- 1School of Pharmaceutical Sciences, Peking University, Beijing, China
- 2Department of Pharmacy, Peking University Third Hospital, Beijing, China
- 3College of Pharmacy, University of Nebraska Medical Center, Omaha, NE, United States
- 4School of Basic Medical Sciences and Clinical Pharmacy, China Pharmaceutical University, Nanjing, China
Background The use of gastric acid suppressants (GASs) has an influence on the exposure of some epidermal growth factor receptor-tyrosine kinase inhibitors (EGFR-TKIs) and therefore may affect the effectiveness and safety of EGFR-TKIs. The impact of GASs, including proton pump inhibitors (PPIs) and histamine type 2 receptor antagonists (H2RAs), on the effectiveness and safety of EGFR-TKIs remains unclear. We conducted a meta-analysis to explore the impact of GASs on the effectiveness and safety of EGFR-TKIs in non–small cell lung cancer (NSCLC) patients.
Method We searched the PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, and Wanfang databases thoroughly from inception to 2nd February 2021, including the studies for NSCLC patients who used GASs, offering the adjusted hazard ratio (HR) of effectiveness outcomes such as overall survival (OS) and progression-free survival (PFS) or adjusted odds ratio (OR) of the adverse drug reaction (ADRs), and the results were calculated with a random effect. Two researchers independently screened the literature, extracted data, and evaluated the quality. Stata 15.0 was used for meta-analysis.
Result Twelve studies were finally included. Nine of them were cohort studies, and three of them were case–control studies. For effectiveness outcomes, the use of GASs was associated with shorter PFS (HR 1.66 [1.40, 1.98]) and OS (HR 1.50 [1.31, 1.72]), and the use of PPIs was associated with shorter OS (HR 1.56 [1.21, 2.02]), regardless of the overlap time and type of EGFR-TKIs. For safety outcomes, the use of GASs (OR 1.98 [1.19, 3.31]) or PPIs (OR 1.91 [1.17, 3.12]) were both associated with an increased risk of hepatotoxicity.
Conclusion The concomitant use of GASs is associated with shorter PFS and OS for NSCLC patients taking EGFR-TKIs and is also associated with a higher risk of hepatotoxicity. The co-administration of GASs should be avoided; if they cannot be avoided, H2RAs is a better choice.
Systematic Review Registration: (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021235018), identifier (PROSPERO 2021 CRD42021235018)
Introduction
Lung cancer is the leading cause of death in patients with malignant tumors worldwide and non–small cell lung cancer (NSCLC) accounts for approximately 85% of all lung cancers (Parkin et al., 2001). Epidermal growth factor receptor (EGFR) mutations present in 10–50% of patients with stage IV NSCLC are most prevalent in women, never-smokers, adenocarcinomas, and East Asian patients (Mitsudomi and Yatabe, 2007; Rosell et al., 2009). Tyrosine kinase inhibitors (TKIs) targeting the EGFR (e.g., gefitinib, erlotinib, afatinib, and osimertinib) are currently indicated as the first-line treatment of sensitizing EGFR mutation-positive stage IV NSCLC (Indini et al., 2020).
The histamine type 2 receptor antagonists (H2RAs) and proton pump inhibitors (PPIs) are the major gastric acid suppressants (GASs) that are widely used for many indications requiring acid suppression, such as peptic ulcer disease and upper gastrointestinal bleeding. Compared to H2RAs, PPIs have shown a stronger acid inhibitory effect by blocking H+/K+ ATPase (Ahmed and Clarke, 2021) on the parietal cells. One study showed that H2RAs could suppress 70% of the mean 24-h acidity after taking them for 2 weeks, while PPIs showed over 90% (Howden and Hunt, 1990).
The co-administration of GASs such as PPIs and H2RAs is reported in 33.2–46.3% of the lung cancer patients (Smelick et al., 2013; Saito et al., 2020). Another study showed that 21.7% of elder lung cancer patients concomitantly use PPIs and erlotinib (Sharma et al., 2019). Since the solubility of some EGFR-TKIs is pH-dependent, the absorption of EGFR-TKIs may be influenced by GASs through altering gastric pH. Preclinical studies showed that gefitinib’s solubility could decrease from 21 mg/ml to <0.001 mg/ml when gastric pH increases from 1 to 7. For erlotinib, its maximal solubility achieves at pH = 4 (Budha et al., 2012). Clinical studies showed that the use of GASs, especially PPIs, can decrease the exposure of gefitinib, erlotinib, and dacomitinib significantly (Kletzl et al., 2015; Ruiz-Garcia et al., 2016; Yokota et al., 2017; Yasumuro et al., 2018). The effect appears to be more robust and durable for PPIs compared with H2RAs (Kletzl et al., 2015; Yokota et al., 2017). The concomitant use of gefitinib and PPIs could result in a sustainable decrease of gefitinib concentration, while the effect time was shortened to 6–24 h, when used together with H2RAs (Yokota et al., 2017).
