- 1Phase I Clinical Trial Center, Beijing Shijitan Hospital Affiliated to Capital Medical University, Beijing, China
- 2Department of Gynecology, Beijing Shijitan Hospital Affiliated to Capital Medical University, Beijing, China
- 3Department of Science and Technology, Beijing Shijitan Hospital Affiliated to Capital Medical University, Beijing, China
- 4Department of Gynecology, Shenzhen Hospital Affiliated to Savaid Medical School, University of Chinese Academy of Sciences, Shenzhen, China
- 5Research Center for Translational Medicine, Shanghai East Hospital, Tongji University School of Medicine, Shanghai, China
Introduction: Which is optimal to treat clomiphene citrate-resistant polycystic ovary syndrome (CCR-PCOS) with LOD or metformin remains a problem. There are three inconsistent or even contradictory views.
Objectives: The present meta-analysis aimed to evaluate the effectiveness and safety of Metformin with or without CC and to compare them with LOD with or without CC (Met/Met-CC vs. LOD/LOD-CC) in women with CCR-PCOS who also have anovulation.
Data source: The PubMed, Cochrane, and Embase databases were searched to identify relevant studies reported between 1 Jan 1966 and 31 Aug 2019; the search was updated on 17 May 2022.
Study eligibility criteria: We included randomized controlled trials (RCTs) of CCR-PCOS that had considered Met/Met-CC and LOD/LOD-CC as the exposure variables and fertility as the main outcome variable.
Study appraisal and synthesis methods: We assessed study quality using the Cochrane risk-of-bias tool. The primary effectiveness outcome was live birth/ongoing pregnancy rate and the primary safety outcome was miscarriage rate. A fixed-effect meta-analysis was performed. The robustness of the results was assessed using sensitivity analyses. Meta-regression and subgroup analysis were performed to examine the reasons for heterogeneity. Publication bias was examined using the funnel plot, Egger linear regression, and Begg rank correlation tests. The quality of this meta-analysis was estimated according to the GRADE approach. This meta-analysis has been registered in PROSPERO (CRD42021240156).
Results: Among 71 potentially relevant studies, we included five RCTs in our meta-analysis. We found no difference in effectiveness between Met-CC and LOD in terms of live birth/ongoing pregnancy (RR = 1.02, 95% CI: 0.87–1.21, z = 0.28; p = 0.780), and miscarriage rates (RR = 0.79, 95% CI: 0.46–1.36, z = 0.86; p = 0.390). I2 tests results revealed moderate or no heterogeneity (I2 = 51.4%, p = 0.083; I2= 0.0%; p = 0.952). Sensitivity analysis confirmed the robustness of the results. Funnel plot, Egger linear regression, and Begg rank correlation tests implied no publication bias (p > 0.05). LOD was more expensive than Met (€1050 vs. €50.16). The evidence quality was moderate.
Conclusion: There is no evidence on the difference in the outcomes between the two interventions regarding ovulation, pregnancy, and live birth. As LOD is an invasive procedure and carries inherent risks, the use of Met/Met-CC should be the second-line treatment for women with CCR-PCOS.
Systematic Review Registration: identifier CRD42021240156.
Introduction
Polycystic ovary syndrome (PCOS), also known as Stein–Leventhal syndrome, was originally described by Stein and Leventhal in 1935. It is characterized by amenorrhea or occasional menometrorrhagia, hirsutism, infertility, and large, pale, polycystic ovaries with thickened capsules (Stein and Leventhal, 1935; Azziz and Adashi, 2016; Greenwood et al., 2018). According to the Rotterdam consensus criteria, PCOS can only be diagnosed when other androgen excess disorders have been excluded and at least two of the following three criteria are present: oligo-ovulation or anovulation, biochemical and/or clinical signs of hyperandrogenism, and polycystic ovaries identified under ultrasound examination (Escobar-Morreale, 2018; Chan et al., 2017; Engmann et al., 2017). PCOS is the most common endocrine disorder, affecting 6%–21% of women of reproductive age (He et al., 2019; Izadi et al., 2019; Makrinou et al., 2020). Approximately 75% of women with PCOS suffer infertility due to anovulation (Costello et al., 2019). One of the primary pharmacological agents used to treat this condition is clomiphene citrate (CC) (Schroeder and American College of Obstetricians and Gynecologists, 2003; Legro et al., 2013; Legro et al., 2014; Amer et al., 2017; Kar, 2012; Bayar et al., 2006; NHRMC, 2018; Teede et al., 2018; Wang et al., 2019; Franik et al., 2018) which induces ovulation in 75%–80% of patients with infertility due to PCOS (Messinis, 2005; Birch Petersen et al., 2016).
Nevertheless, some patients with PCOS show CC resistance (CCR), defined as failure to achieve ovulation after the dose of CC has been gradually increased to 150 or 250 mg/day—the final dosage differed among the studies—in at least three consecutive cycles (Malkawi et al., 2003; Palomba et al., 2004; Palomba et al., 2005; Ashrafinia et al., 2009; Hamed et al., 2010; Palomba et al., 2010; Abu Hashim et al., 2011; Elgafor el sharkwy, 2013). In a previous review, Bordewijk et al. concluded that LOD with or without medication-induced ovulation may result in lower live birth rates than medication-induced ovulation alone in women with anovulatory CCR-PCOS (Bordewijk et al., 2020a). Another review by Yu et al. concluded that no recommendation could be made regarding ovulation induction in patients with CCR-PCOS because the available studies presented low-quality evidence and wide confidence intervals (Yu et al., 2017). The new international evidence-based guideline for PCOS recommended both laparoscopic ovarian drilling (LOD) and combined metformin and CC (Met-CC) as second line therapies to treat CCR-PCOS, without defining any treatment timeline (NHRMC, 2018; Teede et al., 2018).
Prominently, LOD is an invasive procedure and carries inherent risks. Conversely, drug safety is always the center of attention. Metformin has been widely used clinically for >60 years and there is sufficient evidence of its safety and tolerability in most populations (Lv and Guo, 2020). Pregnant and lactating women are considered “therapeutic orphans” because they generally have been excluded from the clinical drug study and new drug development process owing to safety, legal, and ethical concerns. Most medications prescribed for pregnant and lactating women are “off-label” because most of the clinically approved medications do not have appropriate drug labeling information (Ren et al., 2021). The limited evidence indicates the long-term safety of the fetus exposed to metformin excluding mild adverse anthropometric profiles (sex hormone binding globulin levels and long-term body mass index in offspring) (Roy and Sahoo, 2021; Zhu et al., 2022). Although the FDA has not completely ruled out the risk of metformin in pregnancy, “these studies cannot establish the lacking metformin-associated risk because of methodological limitations, including the small sample size and inconsistent comparator groups” (https://packageinserts.bms.com/pi/pi_glucophage.pdf), many of the reviewed studies concluded that metformin is a safe choice at the beginning of pregnancy without persuasive evidence of increased risk for miscarriages or congenital malformations (Quadir, 2021).
In the present study, we conducted a systematic review and meta-analysis of published randomized controlled trials (RCTs) to evaluate the effectiveness and safety of Met, Met-CC, LOD, and LOD combined with CC (LOD-CC), as well as to compare Met/Met-CC (Met with or without CC) with LOD/LOD-CC (LOD with or without CC) in patients with CCR-PCOS who showed anovulation-related infertility. In so doing, we sought to establish which approach should be used as the primary treatment and to confirm which of the above three viewpoints is correct.
Materials and Methods
The present meta-analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) statement protocol (Higgins et al., 2011a) (Supplementary Appendix S1), and was registered in the PROSPERO (Registration number: CRD42021240156).
Information Sources and Search Strategy
PubMed, Embase, and the Cochrane Central Register of RCTs were searched for articles published between 1 Jan 1966 and 31 Aug 2019. In accordance with the PICOS strategy, search terms were formulated as follows: 1) patient population: “polycystic ovarian syndrome”, “polycystic ovary syndrome”, “polycystic ovarian disease”, “PCOS”, and “PCOD”; 2) intervention: “metformin”, “dimethylguanylguanidine”, “dimethylbiguanidine,” “glucophage”, “dimethylbiguanide”, and “DMBG”; 3) control: “laparoscopic ovarian drilling”, “laparoscopic ovarian diathermy”, “LOD”, “laparoscopic ovarian electrocautery”, and “LOE”; 4) outcomes: “ovulation”, “pregnancy”, and “live birth”; 5) study design: “randomized controlled trial”, “random allocation”. Results were restricted to humans and no language restrictions were applied (Supplementary Appendix S2). The reference lists of the included articles were scanned for additional relevant studies. Grey (unpublished) literature was identified by searching the websites of clinical practice guideline collections, clinical trial registries, national and international medical specialty societies, and recent conference abstracts. Full-text articles of potentially relevant studies that were unavailable through the university library were requested from the authors. The literature search was updated on 17 May 2022. The search strategies were formulated by physicians (M-LS and X-HW), gynecologists (G-LG and W-PB), and statisticians (LZ and Q-KS).
