- 1School of Public Health, Lanzhou University, Lanzhou, China
- 2Guangdong Provincial Hospital of Chinese Medicine, Guangdong Provincial, Academy of Chinese Medical Sciences, The Second Clinical School of Guangzhou University of Chinese Medicine, Guangzhou, China
- 3The School of Public Health and Management, Guangzhou University of Chinese Medicine, Guangzhou, China
- 4Guangdong Provincial Key Laboratory of Research on Emergency in Traditional Chinese Medicine, Guangzhou, China
- 5Research Unit of Evidence-Based Evaluation and Guidelines, Chinese Academy of Medical Sciences (2021RU017), School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
- 6Institute of Health Data Science, Lanzhou University, Lanzhou, China
- 7Evidence-Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou, China
- 8WHO Collaborating Centre for Guideline Implementation and Knowledge Translation, Lanzhou, China
- 9Guideline International Network Asia, Lanzhou, China
- 10Key Laboratory of Evidence Based Medicine and Knowledge Translation of Gansu Province, Lanzhou University, Lanzhou, China
- 11Lanzhou University GRADE Center, Lanzhou, China
- 12Lanzhou University, An Affiliate of the Cochrane China Network, Lanzhou, China
Background: Acute infectious diseases constitute the most prevalent public health emergency (PHE) in China. Chinese herbal medicine (CHM) has long been used in the treatment of acute infections, but the overall evidence of its benefit and harm has not been comprehensively and systematically evaluated.
Methods: We searched CBM, CNKI, Wanfang, PubMed, Cochrane Library, embase and preprint platforms to retrieve systematic reviews (SRs) on CHM for acute infectious. Participants with COVID-19, SARS, H1N1, tuberculosis, bacillary dysentery, mumps, herpangina, hand-foot-and-mouth disease (HFMD), and other acute infectious diseases were included. Interventional group consisting of patients treated with CHM combined with Western medicine or CHM alone. The AMSTAR 2 tool was used to assess the methodological quality of the retrieved studies. Information on interventions, control measures and outcomes of the included studies was extracted, and meta-analyses were qualitatively synthesized.
Results: A total of 51 SRs and meta-analyses were eligible for this overview, including 19 for COVID-19, 11 for hand-foot-and-mouth disease, 8 for severe acute respiratory syndrome (SARS), 4 for tuberculosis, 3 for mumps, 2 for bacillary dysentery, 2 for H1N1 influenza and 2 for herpangina. Six systematic reviews were of high quality, all of which were on the use of CHM for COVID-19; 24 were of moderate quality; 10 were of low quality; and 11 were of very low quality. CHM appeared to have potential benefits in improving clinical symptoms and signs for most infections with an acceptable safety profile, and the clinical evidence of the benefits of CHM for acute respiratory infections such as COVID-19, SARS and H1N1 seems more sufficient than that for other acute infections.
Conclusion: Overall, CHM, both decoction and Chinese patent medicine, used alone or in combination with conventional medicine may offer potential benefits to relieving symptoms of people with acute respiratory infections. Full reporting of disease typing, staging, and severity, and intervention details is further required for a better evidence translation to the responses for PHE. Future CHM research should focus mainly on the specific aspects of respiratory infections such as its single use for mild infections, and the adjunct administration for sever infections, and individual CHM prescriptions for well-selected outcomes should be prioritized.
Introduction
Public health emergencies (PHEs) are extraordinary events that are determined to constitute public health risks to other states through the international spread of disease and that potentially require a coordinated international response (World Health Organization, 2005). Acute infectious diseases are among the most common PHEs (World Health Organization, 2017). In China, Chinese herbal medicine (CHM) has a long history of treating acute infections such as smallpox, plague, scarlet fever, cholera, typhoid fever, and malaria (Jiang and Wen, 2021). Given the occurrence and epidemics of infectious diseases across different periods, valuable experience has been accumulated in the use of CHM to fight against infectious diseases, which was often documented in classical literature and monographs (Wang W. et al., 2020). Specifically, Yellow Emperor’s Internal Classic, released in approximately 5,000 years ago, was the first publication to find that the occurrence of infectious diseases was closely related to climate change. Treatise on Cold Attack, released in the Eastern Han Dynasty, was written after a large-scale epidemic of acute infectious diseases. Doctor Zhongjing Zhang summarized the development of infectious diseases in the book and recorded many classical formulas such as Xiaochaihu Decoction and Maxing Shigan Decoction, that have been used since then. In late Ming China, with the further deepening of the understanding of infectious diseases in traditional Chinese medicine (TCM), Systematic Differentiation of Warm Pathogen disease authored by Doctor Jutong Wu, systematically expounded the general laws of the occurrence, development, evolution and treatment of infectious diseases, in which, Yinqiao Powder and Sangju Drink, was first documented, and continues to be used for acute upper respiratory disease.
The clinical effectiveness of some classical CHM prescription has been investigated in rigorous randomised controlled trials (RCTs). For example, a single RCT published in Ann Intern Med in 2011 suggested that a CHM formula combining Maxin Shigan Decoction and Yinqiao Power, alone and in combination with an anti-virus pharmacotherapy oseltamivir, can reduce the time for a fever to resolve in patients with H1N1 influenza infection (Wang et al., 2011). Another outstanding example is artemisia annua L., which was recorded in A Handbook of Prescriptions for Emergencies (Doctor Hong Ge, Eastern Jin Dynasty) for treating malaria. Later, this CHM formula has been developed to artemisinin, and transferred to clinical practice of malaria, for which Tu Youyou won the Nobel Prize (Tu, 2016).
In modern China, CHM continues to be applied to a wide range of emergent infectious diseases, such as severe acute respiratory syndrome (SARS), H1N1 influenza, and Coronavirus disease 2019 (COVID-19). And there are many clinical trials and systematic reviews of CHM that have been published. However, there has been no comprehensive study describing the status of the treatment of acute infectious diseases with CHM in the manner of critical appraisal. Therefore, we conducted this study to provide an overview of systematic reviews (SRs) of the treatment of infectious diseases with CHM that could serve as a reference for decision-making in this field.
Methods
We followed the guidance of overviews of reviews published by Hunt et al. (2018). We also reported this overview according to the PRISMA statement (Moher et al., 2009). We have registered this study with the registration DOI: 10.17605/OSF.IO/VZ4S7.
Inclusion and Exclusion Criteria
Study Types Included in This Overview
Systematic reviews (SRs) and meta-analyses, language limited to Chinese and English.
Participants
Participants with COVID-19, SARS, H1N1, tuberculosis, bacillary dysentery, mumps, herpangina, hand-foot-and-mouth disease (HFMD), and other acute infectious diseases were included, as identified according to the current list of public PHEs in China (Liu et al., 2019).
Interventions
Interventional group consisting of patients treated with CHM combined with Western medicine or CHM alone, where CHM interventions included proprietary Chinese medicine and traditional Chinese medicine decoction. There was no requirement for what should be included in the control group.
Outcomes
Outcomes including effectiveness related outcomes which evaluated by the investigator or reported by patients, laboratory tests and radiological imaging, and safety related outcomes such as adverse events, adverse reactions, and toxic scale. The primary outcomes included effectiveness, mortality and adverse events, and secondary outcomes included symptom score, length of stay, laboratory tests and radiological imaging, etc.
Exclusion Criteria
Studies were excluded from the search when they were conference abstracts, duplicate publications, unpublished data, and those without full details of a SR.
Literature Search and Screening
We searched the Chinese Biomedical Literature database (CBM), China National Knowledge Infrastructure (CNKI), Wanfang database, PubMed, Cochrane Library, embase, medRxiv, bioRxiv, China Association of Chinese Medicine, China Association for Acupuncture and Moxibustion, Chinese Medical Journal Network, and Chinese Medicine Journal Network to retrieve relevant systematic reviews/meta-analyses, and the search time was from the date of database creation to 30 October 2020. Before published of this article, we updated the search time to 31 March 2021. For literature screening, two authors read the title and abstract for the initial screening of the literature, and after downloading the full text, it was read and use to further screen the articles, and the results were submitted to a third author for confirmation and verification. The search strategy was specified in Supplementary 1.