To manage this drug–drug interaction, the package inserts of some EGFR-TKIs recommend separate administration, especially for the H2RAs (FDA, 2004; FDA, 2015; FDA, 2018). The recommendations were primarily based on PK data rather than clinical endpoints. Therefore, it is necessary to explore the influence of GASs on the effectiveness and safety of EGFR-TKIs to provide more information on current recommendations.
Materials and Methods
Literature Search
We searched the PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure, and Wanfang databases. Our search strategy included ((omeprazole or dexlansoprazole or lansoprazole or esomeprazole or pantoprazole or rabeprazole or ilaprazole or “proton pump inhibitor” or PPI or PPIs) or (ranitidine or cimetidine or famotidine or nizatidine or “H2 receptor inhibitor” or H2RA or “histamine 2 receptor antagonist” or H2RAs) or “gastric acid suppressant”) and (gefitinib or erlotinib or afatinib or icotinib or osimertinib or dacomitinib or IRESSA or TARCEVA or GILOTRIF or Conmana or TAGRISSO or VIZIMPRO or EGFR-TKI or EGFR-TKIs). We did not apply any restrictions on publication date, language, or publication status to the searches.
Study Eligibility and Selection
We included studies that 1) recruited NSCLC patients who used EGFR-TKIs; 2) compared the outcomes of patients who used GASs with those who did not; 3) reported the adjusted effectiveness or safety outcomes of EGFR-TKIs such as PFS, OS, and ADR; and 4) were randomized controlled trials (RCTs), cohort studies, or case–control studies. Our exclusion criteria were 1) studies published as an abstract in proceedings of scientific events; 2) studies not in English or Chinese.
Two reviewers (XD and WL) independently screened titles and abstracts and reviewed the full texts of potential studies to determine eligibility. Disagreements were resolved by discussion or by a third reviewer (KC). If an article was not included, the reason was recorded.
Data Collection and the Assessment of the Risk of Bias
Two reviewers (XD and WL) independently extracted data including author names, publication year, study design, the definition of using GASs, sample size, and outcomes. Effectiveness outcomes were mainly PFS and OS. PFS was calculated from the date of initial treatment with EGFR-TKIs until disease progression, clinical deterioration, the date of last contact, or death. OS was calculated from the date of initial treatment with EGFR-TKIs until death. The PFS and OS were measured at the end of the follow-up. Safety outcomes were measured at the end of the follow-up too. Adjusted HR and OR were extracted for effectiveness and safety outcomes, respectively. The data were extracted and summarized into an Excel worksheet.
Two reviewers (ZW and XL) assessed the risk of bias independently using a modified version of the Cochrane criteria for RCTs, a modified version of the Newcastle–Ottawa instrument for cohort studies and an instrument developed specifically for case–control studies. Reviewers judged each criterion as definitely or probably low or high risk of bias and resolved any disagreements by discussion or, if necessary, by consultation with a third reviewer (KC).
Statistical Analysis
We analyzed the results of patients who used the GASs, PPIs, or H2RAs, separately. To account for the expected heterogeneity among studies, the DerSimonian and Laird random-effect model was used for the analysis of adjusted HRs and ORs, and the corresponding forest plots were drawn. The I2 (%) was used to assess the heterogeneity of the selected research studies. The subgroup analysis was performed according to the overlap time of GASs and EGFR-TKIs use, and sensitivity analysis was conducted by eliminating studies one by one and reanalysis. Data analysis was performed by Stata 15.0 software.
