- 1Yueyang Hospital of Integrated Traditional Chinese and Western Medicine Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 2School of Acupuncture-Moxibustion and Tuina, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 3Shanghai University of Traditional Chinese Medicine, Shanghai Traditional Chinese Medicine-Integrated Hospital, Shanghai, China
Background: Chemotherapy-induced peripheral neuropathy (CIPN) is a common adverse reaction of chemotherapy. Many studies have confirmed that traditional Chinese medicine (TCM) has unique advantages for treating CIPN. However, there is no standard TCM prescription in clinical practice or objective outcome index, and similar efficacy varies. Therefore, in this study, a systematic review of randomized controlled trials (RCTs) was performed to evaluate the clinical efficacy of external treatment with Chinese herbal medicine (CHM) for CIPN. This analysis provides evidence-based medical support for the use of CHM for external treatment of CIPN.
Methods: Relevant RCTs assessing CHM external treatment of CIPN were searched in nine electronic databases, including the China National Knowledge Infrastructure Database, China Biology Medicine Disc, China Science and Technology Journal Database, Wanfang Database, PubMed, Cochrane Library, MEDLINE, Web of Science, and OVID, from inception to July 2021. A meta-analysis was performed on these studies using RevMan5.3 software.
Results: Based on the inclusion and exclusion criteria, 33 clinical studies were included, while 1,354 studies were screened out. There were 2,356 patients in total, including 1,208 in the treatment group and 1,148 in the control group. In the treatment group, peripheral neurotoxicity rate, total effect rate, KPS score, TCM syndrome score and efficacy, pain NRS score, and pain relief rate were significantly improved compared with those of the control group (p < 0.01). Furthermore, the peroneal and median nerve conduction velocities were also improved compared with those in the control group (p < 0.05). By creating a funnel plot for the incidence of peripheral neurotoxicity and the total effect rate, we showed that the left and right sides were symmetrical, and that the publication bias was low.
Conclusion: CHM external treatment was found to be an effective method for treating CIPN as it significantly improved clinical symptoms and quality of life in patients with CIPN.
Clinical Trial Registration: identifier ChiCTR1900024617
1 Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) refers to sensory or motor disorders of the extremities due to direct damage to the peripheral nervous system by chemotherapy drugs (Peng Xu et al., 2018). Clinical symptoms include symmetrical pain, numbness, and fine motor impairment at the extremities (Tian and Huang, 2017; Cao et al., 2019), but the main symptom is sensory impairment (Argyriou et al., 2012). CIPN can cause severe disability (Van Hecke et al., 2013), and its advanced development can limit physical activities. The incidence of CIPN closely correlates with the chemotherapy regimen, administration dosage, and evaluation methods (Zis et al., 2017; Avan et al., 2018), and it has been shown that in patients receiving drug combination, CIPN occurs in more than 38% of patients (Waseem et al., 2018). At present, CIPN is often relieved by reducing the dose of chemotherapeutics or by stopping the treatment, which seriously affects treatment efficacy and patient quality of life (Ma et al., 2018). However, there is no specific Western medicine treatment for CIPN. Neuropathic pain management and nutritional supplements are often applied but with poor curative effects (Staff et al., 2017). Therefore, it is vital to identify effective treatments for CIPN. As a traditional Chinese medicine (TCM) therapy, Chinese herbal medicine (CHM) has unique advantages in improving clinical symptoms, enhancing the curative effect of chemotherapy, and reducing its side effects (Liu et al., 2013). This study systematically evaluated the clinical efficacy of external treatment with CHM for CIPN aiming to provide higher-quality clinical evidence and guidance on CHM external treatment of CIPN.
2 Materials and Methods
2.1 Search Strategy
Database retrieval strategies were formulated in accordance with the requirements of the Cochrane Systematic Review Manual, and computer retrieval was the main method. A comprehensive literature search was conducted in the China National Knowledge Infrastructure (CNKI), China Biology Medicine Disc (CBM), China Science and Technology Journal (VIP), Wanfang (WANFANG), PubMed, Cochrane Library, MEDLINE, Web of Science, and OVID databases. The following search terms were used as the search strategy and were modified according to different databases: “chemotherapy-induced peripheral neuropathy,” “chemotherapy-induced peripheral neurotoxicity,” “CIPN,” “peripheral neuropathy,” “peripheral neurotoxicity,” “drugs,” “Chinese herbal,” and traditional Chinese medicine.” In addition, we manually retrieved relevant articles and clinical studies to obtain as much literature as possible. No language or status restriction was set in this review.
2.2 Inclusion Criteria
The following components were included in the present study:
1) Types of studies: Randomized controlled clinical trials (RCTs) that evaluated the efficacy of external treatment with CHM for chemotherapy-induced peripheral neurotoxicity were included.
2) Types of participants: Patients who were pathologically or cytologically diagnosed with malignancy and developed peripheral neuropathy after chemotherapy were considered. No restriction on gender, age, or nationality was set.
3) Types of interventions: Patients in the control group were treated with conventional chemotherapy regimens or further given conventional Western medicine and placebo treatments.
4) Patients in the treatment group were treated with external TCM treatment (external TCM washing, wet dressing, and/or foot bath) in addition to conventional chemotherapy or control treatment.
The following outcome measures were considered:
1) Primary outcomes: incidence of CIPN, overall response rate, nerve conduction velocity, Karnofsky performance score (KPS), TCM syndrome score (Dai et al., 2017), and TCM syndrome effect (Zheng, 2002); clinical recovery: clinical symptoms and signs disappeared or almost disappeared, and TCM syndrome score decreased ≥90%; significant efficacy: clinical symptoms and signs disappeared, improved significantly, and TCM syndrome score were reduced by 70%; efficacy: clinical symptoms and signs are improved, and TCM syndrome score was reduced by 30%; inefficacy: the disappearance of clinical symptoms and signs was not significant, and the TCM syndrome score was reduced by less than 30%. Calculation formula: (score before treatment—score after treatment) ÷ score before treatment × 100%
2) Secondary outcomes: numeric rating scales, myelosuppression, and analgesic rate.
2.3 Exclusion Criteria
The following components were excluded in the present study:
1) Non-randomized controlled trials, case studies without a control group, for example, review, meta-analysis, discussion, conference, cell tissue or animal experiments, and other non-clinical trials.
2) Self–cross-control study.
3) Literature that did not meet outcome measures or failed to present valid data.
4) Repeated publication or repeated detection of literature.
5) Participants whose neurological disorders resulted from electrolyte disorders and other systemic diseases, such as severe cervical spondylosis, severe intervertebral disc compression, severe diabetes, and other non-chemotherapeutic factors.
6) Participants with hand and foot skin diseases and a history of drug allergy.
7) Intervention not including external TCM treatment.
2.4 Study Selection and Data Extraction
Studies were preliminarily independently screened by two researchers based on the inclusion and exclusion criteria, primarily through the title, abstract, and keywords. Then, the full text was read for re-screening, and the results were cross-checked. If a difference occurred, a third party made the final decision. If a decision was not reached, relevant professionals were consulted. The following information was extracted: author, year of publication, number of participants, grouping methods, interventions, and outcome measures.
2.5 Literature Quality Evaluation
A new “risk of bias assessment” tool developed by methodologists, editors, and systematic reviewers was used to evaluate the quality of the included literature as recommended by the Cochrane Collaboration (Higgins et al., 2011). This includes six aspects: 1) random allocation scheme; 2) allocation concealment; 3) blinding of participants and personnel; 4) integrity of the data; 5) selective reporting of research results; and 6) other sources of bias. Each field was assessed as “yes” (low ROB; risk of bias), “no” (high ROB), or “unclear” (unclear ROB).
