- Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, China
Background: A network meta-analysis (NMA) of the current recommended drugs for the treatment of acute heart failure (AHF), was performed to compare the relative efficacy.
Methods: We used PubMed, EMBASE, Cochrane Clinical Trials Register, and Web of Science systems to search studies of randomized controlled trials (RCT) for the treatment of AHF recommended by the guidelines and expert consensus until 1 December 2020. The primary outcome was all-cause mortality within 30 days. The secondary outcomes included 30-days all-cause rehospitalization, rates of HF-related rehospitalization, rates of adverse events, and rates of serious adverse events. A Bayesian NMA based on random effects model was performed.
Results: After screening 14,888 citations, 23 RCTs (17,097 patients) were included, focusing on nesiritide, placebo, serelaxin, rhANP, omecamtiv mecarbil, tezosentan, KW-3902, conivaptan, tolvaptan, TRV027, chlorothiazide, metolazone, ularitide, relaxin, and rolofylline. Omecamtiv mecarbil had significantly lower all-cause mortality rates than the placebo (odds ratio 0.04, 0.01–0.22), rhANP (odds ratio 0.03, 0–0.40), serelaxin (odds ratio 0.05, 0.01–0.38), tezosentan (odds ratio 0.04, 0–0.22), tolvaptan (odds ratio 0.04, 0.01–0.30), and TRV027 (odds ratio 0.03, 0–0.36). No drug was superior to the other drugs for the secondary outcomes and safety outcomes.
Conclusion: No drug was superior to the other drugs for the secondary outcomes and safety outcomes. Current drugs for AHF show similar efficacy and safety.
Introduction
Acute heart failure (AHF) is a clinical syndrome mainly manifested by a marked decrease in myocardial contractility caused by abnormal heart function, an increase in cardiac load, and a sharp decrease in cardiac output (Ponikowski et al., 2016a). The clinical symptoms of AHF include dyspnea, fatigue, and fluid retention, which can adversely affect patient health and quality of life (Arrigo et al., 2020). The mortality caused by AHF during hospitalization in the United States was 4–6% (Adams et al., 2005; Abraham et al., 2008). The readmission rate within 3 months after hospitalization may reach 30% (Ambrosy et al., 2014), and the readmission rate may reach 50% within 4–6 months (Crespo-Leiro et al., 2016). The main drugs recommended for the treatment of AHF include sedatives (i.e., morphine), bronchial antispasmodics (i.e., aminophylline), diuretics (i.e., chlorothiazide), vasodilators (i.e., nesiritide), vasoconstrictors (i.e., epinephrine), and cardiotonics (i.e., dobutamine, Rossignol et al., 2019). However, there are various indicators of the efficacy of the medical treatment of AHF, and there is a lack of comparison of the main outcome indicators in the short term (the evaluation period is either too short or too long). Regarding drug safety, most studies have focused on the number of adverse events and few studies have attempted to determine differences in the incidence of adverse reactions in the treatment of AHF with drugs, especially within 30 days. More importantly, there have been no direct comparisons of AHF-related therapeutic drugs.
AHF is the most common cause of hospitalization in men over 65 years of age (Ponikowski et al., 2016b), and short-term (within 3 months) and long-term (within 1 year) survival rates are not favorable (Chioncel et al., 2017). The pathophysiology of AHF is multifactorial. There are many potential predisposing factors (such as acute coronary syndrome, arrhythmia, kidney damage, and infection), some of which may be related to increased mortality (Arrigo et al., 2017). Despite extensive investigations in prospective randomized trials, no treatment for acute heart failure can prolong survival or reduce mortality (Ferrari et al., 2018). In the absence of treatments to prolong survival or reduce mortality (Ferrari et al., 2018), the main goal of treatment is to stabilize the patient, reduce congestion, relieve symptoms, and reduce organ damage caused by congestion (Harjola et al., 2017). Therefore, to provide a more comprehensive understanding of the impact of treatment on AHF, we reviewed all existing evidence on the medical treatment of AHF, including all drugs recommended by treatment guidelines. We examined all-cause mortality, rates of all-cause rehospitalization, rates of HF-related rehospitalization, rates of adverse events, and the rates of serious adverse events (all outcomes are within 30 days). Among them, all-cause mortality within 30-days was the main efficacy indicator.
Methods
This study used the Cochrane Collaborative Method (Higgins et al., 2011) and PRISMA Statement (Page et al., 2021), as well as the requirements of the Indirect Treatment Working Group of the International Society for Pharmacoeconomics and Outcomes Research (ISPOR, Massarweh et al., 2021). We registered this study with PROSPERO (registration number: CRD42020169369).
Data sources and search strategy
We searched the databases of PubMed, EMBASE, Web of Science, and the Cochrane Clinical Trials Register from their starting dates to 1 December 2020, using the following key words: acute decompensated heart failure OR acute heart failure OR heart failure AND All guideline-recommended and expert consensus-recommended drug classes: Diuretics, Vasopressin V2 receptor antagonist, ACEI, ARB, Natriuretic peptide, beta blockers, Ivabradine, Digitalis, levosimendan, and Phosphodiesterase III inhibitor, administered alone. The article search was limited to studies involving human subjects and published in English. The full search strategies for PubMed are provided in Supplementary Appendix file S1.
Study selection
We excluded the reduplicated studies using Endnote software, and then screened the studies according to their titles or abstracts. Two authors (HHD and HSL) scanned the titles and abstracts of all retrieved articles independently, and irrelevant studies were excluded at this stage (according to inclusion and exclusion criteria). The eligibility of the remaining articles was evaluated for disagreement or uncertainty. Disagreements were resolved by discussion or consensus of a third reviewer.
Data extraction and quality assessment
Data extraction was performed independently by two authors (HHD and HSL), and the data were checked by a third reviewer. Disagreements were settled by discussion. The following information was extracted from each retrieved article: characteristics of included studies (title, first author, publication year, journal, corresponding address, study design, inclusion, and exclusion criteria, doses of the drugs, treatment duration, and pertinent outcomes). Our outcomes included all-cause mortality, rates of all-cause rehospitalization, rates of HF-related rehospitalization, rates of adverse events, and the rates of serious adverse events (all outcomes are limited to a 30-days period). We appraised study validity according to the risk of bias tool recommended by the Cochrane Collaboration (Higgins et al., 2011). Disagreements were resolved by discussion.
