
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Pharmacol., 14 April 2022
Sec. Obstetric and Pediatric Pharmacology
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.771563
This article is part of the Research TopicImproving Efficacy and Safety of drugs in pediatric population: New ChallengesView all 18 articles
Background: Bivalirudin is a direct thrombin inhibitor (DTI) that can be an alternative to unfractionated heparin (UFH). The efficacy and safety of bivalirudin in anticoagulation therapy in extracorporeal membrane oxygenation (ECMO) remain unknown.
Methods: This study followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines. A systematic literature search was performed in PubMed, EMBASE, and The Cochrane Library databases to identify all relevant original studies estimating bivalirudin’s efficacy and safety versus UFH as anticoagulation therapy in ECMO. The time limit for searching is from the search beginning to June 2021. Two researchers independently screened the literature, extracted data and evaluated the risk of bias of the included studies. The meta-analysis (CRD42020214713) was performed via the RevMan version 5.3.5 Software and STATA version 15.1 Software.
Results: Ten articles with 847 patients were included for the quantitative analysis. Bivalirudin can significantly reduce the incidence of major bleeding in children (I2 = 48%, p = 0.01, odd ratio (OR) = 0.17, 95% confidence interval (CI): 0.04–0.66), patient thrombosis (I2 = 0%, p = 0.02, OR = 0.58, 95% CI: 0.37–0.93), in-circuit thrombosis/interventions (I2 = 0%, p = 0.0005, OR = 0.40, 95% CI: 0.24–0.68), and in-hospital mortality (I2 = 0%, p = 0.007, OR = 0.64, 95% CI: 0.46–0.88). Also, comparable clinical outcomes were observed in the incidence of major bleeding in adults (I2 = 48%, p = 0.65, OR = 0.87, 95% CI: 0.46–1.62), 30-day mortality (I2 = 0%, p = 0.61, OR = 0.83, 95% CI: 0.41–1.68), and ECMO duration in adults (I2 = 41%, p = 0.75, mean difference (MD) = −3.19, 95% CI: −23.01–16.63) and children (I2 = 76%, p = 0.65, MD = 40.33, 95% CI:−135.45–216.12).
Conclusions: Compared with UFH, bivalirudin can be a safe and feasible alternative anticoagulant option to UFH as anticoagulation therapy in ECMO, especially for heparin resistance (HR) and heparin-induced thrombocytopenia (HIT) cases.
Extracorporeal membrane oxygenation (ECMO) is a life-supporting system that provides circulatory and/or pulmonary support for patients suffering from severe, life-threatening disease (Karagiannidis et al., 2016), including refractory acute heart failure, ST-segment elevation myocardial infarction (STEMI), or acute respiratory distress syndrome (ARDS). Moreover, ECMO is applied in severe conditions, such as heart transplantation and shock, as well. With the development of medical technology, ECMO complications have reduced significantly, with greatly improved survival rates. In recent studies, ECMO proved its superiority in reducing the mortality in patients with severe respiratory failure from COVID-19 (Shaefi et al., 2021). However, during ECMO treatment, coagulation-related complications (i.e., bleeding or thrombosis) remain the main factors affecting morbidity and mortality. Therefore, clinical researches has focused on the avoidance of those complications.
Blood’s exposure to a foreign surface may render patients vulnerable to thromboembolic events, which can be prevented by the heparinization of blood (Finley and Greenberg, 2013). For decades, unfractionated heparin (UFH) has been the most common anticoagulant and mainstay antithrombotic in ECMO. Nevertheless, its clinical use may be restricted by UFH-related complications, such as heparin resistance (HR), caused by the consumptive deficiency of antithrombin (AT III), and heparin-induced thrombocytopenia (HIT). This devastating event may occur with heparin exposure (Ortel, 2009; Koster et al., 2013). Therefore, replacement of anticoagulation therapy appears crucial.
Bivalirudin is an alternative anticoagulant option. As an oligopeptide analog of hirudin, bivalirudin is a parenteral direct thrombin inhibitor (DTI), inherently independent of AT III. Moreover, bivalirudin is a bivalent DTI that binds specifically to thrombin at two sites without a cofactor (Warkentin et al., 2008). Furthermore, the reversible and transient binding to thrombin makes it a mainstream anticoagulant in the cardiac catheterization room (Warkentin et al., 2008). However, there are no large-scale, randomized controlled trials (RCTs) reporting the incidences of major bleeding, thrombosis, and mortality of bivalirudin versus UFH in the treatment of ECMO. Therefore, we believe it is worthwhile to carefully conduct a meta-analysis to evaluate the efficacy and safety of bivalirudin versus UFH in ECMO anticoagulation therapy.
This is a registered meta-analysis on PROSPERO (https://www.crd.york.ac.uk/prospero/). The registration number is CRD42020214713.
The participants, intervention, comparison, outcome, and study design approach (PCIOS) were used to select clinical studies (Table 1). Reviews, meta-analyses, non-human studies, case reports, and conferences were excluded. Studies that did not compare the clinical outcomes between UFH and bivalirudin were excluded as well. Two authors (S. Liang and J. Zhu) independently searched the PubMed, EMBASE, and The Cochrane Library databases for articles published from inception until 1 June 2021, using the heading terms “heparin,” “unfractionated heparin,” “bivalirudin,” “extracorporeal membrane oxygenation,” “ECMO,” “ECMO treatment,” “ECLS,” or “ECLS treatment”. No language restrictions were used. The references of relevant literature were also searched to look for more eligible studies.
