
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol. , 25 January 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.802123
This article is part of the Research Topic From Clinical Trials to Real-World Data Sciences: Evidence-Based Medicine for Value in Health View all 36 articles
 Zijie Zhan1
Zijie Zhan1 Yiming Ma1
Yiming Ma1 Ke Huang2,3,4
Ke Huang2,3,4 Chen Liang5
Chen Liang5 Xihua Mao5
Xihua Mao5 Yaowen Zhang5
Yaowen Zhang5 Xiaoxia Ren2,3,4
Xiaoxia Ren2,3,4 Jieping Lei2,3,4
Jieping Lei2,3,4 Yan Chen1*
Yan Chen1* Ting Yang2,3,4*
Ting Yang2,3,4* Chen Wang2,3,4,6
Chen Wang2,3,4,6Background: Although medical guidelines discourage the use of methylxanthines in patients with acute exacerbation of chronic obstructive pulmonary disease (AECOPD), they are still widely used in clinical practice. This study investigated the real-world use of methylxanthines in the management of AECOPD.
Methods: Patient data from the Acute exacerbation of Chronic obstructive pulmonary disease Using REgistry data (ACURE, NCT02657525) study database were screened. Enrolled patients were divided into treatment and control groups. Propensity score (PS) matching and Cox regression analyses were used to minimize confounding factors and determine the association between methylxanthine treatment and the length of stay (LOS).
Results: Among the 2088 eligible patients, 1,563 (74.9%) were in the methylxanthine treatment group. Patients treated with methylxanthines had more severe respiratory symptoms and worse lung function than those in the control group. Doxophylline was the most commonly used methylxanthine in both secondary and tertiary hospitals. After PS matching, 966 patients were equally divided into two groups. The LOS of patients in the two groups was similar [median: 8 days, interquartile range (IQR): 7–11 days, p = 0.730]. Patients in the treatment group (median: 8, IQR: 4–12) had a more significant decrease in the COPD Assessment Test score from admission to discharge than those in the control group (median: 6, IQR: 2–10, p < 0.001). Among all matched patients, the LOS was not significantly associated with methylxanthine treatment [adjusted hazard ratio (HR): 1.02, 95% confidence intervals (CIs): 0.89–1.16]. However, in the subgroup analysis, methylxanthines were significantly associated with a short LOS in patients with blood eosinophil count >4% (adjusted HR: 1.56, 95% CIs: 1.12–2.17).
Conclusion: This study revealed that methylxanthines, especially doxophylline, are widely used in China. Methylxanthines were effective in improving symptoms in AECOPD patients. Higher blood eosinophil count may be associated with a better efficacy of methylxanthine treatment.
Chronic obstructive pulmonary disease (COPD) is characterized by chronic respiratory symptoms and persistently inadequate airflow. COPD is highly prevalent worldwide and is associated with high disability and mortality according to the Global Burden of Disease study, making it a major public health challenge. In 2017, 299.4 million COPD cases and 3.2 million COPD-related deaths were reported worldwide (GBD 2017 Causes of Death Collaborators, 2018; GBD 2017 Disease and Injury Incidence and Prevalence Collaborators, 2018). In China, 99.9 million cases were reported, and COPD was the third leading cause of death in 2015 (Zhou et al., 2016; Wang et al., 2018). Acute exacerbation of COPD (AECOPD) is a significant event in the course of the disease, and develops in patients approximately 0.5–3.5 times annually. Further, AECOPD is associated with an accelerated decline of lung function, worse quality of life, and increased risk of mortality (Seemungal et al., 1998; Donaldson et al., 2002; Cai et al., 2014; Müllerova et al., 2015). Therefore, effective pharmacological treatments are required to minimize the impact of this condition on patients’ health and well-being. Methylxanthines, such as theophylline, have been used in the treatment of COPD and asthma for more than 80 years. They are known to promote bronchodilation and enhance inspiratory muscle function (Barnes, 2013). In addition, theophylline has an anti-inflammatory effect and can reverse corticosteroid resistance (Ito et al., 2002; Cosio et al., 2004). However, previous randomized control trials on methylxanthine treatment for AECOPD have indicated frequent side effects and drug interactions (Barr et al., 2003; Duffy et al., 2005). Based on these results, the Global Initiative for Chronic Obstructive Lung Disease (GOLD) does not recommend the use of methylxanthines in AECOPD patients. Similarly, the Chinese expert consensus recommended the use of methylxanthines only in selected patients who have an insufficient response to short-acting bronchodilators or in some severe AECOPD patients (Cai et al., 2014; Global, 2019).
However, methylxanthines, especially doxophylline, which is a methylxanthine derivative, are still widely used for AECOPD in actual clinical settings in China. It has been reported that doxophylline has better efficacy and safety than other types of methylxanthines, such as theophylline and aminophylline (Cazzola et al., 2018). However, the current use of methylxanthines in China and their efficacy and safety in real-world settings have not been described.
In this study, we first described important aspects of current usage of methylxanthines in hospitalized AECOPD patients in China and subsequently analyzed the efficacy of methylxanthine use in such settings.
Data for this multicenter retrospective cohort study were obtained from the Acute exacerbation of Chronic obstructive pulmonary disease Using REgistry (ACURE, NCT02657525) database (Pei et al., 2020). The ACURE study is an ongoing, nationwide, multicenter, prospective study that is investigating the demographic characteristics, clinical features, diagnosis, treatments, disease prognosis, and economic costs associated with hospitalized patients with exacerbations of COPD in real-world settings. Additional details of the ACURE study have been described elsewhere (Pei et al., 2020). The study discussed herein was approved by the Ethics Committee of the China-Japan Friendship Hospital (2015-88) and was conducted in accordance with the ethical standards stated in the Helsinki Declaration.
