- 1Department of Interventional Radiology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 2Cancer Centre, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
- 3General ICU, The First Affiliated Hospital of Zhengzhou University, Henan Key Laboratory of Critical Care Medicine, Zhengzhou, China
- 4Internet Medical and System Applications of National Engineering Laboratory, Zhengzhou, China
- 5Integrated Traditional and Western Medicine, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, China
Aim: To explore the relationship between the use of aspirin and the incidence of hepatocellular carcinoma (HCC).
Methods: MEDLINE, EMBASE, Web of Science and Cochrane CENTRAL databases were searched systematically from the earliest available date to 13 March 2020. The primary outcome was incidence of HCC, and the secondary outcomes were recurrence and mortality of HCC. The results were expressed as the Hazard Ratio (HR) and 95% confidence interval (CI). Based on the heterogeneity evaluated with the I2 statistic, a meta-analysis was performed using either a random- or fixed-effects model.
Results: A total of sixteen articles (2781100 participants) were included. There was lower incidence of HCC in aspirin users than those in non-aspirin users (HR, 0.56; 95% CI, 0.46-0.69; p < 0.001). Subgroup analysis further showed that the incidence of liver cancer in patients with alcoholic cirrhosis (HR, 0.14; 95% CI, 0.09-0.22; p < 0.001) and virus hepatitis (HR, 0.68; 95% CI, 0.62-0.74; p < 0.001) who use aspirin was lower than that of patients who do not use aspirin. In addition, aspirin was found to associate with decreased risk of HCC mortality (HR, 0.71; 95% CI, 0.65-0.78; p < 0.001), not HCC recurrence (HR, 0.52; 95% CI, 0.15-1.76; p = 0.291).
Conclusions: Aspirin use is significantly associated with the low incidence rate of liver cancer.
1 Introduction
Hepatocellular carcinoma (HCC) accounts for a large proportion of cancer deaths worldwide ((Torre et al., 2015; Bray et al., 2018)), and the incidence of HCC is predicted to increase in the future ((Torre et al., 2015; Bray et al., 2018)). HCC can grow at an exponential rate; its recurrence can occur after a therapy and its subsequent metastasis can lead to mortality, making it the second cause of death of cancer patients ((Torre et al., 2015; Bray et al., 2018)). The current diagnosis of HCC remains ineffective; thus, it is important that preventive methods are developed ((Torre et al., 2015; Bray et al., 2018)). The main risk factors for HCC are chronic hepatitis and virus infection, in particular hepatitis B virus (HBV) and hepatitis C virus (HCV), and long-term drinking ((Chen et al., 1991; Degos et al., 2000; Yeh et al., 2010)). Several studies have suggested that chronic hepatitis inflammation could induce HCC((Raza et al., 2011; Hossain et al., 2012; Li et al., 2013a)), especially occurring through the cyclooxygenase-2 (COX-2) pathway ((Cheng et al., 2004)). Thus, modulation of the inflammatory pathways may become a novel method that can restrict HCC development.
Aspirin is a COX inhibitor frequently used to reduce the risk of cardiovascular and cerebrovascular disease-related death, owing to its antiplatelet effect. Moreover, aspirin has been shown to play a role in preventing lung cancer, colorectal cancer and prostate cancer ((Cao et al., 2016; Lapi et al., 2016; Ye et al., 2019)). It has been also shown to have a beneficial effect in liver, such as lowering the risk of hepatic inflammation, fibrosis, and HCC, according to the data from chronic hepatitis animal model ((Cao et al., 2016; Lapi et al., 2016; Ye et al., 2019)). The mechanisms underlying the chemopreventive effect of aspirin, however, remain unknown. Recent studies have explored the associations between HCC and the chemopreventive effect of aspirin that is associated with chronic inflammation. Beside the inflammatory process ((Sitia et al., 2012; Carrat 2014)), aspirin has been suggested to modulate immune response of liver and promote the liver injury mediated by immune and carcinogenesis ((Semple et al., 2011)). This information demonstrates that aspirin plays roles in pathogenesis of HCC.
Given the evidence of aspirin’s chemopreventive effect, it may potentially be prevent HCC. Recent articles have indicated that due to anti-inflammation and immune modulation effect of aspirin ((Sahasrabuddhe et al., 2012; Petrick et al., 2015; Hwang et al., 2018)), number of HCC incidence in patients taking aspirin is lower compared with that in patients without aspirin. Conversely, several studies have reported that use of aspirin has no notable effect on the incidence of HCC ((Sahasrabuddhe et al., 2012; Petrick et al., 2015; Hwang et al., 2018)). Given such equivocal information, it is necessary to compile and evaluate data from available articles to determine whether aspirin is beneficial for preventing the incidence of HCC.
2 Methods
2.1 Search Strategy
The meta-analysis was performed based on the Meta-analysis of Observational Studies in Epidemiology guidelines and the protocol of this study in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) checklist ((Stroup et al., 2000)). And the PRISMA checklist is shown in Supplementary Table S1. Articles written in English language published from the earliest possible date to 13 March 2020 in the MEDLINE, EMBASE, Web of Science and Cochrane CENTRAL databases and were searched using a combination of MeSH/Emtree and title/abstract keywords. The keywords were “Acetylsalicylic acid,” “Aspirin,” “Hepatocellular Carcinoma,” “Liver cancer,” “Hepatic cellular cancer,” and “HCC”. Supplementary Table S2 showed the detailed search strategy.
2.2 Inclusion and Exclusion Criteria
2.2.1 Incidence
The inclusion criteria of this study are as follows (Torre et al., 2015): they enrolled patients using aspirin and non-aspirin for prevention or treatment (Bray et al., 2018); they counted the HCC incidence of aspirin and non-aspirin users (McGlynn and London 2011); they were adults patients (age ≥18 years) (Altekruse et al., 2012); they were observational studies or clinical trials (Villanueva 2019); they were written in English. The articles were excluded when (Torre et al., 2015): These studies lacked the results of the correlation between the use of aspirin and HCC incidence rate and hazard ratio (HR), relative risk, odd ratio (OR) and 95% confidence interval (CI), or did not provide raw data (Bray et al., 2018).; they were reviews, commentaries, editorials, conference abstracts, or animal studies (McGlynn and London 2011); they assessed the impact of aspirin combined with other NSAIDs on the incidence of HCC.
2.2.2 Recurrence and Mortality
The inclusion criteria of this study are as follows (Torre et al., 2015): they enrolled HCC patients who used aspirin and non-aspirin (Bray et al., 2018); they counted the HCC recurrence, mortality of patients using aspirin and non-aspirin (McGlynn and London 2011); they were adults patients (age ≥18 years) (Altekruse et al., 2012); they were observational studies or clinical trials (Villanueva 2019); they were written in English. The articles were excluded when (Torre et al., 2015): the studies lacked outcome data for correlation between the use of aspirin and HCC recurrence, mortality with hazard ratio (HR), relative risk, or odd ratio(OR) value and 95% confidence interval (CI), or not provide the raw data as we can calculate out the result (Bray et al., 2018); they were reviews, commentaries, editorials, conference abstracts, or animal studies (McGlynn and London 2011); they assessed the effect of aspirin in combination with other NSAIDs on the recurrence, mortality of HCC.