Data were extracted by the same two independent readers (S. Liang and J. Zhu) who performed the literature search and study selection; the researchers were not blinded to the authors and institutions of included studies. Disagreements were solved by a third reader (M. Ma). Y. He supervised the whole process. This meta-analysis followed the guidelines for preferred reporting items for systematic reviews and meta-analyses (PRISMA) (Shamseer et al., 2015). The two reviewers extracted the following information independently: the first author, published year, study design (prospective/retrospective), study duration, total patients and number of patients in the bivalirudin and UFH groups, the doses in the bivalirudin and UFH group, and the incidence of thromboses, major bleeding, and mortality (per-patient).
For the observational studies, the Newcastle-Ottawa Scale (NOS) was used to assess the risk of bias. The NOS ranges from 0 (lowest) to 9 (highest), and studies with scores ≥6 are considered high quality. For RCTs, the modified Jadad quality scoring scale is used for the quality assessment, which includes the generation of random sequences, distribution methods, randomized concealment, double-blinding, withdrawals and dropouts. The Jadad score among four to seven is considered as good quality.
Sensitivity analysis of the included studies was conducted via a one-by-one elimination method to evaluate the meta-analysis’s stability. A Galbraith plot was used to find the cause of heterogeneity. Egger’s test was used to test the publication bias via Stata version 15.1 Software (The StataCorp LP, Texas City, United States).
For studies describing the results via median and interquartile range (IQR), Standard deviations (SDs) of the mean differences (MDs) were obtained as described by former researches (Wan et al., 2014; Luo et al., 2018). The RevMan version 5.3.5 Software (The Cochrane Collaboration, Copenhagen, Denmark) and Stata version 15.1 Software (The StataCorp LP, Texas City, United States) was used for all statistical analyses. Statistical heterogeneity was assessed by using the Cochrane Q and the I square statistics. Heterogeneity was interpreted as absent (I2:0–25%), low (I2: 25.1–50%), moderate (I2: 50.1–75%), or high (I2: 75.1–100%) (Higgins et al., 2003). The use of a random-effects model was also considered when the number of studies was relatively small, and a random-effects model was applied to estimate the continuous outcome data for data with a p-value ≤0.1 and an I2-value >50%, which indicated statistical heterogeneity (Higgins et al., 2003). Otherwise, a fixed-effects model was used. The overall log with its 95% CI was used as the summary of the overall effect size. A p-value <0.05 was considered statistically significant.
The literature search produced 125 total findings (101 on EMBASE, 4 on The Cochrane Library, and 20 on PubMed); 80 full texts were retrieved after duplicates were removed. The titles and abstracts of studies were screened, after which 53 articles were excluded due to the following reasons: systematic reviews (n = 2), reviews, letters and editorials (n = 34), case reports (n = 17). A total of 27 full-text articles were reviewed, and 17 were excluded later because they lacked the comparison between UFH and bivalirudin (n = 15) or they are only with abstracts (n = 2). Finally, ten unique retrospective observational studies (Ranucci et al., 2011; Pieri et al., 2013; Ljajikj et al., 2017; Berei et al., 2018; Macielak et al., 2019; Brown et al., 2020; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021; Seelhammer et al., 2021) with 847 patients were included for the quantitative analysis. All articles were published before 1 June 2021. The literature screening process is presented in Figure 1.
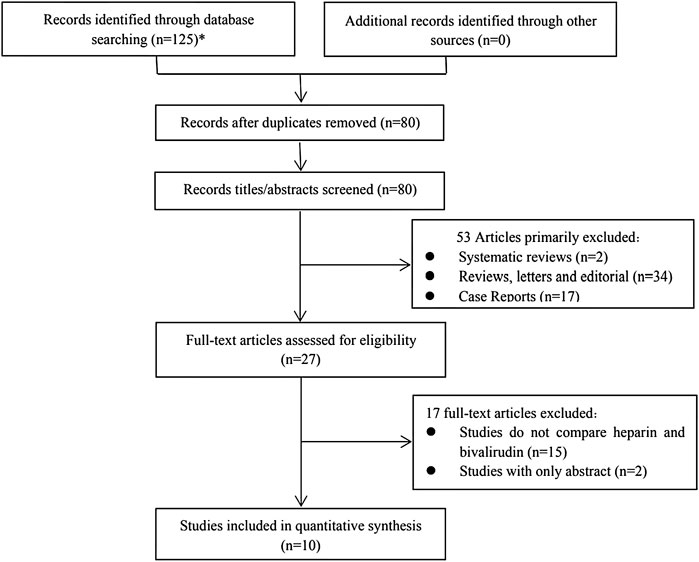
FIGURE 1. Flow chat of study selection (*101 from Embase, 20 from Pubmed and 4 from The Cochrane Library).
Table 2 shows basic information from the included studies; Table 3 shows group definition of the bivalirudin group and clinical outcomes. Generally, these included studies met most NOS quality indicators. However, the control group of all the studies did not meet the standard of “community controls” and “no history of diseases” as the controls was from a hospital. Moreover, as the included studies were all case-control retrospective studies, they were not blinded to the case/control status. According to the NOS, all the included studies were considered as high quality (Supplemental Table S1).
Nine studies (Ranucci et al., 2011; Pieri et al., 2013; Ljajikj et al., 2017; Berei et al., 2018; Macielak et al., 2019; Brown et al., 2020; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021) reported the incidence of major bleeding. Two studies (Macielak et al., 2019; Brown et al., 2020) were not included due to the different ways of expression (per ECMO day). The incidence rate of major bleeding is 0.223 and 0.139 per ECMO day in the UFH and bivalirudin group in Macielak et al. (Macielak et al., 2019)’s study, 0.308 and 0.062 per ECMO day in Brown et al. (Brown et al., 2020)’s study, respectively. Regarding Ljajikj et al. (Ljajikj et al., 2017)’s study, we considered both delayed chest closure and intracranial bleeding as major bleeding because the authors thought that delayed chest closure might also be the result of diffuse persisting bleeding.
