
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Pharmacol. , 12 April 2022
Sec. Drugs Outcomes Research and Policies
Volume 13 - 2022 | https://doi.org/10.3389/fphar.2022.759998
This article is part of the Research Topic Reducing the Harm of Medication - Recent Trends in Pharmacovigilance View all 17 articles
Background and objectives: Highly widespread use of pro re nata (PRN) medicines in various healthcare settings is a potential area for improper medication prescription and administration leading to patient harm. This study aimed to summarize and integrate the findings of all relevant individual studies regarding the practical considerations of PRN medicines management including strategies and interventions by healthcare professionals for safe prescription, dispensing, administration, monitoring, and deprescription of PRN medicines in healthcare settings.
Methods: An integrative systematic review on international databases were performed. Electronic databases including Web of Knowledge, Scopus, PubMed (including MEDLINE), and Cinahl were searched to retrieve articles published until end of May 2021. Original qualitative, quantitative, and mixed methods studies written in English were included with a focus on PRN medicines management in healthcare settings. Research synthesis using the narrative method was performed to summarise the results of included studies.
Results: Thirty-one studies on PRN medicines in healthcare settings by different healthcare providers were included after the screening of the databases based on eligibility criteria. They were published from 1987 to 2021. The majority of studies were from Australia, the United States, Canada, and the United Kingdom and were conducted in psychiatric settings. Given variations in their purposes, methods, and outcomes, the research synthesis was conducted narratively based on diversities and similarities in findings. Eight categories were developed by the authors as follows: “PRN indications and precautionary measures,” “requirements of PRN prescription,” “interventions for PRN administration,” “monitoring and follow up interventions,” “deprescription strategies,” “healthcare professionals’ role,” “participation of patients and families,” and “multidisciplinary collaboration.” Each category consists of several items and describes what factors should be considered by healthcare professionals for PRN medicines management.
Conclusion: The review findings provide insights on the practical considerations of PRN medicines management in clinical practice. The suggested list of considerations in our review can be used by healthcare professionals for optimal PRN medicines management and safeguarding patient care.
Medication therapy is the most common therapeutic intervention (World Health Organization, 2019). Medicines management is the process of the evaluation of the patient’s health status and the need for medications’ prescription and dispensing, and the administration and monitoring of medication’s effectiveness (Car et al., 2017; Mishore et al., 2020). Medication errors are significantly prevalent and happen in up to 67% of patients during hospitalization (Nguyen et al., 2017). They are major international contributors to healthcare complications and the increased costs of healthcare (Acheampong et al., 2014). Therefore, the safe use of medications has a top priority for healthcare systems with an calculated annual burden of $ 42 billion worldwide (World Health Organization, 2018).
Given the frequency and potential association of preventable medication errors with adverse patient outcomes, the development of strategies through medicines management for the reduction of their clinical magnitude are common (Kwan et al., 2013; Basey et al., 2014). Prevention and reduction of medication errors are the primary goals of healthcare organizations through participation in quality improvement initiatives. They are also intertwined with ethical healthcare (Smith, 2013; Pitkänen et al., 2016).
“Pro re nata” (PRN), “when required,” or “as needed” is defined as the prescription and administration of medications based on the immediate patients’ needs instead of prescheduled administration times (Martin et al., 2017). PRN medications often are used for relieving symptoms rather than treating patients’ underlying diseases (Harper et al., 2017). Common medications used as PRN are psycholeptic and psychotropic medications including antipsychotics, anxiolytics, sedatives and hypnotics; painkillers; gastrointestinal medications; and other drugs used for mitigating physical and psychological symptoms (Allers et al., 2017; Dörks et al., 2019; Vaismoradi et al., 2020).
PRN medications are prescribed and administered at least once to 68–83.9% of patients suffering from mental health issues (Vaismoradi et al., 2018). In mental healthcare settings, PRN prescriptions have major contributions to the frequency of dangerous high and combined doses of antipsychotic medications that patients receive (Baker et al., 2008). It has been reported that 62–97% of patients treated in mental health wards receive PRN medications especially antipsychotics and psychotropics (Baker et al., 2008; Fujita et al., 2013; Martin et al., 2017; Hipp et al., 2020; Saito et al., 2020). The use of psychotropic medications as PRN is associated with abuse, polypharmacy, increased risks of morbidity, dependency, and risk of falls, which complicate its safety (Hilton and Whiteford, 2008; Nyborg et al., 2017). Therefore, the potential of patient harm should be carefully evaluated (Stroup and Gray, 2018).
This is the nurse responsibility to administer PRN medications based on the patient health condition after receiving the physician’s prescription order (Dörks et al., 2019). Although PRN medications can improve care efficiency, they are accompanied with potential medication safety issues (Oh et al., 2014). Medication errors can happen during the prescription, dispensing, and administration of PRN medications (Vaismoradi et al., 2019). In the intensive care unit, medication errors have been reported in 89% of PRN medication orders (Alaqqad et al., 2016). Improper prescription and administration of PRN medications can cause medication interactions, adverse drug reactions (ADRs), overuse and abuse (Davies et al., 2007; Vaismoradi et al., 2018). PRN medications increase the complexity of medication regimens (Picton et al., 2021). Frequent administration of PRN medications can hide the signs and symptoms of underlying diseases (Vaismoradi et al., 2018).
PRN is considered an unsafe mechanism for medication delivery because the chain of accountability between the decision to prescribe PRN medications and the decision to administer them is unclear (Price and Baker, 2013). The safety of PRN medicines management is influenced by healthcare professionals’ knowledge and skills, and the healthcare culture (Morkunas et al., 2016). The decision for the use of PRN medications are taken in collaboration with the physician, nurse, patient, and families, but it is accompanied by the risk of errors due to their distinct interpretations of the medication process (Hogan et al., 2019). Ambiguities in PRN medicines management including indication for prescription, method of administration, and complete documentation can adversely impact patient care outcomes, increase the risk of polypharmacy, adverse drug events, and abuse (Friedman et al., 2012; Oh et al., 2014; Hammer et al., 2019). The decision for the administration of PRN medications is a nearly independent component of the nursing role after prescribing, and nurses require a clear articulation in clinical practice associated with PRN medication administration (Molloy et al., 2012).
Discrepancies in medicines management between healthcare professionals in various healthcare settings indicate the potential concerns for the use of PRN medications (Stubbings et al., 2019). Nurses often interpret PRN orders for painkillers to be the least amount of PRN medication use. Also, the practice of PRN medications has setting-specific characteristics rather than being evidenced-based (Lellan, 1997; Sonntag et al., 2006). Moreover, there are disparities in the perspectives of healthcare professions especially nurses with regard to the appropriate indications for PRN medications use in patients with different health conditions (Baker et al., 2007b). Furthermore, the monitoring of PRN medications by nurses after administration and related documentation are not properly performed (Friedman et al., 2012; Ross et al., 2021). Making decisions on the use of PRN medications usually is not guideline-based and rather is based on habits and previous experiences in clinical practice (Douglas-Hall and Whicher, 2015; Walsh et al., 2021).
Due to the highly widespread use of PRN medications in various healthcare settings, and the growing concern regarding the use of PRN medications as the first-line choice for relieving patient’s suffering, there is a need for the introduction of evidenced-based protocols and procedures with regard to prescription, dispensing, administration, and monitoring of PRN medications. Also, reviews on PRN medications in terms of indication, frequency, and interdisciplinary collaborations for PRN medicines management is insufficient (Martin et al., 2017; Vaismoradi et al., 2021a). Therefore, this study aimed to summarize and integrate the findings of all relevant individual studies regarding the practical considerations of PRN medicines management including strategies and interventions by healthcare professionals for prescription, dispensing, administration, monitoring, and deprescription of PRN medicines in healthcare settings. The review question was as follows: What are the practical considerations in terms of strategies and interventions by healthcare professionals including nurses, pharmacists and physicians for PRN medicines management in short-term, long-term and acute healthcare settings?
An integrative systematic review was conducted. This is a review method that allows for the inclusion of studies with qualitative and quantitative methodologies and considers a narrative approach for the synthesis of data from a wide range of research designs (Whittemore and Knafl, 2005; Souza et al., 2010). The review protocol was developed by the authors prior to the study, and all steps of the review were conducted accordingly (Supplementary File S1). In addition, PROSPERO was searched to identify ongoing or recently completed similar systematic reviews.
The review question was framed using the PICO statement as follows:
P (Population): healthcare providers including nurses, pharmacists, and physicians who are involved in PRN medicines management; I (Interest): practical considerations in terms of interventions and strategies by healthcare professionals for prescription, dispensing, administration, monitoring, and deprescription of PRN medicines; Co. (Context): all contexts in healthcare consisting of child, adult, physical and mental health.
The review process was informed by the Preferred Reporting Items Systematic Reviews and Meta-analysis (PRISMA) statement (Liberati et al., 2009) (Supplementary File S2).
Search keywords and phrases were determined by the research team consisting of the nurse (AM, PP, MV), physician (CW), and pharmacist (SJ) through the review of relevant literature and based on a pilot search in general and specialized databases. The Boolean search method was used with the inclusion of the following keywords:
(PRN OR “pro re nata” OR “as needed” OR “as required”) AND (guideline OR “practice guideline” OR “clinical practice guideline” OR “clinical guideline” OR “critical pathway” OR “clinical pathway” OR “critical path” OR “clinical path” OR “patient care planning” OR instruction OR technique OR program*) AND (medication OR drug OR medicines OR “pharmaceutical preparations” OR pharmaceuticals OR “medicines management”).
The online databases of Web of Knowledge, PubMed (including MEDLINE), Cinahl, and Scopus were searched to retrieve empirical studies published by peer-reviewed scientific journals up to end of May 2021. In addition, cross-references from bibliographies and manual search in the references lists of selected studies were performed to expand the search coverage.
Inclusion criteria for the selection of studies to our review were: qualitative, quantitative, and mixed methods studies with a focus on PRN medicines management; use of any practical consideration in terms of interventions and strategies by healthcare professionals for prescription, administration, monitoring, and management of the side effects and ADRs of PRN medications; published in peer-reviewed scientific journals.
Studies without exact relevance to PRN medicines management were excluded. Also, exclusion encompassed non-empirical studies such as reviews, letters, commentaries, conference proceedings, theses, dissertations, books, and governmental documents that did not provide appropriate data to our review.
