- 1University of Lille, Lille, France
- 2Medical Oncology Department, Lille University Hospital, Lille, France
- 3CHU Lille, ULR 7365—GRITA—Groupe de Recherche sur les Formes Injectables et les Technologies Associées, University of Lille, Lille, France
- 4Medical Oncology Department, CNRS, Inserm, CHU Lille, Institut Pasteur de Lille, UMR9020—UMR-S 1277—CANTHER-Cancer Heterogeneity, Plasticity and Resistance to Therapies, CHU Lille, University of Lille, Lille, France
Background: Proton pump inhibitors (PPIs) are one of the most widely used drugs worldwide and are overprescribed in patients with cancer; there is increasing evidence of their effects on cancer development and survival. The objective of this narrative review is to comprehensively identify cancer medications that have clinically meaningful drug–drug interactions (DDIs) with PPIs, including loss of efficacy or adverse effects, and to explore the association between PPIs and cancer.
Methods: A PubMed search of English language studies published from 1 January 2016, to 1 June 2021 was conducted. The search terms included “proton pump inhibitors,” “cancer,” “chemotherapy,” “immunotherapy,” “hormonotherapies,” “targeted therapies,” “tyrosine kinase inhibitors,” and “gut microbiome”. Recent and relevant clinical trials, meta-analyses, and reviews were included.
Results: PPIs may have pro-tumor activity by increasing plasma gastrin levels or anti-tumor activity by inhibiting V-ATPases. However, their impact on cancer survival remains unclear. PPIs may decrease the efficacy of some antineoplastic agents through direct DDIs (e.g., some tyrosine kinase inhibitors, capecitabine, irinotecan, methotrexate). More complex DDIs seem to exist for immunotherapies with indirect interactions through the microbiome. PPIs worsen hypomagnesemia, bone loss, iron, and vitamin B12 deficiencies but may have a protective effect on the renal system.
Discussion/Conclusions: PPIs may interact with the cancer microbiome and the efficacy of various antineoplastic agents, although only a few DDIs involving PPIs are clinically significant. Further pharmaco-epidemiological studies are warranted, but physicians should be aware of the potential consequences of PPI use, which should be dose appropriate and prescribed according to guidelines.
Introduction
Proton pump inhibitors (PPIs) are one of the most commonly prescribed drugs worldwide (Kinoshita et al., 2018). These drugs irreversibly inhibit H+/K+ adenosine triphosphatase pumps in gastric parietal cells and results in the suppression of gastric acid production for >24 h (Yibirin et al., 2021).
The US Food and Drug Administration (FDA) has approved PPIs for a variety of gastric acid‐related conditions, including gastroesophageal reflux disease (GERD), duodenal or gastric ulcers, Helicobacter pylori infections, and Zollinger-Ellison syndrome as well as the prevention of nonsteroidal anti-inflammatory drug (NSAID)-associated gastrointestinal lesions in at-risk patients (aged > 65 years, with a history of gastrointestinal ulcer or with concomitant antiplatelet, anticoagulant, or corticosteroid therapy) (Strand et al., 2017). Long-term treatment is usually required for many of these disorders, which increases the potential for clinically significant drug interactions in patients. In addition, off-label prescribing has been widely reported, particularly in functional dyspepsia and in the prevention of NSAID-induced gastroduodenal lesions in non-at-risk patients (Lassalle et al., 2020).
The use of PPIs has grown in many countries since their market introduction in the late 1980s. For instance, in France, more than 15 million people with health insurance, or almost one-third of the French adult population, were PPI users in 2015 (Singh et al., 2018; Lassalle et al., 2020). In one study, PPI indication could not be established for one-third of the patients, and no measurable risk factor was found for three-quarters of the prophylactic prescriptions associated with NSAIDs (Lassalle et al., 2020). Approximately 20% of patients with cancer use PPIs (Kinoshita et al., 2018; Tvingsholm et al., 2018; Sharma et al., 2019); however, PPIs are often overprescribed in these patients to treat side effects of chemotherapy such as GERD or as prophylaxes in combination therapy with corticosteroids or NSAIDs (Lassalle et al., 2020).
In general, PPIs are believed to have few adverse events, as they are generally well tolerated. However, PPIs have been reported to be associated with gastrointestinal disorders (nausea, abdominal pain, transit disorder), ionic absorption disorders (hypomagnesemia, iron deficiency, vitamin B12 deficiency), kidney failure, infections (pneumonia, Clostridium difficile infections, peritonitis), and bone fractures (Singh et al., 2018; Yibirin et al., 2021).
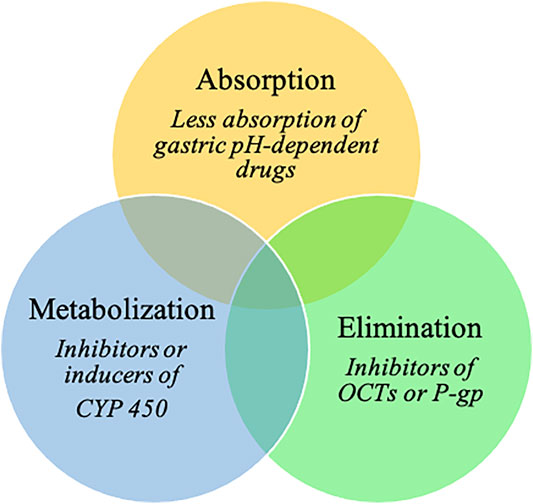
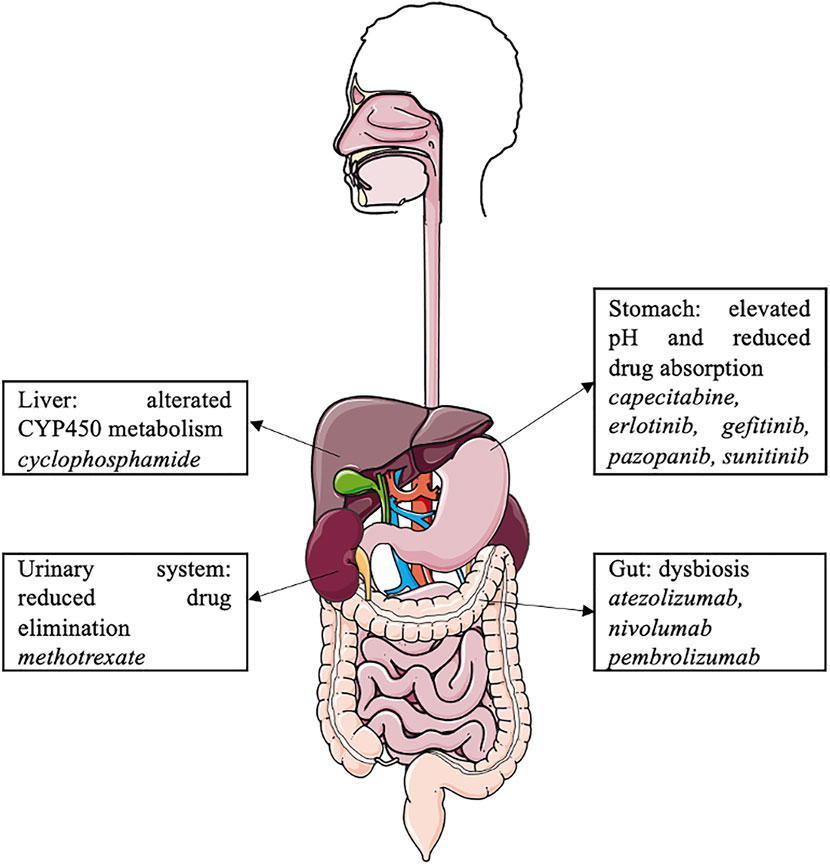
In addition, PPIs are involved in various drug–drug interactions (DDIs) (Wedemeyer and Blume, 2014; Strand et al., 2017; Patel et al., 2020; Uchiyama et al., 2021). By elevating gastric pH, PPIs influence the absorption of gastric pH-dependent drugs. Indeed, an increase in the gastric pH of some weakly basic drugs results in decreases in dissolution and subsequent absorption rates (Wedemeyer and Blume, 2014; Patel et al., 2020). PPIs could potentially also affect drug elimination, as they are potential inhibitors of organic cation transporters (OCTs, which are involved in renal excretion of substrate medications) and P-glycoprotein efflux transporters (Wedemeyer and Blume, 2014; Patel et al., 2020). PPIs are predominantly metabolized in the liver by the cytochrome P450 enzyme (CYP) system, mainly by CYP2C19 and CYP3A4 (Wedemeyer and Blume, 2014). They have the ability to act either as inhibitors or inducers of CYP; the inhibition of CYP increases systemic exposure to a drug (Patel et al., 2020). Omeprazole has considerable DDI potential because of its high affinity for CYP2C19 and moderate affinity for CYP3A4 (Wedemeyer and Blume, 2014). Esomeprazole also inhibits CYP2C19 to a clinically significant degree, whereas CYP2C19 inhibition by other PPIs is not clinically relevant (Patel et al., 2020).
However, only a few DDIs involving PPIs are clinically significant (Wedemeyer and Blume, 2014). Nonetheless, the risk of drug interactions should be considered when choosing a PPI to treat gastric acid-related disorders.