Eligibility Criteria and Study Selection
Two reviewers (ML Sun and L Zheng) independently screened the titles and abstracts to determine whether the articles were relevant to the meta-analysis based on the pre-defined inclusion criteria listed below. The full texts of potentially eligible studies were then reviewed before the final selection. Any disagreements were resolved in consultation with the principal investigator (XH Wang).
The inclusion criteria were as follows: 1) study population of patients with both CCR-PCOS and anovulation-related infertility; 2) intervention of LOD-controlled Met treatment despite continuing CC; 3) reporting of fertility outcomes (ovulation, pregnancy, and live-birth rates); 4) RCT study design. The exclusion criteria were as follows: 1) duplicates; 2) study design other than RCT (e.g., reviews, meta-analyses, case reports, guidelines, trial protocols); 3) absence of comparison between Met/Met-CC and LOD/LOD-CC; 4) absence of fertility outcomes.
Data Extraction
Data were extracted from the included articles by two independent reviewers (M-LS and LZ) using standardized data extraction sheets. Any disagreements were resolved in consultation with the principal investigator (X-HW). If available, the following information was extracted from each article: study period, inclusion, and exclusion criteria, first author, year of publication, subjects’ country of residence, definition of CCR, number of participants in each group, clinical characteristics of the participants, treatment regimens, duration of treatment, follow-up period, and fertility outcomes (ovulation, pregnancy, and live-birth rates). Adverse events (AEs), including miscarriage, multiple pregnancy, ectopic pregnancy, ovarian hyperstimulation syndrome (OHSS), drug-related AEs, and intra- or post-operative complications, were identified according to the data originally documented in the articles.
Assessment of Risk of Bias
Two researchers (M-LS and LZ) independently conducted quality assessment of all the included articles using the Cochrane risk-of-bias tool in the following domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other bias (Higgins et al., 2011a; Higgins et al., 2011b). Each domain was classified as low, unclear, or high risk. If there were discrepancies, the final assessment decision was made in consultation with the principal investigator (X-HW). The overall quality of this meta-analysis was estimated according to the GRADE four-step approach (Higgins et al., 2011b).
Data Synthesis
The overall meta-analysis was performed following the appropriate Cochrane Guidelines (Higgins et al., 2011a). The primary effectiveness outcome was live birth/ongoing pregnancy and the primary safety outcome was miscarriage. The secondary effectiveness outcomes were pregnancy and ovulation-induction, while the secondary safety outcomes included multiple pregnancy, ectopic pregnancy, OHSS, medical-related AEs and surgical complications. We also compared the costs of the two treatments. Effect size was calculated based on the risks (relative risk; RR) provided by each study. A fixed-effect meta-analysis was then conducted (Higgins et al., 2011a).
Investigation of Heterogeneity
Heterogeneity among the included studies was analyzed using the I2 test (Higgins et al., 2011a) as follows: I2 = [(Q–df)/Q] × 100%, where Q is the χ2 heterogeneity statistic and df is the degrees of freedom. I2 values >75% indicate high heterogeneity, whereas values between 50% and 75% indicate moderate heterogeneity. I2 values between 25% and 50% indicate low heterogeneity, and values below 25% indicate no heterogeneity.
Sensitivity Analysis
We conducted sensitivity analyses of the primary effectiveness outcome (live birth/ongoing pregnancy), to determine whether the conclusions were robust to arbitrary decisions made about eligibility, and analysis. Sensitivity analysis was performed using the “random-effects model” and “leave-one-out” methods.
Meta-Regression Analysis
If any heterogeneity occurred, the reasons for it were ascertained using a meta-regression analysis of the primary effectiveness outcome (live birth/ongoing pregnancy).
Subgroup Analysis
Subgroup analysis of the primary effectiveness outcome (live birth/ongoing pregnancy) was also performed to explain expected significant heterogeneity.
Assessment of Reporting Biases
To reduce reporting bias, we were alert to data duplication and ensured that our search for eligible studies was comprehensive. Potential publication bias was examined using the funnel plot, Egger linear regression, and Begg rank correlation tests (Higgins et al., 2011a).
Overall Quality of the Body of Evidence: “Summary of Findings” Table
We generated a “Summary of findings” table using GRADEPro 3.6 software. This table shows the overall quality of the evidence for the main review outcomes according to the GRADE criteria. We justified judgements about evidence quality (high, moderate, low, or very low), and documented and incorporated these judgements into the reporting of results for each outcome.
Statistics and Statistical Software
The statistical significance level was set at p < 0.05. In the meta-analysis, Review Manager 5.3 and one of the Cochrane Collaboration Tools were used to create the risk-of-bias graph; the statistical software package Stata16 (Stata Corp., College Station, TX, United States), and GRADEpro 3.6 software were also used.
Results
Study Selection
Overall, 71 studies were retrieved from the electronic databases. Twenty-three duplicates were removed, resulting in 48 unique titles. Following title and abstract review, we assessed eight full-text articles for eligibility. Five RCTs (Palomba et al., 2004; Palomba et al., 2005; Hamed et al., 2010; Palomba et al., 2010; Abu Hashim et al., 2011) met all the inclusion and exclusion criteria, and were thus included in our main meta-analysis (Figure 1). In the 2010 study by Hamed et al., ovulation induction, pregnancy, and first trimester abortion rate were reported, rather than live-birth rate, so we used the ongoing pregnancy rate in the analysis of live-birth rate (Abu Hashim et al., 2011).
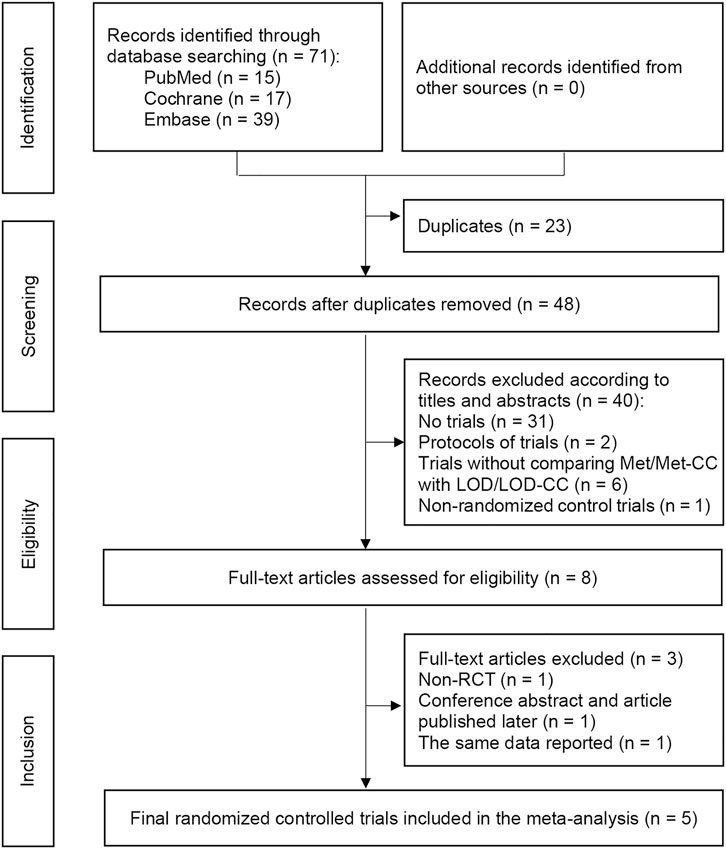
FIGURE 1. Preferred Reporting Items for Systematic Reviews and Meta-analyses flow chart depicting the process of paper selection and the number of papers in each phase. Notes: RCT, randomized controlled trial.