The phases of review were carried out separately by two review authors (AM, MV). They shared results and conducted online conversations to make decisions on the subsequent search steps. The studies’ titles, abstracts and full-texts were screened step by step by them. The review authors held discussions in case of discrepancies in their perspectives to reach agreement, and also sought the perspective of the third review author.
Quality of selected studies was evaluated in terms of the appropriateness of research structure and reporting using the Enhancing the Quality and Transparency of Health Research (EQUATOR). According to the studies’ designs, the following tools were used: 1) the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) for observational and cross-sectional studies; 2) the Standards for Reporting Qualitative Research (SRQR) for qualitative research; 3) Consolidated Standards of Reporting Trials (CONSORT) for experimental and quasi-experimental studies; 4) the Good Reporting of A Mixed Methods Study (GRAMMS) for Mixed-methods studies (EQUATOR Network, 2019).
For making the final decision on whether or not to include studies in the research synthesis, the authors considered scores obtained by the quality appraisal tools and their collective opinions with regard to the significance and the methodological quality of each study.
The Cochrane Collaboration’s tool for assessing the risk of bias for randomized clinical trials was used and the review authors classified their judgments as low, high, and unclear risk of bias (Higgins and Altman, 2011). The Risk of Bias in Non-randomized Studies of Interventions (ROBINS-I) tool was also used along with the categorization of judgments as follows: low, moderate, serious, critical, and no information regarding risk of bias (Sterne et al., 2016). The risk of bias assessment for cross-sectional studies was adapted from the Newcastle-Ottawa Quality Assessment Scale with the judgment’s classification of low, probably low, probably high, and high risk of bias (Herzog et al., 2013).
For data extraction, a table was developed comprising the following sections: 1) the first author’s surname, publication year, and the country where the study was conducted; 2) study design, sample size, and setting; 3) data relating to the practical considerations of PRN medicines management; 4) name and dose of PRN medications and patients’ age group; and 5) healthcare providers involved in PRN medicines management.
To ensure that the data extraction table could gather the required information on the characteristics of selected studies, a pilot test was conducted on a couple of included studies. The review findings were presented narratively, because the presence of heterogeneities in the methods, aims, and results of the studies hindered us to conduct meta-analysis. Therefore, the findings of the included studies were reviewed and based on diversities and similarities in their findings, appropriate categories were developed. The authors discussed to reach agreement on the allocation of the studies’ findings into the relevant categories.
The search results on the databases were reported in Table 1. In total, 4,972 articles were retrieved. After removing irrelevant and duplicate titles and carrying out abstract and full-text readings, 31 studies were picked out to be considered for data analysis and research synthesis.
Figure 1 presents the study flow diagram based on the PRISMA.
The quality appraisal of the selected articles was performed on the full-text of the selected studies (Table 2). Since all studies were judged to have an acceptable level of quality in terms of methodology, theoretical and conceptual framework, no study was ruled out from our review.
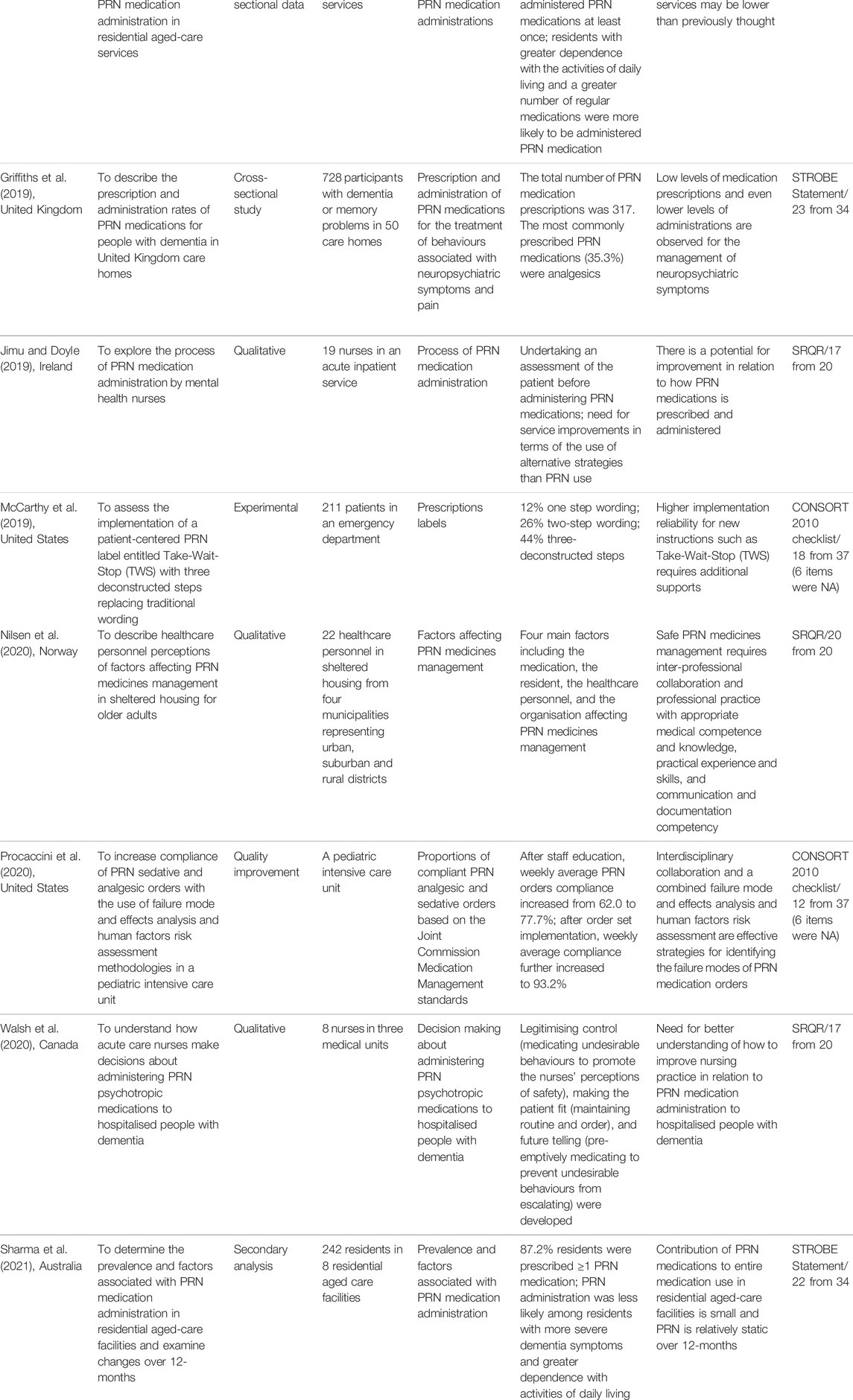
TABLE 2. General characteristics of the included studies to our data analysis and knowledge synthesis.
The results of risk of bias evaluation for two randomized controlled studies (McCarthy et al., 2013; McCarthy et al., 2019) were presented in Supplementary Figure S1. In terms of bias in random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, and selective outcome reporting, the studies presented insufficient data leading to the judgement of unclear risk of bias. In addition, they were judged to have a low risk of bias in terms of bias in incomplete outcome data.
The risk of bias assessment in quasi-experimental (Edwards et al., 2001) and qualitative studies (Procaccini et al., 2020) were described in Supplementary Figure S2. In terms of bias due to confounding and selection of participants into the study, one study was judged to have a low risk of bias and another one had a serious risk of bias. In terms of bias in the classification of interventions and bias due to missing data, one study was judged to have a low risk of bias and another had a critical risk of bias. In addition, both studies had low risk of bias in the view of bias due to deviations from intended interventions and failure to provide information in terms of bias in the measurement of the outcome and bias in the selection of the reported result.
Furthermore, the results of risk of bias for 20 observational studies were presented in Supplementary Figure S3. The selected studies mostly had a low risk of bias in terms of the assessment of exposure (100%), development of the outcome of interest (95%), selection of cases (85%), and controls (85%). In terms of the control of prognostic variable, 40% of the studies had low risk of bias, 20% probably low risk of bias, 15% high risk of bias, and 25% probably high risk of bias.
A summary of selected studies (n = 31) has been presented in Table 2. All studies published in English from 1987 until 2021. Twelve studies were from Australia (Edwards et al., 2001; Geffen et al., 2002; Curtis et al., 2007; Stein-Parbury et al., 2008; Kaur et al., 2009; Usher et al., 2009; Usher et al., 2010; Mullen and Drinkwater, 2011; Russell et al., 2014; Barr et al., 2018; Stasinopoulos et al., 2018; Sharma et al., 2021), five from the United States (Gordon et al., 2008; Carder, 2012; McCarthy et al., 2013; McCarthy et al., 2019; Procaccini et al., 2020), four from Canada (Craven et al., 1987; Swart et al., 2011; Martin et al., 2018a; Walsh et al., 2020), three from the United Kingdom (Di Giulio and Crow, 1997; Baker et al., 2007a; Griffiths et al., 2019), two from Saudi Arabia (Al-Sughayir, 2014; Al-Sughayir, 2017), one from Germany (Dörks et al., 2016), one from Ireland (Jimu and Doyle, 2019), one from Norway (Nilsen et al., 2020), one from Scotland (Akram et al., 2014), and one from Thailand (Chaichan, 2008).
Regarding their methods, 11 studies used the chart review method (Craven et al., 1987; Curtis et al., 2007; Chaichan, 2008; Stein-Parbury et al., 2008; Kaur et al., 2009; Mullen and Drinkwater, 2011; Swart et al., 2011; Akram et al., 2014; Al-Sughayir, 2014; Russell et al., 2014; Al-Sughayir, 2017), six were cross-sectional (Di Giulio and Crow, 1997; Geffen et al., 2002; Gordon et al., 2008; Dörks et al., 2016; Barr et al., 2018; Griffiths et al., 2019), six were qualitative (Usher et al., 2009; Usher et al., 2010; Carder, 2012; Jimu and Doyle, 2019; Nilsen et al., 2020; Walsh et al., 2020), three were interventional (Edwards et al., 2001; McCarthy et al., 2013; McCarthy et al., 2019), two were secondary analysis (Stasinopoulos et al., 2018; Sharma et al., 2021), one was Delphi technique (Baker et al., 2007a), one was mixed-methods (Martin et al., 2018a), and one was qualitative improvement (Procaccini et al., 2020).