PPIs may be involved in many interactions with cancer and cancer-related treatments (Figure 1). Thus, our aims are (i) to comprehensively address the impact of PPI use on cancer occurrence and outcomes and (ii) to pragmatically identify cancer drugs that have clinically meaningful DDIs with PPIs, including loss of efficacy or adverse events.
Review
Methods
This narrative review, with expert opinion, is based on the literature published from 1 January 2016, to 1 June 2021. PubMed searches were limited to English language studies. The search used the following keywords: “proton pump inhibitors,” “cancer,” “chemotherapy,” “immunotherapy,” “hormonotherapies,” “targeted therapies,” “tyrosine kinase inhibitors,” and “gut microbiome” (detailed list in Supplementary Table S1). The search was extended beyond 5 years for specific terms without relevant data in the last 5 years (detailed list in Supplementary Table S2). We selected recent and relevant studies, including clinical trials, meta-analyses, and reviews. Letters to the editor and congress communications were excluded. A total of 98 articles were included in this review.
Results
Cancer Occurrence and Outcomes
One of the hallmarks of cancer is deregulation of the energetic metabolism of tumor cells (Hanahan and Weinberg, 2011). Tumor cells activate the aerobic glycolysis pathways to perform their biosynthesis, which generates an excess of protons and lactates in the intracellular space. V-ATPases are vacuolar proton pumps that maintain a neutral intracellular sector by increasing the acidity of the extracellular medium. These pumps are overexpressed in tumor cells and increase the acidity of the tumor microenvironment, which is believed to be involved in tumorigenesis, tumor proliferation, tumor progression, tumor invasion, and treatment resistance (Whitton et al., 2018; Tozzi et al., 2020).
Several studies have shown that PPIs inhibit V-ATPases in vitro and in vivo (Ikemura et al., 2017a; Tozzi et al., 2020). By decreasing the acidity of the tumor microenvironment, inhibition of V-ATPases slows cell proliferation and induces tumor cell apoptosis. Therefore, PPIs may have anti-tumor activity of their own and may increase the efficacy of anti-tumor therapies via V-ATPase inhibition (Ikemura et al., 2017a; Tvingsholm et al., 2018; Tozzi et al., 2020).
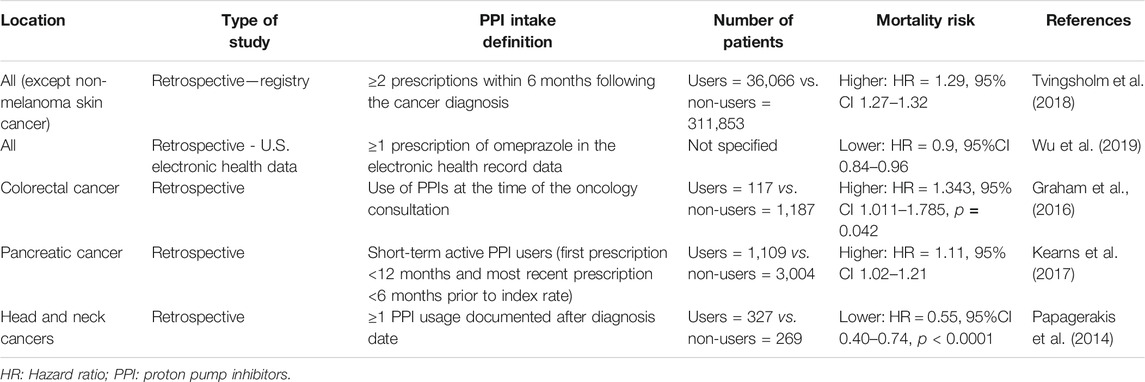
In contrast, PPI administration increases plasma gastrin levels (Kinoshita et al., 2018). Since gastrin promotes the proliferation of gastric enterochromaffin-like cells, PPIs can stimulate the development of gastric neuroendocrine and carcinoid tumors (Kinoshita et al., 2018). Similarly, some observational studies have suggested that PPIs may increase the risk of digestive cancers, such as esophageal, gastric, pancreatic, and colorectal cancer (Brusselaers et al., 2017; Hwang et al., 2017; Kearns et al., 2017; Brusselaers et al., 2018; Kinoshita et al., 2018).
Moreover, PPIs may also affect the prognosis of patients with cancer, but there are contradictory results regarding this issue (Papagerakis et al., 2014; Graham et al., 2016; Kearns et al., 2017; Tvingsholm et al., 2018; Wu et al., 2019). Table 1 summarizes the major studies on cancer-specific mortality among PPI users.
Modulation of Cancer-Related Treatments Side Effects
PPI co-medication may enhance the side effects induced by some anti-tumor treatments.
Clinically, PPIs increase bone loss, which is a risk factor for fractures (Mazziotti et al., 2010; Panday et al., 2014). Therefore, hormonotherapies such as aromatase inhibitors, used in breast cancer, and androgen deprivation therapy, used in prostate cancer, cause bone loss and increase the risk of fractures (Mazziotti et al., 2010; Panday et al., 2014). With concomitant use, the risk of fractures may increase.
Biologically, PPIs might worsen hypomagnesemia induced by therapies such as cisplatin, anti-epithelial growth factor receptor (EGFR) monoclonal antibodies, or tyrosine kinase inhibitors (TKIs) (Abu-Amna and Bar-Sela, 2019). In addition, reduction of gastric acidity decreases the absorption of ferrous iron and vitamin B12, which may lead to anemia (Singh et al., 2018).
In contrast, PPIs may have a renal protective effect by inhibiting OCT2, a renal proximal tubular transmembrane transporter involved in renal elimination of cisplatin (Ikemura et al., 2017a). Its inhibition by PPIs decreases renal accumulation and cisplatin-induced nephrotoxicity. This protective effect was found in a retrospective study involving patients treated with cisplatin and 5-fluorouracil for cancer of the upper aerodigestive tract (Ikemura et al., 2017b). A phase III trial investigating the protective effect of pantoprazole on cisplatin-induced nephrotoxicity in upper aerodigestive tract cancers is currently underway (NCT04217512).
Modulation of Cancer-Related Treatments Efficacy
Oral Chemotherapeutic Agents
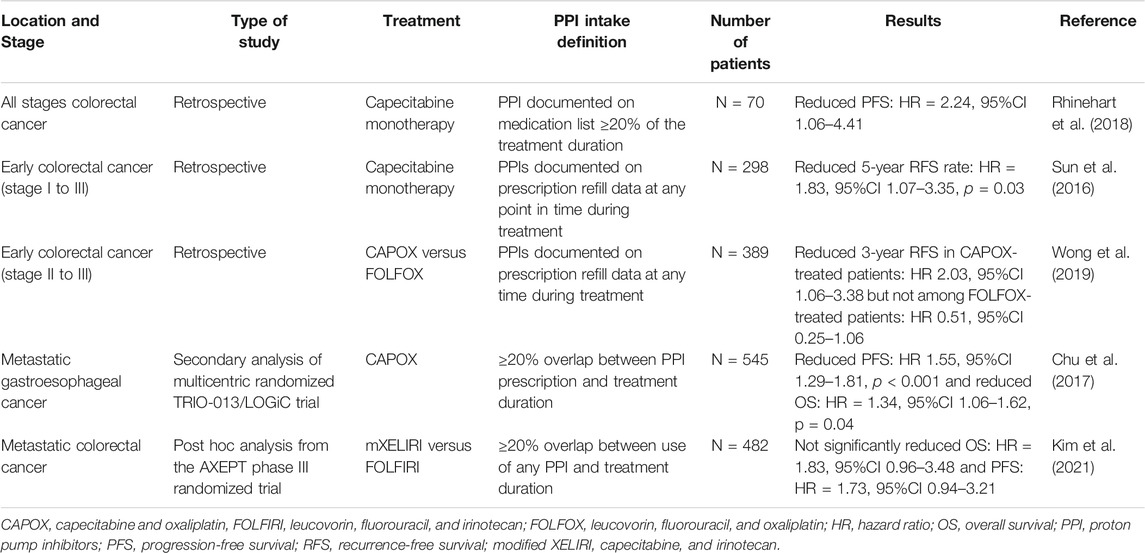
Capecitabine is an oral prodrug of 5-fluorouracil, commonly used in digestive and breast cancers, with optimal absorption under acidic conditions (capecitabine dissociation constant pKa = 1.92). It has been speculated that an increase in the gastric pH may lead to reduced dissolution and absorption of capecitabine tablets, although in vitro data have not confirmed this to date (Cheng et al., 2019; Sekido et al., 2019). However, several studies on colorectal cancer have shown poorer survival when PPIs are combined with capecitabine compared with capecitabine monotherapy (Sun et al., 2016; Chu et al., 2017; Rhinehart et al., 2018; Wong et al., 2019; Kim et al., 2021). Table 2 summarizes the results of the main studies on this topic.
Cyclophosphamide is metabolized by CYP2C19 (Griskevicius et al., 2003). Since PPIs are competitive inhibitors of CYP2C19, DDIs may decrease its efficacy. However, no clinical trials have explored their DDIs.