Seven studies (Ranucci et al., 2011; Pieri et al., 2013; Ljajikj et al., 2017; Berei et al., 2018; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021) were included in the meta-analysis, and moderate heterogeneity was observed (I2 = 59%, p = 0.02), therefore a subgroup analysis was conducted. Five studies (Ranucci et al., 2011; Pieri et al., 2013; Ljajikj et al., 2017; Berei et al., 2018; Kaseer et al., 2020) were included in the adults group, whilst two studies (Hamzah et al., 2020; Schill et al., 2021) were include in the children group (Figure 2). Low heterogeneity was observed in both adults and children group (I2 = 48% and 48%, p = 0.10 and 0.16, respectively), therefore a fixed-effects model was used. The results showed that the difference of pooled incidence of major bleeding was significantly reduced in children (I2 = 48%, p = 0.01, odd ratio (OR) = 0.17, 95% confidence interval (CI): 0.04–0.66) in the bivalirudin group, but not in adults (I2 = 48%, p = 0.65, OR = 0.87, 95% CI: 0.46–1.62). The heterogeneity decreased after subgroup analysis, which indicates that the age maybe one of the sources of heterogeneity.
The sensitivity analysis of the incidence of major bleeding of the included studies showed that all studies’ estimate was within 95% CI of the total effect except for Berei et al. (Berei et al., 2018)’s study, which means the analytical stability may be affected (Supplementary Figure S1). After removing the study the difference of pooled incidence of major bleeding was still not significantly reduced in the adult group (I2 = 0%, p = 0.05, OR = 0.40, 95% CI: 0.16–0.99).
Ten studies (Ranucci et al., 2011; Pieri et al., 2013; Ljajikj et al., 2017; Berei et al., 2018; Macielak et al., 2019; Brown et al., 2020; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021; Seelhammer et al., 2021) reported the incidence of thrombosis. Two studies (Macielak et al., 2019; Brown et al., 2020) were not included due to the different ways of expression (per ECMO day). The incidence rate of thrombosis is 0.207 and 0.089 per ECMO day in the UFH and bivalirudin group in Macielak et al. (Macielak et al., 2019)’s study, 0.043 and 0 per ECMO day in Brown et al. (Brown et al., 2020)’s study, respectively.
Thrombosis can be divided into patient thrombosis and in-circuit thrombosis/interventions. Eight studies (Ranucci et al., 2011; Pieri et al., 2013; Ljajikj et al., 2017; Berei et al., 2018; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021; Seelhammer et al., 2021) reported the incidence of patient thrombosis and six studies (Ranucci et al., 2011; Pieri et al., 2013; Berei et al., 2018; Hamzah et al., 2020; Kaseer et al., 2020; Seelhammer et al., 2021) reported the incidence of in-circuit thrombosis/interventions group (Figure 3). Low heterogeneity was observed in both group (I2 = 0%, p = 0.96 and 0.81, respectively), therefore a fixed-effects model was used. The results showed that both the difference of pooled incidence of patient thrombosis (I2 = 0%, p = 0.02, OR = 0.58, 95% CI: 0.37–0.93) and in-circuit thrombosis/interventions (I2 = 0%, p = 0.0005, OR = 0.40, 95% CI: 0.24–0.68) was significantly reduced in the bivalirudin group. The sensitivity analysis showed that all studies’ estimate was within 95% CI of the total effect, which means the analytical stability was not affected (Supplementary Figure S2)
Seven studies (Ranucci et al., 2011; Pieri et al., 2013; Ljajikj et al., 2017; Berei et al., 2018; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021; Seelhammer et al., 2021) reported the incidence of mortality. Mortality can be divided into in-hospital mortality, 30-day mortality, and 1-year mortality. Seven studies (Ranucci et al., 2011; Pieri et al., 2013; Berei et al., 2018; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021; Seelhammer et al., 2021) were included in the in-hospital mortality group, three studies (Ljajikj et al., 2017; Berei et al., 2018; Kaseer et al., 2020) were included in the 30-day mortality group, and only one study (Ljajikj et al., 2017) was included in the 1-year mortality group (Figure 4). Low heterogeneity was observed in both in-hospital mortality and 30-day mortality group (I2 = 0%, p = 0.58 and 0.53, respectively), therefore a fixed-effects model was used. The results showed that the difference of pooled incidence of in-hospital mortality was significantly reduced in the bivalirudin group (I2 = 0%, p = 0.007, OR = 0.64, 95% CI: 0.46–0.88), but the difference of pooled incidence of 30-day mortality was not significant (I2 = 0%, p = 0.61, OR = 0.83, 95% CI: 0.41–1.68). The sensitivity analysis showed that all studies’ estimate was within 95% CI of the total effect, which means the analytical stability was not affected (Supplementary Figure S3)
Eight studies (Ranucci et al., 2011; Pieri et al., 2013; Berei et al., 2018; Macielak et al., 2019; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021; Seelhammer et al., 2021) reported the ECMO duration, five of which (Pieri et al., 2013; Hamzah et al., 2020; Kaseer et al., 2020; Schill et al., 2021; Seelhammer et al., 2021) described the ECMO duration between the bivalirudin group and the heparin group via IQR (Figure 5). Six studies (Ranucci et al., 2011; Pieri et al., 2013; Berei et al., 2018; Macielak et al., 2019; Kaseer et al., 2020; Seelhammer et al., 2021) were included in the adult group and three studies (Hamzah et al., 2020; Schill et al., 2021; Seelhammer et al., 2021) were included in the children group. Low heterogeneity was observed in the adult group (I2 = 41%, p = 0.13), therefore a fixed-effects model was used. The results showed that the MD of pooled ECMO duration was not significant between the two groups (I2 = 41%, p = 0.75, MD = -3.19, 95% CI: -23.01–16.63).