Characteristics of PRN medicines management including name and dose of PRN medications, patient’s age group, healthcare providers who were involved in PRN medicines management, and related practical considerations for each of the included studies were presented in Table 3.
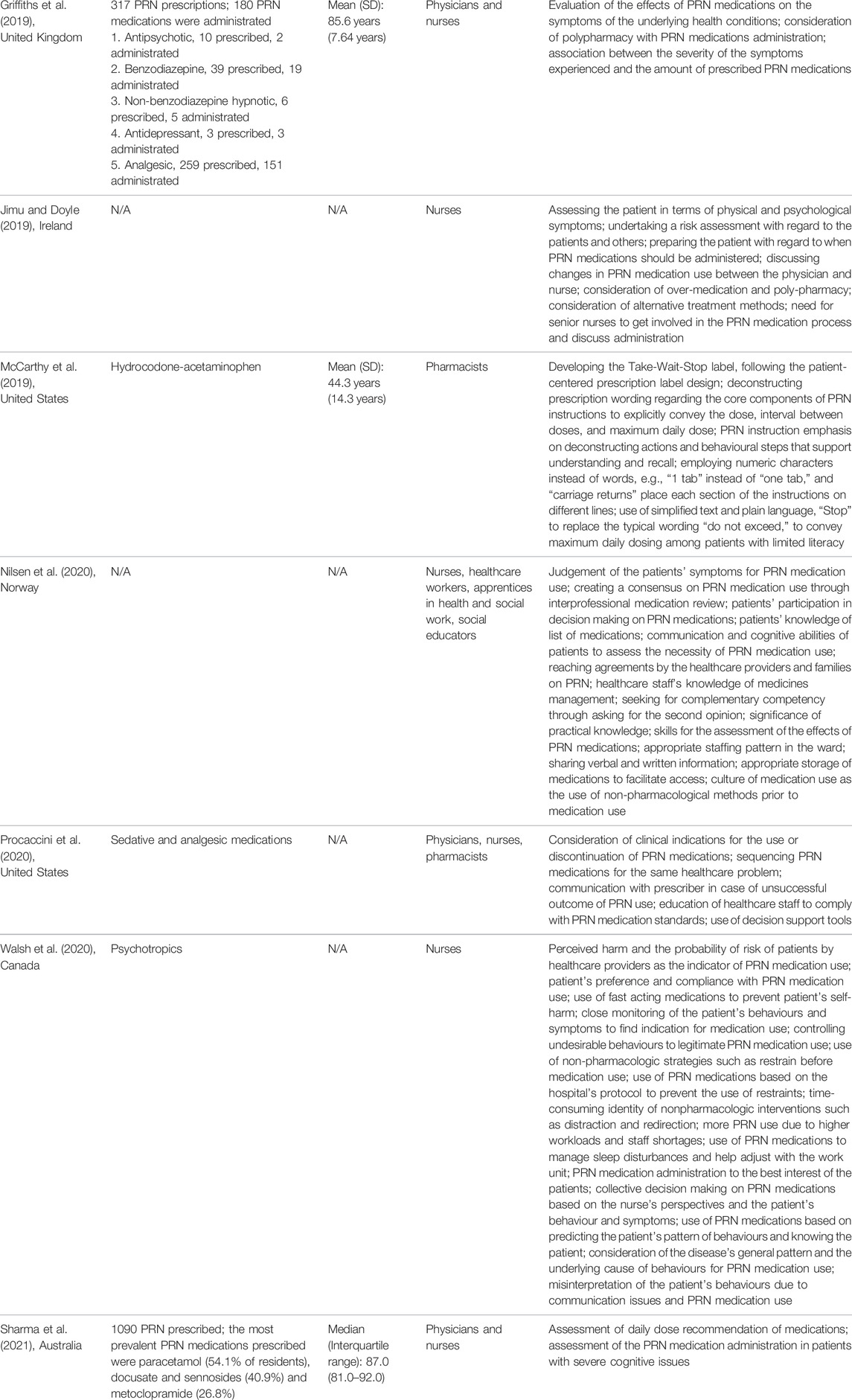
TABLE 3. PRN medicines management and related practical considerations based on the findings of each included study.
During the narrative research synthesis, eight categories in relation to the practical considerations of PRN medicines management were identified: “PRN indications and precautionary measures,” “requirements of PRN prescription,” “interventions for PRN administration,” “monitoring and follow up interventions,” “deprescription strategies,” “healthcare professionals’ role,” “participation of patients and families,” and “multidisciplinary collaboration”. The initial list of items corresponding to each category has been presented in Supplementary File S4.
Prescription of PRN medications should be based on the thorough assessment of patients and collection of data about their medical history (Usher et al., 2009). For the prescription of PRN medications, appropriate indications and purpose of medication use should be specified and fully described (Craven et al., 1987; Baker et al., 2007a). Craven et al. (1987) reported that physicians had not specified any indication for 47% of PRN prescriptions for psychiatric inpatients.
When healthcare providers prescribe a new atypic medication as PRN, they should have more concerns and give more attention to its efficacy and side effects (Usher et al., 2009). Healthcare providers require to find clinical indicators for the continuation or discontinuation of PRN medications in the patient’s treatment plan (Procaccini et al., 2020) and consider underlying health condition and diagnosis for PRN medication prescription (Barr et al., 2018).
The use of regularly prescribed medications in a suitable dose at the time of hospitalization instead of PRN orders has been shown to help with the reduction of the use of PRN medications (Barr et al., 2018). Effectiveness of this method in psychiatric wards has been shown (Al-Sughayir, 2014; Al-Sughayir, 2017).
Medication reconciliation and documentation of current medications should be performed soon after admission to the hospital (Al-Sughayir, 2014; Al-Sughayir, 2017). A specifically designed sheet containing headings for medication name, dose, route of administration, and an empty space for the physician’s instructions should be devised for the prescriptions of PRN medications (Craven et al., 1987). Also, details of the reason for PRN medication use (Chaichan, 2008), the time interval between the doses of medications (Craven et al., 1987; Chaichan, 2008), maximum dosage limit per 24 h (Craven et al., 1987), and sequencing PRN medications for the same healthcare problem, if applicable, (Procaccini et al., 2020), should be specified.
Besides the patient’s health condition, his/her preference should guide healthcare providers for the prescription of appropriate PRN medications. When the patient is at risk of self-harm, the use of fast-acting PRN medications is suggested (Walsh et al., 2020). In addition, the use of PRN medications to reduce agitation in patients who are unable to follow their previous habits including smoke cigarettes, drink alcohol, and access to illicit drugs have been recommended (Barr et al., 2018).
Controlling the undesirable behaviors of patients is a legitimate reason for the prescription and use of PRN medications when non-pharmacological strategies do not work properly (Walsh et al., 2020). Oral PRN medications have been recommended rather than injections when the patient accepts that the required response can be achieved via this method (Al-Sughayir, 2014; Al-Sughayir, 2017).
Having clear goals and ration underpinning the use of PRN medications is required (Baker et al., 2007a; Curtis et al., 2007). The study by Curtis et al. (2007) reported that the rationale for the administration of 42% of PRN psychotropic medications within acute mental health settings was not stated. The PRN medication use should be supported with logic and reasons (Akram et al., 2014). It helps healthcare providers ensure the match between the indication of prescription and administration of PRN medications (Baker et al., 2007a). They require to communicate this ration to the patient and families involved in patient care along with the provision of information about any perceived risks. Answers to their questions should be given and their consent before medication administration should be sought (Baker et al., 2007a).
Timing of PRN medication use should be considered (Mullen and Drinkwater, 2011) and PRN administration should be avoided when the specified minimum time between the doses of the medication would be violated (Chaichan, 2008). Adequate attention should be paid to the interval and dose of re-administration of a similar PRN medication (Gordon et al., 2008).
The route of PRN medication administration and its dose are the important aspects of medicines management. Plasma levels of medications from oral ingestion are notably lower than those of an intramuscular or intravascular injection. Therefore, side effects are more likely to happen when the comparative doses of medications are administrated via injection instead of oral use (Akram et al., 2014).
Being prepared and having PRN medication orders when a patient is involuntarily admitted and is at risk of harm to themselves or others help healthcare providers to administer medications to control a potentially violent incident (Jimu and Doyle, 2019).
Making a decision on PRN medication administration should be based on collected data and the assessment of patients and their healthcare background (Di Giulio and Crow, 1997; Usher et al., 2009). Accordingly, healthcare providers should monitor patients’ physical and psychological symptoms (Usher et al., 2010; Swart et al., 2011; Jimu and Doyle, 2019; Walsh et al., 2020), check their laboratory test results, evaluate their vital signs (Di Giulio and Crow, 1997), assess their allergies (Baker et al., 2007a), consider their behaviors, concerns and requests (Di Giulio and Crow, 1997; Usher et al., 2009; Barr et al., 2018; Walsh et al., 2020). In this respect, the use of both subjective assessment such as interviewing and objective assessment such as observation help with making decisions on PRN medication use (Geffen et al., 2002). Healthcare providers can make a decision on the administration of PRN medications through the interpretation of the patient’s actions and non-verbal clues (Carder, 2012). Specifically, prior to the administration of opioid medications as PRN, the sedation level, pain intensity, respiratory rate, and prior response to medications should be assessed (Gordon et al., 2008). Severity of the patient’s health condition and related symptoms indicate the need for medications (Usher et al., 2009). Therefore, a collective decision-making on PRN medications can be made based on the healthcare provider’s perspectives and the patient’s behaviors and symptoms (Walsh et al., 2020). Perceived harm and the probability of risk should be detected by healthcare providers as an important indicator of PRN medication administration (Walsh et al., 2020).
There is a need to have a closer look at psychological symptoms and having a broader perspective rather than problem-oriented one for the appropriate use of PRN medications (Di Giulio and Crow, 1997). It helps predict the patient’s pattern of behaviors by knowing the patient and empower him/her to safely use PRN medications for the prevention of dangerous behaviors and related harm (Walsh et al., 2020). Healthcare providers’ judgment of the patient’s symptoms is decisive for PRN medication administration. Some symptoms such as heavy breathing or constipation clearly have an obvious cause, which facilitate decision-making regarding PRN medication use. On the other hand, a single night insomnia is not judged to be an indication for the use of hypnotics as PRN (Nilsen et al., 2020).