Results
Study Eligibility and Assessment of the Risk of Bias
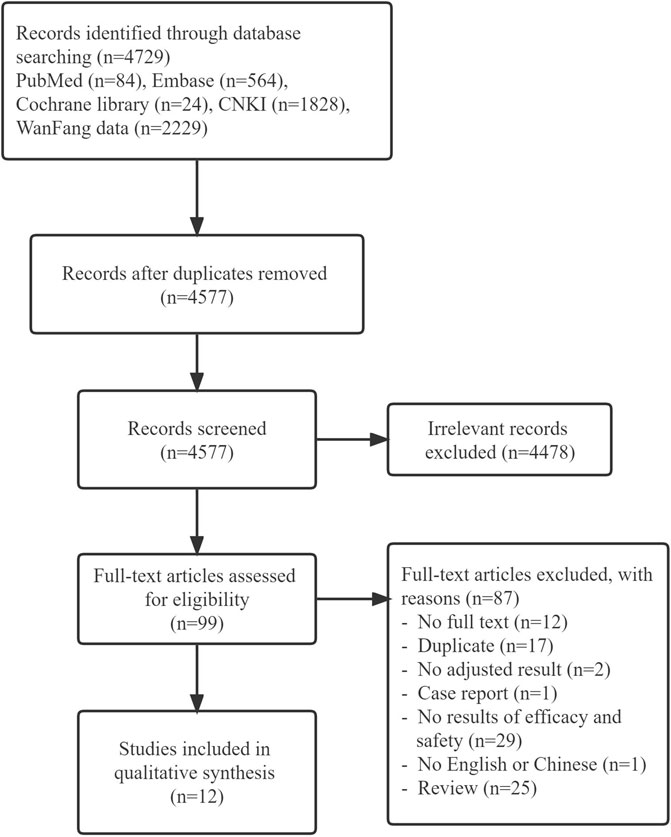
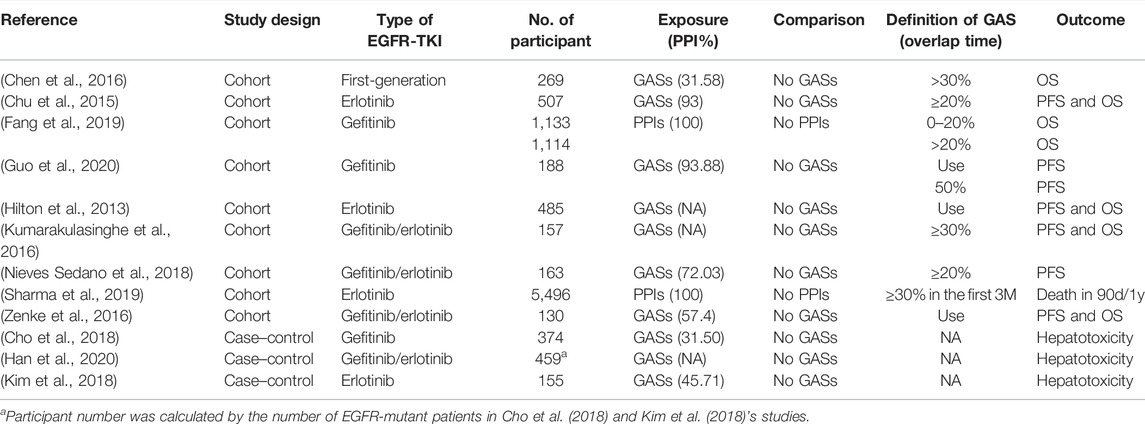
The literature screening process and results are shown in Figure 1. A total of 4,729 related literature were retrieved. After tiered screening, a total of 12 studies met eligibility criteria, including nine cohort and three case–control studies. Nine of 12 studies reported effectiveness outcomes and three studies reported safety outcomes. The quality evaluation showed that all cohort studies and one case–control study were of high quality, while the other two case control studies were of low quality. The main characteristics of the included studies are presented in Table 1, and the quality evaluation is shown in Supplementary Table S1,S2.
Influence on the Effectiveness of EGFR-TKIs
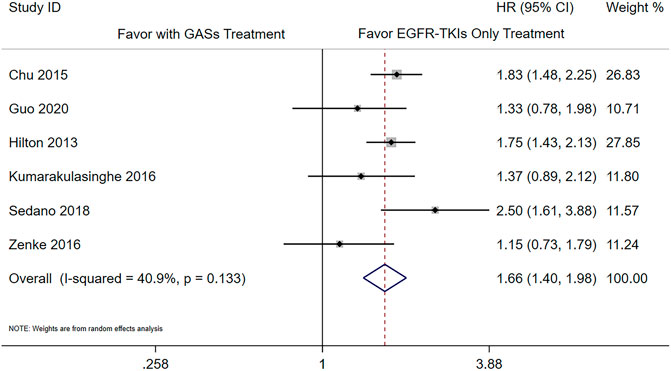
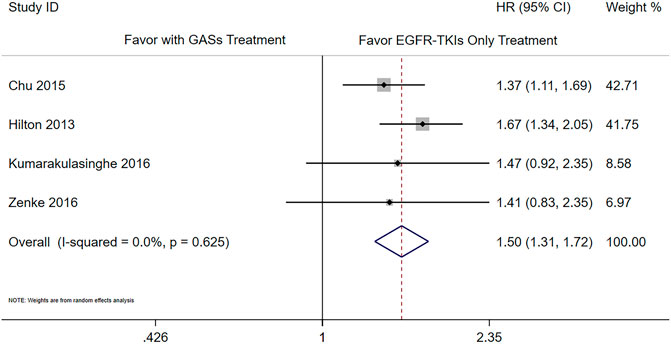
There were six studies that reported the adjusted results of effectiveness outcomes using GASs (Chu et al., 2015; Guo et al., 2020; Hilton et al., 2013; Kumarakulasinghe et al., 2016; Nieves Sedano et al., 2018; Zenke et al., 2016). The meta-analysis results showed that the use of GASs was associated with shorter PFS (HR 1.66 [1.40, 1.98], Figure 2) and OS (HR 1.50 [1.31, 1.72], Figure 3). Three studies focused on PPIs (Sharma et al., 2019; Chen et al., 2016; Fang et al., 2019); two of them reported PFS and OS (Chen et al., 2016; Fang et al., 2019), and the other reported death rate in 90 days and 1 year (Sharma et al., 2019). The meta-analysis of the two studies (Chen et al., 2016; Fang et al., 2019) showed that the use of PPIs was associated with shorter OS (HR 1.56 [1.21, 2.02], Supplementary Figure S1). The other study reported that the use of PPIs was associated with a higher death rate in 90 days (Sharma et al., 2019) (adjusted HR 1.21, [1.07, 1.38]) and in 1 year (adjusted HR 1.11, [1.02, 1.20]). One of the aforementioned studies presented that the use of H2RAs did not influence the OS (Chen et al., 2016) (adjusted HR 1.46, [0.92, 2.33]).