We used data from the ACURE database starting from January 2018 to December 2019 for patients with AECOPD hospitalized at 163 secondary or tertiary hospital sites. The inclusion criteria for patients were the following: patients who 1) were not less than 40 years old; 2) had spirometry test results and met the diagnostic criteria according to the 2020 GOLD report (Global, 2019), which is a ratio of the post-bronchodilator forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) of less than 0.7; and 3) were hospitalized due to AECOPD. Patients were excluded if they: 1) refused or withdrew informed consent; 2) had enrolled in other interventional studies; 3) had a diagnosis of pneumonia or chronic respiratory disease other than COPD, including asthma, lung cancer, bronchiectasis, and pulmonary fibrosis; and 4) were allergic to methylxanthines.
We extracted baseline characteristics of enrolled patients including demographic data, hospital sites of admission, smoking history, body mass index (BMI), AECOPD symptoms at admission, comorbid disease (cardiovascular disease, cerebrovascular disease, and diabetes) history, scores of the modified British Medical Research Council (mMRC) questionnaire, and COPD Assessment Test (CAT) questionnaire at admission, COPD severity, hospitalization history in the previous year, spirometry test results, and blood eosinophil counts. Dyspnea in AECOPD patients was assessed using the mMRC questionnaire, and other symptoms were assessed using the PEACE questionnaire from the Effect of Carbocisteine on Acute Exacerbation of chronic obstructive Pulmonary disease study (Zheng et al., 2008). The severity of COPD was assessed according to the GOLD staging system given in the 2020 GOLD report (Global, 2019). A history of pharmacological treatments undertaken during hospitalization, including the use of methylxanthines, bronchodilators, and corticosteroids, were also extracted. We divided the enrolled patients into treatment and control groups based on the use of methylxanthines.
The primary outcome was the length of stay (LOS), and the secondary outcomes were a change in the CAT score from admission to discharge, total direct costs, intensive care unit (ICU) admission and mortality during hospitalization, the CAT score at the 30-days follow-up visit, all-cause readmissions, and AECOPD-related readmissions within 30 days after discharge. Total direct costs were converted to United States dollars using the average exchange rate in 2019 (1 United States dollar was equivalent to 6.90 Yuan) because most patients were enrolled in this year.
Continuous variables with normal distributions have been presented as a mean with standard deviation, whereas non-normally distributed continuous variables have been presented a median and interquartile range (IQR). Categorical variables have been presented as frequency and percentage. To compare variables in the two groups, Student’s t-test or the Wilcoxon rank sum test were used for continuous variables and the Chi-square test or Fisher’s exact test were used for categorical variables.
To reduce the effect of confounding factors in this non-experimental study, a propensity score (PS) matching was performed, using the “MathIt” package in R software (Brookhart et al., 2013; Zhang, 2017). We calculated the PS using multivariable logistic regression. Demographic characteristics, including the age, sex, and other baseline variables with a p-value <0.1 in univariable analysis were included in the model. In addition, we used the “nearest” method and a caliper equal to 0.05 to conduct the PS match with a 1:1 ratio.
We used the Cox proportional hazards model to estimate the hazard ratio (HR) between methylxanthine treatment and the LOS. The outcome of the event was defined as hospital discharge. Both univariate and multivariate analyses were performed. All factors with a p-value <0.05, following a univariable analysis, were included in the multivariable analysis. HRs > 1 represented early discharge and a short LOS, while HRs < 1 represented late discharge and a long LOS.
In addition, we performed a subgroup analysis to examine the association between methylxanthine treatment and the LOS in subsets of the population. Patients were divided into subgroups according to the age, sex, smoking history, severity of AECOPD symptoms (cough, sputum, dyspnea, and wheezing), CAT score, GOLD stage, hospitalization in the previous year, and blood eosinophil counts. Cough symptoms were considered to be light if patients had no cough or only morning cough, whereas severe cough symptoms were considered to be episodes of cough during the day or nearly continuous cough.
Statistical analyses were performed using the R software (version 4.0.2). All statistical tests were two-sided tests, with significance set at p < 0.05.
A total of 2088 patients met all the eligibility criteria (Figure 1). Among them, 247 patients were from secondary hospitals and 1841 patients were from tertiary hospitals. The median age of the included patients was 70 (IQR: 64–76) years, and 1,679 (80.4%) patients were male. A total of 589 (28.2%) patients were current smokers, 913 (43.7%) were former smokers, and 586 (28.1%) were non-smokers. Among the included patients, 1,563 (74.9%) patients received methylxanthine treatment during hospitalization (treatment group) and 525 (25.1%) patients did not receive methylxanthine treatment (control group).
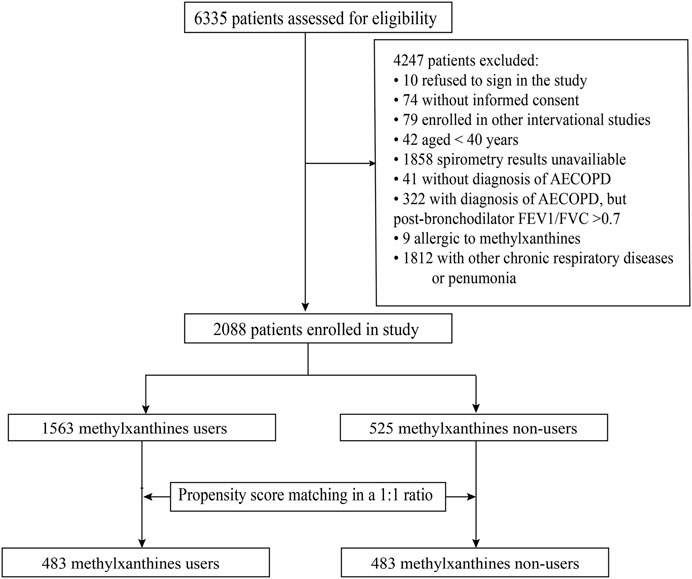
FIGURE 1. The flowchart of patient enrollment process. AECOPD, acute exacerbations of chronic obstructive pulmonary disease, FEV1/FVC, forced expiratory volume in one second/forced vital capacity.