Methodological Quality and Level of Evidence Assessment
The methodological quality of the included studies was evaluated independently by two authors using A MeaSurement Tool to Assess systematic Reviews (AMSTAR 2) (Shea et al., 2017), and a third author assisted in the judgement in cases of disagreement. The methodological quality of AMSTAR2 for systematic review is divided into 16 entries, among which item 2, item 4, item 7, item 9, item 10, item 11, item 13 and item 15 are recommended critical items for determine methodological quality. Considering the specificity of TCM research, we made the following adjustments to the key items. Since some systematic reviews were published before the establishment of the registration platform and the registration platform does not have a Chinese registration language, it was difficult to obtain the protocols of these previous Chinese systematic reviews, so we did not include item 2 as a key entry. Chinese medicine research is mainly published in Chinese language, and most Chinese journal submission systems do not support the presentation of a list of excluded studies, so item 7 was not considered a key entry.
The final evaluation results were classified as 1) “high quality” when there was no or one non-critical weakness, 2) “medium quality” when there was more than one non-critical weakness, 3) “low quality” when there was one critical flaw with or without non-critical weaknesses, or 4) “very low quality” when there was more than one critical flaw with or without non-critical weaknesses.
We also evaluated the level of evidence using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach for primary outcomes.
Data Extraction and Data-Analysis
Two authors independently collected the data on publication information, demographic characteristics, details of the interventions and control measures, outcomes, and statistical results, which were finally checked and confirmed by a third authors. For data analysis, a qualitative integration of the study results was performed for SRs evaluated as having moderate-high quality according to AMSTAR 2.
Results
Results of the Searching and Screening
A total of 46,138 relevant records were obtained from the initial search and 6,468 records were identified from updated search, and after screening, 51 systematic reviews (Liu and Dong, 2021; Liu et al., 2004; Zhang et al., 2004; Zhao et al., 2004; Hao, 2005; Hao et al., 2005; Liu et al., 2005; Chen et al., 2007; Guo et al., 2010; Liu et al., 2012; Ding et al., 2013; Lu et al., 2013; Wang et al., 2013; Xiong et al., 2013; Zhang et al., 2014; Zhang and Wei, 2014; Zhao, 2014; Zhao et al., 2014; Wu et al., 2015; Han, 2016; Li et al., 2016; Liu et al., 2016; Zhang, 2016; Wang et al., 2017; Yan and Gao, 2017; Yue et al., 2017; Jin et al., 2018; Xiong et al., 2019; Ang et al., 2020; Yang et al., 2020a; Yu et al., 2020a; Yang et al., 2020b; Yu et al., 2020b; Fan et al., 2020; Gao et al., 2020; He, 2020; Jin et al., 20201992; Liu et al., 2020; Pang et al., 2020; Qi et al., 2020; Wang et al., 2020; Sun et al., 2020; Wu et al., 2020; Xiong et al., 2020; Yan et al., 2020; Zeng et al., 2020; Zhou et al., 2021a; Zhou et al., 2021b; Luo et al., 2021; Ouyang et al., 2021) were finally included. Among them, 33 (Liu and Dong, 2021; Zhao et al., 2004; Hao, 2005; Hao et al., 2005; Liu et al., 2005; Ding et al., 2013; Lu et al., 2013; Wang et al., 2013; Xiong et al., 2013; Zhang et al., 2014; Zhang and Wei, 2014; Zhao, 2014; Han, 2016; Liu et al., 2016; Zhang, 2016; Wang et al., 2017; Xiong et al., 2019; Yang M. et al., 2020; Yu et al., 2020a; Yang Z. et al., 2020; Yu et al., 2020b; Gao et al., 2020; He, 2020; Qi et al., 2020; Wang S. et al., 2020; Wu et al., 2020; Zhou L. P. et al., 2021; Zhou F. et al., 2021; Ouyang et al., 2021) were written in Chinese, and 18 (Liu et al., 2004; Zhang et al., 2004; Chen et al., 2007; Liu et al., 2012; Zhao et al., 2014; Wu et al., 2015; Li et al., 2016; Ang et al., 2020; Fan et al., 2020; Jin et al., 2020; Liu et al., 2020; Pang et al., 2020; Sun et al., 2020; Xiong et al., 2020; Yan et al., 2020; Zeng et al., 2020; Zhou L. P. et al., 2021; Luo et al., 2021) were written in English. The literature screening process and results are shown in Figure 1. The excluded references are stated in Supplementary 2. The ingredients of the formulas are specified in Supplementary 3.
Basic Characteristics of the Included Literature
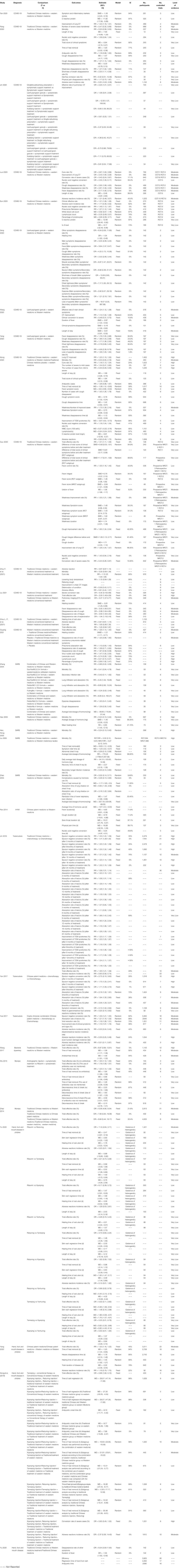
The disease with the largest proportion in the of systematic reviews was COVID-19, with 19 articles (Liu and Dong, 2021; Ang et al., 2020; Yang M. et al., 2020; Fan et al., 2020; Gao et al., 2020; Jin et al., 2020; Liu et al., 2020; Pang et al., 2020; Qi et al., 2020; Wang S. et al., 2020; Sun et al., 2020; Wu et al., 2020; Xiong et al., 2020; Zeng et al., 2020; Zhou L. P. et al., 2021; Zhou F. et al., 2021; Luo et al., 2021; Ouyang et al., 2021), followed by 11 articles on HFMD (Ding et al., 2013; Wang et al., 2013; Xiong et al., 2013; Zhang et al., 2014; Zhang and Wei, 2014; Xiong et al., 2019; Yu et al., 2020a; Yang Z. et al., 2020; Yu et al., 2020b; He, 2020; Yan et al., 2020), 8 for SARS (Liu et al., 2004; Zhang et al., 2004; Zhao et al., 2004; Hao, 2005; Hao et al., 2005; Liu et al., 2005; Chen et al., 2007; Liu et al., 2012), 4 for tuberculosis (Guo et al., 2010; Yan and Gao, 2017; Yue et al., 2017; Jin et al., 2018), 3 for mumps (Zhao, 2014; Wu et al., 2015; Zhang, 2016), 2 for bacterial dysentery (Han, 2016; Wang et al., 2017), 2 for H1N1 (Zhao et al., 2014; Li et al., 2016), and 2 for herpes pharyngitis (Lu et al., 2013; Liu et al., 2016).
The number of RCTs included in each systematic review ranged from 2 to 45. Regarding the type of intervention in the intervention group, TCM combined with Western medicine accounted for the greatest proportion (n = 43, 84.31%) (Liu and Dong, 2021; Fan et al., 2020; Pang et al., 2020; Jin et al., 20201992; Luo et al., 2021; Sun et al., 2020; Zeng et al., 2020; Wang S. et al., 2020; Yang et al., 2020a; Ang et al., 2020; Xiong et al., 2020; Liu et al., 2020; Gao et al., 2020; Qi et al., 2020; Wu et al., 2020; Chen et al., 2007; Liu et al., 2004; Liu et al., 2012; Zhang et al., 2004; Hao, 2005; Hao et al., 2005; Liu et al., 2005; Zhao et al., 2004; Zhao et al., 2014; Li et al., 2016; Jin et al., 2018; Yan and Gao, 2017; Yue et al., 2017; Guo et al., 2010; Wang et al., 2017; Han, 2016; Wu et al., 2015; Zhang, 2016; Zhao, 2014; Lu et al., 2013; Liu et al., 2016; Zhang and Wei, 2014; Zhang et al., 2014; Xiong et al., 2013; Wang et al., 2013; Ding et al., 2013; Yu et al., 2020a; Yang et al., 2020b), with two SRs (3.92%) including studies with CHM alone (Zhao et al., 2014; Yu et al., 2020b) and 6 SRs (11.76%) including studies investigating CHM alone and CHM in combination with Western medicine (Lu et al., 2013; Zhang and Wei, 2014; Zhao, 2014; Liu et al., 2016; Zhang, 2016; Xiong et al., 2019). The most frequently studied herbal preparations were proprietary CHM drugs (n = 37, 80.43%), followed by CHM decoction (n = 20.43.48%). In terms of pre-defined outcomes, the most used for all diseases were the rate of improvement of clinical symptoms or signs such as fever and cough (n = 47, 92.16%), followed by overall effectiveness (n = 25, 49.02%), adverse events (n = 16, 31.37%), mortality (n = 11, 21.57%), and the proportion of lung X-ray shadows absorbed (n = 11, 21.57%). Detailed data are shown in Table 1.