Communication challenges between the patient and healthcare providers contribute to the increased use of PRN medications. It hinders the assessment of patients’ underlying health problems or unmet needs leading to undesirable behaviors. Therefore, the patient’s behaviors due to communication issues should not be misinterpreted and hastily decisions on the use of PRN medications should not be made (Walsh et al., 2020).
Undertaking a formal risk assessment is an important step for making a decision on the use of PRN medications. It consists of the assessment of the risk to the patient themselves, to other patients, and to healthcare providers (Jimu and Doyle, 2019). Relevant assessment tools at the hospital admission determines the need for PRN medication use. For example, the Positive and Negative Syndrome Scale-Excited Component (PANSS-EC) can be used to evaluate the control of agitation and aggression in people with schizophrenia during the first 3 days of admission. Its score influences the decision on the administration of PRN medications (Chaichan, 2008). The study by Chaichan (2008) showed that the mean number of episodes of aggression in patients with schizophrenia during the period of hospitalization was remarkably lower among those assessed with the PANSS-EC. The Positive and Negative Symptom Scale (PANSS) used for the accurate assessment of psychiatric patients with appropriate tools can help determine the need for PRN medications and reduce PRN medication use during hospital stays (Barr et al., 2018). Use of decision support tools for evaluating pain and sedation can optimize PRN medication administration (Procaccini et al., 2020).
In addition to screening tools, the general pattern of the disease, underlying cause of behaviors (Walsh et al., 2020), and underlying diagnosis (Barr et al., 2018) can be helpful for decision-making about PRN medication administration. Nurses have the best position to use their knowledge about patients with long-term health conditions and observe distinctive behavioral patterns and help with the determination of the patient’s needs for PRN medications (Walsh et al., 2020).
Healthcare providers should note that the administration of wrong PRN medications can hamper the diagnosis. Particularly analgesics and antispasmodics can conceal the patient’s symptoms (Di Giulio and Crow, 1997). PRN medication administration should be to the best interest of patients. Healthcare providers should follow a middle ground with regard to how to manage disruptive behaviors using PRN medications without causing medication toxicity (Walsh et al., 2020).
Alternative treatment strategies including non-pharmacologic methods such as redirection and distraction, and physical restraint as the last resort can be considered before the use of PRN medications (Walsh et al., 2020). Restraint, time out, and seclusion can be used to help de-escalation before further PRN medication administration (Usher et al., 2010). Although the use of restrains is outlawed, a balance should be present between the administration of PRN medications and avoiding the use of seclusion, because seclusion is restrictive and has the potential of damaging the therapeutic relationship between healthcare professionals and patients (Barr et al., 2018).
The use and effects of PRN medications should be regularly and systematically evaluated (Baker et al., 2007a). Healthcare providers should be aware of the potential side effects of PRN medications (Baker et al., 2007a) and have a concern about both their ineffectiveness and side effects (Usher et al., 2009). The patient should be regularly checked in terms of physical health before and after PRN medication use (Usher et al., 2010) and probable medication interactions/allergic reactions (Baker et al., 2007a). Side effects of PRN medications can be identified through the monitoring of vital signs and the patient’s symptoms such as extrapyramidal complications (Al-Sughayir, 2014; Al-Sughayir, 2017). In addition, there is a need to assess the effects of PRN medications on the patient’s underlying health condition (Griffiths et al., 2019). The nurse should communicate with the physician as prescriber in case of unsuccessful outcome of PRN medication use (Procaccini et al., 2020). The treating physician should be informed and asked for a medical evaluation in case of any concern regarding the use of PRN medications (Al-Sughayir, 2014; Al-Sughayir, 2017). Assessing the peak time of PRN medication administration during the day can help take appropriate measures to optimize PRN medication use (Akram et al., 2014).
Monitoring and documentation of related data when PRN medications are administered (Al-Sughayir, 2014; Al-Sughayir, 2017; Martin et al., 2018a) and post medication administration are of utmost importance (Akram et al., 2014). Documentation of PRN medications should be clear in terms of the reason (Craven et al., 1987; Martin et al., 2018a) and indication of use (Stein-Parbury et al., 2008), circumstances and symptoms leading to administration (Baker et al., 2007a; Martin et al., 2018a), related effects (Baker et al., 2007a; Curtis et al., 2007; Stein-Parbury et al., 2008; Al-Sughayir, 2014; Al-Sughayir, 2017; Martin et al., 2018a), negative consequences and side effects (Martin et al., 2018a), and the method used for the evaluation of expected outcomes (Curtis et al., 2007). Martin et al. (2018a) in their study reported that in 15% of cases, the administration of psychotropic PRN medications were not documented and in 79% of cases, a reason for it was mentioned. In another study, only in 63.2% of episodes a reason for PRN medication administration was documented (Stein-Parbury et al., 2008). In the study by Curtis et al. (2007), the effect of PRN medications was documented only in 38.8% of occasions.
When PRN medications are used, any additional pre- or post -intervention should be recorded (Curtis et al., 2007). According to the Curtis et al.’s (2007), additional pre- or post -intervention was documented only in 28% of occasions of PRN medication administration. If more than one PRN medications is administered, indications should be clearly explained (Stein-Parbury et al., 2008).
Healthcare providers should take more responsibility for the prescription and administration of PRN medications and should be aware of issues resulting from high doses and polypharmacy especially in patients with mental health problems (Barr et al., 2018; Griffiths et al., 2019; Jimu and Doyle, 2019), and avoid polypharmacy if possible (Al-Sughayir, 2014; Al-Sughayir, 2017).
In those patients who are at the risk of high doses of medications including in long-term care facilities and with severe cognitive issues, the administration of PRN medications and daily recommended dose of PRN medications should be monitored (Sharma et al., 2021). Assessment of over-prescription, over-medication, and polypharmacy of PRN medications should encompass patients with more dependency levels (Stasinopoulos et al., 2018). Monitoring the number of medications in patients with a longer duration of hospital stay is required (Dörks et al., 2016).
The PRN medication regimen should be completed and its use should be discontinued by the treating physician as soon as possible (Al-Sughayir, 2014; Al-Sughayir, 2017). The end date should be clearly stated at the beginning of prescription (Usher et al., 2009). As a rule, PRN medications should be deprescribed when they are no longer needed (Craven et al., 1987). A time-limited prescription of PRN medications requires the regular review of medication use (Baker et al., 2007a). The use of a stop-order policy after 7 days can help avoid unnecessary PRN medication use and the early deprescription of PRNs. Accordingly, the prescriber has to reassess the PRN medication order and decide on the need or for continuation for more than 7 days. In case of the needs for continuation, the prescriber repeats the order with the consideration of the prescription requirements (Craven et al., 1987). Drug dependency and abuse should be considered when making such a decision (Kaur et al., 2009).
Alternative interventions such as non-pharmacologic strategies on appropriate occasions prior or instead of PRN medication administration or in combination with them not only help achieve an optimal response, but also prepare the ground for discontinuation (Geffen et al., 2002; Usher et al., 2009; Swart et al., 2011; Al-Sughayir, 2014; Al-Sughayir, 2017; Martin et al., 2018a; Barr et al., 2018; Jimu and Doyle, 2019). The feasibility of their use depends on that the patient is identified by healthcare professionals to have a low risk level for use along with having a positive attitude toward such interventions (Usher et al., 2009). Behavior therapy, music, counselling, relaxation, redirection, and planned ignoring have been shown helpful in the reduction of PRN medication use and improvement of self-care (Swart et al., 2011; Al-Sughayir, 2014; Al-Sughayir, 2017; Barr et al., 2018). Combination and the simultaneous use of PRN medications with alternative interventions improve the effectiveness of medication use (Akram et al., 2014; Barr et al., 2018). However, the alternative and non-pharmacologic interventions are time-consuming and their practice requires appropriate expertise (Walsh et al., 2020).
Healthcare providers should show their good intentions and positive attitudes toward PRN medication use to be able to perform related caring measures (Edwards et al., 2001). They need to rely on their theoretical and practical knowledge (Di Giulio and Crow, 1997). Having sufficient pharmacotherapeutic knowledge is important for PRN medication use, post-PRN monitoring, and its documentation (Nilsen et al., 2020). Practical experience and knowledge are important and refers to having knowledge about how PRN medications can impact on the patient’s health condition (Nilsen et al., 2020).
Healthcare providers should gain knowledge of any advance directive with regard to PRN medications (Baker et al., 2007a). Appropriate education to healthcare providers can empower them to comply with PRN medication standards such as the dosage guideline (Carder, 2012; Procaccini et al., 2020). Healthcare providers should know about the regulations of PRN medication use in terms of the reason for use, schedule and route, circumstances of use, maximum dose, when to contact the physician, and when to discontinue medications (Carder, 2012). Experienced healthcare providers can teach newly staff regarding the facility-specific systems for PRN medication order, stock, documentation, and administration (Carder, 2012). Personal skills of healthcare providers can contribute to the assessment of the effects of PRN medications (Walsh et al., 2020).
Having access to a senior healthcare provider who is involved in PRN medicines management and discussion on its administration improve medication safety (Jimu and Doyle, 2019). Healthcare providers can seek a second opinion prior to the final decision making regarding PRN medication administration as complementary to their own competence (Nilsen et al., 2020). Sufficient information sharing in both written and oral formats influences PRN medicines management. Quality of the documentation is a significant element in the decision-making process with regard to the PRN medication use. Oral information sharing during shift handoff can inform the next healthcare provider about challenges during the work shift, the patient’s health condition, and how to face issues with PRN medication use (Carder, 2012; Nilsen et al., 2020). Clear, accurate, and up-to-date prescription information avoids uncertainty between the prescriber and the administrator, improves optimal PRN medicines management, and prevents misinterpretations (Usher et al., 2009).
Environmental factors also can influence decisions regarding PRN medication administration. Appropriate staffing on each work shift improves high-quality PRN medicines management (Nilsen et al., 2020). In contrast, staff shortages and heavy workloads increase the inappropriate use of PRN medications (Walsh et al., 2020). When staff shortages are present, healthcare providers are busy and do not have enough time for the patient’s assessment. Therefore, they may give PRN medications more regularly to patients without attempting to take more time and use alternative strategies (Usher et al., 2009; Walsh et al., 2020). Inexperienced healthcare providers who may not be quite familiar with the healthcare setting are the reason for the higher rate of PRN medication use (Usher et al., 2009).
