- 1School of Health Sciences, Jiangsu Food and Pharmaceutical Science College, Huaian, China
- 2Department of Obstetrics and Gynecology, Second Affiliated Hospital to Shandong First Medical University, Taian, China
Endometrial cancer is considered a significant barrier to increasing life expectancy and remains one of the most common malignant cancers among women in many countries worldwide. The increasing mortality rates are potentially proportional to the increasing obesity incidence. Adipose tissue secretes numerous adipocytokines, which may play important roles in endometrial cancer progression. In this scenario, we describe the role of adipocytokines in cell proliferation, cell invasion, cell adhesion, inflammation, angiogenesis, and anti-apoptotic action. A better understanding of the mechanisms of these adipocytokines may open up new therapeutic avenues for women with endometrial cancer. In the future, larger prospective studies focusing on adipocytokines and specific inhibitors should be directed at preventing the rapidly increasing prevalence of gynecological malignancies.
1 Introduction
Endometrial cancer is considered a significant barrier to increasing life expectancy with significantly increased incidence (Morice et al., 2016) and remains one of the most common malignant cancers among women in many countries worldwide, particularly in more developed countries (Oaknin et al., 2022). Worldwide, endometrial cancer, which is classified into two histological subtypes (type I and type II), ranks sixth in incidence among all female cancers (Morice et al., 2016; Sung et al., 2021). Data from the International Agency for Research on Cancer indicate that 417,367 new corpus uteri cancer cases and approximately 97,370 deaths occurred in 2020. According to the results of previous study, the highest incidence rate was noted in North America (21.1 per 100,000) and was approximately 10-fold greater than the lowest rate, which was observed in Middle Africa (2.3 per 100,000) (Sung et al., 2021).
However, the variation in mortality rates in different regions was not as obvious. The lowest mortality rate was observed in Northern Africa (0.7 per 100,000), and the highest was noted in Eastern Europe (3.7 per 100,000) (Sung et al., 2021). In China, the endometrial cancer incidence rate is approximately 7.74/100 000, and the mortality rate is approximately 1.60/100 000 (Sun et al., 2022). The increasing mortality rates are mainly associated with the increasing incidence of obesity, a leading cause of endometrial cancer (Ding et al., 2020; Larsson.et al., 2022; Moukarzel et al., 2022). In adjusted mixed linear models, weight loss is strongly related to the levels of cancer-associated biologically active substances, including reduced interleukin-6 (IL-6) levels and increased adiponectin levels (Linkov et al., 2012).
As a major site for the secretion of protein signals, adipose tissues mainly comprise adipocytes. In addition, as a major endocrine gland, dysfunctional adipose tissue is involved in obesity-related tumorigenesis, which is correlated with its high degree of plasticity (Sakers et al., 2022) and the permissive microenvironment generated by aberrant inflammatory cytokines, adipokines, angiogenic factors, and aromatase (Hefetz-Sela and Scherer 2013). White adipose tissue (WAT), the most abundant adipose form, secretes numerous adipokines and cytokines to regulate whole-body metabolism. Moreover, WAT inflammation, which increases the expression of proinflammatory and proneoplastic genes, is associated with endometrial cancer (Moukarzel et al., 2022). Additionally, it has become helpful to evaluate biomarkers in relation to cancer risk (Linkov et al., 2018).
2 Article types
Review.
3 Manuscript formatting
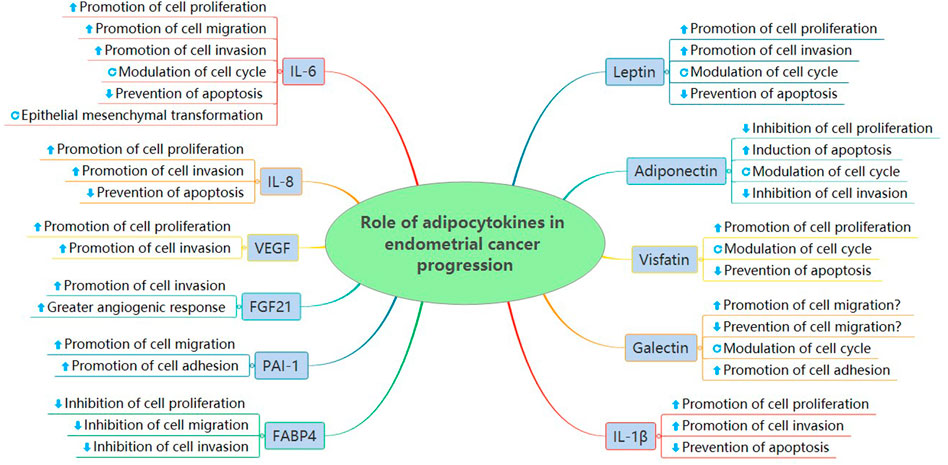
3.1 Role of adipokines in endometrial cancer progression
Adipokines, a diverse group of biologically active substances, are characterized by adipose tissue secretion (Trayhurn and Wood 2004). The levels of various adipokines, such as leptin (Madeddu et al., 2022), visfatin (Wang et al., 2019), galectin (Boutas et al., 2021), resistin (Ozgor et al., 2019), adiponectin (Ellis et al., 2020), and vaspin (Erdogan et al., 2013), are increased or decreased in endometrial cancer and significantly correlated with cancer progression (Ray et al., 2022).
3.1.1 Leptin
Leptin is a 16 kDa cytokine-like hormone encoded by the obesity gene on chromosome 7q31.3, which was first discovered in 1994 (Zhang et al., 1994). The mature leptin protein consists of 146 amino acids and is mainly secreted from white adipose tissue (Zhang et al., 1994). Women with genotype AG of SNP -2548 G/A of leptin are less likely to be at risk for endometrial cancer given that the heterozygote AG is less frequently observed in endometrial cancer patients (Bienkiewicz et al., 2017). Leptin acts on the hypothalamic regions by binding to leptin receptors (Ob-R), which exist in six isoforms with different lengths and C-terminal sequences (Baumann et al., 1996). The AG polymorphic variant of SNP LEP-R c.668A>G (p. Gln223Arg, rs1137101) in the leptin receptor is less frequently observed and considered a protective factor in women with endometrial cancer (Bienkiewicz et al., 2021). By analyzing data from tissue samples and whole blood, overexpression of leptin and its receptors was implicated in endometrial cancer both at the mRNA and protein levels (Boron et al., 2021). In endometrial cancer tissues, Ob-Ra is considered the most common form influencing biological outcomes, not Ob-Rb, which has the same extracellular domain (Yuan et al., 2004). Expression of the long leptin receptor isoform is approximately 5-fold higher in neoplastic tissue compared with normal tissue (Mantzos et al., 2011).
Leptin is involved in endometrial cancer by controlling energy homeostasis and increasing glycolytic capacity. Exposure to leptin could alter endometrial cancer cell morphology. The higher the leptin concentration, the greater the surface roughness (Dabrus et al., 2020). A positive correlation was noted between endometrial cancer and elevated serum leptin levels (Petridou et al., 2002; Tessitore et al., 2004). The incidence rate increased with increasing body mass index (BMI) in endometrial cancer patients (Cymbaluk et al., 2008). The cancer risk of postmenopausal women with the highest tertile of circulating leptin levels was almost three times that noted for women with the lowest tertile (Dallal et al., 2013). In addition, overexpression of leptin and its receptors was observed (Boron et al., 2021). These observations indicated that leptin and its receptors may be potential targets for intervention in the pathophysiology. Furthermore, useful cancer treatment strategies could be designed based on these findings (Boron et al., 2021).
To understand the potential molecular mechanisms of leptin, several studies have been conducted (Bogusiewicz et al., 2006; Carino et al., 2008; Zhou et al., 2015; Daley-Brown et al., 2019). These findings indicated that leptin, a known mitogenic, inflammatory, and angiogenic factor promoted the development of endometrial cancer mainly through the activation of classical biological signalling pathways.
Leptin receptors, including both long and short receptors, can bind to janus-activated kinases and transduce certain signals (Hegyi et al., 2004). Leptin induces two key cell-growth signalling pathways (extracellular signal-regulated kinase (ERK) (Carino et al., 2008) and the serine/threonine kinase (AKT) (Carino et al., 2008)) after rapidly activating the janus-activated kinase (JAK)/signal transducers and activators of transcription (STAT) pathway (Hegyi et al., 2004) (Figure 1). The addition of tyrphostin AG490 abolished leptin-induced proliferation by blocking ERK and AKT phosphorylation (Sharma et al., 2006). Furthermore, the increased phosphorylation of ERK1/2 and leptin-induced stimulation of proliferation were observed upon treatment with 100 ng/ml leptin (Gong et al., 2007). Leptin triggers the phosphatidylinositol3-kinase (PI3K)/AKT pathway by activating the leptin receptor, which is correlated with cell proliferation and invasiveness (Bogusiewicz et al., 2006).
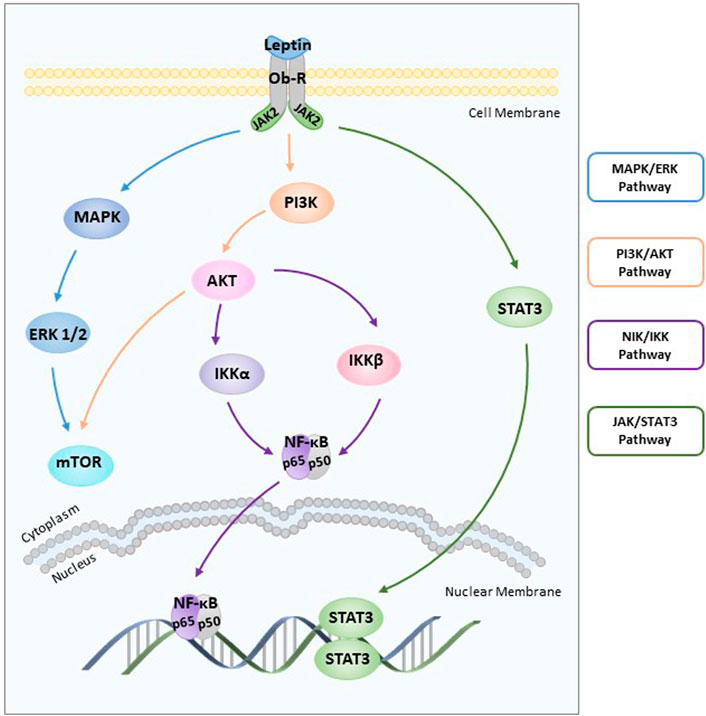
FIGURE 1. Role of leptin in endometrial cancer. Leptin induces two key cell-growth signalling pathways (ERK and AKT) after rapidly activating the JAK/STAT3 pathway. Leptin-induced NF-κB activation inhibits cancer cell apoptosis through the NIK/IKK signalling pathway. ERK: extracellular signal-regulated kinase; AKT: the serine/threonine kinase; JAK: janus-activated kinase; STAT3: signal transducers and activators of transcription 3; NIK: nuclear factor-kappa B inducing kinase; IKK: IKB kinase.
Another pathway involved in cancer progression is nuclear factor-kappaB inducing kinase (NIK)/IKB kinase (IKK), and leptin-induced NIK/IKK phosphorylation inhibits cancer cell apoptosis in carcinoma cells (Zhou et al., 2015) (Figure 1). First discovered in 1986, NF-κB is essentially involved in driving immune and inflammatory responses (Sen and Baltimore 1986). The NF-κB family includes five members and mediates DNA contact by forming homo or heterodimers (May and Ghosh 1997; Caamano and Hunter 2002). The IκB family of proteins, which includes four members, binds to the NF-κB family of proteins to inhibit the activity of transcription factors (Hoesel and Schmid 2013). Nuclear positivity for subunits of NF-κB as well as cytoplasmic staining for three IκB family members was assessed in 57 endometrial carcinoma cases by immunohistochemical evaluation. These data suggest that NF-κB/IκB may be involved in endometrial carcinoma cell proliferation and apoptosis (Pallares et al., 2004). Furthermore, leptin inhibits apoptosis of cancer cells by stimulating phosphorylation of IκBα, IκB kinase α (IKKα), IκB kinase β (IKKβ), and NIK in a dose-dependent manner (Zhou et al., 2015).