2.3 Inclusion of Studies and Data Extraction
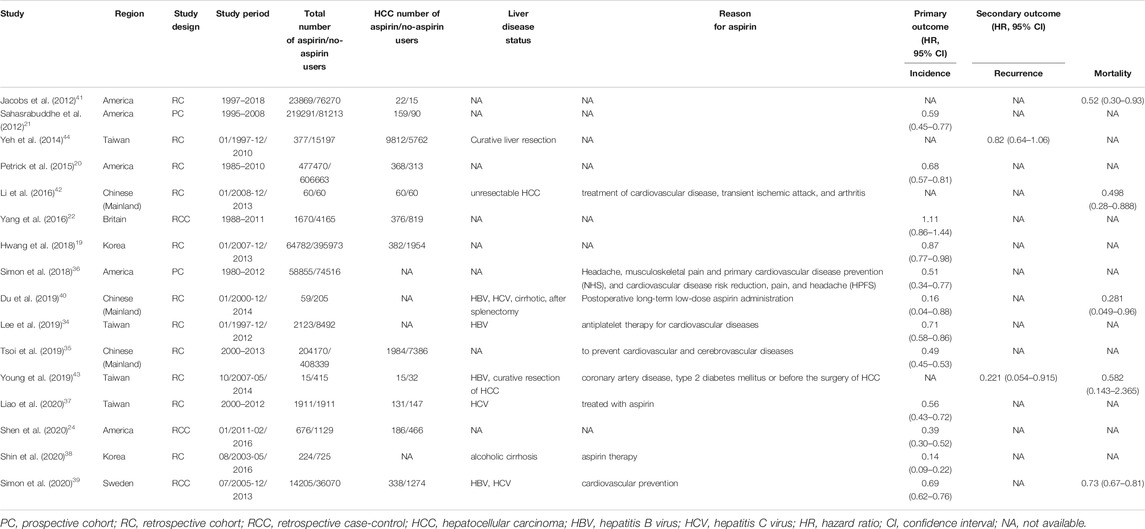
These articles were first extracted based on the inclusion criteria by two investigators; after that, the differences between them were determined by another investigator. The articles that were included into this study contain the following information: first author’s name, year of publication, area where the study was conducted, study design, study period, total number of aspirin/no-aspirin users, HCC number of aspirin/no-aspirin users, liver disease status, primary and secondary outcomes, definition of aspirin user, aspirin dose, adjusted variables. We show these in Table 1 and Table 2. Aspirin users was defined as people who had used aspirin before or after HCC, and the specific information was shown in Table 2. If available, the HR and its related 95% CI were extracted directly from the original article. If not, the HR and 95% CI were calculated according to the raw data in the study. In addition, several case-control studies of this meta-analysis only provide the odd ratio not HR ((Yang et al., 2016; Shen et al., 2020)), so we treated the odd ratio is approximately equivalent to the HR value and then to pooled together because the incidence of HCC is far below 5% (Akinyemiju et al., 2017), namely if research results are rare in all subjects and subgroups, people can usually ignore the differences between various relative risk measures (such as odds ratio, ratio and risk ratio) (Greenland 1987; Siristatidis et al., 2013; Grant 2014).
2.4 Assessment of Risk of Bias
We included thirteen cohort studies and three case-control studies in this meta-analysis. The Newcastle Ottawa scale (NOS) was used to assess the risk of bias for each outcome in all included studies ((BS et al., 2021)). According to the study population selection, comparability, and adequacy of the outcome data, a total of nine points were obtained articles with seven to nine points are considered high-quality articles ((Yang et al., 2016; Shen et al., 2020)) Publication bias was assessed for the primary outcome only. The result of the NOS was shown in Supplementary Table S3.
2.5 Primary and Secondary Outcomes
The main study outcome of this meta-analysis was incidence of HCC, and the secondary outcomes were recurrence and mortality of HCC.
2.6 Statistical Analysis
Heterogeneity between articles was presented as HRs and 95% CIs, and were determined using a random or fixed effect model. Heterogeneity was further assessed by Chi2 and I2 test, in which the percentage of variability was determined (not sampling error) (Higgins and Thompson 2002; Higgins et al., 2003). Articles with p values <0.10 or I2 value >50% was considered having substantial heterogeneity. If there is heterogeneity, subgroup analysis will be conducted to explore the potential sources of heterogeneity and consider whether the random effects model can be used for meta-analysis. In addition, Begg funnel plot and Egger’s linear regression were used to evaluate the potential publication bias of the main results of the included studies ((Begg and Mazumdar 1994; Stuck et al., 1998)). Dissymmetry was evaluated visually by Funnel plots. For Egger’s tests, p < 0.1 indicated a significantly small study size. The robustness of the results for primary outcome was evaluated by one-way sensitivity analysis. All statistical analyses were conducted using STATA 14.0 (College Station, Texas 77845 United States, Serial number: 401406267051). Differences for which p < 0.05 (two-sided) were considered statistically significant.
3 Results
3.1 Included Studies
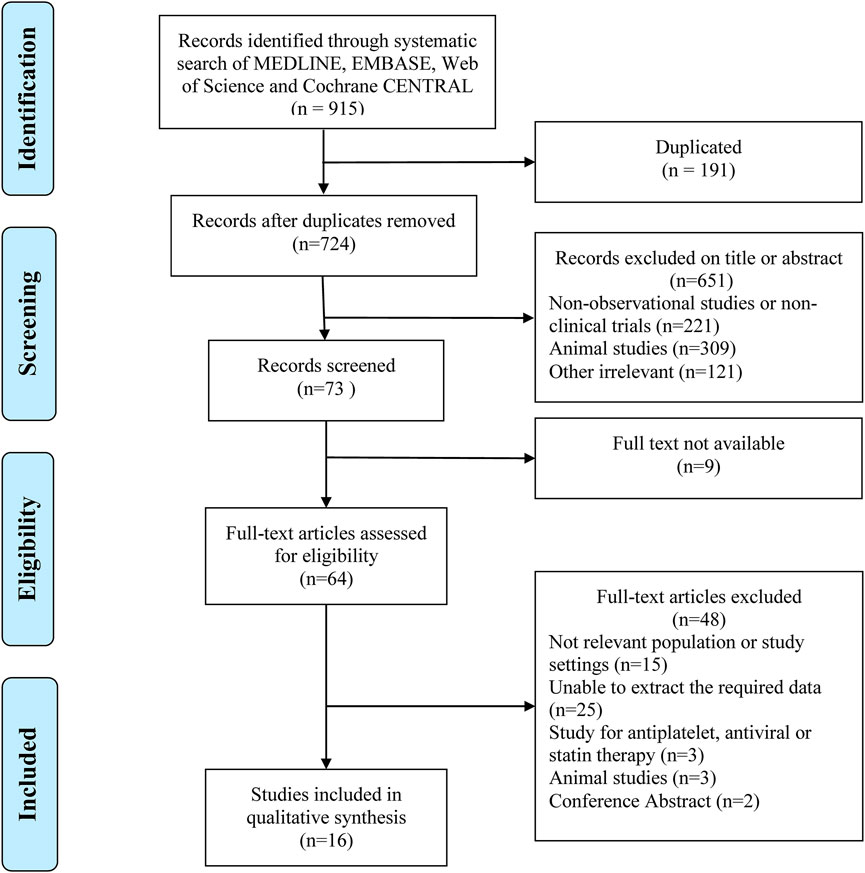
A total of 915 relevant articles were searched, among which 191 articles were duplicates and were removed. After the initial search, 64 articles that discuss problems related to the main goal of this study were selected. Finally, 16 articles ((Sahasrabuddhe et al., 2012; Petrick et al., 2015; Yang et al., 2016; Hwang et al., 2018), (Sahasrabuddhe et al., 2012; Petrick et al., 2015; Yang et al., 2016; Hwang et al., 2018), (Jacobs et al., 2012; Yeh et al., 2015; Li et al., 2016; Simon et al., 2018; Du et al., 2019; Lee et al., 2019; Tsoi et al., 2019; Liao et al., 2020; Shin et al., 2020; Simon et al., 2020; Young et al., 2020)), in which 2781100 patients were involved, and selected (Figure 1).