The appropriate storage of PRN medications can facilitate access to medications and is a contributing component of safe PRN medicines management (Carder, 2012; Nilsen et al., 2020). Other aspects are placing medications in a labeled container inside the locked cabinet, adding a direction about conditions in which the medication can be administered, and informing the healthcare provider about the availability of medications (Carder, 2012). Where PRN medications are in storage and a healthcare provider has the key, other healthcare providers have to discuss with her/him and explain the situation before asking for medications, thereby regulate PRN medicines management (Nilsen et al., 2020).
The culture of applying non-pharmacological interventions before administering PRN medications can prevent inappropriate medication use (Nilsen et al., 2020). Clinical protocols where they restrict the physical restraint policy and the use of chemical restraint when non-pharmacological strategies such as distraction and redirection fail to alleviate unfavorable behaviors are supported (Walsh et al., 2020).
Additionally, healthcare professionals’ disciplines or philosophical perspectives regarding the use of PRN medications impact medication use. Some clinical protocols enforce healthcare providers to administer PRN medications or to apply an alternative strategy. However, some healthcare providers may prefer to discuss with their patients and seek alternative strategies and resolve the problem without the use of PRN medications (Usher et al., 2009). On the other hand, some healthcare providers may use PRN medications to facilitate patients’ adjustment to the requirement of the work environment during hospitalization (Walsh et al., 2020).
Positive attitudes of the patient and his/her family members toward PRN medications influence PRN medicines management (Edwards et al., 2001). The patient’s preference and compliance with PRN medication use influence healthcare provider’s decisions on the administration of PRN medications (Di Giulio and Crow, 1997; Walsh et al., 2020). The patients’ involvement is a substantial aspect of the decision process for PRN medication use (Nilsen et al., 2020). If the patient can reliably express his/her symptoms and request for medications, it is easiest for healthcare providers to decide about PRN medication use (Carder, 2012; Akram et al., 2014). However, patients’ self-request for PRN medications may not be completely to their best interest, specifically for medications that increase the risk of dependence and abuse (Akram et al., 2014). Wherever possible, joint decision-making about the prescription of PRN medications is recommended on translating/agreeing the rational/indication for the prescription into the language of/with the patient (Baker et al., 2007a).
The patients’ knowledge regarding their medications also is important. For instance, when healthcare providers improve their patients’ knowledge of the side effects of a particular PRN medication, it is more likely that the patient accepts non-administration of medications (Nilsen et al., 2020). The patient’s willingness can influence the replacement of medications with alternative and non-pharmacological interventions (Usher et al., 2009).
Communication and cognitive abilities of the patient to assess the necessity of PRN medication use also have been emphasized. For example, the patient’s wellness informs healthcare providers of the patient’s ability to convey the situation that raises the need for the administration of PRN medications (Nilsen et al., 2020). The patient’s ability to cooperate may be influenced by the administration of some PRN medications such as analgesics (Di Giulio and Crow, 1997). Conflicting understanding between healthcare providers and family members regarding the patient’s need for PRN medications should be resolved through reaching agreement by all parties involved in patient care (Nilsen et al., 2020).
When the patient looks unwell and for instance expresses the signs of aggression, agitation, or elated mood, PRN medication use is more likely (Usher et al., 2009). A direct association has been shown between the severity of symptoms and the dose of PRN medications (Griffiths et al., 2019).
For outpatients and those who have to manage PRN medications themselves, applying some instructions and strategies on PRN medication bottles under the name of the Take-Wait-Stop label design is beneficial. Deconstructing prescription wording about PRN instructions can explicitly convey the dose, interval between doses, and maximum daily dose to patients and their families. It consists of explicit, deconstructed instructions, and simplified texts such as numeric characters instead of words, e.g., “1 tab” instead of “one tab,” and carriage returns to place each part of the instructions on separate lines. The use of simplified text and plain language, “Stop” to replace the typical wording “do not exceed,” can inform patients with limited literacy levels about the maximum daily dose (McCarthy et al., 2013; McCarthy et al., 2019).
Collaboration by healthcare professionals is needed from the moment that PRN medications are prescribed. Nurses spent the most time with patients and have the central role for identifying the patient’s need for PRN medications. Physician’s and nurse’s collaboration regarding PRN medication use has been emphasized (Jimu and Doyle, 2019). Interprofessional medication review with the collaboration of pharmacist on the patient’s medication list facilitates updating medications and changing and removing unused ones. It also creates consensus on PRN medication use (Nilsen et al., 2020). Also, involvement of the multidisciplinary team in the management of patients’ behaviors using alternative methods reduces the need for PRN medication use (Barr et al., 2018).
In this systematic review with an integrative approach, the practical considerations of PRN medicines management were suggested. They can help with the improvement of quality and safety of the PRN medication process. Our review findings showed the need for appropriate assessment and planning for safe PRN medication use and inclusion of strategies for the improvement of multidisciplinary collaboration, monitoring of medications’ effects and side effects, deprescription, use of alternative therapies, and involvement of patients and families in medication therapy.
Healthcare professionals’ collaboration for making decisions on the prescription and administration of PRN medications is important. For instance, double-checking by at least two healthcare providers can prevent medication errors (Koyama et al., 2020; Vaismoradi et al., 2020). However, the role of electronic and digital solutions for improving the safety of PRN medicines management has remained unattended. Electronic prescribing and administrating of medications have the potential for reducing the risk of medication errors and adverse drug events (Ammenwerth et al., 2008; Slight et al., 2019). A systematic review and meta-analysis reported a considerable (50%) reduction in preventable adverse drug events when electronic prescribing systems in acute care settings were used in healthcare settings (Nuckols et al., 2014). It can ensure the safety of PRN prescribing through the provision of important capabilities such as decision support, specification of indications for the PRN medication use and the maximum daily dose, provision of appropriate alert, and communication between prescribers and administrators (Donyai et al., 2008; Baysari et al., 2012; Martin et al., 2017).
Since the accurate documentation of patient information is one of the primary competences of healthcare providers and facilities the monitoring of PRN medications, structured report templates regardless of the method of documentation can improve PRN medication documentation (Hammer et al., 2019). The electronic health record with the inclusion of information about effectiveness, side effects, and matching between the indication of PRN prescription and administration contributes to the high quality documentation process (Martin et al., 2017).
In addition, the significance of assessment tools regarding the effectiveness of PRN medication use was not acknowledged in the included studies to this review. In an instrument development study, Silk et al. (2013) suggested that the provision of an accurate evaluation of the effectiveness of PRN medications as a result of decreased subjective and ambiguous language improved the patient outcomes (Silk et al., 2013). The prevention of polypharmacy along with PRN medication use requires appropriate screening tools. Although such a specific tool is not available yet, the STOPP (screening tool of older persons’ potentially inappropriate prescriptions) and START (screening tool to alert doctors to the right treatment) tools can be used to review medications for vulnerable people and identify potentially inappropriate medications (O'Mahony et al., 2015; Vaismoradi et al., 2020).
Our review findings highlighted the deprescription of PRN medications and its replacement with non-pharmacologic methods to prevent polypharmacy and medication abuse. The plan for deprescribing process of PRN medications should be devised based on each patient’s need and under close monitoring (Renn et al., 2018; Vaismoradi et al., 2021a). Also, cost and benefit assessment with regard to the continuation and discontinuation of medications should be performed (Renn et al., 2018). Moreover, the concerns of patients and their informal caregivers about the replacement of medications by alternative therapies that can influence their collaboration with the deprescription plan should be taken seriously (Scott et al., 2015; Vaismoradi et al., 2021a). Given that the use of PRN medications reduce the inclusion of other therapeutic interventions in the therapeutic plan (Hipp et al., 2018), PRN medications should not be used when potential non-pharmacological treatment options are available (Martin et al., 2017).
According to our review findings, healthcare professionals’ competencies for PRN medicines management influenced the safety of the medication process. Their pharmacological competence as having sufficient knowledge and skills to manage real-life medication circumstances and making appropriate decisions (Sulosaari et al., 2011; Salehi et al., 2021) are affected by the complexity of the patient’s medication processes (Sulosaari et al., 2011; Lichtner et al., 2016). Healthcare providers need education and training about the application of alternative and non-pharmacological interventions for relieving patients’ symptoms (Molloy et al., 2012). They should be educated to avoid overreliance on PRN medications (Zeisel et al., 2016; Harper et al., 2017; Martin et al., 2018b).
Patient participation and shared decision-making was a pilar for safe PRN medicines management in our review. Patients play an active role in care planning and should have the opportunity to participate in decision making (Mikesell et al., 2016). Patients eagerly participate in decision-making if they receive sufficient knowledge about their medications, have appropriate understanding of PRN medications (Hipp et al., 2018), and are able to define PRN medications and the rational for their use (Morkunas et al., 2016). It also enhances their compliance to the medication regimen (Fernandez et al., 2006; Mardani et al., 2020). Therefore, the opportunity for asking about PRN medications and giving consent when PRN medications are offered should be given to patients (Hipp et al., 2018; Vaismoradi et al., 2021b).
This systematic review using international databases can improve our understanding of practical considerations that should be applied by healthcare professionals for safe PRN medicines management. We identified relevant literature with qualitative and quantitative research designs by applying multidimensional keywords for a systematic search on international databases. Therefore, our findings provide an extensive overview of the present international knowledge regarding this important clinical topic. However, our review was limited to studies published in the English language due to restriction in translation. Future studies need to consider grey literature and other sources of literature including local guidelines used in clinical settings and in other languages to improve the generatability of our review findings. Also, the majority of retrieved studies in the present systematic review was from Australia, the United States, Canada, and European countries. A limited number of studies from Asia and Africa on PRN medicines management was retrieved. Therefore, PRN medicines management should be addressed in other research contexts to improve our understanding of cultural aspects affecting medication safety.
The current review sought to summarize and integrate practical considerations by healthcare professionals for PRN medicines management in different healthcare settings. The findings of this review demonstrate that PRN medicines management is a complex process and many factors influence its safety. We identified a range of possible practical measures that should be taken for improving the safety of PRN medication therapy.
The synthesised knowledge in our review can be used to develop optimal PRN medicines management guidelines in different clinical settings and to investigate its effect on safe care indicators. A suggested list of practical considerations for PRN medicines management has been developed based on our review findings and has been presented in Table 4. After making it suitable for application in clinical practice, they can be used to guide healthcare professionals in PRN medicines management situations. Along with other medication safety measures, the suggested implications can support healthcare practitioners’ decision making for improving the quality and safety of PRN medication use.