Leptin is involved in endometrial carcinoma cell mitosis, and leptin-mediated effects on endometrial cancer cell cycle progression are concentration-dependent. Leptin reduces the fraction of G0/G1-phase cells and increases S-phase cells by stimulating cyclin D1, a significant cell cycle regulator. Leptin-induced cyclin D1 overexpression increases STAT3-DNA and cAMP-response element binding protein (CREB)-DNA binding activity and recruitment (Catalano et al., 2009).
A positive correlation between overexpression of leptin and hypoxia-inducible factor 1 alpha (HIF-1α), an indicator of tissue hypoxia consisting of two subunits, was clearly observed in endometrial cancer tissues (Koda et al., 2007). Furthermore, leptin overexpression was stimulated through HIF-1α interaction with the leptin gene promotor in hypoxic adipocytes (Grosfeld et al., 2002). Among 48 human endometrioid adenocarcinoma patients, the number of patients positive for STAT3, HIF-1, leptin, and ObR was 36, 38, 29 and 15, respectively. It was clearly demonstrated that leptin induced HIF-1α through STAT3 in response to hypoxia (Wincewicz et al., 2008).
Leptin stimulates cell proliferation by increasing cyclooxygenase-2 (COX-2) protein expression through the JAK2/STAT3, MAPK/ERK, and PI3K/AKT signalling pathways (Gao et al., 2009). COX-2, a rate-limiting enzyme, is of considerable functional importance (Tsujii et al., 1998). The findings of basic in vivo and in vitro studies suggest that COX-2 overexpression is associated with increased susceptibility to endometrial cancer (Chen and Liao 2009; Ma et al., 2015). Increased COX-2 expression was found in higher-grade tumours. Several studies have indicated that functional activation of COX-2 is mediated by JAK2/STAT3 (Peng-Fei et al., 2021), MAPK/ERK (Adderley and Fitzgerald 1999), and PI3K/AKT (Rodriguez-Barbero et al., 2006) signalling pathways. After being treated with inhibitors (AG490, U0126, LY294002, and NS398) respectively, stimulated endometrial cancer cell proliferation and increased COX-2 protein expression induced by leptin were abolished (Gao et al., 2009). Therefore, COX-2 is also considered a significant biomarker for endometrial cancer diagnosis and prognosis (Oplawski et al., 2020).
Leptin-induced aromatase P450 (P450arom) overexpression increases oestrogen formation to promote endometrial cancer progression. P450arom, a key enzyme, is involved in the conversion of androstenedione to oestrogens (Noble et al., 1996). Excessive P450arom activity and transcript levels were found in endometrial cancer tissues. Higher P450arom mRNA and protein expression as well as oestradiol concentrations were observed in endometrial carcinoma cells treated with 100 ng/ml leptin, indicating a strong correlation between leptin and P450arom (Liu et al., 2013).
3.1.2 Adiponectin
Adiponectin, a type of insulin-sensitizing adipokine, is secreted predominantly by WAT (Scherer et al., 1995; Hu et al., 1996; Maeda et al., 1996; Nakano et al., 1996). In addition, recent studies have indicated that adipose-derived stem cell (ASC) is an important source of intracellular adiponectin (Ciortea et al., 2018). In human plasma, Acrp30, a type of full-length adiponectin which consists of 247–amino acid protein is the main adiponectin form found in circulation (Scherer et al., 1995). In a large case‒control study, three SNPs in the ADIPOQ gene (rs3774262, rs1063539, rs12629945) were identified that potentially correlated with energy intake (Chen et al., 2012). Structurally, the adiponectin receptor has two isoforms, both of which include an internal N and an external C-terminus region (Yamauchi et al., 2003). AdipoR1 is ubiquitously expressed but has a higher affinity for globular adiponectin. However, AdipoR2 exhibits intermediate affinity for both globular and full-length adiponectin (Goldstein and Scalia 2004; Kadowaki and Yamauchi 2005). Analysis of endometrial tissues showed that both adiponectin receptors were expressed throughout the menstrual cycle and were especially present at higher levels in the mid-luteal phase (Takemura et al., 2006). Similar to leptin, adiponectin is also correlated with obesity. Higher levels of abdominal fat were found in the endometrial cancer group, and plasma adiponectin level was in a negative linear correlation with the abdominal fat level. (Mihu et al., 2013). Of note, significantly lower adiponectin levels were implicated in endometrial cancer patients (Rzepka-Gorska et al., 2008). Additionally, the abnormal expression of adiponectin receptors was observed in several insulin resistance-related tumours, such as breast cancer (Mocino-Rodriguez et al., 2017; Cicekdal et al., 2020), prostate cancer (Kaklamani et al., 2011; Huang et al., 2021), ovarian cancer (Jin et al., 2016), and endometrial cancer (Petridou et al., 2003; Soliman et al., 2006; Barb et al., 2007). In addition, adiponectin suppresses endometrial cancer proliferation by acting through AdipoRs, which were expressed in both tissue samples and cell lines (Moon et al., 2011). Positive staining was observed in low-grade adenocarcinoma, whereas negative staining was noted in high-grade adenocarcinoma. These results indicate that lower AdipoR expression was strongly correlated with higher histological grade in endometrioid adenocarcinoma (Yamauchi et al., 2012). Data from a study including 60 patients indicated that AdipoR1 levels are related to myometrial invasion (Yunusova et al., 2015). Moreover, another study indicated that the expression of AdipoR-1, not AdipoR-2, exerts suppressive effects on cancer cell proliferation, adhesion, and growth in a group of endometrial carcinoma patients (Yabushita et al., 2014).
Adiponectin directly reduced the viability of normal human endometrial stromal cells without any change in AdipoR1 and AdipoR2 levels (Bohlouli et al., 2013). Moreover, numerous findings showed that serum adiponectin levels were reduced in endometrial cancer patients compared with individuals with no history of endometrial cancer (Soliman et al., 2006; Cust et al., 2007; Ma et al., 2013; Zeng et al., 2015; Ellis et al., 2020). The expression levels of adiponectin and vaspin, which are considered anti-inflammatory molecules, are inversely proportional to endometrial cancer risk even after controlling for potential confounders (Erdogan et al., 2013). In particular, a linear dose-response relationship indicated that the risk was reduced by 3% for every 1 μg/ml increase in adiponectin (Lin et al., 2015). Furthermore, among women younger than 65 years, the odds ratios derived from three different models by multiple logistic regression indicated that the risk was reduced by 50% for a 1 SD increase in adiponectin (Petridou et al., 2003). Additionally, adiponectin concentrations were progressively reduced from grade 1 (19.04 μg/ml) to grade 2 (13.48 μg/ml), and finally grade 3 tumours (12.86 μg/ml). A significant difference was noted between grade 1 and grade 3 tumours but not between grade 1 and grade 2 tumours (Rzepka-Gorska et al., 2008).
Leptin-to-adiponectin ratios (L/A ratios) may be more informative in studies of the risk of endometrial cancer among postmenopausal women (Dallal et al., 2013). Higher L/A ratios were strongly related to endometrial cancer progression even after controlling for the factors of diabetes mellitus and age. The OR of the L/A ratio [6.0 (95% CI: 3.2–11.9)] was higher than those of leptin alone [3.2 (95% CI: 1.8–5.8)] or adiponectin alone [0.5 (95% CI: 0.3–0.9)], suggesting that L/A ratios in individuals may better indicate cancer growth and proliferation (Ashizawa et al., 2010).
Adiponectin exerts an antiproliferative effect on endometrial cancer by increasing the number of G1/G0-phase cells and decreasing the number of S-phase cells. The reduction in cell counts in the HEC-1-A and RL95-2 cell lines reached approximately 30% and 20%, respectively, upon treatment with 40 mg/ml adiponectin. Furthermore, cyclin D1 and cyclin E2 expression was reduced, and 5 adenosine monophosphate-activated protein kinase (AMPK) was rapidly activated within 30 min in human endometrial cancer cell lines (Cong et al., 2007). Moon, H. S et al. (Moon et al., 2011) showed for the first time that adiponectin upregulated the tumour suppressor gene liver kinase B1 (LKB1), an adaptor molecule required for AMPK activation, to stimulate the AMPK/S6 axis. In addition, Wu et al. (Wu et al., 2012) demonstrated that Acrp30 effectively reduced leptin-induced STAT3 phosphorylation by stimulating the MAPK pathway in aggressive SPEC-2 endometrial cancer cells. After Ishikawa cells were treated with 10 μg/ml adiponectin, AMPK phosphorylation was rapidly activated and reached a maximum at 30 min. A 50% reduction in activated ERK and a 40% reduction in AKT expression were observed. Moreover, compound C inhibited adiponectin-induced ERK and AKT phosphorylation, demonstrating that ERK and AKT are downstream targets of AMPK. In addition, 10 μg/ml adiponectin treatment also caused significant reductions in cyclin D1 mRNA (49%), cyclin D1 protein (62%), B-cell lymphoma-2 (Bcl-2) mRNA (45%) and Bcl-2 protein (36%). This result suggested that adiponectin induced mitochondrial dysfunction by decreasing the Bcl-2/bcl-2-associated x (Bax) ratio (Zhang et al., 2015). Cai et al. (Cai et al., 2018) showed that AMPK phosphorylation was significantly induced by adiponectin, whereas mTOR and ribosomal protein S6 kinase-1 protein phosphorylation was inhibited. A considerably reduced proliferation inhibition ratio and enhanced cell migration were found in the inhibitor + adiponectin group than in the adiponectin group without the addition of an inhibitor. Adiponectin may inhibit cell proliferation and migration through the AMPK/(mTOR)/(S6K1) signalling pathway in patients with malignancies (Figure 2).
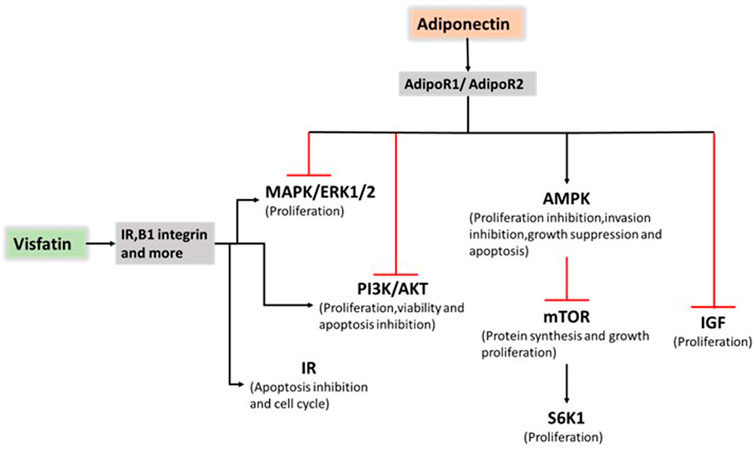
FIGURE 2. Role of adiponectin and visfatin in endometrial cancer progression. Adiponectin exerts an antiproliferative effect on endometrial cancer by stimulating AMPK pathway activation and suppressing PI3K/AKT, MAPK/ERK1/2 and IGF pathway activation. Visfatin promoted cancer progression mainly through PI3K/AKT, and MAPK/ERK1/2 pathway activation and IR. AMP: 5 adenosine monophosphate; AMPK: AMP-activated protein kinase; PI3K: phosphatidylinositol 3-kinase; AKT: the serine/threonine kinase; MAPK: mitogen-activated protein kinase; ERK1/2: extracellular signal-regulated kinases 1 and 2; IGF: insulin-like growth factor; IR: insulin resistance.
