship of Ginsenosides on Hypoxia-Reoxygenation Induced Apoptosis of Cardiomyocytes. Biochem. Biophys. Res. Commun. 494, 556–568. doi:10.1016/j.bbrc.2017.10.056
Fu, D., Gao, T., Liu, M., Li, C., Li, H., Li, H., et al. (2021b). LncRNA TUG1 Aggravates Cardiomyocyte Apoptosis and Myocardial Ischemia/reperfusion Injury. Histol. Histopathol 18381, 1–33. doi:10.14670/HH-18-381
Fu, W., Xu, H., Yu, X., Lyu, C., Tian, Y., Guo, M., et al. (2018). 20(S)-Ginsenoside Rg2 Attenuates Myocardial Ischemia/reperfusion Injury by Reducing Oxidative Stress and Inflammation: Role of SIRT1. RSC Adv. 8, 23947–23962. doi:10.1039/C8RA02316F
Fu, W., Yu, X., Lu, Z., Sun, F., Wang, Y., Zhang, Y., et al. (2016). Protective Effects of Ginsenoside Rb2 on Myocardial Ischemia In Vivo and In Vitro. Int. J. Clin. Exp. Med. 9, 9843–9855.
Fu, X., Zhang, Q., Luo, R., and Wang, P. (2020). Protective Effect of Ginsenoside Rh3 on Myocardial Ischemia-Reperfusion Injury in Rats by Regulation of P38 MAPK/caspase-3 Signaling Pathway. Int. J. Clin. Exp. Med. 13, 3212–3218.
Fu, Y., Zhou, J.-D., Sang, X.-Y., and Zhao, Q.-T. (2021a). Gualou-Xiebai-Banxia Decoction Protects against Type II Diabetes with Acute Myocardial Ischemia by Attenuating Oxidative Stress and Apoptosis via PI3K/Akt/eNOS Signaling. Chin. J. Nat. Medicines 19, 161–169. doi:10.1016/S1875-5364(21)60017-1
Galluzzi, L., Vitale, I., Abrams, J. M., Alnemri, E. S., Baehrecke, E. H., Blagosklonny, M. V., et al. (2012). Molecular Definitions of Cell Death Subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ 19, 107–120. doi:10.1038/cdd.2011.96
Gao, F., Gao, E., Yue, T. L., Ohlstein, E. H., Lopez, B. L., Christopher, T. A., et al. (2002). Nitric Oxide Mediates the Antiapoptotic Effect of Insulin in Myocardial Ischemia-Reperfusion: the Roles of PI3-Kinase, Akt, and Endothelial Nitric Oxide Synthase Phosphorylation. Circulation 105, 1497–1502. doi:10.1161/01.cir.0000012529.00367.0f
Gao, T., Yang, P., Fu, D., Liu, M., Deng, X., Shao, M., et al. (2021). The Protective Effect of Allicin on Myocardial Ischemia-Reperfusion by Inhibition of Ca2+ Overload-Induced Cardiomyocyte Apoptosis via the PI3K/GRK2/PLC-Γ/ip3r Signaling Pathway. Aging (Albany NY) 13, 19643–19656. doi:10.18632/aging.203375
Gao, Y., Yang, J., Wang, Y., and Wang, Q. (2011). Effect of Ginsenoside Re on Rat Myocardial Ischemia-Reperfusion Apoptosis and Caspase-3. J. Liaoning Univ. TCM 13, 123–124.
Geng, J., Dong, J., Ni, H., Lee, M. S., Wu, T., Jiang, K., et al. (2010). Ginseng for Cognition. Cochrane Database Syst. Rev. 2010, CD007769. doi:10.1002/14651858.CD007769.pub2
Gottlieb, R. A., and Engler, R. L. (1999). Apoptosis in Myocardial Ischemia-Reperfusion. Ann. N. Y Acad. Sci. 874, 412–426. doi:10.1111/j.1749-6632.1999.tb09255.x
Gou, D., Pei, X., Wang, J., Wang, Y., Hu, C., Song, C., et al. (2020). Antiarrhythmic Effects of Ginsenoside Rg2 on Calcium Chloride-Induced Arrhythmias without Oral Toxicity. J. Ginseng Res. 44, 717–724. doi:10.1016/j.jgr.2019.06.005
Guan, L., Li, W., and Liu, Z. (2002). Effect of Ginsenoside-Rb1 on Cardiomyocyte Apoptosis after Ischemia and Reperfusion in Rats. J. Huazhong Univ. Sci. Technolog Med. Sci. 22, 212–215. doi:10.1007/BF02828182
Gunata, M., and Parlakpinar, H. (2021). A Review of Myocardial Ischaemia/reperfusion Injury: Pathophysiology, Experimental Models, Biomarkers, Genetics and Pharmacological Treatment. Cell Biochem Funct 39, 190–217. doi:10.1002/cbf.3587
Hall, G., Hasday, J. D., and Rogers, T. B. (2006). Regulating the Regulator: NF-kappaB Signaling in Heart. J. Mol. Cel Cardiol 41, 580–591. doi:10.1016/j.yjmcc.2006.07.006
Hamacher-Brady, A., Brady, N. R., and Gottlieb, R. A. (2006). The Interplay between Pro-death and Pro-survival Signaling Pathways in Myocardial Ischemia/reperfusion Injury: Apoptosis Meets Autophagy. Cardiovasc. Drugs Ther. 20, 445–462. doi:10.1007/s10557-006-0583-7
Hausenloy, D. J., Barrabes, J. A., Bøtker, H. E., Davidson, S. M., Di Lisa, F., Downey, J., et al. (2016). Ischaemic Conditioning and Targeting Reperfusion Injury: a 30 year Voyage of Discovery. Basic Res. Cardiol. 111, 70. doi:10.1038/nrcardio.2016.510.1007/s00395-016-0588-8
Hayden, M. S., and Ghosh, S. (2008). Shared Principles in NF-kappaB Signaling. Cell 132, 344–362. doi:10.1016/j.cell.2008.01.020
Hayden, M. S., and Ghosh, S. (2004). Signaling to NF-kappaB. Genes Dev. 18, 2195–2224. doi:10.1101/gad.1228704