Data synthesis and analysis
R (version 3.4.2, United States) and GeMTC (version 0.14.3, United States) based on a Bayesian model were used for statistical analysis. For direct comparisons, the standard deviation of random effects and the standard deviation of inconsistency were used to determine whether the study was heterogeneous. The odds ratio (OR) was selected as the statistical quantity for the two-class effect size, and a 95% confidence interval (CI) was used. The network meta-analysis adopts the consistency model, and p < 0.05 is considered as statistically significant. The inconsistency test adopts the nodal analysis model (namely, the dot method). A p > 0.05 indicates that there is no evidence proving the inconsistency of the study. The convergence of the meshed Meta was tested using the potential scale reduction parameter (PSRF). IA PSRF close to one means that the convergence of the study is good and the conclusions drawn by the Meta analysis are reliable. Each analysis was based on vague priors for effect sizes (to yield results that are similar to conventional statistical analysis). We used deviance and the deviance information criterion to appraise model fit. We report results of network meta-analysis as odds ratios with 95% credible intervals for categorical outcomes and weighted mean differences with 95% credible intervals for continuous outcomes.
Inclusion criteria and exclusion criteria
The inclusion criteria for the studies were as follows: 1) the study was a randomized, controlled trial (RCT); 2) the study included participants who were adult patients with AHF; 3) the two groups of intervention measures were single drug therapy (medicine for AHF); and 4) the trial had relevant outcomes (occurring within 30 days) including all-cause mortality, rates of all-cause rehospitalization, rates of HF-related rehospitalization, rates of adverse events, and rates of serious adverse events. The exclusion criteria were as follows: 1) observational studies; 2) studies on CHF or failure to report expected results; 3) comparative studies between different doses of the same drug; and 4) observations on the efficacy of drug combinations (> one drug).
Results
Study characteristics and quality
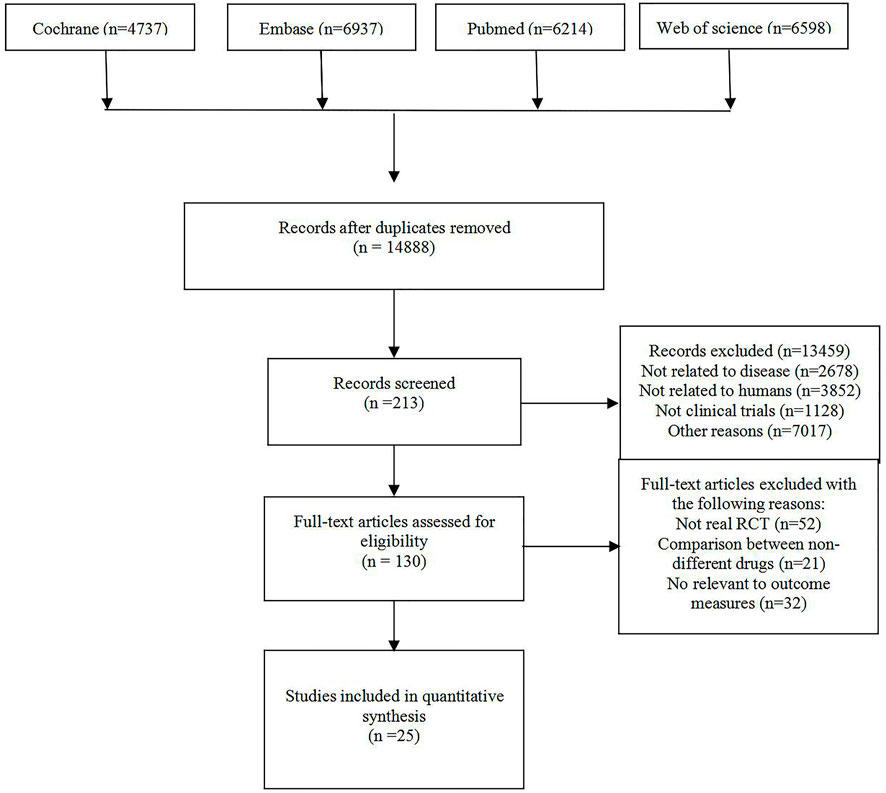
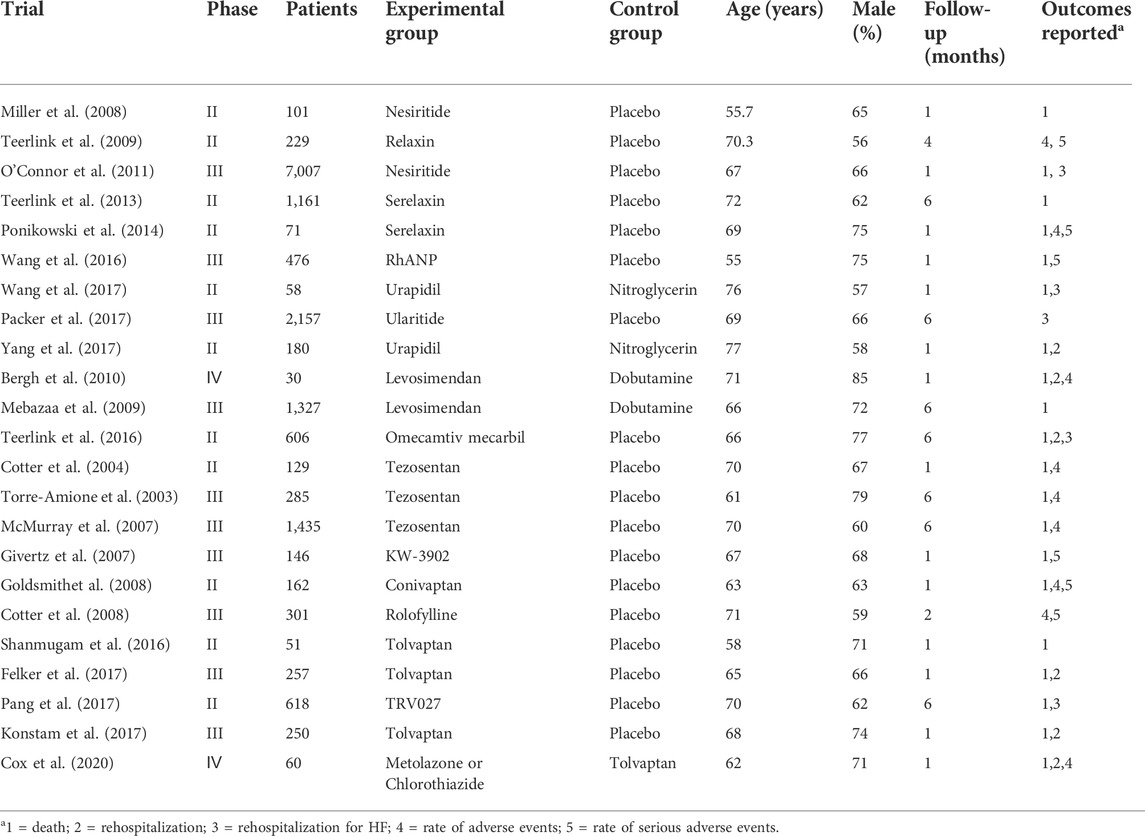
