- 1Department of General Internal Medicine, Hiroshima University Hospital, Hiroshima, Japan
- 2Department of Infectious Disease, Hiroshima University Hospital, Hiroshima, Japan
- 3Department of Virology, Graduate School of Biomedical and Health Sciences, Hiroshima University, Hiroshima, Japan
- 4Kampo Clinical Center, Department of General Internal Medicine, Hiroshima University Hospital, Hiroshima, Japan
Several traditional Japanese Kampo formulas are known to have inhibitory effects on infections with viruses that cause respiratory symptoms. Although some herbs and their components have been reported to suppress SARS-CoV-2 replication in vitro, it is difficult to compare effective Kampo formulas because of the different methods used in studies. Thus, we carried out in vitro experiments on the suppression of SARS-CoV-2 infection by Kampo formulas and crude drugs used for the common cold to compare their suppressive effects on virus infection. After infecting VeroE6/TMPRSS2 cells with SARS-CoV-2, lysates of the Kampo formulas and crude drugs were added, and after 24 h, the infectious titer in the medium was measured by the TCID50 method. Maoto was the most effective among the Kampo formulas, and Ephedrae herba was the most effective among the constituent crude drugs. However, a comparison of the suppressive effects of Ephedrae herba and Kampo formulas containing Ephedrae herba showed that the suppressive effect on virus infection did not depend on the content of Ephedrae herba. Based on the results, we believe that the use of Maoto among Kampo formulas is suitable as a countermeasure against COVID-19.
Introduction
In September 2021, the cumulative number of SARS-CoV-2 infection cases worldwide was more than 200 million and the number of deaths from SARS-CoV-2 infection was more than 4.5 million. There has been no apparent reduction in the number of new infections or deaths (World Health Organization, 2021). Although the efficacy of vaccines has been recognized and vaccination has been accelerated in many countries worldwide, there are still many unclear issues about vaccines as the efficacy of vaccines against emerging mutant strains (Liu et al., 2021). On the other hand, there are few drugs that can be expected to have antiviral effects and most of the drugs are expensive (Hsu, 2020). Therefore, there is an urgent need to find new anti-viral drugs or alternative treatments with drug repositioning.
Kampo medicine is a systematized medical system based on traditional East-Asian medicine and unique drugs. Kampo medicine originated in traditional Chinese medicine, which was introduced to Japan around the 5th century and was refined from the 17th century to the currently used Kampo medicine in Japan. Kampo formulas are mixtures of crude drugs, which are extracts of herbs, insects, minerals, fungi, and other substances (Tsumura and Co., 2016). All Kampo formulas were approved as drugs for humans by Japan’s Ministry of Health, Labour and Welfare in 1986 and are regulated by the National Institute of Health and Welfare (STORK, 2020). Among the medications certified by the Ministry of Health, Labour and Welfare, medical Kampo formulas are comparatively inexpensive, and many of them have versatility in use. On the other hand, herbal medicines themselves are also used as folk remedies not only in Japan but also in many other countries.
Influenza and respiratory syncytial viruses are RNA viruses and the primary symptoms of infections with these viruses are respiratory symptoms that are similar to the symptoms of SARS-CoV-2 infection. In Japan, two Kampo prescriptions, Maoto and Saikokeishito, have been approved for treatment of influenza infection. There have been in vitro studies showing suppression of infection with these viruses by Kampo formulas and crude drug extracts (Mantani et al., 1999; Nomura et al., 2019; Hou et al., 2020). There seems to be a relationship between the clinical effects of Kampo formulas and inhibition of influenza virus growth in cultured cells.
In this study, we examined the suppressive effects of Kampo formulas on SARS-CoV-2 infection using VeroE6/TMPRSS2 cells, which are highly susceptible to SARS-CoV-2 infection (Matsuyama et al., 2020). We selected eight Kampo formulas that have been shown to be effective against influenza virus infections and common cold symptoms and examined their inhibitory effects on SARS-CoV-2 infection. We also investigated the inhibitory effects of six crude constituent drugs constituting those Kampo formulas on SARS-CoV-2 infection.
Materials and Methods
Cells and Viruses
VeroE6/TMPRSS2 cells [African green monkey kidney-derived cells expressing human TMPRSS2, purchased from Japanese Collection of Research Bioresources (JCRB) Cell Bank, JCRB 1819] were propagated in Dulbecco’s modified Eagle’s minimum essential medium (DMEM, Invitrogen) supplemented with 10% fetal calf serum (FCS; Biosera, Kansas City, MO, United States), penicillin G (100 units/ml, Meiji Seika Pharma, Tokyo, Japan), and streptomycin (100 μg/ml, Meiji Seika Pharma). The cells were cultured at 37°C in 5% CO2. SARS-CoV-2/JP/Hiroshima-46059T/2020 (Yamamotoya et al., 2021; B1.1.1, GISAID accession ID: EPI_ISL_6289932, GenBank/DDBJ/EMBL accession number: MZ853926) was used as the test virus.
To prepare virus suspensions, VeroE6/TMPRSS2 cells were infected with the virus and incubated in DMEM. When cytopathic effects were fully developed, the culture supernatant was harvested and filtered through a 0.45-µm filter after low-speed centrifugation. The virus titer was determined by the standard 50% tissue culture infectious dose (TCID50) method. Briefly, a 10-fold serial dilution of the virus was inoculated into cells in a 96-well plate in tetraplicate or octuplicate and incubated for 7 days to check for CPE. Based on this result, infectivity was calculated and expressed as TCID50/ml, as described previously (Nomura et al., 2021).
Reagents
Extract powders of Kampo formulas including Maoto, Saikokeishito, Shomakakkonto, Kakkonto, Shoseiryuto, Senkyuchachosan, Bakumondoto, and Hochuekkito were kindly provided by Tsumura & Co. (Tokyo, Japan) (Table 1). Crude drugs including Glycyrrhiza uralensis Fisch. ex DC [Fabaceae] (Glycyrrhizae radix), Ephedra sinica Stapf [Ephedraceae] (Ephedrae herba), Paeonia lactiflora Pall [Paeoniaceae] (Paeoniae radix), Ligusticum officinale (Makino) Kitag [Apiaceae] (Cnidii rhizoma), Scutellaria baicalensis Georgi [Lamiaceae] (Scutellariae radix), and Bupleurum falcatum L [Apiaceae] (Bupleuri radix) were purchased from Tsumura & Co. The Kampo formulas used in this study and their constituent crude drugs (generic name, scientific name, and percentage included) are shown in detail in Table 1.