He, F., Xu, B. L., Chen, C., Jia, H. J., Wu, J. X., Wang, X. C., et al. (2016). Methylophiopogonanone A Suppresses Ischemia/reperfusion-Induced Myocardial Apoptosis in Mice via Activating PI3K/Akt/eNOS Signaling Pathway. Acta Pharmacol. Sin 37, 763–771. doi:10.1038/aps.2016.14
Holmström, T. H., Schmitz, I., Söderström, T. S., Poukkula, M., Johnson, V. L., Chow, S. C., et al. (2000). MAPK/ERK Signaling in Activated T Cells Inhibits CD95/Fas-Mediated Apoptosis Downstream of DISC Assembly. EMBO J. 19, 5418–5428. doi:10.1093/emboj/19.20.5418
Hotchkiss, R. S., Strasser, A., McDunn, J. E., and Swanson, P. E. (2009). Cell Death. N. Engl. J. Med. 361, 1570–1583. doi:10.1056/NEJMra0901217
Huang, Q., Su, H., Qi, B., Wang, Y., Yan, K., Wang, X., et al. (2021). A SIRT1 Activator, Ginsenoside Rc, Promotes Energy Metabolism in Cardiomyocytes and Neurons. J. Am. Chem. Soc. 143, 1416–1427. doi:10.1021/jacs.0c10836
Hussen, B. M., Azimi, T., Hidayat, H. J., Taheri, M., and Ghafouri-Fard, S. (2021). NF-KappaB Interacting LncRNA: Review of its Roles in Neoplastic and Non-neoplastic Conditions. Biomed. Pharmacother. 139, 111604. doi:10.1016/j.biopha.2021.111604
Hwang, J. W., Park, J. H., Park, B. W., Kim, H., Kim, J. J., Sim, W. S., et al. (2021). Histochrome Attenuates Myocardial Ischemia-Reperfusion Injury by Inhibiting Ferroptosis-Induced Cardiomyocyte Death. Antioxidants (Basel) 10, 1624. doi:10.3390/antiox10101624
Im, D. S., and Nah, S. Y. (2013). Yin and Yang of Ginseng Pharmacology: Ginsenosides vs Gintonin. Acta Pharmacol. Sin 34, 1367–1373. doi:10.1038/aps.2013.100
Jeremias, I., Kupatt, C., Martin-Villalba, A., Habazettl, H., Schenkel, J., Boekstegers, P., et al. (2000). Involvement of CD95/Apo1/Fas in Cell Death after Myocardial Ischemia. Circulation 102, 915–920. doi:10.1161/01.cir.102.8.915
Ji, N., Qi, Z., Wang, Y., Yang, X., Yan, Z., Li, M., et al. (2021). Pyroptosis: A New Regulating Mechanism in Cardiovascular Disease. J. Inflamm. Res. 14, 2647–2666. doi:10.2147/JIR.S308177
Jiang, N., Huang, H., Zhang, Y., Lv, J., Wang, Q., He, Q., et al. (2021). Ginsenoside Rb1 Produces Antidepressant-like Effects in a Chronic Social Defeat Stress Model of Depression through the BDNF-Trkb Signaling Pathway. Front. Pharmacol. 12, 680903. doi:10.3389/fphar.2021.680903
Jneid, H., Addison, D., Bhatt, D. L., Fonarow, G. C., Gokak, S., Grady, K. L., et al. (2017). 2017 AHA/ACC Clinical Performance and Quality Measures for Adults with ST-Elevation and Non-ST-elevation Myocardial Infarction: A Report of the American College of Cardiology/American Heart Association Task Force on Performance Measures. J. Am. Coll. Cardiol. 70, 2048–2090. doi:10.1161/HCQ.000000000000003210.1016/j.jacc.2017.06.032
Junttila, M. R., Li, S. P., and Westermarck, J. (2008). Phosphatase-mediated Crosstalk between MAPK Signaling Pathways in the Regulation of Cell Survival. FASEB J. 22, 954–965. doi:10.1096/fj.06-7859rev
Kim, M. Y., Lee, H., Ji, S. Y., Kim, S. Y., Hwangbo, H., Park, S. H., et al. (2021). Induction of Apoptosis by Isoalantolactone in Human Hepatocellular Carcinoma Hep3B Cells through Activation of the ROS-dependent JNK Signaling Pathway. Pharmaceutics 13, 1627. doi:10.3390/pharmaceutics13101627
Kim, Y. J., Zhang, D., and Yang, D. C. (2015). Biosynthesis and Biotechnological Production of Ginsenosides. Biotechnol. Adv. 33, 717–735. doi:10.1016/j.biotechadv.2015.03.001
Korshunova, A. Y., Blagonravov, M. L., Neborak, E. V., Syatkin, S. P., Sklifasovskaya, A. P., Semyatov, S. M., et al. (2021). BCL2-regulated A-poptotic P-rocess in M-yocardial I-schemia-reperfusion I-njury (Review). Int. J. Mol. Med. 47, 23–36. doi:10.3892/ijmm.2020.4781
Lai, L., Liu, Y., Liu, Y., Zhang, N., Cao, S., Zhang, X., et al. (2020). Role of Endoplasmic Reticulum Oxidase 1α in H9C2 Cardiomyocytes Following Hypoxia/reoxygenation Injury. Mol. Med. Rep. 22, 1420–1428. doi:10.3892/mmr.2020.11217
Lai, T., Shen, Y., Chen, C., Huang, B., Deng, T., Zhao, Z., et al. (2021). Glycyrrhizic Acid Ameliorates Myocardial Ischemia-Reperfusion Injury in Rats through Inhibiting Endoplasmic Reticulum Stress. Eur. J. Pharmacol. 908, 174353. doi:10.1016/j.ejphar.2021.174353
Lee, E. W., Kim, J. H., Ahn, Y. H., Seo, J., Ko, A., Jeong, M., et al. (2012). Ubiquitination and Degradation of the FADD Adaptor Protein Regulate Death Receptor-Mediated Apoptosis and Necroptosis. Nat. Commun. 3, 978. doi:10.1038/ncomms1981