Eighteen systematic reviews on COVID-19 that reported on specific drugs showed that the most used proprietary CHM drugs were Lianhua Qingwen Granule/Capsule (n = 14, 77.78%) and Shufeng Jiedu Capsule (n = 10, 55.56%), and the most used CHM decoction were Qingfei Touxie Fuzheng Decoction (n = 7, 38.89%). Six studies that reported specific drugs for SARS showed that the most used prescription was SARS No.2 formula (n = 6, 75.00%), SARS No.1 formula (n = 5, 62.50%), SARS No.3 formula (n = 5, 62.50%) and SARS No.4 formula (n = 5, 62.50%). The two H1N1 SRs used Lianhua Qingwen Capsule (n = 2,100.00%). The three tuberculosis studies that reported specific drugs showed common use of Astragalus Membranaceus (Chinese pinyin: Huangqi) preparations (n = 2). One SR for bacillary dysentery reported the use of CHM decoctions such as Baitouweng Decoction, Shaoyao Decoction, and Jiawei Dachaihu Decoction. The two SRs for mumps that reported specific drugs used Chuanxinlian injections, externally applied Fuhuang ointment, and Pujixiaodu Decoction. The two SRs for herpangina reported specific drugs, including Pudilan Xiaoyan Oral Solution and Yinqiao Decoction. Ten SRs that reported on specific drugs for HFMD most used herbal injections, such as Xiyanping Injection (n = 7, 70.00%), Reduning Injection (n = 3, 30.00%) and Tanreqing Injection (n = 3, 30.00%). Twenty-three SRs reported safety issues, among which one SR concluded that there were no adverse reactions to CHM. Twenty-one SRs reported adverse events, the most common of which were abdominal distension, diarrhoea, nausea, and vomiting, and poor appetite. Detailed data are shown in Table 1.
Results of AMSTAR2 Quality Assessment
The results of the AMSTAR2 evaluation showed that of the 51 systematic reviews, three (6.52%) were of high quality (Wang S. et al., 2020; Zeng et al., 2020; Luo et al., 2021), 22 (47.83%) were of moderate quality (Zhang et al., 2004; Zhao et al., 2004; Hao, 2005; Hao et al., 2005; Liu et al., 2005; Zhao, 2014; Zhao et al., 2014; Wu et al., 2015; Wang et al., 2017; Yan and Gao, 2017; Yue et al., 2017; Jin et al., 2018; Xiong et al., 2019; Yang M. et al., 2020; Yu et al., 2020a; Yang Z. et al., 2020; Fan et al., 2020; Gao et al., 2020; Jin et al., 2020; Pang et al., 2020; Sun et al., 2020; Xiong et al., 2020), ten (21.74%) were of low quality (Liu et al., 2004; Chen et al., 2007; Guo et al., 2010; Liu et al., 2012; Han, 2016; Li et al., 2016; Ang et al., 2020; Liu et al., 2020; Qi et al., 2020; Wu et al., 2020), and 11 (23.91%) were of very low quality (Liu and Dong, 2021; Zhao et al., 2004; Lu et al., 2013; Liu et al., 2016; Zhang and Wei, 2014; Zhang et al., 2014; Xiong et al., 2013; Wang et al., 2013; Ding et al., 2013; Yan et al., 2020; He, 2020).
Six of the high-quality SRs were on TCMs against COVID-19 (Wang S. et al., 2020; Zeng et al., 2020; Zhou L. P. et al., 2021; Zhou F. et al., 2021; Luo et al., 2021; Ouyang et al., 2021). Most of the medium-quality SRs were on COVID-19 (n = 8, 42.11%) (Liu and Dong, 2021; Fan et al., 2020; Pang et al., 2020; Jin et al., 2020; Sun et al., 2020; Yang M. et al., 2020; Xiong et al., 2020; Gao et al., 2020), followed by SARS (n = 5, 62.50%) (Zhang et al., 2004; Zhao et al., 2004; Hao, 2005; Hao et al., 2005; Liu et al., 2005), HFMD (n = 4, 36.36%) (Xiong et al., 2019; Yu et al., 2020a; Yang Z. et al., 2020; Yu et al., 2020b), tuberculosis (n = 3, 75.00%) (Yan and Gao, 2017; Yue et al., 2017; Jin et al., 2018), mumps (n = 2, 66.67%) (Zhao, 2014; Wu et al., 2015), H1N1 (n = 1, 50.00%) (Zhao et al., 2014) and bacillary dysentery (n = 1, 50.00%) (Wang et al., 2017). Among the lower-quality SRs, COVID-19 was also the most frequent disease (n = 4, 21.05%) (Ang et al., 2020; Liu et al., 2020; Qi et al., 2020; Wu et al., 2020), followed by SARS (n = 3, 37.50%) (Liu et al., 2004; Chen et al., 2007; Liu et al., 2012), H1N1 (n = 1, 50.00%) (Li et al., 2016), tuberculosis (n = 1, 25.00%) (Guo et al., 2010) and bacillary dysentery (n = 1.50.00%) (Han, 2016). The highest number of very low-grade SRs reported on HFMD (n = 7, 63.64%) (Ding et al., 2013; Wang et al., 2013; Xiong et al., 2013; Zhang et al., 2014; Zhang and Wei, 2014; He, 2020; Yan et al., 2020), followed by herpangina (n = 2, 100.00%) (Lu et al., 2013; Liu et al., 2016), COVID-19 (n = 1, 5.26%) (Liu and Dong, 2021), and mumps (n = 1, 33.33%) (Zhang, 2016). The summary of AMSTAR 2 assessment is shown in Figure 2. The details of each evaluation item are shown in Supplementary 4.
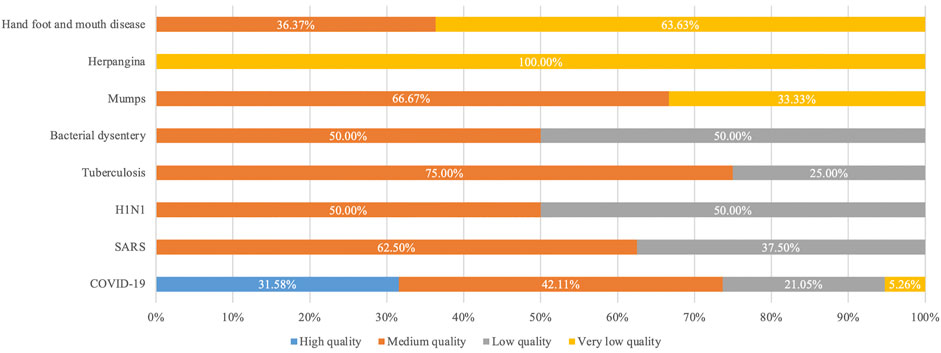
FIGURE 2. Results of the AMSTAR2 methodological quality evaluation. Abbreviations: AMSTAR2: A MeaSurement Tool to Assess Systematic Reviews 2; COVID-19: Coronavirus disease 2019; HFMD: Hand-foot-and-mouth disease; H1N1: Influenza A subtype H1N1; SARS: Severe Acute Respiratory Syndromes.
Qualitatively Analysis of Medium- And-High-Quality Systematic Reviews
The only two SRs on herpangina was excluded from the data-synthesis due to very low quality. SRs of medium- and high-quality for COVID-19, SARS, H1N1 type influenza, tuberculosis, bacillary dysentery, mumps, and HFMD were included to qualitative data-synthesis. Detailed data are shown in Table 2.
COVID-19
Six high-quality SRs (Wang S. et al., 2020; Zeng et al., 2020; Zhou L. P. et al., 2021; Zhou F. et al., 2021; Luo et al., 2021; Ouyang et al., 2021) and eight moderate-quality SRs (Liu and Dong, 2021; Fan et al., 2020; Pang et al., 2020; Jin et al., 2020; Sun et al., 2020; Yang M. et al., 2020; Xiong et al., 2020; Gao et al., 2020) evaluated the efficacy and safety of conventional therapy combined with CHM decoction/proprietary CHM drugs and the results all suggested that this combination therapy was better than conventional therapy alone in improving the overall treatment efficiency for COVID-19 patients.