Study Characteristics
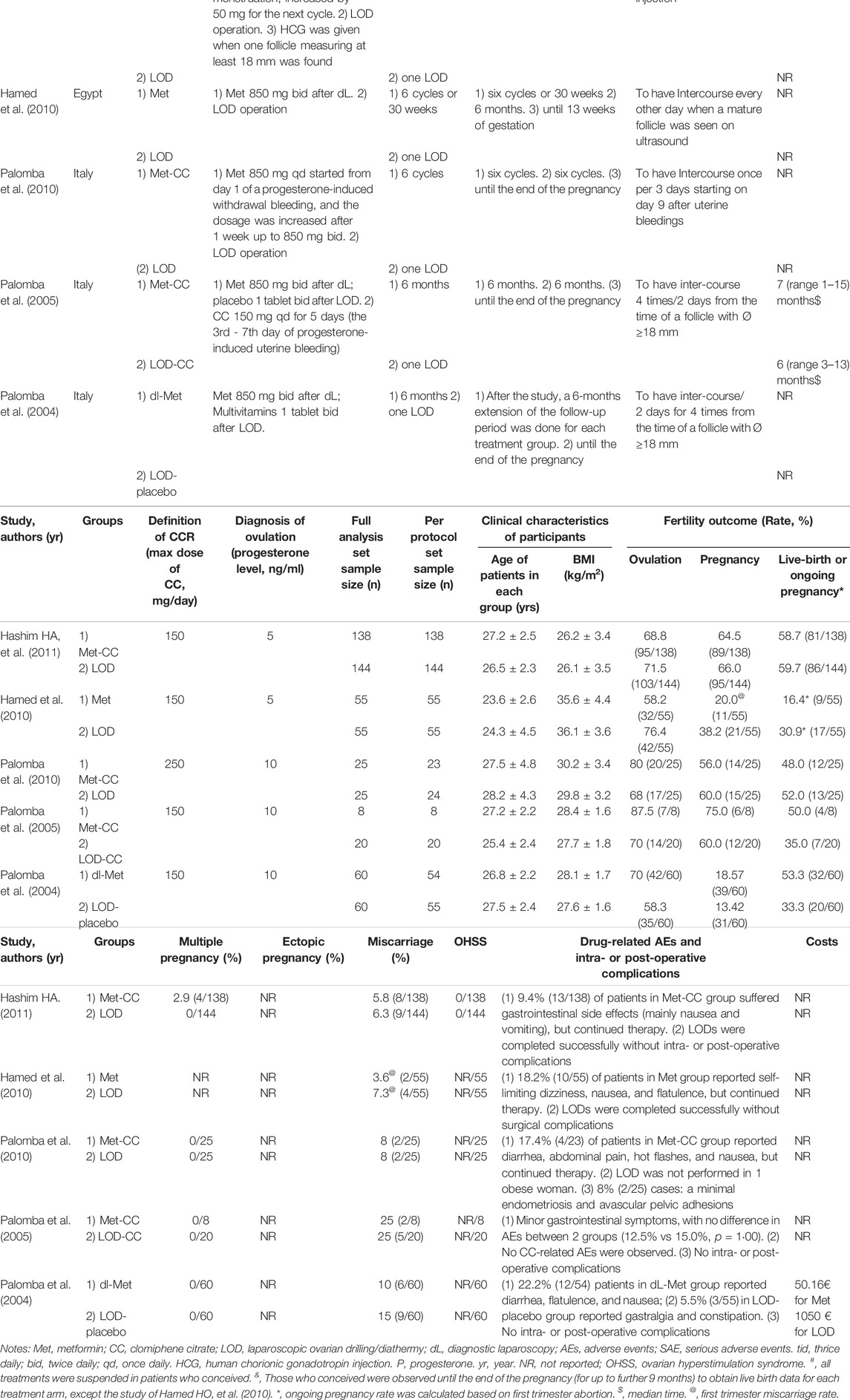
The characteristics of the included RCTs are summarized in Table 1. The studies involved women with CCR-PCOS from two countries: Egypt and Italy.
The included trials had a total of 590 subjects (from 28 to 282 in each study), of which 115 were treated using Met, 171 using Met-CC, 284 using LOD, and 20 using LOD-CC (Palomba et al., 2004; Palomba et al., 2005; Hamed et al., 2010; Palomba et al., 2010; Abu Hashim et al., 2011). Patients were treated using Met for six cycles or followed-up for 6 months or 30 weeks after LOD. Ovulation was identified when the serum progesterone level was ≥5 ng/mL (Hamed et al., 2010; Abu Hashim et al., 2011) or ≥10 ng/ml (Palomba et al., 2004; Palomba et al., 2005; Palomba et al., 2010). Pregnancy was identified based on an increase in β-human chorionic gonadotropin or sonographic evidence of an intrauterine gestational sac. In most of the included studies, subjects who conceived were observed until the end of pregnancy (for up to a further 9 months) to obtain live-birth data for each treatment arm (Palomba et al., 2004; Palomba et al., 2005; Palomba et al., 2010; Abu Hashim et al., 2011) although the study by Hamed et al. only followed up the pregnancy until 13 weeks’ gestation to obtain first trimester abortion and ongoing pregnancy data (Abu Hashim et al., 2011). Ovulation induction, pregnancy, live birth/ongoing pregnancy, and miscarriage rates were calculated as the number of women with ovulation, pregnancy or living baby, and abortion, respectively, divided by the total number of subjects randomized in the same group (See Table 1).
Risk of Bias
The results of the quality assessment are shown in Figure 2. In “randomization and allocation concealment” category, four studies (Palomba et al., 2004; Hamed et al., 2010; Palomba et al., 2010; Abu Hashim et al., 2011) had low risk of bias and one (Palomba et al., 2005) had unclear risk of bias because the randomization and allocation concealment protocols were described unclearly. Although none of the studies described blinding of outcome assessment clearly, a low risk of detection bias was granted to all of them because the outcomes ware objective indicators. The first three studies had a high risk of performance bias because their trial protocols did not involve dual-mode analogue (Hamed et al., 2010; Palomba et al., 2010; Abu Hashim et al., 2011).
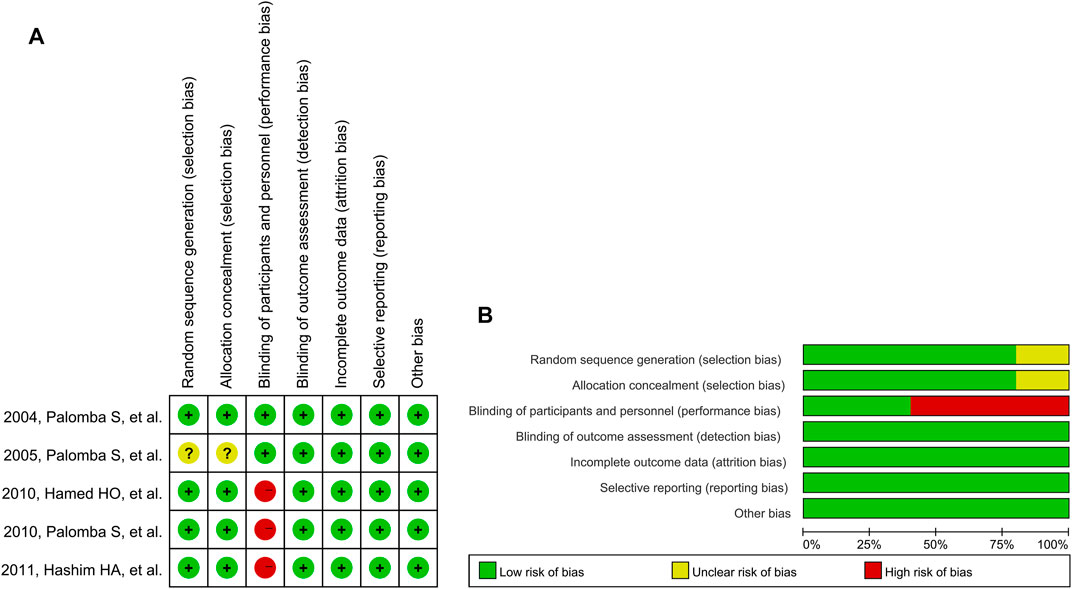
FIGURE 2. Risk of bias graph for reviewing authors’ judgements: (A) Individual studies, and (B) All studies. Note: The colors have the same meaning in both (A) and (B).
Synthesis of Results
To compare Met/Met-CC with LOD/LOD-CC in terms of both effectiveness on fertility outcomes and safety, we pooled data from all subjects in the included trials using the fixed-effects model.
Effectiveness did not differ significantly between Met/Met-CC and LOD/LOD-CC in terms of live birth/ongoing pregnancy (RR = 1.02, 95% confidence interval [CI]: 0.87–1.21, z = 0.28; p = 0.780), pregnancy (RR = 0.98, 95% CI: 0.85–1.12, z = 0.29; p = 0.771), or rate of ovulation induction (RR = 0.99, 95% CI: 0.89–1.10, z = 0.16; p = 0.873; (Figure 3A).