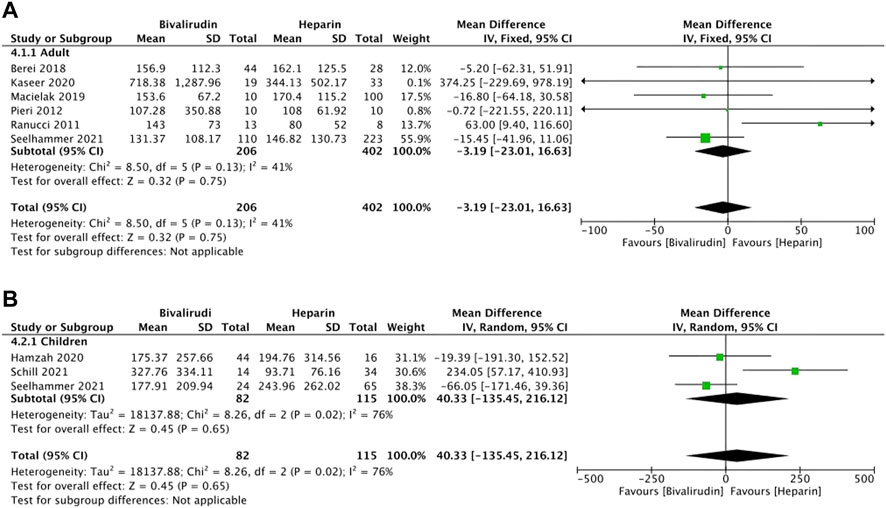
FIGURE 5. The ECMO duration between the bivalirudin group and the heparin group. (A) adult group; (B) children group.
High heterogeneity was observed in the children group (I2 = 76%, p = 0.02), therefore a random-effects model was used. The results showed that the MD of pooled ECMO duration was not significant between the two groups (I2 = 76%, p = 0.65, MD = 40.33, 95% CI: -135.45–216.12).
After removing the study conducted by Schill et al. (2021), the heterogeneity of this outcome decreased significantly (I2 = 0%, p = 0.68), which indicated the main source of heterogeneity. Therefore, a fixed-effects model was used. The results showed that the MD of pooled ECMO duration was not significant between the two groups (I2 = 0%, p = 0.25, MD = -53.30, 95% CI: -143.16–36.56). However, a directional change occurred after removing Schill et al. (2021), indicating that the results of this meta-analysis maybe not that stable, more studies should be included. For adults’ ECMO duration, the sensitivity analysis showed that all studies’ estimate was within 95% CI of the total effect, which means the analytical stability was not affected (Supplementary Figure 4A). For children’s ECMO duration, the sensitivity analysis showed that two studies’ (Schill et al., 2021; Seelhammer et al., 2021) estimate was not within 95% CI of the total effect, which means the analytical stability maybe affected (Supplementary Figure 4B).
Egger’s and Begg’s tests suggested no significant publication bias of the incidence of major bleeding (Egger p = 0.093 and Begg p = 0.368), patient thrombosis (Egger p = 0.116 and Begg p = 0.035), circuit thrombosis (Egger p = 0.503 and Begg p = 0.452), in-hospital mortality (Egger p = 0.551 and Begg p = 0.764), 30-day mortality (Egger p = 0.757 and Begg p = 1), ECMO duration in adults (Egger p = 0.156 and Begg p = 0.133) and children (Egger p = 0.282 and Begg p = 0.296).
Though no large-scale clinical trials have compared the prognosis of anticoagulation therapy with bivalirudin or UFH, bivalirudin has become the first-line anticoagulant therapy strategy for patients with HR, HIT, or those who need surgery. It is widely used in patients undergoing high-risk percutaneous coronary intervention (PCI) and transcatheter aortic valve implantation (TAVI) (Stone et al., 2006; Kastrati et al., 2008; Stone et al., 2008; Han et al., 2015; Ahmad et al., 2017; Villablanca et al., 2017). To our knowledge, this is the first registered meta-analysis exploring the efficacy and safety of bivalirudin versus UFH in anticoagulation therapy in ECMO. The results showed that bivalirudin can significantly reduce the incidence of major bleeding in children, thrombosis in both patients and pumps, and in-hospital mortality. Also, comparable clinical outcomes were observed in the incidence of major bleeding in adults, 30-day mortality, and ECMO duration.
There are great challenges in treating patients receiving ECMO, and finding the balance between anticoagulation therapy and hemorrhagic complications is essential. Major bleeding is one of the most common complications of ECMO, often affecting the mortality of the patients. We found that bivalirudin can reduce the incidence of major bleeding in children, this is the same in Hamzah et al. (Hamzah et al., 2020)’s study. This phenomenon may due to the reason that children’s livers are immature, and their anticoagulant proteins are defective. What is more, children are more prone to develop HR. Hamzah et al. (Hamzah et al., 2020) observed a shorter time to reach treatment anticoagulation levels and fewer bleeding events in the bivalirudin group than that in the UFH group. As an anticoagulant, UFH can stimulate platelet activation in vivo, while bivalirudin can be used as an inhibitor of thrombin-dependent platelet activation and collagen-induced platelet procoagulant activity (Busch et al., 2009; Kimmelstiel et al., 2011). Bivalirudin has better antithrombotic and anticoagulant effects than UFH, with less platelet activation and consumption (Burstein et al., 2019). This may explain children’s lower tendency of major bleeding in the bivalirudin group.