However, contrary to the previous role of adiponectin in suppressing cancer progression, several studies have showed that adiponectin contributes to an increased risk of liver cancer (Aleksandrova et al., 2014). Moreover, a study involving in exploring the relation between cancer and adiponectin underlying the obesity paradox, has showed that exogenous adiponectin significantly inhibited cell apoptosis by up-regulating p-AMPK and Bcl-xL levels in renal cell carcinoma (Ito et al., 2017). This conclusion was consistent with the results of the later study conducted by Lee et al. (Lee et al., 2020). The study conducted in Hong Kong, including 5658 participants, indicated an interesting adiponectin paradox. They demonstrated that higher adiponectin concentrations might be harmful, and positively correlated with the incidence and deaths of cancer in type 2 diabetes (Lee et al., 2020). As the role of adiponectin still remains controversial in various cancers, further studies should be directed to exploring the complex mechanism.
3.1.3 Visfatin
Visfatin, a 52 kDa protein (Fukuhara et al., 2005), plays a significant role in cell growth (Zhang et al., 2011) and insulin resistance (Fukuhara et al., 2005). Recently, accumulating evidence has suggested that visfatin may be a complementary diagnostic and prognostic marker for malignancies, especially those that are strongly related to dysfunctional adipose tissue, such as breast cancer (Rajput et al., 2022), colorectal cancer (Zhao et al., 2020), and endometrial cancer (Mu et al., 2012). Tian et al. (Tian et al., 2020) reported that visfatin protein expression was upregulated by the PI3K/AKT and MAPK/ERK signalling pathways in polycystic ovary syndrome (PCOS) patients with endometrial cancer.
Tian et al. (Tian et al., 2013) suggested that serum visfatin levels were significantly higher in endometrial cancer patients compared with other groups. Furthermore, visfatin expression was measured in tissue samples. Visfatin tissue expression increased gradually from normal proliferative or secretory endometrium (58.1%) and hyperplastic endometrium (66.7%) to endometrial cancer (80.5%). Moreover, visfatin expression was significantly related to serum levels in 50 endometrial cancer patients. High serum visfatin levels represent a key factor correlated with deep myometrial invasion and poor survival (Tian et al., 2013). Visfatin promotes cancer progression mainly through PI3K/AKT and MAPK/ERK1/2 activation as well as insulin resistance (IR) (Figure 2). In 2014, Nergiz Avcioglu et al. (Nergiz Avcioglu et al., 2015) indicated three possible mechanisms (obesity, increased lipolysis, and insulin resistance) to explain the increased serum visfatin levels in endometrial cancer (Nergiz Avcioglu et al., 2015). A study focusing on the molecular mechanisms showed that visfatin exerts pro-proliferative and anti-apoptotic effects by stimulating cell proliferation and increasing the S-phase fraction of cells.
The expression of visfatin and its substrates was upregulated in the context of IR, and maximal levels were noted at 30 min. Increased C-MYC and cyclin D1 expressions as well as decreased caspase-3 expression were also observed with visfatin treatment. To confirm the effect of the PI3K/AKT and MAPK/ERK signalling pathways, Ishikawa cells were treated with 400 ng/ml visfatin. Larger G1 and S-phase fractions were found in Ishikawa cells pretreated with the inhibitor (Wang et al., 2016). Similar results are presented by Cymbaluk-Ploska et al. (Cymbaluk-Ploska et al., 2018). The visfatin concentration was 15.9 ng/ml for the endometrial cancer group and 9.5 ng/ml for the other. Furthermore, a slightly higher visfatin concentration was noted for cases with lower histological differentiation (22.2 and 31.8 ng/ml) compared with well-differentiated cases (17.3 and 22.2 ng/ml). The visfatin level was inversely proportional to the overall survival (OS) of patients (Cymbaluk-Ploska et al., 2018). A retrospective case‒control study showed that the visfatin-adiponectin ratio in 53 endometrial cancer patients was significantly higher than that in the control group (Wang et al., 2019).
3.1.4 Galectin
It is clear that galectins are integrated into the physiological and pathological systems of individuals with a wide range of biological functions (Liu et al., 2002). To date, 11 identified different subtypes have been classified into three subgroups according to structure (prototype, tandem repeat-type, and chimeric-type) (Chou et al., 2018). Among them, four forms (galectin-1, galectin-3, galectin-7, and galectin-9) have been closely linked to gynecological cancer cell biology and immunology (Chetry et al., 2018). Furthermore, multiple studies have indicated that galectin-1, a homodimeric protein involved in angiogenesis (Thijssen and Griffioen 2014) and cross-linking receptors (Hernandez et al., 2006), and galectin-3, a chimaera-type protein associated with cancer metastasis (Fortuna-Costa et al., 2014) and inflammatory regulation (Henderson and Sethi 2009), are mainly involved in endometrial cancer. Galectin-1 expression was observed in endometrioid endometrial adenocarcinoma (EA) tissue (Zinovkin et al., 2019). Higher galectin-1 expression suggested a poorer prognosis (Sun and Dai 2020). In addition, galectin-1 immunoreaction was positively proportional to endometrial cancer grade, increasing from G1 to G3 (Mylonas et al., 2007). The microcystic, elongated and fragmented (MELF) pattern was inversely proportional to endometrial cancer patient survival (Stewart et al., 2009; Zinovkin et al., 2017). The median level of galectin-1 expression among 49 subjects was obviously higher (78.6%) in the positive group. The statistically significant differences analyzed by the Mann‒Whitney test additionally indicated that this marker may be of considerable functional importance in the OS of patients (Zinovkin et al., 2019).
Galectin-1 and galectin-3 immunoperoxidase staining of the uterine carcinoma specimens obtained from Duke University Medical Center was performed and statistically analyzed. Lower scores of galectin-1 expression were found in normal endometrium (scores from 0 to 2), whereas higher scores were found in endometrial carcinomas (scores from 1 to 3). In contrast, galectin-3 expression was significantly decreased in endometrial cancer (van den Brule et al., 1996). This conclusion was consistent with the results of a later study conducted in the Middle East. This finding demonstrated that galectin-3 may play a role in the suppression of cancer progression. Galectin-3 immunoreactivity progressively decreased from normal samples (80%) to endometrial carcinoma (55%), indicating poor prognoses (Al-Maghrabi et al., 2017). Interestingly, deeper invasion of the myometrium was found in cancer cells with only cytoplasmic immunoreactivity (van den Brule et al., 1996). The extent, intensity, and immunohistochemical reactivity of epithelial and stromal galectin-3 expression were reduced in the cancer group. The percentage of the cases with lymph node metastasis negative for galectin-3 expression (64%) was increased almost four-fold compared with cases without lymph node metastasis (18%). This investigation suggests that galectin-3 may be involved in the pathogenesis of endometrial carcinomas and lymph node metastasis (Ege et al., 2011).
However, contradictory results from a study involving 144 patients showed that increased galectin 3 expression was observed in patients with lymphovascular space invasion (Cymbaluk-Ploska., et al., 2020). The mean scores progressively increased from normal endometria (2.58) and atypical hyperplasia (4.77) to clear cell carcinoma (6.71), and significant differences were noted among the various conditions. Based on these findings, Brustmann et al. (Brustmann et al., 2003), assumed that galectin-3 expression was essential to maintain a transformed phenotype in endometrial carcinoma (Brustmann et al., 2003). To investigate the effect of galectin-3 on the endometrial cell cycle and adhesion, multiple analysis methods were used. After seventy-two hours of galectin-3 siRNA transfection, galectin-3 mRNA and protein expression were reduced by 70%–90% in RL95-2 cells. A decrease in S-phase cells and an increase in G1-phase cells were observed. Thus, galectin-3 may be involved in promoting cell adhesion and increasing integrin expression (Lei et al., 2007). Additionally, considering the fact that the environment composed of numerous adipokines and cytokines that promote tumour growth, the different conclusions may be clarified by method sensitivity, case differences, treatment differences, and different sizes of samples. To better understand the effect of galectin-3 and related biological signalling pathways on tumour size, growth, characteristics, and malignancy in endometrial cancers, more studies, such as longitudinal studies and large-scale studies, are needed.
3.2 Role of adipose-secreted inflammatory cytokines in endometrial cancer progression
Inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-8 (IL-8), can modify the immunological network in the endometrium. The primary sources of inflammatory cytokines mainly include inflammatory cells, adipocytes, and cancer cells. Among them, IL-1β and IL-6 are secreted by adipocytes through endocrine and paracrine secretion and are correlated with a modified adipocyte phenotype (Dirat et al., 2011). Inflammatory cytokines are the key factors that explain the difference in the immune microenvironment between normal and malignant endometria. Therefore, understanding the role of inflammatory cytokines in proinflammatory and protumorigenic effects on endometrial cancer progression is crucial.
IL-1β, IL-6, and IL-8, which exhibit a wide range of complex functions, have been extensively examined. Notably, IL-1 is ubiquitously expressed in endometrial tissues (Van Le et al., 1991). However, data from a clinical study revealed a significant increase in IL-8 concentrations, not IL-Iβ and IL-6, which were too low to detect (Chopra et al., 1997). Furthermore, later experiments clearly demonstrated that leptin significantly increased the levels of IL-1 and interleukin-1 receptor tI (IL-1R tI) in a dose-dependent manner. Based on experiments using a kinase inhibitor, the results indicated that leptin-mediated activation of the JAK2/STAT3, PI3K/AKT1, and mTOR signalling pathways was associated with an increase in IL-1β levels in primary endometrial epithelial cells. In contrast, leptin induced IL-1R tI in all endometrial epithelial cells through leptin canonical signalling pathways that generally include JAK2/STAT3, MAPK/ERK1/2, and mTOR without PI3K/AKT1 involvement (Carino et al., 2008).
Adiponectin also stimulated AMPK phosphorylation and suppressed the secretion of IL-6 and IL-8 induced by IL-1β in human endometrial stromal cells (ESCs), suggesting the effect of adiponectin on regulating energy supply and anti-inflammatory function (Takemura et al., 2006).
When assessing endometrial cancer cells using cell invasion assays and statistical analysis, Lipsey et al. (Lipsey et al., 2016) found that Notch, IL-1, and leptin crosstalk outcome (NILCO) was more highly expressed in type II endometrial cancer, the more aggressive form, not type I. Moreover, leptin-induced invasion of endometrial carcinoma cells was significantly reduced in the presence of an inhibitor (Daley-Brown et al., 2017). Remarkably, the levels of Notch receptors, ligands, and targeted molecules were at least a twofold increase compared to basal culture conditions without leptin treatment. After DAPT and anti-IL-1R tI antibodies were added, the results showed that leptin-induced migration of malignancies was abrogated. The role of leptin was more prominent in the more malignant phenotype, such as the more aggressive and poorly differentiated An3CA endometrial cancer cell line. Leptin-induced NILCO molecules in endometrial cancer affect cell proliferation, aggressiveness, and chemoresistance (Daley-Brown et al., 2019). Taken together, these studies indicated the complex crosstalk among Notch, IL-1, and leptin as well as the involvement of IL-1 in inducing inflammatory progression and upregulating leptin expression in endometrial cancer (Figure 3).
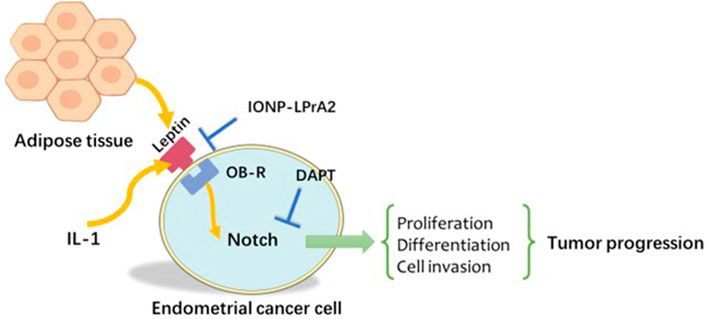
FIGURE 3. Notch, IL-1, and leptin crosstalk outcome (NILCO). Leptin induced Notch receptor, ligand, and targeted molecule expression. The inhibition of Notch and IL-1 signalling in vitro reduced leptin-induced invasion. IL-1: Interleukin-1.