It should be acknowledged that alternative interventions such as non-pharmacologic strategies in appropriate caring occasions have priority over PRN medication use due to fewer side effects. Therefore, healthcare providers should improve their competencies to avoid overreliance on PRN medication use for relieving patients’ symptoms.
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
MV: Conceptualization. AM and MV: Data curation, Formal analysis, Investigation, Methodology; Project administration, Resources, and Software. AM, MV, PP, CW, and SJ: Writing—original draft, Writing-review and editing. All authors have read and agreed to the published version of the manuscript.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.759998/full#supplementary-material
Acheampong, F., Anto, B. P., and Koffuor, G. A. (2014). Medication Safety Strategies in Hospitals-Aa Systematic Review. Int. J. Risk Saf. Med. 26 (3), 117–131. doi:10.3233/JRS-140623
Akram, G., Slavin, A., and Davies, P. (2014). The Administration of Psychotropic PRN Medication in Scottish Psychiatric Intensive Care Units. J. Psychiatr. Intensive Care 10 (2), 64–74. doi:10.1017/S1742646414000028
Al-Sughayir, M. A. (2014). Administered Antipsychotic Pro Re Nata Medications in Psychiatric Inpatients. Pre- and post-accreditation Comparison. Saudi Med. J. 35 (2), 172–177.
Al-Sughayir, M. A. (2017). Does Accreditation Improve Pro Re Nata Benzodiazepines Administration in Psychiatric Inpatients? Pre-post Accreditation Medical Record Comparison. Int. J. Ment. Health Syst. 11 (1), 16–25. doi:10.1186/s13033-017-0124-8
Alaqqad, A., Alharthy, A., and Aldossari, M. (2016). PRN Medications Ordering Practice at a Large Intensive Care Unit in Saudi Arabia. J. Intensive Crit. Care 2 (2), 35. doi:10.21767/2471-8505.100044
Allers, K., Dörks, M., Schmiemann, G., and Hoffmann, F. (2017). Antipsychotic Drug Use in Nursing home Residents with and without Dementia: Keep an Eye on the Pro Re Nata Medication. Int. Clin. Psychopharmacol. 32 (4), 213–218. doi:10.1097/YIC.0000000000000173
Ammenwerth, E., Schnell-Inderst, P., Machan, C., and Siebert, U. (2008). The Effect of Electronic Prescribing on Medication Errors and Adverse Drug Events: a Systematic Review. J. Am. Med. Inform. Assoc. 15 (5), 585–600. doi:10.1197/jamia.M2667
Baker, J. A., Lovell, K., and Harris, N. (2008). A Best-Evidence Synthesis Review of the Administration of Psychotropic Pro Re Nata (PRN) Medication in In-Patient Mental Health Settings. J. Clin. Nurs. 17 (9), 1122–1131. doi:10.1111/j.1365-2702.2007.02236.x
Baker, J. A., Lovell, K., Harris, N., and Campbell, M. (2007a). Multidisciplinary Consensus of Best Practice for Pro Re Nata (PRN) Psychotropic Medications within Acute Mental Health Settings: a Delphi Study. J. Psychiatr. Ment. Health Nurs. 14 (5), 478–484. doi:10.1111/j.1365-2850.2007.01112.x
Baker, J. A., Lovell, K., and Harris, N. (2007b). Mental Health Professionals' Psychotropic Pro Re Nata (p.r.n.) Medication Practices in Acute Inpatient Mental Health Care: a Qualitative Study. Gen. Hosp. Psychiatry 29 (2), 163–168. doi:10.1016/j.genhosppsych.2006.12.005
Barr, L., Wynaden, D., and Heslop, K. (2018). Nurses' Attitudes towards the Use of PRN Psychotropic Medications in Acute and Forensic Mental Health Settings. Int. J. Ment. Health Nurs. 27 (1), 168–177. doi:10.1111/inm.12306
Basey, A. J., Krska, J., Kennedy, T. D., and Mackridge, A. J. (2014). Prescribing Errors on Admission to Hospital and Their Potential Impact: a Mixed-Methods Study. BMJ Qual. Saf. 23 (1), 17–25. doi:10.1136/bmjqs-2013-001978
Baysari, M. T., Reckmann, M. H., Li, L., Day, R. O., and Westbrook, J. I. (2012). Failure to Utilize Functions of an Electronic Prescribing System and the Subsequent Generation of 'technically Preventable' Computerized Alerts. J. Am. Med. Inform. Assoc. 19 (6), 1003–1010. doi:10.1136/amiajnl-2011-000730
Car, J., Tan, W. S., Huang, Z., Sloot, P., and Franklin, B. D. (2017). eHealth in the Future of Medications Management: Personalisation, Monitoring and Adherence. BMC Med. 15 (1), 73–79. doi:10.1186/s12916-017-0838-0
Carder, P. C. (2012). "Learning about Your Residents": How Assisted Living Residence Medication Aides Decide to Administer Pro Re Nata Medications to Persons with Dementia. Gerontologist 52 (1), 46–55. doi:10.1093/geront/gnr099
Chaichan, W. (2008). Evaluation of the Use of the Positive and Negative Syndrome Scale-Excited Component as a Criterion for Administration of p.r.N. Medication. J. Psychiatr. Pract. 14 (2), 105–113. doi:10.1097/01.pra.0000314317.79544.b4
Craven, J. L., Voore, P. M., and Voineskos, G. (1987). PRN Medication for Psychiatric Inpatients. Can. J. Psychiatry 32 (3), 199–203. doi:10.1177/070674378703200308
Curtis, J., Baker, J. A., and Reid, A. R. (2007). Exploration of Therapeutic Interventions that Accompany the Administration of p.r.N. ('as Required') Psychotropic Medication within Acute Mental Health Settings: a Retrospective Study. Int. J. Ment. Health Nurs. 16 (5), 318–326. doi:10.1111/j.1447-0349.2007.00487.x
Davies, S. J., Lennard, M. S., Ghahramani, P., Pratt, P., Robertson, A., and Potokar, J. (2007). PRN Prescribing in Psychiatric Inpatients: Potential for Pharmacokinetic Drug Interactions. J. Psychopharmacol. 21 (2), 153–160. doi:10.1177/0269881107067242
Di Giulio, P., and Crow, R. (1997). Cognitive Processes Nurses and Doctors Use in the Administration of PRN (At Need) Analgesic Drugs. Scand. J. Caring Sci. 11 (1), 12–19. doi:10.1111/j.1471-6712.1997.tb00425.x
Donyai, P., O'Grady, K., Jacklin, A., Barber, N., and Franklin, B. D. (2008). The Effects of Electronic Prescribing on the Quality of Prescribing. Br. J. Clin. Pharmacol. 65 (2), 230–237. doi:10.1111/j.1365-2125.2007.02995.x
Dörks, M., Allers, K., and Hoffmann, F. (2019). Pro Re Nata Drug Use in Nursing home Residents: A Systematic Review. J. Am. Med. Directors Assoc. 20 (3), 287–293. doi:10.1016/j.jamda.2018.10.024
Dörks, M., Schmiemann, G., and Hoffmann, F. (2016). Pro Re Nata (As Needed) Medication in Nursing Homes: the Longer You Stay, the More You Get? Eur. J. Clin. Pharmacol. 72 (8), 995–1001. doi:10.1007/s00228-016-2059-4
Douglas-Hall, P., and Whicher, E. V. (2015). 'As Required' Medication Regimens for Seriously Mentally Ill People in Hospital. Cochrane Database Syst. Rev. 2015 (12), CD003441. doi:10.1002/14651858.CD003441.pub3
Edwards, H. E., Nash, R. E., Yates, P. M., Walsh, A. M., Fentiman, B. J., McDowell, J. K., et al. (2001). Improving Pain Management by Nurses: a Pilot Peer Intervention Program. Nurs. Health Sci. 3 (1), 35–45. doi:10.1046/j.1442-2018.2001.00069.x
EQUATOR Network (2019). EQUATOR Network: Enhancinh the Quality and Transparency of Health Research. Available from http://www.equator-network.org/(Accessed July 25, 2021).
Fernandez, R. S., Evans, V., Griffiths, R. D., and Mostacchi, M. S. (2006). Educational Interventions for Mental Health Consumers Receiving Psychotropic Medication: A Review of the Evidence. Int. J. Ment. Health Nurs. 15 (1), 70–80. doi:10.1111/j.1447-0349.2006.00405.x
Friedman, R., Nurenberg, J. R., Birnbaum, S., and Schleifer, S. J. (2012). Using Structured Clinical Feedback to Encourage Alternatives to Use of "P.R.N." Medication in a State Psychiatric Hospital. J. Psychiatr. Pract. 18 (5), 381–387. doi:10.1097/01.pra.0000419823.69914.c7
Fujita, J., Nishida, A., Sakata, M., Noda, T., and Ito, H. (2013). Excessive Dosing and Polypharmacy of Antipsychotics Caused by Pro Re Nata in Agitated Patients with Schizophrenia. Psychiatry Clin. Neurosci. 67 (5), 345–351. doi:10.1111/pcn.12056
Geffen, J., Cameron, A., Sorensen, L., Stokes, J., Roberts, M. S., and Geffen, L. (2002). Pro Re Nata Medication for Psychoses: the Knowledge and Beliefs of Doctors and Nurses. Aust. N. Z. J. Psychiatry 36 (5), 642–648. doi:10.1046/j.1440-1614.2002.01068.x
Gordon, D. B., Pellino, T. A., Higgins, G. A., Pasero, C., and Murphy-Ende, K. (2008). Nurses' Opinions on Appropriate Administration of PRN Range Opioid Analgesic Orders for Acute Pain. Pain Manag. Nurs. 9 (3), 131–140. doi:10.1016/j.pmn.2008.03.003
Griffiths, A. W., Surr, C. A., Alldred, D. P., Baker, J., Higham, R., Spilsbury, K., et al. (2019). Pro Re Nata Prescribing and Administration for Neuropsychiatric Symptoms and Pain in Long-Term Care Residents with Dementia and Memory Problems: A Cross-Sectional Study. Int. J. Clin. Pharm. 41 (5), 1314–1322. doi:10.1007/s11096-019-00883-7
Hammer, A., Wagner, A., Rieger, M. A., and Manser, T. (2019). Assessing the Quality of Medication Documentation: Development and Feasibility of the MediDocQ Instrument for Retrospective Chart Review in the Hospital Setting. BMJ Open 9 (11), e034609. doi:10.1136/bmjopen-2019-034609
Harper, L., Reddon, J. R., Hunt, C. J., and Royan, H. (2017). PRN Medication Administration in a Geriatric Psychiatric Hospital: Chart Review and Nursing Perspective. Clin. Gerontol. 40 (5), 392–400. doi:10.1080/07317115.2017.1311287
Herzog, R., Álvarez-Pasquin, M. J., Díaz, C., Del Barrio, J. L., Estrada, J. M., and Gil, Á. (2013). Are Healthcare Workers' Intentions to Vaccinate Related to Their Knowledge, Beliefs and Attitudes? A Systematic Review. BMC Public Health 13 (1), 154–217. doi:10.1186/1471-2458-13-154
Higgins, J., and Altman, D. G. E. (2011). “Chapter 8: Assessing Risk of Bias in Included Studies,” in Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. Editors J. P. T. Higgins,, and S. Green. [updated March 2011]. The Cochrane CollaborationAvailable from www.cochrane‐handbook.org.