Although low-dose UFH seems to safely reduce the risk of major bleeding and not increase the risk of thrombosis (Carter et al., 2019; Wood et al., 2020), it may not be practical in patients with HIT and HR. As a way to reduce the UFH dose, a heparin-coating circuit can reduce coagulation activation and the inflammatory reaction, protect platelets and coagulation factors, improve biocompatibility, avoid high-dose systemic heparinization, and reduce the dosage of UFH. Nevertheless, studies have reported that the release of UFH from the circuit may also be responsible for HIT, even in small quantities (Pappalardo et al., 2009). In some department protocols, the heparin-coating circuit’s use was continued even after the diagnosis of HIT (Koster et al., 2000). These findings highlight the pitfalls of UFH and the strengths of bivalirudin. The consumption of platelet and thrombin may lead to consumption coagulopathy, which causes intravasular and extravascular thrombosis. Additionally, the complexity of pharmacokinetic parameters may increase due to the increase of volume distribution and random adsorption of drugs on different parts of the pump, which requires continuous dose titration of UFH (Kato et al., 2021). Compared with UFH, bivalirudin has a more predictable pharmacokinetic profile, a greater reduction in thrombin, and no associated incidence of HIT (Netley et al., 2018). HIT can leads to death in some severe cases (Zhong et al., 2020), which greatly affects the in-hospital mortality. This explains the lower in-hospital mortality in the bivalirudin group.
The activated partial thromboplastin time (APTT) value reflects anticoagulation condition: the higher the values, the higher the risk of bleeding. Kaseer et al. found that compared with UFH, the percentage of time that the APTT was within the therapeutic range was higher with bivalirudin (50 vs. 85.7%; p = 0.007), which means that bivalirudin more consistently maintained the APTT within the therapeutic range in comparison to UFH. Bivalirudin appears to be a reliable alternative anticoagulation option in patients with pediatric ECMO who have failed UFH (Cuker et al., 2018). The researchers recommended an initial bivalirudin dose of 2.5 mcg/kg/min for all patients, checking the APTT 2 hours after the initial dose and then every 4 hours after that (Netley et al., 2018). However, the optimal monitoring strategy remains to be explored (Ryerson et al., 2020). To monitor bivalirudin therapy, APTT hepzyme (HPTT), intrinsic coagulation pathways with heparinase (HEPTEM), and measurement of the clotting time is recommended (Teruya et al., 2020).
Economic factors should also be taken into consideration as comparable clinical outcomes of the incidence of major bleeding in adults, 30-day mortality, and ECMO duration in both groups. For patients with acute myocardial infarction, treatment with bivalirudin may be a cost-effective option rather than heparin plus glycoprotein IIb/IIIa inhibitor (Schwenkglenks et al., 2011; Schwenkglenks et al., 2012). This cost-effectiveness may translate into the ECMO population as well. In ECMO anticoagulation therapy, although bivalirudin is much more expensive than UFH (reportedly $1170 per vial) (Kaseer et al., 2020), the total cost might be lower due to less frequent monitoring, platelet transfusion, etc (Hamzah et al., 2020; Kaseer et al., 2020). Furthermore, Ranucci et al. also reported that the bivalirudin group lost less blood (p = 0.015), and therefore required fewer platelet concentrates (p = 0.008), fresh frozen plasma (p = 0.02), and purified antithrombin (p = 0.048). Thus, the daily cost of ECMO was significantly lower in the patients in the bivalirudin group (Ranucci et al., 2011).
Our study indicated that bivalirudin may provide superior anticoagulation therapy in ECMO compared to that of UFH. For the incidence of major bleeding and thrombosis, patients who received bivalirudin or any other DTI may have done so because of the HIT potential, a hypercoagulable syndrome already predisposing patients to worse outcomes, especially regarding potential thrombotic sequela. For mortality, the underlying cause of patients requiring ECMO is likely the major determinant of outcomes and is already associated with an extremely high risk of adverse events. For the ECMO duration, weaning from ECMO differs between centers, and there is no specific information about standardized weaning protocols (Lüsebrink et al., 2020), more studies are truly requested. From our perspective, to rationally use bivalirudin in ECMO, the baseline APTT value, the presence of renal and/or liver insufficiency, the use of other drugs (e.g., argatroban) (Geli et al., 2021), the possibility of bivalirudin resistance, and the methods of operation should all be taken into consideration.
Compared with the former systematic review (Sanfilippo et al., 2017), our study introduced new clinical studies and expanded the sample size, and we conducted the first meta-analysis. However, there are still some limitations in our study. Initially, the studies included were retrospective small-size studies, which means the argumentation intensity may not be strong enough, and only a hypothesis can be generated. We hope that there will be more large-scale RCTs in the future. Secondly, though sensitivity and subgroup analyses were performed, the patients’ variable character may lead to heterogeneity, which may affect the stability of the results. Future research is essential to ensure the homogeneity of the population as much as possible. Finally, the lack of specific results or available research data may restrict our subgroup analysis, such as the use of bivalirudin versus UFH in a different type of ECMOs (VV or VA), and different indications (STEMI, ARDS, heart transplantation, and so forth); these can be further investigated in future studies and enrolled in the sensitivity analysis. Despite these limitations, our meta-analysis provides valuable insight into the use of bivalirudin in the anticoagulation therapy of ECMO.