Li, C. Y., Yang, P., Jiang, Y. L., Lin, Z., Pu, Y. W., Xie, L. Q., et al. (2020a). Ginsenoside Rb1 Attenuates Cardiomyocyte Apoptosis Induced by Myocardial Ischemia Reperfusion Injury through mTOR Signal Pathway. Biomed. Pharmacother. 125, 109913. doi:10.1016/j.biopha.2020.109913
Li, G., Qian, W., and Zhao, C. (2016). Analyzing the Anti-ischemia-reperfusion Injury Effects of Ginsenoside Rb1 Mediated through the Inhibition of P38α MAPK. Can. J. Physiol. Pharmacol. 94, 97–103. doi:10.1139/cjpp-2014-0164
Li, J., Yang, Y. L., Li, L. Z., Zhang, L., Liu, Q., Liu, K., et al. (2017). Succinate Accumulation Impairs Cardiac Pyruvate Dehydrogenase Activity through GRP91-dependent and Independent Signaling Pathways: Therapeutic Effects of Ginsenoside Rb1. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 2835–2847. doi:10.1016/j.bbadis.2017.07.017
Li, L., Pan, C. S., Yan, L., Cui, Y. C., Liu, Y. Y., Mu, H. N., et al. (2018). Ginsenoside Rg1 Ameliorates Rat Myocardial Ischemia-Reperfusion Injury by Modulating Energy Metabolism Pathways. Front. Physiol. 9, 78. doi:10.3389/fphys.2018.00078
Li, L., Wang, Y., Guo, R., Li, S., Ni, J., Gao, S., et al. (2020b). Ginsenoside Rg3-Loaded, Reactive Oxygen Species-Responsive Polymeric Nanoparticles for Alleviating Myocardial Ischemia-Reperfusion Injury. J. Control. Release 317, 259–272. doi:10.1016/j.jconrel.2019.11.032
Li, L. (2014). The Effect and Mechanism of Ginsenoside Rg1 in Improving Myocardial Ischemia/Reperfusion Injury. Beijing (China): Beijing University of Chinese Medicine.
Li, Y., Chen, B., Yang, X., Zhang, C., Jiao, Y., Li, P., et al. (2019). S100a8/a9 Signaling Causes Mitochondrial Dysfunction and Cardiomyocyte Death in Response to Ischemic/Reperfusion Injury. Circulation 140, 751–764. doi:10.1161/CIRCULATIONAHA.118.039262
Liao, S., Apaijai, N., Luo, Y., Wu, J., Chunchai, T., Singhanat, K., et al. (2021). Cell Death Inhibitors Protect against Brain Damage Caused by Cardiac Ischemia/reperfusion Injury. Cell Death Discov 7, 312. doi:10.1038/s41420-021-00698-4
Liu, C., Wu, S., and Ye, G. (2012b). Mechanism of Ginsenoside Rb1 against Myocardial Apoptosis during Ischemia-Reperfusion Injury in Diabetic Rats. JETCM 21, 1080–1081.
Liu, J., Sui, H., Zhao, J., and Wang, Y. (2017). Osmotin Protects H9c2 Cells from Simulated Ischemia-Reperfusion Injury through AdipoR1/PI3K/AKT Signaling Pathway. Front. Physiol. 8, 611. doi:10.3389/fphys.2017.00611
Liu, P., Xu, Y., Yin, H., Wang, J., Chen, K., and Li, Y. (2005). Developmental Toxicity Research of Ginsenoside Rb1 Using a Whole Mouse Embryo Culture Model. Birth Defects Res. B Dev. Reprod. Toxicol. 74, 207–209. doi:10.1002/bdrb.20038
Liu, P., Yin, H., Xu, Y., Zhang, Z., Chen, K., and Li, Y. (2006). Effects of Ginsenoside Rg1 on Postimplantation Rat and Mouse Embryos Cultured In Vitro. Toxicol. Vitro 20, 234–238. doi:10.1016/j.tiv.2005.06.029
Liu, S. X., Zhang, Y., Wang, Y. F., Li, X. C., Xiang, M. X., Bian, C., et al. (2012a). Upregulation of Heme Oxygenase-1 Expression by Hydroxysafflor Yellow A Conferring protection from Anoxia/reoxygenation-Induced Apoptosis in H9c2 Cardiomyocytes. Int. J. Cardiol. 160, 95–101. doi:10.1016/j.ijcard.2011.03.033
Liu, X., Jiang, Y., Fu, W., Yu, X., and Sui, D. (2020). Combination of the Ginsenosides Rb3 and Rb2 Exerts Protective Effects against Myocardial Ischemia Reperfusion Injury in Rats. Int. J. Mol. Med. 45, 519–531. doi:10.3892/ijmm.2019.4414
Liu, X., Jiang, Y., Yu, X., Fu, W., Zhang, H., and Sui, D. (2014). Ginsenoside-Rb3 Protects the Myocardium from Ischemia-Reperfusion Injury via the Inhibition of Apoptosis in Rats. Exp. Ther. Med. 8, 1751–1756. doi:10.3892/etm.2014.2007
Liu, X., Simpson, J. A., Brunt, K. R., Ward, C. A., Hall, S. R., Kinobe, R. T., et al. (2007). Preemptive Heme Oxygenase-1 Gene Delivery Reveals Reduced Mortality and Preservation of Left Ventricular Function 1 Yr after Acute Myocardial Infarction. Am. J. Physiol. Heart Circ. Physiol. 293, H48–H59. doi:10.1152/ajpheart.00741.2006
Liu, X., Zhang, C., Qian, L., Zhang, C., Wu, K., Yang, C., et al. (2015). NF45 Inhibits Cardiomyocyte Apoptosis Following Myocardial Ischemia-Reperfusion Injury. Pathol. Res. Pract. 211, 955–962. doi:10.1016/j.prp.2015.09.018
Liu, X. (2014). The Study on the Protective Effects and Mechanisms of Ginsenoside Rb3 and Rb2 Combination on Myocardial Ischemia Reperfusion Injury. Changchun (China): Jilin University of China. [Doctor's thesis].