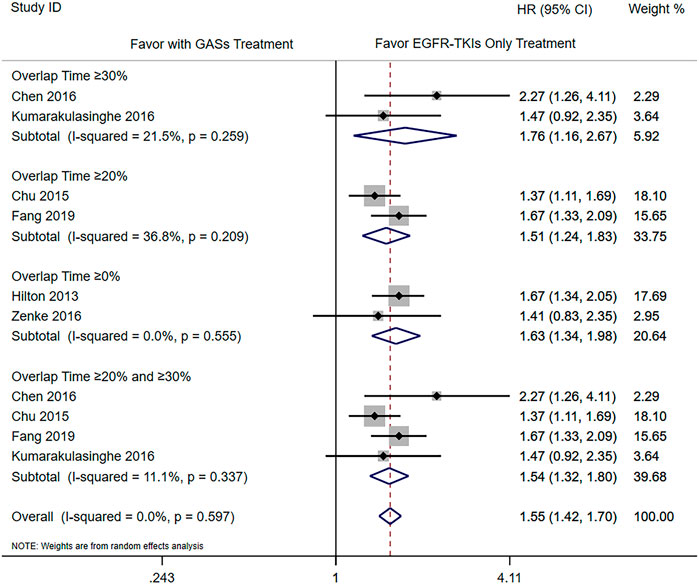
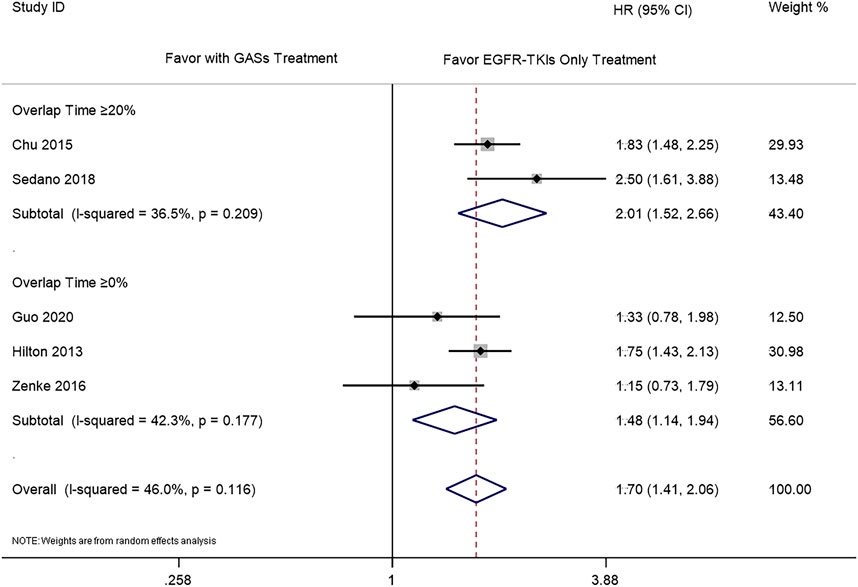
The subgroup analysis was carried out according to the different overlap times of GASs and EGFR-TKIs. Since the previous result showed that the use of H2RAs did not influence the OS, we only combined the results of PPIs and GASs. The included studies used different overlap times such as ≥0% (Hilton et al., 2013; Zenke et al., 2016; Guo et al., 2020), 0–20% (Guo et al., 2020), ≥20% (Chu et al., 2015; Nieves Sedano et al., 2018; Fang et al., 2019), ≥30% (Chen et al., 2016; Kumarakulasinghe et al., 2016), and ≥50% (Guo et al., 2020), respectively. For the OS, different overlap times such as ≥30% (HR 1.76 [1.16, 2.67]), ≥20% (HR 1.51 [1.24, 1.83]), ≥0% (HR 1.63 [1.34, 1.98]), and ≥20% and ≥30% (HR 1.54 [1.32, 1.80]) were all associated with a shorter OS (Figure 4). For PFS, the results showed that the overlap time of both ≥0% (HR 1.48 [1.14, 1.94]) and ≥20% (HR 2.01 [1.52, 2.66]) were associated with a shorter PFS; the higher overlap time showed a greater influence (Figure 5).