3.3 Role of adipose-secreted angiogenic factors in endometrial cancer progression
Adipose-secreted angiogenic factors, such as vascular endothelial growth factor (VEGF) and fibroblast growth Factor 21 (FGF21), play significant roles in stimulating angiogenesis and forming the proangiogenic microenvironment. Potential therapeutic implications targeting VEGF and FGF21 may open up new avenues for endometrial cancer women with cancer cell metastasis.
VEGF, a multiple proangiogenic factor observed across endometrioid endometrial adenocarcinoma (EA) cells in different stages, was correlated with abnormal vasculature formation, insulin sensitivity, and adipocyte death (Sun et al., 2012; Goel and Mercurio 2013). Moderate VEGF expression was positively correlated with EA progression as well as an elongated and fragmented (MELF) pattern. However, this parameter was inversely proportional to the number of survival days (Zinovkin et al., 2019). A preliminary study suggested that leptin significantly increased the levels of VEGF and vascular endothelial growth factor receptor 2 (VEGFR2) through the MAPK/ERK1/2 and mTOR signalling pathways (Carino et al., 2008). In addition, overexpression of VEGF and its receptors in uterine tissue appeared to be affected by cotreatment (leptin and oestradiol) probably through the ERK1/2 and STAT3 pathways (Shetty et al., 2020). Interestingly, additional experiments clearly showed that leptin-induced angiogenesis was probably attributed to activating VEGFR-Notch signalling crosstalk in overweight cancer patients with increased expression of VEGF, VEGFR-2, and Notch (Lanier et al., 2016).
Fibroblast growth Factor 21 (FGF21) belongs to the sixth subfamily of FGFs and mainly modulates the storage of carbohydrates (Beenken and Mohammadi 2009). Based on comparative analysis, high FGF21 concentrations were positively related to high leptin levels. Taken together, the results showed that FGF21 concentrations were higher in poorly and moderately differentiated tumours compared with highly differentiated tumours. In addition, the area under the receiver operator characteristic curve (AUC) for FGF21 was 0.81, indicating that FGF21 was a promising diagnostic biomarker with good sensitivity and specificity through FGFR 2 and the PI3K/AKT and mTOR signalling pathways (Cymbaluk-Ploska et al., 2020).
3.4 Role of other adipocytokines in endometrial cancer progression
On review of the recent studies, other identified adipocytokines, including plasminogen activator inhibitor-1 (PAI-1) (Wang et al., 2021), and fatty acid-binding protein 4 (FABP4) (Wu et al., 2021), also play important roles in regulating various physiological processes. Of note, these adipocytokines have considerable consequences for promoting the proliferation and migration of endometrial cancer cells and may be possible targets for the therapy.
PAI-1, a promising prognostic factor involving in selective degradation of extracellular matrix components (Andreasen et al., 1997; Fredstorp-Lidebring et al., 2001), has been found to be associated with neovascularization, invasion, and migration in breast (Schmitt et al., 2010), prostate (Almasi et al., 2011), colorectal (Markl et al., 2017), ovarian (Zhang et al., 2013), and endometrial cancers (Tecimer et al., 2001). Women with PAI-1 rs1799889 4G/4G genotype are more likely to be at risk for endometrial cancer and the susceptibility to cancer may be associated with the 4G allele. (Su et al., 2011; Xu et al., 2012). Compared to normal endometrium, concentrations of PAI-1 in cytosols of endometrial cancer were significantly higher (Kohler et al., 1997; Osmak et al., 2001). In addition, expression of PAI-1 was regulated by estrogen and progesterone, and appeared negatively correlated with estrogen and progesterone receptor levels (Fujimoto et al., 1996; Steiner et al., 2008). The potential of sex steroids-dependent metastasis plays significant roles in cancer progression (Gotte et al., 2010; Fujimoto and Sato 2011). Previous studies showed that PAI-1 was positively correlated with cancer stage, but negatively correlated with relapse free time and OS of patients (Tecimer et al., 2001; Steiner et al., 2008). As one of the most abundant adipocytokines in adipose stromal cells (ASCs), PAI-1 could diminish transforming growth factor β (TGF-β)-mediated tumor suppressor activity through the TGF-β/SMAD pathway (Lin et al., 2020).
FABP4, belonging to the fatty acid binding proteins (FABPs) family, has a central role in tumour metastasis and endothelial migration by regulating metabolic and inflammatory pathways (Hotamisligil and Bernlohr 2015). As a marker involved in adipocyte differentiation (Bernlohr et al., 1984), FABP4 promotes the progression of feminine cancers, such as ovarian cancer, and cervical cancer (Gharpure et al., 2018; Jin et al., 2018). However, a recent study showed that FABP4 might play a possible suppressive role in endometrial cancer cell proliferation, migration, and invasion through the PI3K/AKT pathway (Wu et al., 2021). These studies have showed that the effects FABP4 exerts on cancers may be related to tumor type and signaling pathways. To further explore the decreased expression of FABP4 in endometrial cancer, more researches are required.
3.5 Possible role of adipocytokines in the treatment of endometrial cancer
Adipocytokines, including adipokines, inflammatory cytokines, and angiogenic factors, are significant biomarkers in various cancers, particularly endometrial cancer. To date, among the identified adipocytokines, some have been found to be good prognostic factors with a wide range of biological functions, including suppression of cell proliferation, induction of apoptosis, and reduced cell invasion. However, other adipocytokines, such as leptin, galectin, and visfatin, are considered poor prognostic factors associated with the promotion of cell proliferation, inhibition of apoptosis, and increased cell invasion (Figure 4). Leptin and adiponectin are the two main adipocytokines involved in most studies in endometrial cancer. However, studies on the potential molecular mechanisms of other adipocytokines, such as resistin, galectin, and visfatin, are limited. Particularly, contradictory results have been reported from different studies on galectin-3 concentrations and expression in endometrial cancers. The main inflammatory pathways predominantly reported include the MAPK/ERK1/2, JAK/STAT3, PI3K/AKT/mTOR, Notch, IR, IGF, AMPK/ERK, and AKT signalling pathways. When using specific inhibitors, endometrial cancer cell proliferation, invasion, and migration were reduced.
Based on the roles of significant adipocytokines and specific inhibitors, the use of targeted treatments in cancers has been studied in various experiments. Metformin, a potential anti-cancer drug, could induce cell cycle arrest and apoptosis through the AMPK and mTOR signalling pathways (Jalving et al., 2010). Furthermore, compared to metformin (Met) alone, metformin and silibinin (Sil) in magnetic PLGA/PEG nanoparticles (NPs) kill lung cancer cells more rapidly by reducing the expression of leptin and its receptor (Salmani Javan et al., 2022). Thiazolidinediones (TZDs) (rosiglitazone, pioglitazone) are also reported to increase adiponectin levels and decrease leptin, tumor necrosis factor-α (TNF-α), and IL-6 levels through modulatory mechanisms (Garikapati et al., 2019; Biondo et al., 2020). Moreover, TZDs have played important roles in preventing progression of hepatocellular carcinoma (HCC) (Arvind et al., 2021), colon cancer (Yoon et al., 2020), and lung cancer (Konieczna et al., 2015). Atorvastatin reduces cardiovascular mortality by increasing levels of adiponectin, which is involved in insulin resistance (Koh et al., 2005; Ando et al., 2008). Atorvastatin has been reported to be used as a kind of important therapy in oesophageal adenocarcinoma by suppressing leptin-induced activation of cdc42 and AKT (Beales and Ogunwobi 2021).
Furthermore, recent studies have showed that mild obesity (BMI≥ 25.0,≤ 29.9) is correlated to an improved immunotherapy response (Li and Kalantar-Zadeh 2013; Li et al., 2017). The cancers who have mild obesity are more likely to reach a balance between pro- and anti-inflammatory cytokines (Assumpcao et al., 2022). Compared with poor response to chemotherapy in obese patients (Horowitz and Wright 2015), immunotherapy may be a more favorable therapeutic approach for the obesity (Waldman et al., 2020). The dysregulation of the secretion of adipocytokines, which involves in T cell modulation, macrophage polarization, and binding of adipocyte PD-L1 to anti-PD-L1 antibodies, affects immune checkpoint inhibitor therapy (Assumpcao et al., 2022). By using checkpoint inhibitor (anti–CTLA-4 mAb), Murphy et al. (Murphy et al., 2018) have found that leptin was a contributor to the failure of tumor immunotherapy. It implicated the potential role of leptin in the efficacy of immunotherapy.
4 Conclusion
Adipocytokines, regulating various physiological and pathological processes, play crucial roles in endometrial cancer progression. Larger prospective studies focusing on adipocytokines and specific inhibitors, particularly immune checkpoint inhibitor therapy, should be directed at preventing the rapidly increasing prevalence of gynecological malignancies.
Author contributions
Study concepts: GL and XN. Study design: RL and LZ. Manuscript preparation: RL and FD. Manuscript editing: RL, LZ, and GL. Manuscript review: RL, LZ, FD, XN, and GL.
Funding
This work was supported by the National Natural Science Foundation of China (81200011).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adderley, S. R., and Fitzgerald, D. J. (1999). Oxidative damage of cardiomyocytes is limited by extracellular regulated kinases 1/2-mediated induction of cyclooxygenase-2. J. Biol. Chem. 274, 5038–5046. doi:10.1074/jbc.274.8.5038
Al-Maghrabi, J., Abdelrahman, A. S., Ghabrah, T., Butt, N. S., Al-Maghrabi, B., and Khabaz, M. N. (2017). Immunohistochemical expression of galectin-3 is significantly associated with grade, stage and differentiation of endometrial carcinomas. Pathol. Res. Pract. 213, 348–352. doi:10.1016/j.prp.2017.01.012
Aleksandrova, K., Boeing, H., Nothlings, U., Jenab, M., Fedirko, V., Kaaks, R., et al. (2014). Inflammatory and metabolic biomarkers and risk of liver and biliary tract cancer. Hepatology 60, 858–871. doi:10.1002/hep.27016
Almasi, C. E., Brasso, K., Iversen, P., Pappot, H., Hoyer-Hansen, G., Dano, K., et al. (2011). Prognostic and predictive value of intact and cleaved forms of the urokinase plasminogen activator receptor in metastatic prostate cancer. Prostate 71, 899–907. doi:10.1002/pros.21306
Ando, H., Sugimoto, K., Yanagihara, H., Tsuruoka, S., Saito, T., Takamura, T., et al. (2008). Effects of atorvastatin and pravastatin on glucose tolerance, adipokine levels and inflammatory markers in hypercholesterolaemic patients. Clin. Exp. Pharmacol. Physiol. 35, 1012–1017. doi:10.1111/j.1440-1681.2008.04945.x
Andreasen, P. A., Kjoller, L., Christensen, L., and Duffy, M. J. (1997). The urokinase-type plasminogen activator system in cancer metastasis: A review. Int. J. Cancer 72, 1–22. doi:10.1002/(sici)1097-0215(19970703)72:1<1::aid-ijc1>3.0.co;2-z
Arvind, A., Memel, Z. N., Philpotts, L. L., Zheng, H., Corey, K. E., and Simon, T. G. (2021). Thiazolidinediones, alpha-glucosidase inhibitors, meglitinides, sulfonylureas, and hepatocellular carcinoma risk: A meta-analysis. Metabolism. 120, 154780. doi:10.1016/j.metabol.2021.154780
Ashizawa, N., Yahata, T., Quan, J., Adachi, S., Yoshihara, K., and Tanaka, K. (2010). Serum leptin-adiponectin ratio and endometrial cancer risk in postmenopausal female subjects. Gynecol. Oncol. 119, 65–69. doi:10.1016/j.ygyno.2010.07.007
Assumpcao, J. A. F., Pasquarelli-do-Nascimento, G., Duarte, M. S. V., Bonamino, M. H., and Magalhaes, K. G. (2022). The ambiguous role of obesity in oncology by promoting cancer but boosting antitumor immunotherapy. J. Biomed. Sci. 29, 12. doi:10.1186/s12929-022-00796-0
Barb, D., Williams, C. J., Neuwirth, A. K., and Mantzoros, C. S. (2007). Adiponectin in relation to malignancies: A review of existing basic research and clinical evidence. Am. J. Clin. Nutr. 86, s858–s866. doi:10.1093/ajcn/86.3.858S
Baumann, H., Morella, K. K., White, D. W., Dembski, M., Bailon, P. S., Kim, H., et al. (1996). The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc. Natl. Acad. Sci. U. S. A. 93, 8374–8378. doi:10.1073/pnas.93.16.8374
Beales, I. L. P., and Ogunwobi, O. O. (2021). Leptin activates Akt in oesophageal cancer cells via multiple atorvastatin-sensitive small GTPases. Mol. Cell. Biochem. 476, 2307–2316. doi:10.1007/s11010-021-04067-8
Beenken, A., and Mohammadi, M. (2009). The FGF family: Biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 8, 235–253. doi:10.1038/nrd2792
Bernlohr, D. A., Angus, C. W., Lane, M. D., Bolanowski, M. A., and Kelly, T. J. (1984). Expression of specific mRNAs during adipose differentiation: Identification of an mRNA encoding a homologue of myelin P2 protein. Proc. Natl. Acad. Sci. U. S. A. 81, 5468–5472. doi:10.1073/pnas.81.17.5468
Bienkiewicz, J., Romanowicz, H., Malinowski, A., and Smolarz, B. (2017). Association of Single Nucleotide Polymorphism -2548 G/A (rs12112075) of leptin gene with endometrial cancer and uterine leiomyomas. Eur. J. Obstet. Gynecol. Reprod. Biol. 218, 113–118. doi:10.1016/j.ejogrb.2017.09.022
Bienkiewicz, J., Romanowicz, H., Wilczynski, M., Jablonski, G., Stepowicz, A., Oblekowska, A., et al. (2021). Association of Single Nucleotide Polymorphism LEP-R c.668A>G (p.Gln223Arg, rs1137101) of leptin receptor gene with endometrial cancer. BMC Cancer 21, 925. doi:10.1186/s12885-021-08620-y
Biondo, L. A., Teixeira, A. A. S., Ferreira K, O. S., and Neto, J. C. R. (2020). Pharmacological strategies for insulin sensitivity in obesity and cancer: Thiazolidinediones and metformin. Curr. Pharm. Des. 26, 932–945. doi:10.2174/1381612826666200122124116
Bogusiewicz, M., Semczuk, A., Gogacz, M., Skomra, D., Jakowicki, J. A., and Rechberger, T. (2006). Lack of correlation between leptin receptor expression and PI3-K/Akt signaling pathway proteins immunostaining in endometrioid-type endometrial carcinomas. Cancer Lett. 238, 61–68. doi:10.1016/j.canlet.2005.06.028
Bohlouli, S., Khazaei, M., Teshfam, M., and Hassanpour, H. (2013). Adiponectin effect on the viability of human endometrial stromal cells and mRNA expression of adiponectin receptors. Int. J. Fertil. Steril. 7, 43–48.