3.2 Characteristics of Included Studies
The included articles were observational studies that reported the relationship between aspirin and HCC. Among which, twelve articles reported the relationship between aspirin and incidence of HCC((Sahasrabuddhe et al., 2012; Petrick et al., 2015; Yang et al., 2016; Hwang et al., 2018), (Sahasrabuddhe et al., 2012; Petrick et al., 2015; Yang et al., 2016; Hwang et al., 2018), (Simon et al., 2018; Du et al., 2019; Lee et al., 2019; Tsoi et al., 2019; Liao et al., 2020; Shin et al., 2020; Simon et al., 2020)), two articles showed the relation between aspirin and recurrence of HCC((Yeh et al., 2015; Young et al., 2020)), and five articles indicated the connection between aspirin and mortality of HCC((Jacobs et al., 2012; Li et al., 2016; Du et al., 2019; Simon et al., 2020; Young et al., 2020)). All the included articles gained NOS of greater than or equal to 7 points, suggesting that there is a low risk of bias. The characteristics of the included articles are shown as Table 1 and Table 2.
3.3 Primary and Secondary Outcome
A random-effects model was used to perform in this meta-analysis for incidence and recurrence due to substantial heterogeneity (I2 = 92.5%, 68.7%, respectively), and fixed-effects model was used to perform in this meta-analysis for mortality due to mild heterogeneity (I2 = 26.4%) between studies. The pooled results from these studies showed that aspirin is associated with lower incidence of HCC (HR, 0.56; 95% CI, 0.46-0.69, p < 0.001; I2 = 92.5%; Figure 2). These articles suggest that aspirin is associated with a lower risk of HCC mortality (HR, 0.71; 95% CI, 0.65-0.78; p < 0.001; I2 = 26.4%; Figure 3), not HCC recurrence (HR, 0.52; 95% CI, 0.15-1.76; p = 0.291; I2 = 68.7%; Figure 4).
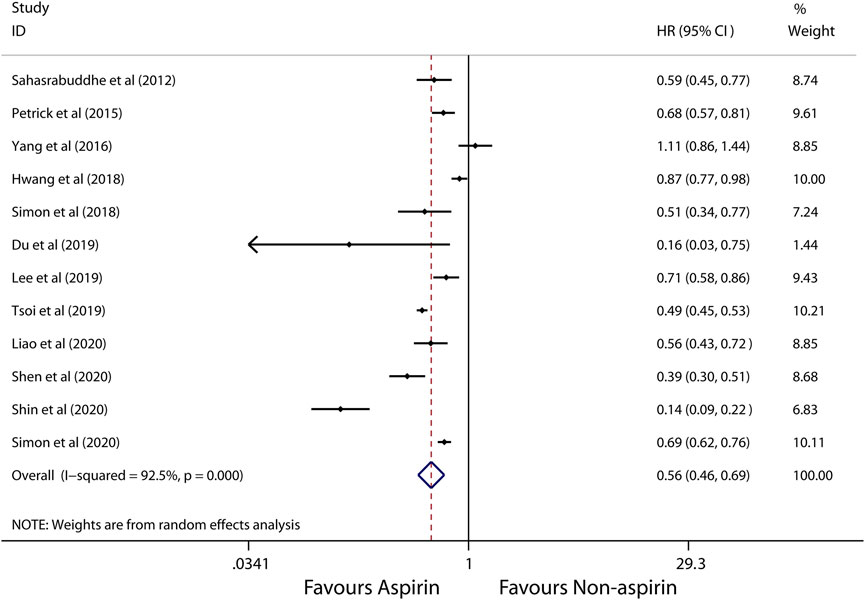
FIGURE 2. Meta-analysis of overall pooled HRs with 95% CIs across studies for primary outcomes. Forest plot showing the significance of the relationship between aspirin use and incidence risk of HCC according to the random-effects model.
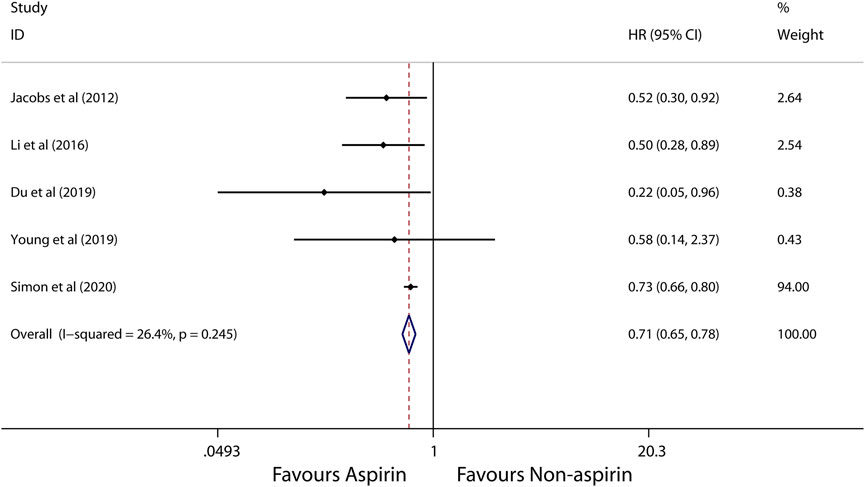
FIGURE 3. Meta-analysis of overall pooled HRs with 95% CIs across studies for secondary outcomes. Forest plot showing the significance of the relationship between aspirin use and mortality of HCC according to the fixed-effects model.
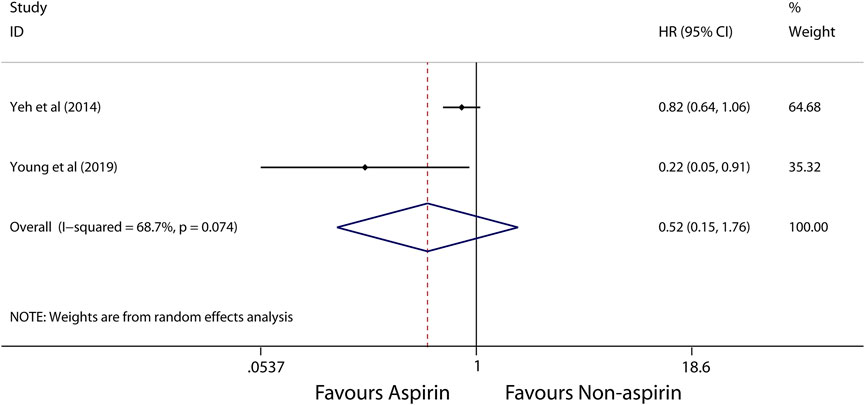
FIGURE 4. Meta-analysis of overall pooled HRs with 95% CIs across studies for secondary outcomes. Forest plot showing the significance of the relationship between aspirin use and recurrence of HCC according to the random-effects model.