After screening 14,888 citations (Figure 1), we included 23 studies, with a total of 17,097 patients, treated with diuretics (conivaptan, tolvaptan, metolazone, chlorothiazide, KW-3902, rolofylline), vasodilators (nesiritide, rhANP, urapidil, ularitide, relaxin, serelaxin, tezosentan, TRV027, nitroglycerin), cardiotonics (levosimendan, dobutamine, omecamtiv mecarbil) or placebo (Table 1). Patients had a median age of 67.5 years and 66% were males. Patients were followed for a median of 2.7 months. Some drugs in the study could not be linked to other study drugs, so they were not included in the analysis (Figure 2 and Figure 3). All trials had a low risk of bias according to Cochrane metrics (Supplementary Appendix Figure AS1). Only two trials directly compared one drug to another, which means that only a comparison with a placebo could provide direct evidence. Therefore, the effects of drugs could only be compared with each other using indirect evidence.
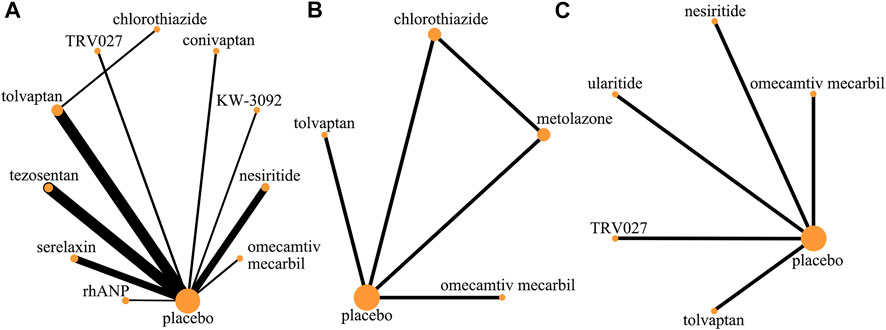
FIGURE 2. Evidence network (death and rehospitalization). (A) All-cause mortality within 30 days; (B) all-cause readmission rate within 30 days; (C) HF-related readmission rate within 30 days.
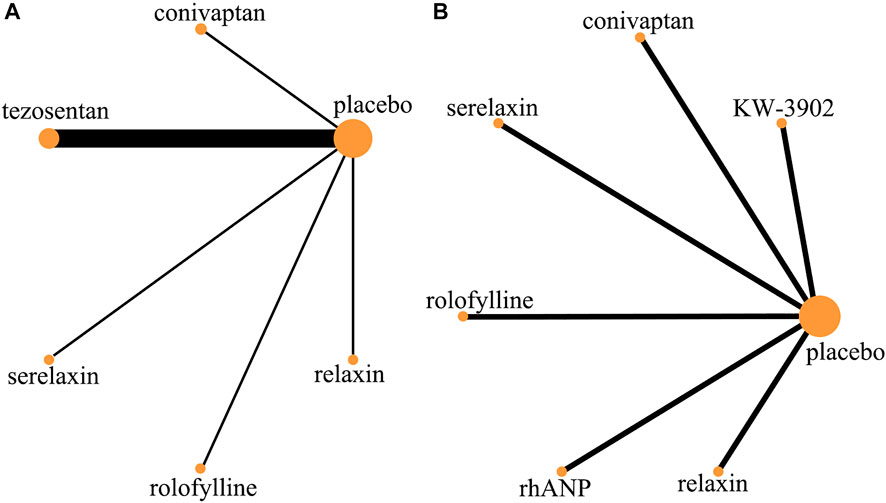
FIGURE 3. Evidence network (adverse event). (A) Rate of adverse event; (B) rate of serious adverse event.
Effect of drugs on mortality within 30 days
In all-cause mortality within 30 days, a total of 19 trials (3 trials could not be compared with other drugs), 10 drugs (including one diuretic, three vasodilators, and five vasoconstrictors, and one cardiotonic agent), and 12,777 patients were involved (Table 2). The comparison of the results of the consistency model and the inconsistency model showed that the results were heterogeneous but the sensitivity analysis that excludes one by one showed that the results were stable. The results showed that omecamtiv mecarbil had significantly lower all-cause mortality rates than the placebo (odds ratio 0.04, 0.01–0.22), rhANP (odds ratio 0.03, 0–0.40), serelaxin (odds ratio 0.05, 0.01–0.38), tezosentan (odds ratio 0.04, 0–0.22), tolvaptan (odds ratio 0.04, 0.01–0.30), and TRV027 (odds ratio 0.03, 0–0.36). Conivaptan, KW-3902, and nesiritide had significantly higher all-cause mortality rates than omecamtiv mecarbil. The pairwise comparison results of the other drugs were not significant. Generally, the effects on all-cause mortality within 30 days, from low to high, were omecamtiv mecarbil, conivaptan, KW-3902, chlorothiazide, nesiritide, tezosentan, serelaxin, rhANP, tolvaptan, and TRV027. Among them, omecamtiv mecarbil, conivaptan, and KW-3902 may have a lower impact on all-cause mortality within 30 days than the placebo, while chlorothiazide, nesiritide, tezosentan, serelaxin, rhANP, tolvaptan, and TRV027 may have a higher impact than the placebo (Supplementary Appendix Figure AS2).