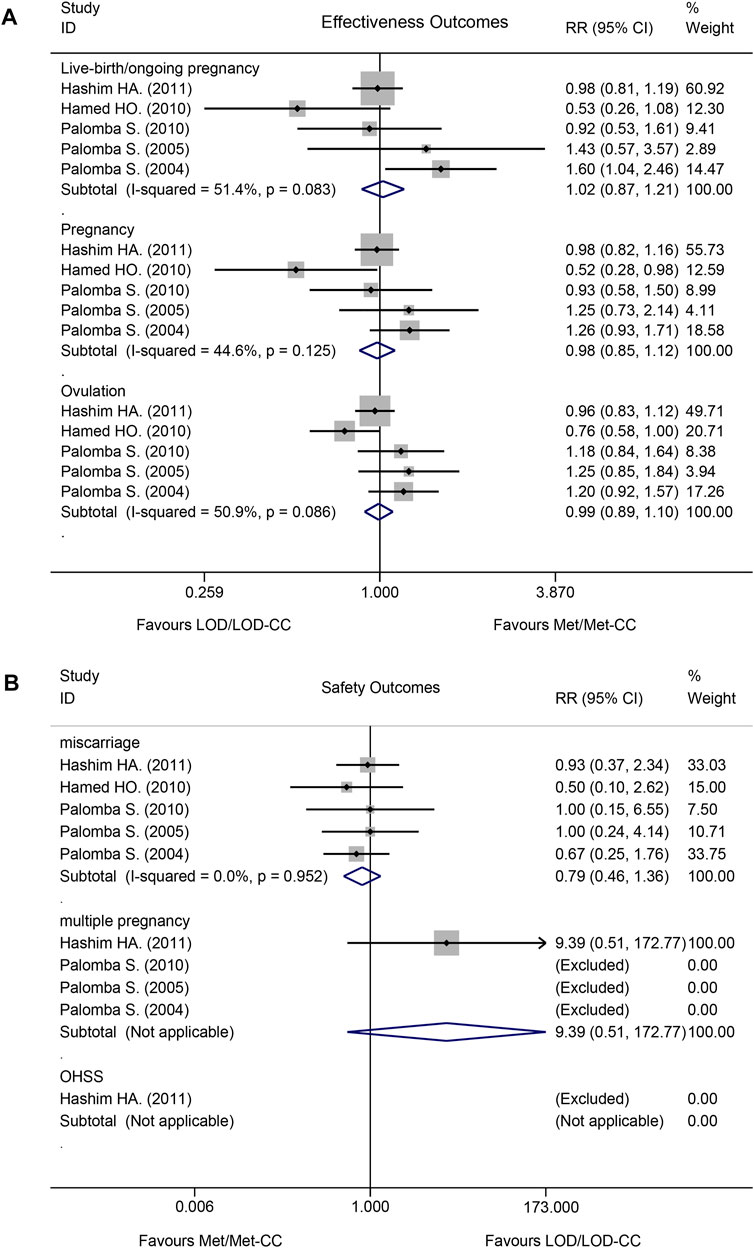
FIGURE 3. Forest plot of effectiveness and safety outcomes in the Met/Met-CC vs LOD/LOD-CC comparison using the fixed-effect model: (A) effectiveness; and (B) safety. Notes: Met, metformin, CC, clomiphene citrate, LOD, laparoscopic ovarian drilling.
Safety did not differ significantly between Met/Met-CC and LOD/LOD-CC in terms of miscarriage (RR = 0.79, 95% CI: 0.46–1.36, z = 0.86, p = 0.390), multiple pregnancy rates (RR = 9.39, 95% CI: 0.51–172.77, z = 1.51; p = 0.132). OHSS was rarely reported (Figure 3B). No data about ectopic pregnancy were provided in any of the five RCTs.
Heterogeneity
The I2 test results revealed moderate heterogeneity with regards to live birth/ongoing pregnancy (I2 = 51.4%; p = 0.083) and rate of ovulation induction (I2 = 50.9%; p < 0.086), but low heterogeneity regarding pregnancy rate (I2 = 44.6%; p = 0.125; Figure 3A).
The I2 test result revealed no heterogeneity with regards to miscarriage (I2 = 0.0%; p = 0.952). A multiple pregnancy rate of 2.9% (4/138 women) was reported in the Met-CC group of one study (Abu Hashim et al., 2011) (Figure 3B).
Sensitivity Analysis
Pooling based on a random-effects model (M-H heterogeneity) resulted in similar live-birth/ongoing pregnancy rates (RR = 1.04, 95% CI: 0.76–1.42, z = 0.25, p = 0.805; I2 = 51.4%, Tau2 = 0.059, p = 0.083), pregnancy (RR = 1.00, 95% CI: 0.80–1.25, z = 0.02, p = 0.984; I2 = 44.6%, Tau2 = 0.027, p = 0.125), and rate of ovulation induction (RR = 1.02, 95% CI 0.86–1.21, z = 0.28, p = 0.776; I2 = 50.9%, Tau2 = 0.018, p = 0.086; (Supplementary Appendix S3, Figure 1A). Sensitivity analysis using the leave-one-out method revealed that the results when omitting each study were similar to those obtained when all studies were included (Supplementary Appendix S3, Figure 1B). As such, sensitivity analysis indicated that the results were stable.
Meta-Regression Analysis to Explore Sources of Heterogeneity
We assessed the treatment methods, follow-up period after pregnancy, subjects’ country of residence, and samples using logistic meta-regression analysis. The results implied that different follow-up periods contributed to 15.43% of the heterogeneity (I2 residual = 59.84%, Tau2 = 0.073; p = 0.271), and that the different countries of the participants accounted for 26.17% of the heterogeneity (I2 residual = 40.43%, Tau2 = 0.064; p = 0.245). The adjusted R2 values of the regression results were negative for both treatment used and sample size since the number of included studies was small (Supplementary Appendix S4).
Subgroup Analysis and Investigation of Heterogeneity
The included studies were stratified by treatment methods. No significant difference occurred between Met-CC and LOD (RR = 0.97, 95% CI: 0.81–1.17, z = 0.27, p = 0.785; I2 = 0.0%, p = 0.834) or between Met and LOD (RR = 1.11, 95% CI: 0.77–1.59, z = 0.56, p = 0.576; I2 = 85.5%, p = 0.009). Only one RCT analyzing Met-CC vs LOD-CC was included in the present meta-analysis (Supplementary Appendix S5, Figure 2A).
The included studies were then divided into subgroups according to follow-up period. After excluding the study by Hamed et al., in which the pregnancy was only followed-up until 13 weeks’ gestation to obtain first trimester abortion and ongoing pregnancy data, no significant effect was found (RR = 1.09, 95% CI: 0.93–1.29, z = 1.04; p = 0.296), and there was low RR variation due to heterogeneity (I2 = 38.4%; p = 0.182) in the live birth subgroup (Supplementary Appendix S5, Figure 2B).
Next, the participants were divided into subgroups based on the participants’ countries. No significant difference occurred between any of the treatment methods in either Egypt (RR = 0.91, 95% CI: 0.75–1.10, z = 1.00, p = 0.316; I2 = 64.7%, p = 0.092) or Italy (RR = 1.34, 95% CI: 0.98–1.85, z = 1.83, p = 0.068; I2 = 17.0%, p = 0.300; Supplementary Appendix S5, Figure 2C).
Meta-Analysis Was Reperformed After Excluding a Study With a Concern Note by the Journal
The RCT published by Hashim HA (2011), which included the largest number of patients and cited by two reviews (Yu et al., 2017; Bordewijk et al., 2020a) and Guidelines (Teede et al., 2018) are essential and had recently been suspected by rationalization on the study and data integrity (Bordewijk et al., 2020b; Bordewijk et al., 2020c; Bordewijk et al., 2020d). Although Hashim HA. has explained the data and opposed undue stigmatization (Abu Hashim, 2020) a meta-analysis was reperformed to be rigorous after excluding Hashim HA (2011) before a final decision by the journal to retract the paper or concern note. (Supplementary Appendix S6).
Assessment of Reporting Bias
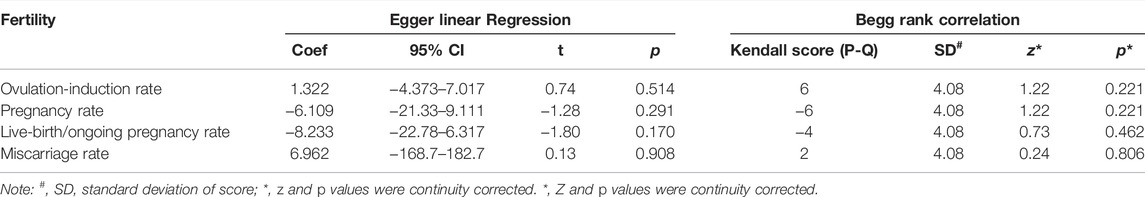
The funnel plot in Figure 4 shows roughly symmetrical distributions when fertility outcome was compared among the five included RCTs. No significant evidence of publication bias was found in the present meta-analysis using Egger linear regression (p = 0.170, 0.291, 0.514, and 0.908 for live birth/ongoing pregnancy, pregnancy, ovulation induction, and miscarriage rates, respectively) or the Begg rank correlation test (Kendall score, z and p value with continuity correction = −4, −0.73, and 0.462 in live birth/ongoing pregnancy; −6, 1.22, and 0.221 in pregnancy; 6, 1.22, and 0.221 in ovulation induction; and 2, 0.24, 0.806 in miscarriage rates, respectively; Table 2).