3.4 Subgroup Analysis
Subgroup analysis further showed that use of aspirin is linked to a lower incidence of HCC in patients with alcoholic cirrhosis (HR, 0.14; 95% CI, 0.09-0.22; p < 0.001; I2 = 0%; Figure 5), virus hepatitis (HR, 0.68; 95% CI, 0.62-0.74; p < 0.001; I2 = 48.1%; Figure 6). Obviously, aspirin is associated with a lower incidence rate of liver cancer in alcoholic cirrhosis.
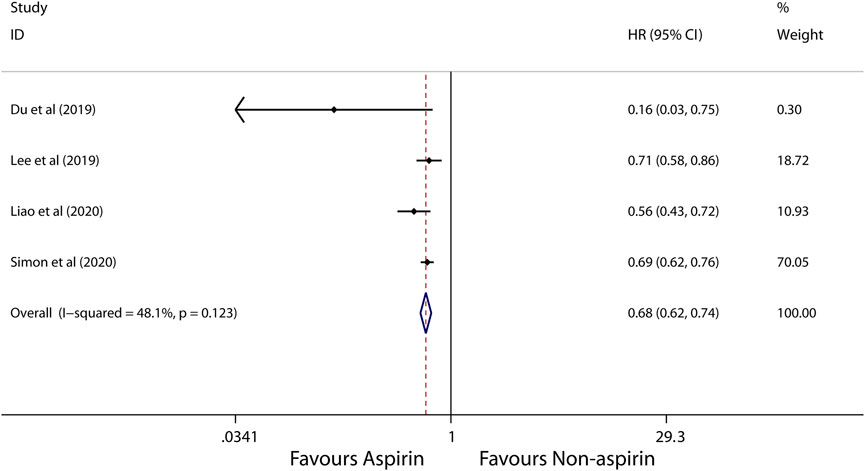
FIGURE 5. Meta-analysis of overall pooled HRs with 95% CIs across studies for primary outcomes in subgroup analyses. Forest plot showing the significance of the relationship between aspirin use and incidence risk of HCC in alcoholic cirrhosis patients according to the fixed-effects model.
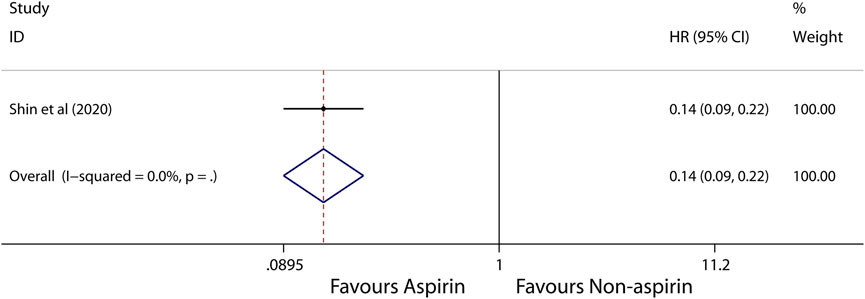
FIGURE 6. Meta-analysis of overall pooled HRs with 95% CIs across studies for primary outcomes in subgroup analyses. Forest plot showing the significance of the relationship between aspirin use and incidence risk of HCC in virus hepatitis patients according to the fixed-effects model.
3.5 Sensitivity Analyses
Since the included studies were observational studies, the risk of bias was low (Supplementary Table S3), we did not conduct a sensitivity analysis of the methodological criteria. So we conduct a sensitivity analysis to evaluate the effect of any one study on the pooled HRs and 95% CIs by removing one individual study at a time. The results showed that the meta-analysis is robust and reliable (Supplementary Figure S1).
3.6 Publication Bias
We employed the funnel plots (Supplementary Figure S2) and the egger’s regression asymmetry tests (Supplementary Figure S3) to evaluate potential publication bias of the included studies. We observed a slight asymmetry in the funnel plots and the Egger’s Publication bias plot. However the results of Egger’s regression asymmetry tests confirmed no publication bias exist in these studies (p = 0.594).
4 Discussion
Evaluation of studies that enrolled 2781100 participants demonstrated that the incidence of HCC was lower in patients with alcoholic cirrhosis and virus hepatitis who use aspirin than that of patients who do not use aspirin, but also the mortality of HCC was lower in aspirin users than those in non-aspirin users. This finding suggests that the use of aspirin was associated with decreased risk of HCC and the risk of HCC mortality. However, clinical trials should be further conducted to confirm this suggestion.
Aspirin is a first-line nonsteroidal anti-inflammatory drug (NSAID), which has been reported to have chemo-protective effects, according to epidemiological researches. Studies ((Gann et al., 1993; Cook et al., 2005; Flossmann and Rothwell 2007; Burn et al., 2011)), in which patients with different genders and are from different regions were enrolled, have indicated the association between aspirin and cancer risk. While some recent studies have reported that NSAID ((Sahasrabuddhe et al., 2012; Petrick et al., 2015)), especially for aspirin, may be able to protect against incidence risk of HCC, other studies have indicated that non-aspirin NSAID could also reduce the number of HCC incidence ((Pang et al., 2017; Tao et al., 2018)). Thus, to further clarify this, we evaluated several studies ((Pang et al., 2017; Tao et al., 2018), (Simon et al., 2018; Liao et al., 2020; Shin et al., 2020), (Oh et al., 2017)), in which aspirin users and non-aspirin users were compared. Our investigation suggested that aspirin was associated with a reduced risk of HCC. And the subgroup analysis result of our meta-analysis also indicated that aspirin use was associated with a reduced incidence of HCC in virus hepatitis and alcoholic cirrhosis patients. Similar effects or mechanisms have also been observed in chronic hepatitis patients ((Maini and Schurich 2012; Sitia et al., 2013)). The presence of hepatitis virus causes CD8+ lymphocytes to secret many inflammatory factors involving in the dealing with infection; inability to timely clear the virus can lead to failure of the liver. Although numerous articles have demonstrated that aspirin users has a significantly lower incidence of HCC compared with non-aspirin users ((Maini and Schurich 2012; Sitia et al., 2013), (Maini and Schurich 2012; Sitia et al., 2013), (Maini and Schurich 2012; Sitia et al., 2013), (Maini and Schurich 2012; Sitia et al., 2013), (Simon et al., 2019), (Lee et al., 2017)), contradictory results have been reported by some studies ((Chiu et al., 2011; Kim et al., 2017), (Chiu et al., 2011; Kim et al., 2017)). Moreover, a meta-analysis that included 5 studies for incidence and 2 studies for mortality reported aspirin use could reduce the incidence risk of HCC((Chiu et al., 2011; Kim et al., 2017)), and also the 2-years and 4-years mortalities in patients with HCC. And the result is consistent with our meta-analysis. However another meta-analysis that included 5 studies reported aspirin use could not reduce the incidence risk of HCC((Chiu et al., 2011; Kim et al., 2017)). In addition, A recent meta-analysis included 8 cohort studies by Wang et al. focused on the dose-response effect of aspirin use and incidence risk of HCC((Chiu et al., 2011; Kim et al., 2017)), and which reported that the higher the aspirin dose, the lower incidence risk of HCC. However, all these three previous meta-analysis may lead to the in-reliable result as their small number of included studies ((Pang et al., 2017; Tao et al., 2018; Wang et al., 2020)). Besides, we also look forward to more studies on the dose dependence or time dependence between aspirin and HCC occur. In all, the majority of evidences shown above indicate that use of aspirin could reduce the incidence risk of HCC, also aspirin use and lower mortality in HCC patients.