Boron, D., Nowakowski, R., Grabarek, B. O., Zmarzly, N., and Oplawski, M. (2021). Expression pattern of leptin and its receptors in endometrioid endometrial cancer. J. Clin. Med. 10, 2787. doi:10.3390/jcm10132787
Boutas, I., Kontogeorgi, A., Dimitrakakis, C., and Kalantaridou, S. N. (2021). The expression of galectin-3 in endometrial cancer: A systematic review of the literature. Mol. Biol. Rep. 48, 5699–5705. doi:10.1007/s11033-021-06536-1
Brustmann, H., Riss, D., and Naude, S. (2003). Galectin-3 expression in normal, hyperplastic, and neoplastic endometrial tissues. Pathol. Res. Pract. 199, 151–158. doi:10.1078/0344-0338-00368
Caamano, J., and Hunter, C. A. (2002). NF-kappaB family of transcription factors: Central regulators of innate and adaptive immune functions. Clin. Microbiol. Rev. 15, 414–429. doi:10.1128/cmr.15.3.414-429.2002
Cai, Z. F., Deng, L., Wang, M. M., Zhang, J. Q., and Li, L. (2018). [Effect of AMPK/mTOR/S6K1 pathways and the insulin-sensitizing effect for adiponectin in endometrial cancer cells]. Zhonghua Fu Chan Ke Za Zhi 53, 554–560. doi:10.3760/cma.j.issn.0529-567x.2018.08.008
Carino, C., Olawaiye, A. B., Cherfils, S., Serikawa, T., Lynch, M. P., Rueda, B. R., et al. (2008). Leptin regulation of proangiogenic molecules in benign and cancerous endometrial cells. Int. J. Cancer 123, 2782–2790. doi:10.1002/ijc.23887
Catalano, S., Giordano, C., Rizza, P., Gu, G., Barone, I., Bonofiglio, D., et al. (2009). Evidence that leptin through STAT and CREB signaling enhances cyclin D1 expression and promotes human endometrial cancer proliferation. J. Cell. Physiol. 218, 490–500. doi:10.1002/jcp.21622
Chen, J. Y., and Liao, Q. P. (2009). [Expression and function of cyclooxygenase-2 in endometrial carcinoma]. Beijing Da Xue Xue Bao Yi Xue Ban. 41, 657–663.
Chen, X., Xiang, Y. B., Long, J. R., Cai, H., Cai, Q., Cheng, J., et al. (2012). Genetic polymorphisms in obesity-related genes and endometrial cancer risk. Cancer 118, 3356–3364. doi:10.1002/cncr.26552
Chetry, M., Thapa, S., Hu, X., Song, Y., Zhang, J., Zhu, H., et al. (2018). The role of galectins in tumor progression, treatment and prognosis of gynecological cancers. J. Cancer 9, 4742–4755. doi:10.7150/jca.23628
Chopra, V., Dinh, T. V., and Hannigan, E. V. (1997). Serum levels of interleukins, growth factors and angiogenin in patients with endometrial cancer. J. Cancer Res. Clin. Oncol. 123, 167–172. doi:10.1007/BF01214669
Chou, F. C., Chen, H. Y., Kuo, C. C., and Sytwu, H. K. (2018). Role of galectins in tumors and in clinical immunotherapy. Int. J. Mol. Sci. 19, 430. doi:10.3390/ijms19020430
Cicekdal, M. B., Kazan, B. T., Tuna, B. G., Ozorhan, U., Ekici, I. D., Zhu, F., et al. (2020). Effects of two types of calorie restriction on methylation levels of adiponectin receptor 1 (AdipoR1) and leptin receptor overlapping transcript (leprot) in a MMTV-TGF-alpha breast cancer mouse model. Br. J. Nutr. 123, 1079. doi:10.1017/S0007114520000471
Ciortea, R., Susman, S., Malutan, A. M., Berceanu, C., Mocan-Hognogi, R. F., Bucuri, C. E., et al. (2018). Mesenchymal stem cells derived from adipose tissue and Ishikawa cells co-culture highlight the role of adiponectin in endometrial cancer pathogenesis. Rom. J. Morphol. Embryol. 59, 1165–1172.
Cong, L., Gasser, J., Zhao, J., Yang, B., Li, F., and Zhao, A. Z. (2007). Human adiponectin inhibits cell growth and induces apoptosis in human endometrial carcinoma cells, HEC-1-A and RL95 2. Endocr. Relat. Cancer 14, 713–720. doi:10.1677/ERC-07-0065
Cust, A. E., Kaaks, R., Friedenreich, C., Bonnet, F., Laville, M., Lukanova, A., et al. (2007). Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J. Clin. Endocrinol. Metab. 92, 255–263. doi:10.1210/jc.2006-1371
Cymbaluk, A., Chudecka-Glaz, A., and Rzepka-Gorska, I. (2008). Leptin levels in serum depending on Body Mass Index in patients with endometrial hyperplasia and cancer. Eur. J. Obstet. Gynecol. Reprod. Biol. 136, 74–77. doi:10.1016/j.ejogrb.2006.08.012
Cymbaluk-Ploska, A., Chudecka-Glaz, A., Pius-Sadowska, E., Sompolska-Rzechula, A., Machalinski, B., and Menkiszak, J. (2018). Circulating serum level of visfatin in patients with endometrial cancer. Biomed. Res. Int. 2018, 8576179. doi:10.1155/2018/8576179
Cymbaluk-Ploska, A., Gargulinska, P., Chudecka-Glaz, A., Kwiatkowski, S., Pius-Sadowska, E., and Machalinski, B. (2020). The suitability of FGF21 and FGF23 as new biomarkers in endometrial cancer patients. Diagnostics 10, 414. doi:10.3390/diagnostics10060414
Cymbaluk-Ploska, A., Gargulinska, P., Kwiatkowski, S., Pius-Sadowska, E., and Machalinski, B. (2020). Could galectin 3 Be a good prognostic factor in endometrial cancer?. Diagnostics, 10, 635. doi:10.3390/diagnostics10090635
Dabrus, D., Kielbasinski, R., Grabarek, B. O., and Boron, D. (2020). Evaluation of the impact of cisplatin on variances in the expression pattern of leptin-related genes in endometrial cancer cells. Int. J. Mol. Sci. 21, 4135. doi:10.3390/ijms21114135
Daley-Brown, D., Harbuzariu, A., Kurian, A. A., Oprea-Ilies, G., and Gonzalez-Perez, R. R. (2019). Leptin-induced Notch and IL-1 signaling crosstalk in endometrial adenocarcinoma is associated with invasiveness and chemoresistance. World J. Clin. Oncol. 10, 222–233. doi:10.5306/wjco.v10.i6.222
Daley-Brown, D., Oprea-Iles, G., Vann, K. T., Lanier, V., Lee, R., Candelaria, P. V., et al. (2017). Type II endometrial cancer overexpresses NILCO: A preliminary evaluation. Dis. Markers 2017, 8248175. doi:10.1155/2017/8248175
Dallal, C. M., Brinton, L. A., Bauer, D. C., Buist, D. S., Cauley, J. A., Hue, T. F., et al. (2013). Obesity-related hormones and endometrial cancer among postmenopausal women: A nested case-control study within the B∼FIT cohort. Endocr. Relat. Cancer 20, 151–160. doi:10.1530/ERC-12-0229
Ding, S., Madu, C. O., and Lu, Y. (2020). The impact of hormonal imbalances associated with obesity on the incidence of endometrial cancer in postmenopausal women. J. Cancer 11, 5456–5465. doi:10.7150/jca.47580
Dirat, B., Bochet, L., Dabek, M., Daviaud, D., Dauvillier, S., Majed, B., et al. (2011). Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 71, 2455–2465. doi:10.1158/0008-5472.CAN-10-3323
Ege, C. B., Akbulut, M., Zekioglu, O., and Ozdemir, N. (2011). Investigation of galectin-3 and heparanase in endometrioid and serous carcinomas of the endometrium and correlation with known predictors of survival. Arch. Gynecol. Obstet. 284, 1231–1239. doi:10.1007/s00404-010-1766-9
Ellis, P. E., Barron, G. A., and Bermano, G. (2020). Adipocytokines and their relationship to endometrial cancer risk: A systematic review and meta-analysis. Gynecol. Oncol. 158, 507–516. doi:10.1016/j.ygyno.2020.05.033
Erdogan, S., Sezer, S., Baser, E., Gun-Eryilmaz, O., Gungor, T., Uysal, S., et al. (2013). Evaluating vaspin and adiponectin in postmenopausal women with endometrial cancer. Endocr. Relat. Cancer 20, 669–675. doi:10.1530/ERC-13-0280
Fortuna-Costa, A., Gomes, A. M., Kozlowski, E. O., Stelling, M. P., and Pavao, M. S. (2014). Extracellular galectin-3 in tumor progression and metastasis. Front. Oncol. 4, 138. doi:10.3389/fonc.2014.00138
Fredstorp-Lidebring, M., Bendahl, P. O., Brunner, N., Casslen, B., Hogberg, T., Langstrom-Einarsson, E., et al. (2001). Urokinase plasminogen activator and its inhibitor, PAI-1, in association with progression-free survival in early stage endometrial cancer. Eur. J. Cancer 37, 2339–2348. doi:10.1016/s0959-8049(01)00306-9
Fujimoto, J., Hori, M., Ichigo, S., and Tamaya, T. (1996). Sex steroids regulate the expression of plasminogen activator inhibitor-1 (PAI-1) and its mRNA in uterine endometrial cancer cell line Ishikawa. J. Steroid Biochem. Mol. Biol. 59, 1–8. doi:10.1016/s0960-0760(96)00084-2
Fujimoto, J., and Sato, E. (2011). Sex steroids in uterine endometrial cancers. Horm. Mol. Biol. Clin. Investig. 5, 143–151. doi:10.1515/HMBCI.2010.049
Fukuhara, A., Matsuda, M., Nishizawa, M., Segawa, K., Tanaka, M., Kishimoto, K., et al. (2005). Visfatin: A protein secreted by visceral fat that mimics the effects of insulin. Science 307, 426–430. doi:10.1126/science.1097243
Gao, J., Tian, J., Lv, Y., Shi, F., Kong, F., Shi, H., et al. (2009). Leptin induces functional activation of cyclooxygenase-2 through JAK2/STAT3, MAPK/ERK, and PI3K/AKT pathways in human endometrial cancer cells. Cancer Sci. 100, 389–395. doi:10.1111/j.1349-7006.2008.01053.x
Garikapati, K. K., Ammu, Vvvrk, Krishnamurthy, P. T., Chintamaneni, P. K., and Pindiprolu., S. (2019). Possible role of thiazolidinedione in the management of type-II endometrial cancer. Med. Hypotheses 126, 78–81. doi:10.1016/j.mehy.2019.03.014
Gharpure, K. M., Pradeep, S., Sans, M., Rupaimoole, R., Ivan, C., Wu, S. Y., et al. (2018). FABP4 as a key determinant of metastatic potential of ovarian cancer. Nat. Commun. 9, 2923. doi:10.1038/s41467-018-04987-y
Goel, H. L., and Mercurio, A. M. (2013). VEGF targets the tumour cell. Nat. Rev. Cancer 13, 871–882. doi:10.1038/nrc3627
Goldstein, B. J., and Scalia, R. (2004). Adiponectin: A novel adipokine linking adipocytes and vascular function. J. Clin. Endocrinol. Metab. 89, 2563–2568. doi:10.1210/jc.2004-0518
Gong, C., Liu, Y., Xiao, W., Yin, J., Wang, D. H., and Sheng, H. (2007). The role of ERK1/2 in leptin promoting the proliferation of human endometrial cancer cell line Ishikawa. Ai Zheng 26, 1211–1214.