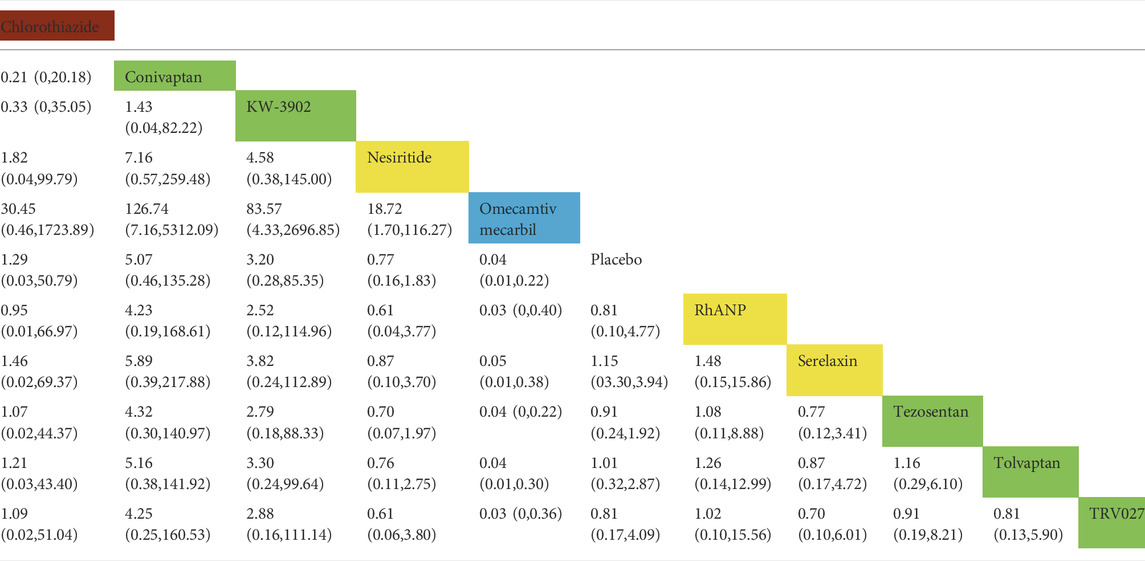
TABLE 2. Direct comparisons among different drugs of all-cause mortality within 30 days (reported as point estimates of odds ratios or weighted mean differences with 95% credible intervals, with number of studies contributing to network). Among them, those marked as red are diuretics, those marked as yellow are vasodilators, those marked as blue are cardiotonics, those marked as green are vasoconstrictors, and those marked as white are placebos.
Effect of drugs on the rates of rehospitalization within 30 days
In the rate of all-cause rehospitalization within 30 days, there are five trials (2 trials could not be compared with other drugs), five drugs (including two diuretics, one vasoconstrictor, and one cardiotonic), and 1,163 patients were involved (Table 3 and Table 4). Comparison of the consistency model and the inconsistency model results showed good agreement (0.23 vs. 0.22). Although the results of the pairwise comparison of the drugs were not statistically significant, the effects on the rates of all-cause rehospitalization within 30 days, from low to high, were chlorothiazide, metolazone, omecamtiv mecarbil and tolvaptan. Among them, the effect of chlorothiazide and metolazone on the rate of all-cause rehospitalization within 30 days may be lower than that of placebo, while the effect of omecamtiv mecarbil and tolvaptan may be higher than that of placebo. In the rates of HF-related rehospitalization within 30 days, a total of six trials (one trial could not be compared with other drugs), five drugs (including one vasodilator, two vasoconstrictors, and two cardiotonics), and 10,473 patients were involved. Comparison of the consistency model and the inconsistency model results showed good agreement (0.24 vs. 0.23). There was no statistical difference in the impact of these five drugs on the rates of HF-related rehospitalization within 30 days. The risk probabilities, from low to high, were TRV027, tolvaptan, omecamtiv mecarbil, nesiritide, and ularitide. Among them, the effects of TRV027 and tolvaptan on the rate of HF-related rehospitalization within 30 days may be lower than the placebo, whereas the effects of omecamtiv mecarbil, nesiritide, and ularitide may be higher than placebo.
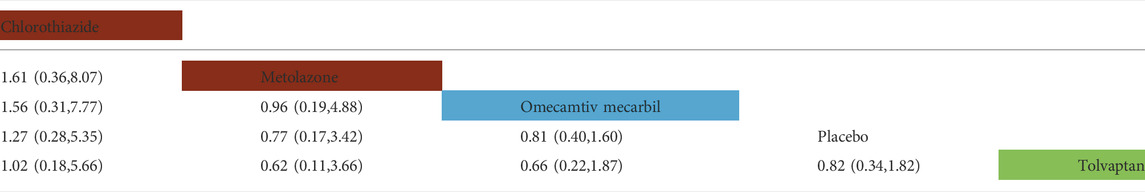
TABLE 3. Direct comparisons between different drugs of all-cause readmission rate within 30 days (reported as point estimates of odds ratios or weighted mean differences with 95% credible intervals, with number of studies contributing to network). Among them, those marked as red are diuretics, those marked as blue are cardiotonics, those marked as green are vasoconstrictors, and those marked as white are placebos.
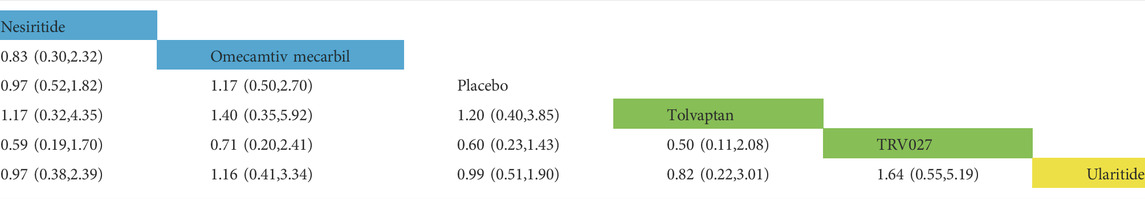
TABLE 4. Direct comparisons between different drugs of HF-related readmission rate within 30 days (reported as point estimates of odds ratios or weighted mean differences with 95% credible intervals, with number of studies contributing to network). Among them, those marked as yellow are vasodilators, those marked as blue are cardiotonics, those marked as green are vasoconstrictors, and those marked as white are placebos.