Several mechanisms may support the beneficial effects of aspirin on the incidence risk of HCC. Aspirin has a very short half-life in the serum; it selectively inhibits the platelet COX-1. Patients with inflammation-related cancer, including HCC, have increased levels of pro-inflammatory factor, COX-2 enzyme ((Lim et al., 2000; Yip-Schneider et al., 2000; Singh et al., 2005; Chan et al., 2007)). Animal studies have suggested that aspirin at high doses is required to completely inhibit COX-2 ((Kern et al., 2002; Foderà et al., 2004; Chen et al., 2017)). Additionally, higher COX-2 levels can induce the hepatocarcinogenesis-related inflammatory factor cascades, such as protein kinase 3 (PK-3) and nuclear factor κB (NF-κB) pathways ((Iannacone et al., 2005)). Aspirin at high doses could block the NF-κB and PK-3 pathways ((Chan et al., 1998; Leng et al., 2003; Patrono et al., 2005)). The mechanisms in which use of aspirin may benefit HCC patients with HBV are as follows: while platelets accelerate HBV-related liver injury by retaining inflammation (69), aspirin exerts its anti-inflammation by inhibiting the production of thromboxane A2 and relevant platelet activation pathways ((Cattaneo 2004; Aiolfi and Sitia 2015)). Moreover, the use of aspirin antiplatelet therapy reduces the frequency of platelet immune cell interaction and intrahepatic platelet accumulation, thereby limiting the transport of hepatic immune cells, which can prevent the development of non-alcoholic steatohepatitis (NASH) and subsequent HCC((Malehmir et al., 2019)). Other studies have suggested that anti-platelet therapy can diminish not only the intrahepatic HBV-specific CD8 T cells ((Cattaneo 2004; Aiolfi and Sitia 2015)), but also normal inflammatory cells; as a result, HCC is developing in the construction of HBV transgenic animal model. A study of a xenograft nude mouse model have also indicated that use of aspirin could inhibit growth and/or death of HCC tumor ((Cattaneo 2004; Aiolfi and Sitia 2015), (Li et al., 2013b)).
This study has several advantages. First, the total sample sizes are sufficient for this meta-analysis; therefore, the bias from sample sizes can be diminished; Second, it is the first systematic review and meta-analysis to analyses the incidence of HCC in aspirin users in patients with virus hepatitis and alcoholic cirrhosis. Third, the results shown in the NOS quality list showed that this article may have a risk of bias, suggesting the methodology used in the original studies has higher quality; Moreover, the outcome data in the included studies were adjusted into the primary and secondary outcomes; hence, analyses of effects of aspirin on the incidence, and recurrence and mortality of HCC were conducted separately. Furthermore, the synthetic HR and its 95% CI were obtained using the generic inverse variance methods of random-effect models; thus, the obtained HR indicates the sum effect of aspirin on HCC incidence. More importantly, although all the included studies are observational studies, the main confounding variables that impacted the primary results that were adjusted in most of original articles. Finally the sensitivity analysis results suggested that the meta-analysis employed in this study is robust and reliable.
Despite the above strengths, this study has some limitation: First, high heterogeneity was observed in the sum effect of aspirin on the incidence of HCC, suggesting the results presented here may be different from those in the original articles (NOS assessment, however, indicated that the conclusions presented here is reliable); Second, aspirin dose and period of use were varied in the original studies, which may affect the results shown in this study and may be the source of heterogeneity; therefore, further researches are needed; Third, the small included studies for aspirin use and incidence of HCC in alcoholic cirrhosis patients may lead to the in-reliable result. However, it is clear that use of aspirin has a significant reduction in the incidence of HCC in patients with alcoholic cirrhosis and virus hepatitis. Fourth, this study evaluate only the preventive or therapeutic effect of aspirin on HCC patients, not its safety, administration initiation or continuation; Finally, more importantly, although the included articles have a low risk of bias, this study did not include randomized controlled trials, which may be one of the most important disadvantages.
This article presents the first meta-analysis that elaborate on the beneficial effect of aspirin on HCC incidence in patients with or without virus hepatitis and alcoholic cirrhosis. The analysis indicated that aspirin could decrease the incidence risk of HCC including virus hepatitis, especially for patients with alcoholic cirrhosis, and also the mortality of HCC. However, further clinical trials are encouraged to be conducted to support the present results.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author Contributions
Conceptualization, ZF and LL; Data curation, XZ; Formal analysis, XZ; Funding acquisition, ZF and LL; Investigation, LM and YS; Methodology, YS and CL; Project administration, XZ and YS; Software, YZ and LM; Validation, LL; Visualization, LM; Writing–original draft, XD and YZ; Writing–review and editing, XZ.
Funding
This study was supported by the Special Project of Traditional Chinese Medicine Research in Henan Province (Grant No. 20-21ZY2311), Henan Province Medical Science and Technology Research Project Joint Construction Project (Grant No. LHGJ20190003, LHGJ20190055), Young and Middle-aged Health Science and Technology Innovation Talents in 2020 (Grant No. YXKC2020049), and Key scientific research projects of higher education institutions in Henan Province (Grant No. 21A320036).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Chinese Evidence Based Medicine Center, West China Hospital, Sichuan University provided the Stata 14.0 statistical software.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.764854/full#supplementary-material
References
Aiolfi, R., and Sitia, G. (2015). Chronic Hepatitis B: Role of Anti-platelet Therapy in Inflammation Control. Cell Mol Immunol 12 (3), 264–268. doi:10.1038/cmi.2014.124
Akinyemiju, T., Akinyemiju, T., Abera, S., Ahmed, M., Alam, N., Alemayohu, M. A., et al. (2017). The Burden of Primary Liver Cancer and Underlying Etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 3 (12), 1683–1691. doi:10.1001/jamaoncol.2017.3055
Altekruse, S. F., McGlynn, K. A., Dickie, L. A., and Kleiner, D. E. (2012). Hepatocellular Carcinoma Confirmation, Treatment, and Survival in Surveillance, Epidemiology, and End Results Registries, 1992-2008. Hepatology 55 (2), 476–482. doi:10.1002/hep.24710
Begg, C. B., and Mazumdar, M. (1994). Operating Characteristics of a Rank Correlation Test for Publication Bias. Biometrics 50 (4), 1088–1101. doi:10.2307/2533446
Bray, F., Ferlay, J., Soerjomataram, I., Siegel, R. L., Torre, L. A., and Jemal, A. (2018). Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 68 (6), 394–424. doi:10.3322/caac.21492
Bs, G. W., O’Connell, D., Robertson, J., Peterson, J., Welch, V., Losos, M., et al. (2021).The Newcastle-Ottawa Scale (NOS) for Assesing the Quality of Nonrandomized Studies in Meta-Analysis. (Accessed November 18, 2019).