Gotte, M., Mohr, C., Koo, C. Y., Stock, C., Vaske, A. K., Viola, M., et al. (2010). miR-145-dependent targeting of junctional adhesion molecule A and modulation of fascin expression are associated with reduced breast cancer cell motility and invasiveness. Oncogene 29, 6569–6580. doi:10.1038/onc.2010.386
Grosfeld, A., Andre, J., Hauguel-De Mouzon, S., Berra, E., Pouyssegur, J., and Guerre-Millo, M. (2002). Hypoxia-inducible factor 1 transactivates the human leptin gene promoter. J. Biol. Chem. 277, 42953–42957. doi:10.1074/jbc.M206775200
Hefetz-Sela, S., and Scherer, P. E. (2013). Adipocytes: Impact on tumor growth and potential sites for therapeutic intervention. Pharmacol. Ther. 138, 197–210. doi:10.1016/j.pharmthera.2013.01.008
Hegyi, K., Fulop, K., Kovacs, K., Toth, S., and Falus, A. (2004). Leptin-induced signal transduction pathways. Cell Biol. Int. 28, 159–169. doi:10.1016/j.cellbi.2003.12.003
Henderson, N. C., and Sethi, T. (2009). The regulation of inflammation by galectin-3. Immunol. Rev. 230, 160–171. doi:10.1111/j.1600-065X.2009.00794.x
Hernandez, J. D., Nguyen, J. T., He, J., Wang, W., Ardman, B., Green, J. M., et al. (2006). Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J. Immunol. 177, 5328–5336. doi:10.4049/jimmunol.177.8.5328
Hoesel, B., and Schmid, J. A. (2013). The complexity of NF-κB signaling in inflammation and cancer. Mol. Cancer 12, 86. doi:10.1186/1476-4598-12-86
Horowitz, N. S., and Wright, A. A. (2015). Impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol. Oncol. 138, 201–206. doi:10.1016/j.ygyno.2015.04.002
Hotamisligil, G. S., and Bernlohr, D. A. (2015). Metabolic functions of FABPs-mechanisms and therapeutic implications. Nat. Rev. Endocrinol. 11, 592–605. doi:10.1038/nrendo.2015.122
Hu, E., Liang, P., and Spiegelman, B. M. (1996). AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 271, 10697–10703. doi:10.1074/jbc.271.18.10697
Huang, Q., Peng, L., Sun, Y., Huang, J., Han, T., Li, Y., et al. (2021). miR-593-3p promotes proliferation and invasion in prostate cancer cells by targeting ADIPOR1. Onco. Targets. Ther. 14, 3729–3737. doi:10.2147/OTT.S310198
Ito, R., Narita, S., Huang, M., Nara, T., Numakura, K., Takayama, K., et al. (2017). The impact of obesity and adiponectin signaling in patients with renal cell carcinoma: A potential mechanism for the "obesity paradox". PLoS One 12, e0171615. doi:10.1371/journal.pone.0171615
Jalving, M., Gietema, J. A., Lefrandt, J. D., de Jong, S., Reyners, A. K., Gans, R. O., et al. (2010). Metformin: Taking away the candy for cancer? Eur. J. Cancer 46, 2369–2380. doi:10.1016/j.ejca.2010.06.012
Jin, J. H., Kim, H. J., Kim, C. Y., Kim, Y. H., Ju, W., and Kim, S. C. (2016). Association of plasma adiponectin and leptin levels with the development and progression of ovarian cancer. Obstet. Gynecol. Sci. 59, 279–285. doi:10.5468/ogs.2016.59.4.279
Jin, J., Zhang, Z., Zhang, S., Chen, X., Chen, Z., Hu, P., et al. (2018). Fatty acid binding protein 4 promotes epithelial-mesenchymal transition in cervical squamous cell carcinoma through AKT/GSK3β/Snail signaling pathway. Mol. Cell. Endocrinol. 461, 155–164. doi:10.1016/j.mce.2017.09.005
Kadowaki, T., and Yamauchi, T. (2005). Adiponectin and adiponectin receptors. Endocr. Rev. 26, 439–451. doi:10.1210/er.2005-0005
Kaklamani, V., Yi, N., Zhang, K., Sadim, M., Offit, K., Oddoux, C., et al. (2011). Polymorphisms of ADIPOQ and ADIPOR1 and prostate cancer risk. Metabolism. 60, 1234–1243. doi:10.1016/j.metabol.2011.01.005
Koda, M., Sulkowska, M., Wincewicz, A., Kanczuga-Koda, L., Musiatowicz, B., Szymanska, M., et al. (2007). Expression of leptin, leptin receptor, and hypoxia-inducible factor 1 alpha in human endometrial cancer. Ann. N. Y. Acad. Sci. 1095, 90–98. doi:10.1196/annals.1397.013
Koh, K. K., Han, S. H., and Quon, M. J. (2005). Inflammatory markers and the metabolic syndrome: Insights from therapeutic interventions. J. Am. Coll. Cardiol. 46, 1978–1985. doi:10.1016/j.jacc.2005.06.082
Kohler, U., Hiller, K., Martin, R., Langanke, D., Naumann, G., Bilek, K., et al. (1997). Tumor-associated proteolytic factors uPA and PAI-1 in endometrial carcinoma. Gynecol. Oncol. 66, 268–274. doi:10.1006/gyno.1997.4751
Konieczna, A., Novakova, V., Medalova, J., Erceg, S., and Klabusay, M. (2015). Thiazolidinediones regulate the level of ABC transporters expression on lung cancer cells. Klin. Onkol. 28, 431–438. doi:10.14735/amko2015431
Lanier, V., Gillespie, C., Leffers, M., Daley-Brown, D., Milner, J., Lipsey, C., et al. (2016). Leptin-induced transphosphorylation of vascular endothelial growth factor receptor increases Notch and stimulates endothelial cell angiogenic transformation. Int. J. Biochem. Cell Biol. 79, 139–150. doi:10.1016/j.biocel.2016.08.023
Larsson, S. C., Spyrou, N., and Mantzoros, C. S. (2022). Body fatness associations with cancer: Evidence from recent epidemiological studies and future directions. Metabolism. 137, 155326. doi:10.1016/j.metabol.2022.155326
Lee, C. H., Lui, D. T. W., Cheung, C. Y. Y., Fong, C. H. Y., Yuen, M. M. A., Chow, W. S., et al. (2020). Response to letter to the editor: "Higher circulating adiponectin concentrations predict incident cancer in type 2 diabetes - the adiponectin paradox". J. Clin. Endocrinol. Metab. 105, dgaa386. doi:10.1210/clinem/dgaa386
Lei, C., Zhang, W., Sun, X., Du, G., Wang, L., and Liu, Y. (2007). Effects of galectin-3 inhibition on endometrial cell cycle and adhesion. Front. Med. China 1, 390–397. doi:10.1007/s11684-007-0076-5
Li, L., and Kalantar-Zadeh, K. (2013). Obesity that makes kidney cancer more likely but helps fight it more strongly. J. Natl. Cancer Inst. 105, 1848–1849. doi:10.1093/jnci/djt348
Li, S., Wang, Z., Huang, J., Fan, J., Du, H., Liu, L., et al. (2017). Systematic review of prognostic roles of body mass index for patients undergoing lung cancer surgery: Does the obesity paradox really exist? Eur. J. Cardiothorac. Surg. 51, 817–828. doi:10.1093/ejcts/ezw386
Lin, L. L., Kost, E. R., Lin, C. L., Valente, P., Wang, C. M., Kolonin, M. G., et al. (2020). PAI-1-Dependent inactivation of SMAD4-modulated junction and adhesion complex in obese endometrial cancer. Cell Rep. 33, 108253. doi:10.1016/j.celrep.2020.108253
Lin, T., Zhao, X., and Kong, W. M. (2015). Association between adiponectin levels and endometrial carcinoma risk: Evidence from a dose-response meta-analysis. BMJ Open 5, e008541. doi:10.1136/bmjopen-2015-008541
Linkov, F., Goughnour, S. L., Adambekov, S., Lokshin, A., Kelley, J. L., Sukumvanich, P., et al. (2018). Inflammatory biomarker in adipose stem cells of women with endometrial cancer. Biomark. Med. 12, 945–952. doi:10.2217/bmm-2017-0355
Linkov, F., Maxwell, G. L., Felix, A. S., Lin, Y., Lenzner, D., Bovbjerg, D. H., et al. (2012). Longitudinal evaluation of cancer-associated biomarkers before and after weight loss in RENEW study participants: Implications for cancer risk reduction. Gynecol. Oncol. 125, 114–119. doi:10.1016/j.ygyno.2011.12.439
Lipsey, C. C., Harbuzariu, A., Daley-Brown, D., and Gonzalez-Perez, R. R. (2016). Oncogenic role of leptin and Notch interleukin-1 leptin crosstalk outcome in cancer. World J. Methodol. 6, 43–55. doi:10.5662/wjm.v6.i1.43
Liu, F. T., Patterson, R. J., and Wang, J. L. (2002). Intracellular functions of galectins. Biochim. Biophys. Acta 1572, 263–273. doi:10.1016/s0304-4165(02)00313-6
Liu, L., Wang, L., Zheng, J., and Tang, G. (2013). Leptin promotes human endometrial carcinoma cell proliferation by enhancing aromatase (P450arom) expression and estradiol formation. Eur. J. Obstet. Gynecol. Reprod. Biol. 170, 198–201. doi:10.1016/j.ejogrb.2013.04.004
Ma, X., Hui, Y., Lin, L., Wu, Y., Zhang, X., and Liu, P. (2015). Clinical significance of COX-2, GLUT-1 and VEGF expressions in endometrial cancer tissues. Pak. J. Med. Sci. 31, 280–284. doi:10.12669/pjms.312.6604
Ma, Y., Liu, Z., Zhang, Y., and Lu, B. (2013). Serum leptin, adiponectin and endometrial cancer risk in Chinese women. J. Gynecol. Oncol. 24, 336–341. doi:10.3802/jgo.2013.24.4.336
Madeddu, C., Sanna, E., Gramignano, G., Tanca, L., Cherchi, M. C., Mola, B., et al. (2022). Correlation of leptin, proinflammatory cytokines and oxidative stress with tumor size and disease stage of endometrioid (type I) endometrial cancer and review of the underlying mechanisms. Cancers (Basel) 14, 268. doi:10.3390/cancers14020268
Maeda, K., Okubo, K., Shimomura, I., Funahashi, T., Matsuzawa, Y., and Matsubara, K. (1996). cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1). Biochem. Biophys. Res. Commun. 221, 286–289. doi:10.1006/bbrc.1996.0587
Mantzos, F., Vanakara, P., Samara, S., Wozniak, G., Kollia, P., Messinis, I., et al. (2011). Leptin receptor expression in neoplastic and normal ovarian and endometrial tissue. Eur. J. Gynaecol. Oncol. 32, 84–86.