2.6 Data Analysis
Review Manager (RevMan, Version 5.3, Copenhagen: The Nordic Cochrane Center, The Cochrane Collaboration, 2014) was utilized to conduct the data analysis. A heterogeneity test was performed on the results of each included study. No statistical significance (p > 0.05, I2 < 50%) indicated that there was no significant statistical heterogeneity in the included studies, and a fixed-effects model was used for the analysis. Statistical significance (p < 0.05, I2 > 50%) indicated that heterogeneity existed in the included studies, and further analysis was needed to determine the source of heterogeneity and whether a random-effects model could be used for the analysis. The odds ratios (ORs) and 95% confidence intervals (CIs) were utilized for efficacy analysis of dichotomous variables, while standardized mean difference (SMD) and 95% CIs were utilized for efficacy analysis of continuous variables.
3 Results
3.1 Literature Retrieval Results
The process of literature identification and screening is described in the flowchart (Figure 1). In total, 1,354 related studies were derived from electronic databases. After removing 306 duplicates, 1,048 studies were rescreened through titles and abstracts. Then, 894 were excluded, while 154 remained to be assessed in full text. Ultimately, 33 eligible RCTs that satisfied the inclusion and exclusion criteria were included.
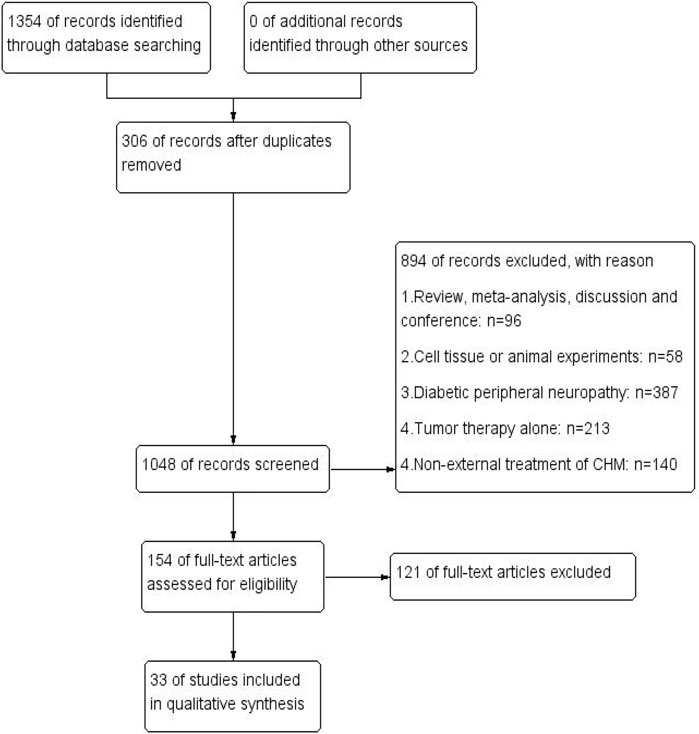
FIGURE 1. Flowchart of literature identification and screening. PubMed (n = 49), Cochrane library (n = 44), Web of Science (n = 79), MEDLINE (n = 3), OVID (n = 8), CNKI (n = 234), CBM (n = 528), VIP (n = 40), and WANFANG (n = 369).
3.2 Basic Features and Quality Evaluation
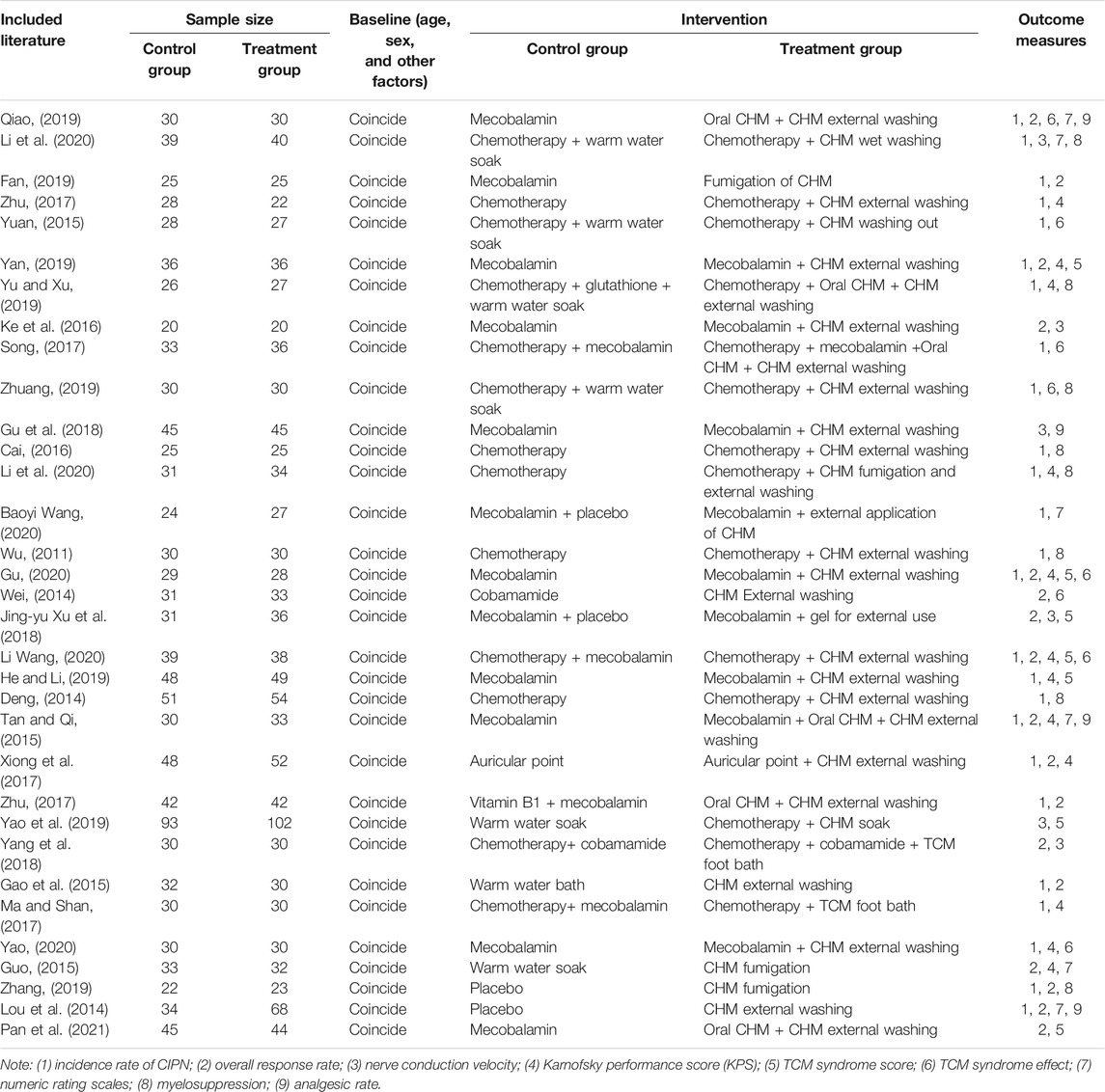
In total, 33 RCTs with a total of 2,356 patients were included. There were 1,208 patients in the treatment group and 1,148 in the control group. The baseline criteria (age, sex, and other factors) in each included study were balanced, indicating comparability between the treatment and control groups (Table 1).