Burn, J., Gerdes, A. M., Macrae, F., Mecklin, J. P., Moeslein, G., Olschwang, S., et al. (2011). Long-term Effect of Aspirin on Cancer Risk in Carriers of Hereditary Colorectal Cancer: an Analysis from the CAPP2 Randomised Controlled Trial. Lancet 378 (9809), 2081–2087. doi:10.1016/s0140-6736(11)61049-0
Cao, Y., Nishihara, R., Wu, K., Wang, M., Ogino, S., Willett, W. C., et al. (2016). Population-wide Impact of Long-Term Use of Aspirin and the Risk for Cancer. JAMA Oncol. 2 (6), 762–769. doi:10.1001/jamaoncol.2015.6396
Carrat, F. (2014). Statin and Aspirin for Prevention of Hepatocellular Carcinoma: what Are the Levels of Evidence? Clin. Res. Hepatol. Gastroenterol. 38 (1), 9–11. doi:10.1016/j.clinre.2013.09.007
Cattaneo, M. (2004). Aspirin and Clopidogrel: Efficacy, Safety, and the Issue of Drug Resistance. Arterioscler Thromb. Vasc. Biol. 24 (11), 1980–1987. doi:10.1161/01.ATV.0000145980.39477.a9
Chan, A. T., Ogino, S., and Fuchs, C. S. (2007). Aspirin and the Risk of Colorectal Cancer in Relation to the Expression of COX-2. N. Engl. J. Med. 356 (21), 2131–2142. doi:10.1056/NEJMoa067208
Chan, T. A., Morin, P. J., Vogelstein, B., and Kinzler, K. W. (1998). Mechanisms Underlying Nonsteroidal Antiinflammatory Drug-Mediated Apoptosis. Proc. Natl. Acad. Sci. U S A. 95 (2), 681–686. doi:10.1073/pnas.95.2.681
Chen, C. J., Liang, K. Y., Chang, A. S., Chang, Y. C., Lu, S. N., Liaw, Y. F., et al. (1991). Effects of Hepatitis B Virus, Alcohol Drinking, Cigarette Smoking and Familial Tendency on Hepatocellular Carcinoma. Hepatology 13 (3), 398–406. doi:10.1002/hep.1840130303
Chen, H., Cai, W., Chu, E. S. H., Tang, J., Wong, C. C., Wong, S. H., et al. (2017). Hepatic Cyclooxygenase-2 Overexpression Induced Spontaneous Hepatocellular Carcinoma Formation in Mice. Oncogene 36 (31), 4415–4426. doi:10.1038/onc.2017.73
Cheng, A. S., Chan, H. L., Leung, W. K., To, K. F., Go, M. Y., Chan, J. Y., et al. (2004). Expression of HBx and COX-2 in Chronic Hepatitis B, Cirrhosis and Hepatocellular Carcinoma: Implication of HBx in Upregulation of COX-2. Mod. Pathol. 17 (10), 1169–1179. doi:10.1038/modpathol.3800196
Chiu, H. F., Ho, S. C., Chen, C. C., and Yang, C. Y. (2011). Statin Use and the Risk of Liver Cancer: a Population-Based Case–Control Study. Am. J. Gastroenterol. 106 (5), 894–898. doi:10.1038/ajg.2010.475
Cook, N. R., Lee, I. M., Gaziano, J. M., Gordon, D., Ridker, P. M., Manson, J. E., et al. (2005). Low-dose Aspirin in the Primary Prevention of Cancer: the Women's Health Study: a Randomized Controlled Trial. Jama 294 (1), 47–55. doi:10.1001/jama.294.1.47
Degos, F., Christidis, C., Ganne-Carrie, N., Farmachidi, J. P., Degott, C., Guettier, C., et al. (2000). Hepatitis C Virus Related Cirrhosis: Time to Occurrence of Hepatocellular Carcinoma and Death. Gut 47 (1), 131–136. doi:10.1136/gut.47.1.131
Du, Z. Q., Zhao, J. Z., Dong, J., Bi, J. B., Ren, Y. F., Zhang, J., et al. (2019). Effect of Low-Dose Aspirin Administration on Long-Term Survival of Cirrhotic Patients after Splenectomy: A Retrospective Single-center Study. World J. Gastroenterol. 25 (28), 3798–3807. doi:10.3748/wjg.v25.i28.3798
Flossmann, E., and Rothwell, P. M. (2007). Effect of Aspirin on Long-Term Risk of Colorectal Cancer: Consistent Evidence from Randomised and Observational Studies. Lancet 369 (9573), 1603–1613. doi:10.1016/s0140-6736(07)60747-8
Foderà, D., D'Alessandro, N., Cusimano, A., Poma, P., Notarbartolo, M., Lampiasi, N., et al. (2004). Induction of Apoptosis and Inhibition of Cell Growth in Human Hepatocellular Carcinoma Cells by COX-2 Inhibitors. Ann. N. Y Acad. Sci. 1028, 440–449. doi:10.1196/annals.1322.052
Gann, P. H., Manson, J. E., Glynn, R. J., Buring, J. E., and Hennekens, C. H. (1993). Low-dose Aspirin and Incidence of Colorectal Tumors in a Randomized Trial. J. Natl. Cancer Inst. 85 (15), 1220–1224. doi:10.1093/jnci/85.15.1220
Grant, R. L. (2014). Converting an Odds Ratio to a Range of Plausible Relative Risks for Better Communication of Research Findings. BMJ 348, f7450. doi:10.1136/bmj.f7450
Greenland, S. (1987). Quantitative Methods in the Review of Epidemiologic Literature. Epidemiol. Rev. 9, 1–30. doi:10.1093/oxfordjournals.epirev.a036298
Higgins, J. P., Thompson, S. G., Deeks, J. J., and Altman, D. G. (2003). Measuring Inconsistency in Meta-Analyses. BMJ 327 (7414), 557–560. doi:10.1136/bmj.327.7414.557
Higgins, J. P., and Thompson, S. G. (2002). Quantifying Heterogeneity in a Meta-Analysis. Stat. Med. 21 (11), 1539–1558. doi:10.1002/sim.1186
Hossain, M. A., Kim, D. H., Jang, J. Y., Kang, Y. J., Yoon, J. H., Moon, J. O., et al. (2012). Aspirin Induces Apoptosis In Vitro and Inhibits Tumor Growth of Human Hepatocellular Carcinoma Cells in a Nude Mouse Xenograft Model. Int. J. Oncol. 40 (4), 1298–1304. doi:10.3892/ijo.2011.1304
Hwang, I. C., Chang, J., Kim, K., and Park, S. M. (2018). Aspirin Use and Risk of Hepatocellular Carcinoma in a National Cohort Study of Korean Adults. Sci. Rep. 8 (1), 4968. doi:10.1038/s41598-018-23343-0
Iannacone, M., Sitia, G., Isogawa, M., Marchese, P., Castro, M. G., Lowenstein, P. R., et al. (2005). Platelets Mediate Cytotoxic T Lymphocyte-Induced Liver Damage. Nat. Med. 11 (11), 1167–1169. doi:10.1038/nm1317
Jacobs, E. J., Newton, C. C., Gapstur, S. M., and Thun, M. J. (2012). Daily Aspirin Use and Cancer Mortality in a Large US Cohort. J. Natl. Cancer Inst. 104 (16), 1208–1217. doi:10.1093/jnci/djs318