Solutions of the Kampo formulas for testing were prepared as described previously (Nomura et al., 2019). The powder of each Kampo formula was mixed with DMEM to a concentration of 20 mg/ml. The powder was dissolved at 50°C for 1 h and the mixture was centrifuged at a low speed and then the supernatant was filter-sterilized through a 0.22-μm filter. The crude drugs were prepared in a similar way. For both the Kampo formulas and the crude drugs, little insoluble material was found after low-speed centrifugation, and the weight of the initial powder was therefore used as the weight of the solute.
Cytotoxicity Assay
VeroE6/TMPRSS2 cells were cultured in DMEM with the specified concentrations of reagents for 24 h, and lactate dehydrogenase (LDH), which was released from the cells into the medium, was assayed with a colorimetric method using the Cytotoxicity LDH Assay Kit-WST (Dojindo Laboratories, Kumamoto, Japan) by measuring absorbance at 490 nm in the TriStar LB 941 plate reader (Bertohold Technologies, Wildbad, Germany). The cytotoxicity of the reagents was calculated from the absorbance measurements as 100% for the high control (cell lysis by surfactant) and 0% for the low control (culture medium only).
Replication of SARS-CoV-2 in vitro
Confluent monolayers of VeroE6/TMPRSS2 cells in a 96-well plate were infected with 50 µl/well of the virus at an input multiplicity of infection (m.o.i.) of 0.05 or 10. After adsorption for 2 h, the inoculated viruses were removed, and the cells were further cultured in 100 µl/well of DMEM containing different concentrations of Kampo formulas or crude drugs. The conditions of m.o.i. and virus adsorption time were based on the conditions used in a previous study for effective infection of cells (Wang et al., 2020). The medium was harvested after 24 h, and viral infectivity was assayed by the TCID50 method. The logarithm of infectivity titer and reagent concentration in the medium were plotted and an approximation straight-line was drawn to calculate the 50% inhibitory concentration (IC50), as described previously (Nomura et al., 2021).
We conducted infection experiments under two conditions: m o.i. of 0.05 and m.o.i. of 10. At m.o.i. of 10, all cells are infected at once, allowing us to observe the process of virus entry and replication (one-step replication). When m.o.i. of 0.05, on the other hand, 20 cells are infected with a single virus. In addition to the entry and replication of the virus into the cell, the progeny virus is released from the cell and further infects the surrounding cells (multi-step replication).
Assay for Inactivation of Viral Particles
For the Kampo formulas, the solution was mixed with 90 µl of the drug at a concentration of 20 mg/ml and 10 µl of the virus solution at 2.0 × 109 TCID50/ml and incubated for 3 min at room temperature. The mixture was then serially diluted 10-fold in DMEM, and adsorbed on VeroE6/TMPRSS cells for 1 h. The inoculum was removed, and cell culture medium was added and incubated for 7 days. The infectivity of the solution was determined by the TCID50 method. For crude drugs, reagents at 1.25–10 mg/ml concentrations were used considering their cytotoxicity. Phosphate-buffered saline (PBS) was used as an untreated control, and 70% (w/w) ethanol was used as an inactivation control as described previously (Nomura et al., 2021).