Liu, Z., Li, Z., and Liu, X. (2002). Effect of Ginsenoside Re on Cardiomyocyte Apoptosis and Expression of Bcl-2/Bax Gene after Ischemia and Reperfusion in Rats. J. Huazhong Univ. Sci. Technolog Med. Sci. 22, 305–309. doi:10.1007/BF02896771
Lu, D., Liu, J., Zhao, W., and Li, P. (2012). Chronic Toxicity of Ginsenoside Re on Sprague-Dawley Rats. J. Ethnopharmacol 144, 656–663. doi:10.1016/j.jep.2012.10.007
Lu, S., Zhang, Y., Zhong, S., Gao, F., Chen, Y., Li, W., et al. (2017). N-n-butyl Haloperidol Iodide Protects against Hypoxia/Reoxygenation Injury in Cardiac Microvascular Endothelial Cells by Regulating the ROS/MAPK/Egr-1 Pathway. Front. Pharmacol. 7, 520. doi:10.3389/fphar.2016.00520
Lu, X. (2009). Studies on Influence of Ginsenoside-Re on Myocardium Ischemia-Reperfusion Injury and Apoptosis Pathway of Mitochondrial. Jilin (China): Jinlin University. [Master's Thesis].
Luan, Y., Sun, C., Wang, J., Jiang, W., Xin, Q., Zhang, Z., et al. (2019). Baicalin Attenuates Myocardial Ischemia-Reperfusion Injury through Akt/NF-Κb Pathway. J. Cel Biochem 120, 3212–3219. doi:10.1002/jcb.27587
Lv, S., Li, X., Zhao, S., Liu, H., and Wang, H. (2021). The Role of the Signaling Pathways Involved in the Protective Effect of Exogenous Hydrogen Sulfide on Myocardial Ischemia-Reperfusion Injury. Front Cel Dev Biol 9, 723569. doi:10.3389/fcell.2021.723569
Ma, L., Liu, H., Xie, Z., Yang, S., Xu, W., Hou, J., et al. (2014). Ginsenoside Rb3 Protects Cardiomyocytes against Ischemia-Reperfusion Injury via the Inhibition of JNK-Mediated NF-Κb Pathway: a Mouse Cardiomyocyte Model. PLoS One 9, e103628. doi:10.1371/journal.pone.0103628
Mancuso, C., and Santangelo, R. (2017). Panax Ginseng and Panax Quinquefolius: From Pharmacology to Toxicology. Food Chem. Toxicol. 107, 362–372. doi:10.1016/j.fct.2017.07.019
Mann, G. E., Niehueser-Saran, J., Watson, A., Gao, L., Ishii, T., de Winter, P., et al. (2007). Nrf2/ARE Regulated Antioxidant Gene Expression in Endothelial and Smooth Muscle Cells in Oxidative Stress: Implications for Atherosclerosis and Preeclampsia. Sheng Li Xue Bao 59, 117–127.
Meloche, S., and Pouysségur, J. (2007). The ERK1/2 Mitogen-Activated Protein Kinase Pathway as a Master Regulator of the G1- to S-phase Transition. Oncogene 26, 3227–3239. doi:10.1038/sj.onc.1210414
Moens, A. L., Claeys, M. J., Timmermans, J. P., and Vrints, C. J. (2005). Myocardial Ischemia/reperfusion-Injury, a Clinical View on a Complex Pathophysiological Process. Int. J. Cardiol. 100, 179–190. doi:10.1016/j.ijcard.2004.04.013
Neamatallah, T., El-Shitany, N. A., Abbas, A. T., Ali, S. S., and Eid, B. G. (2018). Honey Protects against Cisplatin-Induced Hepatic and Renal Toxicity through Inhibition of NF-Κb-Mediated COX-2 Expression and the Oxidative Stress Dependent BAX/Bcl-2/caspase-3 Apoptotic Pathway. Food Funct. 9, 3743–3754. doi:10.1039/c8fo00653a
Neri, M., Fineschi, V., Di Paolo, M., Pomara, C., Riezzo, I., Turillazzi, E., et al. (2015). Cardiac Oxidative Stress and Inflammatory Cytokines Response after Myocardial Infarction. Curr. Vasc. Pharmacol. 13, 26–36. doi:10.2174/15701611113119990003
Neri, M., Riezzo, I., Pascale, N., Pomara, C., and Turillazzi, E. (2017). Ischemia/Reperfusion Injury Following Acute Myocardial Infarction: A Critical Issue for Clinicians and Forensic Pathologists. Mediators Inflamm. 2017, 7018393. doi:10.1155/2017/7018393
Pai, P. Y., Lin, Y. Y., Yu, S. H., Lin, C. Y., Liou, Y. F., Wu, X. B., et al. (2021). Angiotensin II Receptor Blocker Irbesartan Attenuates Sleep Apnea-Induced Cardiac Apoptosis and Enhances Cardiac Survival and Sirtuin 1 Upregulation. Sleep Breath, 1–12. doi:10.1007/s11325-021-02499-6
Pasdois, P., Parker, J. E., Griffiths, E. J., and Halestrap, A. P. (2011). The Role of Oxidized Cytochrome C in Regulating Mitochondrial Reactive Oxygen Species Production and its Perturbation in Ischaemia. Biochem. J. 436, 493–505. doi:10.1042/BJ20101957
Peng, L., Lei, Z., Rao, Z., Yang, R., Zheng, L., Fan, Y., et al. (2021). Cardioprotective Activity of Ethyl Acetate Extract of Cinnamomi Ramulus against Myocardial Ischemia/reperfusion Injury in Rats via Inhibiting NLRP3 Inflammasome Activation and Pyroptosis. Phytomedicine 93, 153798. doi:10.1016/j.phymed.2021.153798
Peng, Y., Huang, S., Wu, Y., Cheng, B., Nie, X., Liu, H., et al. (2013). Platelet Rich Plasma Clot Releasate Preconditioning Induced PI3K/AKT/NFκB Signaling Enhances Survival and Regenerative Function of Rat Bone Marrow Mesenchymal Stem Cells in Hostile Microenvironments. Stem Cell Dev 22, 3236–3251. doi:10.1089/scd.2013.0064
Pu, J., Yuan, A., Shan, P., Gao, E., Wang, X., Wang, Y., et al. (2013). Cardiomyocyte-expressed Farnesoid-X-Receptor Is a Novel Apoptosis Mediator and Contributes to Myocardial Ischaemia/reperfusion Injury. Eur. Heart J. 34, 1834–1845. doi:10.1093/eurheartj/ehs011
Qian, S., Kan, J., and Yang, Y. (2019). Protective Effects of the Combination of Ginsenoside Rgl and Resveratrol against Ischemia Reperfusion Injury of Cardiomyocytes. J. Liaoning Univ. TCM 21, 47–49.