Demographic characteristics and clinical factors at admission of all enrolled patients before and after PS matching are given in Table 1, and treatment course during hospitalization has been summarized in Table 2.
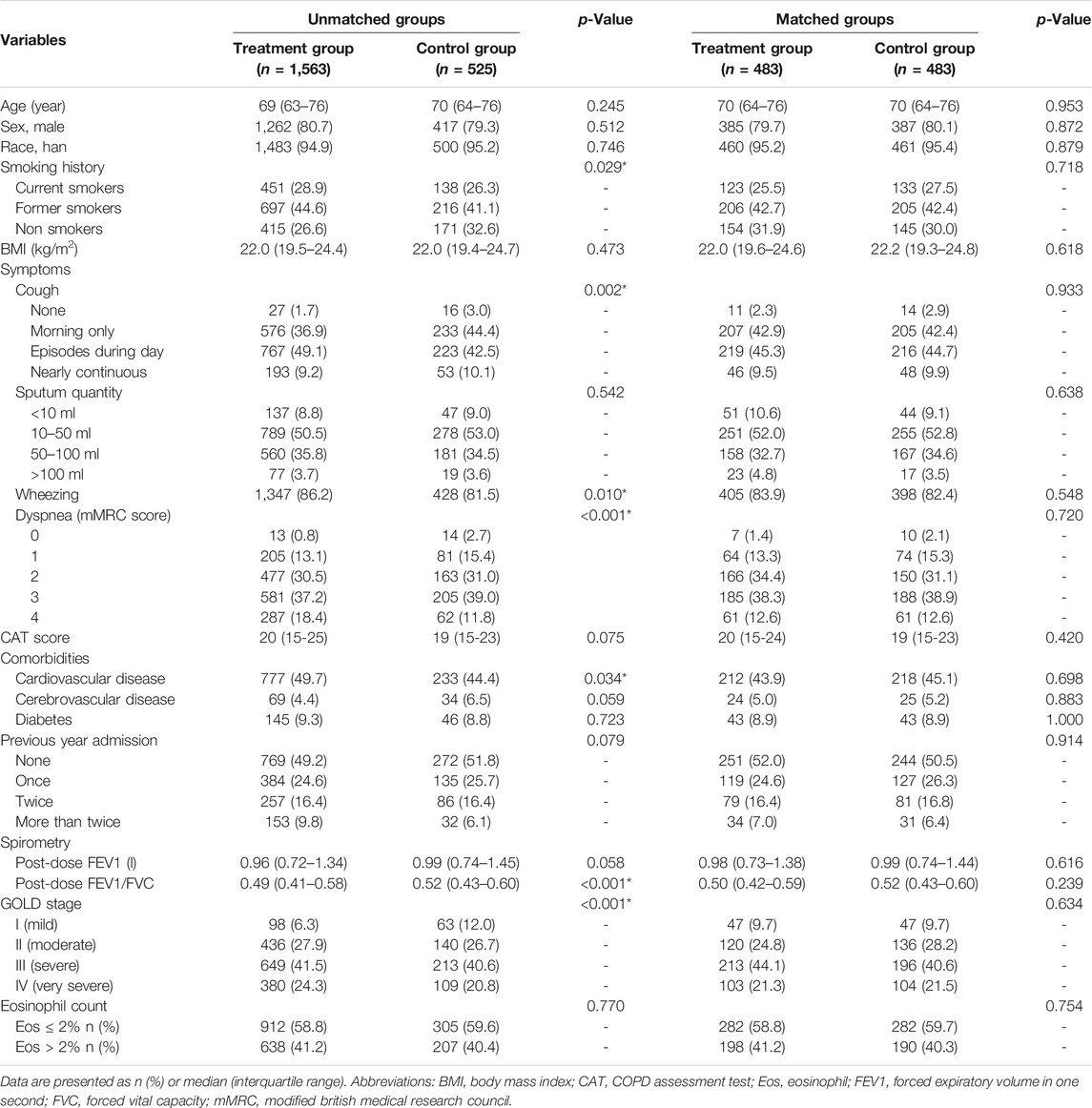
TABLE 1. Baseline characteristics of hospitalized acute exacerbation of chronic obstructive pulmonary disease patients at admission.