Kern, M. A., Schubert, D., Sahi, D., Schöneweiss, M. M., Moll, I., Haugg, A. M., et al. (2002). Proapoptotic and Antiproliferative Potential of Selective Cyclooxygenase-2 Inhibitors in Human Liver Tumor Cells. Hepatology 36 (4 Pt 1), 885–894. doi:10.1053/jhep.2002.36125
Kim, G., Jang, S. Y., Han, E., Lee, Y. H., Park, S. Y., Nam, C. M., et al. (2017). Effect of Statin on Hepatocellular Carcinoma in Patients with Type 2 Diabetes: A Nationwide Nested Case-Control Study. Int. J. Cancer 140 (4), 798–806. doi:10.1002/ijc.30506
Lapi, F., Levi, M., Simonetti, M., Cancian, M., Parretti, D., Cricelli, I., et al. (2016). Risk of Prostate Cancer in Low-Dose Aspirin Users: A Retrospective Cohort Study. Int. J. Cancer 139 (1), 205–211. doi:10.1002/ijc.30061
Lee, M., Chung, G. E., Lee, J. H., Oh, S., Nam, J. Y., Chang, Y., et al. (2017). Antiplatelet Therapy and the Risk of Hepatocellular Carcinoma in Chronic Hepatitis B Patients on Antiviral Treatment. Hepatology 66 (5), 1556–1569. doi:10.1002/hep.29318
Lee, T. Y., Hsu, Y. C., Tseng, H. C., Yu, S. H., Lin, J. T., Wu, M. S., et al. (2019). Association of Daily Aspirin Therapy with Risk of Hepatocellular Carcinoma in Patients with Chronic Hepatitis B. JAMA Intern. Med. 179 (5), 633–640. doi:10.1001/jamainternmed.2018.8342
Leng, J., Han, C., Demetris, A. J., Michalopoulos, G. K., and Wu, T. (2003). Cyclooxygenase-2 Promotes Hepatocellular Carcinoma Cell Growth through Akt Activation: Evidence for Akt Inhibition in Celecoxib-Induced Apoptosis. Hepatology 38 (3), 756–768. doi:10.1053/jhep.2003.50380
Li, G., Zhang, S., Fang, H., Yan, B., Zhao, Y., Feng, L., et al. (2013). Aspirin Overcomes Navitoclax-Resistance in Hepatocellular Carcinoma Cells through Suppression of Mcl-1. Biochem. Biophys. Res. Commun. 434 (4), 809–814. doi:10.1016/j.bbrc.2013.04.018
Li, J. H., Wang, Y., Xie, X. Y., Yin, X., Zhang, L., Chen, R. X., et al. (2016). Aspirin in Combination with TACE in Treatment of Unresectable HCC: a Matched-Pairs Analysis. Am. J. Cancer Res. 6 (9), 2109–2116.
Li, T., Dong, Z. R., Guo, Z. Y., Wang, C. H., Tang, Z. Y., Qu, S. F., et al. (2013). Aspirin Enhances IFN-α-Induced Growth Inhibition and Apoptosis of Hepatocellular Carcinoma via JAK1/STAT1 Pathway. Cancer Gene Ther. 20 (6), 366–374. doi:10.1038/cgt.2013.29
Liao, Y. H., Hsu, R. J., Wang, T. H., Wu, C. T., Huang, S. Y., Hsu, C. Y., et al. (2020). Aspirin Decreases Hepatocellular Carcinoma Risk in Hepatitis C Virus Carriers: a Nationwide Cohort Study. BMC Gastroenterol. 20 (1), 6. doi:10.1186/s12876-020-1158-y
Lim, H. Y., Joo, H. J., Choi, J. H., Yi, J. W., Yang, M. S., Cho, D. Y., et al. (2000). Increased Expression of Cyclooxygenase-2 Protein in Human Gastric Carcinoma. Clin. Cancer Res. 6 (2), 519–525.
Maini, M. K., and Schurich, A. (2012). Platelets Harness the Immune Response to Drive Liver Cancer. Proc. Natl. Acad. Sci. U S A. 109 (32), 12840–12841. doi:10.1073/pnas.1210296109
Malehmir, M., Pfister, D., Gallage, S., Szydlowska, M., Inverso, D., Kotsiliti, E., et al. (2019). Platelet GPIbα Is a Mediator and Potential Interventional Target for NASH and Subsequent Liver Cancer. Nat. Med. 25 (4), 641–655. doi:10.1038/s41591-019-0379-5
McGlynn, K. A., and London, W. T. (2011). The Global Epidemiology of Hepatocellular Carcinoma: Present and Future. Clin. Liver Dis. 15 (2), 223–x. doi:10.1016/j.cld.2011.03.006
Oh, S., Shin, S., Lee, S. H., Kim, T. S., Nam, S.-J., Park, J. M., et al. (2017). Aspirin and the Risk of Hepatocellular Carcinoma Development in Patients with Compensated Alcoholic Cirrhosis. J. Hepatol. 66 (1), S629–S630. doi:10.1016/s0168-8278(17)31706-3
Pang, Q., Jin, H., Qu, K., Man, Z., Wang, Y., Yang, S., et al. (2017). The Effects of Nonsteroidal Anti-inflammatory Drugs in the Incident and Recurrent Risk of Hepatocellular Carcinoma: a Meta-Analysis. Onco Targets Ther. 10, 4645–4656. doi:10.2147/ott.s143154
Patrono, C., García Rodríguez, L. A., Landolfi, R., and Baigent, C. (2005). Low-dose Aspirin for the Prevention of Atherothrombosis. N. Engl. J. Med. 353 (22), 2373–2383. doi:10.1056/NEJMra052717
Petrick, J. L., Sahasrabuddhe, V. V., Chan, A. T., Alavanja, M. C., Beane-Freeman, L. E., Buring, J. E., et al. (2015). NSAID Use and Risk of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma: The Liver Cancer Pooling Project. Cancer Prev. Res. (Phila) 8 (12), 1156–1162. doi:10.1158/1940-6207.capr-15-0126
Raza, H., John, A., and Benedict, S. (2011). Acetylsalicylic Acid-Induced Oxidative Stress, Cell Cycle Arrest, Apoptosis and Mitochondrial Dysfunction in Human Hepatoma HepG2 Cells. Eur. J. Pharmacol. 668 (1-2), 15–24. doi:10.1016/j.ejphar.2011.06.016
Sahasrabuddhe, V. V., Gunja, M. Z., Graubard, B. I., Trabert, B., Schwartz, L. M., Park, Y., et al. (2012). Nonsteroidal Anti-inflammatory Drug Use, Chronic Liver Disease, and Hepatocellular Carcinoma. J. Natl. Cancer Inst. 104 (23), 1808–1814. doi:10.1093/jnci/djs452
Semple, J. W., Italiano, J. E., and Freedman, J. (2011). Platelets and the Immune Continuum. Nat. Rev. Immunol. 11 (4), 264–274. doi:10.1038/nri2956
Shen, Y., Risch, H., Lu, L., Ma, X., Irwin, M. L., Lim, J. K., et al. (2020). Risk Factors for Hepatocellular Carcinoma (HCC) in the Northeast of the United States: Results of a Case-Control Study. Cancer Causes Control 31 (4), 321–332. doi:10.1007/s10552-020-01277-1
Shin, S., Lee, S. H., Lee, M., Kim, J. H., Lee, W., Lee, H. W., et al. (2020). Aspirin and the Risk of Hepatocellular Carcinoma Development in Patients with Alcoholic Cirrhosis. Medicine (Baltimore) 99 (9), e19008. doi:10.1097/md.0000000000019008