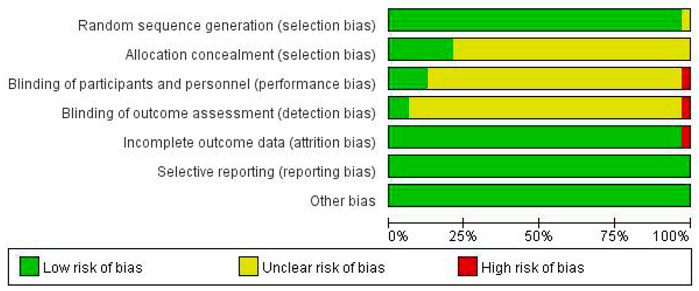
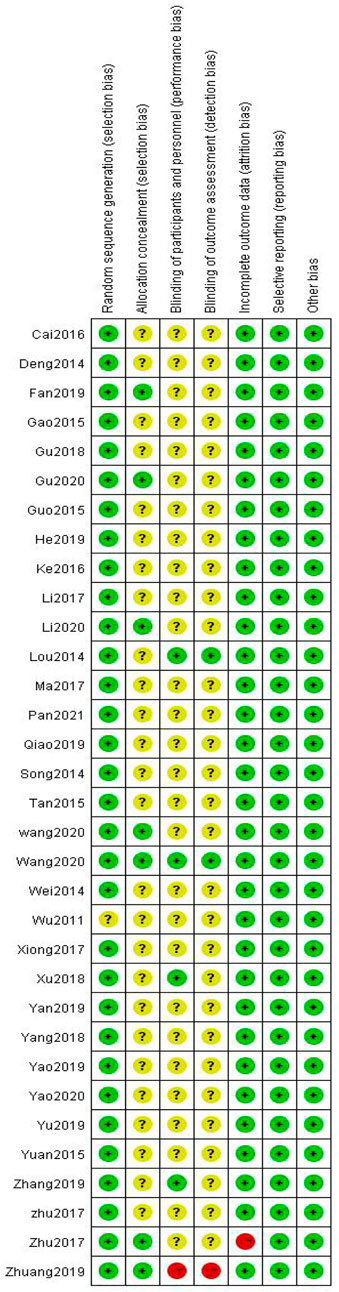
Among the 33 included studies, 20 applied a random-number table for grouping (Deng, 2014; Wei, 2014; Guo, 2015; Tan and Qi, 2015; Yuan, 2015; Ke et al., 2016; Zhu et al., 2017; Jing-yu Xu et al., 2018; Gu et al., 2018; Yang et al., 2018; He and Li, 2019; Yao et al., 2019; Yu and Xu, 2019; Zhang, 2019; Zhuang, 2019; Li Wang, 2020; Gu, 2020; Li et al., 2020; Yao, 2020; Pan et al., 2021); 10 only mentioned random allocation (Gao et al., 2015; Cai, 2016; Li and Cai, 2017; Ma and Shan, 2017; Song, 2017; Zhu, 2017; Fan, 2019; Qiao, 2019; Yan, 2019; Baoyi Wang, 2020); two used a number-parity method and stratified random-block method (Lou et al., 2014; Xiong et al., 2017); and one study did not provide any information on this aspect (Wu, 2011). Six studies used allocation concealment (Fan, 2019; Zhuang, 2019; Li Wang, 2020; Baoyi Wang, 2020; Gu, 2020; Li et al., 2020), and the remaining studies did not mention the use of allocation concealment. Five studies involved blinding (Lou et al., 2014; Zhu, 2017; Jing-yu Xu et al., 2018; Zhang, 2019; Baoyi Wang, 2020); one did not (Zhuang, 2019); and the remaining did not mention blinding (Zhu, 2017). All of the 33 studies fully reported the results without any selection or other bias, while only one study was incomplete due to subject dropout (Figures 2,3).
3.3 Results of the Meta-analysis
3.3.1 Primary Outcomes
3.3.1.1 Incidence of Peripheral Neurotoxicity
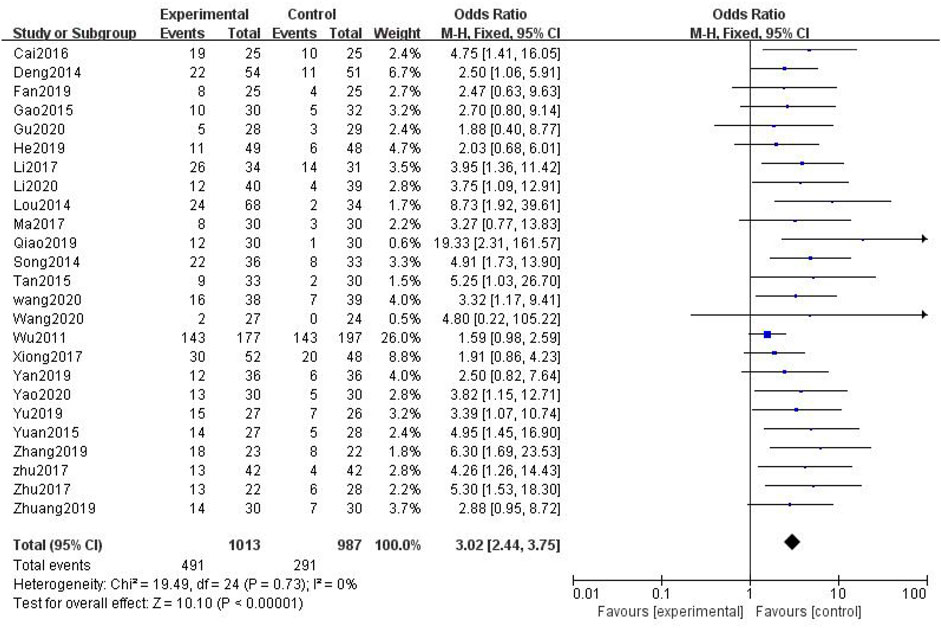
In total, 25 studies with 2,000 patients were included (Wu, 2011; Deng, 2014; Lou et al., 2014; Gao et al., 2015; Tan and Qi, 2015; Yuan, 2015; Cai, 2016; Li and Cai, 2017; Ma and Shan, 2017; Song, 2017; Xiong et al., 2017; Zhu, 2017; Zhu et al., 2017; Fan, 2019; He and Li, 2019; Qiao, 2019; Yan, 2019; Yu and Xu, 2019; Zhang, 2019; Zhuang, 2019; Li Wang, 2020; Baoyi Wang, 2020; Gu, 2020; Li et al., 2020; Yao, 2020), with 1,013 in the treatment group and 987 in the control group. The heterogenicity test showed homogeneity among the 25 studies (p > 0.05, I2 = 0%). Therefore, the fixed-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the incidence of peripheral neurotoxicity between the treatment and control groups (OR = 3.02, 95% CI [2.44, 3.75], p < 0.01, Figure 4).