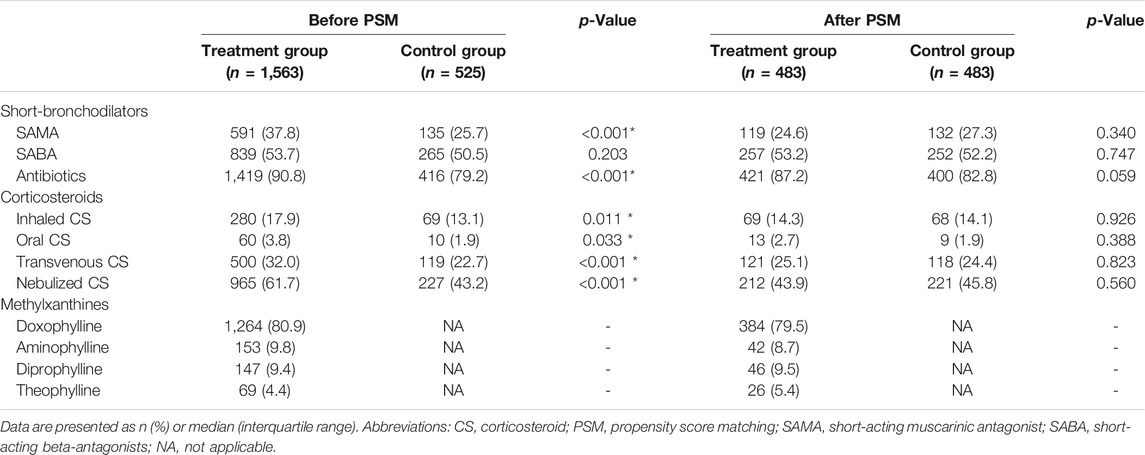
TABLE 2. In-hospital treatment of acute exacerbation of chronic obstructive pulmonary disease patients with or without methylxanthines.
The groups were imbalanced for the smoking history, AECOPD symptoms, including cough, wheezing, and dyspnea, comorbid cardiovascular disease, post-dose FEV1/FVC, GOLD stage, and use of bronchodilators, antibiotics and corticosteroids. Patients who received methylxanthines seemed to have severe AECOPD; they had severe cough (p = 0.002), dyspnea (p < 0.001), and wheezing (p = 0.010) at admission. Patients in the treatment group had a history of smoking (p = 0.029) and comorbid cardiovascular disease (p = 0.034). Spirometry test results showed that patients in the treatment group had a severe deterioration of lung function, as characterized by the FEV1/FVC (p < 0.001) and GOLD stage (p < 0.001). The CAT score in the treatment group (median: 20, IQR: 15–25) was slightly higher than that in the control group (median: 19, IQR: 15–23); however, the difference was statistically insignificant (p = 0.075). Among all AECOPD patients, 1,562 (74.8%) received either short-acting muscarinic antagonists (SAMA) or short-acting beta-antagonists (SABA). Further, 1,233 of the 1,563 (78.9%) patients in the treatment group received either SAMA or SABA; this proportion was significantly higher than that in the control group (329, 62.7%) (p < 0.001). Nearly half of the enrolled AECOPD patients received SABA during hospitalization, and there was no significant difference between groups; however, more patients in the treatment group (591, 37.8%) received SAMA than in the control group (135, 25.7%) (p < 0.001). Corticosteroids were commonly used for patients in the treatment group, regardless of the administration route. Methylxanthines were commonly used in both secondary and tertiary hospitals, and doxophylline was the most popular methylxanthine drug (63.49% in secondary hospitals and 57.92% in tertiary hospitals; Figure 2). The type of methylxanthine use was not significantly different between the two hospital levels (p = 0.226).
After PS matching, a total of 966 patients were divided equally into two groups, and all baseline characteristics were balanced. A total of 772 (79.9%) patients in the matched cohort were male, and the median age was 70 years (IQR: 64–76).
After PS matching, the median LOS for both the treatment and control groups was approximately 8 days (IQR: 7–11), without any significant difference (p = 0.730). However, a significant decrease was observed in the CAT score between the groups (treatment: median, 8; IQR: 4–12 vs. control: median: 6, IQR: 2–10; p < 0.001). The CAT score at discharge was also lower in the treatment group (median: 10, IQR: 8-14) than the control group (median 12, IQR: 8–16) (p < 0.001). There was no significant difference in the total cost, ICU admission, and in-hospital mortality between the groups (Table 3).
In the univariable Cox regression analysis, the use of methylxanthines was not significantly associated with the LOS (HR 0.97, 95% CI: 0.86–1.10, p = 0.644). After adjusting for the age, sex, BMI, sputum history, wheezing history, mMRC score, CAT score, comorbid cardiovascular and cerebrovascular diseases, history of hospitalization in the previous year, post-dose FEV1/FVC, use of antibiotics, and use of oral and transvenous corticosteroids, the use of methylxanthines was not significantly associated with the LOS (adjusted HR 1.02, 95% CI: 0.89–1.16, p = 0.783) (Table 4).
Among the 2088 enrolled patients, 30-days follow-up data was available for 1,246 (59.7%) patients after discharge. Baseline characteristics were similar between patients with and without 30-days follow-up data (Supplementary Table S1). After PS matching, 5 of 273 (1.8%) patients were readmitted to the hospital within 30 days with any cause in the treatment group. Only 1 of 300 (0.3%) patients were readmitted to the hospital within 30 days with any cause in the control group, and there was no significant difference between these groups (p = 0.107). Four of 273 (1.5%) patients were readmitted to the hospital because of AECOPD within 30 days in the treatment group, and there were no cases in the control group that had 30-days AECOPD-related readmission. However, there was no significant difference in AECOPD-related readmissions between the two groups (p = 0.051). The CAT score of the treatment group (median, 10, IQR: 8–14) at 30 days after discharge was still significantly lower than that of the control group (median, 12, IQR: 8–17, p = 0.006).
In the subgroup analysis of the matched cohort for LOS, the association between the use of methylxanthines and LOS remained insignificant in AECOPD patients across different categories of the age, sex, smoking history, severity of AECOPD symptoms, CAT score, GOLD stage, and history of hospitalization in the previous year. The use of methylxanthines was significantly associated with a short LOS only in patients with blood eosinophil counts >4% (adjusted HR: 1.56, 95% CI: 1.12–2.17) (Figure 3).