Influence on the Safety of EGFR-TKIs
For GASs, three studies reported adjusted safety results (Cho et al., 2018; Kim et al., 2018; Han et al., 2020). Two of them (Cho et al., 2018; Kim et al., 2018) reported all-grade hepatotoxicity, while the other (Han et al., 2020) analyzed grade 3–4 hepatotoxicity whose data came from the former two studies. The results showed that the use of GASs was associated with a higher risk of all-grade hepatotoxicity (OR 1.98 [1.19, 3.31], Supplementary Figure S2) as well as grade 3–4 hepatotoxicity (adjusted OR 3.757 [1.642, 8.601]). The use of PPIs was associated with both all-grade hepatotoxicity (OR 1.91 [1.17, 3.12], Supplementary Figure S3) and grade 3–4 hepatotoxicity (adjusted OR 3.365 [1.376, 8.227]).
For H2RAs, there were two studies (Cho et al., 2018; Han et al., 2020) that reported all-grade hepatotoxicity and grade 3–4 hepatotoxicity, respectively. The results showed that the use of H2RAs was associated with all-grade hepatotoxicity (OR 1.566, [1.044, 2.348]) and grade 3–4 hepatotoxicity (adjusted OR 2.823, [1.279, 6.232]).
Sensitivity
Sensitivity analysis showed that the impact of using GASs on PFS and OS was stable, while for the use of PPIs, it had no significant influence on the OS when the result of Fang (overlap time >20%) was excluded.
Discussion
This is the first meta-analysis exploring the impact of PPIs and H2RAs on both effectiveness and safety of EGFR-TKIs using the adjusted results and also explored the impact according to different overlap times. According to the results, the use of GASs was found to be associated with decreased OS and PFS, even with an overlap of co-administration time shorter than 20%. In addition, a higher risk of all-grade hepatotoxicity was observed regardless of the use of GASs, PPIs or H2RAs.
A previous meta-analysis (Indini et al., 2020) focused on multiple cancer types, which only included five studies for NSCLC. Our study included 12 studies for NSCLC patients reporting adjusted outcomes. The reason why we restricted to adjusted data is that the survival time of cancer patients was influenced by numerous factors, which should be appropriately accounted for during statistical analysis to reduce possible confounding effects.
Different overlap times may affect the result. Guo et al. (2020) used a lower overlap time and showed an insignificant impact with overlap time ≥0% (HR 1.33, [0.784, 1.98]), and the impact turned to be significant when the overlap time was ≥50% (HR 0.067, [0.033, 0.136]).
There may be several reasons that H2RAs did not influence the OS. First, there is only one study reporting the influence of H2RAs on OS with inadequate power to detect the significance. Second, as mentioned earlier, H2RAs have a shorter effect on acid suppression compared to PPIs. Third, some H2RAs such as ranitidine and cimetidine are CYP3A inhibitors, while most of EGFR-TKIs are metabolized by CYP3A4 (Hakkola et al., 2020). Therefore, the use of H2RAs could increase the EGFR-TKI concentration which may offset some of the influence from the altered pH.
A higher risk of all-grade hepatotoxicity was observed in patients using any kind of GASs. It might be due to the interaction of transporters and enzymes. Nearly all of the EGFR-TKIs are substrates for P-glycoprotein (P-gp) and breast cancer resistance protein (BCRP), while the PPIs are P-gp and BCRP inhibitors. Moreover, the therapeutic concentration of some PPIs such as omeprazole and pantoprazole could be higher than their half-maximal inhibitory concentrations (IC50) of P-gp and BCRP (Stedman and Barclay, 2000; Pauli-Magnus et al., 2001; Suzuki et al., 2009). For omeprazole, the IC50 values of P-gp and BCRP are 17.7 and 17.6 μM, separately, while its Cmax could reach 8 μg/ml (23.2 μM). For pantoprazole, the IC50 of BCRP is 5.5 μM, and its Cmax could reach 3.3 μg/ml (8.62 μM). This may result in the increased liver concentration of EGFR-TKIs, although this effect may not be huge enough to offset the decreased absorption (we still see the decreased plasma concentration), but we speculated the increased local concentration may result in hepatotoxicity.
According to the results, we suggested the co-administration of EGFR-TKIs and GASs should be avoided if possible, even with intake–separation. Kwok et al. (2020) carried out a study and showed that even administrating GASs and EGFR-TKIs with a separation of 12 h could still lead to a shorter PFS and a shorter OS in patients using gefitinib. Therefore, the antacids may be a better choice because of the relatively short duration of anti-acid effects that could be easily separated from the EGFR-TKIs usage. If the use of H2RAs or PPIs cannot be avoided, the H2RAs may be the choice compared with the PPIs, providing strict monitoring of the liver function.