One single high-quality SR including 19 controlled trials (Luo et al., 2021) identified the efficacy and safety of conventional therapy combined with TCM/tonics, the results showed that the combined with TCM/tonics could improve the appearance of pulmonary CT lesions and the nucleic acid conversion rate, improve the alleviation of symptoms such as fever, cough, malaise, reduce hospitalization time and the rate of clinical cases from mild to severe. However, there was no difference in the incidence of adverse events between the treatments.
Specific to Lianhuaqingwen Capsule, a proprietary CHM drug, a moderate quality SR involving seven RCTs (Wang S. et al., 2020) identified the CHM combined with conventional therapy vs. conventional therapy to treat the COVID-19 patients, and the results suggested that the CHM combined with conventional therapy could improve the appearance of pulmonary CT lesions, shorten the fever duration and the time in hospital, and reduce the possibility being worsening. As for safety, no adverse events were reported.
One moderate quality SR including 12 RCTs with mild and ordinary COVID-19 patients (Gao et al., 2020) suggested that the combined with CHM decoction/proprietary CHM drugs could reduce the duration of fever, fatigue, and cough, improve the appearance of pulmonary CT lesions and the nucleic acid conversion rate, and reduce the rate of clinical cases from mild to severe. However, another high-quality systematic review (Ouyang et al., 2021) including six RCTs and four cohort studies identified the efficacy and safety of TCM in the treatment of common or mild COVID-19 patients, showing that TCM was superior to the control group in improving efficiency and reducing the duration of fever, but there was no difference in the relief of related symptoms such as fever and malaise and the incidence of adverse effects between the two groups.
One moderate quality SR involving seven RCTs (Fan et al., 2020) identified the CHM combined with conventional therapy vs. conventional therapy to treat the COVID-19 patients ranging from being mild to severe, and the results suggested that the CHM combined with conventional therapy could improve the appearance of pulmonary CT lesions and reduce C-reactive protein. As for safety, no adverse events were reported.
One single moderate-quality SR including three RCTs (Yang M. et al., 2020) evaluated the efficacy and safety of Lianhuaqingwen capsule, and the results suggested that in combination with conventional treatment, they could improve the alleviation of symptoms such as fever, cough, fatigue, and chest tightness, dyspnoea, and loss of appetite in ordinary COVID-19 patients better than conventional treatment alone. Regarding safety, there was no difference in the incidence of adverse events between the treatments.
One high-quality network meta-analysis including five RCTs (Jin et al., 2020) evaluated the efficacy of four CHM prescripts, namely, Qingfei Touxie Fuzheng Decoction, Lianhua Qingwen Granule, Lianhua Qingke Granule, and Xuebijing Injections, and the results suggested that the combination of symptomatic and supportive treatment with either one of four prescriptions could better improve the appearance of the lungs on pulmonary CT than symptomatic treatment alone. Among them, the combination of symptomatic and supportive care with Lianhua Qingke Granule had the highest surface under the cumulative ranking (SUCRA) value, suggesting it had the highest overall effectiveness.
Two high-quality systematic reviews (Zhou L. P. et al., 2021; Zhou F. et al., 2021) identified the add-on effect of TCM for COVID-19. One included 10 RCTs and the other included 6 RCTs, and both studies suggested that TCM may be an effective auxiliary treatment for COVID-19 patients, which is likely to help improve the main symptoms, such as fever, cough, and fatigue, shorten the hospital stay and reduce disease progression.
SARS
Five moderate-quality SRs (Zhang et al., 2004; Zhao et al., 2004; Hao, 2005; Hao et al., 2005; Liu et al., 2005) evaluated the effectiveness of CHM combined with Western medicine for SARS, and the results all suggested that the combination better improved the clinical progression of SARS patients; however, the benefits to specific outcomes varied across SRs.
One moderate-quality SR including eight controlled trials (Liu et al., 2005) suggested that the additional use of CHM reduced the mortality, the incidence of secondary fungal infections in the lungs, shorten the duration of fever, the persisting clinical symptoms and the time for Chest X-ray to return normal appearance. There were no adverse events for the combination treatments.
Another moderate-quality SR including six RCTs with mild-to-sever patients (Zhang et al., 2004) showed that the improvement of the appearance of abnormal chest X-ray shadows was better in the group treated with CHM decoction and conventional medicine than the conventional treatment alone. However, there was no statistical difference in the reduction of mortality, and dose of corticosteroids, and the alleviation of cough and dyspnoea between two groups.
Two other moderate-quality SR (Hao, 2005; Hao et al., 2005) supported the conclusion the combination of CHM and conventional medicine was better in reducing the duration of fever and mortality among the patients with SARS; however, the use of corticosteroids had not been reduced due to the additional use of CHM.
Another moderate-quality SR (Zhao et al., 2004) did not support the benefits to improving Chest X-ray imaging among the SARs patients when CHM was used alongside conventional medicine; it confirmed the superiority of CHM in reducing the duration of fever, mortality dose of corticosteroids and complications due to overuse of corticosteroids as well as improving clinical symptoms.
H1N1 Influenza
One moderate-quality SR including five RCTs (Zhao et al., 2014) suggested that the use of Lianhua Qingwen Capsule was better at reducing the duration of symptoms such as fever, cough, sore throat, and body pain in H1N1 patients compared with the use of ooseltamivir. However, there was no statistical difference of the time to conversion to nucleic acid negativity between two treatments. Regarding safety, no details of adverse events were reported.
Tuberculosis
One moderate-quality SR (Jin et al., 2018) evaluated the efficacy of CHM decoction/proprietary CHM drugs combined with chemotherapy, and the results suggested that the combination better improved the negative conversion rate of sputum bacteria, lesion absorption rate, lung cavity closure rate, clinical symptom improvement rate, and overall effectiveness of patients with multi-drug-resistant tuberculosis over chemotherapy alone. In terms of safety, the incidence of adverse events was more reduced with the combination treatment.
Specifically, a moderate-quality SR including 16 RCTs (Yan and Gao, 2017) suggested that the proprietary CHM drugs Jiehe Pills in combination of chemotherapy better improved the rate of sputum conversion and lesion resorption and alleviated clinical symptoms and signs such as cough, haemoptysis, fever, emaciation, fatigue, and night sweats in tuberculosis patients over chemotherapy alone. In terms of safety, the incidence of digestive discomforts was more reduced with the combination treatment. Another moderate-quality SR including 20 RCTs (Yue et al., 2017) evaluated the efficacy of oral proprietary CHM drugs including Astragalus membranaceus in combination with chemotherapy better improved the rate of sputum conversion and lesion resorption, with less adverse events related to digestive discomforts, liver injury and the occurrence of rash.
Bacillary Dysentery
One moderate-quality SR (Wang et al., 2017) evaluated the efficacy of the combined use of CHM decoction and Western conventional therapy, and the results suggested that the combination better improved the overall effectiveness and shortened the time to fever and to diarrhoeal alleviation in adults with bacillary dysentery over Western conventional therapy alone; in terms of safety, digestive disorders were observed (intervention: control: 2 cases versus 5 cases).
Mumps
One moderate-quality SR including 11 RCTs (Wu et al., 2015) evaluated the effectiveness of the combined use of Chuanhuning Injection versus anti-virus pharmacotherapy ribavirin, and the results suggested that the combined use of Chuanhuning Injection and routine care better improved the overall effectiveness, shortened the time to fever and cheek swelling reduction, and reduced the occurrence of complications in children with mumps over ribavirin combined with routine care. In terms of safety, no adverse events occurred in the intervention group compared with the control including 4 cases of adverse events.
Another moderate-quality SR (Zhao, 2014) evaluated the effect of treatment with CHM alone, and the results suggested that internal and external treatment with CHM better improved the overall effectiveness, over proprietary CHM drugs alone; the external use of CHM outperformed the oral treatment. For safety, adverse events were observed, but no details were provided for individual groups.
Hand-Foot-And-Mouth Disease
A moderate-quality SR (Xiong et al., 2019) evaluated the effectiveness of proprietary CHM injections alone or in combination with conventional treatment, and the results suggested the monotherapy or the adjunct use of CHM injections reduced the time to fever and rash reduction, and improved the overall clinical effectiveness in children with HFMD. However, there was no difference in the incidence of adverse events and severe case conversion rate between treatments.
A moderate-quality SR including 24 RCTs (Yang Z. et al., 2020) evaluated the effectiveness of using oral proprietary CHM drug Lanqin Oral Solution in addition to conventional treatment, and the results suggested that the combination treatment better reduced the time to fever and rash reduction and oral ulcer healing and shortened the total duration of illness in children with HFMD. In terms of safety, there was no difference in the incidence of adverse events between treatments.