Markl, B., Hardt, J., Franz, S., Schaller, T., Schenkirsch, G., Kriening, B., et al. (2017). Tumor budding, uPA, and PAI-1 in colorectal cancer: Update of a prospective study. Gastroenterol. Res. Pract. 2017, 6504960. doi:10.1155/2017/6504960
May, M. J., and Ghosh, S. (1997). Rel/NF-kappa B and I kappa B proteins: An overview. Semin. Cancer Biol. 8, 63–73. doi:10.1006/scbi.1997.0057
Mihu, D., Ciortea, R., and Mihu, C. M. (2013). Abdominal adiposity through adipocyte secretion products, a risk factor for endometrial cancer. Gynecol. Endocrinol. 29, 448–451. doi:10.3109/09513590.2012.752452
Mocino-Rodriguez, M. D., Santillan-Benitez, J. G., Dozal-Dominguez, D. S., Hernandez-Navarro, M. D., Flores-Merino, M. V., Sandoval-Cabrera, A., et al. (2017). Expression of AdipoR1 and AdipoR2 receptors as leptin-breast cancer regulation mechanisms. Dis. Markers 2017, 4862016. doi:10.1155/2017/4862016
Moon, H. S., Chamberland, J. P., Aronis, K., Tseleni-Balafouta, S., and Mantzoros, C. S. (2011). Direct role of adiponectin and adiponectin receptors in endometrial cancer: In vitro and ex vivo studies in humans. Mol. Cancer Ther. 10, 2234–2243. doi:10.1158/1535-7163.MCT-11-0545
Morice, P., Leary, A., Creutzberg, C., Abu-Rustum, N., and Darai, E. (2016). Endometrial cancer. Lancet 387, 1094–1108. doi:10.1016/S0140-6736(15)00130-0
Moukarzel, L. A., Ferrando, L., Stylianou, A., Lobaugh, S., Wu, M., Nobre, S. P., et al. (2022). Impact of obesity and white adipose tissue inflammation on the omental microenvironment in endometrial cancer. Cancer 128, 3297–3309. doi:10.1002/cncr.34356
Mu, N., Zhu, Y., Wang, Y., Zhang, H., and Xue, F. (2012). Insulin resistance: A significant risk factor of endometrial cancer. Gynecol. Oncol. 125, 751–757. doi:10.1016/j.ygyno.2012.03.032
Murphy, K. A., James, B. R., Sjaastad, F. V., Kucaba, T. A., Kim, H., Brincks, E. L., et al. (2018). Cutting edge: Elevated leptin during diet-induced obesity reduces the efficacy of tumor immunotherapy. J. Immunol. 201, 1837–1841. doi:10.4049/jimmunol.1701738
Mylonas, I., Mayr, D., Walzel, H., Shabani, N., Dian, D., Kuhn, C., et al. (2007). Mucin 1, thomsen-friedenreich expression and galectin-1 binding in endometrioid adenocarcinoma: An immunohistochemical analysis. Anticancer Res. 27, 1975–1980.
Nakano, Y., Tobe, T., Choi-Miura, N. H., Mazda, T., and Tomita, M. (1996). Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J. Biochem. 120, 803–812. doi:10.1093/oxfordjournals.jbchem.a021483
Nergiz Avcioglu, S., Altinkaya, S. O., Kucuk, M., Yuksel, H., Omurlu, I. K., and Yanik, S. (2015). Visfatin concentrations in patients with endometrial cancer. Gynecol. Endocrinol. 31, 202–207. doi:10.3109/09513590.2014.975687
Noble, L. S., Simpson, E. R., Johns, A., and Bulun, S. E. (1996). Aromatase expression in endometriosis. J. Clin. Endocrinol. Metab. 81, 174–179. doi:10.1210/jcem.81.1.8550748
Oaknin, A., Bosse, T. J., Creutzberg, C. L., Giornelli, G., Harter, P., Joly, F., et al. Esmo Guidelines Committee (2022). Electronic address:Y2xpbmljYWxndWlkZWxpbmVzQGVzbW8ub3JnRW5kb21ldHJpYWw=cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 33, 860–877. doi:10.1016/j.annonc.2022.05.009
Oplawski, M., Dziobek, K., Zmarzly, N., Grabarek, B. O., Kielbasinski, R., Kieszkowski, P., et al. (2020). Variances in the level of COX-2 and iNOS in different grades of endometrial cancer. Curr. Pharm. Biotechnol. 21, 52–59. doi:10.2174/1389201020666190918104105
Osmak, M., Babic, D., Abramic, M., Milicic, D., Vrhovec, I., and Skrk, J. (2001). Plasminogen activator inhibitor type 2: Potential prognostic factor for endometrial carcinomas. Neoplasma 48, 462–467.
Ozgor, B. Y., Iyibozkurt, C., Bastu, E., Berkman, S., Yalcin, O., Cakmakoglu, B., et al. (2019). Investigation of resistin 420 and 62 gene polymorphism in patients with endometrial cancer. Taiwan. J. Obstet. Gynecol. 58, 164–167. doi:10.1016/j.tjog.2018.11.030
Pallares, J., Martinez-Guitarte, J. L., Dolcet, X., Llobet, D., Rue, M., Palacios, J., et al. (2004). Abnormalities in the NF-kappaB family and related proteins in endometrial carcinoma. J. Pathol. 204, 569–577. doi:10.1002/path.1666
Peng-Fei, H., Ru Na, A., Hui, C., Hong-Yu, W., and Jin-Shan, C. (2021). Activation of alpha7 nicotinic acetylcholine receptor protects bovine endometrial tissue against LPS-induced inflammatory injury via JAK2/STAT3 pathway and COX-2 derived prostaglandin E(2). Eur. J. Pharmacol. 900, 174067. doi:10.1016/j.ejphar.2021.174067
Petridou, E., Belechri, M., Dessypris, N., Koukoulomatis, P., Diakomanolis, E., Spanos, E., et al. (2002). Leptin and body mass index in relation to endometrial cancer risk. Ann. Nutr. Metab. 46, 147–151. doi:10.1159/000063081
Petridou, E., Mantzoros, C., Dessypris, N., Koukoulomatis, P., Addy, C., Voulgaris, Z., et al. (2003). Plasma adiponectin concentrations in relation to endometrial cancer: A case-control study in Greece. J. Clin. Endocrinol. Metab. 88, 993–997. doi:10.1210/jc.2002-021209
Rajput, P. K., Sharma, J. R., and Yadav, U. C. S. (2022). Cellular and molecular insights into the roles of visfatin in breast cancer cells plasticity programs. Life Sci. 304, 120706. doi:10.1016/j.lfs.2022.120706
Ray, I., Meira, L. B., Michael, A., and Ellis, P. E. (2022). Adipocytokines and disease progression in endometrial cancer: A systematic review. Cancer Metastasis Rev. 41, 211–242. doi:10.1007/s10555-021-10002-6
Rodriguez-Barbero, A., Dorado, F., Velasco, S., Pandiella, A., Banas, B., and Lopez-Novoa, J. M. (2006). TGF-beta1 induces COX-2 expression and PGE2 synthesis through MAPK and PI3K pathways in human mesangial cells. Kidney Int. 70, 901–909. doi:10.1038/sj.ki.5001626
Rzepka-Gorska, I., Bedner, R., Cymbaluk-Ploska, A., and Chudecka-Glaz, A. (2008). Serum adiponectin in relation to endometrial cancer and endometrial hyperplasia with atypia in obese women. Eur. J. Gynaecol. Oncol. 29, 594–597.
Sakers, A., De Siqueira, M. K., Seale, P., and Villanueva, C. J. (2022). Adipose-tissue plasticity in health and disease. Cell 185, 419–446. doi:10.1016/j.cell.2021.12.016
Salmani Javan, E., Lotfi, F., Jafari-Gharabaghlou, D., Mousazadeh, H., Dadashpour, M., and Zarghami, N. (2022). Development of a magnetic nanostructure for Co-delivery of metformin and silibinin on growth of lung cancer cells: Possible action through leptin gene and its receptor regulation. Asian pac. J. Cancer Prev. 23, 519–527. doi:10.31557/APJCP.2022.23.2.519
Scherer, P. E., Williams, S., Fogliano, M., Baldini, G., and Lodish, H. F. (1995). A novel serum protein similar to C1q, produced exclusively in adipocytes. J. Biol. Chem. 270, 26746–26749. doi:10.1074/jbc.270.45.26746
Schmitt, M., Mengele, K., Napieralski, R., Magdolen, V., Reuning, U., Gkazepis, A., et al. (2010). Clinical utility of level-of-evidence-1 disease forecast cancer biomarkers uPA and its inhibitor PAI-1. Expert Rev. Mol. diagn. 10, 1051–1067. doi:10.1586/erm.10.71
Sen, R., and Baltimore, D. (1986). Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 46, 705–716. doi:10.1016/0092-8674(86)90346-6
Sharma, D., Saxena, N. K., Vertino, P. M., and Anania, F. A. (2006). Leptin promotes the proliferative response and invasiveness in human endometrial cancer cells by activating multiple signal-transduction pathways. Endocr. Relat. Cancer 13, 629–640. doi:10.1677/erc.1.01169
Shetty, A., Venkatesh, T., Tsutsumi, R., and Suresh, P. S. (2020). Gene expression changes and promoter methylation with the combined effects of estradiol and leptin in uterine tissue of the ovariectomized mice model of menopause. Mol. Biol. Rep. 47, 151–168. doi:10.1007/s11033-019-05116-8
Soliman, P. T., Wu, D., Tortolero-Luna, G., Schmeler, K. M., Slomovitz, B. M., Bray, M. S., et al. (2006). Association between adiponectin, insulin resistance, and endometrial cancer. Cancer 106, 2376–2381. doi:10.1002/cncr.21866
Steiner, E., Pollow, K., Hasenclever, D., Schormann, W., Hermes, M., Schmidt, M., et al. (2008). Role of urokinase-type plasminogen activator (uPA) and plasminogen activator inhibitor type 1 (PAI-1) for prognosis in endometrial cancer. Gynecol. Oncol. 108, 569–576. doi:10.1016/j.ygyno.2007.11.025
Stewart, C. J., Brennan, B. A., Leung, Y. C., and Little, L. (2009). MELF pattern invasion in endometrial carcinoma: Association with low grade, myoinvasive endometrioid tumours, focal mucinous differentiation and vascular invasion. Pathology 41, 454–459. doi:10.1080/00313020903041135
Su, C. K., Yeh, K. T., Yeh, C. B., Wang, P. H., Ho, E. S., Chou, M. C., et al. (2011). Genetic polymorphism of the plasminogen activator inhibitor-1 is associated with an increased risk of endometrial cancer. J. Surg. Oncol. 104, 755–759. doi:10.1002/jso.22035
Sun, K., Wernstedt Asterholm, I., Kusminski, C. M., Bueno, A. C., Wang, Z. V., Pollard, J. W., et al. (2012). Dichotomous effects of VEGF-A on adipose tissue dysfunction. Proc. Natl. Acad. Sci. U. S. A. 109, 5874–5879. doi:10.1073/pnas.1200447109
Sun, K. X., Zheng, R. S., Zuo, J., Zhang, S. W., Zeng, H. M., Wang, S. M., et al. (2022). [The incidence and mortality of endometrial cancer in China, 2015. Zhonghua Yi Xue Za Zhi 102, 1987–1992. doi:10.3760/cma.j.cn112137-20211029-02403
Sun, X. F., and Dai, S. Y. (2020). The significance of galectin-1 and galectin-9 expression in endometrial carcinoma. Gynecol. Obstet. Invest. 85, 34–40. doi:10.1159/000502787
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca. Cancer J. Clin. 71, 209–249. doi:10.3322/caac.21660
Takemura, Y., Osuga, Y., Yamauchi, T., Kobayashi, M., Harada, M., Hirata, T., et al. (2006). Expression of adiponectin receptors and its possible implication in the human endometrium. Endocrinology 147, 3203–3210. doi:10.1210/en.2005-1510
Tecimer, C., Doering, D. L., Goldsmith, L. J., Meyer, J. S., Abdulhay, G., and Wittliff, J. L. (2001). Clinical relevance of urokinase-type plasminogen activator, its receptor, and its inhibitor type 1 in endometrial cancer. Gynecol. Oncol. 80, 48–55. doi:10.1006/gyno.2000.6015
Tessitore, L., Vizio, B., Pesola, D., Cecchini, F., Mussa, A., Argiles, J. M., et al. (2004). Adipocyte expression and circulating levels of leptin increase in both gynaecological and breast cancer patients. Int. J. Oncol. 24, 1529–1535.