This meta-analysis has limitations. Our data was limited; therefore, some of the subgroup analyses such as comparing the different influence of PPIs and H2RAs and different overlap times were not feasible. In addition, the included studies were all about gefitinib or erlotinib. We will have to wait for more studies of the newer EGFR-TKIs. Third, both PPIs and H2RAs are available as over-the-counter medications in some countries, and there may be some usage that is not recorded that could influence the results of the original studies and also our results.
Conclusion
The use of GASs, especially PPIs, could influence the effectiveness and safety of EGFR-TKIs. The PFS and OS of NSCLC patients taking EGFR-TKIs are shorter when co-administered with GASs. When the overlap time exceeds 20%, the negative effect of GASs on OS becomes greater. The concomitant use of GASs is associated with a higher risk of all-grade hepatotoxicity. The co-administration of GASs and EGFR-TKIs should be avoided. If it cannot be avoided, H2RAs may be a better choice, with close monitoring of the liver function during the co-administration, especially for gefitinib or erlotinib. However, the results still have some limitations, and further studies with improved designs are necessary to confirm these results.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
XD, WL, KC, LY, and XXH contributed to the conception or design of the study. XD, WL, KC, ZW and XL acquired, analyzed, or interpreted the data. XD, WL, KC and XX drafted the manuscript or substantively revised it. LY and WL offered the funding acquisition. XD and WL contributed equally to this work. All authors read and approved the final manuscript and agreed to be personally accountable for the author’s own contributions and to ensure the questions related to the accuracy or integrity of any part of the work.
Funding
This work was supported by the 13th Five-Year Plan National Sciences and Technology Major Project (Nos. 2017ZX09304012-006 and 2017ZX09304012-007, Ministry of Science of Technology of China).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.796538/full#supplementary-material
Abbreviations
NSCLC, non–small cell lung cancer; EGFR-TKIs, epidermal growth factor receptor tyrosine kinase knhibitors; GASs, gastric acid suppressants; PPIs, proton pump inhibitors; H2RAs, histamine type 2 receptor antagonists; EGFR, epidermal growth factor receptor; TKIs, tyrosine kinase inhibitors; PFS, progression-free survival; OS, overall survival; ADRs, adverse drug reactions; RCTs, randomized controlled trials; HR, hazard ratio; OR, odds ratio; P-gp, P-glycoprotein; BCRP, breast cancer resistance protein; IC50, half-maximal inhibitory concentrations.
References
Ahmed, A., and Clarke, J. O. (2022). Proton Pump Inhibitors (PPI). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
Budha, N. R., Frymoyer, A., Smelick, G. S., Jin, J. Y., Yago, M. R., Dresser, M. J., et al. (2012). Drug Absorption Interactions between Oral Targeted Anticancer Agents and PPIs: Is pH-dependent Solubility the Achilles Heel of Targeted Therapy? Clin. Pharmacol. Ther. 92 (2), 203–213. doi:10.1038/clpt.2012.73
Chen, Y. M., Lai, C. H., Chang, H. C., Chao, T. Y., Tseng, C. C., Fang, W. F., et al. (2016). Antacid Use and De Novo Brain Metastases in Patients with Epidermal Growth Factor Receptor-Mutant Non-small Cell Lung Cancer Who Were Treated Using First-Line First-Generation Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors. PLoS One 11 (2), e0149722. doi:10.1371/journal.pone.0149722
Cho, S., Yee, J., Kim, J. Y., Jeong Rhie, S., and Gwak, H. S. (2018). Effects of Concomitant Medication Use on Gefitinib-Induced Hepatotoxicity. J. Clin. Pharmacol. 58 (2), 263–268. doi:10.1002/jcph.1010
Chu, M. P., Ghosh, S., Chambers, C. R., Basappa, N., Butts, C. A., Chu, Q., et al. (2015). Gastric Acid Suppression Is Associated with Decreased Erlotinib Efficacy in Non-small-cell Lung Cancer. Clin. Lung Cancer 16 (1), 33–39. doi:10.1016/j.cllc.2014.07.005
Fang, Y. H., Yang, Y. H., Hsieh, M. J., Hung, M. S., and Lin, Y. C. (2019). Concurrent Proton-Pump Inhibitors Increase Risk of Death for Lung Cancer Patients Receiving 1st-Line Gefitinib Treatment - a Nationwide Population-Based Study. Cancer Manag. Res. 11, 8539–8546. doi:10.2147/CMAR.S222278
Guo, Z., Du, Q., Ye, X., Gao, F., You, Y., Yu, B., et al. (2020). Concomitant Administration of Gastric Acid Suppression Might Attenuates the Clinical Efficacy of Gefitinib: A Single Cancer Center Retrospective Study[J]. J. Chin. Pharm. Sci. 29 (3), 192–198.