3.3.1.2 Overall Response Rate
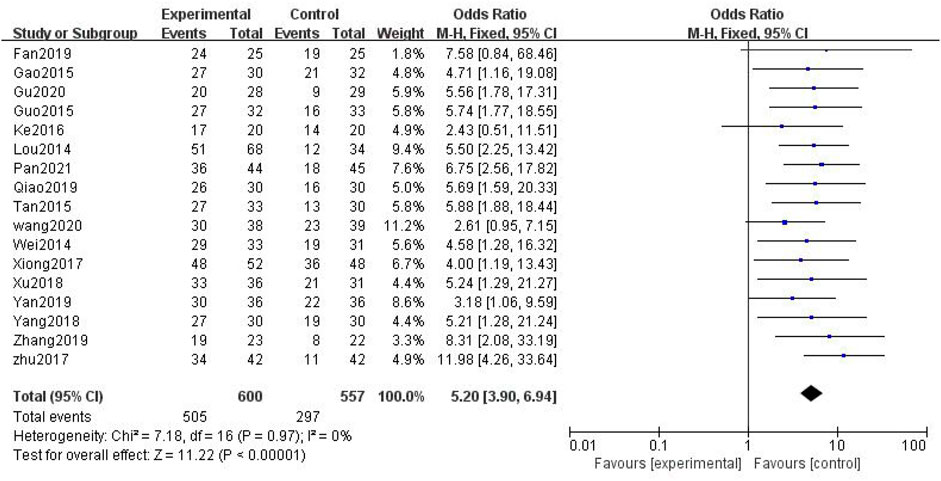
In total, 17 studies with 1,157 patients were included (Lou et al., 2014; Wei, 2014; Gao et al., 2015; Guo, 2015; Tan and Qi, 2015; Ke et al., 2016; Xiong et al., 2017; Zhu et al., 2017; Jing-yu Xu et al., 2018; Yang et al., 2018; Fan, 2019; Qiao, 2019; Yan, 2019; Zhang, 2019; Li Wang, 2020; Gu, 2020; Pan et al., 2021), with 600 in the treatment group and 557 in the control group. The heterogenicity test showed homogeneity among the 17 studies (p > 0.05, I2 = 0%). Therefore, the fixed-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the overall response rate between the treatment and control groups (OR = 5.20, 95% CI [3.90, 6.94], p < 0.01, Figure 5).
3.3.1.3 Peroneal Nerve Conduction Velocity
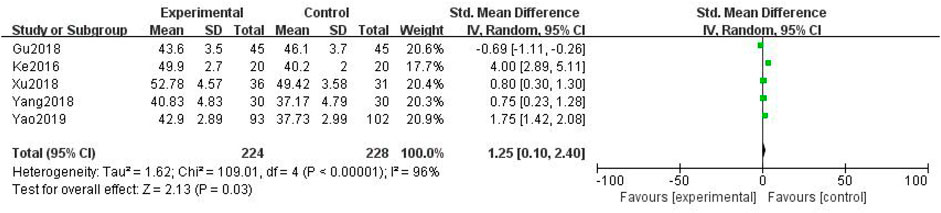
In total, five studies with 452 patients were included (Ke et al., 2016; Jing-yu Xu et al., 2018; Gu et al., 2018; Yang et al., 2018; Yao et al., 2019), with 224 in the treatment group and 228 in the control group. The heterogenicity test showed homogeneity among the five studies (p < 0.05, I2 = 96%). Therefore, the random-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the peroneal nerve conduction velocity between the treatment and control groups (SMD = 1.25, 95% CI [0.10, 2.40], p < 0.05, Figure 6).
3.3.1.4 Median Nerve Conduction Velocity
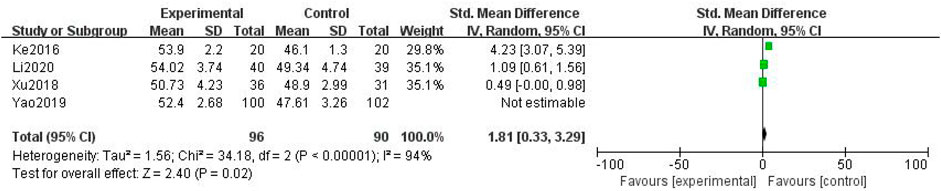
In total, four studies with 388 patients were included (Jing-yu Xu et al., 2018; Yao et al., 2019; Gu, 2020; Li et al., 2020), with 196 in the treatment group and 192 in the control group. The heterogenicity test showed homogeneity among the four studies (p < 0.05, I2 = 94%). Therefore, the random-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the median nerve conduction velocity between the treatment and control groups (SMD = 1.81, 95% CI [0.33, 3.29], p < 0.05, Figure 7).
3.3.1.5 KPS
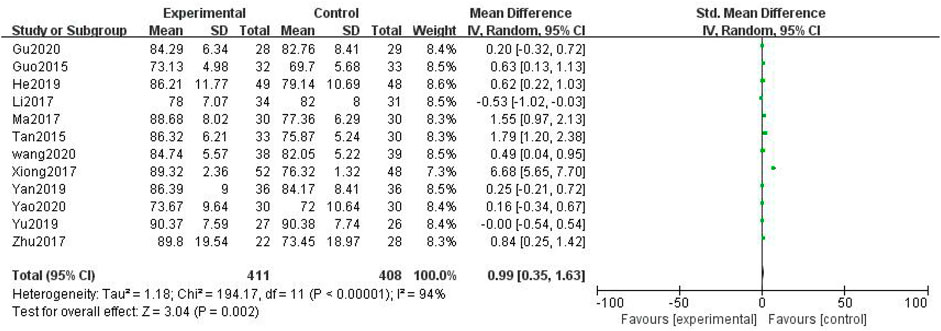
In total, 12 studies with 819 patients were included (Guo, 2015; Tan and Qi, 2015; Li and Cai, 2017; Ma and Shan, 2017; Xiong et al., 2017; Zhu, 2017; He and Li, 2019; Yan, 2019; Yu and Xu, 2019; Li Wang, 2020; Gu, 2020; Yao, 2020), with 411 in the treatment group and 408 in the control group. The heterogenicity test showed homogeneity among the 12 studies (p < 0.05, I2 = 94%). Therefore, the random-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the KPS between the treatment and control groups (SMD = 0.99, 95% CI [0.35, 1.63], p < 0.01, Figure 8).
3.3.1.6 Traditional Chinese Medicine Syndrome Score
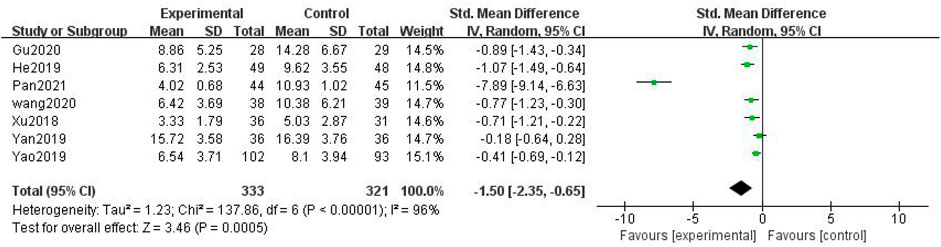
In total, seven studies with 654 patients were included (Jing-yu Xu et al., 2018; He and Li, 2019; Yan, 2019; Yao et al., 2019; Li Wang, 2020; Gu, 2020; Pan et al., 2021), with 333 in the treatment group and 321 in the control group. The heterogenicity test showed homogeneity among the seven studies (p < 0.05, I2 = 96%). Therefore, the random-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the TCM syndrome score between the treatment and control groups (SMD = –1.50, 95% CI [–2.35, –0.65], p < 0.01, Figure 9).
3.3.1.7 Traditional Chinese Medicine Syndrome Effect
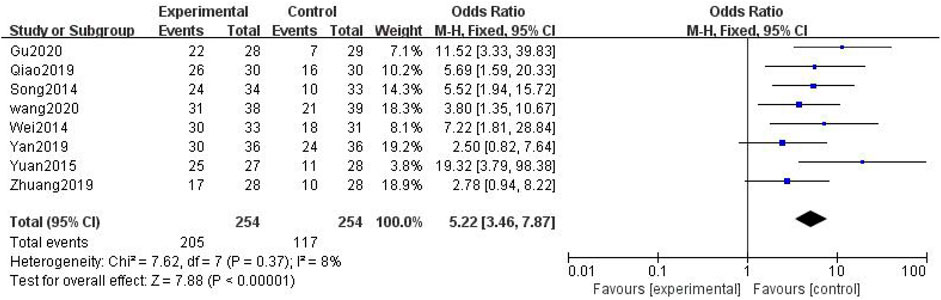
In total, eight studies with 508 patients were included (Deng, 2014; Wei, 2014; Guo, 2015; Tan and Qi, 2015; Yuan, 2015; Song, 2017; Zhu et al., 2017; Yang et al., 2018; He and Li, 2019; Qiao, 2019; Yan, 2019; Yao et al., 2019; Zhang, 2019; Zhuang, 2019; Li Wang, 2020; Gu, 2020; Yao, 2020; Pan et al., 2021), with 254 in the treatment group and 254 in the control group. The heterogenicity test showed homogeneity among the eight studies (p > 0.05, I2 = 8%). Therefore, the fixed-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the TCM syndrome effect velocity between the treatment and control groups (OR = 5.22, 95% CI [3.46, 7.87], p < 0.01, Figure 10).