One moderate-quality SR including 17 RCTs (Yu et al., 2020a) conducted a network meta-analysis of proprietary CHM drugs for HFMD. The results suggested that the Yanhuning Injection, Reduning Injection, Xiyanping injection and Tanreqing injection were significantly better than Ribavirin in improving the total clinical effectiveness; as for oral ulcer healing time and hospitalization time, Xiyanping and Reduning were significantly shorter than ribavirin; in terms of safety, Reduning and Xiyanping were significantly higher than ribavirin.
Another moderate-quality SR (Yu et al., 2020b) conducted a network meta-analysis to identify the effectiveness and safety of Qingre Jiedu TCM oral liquid in the treatment of HFMD. They concluded that seven TCM oral liquids, including Lanqin oral liquid, Pudilan oral liquid, Yellow Gardenia liquid, Fuganlin oral liquid, Kangbindu oral liquid, Huangqing oral liquid, and Shuanghuanglian oral liquid, had good therapeutic effects in clinical efficacy and recovery time of related symptoms. In the adverse reactions aspect, Pudilan oral liquid had the highest clinical safety.
Supplementary 5 detailed the amount of each drug in a polyherbal preparation, and the complete species and drug name of the included SRs.
Discussion
This study provides a broad review of the efficacy and safety of CHM in the treatment of acute infectious diseases. After a systematic search and screening, we included 46 systematic reviews, and meta-analysis of moderate-to-high-quality showed that CHM alone or in combination with Western medicine was effective in treating acute and emergent respiratory diseases such as COVID-19, H1N1, and SARS in terms of symptom improvement such as fever, cough and dyspnoea, without serious adverse events. When combined with Western medicine, CHM shows potential in improving certain outcomes, such as mortality, but the evidence is not yet sufficient. In addition, some studies showed that CHM combined with Western medicine can also improve some intermediate outcomes including white blood cell count, nucleic acid negativity conversion rate, lung CT improvement rate. The adjunct use of CHM may be accounted for treating children with acute infections such as HFMD, bacillary dysentery and mumps; however, safety should be closely monitored before and after the treatment.
In the treatment of COVID-19, several moderate-to-high quality systematic reviews and meta-analyses (Yang M. et al., 2020; Fan et al., 2020; Gao et al., 2020; Jin et al., 2020; Pang et al., 2020; Wang S. et al., 2020; Sun et al., 2020; Xiong et al., 2020; Zeng et al., 2020; Luo et al., 2021) showed that combination therapy had a good overall efficiency and nucleic acid negativity conversion rate and alleviated disease symptoms and that CHM may effectively control cytokine storms by inhibiting the excessive activation of immune cells and reducing inflammatory cytokines in relieving COVID-19 symptoms. According to the current overview, the most common drug in the SRs included in this study was Lianhua Qingwen Capsule, a proprietary CHM drug composed of 13 herbs, namely, the dry fruit of Forsythia suspensa (Thunb.) Vahl, the dry buds or with blooming flowers of Lonicera japonica Thunb., the dry caudex of Ephedra sinica Stapf, Ephedra intermedia Schrenk et C.A.Mey. or Ephedra equisetina Bge., the dry matured seeds of Prunus armeniaca L. var.ansu Maxim., Prunus sibirica L. or Prunus mandshurica (Maxim.) Koehne or Prunus armeniaca L., Gypsum Fibrosum, the dry roots of Isatis indigotica Fort., the dry roots of Dryopteris crassirhizoma Nakai., the dry aboveground part of Houttuynia cordata Thunb., the dry aboveground part of Pogostemon cablin (Blanco) Benth, the dry roots of Rheum palmatum L., the dry roots of Rhodiola crenulate (Hook. f. et Thoms.) H. Ohba, the fresh stem of Mentha haplocalyx Briq., and the dry roots and rhizomes of Glycorrhiza uralensis Fisch., Glycorrhiza inflata Bat. or Glycorrhiza glabra L. Its benefits for people infected by H1N1 virus and SARS-CoV-2 has been determined by randomised, large-sample, controlled clinical trials, and explained by its capacity of anti-inflammation and immunoregulation in pharmacological experiments (Duan et al., 2011; Huang et al., 2020; Hu et al., 2021). However, some important CHM interventions, for which no SRs have been published yet, probably due to the urgency of the fight against the epidemic, have been published as original studies, while drugs for which clinical studies have been conducted including Xuebijing Injection, Xuanfeibaidu Decoction, Qinfeipaidu Decoction, and Huashibaidu Decoction (Wang L. et al., 2020; Xiao et al., 2020; Hu et al., 2021). Substantial publications on prospective/retrospective cohort studies for these CHM prescriptions should be included in future updates of SRs on CHM for acute infections.
For other diseases, a moderate-quality systematic review found that CHM combined with Western medicine for epidemic parotitis shortened the time to fever reduction and improved the overall efficiency, with no significant differences in safety. The main modalities of TCM treatment for mumps include both external and internal application, but validation of the efficacy of these regimens is challenging when designing blinded clinical trials. To enhance and promote exploration of this aspect of the study, some objective outcomes can be selected to be measured as much as possible. Additionally, appropriate reporting guidelines can be selected, such as the CONSORT for Non-Pharmacologic Treatment Interventions (Boutron et al., 2017) and the CONSORT for Chinese Herbal Medicine Formulas (Cheng et al., 2017), to enhance the convenience and operability in conducting systematic reviews.
In addition, the systematic reviews included in this study showed that CHM injections improved the overall clinical effectiveness and severe conversion rate, reduced the time to fever and rash remission and the time for healing of oral ulcers, and shortened the total duration of illness in patients with HFMD. However, none of these SRs reported the occurrence of adverse reactions. HFMD is most prevalent in children, who are a vulnerable group, and there are challenges in conducting clinical studies for this population. Overall, the safety of CHM injections, particularly regarding the amounts used, continues to be of concern. When using CHM injections, one needs to determine whether they are worth using, and if so, their safety needs to be monitored closely.
To the best of our knowledge, this study is the first overview to analyse and evaluate CHM for acute infectious diseases. We systematically assessed 46 systematic reviews and meta-analyses to describe the status of CHM in the treatment of acute infectious diseases. However, the systematic reviews and meta-analyses of CHM alone or in combination with Western medicine for acute infectious diseases were generally plagued with several problems. First, many clinical trials and systematic reviews on Chinese medicine for acute infectious diseases have been published, but most of they are lacking rigorous design and strict quality control. Though time is pressed for fighting against public health emergencies, complying with relevant regulations and methodological consensuses such as “Best practice in research–overcoming common challenges in phytopharmacological research”, is necessary for conducting an ethical and high-quality studies. Theses quality-improving issues should be considered in the future research (Heinrich et al., 2020). Second, we only included studies published in Chinese and English, which may lead to publication bias. Last, we are not able to recommend any specific kind of TCM to be used in public health emergencies as the comparative effectiveness between CHM decoction and Chinese patent medicine is to be determined in future studies.
In general, the clinical applicability of existing SRs on the treatment of acute infectious diseases in CHM is not good, and it is suggested that future studies should focus on the staging and typing of diseases, the type of drugs used, and the singularity of interventions. Second, the reporting of outcomes of these systematic reviews is not standardized, and references can be made to the core set of outcomes in TCM for reporting, such as the COVID-19 core outcome set (COS) (Jin et al., 2020; Qiu et al., 2020). In addition, the low quality of reviews can be addressed by strictly following the standards of PRISMA 2020 (Page et al., 2021) and AMSTAR 2 (Shea et al., 2017) when producing future systematic reviews, thus improving the overall quality in the field. Last but not the least, the precise and appropriate use of botanical scientific nomenclature in CHM SRs is further required to avoid ambiguities and error (Rivera et al., 2014).
Although PHEs are a worldwide issue, China has achieved excellent results by applying CHM and Western medicine. For countries that use traditional medicine, there should be more benefits from applying the wisdom of traditional medicine, especially when there is no drug treatment for new and emergency infectious diseases. Moreover, the richness of traditional medicine may also be a source for developing new drugs for emergency infectious diseases, and it would be worthwhile to conduct in-depth research on drugs with a long history of application and clinical effectiveness. However, due to lack of rigorous regulation, the efficacy, safety and quality of some CHM products need to be proved by more high quality, large sample, unbiased randomized trials.