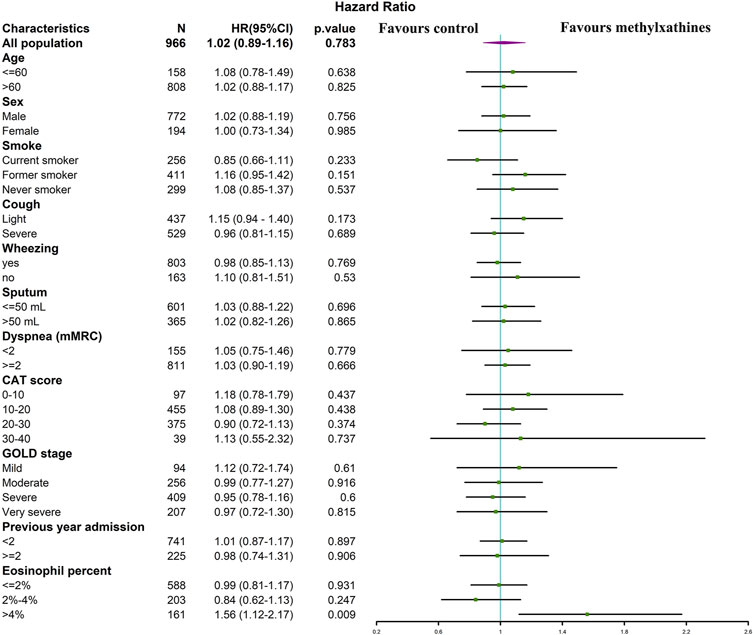
FIGURE 3. The association between LOS and methylxanthines use in subgroups of matched cohort. Variables increase the length of stay if HR < 1, whereas variables reduce the length of stay if HR > 1.
To the best of our knowledge, this is the first multicenter study that describes methylxanthine use in China and investigates the efficacy of methylxanthine treatment for hospitalized AECOPD patients. In our study, we found that a large number of hospitalized AECOPD patients around China received methylxanthine treatment. Among the commonly used methylxanthine drugs, doxophylline was the most common in both secondary and tertiary hospitals. Patients receiving add-on methylxanthine treatment tend to have severe disease as indicated by severe symptoms, spirometry tests, and comorbid diseases. We performed a 1:1 PS matching analysis to estimate the efficacy of methylxanthines. The matched cohort analysis showed significant symptomatic relief in patients who received methylxanthines during hospitalization. However, we did not find significant differences in the LOS, total cost of hospitalization, ICU admission, and in-hospital mortality. To investigate the subgroup of AECOPD patients who could benefit from methylxanthine treatment, we performed a subgroup analysis between methylxanthine treatment and the LOS in a subset of patients across categories of variables of interest. Interestingly, we found that patients with higher blood eosinophil counts may be associated with a shorter LOS and could benefit from methylxanthine treatment.
The methylxanthine family of drugs includes theophylline, aminophylline, diprophylline, and doxophylline. Theophylline is a classic type of methylxanthine, and several molecular mechanisms have been proposed for its therapeutic effect. It may inhibit phosphodiesterase (PDE) non-selectively, be antagonistic to adenosine receptors, and activate certain histone acetylases (Barnes, 2013). Recently, researchers have investigated the potential use of low-dose theophylline to reverse corticosteroid resistance (Cosio et al., 2004). Several randomized control trials using theophylline as an add-on therapy to corticosteroids for stable COPD patients have been conducted. However, the efficacy of low-dose theophylline add-on therapy is limited (Devereux et al., 2018; Devereux et al., 2019; Jenkins et al., 2020). As for management of acute exacerbations, an early meta-analysis revealed that treatment with theophylline or aminophylline did not improve the FEV1 during hospitalization and LOS. Inversely, some side effects such as nausea and vomiting were observed in patients in the treatment group (Barr et al., 2003). As a result, the GOLD report did not recommend theophylline or aminophylline for the management of AECOPD patients. Consistent with guidelines, our study found limited use of theophylline or aminophylline at both secondary and tertiary hospitals.
Doxophylline is another xanthine derivative with anti-inflammatory and bronchodilator effects. The molecular mechanisms of doxophylline are partially different from those of theophylline. It has decreased affinity toward adenosine A1 and A2 receptors, which may contribute to its better safety profile (Matera et al., 2017; Cazzola and Matera, 2020). Studies comparing the safety profile of doxophylline with other types of methylxanthines revealed fewer side effects than those with theophylline and aminophylline in COPD patients. In addition, it seems that doxophylline has significant efficacy in improving lung function, similar to the therapeutic effects of theophylline and aminophylline (Cazzola et al., 2018). Furthermore, although doxophylline has no significant effect on activation of certain histone acetylases, a recent study using a combination of doxophylline and dexamethasone in a murine model of lung inflammation indicated a corticosteroid-sparing effect at a low dose (Riffo-Vasquez et al., 2018). Thus, because of its good safety profile and promising efficacy, doxophylline was the most common drug used among the hospitalized AECOPD patients receiving methylxanthine treatment in our study. Similar to our study findings, methylxanthines in COPD patients are also widely used in Korea. In 2013, 63.4% of patients with COPD prescribed methylxanthines in Korea. However, the researchers did not analyze the type of methylxanthine and the disease stage for COPD patients (Lee et al., 2017).
In our study, we also designed a PS-matched cohort to investigate the efficacy of methylxanthines in real-world conditions. Unlike the results of a previous meta-analysis that indicated a non-significant change in symptom scores for theophylline and aminophylline treatment in AECOPD patients (Barr et al., 2003), we found significant symptomatic relief in patients who were administered methylxanthine treatment, as assessed by the CAT score. However, these patients did not show a significant reduction in the LOS. Interestingly, further subgroup analysis revealed a significant reduction in LOS for patients with blood eosinophil count >4%.