Qin, L., Fan, S., Jia, R., and Liu, Y. (2018). Ginsenoside Rg1 Protects Cardiomyocytes from Hypoxia-Induced Injury through the PI3K/AKT/mTOR Pathway. Pharmazie 73, 349–355. doi:10.1691/ph.2018.8329
Querio, G., Antoniotti, S., Geddo, F., Tullio, F., Penna, C., Pagliaro, P., et al. (202110692). Ischemic Heart Disease and Cardioprotection: Focus on Estrogenic Hormonal Setting and Microvascular Health. Vasc. Pharmacol. 141, 106921. doi:10.1016/j.vph.2021.106921
Ren, Y., Lin, S., Liu, W., and Ding, H. (2021). Hepatic Remote Ischemic Preconditioning (RIPC) Protects Heart Damages Induced by Ischemia Reperfusion Injury in Mice. Front. Physiol. 12, 713564. doi:10.3389/fphys.2021.713564
Sabouri-Rad, S., Sabouri-Rad, S., Sahebkar, A., and Tayarani-Najaran, Z. (2017). Ginseng in Dermatology: A Review. Curr. Pharm. Des. 23, 1649–1666. doi:10.2174/1381612822666161021152322
Shaukat, A., Shaukat, I., Rajput, S. A., Shukat, R., Hanif, S., Jiang, K., et al. (2021). Ginsenoside Rb1 Protects from Staphylococcus Aureus-Induced Oxidative Damage and Apoptosis through Endoplasmic Reticulum-Stress and Death Receptor-Mediated Pathways. Ecotoxicol Environ. Saf. 219, 112353. doi:10.1016/j.ecoenv.2021.112353
Shen, W., Li, Y., and Yang, S. (2017). Effects of Ginsenoside Rg1 on Arrhythmias Induced by Ischemic/reperfusion Injury in Rats. J. Clin. Cardiol. 33, 465–469.
Shi, H., Zhao, T., Li, Y., Xiao, X., Wu, J., Zhang, H., et al. (2021). Velvet Antler Ameliorates Cardiac Function by Restoring Sarcoplasmic Reticulum Ca2+-ATPase Activity in Rats with Heart Failure after Myocardial Infarction. Front. Pharmacol.Front Pharmaco 12. doi:10.3389/fphar.2021.621194
Son, S. M., Byun, J., Roh, S. E., Kim, S. J., and Mook-Jung, I. (2014). Reduced IRE1α Mediates Apoptotic Cell Death by Disrupting Calcium Homeostasis via the InsP3 Receptor. Cell Death Dis 5, e1188. doi:10.1038/cddis.2014.129
Song, N., Ma, J., Meng, X. W., Liu, H., Wang, H., Song, S. Y., et al. (2020). Heat Shock Protein 70 Protects the Heart from Ischemia/Reperfusion Injury through Inhibition of P38 MAPK Signaling. Oxid Med. Cel Longev 2020, 3908641. doi:10.1155/2020/3908641
Sugiyama, A., Shimizu, Y., Okada, M., Otani, K., and Yamawaki, H. (2021). Preventive Effect of Canstatin against Ventricular Arrhythmia Induced by Ischemia/Reperfusion Injury: A Pilot Study. Int. J. Mol. Sci. 22, 1004. doi:10.3390/ijms22031004
Sun, J., Sun, G., Meng, X., Wang, H., Wang, M., Qin, M., et al. (20132013). Ginsenoside RK3 Prevents Hypoxia-Reoxygenation Induced Apoptosis in H9c2 Cardiomyocytes via AKT and MAPK Pathway. Evid. Based Complement. Alternat Med. 2013, 690190. doi:10.1155/2013/690190
Sun, J., Yu, X., Huangpu, H., and Yao, F. (2019). Ginsenoside Rb3 Protects Cardiomyocytes against Hypoxia/reoxygenation Injury via Activating the Antioxidation Signaling Pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 109, 254–261. doi:10.1016/j.biopha.2018.09.002
Sun, J. (2013). Research on Active Ingredients of Chinese Medicine and Molecular Mechanisms Based on the Myocardial Protective Effec. Beijing (China): Chinese Academy of Medical Sciences & Peking Union Medical College. [Doctor's Thesis].
Sun, Y., Liu, Y., and Chen, K. (2016). Roles and Mechanisms of Ginsenoside in Cardiovascular Diseases: Progress and Perspectives. Sci. China Life Sci. 59, 292–298. doi:10.1007/s11427-016-5007-8
Syamsunarno, M. R. A., Safitri, R., and Kamisah, Y. (2021). Protective Effects of Caesalpinia Sappan Linn. And its Bioactive Compounds on Cardiovascular Organs. Front. Pharmacol. 12, 725745. doi:10.3389/fphar.2021.725745
Syeda, M. Z., Fasae, M. B., Yue, E., Ishimwe, A. P., Jiang, Y., Du, Z., et al. (2019). Anthocyanidin Attenuates Myocardial Ischemia Induced Injury via Inhibition of ROS-JNK-Bcl-2 Pathway: New Mechanism of Anthocyanidin Action. Phytother Res. 33, 3129–3139. doi:10.1002/ptr.6485
Tian, F., and Zhang, Y. (2021). Overexpression of SERCA2a Alleviates Cardiac Microvascular Ischemic Injury by Suppressing Mfn2-Mediated ER/Mitochondrial Calcium Tethering. Front. Cel Dev Biol 9, 636553. doi:10.3389/fcell.2021.636553
Tian, J., Zheng, S., Guo, W., Ye, J., and Li, L. (2004). Ginsenoside Rg2 protection against Apoptosis in Ischemia and Reperfusion Rat Myocardium. CHinese Pharmacol. Bull. 20, 480.