Effect of drugs on the rates of adverse events within 30 days
In the rates of adverse events within 30 days, a total of nine trials (2 trials were not comparable with other drugs), five drugs (including two vasodilators and three vasoconstrictors), and 2,725 patients were involved (Table 5 and Table 6). Comparison of the consistency model and the inconsistency model results showed good agreement (0.60 vs. 0.54). There was no statistical difference in the effects of these five drugs on the rates of adverse events within 30 days. The risk probabilities, from low to high, were conivaptan, tezosentan, serelaxin, relaxin, and rolofylline. Among them, conivaptan, tezosentan, and serelaxin may have a lower impact on the rates of adverse events within 30 days than the placebo, while the impact of relaxin and rolofylline may be higher than the placebo. In the rates of serious adverse events within 30 days, a total of six trials, six drugs (including three vasodilators and three vasoconstrictors), and 1,386 patients were involved. Comparison of the consistency model and the inconsistency model results showed that they were in good agreement (0.49 vs. 0.48). Similar to the rates of adverse events, there was no statistical difference between the effects of drugs on the rates of adverse events. The risk probability, from low to high, was serelaxin, rhANP, KW-3902, rolofylline, relaxin, and conivaptan. Among them, serelaxin and rhANP may have a higher impact on the incidence of serious adverse events within 30 days than the placebo, while KW-3902, rolofylline, relaxin, and conivaptan may have a lower impact than the placebo (Supplementary Appendix Figure AS3).
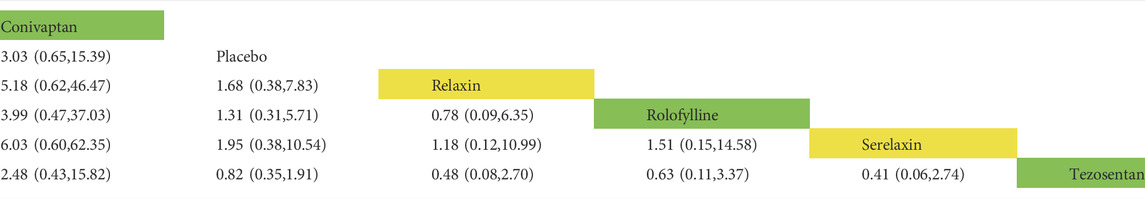
TABLE 5. Direct comparisons between different drugs of the rate of adverse event within 30 days (reported as point estimates of odds ratios or weighted mean differences with 95% credible intervals, with number of studies contributing to network). Among them, those marked as yellow are vasodilators, those marked as green are vasoconstrictors, and those marked as white are placebos.
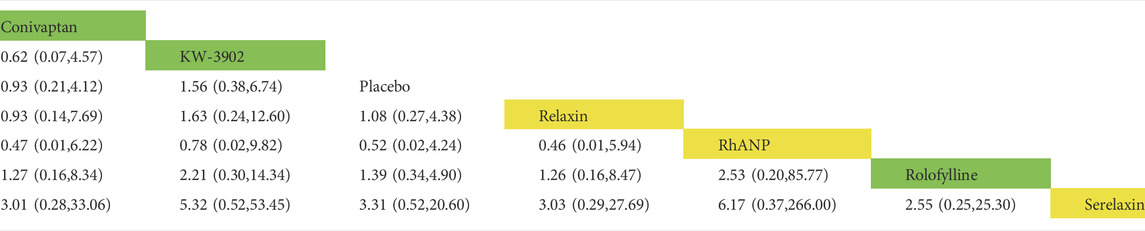
TABLE 6. Direct comparisons between different drugs in the rate of serious adverse events within 30 days (reported as point estimates of odds ratios or weighted mean differences with 95% credible intervals, with number of studies contributing to network). Among them, those marked as yellow are vasodilators, those marked as green are vasoconstrictors, and those marked as white are placebos.
Discussion
The results of the study showed that, for all-cause mortality within 30 days, omecamtiv mecarbil was somewhat better than most of the other drugs that we included. Omecamtiv mecarbil is a myosin exercise activator. It can directly act on cardiac myosin, resulting in increased strength of the heart muscle and prolonged contraction time (Nanasi et al., 2018). However, there was only one RCT study involving omecamtiv mecarbil that met our inclusion criteria and the results of the study may be biased. Within 30 days, the rates of all-cause rehospitalization, the rates of HF-related rehospitalization, the rates of adverse events, and the rates of serious adverse events, showed no significant differences associated with the effects of each drug, which was the same as the previous results (Curfman 2019). The difference is that we have provided the risk probability of each drug to the research outcome and this has reference value for clinicians. During the process of screening related studies, we found that the drugs reported for treating AHF in the RCT studies mainly include vasoconstrictors, vasodilators, cardiotonics and diuretics. However, only a few studies reported the rate of rehospitalization or mortality within 30 days. Most studies focused on specific indicators to determine the efficacy of the drug and did not report key outcome indicators. For adverse events, most studies reported the number of adverse events but did not report the incidence and this made our study more difficult.
Our analysis was limited by the data in the research and the data structure of the reports. Our criteria for inclusion and exclusion were relatively strict, resulting in a relatively small number final number of documents. Because of this, caution is needed when interpreting the results. Most of the studies we included were efficacy comparisons between drugs and placebo. There are few direct comparisons between drugs and this certainly impacted the final results. Although we have confidence in our search strategy, some experiments, such as some non-English language experiments, were excluded. The results are also limited by modeling assumptions. Notably, the present meta-analyses did not include studies of sodium glucose co-transporter-2 inhibitors (SGLT2i). SGLT2i have recently been recommended as a first-line foundational treatment for chronic heart failure. SGLT2i (Sotagliflozin) was also found to improve acute heart failure prognosis in the SOLOIST trial (Bhatt et al., 2021) and is being further investigated in the EMPULSE trial (empagliflozin for acute heart failure, Voors et al., 2022). It is reasonable to believe that SGLT2i may decrease cardiovascular mortality and heart failure readmissions regardless of heart failure acuity. These benefits could potentially stem from the following mechanisms: 1) diuretic and natriuretic effects (including inhibition of the Na +/H + exchanger, Griffin et al., 2020); 2) restoration of myocardial energetics (Garcia-Ropero et al., 2019); 3) improvement in diastolic heart function (Santos-Gallego et al., 2020); 4) improvement in aortic stiffness and systemic vascular resistance (Requena-Ibáñez et al., 2021), and 5) inhibition of the NHE (Uthman et al., 2018). As the evidence for SGLT2i for heart failure outcomes is evolving and their therapeutic mechanisms are being better elucidated, future meta-analyses should include studies of SGLT2i.