Other commonly used oral chemotherapeutic agents include etoposide, temozolomide, topotecan, and vinorelbine. There are no DDIs between these drugs and PPIs described in the present literature.
Intravenous Chemotherapeutic Agents
PPIs are also thought to be involved in DDIs with two intravenous agents, irinotecan, a topoisomerase I inhibitor, and methotrexate, an antifolate agent. One of the mechanisms of resistance to irinotecan is the rapid degradation of topoisomerase I. Topoisomerase I degradation occurs in the proteasome following phosphorylation by DNA-PKc. CTDSP1 nuclear phosphatase is believed to negatively regulate the activation of DNA-PKc. Therefore, high expression of CTDSP1 inhibits DNA-PKc activation and limits topoisomerase I degradation (Matsuoka et al., 2020). PPIs such as rabeprazole inhibit the activity of CTDSP1. Consequently, DNA-PKc is activated, and the degradation of topoisomerase I is enhanced. A retrospective study found a poor clinical response to irinotecan in patients with colorectal cancer when used in combination with rabeprazole (Matsuoka et al., 2020). However, in a pharmacological study, omeprazole co-medication did not affect the main pharmacokinetic parameters of irinotecan and its main metabolites (van der Bol et al., 2011). The observed changes may be related to mechanisms other than pharmacokinetic alterations.
High-dose methotrexate, usually defined as >1 g/m2, is widely used to treat a variety of malignancies, including lymphoma, acute leukemia, and osteosarcoma (Bezabeh et al., 2012). Methotrexate is eliminated by active tubular secretion through the organic anion transporter 3 (OAT3) (Narumi et al., 2017). PPIs may inhibit OAT3 and therefore decrease methotrexate clearance, resulting in elevated serum levels of methotrexate and its metabolite hydroxymethotrexate and may induce methotrexate toxicity (Bezabeh et al., 2012). However, the mechanism of interaction is not well understood, and current data remain controversial regarding this DDI (Ranchon et al., 2018; Wang et al., 2020). The FDA recommends that clinicians “use caution when administering high-dose methotrexate to patients receiving proton pump inhibitor therapy” (Bezabeh et al., 2012).
No DDIs were found between PPIs and other parenteral chemotherapies.
Targeted Therapies
A well-known DDI between PPIs and cancer treatment concerns several TKIs. TKIs are oral antineoplastic treatments used in various solid and hematological tumors. By increasing the gastric pH, PPIs decrease the absorption of some TKIs. TKIs are weak bases and can be present in either the ionized or non-ionized form according to the pH in the stomach. When a TKI is co-administered with a PPI, the pH in the stomach rises from 1 to 4, and the equilibrium of ionized and non-ionized drugs shifts to the less soluble non-ionized form, resulting in a decrease in the bioavailability of the TKI (van Leeuwen et al., 2014).
Several studies have investigated the DDIs between PPIs and TKIs; the results are contradictory concerning PPI interaction with the bioavailability and activity of TKIs. A summary of these DDIs is presented in Table 3 (Rugo et al., 2005; Egorin et al., 2009; Yin et al., 2010; Yin et al., 2012; Takahashi et al., 2012; Hilton et al., 2013; Abbas et al., 2013; Musib et al., 2013; Tan et al., 2013; Johansson et al., 2014; Narasimhan et al., 2014; Chu et al., 2015; Kletzl et al., 2015; Ha et al., 2015; Iurlo et al., 2016; Kumarakulasinghe et al., 2016; Lam et al., 2016; Zenke et al., 2016; Chu et al., 2017; Lalani et al., 2017; Lacy et al., 2017; Lau et al., 2017; Morcos et al., 2017; Yokota et al., 2017; de Jong et al., 2018; Nieves Sedano et al., 2018; Ohgami et al., 2018; Fang et al., 2019; Mir et al., 2019; Vishwanathan et al., 2018; Pisano et al., 2019; de Man et al., 2019; Koutake et al., 2020; Ruanglertboon et al., 2020; Van De Sijpe et al., 2020; Chen et al., 2021; Rassy et al., 2021). Table 4 summarizes the main studies reporting reductions in the survival of patients receiving this combination of medication (Chu et al., 2015; Ha et al., 2015; Fang et al., 2019; Mir et al., 2019).
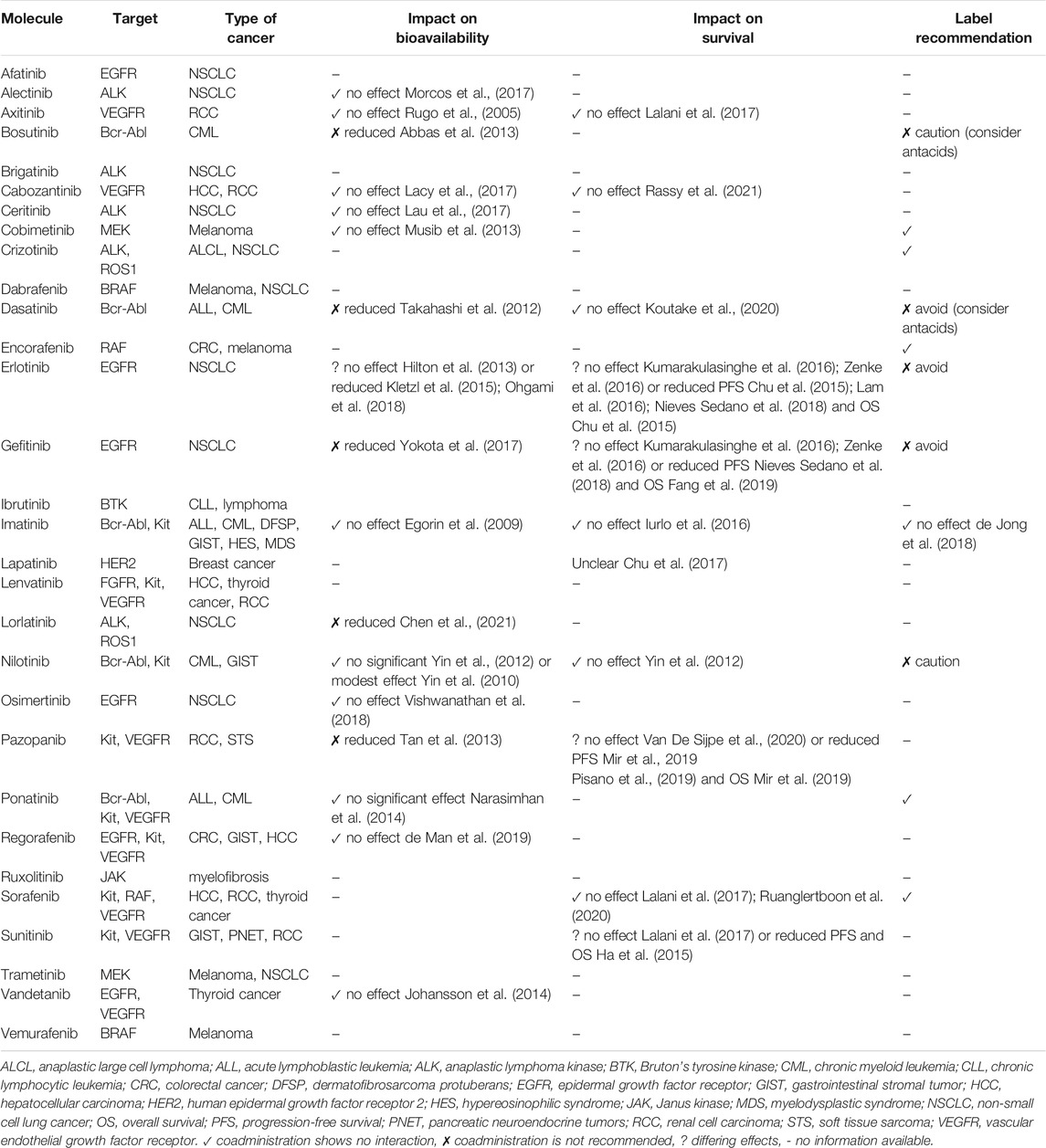
TABLE 3. Summary of drug–drug interactions between proton pump inhibitors and tyrosine kinase inhibitors.
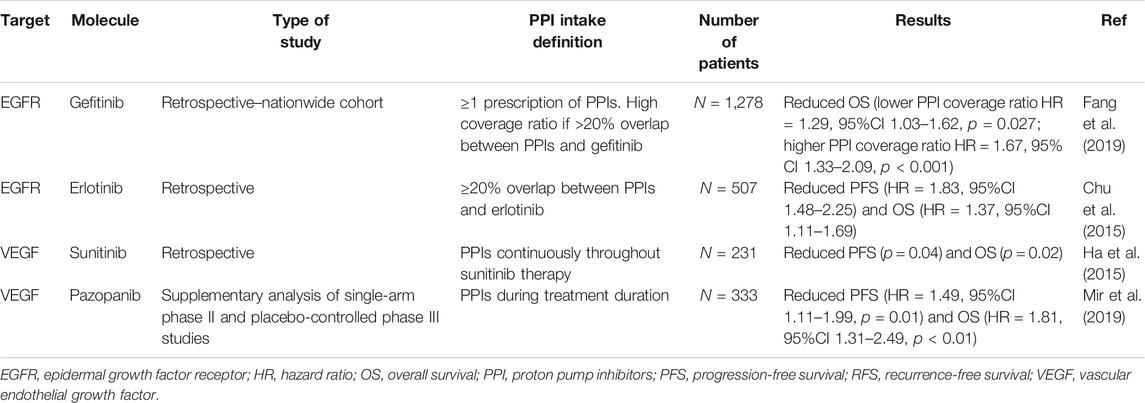
TABLE 4. Main studies reporting a decrease in survival of patients receiving proton pump inhibitors with tyrosine kinase inhibitors.