Tribulova, N., Knezl, V., Szeiffova Bacova, B., Egan Benova, T., Viczenczova, C., Gonçalvesova, E., et al. (2016). Disordered Myocardial Ca(2+) Homeostasis Results in Substructural Alterations that May Promote Occurrence of Malignant Arrhythmias. Physiol. Res. 65 Suppl 1, S139–S148. doi:10.33549/physiolres.933388
Vilela, E. M., and Fontes-Carvalho, R. (2021). Inflammation and Ischemic Heart Disease: The Next Therapeutic Target? Rev. Port Cardiol. (Engl Ed. 40, 785–796. doi:10.1016/j.repc.2021.02.01110.1016/j.repce.2021.10.010
Virani, S. S., Alonso, A., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., Carson, A. P., et al. (2020). Heart Disease and Stroke Statistics-2020 Update: A Report from the American Heart Association. Circulation 141, e139–e596. doi:10.1161/CIR.0000000000000757
Wang, F., and Roh, Y. S. (2020). Mitochondrial Connection to Ginsenosides. Arch. Pharm. Res. 43, 1031–1045. doi:10.1007/s12272-020-01279-2
Wang, F., Yuan, Q., Chen, F., Pang, J., Pan, C., Xu, F., et al. (2021b). Fundamental Mechanisms of the Cell Death Caused by Nitrosative Stress. Front. Cel Dev Biol 9, 742483. doi:10.3389/fcell.2021.742483
Wang, G., Hao, M., Liu, Q., Jiang, Y., Huang, H., Yang, G., et al. (2021d). Protective Effect of Recombinant Lactobacillus Plantarum against H2O2-Induced Oxidative Stress in HUVEC Cells. J. Zhejiang Univ. Sci. B 22, 348–365. doi:10.1631/jzus.B2000441
Wang, H., Zheng, Y., Sun, Q., Zhang, Z., Zhao, M., Peng, C., et al. (2021a). Ginsenosides Emerging as Both Bifunctional Drugs and Nanocarriers for Enhanced Antitumor Therapies. J. Nanobiotechnol 19, 322. doi:10.1186/s12951-021-01062-5
Wang, J., Wang, H., Mou, X., Luan, M., Zhang, X., He, X., et al. (2020). The Advances on the Protective Effects of Ginsenosides on Myocardial Ischemia and Ischemia-Reperfusion Injury. Mini Rev. Med. Chem. 20, 1610–1618. doi:10.2174/1389557520666200619115444
Wang, J., Cui, Y., Wang, J., Li, H., Dai, J., and Ma, H. (2016b). Effect of Ginsenoside Rh3 Pretreatment on Expression of Myocardial Sarcoplasmic Reticulum Ca2+-ATP in Rats Following I/R Injury. Chin. J. Geriatr. Heart Brain Vessel Dis. 18, 1077–1081.
Wang, J., Cui, Y., Wang, J., Li, H., Dai, J., Ma, H., et al. (2017b). Ginseng Saponin Rh3 Pretreatment to Protect Myocardial Ischemia-Reperfusion Injury in Rats. Chin. Arch. Traditional Chin. Med. 35, 2783–2786.
Wang, J., Cui, Y., Wang, J., Li, H., Dai, J., and Ma, H. (2019). Protective Effects of the Ginseng Saponin Rh1 Pretreatment to Myocardial Ischemia-Reperfusion Injury in Rats. J. Liaoning Univ. TCM 1, 42–45.
Wang, J., Cui, Y., Wang, J., Li, H., Dai, J., and Ma, H. (2017c). Protective Effects of the Ginseng Saponin Rh3 Pretreatment to Caspase-3 of Myocardial Ischemia-Reperfusion Injury in Rats. J. Changchun Univ. Chin. Med. 33, 13–15.
Wang, K., Li, Y., Qiang, T., Chen, J., and Wang, X. (2021f). Role of Epigenetic Regulation in Myocardial Ischemia/reperfusion Injury. Pharmacol. Res. 170, 105743. doi:10.1016/j.phrs.2021.105743
Wang, M., and Su, P. (2018). The Role of the Fas/FasL Signaling Pathway in Environmental Toxicant-Induced Testicular Cell Apoptosis: An Update. Syst. Biol. Reprod. Med. 64, 93–102. doi:10.1080/19396368.2017.1422046
Wang, M., Yang, D., Hu, Z., Shi, Y., Ma, Y., Cao, X., et al. (2021e). Extracorporeal Cardiac Shock Waves Therapy Improves the Function of Endothelial Progenitor Cells after Hypoxia Injury via Activating PI3K/Akt/eNOS Signal Pathway. Front. Cardiovasc. Med. 8, 747497. doi:10.3389/fcvm.2021.747497
Wang, Q., Fu, W., Yu, X., Xu, H., Sui, D., and Wang, Y. (2021c). Ginsenoside Rg2 Alleviates Myocardial Fibrosis by Regulating TGF-β1/Smad Signalling Pathway. Pharm. Biol. 59, 106–113. doi:10.1080/13880209.2020.1867197
Wang, Q., Zhang, L., Yuan, X., Ou, Y., Zhu, X., Cheng, Z., et al. (2016a). The Relationship between the Bcl-2/Bax Proteins and the Mitochondria-Mediated Apoptosis Pathway in the Differentiation of Adipose-Derived Stromal Cells into Neurons. PLoS One 11, e0163327. doi:10.1371/journal.pone.0163327
Wang, Y., Gao, W., Shi, X., Ding, J., Liu, W., He, H., et al. (2017a). Chemotherapy Drugs Induce Pyroptosis through Caspase-3 Cleavage of a Gasdermin. Nature 547, 99–103. doi:10.1038/nature22393
Wang, Y., Hu, Z., Sun, B., Xu, J., Jiang, J., and Luo, M. (2015). Ginsenoside Rg3 Attenuates Myocardial Ischemia/reperfusion Injury via Akt/endothelial Nitric Oxide Synthase Signaling and the B-cell lymphoma/B-cell L-ymphoma-associated X P-rotein P-athway. Mol. Med. Rep. 11, 4518–4524. doi:10.3892/mmr.2015.3336
Wang, Y., Li, X., Wang, X., Lau, W., Wang, Y., Xing, Y., et al. (2013). Ginsenoside Rd Attenuates Myocardial Ischemia/reperfusion Injury via Akt/GSK-3β Signaling and Inhibition of the Mitochondria-dependent Apoptotic Pathway. PLoS One 8, e70956. doi:10.1371/journal.pone.0070956
Wang, Y., and Zhang, Y. (2016). The Effect and Mechanism of Ginsenoside Rb1 in Improving Myocardial Ischemia/reperfusion Injury via P38MAPK Pathway. World Latest Med. Inf. 16, 89–90.