3.3.2 Secondary Outcomes
3.3.2.1 Numerical Rating Scale
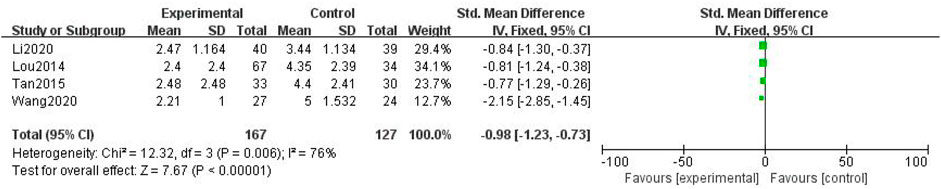
In total, four studies with 294 patients were included (Lou et al., 2014; Tan and Qi, 2015; Baoyi Wang, 2020; Li et al., 2020), with 167 in the treatment group and 127 in the control group. The heterogenicity test showed homogeneity among the four studies (p > 0.05, I2 = 76%). Therefore, the fixed-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the numerical rating scale between the treatment and control groups (SMD = –0.98, 95% CI [–1.23, –0.73], p < 0.01, Figure 11).
3.3.2.2 Myelosuppression
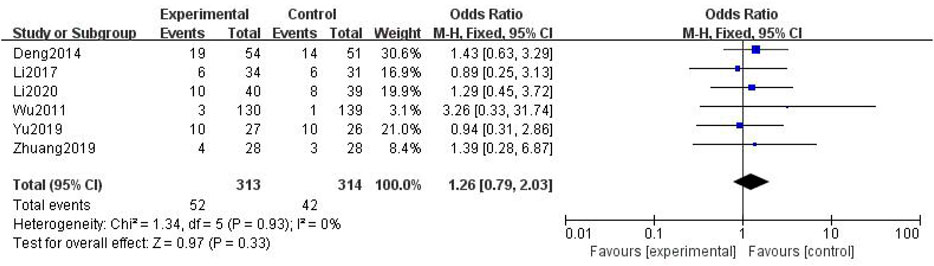
In total, six studies with 627 patients were included (Wu, 2011; Deng, 2014; Li and Cai, 2017; Yu and Xu, 2019; Zhuang, 2019; Li et al., 2020), with 313 in the treatment group and 314 in the control group. The heterogenicity test showed homogeneity among the four studies (p > 0.05, I2 = 0%). Therefore, the fixed-effect model was used for data analysis. The meta-analysis showed no statistically significant difference in myelosuppression between the treatment and control groups (OR = 1.26, 95% CI [0.79, 2.03], p > 0.05, Figure 12).
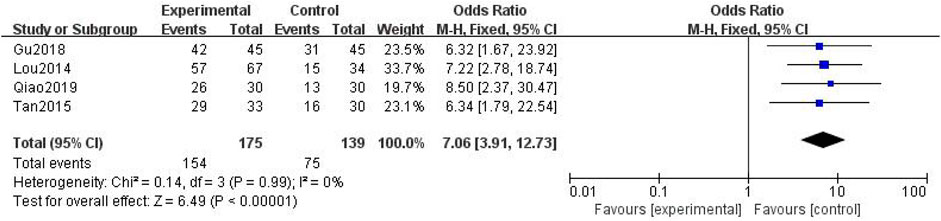
3.3.2.3 Analgesic Rate
In total, four studies with 314 patients were included (Lou et al., 2014; Tan and Qi, 2015; Gu et al., 2018; Qiao, 2019), with 175 in the treatment group and 139 in the control group. The heterogenicity test showed that there was homogeneity among the four studies (p > 0.05, I2 = 0%). Therefore, the fixed-effect model was used for data analysis. The meta-analysis showed a statistically significant difference in the analgesic rate between the treatment and control groups (OR = 7.06, 95% CI [3.91, 12.73], p < 0.01, Figure 13).
3.3.3 Sensitivity Analysis
When heterogeneity was identified among studies in the outcome index, the causes of heterogeneity were analyzed. Examination of the impact of each study on this meta-analysis and sensitivity analysis was conducted; each study was eliminated to calculate the heterogeneity and effective size of the remaining studies.
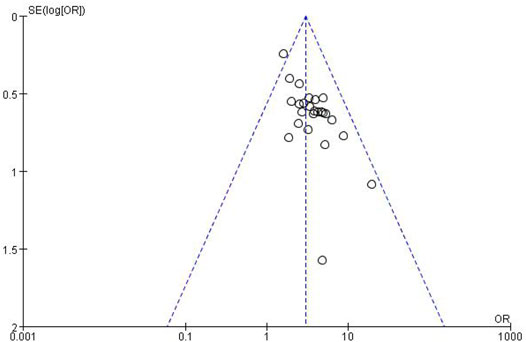
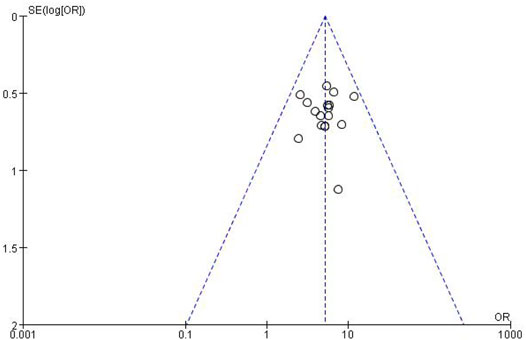
3.3.4 Publication Bias Assessment
The funnel plots for the incidence of peripheral neurotoxicity and the overall effect rate (Figures 14, 15) showed that the left and right sides were symmetric, indicating low publication bias. The results suggest that CHM external treatment decreased the incidence of peripheral neurotoxicity, increased clinical efficacy, and improved quality of life in patients with CIPN.
4 Discussion
CIPN is characterized by limb pain and numbness, which is referred to as “Bi syndrome” in TCM. Chemotherapeutic drugs belong to “toxic evil” in TCM; this is equivalent to anticancer CHM (Ren, 2015) and can easily damage the healthy qi of the human body. In addition, cancer patients lack essence and healthy qi, resulting in a deficiency of qi and blood, phlegm congealing, and blood stasis (Ma and Huo, 2019). Therefore, the basic principles of treatment should focus on tonifying qi, replenishing blood, promoting blood circulation, and dredging collaterals (Ji and Wu, 2016).