Eosinophils were recently regarded as an essential biomarker in COPD (Bafadhel et al., 2017). High eosinophils either in blood, sputum or airway were independently associated with frequent exacerbation, worse quality of life, and mortality (Hospers et al., 1999; Hospers et al., 2000; Hastie et al., 2017). It was argued that patients with high eosinophils share some common features with asthma, such as more reversibility to bronchodilators, increased FENO, and better treatment response to corticosteroids (Barnes, 2019). There were several well-designed randomized control trials investigating the role of eosinophils in corticosteroid treatment. For example, when comparing with dual bronchodilator therapy, a randomized clinical trial showed inhaled triple therapy adding beclomethasone dipropionate was associated with a significant reduction of moderate to severe exacerbation in patients with blood eosinophils >2% (Papi et al., 2018). Another hoc analysis of three randomized trials also indicated the exacerbation rate was significantly reduced in the group with blood eosinophils >100 cells/ul, while the efficacy increased at higher blood eosinophil level (Bafadhel et al., 2018). A meta-analysis including 5 randomized control trials indicated 17% reduction in exacerbation rate in patients with blood eosinophils >2% (Cheng, 2018).
Corticosteroids were widely used potent anti-inflammatory agents. They have been reported to induce eosinophil apoptosis through inhibiting the effects of IL-5 and GM-CSF on eosinophil survival and production of cytokines from cytokine-producing cells, such as T cells by means of apoptosis. Interestingly, theophylline, one typical type of methylxanthine, showed some addictive effect on induction of eosinophil apoptosis by functioning as a PDE inhibitor to increase intracellular cyclic adenosine monophosphate on activated eosinophils, the latter can induce apoptosis in eosinophils with some cytokines, such as IL-5 and GM-CSF (Hallsworth et al., 1992; Ohta and Yamashita, 1999). Even though there was no previous studies which investigated the role of methylxanthines in eosinophilic COPD, there was one study about the association between roflumilast, a selective PDE4 inhibitor, and eosinophils which revealed an improved efficacy in high blood eosinophil groups (Martinez et al., 2018). It was also reported that treatment with roflumilast reduced sputum neutrophil and eosinophil numbers by targeting PDE4 (Grootendorst et al., 2007). Thus, it was reasonable to infer that methylxanthines may have a better efficacy in patients with higher blood eosinophils.
An important concern regarding methylxanthine use is the risk of adverse effects. However, the ACURE study lacked data on side effects experienced by patients. Therefore, we could not analyze the side effects that occurred in real-world conditions. There were also other limitations to our study. First, follow-up data after 30 days was not available for all patients in our study. However, we compared the baseline characteristics between those with and those without follow-up data, and we did not find a significant difference, which reduced potential selective bias. Second, since the ACURE study is ongoing we did not have long-term follow-up data. This information will be available in the future and then analyzed. Third, the number of cases with 30-days readmission was limited, and the impact of methylxanthines on short-term readmission needs further study. Finally, for some sub-group analyses, the number of enrolled patients was limited, and further studies for particular AECOPD patients should be conducted.
This study revealed that methylxanthines, especially doxophylline, are widely used in China for the treatment of AECOPD. Methylxanthines were found to be effective in improving AECOPD symptoms. Higher blood eosinophil levels may be associated with better efficacy of methylxanthine treatment. Further studies are needed to determine the association between eosinophil count and the efficacy of methylxanthines in a large population.
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding authors.
The studies involving human participants were reviewed and approved by the Ethics Committee of the China-Japan Friendship Hospital (2015-88). The patients/participants provided their written informed consent to participate in this study.
Concept and design of the study by ZZ, CL, KH, TY, YC, and CW. Data collection and management of the study by ZZ, YM, CL, KH, XM, YZ, XR, JL, YC, TY, and CW. Statistical anlysis by ZZ, CL, XM, and YZ. Drafting of the manuscript by ZZ. All authors contributed to the article and approved the submitted version.