In particular, another DDI concerns PPIs and cyclin-dependent kinases (CDKs), which are major enzymes that control the cell cycle and cell division. CDK 4/6 inhibitors, such as palbociclib and ribociclib, have been used with success to treat breast cancer. Palbociclib is a weak base with gastric pH-dependent solubility, and PPIs decrease their bioavailability under fasting conditions. However, the impact of PPIs on the bioavailability of palbociclib is mitigated by food intake (Sun et al., 2017). No study has investigated the interaction between PPIs and palbociclib on survival. However, PPIs do not affect the bioavailability of ribociclib (Samant et al., 2018).
There are no data or clinical relevance regarding any interaction between PPIs and targeted therapies such as mTOR inhibitors, PARP inhibitors, or PI3K inhibitors (Patel et al., 2020; Uchiyama et al., 2021).
Hormonotherapy
No interaction between PPIs and second-generation antiandrogens, such as abiraterone acetate or enzalutamide, has been described. However, DDIs may occur in patients with prostate cancer because of the inhibition of CYP2C8 and 2D6 by abiraterone and induction of CYP3A4, 2C9, and 2C19 by enzalutamide (Del Re et al., 2017). As CYP2C19, and to a lesser degree CYP3A4, clear the PPIs metabolically (Ward and Kearns, 2013), there is a potential for DDIs between PPIs and enzalutamide or apalutamide. Further studies are needed to address this topic.
No DDI is described between PPIs and breast cancer endocrine therapies.
Immunotherapy
There is growing evidence that the gut microbiome has a central role in controlling both the antitumor immune response in digestive organs and the host immune system response to anti-cancer therapies (Gopalakrishnan et al., 2018). An imbalance of the microbiota, called dysbiosis, disturbs the anti-tumor immune response to immune checkpoint inhibitors (ICIs) (Gopalakrishnan et al., 2018; Rossi et al., 2019).
The reduction of gastric acidity secondary to PPIs leads to a decrease in the gastric bactericidal effect and a subsequent change in the gut microbiome. Bacteria that are naturally present in the oral cavity and usually destroyed in the gastric area emerge in the digestive tract (e.g., Streptococcaceae, Enterococcaceae) (Naito et al., 2018). The concentration of bacteria in the small intestine subsequently increases (e.g., Salmonella, Campylobacter, and C. difficile). Small intestinal bacterial overgrowth (SIBO) is the presence of 100,000 bacterial colonies/mL in the small intestinal content. PPI administration is considered a risk factor for SIBO (Kinoshita et al., 2018; Naito et al., 2018).
First, retrospective studies have not found statistically significant differences in the clinical activity of ICIs in terms of progression-free survival (PFS) and overall survival (OS) in different solid tumors (Mukherjee et al., 2018; Zhao et al., 2019). Two retrospective analyses of two randomized control trials found a major impact on survival (Chalabi et al., 2020; Hopkins et al., 2020). The first was Chalabi’s study (pooled POPLAR and OAK studies), which found reduced OS and PFS in patients with advanced or metastatic non-small cell lung cancer treated with atezolizumab and concomitant PPIs compared with survival in non-PPI recipients (HR 1.45, 95% CI 1.20–1.75, p = 0.0001 and HR 1.30, 95% CI 1.10–1.53, p = 0.001, respectively) (Chalabi et al., 2020). Similar results were found by Hopkins et al. in advanced or metastatic urothelial cancer treated with atezolizumab (HR 1.52, 95%CI 1.27–1.83, p < 0.001 and HR 1.38, 95% CI 1.18–1.62, p < 0.001) (Hopkins et al., 2020).
Two recent meta-analyses reported that PPI use was not associated with reduced survival in patients undergoing ICI treatment (Li et al., 2020a; Li et al., 2020b). However, these meta-analyses included only five and seven studies. Since then, many studies have continued to explore this DDI, and Table 5 summarizes the latest ones (Mukherjee et al., 2018; Zhao et al., 2019; Chalabi et al., 2020; Cortellini et al., 2020; Hopkins et al., 2020; Iglesias-Santamaría, 2020; Buti et al., 2021; Cortellini et al., 2021; Gaucher et al., 2021; Jun et al., 2021; Rounis et al., 2021; Ruiz-Bañobre et al., 2021). Robust recommendations for PPI use cannot be inferred given the retrospective nature of the currently available evidence, but caution should be exercised with ICIs (Rossi et al., 2019; Hussain et al., 2021). Further prospective studies on ICI and PPI DDIs are warranted.
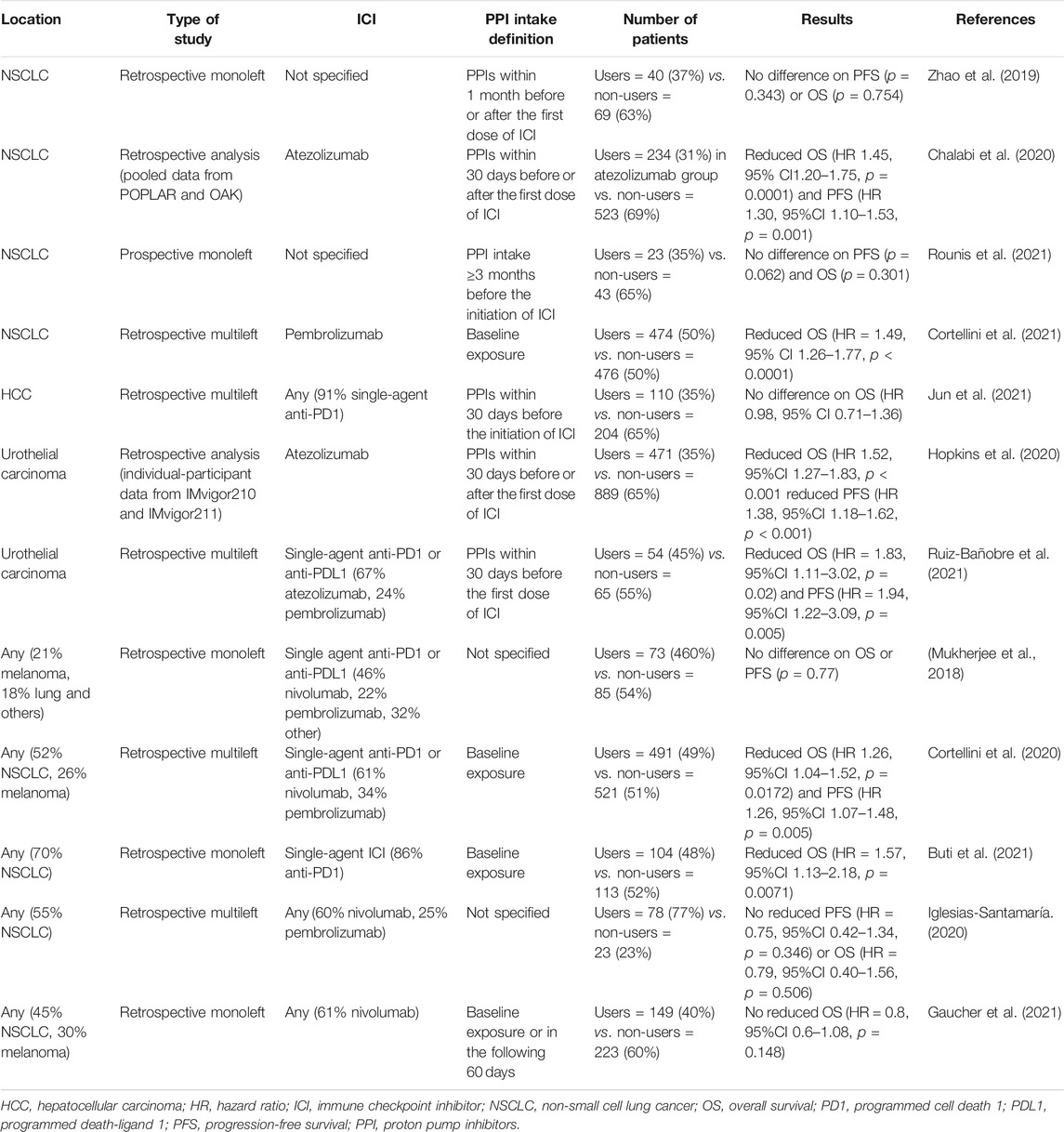
TABLE 5. Main studies on interaction between immune checkpoint inhibitors and co-medication with proton pump inhibitors.