Conclusion
We analyzed clinical trials that included all drugs recommended by the guidelines for the treatment of AHF and used Bayesian network meta-analysis to compare drug efficacy. Omecamtiv mecarbil provided a greater reduction in all-cause mortality within 30 days, compared with the other drugs. Within 30 days, the rates of all-cause rehospitalization, rates of HF-related rehospitalization, rates of adverse events, and rates of serious adverse events were all similar among the various drugs. The individual advantages of these drugs cannot be determined with currently available data. A more comprehensive comparison of individual drugs is needed to determine if any of these drugs provide significant advantages. AHF is a life-threatening condition. It is important to merge data or compare outcomes between RCTs that use different interventions. However, a comparison and meta-analyses were hampered because of the heterogeneity of outcome reporting in systematic reviews (Kewcharoen et al., 2019). To improve the consistency of outcomes, it is necessary to develop a core outcome set (COS) for AHF in future studies.
Author contributions
HD and HL wrote original draft, review and editing. BW completed the drawing of charts of network meta-analysis. JZ and YC searched and sort out RCTs related to topics. XZ extracted the key information in RCTs. YL and HS supervised and edited the final manuscript.
Funding
This work was supported by the National Key Research and Development Programmes of China (2017YFC1700400, 2017YFC1700403) and National Natural Science Foundation of China (82174233).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.677589/full#supplementary-material
References
Abraham, W. T., Fonarow, G. C., Albert, N. M., Stough, W. G., Gheorghiade, M., Greenberg, B. H., et al. (2008). Predictors of in-hospital mortality in patients hospitalized for heart failure: Insights from the organized Program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). J. Am. Coll. Cardiol. 52 (5), 347–356. doi:10.1016/j.jacc.2008.04.028
Adams, K. F., Fonarow, G. C., Emerman, C. L., LeJemtel, T. H., Costanzo, M. R., Abraham, W. T., et al. (2005). Characteristics and outcomes of patients hospitalized for heart failure in the United States: Rationale, design, and preliminary observations from the first 100, 000 cases in the acute decompensated heart failure national registry (ADHERE). Am. Heart J. 149 (2), 209–216. doi:10.1016/j.ahj.2004.08.005
Ambrosy, A. P., Fonarow, G. C., Butler, J., Chioncel, O., Greene, S. J., Vaduganathan, M., et al. (2014). The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 63 (12), 1123–1133. doi:10.1016/j.jacc.2013.11.053
Arrigo, M., Gayat, E., Parenica, J., Ishihara, S., Zhang, J., Choi, D. J., et al. (2017). Precipitating factors and 90-day outcome of acute heart failure: A report from the intercontinental GREAT registry. Eur. J. Heart Fail. 19, 201–208. doi:10.1002/ejhf.682
Arrigo, M., Jessup, M., Mullens, W., Reza, N., Shah, A. M., Sliwa, K., et al. (2020). Acute heart failure. Nat. Rev. Dis. Prim. 6 (1), 16. doi:10.1038/s41572-020-0151-7
Bergh, C. H., Andersson, B., Dahlström, U., Forfang, K., Kivikko, M., Sarapohja, T., et al. (2010). Intravenous levosimendan vs. dobutamine in acute decompensated heart failure patients on beta-blockers. Eur. J. Heart Fail. 12 (4), 404–410. doi:10.1093/eurjhf/hfq032
Bhatt, D. L., Szarek, M., Steg, P. G., Cannon, C. P., Leiter, L. A., McGuire, D. K., et al. (2021). Sotagliflozin in patients with diabetes and recent worsening heart failure. N. Engl. J. Med. 384 (2), 117–128. doi:10.1056/NEJMoa2030183
Chioncel, O., Mebazaa, A., Harjola, V. P., Coats, A. J., Piepoli, M. F., Crespo-Leiro, M. G., et al. (2017). Clinical phenotypes and outcome of patients hospitalized for acute heart failure: The ESC heart failure long-term registry. Eur. J. Heart Fail. 19 (10), 1242–1254. doi:10.1002/ejhf.890
Cotter, G., Dittrich, H. C., Weatherley, B. D., Bloomfield, D. M., O'Connor, C. M., Metra, M., et al. (2008). The PROTECT pilot study: A randomized, placebo-controlled, dose-finding study of the adenosine A1 receptor antagonist rolofylline in patients with acute heart failure and renal impairment. J. Card. Fail. 14 (8), 631–640. doi:10.1016/j.cardfail.2008.08.010
Cotter, G., Kaluski, E., Stangl, K., Pacher, R., Richter, C., Milo-Cotter, O., et al. (2004). The hemodynamic and neurohormonal effects of low doses of tezosentan (an endothelin A/B receptor antagonist) in patients with acute heart failure. Eur. J. Heart Fail. 6 (5), 601–609. doi:10.1016/j.ejheart.2004.05.004
Cox, Z. L., Hung, R., Lenihan, D. J., and Testani, J. M. (2020). Diuretic strategies for loop diuretic resistance in acute heart failure: The 3T trial. JACC. Heart Fail. 8 (3), 157–168. doi:10.1016/j.jchf.2019.09.012
Crespo-Leiro, M. G., Anker, S. D., Maggioni, A. P., Coats, A. J., Filippatos, G., Ruschitzka, F., et al. (2016). European society of cardiology heart failure long-term registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 18 (6), 613–625. doi:10.1002/ejhf.566
Curfman, G. (2019). Vasodilator therapy in acute heart failure. JAMA 322 (23), 2288–2289. doi:10.1001/jama.2019.20285