Results
Suppressive Effects of Kampo Formulas on SARS-CoV-2 Infection
Eight Kampo formulas used to treat respiratory symptoms, including respiratory symptoms of influenza and common cold infections, were investigated for their inhibitory effects on SARS-CoV-2 infection in vitro. Table 1 shows the clinical indications of each Kampo formula, the crude drugs included, and the proportions of the crude drugs.
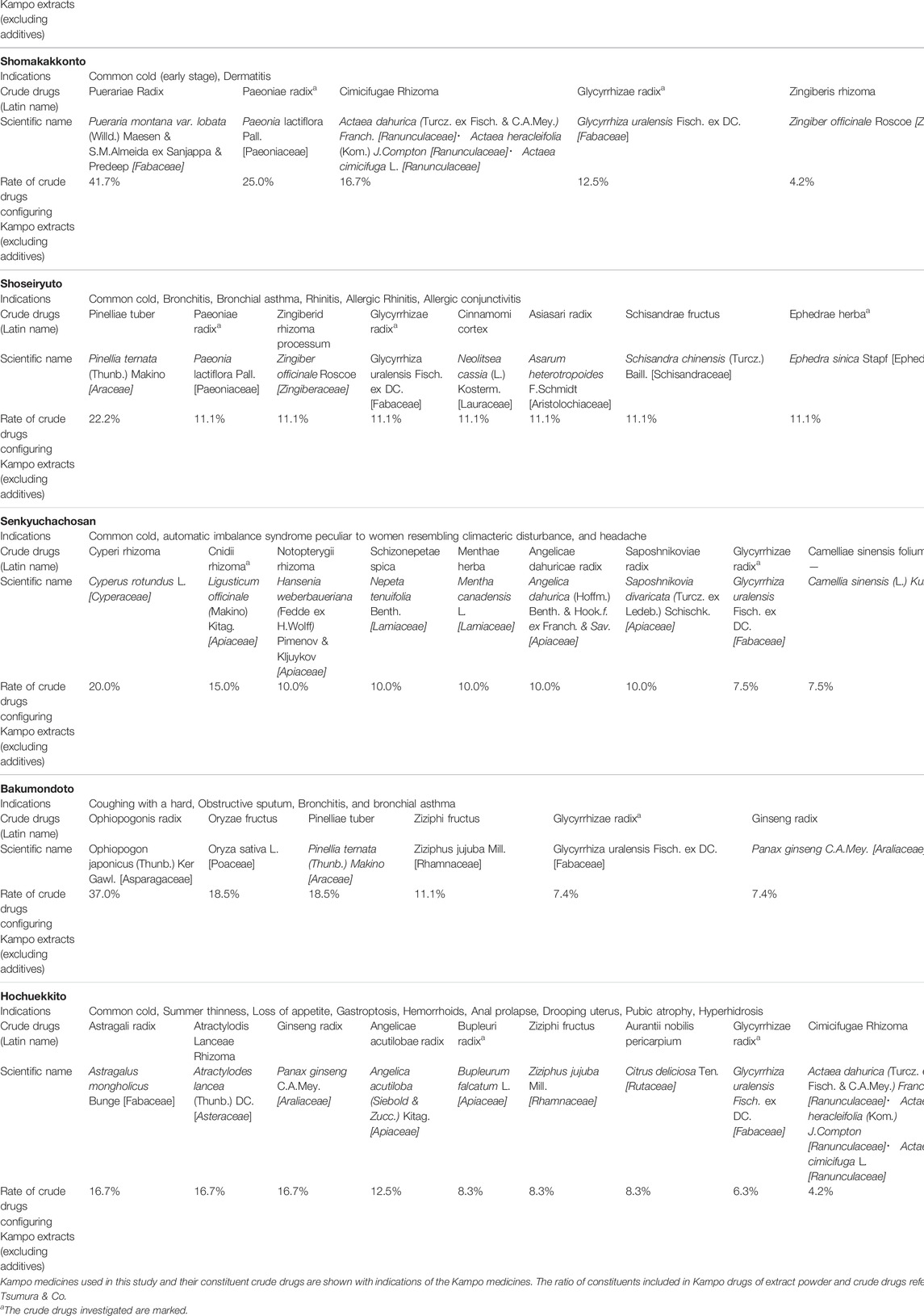
Initially, the cytotoxicity of each Kampo formula was examined by an LDH assay, and the values were plotted on a graph (Figure 1, △ marker, right Y-axis). Twenty mg/ml of each Kampo formula of Maoto (Figure 1A), Saikokeishito (Figure 1B), Kakkonto (Figure 1C), and Shomakakkonto (Figure 1D) showed more than 30% cytotoxicity. However, at concentrations below 10 mg/ml, none of the Kampo formulas showed apparent cytotoxicity. The results at concentrations below 10 mg/ml could be interpreted without considering the effect of cytotoxicity, while at 20 mg/ml, the results should be interpreted with caution.
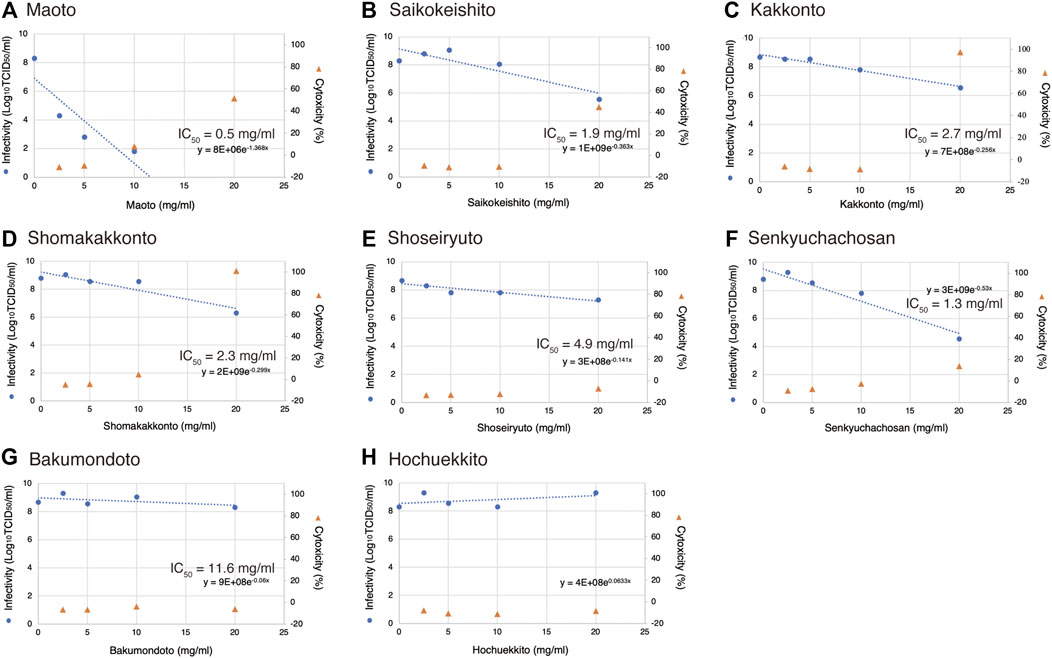
FIGURE 1. Inhibition of SARS-CoV-2 replication by Kampo formulas. (A-H) The Kampo formula used is noted at the top of each panel. VeroE6/TMPRSS2 cells were infected with SARS-CoV-2 at an m.o.i. of 0.05. After 2-h adsorption, the inoculum was removed and the cells were cultured in DMEM containing different concentrations of Kampo formulas for 24 h. The viral infectivity in the medium was assayed by the TCID50 method. The left y-axis of the graph is the infectivity for each concentration. An exponential approximation was made by Excel to calculate the drug concentration that reduces the infectivity in the absence of a drug to 50% (IC50). The IC50 values and approximation equations are shown in the graph. If there is no decrease, the IC50 is not shown (Panel 1). VeroE6/TMPRSS2 cells were incubated in DMEM supplemented with the designated concentrations of a Kampo formula for 24 h, and LDH values in the media were then measured to evaluate cytotoxicity. The LDH value from detergent-treated cells was set at 100%, and the right y-axis is the percent inhibition of cytotoxicity for each concentration.
VeroE6/TMPRSS2 cells were infected with SARS-CoV-2 at an m.o.i. of 0.05, and each Kampo formula was added to the medium. After 24 h, the infection titer was measured by the TCID50 method and plotted on a graph against the concentration of the Kampo formula (Figure 1, ○ marker, left Y-axis). In the case of Maoto, viral replication was inhibited as the concentration was increased and was almost completely inhibited at a concentration of 10 mg/ml (Figure 1A). An approximate line of these points was drawn, and the equation is shown in the graph. The 50% inhibitory concentration (IC50) that was calculated from the equation is also shown in the graph (Figure 1A). In the case of Hochuekkito, there was little change even when the drug concentration was increased (Figure 1H). According to the IC50 data shown in Figure 1, both Maoto and Senkyuchachosan had strong suppressive effects on SARS-CoV-2 infection. On the other hand, no suppressive effects of Bakumondo and Hochuekkito on virus infection were observed.