Thijssen, V. L., and Griffioen, A. W. (2014). Galectin-1 and -9 in angiogenesis: A sweet couple. Glycobiology 24, 915–920. doi:10.1093/glycob/cwu048
Tian, W., Zhang, H., Zhang, Y., Wang, Y., Zhang, Y., Xue, F., et al. (2020). High level of visfatin and the activation of Akt and ERK1/2 signaling pathways are associated with endometrium malignant transformation in polycystic ovary syndrome. Gynecol. Endocrinol. 36, 156–161. doi:10.1080/09513590.2019.1650340
Tian, W., Zhu, Y., Wang, Y., Teng, F., Zhang, H., Liu, G., et al. (2013). Visfatin, a potential biomarker and prognostic factor for endometrial cancer. Gynecol. Oncol. 129, 505–512. doi:10.1016/j.ygyno.2013.02.022
Trayhurn, P., and Wood, I. S. (2004). Adipokines: Inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 92, 347–355. doi:10.1079/bjn20041213
Tsujii, M., Kawano, S., Tsuji, S., Sawaoka, H., Hori, M., and DuBois, R. N. (1998). Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell 93, 705–716. doi:10.1016/s0092-8674(00)81433-6
van den Brule, F. A., Buicu, C., Berchuck, A., Bast, R. C., Deprez, M., Liu, F. T., et al. (1996). Expression of the 67-kD laminin receptor, galectin-1, and galectin-3 in advanced human uterine adenocarcinoma. Hum. Pathol. 27, 1185–1191. doi:10.1016/s0046-8177(96)90313-5
Van Le, L., Haskill, S., Jaffe, G. J., and Fowler, W. C. (1991). Expression of interleukin-1 and interleukin-1 receptor antagonists in endometrial cancer. Gynecol. Oncol. 42, 161–164. doi:10.1016/0090-8258(91)90338-6
Waldman, A. D., Fritz, J. M., and Lenardo, M. J. (2020). A guide to cancer immunotherapy: from T cell basic science to clinical practice. Nat. Rev. Immunol. 20, 651–668. doi:10.1038/s41577-020-0306-5
Wang, J., Peng, Y., Guo, H., and Li, C. (2021). PAI-1 polymorphisms have significant associations with cancer risk, especially feminine cancer. Technol. Cancer Res. Treat. 20, 15330338211037813. doi:10.1177/15330338211037813
Wang, Y., Gao, C., Zhang, Y., Gao, J., Teng, F., Tian, W., et al. (2016). Visfatin stimulates endometrial cancer cell proliferation via activation of PI3K/Akt and MAPK/ERK1/2 signalling pathways. Gynecol. Oncol. 143, 168–178. doi:10.1016/j.ygyno.2016.07.109
Wang, Z., Gao, S., Sun, C., Li, J., Gao, W., and Yu, L. (2019). Clinical significance of serum adiponectin and visfatin levels in endometrial cancer. Int. J. Gynaecol. Obstet. 145, 34–39. doi:10.1002/ijgo.12772
Wincewicz, A., Koda, M., Sulkowska, M., Kanczuga-Koda, L., and Sulkowski, S. (2008). Comparison of STAT3 with HIF-1alpha, Ob and ObR expressions in human endometrioid adenocarcinomas. Tissue Cell 40, 405–410. doi:10.1016/j.tice.2008.04.004
Wu, X., Yan, Q., Zhang, Z., Du, G., and Wan, X. (2012). Acrp30 inhibits leptin-induced metastasis by downregulating the JAK/STAT3 pathway via AMPK activation in aggressive SPEC-2 endometrial cancer cells. Oncol. Rep. 27, 1488–1496. doi:10.3892/or.2012.1670
Wu, Z., Jeong, J. H., Ren, C., Yang, L., Ding, L., Li, F., et al. (2021). Fatty acid-binding protein 4 (FABP4) suppresses proliferation and migration of endometrial cancer cells via PI3K/akt pathway. Onco. Targets. Ther. 14, 3929–3942. doi:10.2147/OTT.S311792
Xu, X., Xie, Y., Lin, Y., Xu, X., Zhu, Y., Mao, Y., et al. (2012). PAI-1 promoter 4G/5G polymorphism (rs1799768) contributes to tumor susceptibility: Evidence from meta-analysis. Exp. Ther. Med. 4, 1127–1133. doi:10.3892/etm.2012.734
Yabushita, H., Iwasaki, K., Obayashi, Y., and Wakatsuki, A. (2014). Clinicopathological roles of adiponectin and leptin receptors in endometrial carcinoma. Oncol. Lett. 7, 1109–1117. doi:10.3892/ol.2014.1846
Yamauchi, N., Takazawa, Y., Maeda, D., Hibiya, T., Tanaka, M., Iwabu, M., et al. (2012). Expression levels of adiponectin receptors are decreased in human endometrial adenocarcinoma tissues. Int. J. Gynecol. Pathol. 31, 352–357. doi:10.1097/PGP.0b013e3182469583
Yamauchi, T., Kamon, J., Ito, Y., Tsuchida, A., Yokomizo, T., Kita, S., et al. (2003). Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 423, 762–769. doi:10.1038/nature01705
Yoon, J. K., Byeon, H. E., Ko, S. A., Park, B. N., An, Y. S., Lee, H. Y., et al. (2020). Cell cycle synchronisation using thiazolidinediones affects cellular glucose metabolism and enhances the therapeutic effect of 2-deoxyglucose in colon cancer. Sci. Rep. 10, 4713. doi:10.1038/s41598-020-61661-4
Yuan, S. S., Tsai, K. B., Chung, Y. F., Chan, T. F., Yeh, Y. T., Tsai, L. Y., et al. (2004). Aberrant expression and possible involvement of the leptin receptor in endometrial cancer. Gynecol. Oncol. 92, 769–775. doi:10.1016/j.ygyno.2003.11.043
Yunusova, N. V., Kondakova, I. V., Kolomiets, L. A., Afanasiev, S. G., Chernyshova, A. L., Shatokhina, O. V., et al. (2015). [Serum adipokines and their receptors in endometrial and colon cancer patients: Relationship with tumor invasion and metastasis]. Vopr. Onkol. 61, 619–623.
Zeng, F., Shi, J., Long, Y., Tian, H., Li, X., Zhao, A. Z., et al. (2015). Adiponectin and endometrial cancer: A systematic review and meta-analysis. Cell. Physiol. biochem. 36, 1670–1678. doi:10.1159/000430327
Zhang, L. Q., Heruth, D. P., and Ye, S. Q. (2011). Nicotinamide phosphoribosyltransferase in human diseases. J. Bioanal. Biomed. 3, 13–25. doi:10.4172/1948-593X.1000038
Zhang, L., Wen, K., Han, X., Liu, R., and Qu, Q. (2015). Adiponectin mediates antiproliferative and apoptotic responses in endometrial carcinoma by the AdipoRs/AMPK pathway. Gynecol. Oncol. 137, 311–320. doi:10.1016/j.ygyno.2015.02.012
Zhang, W., Ling, D., Tan, J., Zhang, J., and Li, L. (2013). Expression of urokinase plasminogen activator and plasminogen activator inhibitor type-1 in ovarian cancer and its clinical significance. Oncol. Rep. 29, 637–645. doi:10.3892/or.2012.2148
Zhang, Y., Proenca, R., Maffei, M., Barone, M., Leopold, L., and Friedman, J. M. (1994). Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432. doi:10.1038/372425a0
Zhao, Q., Li, J. Y., Zhang, J., Long, Y. X., Li, Y. J., Guo, X. D., et al. (2020). Role of visfatin in promoting proliferation and invasion of colorectal cancer cells by downregulating SDF-1/CXCR4-mediated miR-140-3p expression. Eur. Rev. Med. Pharmacol. Sci. 24, 5367–5377. doi:10.26355/eurrev_202005_21320
Zhou, X., Li, H., Chai, Y., and Liu, Z. (2015). Leptin inhibits the apoptosis of endometrial carcinoma cells through activation of the nuclear factor κB-inducing kinase/i?b kinase pathway. Int. J. Gynecol. Cancer 25, 770–778. doi:10.1097/IGC.0000000000000440
Zinovkin, D. A., Achinovich, S. L., Zubritskiy, M. G., Whatmore, J. L., and Pranjol, M. Z. I. (2019). High expression of galectin-1, VEGF and increased microvessel density are associated with MELF pattern in stage I-iii endometrioid endometrial adenocarcinoma. J. Pathol. Transl. Med. 53, 280–288. doi:10.4132/jptm.2019.05.13
Zinovkin, D. A., Pranjol, M. Z. I., Petrenyov, D. R., Nadyrov, E. A., and Savchenko, O. G. (2017). The potential roles of MELF-pattern, microvessel density, and VEGF expression in survival of patients with endometrioid endometrial carcinoma: A morphometrical and immunohistochemical analysis of 100 cases. J. Pathol. Transl. Med. 51, 456–462. doi:10.4132/jptm.2017.07.19
Keywords: endometrial cancer, signalling pathway, adipokines, inflammatory cytokines, angiogenic factors
Citation: Li R, Dong F, Zhang L, Ni X and Lin G (2022) Role of adipocytokines in endometrial cancer progression. Front. Pharmacol. 13:1090227. doi: 10.3389/fphar.2022.1090227
Received: 05 November 2022; Accepted: 28 November 2022;
Published: 12 December 2022.
Edited by:
Hai-long Piao, Dalian Institute of Chemical Physics (CAS), ChinaReviewed by:
Huai-Qiang Ju, Sun Yat-sen University, ChinaJunli Liu, Shanghai Jiao Tong University, China
Wei Yang, Southern Medical University, China
Copyright © 2022 Li, Dong, Zhang, Ni and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guozhi Lin, dHlmeWxnekAxNjMuY29t
 Ran Li
Ran Li Fang Dong1
Fang Dong1