Felker, G. M., Mentz, R. J., Cole, R. T., Adams, K. F., Egnaczyk, G. F., Fiuzat, M., et al. (2017). Efficacy and safety of tolvaptan in patients hospitalized with acute heart failure. J. Am. Coll. Cardiol. 69 (11), 1399–1406. doi:10.1016/j.jacc.2016.09.004
Ferrari, R., Bueno, H., Chioncel, O., Cleland, J. G., Stough, W. G., Lettino, M., et al. (2018). Acute heart failure: Lessons learned, roads ahead. Eur. J. Heart Fail. 20, 842–850. doi:10.1002/ejhf.1169
Garcia-Ropero, A., Santos-Gallego, C. G., Zafar, M. U., and Badimon, J. J. (2019). Metabolism of the failing heart and the impact of SGLT2 inhibitors. Expert Opin. Drug Metab. Toxicol. 15 (4), 275–285. doi:10.1080/17425255.2019.1588886
Givertz, M. M., Massie, B. M., Fields, T. K., Pearson, L. L., and Dittrich, H. C. (2007). CKI-201 and CKI-202 InvestigatorsThe effects of KW-3902, an adenosine A1-receptor antagonist, on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J. Am. Coll. Cardiol. 50 (16), 1551–1560. doi:10.1016/j.jacc.2007.07.019
Goldsmith, S. R., Elkayam, U., Haught, W. H., Barve, A., and He, W. (2008). Efficacy and safety of the vasopressin V1A/V2-receptor antagonist conivaptan in acute decompensated heart failure: A dose-ranging pilot study. J. Card. Fail. 14 (8), 641–647. doi:10.1016/j.cardfail.2008.06.003
Griffin, M., Rao, V. S., Ivey-Miranda, J., Fleming, J., Mahoney, D., Maulion, C., et al. (2020). Empagliflozin in heart failure: Diuretic and cardiorenal effects. Circulation 142 (11), 1028–1039. doi:10.1161/CIRCULATIONAHA.120.045691
Harjola, V. P., Mullens, W., Banaszewski, M., Bauersachs, J., Brunner-La Rocca, H. P., Chioncel, O., et al. (2017). Organ dysfunction, injury and failure in acute heart failure: From pathophysiology to diagnosis and management. A review on behalf of the acute heart failure committee of the heart failure association (hfa) of the European society of cardiology (esc). Eur. J. Heart Fail. 19, 821–836. doi:10.1002/ejhf.872
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Kewcharoen, J., Trongtorsak, A., Kanitsoraphan, C., Prasitlumkum, N., Mekritthikrai, R., Techorueangwiwat, C., et al. (2019). Cognitive impairment and 30-day rehospitalization rate in patients with acute heart failure: A systematic review and meta-analysis. Indian Heart J. 71, 52–59. doi:10.1016/j.ihj.2018.12.006
Konstam, M. A., Kiernan, M., Chandler, A., Dhingra, R., Mody, F. V., Eisen, H., et al. (2017). Short-term effects of tolvaptan in patients with acute heart failure and volume overload. J. Am. Coll. Cardiol. 69 (11), 1409–1419. doi:10.1016/j.jacc.2016.12.035
Massarweh, N. N., Haukoos, J. S., and Ghaferi, A. A. (2021). ISPOR reporting guidelines for comparative effectiveness research. JAMA Surg. 156 (7), 673–674. doi:10.1001/jamasurg.2021.0534
McMurray, J. J., Teerlink, J. R., Cotter, G., Bourge, R. C., Cleland, J. G., Jondeau, G., et al. (2007). Effects of tezosentan on symptoms and clinical outcomes in patients with acute heart failure: The VERITAS randomized controlled trials. JAMA 298 (17), 2009–2019. doi:10.1001/jama.298.17.2009
Mebazaa, A., Nieminen, M. S., Filippatos, G. S., Cleland, J. G., Salon, J. E., Thakkar, R., et al. (2009). Levosimendan vs. dobutamine: Outcomes for acute heart failure patients on beta-blockers in SURVIVE. Eur. J. Heart Fail. 11 (3), 304–311. doi:10.1093/eurjhf/hfn045
Miller, A. H., Nazeer, S., Pepe, P., Estes, B., Gorman, A., and Yancy, C. W. (2008). Acutely decompensated heart failure in a county emergency department: A double-blind randomized controlled comparison of nesiritide versus placebo treatment. Ann. Emerg. Med. 51 (5), 571–578. doi:10.1016/j.annemergmed.2007.12.003
Nanasi, P., Komaromi, I., Gaburjakova, M., and Almassy, J. (2018). Omecamtiv mecarbil: A myosin motor activator agent with promising clinical performance and new in vitro results. Curr. Med. Chem. 25 (15), 1720–1728. doi:10.2174/0929867325666171222164320
O'Connor, C. M., Starling, R. C., Hernandez, A. F., Armstrong, P. W., Dickstein, K., Hasselblad, V., et al. (2011). Effect of nesiritide in patients with acute decompensated heart failure. N. Engl. J. Med. 365 (8), 32–43. doi:10.1056/NEJMoa1100171
Packer, M., O'Connor, C., McMurray, J. J. V., Wittes, J., Abraham, W. T., Anker, S. D., et al. (2017). Effect of ularitide on cardiovascular mortality in acute heart failure. N. Engl. J. Med. 376 (20), 1956–1964. doi:10.1056/NEJMoa1601895
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, n71. doi:10.1136/bmj.n71
Pang, P. S., Butler, J., Collins, S. P., Cotter, G., Davison, B. A., Ezekowitz, J. A., et al. (2017). Biased ligand of the angiotensin II type 1 receptor in patients with acute heart failure: A randomized, double-blind, placebo-controlled, phase IIB, dose ranging trial (BLAST-AHF). Eur. Heart J. 38 (30), 2364–2373. doi:10.1093/eurheartj/ehx196
Ponikowski, P., Mitrovic, V., Ruda, M., Fernandez, A., Voors, A. A., Vishnevsky, A., et al. (2014). A randomized, double-blind, placebo-controlled, multicentre study to assess haemodynamic effects of serelaxin in patients with acute heart failure. Eur. Heart J. 35 (7), 431–441. doi:10.1093/eurheartj/eht459