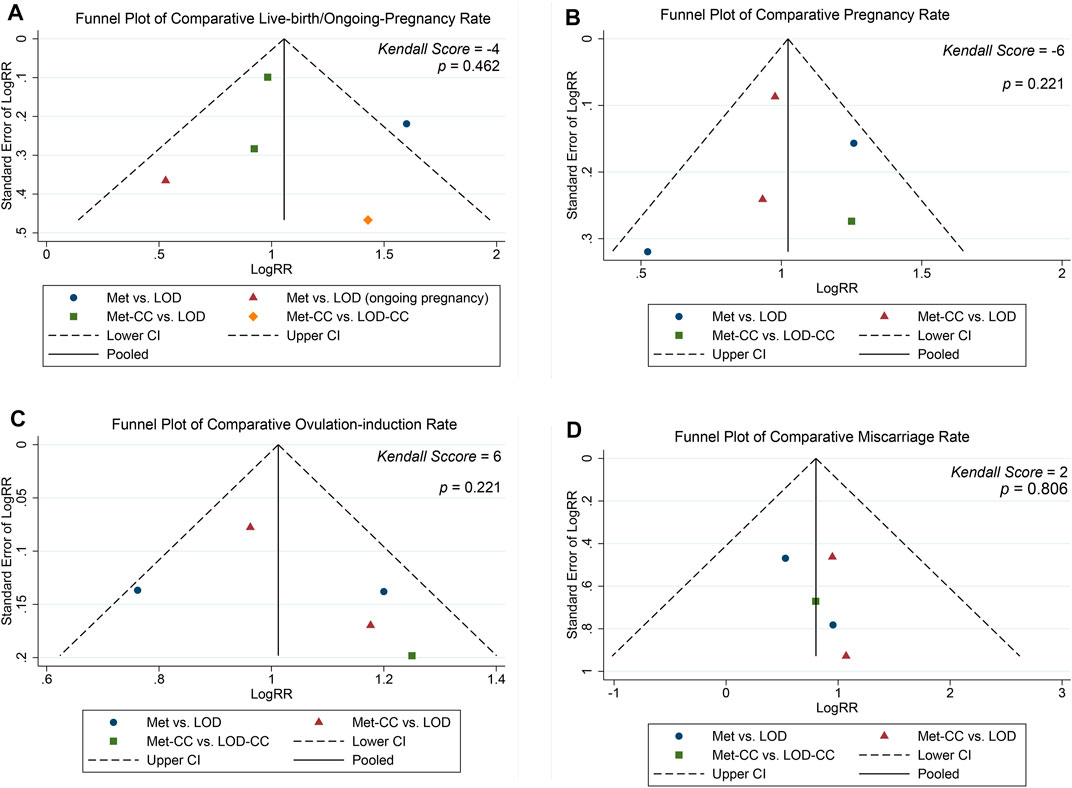
FIGURE 4. Funnel plot of (A) live birth/ongoing pregnancy, (B) pregnancy (C) ovulation induction, and (D) miscarriage rates.
Comparison of Other Safety Outcomes and Costs
Although drug-related AEs occurred at rates between 5.5% and 22.2%; the symptoms were self-limiting and did not affect treatment continuation. LOD was completed successfully without any intra- or post-operative complications in all but three patients: one with obesity who failed the laparoscopy and two who showed minimal endometriosis and avascular pelvic adhesions (Palomba et al., 2010). Costs were reported in one included trial (€50.16 for Met vs €1050 for LOD; Table 1). (Palomba et al., 2004)
Quality of the Body of Evidence
We found no evidence of a difference between Met/Met-CC and LOD, as well as between Met-CC and LOD-CC in terms of live birth/ongoing pregnancy, pregnancy, ovulation induction, and miscarriage rates. The “summary of findings” table showed that the quality of evidence was moderate in the Met-CC vs. LOD comparison, low for the pregnancy and ovulation induction rates of the Met vs. LOD comparison, and very low for the Met-CC vs. LOD-CC comparison, live birth/ongoing pregnancy, and miscarriage rates of the Met vs. LOD comparison (Supplementary Appendix S7, Table 1).
Comments
Summary of Main Findings
In patients with anovulatory, infertility-related CCR-PCOS, our main analysis including all studies indicated no significant difference in effectiveness or safety between Met/Met-CC and LOD, as well as between Met-CC and LOD-CC. The robustness of this result was evaluated using sensitivity analysis. The quality of evidence was very low to moderate.
Moderate-quality evidence suggested that Met-CC and LOD should be recommended as parallel treatments for CCR-PCOS. The live-birth rates associated with Met-CC and LOD were 57.06% and 58.58%, respectively. The miscarriage rate was similar between the two (6.13% vs. 6.51%). The I2 tests results revealed no heterogeneity. With regards to costs, Met-CC is slightly superior to LOD. These findings confirmed the latest international guideline that Met-CC and LOD should be recommended as second-line treatments, with no definitive treatment timeline specified (NHRMC, 2018; Teede et al., 2018).
Very-low quality evidence indicated that there was uncertainty about the effectiveness and safety of Met and LOD in terms of live birth/ongoing pregnancy and miscarriage rates. Similarly, the effectiveness and safety of Met-CC and LOD-CC were unclear in terms of live birth, pregnancy, ovulation induction, and miscarriage rates. I2 tests results indicated high heterogeneity in live birth/ongoing pregnancy rate between Met and LOD. Low-quality evidence indicated no difference in pregnancy or ovulation induction rates between Met and LOD. Our results showed that Met is a promising drug and were consistent with the international evidence-based guideline, which recommend Met alone to manage PCOS.
Comparison With Existing Literature
The results of Yu et al. (2017) showed no difference in live birth, pregnancy, ovulation induction, or miscarriage rates between Met/Met-CC and LOD (Yu et al., 2017). The same authors concluded that no recommendation could be made regarding ovulation induction in patients with CCR-PCOS because the quality of evidence was low and the confidence intervals wide. The present meta-analysis showed similar results, with some differences. For example, we included more RCTs comparing Met-CC with LOD-CC. Moreover, Yu et al. compared pregnancy per cycle, abortion rate per pregnancy, and multiple pregnancy rate per pregnancy as their outcomes. Yu et al. also classified the 2011 study by Hashim et al. as having high risk of bias because it failed to blind outcome assessment, failing to consider that all outcomes were objective indicators. As such, the evidence quality was assessed differently between the study by Yu et al. and the present study.
Bordewijk et al. (2020) concluded that LOD/LOD-CC medical ovulation induction may lead to lower live birth rates in women with anovulatory CCR-PCOS than medical ovulation induction alone (Bordewijk et al., 2020a). This conclusion was inconsistent with the present meta-analysis. Bordewijk et al. included three non-RCTs (Malkawi et al., 2003; Ashrafinia et al., 2009; Elgafor el sharkwy, 2013) and three RCTs (Palomba et al., 2004; Palomba et al., 2010; Abu Hashim et al., 2011) to compare the effectiveness of Met/Met-CC with that of LOD. Furthermore, we concluded that the 2004 study by Palomba et al. should be classified as a comparison of Met with LOD rather than of Met-CC with LOD, because CC was only given to women who failed to achieve ovulation after the trial in that study (Palomba et al., 2004).
Strengths and Limitations
The strengths of the present systematic review included the extensive search strategy, as well as the meta-regression, subgroup, and sensitivity analyses. All included studies were RCTs with no publication bias, and the robustness of their results was confirmed by sensitivity analysis.
However, the present meta-analysis also had several limitations. For example, the number of included RCTs and participants was limited. In addition, the treatment methods were potential source of heterogeneity. The two RCTs comparing Met with LOD were highly heterogeneous, while the one comparing Met-CC with LOD-CC had a small sample size. No RCT compared Met with LOD-CC. Because the quality of evidence was low or very low, no conclusion could be drawn regarding the comparison of Met with LOD or of Met-CC with LOD-CC. Lastly, variation in the follow-up periods after pregnancy may have impacted fertility results. That said, the two groups in the trials were subject to the same experimental conditions in terms of follow-up time, which may have reduced heterogeneity.