The cytotoxicity of Saikokeishito at 20 mg/ml was 44.7%, and we cannot deny the possibility that its suppressive effect on virus infection is due to its cytotoxicity. Since Kakkonto and Maoto showed even higher cytotoxicity at a concentration of 20 mg/ml, it was difficult to clearly determine their suppressive effects on virus infection at that concentration. Still, it can be considered that they have clear suppressive effects on virus infection at concentrations below 10 mg/ml.
When the highest concentration (20 mg/ml) of each Kampo formula was mixed with the virus and the infectious titer was measured, none of the Kampo formulas decreased the infectious titer. This suggests that there was no direct inactivating effect of each Kampo formula on the virus particles (Figure 2).
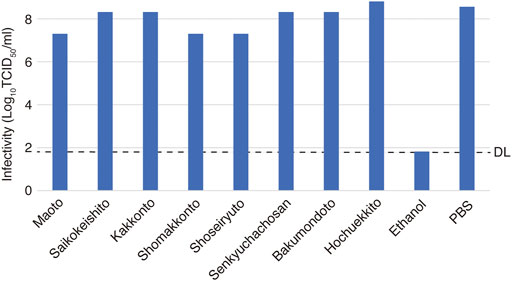
FIGURE 2. Effects of Kampo formulas on virus particle inactivation. A solution of the Kampo formula (20 mg/ml, 90 µl) and 10 µl of the virus solution at 2.0 × 109 TCID50/ml was incubated for 3 min at room temperature. The mixture was then serially diluted 10-fold in DMEM, and the infectivity was determined by the TCID50 method. Phosphate-buffered saline (PBS) was used as an untreated control, and ethanol [70% (w/w)] was used as an inactivation control. The dotted line indicates the detection limit (DL) of the infectivity assay.
In this experiment, the virus was inoculated into cells at an m.o.i. of 0.05. If the virus is inhibited by the Kampo formula, then the virus may be suppressed at one of the following stages: intracellular multiplication, release from the cell, or reinfection of neighboring cells. From the results of the experiment described above (Figure 2), it is unlikely that the viral particles are directly inactivated, and intracellular proliferation or release from the cell may therefore be impaired.
Suppressive Effects of Crude Drugs on SARS-CoV-2 Infection
We investigated the suppressive effects on virus infection of the available crude drugs with high percentages of composition among the crude drugs constituting Maoto, Senkyuchachosan, and Saikokeishito, which had strong inhibitory effects on SARS-CoV-2 infection. The crude drugs tested were Ephedrae herba, Glycyrrhizae radix, Scutellariae radix, Paeoniae radix, Bupleuri radix, and Cnidii rhizoma (Figure 3). Ephedrae herba showed cytotoxicity at concentration of 2.5 mg/ml and above, Bupleuri radix showed cytotoxicity at concentrations of 10 mg/ml and above, and Scutellariae radix showed cytotoxicity at concentrations of 5 mg/ml and above. Therefore, the effects of these crude drugs at lower concentrations were evaluated.
FIGURE 3. Inhibition of SARS-CoV-2 replication by crude drugs. (A-F) The crude drug used is noted at the top of each panel. VeroE6/TMPRSS2 cells were infected with SARS-CoV-2 at an m.o.i. of 0.05 or 10. After 2-h adsorption, the inoculum was removed and the cells were cultured in DMEM containing different concentrations of crude drugs for 24 h. The viral infectivity in the medium was assayed by the TCID50 method. The left y-axis of the graph is the infectivity for each concentration. An exponential or linear approximation was made by Excel to calculate the drug concentration that reduces the infectivity in the absence of a drug to 50% (IC50). The IC50 values and approximation equations are shown in the graph. If there is little or no decrease, the IC50 is not shown (E,F). VeroE6/TMPRSS2 cells were incubated in DMEM supplemented with the designated concentrations of a Kampo fomula for 24 h, and LDH values in the media were then measured to evaluate cytotoxicity. The LDH value from detergent-treated cells was set at 100%, and the right y-axis is the percent inhibition of cytotoxicity for each concentration.
The cells were infected with the virus at an m.o.i. of 0.05, and the infectious titer was measured by the TCID50 method (Figure 3). Ephedrae herba showed a strong inhibitory effect on virus infection, while Paeoniae radix, Scutellariae radix, and Glycyrrhizae radix showed weaker inhibitory effects (Figure 3).
To investigate the mechanisms by which the crude drugs suppress virus infection, we conducted infection experiments using crude drugs (Ephedrae herba, Scutellariae radix, Paeoniae radix, Glycyrrhizae radix) at an m.o.i. of 10 so that all of the cells would be infected (Figure 3). Ephedrae herba showed a strong suppressive effect on virus infection even at an m.o.i. of 10, and Paeoniae radix and Scutellariae radix also showed suppressive effects (Figure 3). These results suggest that Ephedrae herba, Paeoniae radix, and Scutellariae radix act on virus-infected cells to inhibit viral replication in the cells. On the other hand, Glycyrrhizae radix showed little inhibitory effect in the infection experiment with an m.o.i. of 10 (Figure 3), suggesting that the antiviral effect of Glycyrrhizae radix is due to its inhibition of spread of the virus to neighboring uninfected cells.
The inactivating effect of each of the crude drugs on virus particles was investigated by mixing the crude drug at the highest concentration used in the virus infection experiment and measuring the infectivity titer (Figure 4). Ephedrae herba decreased the infections titer by 2.5 Log10 (TCID50/ml) compared to the control, suggesting that Ephedrae herba may act directly on the virus particles to inactivate them. The other crude drugs did not reduce the infectious titer, indicating that they did not inactivate the virus particles (Figure 4).