Hakkola, J., Hukkanen, J., Turpeinen, M., and Pelkonen, O. (2020). Inhibition and Induction of CYP Enzymes in Humans: an Update. Arch. Toxicol. 94 (11), 3671–3722. doi:10.1007/s00204-020-02936-7
Han, J. M., Han, H. W., Yee, J., Kim, M. K., Moon, J. Y., Cho, S., et al. (2020). Factors Affecting High-Grade Hepatotoxicity of Tyrosine Kinase Inhibitors in Cancer Patients: a Multi-Center Observational Study. Eur. J. Clin. Pharmacol. 76 (8), 1183–1191. doi:10.1007/s00228-020-02897-x
Hilton, J. F., Tu, D., Seymour, L., Shepherd, F. A., and Bradbury, P. A. (2013). An Evaluation of the Possible Interaction of Gastric Acid Suppressing Medication and the EGFR Tyrosine Kinase Inhibitor Erlotinib. Lung Cancer 82 (1), 136–142. doi:10.1016/j.lungcan.2013.06.008
Howden, C. W., and Hunt, R. H. (1990). The Relationship between Suppression of Acidity and Gastric Ulcer Healing Rates. Aliment. Pharmacol. Ther. 4 (1), 25–33. doi:10.1111/j.1365-2036.1990.tb00445.x
Indini, A., Petrelli, F., Tomasello, G., Rijavec, E., Facciorusso, A., Grossi, F., et al. (2020). Impact of Use of Gastric-Acid Suppressants and Oral Anti-cancer Agents on Survival Outcomes: A Systematic Review and Meta-Analysis. Cancers (Basel) 12 (4), 998. doi:10.3390/cancers12040998
Kim, M. K., Yee, J., Cho, Y. S., Jang, H. W., Han, J. M., and Gwak, H. S. (2018). Risk Factors for Erlotinib-Induced Hepatotoxicity: a Retrospective Follow-Up Study. BMC Cancer 18 (1), 988. doi:10.1186/s12885-018-4891-7
Kletzl, H., Giraudon, M., Ducray, P. S., Abt, M., Hamilton, M., and Lum, B. L. (2015). Effect of Gastric pH on Erlotinib Pharmacokinetics in Healthy Individuals: Omeprazole and Ranitidine. Anticancer Drugs 26 (5), 565–572. doi:10.1097/CAD.0000000000000212
Kumarakulasinghe, N. B., Syn, N., Soon, Y. Y., Asmat, A., Zheng, H., Loy, E. Y., et al. (2016). EGFR Kinase Inhibitors and Gastric Acid Suppressants in EGFR-Mutant NSCLC: a Retrospective Database Analysis of Potential Drug Interaction. Oncotarget 7 (51), 85542–85550. doi:10.18632/oncotarget.13458
Kwok, K. C., Ho, J. C. M., Lam, D. C. L., Liu, M. M. S., Ip, M. S. M., and Tam, T. C. C. (2020). Clinical Impact of Gastric Acid Suppressants Use on the Efficacy of Gefitinib in Patients with Advanced Adenocarcinoma of the Lung Harboring Common EGFR Mutations. Clinical Cancer Drugs 7 (1), 57–61. doi:10.2174/2212697x06666191021155535
Mitsudomi, T., and Yatabe, Y. (2007). Mutations of the Epidermal Growth Factor Receptor Gene and Related Genes as Determinants of Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitors Sensitivity in Lung Cancer. Cancer Sci. 98 (12), 1817–1824. doi:10.1111/j.1349-7006.2007.00607.x
Nieves Sedano, M., Manuel Caro Teller, J., García Muñoz, C., Fernandez Redondo, D., Ponce Aix, S., Menéndez Orenga, M., et al. (2018). Clinical Impact of Gastric Acid Suppressing Medication on the Effectiveness of Tyrosine Kinase Inhibitors in Lung Cancer Patients. J. BUON 23 (3), 647–653.