Conclusion
Proton pump inhibitors may interact with the cancer microbiome and various antineoplastic agents, such as oral and intravenous chemotherapy, tyrosine kinase inhibitors, and immune checkpoint inhibitors, and modulate their efficacy (Figure 2). However, due to the limitations of retrospective cohort studies with a small number of patients, data on these drug–drug interactions are limited, and further pharmaco-epidemiological studies are warranted. In the context of cancer-related treatment, oncologists should consider the pathophysiological consequences of PPI use, with significant drug–drug interactions and dysbiosis. PPIs should be dose appropriate and prescribed in accordance with the guidelines.
Author Contributions
MB, and AT did the concept of the review. MB acquired the data and select the articles of interest in the literature. AT controlled the quality of data. MB and AT prepared the manuscript. NS reviewed the manuscript.
Conflict of Interest
AT has served in a consulting/advisory role and/or received honoraria from Amgen, Merck, Servier, Mylan, and has received travel, accommodations, and expenses from Pfizer, Sanofi.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Lille Faculty of Medicine and Editage (www.editage.com) for the English language editing.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.798272/full#supplementary-material
References
Abbas, R., Leister, C., and Sonnichsen, D. (2013). A Clinical Study to Examine the Potential Effect of Lansoprazole on the Pharmacokinetics of Bosutinib when Administered Concomitantly to Healthy Subjects. Clin. Drug Investig. 33 (8), 589–595. doi:10.1007/s40261-013-0103-z
Abu-Amna, M., and Bar-Sela, G. (2019). Increase in Cetuximab-Induced Skin Rash and Hypomagnesemia in Patients Receiving Concomitant Treatment with Proton Pump Inhibitors (PPIs): a Possible Drug Interaction? Cancer Chemother. Pharmacol. 83 (3), 545–550. doi:10.1007/s00280-018-3758-6
Bezabeh, S., Mackey, A. C., Kluetz, P., Jappar, D., and Korvick, J. (2012). Accumulating Evidence for a Drug-Drug Interaction between Methotrexate and Proton Pump Inhibitors. Oncologist 17 (4), 550–554. doi:10.1634/theoncologist.2011-0431
Brusselaers, N., Engstrand, L., and Lagergren, J. (2018). Maintenance Proton Pump Inhibition Therapy and Risk of Oesophageal Cancer. Cancer Epidemiol. 53, 172–177. doi:10.1016/j.canep.2018.02.004
Brusselaers, N., Wahlin, K., Engstrand, L., and Lagergren, J. (2017). Maintenance Therapy with Proton Pump Inhibitors and Risk of Gastric Cancer: a Nationwide Population-Based Cohort Study in Sweden. BMJ Open 7 (10), e017739. doi:10.1136/bmjopen-2017-017739
Buti, S., Bersanelli, M., Perrone, F., Tiseo, M., Tucci, M., Adamo, V., et al. (2021). Effect of Concomitant Medications with Immune-Modulatory Properties on the Outcomes of Patients with Advanced Cancer Treated with Immune Checkpoint Inhibitors: Development and Validation of a Novel Prognostic index. Eur. J. Cancer 142, 18–28. doi:10.1016/j.ejca.2020.09.033
Chalabi, M., Cardona, A., Nagarkar, D. R., Dhawahir Scala, A., Gandara, D. R., Rittmeyer, A., et al. (2020). Efficacy of Chemotherapy and Atezolizumab in Patients with Non-small-cell Lung Cancer Receiving Antibiotics and Proton Pump Inhibitors: Pooled Post Hoc Analyses of the OAK and POPLAR Trials. Ann. Oncol. 31 (4), 525–531. doi:10.1016/j.annonc.2020.01.006
Chen, J., Houk, B., Pithavala, Y. K., and Ruiz‐Garcia, A. (2021). Population Pharmacokinetic Model with Time‐varying Clearance for Lorlatinib Using Pooled Data from Patients with Non‐small Cell Lung Cancer and Healthy Participants. CPT Pharmacometrics Syst. Pharmacol. 10 (2), 148–160. doi:10.1002/psp4.12585
Cheng, V., Lemos, M., Hunter, N., Badry, N., and Lemos, J. (2019). Concomitant Use of Capecitabine and Proton Pump Inhibitors - Is it Safe? J. Oncol. Pharm. Pract. 25 (7), 1705–1711. doi:10.1177/1078155219846952
Chu, M. P., Ghosh, S., Chambers, C. R., Basappa, N., Butts, C. A., Chu, Q., et al. (2015). Gastric Acid Suppression Is Associated with Decreased Erlotinib Efficacy in Non-small-cell Lung Cancer. Clin. Lung Cancer 16 (1), 33–39. doi:10.1016/j.cllc.2014.07.005
Chu, M. P., Hecht, J. R., Slamon, D., Wainberg, Z. A., Bang, Y. J., Hoff, P. M., et al. (2017). Association of Proton Pump Inhibitors and Capecitabine Efficacy in Advanced Gastroesophageal Cancer: Secondary Analysis of the TRIO-013/LOGiC Randomized Clinical Trial. JAMA Oncol. 3 (6), 767–773. doi:10.1001/jamaoncol.2016.3358
Cortellini, A., Di Maio, M., Nigro, O., Leonetti, A., Cortinovis, D. L., Aerts, J. G., et al. (2021). Differential Influence of Antibiotic Therapy and Other Medications on Oncological Outcomes of Patients with Non-small Cell Lung Cancer Treated with First-Line Pembrolizumab versus Cytotoxic Chemotherapy. J. Immunother. Cancer 9 (4). doi:10.1136/jitc-2021-002421
Cortellini, A., Tucci, M., Adamo, V., Stucci, L. S., Russo, A., Tanda, E. T., et al. (2020). Integrated Analysis of Concomitant Medications and Oncological Outcomes from PD-1/pd-L1 Checkpoint Inhibitors in Clinical Practice. J. Immunother. Cancer 8 (2). doi:10.1136/jitc-2020-001361
de Jong, J., Haddish-Berhane, N., Hellemans, P., Jiao, J., Sukbuntherng, J., and Ouellet, D. (2018). The pH-Altering Agent Omeprazole Affects Rate but Not the Extent of Ibrutinib Exposure. Cancer Chemother. Pharmacol. 82 (2), 299–308. doi:10.1007/s00280-018-3613-9
de Man, F. M., Hussaarts, K. G. A. M., de With, M., Oomen-de Hoop, E., de Bruijn, P., van Halteren, H. K., et al. (2019). Influence of the Proton Pump Inhibitor Esomeprazole on the Bioavailability of Regorafenib: A Randomized Crossover Pharmacokinetic Study. Clin. Pharmacol. Ther. 105 (6), 1456–1461. doi:10.1002/cpt.1331
Del Re, M., Fogli, S., Derosa, L., Massari, F., De Souza, P., Crucitta, S., et al. (2017). The Role of Drug-Drug Interactions in Prostate Cancer Treatment: Focus on Abiraterone Acetate/prednisone and Enzalutamide. Cancer Treat. Rev. 55, 71–82. doi:10.1016/j.ctrv.2017.03.001
Egorin, M. J., Shah, D. D., Christner, S. M., Yerk, M. A., Komazec, K. A., Appleman, L. R., et al. (2009). Effect of a Proton Pump Inhibitor on the Pharmacokinetics of Imatinib. Br. J. Clin. Pharmacol. 68 (3), 370–374. doi:10.1111/j.1365-2125.2009.03466.x
Fang, Y. H., Yang, Y. H., Hsieh, M. J., Hung, M. S., and Lin, Y. C. (2019). Concurrent Proton-Pump Inhibitors Increase Risk of Death for Lung Cancer Patients Receiving 1st-Line Gefitinib Treatment - a Nationwide Population-Based Study. Cancer Manag. Res. 11, 8539–8546. doi:10.2147/CMAR.S222278
Gaucher, L., Adda, L., Séjourné, A., Joachim, C., Guillaume, C., Poulet, C., et al. (2021). Associations between Dysbiosis-Inducing Drugs, Overall Survival and Tumor Response in Patients Treated with Immune Checkpoint Inhibitors. Ther. Adv. Med. Oncol. 13, 17588359211000591. doi:10.1177/17588359211000591