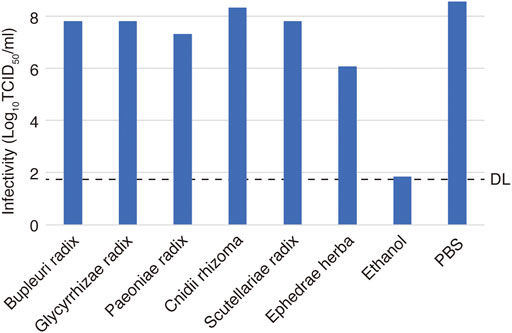
FIGURE 4. Effects of crude drugs on virus particle inactivation. A solution of a crude drug (90 µl) and 10 µl of the virus solution at 2.0 × 109 TCID50/ml was incubated for 3 min at room temperature. Reagents at 1.25–10 mg/ml concentrations were used considering their cytotoxicity. The mixture was then serially diluted 10-fold in DMEM, and the infectivity was determined by the TCID50 method. Phosphate-buffered saline (PBS) was used as an untreated control, and ethanol [70% (w/w)] was used as an inactivation control. The dotted line indicates the detection limit (DL) of the infectivity assay.
Contribution of Epherae Herba in Kampo Formulas
In this study, we found that Ephedrae herba has a strong inhibitory effect on SARS-CoV-2 infection. Ephedrae herba is found in several Kampo formulas, and to verify the role of Ephedrae herba in inactivation of SARS-CoV-2 by the herbal medicines, we recalculated the effect of Ephedrae herba on SARS-CoV-2 based on the amount of Ephedrae herba in each Kampo formula.
The IC50 value of Ephedrae herba alone was 61.1 μg/ml (Figures 3A, 5A). Maoto contains 32.3% of Ephedrae herba (Table 1), and the IC50 value was calculated by plotting the amount of Ephedrae herba in Maoto on the horizontal axis of the graph (Figure 5B) to be 163.6 μg/ml (Figure 5B). Thus, although it should be the same amount of Ephedrae herba, Ephedrae herba in the form of Maoto was weakened, suggesting that other components of Maoto may be inhibiting the effect Ephedrae herba. The IC50 value of Maoto itself was calculated to be 0.5 mg/ml (Figure 1A), being consistent with this hypothesis.
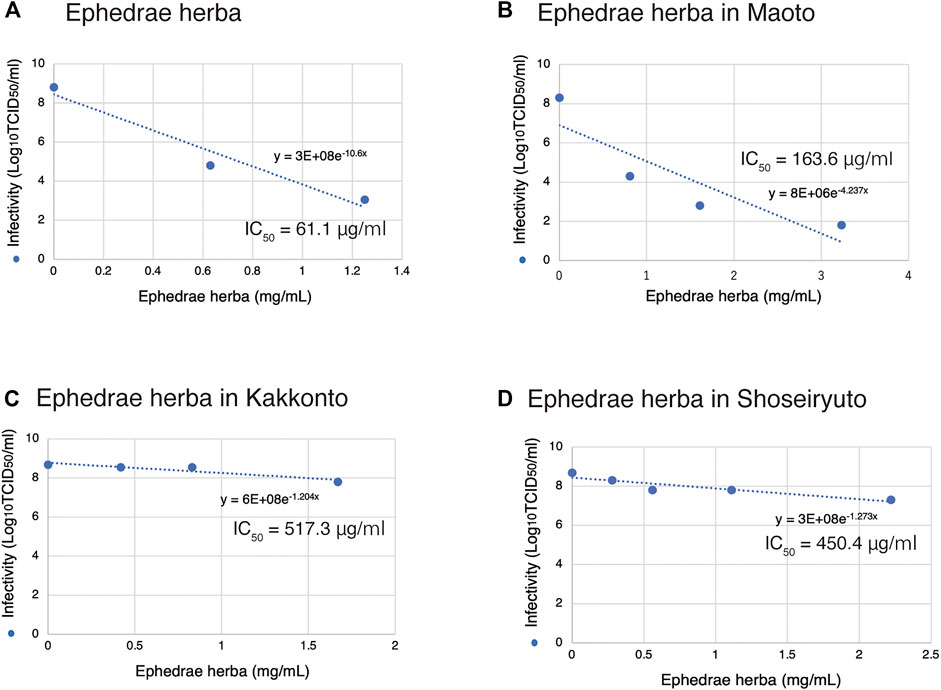
FIGURE 5. Inhibition of SARS-CoV-2 replication based on the amount of Ephedrae herba contained. (A-D) The origin of Ephedra herba is noted at the top of each panel. The results for growth inhibition of SARS-CoV-2 by Ephedrae herba and Kampo formulas containing Ephedrae herba (Figure 1) are re-plotted in a graph based on the amount of Ephedrae herba contained. An exponential approximation was made by Excel to calculate the drug concentration that reduces the infectivity in the absence of a drug to 50% (IC50). The IC50 values and approximation equations are shown in the graph.
Similarly, in the case of Kakkonto containing 16.7% of Ephedrae herba (Table 1), the IC50 value was calculated to be as high as 517.3 μg/ml based on the amount of Ephedrae herba (Figure 5C). The IC50 value of Kakkonto itself was 2.7 mg/ml (Figure 1C). Shoseiryuto contained 11.1% of Ephedrae herba (Table 1), and the IC50 value of Ephedrae herba was as high as 450.4 μg/ml based on Ephedrae herba (Figure 5D). The IC50 value of Shoseiryuto itself was 4.9 mg/ml (Figure 1E). These results suggest that the inhibitory effect of Ephedrae herba in the Kampo formulas on SARS-CoV-2 infection is weakened by other components.
Discussion
Kampo formulas consist of various combinations of crude drugs, traditionally regarded as units, and are often used on the base of ancient experience (Tanaka et al., 1995; Odaguchi et al., 2019). In Japan, Kampo formulas are covered by the national health insurance system and are prescribed by doctors under the condition of government subsidies. Some Kampo formulas are also available as over-the-counter drugs that can be purchased at pharmacies without a doctor’s prescription. The extensive experience of using Kampo formulas in Japan has led to their safe and easy use in medical practice. In recent years, scientific analysis of the effects of Kampo formulas has provided evidence of their clinical benefits, and their clinical usefulness for the treatment of COVID-19 has also been suggested (Motoo et al., 2014; Takayama et al., 2021).