Parkin, D. M., Bray, F. I., and Devesa, S. S. (2001). Cancer Burden in the Year 2000. The Global Picture. Eur. J. Cancer 37 (Suppl. 8), S4–S66. doi:10.1016/s0959-8049(01)00267-2
Pauli-Magnus, C., Rekersbrink, S., Klotz, U., and Fromm, M. F. (2001). Interaction of Omeprazole, Lansoprazole and Pantoprazole with P-Glycoprotein. Naunyn Schmiedeb. Arch. Pharmacol. 364 (6), 551–557. doi:10.1007/s00210-001-0489-7
Rosell, R., Moran, T., Queralt, C., Porta, R., Cardenal, F., Camps, C., et al. (2009). Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N. Engl. J. Med. 361 (10), 958–967. doi:10.1056/NEJMoa0904554
Ruiz-Garcia, A., Masters, J. C., Mendes da Costa, L., LaBadie, R. R., Liang, Y., Ni, G., et al. (2016). Effect of Food or Proton Pump Inhibitor Treatment on the Bioavailability of Dacomitinib in Healthy Volunteers. J. Clin. Pharmacol. 56 (2), 223–230. doi:10.1002/jcph.588
Saito, Y., Takekuma, Y., Kobayashi, M., Shinagawa, N., Shimizu, Y., Kinoshita, I., et al. (2020). Impact of Histamine Type-2 Receptor Antagonists on the Anticancer Efficacy of Gefitinib in Patients with Non-small Cell Lung Cancer. Eur. J. Clin. Pharmacol. 77 (3), 381–388. doi:10.1007/s00228-020-03013-9
Sharma, M., Holmes, H. M., Mehta, H. B., Chen, H., Aparasu, R. R., Shih, Y. T., et al. (2019). The Concomitant Use of Tyrosine Kinase Inhibitors and Proton Pump Inhibitors: Prevalence, Predictors, and Impact on Survival and Discontinuation of Therapy in Older Adults with Cancer. Cancer 125 (7), 1155–1162. doi:10.1002/cncr.31917
Smelick, G. S., Heffron, T. P., Chu, L., Dean, B., West, D. A., Duvall, S. L., et al. (2013). Prevalence of Acid-Reducing Agents (ARA) in Cancer Populations and ARA Drug-Drug Interaction Potential for Molecular Targeted Agents in Clinical Development. Mol. Pharm. 10 (11), 4055–4062. doi:10.1021/mp400403s
Stedman, C. A., and Barclay, M. L. (2000). Review Article: Comparison of the Pharmacokinetics, Acid Suppression and Efficacy of Proton Pump Inhibitors. Aliment. Pharmacol. Ther. 14 (8), 963–978. doi:10.1046/j.1365-2036.2000.00788.x
Suzuki, K., Doki, K., Homma, M., Tamaki, H., Hori, S., Ohtani, H., et al. (2009). Co-administration of Proton Pump Inhibitors Delays Elimination of Plasma Methotrexate in High-Dose Methotrexate Therapy. Br. J. Clin. Pharmacol. 67 (1), 44–49. doi:10.1111/j.1365-2125.2008.03303.x
Yasumuro, O., Uchida, S., Kashiwagura, Y., Suzuki, A., Tanaka, S., Inui, N., et al. (2018). Changes in Gefitinib, Erlotinib and Osimertinib Pharmacokinetics under Various Gastric pH Levels Following Oral Administration of Omeprazole and Vonoprazan in Rats. Xenobiotica 48 (11), 1106–1112. doi:10.1080/00498254.2017.1396379
Yokota, H., Sato, K., Okuda, Y., Kobayashi, H., Takeda, M., Asano, M., et al. (2017). Effects of Histamine 2-receptor Antagonists and Proton Pump Inhibitors on the Pharmacokinetics of Gefitinib in Patients with Non-small-cell Lung Cancer. Clin. Lung Cancer 18 (6), e433–e39. doi:10.1016/j.cllc.2017.05.010
Zenke, Y., Yoh, K., Matsumoto, S., Umemura, S., Niho, S., Ohmatsu, H., et al. (2016). Clinical Impact of Gastric Acid-Suppressing Medication Use on the Efficacy of Erlotinib and Gefitinib in Patients with Advanced Non-small-cell Lung Cancer Harboring EGFR Mutations. Clin. Lung Cancer 17 (5), 412–418. doi:10.1016/j.cllc.2016.01.006
Keywords: EGFR-TKIs, GASs, drug–drug interaction, effectiveness, safety, NSCLC
Citation: Du X, Liu W, Chen K, Wang Z, Li X, Yang L and Xie X (2022) Impact of the Gastric Acid Suppressant Use on the Safety and Effectiveness of EGFR-TKIs: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:796538. doi: 10.3389/fphar.2022.796538
Received: 17 October 2021; Accepted: 27 April 2022;
Published: 20 June 2022.
Edited by:
William K.K. Wu, Chinese University of Hong Kong, ChinaReviewed by:
Raseen Tariq, Mayo Clinic, United StatesYu Chen, Nanhai Hospital, Southern Medical University, China
Masatomo Miura, Akita University Hospital, Japan
Copyright © 2022 Du, Liu, Chen, Wang, Li, Yang and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Yang, bGlsaWFueWFuZ2xpQDE2My5jb20=; Xiaohui Xie, eHhocmVuZWVAYmptdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Xin Du
Xin Du Wei Liu
Wei Liu Ken Chen3
Ken Chen3 Ziyu Wang
Ziyu Wang Xinyi Li
Xinyi Li Li Yang
Li Yang Xiaohui Xie
Xiaohui Xie