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G. F., Coats, A. J. S., et al. (2016a). 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Rev. Esp. Cardiol. 37 (27), 1167–1200. doi:10.1016/j.rec.2016.11.005
Ponikowski, P., Voors, A. A., Anker, S. D., Bueno, H., Cleland, J. G. F., Coats, A. J. S., et al. (2016b). 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European society of cardiology (ESC). Developed with the special contribution of the heart failure association (HFA) of the ESC. Eur. J. Heart Fail. 18, 891–975. doi:10.1002/ejhf.592
Requena-Ibáñez, J. A., Santos-Gallego, C. G., Rodriguez-Cordero, A., Vargas-Delgado, A. P., Mancini, D., Sartori, S., et al. (2021). Mechanistic insights of empagliflozin in nondiabetic patients with HFrEF: From the EMPA-TROPISM study. JACC. Heart Fail. 9 (8), 578–589. doi:10.1016/j.jchf.2021.04.014
Rossignol, P., Hernandez, A. F., Solomon, S. D., and Zannad, F. (2019). Heart failure drug treatment. Lancet 393 (10175), 1034–1044. doi:10.1016/S0140-6736(18)31808-7
Santos-Gallego, C. G., Requena-Ibanez, J. A., San Antonio, R., Garcia-Ropero, A., Ishikawa, K., Watanabe, S., et al. (2020). Empagliflozin ameliorates diastolic dysfunction and left ventricular fibrosis/stiffness in nondiabetic heart failure: A multimodality study. JACC. Cardiovasc. Imaging 14 (2), 393–407. doi:10.1016/j.jcmg.2020.07.042
Shanmugam, E., Doss, C. R., George, M., Jena, A., Rajaram, M., Ramaraj, B., et al. (2016). Effect of tolvaptan on acute heart failure with hyponatremia--a randomized, double blind, controlled clinical trial. Indian Heart J. 68, S15–S21. doi:10.1016/j.ihj.2015.07.006
Teerlink, J. R., Cotter, G., Davison, B. A., Felker, G. M., Filippatos, G., Greenberg, B. H., et al. (2013). Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): A randomised, placebo-controlled trial. Lancet 381 (9860), 29–39. doi:10.1016/S0140-6736(12)61855-8
Teerlink, J. R., Felker, G. M., McMurray, J. J. V., Ponikowski, P., Metra, M., Filippatos, G. S., et al. (2016). Acute treatment with omecamtiv mecarbil to increase contractility in acute heart failure: The ATOMIC-AHF study. J. Am. Coll. Cardiol. 67 (12), 1444–1455. doi:10.1016/j.jacc.2016.01.031
Teerlink, J. R., Metra, M., Felker, G. M., Ponikowski, P., Voors, A. A., Weatherley, B. D., et al. (2009). Relaxin for the treatment of patients with acute heart failure (Pre-RELAX-AHF): A multicentre, randomised, placebo-controlled, parallel-group, dose-finding phase IIb study. Lancet 373 (9673), 1429–1439. doi:10.1016/S0140-6736(09)60622-X
Torre-Amione, G., Young, J. B., Colucci, W. S., Lewis, B. S., Pratt, C., Cotter, G., et al. (2003). Hemodynamic and clinical effects of tezosentan, an intravenous dual endothelin receptor antagonist, in patients hospitalized for acute decompensated heart failure. J. Am. Coll. Cardiol. 42 (1), 140–147. doi:10.1016/s0735-1097(03)00556-4
Uthman, L., Baartscheer, A., Bleijlevens, B., Schumacher, C. A., Fiolet, J. W. T., Koeman, A., et al. (2018). Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: Inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 61 (3), 722–726. doi:10.1007/s00125-017-4509-7
Voors, A. A., Angermann, C. E., Teerlink, J. R., Collins, S. P., Kosiborod, M., Biegus, J., et al. (2022). The SGLT2 inhibitor empagliflozin in patients hospitalized for acute heart failure: A multinational randomized trial. Nat. Med. 28 (3), 568–574. doi:10.1038/s41591-021-01659-1
Wang, G, Wang, P, Li, Y, Liu, W, Bai, S, Zhen, Y, et al. (2016). Efficacy and Safety of 1-Hour Infusion of Recombinant Human Atrial Natriuretic Peptide in Patients With Acute Decompensated Heart Failure: A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Medicine (Baltimore). 95(9):e2947. doi:10.1097/MD.0000000000002947
Wang, Z., Hua, Q., Tan, J., Zhou, Y., Fu, Y., Qin, J., et al. (2017). Comparison of therapeutic effects between urapidil and nitroglycerin for treatment of acute heart failure with hypertension and atrial fibrillation in elderly patients: A randomized multi-center parallel-control study in China. Int. J. Clin. Exp. Med. 10, 7020–7029.
Keywords: acute heart failure, network meta-analysis, randomized controlled trial, drug therapy, all-cause mortality
Citation: Dai H, Li H, Wang B, Zhang J, Chen Y, Zhang X, Liu Y and Shang H (2022) Efficacy of pharmacologic therapies in patients with acute heart failure: A network meta-analysis. Front. Pharmacol. 13:677589. doi: 10.3389/fphar.2022.677589
Received: 08 March 2021; Accepted: 22 August 2022;
Published: 23 September 2022.
Edited by:
Nelson Chong, Nottingham Trent University, United KingdomReviewed by:
Juan Badimon, Icahn School of Medicine at Mount Sinai, United StatesMaria Jelinic, La Trobe University, Australia
Copyright © 2022 Dai, Li, Wang, Zhang, Chen, Zhang, Liu and Shang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yan Liu, c2FzbGl1QHllYWgubmV0; Hongcai Shang, c2hhbmdob25nY2FpQDEyNi5jb20=
†These authors have contributed equally to this work and share first authorship.
 Hengheng Dai
Hengheng Dai Haisong Li†
Haisong Li† Xuecheng Zhang
Xuecheng Zhang Yan Liu
Yan Liu Hongcai Shang
Hongcai Shang