Some advantages of Kampo formulas are that they can be taken orally, are relatively inexpensive, and can be taken at an early stage. For example, Maoto, one of the Kampo formulas examined in this study, can be used to treat common colds in infants even before the onset of cold symptoms such as fever and nasal discharge (Kubo and Nishimura, 2007; Nagai et al., 2014). On the other hand, antivirals, as shown in clinical trials for influenza (Muthuri et al., 2014; Dobson et al., 2015), need to be taken after the onset of illness and before the peak of viral replication. The clinical course of COVID-19 is longer than that of influenza, and viral replication in patients with COVID-19 likely to continue for a long time. Although existing antivirals such as remdesivir may be effective if treatment with the antivirals started after the diagnosis of COVID-19, consideration should be given to the use of Kampo formulas, which can be administered earlier.
Kampo formulas that are used for the treatment of influenza have been reported to inhibit the growth of influenza viruses in cultured cells (Mantani et al., 1999; Nomura et al., 2019; Hou et al., 2020). Although the relationship between inhibition of influenza virus replication in cultured cells and clinical efficacy is not entirely clear, the therapeutic effect of Kampo formulas may be elicited by suppression of the ability of the virus of replicate in the human respiratory tract. In addition, Lian hua qing wen, which contains Ephedrae herba and is one of the therapeutic agents used for influenza viruses in traditional Chinese medicine, has been shown in vitro experiments to have an inhibitory effect on SARS-CoV-2 infection and has also been sown to be effective for COVID-19 in a clinical setting, (Su et al., 2020; Hu et al., 2021). Therefore, we investigated the effects of Kampo formulas on SARS-CoV-2 infection in vitro experiments.
Since commercial Kampo formulas contain additives and the types and amounts of additives are not uniform, we used the same concentrations of Kampo formulas without additives in this study. We found that virus infection was inhibited by the Kampo formulas Maoto, Saikokeishito, and Senkyuchachosan but not by the Kampo formulas Bakumondoto and Hochuekkito. We also examined the viral inhibitory effects of crude drugs as constituents of the Kampo formulas that had strong inhibitory effects on SARS-CoV-2 infection. Ephedrae herba had the strongest inhibitory effect on SARS-CoV-2 infection. Among the six crude drugs examined, Ephedrae herba, Paeoniae radix, Scutellariae radix, and Glycyrrhizae radix had the strongest inhibitory effects in that order.
Ephedrae herba showed direct inactivating effects on virus particles as well as on infected cells. Previous studies have shown that tannins, which are components of Ephedrae herba, inhibit influenza virus infection (Mantani et al., 1999) and that tannins extracted from plants such as persimmon have inactivating effects on various viruses (Ueda et al., 2013). In the present study, it was also thought that tannins in Ephedrae herba may have direct inhibitory effects on virus infection. However, in the infection experiments to investigate the suppressive effects of crude drugs, on virus infection, it was found that Ephedra herba had a suppressive effect even under the condition of an m.o.i. of 10, in which all cells were infected at once, and no virus particle formation process was involved. Therefore, Ephedrae herba may have both a direct inactivation effect on virus particles and a suppressive effect on infection of cells, suggesting a combined virus suppression mechanism.
However, it was found that Kampo formulas containing Ephedrae herba had little direct inactivation effect on virus particles. The contents of Ephedrae herba in the Kampo formulas used in the virus direct inactivation test (20 mg/ml) were 6.45 mg/ml in Maoto, 3.33 mg/ml in Kakkonto, and 2.22 mg/ml in Shoseiryuto. These concentrations were higher than the concentration of Ephedrae herba tested as a crude drug (1.25 mg/ml), which was found to be effective in the virus particle direct inactivation test. Furthermore, when the IC50 values of Kampo formulas containing Ephedrae herba and Ephedrae herba alone were compared on the basis of the content of Ephedrae herba, the effects of all Kampo formulas were weaker than the effect of Ephedrae herba alone. These results suggest that the combination of Ephedrae herba with other crude drugs in Kampo formulas has a weaker antiviral effect than that of Ephedrae herba. Ephedra herba has various side effects, and Kampo formulas are shown to contain crude drugs that alleviate the side effects of the strong Ephedra herba (Odaguchi et al., 2019). The reduced suppressive effect of Ephedra herba on virus infection may mean that its side effects on the human body are reduced.
Among the Kampo formulas that do not contain Ephedrae herba, Saikokeishito and Senkyuchachosan showed efficacy. These Kampo formulas have been reported to inhibit influenza virus in vitro (Shirayama et al., 2016; Nomura et al., 2019), and the Ministry of Health, Labour, and Welfare has approved Saikokeishito for treatment of influenza. Therefore, it would not be surprising if these Kampo formulas also have inhibitory effects on SARS-CoV-2 infection. Furthermore, viral inhibitory effects of Glycyrrhizae radix, Paeoniae radix, and Scutellariae radix as crude drugs contained in these Ephedrae herba-free Kampo formulas were confirmed. It was shown in an in vitro study that rhizoma had an inhibitory effect on influenza virus infecion (Nomura et al., 2019), but it had no inhibitory effect on SARS-CoV-2 infection.
Glycyrrhizae radix is a crude drug in many Kampo formulas. All of the Kampo formulas used in this study contained Glycyrrhizae radix (Maoto: 9.7%, Saikokeishito: 9.1%, Kakkonto: 11%, Shomakakkonto: 12.5%, Shoseiryuto: 11%, Senkyuchachosan: 7.5%, Bakumondoto: 7.4%, Hochuekkito: 6.3%; Table 1). Although a suppressive effect of Glycyrrhizae radix alone on SARS-CoV-2 infection was shown in a previous study (van de Sand et al., 2021), not all of the Kampo formulas containing Glycyrrhizae radix showed suppressive effects on virus infection, and some Kampo formulas showed no effect at all. Therefore, other crude drug components in the Kampo formulas may weaken the effect of Glycyrrhizae radix. Alternatively, the amount of Glycyrrhizae radix in the Kampo formulas may have been too small to reach the threshold for suppression of virus infection.