Bivalirudin can significantly reduce the incidence of major bleeding in children, patient thrombosis, in-circuit thrombosis/interventions, and in-hospital mortality. Though comparable clinical outcomes were observed in the incidence of major bleeding in adults, 30-day mortality, and ECMO duration, the incidence of the aforementioned complications is seemingly lower in the bivalirudin group. Compared with UFH, bivalirudin is safer, more practical, and dependable, which can be a safe and feasible alternative anticoagulant option to UFH as anticoagulation therapy in ECMO, especially for patients at risk for HR and HIT.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
MM and SL contributed equally to this work. MM, SL, JZ, ZJ, MD, HH, and YH conceived the study, participated in the design, collected the data, performed statistical analyses and drafted the manuscript. SL and JZ performed statistical analyses and helped to draft the manuscript. SL and JZ collected the data and revised the manuscript critically for important intellectual content. ZJ and MD helped for the language editing and data revision. MM, HH, and YH helped to revise the manuscript critically for important intellectual content. All authors read and approved the final manuscript.
This work was supported by the Applied and fundamental study of Sichuan Province (No.2017JY0026), the Fellowship of China Postdoctoral Science Foundation (No. 2020M683325), the Post-Doctor Research Project, West China Hospital, Sichuan University (No.2020HXBH048), and the Innovative scientific research project of medical youth in Sichuan Province (No.Q20061).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.771563/full#supplementary-material
Ahmad, A. H., Dali, A. F., Mat Nuri, T. H., Saleh, M. S., Ajmi, N. N., Neoh, C. F., et al. (2017). Safety and Effectiveness of Bivalirudin in Patients Undergoing Percutaneous Coronary Intervention: A Systematic Review and Meta-Analysis. Front. Pharmacol. 8, 410. doi:10.3389/fphar.2017.00410
Berei, T. J., Lillyblad, M. P., Wilson, K. J., Garberich, R. F., and Hryniewicz, K. M. (2018). Evaluation of Systemic Heparin versus Bivalirudin in Adult Patients Supported by Extracorporeal Membrane Oxygenation. ASAIO J. 64 (5), 623–629. doi:10.1097/MAT.0000000000000691
Brown, M. A., Najam, F., Pocock, E. S., Munoz, P. F., Farrar, K. A., and Yamane, D. P. (2020). A Comparison of Bivalirudin and Heparin for Patients on Extracorporeal Membrane Oxygenation. Thromb. Res. 190, 76–78. doi:10.1016/j.thromres.2020.04.009
Burstein, B., Wieruszewski, P. M., Zhao, Y. J., and Smischney, N. (2019). Anticoagulation with Direct Thrombin Inhibitors during Extracorporeal Membrane Oxygenation. World J. Crit. Care Med. 8 (6), 87–98. doi:10.5492/wjccm.v8.i6.87
Busch, G., Steppich, B., Sibbing, D., Braun, S. L., Stein, A., Groha, P., et al. (2009). Bivalirudin Reduces Platelet and Monocyte Activation after Elective Percutaneous Coronary Intervention. Thromb. Haemost. 101 (2), 340–344. doi:10.1160/th08-09-0582
Carter, K. T., Kutcher, M. E., Shake, J. G., Panos, A. L., Cochran, R. P., Creswell, L. L., et al. (2019). Heparin-Sparing Anticoagulation Strategies Are Viable Options for Patients on Veno-Venous ECMO. J. Surg. Res. 243, 399–409. doi:10.1016/j.jss.2019.05.050
Cuker, A., Arepally, G. M., Chong, B. H., Cines, D. B., Greinacher, A., Gruel, Y., et al. (2018). American Society of Hematology 2018 Guidelines for Management of Venous Thromboembolism: Heparin-Induced Thrombocytopenia. Blood Adv. 2 (22), 3360–3392. doi:10.1182/bloodadvances.2018024489
Finley, A., and Greenberg, C. (2013). Review Article: Heparin Sensitivity and Resistance: Management during Cardiopulmonary Bypass. Anesth. Analg 116 (6), 1210–1222. doi:10.1213/ANE.0b013e31827e4e62
Geli, J., Capoccia, M., Maybauer, D. M., and Maybauer, M. O. (2021). Argatroban Anticoagulation for Adult Extracorporeal Membrane Oxygenation: A Systematic Review. J. Intensive Care Med. 37, 459–471. doi:10.1177/0885066621993739
Greinacher, A., Koster, A., and Warkentin, T. (2008). Bivalirudin. Thromb. Haemost. 99 (5), 830–839. doi:10.1160/TH07-10-0644
Hamzah, M., Jarden, A. M., Ezetendu, C., and Stewart, R. (2020). Evaluation of Bivalirudin as an Alternative to Heparin for Systemic Anticoagulation in Pediatric Extracorporeal Membrane Oxygenation. Pediatr. Crit. Care Med. 21 (9), 827–834. doi:10.1097/PCC.0000000000002384
Han, Y., Guo, J., Zheng, Y., Zang, H., Su, X., Wang, Y., et al. BRIGHT Investigators(2015). Bivalirudin vs Heparin with or without Tirofiban during Primary Percutaneous Coronary Intervention in Acute Myocardial Infarction: the BRIGHT Randomized Clinical Trial. JAMA 313 (13), 1336–1346. doi:10.1001/jama.2015.2323