Conclusion and Implications
Although moderate-quality evidence suggested that Met-CC and LOD should be recommended as parallel, second-line therapies for patients with CCR-PCOS, the present study indicates that Met-CC should be recommended as the optimum treatment for patients with CCR-PCOS because it is cheap, safe, and different from LOD, whose effect depends on operator proficiency; LOD intervention might be the first choice for patients with CCR-PCOS if they are willing to undergo diagnostic laparoscopy.
Very low-to low-quality evidence has suggested that there is little or no difference in effectiveness or safety between Met and LOD, as well as between Met-CC and Met-CC in women with CCR-PCOS, but our results showed that Met is a promising drug that could play a more important role in CCR-PCOS treatment because it is effective, safe, and cheap. Specifically, Met is an old drug with new applications, including PCOS treatment (Wang et al., 2017; Zhou et al., 2018; Sharpe et al., 2019) and Met treatment during pregnancy does not influence metabolic profile in women with PCOS (Underdal et al., 2018). Furthermore, although a recently published follow-up study of two RCTs suggested that in utero exposure to Met increases the risk of overweightness in early childhood (Greenhill, 2018; Hanem et al., 2018) there is fair evidence that Met alone does not increase rates of miscarriage when stopped at the initiation of pregnancy (Practice Committee of the American Society for Reproductive Medicine, 2017).
LOD reduces both testosterone and the luteinizing hormone/follicle-stimulating hormone ratio in women with PCOS, and it improves clinical outcome (Sinha et al., 2019). Currently, LOD can be performed using monopolar, bipolar, or laser diathermy; even ultrasound-guided transvaginal ovarian needle drilling has been used to induce ovulation in anovulatory PCOS (Farquhar et al., 2007; Rezk et al., 2016; Kandil et al., 2018). With developing technology, LOD has emerged as an ideal alternative to letrozole, maximizing ovulation induction and pregnancy benefits without the problems associated with ovarian wedge resection (Abu Hashim et al., 2013; Zahiri Sorouri et al., 2015; El-Sayed et al., 2017; Debras et al., 2019; Yu et al., 2019; Zhang et al., 2019). Currently, the latest international evidence-based guideline recommended letrozole as first line pharmacological treatment for anovulation women with PCOS to improve ovulation, pregnancy and live-birth (NHRMC, 2018). The results of present meta-analysis did not found difference in effectiveness or safety between Met/Met-CC and LOD/LOD-CC, and thus we could safely deduce that Met is a promising drug.
Additional large RCTs with adequate blinding are needed to more precisely estimate the difference between Met/Met-CC and LOD/LOD-CC. Such trials should comprehensively evaluate outcomes, including live birth, pregnancy, ovulation induction, AEs (multiple pregnancy, miscarriage, ectopic pregnancy, OHSS, drug-related AEs, and surgical complications), costs, patient satisfaction, long-term benefits (spontaneous resumption of ovulation and menstruation), as well as the potential risks of LOD (such as premature ovarian failure).
In conclusion, there is no evidence on the difference in the outcomes between the two interventions regarding ovulation, pregnancy, and live birth. As LOD is an invasive procedure and carries inherent risks, the use of metformin with or without clomiphene should be the second-line treatment for women with polycystic ovary syndrome (PCOS) who do not ovulate only by clomiphene citrate.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
All authors took part in the research design. M-LS and LZ performed the article screening and data extraction. M-LS wrote the first and successive versions of the manuscript. W-PB, H-YW and Q-KS contributed many constructive opinions and suggestions. All authors contributed to the interpretation of the results, intellectual content, critical revisions of the drafts of the paper, and approved the final version of the paper. As the principal investigators, X-HW, LZ, and G-LG had full access to all data in the study and had final responsibility for the decision to submit for publication.
Funding
This research was supported by the Open Research Funding of Central Laboratory, Beijing Shijitan Hospital Affiliated to Capital Medical University (Grant No. 2020-KF28). This work was also supported in part by the National Science and Technology Major Project for Significant New Drugs Creation (Grant No. 2017ZX09304026). The funders of the study had no role in data collection, data analysis, data interpretation, or writing of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We would like to thank Yong Wang for his contributions to formulating the search string of the systematic literature search.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.576458/full#supplementary-material
References
Abu Hashim, H., Al-Inany, H., De Vos, M., and Tournaye, H. (2013). Three Decades after Gjönnaess's Laparoscopic Ovarian Drilling for Treatment of PCOS; what Do We Know? an Evidence-Based Approach. Arch. Gynecol. Obstet. 288 (2), 409–422. doi:10.1007/s00404-013-2808-x
Abu Hashim, H. (2020). Data Integrity of 35 Randomized Controlled Trials: An Incorrect Rejection of Data with Undue Author Stigmatization. Eur. J. Obstet. Gynecol. Reprod. Biol. 255, 261. doi:10.1016/j.ejogrb.2020.08.056
Abu Hashim, H., El Lakany, N., and Sherief, L. (2011). Combined Metformin and Clomiphene Citrate versus Laparoscopic Ovarian Diathermy for Ovulation Induction in Clomiphene-Resistant Women with Polycystic Ovary Syndrome: a Randomized Controlled Trial. J. Obstet. Gynaecol. Res. 37 (3), 169–177. doi:10.1111/j.1447-0756.2010.01383.x
Amer, S. A., Smith, J., Mahran, A., Fox, P., and Fakis, A. (2017). Double-blind Randomized Controlled Trial of Letrozole versus Clomiphene Citrate in Subfertile Women with Polycystic Ovarian Syndrome. Hum. Reprod. 32 (8), 1631–1638. doi:10.1093/humrep/dex227
Ashrafinia, M., Hosseini, R., Moini, A., Eslami, B., and Asgari, Z. (2009). Comparison of Metformin Treatment and Laparoscopic Ovarian Diathermy in Patients with Polycystic Ovary Syndrome. Int. J. Gynaecol. Obstet. 107 (3), 236–239. doi:10.1016/j.ijgo.2009.06.022
Azziz, R., and Adashi, E. Y. (2016). Stein and Leventhal: 80 Years on. Am. J. Obstet. Gynecol. 214 (2), 247.e1–247.e11. doi:10.1016/j.ajog.2015.12.013
Bayar, U., Basaran, M., Kiran, S., Coskun, A., and Gezer, S. (2006). Use of an Aromatase Inhibitor in Patients with Polycystic Ovary Syndrome: a Prospective Randomized Trial. Fertil. Steril. 86 (5), 1447–1451. doi:10.1016/j.fertnstert.2006.04.026
Birch Petersen, K., Pedersen, N. G., Pedersen, A. T., Lauritsen, M. P., and la Cour Freiesleben, N. (2016). Mono-ovulation in Women with Polycystic Ovary Syndrome: a Clinical Review on Ovulation Induction. Reprod. Biomed. Online 32 (6), 563–583. doi:10.1016/j.rbmo.2016.03.006
Bordewijk, E. M., Ng, K. Y. B., Rakic, L., Mol, B. W. J., Brown, J., Crawford, T. J., et al. (2020). Laparoscopic Ovarian Drilling for Ovulation Induction in Women with Anovulatory Polycystic Ovary Syndrome. Cochrane Database Syst. Rev. 2 (2), CD001122. doi:10.1002/14651858.CD001122.pub5