Paeoniae radix was suggested to be effective against SARS-CoV-2 infection by molecular modeling predictions (Ma et al., 2020). It was also shown to be effective against influenza virus infection in cell infection experiments (Ho et al., 2014). Scutellariae radix is one of the crude drugs contained in Saikokeishito, which was effective against SARS-CoV-2 infection. In addition, Baicalin, a component of Scutellariae radix, has been shown to have an inhibitory effect on SARS-CoV-2 infection in cell and animal experiments (Song et al., 2021). In the present study, Glycyrrhizae radix, Paeoniae radix, and Scutellariae radix showed inhibitory effects on SARS-CoV-2 infection, but their effects were inferior to the effect of Ephedrae herba.
Conclusion
We performed in vitro experiments to determine the inhibitory effects on SARS-CoV-2 infection of Kampo formulas and their constituent crude drugs that are used to treat respiratory symptoms including influenza and common cold symptoms. We found that Maoto among the Kampo formulas and Ephedrae herba among the crude drugs had the strongest inhibitory effects on SARS-CoV-2 infection. Some other Kampo formulas and crude drugs also showed suppressive effects on virus infection. Although further analysis and evidence are needed, Kampo formulas might contribute to the treatment of COVID-19.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
MK, TNo and TNa. developed the study design, performed the experiments, and analyzed the data. HK, KO-O, HO and TS provided assistance in developing the study design and experiments. MK, TNo, TNa and TS wrote the manuscript. KK, KO-O, HO and MI provided advice on interpretation of data and revised the manuscript. All authors read and approved the manuscript.
Funding
This study was supported by the Research Grants for COVID-19 from AMED, Japan (to HO and TS) and a grant from the Government-Academia Collaboration of Hiroshima Prefecture (to HO and TS). This study also received funding from the Sumitomo Mitsui Trust Bank COVID-19 Research Fund (to TS) and the Otsuka Toshimi Scholarship Foundation (to TNa.). The funders were not involved in the study design, collection, analysis, and interpretation of data, writing of this article, or the decision to submit it for publication. All authors declare no other competing interests.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank Rie Hirakawa and Chika Masaki for manuscript preparation. We also thank the staff of the Analysis Center of Life Science, Hiroshima University for the use of their facilities.
References
Dobson, J., Whitley, R. J., Pocock, S., and Monto, A. S. (2015). Oseltamivir Treatment for Influenza in Adults: a Meta-Analysis of Randomised Controlled Trials. Lancet 385, 1729–1737. doi:10.1016/S0140-6736(14)62449-1
Ho, J. Y., Chang, H. W., Lin, C. F., Liu, C. J., Hsieh, C. F., and Horng, J. T. (2014). Characterization of the Anti-influenza Activity of the Chinese Herbal Plant Paeonia Lactiflora. Viruses 6, 1861–1875. doi:10.3390/v6041861
Hou, S., Xu, X., Wang, Y., and Yang, Y. (2020). Ephedrannin B Exerts Anti-viral and Anti-inflammatory Properties in BEAS-2B Cells Infected with Respiratory Syncytial Virus. J. Biosci. 45, 46. doi:10.1007/s12038-020-0016-y
Hu, K., Guan, W. J., Bi, Y., Zhang, W., Li, L., Zhang, B., et al. (2021). Efficacy and Safety of Lianhuaqingwen Capsules, a Repurposed Chinese Herb, in Patients with Coronavirus Disease 2019: A Multicenter, Prospective, Randomized Controlled Trial. Phytomedicine 85, 153242. doi:10.1016/j.phymed.2020.153242
Kubo, T., and Nishimura, H. (2007). Antipyretic Effect of Mao-To, a Japanese Herbal Medicine, for Treatment of Type A Influenza Infection in Children. Phytomedicine 14, 96–101. doi:10.1016/j.phymed.2006.09.015
Liu, Y., Arase, N., Kishikawa, J., Hirose, M., Lie, S., Tada, A., et al. (2021). The SARS-CoV-2 Delta Variant Is Poised to Acquire Complete Resistance to Wild-type Spike Vaccines. bioRxiv [Preprint]. Available at: https://www.biorxiv.org/content/10.1101/2021.08.22.457114v1 (Accessed September 8, 2021).
Ma, J., Huo, X. Q., Chen, X., Zhu, W. X., Yao, M. C., Qiao, Y. J., et al. (2020). Study on Screening Potential Traditional Chinese Medicines against 2019-nCoV Based on Mpro and PLP. Zhongguo Zhong Yao Za Zhi 45, 1219–1224. (in Chinese). doi:10.19540/j.cnki.cjcmm.20200216.401
Mantani, N., Andoh, T., Kawamata, H., Terasawa, K., and Ochiai, H. (1999). Inhibitory Effect of Ephedrae Herba, an oriental Traditional Medicine, on the Growth of Influenza A/PR/8 Virus in MDCK Cells. Antivir. Res 44, 193–200. doi:10.1016/s0166-3542(99)00067-4
Matsuyama, S., Nao, N., Shirato, K., Kawase, M., Saito, S., Takayama, I., et al. (2020). Enhanced Isolation of SARS-CoV-2 by TMPRSS2-Expressing Cells. Proc. Natl. Acad. Sci. U S A. 117, 7001–7003. doi:10.1073/pnas.2002589117
Motoo, Y., Arai, I., and Tsutani, K. (2014). Use of Kampo Diagnosis in Randomized Controlled Trials of Kampo Products in Japan: a Systematic Review. PLoS One 9, e104422. doi:10.1371/journal.pone.0104422