Conclusion
Overall, CHM, both decoction and Chinese patent medicine, used alone or in combination with conventional medicine may offer potential benefits to relieving symptoms of people with acute respiratory infections. Full reporting of disease typing, staging, and severity, and intervention details is further required for a better evidence translation to the responses for PHE. Future CHM research should focus mainly on the specific aspects of respiratory infections such as its single use for mild infections, and the adjunct administration for sever infections, and individual CHM prescriptions for well-selected outcomes should be prioritized.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Authors Contributions
YC and XN conceived the study. XN, XL and YZ drafted the manuscript. XL validated the data and contributed to the methodology. XN designed the study and analyzed the data. YZ, HL, YLL, MR, YWL, YZ, ZK contributed to the literature search, data collection and quality assessment. YC, and XN interpreted the result from the perspective of Chinese medicine practitioner and clinical investigator. XN interpreted the data from the perspective of public health emergency. YC and XL interpreted the result from the perspective of methodology. All authors provided critical review to the manuscript and approved the submission.
Funding
This study was supported by the internal funding from Guangdong Provincial Key Laboratory of Research on Emergency in TCM (No. 2017B030314176), the external funding from National Natural Science Foundation of China (No. 82104685) and Lanzhou City Talent Innovation and Entrepreneurship Project (Evaluation and Translation of Clinical Evidence of Dominant Diseases of Traditional Chinese Medicine in Gansu Province, No. 2016-RC-1). The funding body was not involved in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledged the contribution from Jingyan Lin, Yuan Liu, Jiahui Lin, and Yidan Zhang, Ping Zeng, and Sichu Xiong from The Second Clinical School of Guangzhou University of Chinese Medicine for their assistance with the preliminary search and data sorting.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.752978/full#supplementary-material
Abbreviations
AMSTAR 2, A MeaSurement Tool to Assess systematic Reviews 2; CBM, Chinese Biomedical Literature database; CD4, Cluster of differentiation 4; CHM, Chinese herbal medicine; COS, Core Outcome Set; CNKI, China National Knowledge Infrastructure; COVID-19, Coronavirus disease 2019; CONSORT, Consolidated Standards of Reporting Trials; H1N1, Influenza A H1N1 influenza; HFMD, Hand-foot-and-mouth disease; IL-6 level, Interleukin-6 level; IFN-α, Interferon-alpha; MD, Mean difference; OR, Odds ratio; PHE, Public health emergency; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, Randomised controlled trial; RD, Rate difference, Risk difference; RoB, Risk of Bias; RR, Relative risk; SARS, Severe Acute Respiratory Syndrome; SMD, Standardized mean difference; SR, Systematic review; SUCRA, Surface under the cumulative ranking; TCM, Traditional Chinese Medicine.
References
Ang, L., Song, E., Lee, H. W., and Lee, M. S. (2020). Herbal Medicine for the Treatment of Coronavirus Disease 2019 (COVID-19): A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 9, 1583. doi:10.3390/jcm9051583
Boutron, I., Altman, D. G., Moher, D., Schulz, K. F., Ravaud, P., and Consort Npt Group., (2017). CONSORT Statement for Randomized Trials of Nonpharmacologic Treatments: A 2017 Update and a CONSORT Extension for Nonpharmacologic Trial Abstracts. Ann. Intern. Med. 167 (1), 40–47. doi:10.7326/M17-0046
Chen, Y., Guo, J. J., Healy, D. P., and Zhan, S. (2007). Effect of Integrated Traditional Chinese Medicine and Western Medicine on the Treatment of Severe Acute Respiratory Syndrome: A Meta-Analysis. Pharm. Pract. (Granada) 5 (1), 1–9. doi:10.4321/s1886-36552007000100001
Cheng, C. W., Wu, T. X., Shang, H. C., Li, Y. P., Altman, D. G., Moher, D., et al. (2017). CONSORT Extension for Chinese Herbal Medicine Formulas 2017: Recommendations, Explanation, and Elaboration (Simplified Chinese Version). Ann. Intern. Med. 167 (2), W21–W34. doi:10.7326/IsTranslatedFrom_M17-2977_2
Ding, J., Zhang, J., Tian, Y., Liu, D., and Li, X. (2013). Meta-analysis of Xiyanping Injection in the Treatment of Hand-Foot-Mouth Disease. Glob. Traditional Chin. Med. 6 (8), 585–588. doi:10.3969/j.issn.1674-1749.2013.08.007
Duan, Z. P., Jia, Z. H., Zhang, J., Liu, S., Chen, Y., Liang, L. C., et al. (2011). Natural Herbal Medicine Lianhuaqingwen Capsule Anti-influenza A (H1N1) Trial: a Randomized, Double Blind, Positive Controlled Clinical Trial. Chin. Med. J. (Engl) 124 (18), 2925–2933. doi:10.3760/cma.j.issn.0366-6999.2011.18.024
Fan, A. Y., Gu, S., and Alemi, S. F.Research Group for Evidence-based Chinese Medicine (2020). Chinese Herbal Medicine for COVID-19: Current Evidence with Systematic Review and Meta-Analysis. J. Integr. Med. 18 (5), 385–394. doi:10.1016/j.joim.2020.07.008
Gao, C., Song, C., Fu, Y., and Zhang, J. (2020). The Curative Effect on Treating COVID -19 by Integrated Medicine: A Systematic Review. J. Shaanxi Univ. Chin. Med. 44 (1), 1–9. doi:10.13424/j.cnki.jsctcm.2021.01.001
Guo, X., Zhang, H., Wu, D., Ni, W., Lu, Z., and Geng, P. (2010). Systematic Review of Randomized Controlled Trials of Integrated Traditional Chinese and Western Medicine in the Treatment of Multi Drug Resistant Pulmonary Tuberculosis. J. traditional Chin. Med. 51, 159–160. doi:10.13288/j.11-2166/r.2010.s2.229
Han, S. (2016). The System Evaluation for the Treatment of Acute Bacterial Dysentery in Children by TCM and Chinese and Western Medicine. Shijiazhuang: Hebei University of traditional Chinese Medicine. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201701&filename=1016324190.
Hao, Y., Hong, J., Kou, C., and Shi, L. (2005). Meta-analysis of Integrated Traditional Chinese and Western Medicine and Simple Western Medicine in the Treatment of SARS. Chin. J. Public Health 21 (5), 525–526.
Hao, Y. (2005). Meta-analysis of Comparative Studies of lntegrative Traditional Chinese Medicine with Western Medicine and Western Medicine Alone for SARS. Changchun: Jilin University. http://kns.cnki.net/KCMS/detail.aspx?dbname=CMFD0506&filename=2005105734.
He, Q. (2020). Meta-analysis of Xiyanping Combined with Chinese Patent Medicine in the Treatment of Hand Foot Mouth Disease in Children. BABY AND ME 10 (7), 79–82.