Bordewijk, E. M., Wang, R., Askie, L. M., Gurrin, L. C., Thornton, J. G., van Wely, M., et al. (2020). Letter of Response - Data Integrity of 35 Randomised Controlled Trials in Women' Health. Eur. J. Obstet. Gynecol. Reprod. Biol. 255, 260–283. doi:10.1016/j.ejogrb.2020.06.007
Bordewijk, E. M., Wang, R., Askie, L. M., Gurrin, L. C., Thornton, J. G., van Wely, M., et al. (2020). Letter of Response - Data Integrity of 35 Randomised Controlled Trials in Women' Health. Eur. J. Obstet. Gynecol. Reprod. Biol. 255, 260. doi:10.1016/j.ejogrb.2020.06.007
Bordewijk, E. M., Wang, R., Askie, L. M., Gurrin, L. C., Thornton, J. G., van Wely, M., et al. (2020). Letter of Response (2) - Data Integrity of 35 Randomised Controlled Trials in Women' Health. Eur. J. Obstet. Gynecol. Reprod. Biol. 255, 261–262. doi:10.1016/j.ejogrb.2020.08.050
Chan, J. L., Kar, S., Vanky, E., Morin-Papunen, L., Piltonen, T., Puurunen, J., et al. (2017). Racial and Ethnic Differences in the Prevalence of Metabolic Syndrome and its Components of Metabolic Syndrome in Women with Polycystic Ovary Syndrome: a Regional Cross-Sectional Study. Am. J. Obstet. Gynecol. 217 (2), 189.e1–189.e8. doi:10.1016/j.ajog.2017.04.007
Costello, M. F., Misso, M. L., Balen, A., Boyle, J., Devoto, L., Garad, R. M., et al. (2019). A Brief Update on the Evidence Supporting the Treatment of Infertility in Polycystic Ovary Syndrome. Aust. N. Z. J. Obstet. Gynaecol. 59 (6), 867–873. doi:10.1111/ajo.13051
Debras, E., Fernandez, H., Neveu, M. E., Deffieux, X., and Capmas, P. (2019). Ovarian Drilling in Polycystic Ovary Syndrome: Long Term Pregnancy Rate. Eur. J. Obstet. Gynecol. Reprod. Biol. X 4, 100093. doi:10.1016/j.eurox.2019.100093
El-Sayed, M. L. M., Ahmed, M. A., Mansour, M. A. A., and Mansour, S. A. A. (2017). Unilateral Versus Bilateral Laparoscopic Ovarian Drilling Using Thermal Dose Adjusted According to Ovarian Volume in CC-Resistant PCOS, A Randomized Study. J. Obstet. Gynaecol. India 67 (5), 356–362. doi:10.1007/s13224-017-1010-7
Elgafor el sharkwy, I. A. (2013). Metformin versus Laparoscopic Unilateral Ovarian Drilling in Clomiphene Resistant Women with Polycystic Ovary Syndrome. Middle East Fertil. Soc. J. 18 (3), 202–207. doi:10.1016/j.mefs.2012.12.008
Engmann, L., Jin, S., Sun, F., Legro, R. S., Polotsky, A. J., Hansen, K. R., et al. (2017). Racial and Ethnic Differences in the Polycystic Ovary Syndrome Metabolic Phenotype. Am. J. Obstet. Gynecol. 216 (5), 493.e1. doi:10.1016/j.ajog.2017.01.003
Escobar-Morreale, H. F. (2018). Polycystic Ovary Syndrome: Definition, Aetiology, Diagnosis and Treatment. Nat. Rev. Endocrinol. 14 (5), 270–284. doi:10.1038/nrendo.2018.24
Farquhar, C., Lilford, R. J., Marjoribanks, J., and Vandekerckhove, P. (2007). Laparoscopic "drilling" by Diathermy or Laser for Ovulation Induction in Anovulatory Polycystic Ovary Syndrome. Cochrane Database Syst. Rev. (3), CD001122. doi:10.1002/14651858.CD001122
Franik, S., Eltrop, S. M., Kremer, J. A., Kiesel, L., and Farquhar, C. (2018). Aromatase Inhibitors (Letrozole) for Subfertile Women with Polycystic Ovary Syndrome. Cochrane Database Syst. Rev. 5 (5), CD010287. doi:10.1002/14651858.CD010287.pub3
Greenhill, C. (2018). PCOS: Metformin Risk for Offspring. Nat. Rev. Endocrinol. 14 (5), 253. doi:10.1038/nrendo.2018.34
Greenwood, E. A., Pasch, L. A., Cedars, M. I., Legro, R. S., and Huddleston, H. G.Eunice Kennedy Shriver National Institute of Child Health and Human Development Reproductive Medicine Network (2018). Association Among Depression, Symptom Experience, and Quality of Life in Polycystic Ovary Syndrome. Am. J. Obstet. Gynecol. 219 (3), 279–e7. doi:10.1016/j.ajog.2018.06.017
Hamed, H. O., Hasan, A. F., Ahmed, O. G., and Ahmed, M. A. (2010). Metformin versus Laparoscopic Ovarian Drilling in Clomiphene- and Insulin-Resistant Women with Polycystic Ovary Syndrome. Int. J. Gynaecol. Obstet. 108 (2), 143–147. doi:10.1016/j.ijgo.2009.08.033
Hanem, L. G. E., Stridsklev, S., Júlíusson, P. B., Salvesen, Ø., Roelants, M., Carlsen, S. M., et al. (2018). Metformin Use in PCOS Pregnancies Increases the Risk of Offspring Overweight at 4 Years of Age: Follow-Up of Two RCTs. J. Clin. Endocrinol. Metab. 103 (4), 1612–1621. doi:10.1210/jc.2017-02419
He, Y., Lu, Y., Zhu, Q., Wang, Y., Lindheim, S. R., Qi, J., et al. (2019). Influence of Metabolic Syndrome on Female Fertility and In Vitro Fertilization Outcomes in PCOS Women. Am. J. Obstet. Gynecol. 221 (2), 138.e1. doi:10.1016/j.ajog.2019.03.011
Higgins, J. P., Altman, D. G., Gøtzsche, P. C., Jüni, P., Moher, D., Oxman, A. D., et al. (2011). The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ 343, d5928. doi:10.1136/bmj.d5928
Higgins, J. P. T., and Green, S.The Cochrane Collaboration (2011). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. Available at:http://www.cochrane-handbook.org (updated March, 2011).
Izadi, A., Ebrahimi, S., Shirazi, S., Taghizadeh, S., Parizad, M., Farzadi, L., et al. (2019). Hormonal and Metabolic Effects of Coenzyme Q10 And/or Vitamin E in Patients with Polycystic Ovary Syndrome. J. Clin. Endocrinol. Metab. 104 (2), 319–327. doi:10.1210/jc.2018-01221
Kandil, M., Rezk, M., Al-Halaby, A., Emarh, M., and El-Nasr, I. S. (2018). Impact of Ultrasound-Guided Transvaginal Ovarian Needle Drilling Versus Laparoscopic Ovarian Drilling on Ovarian Reserve and Pregnancy Rate in Polycystic Ovary Syndrome: A Randomized Clinical Trial. J. Minim. Invasive Gynecol. 25 (6), 1075–1079. doi:10.1016/j.jmig.2018.01.036
Kar, S. (2012). Clomiphene Citrate or Letrozole as First-Line Ovulation Induction Drug in Infertile PCOS Women: A Prospective Randomized Trial. J. Hum. Reprod. Sci. 5 (3), 262–265. doi:10.4103/0974-1208.106338
Legro, R. S., Arslanian, S. A., Ehrmann, D. A., Hoeger, K. M., Murad, M. H., Pasquali, R., et al. (2013). Diagnosis and Treatment of Polycystic Ovary Syndrome: an Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 98 (12), 4565–4592. doi:10.1210/jc.2013-2350
Legro, R. S., Brzyski, R. G., Diamond, M. P., Coutifaris, C., Schlaff, W. D., Casson, P., et al. (2014). Letrozole versus Clomiphene for Infertility in the Polycystic Ovary Syndrome. N. Engl. J. Med. 371 (2), 119–129. doi:10.1056/NEJMoa1313517
Lv, Z., and Guo, Y. (2020). Metformin and its Benefits for Various Diseases. Front. Endocrinol. (Lausanne) 11, 191. doi:10.3389/fendo.2020.00191
Makrinou, E., Drong, A. W., Christopoulos, G., Lerner, A., Chapa-Chorda, I., Karaderi, T., et al. (2020). Genome-wide Methylation Profiling in Granulosa Lutein Cells of Women with Polycystic Ovary Syndrome (PCOS). Mol. Cell Endocrinol. 500, 110611. doi:10.1016/j.mce.2019.110611
Malkawi, H. Y., Qublan, H. S., and Hamaideh, A. H. (2003). Medical vs. Surgical Treatment for Clomiphene Citrate-Resistant Women with Polycystic Ovary Syndrome. J. Obstet. Gynaecol. 23 (3), 289–293. doi:10.1080/01443610310000100123
Messinis, I. E. (2005). Ovulation Induction: a Mini Review. Hum. Reprod. 20 (10), 2688–2697. doi:10.1093/humrep/dei128
NHRMC. (2018). International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Available at: https://www.monash.edu/__data/assets/pdf_file/0004/1412644/PCOS_Evidence-Based-Guidelines_20181009.pdf.