Muthuri, S. G., Venkatesan, S., Myles, P. R., Leonardi-Bee, J., Al Khuwaitir, T. S., Al Mamun, A., et al. (2014). Effectiveness of Neuraminidase Inhibitors in Reducing Mortality in Patients Admitted to Hospital with Influenza A H1N1pdm09 Virus Infection: a Meta-Analysis of Individual Participant Data. Lancet Respir. Med. 2, 395–404. doi:10.1016/S2213-2600(14)70041-4
Nagai, T., Kataoka, E., Aoki, Y., Hokari, R., Kiyohara, H., and Yamada, H. (2014). Alleviative Effects of a Kampo (A Japanese Herbal) Medicine "Maoto (Ma-Huang-Tang)" on the Early Phase of Influenza Virus Infection and its Possible Mode of Action. Evid. Based Complement. Alternat Med. 2014, 187036. doi:10.1155/2014/187036
Nomura, T., Fukushi, M., Oda, K., Higashiura, A., Irie, T., and Sakaguchi, T. (2019). Effects of Traditional Kampo Drugs and Their Constituent Crude Drugs on Influenza Virus Replication In Vitro: Suppression of Viral Protein Synthesis by Glycyrrhizae Radix. Evid. Based Complement. Alternat Med. 2019, 3230906. doi:10.1155/2019/3230906
Nomura, T., Nazmul, T., Yoshimoto, R., Higashiura, A., Oda, K., and Sakaguchi, T. (2021). Ethanol Susceptibility of SARS-CoV-2 and Other Enveloped Viruses. Biocontrol Sci. 26, 177–180. doi:10.4265/bio.26.177
Odaguchi, H., Hyuga, S., Sekine, M., Nakamori, S., Takemoto, H., Huang, X., et al. (2019). The Adverse Effects of Ephedra Herb and the Safety of Ephedrine Alkaloids-free Ephedra Herb Extract (EFE). Yakugaku zasshi 139, 1417–1425. (in Japanese). doi:10.1248/yakushi.19-00122
Shirayama, R., Shoji, M., Sriwilaijaroen, N., Hiramatsu, H., Suzuki, Y., and Kuzuhara, T. (2016). Inhibition of PA Endonuclease Activity of Influenza Virus RNA Polymerase by Kampo Medicines. Drug Discov. Ther. 10, 109–113. doi:10.5582/ddt.2016.01010
Song, J., Zhang, L., Xu, Y., Yang, D., Zhang, L., Yang, S., et al. (2021). The Comprehensive Study on the Therapeutic Effects of Baicalein for the Treatment of COVID-19 In Vivo and In Vitro. Biochem. Pharmacol. 183, 114302. doi:10.1016/j.bcp.2020.114302
STORK (2020). Standards of Reporting Kampo Products, ver. 4.3. Department of Pharmacognosy, Phytochemistry and Narcotics (DPPN), National Institute of Health Sciences (NIHS) of Japan and National Institutes of Biomedical Innovation, Health and Nutrition (NIBIOHN). Available at: http://mpdb.nibiohn.go.jp/stork/index.html.(Accessed September 1, 2021)
Su, H. X., Yao, S., Zhao, W. F., Li, M. J., Liu, J., Shang, W. J., et al. (2020). Anti-SARS-CoV-2 Activities In Vitro of Shuanghuanglian Preparations and Bioactive Ingredients. Acta Pharmacol. Sin 41 (9), 1167–1177. doi:10.1038/s41401-020-0483-6
Takayama, S., Namiki, T., Odaguchi, H., Arita, R., Hisanaga, A., Mitani, K., et al. (2021). Prevention and Recovery of COVID-19 Patients with Kampo Medicine: Review of Case Reports and Ongoing Clinical Trials. Front. Pharmacol. 12, 656246. doi:10.3389/fphar.2021.656246
Tanaka, T., Obha, K., Lawaahara, K., and Sakai, E. (1995). Comparison of the Constituents of Ephedra Herbs from Various Countries on Ephedrine Type Alkaloids. Nat. Med 49, 418–424.
Tsumura & Co. (2016). About Kampo. Available at: https://www.tsumura.co.jp/english/kampo/02.htm (Accessed September 1, 2021).
Ueda, K., Kawabata, R., Irie, T., Nakai, Y., Tohya, Y., and Sakaguchi, T. (2013). Inactivation of Pathogenic Viruses by Plant-Derived Tannins: strong Effects of Extracts from Persimmon (Diospyros Kaki) on a Broad Range of Viruses. PLoS One 8, e55343. doi:10.1371/journal.pone.0055343
van de Sand, L., Bormann, M., Alt, M., Schipper, L., Heilingloh, C. S., Steinmann, E., et al. (2021). Glycyrrhizin Effectively Inhibits SARS-CoV-2 Replication by Inhibiting the Viral Main Protease. Viruses 13, 609. doi:10.3390/v13040609
Wang, M., Cao, R., Zhang, L., Yang, X., Liu, J., Xu, M., et al. (2020). Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-nCoV) In Vitro. Cell Res 30, 269–271. doi:10.1038/s41422-020-0282-0
World Health Organization. (2021). WHO Coronavirus (COVID-19) Dashboard. Available at: https://covid19.who.int (Accessed September 1, 2021).
Keywords: anti-viral, COVID-19, SARS-CoV-2, herbal medicine, kampo medicine, crude drug
Citation: Kakimoto M, Nomura T, Nazmul T, Kitagawa H, Kanno K, Ogawa-Ochiai K, Ohge H, Ito M and Sakaguchi T (2022) In vitro Suppression of SARS-CoV-2 Infection by Existing Kampo Formulas and Crude Constituent Drugs Used for Treatment of Common Cold Respiratory Symptoms. Front. Pharmacol. 13:804103. doi: 10.3389/fphar.2022.804103
Received: 28 October 2021; Accepted: 07 March 2022;
Published: 29 March 2022.
Edited by:
Kenji Watanabe, Yokohama College of Pharmacy, JapanReviewed by:
Xuepeng Gong, Huazhong University of Science and Technology, ChinaTheresa Li-Yun Chang, Rutgers, The State University of New Jersey, United States
Copyright © 2022 Kakimoto, Nomura, Nazmul, Kitagawa, Kanno, Ogawa-Ochiai, Ohge, Ito and Sakaguchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Takemasa Sakaguchi, dHNha2FAaGlyb3NoaW1hLXUuYWMuanA=
 Masaki Kakimoto
Masaki Kakimoto Toshihito Nomura2,3
Toshihito Nomura2,3 Keishi Kanno
Keishi Kanno Takemasa Sakaguchi
Takemasa Sakaguchi