CHM external and internal treatments show similar efficacy with different features. CHM external treatment is based on the meridian theory, which can strengthen the penetration of drugs through the skin to the interior of the body. It not only helps the effective CHM ingredients to directly cure the illness and better relieve symptoms but also promotes blood circulation, improves immunity, and regulates the function of the viscera (Wang and Zhu, 2015). Furthermore, it solves the problem of a poor curative effect when patients fail to take oral medications for various reasons (Yan, 2019). The treatment reflects holism in TCM (Liu and Zuo, 2012). Previous studies have indicated that external treatment with CHM can effectively decrease the incidence of peripheral neurotoxicity, improve clinical efficacy and clinical symptoms, prevent the occurrence of adverse reactions, and improve patient quality of life (Ji et al., 2017). Li et al. (2020) found that wet application of CHM can prevent the occurrence of peripheral neurotoxicity, improve nerve conduction velocity, reduce NRS score, and effectively relieve pain symptoms. Ye and Chen (2019) have found that TCM fumigation and washing can significantly reduce the incidence of peripheral neurotoxicity, effectively improve adverse reactions such as bone marrow suppression, and improve quality of life, further confirming the effectiveness and safety of CHM for the treatment of CIPN.
Among the 33 included studies, Guizhi was the most frequently used CHM followed by Huangqi and Honghua, which is consistent with CIPN’s previous treatment principle of “benefiting qi, promoting blood circulation, removing blood stasis, warming Yang, and dredging collaterals” (Zheng et al., 2015). Guizhi lubricates the joints and warms the meridian, curing numbness and pain in the limbs. Previous studies have shown that Guizhi exhibits anticancer activity and promotes vascular protection by accelerating blood flow and inhibiting platelet aggregation and thrombosis (Huang et al., 2007). Huangqi can dilate blood vessels and improve peripheral blood supply, thereby promoting limb blood circulation (Chen, 2018). Honghua can activate blood, unblock meridians, disperse blood stasis, and relieve pain. Modern pharmacology shows that Honghua can protect vascular endothelial cells, inhibit vascular smooth muscle, nourish nerves, and improve nerve conduction velocity (Shi and Li, 2017).
In total, 33 studies were included in this study. The results showed that the treatment group had lower incidence of peripheral neurotoxicity and greater pain relief (p < 0.01), overall response rate, KPS, TCM syndrome effect (p < 0.01), peroneal nerve conduction velocity, and median nerve conduction velocity (p < 0.05), and reduced TCM syndrome score and NRS (p < 0.01) than that of the control group. However, there was no difference in myelosuppression between the two groups.
In summary, the treatment of CIPN using TCM showed preventive and control effects by decreasing the incidence of peripheral neurotoxicity, alleviating clinical symptoms, and improving the quality of life. However, there was no significant improvement in bone marrow inhibition. Bone marrow inhibition is a common adverse reaction in chemotherapy patients; it is caused by various mechanisms and leads to decline in the activity and function of hematopoietic stem cells in the bone marrow, resulting in inability to produce enough blood cells. It manifests as a decrease in white blood cells and platelet counts, resulting in a decline in the body’s immunity, and thus significantly affecting the compliance of chemotherapy patients and chemotherapy efficacy. Therefore, the results of this study suggest that TCM should be selected purposely based on the characteristic of bone marrow inhibition, which has important clinical significance. In addition, although the safety of Chinese medicine is high, and adverse reactions are relatively rare, attention is still needed. In the process of Chinese medicine treatment, doctors should pay close attention to the patient’s vital signs and physical condition. If discomfort exists, treatment should be immediately stopped, and appropriate measures should be taken. When dizziness or panic occurs, windows should be opened for ventilation, and patients should rest in bed. When allergic reactions occur, patients should take anti-allergy drugs under the guidance of a doctor and avoid scratching their skin.
5 Limitations
This study systematically evaluated the clinical efficacy of external CHM treatment of CIPN. CHM external treatment significantly reduced the incidence of peripheral neurotoxicity and improved clinical symptoms and quality of life. However, there were also some limitations to this study. First, cancer patients often suffer from a deficiency of qi and blood. Qi stagnation and blood stasis as clinical manifestations, coupled with the use of chemotherapy drugs, further consume qi and injure blood, thereby aggravating the symptoms or leading to various complications. This makes the diagnosis of CIPN difficult and results in incomplete or poor-quality literature that may have been included in this study. Second, different types of external treatment, treatment frequency, duration, and other factors have caused heterogeneity among the various studies. Finally, the quality of the methodology and applied method reports included in this study were poor, thereby creating a risk of bias. If the allocation results were not hidden, there may have been a selection bias. If the blinding was not implemented, that is, the subjects, researchers, and testers were not blinded, selection, implementation, and measurement bias were likely (Liu, 2012).
6 Conclusion
The results of this study indicated that compared with other therapies, external treatment with CHM for CIPN has obvious advantages; it may significantly decrease the incidence of peripheral neurotoxicity and clinical symptoms and improve quality of life. These results may guide clinical practice. Due to the limitations of this study, further rigorously designed, large-scale, multicenter RCT trials are needed to fully evaluate the efficacy of CHM external treatment for CIPN to provide more reliable evidence.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author Contributions
SJ obtained funding for the study.SJ, LQ-Y, CF-H, LY, LH,WX, and LF-L designed this trial. LQ-Y and CF-H wrote the first draft of the manuscript, and the remaining coauthors participated in the revision of the subsequent draft. All authors approved the final version of the manuscript.
Funding
Shanghai Science and Technology Commission TCM Guide Project (18401905100); Shanghai Charity Cancer Research Center Fund Project (2017-2020).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors would like to thank all the researchers in our working group. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
Argyriou, A. A., Bruna, J., Marmiroli, P., and Cavaletti, G. (2012). Chemotherapy-induced Peripheral Neurotoxicity (CIPN): an Update. Crit. Rev. Oncol. Hematol. 82 (1), 51–77. doi:10.1016/j.critrevonc.2011.04.012
Avan, R., Janbabaei, G., Hendouei, N., Alipour, A., Borhani, S., Tabrizi, N., et al. (2018). The Effect of Pregabalin and Duloxetine Treatment on Quality of Life of Breast Cancer Patients with Taxane-Induced Sensory Neuropathy: A Randomized Clinical Trial. J. Res. Med. Sci. 23, 52. doi:10.4103/jrms.JRMS_1068_17
Cai, Z. (2016). Clinical Observation of Prevention of Oxaliplatin-Related Neurotoxicity by External bath of “Wen Jing Huo Xue Decoction”[D]. Guangzhou, China: Guangzhou University of Chinese Medicine.
Cao, W., Wei, G., Li, L., Ma, J., Li, D., Sun, Y., et al. (2019). Advances in Treatment of Chemotherapy-Related Peripheral Neurotoxicity and Prevention and Treatment Strategies of Traditional Chinese Medicine[J]. World Sci. Technology/Modernization Traditional Chin. Med. Materia Med. 21 (07), 1458–1466.
Chen, S. (2018). Prevention of Oxaliplatin-Induced Peripheral Neuropathy by Huangqi Guizhi Wuwu Decoction: A Systematic Review and Meta-analysis[D]. Nanjing: Nanjing University of Chinese Medicine.
Dai, T., Chen, C., Pei, J., Wang, Y., Lv, W., Yang, X., et al. (2017). Clinical Study on Maqianzi Capsules Treating Bortezomib-Induced Peripheral Neuropathy[J]. Chin. Arch. Traditional Chin. Med. 35 (6), 1405–1409. doi:10.13193/j.issn.1673-7717.2017.06.015
Deng, C. (2014). Clinical Research on the Prevention and Treatment of Neurotoxicity Induced by Oxaliplatin through Nourishing Blood and Warming Channel for Dredging Collateral with Chinese Herbal Medicine Soaking[D]. Beijing, China: Capital medical University.
Fan, J. (2019). Clinical Observation on Fumigation Treatment of Huangqi Guizhi Wuwu Decoction and Wenjing Tongluo Decoction for Peripheral Neurotoxicity after Chemotherapy in Tumor Patients[J]. Chin. J. Cancer Prev. Treat. 26 (S1), 164–165.