Wang, Z., Zheng, Q., Liu, K., Li, G., and Zheng, R. (2006). Ginsenoside Rh(2) Enhances Antitumour Activity and Decreases Genotoxic Effect of Cyclophosphamide. Basic Clin. Pharmacol. Toxicol. 98, 411–415. doi:10.1111/j.1742-7843.2006.pto_348.x
Wang, Z. (2008). Protective Effects of Ginsenoside Rb1 on Myocardial Ischemia-Reperfusion Injury and its Mechanisms. Guangzhou (China): Sun Yat-sen University. [Doctor's Thesis].
WHO (2020). The Top 10 Causes of Death. Available at: https://www.who.int/en/news-room/fact-sheets/detail/the-top-10-causes-of-death (Accessed October 30, 2021).
Wu, Y., Xia, Z. Y., Dou, J., Zhang, L., Xu, J. J., Zhao, B., et al. (2011). Protective Effect of Ginsenoside Rb1 against Myocardial Ischemia/reperfusion Injury in Streptozotocin-Induced Diabetic Rats. Mol. Biol. Rep. 38, 4327–4335. doi:10.1007/s11033-010-0558-4
Wu, Y. (2011). An Experimental Study on the Effects of Ginsenoside Rb1 against Myocardial Ischemia/reperfusion Injury in Streptozotocin-Induced Diabetic Rats. Wuhan (China): Wuhan University. [Doctor's Thesis].
Xin, G. (2020). Study on the Mechanism of Shuangshen Ningxin Apsule Regulating FUNDC1 Mediated Mitochondrial Autophagy to Protect Myocardial Ischemia-Reperfusion Injury. Beijing (China): China Academy of Chinese Medical Sciences. [Master's Thesis].
Xu, J. F., Wan, Y., Tang, F., Chen, L., Yang, Y., Xia, J., et al. (2021a). Emerging Significance of Ginsenosides as Potentially Reversal Agents of Chemoresistance in Cancer Therapy. Front. Pharmacol. 12, 720474. doi:10.3389/fphar.2021.720474
Xu, T., Zhang, K., Kan, F., Li, F., Yu, B., Du, W., et al. (2019). Adeno-associated Virus 9-mediated Small RNA Interference of TLR4 Alleviates Myocardial Ischemia and Reperfusion Injury by Inhibition of the NF-Κb and MAPK Signaling Pathways in Rats. Curr. Mol. Med. 19, 127–135. doi:10.2174/1566524019666190311122521
Xu, X. N., Jiang, Y., Yan, L. Y., Yin, S. Y., Wang, Y. H., Wang, S. B., et al. (2021b). Aesculin Suppresses the NLRP3 Inflammasome-Mediated Pyroptosis via the Akt/GSK3β/NF-Κb Pathway to Mitigate Myocardial Ischemia/reperfusion Injury. Phytomedicine 92, 153687. doi:10.1016/j.phymed.2021.153687
Xue, Y., Fu, W., Liu, Y., Yu, P., Sun, M., Li, X., et al. (2020). Ginsenoside Rb2 Alleviates Myocardial Ischemia/reperfusion Injury in Rats through SIRT1 Activation. J. Food Sci. 85, 4039–4049. doi:10.1111/1750-3841.15505
Yan, X., Liu, J., Wu, H., Liu, Y., Zheng, S., Zhang, C., et al. (2016). Impact of miR-208 and its Target Gene Nemo-like Kinase on the Protective Effect of Ginsenoside Rb1 in Hypoxia/Ischemia Injuried Cardiomyocytes. Cell Physiol Biochem 39, 1187–1195. doi:10.1159/000447825
Yan, X., Xue, J., Wu, H., Wang, S., Liu, Y., Zheng, S., et al. (2015). Ginsenoside-Rb1 Protects Hypoxic- and Ischemic-Damaged Cardiomyocytes by Regulating Expression of miRNAs. Evid. Based Complement. Alternat Med. 2015, 171306. doi:10.1155/2015/171306
Yang, W., Lai, Q., Zhang, L., Zhang, Y., Zhang, Y., Yu, B., et al. (2021). Mechanisms Dissection of the Combination GRS Derived from ShengMai Preparations for the Treatment of Myocardial Ischemia/reperfusion Injury. J. Ethnopharmacol 264, 113381. doi:10.1016/j.jep.2020.113381
Yang, Y. (2013). Influences of Ginsenoside Rbi on Mitochondrial Dynamics in NRVMs Hypoxia/reoxyenation Model. Shantou (China): Shantou University. [Master's Thesis].
Yi-Dan, H., Ying-Xin, Z., Shi-Wei, Y., and Yu-Jie, Z. (2021). High-Energy Phosphates and Ischemic Heart Disease: From Bench to Bedside. Front. Cardiovasc. Med. 8, 675608. doi:10.3389/fcvm.2021.675608
Yuan, C., Wang, H., and Yuan, Z. (2019). Ginsenoside Rg1 Inhibits Myocardial Ischaemia and Reperfusion Injury via HIF-1 α-ERK Signalling Pathways in a Diabetic Rat Model. Pharmazie 74, 157–162. doi:10.1691/ph.2019.8858
Zhai, M., Li, B., Duan, W., Jing, L., Zhang, B., Zhang, M., et al. (2017). Melatonin Ameliorates Myocardial Ischemia Reperfusion Injury through SIRT3-dependent Regulation of Oxidative Stress and Apoptosis. J. Pineal Res. 63, e12419. doi:10.1111/jpi.12419
Zhang, C., He, M., Ni, L., He, K., Su, K., Deng, Y., et al. (2020). The Role of Arachidonic Acid Metabolism in Myocardial Ischemia-Reperfusion Injury. Cell Biochem Biophys 78, 255–265. doi:10.1007/s12013-020-00928-z
Zhang, F. L., Liu, X. Y., Linn, Y. C., and Zhang, K. Z. (2002). Insulin Resistance Model Induced by Dexamethasone in Rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 18, 98–100.