Palomba, S., Falbo, A., Battista, L., Russo, T., Venturella, R., Tolino, A., et al. (2010). Laparoscopic Ovarian Diathermy vs Clomiphene Citrate Plus Metformin as Second-Line Strategy for Infertile Anovulatory Patients with Polycystic Ovary Syndrome: a Randomized Controlled Trial. Am. J. Obstet. Gynecol. 202 (6), 577.e1–8. doi:10.1016/j.ajog.2009.11.042
Palomba, S., Orio, F., Falbo, A., Russo, T., Caterina, G., Manguso, F., et al. (2005). Metformin Administration and Laparoscopic Ovarian Drilling Improve Ovarian Response to Clomiphene Citrate (CC) in Oligo-Anovulatory CC-Resistant Women with Polycystic Ovary Syndrome. Clin. Endocrinol. (Oxf) 63 (6), 631–635. doi:10.1111/j.1365-2265.2005.02392.x
Palomba, S., Orio, F., Nardo, L. G., Falbo, A., Russo, T., Corea, D., et al. (2004). Metformin Administration versus Laparoscopic Ovarian Diathermy in Clomiphene Citrate-Resistant Women with Polycystic Ovary Syndrome: a Prospective Parallel Randomized Double-Blind Placebo-Controlled Trial. J. Clin. Endocrinol. Metab. 89 (10), 4801–4809. doi:10.1210/jc.2004-0689
Practice Committee of the American Society for Reproductive Medicine, (2017). Role of Metformin for Ovulation Induction in Infertile Patients with Polycystic Ovary Syndrome (PCOS): a Guideline. Fertil. Steril. 108 (3), 426–441. doi:10.1016/j.fertnstert.2017.06.026
Quadir, H. (2021). Current Therapeutic Use of Metformin during Pregnancy: Maternal Changes, Postnatal Effects and Safety. Cureus 13 (10), e18818. doi:10.7759/cureus.18818
Ren, Z., Bremer, A. A., and Pawlyk, A. C. (2021). Drug Development Research in Pregnant and Lactating Women. Am. J. Obstet. Gynecol. 225 (1), 33–42. doi:10.1016/j.ajog.2021.04.227
Rezk, M., Sayyed, T., and Saleh, S. (2016). Impact of Unilateral versus Bilateral Laparoscopic Ovarian Drilling on Ovarian Reserve and Pregnancy Rate: a Randomized Clinical Trial. Gynecol. Endocrinol. 32 (5), 399–402. doi:10.3109/09513590.2015.1124262
Roy, A., and Sahoo, J. (2021). Long-term Effects of Metformin Use in Gestational Diabetes Mellitus on Offspring Health. World J. Diabetes 12 (11), 1812–1817. doi:10.4239/wjd.v12.i11.1812
Schroeder, B. M.American College of Obstetricians and Gynecologists (2003). ACOG Releases Guidelines on Diagnosis and Management of Polycystic Ovary Syndrome. Am. Fam. Physician 67 (7), 1619–1622.
Sharpe, A., Morley, L. C., Tang, T., Norman, R. J., and Balen, A. H. (2019). Metformin for Ovulation Induction (Excluding Gonadotrophins) in Women with Polycystic Ovary Syndrome. Cochrane Database Syst. Rev. 12 (12), CD013505. doi:10.1002/14651858.CD013505
Sinha, P., Chitra, T., Papa, D., and Nandeesha, H. (2019). Laparoscopic Ovarian Drilling Reduces Testosterone and Luteinizing Hormone/Follicle-Stimulating Hormone Ratio and Improves Clinical Outcome in Women with Polycystic Ovary Syndrome. J. Hum. Reprod. Sci. 12 (3), 224–228. doi:10.4103/jhrs.JHRS_161_18
Stein, I. F., and Leventhal, M. L. (1935). Amenorrhea Associated with Bilateral Polycystic Ovaries. Am. J. Obstetrics Gynecol. 29, 181–191. doi:10.1016/S0002-9378(15)30642-6
Teede, H. J., Misso, M. L., Costello, M. F., Dokras, A., Laven, J., Moran, L., et al. (2018). Recommendations from the International Evidence-Based Guideline for the Assessment and Management of Polycystic Ovary Syndrome. Clin. Endocrinol. (Oxf) 89 (3), 251–268. doi:10.1093/humrep/dey256
Underdal, M. O., Stridsklev, S., Oppen, I. H., Høgetveit, K., Andersen, M. S., and Vanky, E. (2018). Does Metformin Treatment during Pregnancy Modify the Future Metabolic Profile in Women with PCOS? J. Clin. Endocrinol. Metab. 103 (6), 2408–2413. doi:10.1210/jc.2018-00485
Wang, R., Li, W., Bordewijk, E. M., Legro, R. S., Zhang, H., Wu, X., et al. (2019). First-line Ovulation Induction for Polycystic Ovary Syndrome: an Individual Participant Data Meta-Analysis. Hum. Reprod. Update 25 (6), 717–732. doi:10.1093/humupd/dmz029
Wang, Y. W., He, S. J., Feng, X., Cheng, J., Luo, Y. T., Tian, L., et al. (2017). Metformin: a Review of its Potential Indications. Drug Des. Devel Ther. 11, 2421–2429. doi:10.2147/DDDT.S141675
Yu, Q., Hu, S., Wang, Y., Cheng, G., Xia, W., and Zhu, C. (2019). Letrozole versus Laparoscopic Ovarian Drilling in Clomiphene Citrate-Resistant Women with Polycystic Ovary Syndrome: a Systematic Review and Meta-Analysis of Randomized Controlled Trials. Reprod. Biol. Endocrinol. 17 (1), 17. doi:10.1186/s12958-019-0461-3
Yu, Y., Fang, L., Zhang, R., He, J., Xiong, Y., Guo, X., et al. (2017). Comparative Effectiveness of 9 Ovulation-Induction Therapies in Patients with Clomiphene Citrate-Resistant Polycystic Ovary Syndrome: a Network Meta-Analysis. Sci. Rep. 7 (1), 3812. doi:10.1038/s41598-017-03803-9
Zahiri Sorouri, Z., Sharami, S. H., Tahersima, Z., and Salamat, F. (2015). Comparison between Unilateral and Bilateral Ovarian Drilling in Clomiphene Citrate Resistance Polycystic Ovary Syndrome Patients: A Randomized Clinical Trial of Efficacy. Int. J. Fertil. Steril. 9 (1), 9–16. doi:10.22074/ijfs.2015.4202
Zhang, J., Tang, L., Kong, L., Wu, T., Xu, L., Pan, X., et al. (2019). Ultrasound-guided Transvaginal Ovarian Needle Drilling for Clomiphene-Resistant Polycystic Ovarian Syndrome in Subfertile Women. Cochrane Database Syst. Rev. 7 (7), CD008583. doi:10.1002/14651858.CD008583.pub2
Zhou, J., Massey, S., Story, D., and Li, L. (2018). Metformin: An Old Drug with New Applications. Int. J. Mol. Sci. 19 (10), 2863. doi:10.3390/ijms19102863
Zhu, D., Chen, Y., Huang, J., Deng, H., Shen, X., Lu, D., et al. (2022). Effects of Metformin on Pregnancy Outcome, Metabolic Profile, and Sex Hormone Levels in Women with Polycystic Ovary Syndrome and Their Offspring: a Systematic Review and Meta-Analysis. Ann. Transl. Med. 10 (7), 418. doi:10.21037/atm-22-909
Keywords: metformin, laparoscopic ovarian drilling, clomiphene citrate resistance, live-birth, meta-analysis
Citation: Sun M-L, Bai W-P, Song Q-K, Wang H-Y, Gao G-L, Zheng L and Wang X-H (2022) Metformin With or Without Clomiphene Citrate Versus Laparoscopic Ovarian Drilling With or Without Clomiphene Citrate to Treat Patients With Clomiphene Citrate-Resistant Polycystic Ovary Syndrome: A Systematic Review and Meta-Analysis. Front. Pharmacol. 13:576458. doi: 10.3389/fphar.2022.576458
Received: 04 September 2020; Accepted: 27 May 2022;
Published: 22 June 2022.
Edited by:
Judith Ann Smith, University of Texas Health Science Center at Houston, United StatesReviewed by:
Ahmed Gibreel, Mansoura University, EgyptIbrahim A. Abdelazim, Ain Shams University, Egypt
Copyright © 2022 Sun, Bai, Song, Wang, Gao, Zheng and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guo-Lan Gao, Z3VvbGFuX2dhb0AxNjMuY29t; Liang Zheng, emhlbmdsaWFuZzk2MzI1OEAxMjYuY29t; Xing-He Wang, d2FuZ3hoQGJqc2p0aC5jbg==
†These authors have contributed equally to this work
 Ming-Li Sun
Ming-Li Sun Wen-Pei Bai
Wen-Pei Bai Qing-Kun Song
Qing-Kun Song Hui-Ying Wang2
Hui-Ying Wang2 Xing-He Wang
Xing-He Wang