Gao, J., Li, D., and Yan, S. (2015). Clinical Observation on 30 Cases of Chinese Medicine Soaking Treatment for Oxaliplatin-Induced Neurotoxicity[J]. Hebei J. Traditional Chin. Med. 37 (11), 1638–1640.
Gu, N., Wang, F., Xu, Y., and Li, Z. (2018). Clinical Study on the Treatment of Peripheral Nerve Injury after Chemotherapy by Self-Made Tongluo Huoxue Tang[J]. ACTA CHINESE MEDICINE 33 (01), 22–26. doi:10.16368/j.issn.1674-8999.2018.01.006
Gu, Y. (2020). The Clinical Observation on the Treatment of Cold Dampness Stasis Type Peripheral Neuropathy Caused by Taxanes or Platinum with External Washing of Wenyang Huashi Tongluo Formula Combined with MeCobalamin tablets[D]. Nanjing, China: Nanjing University of Traditional Chinese Medicine.
Guo, Y. (2015). The Clinical Observation on NO. 1 Chinese Traditional Fumigation and Washing Recipe Prevent Peripheral Neurotoxicity Caused by oxaliplatin[D]. Nanjing, China: Nanjing University of Chinese Medicine.
He, X., and Li, S. (2019). Clinical Observation of Yanghe Decoction on Peripheral Neuropathy of Oxaliplatin Chemotherapy in Colorectal Cancer[J]. J. Sichuan Traditional Chin. Med. 37 (09), 101–104.
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Huang, J., Wang, S., Luo, X., Xie, Y., and Shi, X. (2007). Cinnamaldehyde Reduction of Platelet Aggregation and Thrombosis in Rodents. Thromb. Res. 119, 337–342. doi:10.1016/j.thromres.2006.03.001
Ji, Y., Li, G., Li, L., Shen, Z., Li, W., and Wu, M. (2017). Prevention of Oxaliplatin-Induced Peripheral Neurotoxicity by External Washing of Traditional Chinese Medicine: A Systematic Review and Meta-Analysis[J]. Chin. Arch. Traditional Chin. Med. 35 (02), 335–340. doi:10.13193/j.issn.1673-7717.2017.02.022
Ji, Y., and Wu, M. (2016). Research Progress on Traditional Chinese Medicine Preventing and Treating Peripheral Neurotoxicity Induced by Chemotherapy for Malignant Tumor[J]. Shandong J. Traditional Chin. Med. 35 (10), 924–926. doi:10.16295/j.cnki.0257-358x.2016.10.029
Ke, Y., Mo, M., Chen, C., and Hu, Z. (2016). 20 Cases of Empirical External Washing Formula Treating Peripheral Neuropathy after Chemotherapy for Multiple Myeloma [J]. J. Shaanxi Univ. Chin. Med. 39 (01), 56–58. doi:10.13424/j.cnki.jsctcm.2016.01.024
Li, D., and Cai, Z. (2017). Clinical Study of External Bath with "Wenjing Huoxue Prescription" on Peripheral Neurotoxicity Due to Chemotherapy[J]. Acta Chin. Med. 32 (07), 1148–1150. doi:10.16368/j.issn.1674-8999.2017.07.301
Li, M., Wang, W., and Jin, S. (2020). Clinical Study on Prevention and Treatment of Oxaliplatin-Induced Neuropathic Pain with Huangqi Guizhi Wuwu Decoction Combined with Wax Therapy [J]. J. Liaoning Univ. TCM 22 (04), 91–94. doi:10.13194/j.issn.1673-842x.2020.04.023
Liu, M. (2012). Systematic Review, Meta-Analysis Design and Implementation Methods [M]. Beijing: People's Medical Publishing House, 68–71.
Liu, X., and Zuo, M. (2012). Treatment of Oxaliplatin-Induced Peripheral Neurotoxicity with Soaking-Washing with Chinese Medicinals[J]. Mod. Chin. Clin. Med. 19 (5), 23–26.
Liu, Z., Chen, S., Cai, J., Zhang, E., Lan, L., Zheng, J., et al. (2013). Traditional Chinese Medicine Syndrome-Related Herbal Prescriptions in Treatment of Malignant Tumors. J. Tradit Chin. Med. 33, 19–26. doi:10.1016/s0254-6272(13)60095-3
Lou, Y., Tian, A., Zhang, X., Feng, L., Tan, H., Zhu, S., et al. (2014). Randomized, multi-center, Double-Blinded, Controlled Clinical Trial of External Treatment of TCM on Peripheral Neuropathy Caused by Chemotherapy[J]. China J. Traditional Chin. Med. Pharm. 29 (08), 2682–2685.
Ma, J., and Huo, J. (2019). The Clinical Research Status of Chemotherapy - Induced Peripheral Neuropathy[J]. J. Mod. Oncol. 27 (13), 2415–2420.
Ma, J., Kavelaars, A., Dougherty, P. M., and Heijnen, C. J. (2018). Beyond Symptomatic Relief for Chemotherapy-Induced Peripheral Neuropathy: Targeting the Source. Cancer 124 (11), 2289–2298. doi:10.1002/cncr.31248
Ma, J., and Shan, H. (2017). Clinical Effect Observation on Chinese Medicine External Treatment for Oxaliplatin-Induced Peripheral Neurotoxicity[J]. Shanxi J. Traditional Chin. Med. 33 (01), 24+26.
Pan, C., Yao, Q., Zhu, L., and Shen, K. (2021). Effect of Oral Administration of Qiteng Tongluo Formula Combined with Hong'ai Decoction External Bath on Oxaliplatin-Induced Peripheral Neuropathy[J]. Liaoning J. Traditional Chin. Med. 48 (06), 140–142. doi:10.13192/j.issn.1000-1719.2021.06.039
Qiao, X. (2019). Clinical Study on Danggui Sini Decoction Combined with Traditional Chinese Medicine External Washing in Treating Peripheral Neuropathy[D]. Hebei, China: Heibei University.
Ren, J. (2015). Clinical Observation on Auricular Pressure and Acupoint Massage Preventing and Treating Nausea and Vomiting after Chemotherapy[J]. New Chin. Med. 47 (12), 207–208. doi:10.13457/j.cnki.jncm.2015.12.093
Shi, Y., and Li, C. (2017). Advances of Chinese and Western Medicine in Treating Chemotherapy-Induced Peripheral Neurotoxicity[J]. Clin. J. Traditional Chin. Med. 29 (03), 327–330. doi:10.16448/j.cjtcm.2017.0107
Song, Y. (2017). Prevention and Treatment of Oxaliplatin Neurotoxicity by of Clinical Research[D]. Shandong, China: Shandong University of Chinese Medicine.
Staff, N. P., Grisold, A., Grisold, W., and Windebank, A. J. (2017). Chemotherapy-induced Peripheral Neuropathy: A Current Review. Ann. Neurol. 81 (6), 772–781. doi:10.1002/ana.24951
Tan, Z., and Qi, Y. (2015). Clinical Study on Gastric Cancer Patients with Chemotherapy-Induced Neurotoxicity Treated with Yiqi Wenyang Huoxue Method [J]. Shandong J. Traditional Chin. Med. 34 (02), 101–103. doi:10.16295/j.cnki.0257-358x.2015.02.002
Tian, M., and Huang, H. (2017). The Therapeutic Effect of Modified Huangqi Guizhi Wuwu Tang for Multiple Myeloma: An 18-year Follow-Up Case Report. Medicine (Baltimore) 96 (49), e9074. doi:10.1097/MD.0000000000009074
Van Hecke, O., Torrance, N., and Smith, B. H. (2013). Chronic Pain Epidemiology and its Clinical Relevance. Br. J. Anaesth. 111, 13–18. doi:10.1093/bja/aet123
Wang, B. (2020). Clinical Observation on Peripheral Neurotoxicity Induced by Paclitaxel Treated with Wenluotong Granules Clinical Observation[D]. Beijing, China: Beijing University of Chinese Medicine.