Heinrich, M., Appendino, G., Efferth, T., Fürst, R., Izzo, A. A., Kayser, O., et al. (2020). Best Practice in Research - Overcoming Common Challenges in Phytopharmacological Research. J. Ethnopharmacol 246, 112230. doi:10.1016/j.jep.2019.112230
Hu, K., Guan, W. J., Bi, Y., Zhang, W., Li, L., Zhang, B., et al. (2021). Efficacy and Safety of Lianhuaqingwen Capsules, a Repurposed Chinese Herb, in Patients with Coronavirus Disease 2019: A Multicenter, Prospective, Randomized Controlled Trial. Phytomedicine 85, 153242. doi:10.1016/j.phymed.2020.153242
Huang, Y. F., Bai, C., He, F., Xie, Y., and Zhou, H. (2020). Review on the Potential Action Mechanisms of Chinese Medicines in Treating Coronavirus Disease 2019 (COVID-19). Pharmacol. Res. 158, 104939. doi:10.1016/j.phrs.2020.104939
Hunt, H., Pollock, A., Campbell, P., Estcourt, L., and Brunton, G. (2018). An Introduction to Overviews of Reviews: Planning a Relevant Research Question and Objective for an Overview. Syst. Rev. 7 (1), 39. doi:10.1186/s13643-018-0695-8
Jiang, D., and Wen, X. (2021). Investigation on Origin and Development of Epidemic Disease. J. Liaoning Univ. Traditional Chin. Med. 23 (2), 1–4. doi:10.13194/j.issn.1673-842x.2021.02.001
Jin, L., Xu, Y., and Yuan, H. (2020). Effects of Four Types of Integrated Chinese and Western Medicines for the Treatment of COVID-19 in China: a Network Meta-Analysis. Rev. Assoc. Med. Bras 66 (6), 771–777. doi:10.1590/1806-9282.66.6.771
Jin, X., Pang, B., Zhang, J., Liu, Q., Yang, Z., Feng, J., et al. (2020). Core Outcome Set for Clinical Trials on Coronavirus Disease 2019 (COS-COVID). Engineering (Beijing) 6 (10), 1147–1152. doi:10.1016/j.eng.2020.03.002
Jin, X., Xie, H., Zeng, H., Liu, Y., Zhang, T., Cao, S., et al. (2018). Combined Traditional Chinese and Western Medicine Treatment for Multidrug-Resistant Pulmonary Tuberculosis: A Meta-Analysis of Randomized Controlled Trials. Chin. Med. Guide 24 (1), 84–95. doi:10.13862/j.cnki.cn43-1446/r.2018.01.028
Li, J. H., Wang, R. Q., Guo, W. J., and Li, J. S. (2016). Efficacy and Safety of Traditional Chinese Medicine for the Treatment of Influenza A (H1N1): A Meta-Analysis. J. Chin. Med. Assoc. 79 (5), 281–291. doi:10.1016/j.jcma.2015.10.009
Liu, A., and Dong, J. (2021). Meta-analysis of the Clinical Efficacy of Chinese Patent Medicine Alone or Combined with Western Medicine in the Treatment of Covid-19. Chin. Traditional Patent Med. 43 (3), 836–840. doi:10.3969/j.issn.1001-1528.2021.03.054
Liu, J., Manheimer, E., Shi, Y., and Gluud, C. (2004). Chinese Herbal Medicine for Severe Acute Respiratory Syndrome: a Systematic Review and Meta-Analysis. J. Altern. Complement. Med. 10 (6), 1041–1051. doi:10.1089/acm.2004.10.1041
Liu, J. P., Manheimer, E., and Shi, Y. (2005). [Systematic Review and Meta-Analysis on the Integrative Traditional Chinese and Western Medicine in Treating SARS]. Zhongguo Zhong Xi Yi Jie He Za Zhi 25 (12), 1082–1088. doi:10.3321/j.issn:1003-5370.2005.12.006
Liu, L., Zhang, X., Qu, W., Jing, W., and Wang, Y. (2016). Meta-analysis of Chinese Medicine on Herpangina in Children. Guid J. Tradit Chin. Med. Pharm. 22 (22), 88–94. doi:10.13862/j.cnki.cn43-1446/r.2016.22.031
Liu, M., Gao, Y., Yuan, Y., Yang, K., Shi, S., Zhang, J., et al. (2020). Efficacy and Safety of Integrated Traditional Chinese and Western Medicine for Corona Virus Disease 2019 (COVID-19): a Systematic Review and Meta-Analysis. Pharmacol. Res. 158, 104896. doi:10.1016/j.phrs.2020.104896
Liu, W., Chen, Z., Liu, Y., Li, M., Ma, Y., Lu, J., et al. (2019). Epidemiological Analysis of Public Health Emergencies in Guangzhou from 2004 to 2017. Int. J. Virol. 4, 265–268. doi:10.3760/cma.j.issn.1673-4092.2019.04.014
Liu, X., Zhang, M., He, L., and Li, Y. (2012). Chinese Herbs Combined with Western Medicine for Severe Acute Respiratory Syndrome (SARS). Cochrane Database Syst. Rev. 10 (10), CD004882. doi:10.1002/14651858.CD004882.pub3
Lu, H., Chen, L., Sha, W., Hu, X., and Hu, Z. (2013). Systematic Evaluation on Pudilan Xiaoyan Oral Liquid for Treating Pediatric Herpangina. China Pharmaceuticals 22 (15), 24–27. doi:10.3969/j.issn.1006-4931.2013.15.011
Luo, X., Ni, X., Lin, J., Zhang, Y., Wu, L., Huang, D., et al. (2021). The Add-On Effect of Chinese Herbal Medicine on COVID-19: A Systematic Review and Meta-Analysis. Phytomedicine 85, 153282. doi:10.1016/j.phymed.2020.153282
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G., and Prisma Group., (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA Statement. BMJ 339 (7), b2535. doi:10.1136/bmj.b2535
Ouyang, J., Jiang, Z., Zhang, M., Wang, Y., Wang, Z., Bai, R., et al. (2021). Efficacy and Safety of Traditional Chinese Medicine for Patients with Mild or Common COVID-19: A Meta-Analysis. J. Emerg. Traditional Chin. Med. 30 (1), 17–26. doi:10.3969/j.issn.1004-745X.2021.01.006
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ (Clinical research ed.). 372, 71. doi:10.1136/bmj.n71
Pang, W., Liu, Z., Li, N., Li, Y., Yang, F., Pang, B., et al. (2020). Chinese Medical Drugs for Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Integr. Med. Res. 9 (3), 100477. doi:10.1016/j.imr.2020.100477
Qi, G., Qi, W., Jiang, Q., Shen, K., Zhang, X., and Zhang, L. (2020). The Efficacy of Lianhua Qingwen Combined with Western Medicine Scheme on COVID-19 General Type Patients: a Systematic Review. Clin. J. Traditional Chin. Med. 32, 1195–1199. doi:10.16448/j.cjtcm.2020.0701
Qiu, R., Zhao, C., Liang, T., Hao, X., Huang, Y., Zhang, X., et al. (2020). Core Outcome Set for Clinical Trials of COVID-19 Based on Traditional Chinese and Western Medicine. Front. Pharmacol. 11, 781. doi:10.3389/fphar.2020.00781
Rivera, D., Allkin, R., Obón, C., Alcaraz, F., Verpoorte, R., and Heinrich, M. (2014). What Is in a Name? the Need for Accurate Scientific Nomenclature for Plants. J. Ethnopharmacol 152 (3), 393–402. doi:10.1016/j.jep.2013.12.022
Shea, B. J., Reeves, B. C., Wells, G., Thuku, M., Hamel, C., Moran, J., et al. (2017). AMSTAR 2: a Critical Appraisal Tool for Systematic Reviews that Include Randomised or Non-randomised Studies of Healthcare Interventions, or Both. BMJ 358, j4008. doi:10.1136/bmj.j4008
Sun, C. Y., Sun, Y. L., and Li, X. M. (2020). The Role of Chinese Medicine in COVID-19 Pneumonia: A Systematic Review and Meta-Analysis. Am. J. Emerg. Med. 38 (10), 2153–2159. doi:10.1016/j.ajem.2020.06.069
Tu, Y. (2016). Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angew. Chem. Int. Ed. Engl. 55 (35), 10210–10226. doi:10.1002/anie.201601967
Wang, C., Cao, B., Liu, Q. Q., Zou, Z. Q., Liang, Z. A., Gu, L., et al. (2011). Oseltamivir Compared with the Chinese Traditional Therapy Maxingshigan-Yinqiaosan in the Treatment of H1N1 Influenza: a Randomized Trial. Ann. Intern. Med. 155 (4), 217–225. doi:10.7326/0003-4819-155-4-201108160-00005
Wang, J., Ren, J. X., Xie, Y. M., Wang, W. W., Hu, J., and Liao, X. (2013). [Systematic Review of Xiyanping Injection for Hand Foot Mouth Disease]. Zhongguo Zhong Yao Za Zhi 38 (18), 3215–3222. doi:10.4268/cjcmm20131851
Wang, W., Liang, Q., and Zhang, H. (2020). The Ancient Literature Research about Traditional Chinese Medicine Treatment of Cold Epidemic. Acta Chin. Med. Pharmacol. 48, 1–4. doi:10.19664/j.cnki.1002-2392.200115
Wang, L., Yang, Z., Zhang, H., Yu, H., Yang, K., Fu, B., et al. (2020). Pharmacologic Study and Preliminary Study on Treatment of New Coronavirus (2019-nCoV) Pneumonia with Lianhua Qingwen. J. Chin. Med. Mater. 43, 772–778. doi:10.13863/j.issn1001-4454.2020.03.049
Wang, S., Li, M., Chen, X., Ma, M., and Hu, J. (2020c). Clinical Efficacy of Lianhua Qingwen Integrated with Western Medicine on COVID-19 by Meta-Analysis. Chin. Traditional Herbal Drugs 51 (14), 3763–3769. doi:10.7501/j.issn.0253-2670.2020.14.021
Wang, Y., Su, R., Han, J., Wang, Y., and Fan, J. (2017). Intergrated Chinese Medicine Compound and Western Medicine for Acute Bacillary DysenteryA Meta-Analysis. J. Liaoning Univ. Tcm 19 (1), 81–84. doi:10.13194/j.issn.1673-842x.2017.01.024
World Health Organization (2005). International Health Regulations 2005. 2nd edition. Geneva: The Network.