Gopalakrishnan, V., Helmink, B. A., Spencer, C. N., Reuben, A., and Wargo, J. A. (2018). The Influence of the Gut Microbiome on Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell 33 (4), 570–580. doi:10.1016/j.ccell.2018.03.015
Graham, C., Orr, C., Bricks, C. S., Hopman, W. M., Hammad, N., and Ramjeesingh, R. (2016). A Retrospective Analysis of the Role of Proton Pump Inhibitors in Colorectal Cancer Disease Survival. Curr. Oncol. 23 (6), e583–8. doi:10.3747/co.23.3204
Griskevicius, L., Yasar, U., Sandberg, M., Hidestrand, M., Eliasson, E., Tybring, G., et al. (2003). Bioactivation of Cyclophosphamide: the Role of Polymorphic CYP2C Enzymes. Eur. J. Clin. Pharmacol. 59 (2), 103–109. doi:10.1007/s00228-003-0590-6
Ha, V. H., Ngo, M., Chu, M. P., Ghosh, S., Sawyer, M. B., and Chambers, C. R. (2015). Does Gastric Acid Suppression Affect Sunitinib Efficacy in Patients with Advanced or Metastatic Renal Cell Cancer? J. Oncol. Pharm. Pract. 21 (3), 194–200. doi:10.1177/1078155214527145
Hanahan, D., and Weinberg, R. A. (2011). Hallmarks of Cancer: the Next Generation. Cell 144 (5), 646–674. doi:10.1016/j.cell.2011.02.013
Hilton, J. F., Tu, D., Seymour, L., Shepherd, F. A., and Bradbury, P. A. (2013). An Evaluation of the Possible Interaction of Gastric Acid Suppressing Medication and the EGFR Tyrosine Kinase Inhibitor Erlotinib. Lung Cancer 82 (1), 136–142. doi:10.1016/j.lungcan.2013.06.008
Hopkins, A. M., Kichenadasse, G., Karapetis, C. S., Rowland, A., and Sorich, M. J. (2020). Concomitant Proton Pump Inhibitor Use and Survival in Urothelial Carcinoma Treated with Atezolizumab. Clin. Cancer Res. 26 (20), 5487–5493. doi:10.1158/1078-0432.CCR-20-1876
Hussain, N., Naeem, M., and Pinato, D. J. (2021). Concomitant Medications and Immune Checkpoint Inhibitor Therapy for Cancer: Causation or Association? Hum. Vaccin. Immunother. 17 (1), 55–61. doi:10.1080/21645515.2020.1769398
Hwang, I. C., Chang, J., and Park, S. M. (2017). Emerging hazard Effects of Proton Pump Inhibitor on the Risk of Colorectal Cancer in Low-Risk Populations: A Korean Nationwide Prospective Cohort Study. PLoS One 12 (12), e0189114. doi:10.1371/journal.pone.0189114
Iglesias-Santamaría, A. (2020). Impact of Antibiotic Use and Other Concomitant Medications on the Efficacy of Immune Checkpoint Inhibitors in Patients with Advanced Cancer. Clin. Transl Oncol. 22 (9), 1481
Ikemura, K., Hiramatsu, S., and Okuda, M. (2017a). Drug Repositioning of Proton Pump Inhibitors for Enhanced Efficacy and Safety of Cancer Chemotherapy. Front. Pharmacol. 8, 911. doi:10.3389/fphar.2017.00911
Ikemura, K., Oshima, K., Enokiya, T., Okamoto, A., Oda, H., Mizuno, T., et al. (2017b). Co-administration of Proton Pump Inhibitors Ameliorates Nephrotoxicity in Patients Receiving Chemotherapy with Cisplatin and Fluorouracil: a Retrospective Cohort Study. Cancer Chemother. Pharmacol. 79 (5), 943–949. doi:10.1007/s00280-017-3296-7
Iurlo, A., Nobili, A., Latagliata, R., Bucelli, C., Castagnetti, F., Breccia, M., et al. (2016). Imatinib and Polypharmacy in Very Old Patients with Chronic Myeloid Leukemia: Effects on Response Rate, Toxicity and Outcome. Oncotarget 7 (48), 80083–80090. doi:10.18632/oncotarget.11657
Johansson, S., Read, J., Oliver, S., Steinberg, M., Li, Y., Lisbon, E., et al. (2014). Pharmacokinetic Evaluations of the Co-administrations of Vandetanib and Metformin, Digoxin, Midazolam, Omeprazole or Ranitidine. Clin. Pharmacokinet. 53 (9), 837–847. doi:10.1007/s40262-014-0161-2
Jun, T., Ozbek, U., Dharmapuri, S., Hardy-Abeloos, C., Zhu, H., Lin, J. Y., et al. (2021). Antacid Exposure and Immunotherapy Outcomes Among Patients with Advanced Hepatocellular Carcinoma. Ther. Adv. Med. Oncol. 13, 17588359211010937. doi:10.1177/17588359211010937
Kearns, M. D., Boursi, B., and Yang, Y. X. (2017). Proton Pump Inhibitors on Pancreatic Cancer Risk and Survival. Cancer Epidemiol. 46, 80–84. doi:10.1016/j.canep.2016.12.006
Kim, S. Y., Lee, J. S., Kang, J., Morita, S., Park, Y. S., Sakamoto, J., et al. (2021). Proton Pump Inhibitor Use and the Efficacy of Chemotherapy in Metastatic Colorectal Cancer: A Post Hoc Analysis of a Randomized Phase III Trial (AXEPT). Oncologist. doi:10.1002/onco.13735
Kinoshita, Y., Ishimura, N., and Ishihara, S. (2018). Advantages and Disadvantages of Long-Term Proton Pump Inhibitor Use. J. Neurogastroenterol Motil. 24 (2), 182–196. doi:10.5056/jnm18001
Kletzl, H., Giraudon, M., Ducray, P. S., Abt, M., Hamilton, M., and Lum, B. L. (2015). Effect of Gastric pH on Erlotinib Pharmacokinetics in Healthy Individuals: Omeprazole and Ranitidine. Anticancer Drugs 26 (5), 565–572. doi:10.1097/CAD.0000000000000212
Koutake, Y., Taniguchi, J., Yasumori, N., Nagaishi, H., Eto, T., Nakashima, K., et al. (2020). Influence of Proton Pump Inhibitors and H2-Receptor Antagonists on the Efficacy and Safety of Dasatinib in Chronic Myeloid Leukemia Patients. Int. J. Hematol. 111 (6), 826–832. doi:10.1007/s12185-020-02845-6
Kumarakulasinghe, N. B., Syn, N., Soon, Y. Y., Asmat, A., Zheng, H., Loy, E. Y., et al. (2016). EGFR Kinase Inhibitors and Gastric Acid Suppressants in EGFR-Mutant NSCLC: a Retrospective Database Analysis of Potential Drug Interaction. Oncotarget 7 (51), 85542–85550. doi:10.18632/oncotarget.13458
Lacy, S. A., Miles, D. R., and Nguyen, L. T. (2017). Clinical Pharmacokinetics and Pharmacodynamics of Cabozantinib. Clin. Pharmacokinet. 56 (5), 477–491. doi:10.1007/s40262-016-0461-9
Lalani, A. A., McKay, R. R., Lin, X., Simantov, R., Kaymakcalan, M. D., and Choueiri, T. K. (2017). Proton Pump Inhibitors and Survival Outcomes in Patients with Metastatic Renal Cell Carcinoma. Clin. Genitourin Cancer 15 (6), 724–732. doi:10.1016/j.clgc.2017.05.019
Lam, L. H., Capparelli, E. V., and Kurzrock, R. (2016). Association of Concurrent Acid-Suppression Therapy with Survival Outcomes and Adverse Event Incidence in Oncology Patients Receiving Erlotinib. Cancer Chemother. Pharmacol. 78 (2), 427–432. doi:10.1007/s00280-016-3087-6
Lassalle, M., Le Tri, T., Bardou, M., Biour, M., Kirchgesner, J., Rouby, F., et al. (2020). Use of Proton Pump Inhibitors in Adults in France: a Nationwide Drug Utilization Study. Eur. J. Clin. Pharmacol. 76 (3), 449–457. doi:10.1007/s00228-019-02810-1
Lau, Y. Y., Gu, W., Lin, T., Viraswami-Appanna, K., Cai, C., Scott, J. W., et al. (2017). Assessment of Drug-Drug Interaction Potential between Ceritinib and Proton Pump Inhibitors in Healthy Subjects and in Patients with ALK-Positive Non-small Cell Lung Cancer. Cancer Chemother. Pharmacol. 79 (6), 1119–1128. doi:10.1007/s00280-017-3308-7
Li, C., Xia, Z., Li, A., and Meng, J. (2020). The Effect of Proton Pump Inhibitor Uses on Outcomes for Cancer Patients Treated with Immune Checkpoint Inhibitors: a Meta-Analysis. Ann. Transl Med. 8 (24), 1655. doi:10.21037/atm-20-7498