Wang, H., and Zhu, Y. (2015). Research Progress on Prevention and Treatment of Oxaliplatin Induced Peripheral Neurotoxicity by Traditional Chinese Medicine[J]. Med. Pharm. J. Chin. People's Liberation Army 27 (4), 76–80.
Wang, L. (2020). Clinical Observation of the Traditional Chinese Xionggui Juanbi Decoction on the Prevention and Treatment of Peripheral Neuropathy Caused by Vinblastine[D]. Gansu, China: Gansu University of Chinese Medicine.
Waseem, M., Kaushik, P., Tabassum, H., and Parvez, S. (2018). Role of Mitochondrial Mechanism in Chemotherapy-Induced Peripheral Neuropathy. Curr. Drug Metab. 19 (1), 47–54. doi:10.2174/1389200219666171207121313
Wei, M. (2014). Clinical Observation the Therapeutic Effects of External Using Wen Yang Tong Bi Fang on the Treatment of Chemotherapy-Induced Peripheral Neuropathy [D]. Beijing, China: Beijing University of Chinese Medicine.
Wu, S. (2011). Clinical Observation of Prevention of Oxaliplatin-Related Neurotoxicity by External bath of “Wen Tong 1 Decotion”[D]. Guangzhou, China: Guangzhou University of Chinese Medicine.
Xiong, B., Zhang, M., Huang, X., Ren, J., Ni, X., Lai, C., et al. (2017). Clinical Observation of Foot and Hand Bathing with Chinese Medicine Combined with Auricular Point Sticking in Treating Peripheral Neurotoxicity after Chemotherapy [J]. J. New Chin. Med. 49 (02), 129–131. doi:10.13457/j.cnki.jncm.2017.02.045
Xu, J., Zhang, X., Wang, X. W., Qin, Z., Tang, J., Lu, Y., et al. (2018). Clinical Observation of Xiaotan Tongluo Gel Combined with Mecobalamin Tablets in Treating Peripheral Neurotoxicity Induced by Chemotherapy [J]. Chin. J. Inf. TCM 25 (07), 11–15.
Xu, P., Xue, P., Li, L., Zhu, S., and He, S. (2018). Clinical Research Progress of Traditional Chinese Medicine and Western Medicine in the Treatment of Chemotherapy-Induced Peripheral Neuropathy[J]. J. Shandong Univ. Traditional Chin. Med. 42 (06), 565–568. doi:10.16294/j.cnki.1007-659x.2018.06.024
Yan, W. (2019). Huoxue Tongluo Zhitong Decoction for the Treatment of Oxaliplatin Clinical Study of Peripheral neuropathy[D]. Changchun, China: Changchun University of Chinese Medicine.
Yang, H., Zhang, J., Li, F., Zhou, M., and Han, Y. (2018). Application of Traditional Chinese Medicine Foot Bath in Peripheral Neuropathy Caused by Velcade Multiple Myeloma Patients[J]. J. Clin. Res. 35 (9), 1693–1696.
Yao, H. (2020). Observation on the Curative Effect of Traditional Chinese Medicine External bath Combined with Mecobalamine in the Treatment of Oxaliplatin-Induced Peripheral neuropathy[D]. Chengdu, China: Chengdu University of Traditional Chinese Medicine.
Yao, Q., Shen, K., and Gu, X. (2019). Clinical Research on the Neurotoxicity of Oxaliplatin Treated by External Washing of Traditional Chinese Medicine [J]. Mod. Digestion Intervention 2019 (A01), 0882.
Ye, L., and Chen, W. (2019). Clinical Effect of Qiteng Tongbi Fumigant Combined with Ganglioside in Preventing Neurotoxicity Caused by mFOLFOX6 Chemotherapy: A Randomized Controlled Study[J]. J. Chongqing Med. Univ. 44 (07), 938–943. doi:10.13406/j.cnki.cyxb.002146
Yu, B., and Xu, P. P. (2019). Clinical Research on the Prevention and Treatment of Oxaliplatin Induced Peripheral Neurotoxicity by Jiawei Wutou Decoction[J]. Mod. Traditional Chin. Med. 39 (02), 63–67. doi:10.13424/j.cnki.mtcm.2019.02.020
Yuan, Y. (2015). Clinical Research on the Circulation of Menstruation Soup Soak Prevention and Control of Traditional Chinese Medicine for External Use Only Oxaliplatin into the Curative Effect of Peripheral Nerve Toxicity Caused by Chemotherapy[D]. Liaoning, China: Liaoning University of Traditional Chinese Medicine.
Zhang, W. (2019). Clinical Study on the Treatment of Fumigation and Washing with Chinese Herbal Medicine for Peripheral Neurotoxicity Induced by Chemotherapy[J]. J. Hunan Univ. Chin. Med. 39 (12), 1517–1520.
Zheng, L., Mary, Y., Liu, M., and Jia, L. (2015). Literature Analysis of the Law of Drug Use in the Treatment of Chemotherapy Peripheral Neuropathy by Chinese Medicine. J. Traditional Chin. Med. 56 (17), 1509–1512. doi:10.13288/j.11-2166/r.2015.17.019
Zheng, X. (2002). Guiding Principles for Clinical Research of the New-Made of Chinese Herbal Medicine (Trial)[M], 174-175. Beijing: China Medical Science Press, 177–180.
Zhu, L. (2017). Application of Blood-Activating and Stasis-Dissolving Medicine in Oxaliplatin Chemotherapy[J]. Shenzhen J. Integrated Traditional Chin. West. Med. 27 (04), 28–30. doi:10.16458/j.cnki.1007-0893.2017.04.013
Zhu, Z., Wang, S., and Hu, Y. (2017). Oral Administration and External bath of TCM for Cumulative Peripheral Neurotoxicity Induced by Oxaliplatin: a Report of 42 Cases [J]. Anhui Med. Pharm. J. 21 (09), 1696–1698.
Zhuang, J. (2019). Curative Effect Observation of Shuangjinlong External Washing Prescription on Peripheral Neurotoxicity in Patients with Colorectal Cancer Induced by oxaliplatin[D]. Guangzhou, China: Guangzhou University of Traditional Chinese Medicine.
Keywords: traditional Chinese medicine, Chinese herbal medicine, chemotherapy-induced peripheral neuropathy, systematic review, meta-analysis
Citation: Li Q-y, Cai F-h, Lu Y, Liu H, Wang X, Li F-l and Shi J (2022) External Treatment With Chinese Herbal Medicine for Chemotherapy-Induced Peripheral Neuropathy: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:764473. doi: 10.3389/fphar.2022.764473
Received: 07 September 2021; Accepted: 28 January 2022;
Published: 18 February 2022.
Edited by:
Juei-Tang Cheng, Chang Jung Christian University, TaiwanReviewed by:
Saeideh Momtaz, Tehran University of Medical Sciences, IranJanet Schloss, Southern Cross University, Australia
Copyright © 2022 Li, Cai, Lu, Liu, Wang, Li and Shi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Shi, c2hpanVuZG9jdG9yQDE2My5jb20=
†These authors share first authorship
 Quan-yao Li
Quan-yao Li Fei-hong Cai
Fei-hong Cai Ying Lu3
Ying Lu3 Hui Liu
Hui Liu