Hilton, M. F., and Whiteford, H. A. (2008). Pro Re Nata Medication for Psychiatric Inpatients: Time to Act. Aust. N. Z. J. Psychiatry 42 (7), 555–564. doi:10.1080/00048670802119804
Hipp, K., Kuosmanen, L., Repo-Tiihonen, E., Leinonen, M., Louheranta, O., and Kangasniemi, M. (2018). Patient Participation in Pro Re Nata Medication in Psychiatric Inpatient Settings: An Integrative Review. Int. J. Ment. Health Nurs. 27 (2), 536–554. doi:10.1111/inm.12427
Hipp, K., Repo-Tiihonen, E., Kuosmanen, L., Katajisto, J., and Kangasniemi, M. (2020). PRN Medication Events in a Forensic Psychiatric Hospital: A Document Analysis of the Prevalence and Reasons. Int. J. Forensic Ment. Health 19 (4), 329–340. doi:10.1080/14999013.2020.1774686
Hogan, K. A., Lapenskie, J., Leclair, J., Thick, N., Gazarin, M., Webster, P., et al. (2019). Implementing Safe Practices in Administering Pro Re Nata Medications in a Rural Hospital. J. Patient Saf. 15 (4), 302–304. doi:10.1097/PTS.0000000000000276
Jimu, M., and Doyle, L. (2019). The Administration of Pro Re Nata Medication by Mental Health Nurses: A Thematic Analysis. Issues Ment. Health Nurs. 40 (6), 511–517. doi:10.1080/01612840.2018.1543739
Kaur, S., Daffern, M., and Thomas, S. (2009). Are Patients with a History of Illicit Drug Use Perceived to Be 'drug-Seeking' when They Requestpro Re Natamedication and Does This Impact on its Administration? Ment. Health Substance Use 2 (2), 111–119. doi:10.1080/17523280902930114
Koyama, A. K., Maddox, C. S., Li, L., Bucknall, T., and Westbrook, J. I. (2020). Effectiveness of Double Checking to Reduce Medication Administration Errors: a Systematic Review. BMJ Qual. Saf. 29 (7), 595–603. doi:10.1136/bmjqs-2019-009552
Kwan, J. L., Lo, L., Sampson, M., and Shojania, K. G. (2013). Medication Reconciliation during Transitions of Care as a Patient Safety Strategy: a Systematic Review. Ann. Intern. Med. 158 (5_Part_2), 397–403. doi:10.7326/0003-4819-158-5-201303051-00006
Lellan, K. M. (1997). A Chart Audit Reviewing the Prescription and Administration Trends of Analgesia and the Documentation of Pain, after Surgery. J. Adv. Nurs. 26 (2), 345–350. doi:10.1046/j.1365-2648.1997.1997026345.x
Liberati, A., Altman, D. G., Tetzlaff, J., Mulrow, C., Gøtzsche, P. C., Ioannidis, J. P., et al. (2009). The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies that Evaluate Health Care Interventions: Explanation and Elaboration. Plos Med. 6 (10), e1000100–e34. doi:10.1016/j.jclinepi.2009.06.00610.1371/journal.pmed.1000100
Lichtner, V., Dowding, D., Allcock, N., Keady, J., Sampson, E. L., Briggs, M., et al. (2016). The Assessment and Management of Pain in Patients with Dementia in Hospital Settings: a Multi-Case Exploratory Study from a Decision Making Perspective. BMC Health Serv. Res. 16 (1), 427–515. doi:10.1186/s12913-016-1690-1
Mardani, A., Griffiths, P., and Vaismoradi, M. (2020). The Role of the Nurse in the Management of Medicines during Transitional Care: a Systematic Review. J. Multidiscip Healthc. 13, 1347–1361. doi:10.2147/JMDH.S276061
Martin, K., Arora, V., Fischler, I., and Tremblay, R. (2017). Descriptive Analysis of Pro Re Nata Medication Use at a Canadian Psychiatric Hospital. Int. J. Ment. Health Nurs. 26 (4), 402–408. doi:10.1111/inm.12265
Martin, K., Ham, E., and Hilton, N. Z. (2018a). Documentation of Psychotropic Pro Re Nata Medication Administration: An Evaluation of Electronic Health Records Compared with Paper Charts and Verbal Reports. J. Clin. Nurs. 27 (15-16), 3171–3178. doi:10.1111/jocn.14511
Martin, K., Ham, E., and Hilton, N. Z. (2018b). Staff and Patient Accounts of PRN Medication Administration and Non-pharmacological Interventions for Anxiety. Int. J. Ment. Health Nurs. 27 (6), 1834–1841. doi:10.1111/inm.12492
McCarthy, D. M., Davis, T. C., King, J. P., Mullen, R. J., Bailey, S. C., Serper, M., et al. (2013). Take-Wait-Stop: a Patient-Centered Strategy for Writing PRN Medication Instructions. J. Health Commun. 18 (Suppl. 1), 40–48. doi:10.1080/10810730.2013.825675
McCarthy, D. M., Russell, A. M., Eifler, M. R., Opsasnick, L. A., Lyden, A. E., Gravenor, S. J., et al. (2019). Implementation Fidelity of Patient-Centered Prescription Label to Promote Opioid Safe Use. Pharmacoepidemiol. Drug Saf. 28 (9), 1251–1257. doi:10.1002/pds.4795
Mikesell, L., Bromley, E., Young, A. S., Vona, P., and Zima, B. (2016). Integrating Client and Clinician Perspectives on Psychotropic Medication Decisions: Developing a Communication-Centered Epistemic Model of Shared Decision Making for Mental Health Contexts. Health Commun. 31 (6), 707–717. doi:10.1080/10410236.2014.993296
Mishore, K. M., Girma, Y., Tola, A., Mekuria, A. N., and Ayele, Y. (2020). Evaluation of Medication Use Pattern Among Patients Presenting to the Emergency Department of Hiwot Fana Specialized University Hospital, Using WHO Prescribing Indicators. Front. Pharmacol. 11, 509. doi:10.3389/fphar.2020.00509
Molloy, L., Field, J., Beckett, P., and Holmes, D. (2012). PRN Psychotropic Medication and Acute Mental Health Nursing: Reviewing the Evidence. J. Psychosoc Nurs. Ment. Health Serv. 50 (8), 12–15. doi:10.3928/02793695-20120703-03
Morkunas, B., Porritt, K., and Stephenson, M. (2016). Experiences of Mental Health Professionals and Patients in the Use of Pro Re Nata Medication in Acute Adult Mental Healthcare Settings: a Systematic Review. JBI Database Syst. Rev Implement Rep 14 (10), 209–250. doi:10.11124/JBISRIR-2016-003167
Mullen, A., and Drinkwater, V. (2011). Pro Re Nata Use in a Psychiatric Intensive Care Unit. Int. J. Ment. Health Nurs. 20 (6), 409–417. doi:10.1111/j.1447-0349.2011.00746.x
Nguyen, T. L., Leguelinel-Blache, G., Kinowski, J. M., Roux-Marson, C., Rougier, M., Spence, J., et al. (2017). Improving Medication Safety: Development and Impact of a Multivariate Model-Based Strategy to Target High-Risk Patients. PLoS One 12 (2), e0171995. doi:10.1371/journal.pone.0171995
Nilsen, M. K., Sletvold, H., and Olsen, R. M. (2020). 'To Give or Not to Give Medication, that Is the question.' Healthcare Personnel's Perceptions of Factors Affecting Pro Re Nata Medication in Sheltered Housing for Older Adults - a Focus-Group Interview Study. BMC Health Serv. Res. 20 (1), 622–711. doi:10.1186/s12913-020-05439-4
Nuckols, T. K., Smith-Spangler, C., Morton, S. C., Asch, S. M., Patel, V. M., Anderson, L. J., et al. (2014). The Effectiveness of Computerized Order Entry at Reducing Preventable Adverse Drug Events and Medication Errors in Hospital Settings: a Systematic Review and Meta-Analysis. Syst. Rev. 3 (1), 56–12. doi:10.1186/2046-4053-3-56
Nyborg, G., Brekke, M., Straand, J., Gjelstad, S., and Romøren, M. (2017). Potentially Inappropriate Medication Use in Nursing Homes: an Observational Study Using the NORGEP-NH Criteria. BMC Geriatr. 17 (1), 220–311. doi:10.1186/s12877-017-0608-z
O'Mahony, D., O'Sullivan, D., Byrne, S., O'Connor, M. N., Ryan, C., and Gallagher, P. (2015). STOPP/START Criteria for Potentially Inappropriate Prescribing in Older People: Version 2. Age Ageing 44 (2), 213–218. doi:10.1093/ageing/afu145
Oh, S. H., Woo, J. E., Lee, D. W., Choi, W. C., Yoon, J. L., and Kim, M. Y. (2014). Pro Re Nata Prescription and Perception Difference between Doctors and Nurses. Korean J. Fam. Med. 35 (4), 199–206. doi:10.4082/kjfm.2014.35.4.199
Picton, L., Ilomäki, J., Keen, C. S., Lalic, S., Adams, B., Clinnick, L. M., et al. (2021). Rates of PRN Medication Administration in Australian Residential Aged Care. J. Am. Med. Dir. Assoc. 22 (1), 117–e1. e111. doi:10.1016/j.jamda.2020.04.033
Pitkänen, A., Teuho, S., Uusitalo, M., and Kaunonen, M. (2016). Improving Medication Safety Based on Reports in Computerized Patient Safety Systems. Comput. Inform. Nurs. 34 (3), 122–127. doi:10.1097/CIN.0000000000000208