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Karagiannidis, C., Brodie, D., Strassmann, S., Stoelben, E., Philipp, A., Bein, T., et al. (2016). Extracorporeal Membrane Oxygenation: Evolving Epidemiology and Mortality. Intensive Care Med. 42 (5), 889–896. doi:10.1007/s00134-016-4273-z
Kaseer, H., Soto-Arenall, M., Sanghavi, D., Moss, J., Ratzlaff, R., Pham, S., et al. (2020). Heparin vs Bivalirudin Anticoagulation for Extracorporeal Membrane Oxygenation. J. Card. Surg. 35 (4), 779–786. doi:10.1111/jocs.14458
Kastrati, A., Neumann, F. J., Mehilli, J., Byrne, R. A., Iijima, R., Büttner, H. J., et al. ISAR-REACT 3 Trial Investigators (2008). Bivalirudin versus Unfractionated Heparin during Percutaneous Coronary Intervention. N. Engl. J. Med. 359 (7), 688–696. doi:10.1056/NEJMoa0802944
Kato, C., Oakes, M., Kim, M., Desai, A., Olson, S. R., Raghunathan, V., et al. (2021). Anticoagulation Strategies in Extracorporeal Circulatory Devices in Adult Populations. Eur. J. Haematol. 106 (1), 19–31. doi:10.1111/ejh.13520
Kimmelstiel, C., Zhang, P., Kapur, N. K., Weintraub, A., Krishnamurthy, B., Castaneda, V., et al. (2011). Bivalirudin Is a Dual Inhibitor of Thrombin and Collagen-dependent Platelet Activation in Patients Undergoing Percutaneous Coronary Intervention. Circ. Cardiovasc. Interv. 4 (2), 171–179. doi:10.1161/CIRCINTERVENTIONS.110.959098
Koster, A., Sänger, S., Hansen, R., Sodian, R., Mertzlufft, F., Harke, C., et al. (2000). Prevalence and Persistence of Heparin/platelet Factor 4 Antibodies in Patients with Heparin Coated and Noncoated Ventricular Assist Devices. ASAIO J. 46 (3), 319–322. doi:10.1097/00002480-200005000-00015
Koster, A., Zittermann, A., and Schirmer, U. (2013). Heparin Resistance and Excessive Thrombocytosis. Anesth. Analg 117 (5), 1262. doi:10.1213/ANE.0b013e3182a5392f
Ljajikj, E., Zittermann, A., Morshuis, M., Börgermann, J., Ruiz-Cano, M., Schoenbrodt, M., et al. (2017). Erratum to: "Bivalirudin Anticoagulation for Left Ventricular Assist Device Implantation on an Extracorporeal Life Support System in Patients with Heparin-Induced Thrombocytopenia antibodies" [Interact CardioVasc Thorac Surg 2017:1-7; doi:10.1093/icvts/ivx251]. Interact Cardiovasc. Thorac. Surg. 25 (6), 675–904. doi:10.1093/icvts/ivx25110.1093/icvts/ivx300
Luo, D., Wan, X., Liu, J., and Tong, T. (2018). Optimally Estimating the Sample Mean from the Sample Size, Median, Mid-range, And/or Mid-quartile Range. Stat. Methods Med. Res. 27 (6), 1785–1805. doi:10.1177/0962280216669183
Lüsebrink, E., Stremmel, C., Stark, K., Joskowiak, D., Czermak, T., Born, F., et al. (2020). Update on Weaning from Veno-Arterial Extracorporeal Membrane Oxygenation. J. Clin. Med. 9 (4), 992. doi:10.3390/jcm9040992
Macielak, S., Burcham, P., Whitson, B., Abdel-Rasoul, M., and Rozycki, A. (2019). Impact of Anticoagulation Strategy and Agents on Extracorporeal Membrane Oxygenation Therapy. Perfusion 34 (8), 671–678. doi:10.1177/0267659119842809
Netley, J., Roy, J., Greenlee, J., Hart, S., Todt, M., and Statz, B. (2018). Bivalirudin Anticoagulation Dosing Protocol for Extracorporeal Membrane Oxygenation: A Retrospective Review. J. Extra Corpor Technol. 50 (3), 161–166.
Ortel, T. L. (2009). Heparin-induced Thrombocytopenia: when a Low Platelet Count Is a Mandate for Anticoagulation. Hematol. Am Soc Hematol Educ Program, 225–232. doi:10.1182/asheducation-2009.1.225
Pappalardo, F., Maj, G., Scandroglio, A., Sampietro, F., Zangrillo, A., and Koster, A. (2009). Bioline Heparin-Coated ECMO with Bivalirudin Anticoagulation in a Patient with Acute Heparin-Induced Thrombocytopenia: the Immune Reaction Appeared to Continue Unabated. Perfusion 24 (2), 135–137. doi:10.1177/02610.1177/0267659109106773
Pieri, M., Agracheva, N., Bonaveglio, E., Greco, T., De Bonis, M., Covello, R. D., et al. (2013). Bivalirudin versus Heparin as an Anticoagulant during Extracorporeal Membrane Oxygenation: a Case-Control Study. J. Cardiothorac. Vasc. Anesth. 27 (1), 30–34. doi:10.1053/j.jvca.2012.07.019
Ranucci, M., Ballotta, A., Kandil, H., Isgrò, G., Carlucci, C., Baryshnikova, E., et al. Surgical and Clinical Outcome Research Group (2011). Bivalirudin-based versus Conventional Heparin Anticoagulation for Postcardiotomy Extracorporeal Membrane Oxygenation. Crit. Care 15 (6), R275. doi:10.1186/cc10556
Ryerson, L. M., Balutis, K. R., Granoski, D. A., Nelson, L. R., Massicotte, M. P., Lequier, L. L., et al. (2020). Prospective Exploratory Experience with Bivalirudin Anticoagulation in Pediatric Extracorporeal Membrane Oxygenation. Pediatr. Crit. Care Med. 21 (11), 975–985. doi:10.1097/PCC.0000000000002527