Wu, J. R., Zhang, X. M., and Zhang, B. (2015). Potassium Dehydroandrographolide Succinate Injection for the Treatment of Child Epidemic Parotitis: A Systematic Review and Meta-Analysis. Chin. J. Integr. Med. 21 (11), 866–873. doi:10.1007/s11655-014-1895-2
Wu, Y., Zou, L., Yu, X., Sun, D., Li, S., Tang, L., et al. (2020). Clinical Effects of Integrated Treatment of Traditional Chinese and Western Medicine on COVID-19: a Systematic Review. SH. J. TCM. 54 (6), 29–36. doi:10.16305/j.1007-1334.2020.06.093
Xiao, M., Tian, J., Zhou, Y., Xu, X., Min, X., Lv, Y., et al. (2020). Efficacy of Huoxiang Zhengqi Dropping Pills and Lianhua Qingwen Granules in Treatment of COVID-19: A Randomized Controlled Trial. Pharmacol. Res. 161, 105126. doi:10.1016/j.phrs.2020.105126
Xiong, F., Zhang, J., Tang, H., Cai, Q., and Qiu, Z. (2019). Meta-analysis of Three Kinds of Heat-Clearing and Detoxifying Traditional Chinese Medicine Injections in the Treatment of Children with Hand-Foot-Mouth Disease. China Pharmacist 22 (10), 1850–1855. doi:10.3969/j.issn.1008-049X.2019.10.018
Xiong, X., Wang, P., Su, K., Cho, W. C., and Xing, Y. (2020). Chinese Herbal Medicine for Coronavirus Disease 2019: A Systematic Review and Meta-Analysis. Pharmacol. Res. 160, 105056. doi:10.1016/j.phrs.2020.105056
Xiong, X., Yuan, K., Yan, S., Tang, J., Chen, P., and Jiang, Z. (2013). Meta-analysis of Xiyanping Injection in the Treatment of Children with Hand Foot and Mouth Disease. Hubei J. TCM 35 (5), 10–12. doi:10.3969/j.issn.1000-0704.2013.05.004
Yan, B., and Gao, Y. (2017). Meta-analysis of Conventional Anti Tuberculosis Chemotherapy Combined with Traditional Chinese Medicine Tuberculous Pill in the Treatment of Pulmonary Tuberculosis. Sci. Technol. Chin. Med. 24 (4), 533–537.
Yan, S., Lu, Y., Zhang, G., Li, X., Wang, Z., Yao, C., et al. (2020). Effect of Heat-Clearing and Detoxifying Chinese Medicines Combined with Conventional Therapy on Mild Hand, Foot, and Mouth Disease with Fever: An Individual Patient Data Meta-Analysis. Medicine (Baltimore) 99 (23), e20473. doi:10.1097/MD.0000000000020473
Yang, M., Yang, S., Yang, M., and You, D. (2020). Systematic Review on the Treatment of Novel Coronavirus Pneumonia with Chinese Herbal Lianhua Qingwen. Chin. J. Drug Eval. 37 (2), 126–130.
Yang, Z., Lyu, J., Sun, M. H., Zhi, Y. J., and Xie, Y. M. (2020). [Systematic Review on Efficacy and Safety of Lanqin Oral Liquid in Treatment of Hand, Foot and Mouth Disease]. Zhongguo Zhong Yao Za Zhi 45 (15), 3547–3555. doi:10.19540/j.cnki.cjcmm.20200522.501
Yu, Y., Zhang, G., Han, T., and Huang, H. (2020a). Network Meta-Analysis of Four Kinds of Traditional Chinese Medicine Injection in Treatment of Hand-Foot-Mouth Disease. J. Shandong Univ. Tcm 44 (2), 156–163. doi:10.16294/j.cnki.1007-659x.2020.02.009
Yu, Y., Zhang, G., Han, T., and Huang, H. (2020b). A Network Meta-Analysis of Qingre Jiedu Traditional Chinese Medicine Oral Liquid in the Treatment of Hand Foot Mouth Disease. J. Basic Chin. Med. 26 (11), 1665–1670. doi:10.3969/j.issn.1006-3250.2020.11.027
Yue, J., Xiong, L., Wang, C., Wen, Q., Tang, M., Chen, L., et al. (2017). Meta-analysis on Synergy and Attenuation of Astragalus Membranaceus Elated Oral Preparations of Traditional Chinese Medicine in the Adjuvant Therapy for Pulmonary Tuberculosis. PJCCPVD 25 (4), 1–7. doi:10.3969/j.issn.1008-5971.2017.04.001
Zeng, M., Li, L., and Wu, Z. (2020). Traditional Chinese Medicine Lianhua Qingwen Treating corona Virus Disease 2019(COVID-19): Meta-Analysis of Randomized Controlled Trials. PLoS One 15 (9), e0238828. doi:10.1371/journal.pone.0238828
Zhang, G., and Wei, C. (2014). Meta-Analysis on Hand-Foot-Mouth Disease Treated with Traditional Chinese Medicine. World J. Integrated Traditional West. Med. 9 (2), 122–129. doi:10.3969/j.issn.1673-6613.2014.02.005
Zhang, M. M., Liu, X. M., and He, L. (2004). Effect of Integrated Traditional Chinese and Western Medicine on SARS: a Review of Clinical Evidence. World J. Gastroenterol. 10 (23), 3500–3505. doi:10.3748/wjg.v10.i23.3500
Zhang, Y. (2016). Research on the Prevention and Treatment of Viral Diseases in TCM Based on Meta-Analysis. Jinan: Shandong University of traditional Chinese Medicine. http://kns.cnki.net/KCMS/detail.aspx?dbname=CMFD201701&filename=1016322627.
Zhang, Y., Zhu, Z., and Huang, M. (2014). System Evaluation of Chinese and Western Medicine Combined Treatment of Hand-Foot-Mouth Disease. J. Trop. Med. 14 (9), 1151–1155.
Zhao, F. (2014). Systematic Review of Modern Literature of Traditional Chinese Medicine Used in the Mumps. Jinan: Shandong University of traditional Chinese Medicine. https://kns.cnki.net/KCMS/detail/detail.aspx?dbname=CMFD201501&filename=1015506713.
Zhao, N., Dong, B., Xu, J., Wu, T., and Liu, G. (2004). Systematic Assessment on the Effect of Integrated Chinese Traditional Medicine with Western Medicine in the Treatment of Severe Acute Respiratory Syndrome (SARS). West China Med. J. 19 (3), 353–357. doi:10.3969/j.issn.1002-0179.2004.03.001
Zhao, P., Yang, H. Z., Lv, H. Y., and Wei, Z. M. (2014). Efficacy of Lianhuaqingwen Capsule Compared with Oseltamivir for Influenza A Virus Infection: a Meta-Analysis of Randomized, Controlled Trials. Altern. Ther. Health Med. 20 (2), 25–30.
Zhou, F., Pu, L., Rong, X., Liu, J., Yang, Y., and Liu, W. (2021). Efficacy and Safety of Chinese Herbal Decoction Combined with Western Medicine in Treatment of COVID⁃19: A Meta⁃analysis. J. Pract. Med. 37 (5), 564–568. doi:10.3969/j.issn.1006-5725.2021.05.002
Keywords: Chinese herbal medicine, acute infectious diseases, overview of systematic reviews, COVID-19, public health emergency
Citation: Luo X, Zhang Y, Li H, Ren M, Liu Y, Liu Y, Zhang Y, Kuang Z, Cai Y, Chen Y and Ni X (2022) Clinical Evidence on the Use of Chinese Herbal Medicine for Acute Infectious Diseases: An Overview of Systematic Reviews. Front. Pharmacol. 13:752978. doi: 10.3389/fphar.2022.752978
Received: 04 August 2021; Accepted: 24 January 2022;
Published: 25 February 2022.
Edited by:
Anthony Booker, University of Westminster, United KingdomReviewed by:
Linzi Long, China Academy of Chinese Medical Sciences, ChinaHong Li, Southern Medical University, China
Copyright © 2022 Luo, Zhang, Li, Ren, Liu, Liu, Zhang, Kuang, Cai, Chen and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaojia Ni, Z3JhY2UxOTg0MzI1QDEyNi5jb20=; Yaolong Chen, Y2hlbnlhb2xvbmdAdmlwLjE2My5jb20=
†These authors have contributed equally to this work
 Xufei Luo
Xufei Luo Yikai Zhang
Yikai Zhang Huishan Li
Huishan Li Mengjuan Ren1
Mengjuan Ren1 Xiaojia Ni
Xiaojia Ni