This project was supported by CAMS Innovation Fund for Medical Sciences (CIFMS) (2020-I2M-2-008), Major Program of National Natural Science Foundation of China (82090010, 82090011), Respiratory Disease Clinical Research Public Welfare Program of China Soong Ching Ling Foundation (2018MZFC-032), and National Key R&D Program of China (2018YFC1315100).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We would like to thank all participants in the ACURE study that provided consent for the data used in this clinical study.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.802123/full#supplementary-material
Bafadhel, M., Pavord, I. D., and Russell, R. E. K. (2017). Eosinophils in COPD: Just Another Biomarker?. Lancet Respir. Med. 5, 747–759. doi:10.1016/S2213-2600(17)30217-5
Bafadhel, M., Peterson, S., De Blas, M. A., Calverley, P. M., Rennard, S. I., Richter, K., et al. (2018). Predictors of Exacerbation Risk and Response to Budesonide in Patients with Chronic Obstructive Pulmonary Disease: a post-hoc Analysis of Three Randomised Trials. Lancet Respir. Med. 6, 117–126. doi:10.1016/S2213-2600(18)30006-7
Barnes, P. J. (2013). Theophylline. Am. J. Respir. Crit. Care Med. 188 (8), 901–906. doi:10.1164/rccm.201302-0388pp
Barr, R. G., Rowe, B. H., and Camargo, C. A. (2003). Methylxanthines for Exacerbations of Chronic Obstructive Pulmonary Disease: Meta-Analysis of Randomised Trials. BMJ 327 (7416), 643. doi:10.1136/bmj.327.7416.643
Brookhart, M. A., Wyss, R., Layton, J. B., and Stürmer, T. (2013). Propensity Score Methods for Confounding Control in Nonexperimental Research. Circ. Cardiovasc. Qual. Outcomes 6 (5), 604–611. doi:10.1161/CIRCOUTCOMES.113.000359
Cai, B. Q., Cai, S. X., Chen, R. C., Cui, L. Y., Feng, Y. L., Gu, Y. T., et al. (2014). Expert Consensus on Acute Exacerbation of Chronic Obstructive Pulmonary Disease in the People's Republic of China. Int. J. Chron. Obstruct Pulmon Dis. 9, 381–395. doi:10.2147/COPD.S58454
Cazzola, M., Calzetta, L., Barnes, P. J., Criner, G. J., Martinez, F. J., Papi, A., et al. (2018). Efficacy and Safety Profile of Xanthines in COPD: a Network Meta-Analysis. Eur. Respir. Rev. 27 (148). doi:10.1183/16000617.0010-2018
Cazzola, M., and Matera, M. G. (2020). The Effect of Doxofylline in Asthma and COPD. Respir. Med. 164, 105904. doi:10.1016/j.rmed.2020.105904
Cheng, S. L. (2018). Blood Eosinophils and Inhaled Corticosteroids in Patients with COPD: Systematic Review and Meta-Analysis. Int. J. Chron. Obstruct Pulmon Dis. 13, 2775–2784. doi:10.2147/COPD.S175017
Cosio, B. G., Tsaprouni, L., Ito, K., Jazrawi, E., Adcock, I. M., and Barnes, P. J. (2004). Theophylline Restores Histone Deacetylase Activity and Steroid Responses in COPD Macrophages. J. Exp. Med. 200 (5), 689–695. doi:10.1084/jem.20040416
Devereux, G., Cotton, S., Fielding, S., McMeekin, N., Barnes, P. J., Briggs, A., et al. (2018). Effect of Theophylline as Adjunct to Inhaled Corticosteroids on Exacerbations in Patients with COPD: A Randomized Clinical Trial. JAMA 320 (15), 1548–1559. doi:10.1001/jama.2018.14432
Devereux, G., Cotton, S., Fielding, S., McMeekin, N., Barnes, P. J., Briggs, A., et al. (2019). Low-dose Oral Theophylline Combined with Inhaled Corticosteroids for People with Chronic Obstructive Pulmonary Disease and High Risk of Exacerbations: a RCT. Health Technol. Assess. 23 (37), 1–146. doi:10.3310/hta23370
Donaldson, G. C., Seemungal, T. A., Bhowmik, A., and Wedzicha, J. A. (2002). Relationship between Exacerbation Frequency and Lung Function Decline in Chronic Obstructive Pulmonary Disease. Thorax 57 (10), 847–852. doi:10.1136/thorax.57.10.847
Duffy, N., Walker, P., Diamantea, F., Calverley, P. M., and Davies, L. (2005). Intravenous Aminophylline in Patients Admitted to Hospital with Non-acidotic Exacerbations of Chronic Obstructive Pulmonary Disease: a Prospective Randomised Controlled Trial. Thorax 60 (9), 713–717. doi:10.1136/thx.2004.036046
Global (2019) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2019 Report. Available from: http://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020- REPORT-ver1.1wms.pdf. Accessed Dec 6, 2019.
GBD 2017 Causes of Death Collaborators (2018). Global, Regional, and National Age-sex-specific Mortality for 282 Causes of Death in 195 Countries and Territories, 1980-2017: a Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 392 (10159), 1736–1788. doi:10.1016/S0140-6736(18)32203-7
GBD 2017 Disease and Injury Incidence and Prevalence Collaborators (2018). Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 354 Diseases and Injuries for 195 Countries and Territories, 1990-2017: a Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 392 (10159), 1789–1858. doi:10.1016/S0140-6736(18)32279-7
Grootendorst, D. C., Gauw, S. A., Verhoosel, R. M., Sterk, P. J., Hospers, J. J., Bredenbröker, D., et al. (2007). Reduction in Sputum Neutrophil and Eosinophil Numbers by the PDE4 Inhibitor Roflumilast in Patients with COPD. Thorax 62, 1081–1087. doi:10.1136/thx.2006.075937
Hallsworth, M. P., Litchfield, T. M., and Lee, T. H. (1992). Glucocorticoids Inhibit Granulocyte-Macrophage colony-stimulating Factor-1 and Interleukin-5 Enhanced In Vitro Survival of Human Eosinophils. Immunology 75, 382–385.