Price, O., and Baker, J. A. (2013). Resistance to Changing Practice from Pro Re Nata Prescriptions to Patient Group Directions in Acute Mental Health Settings. J. Psychiatr. Ment. Health Nurs. 20 (7), 623–630. doi:10.1111/j.1365-2850.2012.01960.x
Procaccini, D., Rapaport, R., Petty, B., Moore, D., Lee, D., and Kudchadkar, S. R. (2020). Design and Implementation of an Analgesia, Sedation, and Paralysis Order Set to Enhance Compliance of Pro Re Nata Medication Orders with Joint Commission Medication Management Standards in a Pediatric ICU. Jt. Comm. J. Qual. Patient Saf. 46 (12), 706–714. doi:10.1016/j.jcjq.2020.06.003
Renn, B. N., Asghar-Ali, A. A., Thielke, S., Catic, A., Martini, S. R., Mitchell, B. G., et al. (2018). A Systematic Review of Practice Guidelines and Recommendations for Discontinuation of Cholinesterase Inhibitors in Dementia. Am. J. Geriatr. Psychiatry 26 (2), 134–147. doi:10.1016/j.jagp.2017.09.027
Ross, S. L., Bhushan, Y., Davey, P., and Grant, S. (2021). Improving Documentation of Prescriptions for As-Required Medications in Hospital Inpatients. BMJ Open Qual. 10 (3), e001277. doi:10.1136/bmjoq-2020-001277
Russell, B. J., Rowett, D., and Currow, D. C. (2014). Pro Re Nata Prescribing in a Population Receiving Palliative Care: a Prospective Consecutive Case Note Review. J. Am. Geriatr. Soc. 62 (9), 1736–1740. doi:10.1111/jgs.12981
Saito, E., Eng, S., Grosso, C., Ozinci, Z., and Van Meter, A. (2020). Pro Re Nata Medication Use in Acute Care Adolescent Psychiatric Unit. J. Child. Adolesc. Psychopharmacol. 30 (4), 250–260. doi:10.1089/cap.2019.0131
Salehi, T., Seyedfatemi, N., Mirzaee, M. S., Maleki, M., and Mardani, A. (2021). Nurses' Knowledge, Attitudes, and Practice in Relation to Pharmacovigilance and Adverse Drug Reaction Reporting: A Systematic Review. Biomed. Res. Int. 2021, 6630404. doi:10.1155/2021/6630404
Scott, I. A., Hilmer, S. N., Reeve, E., Potter, K., Le Couteur, D., Rigby, D., et al. (2015). Reducing Inappropriate Polypharmacy: the Process of Deprescribing. JAMA Intern. Med. 175 (5), 827–834. doi:10.1001/jamainternmed.2015.0324
Sharma, M., Wong, X. Y., Bell, J. S., Corlis, M., Hogan, M., and Sluggett, J. K. (2021). Trajectories of Pro Re Nata (PRN) Medication Prescribing and Administration in Long-Term Care Facilities. Res. Soc. Adm Pharm 17 (8), 1463–1468. doi:10.1016/j.sapharm.2020.11.003
Silk, L., Watt, J., Pilon, N., and Draper, C. (2013). Development of a Psychotropic PRN Medication Evaluative Tool. J. Ment. Health Res. Intellect. Disabilities 6 (1), 29–41. doi:10.1080/19315864.2011.640096
Slight, S. P., Tolley, C. L., Bates, D. W., Fraser, R., Bigirumurame, T., Kasim, A., et al. (2019). Medication Errors and Adverse Drug Events in a UK Hospital during the Optimisation of Electronic Prescriptions: a Prospective Observational Study. Lancet Digit Health 1 (8), e403–e412. doi:10.1016/S2589-7500(19)30158-X
Smith, M. L. (2013). Thinking Ethically about Medical Mistakes. J. Child. Neurol. 28 (6), 809–811. doi:10.1177/0883073813477690
Sonntag, A., Matschinger, H., Angermeyer, M. C., and Riedel-Heller, S. G. (2006). Does the Context Matter? Utilization of Sedative Drugs in Nursing Homes-Aa Multilevel Analysis. Pharmacopsychiatry 39 (04), 142–149. doi:10.1055/s-2006-946704
Souza, M. T., Silva, M. D., and Carvalho, R. D. (2010). Integrative Review: what Is it? How to Do it? Einstein (Sao Paulo) 8 (1), 102–106. doi:10.1590/s1679-45082010rw1134
Stasinopoulos, J., Bell, J. S., Ryan-Atwood, T. E., Tan, E. C. K., Ilomäki, J., Cooper, T., et al. (2018). Frequency of and Factors Related to Pro Re Nata (PRN) Medication Use in Aged Care Services. Res. Soc. Adm Pharm 14 (10), 964–967. doi:10.1016/j.sapharm.2017.11.004
Stein-Parbury, J., Reid, K., Smith, N., Mouhanna, D., and Lamont, F. (2008). Use of Pro Re Nata Medications in Acute Inpatient Care. Aust. N. Z. J. Psychiatry 42 (4), 283–292. doi:10.1080/00048670701881553
Sterne, J. A., Hernán, M. A., Reeves, B. C., Savović, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: a Tool for Assessing Risk of Bias in Non-randomised Studies of Interventions. BMJ 355, i4919. doi:10.1136/bmj.i4919
Stroup, T. S., and Gray, N. (2018). Management of Common Adverse Effects of Antipsychotic Medications. World Psychiatry 17 (3), 341–356. doi:10.1002/wps.20567
Stubbings, D. R., Hughes, K., and Limbert, C. (2019). Staff Perceptions of PRN Medication in a Residential Care Setting. Jmhtep 14 (6), 469–479. doi:10.1108/JMHTEP-10-2018-0059
Sulosaari, V., Suhonen, R., and Leino-Kilpi, H. (2011). An Integrative Review of the Literature on Registered Nurses' Medication Competence. J. Clin. Nurs. 20 (3‐4), 464–478. doi:10.1111/j.1365-2702.2010.03228.x
Swart, G. T., Siman, E., and Stewart, S. L. (2011). The Use of Pro Re Nata or Statim Medications for Behavioral Control: a Summary of Experience at a Tertiary Care Children's Mental Health center. J. Child. Adolesc. Psychopharmacol. 21 (1), 67–77. doi:10.1089/cap.2010.0010
Usher, K., Baker, J. A., Holmes, C., and Stocks, B. (2009). Clinical Decision-Making for 'as Needed' Medications in Mental Health Care. J. Adv. Nurs. 65 (5), 981–991. doi:10.1111/j.1365-2648.2008.04957.x
Usher, K., Baker, J. A., and Holmes, C. A. (2010). Understanding Clinical Decision Making for PRN Medication in Mental Health Inpatient Facilities. J. Psychiatr. Ment. Health Nurs. 17 (6), 558–564. doi:10.1111/j.1365-2850.2010.01565.x
Vaismoradi, M., Amaniyan, S., and Jordan, S. (2018). Patient Safety and Pro Re Nata Prescription and Administration: A Systematic Review. Pharmacy (Basel) 6 (3), 95. doi:10.3390/pharmacy6030095
Vaismoradi, M., Jamshed, S., Lorenzl, S., and Paal, P. (2021a). PRN Medicines Management for Older People with Long-Term Mental Health Disorders in Home Care. Risk Manag. Healthc. Pol. 14, 2841–2849. doi:10.2147/RMHP.S316744
Vaismoradi, M., Jordan, S., Vizcaya-Moreno, F., Friedl, I., and Glarcher, M. (2020). PRN Medicines Optimization and Nurse Education. Pharmacy (Basel) 8 (4), 201. doi:10.3390/pharmacy8040201
Vaismoradi, M., Vizcaya Moreno, F., Sletvold, H., and Jordan, S. (2019). PRN Medicines Management for Psychotropic Medicines in Long-Term Care Settings: A Systematic Review. Pharmacy (Basel) 7 (4), 157. doi:10.3390/pharmacy7040157
Vaismoradi, M., Fredriksen Moe, C., Vizcaya-Moreno, M. F., and Paal, P. (2021b). Administration of Pro Re Nata Medications by the Nurse to Incapacitated Patients: An Ethical Perspective. Clin. Ethics 17 (0), 5–13. doi:10.1177/14777509211034146
Walsh, B., Dahlke, S., O’Rourke, H., and Hunter, K. F. (2020). Exploring Acute Care Nurses' Decision‐making in Psychotropic PRN Use in Hospitalised People with Dementia. J. Clin. Nurs. 1, 1. doi:10.1111/jocn.15477
Walsh, B., Dahlke, S., O’Rourke, H., Hunter, K. F., and Knafl, K. (2021). Nurses’ Decision‐making Related to Administering as Needed Psychotropic Medication to Persons with Dementia: an Empty Systematic reviewThe Integrative Review: Updated Methodology. Int. J. Old. People NursJ Adv Nurs 1652 (15), e12350546–553. doi:10.1111/opn.12350
Whittemore, R., and Knafl, K. (2005). The Integrative Review: Updated Methodology. J. Adv. Nurs. 52 (5), 546–553. doi:10.1111/j.1365-2648.2005.03621.x
Keywords: clinical practice, medication, medicines management, patient safety, pro re nata
Citation: Mardani A, Paal P, Weck C, Jamshed S and Vaismoradi M (2022) Practical Considerations of PRN Medicines Management: An Integrative Systematic Review. Front. Pharmacol. 13:759998. doi: 10.3389/fphar.2022.759998
Received: 17 August 2021; Accepted: 16 March 2022;
Published: 12 April 2022.
Edited by:
Chanda Kulkarni, Sakra World Hospital, IndiaReviewed by:
Kurt Neumann, Independent researcher, HungaryCopyright © 2022 Mardani, Paal, Weck, Jamshed and Vaismoradi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mojtaba Vaismoradi, bW9qdGFiYS52YWlzbW9yYWRpQG5vcmQubm8=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.