Li, M., Zeng, C., Yao, J., Ge, Y., and An, G. (2020). The Association between Proton Pump Inhibitors Use and Clinical Outcome of Patients Receiving Immune Checkpoint Inhibitors Therapy. Int. Immunopharmacol 88, 106972. doi:10.1016/j.intimp.2020.106972
Matsuoka, H., Ando, K., Swayze, E. J., Unan, E. C., Mathew, J., Hu, Q., et al. (2020). CTDSP1 Inhibitor Rabeprazole Regulates DNA-PKcs Dependent Topoisomerase I Degradation and Irinotecan Drug Resistance in Colorectal Cancer. PLoS One 15 (8), e0228002. doi:10.1371/journal.pone.0228002
Mazziotti, G., Canalis, E., and Giustina, A. (2010). Drug-induced Osteoporosis: Mechanisms and Clinical Implications. Am. J. Med. 123 (10), 877–884. doi:10.1016/j.amjmed.2010.02.028
Mir, O., Touati, N., Lia, M., Litière, S., Le Cesne, A., Sleijfer, S., et al. (2019). Impact of Concomitant Administration of Gastric Acid-Suppressive Agents and Pazopanib on Outcomes in Soft-Tissue Sarcoma Patients Treated within the EORTC 62043/62072 Trials. Clin. Cancer Res. 25 (5), 1479–1485. doi:10.1158/1078-0432.CCR-18-2748
Morcos, P. N., Guerini, E., Parrott, N., Dall, G., Blotner, S., Bogman, K., et al. (2017). Effect of Food and Esomeprazole on the Pharmacokinetics of Alectinib, a Highly Selective ALK Inhibitor, in Healthy Subjects. Clin. Pharmacol. Drug Dev. 6 (4), 388–397. doi:10.1002/cpdd.296
Mukherjee, S., Ibrahimi, S., Khalid, B., Roman, D., Zhao, D., and Aljumaily, R. (2018). Do Proton Pump Inhibitors Modulate the Efficacy of Anti-PD-1/PD-L1 Therapy? A Retrospective Study. J. Oncol. Pharm. Pract. 25 (3), 762–764. doi:10.1177/1078155218771152
Musib, L., Choo, E., Deng, Y., Eppler, S., Rooney, I., Chan, I. T., et al. (2013). Absolute Bioavailability and Effect of Formulation Change, Food, or Elevated pH with Rabeprazole on Cobimetinib Absorption in Healthy Subjects. Mol. Pharm. 10 (11), 4046–4054. doi:10.1021/mp400383x
Naito, Y., Kashiwagi, K., Takagi, T., Andoh, A., and Inoue, R. (2018). Intestinal Dysbiosis Secondary to Proton-Pump Inhibitor Use. Digestion 97 (2), 195–204. doi:10.1159/000481813
Narasimhan, N. I., Dorer, D. J., Davis, J., Turner, C. D., and Sonnichsen, D. (2014). Evaluation of the Effect of Multiple Doses of Lansoprazole on the Pharmacokinetics and Safety of Ponatinib in Healthy Subjects. Clin. Drug Investig. 34 (10), 723–729. doi:10.1007/s40261-014-0225-y
Narumi, K., Sato, Y., Kobayashi, M., Furugen, A., Kasashi, K., Yamada, T., et al. (2017). Effects of Proton Pump Inhibitors and Famotidine on Elimination of Plasma Methotrexate: Evaluation of Drug-Drug Interactions Mediated by Organic Anion Transporter 3. Biopharm. Drug Dispos 38 (9), 501–508. doi:10.1002/bdd.2091
Nieves Sedano, M., Manuel Caro Teller, J., García Muñoz, C., Fernandez Redondo, D., Ponce Aix, S., Menéndez Orenga, M., et al. (2018). Clinical Impact of Gastric Acid Suppressing Medication on the Effectiveness of Tyrosine Kinase Inhibitors in Lung Cancer Patients. J. BUON 23 (3), 647
Ohgami, M., Kaburagi, T., Kurosawa, A., Doki, K., Shiozawa, T., Hizawa, N., et al. (2018). Effects of Proton Pump Inhibitor Coadministration on the Plasma Concentration of Erlotinib in Patients with Non-small Cell Lung Cancer. Ther. Drug Monit. 40 (6), 699–704. doi:10.1097/FTD.0000000000000552
Panday, K., Gona, A., and Humphrey, M. B. (2014). Medication-induced Osteoporosis: Screening and Treatment Strategies. Ther. Adv. Musculoskelet. Dis. 6 (5), 185–202. doi:10.1177/1759720X14546350
Papagerakis, S., Bellile, E., Peterson, L. A., Pliakas, M., Balaskas, K., Selman, S., et al. (2014). Proton Pump Inhibitors and Histamine 2 Blockers Are Associated with Improved Overall Survival in Patients with Head and Neck Squamous Carcinoma. Cancer Prev. Res. (Phila) 7 (12), 1258–1269. doi:10.1158/1940-6207.CAPR-14-0002
Patel, D., Bertz, R., Ren, S., Boulton, D. W., and Någård, M. (2020). A Systematic Review of Gastric Acid-Reducing Agent-Mediated Drug-Drug Interactions with Orally Administered Medications. Clin. Pharmacokinet. 59 (4), 447–462. doi:10.1007/s40262-019-00844-3
Pisano, S. G., Hoffman, S. E., Legasto, C. S., McLaughlin, E. M., Porter, K., and Chen, J. L. (2019). Effect of Acid-Suppressive Strategies on Pazopanib Efficacy in Patients with Soft-Tissue Sarcoma. Clin. Transl Sci. 12 (5), 529–533. doi:10.1111/cts.12648
Ranchon, F., Vantard, N., Henin, E., Bachy, E., Sarkozy, C., Karlin, L., et al. (2018). Delayed Methotrexate Elimination: Incidence, Interaction with Antacid Drugs, and Clinical Consequences? Hematol. Oncol. 36 (2), 399–406. doi:10.1002/hon.2479
Rassy, E., Cerbone, L., Auclin, E., Benchimoll-Zouari, A., Flippot, R., Alves Costa Silva, C., et al. (2021). The Effect of Concomitant Proton Pump Inhibitor and Cabozantinib on the Outcomes of Patients with Metastatic Renal Cell Carcinoma. Oncologist 26 (5), 389–396. doi:10.1002/onco.13711
Rhinehart, H. E., Phillips, M. A., Wade, N., and Baran, A. (2018). Evaluation of the Clinical Impact of Concomitant Acid Suppression Therapy in Colorectal Cancer Patients Treated with Capecitabine Monotherapy. J. Oncol. Pharm. Pract. 25 (8), 1839–1845. doi:10.1177/1078155218818237
Rossi, G., Pezzuto, A., Sini, C., Tuzi, A., Citarella, F., McCusker, M. G., et al. (2019). Concomitant Medications during Immune Checkpoint Blockage in Cancer Patients: Novel Insights in This Emerging Clinical Scenario. Crit. Rev. Oncol. Hematol. 142, 26–34. doi:10.1016/j.critrevonc.2019.07.005
Rounis, K., Makrakis, D., Papadaki, C., Monastirioti, A., Vamvakas, L., Kalbakis, K., et al. (2021). Prediction of Outcome in Patients with Non-small Cell Lung Cancer Treated with Second Line PD-1/PDL-1 Inhibitors Based on Clinical Parameters: Results from a Prospective, Single Institution Study. PLOS ONE 16 (6), e0252537. doi:10.1371/journal.pone.0252537
Ruanglertboon, W., Sorich, M. J., Logan, J. M., Rowland, A., and Hopkins, A. M. (2020). The Effect of Proton Pump Inhibitors on Survival Outcomes in Advanced Hepatocellular Carcinoma Treated with Sorafenib. J. Cancer Res. Clin. Oncol. 146 (10), 2693–2697. doi:10.1007/s00432-020-03261-3
Rugo, H. S., Herbst, R. S., Liu, G., Park, J. W., Kies, M. S., Steinfeldt, H. M., et al. (2005). Phase I Trial of the Oral Antiangiogenesis Agent AG-013736 in Patients with Advanced Solid Tumors: Pharmacokinetic and Clinical Results. J. Clin. Oncol. 23 (24), 5474–5483. doi:10.1200/JCO.2005.04.192
Ruiz-Bañobre, J., Molina-Díaz, A., Fernández-Calvo, O., Fernández-Núñez, N., Medina-Colmenero, A., Santomé, L., et al. (2021). Rethinking Prognostic Factors in Locally Advanced or Metastatic Urothelial Carcinoma in the Immune Checkpoint Blockade Era: A Multicenter Retrospective Study. ESMO Open [Internet]. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7980066/.