Sanfilippo, F., Asmussen, S., Maybauer, D. M., Santonocito, C., Fraser, J. F., Erdoes, G., et al. (2017). Bivalirudin for Alternative Anticoagulation in Extracorporeal Membrane Oxygenation: A Systematic Review. J. Intensive Care Med. 32 (5), 312–319. doi:10.1177/0885066616656333
Schill, M. R., Douds, M. T., Burns, E. L., Lahart, M. A., Said, A. S., and Abarbanell, A. M. (2021). Is Anticoagulation with Bivalirudin Comparable to Heparin for Pediatric Extracorporeal Life Support? Results from a High‐volume center. Artif. Organs 45 (1), 15–21. doi:10.1111/aor.13758
Schwenkglenks, M., Brazier, J. E., Szucs, T. D., and Fox, K. A. (2011). Cost-effectiveness of Bivalirudin versus Heparin Plus Glycoprotein IIb/IIIa Inhibitor in the Treatment of Non-ST-segment Elevation Acute Coronary Syndromes. Value Health 14 (1), 24–33. doi:10.1016/j.jval.2010.10.025
Schwenkglenks, M., Toward, T. J., Plent, S., Szucs, T. D., Blackman, D. J., and Baumbach, A. (2012). Cost-effectiveness of Bivalirudin versus Heparin Plus Glycoprotein IIb/IIIa Inhibitor in the Treatment of Acute ST-Segment Elevation Myocardial Infarction. Heart 98 (7), 544–551. doi:10.1136/heartjnl-2011-301323
Seelhammer, T. G., Bohman, J. K., Schulte, P. J., Hanson, A. C., and Aganga, D. O. (2021). Comparison of Bivalirudin versus Heparin for Maintenance Systemic Anticoagulation during Adult and Pediatric Extracorporeal Membrane Oxygenation. Crit. Care Med. 49 (9), 1481–1492. doi:10.1097/CCM.0000000000005033
Shaefi, S., Brenner, S. K., Gupta, S., O'Gara, B. P., Krajewski, M. L., Charytan, D. M., et al. (2021). Extracorporeal Membrane Oxygenation in Patients with Severe Respiratory Failure from COVID-19. Intensive Care Med. 47 (2), 208–221. doi:10.1007/s00134-020-06331-9
Shamseer, L., Moher, D., Clarke, M., Ghersi, D., Liberati, A., Petticrew, M., et al. PRISMA-P Group (2015). Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. Bmj 349–g7647. doi:10.1136/bmj.g7647
Stone, G. W., McLaurin, B. T., Cox, D. A., Bertrand, M. E., Lincoff, A. M., Moses, J. W., et al. (2006). Bivalirudin for Patients with Acute Coronary Syndromes. N. Engl. J. Med. 355 (21), 2203–2216. doi:10.1056/NEJMoa062437
Stone, G. W., Witzenbichler, B., Guagliumi, G., Peruga, J. Z., Brodie, B. R., Dudek, D., et al. HORIZONS-AMI Trial Investigators (2008). Bivalirudin during Primary PCI in Acute Myocardial Infarction. N. Engl. J. Med. 358 (21), 2218–2230. doi:10.1056/NEJMoa0708191
Teruya, J., Hensch, L., Bruzdoski, K., Adachi, I., Hui, S. R., and Kostousov, V. (2020). Monitoring Bivalirudin Therapy in Children on Extracorporeal Circulatory Support Devices: Thromboelastometry versus Routine Coagulation Testing. Thromb. Res. 186, 54–57. doi:10.1016/j.thromres.2019.12.007
Villablanca, P. A., Al-Bawardy, R., Mohananey, D., Maraboto, C., Weinreich, M., Gupta, T., et al. (2017). Bivalirudin versus Heparin in Patients Undergoing Percutaneous Transcatheter Aortic Valve Interventions: A Systematic Review and Meta-Analysis. J. Interv. Cardiol. 30 (6), 586–594. doi:10.1111/joic.12428
Wan, X., Wang, W., Liu, J., and Tong, T. (2014). Estimating the Sample Mean and Standard Deviation from the Sample Size, Median, Range And/or Interquartile Range. BMC Med. Res. Methodol. 14, 135. doi:10.1186/1471-2288-14-135
Wood, K. L., Ayers, B., Gosev, I., Kumar, N., Melvin, A. L., Barrus, B., et al. (2020). Venoarterial-Extracorporeal Membrane Oxygenation without Routine Systemic Anticoagulation Decreases Adverse Events. Ann. Thorac. Surg. 109 (5), 1458–1466. doi:10.1016/j.athoracsur.2019.08.040
Zhong, H., Zhu, M. L., Yu, Y. T., Li, W., Xing, S. P., Zhao, X. Y., et al. (2020). Management of Bivalirudin Anticoagulation Therapy for Extracorporeal Membrane Oxygenation in Heparin-Induced Thrombocytopenia: A Case Report and a Systematic Review. Front. Pharmacol. 11, 565013. doi:10.3389/fphar.2020.5610.3389/fphar.2020.565013
Keywords: extracorporeal membrane oxygenation, heparin, bivalirudin, meta-analysis, systematic review
Citation: Ma M, Liang S, Zhu J, Dai M, Jia Z, Huang H and He Y (2022) The Efficacy and Safety of Bivalirudin Versus Heparin in the Anticoagulation Therapy of Extracorporeal Membrane Oxygenation: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:771563. doi: 10.3389/fphar.2022.771563
Received: 01 October 2021; Accepted: 29 March 2022;
Published: 14 April 2022.
Edited by:
Annalisa Capuano, University of Campania Luigi Vanvitelli, ItalyCopyright © 2022 Ma, Liang, Zhu, Dai, Jia, Huang and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: He Huang, aHVhbmdoZUB3Y2hzY3UuY24=; Yong He, aGV5b25nX2h1YXhpQDE2My5jb20=
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.