Hastie, A. T., Martinez, F. J., Curtis, J. L., Doerschuk, C. M., Hansel, N. N., Christenson, S., et al. (2017). Association of Sputum and Blood Eosinophil Concentrations with Clinical Measures of COPD Severity: an Analysis of the SPIROMICS Cohort. Lancet Respir. Med. 5, 956–967. doi:10.1016/S2213-2600(17)30432-0
Hospers, J. J., Rijcken, B., Schouten, J. P., Postma, D. S., and Weiss, S. T. (1999). Eosinophilia and Positive Skin Tests Predict Cardiovascular Mortality in a General Population Sample Followed for 30 Years. Am. J. Epidemiol. 150, 482–491. doi:10.1093/oxfordjournals.aje.a010037
Hospers, J. J., Schouten, J. P., Weiss, S. T., Postma, D. S., and Rijcken, B. (2000). Eosinophilia Is Associated with Increased All-Cause Mortality after a Follow-Up of 30 Years in a General Population Sample. Epidemiology 11, 261–268. doi:10.1097/00001648-200005000-00006
Ito, K., Lim, S., Caramori, G., Cosio, B., Chung, K. F., Adcock, I. M., et al. (2002). A Molecular Mechanism of Action of Theophylline: Induction of Histone Deacetylase Activity to Decrease Inflammatory Gene Expression. Proc. Natl. Acad. Sci. U S A. 99 (13), 8921–8926. doi:10.1073/pnas.132556899
Jenkins, C. R., Wen, F.-Q., Martin, A., Barnes, P. J., Celli, B., Zhong, N.-S., et al. (2020). The Effect of Low Dose Corticosteroids and Theophylline on the Risk of Acute Exacerbations of COPD. The TASCS Randomised Controlled Trial. Eur. Respir. J. 57 (6), 2003338. doi:10.1183/13993003.03338-2020
Lee, J., Lee, J. H., Kim, J. A., and Rhee, C. K. (2017). Trend of Cost and Utilization of COPD Medication in Korea. Int. J. Chron. Obstruct Pulmon Dis. 12, 27–33. doi:10.2147/COPD.S121687
Martinez, F. J., Rabe, K. F., Calverley, P. M. A., Fabbri, L. M., Sethi, S., Pizzichini, E., et al. (2018). Determinants of Response to Roflumilast in Severe Chronic Obstructive Pulmonary Disease. Pooled Analysis of Two Randomized Trials. Am. J. Respir. Crit. Care Med. 198, 1268–1278. doi:10.1164/rccm.201712-2493OC
Matera, M. G., Page, C., and Cazzola, M. (2017). Doxofylline Is Not Just Another Theophylline!. Int. J. Chron. Obstruct Pulmon Dis. 12, 3487–3493. doi:10.2147/COPD.S150887
Müllerova, H., Maselli, D. J., Locantore, N., Vestbo, J., Hurst, J. R., Wedzicha, J. A., et al. (2015). Hospitalized Exacerbations of COPD: Risk Factors and Outcomes in the ECLIPSE Cohort. Chest 147 (4), 999–1007. doi:10.1378/chest.14-065
Ohta, K., and Yamashita, N. (1999). Apoptosis of Eosinophils and Lymphocytes in Allergic Inflammation. J. Allergy Clin. Immunol. 104, 14–21. doi:10.1016/s0091-6749(99)70107-7
Papi, A., Vestbo, J., Fabbri, L., Corradi, M., Prunier, H., Cohuet, G., et al. (2018). Extrafine Inhaled Triple Therapy versus Dual Bronchodilator Therapy in Chronic Obstructive Pulmonary Disease (TRIBUTE): a Double-Blind, Parallel Group, Randomised Controlled Trial. Lancet 391, 1076–1084. doi:10.1016/S0140-6736(18)30206-X
Pei, Z., Sun, Y., Wang, S., Chen, Y., Yang, T., Huang, K., et al. (2020). Estimating Mortality Among Inpatients with Acute Exacerbation of Chronic Obstructive Pulmonary Disease Using Registry Data. NPJ Prim. Care Respir. Med. 30 (1), 28. doi:10.1038/s41533-020-0186-y
Riffo-Vasquez, Y., Venkatasamy, R., and Page, C. P. (2018). Steroid Sparing Effects of Doxofylline. Pulm. Pharmacol. Ther. 48, 1–4. doi:10.1016/j.pupt.2017.10.008
Seemungal, T. A., Donaldson, G. C., Paul, E. A., Bestall, J. C., Jeffries, D. J., and Wedzicha, J. A. (1998). Effect of Exacerbation on Quality of Life in Patients with Chronic Obstructive Pulmonary Disease. Am. J. Respir. Crit. Care Med. 157 (5 Pt 1), 1418–1422. doi:10.1164/ajrccm.157.5.9709032
Wang, C., Xu, J., Yang, L., Xu, Y., Zhang, X., Bai, C., et al. (2018). Prevalence and Risk Factors of Chronic Obstructive Pulmonary Disease in China (The China Pulmonary Health [CPH] Study): a National Cross-Sectional Study. Lancet 391 (10131), 1706–1717. doi:10.1016/S0140-6736(18)30841-9
Zhang, Z. (2017). Propensity Score Method: a Non-parametric Technique to Reduce Model Dependence. Ann. Transl Med. 5 (1), 7. doi:10.21037/atm.2016.08.57
Zheng, J. P., Kang, J., Huang, S. G., Chen, P., Yao, W. Z., Yang, L., et al. (2008). Effect of Carbocisteine on Acute Exacerbation of Chronic Obstructive Pulmonary Disease (PEACE Study): a Randomised Placebo-Controlled Study. Lancet 371 (9629), 2013–2018. doi:10.1016/S0140-6736(08)60869-7
Keywords: chronic obstructive pulmonary disease, acute exacerbation, hospitalization, methylxanthine, length of stay
Citation: Zhan Z, Ma Y, Huang K, Liang C, Mao X, Zhang Y, Ren X, Lei J, Chen Y, Yang T and Wang C (2022) Methylxanthine Treatment in Patients Hospitalized for Acute Exacerbation of Chronic Obstructive Pulmonary Disease in China: A Real-World Study Using Propensity Score Matching Analysis. Front. Pharmacol. 13:802123. doi: 10.3389/fphar.2022.802123
Received: 26 October 2021; Accepted: 06 January 2022;
Published: 25 January 2022.
Edited by:
Brian Godman, University of Strathclyde, United KingdomReviewed by:
Antonio Molino, University of Naples Federico II, ItalyCopyright © 2022 Zhan, Ma, Huang, Liang, Mao, Zhang, Ren, Lei, Chen, Yang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Chen, Y2hlbnlhbjk5NzI3QGNzdS5lZHUuY24=; Ting Yang, enJ5eXlhbmd0aW5nQDE2My5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.