Samant, T. S., Dhuria, S., Lu, Y., Laisney, M., Yang, S., Grandeury, A., et al. (2018). Ribociclib Bioavailability Is Not Affected by Gastric pH Changes or Food Intake: In Silico and Clinical Evaluations. Clin. Pharmacol. Ther. 104 (2), 374–383. doi:10.1002/cpt.940
Sekido, M., Fujita, K. I., Kubota, Y., Ishida, H., Takahashi, T., Ohkuma, R., et al. (2019). Rabeprazole Intake Does Not Affect Systemic Exposure to Capecitabine and its Metabolites, 5'-Deoxy-5-Fluorocytidine, 5'-Deoxy-5-Fluorouridine, and 5-fluorouracil. Cancer Chemother. Pharmacol. 83 (6), 1127–1135. doi:10.1007/s00280-019-03837-y
Sharma, M., Holmes, H. M., Mehta, H. B., Chen, H., Aparasu, R. R., Shih, Y. T., et al. (2019). The Concomitant Use of Tyrosine Kinase Inhibitors and Proton Pump Inhibitors: Prevalence, Predictors, and Impact on Survival and Discontinuation of Therapy in Older Adults with Cancer. Cancer 125 (7), 1155–1162. doi:10.1002/cncr.31917
Singh, A., Cresci, G. A., and Kirby, D. F. (2018). Proton Pump Inhibitors: Risks and Rewards and Emerging Consequences to the Gut Microbiome. Nutr. Clin. Pract. 33 (5), 614–624. doi:10.1002/ncp.10181
Strand, D. S., Kim, D., and Peura, D. A. (2017). 25 Years of Proton Pump Inhibitors: A Comprehensive Review. Gut Liver 11 (1), 27–37. doi:10.5009/gnl15502
Sun, J., Ilich, A. I., Kim, C. A., Chu, M. P., Wong, G. G., Ghosh, S., et al. (2016). Concomitant Administration of Proton Pump Inhibitors and Capecitabine Is Associated with Increased Recurrence Risk in Early Stage Colorectal Cancer Patients. Clin. Colorectal Cancer 15, 257–263. doi:10.1016/j.clcc.2015.12.008
Sun, W., Klamerus, K. J., Yuhas, L. M., Pawlak, S., Plotka, A., O'Gorman, M., et al. (2017). Impact of Acid-Reducing Agents on the Pharmacokinetics of Palbociclib, a Weak Base with pH-dependent Solubility, with Different Food Intake Conditions. Clin. Pharmacol. Drug Dev. 6 (6), 614–626. doi:10.1002/cpdd.356
Takahashi, N., Miura, M., Niioka, T., and Sawada, K. (2012). Influence of H2-Receptor Antagonists and Proton Pump Inhibitors on Dasatinib Pharmacokinetics in Japanese Leukemia Patients. Cancer Chemother. Pharmacol. 69 (4), 999–1004. doi:10.1007/s00280-011-1797-3
Tan, A. R., Gibbon, D. G., Stein, M. N., Lindquist, D., Edenfield, J. W., Martin, J. C., et al. (2013). Effects of Ketoconazole and Esomeprazole on the Pharmacokinetics of Pazopanib in Patients with Solid Tumors. Cancer Chemother. Pharmacol. 71 (6), 1635–1643. doi:10.1007/s00280-013-2164-3
Tozzi, M., Sørensen, C. E., Magni, L., Christensen, N. M., Bouazzi, R., Buch, C. M., et al. (2020). Proton Pump Inhibitors Reduce Pancreatic Adenocarcinoma Progression by Selectively Targeting H+, K+-ATPases in Pancreatic Cancer and Stellate Cells. Cancers (Basel) 12 (3), 640. doi:10.3390/cancers12030640
Tvingsholm, S. A., Dehlendorff, C., Østerlind, K., Friis, S., and Jäättelä, M. (2018). Proton Pump Inhibitor Use and Cancer Mortality. Int. J. Cancer 143, 1315. doi:10.1002/ijc.31529
Uchiyama, A. A. T., Silva, P. A. I. A., Lopes, M. S. M., Yen, C. T., Ricardo, E. D., Mutão, T., et al. (2021). Proton Pump Inhibitors and Oncologic Treatment Efficacy: A Practical Review of the Literature for Oncologists. Curr. Oncol. 28 (1), 783–799. doi:10.3390/curroncol28010076
Van De Sijpe, G., Beuselinck, B., Van Nieuwenhuyse, T., Poncelet, R., Bechter, O., Albersen, M., et al. (2020). Impact of Concomitant Acid Suppressive Therapy on Pazopanib Efficacy and Dose Reductions in Patients with Metastatic Renal Cell Carcinoma. Eur. J. Clin. Pharmacol. 76 (9), 1273–1280. doi:10.1007/s00228-020-02902-3
van der Bol, J. M., Loos, W. J., de Jong, F. A., van Meerten, E., Konings, I. R., Lam, M. H., et al. (2011). Effect of Omeprazole on the Pharmacokinetics and Toxicities of Irinotecan in Cancer Patients: a Prospective Cross-Over Drug-Drug Interaction Study. Eur. J. Cancer 47 (6), 831–838. doi:10.1016/j.ejca.2010.11.030
van Leeuwen, R. W., van Gelder, T., Mathijssen, R. H., and Jansman, F. G. (2014). Drug-drug Interactions with Tyrosine-Kinase Inhibitors: a Clinical Perspective. Lancet Oncol. 15 (8), e315–26. doi:10.1016/S1470-2045(13)70579-5
Vishwanathan, K., Dickinson, P. A., Bui, K., Cassier, P. A., Greystoke, A., Lisbon, E., et al. (2018). The Effect of Food or Omeprazole on the Pharmacokinetics of Osimertinib in Patients with Non-small-cell Lung Cancer and in Healthy Volunteers. J. Clin. Pharmacol. 58 (4), 474–484. doi:10.1002/jcph.1035
Wang, X., Song, Y., Wang, J., He, J., Liu, R., Li, X., et al. (2020). Effect of Proton Pump Inhibitors on High-Dose Methotrexate Elimination: a Systematic Review and Meta-Analysis. Int. J. Clin. Pharm. 42 (1), 23–30. doi:10.1007/s11096-019-00958-5
Ward, R. M., and Kearns, G. L. (2013). Proton Pump Inhibitors in Pediatrics : Mechanism of Action, Pharmacokinetics, Pharmacogenetics, and Pharmacodynamics. Paediatr. Drugs 15 (2), 119–131. doi:10.1007/s40272-013-0012-x
Wedemeyer, R. S., and Blume, H. (2014). Pharmacokinetic Drug Interaction Profiles of Proton Pump Inhibitors: an Update. Drug Saf. 37 (4), 201–211. doi:10.1007/s40264-014-0144-0
Whitton, B., Okamoto, H., Packham, G., and Crabb, S. J. (2018). Vacuolar ATPase as a Potential Therapeutic Target and Mediator of Treatment Resistance in Cancer. Cancer Med. 7 (8), 3800–3811. doi:10.1002/cam4.1594
Wong, G. G., Ha, V., Chu, M. P., Dersch-Mills, D., Ghosh, S., Chambers, C. R., et al. (2019). Effects of Proton Pump Inhibitors on FOLFOX and CapeOx Regimens in Colorectal Cancer. Clin. Colorectal Cancer 18 (1), 72–79. doi:10.1016/j.clcc.2018.11.001
Wu, Y., Warner, J. L., Wang, L., Jiang, M., Xu, J., Chen, Q., et al. (2019). Discovery of Noncancer Drug Effects on Survival in Electronic Health Records of Patients with Cancer: A New Paradigm for Drug Repurposing. JCO Clin. Cancer Inform. 3, 1–9. doi:10.1200/CCI.19.00001
Yibirin, M., De Oliveira, D., Valera, R., Plitt, A. E., and Lutgen, S. (2021). Adverse Effects Associated with Proton Pump Inhibitor Use. Cureus 13 (1), e12759. doi:10.7759/cureus.12759
Yin, O. Q., Gallagher, N., Fischer, D., Demirhan, E., Zhou, W., Golor, G., et al. (2010). Effect of the Proton Pump Inhibitor Esomeprazole on the Oral Absorption and Pharmacokinetics of Nilotinib. J. Clin. Pharmacol. 50 (8), 960–967. doi:10.1177/0091270009346061
Yin, O. Q., Giles, F. J., Baccarani, M., le Coutre, P., Chiparus, O., Gallagher, N., et al. (2012). Concurrent Use of Proton Pump Inhibitors or H2 Blockers Did Not Adversely Affect Nilotinib Efficacy in Patients with Chronic Myeloid Leukemia. Cancer Chemother. Pharmacol. 70 (2), 345–350. doi:10.1007/s00280-012-1881-3
Yokota, H., Sato, K., Okuda, Y., Kobayashi, H., Takeda, M., Asano, M., et al. (2017). Effects of Histamine 2-receptor Antagonists and Proton Pump Inhibitors on the Pharmacokinetics of Gefitinib in Patients with Non-small-cell Lung Cancer. Clin. Lung Cancer 18 (6), e433–9. doi:10.1016/j.cllc.2017.05.010
Zenke, Y., Yoh, K., Matsumoto, S., Umemura, S., Niho, S., Ohmatsu, H., et al. (2016). Clinical Impact of Gastric Acid-Suppressing Medication Use on the Efficacy of Erlotinib and Gefitinib in Patients with Advanced Non-small-cell Lung Cancer Harboring EGFR Mutations. Clin. Lung Cancer 17 (5), 412–418. doi:10.1016/j.cllc.2016.01.006
Keywords: cancer, proton-pump inhibitors, chemotherapy, targeted therapies, drug interactions
Citation: Bridoux M, Simon N and Turpin A (2022) Proton Pump Inhibitors and Cancer: Current State of Play. Front. Pharmacol. 13:798272. doi: 10.3389/fphar.2022.798272
Received: 14 November 2021; Accepted: 04 February 2022;
Published: 14 March 2022.
Edited by:
Alvaro Francisco Lopes Sousa, University of São Paulo, BrazilReviewed by:
Kurt Neumann, Independent researcher, Kerékteleki, HungaryHelge Waldum, Norwegian University of Science and Technology, Norway
Copyright © 2022 Bridoux, Simon and Turpin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anthony Turpin, QW50aG9ueS50dXJwaW5AY2hydS1saWxsZS5mcg==, b3JjaWQub3JnLzAwMDAtMDAwMi0yMjgyLTAxMDE=
 Marie Bridoux1,2
Marie Bridoux1,2 Anthony Turpin
Anthony Turpin