Zhang, H., Wang, X., Ma, Y., and Shi, Y. (2019b2019). The Effect of Ginsenoside RB1, Diazoxide, and 5-Hydroxydecanoate on Hypoxia-Reoxygenation Injury of H9C2 Cardiomyocytes. Evid. Based Complement. Alternat Med. 2019, 6046405. doi:10.1155/2019/6046405
Zhang, H. (2020). Ginsenoside Rb1 Attenuates HR Injury of H9c2 Cells through Mitochondrial ATP-Sensitive Potassium Channels. Jinzhou (China): Jinzhou Medical University. [Master's Thesis].
Zhang, J. H., Yang, H. Z., Su, H., Song, J., Bai, Y., Deng, L., et al. (2021a). Berberine and Ginsenoside Rb1 Ameliorate Depression-like Behavior in Diabetic Rats. Am. J. Chin. Med. 49, 1195–1213. doi:10.1142/S0192415X21500579
Zhang, J., Zhuge, Y., Rong, X., Ni, C., Niu, C., Wen, Z., et al. (2021c). Protective Roles of Xijiao Dihuang Tang on Coronary Artery Injury in Kawasaki Disease. Cardiovasc. Drugs Ther., 1–14. doi:10.1007/s10557-021-07277-w
Zhang, L. (2017). Effects and Mechanism of Alprostadil and Ginsenoside Rg3 on Myocardial Ischemia/reperfusion Injury in Rats. Jilin (China): Jinlin University. [Doctor's Thesis].
Zhang, L. P., Jiang, Y. C., Yu, X. F., Xu, H. L., Li, M., Zhao, X. Z., et al. (20162016). Ginsenoside Rg3 Improves Cardiac Function after Myocardial Ischemia/Reperfusion via Attenuating Apoptosis and Inflammation. Evid. Based Complement. Alternat Med. 2016, 6967853. doi:10.1155/2016/6967853
Zhang, Q., Liu, J., Zhang, M., Wei, S., Li, R., Gao, Y., et al. (2019a). Apoptosis Induction of Fibroblast-like Synoviocytes Is an Important Molecular-Mechanism for Herbal Medicine along with its Active Components in Treating Rheumatoid Arthritis. Biomolecules 9, 795. doi:10.3390/biom9120795
Zhang, X., Agborbesong, E., and Li, X. (2021b). The Role of Mitochondria in Acute Kidney Injury and Chronic Kidney Disease and its Therapeutic Potential. Int. J. Mol. Sci. 22, 11253. doi:10.3390/ijms222011253
Zhang, Z. L., Fan, Y., and Liu, M. L. (2012). Ginsenoside Rg1 Inhibits Autophagy in H9c2 Cardiomyocytes Exposed to Hypoxia/reoxygenation. Mol. Cel Biochem 365, 243–250. doi:10.1007/s11010-012-1265-3
Zhao, H., Zhang, D., Pang, L., Zhang, H., and Yu, R. (2021). Effects of Astragaloside IV and Ginsenoside Rg1 on Myocardial Apoptosis after Myocardial Ischemia Reperfusion Injury Hyperlipidemia Rats. Liaoning J. Traditional Chin. Med. 48, 188–191.
Zheng, T., Yang, J., Zhang, J., Yang, C., Fan, Z., Li, Q., et al. (2021). Downregulated MicroRNA-327 Attenuates Oxidative Stress-Mediated Myocardial Ischemia Reperfusion Injury through Regulating the FGF10/Akt/Nrf2 Signaling Pathway. Front. Pharmacol. 12, 669146. doi:10.3389/fphar.2021.669146
Zhou, C. (2009). The Protective Effect of Rg2 on Hypoxic Myocardial Cells. Qingdao (China): Qingdao University. [Master's Thesis].
Zhou, Q. L., Teng, F., Zhang, Y. S., Sun, Q., Cao, Y. X., and Meng, G. W. (2018). FPR1 Gene Silencing Suppresses Cardiomyocyte Apoptosis and Ventricular Remodeling in Rats with Ischemia/reperfusion Injury through the Inhibition of MAPK Signaling Pathway. Exp. Cel Res 370, 506–518. doi:10.1139/cjpp-2021-028910.1016/j.yexcr.2018.07.016
Zhu, H., Jia, Z., Misra, B. R., Zhang, L., Cao, Z., Yamamoto, M., et al. (2008). Nuclear Factor E2-Related Factor 2-dependent Myocardiac Cytoprotection against Oxidative and Electrophilic Stress. Cardiovasc. Toxicol. 8, 71–85. doi:10.1007/s12012-008-9016-0
Keywords: ginsenosides, apoptosis, myocardial ischemia/reperfusion injury, Panax ginseng, review
Citation: Chen Z, Wu J, Li S, Liu C and Ren Y (2022) Inhibition of Myocardial Cell Apoptosis Is Important Mechanism for Ginsenoside in the Limitation of Myocardial Ischemia/Reperfusion Injury. Front. Pharmacol. 13:806216. doi: 10.3389/fphar.2022.806216
Received: 31 October 2021; Accepted: 09 February 2022;
Published: 01 March 2022.
Edited by:
Xin Luan, Shanghai University of Traditional Chinese Medicine, ChinaReviewed by:
Sheng-feng Lu, Nanjing University of Chinese Medicine, ChinaYanping Gong, Chinese PLA General Hospital, China
Copyright © 2022 Chen, Wu, Li, Liu and Ren. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yulan Ren, cnlsQGNkdXRjbS5lZHUuY24=
†These authors have contributed equally to this work
 Zhihan Chen
Zhihan Chen Jingping Wu2†
Jingping Wu2†