Simon, T. G., Duberg, A-S., Aleman, S., Chung, R. T., and Ludvigsson, J. F. (2019). Aspirin use is Associated with Reduced Risk for Incident Hepatocellular Carcinoma in Patients with Chronic Viral Hepatitis: Results from a Nationwide Population. Gastroenterology 156 (6), S1200. doi:10.1016/s0016-5085(19)39981-0
Simon, T. G., Duberg, A. S., Aleman, S., Chung, R. T., Chan, A. T., and Ludvigsson, J. F. (2020). Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 382 (11), 1018–1028. doi:10.1056/NEJMoa1912035
Simon, T. G., Ma, Y., Ludvigsson, J. F., Chong, D. Q., Giovannucci, E. L., Fuchs, C. S., et al. (2018).Association between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol. 4(12), 1683–1690. doi:10.1001/jamaoncol.2018.4154
Singh, B., Berry, J. A., Shoher, A., Ramakrishnan, V., and Lucci, A. (2005). COX-2 Overexpression Increases Motility and Invasion of Breast Cancer Cells. Int. J. Oncol. 26 (5), 1393–1399. doi:10.3892/ijo.26.5.1393
Siristatidis, C., Sergentanis, T. N., Kanavidis, P., Trivella, M., Sotiraki, M., Mavromatis, I., et al. (2013). Controlled Ovarian Hyperstimulation for IVF: Impact on Ovarian, Endometrial and Cervical Cancer-Aa Systematic Review and Meta-Analysis. Hum. Reprod. Update 19 (2), 105–123. doi:10.1093/humupd/dms051
Sitia, G., Aiolfi, R., Di Lucia, P., Mainetti, M., Fiocchi, A., Mingozzi, F., et al. (2012). Antiplatelet Therapy Prevents Hepatocellular Carcinoma and Improves Survival in a Mouse Model of Chronic Hepatitis B. Proc. Natl. Acad. Sci. U S A. 109 (32), E2165–E2172. doi:10.1073/pnas.1209182109
Sitia, G., Iannacone, M., and Guidotti, L. G. (2013). Anti-platelet Therapy in the Prevention of Hepatitis B Virus-Associated Hepatocellular Carcinoma. J. Hepatol. 59 (5), 1135–1138. doi:10.1016/j.jhep.2013.05.040
Stroup, D. F., Berlin, J. A., Morton, S. C., Olkin, I., Williamson, G. D., Rennie, D., et al. (2000). Meta-analysis of Observational Studies in Epidemiology: a Proposal for Reporting. Meta-Analysis of Observational Studies in Epidemiology (MOOSE) Group. Jama 283 (15), 2008–2012. doi:10.1001/jama.283.15.2008
Stuck, A. E., Rubenstein, L. Z., and Wieland, D. (1998). Bias in Meta-Analysis Detected by a Simple, Graphical Test. Asymmetry Detected in Funnel Plot Was Probably Due to True Heterogeneity. BMJ 316 (7129), 469–471. doi:10.1136/bmj.316.7129.469
Tao, Y., Li, Y., Liu, X., Deng, Q., Yu, Y., and Yang, Z. (2018). Nonsteroidal Anti-inflammatory Drugs, Especially Aspirin, Are Linked to Lower Risk and Better Survival of Hepatocellular Carcinoma: a Meta-Analysis. Cancer Manag. Res. 10, 2695–2709. doi:10.2147/cmar.s167560
Torre, L. A., Bray, F., Siegel, R. L., Ferlay, J., Lortet-Tieulent, J., and Jemal, A. (2015). Global Cancer Statistics, 2012. CA Cancer J. Clin. 65 (2), 87–108. doi:10.3322/caac.21262
Tsoi, K. K. F., Ho, J. M. W., Chan, F. C. H., and Sung, J. J. Y. (2019). Long-term Use of Low-Dose Aspirin for Cancer Prevention: A 10-year Population Cohort Study in Hong Kong. Int. J. Cancer 145 (1), 267–273. doi:10.1002/ijc.32083
Villanueva, A. (2019). Hepatocellular Carcinoma. N. Engl. J. Med. 380 (15), 1450–1462. doi:10.1056/NEJMra1713263
Wang, S., Yu, Y., Ryan, P. M., Dang, M., Clark, C., Kontogiannis, V., et al. (2020). Association of Aspirin Therapy with Risk of Hepatocellular Carcinoma: A Systematic Review and Dose-Response Analysis of Cohort Studies with 2.5 Million Participants. Pharmacol. Res. 151, 104585. doi:10.1016/j.phrs.2019.104585
Yang, B., Petrick, J. L., Chen, J., Hagberg, K. W., Sahasrabuddhe, V. V., Graubard, B. I., et al. (2016). Associations of NSAID and Paracetamol Use with Risk of Primary Liver Cancer in the Clinical Practice Research Datalink. Cancer Epidemiol. 43, 105–111. doi:10.1016/j.canep.2016.06.009
Ye, S., Lee, M., Lee, D., Ha, E. H., and Chun, E. M. (2019). Association of Long-Term Use of Low-Dose Aspirin as Chemoprevention with Risk of Lung Cancer. JAMA Netw. Open 2 (3), e190185. doi:10.1001/jamanetworkopen.2019.0185
Yeh, C. C., Lin, J. T., Jeng, L. B., Ho, H. J., Yang, H. R., Wu, M. S., et al. (2015). Nonsteroidal Anti-inflammatory Drugs Are Associated with Reduced Risk of Early Hepatocellular Carcinoma Recurrence after Curative Liver Resection: a Nationwide Cohort Study. Ann. Surg. 261 (3), 521–526. doi:10.1097/sla.0000000000000746
Yeh, M. M., Daniel, H. D., and Torbenson, M. (2010). Hepatitis C-Associated Hepatocellular Carcinomas in Non-cirrhotic Livers. Mod. Pathol. 23 (2), 276–283. doi:10.1038/modpathol.2009.174
Yip-Schneider, M. T., Barnard, D. S., Billings, S. D., Cheng, L., Heilman, D. K., Lin, A., et al. (2000). Cyclooxygenase-2 Expression in Human Pancreatic Adenocarcinomas. Carcinogenesis 21 (2), 139–146. doi:10.1093/carcin/21.2.139
Keywords: hepatocellular carcinoma, aspirin, HCC, meta-analysis, systematic review 3
Citation: Zhou X, Zhang T, Sun Y, Li C, Ding X, Zhu Y, Li L and Fan Z (2022) Systematic Review and Meta-analysis: Association of Aspirin With Incidence of Hepatocellular Carcinoma. Front. Pharmacol. 13:764854. doi: 10.3389/fphar.2022.764854
Received: 26 August 2021; Accepted: 31 January 2022;
Published: 01 March 2022.
Edited by:
David Sacerdoti, University of Verona, ItalyReviewed by:
Zhipeng Liu, Purdue University, United StatesAndrea Dalbeni, Verona University Hospital, Italy
Copyright © 2022 Zhou, Zhang, Sun, Li, Ding, Zhu, Li and Fan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhirui Fan, fanzhirui88@126.com; Lifeng Li, lilifeng0317@163.com
†These authors have equally contributed to this work.
 Xueliang Zhou
Xueliang Zhou Tengfei Zhang
Tengfei Zhang Yali Sun
Yali Sun Chunwei Li4
Chunwei Li4 Xianfei Ding
Xianfei Ding Lifeng Li
Lifeng Li